Abstract
Background
There is growing evidence that disturbances in cholesterol metabolism may be involved in major depressive disorder (MDD). However, it is not known if cholesterol metabolites present in the brain and periphery can be used to diagnose and predict an MDD patient’s response to antidepressant treatment.
Methods
A total of 176 subjects (85 patients with MDD and 91 healthy control subjects) were included in this study. The expression of peripheral and brain-specific oxysterols and related gene polymorphisms were investigated in all subjects. The severity of depression was measured using the 17-item Hamilton Depression Rating Scale, 16-item Quick Inventory of Depressive Symptoms–Self-Report, and Patient Health Questionnaire-9 for all patients with MDD before and after 12 weeks of antidepressant treatment.
Results
Patients with MDD expressed higher plasma levels of 24(S)-hydroxycholesterol (24OHC) (mainly secreted from the brain) compared with healthy control subjects, and the higher levels of 24OHC were associated with 24OHC synthetase (CYP46A1) gene polymorphisms. In patients with MDD, an improved response to the 12-week antidepressant treatment was associated with a reduction of both 24OHC and 27OHC (mainly secreted from the peripheral system) levels relative to baseline levels. Nonresponders exhibited increased levels of oxysterols at the end of treatment compared with baseline. The superior reduction in oxysterol levels correlated with better outcomes from the antidepressant treatment.
Conclusions
These data suggest a potential role for oxysterols as diagnostic and treatment response–related indicators for MDD.
Keywords: 24(S)-Hydroxycholesterol, 27-Hydroxycholesterol, Major depressive disorder, Treatment response
A disruption in cholesterol metabolism has been implicated in the development of major depressive disorder (MDD) (1, 2, 3). Synthesis and metabolism of cholesterol are relatively independent in the brain and peripheral system due to the blood-brain barrier (BBB) (4). However, it has not been established if and how the two tissue-specific cholesterols are involved in the pathogenesis of MDD and if these metabolites are good indicators to predict a patient’s response to antidepressant treatment.
Previous studies demonstrated that peripheral cholesterol levels might serve as a biological marker for MDD (5, 6, 7). However, the association between circulating cholesterol levels and MDD remains controversial. For example, some studies showed that lower circulating cholesterol is related to a higher risk of MDD (8, 9, 10), but other studies demonstrated opposite results (3,11). Furthermore, an increase in levels of total circulating cholesterols induced by antidepressant administration or electroconvulsive therapy was also reported in patients with MDD (12,13). Meanwhile, a different study reported on lower levels of cholesterol following antidepressant treatment (14). More importantly, hypocholesterolemic drugs reduced the risk of MDD (15). Taken together, the evidence suggests an important role for cholesterol in MDD. It is hypothesized that the contradictory reports of circulating cholesterol levels and their association with MDD might be related to tissue-specific cholesterol, metabolism, and genetic and environmental factors (16, 17, 18, 19). Therefore, to understand the impact of cholesterol on MDD, there is an urgent need to investigate the central or peripheral cholesterol metabolism.
As the major circulating oxysterol in the bloodstream, peripheral cholesterol can be oxidized by cytochrome P450 CYP27A1 into 27-hydroxycholesterol (27OHC), which can cross the BBB to gain entry into the brain by diffusion (20). Both CYP27A1 and 27OHC have been implicated in several neuropsychiatric diseases (20, 21, 22, 23). Changes in the circulating levels of 27OHC are induced by various insults such as oxidative stress and hypercholesteremia (24,25). An increase in 27OHC levels might promote oxidative stress–induced cell damage and contribute to the development of neuropsychiatric diseases (26,27).
The brain is the most cholesterol-rich organ and contains 25% of the body’s cholesterol content (28). Because cholesterol cannot cross the BBB, the brain and peripheral system are able to independently synthesize and metabolize cholesterol (4). For example, brain cholesterol is mainly oxidized into 24(S)-hydroxycholesterol (24OHC), which is catalyzed by the brain-specific enzyme CYP46A1 and released into the bloodstream (29). More than 90% of plasma 24OHC can be attributed to the brain (24,25); therefore, circulating 24OHC reflects brain-specific metabolized cholesterol (4). The source of residual 24OHC in the blood has not been determined but may result from the conversion of cholesterol by CYP27A1 to produce 24OHC, 25OHC, and 27OHC (30). It has been reported that the ratio of 24OHC to total cholesterol in plasma is similar to that in cerebrospinal fluid (r2 = 0.94) (31). 24OHC plays a critical role in the physiological cholesterol homeostasis within the brain, and abnormal changes in 24OHC levels are associated with diseases such as Alzheimer's disease (32, 33, 34), amyotrophic lateral sclerosis (35), Parkinson's disease (14), and autism spectrum disorders (36). Our previous study also indicated an important role of oxysterols in schizophrenia (37). However, there have been limited studies on brain cholesterol metabolism in MDD (5,10). Importantly, a human postmortem study showed a significant increase in 24OHC in the brain of patients with MDD who had committed suicide (38), while another postmortem study indicated no significant change in 24OHC levels associated with the cortex of depressive patients (39). Together, both postmortem human studies proposed an important role for brain cholesterol turnover in MDD, which might differ from changes in peripheral cholesterol levels. The aim of this study was to determine if central and/or peripheral originated oxysterols are involved in the pathogenesis of MDD, and if the levels are able to predict the efficacy and outcome of 12-week antidepressant treatment in patients with MDD.
Methods and Materials
Participants
The participants in this study were part of a multicenter, randomized clinical trial (ChiCTR-OOC-17012566). A total of 176 participants were recruited from the Beijing Anding Hospital of Capital Medical University in China from May 2017 to December 2020 (40). The subjects comprised 85 patients with MDD and 91 age- and sex-matched healthy control (HC) subjects. The study was approved by the Independent Ethics Committee of Beijing Anding Hospital (no. 2017-24) (14). All participants completed written informed consent before being included in the study. Figure 1 represents an overview of the study undertaken.
Figure 1.
An overview of participant selection and treatment protocols. HAMD-17, 17-item Hamilton Depression Rating Scale; HC, healthy control; MDD, major depressive disorder; QIDS-SR16, 16-item Quick Inventory of Depressive Symptoms–Self-Report.
The enrolled patients met the following criteria (14): 1) was an outpatient between 18 and 65 years of age; 2) met the DSM-IV criteria for MDD; and 3) scored 11 or greater on the 16-item Quick Inventory of Depressive Symptoms–Self-Report (QIDS-SR16) (41), and scored 14 or greater on the 17-item Hamilton Depression Rating Scale (HAMD-17) (42). Clinical evaluations were performed through interviews conducted by at least 2 experienced research psychiatrists. The exclusion criteria were the following: 1) had a lifetime history of other psychiatric disorders such as bipolar, schizophrenia, or schizoaffective; 2) had used antidepressant medication over a period of 7 days or longer 14 days prior to their recruitment; 3) had a history of drug or alcohol abuse; 4) was pregnant or breastfeeding; 5) had a serious suicide risk (score of ≥3 on item 3 of the HAMD-17); 6) had severe chronic diseases with ongoing treatment; and 7) had significant medical conditions and unstable psychiatric condition.
The HC subjects were recruited through advertisements and were deemed as mentally healthy based on the criteria of any DSM-IV Axis I psychiatric disorder. In addition, HC participants were excluded if they: 1) had a history of any type of mental disorder; 2) had significant medical conditions; and 3) were pregnant or breastfeeding.
Clinical Treatment and Evaluation
After enrollment, all patients were treated with escitalopram, a commonly used selective serotonin reuptake inhibitor, for 12-weeks to evaluate the effectiveness of the antidepressant. The starting dose of escitalopram was 5 to 10 mg/day based on the individual clinical evaluation, and the average dose was 15.83 ± 4.12 mg/day by the end of the 12th week. During the 12-week treatment period, the severity of depression was assessed with the HAMD-17, QIDS-SR16, and Patient Health Questionnaire-9 (43) at the 4th, 8th, and 12th weeks after initiating treatment. An additional evaluation on the severity of depression was performed using the QIDS-SR16 at the second week. Two clinically experienced psychiatrists who were not informed of the antidepressant treatment assessed the patients’ symptoms. Before subject enrollment, all psychiatrists were trained on the consistency of the scale. The clinical assessment was performed and drug administration recorded at each visit. In addition, all subjects completed self-assessment and mood mapping on the mobile app every day. Patients with MDD who showed a good response to the treatment (a reduced HAMD-17 score by at least 50% posttreatment compared with the initial assessment) were recorded as responders (44,45) and others were identified as nonresponders.
Sample Collection
Plasma samples were collected before treatment (baseline) and at the 2nd and 12th weeks after initiating treatment. In brief, blood samples were collected in EDTA tubes. For DNA isolation, the blood samples were stored at −80 °C until required. For plasma preparation, blood was centrifuged at 1500g for 10 minutes and the plasma was liquated and stored at −80 °C.
Oxysterol Analysis
Plasma cholesterol levels were assessed in the Clinical Laboratory of Beijing Anding Hospital using enzymatic methods (Modular; Roche Diagnostics) as described by Hummel et al. (46). Plasma oxysterol levels were detected using high-performance liquid chromatography–mass spectrometry as described previously (37). In general, 50 μL of plasma or standard sample (with 5% bovine serum albumin) was mixed with 200 μL of acidic buffer solution (50 mM ammonium acetate, 1% formic acid, pH 3) and 1 mL methyl tert-butyl ether in a microcentrifuge tube, while 100 ng D5/D7 deuterium cholesterol was spiked in as an internal standard. The supernatant was harvested after fully mixed and dried down with nitrogen at 30 °C. For the derivatization, the dried samples were reacted with 12.6 g/L N,N′-diisopropylcarbodiimide, 12.4 g/L nicotinamide, and 12.2 g/L 4-dimethylaminopyridine in 50 μL of chloroform solution at 35 °C for 2 hours. The solution was then dried and dissolved in 100 μL of methanol for high-performance liquid chromatography–mass spectrometry analysis.
Genotyping
Genomic DNA was extracted from whole blood using standard methods (QIAGEN). The ratio of optical density 260/optical density 280 was calculated to evaluate the quality of the extracted DNA. DNA extracted from one particular patient was excluded due to the poor quality of the extracted genomic DNA. DNA samples that passed quality control were submitted for genotyping. Genotyping was carried out with the Capital Biotechnology Precision Medicine Research Array Kit (Thermo Fisher Scientific), which is a customized chip based on the Axiom 2.0 platform (Thermo Fisher Scientific). This microarray contains 787,400 single nucleotide polymorphisms (SNPs) and includes 50,000 novel markers specifically for East and South Asian populations, particularly Chinese, based on the human genome version 19 (Genome Reference Consortium Human Build 37). A series of different criteria were used to ensure quality control, including call rate (≥95%), minor allele frequency (≥0.01), and Hardy-Weinberg equilibrium p values (≥1 × 10−6).
Statistical Analysis
Using α = 0.05 and power = 0.90 (Power Analysis and Sample Size, PASS), we projected that the sample size needed to detect differences in oxysterol levels between patients with MDD and HC subjects would be 78 (n = 39 per group). All variables were analyzed using SAS version 9.4 (SAS Institute). The variables were expressed as mean ± SD. Differences in the variables between the HC and MDD groups were analyzed using χ2 test, independent-sample t test, or Wilcoxon rank sum test. Comparison of the variables between HC subjects and MDD responders and nonresponders at baseline was performed using one-way analysis of variance followed by post hoc Bonferroni multiple comparison. Changes in oxysterol levels at baseline and the 2nd and 12th weeks were analyzed via paired t test. The corrected p value < .0167 (.05/3) was considered statistically significant after Bonferroni correction for multiple comparison in oxysterols and cholesterol. Multiple linear regression analysis was used to assess the relationship between changes in oxysterol levels and severity of depression in patients with MDD from baseline to the endpoint of treatment with age, sex, and body mass index as covariates. Two-way repeated-measures analysis of variance with Bonferroni adjustment was used to compare the oxysterol levels between responder and nonresponder groups. The differences in clinical assessments and oxysterol levels between genotypes were analyzed via independent sample t test. The receiver-operating characteristic (ROC) curve and the area under the ROC curve were used to evaluate the diagnosis value of oxysterol levels in patients with MDD. The genotyping analysis was assessed with PLINK 1.9 (https://www.cog-genomics.org/plink/1.9/). The Hardy-Weinberg equilibrium was conducted by χ2 test for each SNP of every participant. The multivariate logistic regression analysis was used to evaluate the association between SNPs and MDD susceptibility or plasma 24OHC levels at baseline, adjusting for age and sex. The multiple-corrected p value of <1.33 × 10−7 was considered statistically significant after Bonferroni correction for the gene analysis. In other cases, the level of significance (p value) of <.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
The sociodemographic and clinical characteristics of the participants are presented in Table 1. There were no significant differences for all demographic data between patients with MDD and HC subjects, despite the MDD group showing a trend of higher body mass index than HC subjects.
Table 1.
Demographic and Clinical Characteristics
| HC, n = 91 | MDD, n = 85 | Statistic | p | |
|---|---|---|---|---|
| Demographic Data | ||||
| Age, years | 29.31 (6.98) | 28.95 (7.56) | t174 = 0.336 | .737 |
| Sex, female/male | 57/34 | 53/32 | χ21 = 0.002 | .969 |
| Body mass index | 21.78 (3.87) | 22.68 (3.45) | t174 = −1.625 | .106 |
| Clinical Characteristics | ||||
| Family history, MDD, yes/no | 0/91 | 20/65 | χ21 = 24.157 | <.001 |
| Onset age, years | NA | 25.08 (7.18) | NA | NA |
| Duration of illness, years | NA | 2.91 (4.04) | NA | NA |
| Clinical Assessments | ||||
| HAMD-17 score at baseline | NA | 20.60 (4.26) | NA | NA |
| HAMD-17 score at endpoint | NA | 7.63 (6.28) | NA | NA |
| QIDS-SR16 score at baseline | NA | 15.21 (3.38) | NA | NA |
| QIDS-SR16 score at endpoint | NA | 6.91 (4.63) | NA | NA |
| PHQ-9 score at baseline | NA | 16.55 (4.34) | NA | NA |
| PHQ-9 score at endpoint | NA | 6.41 (5.99) | NA | NA |
Values are mean (SD) or n.
HAMD-17, 17-item Hamilton Depression Rating Scale; HC, healthy control; MDD, major depressive disorder; NA, not available; PHQ-9, Patient Health Questionnaire-9; QIDS-SR16, 16-item Quick Inventory of Depressive Symptoms–Self-Report.
Higher Levels of Cholesterol and 24OHC in Patients With MDD Compared With HC Subjects
As shown in Table 2, plasma cholesterol levels were significantly higher in patients with MDD compared with HC subjects (p < .001). At same time, plasma 24OHC levels were also higher in patients with MDD compared with HC subjects (p < .001). Hence, the 24OHC/cholesterol ratio was significantly higher in patients with MDD than control subjects (p = .009). On the other hand, no significant difference was observed in plasma 27OHC levels between patients with MDD and control subjects (p = .799). There was also no difference in the 27OHC/cholesterol ratios between patients and control subjects (p = .080).
Table 2.
Comparison of Cholesterol Metabolism Between Patients With MDD and HC Subjects and Patients Prior to and Post Antidepressant Treatment
| HC |
MDD |
HC vs. MDD (Baseline) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Baseline (W0) | W2 | W12 | p1 | p2 | p3 | Statistic | p | |
| 24OHC, ng/mL | 33.11 (11.49) | 44.08 (17.31) | 43.54 (17.41) | 42.66 (16.93) | .644 | .364 | .712 | t174 = −4.990 | <.001a |
| 27OHC, ng/mL | 58.38 (38.53) | 57.08 (28.20) | 55.54 (26.70) | 53.91 (26.77) | .496 | .047 | .396 | z = 0.255b | .799 |
| Cholesterol, mmol/L | 4.27 (0.91) | 4.85 (1.15) | 4.70 (0.99) | 4.82 (0.95) | .114 | .747 | .263 | t172 = −3.759 | <.001a |
| 24OHC/Cholesterol Ratio, mg/g | 20.71 (6.80) | 23.84 (8.71) | 24.63 (10.57) | 23.26 (9.02) | .348 | .572 | .319 | t172 = −2.649 | .009a |
| 27OHC/Cholesterol Ratio, mg/g | 37.21 (25.81) | 31.38 (16.46) | 31.87 (16.10) | 29.81 (15.33) | .398 | .124 | .118 | z172 = 1.760b | .080 |
Values are mean (SD). A corrected p value < .0167 (.05/3) was considered statistically significant after Bonferroni correction. p1: W0 vs. W2; p2: W0 vs. W12; p3: W2 vs. W12.
24OHC, 24(S)-hydroxycholesterol; 27OHC, 27-hydroxycholesterol; HC, healthy control; MDD, major depressive disorder; W, week.
Statistically significant differences.
Wilcoxon rank sum test.
To examine the disease specificity of plasma 24OHC levels in MDD, we subsequently performed an ROC curve analysis. The area under the ROC curve obtained by the ROC analysis was 0.783 (95% CI, 0.717–0.849) when the indicators of age, sex, and body mass index were combined, denoting the utility of plasma 24OHC levels to differentiate patients with MDD from HC subjects. The best area under the ROC curve result showed a sensitivity of 74.7% and specificity of 72.5%, suggesting that plasma 24OHC levels might be a potential diagnostic biomarker of MDD.
CYP46A1 Gene Polymorphisms and 24OHC Levels in Patients With MDD and HC Subjects
To explore the mechanism of increase in 24OHC levels in patients with MDD, we conducted a gene polymorphisms analysis in subjects. Unfortunately, we found no significant difference in SNPs (n = 375,184) between patients with MDD and HC subjects (all ps > 1.33 × 10−7 [.05/375,184] after Bonferroni correction) (Table S1). Considering that CYP46A1 gene polymorphisms might be an important factor to 24OHC levels, we analyzed the SNPs in CYP46A1 (n = 100) between patients with MDD and HC subjects to explore the linkage between 24OHC levels and oxysterol-related gene polymorphisms (Table S2). Our data showed 6 SNPs in the CYP46A1 gene potentially associated with susceptibility to MDD (Table 3 and Table S2); however, these differences did not reach statistical significance after Bonferroni correction (all ps > 5 × 10−4 [.05/100]). We conducted linear regression analyses on the 6 SNPs and levels of 24OHC to explore whether the disease-associated variants could account for the difference in 24OHC levels among individuals. Interestingly, we observed that an increase in the minor allele (CA) dose of rs35267715 was associated with increments in 24OHC levels (β = 5.045; 95% CI = 1.331–8.760; t = 2.662, p = .009) in HC subjects. Coincidentally, more patients with MDD carried the CA allele (56%) than HC subjects (46%) (Table 3). However, any association between the minor allele (CA) dose of rs35267715 and plasma 24OHC levels was not evident for patients with MDD (p > .05) (Figure S1).
Table 3.
Associations of CYP46A1 Gene Polymorphisms With the Susceptibility to MDD
| SNP | Allelea | HCb | MDDb | MAFc | Additive Modeld |
Dominant Modeld |
Recessive Modeld |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |||||
| rs4905883 | G/T | 0/4/87 | 2/12/70 | 0.02/0.10 | 4.245 (1.39–12.96) | .011 | 4.500 (1.40–14.44) | .012 | NA | NA |
| rs1957512 | G/A | 1/7/83 | 2/17/65 | 0.05/0.13 | 2.542 (1.15–5.64) | .022 | 3.062 (1.26–7.46) | .014 | 2.222 (0.19–25.36) | .520 |
| rs1957513 | T/C | 1/7/83 | 2/16/66 | 0.05/0.12 | 2.387 (1.08–5.29) | .032 | 2.849 (1.16–6.98) | .022 | 2.222 (0.19–25.36) | .520 |
| rs1957515 | C/A, G, T | 1/8/82 | 2/18/64 | 0.05/0.13 | 2.439 (1.13–5.26) | .023 | 2.873 (1.22–6.75) | .015 | 2.222 (0.19–25.36) | .520 |
| rs6575744 | G/C | 1/7/83 | 2/16/66 | 0.05/0.12 | 2.387 (1.08–5.29) | .032 | 2.849 (1.16–6.98) | .022 | 2.222 (0.19–25.36) | .520 |
| rs35267715 | CA/C | 6/36/49 | 14/33/37 | 0.26/0.36 | 1.571 (0.99–2.48) | .052 | 1.489 (0.81–2.72) | .196 | 2.976 (1.06–8.36) | .038 |
HC, healthy control; MAF, minor allele frequency; MDD, major depressive disorder; NA, not available; OR, odds ratio; SNP, single nucleotide polymorphism.
Minor/major allele.
Rare homozygote/heterozygote/major homozygote.
HC subjects/patients with MDD.
Logistic regression with adjustment for age and sex.
Association of Changes in Cholesterol and Oxysterol Levels and Improvement in Depressive Symptoms During 12 Weeks of Antidepressant Treatment
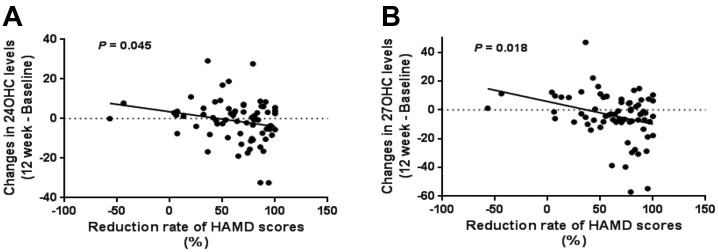
A multiple linear regression analysis was used to assess the association between the changes in oxysterol levels and changes in depression severity after the 12-week antidepressant treatment. As shown in Table 4 and Figure 2, changes in oxysterol levels were significantly associated with reduced scores for the HAMD-17 (24OHC: p = .045; 27OHC: p = .018) and QIDS-SR16 (24OHC: p = .088; 27OHC: p = .048).
Table 4.
Associations of the Changes in Oxysterol Levels and Improvement of Depressive Symptoms After Antidepressant Treatment
| HAMD-17 |
QIDS-SR16 |
PHQ-9 |
||||
|---|---|---|---|---|---|---|
| t | p | t | p | t | p | |
| 24OHC | ||||||
| Baseline | 1.092 | .278 | 1.14 | .269 | 0.974 | .333 |
| Week 2 – baseline | 0.220 | .826 | 0.497 | .621 | 0.176 | .861 |
| Week 12 – baseline | −2.048 | .045a | −1.733 | .088 | −0.899 | .372 |
| 27OHC | ||||||
| Baseline | 1.495 | .139 | 1.699 | .094 | 0.629 | .531 |
| Week 2 – baseline | 0.030 | .976 | 0.72 | .474 | 0.843 | .402 |
| Week 12 – baseline | −2.432 | .018a | −2.011 | .048a | −0.365 | .716 |
| Cholesterol | ||||||
| Baseline | 1.081 | .283 | 1.071 | .288 | 1.157 | .251 |
| Week 2 – baseline | 0.175 | .861 | 0.671 | .449 | 0.748 | .457 |
| Week 12 – baseline | −0.641 | .524 | −0.435 | .665 | −0.652 | .517 |
24OHC, 24(S)-hydroxycholesterol; 27OHC, 27-hydroxycholesterol; HAMD-17, 17-item Hamilton Depression Rating Scale; PHQ-9, Patient Health Questionnaire-9; QIDS-SR16, 16-item Quick Inventory of Depressive Symptoms–Self-Report.
Statistically significant differences.
Figure 2.
Multiple regression analysis of oxysterols and improvement in depressive symptoms of patients with major depressive disorder. The regression curves of plasma (A) 24(S)-hydroxycholesterol (24OHC) and (B) 27-hydroxycholesterol (27OHC) levels and Hamilton Depression Rating Scale (HAMD) reduction rates in patients with major depressive disorder. n = 85.
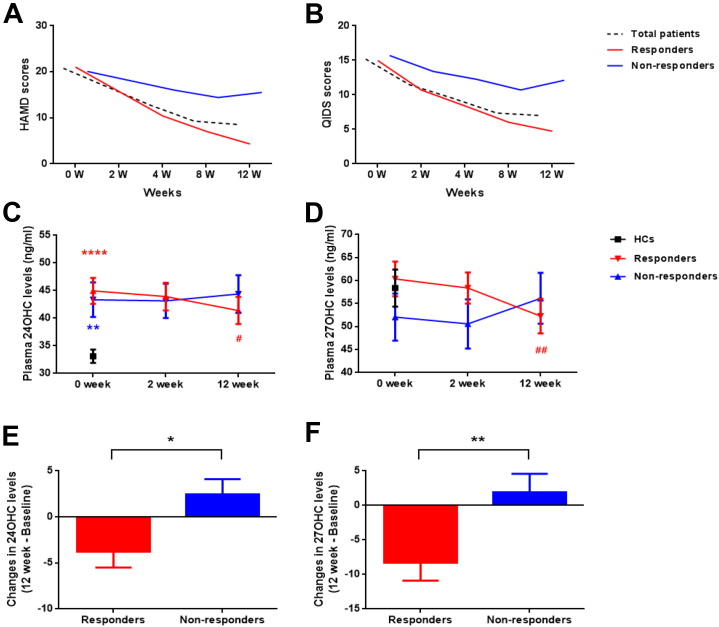
Changes in Oxysterol Levels With Different Treatment Outcomes
To further clarify the relationship between oxysterol levels and the effectiveness of treatment, patients with MDD were subgrouped as responders (n = 62 [72.9%]) and nonresponders (n = 23 [27.1%]). However, a total of 15 patients were excluded from the analysis, as their 12-week blood samples were not available; hence, all further analysis is based on 51 responders and 19 nonresponders (Figure 3A, B). In general, 12 weeks of antidepressant treatment did not result in any significant changes in average total plasma cholesterol or oxysterol levels in patients with MDD (Table 2). However, at the end of the 12-week treatment, only the responders exhibited a significant reduction in both 24OHC and 27OHC levels when compared with baseline levels, as shown in Figure 3C and D. In contrast, the nonresponders demonstrated elevated 24OHC and 27OHC levels after completing the treatment (Figure 3E, F). Repeated-measures analysis of variance showed that there was no significant main effect for time (F1 = 0.002, p = .961), group assignment (F1 = 0.335, p = .565), or interaction (F1 = 2.747, p = .102) in 24OHC levels. However, we found a significant interaction effect (F1 = 8.498, p = .005) in 27OHC but no significant main effect for time (F1 = 0.276, p = .601) or group assignment (F1 = 0.072, p = .789).
Figure 3.
Different changes in depressive symptoms and oxysterol levels between the responders and nonresponders after antidepressant therapy. The different alteration of (A) Hamilton Depression Rating Scale (HAMD) and (B) Quick Inventory of Depressive Symptoms (QIDS) scores in the responders and nonresponders. The difference in levels of (C) 24(S)-hydroxycholesterol (24OHC) and (D) 27-hydroxycholesterol (27OHC) in responders, nonresponders, and healthy control (HC) subjects after antidepressant treatment. A contradictory change in both 24OHC and 27OHC levels after treatment was noted in responders and nonresponders. The reduction of (E) 24OHC and (F) 27OHC levels from baseline to week 12 in responders and nonresponders. ∗∗p < .01 and ∗∗∗∗p < .001 vs. HC subjects at baseline in panel (C); #p < .05 vs. responders at baseline in panel (C); ##p < .01 vs. responders at baseline in panel (D); and ∗p < .05 and ∗∗p < .01 vs. responders in panels (E) and (F), respectively. n = 51 in the responder group; n = 19 in the nonresponder group; n = 91 in the HC group.
Discussion
Given the previous association between cholesterol levels and the pathogenesis of MDD, this study was initiated to explore tissue-specific cholesterol metabolism in MDD and its relationship with the effectiveness of antidepressant treatment. We noted elevated brain cholesterol metabolism in patients with MDD, while changes in both brain and peripheral cholesterol metabolism were associated with antidepressant treatment. We demonstrated that only responders had reduced 24OHC and 27OHC levels compared with pretreatment or baseline levels. On the other hand, the opposite pattern was observed in the nonresponders after 12 weeks of antidepressant treatment.
First, we analyzed levels of the brain cholesterol metabolite 24OHC and peripheral oxysterol 27OHC in plasma to examine whether patients with MDD expressed any disruption in cholesterol metabolism in the brain and peripheral region. We found that plasma 24OHC levels, but not 27OHC levels, were significantly higher in patients with MDD compared with HC subjects. Because circulating levels of 24OHC might reflect general brain cholesterol hemostasis, the higher levels of plasma 24OHC could also be a result of neuronal damage–induced accumulation of cholesterol in the brain (29), or they could be involved in the pathogenesis of brain disorders. It is reasonable to hypothesize that elevated 24OHC plasma levels in patients with MDD might be related to neuropathology of MDD because brain atrophy and white matter lesions are often found in patients with MDD compared with HC subjects (47, 48, 49, 50, 51). Another possible regulator for brain-specific cholesterol levels is the activity of enzyme involved in cholesterol oxidization, such as CYP46A1 (52). In this study, HC subjects showed an increase in the minor allele (CA) dose of rs35267715 in the CYP46A1 gene, which was positively associated with increments in 24OHC levels. Interestingly, our data showed no such relationship between the CA allele with 24OHC levels in patients with MDD, even with a higher frequency of MDD CA carriers compared with HC subjects. While the impact of the CYP46A1 gene polymorphism rs35267715 on MDD pathology remains unresolved, the CYP46A1 gene rs754203 mutation has been reported to confer a high risk of Alzheimer’s disease as well as reduced circulating 24OHC levels in patients with Alzheimer’s disease (53,54). However, we were not able to identify any impact of rs754203 on 24OHC levels and risk of MDD (Table S2). Our data suggest that the higher levels of 24OHC in patients with MDD might be related to MDD brain pathologies, rather than to CYP46A1 polymorphisms. Another possible reason for the higher levels of 24OHC in MDD might be due to the higher levels of cholesterol in patients with MDD (Table 2). However, 27OHC levels or the ratio of 27OHC/cholesterol were similar in both patients with MDD and HC subjects; therefore, it seems unlikely that plasma cholesterol is responsible for the higher 24OHC level in patients with MDD.
While the underlying mechanism involved in the higher levels of circulating 24OHC in MDD remains unclear, studies have shown that enriched 24OHC levels might lead to many neurotoxic effects, such as inducing oxidative stress, inflammation, apoptosis, and excitatory toxicity (33,55, 56, 57, 58, 59). Furthermore, previous studies have shown that 24OHC might also serve as a potent positive allosteric modulator for the synaptic excitatory protein NMDA receptor to increase synaptic plasticity (60, 61, 62). In addition, elevated 24OHC levels might impair cholesterol synthesis and promote cholesterol excretion from the body by the liver X receptor (51). However, in our study, the higher levels of cholesterol observed in patients with MDD compared with control subjects suggests a limited role for liver X receptor.
Although strong evidence indicates that 27OHC is toxic to cells, including causing inflammatory reactions (53,59), promoting oxidative stress (27), and serving as an endogenous selective estrogen receptor modulator to link oxysterols and disease (63,64), we found no differences in 27OHC levels between MDD and HC subjects. It seems likely that cholesterol metabolism in the peripheral system might not contribute as much oxysterol as the central region toward the risk of experiencing MDD. A previous study indicated that 27OHC might promote less insult-induced neurotoxicity than 24OHC (65), suggesting a role for brain cholesterol metabolism in MDD. Further investigation into tissue-specific oxysterols together with a full panel of lipid metabolism in MDD might be important.
Another important and interesting finding from this study is that the changes to oxysterol levels were associated with an improvement in the depressive symptoms exhibited by patients with MDD. While all 3 clinical scores (HAMD-17, Patient Health Questionnaire-9, QIDS-SR16) were used in the evaluation of depression, only improvement of HAMD-17 scores showed significant association with changes in 24OHC levels. As previous studies indicated that the HAMD-17 is the most sensitive and reliable scale for depressive symptoms evaluation (66,67), a potential explanation of our result might be due to the individual feelings of the patients when performed, as the self-assessment scales varied greatly. In this study, we found that oxysterols, especially 27OHC, were reduced in the responders but increased in the nonresponders after antidepressant treatment when compared with levels at the baseline. We speculate that the relationship between oxysterols and the effectiveness of antidepressant treatment is dependent on the integrity of lipid rafts in the plasma membrane. It is known that localization of serotonin transporters into lipid raft microdomains is critical for serotonin uptake and is associated with the success of antidepressant treatment (34,68). Indeed, escitalopram is associated with the translocation of G protein alpha (Gsα) from lipid rafts (69,70). Both 24OHC and 27OHC are found in lipid raft microdomains. It is thought that an increase in 24OHC concentration could induce protein distribution within lipid raft microdomains to influence the lipid raft function (71), and an increase in 27OHC levels disrupted the lipid raft function through reducing the cholesterol density in the raft (45). These data imply that the increase in 27OHC after escitalopram treatment might have resulted from disrupting the integrity of lipid microdomains in the plasma membrane to dampen the antidepressant effect in the nonresponders. In addition, excess plasma 27OHC can cross the BBB into the brain, where it can activate nuclear factor-κB, oxidative stress, and inflammation, all of which are hallmarks of MDD (43,72, 73, 74, 75, 76).
This study has several limitations. First, we were able to complete the genotype analysis of only 84 patients, and future studies with larger sample sizes are needed to clarify the association between CYP46A1 polymorphisms and MDD as well as 24OHC levels in patients with MDD. Second, we only treated patients with MDD with escitalopram for 12 weeks, while a long treatment trial with follow-up investigation might provide more insight on tissue-specific oxysterols in patients with MDD (77). Third, we did not measure the levels of escitalopram and its metabolites in the blood to monitor the medication of patients. Last, the patients enrolled in this study mainly presented with mild forms of depression. Whether oxysterols play a role in patients with mild and severe symptoms should be investigated in the future.
To our knowledge, this is the first study to provide evidence of a relationship between MDD and plasma oxysterol levels following antidepressant treatment. Taken together, our findings suggest a potential role for oxysterols as diagnostic and treatment response–related markers for MDD and might represent targets for intervention in an attempt to slow disease progression and improve antidepressant treatment.
Acknowledgments and Disclosures
This work was supported by the National Natural Science Foundation of China (Grant No. 82171525 [to ZS]), National Key Research and Development Program of China (Grant No. 2016YFC1307200 [to GW]), Beijing Hospitals Authority Youth Programme (Grant No. QML20181901 [to JY]), Beijing Young Top-Notch Talent Support Project (Grant No. 2018000021223ZK36 [to JY]), and Beijing Biobank of Clinical Resources-Mental Disorders. The grant provider had no role in study design, data collection, and decision to publish the article.
The authors report no biomedical financial interests or potential conflicts of interest.
Chinese Clinical Trial Registry: Appropriate technology study of MDD diagnosis and treatment based on objective indicators and measurement; http://www.chictr.org.cn/showproj.aspx?proj=21377; ChiCTR-OOC-17012566.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.01.004.
Contributor Information
Gang Wang, Email: gangwangdoc@ccmu.edu.cn.
Rena Li, Email: renali@ccmu.edu.cn.
Supplementary Material
References
- 1.Parekh A., Smeeth D., Milner Y., Thure S. The role of lipid biomarkers in major depression. Healthcare (Basel) 2017;5:5. doi: 10.3390/healthcare5010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persons J.E., Fiedorowicz J.G. Depression and serum low-density lipoprotein: A systematic review and meta-analysis. J Affect Disord. 2016;206:55–67. doi: 10.1016/j.jad.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner C.J., Musenbichler C., Bohm L., Farber K., Fischer A.I., von Nippold F., et al. LDL cholesterol relates to depression, its severity, and the prospective course. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:405–411. doi: 10.1016/j.pnpbp.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkhem I. Crossing the barrier: Oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 5.Ong K.L., Morris M.J., McClelland R.L., Maniam J., Allison M.A., Rye K.A. Lipids, lipoprotein distribution and depressive symptoms: The Multi-Ethnic Study of Atherosclerosis. Transl Psychiatry. 2016;6:e962. doi: 10.1038/tp.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bot M., Milaneschi Y., Al-Shehri T., Amin N., Garmaeva S., Onderwater G.L.J., et al. Metabolomics profile in depression: A pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol Psychiatry. 2020;87:409–418. doi: 10.1016/j.biopsych.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nobis A., Zalewski D., Waszkiewicz N. Peripheral markers of depression. J Clin Med. 2020;9 doi: 10.3390/jcm9123793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabe-Jablonska J., Poprawska I. Levels of serum total cholesterol and LDL-cholesterol in patients with major depression in acute period and remission. Med Sci Monit. 2000;6:539–547. [PubMed] [Google Scholar]
- 9.Wu S., Ding Y., Wu F., Xie G., Hou J., Mao P. Serum lipid levels and suicidality: A meta-analysis of 65 epidemiological studies. J Psychiatry Neurosci. 2016;41:56–69. doi: 10.1503/jpn.150079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messaoud A., Mensi R., Mrad A., Mhalla A., Azizi I., Amemou B., et al. Is low total cholesterol levels associated with suicide attempt in depressive patients? Ann Gen Psychiatry. 2017;16:20. doi: 10.1186/s12991-017-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y.J., Zhou Y.J., Wang D.F., Li Y., Wang D.M., Liu T.Q., et al. Association of lipid profile and suicide attempts in a large sample of first episode drug-naive patients with major depressive disorder. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.543632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabriel A. Changes in plasma cholesterol in mood disorder patients: Does treatment make a difference? J Affect Disord. 2007;99:273–278. doi: 10.1016/j.jad.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Aksay S.S., Bumb J.M., Janke C., Biemann R., Borucki K., Lederbogen F., et al. Serum lipid profile changes after successful treatment with electroconvulsive therapy in major depression: A prospective pilot trial. J Affect Disord. 2016;189:85–88. doi: 10.1016/j.jad.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Stuchtey F.C., Block A., Osei F., Wippert P.M. Lipid biomarkers in depression: Does antidepressant therapy have an impact? Healthcare (Basel) 2022;10:333. doi: 10.3390/healthcare10020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsaik A.K., Singh B., Murad M.H., Singh K., Mascarenhas S.S., Williams M.D., et al. Statins use and risk of depression: A systematic review and meta-analysis. J Affect Disord. 2014;160:62–67. doi: 10.1016/j.jad.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Bortolasci C.C., Vargas H.O., Souza-Nogueira A., Barbosa D.S., Moreira E.G., Nunes S.O., et al. Lowered plasma paraoxonase (PON)1 activity is a trait marker of major depression and PON1 Q192R gene polymorphism-smoking interactions differentially predict the odds of major depression and bipolar disorder. J Affect Disord. 2014;159:23–30. doi: 10.1016/j.jad.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Casarotto A.A.F., Galera B.B., Sumiyoshi L.M., Floor T.M. Polymorphism rs7895833 in the SIRT1 gene and its association with dyslipidaemia in the elderly. Rev Esp Geriatr Gerontol. 2019;54:214–219. doi: 10.1016/j.regg.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J., Zhang C., Wu X., Xie Q., Li L., Chen Y., et al. Identification of genes and pathways related to atherosclerosis comorbidity and depressive behavior via RNA-seq and bioinformation analysis in ApoE(-/-) mice. Ann Transl Med. 2019;7:733. doi: 10.21037/atm.2019.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rey M., Kruse M.S., Magrini-Huaman R.N., Coirini H. High-fat diets and LXRs expression in rat liver and hypothalamus. Cell Mol Neurobiol. 2019;39:963–974. doi: 10.1007/s10571-019-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao S., Liao W., Xu N., Xu H., Yu C., Liu X., et al. Polar metabolite of cholesterol induces rat cognitive dysfunctions. Neuroscience. 2009;164:398–403. doi: 10.1016/j.neuroscience.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Heverin M., Maioli S., Pham T., Mateos L., Camporesi E., Ali Z., et al. 27-hydroxycholesterol mediates negative effects of dietary cholesterol on cognition in mice. Behav Brain Res. 2015;278:356–359. doi: 10.1016/j.bbr.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Wang H.-L., Wang Y.-Y., Liu X.-G., Kuo S.-H., Liu N., Song Q.-Y., et al. Cholesterol, 24-hydroxycholesterol, and 27-hydroxycholesterol as surrogate biomarkers in cerebrospinal fluid in mild cognitive impairment and Alzheimer's disease: A meta-analysis. Journal of Alzheimers Disease. 2016;51:45–55. doi: 10.3233/JAD-150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schommer J., Marwarha G., Schommer T., Flick T., Lund J., Ghribi O. 27-Hydroxycholesterol increases alpha-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci. 2018;19:17. doi: 10.1186/s12868-018-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Iuliano L., Crick P.J., Zerbinati C., Tritapepe L., Abdel-Khalik J., Poirot M., et al. Cholesterol metabolites exported from human brain. Steroids. 2015;99:189–193. doi: 10.1016/j.steroids.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leoni V. Oxysterols as markers of neurological disease--A review. Scand J Clin Lab Invest. 2009;69:22–25. doi: 10.1080/00365510802651858. [DOI] [PubMed] [Google Scholar]
- 26.Liu J., Liu Y., Chen J., Hu C., Teng M., Jiao K., et al. The ROS-mediated activation of IL-6/STAT3 signaling pathway is involved in the 27-hydroxycholesterol-induced cellular senescence in nerve cells. Toxicol In Vitro. 2017;45:10–18. doi: 10.1016/j.tiv.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Ma W.-W., Li C.-Q., Yu H.-L., Zhang D.-D., Xi Y.-D., Han J., et al. The oxysterol 27-hydroxycholesterol increases oxidative stress and regulate Nrf2 signaling pathway in astrocyte cells. Neurochem Res. 2015;40:758–766. doi: 10.1007/s11064-015-1524-2. [DOI] [PubMed] [Google Scholar]
- 28.Bjorkhem I., Meaney S. Brain cholesterol: Long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 29.Hughes T.M., Rosano C., Evans R.W., Kuller L.H. Brain cholesterol metabolism, oxysterols, and dementia. J Alzheimers Dis. 2013;33:891–911. doi: 10.3233/JAD-2012-121585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund E.G., Xie C., Kotti T., Turley S.D., Dietschy J.M., Russell D.W. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem. 2003;278:22980–22988. doi: 10.1074/jbc.M303415200. [DOI] [PubMed] [Google Scholar]
- 31.Lutjohann D., Breuer O., Ahlborg G., Nennesmo I., Siden A., Diczfalusy U., et al. Cholesterol homeostasis in human brain: Evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci U S A. 1996;93:9799–9804. doi: 10.1073/pnas.93.18.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarrouk A., Debbabi M., Bezine M., Karym E.M., Badreddine A., Rouaud O., et al. Lipid biomarkers in Alzheimer's disease. Curr Alzheimer Res. 2018;15:303–312. doi: 10.2174/1567205014666170505101426. [DOI] [PubMed] [Google Scholar]
- 33.Testa G., Staurenghi E., Zerbinati C., Gargiulo S., Iuliano L., Giaccone G., et al. Changes in brain oxysterols at different stages of Alzheimer's disease: Their involvement in neuroinflammation. Redox Biol. 2016;10:24–33. doi: 10.1016/j.redox.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponleitner M., Szollosi D., El-Kasaby A., Koban F., Freissmuth M., Stockner T. Thermal unfolding of the human serotonin transporter: Differential effect by stabilizing and destabilizing mutations and cholesterol on thermodynamic and kinetic stability. Mol Pharmacol. 2022;101:95–105. doi: 10.1124/molpharm.121.000413. [DOI] [PubMed] [Google Scholar]
- 35.Vejux A., Namsi A., Nury T., Moreau T., Lizard G. Biomarkers of amyotrophic lateral sclerosis: Current status and interest of oxysterols and phytosterols. Front Mol Neurosci. 2018;11:12. doi: 10.3389/fnmol.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grayaa S., Zerbinati C., Messedi M., Hadjkacem I., Chtourou M., Ben Touhemi D., et al. Plasma oxysterol profiling in children reveals 24-hydroxycholesterol as a potential marker for autism spectrum disorders. Biochimie. 2018;153:80–85. doi: 10.1016/j.biochi.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Sun Z., Zhao L., Bo Q., Mao Z., He Y., Jiang T., et al. Brain-specific oxysterols and risk of schizophrenia in clinical high-risk subjects and patients with schizophrenia. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.711734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freemantle E., Chen G.G., Cruceanu C., Mechawar N., Turecki G. Analysis of oxysterols and cholesterol in prefrontal cortex of suicides. International Journal of Neuropsychopharmacology. 2013;16:1241–1249. doi: 10.1017/S1461145712001587. [DOI] [PubMed] [Google Scholar]
- 39.Beasley C.L., Honer W.G., Bergmann K., Falkai P., Lutjohann D., Bayer T.A. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7:449–455. doi: 10.1111/j.1399-5618.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J., Wang X., Yang J., Zhu X., Xiao L., Feng L., et al. Optimization of measurement-based care (OMBC) for depression based on all-round and continuous assessment: Rationale and protocol for a multicenter randomized control clinical trial. Trials. 2022;23:367. doi: 10.1186/s13063-022-06295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rush A.J., Trivedi M.H., Ibrahim H.M., Carmody T.J., Arnow B., Klein D.N., et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman M., Martinez J.H., Young D., Chelminski I., Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150:384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis A.K., Barrett F.S., May D.G., Cosimano M.P., Sepeda N.D., Johnson M.W., et al. Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry. 2021;78:481–489. doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR∗D: Implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 46.Hummel J., Westphal S., Weber-Hamann B., Gilles M., Lederbogen F., Angermeier T., et al. Serum lipoproteins improve after successful pharmacologic antidepressant treatment: A randomized open-label prospective trial. J Clin Psychiatry. 2011;72:885–891. doi: 10.4088/JCP.09m05853blu. [DOI] [PubMed] [Google Scholar]
- 47.Repple J., Mauritz M., Meinert S., de Lange S.C., Grotegerd D., Opel N., et al. Severity of current depression and remission status are associated with structural connectome alterations in major depressive disorder. Mol Psychiatry. 2019;25:1550–1558. doi: 10.1038/s41380-019-0603-1. [DOI] [PubMed] [Google Scholar]
- 48.Wang L., Leonards C.O., Sterzer P., Ebinger M. White matter lesions and depression: A systematic review and meta-analysis. J Psychiatr Res. 2014;56:56–64. doi: 10.1016/j.jpsychires.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Elbejjani M., Fuhrer R., Abrahamowicz M., Mazoyer B., Crivello F., Tzourio C., et al. Depression, depressive symptoms, and rate of hippocampal atrophy in a longitudinal cohort of older men and women. Psychol Med. 2015;45:1931–1944. doi: 10.1017/S0033291714003055. [DOI] [PubMed] [Google Scholar]
- 50.Yao Z., Fu Y., Wu J., Zhang W., Yu Y., Zhang Z., et al. Morphological changes in subregions of hippocampus and amygdala in major depressive disorder patients. Brain Imaging Behav. 2018;14:653–667. doi: 10.1007/s11682-018-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pannu P.S., Allahverdian S., Francis G.A. Oxysterol generation and liver X receptor-dependent reverse cholesterol transport: Not all roads lead to Rome. Mol Cell Endocrinol. 2013;368:99–107. doi: 10.1016/j.mce.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 52.McGraw J., Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol. 2012;8:371–382. doi: 10.1517/17425255.2012.657626. [DOI] [PubMed] [Google Scholar]
- 53.Asghari A., Ishikawa T., Hiramitsu S., Lee W.R., Umetani J., Bui L., et al. 27-Hydroxycholesterol promotes adiposity and mimics adipogenic diet-induced inflammatory signaling. Endocrinology. 2019;160:2485–2494. doi: 10.1210/en.2019-00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrov A.M., Pikuleva I.A. Cholesterol 24-hydroxylation by CYP46A1: Benefits of modulation for brain diseases. Neurotherapeutics. 2019;16:635–648. doi: 10.1007/s13311-019-00731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarrouk A., Hammami M., Moreau T., Lizard G. Accumulation of 24S-hydroxycholesterol in neuronal SK-N-BE cells treated with hexacosanoic acid (C26:0): Argument in favor of 24S-hydroxycholesterol as a potential biomarker of neurolipotoxicity. Rev Neurol (Paris) 2015;171:125–129. doi: 10.1016/j.neurol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Bezine M., Debbabi M., Nury T., Ben-Khalifa R., Samadi M., Cherkaoui-Malki M., et al. Evidence of K+ homeostasis disruption in cellular dysfunction triggered by 7-ketocholesterol, 24S-hydroxycholesterol, and tetracosanoic acid (C24:0) in 158N murine oligodendrocytes. Chem Phys Lipids. 2017;207:135–150. doi: 10.1016/j.chemphyslip.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Mast N., Lin J.B., Anderson K.W., Bjorkhem I., Pikuleva I.A. Transcriptional and post-translational changes in the brain of mice deficient in cholesterol removal mediated by cytochrome P450 46A1 (CYP46A1) PLoS One. 2017;12 doi: 10.1371/journal.pone.0187168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun M.Y., Taylor A., Zorumski C.F., Mennerick S. 24S-hydroxycholesterol and 25-hydroxycholesterol differentially impact hippocampal neuronal survival following oxygen-glucose deprivation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houben T., Bitorina A.V., Oligschlaeger Y., Jeurissen M.L., Rensen S., Kohler S.E., et al. Sex-opposed inflammatory effects of 27-hydroxycholesterol are mediated via differences in estrogen signaling. J Pathol. 2020;251:429–439. doi: 10.1002/path.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasimov M.R., Fatkhrakhmanova M.R., Mukhutdinova K.A., Petrov A.M. 24S-Hydroxycholesterol enhances synaptic vesicle cycling in the mouse neuromuscular junction: Implication of glutamate NMDA receptors and nitric oxide. Neuropharmacology. 2017;117:61–73. doi: 10.1016/j.neuropharm.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 61.Paul S.M., Doherty J.J., Robichaud A.J., Belfort G.M., Chow B.Y., Hammond R.S., et al. The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-D-aspartate receptors. J Neurosci. 2013;33:17290–17300. doi: 10.1523/JNEUROSCI.2619-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei X., Nishi T., Kondou S., Kimura H., Mody I. Preferential enhancement of GluN2B-containing native NMDA receptors by the endogenous modulator 24S-hydroxycholesterol in hippocampal neurons. Neuropharmacology. 2019;148:11–20. doi: 10.1016/j.neuropharm.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He S.S., Nelson E.R. 27-Hydroxycholesterol, an endogenous selective estrogen receptor modulator. Maturitas. 2017;104:29–35. doi: 10.1016/j.maturitas.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vini R., Rajavelu A., Sreeharshan S. 27-Hydroxycholesterol, the estrogen receptor modulator, alters DNA methylation in breast cancer. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.783823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gamba P., Leonarduzzi G., Tamagno E., Guglielmotto M., Testa G., Sottero B., et al. Interaction between 24-hydroxycholesterol, oxidative stress, and amyloid-beta in amplifying neuronal damage in Alzheimer's disease: Three partners in crime. Aging Cell. 2011;10:403–417. doi: 10.1111/j.1474-9726.2011.00681.x. [DOI] [PubMed] [Google Scholar]
- 66.Feng Y., Huang W., Tian T.F., Wang G., Hu C., Chiu H.F., et al. The psychometric properties of the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR) and the Patient Health Questionnaire-9 (PHQ-9) in depressed inpatients in China. Psychiatry Res. 2016;243:92–96. doi: 10.1016/j.psychres.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 67.Ma S., Kang L., Guo X., Liu H., Yao L., Bai H., et al. Discrepancies between self-rated depression and observed depression severity: The effects of personality and dysfunctional attitudes. Gen Hosp Psychiatry. 2021;70:25–30. doi: 10.1016/j.genhosppsych.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Magnani F., Tate C.G., Wynne S., Williams C., Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- 69.Donati R.J., Schappi J., Czysz A.H., Jackson A., Rasenick M.M. Differential effects of antidepressants escitalopram versus lithium on Gs alpha membrane relocalization. BMC Neurosci. 2015;16:40. doi: 10.1186/s12868-015-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erb S.J., Schappi J.M., Rasenick M.M. Antidepressants accumulate in lipid rafts independent of monoamine transporters to modulate redistribution of the G protein, Galphas. J Biol Chem. 2016;291:19725–19733. doi: 10.1074/jbc.M116.727263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gambert S., Gabrielle P.H., Masson E., Leger-Charnay E., Ferrerro A., Vannier A., et al. Cholesterol metabolism and glaucoma: Modulation of Muller cell membrane organization by 24S-hydroxycholesterol. Chem Phys Lipids. 2017;207:179–191. doi: 10.1016/j.chemphyslip.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Nunes V.S., Panzoldo N.B., Leanca C.C., Parra E.S., Zago V.S., da Silva E.J., et al. Increased 27-hydroxycholesterol plasma level in men with low high density lipoprotein-cholesterol may circumvent their reduced cell cholesterol efflux rate. Clin Chim Acta. 2014;433:169–173. doi: 10.1016/j.cca.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 73.Hirayama T., Mizokami Y., Honda A., Homma Y., Ikegami T., Saito Y., et al. Serum concentration of 27-hydroxycholesterol predicts the effects of high-cholesterol diet on plasma LDL cholesterol level. Hepatol Res. 2009;39:149–156. doi: 10.1111/j.1872-034X.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 74.Korytowski W., Wawak K., Pabisz P., Schmitt J.C., Chadwick A.C., Sahoo D., et al. Impairment of macrophage cholesterol efflux by cholesterol hydroperoxide trafficking: Implications for atherogenesis under oxidative stress. Arterioscler Thromb Vasc Biol. 2015;35:2104–2113. doi: 10.1161/ATVBAHA.115.306210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cardenia V., Rodriguez-Estrada M.T., Lorenzini A., Bandini E., Angeloni C., Hrelia S., et al. Effect of broccoli extract enriched diet on liver cholesterol oxidation in rats subjected to exhaustive exercise. J Steroid Biochem Mol Biol. 2017;169:137–144. doi: 10.1016/j.jsbmb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Marwarha G., Dasari B., Ghribi O. Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell Signal. 2012;24:484–492. doi: 10.1016/j.cellsig.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schapir L., Weizman A., Golubchik P. The impact of prolonged, selective, serotonin reuptake inhibitor treatment on serum lipid and glucose levels in children and adolescents: A preliminary prospective study. J Child Adolesc Psychopharmacol. 2018;28:485–487. doi: 10.1089/cap.2018.0055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.