Abstract
Background
Intraindividual variability (IIV) during cognitive task performance is a key behavioral index of attention and a consistent marker of attention-deficit/hyperactivity disorder. In adults, lower IIV has been associated with anticorrelation between the default mode network (DMN) and dorsal attention network (DAN)—thought to underlie effective allocation of attention. However, whether these behavioral and neural markers of attention are 1) associated with each other and 2) can predict future attention-related deficits has not been examined in a developmental, population-based cohort.
Methods
We examined relationships at the baseline visit between IIV on 3 cognitive tasks, DMN-DAN anticorrelation, and parent-reported attention problems using data from the Adolescent Brain Cognitive Development (ABCD) Study (N = 11,878 participants, ages 9 to 10 years, female = 47.8%). We also investigated whether behavioral and neural markers of attention at baseline predicted attention problems 1, 2, and 3 years later.
Results
At baseline, greater DMN-DAN anticorrelation was associated with lower IIV across all 3 cognitive tasks (B = 0.22 to 0.25). Older age at baseline was associated with stronger DMN-DAN anticorrelation and lower IIV (B = −0.005 to −0.0004). Weaker DMN-DAN anticorrelation and IIV were cross-sectionally associated with attention problems (B = 1.41 to 7.63). Longitudinally, lower IIV at baseline was associated with less severe attention problems 1 to 3 years later, after accounting for baseline attention problems (B = 0.288 to 0.77).
Conclusions
The results suggest that IIV in early adolescence is associated with worsening attention problems in a representative cohort of U.S. youth. Attention deficits in early adolescence may be important for understanding and predicting future cognitive and clinical outcomes.
Keywords: ABCD Study, Adolescence, Attention, Neurocognitive performance, Resting-state functional connectivity
Momentary lapses in attention can interfere with goal-directed behaviors. In individuals with attention deficits, these lapses are persistent and can hinder task completion at work or concentrating in school. A key metric of attentional lapses is intraindividual variability (IIV), which is defined as trial-by-trial fluctuation in reaction time during a timed cognitive task (1). Higher IIV is a robust behavioral marker of attention-deficit/hyperactivity disorder (ADHD) (Hedges’ g = 0.76; individuals with ADHD vs. control subjects) (2,3). Across the lifespan, IIV exhibits a U-shaped trajectory, with dramatic reductions during childhood and adolescence corresponding with a major developmental shift in sustained attention (4, 5, 6, 7). At the same time, adolescence is a sensitive period involving increased risk for onset of many psychiatric disorders (8). Investigating the spectrum of attention variability at this juncture of development may be important for understanding and predicting future cognitive and clinical outcomes.
While greater IIV has been established as a robust marker of attention deficits, its neural bases remain an open question. Studies using resting-state functional magnetic resonance imaging (fMRI) implicate dysfunction of functional brain networks in greater IIV and attention problems (9, 10, 11, 12, 13, 14). In particular, resting-state fMRI studies have identified the default mode network (DMN) and task-positive networks, including the dorsal attention network (DAN), and the balance between these networks as key for attention allocation (11). The DMN is engaged during internally directed processes, like self-referential processing, and its activity is typically reduced when individuals complete tasks requiring visuospatial attention (15, 16, 17, 18). The DMN includes nodes in the medial prefrontal cortex, posterior cingulate cortex, temporoparietal junction, and lateral and medial temporal lobes (15,17,18). The DAN includes nodes in the frontal eye fields and the inferior parietal sulcus and is involved in top-down attention (19, 20, 21).
The DMN and DAN typically exhibit negatively correlated (anticorrelated) patterns of activity: as activity in the regions of the DAN increases when engaging in an attention-demanding task, activity in the DMN decreases (17,22, 23, 24, 25). Evidence from fMRI and electroencephalography studies suggests that excess DMN activity during tasks is associated with less efficient cognitive performance (10,26, 27, 28). Furthermore, DMN deactivation during attentional tasks is associated with better cognitive performance (29,30). The anticorrelation of these two networks may be a fundamental property of brain organization that supports effective attention allocation by focusing on the task at hand and suppressing internally directed thoughts to support neurocognitive performance (11,17,31).
Consistent evidence from resting-state fMRI research across the lifespan and studies of ADHD links DMN-DAN anticorrelation with attention allocation. These networks become increasingly anticorrelated in infancy through adolescence and less anticorrelated through older adulthood (32, 33, 34). Kelly et al. (11) were the first to link DMN-DAN anticorrelation during rest and task with reduced IIV in healthy adults, which was later replicated and extended through task-based fMRI and electroencephalography studies (33,35, 36, 37, 38, 39). Recently, Owens et al. (40) linked DMN-DAN anticorrelation with less severe parent-reported attention problems using baseline data from the Adolescent Brain Cognitive Development (ABCD) Study. From the ADHD literature, a meta-analysis found that anticorrelation between DMN and task-positive networks was diminished in children and adolescents with ADHD compared with typically developing youth (41). Furthermore, evidence from dynamic functional connectivity (FC) analyses, a method that measures moment-to-moment shifts in connectivity (42), suggests that children with ADHD spent less total time in, and switched out of, anticorrelated states involving the DMN and task-relevant networks more frequently compared with typically developing children (43).
While this cogent body of literature supports the notion that DMN-DAN anticorrelation is linked with IIV, several clinically relevant questions and literature gaps remain. While IIV is a robust marker of ADHD, it is unclear whether IIV can be used in the general population to predict future attention problems (3). Case-control study design does not capture the full spectrum of attention problems because individuals with milder symptoms that do not reach clinical impairment may be excluded (39,43,44). Importantly, studies linking DMN-DAN anticorrelation at rest to IIV have only been established in adulthood and are relatively small (11,36). Yet, studying IIV in early adolescence is particularly important. Evidence from executive function task performance from N > 10,000 participants, ages 8 to 35 years, has shown that executive function (including inhibitory attention) undergoes the most rapid development in early adolescence (45). Whether DMN-DAN anticorrelation develops alongside, prior to, or in response to this dynamic development is unknown. Last, whether DMN-DAN anticorrelation can be used to predict future attention problems has not been examined in a large, population-based sample. Deviations in these neural or behavioral markers of attention during this important stage of cognitive development may lead to poorer cognitive outcomes in adulthood.
To address these questions, we examined the relationship between IIV in timed cognitive tasks and resting-state FC from the ABCD Study, a population-based, longitudinal cohort of 11,878 children ages 9 to 10. First, in contrast to previous work, this study captured the full spectrum of attention problems during a period of dynamic cognitive and neural development (46,47). The main hypotheses tested using cross-sectional data at baseline were the following: 1a) stronger DMN-DAN anticorrelation would be associated with lower IIV, across cognitive tasks; 1b) within these models, older baseline age would be associated with stronger DMN-DAN anticorrelation; and 1c) within these models, older baseline age would be associated with lower IIV. Second, we examined the cross-sectional relationship of lab-based cognitive and neuroimaging measures (IIV and DMN-DAN anticorrelation) with the parent report of their child’s attention problems. We predicted that both 2a) weaker DMN-DAN anticorrelation and 2b) higher IIV would be associated with more severe attention problems. Last, we tested 2 hypotheses using longitudinal data: 3a) weaker DMN-DAN anticorrelation and/or 3b) greater IIV would be associated with future attention symptoms at 1-, 2-, and 3-year follow-up time points, after accounting for baseline attention symptoms.
In addition to these a priori hypotheses based on work conducted in adults (11), we also conducted 3 exploratory analyses. First, we examined brain-behavior associations via ex-Gaussian distribution modeling that parses the reaction time distribution into the variability of extremely slow (tau) versus fast (sigma) responses. Individuals with ADHD exhibit greater tau and greater sigma on reaction time tasks, and neural mechanisms supporting tau and sigma may differ because they follow different developmental patterns (1,5,48, 49, 50, 51). Therefore, we investigated whether DMN-DAN anticorrelation was differentially associated with tau or sigma, to better characterize the nature of attentional variability. Second, we evaluated additional putative anticorrelated networks (DMN–cingulo-opercular network, DMN–frontoparietal network, DMN–ventral attention network) and their behavioral associations. Last, we evaluated whether IIV and/or DMN-DAN anticorrelation are linked with externalizing symptoms, as deficits in attention may contribute to problems with self-regulation more broadly (52, 53, 54).
Methods and Materials
Participants
Data from 11,878 participants were obtained through the ABCD Study, a prospective, longitudinal study tracking 9- to 11-year-olds for the following 10 years, across 20 research collection sites in the United States (55). Each site obtained informed consent from parents and assent from children, approved by each site’s Institutional Review Board, with centralized Institutional Review Board approval at the University of California San Diego. All de-identified raw and processed data for these analyses were from ABCD Data Release 4.0, accessed through the National Institute of Mental Health Data Archive (Collection 2573). Due to use of de-identified data, this study was exempt from Institutional Review Board approval. Exclusion criteria included 1) missing demographic information, such as parental income (n = 1022) or race/ethnicity (n = 2); 2) missing trialwise data for the flanker (n = 1310), dimensional change card sort (n = 1687), or pattern comparison processing speed (n = 1316) tasks; or 3) missing psychiatric symptom data at baseline (n = 10). In concordance with previous work, further exclusion criteria for neuroimaging analyses can be found in the Supplement (56, 57, 58). Baseline demographic characteristics are reported in Table 1; see the Supplement for demographic tables for years 1, 2, and 3 follow-up time points.
Table 1.
Baseline Demographics, Based on Imaging Inclusion Criteria (N = 8446)
| Characteristic | Mean (SD) or n (%) |
|---|---|
| Sex | |
| Female | 4201 (49.75%) |
| Male | 4245 (50.25%) |
| Age, Years | 9.94 (0.63) |
| Parent Report of Child’s Race/Ethnicity | |
| Asian | 164 (1.94%) |
| Black | 1202 (14.23%) |
| Hispanic | 1698 (20.11%) |
| White | 4474 (52.97%) |
| Other | 906 (10.73%) |
| Income Category | |
| <$50,000 | 2219 (26.28%) |
| $50,000–$99,999 | 2232 (26.43%) |
| $100,000+ | 3321 (39.31%) |
| Refuse to report income | 332 (3.93%) |
| Don't know income | 340 (4.03%) |
| Parental Education, Years | 16.67 (2.67) |
| CBCL Attention T Score | 53.73 (6.04) |
| CBCL Externalizing T Score | 45.57 (10.24) |
| Framewise Displacement, Mean (SD) | 0.22 (2.34 × 10−5) |
CBCL, Child Behavior Checklist.
Measures
Neuropsychological Test Battery
Participants completed a developmentally appropriate neurocognitive battery, the NIH Toolbox (55), which includes 7 tasks covering episodic memory, executive function, attention, working memory, processing speed, and language abilities. This neurocognitive battery was designed to comprehensively assess these domains, has been used in longitudinal studies across childhood and adolescence, and is psychometrically sound (55).
The current study used the timed reaction time tasks from NIH Toolbox, which include the flanker, pattern comparison processing speed, and dimensional change card sort tasks. The flanker and dimensional change card sort task show excellent test-retest reliability (intraclass correlation coefficient = 0.92 for both measures) and the processing speed task shows good test-retest reliability (intraclass correlation coefficient = 0.84) in children and adolescents over a 2-week interval (59,60). Details of these tasks have been published elsewhere (55,61).
IIV for each task and participant was operationalized as the standard deviation in reaction time across all correct trials. To remove extreme outliers that suggest invalid task performance, IIV was winsorized at 3 standard deviations for each task. Because the flanker task assesses attention, we focused first on testing our analyses using flanker IIV. Then, we examined associations using IIV from the dimensional change card sort and the pattern comparison processing speed tasks, to assess whether the associations would generalize to these related cognitive domains. To verify these results, we also analyzed these associations using the raw reaction time data to derive IIV (Supplement). Last, we generated ex-Gaussian parameters (tau, sigma, and mu) from the raw reaction time data for each task and participant by applying the retimes package in R version 4.1.1 (R Foundation for Statistical Computing).
Imaging Procedure: Acquisition
ABCD imaging collection, acquisition, and analysis has been previously described (62, 63, 64). All participants were scanned on 3T scanners including Prisma (Siemens), Discovery MR750 (GE Healthcare), and Achieva dStream or Ingenia CX (Philips) with a 32-channel head coil. Participants completed T1-weighted and T2-weighted structural scans, as well as four 5-minute resting-state blood oxygen level–dependent scans, with their eyes open, fixed at a crosshair. Further details about the resting-state imaging acquisition that varied by 3T scanner have been detailed previously (62).
Imaging Procedure: Processing
The ABCD Data Analysis, Informatics and Research Center performs centralized processing of MRI data from the ABCD Study using the multi-modal processing stream (see Supplement) (64). After processing, between-network connectivity was calculated by computing pairwise correlations between each region of interest within a given network and each region of interest within another network, defined by the Gordon parcellation (65) These correlations were averaged and Fisher z transformed to generate a summary metric of between-network connectivity strength, which we considered anticorrelation if significantly negatively correlated.
Attention Problems and Externalizing Symptoms
Caregivers of the participants completed the Child Behavior Checklist (CBCL), a 112-item questionnaire used to detect emotional and behavioral problems in youth (66). The longitudinal analyses use the CBCL attention and externalizing T scores from follow-up years 1, 2, and 3. Demographic characteristics of participants by follow-up year, based on availability of the CBCL data, are included in the Supplement.
Statistical Analyses
Linear mixed models were conducted in R version 4.1.1 lmer package, with research site and family unit (nested within site, to account for 1336 twins and 23 triplets in this study sample) as random effects and age, sex, race/ethnicity, parental income, and parental education modeled as covariates (67). Imaging analyses additionally included mean framewise displacement as a covariate. All results reported were false discovery rate corrected.
Linear mixed models were used to test the following cross-sectional predictions: 1a) across cognitive tasks (each in a separate model), lower IIV would be associated with stronger DMN-DAN anticorrelation; 1b) there would be an age effect, such that greater baseline age would be associated with stronger DMN-DAN anticorrelation; and 1c) there would be an age effect, such that greater baseline age would be associated with lower IIV. Furthermore, we tested the following hypotheses regarding associations between the lab-based measures and neurobehavioral symptoms: 2a) greater IIV and/or 2b) weaker DMN-DAN anticorrelation would be linked with worse attention symptoms at baseline. Last, we tested the following predictions using longitudinal data: 3a) across cognitive tasks (each in a separate model), greater IIV and 3b) weaker DMN-DAN anticorrelation, all assessed at baseline, would be associated with subsequent attention problems, 1, 2, and 3 years later, after accounting for baseline CBCL attention problems. Results are expressed as standardized coefficients with 95% confidence intervals. As a control analysis, we hypothesized that IIV and parent-reported attention problems would not be associated with between-network connectivity between auditory and retrosplenial networks, as the flanker, dimensional change card sort, and pattern comparison processing speed tasks are not thought to engage either network (68). When investigating behavioral and neural measures of attentional variability, we examined other key factors associated with these variables, including sex, race/ethnicity, and parental income, to better contextualize these associations.
Results
DMN-DAN Anticorrelation and Behavioral Variability
As hypothesized, lower IIV across all 3 neurocognitive tasks, modeled in separate linear mixed models, was associated with stronger anticorrelation between the DMN and DAN (Figure 1 and Table 2; see the Supplement for estimated marginal means visualization). Within this model, older baseline age was associated with stronger DMN-DAN anticorrelation. Of the other covariates, head motion, sex, race/ethnicity, and family income were associated with DMN-DAN anticorrelation across models.
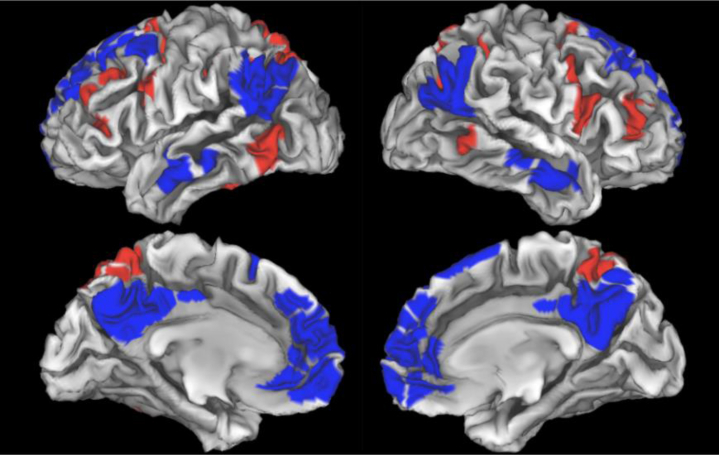
Figure 1.
Default mode network (blue) and dorsal attention network (red) from the Gordon parcellation.
Table 2.
Associations Between IIV and DMN-DAN Functional Connectivity
| Predictor | Flanker IIV |
DCCS IIV |
Pattern Comparison Processing Speed IIV |
|||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| DMN-DAN Anticorrelation | 0.04a | 0.02 to 0.06 | 0.05b | 0.02 to 0.07 | 0.03c | 0.01 to 0.05 |
| Age | −0.09b | −0.11 to −0.07 | −0.13b | −0.15 to −0.11 | −0.08b | −0.11 to −0.06 |
| Sex | 0.02 | −0.02 to 0.06 | 0.07a | 0.02 to 0.11 | 0.12b | 0.07 to 0.16 |
| Mean FD | 0.10b | 0.07 to 0.12 | 0.11b | 0.09 to 0.13 | 0.07b | 0.05 to 0.09 |
| Income $50,000–$99,999 | −0.23b | −0.29 to −0.17 | −0.30b | −0.36 to −0.23 | −0.11b | −0.17 to −0.04 |
| Income $100,000+ | −0.34b | −0.40 to −0.28 | −0.35b | −0.41 to −0.29 | −0.15b | −0.22 to −0.09 |
| Non-White | 0.19b | 0.13 to 0.24 | 0.17b | 0.12 to 0.22 | 0.12b | 0.07 to 0.18 |
| Parental Education | −0.02 | −0.04 to 0.00 | −0.02 | −0.04 to 0.01 | −0.00 | −0.03 to 0.02 |
Reference categories: sex (female), income (<$50,000), race/ethnicity (White). Greater magnitude in negative values indicate stronger DMN-DAN anticorrelation.
DAN, dorsal attention network; DCCS, dimensional change card sort; DMN, default mode network; FD, framewise displacement; FDR, false discovery rate; IIV, intraindividual variability.
p < .01, FDR corrected.
p < .001, FDR corrected.
p < .05, FDR corrected.
Age and Behavioral Variability
We replicated the neuropsychological literature showing that younger baseline age was also associated with greater IIV, across all 3 tasks after adjusting for covariates (4,5). Within these 3 models, there were also effects of race/ethnicity, parental income, and sex (Supplement). In a supplemental analysis, greater baseline age was also related to greater accuracy (Supplement).
Behavioral and Neural Associations With Attentional and Externalizing Symptoms at Baseline
Higher IIV across the flanker, pattern comparison processing speed, and dimensional change card sort tasks was associated with more severe baseline attention symptoms (Table 3). Furthermore, weaker DMN-DAN anticorrelation was associated with more severe attention symptoms (Table 3).
Table 3.
Behavioral and Neural Associations With Attention Problems at Baseline
| Predictor | CBCL Attention BV |
CBCL Attention BV |
CBCL Attention BV |
CBCL Attention BV |
||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Flanker IIV | 0.12a | 0.10 to 0.14 | – | – | – | – | – | – |
| DCCS IIV | – | – | 0.13a | 0.11 to 0.15 | – | – | – | – |
| Pattern Comparison Processing Speed IIV | – | – | – | – | 0.11a | 0.09 to 0.13 | – | – |
| DMN-DAN Anticorrelation | – | – | – | – | – | – | 0.07a | 0.05 to 0.09 |
| Mean FD | – | – | – | – | – | – | 0.05a | 0.02 to 0.07 |
| Age | 0.01 | −0.01 to 0.03 | 0.01 | −0.01 to 0.03 | 0.00 | −0.01 to 0.02 | 0.01 | −0.02 to 0.03 |
| Sex | 0.11a | 0.07 to 0.15 | 0.10a | 0.06 to 0.14 | 0.10a | 0.06 to 0.14 | 0.06b | 0.02 to 0.11 |
| Income $50,000–$99,999 | −0.15a | −0.21 to −0.10 | −0.14a | −0.20 to −0.08 | −0.17a | −0.23 to −0.12 | −0.17a | −0.23 to −0.11 |
| Income $100,000+ | −0.25a | −0.30 to −0.19 | −0.24a | −0.30 to −0.19 | −0.28a | −0.33 to −0.22 | −0.28a | −0.34 to −0.22 |
| Non-White | −0.01 | −0.05 to 0.04 | −0.01 | −0.06 to 0.03 | −0.00 | −0.05 to 0.04 | −0.01 | −0.06 to 0.04 |
| Parental Education | −0.01 | −0.03 to 0.01 | −0.01 | −0.03 to 0.01 | −0.01 | −0.03 to 0.01 | – | – |
Reference categories: sex (female), income (<$50,000), race/ethnicity (White). Greater magnitude in negative values indicate stronger DMN-DAN anticorrelation.
BV, baseline visit; DAN, dorsal attention network; DCCS, dimensional change card sort; DMN, default mode network; FD, framewise displacement; FDR, false discovery rate; IIV, intraindividual variability.
p < .001, FDR corrected.
p < .01, FDR corrected.
In an exploratory analysis, higher IIV across all cognitive tasks and weaker (less negative) DMN-DAN anticorrelation was associated with more severe externalizing symptoms, all measured contemporaneously at baseline (Supplement). Of the covariates, several effects are consistent across all 8 models (Table 3 for attentional symptoms and the Supplement for externalizing symptoms); sex, parental income, and race/ethnicity were associated with attention and externalizing symptoms.
Prospective Behavioral and Neural Associations With Attentional Symptoms, 1 to 3 Years Later
Next, we evaluated if either IIV or DMN-DAN connectivity at baseline was predictive of attention symptoms 1, 2, and 3 years after the baseline visit. For all 3 tasks, higher IIV at baseline predicted more severe attention symptoms at the participants’ 1-, 2-, and 3-year follow-up visits, after controlling for baseline attention problems (the flanker task in Table 4, other tasks in the Supplement). After accounting for baseline attention problems and covariates included in baseline models, DMN-DAN connectivity did not predict future attention symptoms (Supplement).
Table 4.
Prospective Behavioral Associations With Attentional Symptoms, 1 to 3 Years Later
| Predictor | CBCL Attention Y1 |
CBCL Attention Y2 |
CBCL Attention Y3 |
|||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| (Intercept) | 0.05a | 0.01 to 0.09 | 0.08b | 0.02 to 0.13 | 0.08a | 0.01 to 0.15 |
| Flanker IIV | 0.02b | 0.01 to 0.03 | 0.03b | 0.01 to 0.04 | 0.03a | 0.01 to 0.05 |
| Baseline CBCL Attention | 0.73c | 0.71 to 0.74 | 0.68c | 0.66 to 0.70 | 0.61c | 0.59 to 0.63 |
| Age | −0.00 | −0.02 to 0.01 | 0.01 | −0.01 to 0.03 | 0.01 | −0.01 to 0.03 |
| Sex | 0.01 | −0.02 to 0.04 | −0.01 | −0.05 to 0.02 | −0.06a | −0.10 to −0.01 |
| Income $50,000–$99,999 | −0.02 | −0.06 to 0.02 | −0.06a | −0.10 to −0.01 | −0.04 | −0.10 to 0.03 |
| Income $100,000+ | −0.07c | −0.11 to −0.04 | −0.06a | −0.11 to −0.01 | −0.06a | −0.13 to −0.00 |
| Non-White | −0.04a | −0.07 to −0.01 | −0.07c | −0.11 to −0.03 | −0.05 | −0.10 to 0.00 |
| Parental Education | 0.00 | −0.01 to 0.02 | 0.00 | −0.02 to 0.02 | 0.01 | −0.01 to 0.04 |
Reference categories: sex (female), income (<$50,000), race/ethnicity (White). Greater magnitude in negative values indicate stronger DMN-DAN anticorrelation.
DAN, dorsal attention network; CBCL, Child Behavior Checklist; DMN, default mode network; FDR, false discovery rate; IIV, intraindividual variability; Y1, year 1 follow-up; Y2, year 2 follow-up; Y3, year 3 follow-up.
p < .05, FDR corrected.
p < .01, FDR corrected.
p < .001, FDR corrected.
Prospective Behavioral and Neural Associations With Externalizing Symptoms, 1 to 3 Years Later
Higher IIV at baseline was also associated with increased externalizing symptoms at the 1-year follow-up visit after controlling for baseline externalizing symptom scores but not at later time points (Supplement). Furthermore, DMN-DAN anticorrelation did not predict future externalizing symptoms at any time point after controlling for baseline externalizing symptoms.
Ex-Gaussian Distribution Modeling
Using ex-Gaussian approaches to parse reaction time distribution into variability in slow (tau) versus fast (sigma) responses, we found that stronger DMN-DAN anticorrelation was associated with both lower sigma and lower tau, across all 3 tasks, after controlling for covariates (Supplement). We also found that DMN-CON anticorrelation was specifically associated with lower tau and lower sigma in the dimensional change card sort task but not the other 2 tasks.
Specificity and Robustness Analyses at Baseline
To demonstrate specificity, we tested an association between IIV and between-network connectivity between the auditory and retrosplenial networks. The cognitive tasks require visuospatial attention but not auditory processing. Furthermore, these tasks require in-the-moment attention, and not the range of cognitive functions linked with the retrosplenial network, including episodic memory, navigation, imagination, and planning for the future (68). The auditory-retrosplenial network connectivity was not associated with the flanker IIV (p = .753) or CBCL attention T score (p = .857) at baseline, after accounting for the covariates included in previous models (Supplement).
To evaluate the robustness of these results, we also formally tested 1) the effect of site and examined our hypotheses using 2) raw reaction time data to calculate IIV and 3) using a subset of low-motion subjects. First, we modeled site as a predictor using general linear models to compare site effects with the main effects in aims 1 to 3. Our main findings remained significant, though a minority of sites were significant in these models (Supplement). Using raw nonwinsorized data, all the results remain significant, with the only exception being that pattern comparison processing speed IIV was no longer associated with DMN-DAN anticorrelation (Supplement). Last, we applied a more stringent motion threshold that only included participants with 60% of resting-state frames or higher with framewise displacement <0.2 mm (n = 7003). Greater DMN-DAN anticorrelation remained significantly associated with lower IIV in the flanker and dimensional change card sort tasks, greater baseline age, and less severe baseline attention symptoms (Supplement).
Discussion
The current study provides the first evidence linking anticorrelation between brain networks at rest and neurocognitive measures of attentional variability in early adolescence. Importantly, both lab-based resting-state FC and neurocognitive markers of attentional variability were associated with parent-reported attention symptoms at baseline. We also provide novel evidence that worse scores on lab-based measures of attentional variability (IIV) at ages 9 to 10 predict subsequent parent-reported attentional problems at 1-, 2-, and 3-year follow-up visits. Leveraging the ABCD cohort to evaluate longitudinal associations between functional connectivity, neurocognitive measures, and symptoms allows us to evaluate potential markers for attentional dysfunction in the general population and to understand the relationships between neural and behavioral development in adolescence.
We provide the first evidence linking stronger DMN-DAN anticorrelation and lower IIV in early adolescence, connecting resting-state and neurocognitive literature, which have been largely investigated independently as measures of attention variability. Previous studies have shown that the DMN and DAN have an intrinsically antiphase relationship, first established 1 year after birth, increasing during development, and decreasing in older adulthood (17,22,32, 33, 34,69). Kelly et al. (11) were the first to link stronger DMN-DAN anticorrelation with lower flanker IIV in 20 adults. The current results extend this work to early adolescence and to other goal-directed, cognitive tasks, showing that stronger DMN-DAN anticorrelation is associated with greater baseline age and lower IIV across cognitive domains. Furthermore, using ex-Gaussian distribution modeling, stronger DMN-DAN anticorrelation is also related to both lower tau and lower sigma across all tasks, indicating lower variability of slow and fast responses, respectively. Taken together, both Gaussian and ex-Gaussian approaches suggest that segregation of the DMN and DAN may be relevant to cognitive development during early adolescence.
These results also indicate a remarkably consistent effect of age on neural and behavioral markers of attention, in a narrow age range from 9 to 11 years that aligns with previous work in smaller samples (4, 5, 6, 7). The neuropsychological task literature indicates that IIV decreases dramatically during childhood and adolescence, in conjunction with improved sustained attention (4, 5, 6, 7). However, most of this literature has focused on IIV across the lifespan. Here, we focused on ages 9 to 11 and found that older baseline age was associated with lower IIV across the flanker, pattern comparison processing speed, and dimensional change card sort tasks. These consistent behavioral findings suggest that attentional variability is improving across different cognitive demands in early adolescence, even within the narrow age band of 9 to 11 years.
The current study also found that higher IIV across 3 cognitive tasks was cross-sectionally associated with more severe attention symptoms. Previous work indicated that higher IIV robustly identifies ADHD versus typically developing youth (2,3)—a binary approach to attention problems (39,46,48). However, continuous approaches are closer to the phenomena of attention and its underlying neurobiological processes (70, 71, 72, 73). As such, this study takes a dimensional approach to include the full spectrum of attention problems in the ABCD sample (3,74). The current findings are consistent with previous work in children with ADHD and generalize to a representative sample of U.S. youth (2).
The current results are the first to show that IIV assessed at ages 9 to 10 is a consistent, proximal marker of worsening attention during early adolescence (1, 2, and 3 years later). These results align with 2 longitudinal, developmental studies (nondiverse, smaller samples) that controlled for attention symptoms at baseline and used IIV to predict overall functioning and symptoms of inattention (75,76). By controlling for attention problems at baseline, the current results highlight the relationship between a lab-based measure of attentional variability in early adolescence and future worsening of attention symptoms, over and above early parent-reported attention deficits. In early adolescence, worsening attention problems may be a broad indicator of functional impairment, regardless of specific etiology or diagnoses that may emerge later (8). Research on other neurodevelopmental disorders with onset in late adolescence (i.e., psychotic disorders) indicates that early intervention can be key for improving future symptom trajectories (77, 78, 79, 80, 81, 82). Because the ABCD Study will continue to collect data prospectively on participants through late adolescence, future work could examine IIV as a predictor of altered trajectories of cognitive development and mental health outcomes (8).
While we found that greater DMN-DAN anticorrelation was linked with lower contemporaneous attention problems, we did not find an association between DMN-DAN connectivity and future attention symptoms. The cross-sectional DMN-DAN FC -attention symptom association aligns with work using a previous release of the ABCD data (40). However, this work also stands in contrast with previous studies that have found no association between anticorrelated networks and attention symptoms, albeit in much smaller adult samples (83,84). Using multivariate approaches may improve prediction abilities (85). Notably, Rosenberg et al. (86) used a connectome-based predictive model to predict future sustained attention in adults. Therefore, other metrics of resting-state FC may be important neural predictors of symptom onset or progression.
In exploratory analyses, we evaluated whether additional task-positive networks were anticorrelated with the DMN and determined that only the DMN and CON were significantly anticorrelated. Because DMN-CON anticorrelation was significantly associated with Gaussian and ex-Gaussian measures of variability for the dimensional change card sort task but not the other 2 tasks, these findings may suggest that this circuitry specifically supports fluctuation in cognitive flexibility, as opposed to variability in cognitive performance broadly. In addition to attention problems, we found that more severe externalizing symptoms were cross-sectionally associated with greater IIV and weaker DMN-DAN anticorrelation. Further, greater IIV at baseline predicted more severe externalizing symptoms at the following year, after controlling for externalizing symptoms at baseline. These results give credence to the possibility that attention is an important feature in self-regulation. Focusing on the task at hand, while suppressing internally directed cognition, may be critical for controlling one’s behavior in relation to current and future goals (52, 53, 54).
Limitations and Future Directions
This study leverages the largest imaging and behavior study of adolescence to date to examine a key developmental shift in attentional variability and its neural correlates; however, some limitations should be noted. Global signal regression (GSR) is included in the standard ABCD data analysis pipeline, used in the current analyses. While GSR is a powerful tool to remove spurious artifacts due to motion, respiration, and physiological noise that is prominent in developmental populations (87), it also can introduce negative correlations into the data. This has called into question whether DMN-DAN anticorrelation may be an artifact of GSR (88,89). However, anticorrelation between the DMN and task-positive networks has been detected in studies both with and without GSR, indicating that that anticorrelation is not a result of GSR (89, 90, 91). Because of the consistency of anticorrelated networks, whether GSR is included or not, we have chosen to replicate and extend the majority of studies using GSR that show 1) development of anticorrelated networks or 2) that anticorrelation is linked with superior cognitive performance (11,32, 33, 34,39,46). Furthermore, Nomi et al. (92) discovered complex linear and quadratic associations between GSR and age with different brain regions and networks. Though the impact of GSR is minimal on the linear and quadratic associations of the DMN-DAN anticorrelation with age, this question warrants future investigation in lifespan studies of functional connectivity. Last, we note that although large-scale brain networks undergo development during adolescence, no adolescent-specific brain parcellations exist to date. Ongoing work addresses this issue by creating age-specific parcellations (93).
With regard to capturing attention problems, the CBCL inattention scale includes a variety of attention symptoms, including both hyperactivity and inattentiveness, which may warrant separate investigation in future studies (66). As a parent-report measure, the CBCL may be biased due to rater; multiple informants including teachers would be ideal to capture inattentive behavior across contexts. Though ABCD used a structured interview to assess categorical psychiatric disorders, ADHD diagnoses were not available in the current data release due to errors in the programming algorithm. Regardless, our current work focuses on dimensional phenotypes of attention dysfunction. In the future, we will investigate these questions in the context of ADHD when accurate diagnoses are available.
The results of the current study demonstrate the functional significance of lab-based measures of attentional variability, linking them to attentional impairment in early adolescence. Increased IIV across 3 goal-directed behavioral tasks was robustly linked with more severe attention deficits at baseline as well as 1, 2, and 3 years later. While higher IIV is a well-known hallmark of current ADHD (2,3), these results show that IIV may be a useful index to identify worsening attention symptoms in the general population. Further, we have shown that stronger negative anticorrelation between the DMN and DAN is cross-sectionally associated with lower IIV across multiple tasks, better attention, and older age. These results suggest that the segregation of brain networks is associated with developmental improvements in cognition. In future studies, focusing on altered longitudinal patterns of DMN-DAN anticorrelation may be informative for predicting functional outcome.
Acknowledgments and Disclosures
The ABCD study is crossfunded by several National Institutes of Health institutes and federal partners and is supported by U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041025, U01DA041093, U24DA041123, and U24DA041147. The full list of federal partners can be found at https://abcdstudy.org/about/federal-partners/. This work was supported by The Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant No. 1T32HD091059-01A1 [to SEC]) and the National Institute of Mental Health (Grant No. U01MH124639 [to CEB]).
We thank Drs. Andrew Fuligni, Mirella Dapretto, and Adriana Galván for their feedback on these analyses and for their mentorship through the T32 for Brain and Behavioral Development during Adolescence.
A previous version of this article was published as a preprint on bioRxiv: https://www.biorxiv.org/content/10.1101/2022.07.07.499196v1.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.11.003.
Supplementary Material
References
- 1.Leth-Steensen C., Elbaz Z.K., Douglas V.I. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychol. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 2.Castellanos F.X., Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 3.Kofler M.J., Rapport M.D., Sarver D.E., Raiker J.S., Orban S.A., Friedman L.M., Kolomeyer E.G. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald S.W.S., Nyberg L., Bäckman L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Williams B.R., Hultsch D.F., Strauss E.H., Hunter M.A., Tannock R. Inconsistency in reaction time across the life span. Neuropsychology. 2005;19:88–96. doi: 10.1037/0894-4105.19.1.88. [DOI] [PubMed] [Google Scholar]
- 6.Tamnes C.K., Fjell A.M., Westlye L.T., Østby Y., Walhovd K.B. Becoming consistent: Developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 2012;32:972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykiert D., Der G., Starr J.M., Deary I.J. Sex differences in reaction time mean and intraindividual variability across the life span. Dev Psychol. 2012;48:1262–1276. doi: 10.1037/a0027550. [DOI] [PubMed] [Google Scholar]
- 8.Solmi M., Radua J., Olivola M., Croce E., Soardo L., Salazar de Pablo G., et al. Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2021;27:281–295. doi: 10.1038/s41380-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosazza C., Minati L. Resting-state brain networks: Literature review and clinical applications. Neurol Sci. 2011;32:773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- 10.Sonuga-Barke E.J.S., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Kelly A.M.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Taghia J., Ryali S., Chen T., Supekar K., Cai W., Menon V. Bayesian switching factor analysis for estimating time-varying functional connectivity in fMRI. Neuroimage. 2017;155:271–290. doi: 10.1016/j.neuroimage.2017.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: A meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., et al. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margulies D.S., Ghosh S.S., Goulas A., Falkiewicz M., Huntenburg J.M., Langs G., et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A. 2016;113:12574–12579. doi: 10.1073/pnas.1608282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 20.Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan J., McCandliss B.D., Fossella J., Flombaum J.I., Posner M.I. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai X.J., Castañón A.N., Ongür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito R., Cieri F., Chiacchiaretta P., Cera N., Lauriola M., Di Giannantonio M., et al. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: Comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2018;12:127–141. doi: 10.1007/s11682-017-9686-y. [DOI] [PubMed] [Google Scholar]
- 25.Raichle M.E. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 26.Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 27.Fassbender C., Zhang H., Buzy W.M., Cortes C.R., Mizuiri D., Beckett L., Schweitzer J.B. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helps S.K., Broyd S.J., James C.J., Karl A., Chen W., Sonuga-Barke E.J.S. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 29.McKiernan K.A., Kaufman J.N., Kucera-Thompson J., Binder J.R. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 30.Singh K.D., Fawcett I.P. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Kucyi A., Raya J., Nielsen A.N., Nomi J.S., Damoiseaux J.S., et al. What have we really learned from functional connectivity in clinical populations? Neuroimage. 2021;242 doi: 10.1016/j.neuroimage.2021.118466. [DOI] [PubMed] [Google Scholar]
- 32.Gao W., Gilmore J.H., Shen D., Smith J.K., Zhu H., Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex. 2013;23:594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai X.J., Ofen N., Gabrieli J.D.E., Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci. 2014;26:501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spreng R.N., Schacter D.L. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golland Y., Bentin S., Gelbard H., Benjamini Y., Heller R., Nir Y., et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- 36.Thompson G.J., Magnuson M.E., Merritt M.D., Schwarb H., Pan W.-J., McKinley A., et al. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp. 2013;34:3280–3298. doi: 10.1002/hbm.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisz N., Wühle A., Monittola G., Demarchi G., Frey J., Popov T., Braun C. Prestimulus oscillatory power and connectivity patterns predispose conscious somatosensory perception. Proc Natl Acad Sci U S A. 2014;111:E417–E425. doi: 10.1073/pnas.1317267111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadaghiani S., Poline J.-B., Kleinschmidt A., D’Esposito M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc Natl Acad Sci U S A. 2015;112:8463–8468. doi: 10.1073/pnas.1420687112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber A.D., Jacobson L.A., Wexler J.L., Nebel M.B., Caffo B.S., Pekar J.J., Mostofsky S.H. Connectivity supporting attention in children with attention deficit hyperactivity disorder. Neuroimage Clin. 2015;7:68–81. doi: 10.1016/j.nicl.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens M.M., Yuan D., Hahn S., Albaugh M., Allgaier N., Chaarani B., et al. Investigation of psychiatric and neuropsychological correlates of default mode network and dorsal attention network anticorrelation in children. Cereb Cortex. 2020;30:6083–6096. doi: 10.1093/cercor/bhaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutcubasi B., Metin B., Kurban M.K., Metin Z.E., Beser B., Sonuga-Barke E. Resting-state network dysconnectivity in ADHD: A system-neuroscience-based meta-analysis. World J Biol Psychiatry. 2020;21:662–672. doi: 10.1080/15622975.2020.1775889. [DOI] [PubMed] [Google Scholar]
- 42.Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shappell H.M., Duffy K.A., Rosch K.S., Pekar J.J., Mostofsky S.H., Lindquist M.A., Cohen J.R. Children with attention-deficit/hyperactivity disorder spend more time in hyperconnected network states and less time in segregated network states as revealed by dynamic connectivity analysis. Neuroimage. 2021;229 doi: 10.1016/j.neuroimage.2021.117753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Querne L., Fall S., Le Moing, A-G, Bourel-Ponchel E., Delignières A., Simonnot A., et al. Effects of methylphenidate on default-mode network/task-positive network synchronization in children with ADHD. J Atten Disord. 2017;21:1208–1220. doi: 10.1177/1087054713517542. [DOI] [PubMed] [Google Scholar]
- 45.Tervo-Clemmens B., Calabro F.J., Parr A.C., Fedor J., Foran W., Luna B. A canonical trajectory of executive function maturation during the transition from adolescence to adulthood. psyRxiv. 2022 doi: 10.31234/osf.io/73yfv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Halloran L., Cao Z., Ruddy K., Jollans L., Albaugh M.D., Aleni A., et al. Neural circuitry underlying sustained attention in healthy adolescents and in ADHD symptomatology. Neuroimage. 2018;169:395–406. doi: 10.1016/j.neuroimage.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Bernanke J., Luna A., Chang L., Bruno E., Dworkin J., Posner J. Structural brain measures among children with and without ADHD in the Adolescent Brain and Cognitive Development Study cohort: A cross-sectional US population-based study. Lancet Psychiatry. 2022;9:222–231. doi: 10.1016/S2215-0366(21)00505-8. [DOI] [PubMed] [Google Scholar]
- 48.van Belle J., van Raalten T., Bos D.J., Zandbelt B.B., Oranje B., Durston S. Capturing the dynamics of response variability in the brain in ADHD. Neuroimage Clin. 2015;7:132–141. doi: 10.1016/j.nicl.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geurts H.M., Grasman R.P.P.P., Verté S., Oosterlaan J., Roeyers H., van Kammen S.M., Sergeant J.A. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia. 2008;46:3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Hervey A.S., Epstein J.N., Curry J.F., Tonev S., Arnold L.E., Conners C.K., et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- 51.McAuley T., Yap M., Christ S.E., White D.A. Revisiting inhibitory control across the life span: Insights from the ex-Gaussian distribution. Dev Neuropsychol. 2006;29:447–458. doi: 10.1207/s15326942dn2903_4. [DOI] [PubMed] [Google Scholar]
- 52.Rueda M.R., Posner M.I., Rothbart M.K. In: Handbook of Self-Regulation: Research, Theory, and Applications. Baumeister R.F., Vohs K.D., editors. Guilford Press; New York: 2004. Attentional control and self-regulation; pp. 284–299. [Google Scholar]
- 53.Berger A., Kofman O., Livneh U., Henik A. Multidisciplinary perspectives on attention and the development of self-regulation. Prog Neurobiol. 2007;82:256–286. doi: 10.1016/j.pneurobio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Posner M.I., Rothbart M.K. Toward a physical basis of attention and self regulation. Phys Life Rev. 2009;6:103–120. doi: 10.1016/j.plrev.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luciana M., Bjork J.M., Nagel B.J., Barch D.M., Gonzalez R., Nixon S.J., Banich M.T. Adolescent neurocognitive development and impacts of substance use: Overview of the Adolescent Brain Cognitive Development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018;32:67–79. doi: 10.1016/j.dcn.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marek S., Tervo-Clemmens B., Nielsen A.N., Wheelock M.D., Miller R.L., Laumann T.O., et al. Identifying reproducible individual differences in childhood functional brain networks: An ABCD study. Dev Cogn Neurosci. 2019;40 doi: 10.1016/j.dcn.2019.100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai Y., Elsayed N.M., Barch D.M. Contributions from resting state functional connectivity and familial risk to early adolescent-onset MDD: Results from the Adolescent Brain Cognitive Development study. J Affect Disord. 2021;287:229–239. doi: 10.1016/j.jad.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 59.Zelazo P.D., Anderson J.E., Richler J., Wallner-Allen K., Beaumont J.L., Weintraub S. II. NIH Toolbox Cognition Battery (CB): Measuring executive function and attention. Monogr Soc Res Child Dev. 2013;78:16–33. doi: 10.1111/mono.12032. [DOI] [PubMed] [Google Scholar]
- 60.Carlozzi N.E., Tulsky D.S., Kail R.V., Beaumont J.L. VI. NIH Toolbox Cognition Battery (CB): Measuring processing speed. Monogr Soc Res Child Dev. 2013;78:88–102. doi: 10.1111/mono.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anokhin A.P., Luciana M., Banich M., Barch D., Bjork J.M., Gonzalez M.R., et al. Age-related changes and longitudinal stability of individual differences in ABCD neurocognition measures. Dev Cogn Neurosci. 2022;54 doi: 10.1016/j.dcn.2022.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., et al. Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagler D.J., Jr., Hatton S., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Achenbach T.M. In: The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 2nd ed. Maruish M.E., editor. Erlbaum; Mahwah, NJ: 1999. The Child Behavior Checklist and related instruments; pp. 429–466. [Google Scholar]
- 67.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 68.Vann S.D., Aggleton J.P., Maguire E.A. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 69.Buckner R.L., Krienen F.M., Yeo B.T.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 70.Hudziak J.J., Achenbach T.M., Althoff R.R., Pine D.S. A dimensional approach to developmental psychopathology. Int J Methods Psychiatr Res. 2007;16:S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chabernaud C., Mennes M., Kelly C., Nooner K., Di Martino A., Castellanos F.X., Milham M.P. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:434–442. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karalunas S.L., Nigg J.T. Heterogeneity and subtyping in attention-deficit/hyperactivity disorder—Considerations for emerging research using person-centered computational approaches. Biol Psychiatry. 2020;88:103–110. doi: 10.1016/j.biopsych.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pruim R.H.R., Beckmann C.F., Oldehinkel M., Oosterlaan J., Heslenfeld D., Hartman C.A., et al. An integrated analysis of neural network correlates of categorical and dimensional models of attention-deficit/hyperactivity disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:472–483. doi: 10.1016/j.bpsc.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Tamm L., Narad M.E., Antonini T.N., O’Brien K.M., Hawk L.W., Jr., Epstein J.N. Reaction time variability in ADHD: A review. Neurotherapeutics. 2012;9:500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Lieshout M., Luman M., Twisk J.W.R., Faraone S.V., Heslenfeld D.J., Hartman C.A., et al. Neurocognitive predictors of ADHD outcome: A 6-year follow-up study. J Abnorm Child Psychol. 2017;45:261–272. doi: 10.1007/s10802-016-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sjöwall D., Bohlin G., Rydell A.-M., Thorell L.B. Neuropsychological deficits in preschool as predictors of ADHD symptoms and academic achievement in late adolescence. Child Neuropsychol. 2017;23:111–128. doi: 10.1080/09297049.2015.1063595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGorry P.D., Killackey E., Yung A. Early intervention in psychosis: Concepts, evidence and future directions. World Psychiatry. 2008;7:148–156. doi: 10.1002/j.2051-5545.2008.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGorry P.D. Early intervention in psychosis: Obvious, effective, overdue. J Nerv Ment Dis. 2015;203:310–318. doi: 10.1097/NMD.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aceituno D., Vera N., Prina A.M., McCrone P. Cost-effectiveness of early intervention in psychosis: Systematic review. Br J Psychiatry. 2019;215:388–394. doi: 10.1192/bjp.2018.298. [DOI] [PubMed] [Google Scholar]
- 80.Woods S.W., Bearden C.E., Sabb F.W., Stone W.S., Torous J., Cornblatt B.A., et al. Counterpoint. Early intervention for psychosis risk syndromes: Minimizing risk and maximizing benefit. Schizophr Res. 2021;227:10–17. doi: 10.1016/j.schres.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Csillag C., Nordentoft M., Mizuno M., McDaid D., Arango C., Smith J., et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12:757–764. doi: 10.1111/eip.12514. [DOI] [PubMed] [Google Scholar]
- 82.Malla A., McGorry P. Early intervention in psychosis in young people: A population and public health perspective. Am J Public Health. 2019;109:S181–S184. doi: 10.2105/AJPH.2019.305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sidlauskaite J., Sonuga-Barke E., Roeyers H., Wiersema J.R. Altered intrinsic organisation of brain networks implicated in attentional processes in adult attention-deficit/hyperactivity disorder: A resting-state study of attention, default mode and salience network connectivity. Eur Arch Psychiatry Clin Neurosci. 2016;266:349–357. doi: 10.1007/s00406-015-0630-0. [DOI] [PubMed] [Google Scholar]
- 84.McCarthy H., Skokauskas N., Mulligan A., Donohoe G., Mullins D., Kelly J., et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- 85.Yoo K., Rosenberg M.D., Noble S., Scheinost D., Constable R.T., Chun M.M. Multivariate approaches improve the reliability and validity of functional connectivity and prediction of individual behaviors. Neuroimage. 2019;197:212–223. doi: 10.1016/j.neuroimage.2019.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenberg M.D., Scheinost D., Greene A.S., Avery E.W., Kwon Y.H., Finn E.S., et al. Functional connectivity predicts changes in attention observed across minutes, days, and months. Proc Natl Acad Sci U S A. 2020;117:3797–3807. doi: 10.1073/pnas.1912226117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang C., Glover G.H. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nomi J.S., Bzdok D., Li J., Bolt T., Kornfeld S., Goodman Z.T., et al. Global signal topography in the human brain differs systematically across the lifespan. bioRxiv. 2022 doi: 10.1101/2022.07.27.501804v1. [DOI] [Google Scholar]
- 93.Uddin L.Q., Betzel R.F., Cohen J.R., Damoiseaux J.S., Brigard F.D., Eickhoff S.B., et al. Controversies and current progress on large-scale brain network nomenclature from OHBM WHATNET: Workgroup for HArmonized Taxonomy of NETworks. https://osf.io/25za6/ Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.