Abstract
Background
This study examined whether C-reactive protein (CRP), a marker of low-grade systemic inflammation, mediates the association between vascular risk factor (VRF) burden and depressive symptoms.
Methods
We drew on the prospective design of the UK Biobank to include participants with longitudinal data on VRF burden, CRP, and depressive symptoms. Total, direct, and indirect effects were estimated using regression-based mediation models while controlling for confounding by sociodemographic factors, baseline CRP, and baseline depression. Sensitivity analyses probed the robustness of results to unmeasured confounding.
Results
We analyzed data from 10,470 participants from the UK Biobank (mean age = 56.75 years at baseline). Net of covariates, VRFs at baseline were associated with higher depressive symptoms at follow-up (total effect = 0.099; 95% CI, 0.002–0.163). CRP mediated this association (indirect effect = 0.010; 95% CI, 0.004–0.017), accounting for 10.0% (95% CI, 0.3%–30.0%) of the total effect of VRF burden on depressive symptoms. Exploratory analyses suggested that the total and indirect effects pertained to somatic depressive symptoms (tiredness and appetite).
Conclusions
These results suggest that inflammation-promoting effects of VRFs may contribute to depressive symptoms in mid- and later life. However, the mediating pathway via CRP explains only a small part of the association between VRFs and depression after accounting for important covariates and may pertain to specific depressive symptoms. Future studies leveraging similar longitudinal designs are needed to further disentangle the time-varying effects between VRFs, inflammation, and certain depressive symptoms while addressing important confounders.
Keywords: Depression, Inflammation, Mediation, UK Biobank, Vascular depression, Vascular risk factors
Hypertension, overweight and obesity, hypercholesterolemia, diabetes, and smoking are important modifiable risk factors for vascular diseases and major contributors to global disease burden (1). Vascular risk factors (VRFs) have also been implicated in the etiology of mood disorders, particularly in later-life depression (2, 3, 4). Although findings have not been entirely consistent (5, 6, 7, 8), a number of prospective population-based studies support that people with VRFs are at higher risk for developing depression and depressive symptoms (9, 10, 11, 12, 13). This association is particularly strong for burden from multiple VRFs (10), which often tend to accumulate (14). However, mechanistic explanations as to why VRF burden and depressive symptoms are linked remain debated (6,15,16).
In recent years, low-grade systemic inflammation has gained considerable attention as a potential link between vascular and mental health (15,17, 18, 19). Low-grade systemic inflammation reflects a chronic manifestation of the body’s natural inflammatory response to physical injury or infection (19). While an acute inflammatory response—as a temporally and spatially restricted activation of immune cells—is typically adaptive and resolves once the threat has passed (20,21), low-grade systemic inflammation reflects a prolonged and unresolved activation of the immune system. Low-grade inflammation is thus characterized by the systemic elevation of inflammatory markers and has been connected to a broad range of chronic diseases (19,21,22).
Increasing evidence supports that inflammatory processes are involved in the pathophysiology of depression (23, 24, 25). Earlier experimental evidence has shown that acute inflammation is associated with protective behavioral responses (“sickness behavior”), such as sadness, anhedonia, fatigue, or social withdrawal (26,27), that mimic depressive symptoms. Furthermore, mounting evidence from observational studies has implicated low-grade systemic inflammation in the development of depression (20,24,25). For example, elevated levels of C-reactive protein (CRP), a hallmark of low-grade systemic inflammation, have been associated with higher depressive symptoms in population-based studies (28, 29, 30, 31) and shown to be present in a considerable proportion of patients with depression (32). Recent findings have also highlighted that these processes may not be general but rather specific to certain somatic symptoms of depression (30,31,33, 34, 35).
VRFs have been discussed as one of the main sources of low-grade systemic inflammation (36,37). Smoking, diabetes, and obesity especially have been linked to elevated plasma levels of inflammatory proteins (38, 39, 40, 41). For example, compounds consumed from smoking tobacco increase proinflammatory cytokines and proteins due to the burden of free radicals and induction of local inflammation in the lung parenchyma (37,42). These inflammatory processes may in turn contribute to the development of depressive symptoms (15,17,18).
However, evidence on the mediating effect of inflammation on the link between vascular risk and depressive symptoms is still scarce. Previous studies have mostly been cross-sectional (43, 44, 45), and few studies have tested or quantified the mediating role of inflammation in the association between VRFs and depression (46). Investigating the contribution of inflammation to the association between VRFs and depressive symptoms is furthermore complicated in that baseline levels of CRP and depressive symptoms may act as important confounders. For example, there is increasing evidence that CRP and depression are independent risk factors for poor vascular health (37,47,48). To tease apart the mediating effect of inflammation on the relationship between VRFs and depressive symptoms, longitudinal data are needed that establish the temporal order of the variables while also addressing confounding by previous levels of CRP and depression (49,50).
In the current study, we drew on the prospective design of the UK Biobank and applied regression-based mediation to estimate the contribution of CRP as a marker of low-grade systemic inflammation to the association between VRF burden and depressive symptoms. Using longitudinal data allowed us to estimate total, direct, and indirect effects based on temporal ordering of VRFs (measured at baseline), CRP (measured about 4 years later), and depressive symptoms (measured about 8 years after baseline). Importantly, this approach also allowed us to control for possible confounding by sociodemographic variables, baseline CRP, and baseline depression. We also performed sensitivity analyses to probe the robustness of our results against unmeasured confounding. Finally, given studies suggesting symptom-specific associations with VRFs as well as inflammation (30,31,33, 34, 35,51), exploratory analyses were conducted to test whether these mediating effects of CRP are pertinent to specific depressive symptoms.
Methods and Materials
Participants
The UK Biobank is an ongoing cohort study that initially recruited about 500,000 community-dwelling adults ages 37 to 73 years from across the United Kingdom (52). To ensure temporal ordering of the variables under study, the current study included data from 11,540 UK Biobank participants, who consecutively underwent a baseline assessment between 2006 and 2010 (time [T] 1), a follow-up assessment between 2012 and 2013 (T2), and an online mental health assessment between 2016 and 2017 (T3). From among those participants, we excluded 1041 individuals with manifest vascular diseases, autoimmune disorders, neurodegenerative or neuropsychiatric disorders, or a previous diagnosis of depression at baseline. Although participants with CRP levels exceeding 10 mg/L are often excluded from analyses [e.g., (27,34)] because these CRP levels may indicate an acute infection instead of chronic low-grade inflammation, this exclusion criterion has been criticized if researchers do not have additional information about a participant’s acute health status (53,54). To avoid excluding the data of such participants, we opted to use all available data points but to winsorize CRP data. A detailed flow chart of participant inclusion and exclusion is shown in Figure S1. Details on the variables used for exclusion are provided in Table S1.
The UK Biobank received ethical approval from the North West Multi-centre Research Ethics Committee (reference 11/NW/0382). All participants provided informed consent.
VRF Burden
VRF burden was defined as the presence of 5 VRFs that have been linked to an increased risk of vascular diseases: hypertension, overweight or obesity, hypercholesterolemia, diabetes, and smoking (55). Hypertension was defined as either reporting the presence of diagnosed hypertension or having systolic blood pressure ≥ 130 mm Hg and diastolic blood pressure ≥ 80 mm Hg (56). Overweight/obesity was defined as having a body mass index ≥ 25 (57). Hypercholesterolemia and diabetes were defined as reporting the presence of diagnosed high cholesterol and diabetes, respectively. Smoking was defined as reporting (occasional) current smoking of tobacco. All 5 VRFs were coded as either present (1) or absent (0) and summed into a composite score with a higher score indicating higher VRF burden (total score range 0–5). Details on the assessment of these variables used can be found in Table S2.
C-Reactive Protein
Serum concentrations of high-sensitivity CRP were used as a marker of low-grade systemic inflammation. Detailed information on the blood sampling and the serum CRP measurement can be found in the UK Biobank documentation (58, 59, 60). In brief, participants provided a nonfasting blood sample on the assessment day. Serum CRP levels were later determined in stored samples using an immunoturbidimetric method (Beckman Coulter AU5800) with a reportable range of high-sensitivity serum CRP from 0.08 to 80 mg/L. For all analyses, we used the untransformed CRP values provided (61,62). To potentially ease comparison with other studies, we provide sensitivity analyses with log-transformed CRP values. In addition, we included all participants with available CRP data, but winsorized CRP with an upper boundary of 10 mg/L to limit the influence of outliers due to potential acute infections.
Depressive Symptoms
Depressive symptoms were assessed at T3 using the Patient Health Questionnaire-9, which is a well-established screening instrument for diagnosing and monitoring the severity of depression (63). Participants answered 9 items following the DSM-5 criteria for major depressive disorder: 1) depression, 2) inadequacy, 3) tiredness or low energy, 4) lack of interest or pleasure in doing things, 5) poor appetite or overeating, 6) thought of suicide or self-harm, 7) concentration problems, 8) sleep problems, and 9) changes in speed. Answers were given on a Likert scale ranging from 0 (not at all) to 3 (nearly every day). For our analyses of overall depressive symptom severity, we calculated a sum score (ranging from 0 to 27), with higher values indicating more severe depressive symptoms.
Covariates
We considered sociodemographic factors (age, gender, racial-ethnic background, educational attainment, household income, and Townsend deprivation index, all assessed at baseline), as well as baseline CRP, baseline depressive symptoms, and a potential history of mood disorder as covariates. Age (in years) was calculated by subtracting the date of birth from the date of the baseline assessment. Gender was defined as a dichotomous variable due to its binary assessment (woman/man). We defined racial-ethnic background as a dichotomous variable (White/person of color [including people who identified as Asian or Asian British, Black or Black British, Chinese, Mixed, or Other]) because most of the sample identified themselves as White. Educational attainment was defined as a dichotomous variable (college or university degree/no college or university degree). Household income was assessed as a categorial variable indicating total pretax household income in British pounds sterling (<18,000/18,000–30,999/31,000–51,999/52,000–100,000/>100,000). The Townsend deprivation index reflects material deprivation within a population and was assessed as a continuous variable with higher values indicating greater deprivation (64,65). Baseline depressive symptoms were calculated as the sum score of 4 items assessing mood symptoms at baseline: frequency of depressed mood, frequency of unenthusiasm/disinterest, frequency of tenseness/restlessness, frequency of tiredness (answers ranged from 0 [not at all] to 3 [nearly every day]). A potential history of mood disorder was assessed at baseline by asking participants whether they had ever seen a general practitioner for nerves, anxiety, tension, or depression (yes vs. no). CRP at T1 as a covariate was assessed as described above for CRP at T2. More details on the specific variables used are provided in Table S2.
Statistical Analyses
Mediation Analysis
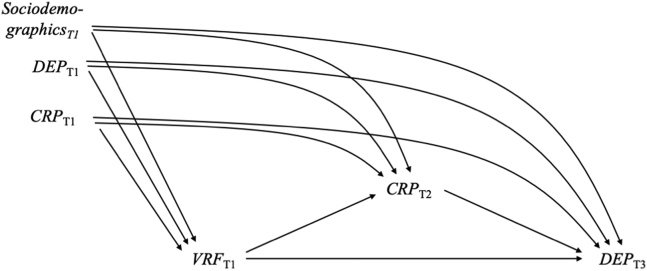
The proposed diagram for the relationship between VRF burden, CRP, and depressive symptoms is shown in Figure 1. We assumed that VRF burden at T1 (X) would lead to elevated levels of CRP at T2 (M), which would result in higher levels of depressive symptoms at T3 (Y). Sociodemographic and socioeconomic variables, baseline CRP, and baseline depression may confound the effects between VRF burden, CRP, and depressive symptoms.
Figure 1.
Directed acyclic graph showing the assumed causal structure of the effects between vascular risk factor (VRF) burden, C-reactive protein (CRP), and depressive symptoms (DEP). The exposure VRF burden was measured at time (T) 1 and assumed to affect CRP at T2, which in turn affects DEP at T3. Sociodemographic covariates include age, gender, racial-ethnic background, educational attainment, household income, and Townsend deprivation index measured at T1; depression at T1 was assumed to be a confounder and was measured by depressive symptoms at T1 and a potential history of mood disorder; CRP at T1 was also assumed to be a confounder.
To estimate the mediation models, we relied on a path-analytic framework, which simultaneously estimates the direct, indirect, and total effects (66). In these models, the direct effect c reflects the association between the exposure VRF burden at T1 (X) and the outcome depressive symptoms at T3 (Y). It is the regression coefficient estimated by regressing X on Y while accounting for covariates. The indirect effect reflects the part of the association between X and Y that is mediated by serum CRP measured at T2 (M). It is the product of 2 path coefficients: the coefficient from regressing X on M (a) and the coefficient from regressing M on Y while accounting for X and all covariates (b). The total effect is the sum of the direct effect (c) and the indirect effect (a × b) and reflects the association between X and Y that is direct and mediated via M (67).
All mediation models were fitted using the structural equation modeling package lavaan (68) in R version 4.1.1. To account for missing data, we used the robust full information maximum likelihood estimation, which assumes that data are missing at random (69). While this assumption is impossible to test formally in practice, full information maximum likelihood estimation can still provide less biased estimates even if data are not missing at random and is preferable to other missing data techniques, such as listwise deletion (70). In all models, 95% CIs were computed using bootstrapping (with 5000 bootstrapping samples) to test the direct, total, and indirect effects. Effects were considered significant when the 95% CI did not contain 0. The ratio of the indirect effect to the total effect was used to quantify the proportion of the total effect that was mediated by the indirect effect (71), and this proportion mediated was calculated when both the total and indirect effects were significantly different from 0 and were in the same direction (72).
Sensitivity Analyses
To test the sensitivity of the results of the fully adjusted model (model 3) to unmeasured confounding, we conducted a sensitivity analysis by examining how the size of the indirect effect changed when we included an (unobserved) confounder of the mediator-outcome relation of a small (r = 0.1), a moderate (r = 0.3), and a large (r = 0.5) magnitude (73). To test the sensitivity of the results of the fully adjusted model (model 3) to the winsorization of CRP, we reran model 3 with unwinsorized CRP data. Finally, to allow comparison with other studies in the field, we also estimated model 3 with log-transformed CRP.
Exploratory Analyses
Given findings of symptom-specific associations of depressive symptoms with VRFs and CRP, we performed a range of exploratory models with single depressive symptoms as outcomes. All single-symptom models included the full set of covariates.
Results
Sample Characteristics
The sample included 10,470 participants with longitudinal data for VRF burden, CRP, and depressive symptoms. The baseline characteristics of participants are shown in Table 1. Table S3 shows zero-order correlations between all study variables. On average, participants were 56.75 years old at baseline (SD = 7.30, range 40–70). Of the participants, 5109 participants (48.80%) were women, 10,269 (98.08%) identified themselves as White, and 4890 (46.70%) held a university degree.
Table 1.
Baseline Characteristics of Study Participants, N = 10,470
| Variable | Missing Data, n (%) | n (%) or Mean ± SD |
|---|---|---|
| Age at T1, Years | 0 (0%) | 56.75 ± 7.30 |
| Gender | 0 (0%) | |
| Woman | 5109 (48.80%) | |
| Man | 5361 (51.20%) | |
| Racial-Ethnic Background | 21 (<0.01%) | |
| White | 10,269 (98.08%) | |
| Person of colora | 180 (0.02%) | |
| Educational Attainment | 290 (0.03%) | |
| University degree | 4890 (46.70%) | |
| No university degree | 5290 (50.52%) | |
| Household Income, £ | 1098 (10.49%) | |
| <18,000 | 1237 (11.81%) | |
| 18,000–30,999 | 2412 (23.04%) | |
| 31,000–51,999 | 2890 (27.60%) | |
| 52,000–100,000 | 2304 (22.00%) | |
| >100,000 | 529 (5.05%) | |
| TDI | 5 (0.05%) | −2.23 ± 2.54 |
| VRF Burden | 0 (0%) | 1.33 ± 0.94 |
| Depressive Symptoms (T1) | 0 (0%) | 0.99 ± 1.37 |
| Doctor Visit Mood Disorder | 2 (<0.01%) | |
| Yes | 2988 (28.54%) | |
| No | 7480 (71.44%) | |
| CRP (T1), mg/L | 802 (7.66%) | 1.94 ± 2.21 |
| T1→T2, Days | 0 (0%) | 1547 ± 335 |
| T2→T3, Days | 0 (0%) | 1344 ± 94 |
CRP, C-reactive protein; T, time; TDI, Townsend deprivation index; VRF, vascular risk factor.
Includes people who identified as Asian or Asian British, Black or Black British, Chinese, Mixed, or Other ethnic groups.
Mediation Analysis
The estimated total, direct, and indirect effects are depicted in Figure 2. The total effects showed a significant association between VRF burden and depressive symptoms in all 3 models with increasing covariate control, although increasing covariate adjustment led to an attenuation of the estimated total effects (model 1: total effect = 0.219, 95% CI, 0.155–0.284; model 2: total effect = 0.123, 95% CI, 0.063–0.184; model 3: total effect = 0.099, 95% CI, 0.037–0.163). This suggests that after accounting for sociodemographic and socioeconomic covariates, baseline mediator, and baseline outcome, 1 more VRF at baseline was associated with 0.099 (95% CI, 0.037–0.163) additional depressive symptoms about 8 years later (Figure 2A).
Figure 2.
Results of mediation models estimating the effects between vascular risk factor burden, C-reactive protein (CRP), and depressive symptoms (Dep). (A) Direct, indirect, and total effect of 3 models with increasing covariate control (estimates and 95% CIs). Effects of model 1 (sociodemographic and socioeconomic covariates only) are plotted in light green; effects of model 2 (sociodemographic covariates, depressive symptoms at time (T) 1, and potential history of mood disorder) are plotted in forest green; effects of model 3 (sociodemographic covariates, depressive symptoms at T1, potential history of mood disorder, and CRP at T1) are plotted in blue. (B) Proportion mediated of 3 models with increasing covariate control, which were calculated as the relation between the indirect and the total effect. The colored sections (same colors as in plotting models 1, 2, and 3) represent the portion of the total effect between vascular risk factors and depressive symptoms that is accounted for by the indirect effect via CRP, while the white sections indicate the unexplained portion of the total effect. sociodem. cov, sociodemographic covariates.
The estimated indirect effects showed that CRP mediated a small but significant part of the association between VRFs and depressive symptoms in all 3 models with increasing covariate control, although increasing covariate adjustment also slightly attenuated the strength of the indirect effects (model 1: indirect effect = 0.034, 95% CI, 0.021–0.048; model 2: indirect effect = 0.025, 95% CI, 0.014–0.037; model 3: indirect effect = 0.010, 95% CI, 0.004–0.017). Thus, in model 3, with adjustment for the full set of covariates, the estimated indirect effect via CRP was 0.010 (95% CI, 0.004–0.017), which accounted for 10.0% (95% CI, 3.4%–30.0%) of the association between VRFs and depressive symptoms (Figure 2A, B).
Sensitivity Analyses
Sensitivity analyses building on the fully adjusted model (model 3) were used to probe the indirect effect to an unobserved confounder. The results indicated that the indirect effect (−0.004, 95% CI −0.010 to 0.003) was not significantly different from zero in the presence of an unobserved confounder with a medium-sized effect. Additional sensitivity analyses with unwinsorized CRP data showed similar results as the main analyses (Table S4). Finally, sensitivity analyses with log-transformed CRP data also showed similar results as the main analyses (Table S5).
Exploratory Analyses
Additional analyses were used to examine the mediating role of CRP in the association between VRFs and specific depressive symptoms while controlling for all confounding variables. The full results of all symptom-specific mediation models are shown in Table 2. Significant total effects of VRF burden were found on feelings of tiredness (0.036, 95% CI, 0.021–0.051), changes in appetite (0.041, 95% CI, 0.030–0.052), and sleep problems (0.021, 95% CI, 0.003–0.039). Significant indirect effects of CRP were found for tiredness (0.003, 95% CI, 0.002–0.009), where CRP mediated 9.2% (95% CI, 4.7%–18.2%) of the association between VRFs and depressive symptoms, and changes in appetite (0.002, 95% CI, 0.001–0.004), where CRP mediated 5.5% (95% CI, 2.3%–9.6%) of the association between VRFs and depressive symptoms.
Table 2.
Results of Longitudinal Mediation Analyses of VRF Burden, Log CRP, and Single Depressive Symptoms
| Symptom | Total Effect (95% CI) | Direct Effect (95% CI) | Indirect Effect (95% CI) | Proportion Mediateda (95% CI) |
|---|---|---|---|---|
| Feelings of Depression | −0.002 (−0.012 to 0.008) | −0.002 (−0.012 to 0.008) | 0.000 (−0.001 to 0.001) | – |
| Inadequacy | −0.001 (−0.012 to 0.009) | −0.002 (−0.013 to 0.008) | 0.001 (−0.000 to 0.002) | – |
| Tiredness | 0.036 (0.021 to 0.051) | 0.033 (0.018 to 0.048) | 0.003 (0.002 to 0.005) | 9.6% (4.7% to 18.2%) |
| Lack of Interest | 0.002 (−0.009 to 0.012) | 0.001 (−0.009 to 0.011) | 0.001 (−0.000 to 0.001) | – |
| Changes in Appetite | 0.041 (0.030 to 0.052) | 0.039 (0.028 to 0.050) | 0.002 (0.001 to 0.004) | 5.5% (2.3% to 9.6%) |
| Thoughts of Suicide | 0.004 (−0.001 to 0.009) | 0.004 (−0.001 to 0.008) | 0.000 (−0.001 to 0.001) | – |
| Concentration Problems | 0.001 (−0.009 to 0.012) | 0.000 (−0.010 to 0.011) | 0.001 (−0.000 to 0.002) | – |
| Sleep Problems | 0.021 (0.003 to 0.039) | 0.020 (0.003 to 0.039) | 0.001 (−0.001 to 0.002) | – |
| Changes in Speed | −0.002 (−0.008 to 0.004) | −0.003 (−0.009 to 0.003) | 0.001 (−0.000 to 0.002) | – |
All associations are adjusted for covariates (age, gender, racial-ethnic background, educational attainment, household income, Townsend deprivation index, baseline depressive symptoms, potential history of mood disorder, and CRP at baseline).
CRP, C-reactive protein; VRF, vascular risk factor.
Proportion mediated was only calculated when total and indirect effects were significantly different from 0.
Discussion
By drawing on the prospective design of the UK Biobank, we investigated the extent to which CRP, as a marker of low-grade systemic inflammation, might underly the association between VRFs and depressive symptoms. Our results suggested that after accounting for important covariates, middle-aged and older adults with higher VRF burden at baseline had more severe depressive symptoms about 8 years later and that a small part of this association was mediated by serum CRP. Exploratory analyses revealed that the mediating effect of CRP was most prominent for 2 somatic symptoms, feelings of tiredness and changes in appetite. However, our findings also suggest that these results must be interpreted with caution given that the estimated effects are relatively small and may be somewhat sensitive to residual confounding.
The finding that VRF burden is associated with an increased risk for depressive symptoms at follow-up in middle-aged and older adults is consistent with previous work linking vascular and mental health in later life (10,12,13). However, the reasons why VRFs may increase the risk for depressive symptoms are still being debated (6,15,16). Several authors previously proposed inflammation as a possible mechanism linking vascular and mental health (15,17, 18, 19). However, studies disentangling the mediational effect of inflammation on the relationship between VRF burden and depressive symptoms are scarce and mainly focused on cross-sectional data or bivariate associations (44,46,74). A strength of the current study is the leveraging of longitudinal data from a large cohort study to establish temporal ordering between VRFs, CRP, and depressive symptoms while strengthening causal inference by controlling for baseline CRP and baseline depression. By doing so, the results of our study provide putative support for the hypothesis that a small part of the depressogenic effect of VRFs may indeed be due to their inflammation-promoting characteristics.
While our findings add to the literature on mechanisms underlying the association between VRFs and depression, they also suggest that the effect is small after accounting for baseline CRP and depression and may be somewhat sensitive to residual confounding. An advantage of our study compared with cross-sectional studies is that the longitudinal study design allowed us to provide more precise estimates of these associations while considering important confounders. Although the observed effects from this observational study may not be directly applicable to clinical outcomes, our study provides an important perspective on how inflammation may contribute to the link between vascular risk and depressive symptoms that could inform current clinical research and the development of therapeutic approaches. Future population-based and clinical studies are necessary to replicate and extend our findings to make clear policy or clinical recommendations.
Further high-quality research replicating and extending our findings is also important given the bidirectional effects among the variables under study. Several lines of research support potential bidirectional pathways: Depression, especially in adolescence and young adulthood, may increase the risk of inflammatory processes and the development of higher vascular risk (75,76). Inflammation also increases the risk of manifest cardiovascular diseases independent of classical VRFs (37,47). Some studies have even suggested that inflammation may explain part of the increased risk of manifest vascular diseases (e.g., heart disease, stroke) in depression, although results have been inconsistent (77, 78, 79, 80). We aimed to minimize confounding by these bidirectional effects by accounting for baseline CRP and baseline depression when testing our mediational hypothesis (36,44,74). However, future studies will benefit from going one step further to elucidate the temporal ordering and causality between VRFs, inflammation, and depressive symptoms. For example, a study with at least 3 waves of data among the variables under study would allow extension of the longitudinal design and use of advanced causal inference methods, such as marginal structural models or g-estimation, which would more appropriately address time-varying effects of VRFs, CRP, and depressive symptoms (81,82). While the current study aimed to test one specific pathway and was limited to the available data from the UK Biobank, such approaches would be a fruitful avenue for future research.
Causal inference could also be strengthened in future research by taking an interventionist approach. Indeed, alternative designs based on interventional effects and intensive sampling approaches have also been called for recently to better understand the temporal sequence and causal relationships in this area (83). For example, our results point to the possibility that VRF burden may be a promising shared upstream target for preventing depressive symptoms and inflammatory processes (84). One avenue for future research will be to investigate whether interventions or policies aimed at reducing VRF burden (and preventing vascular diseases) could alleviate low-grade systemic inflammation and depressive symptoms. Large-scale randomized controlled trials that could answer these research questions in the future are underway with multiple components of interventions in middle-aged and older adults (85,86). Moreover, it remains to be investigated whether inflammation-reducing treatments are an effective complementary treatment for depressed patients with high VRF burden (87).
Importantly, the symptom-specific associations observed in our study are consistent with recent work suggesting that depression is not a homogeneous construct but rather an interplay of symptoms that can have different risk factors (88,89). Several previous studies have supported that VRFs as well as inflammation have distinct associations with specific depressive symptoms that are related to somatic or metabolic processes (30,31,33,51,74). Our findings are consistent with this research, supporting the notion that VRFs and low-grade inflammation may promote a specific symptom profile in depression. Thus, our findings may be informative for novel research on the recently proposed immuno-metabolic subtype of depression, which has been characterized by altered vascular and metabolic functions, disturbances in immune function, and specific behavioral symptoms (90).
There are several limitations to our study. One limitation is that our study sample may have limited generalizability. While the UK Biobank offers a unique data source with extensive health data and a longitudinal design, the sample has been shown to be selective and healthier than the general UK population (91). The UK Biobank is known to be less diverse with respect to racial-ethnic identity than the general population of the UK (92). The generalizability of our results is further constrained by the restriction to a longitudinal sample, which was even less diverse and more highly educated than the UK Biobank sample overall. To better understand mechanisms between VRFs, inflammation, and depression, future studies using large, representative, and more diverse samples—especially with a particular focus on retaining people in the study longitudinally—are needed. Moreover, future studies are needed to replicate our results. This is especially important for the single-symptom analyses, which were not corrected for multiple comparisons due to their exploratory nature. Another limitation is that we were unable to include data on VRFs, CRP, and depressive symptoms at all 3 time points because these variables were not consistently assessed across measurement occasions in the UK Biobank. Therefore, although we included a covariate of CRP and depression at baseline, we were not able to disentangle more complex, bidirectional associations over time. Finally, given the age range of UK Biobank participants, we were not able to include data from before midlife. While middle-aged and older adults are at increased risk for VRFs and inflammation (93,94), the underlying pathological processes probably start much earlier in life. For example, recent studies have demonstrated that VRFs are associated with depressive symptoms during the first 18 years of life (75,95) and that inflammation is already linked to depressive symptoms in younger adults (96). Future studies including assessments during childhood and adolescence would be needed to extend our findings and disentangle the associations between VRFs, inflammation, and depressive symptoms across the life span.
In this longitudinal study, we showed that the association between VRFs and depressive symptoms in mid- and later life may be mediated to a small extent by low-grade systemic inflammation. Therefore, the current study adds some weight to the notion that inflammation plays a role in linking vascular and mental health. Furthermore, exploratory results suggest that vascular and inflammatory processes pertain to a certain (somatic) subgroup of depression.
Acknowledgments and Disclosures
This work was not supported by any specific funding sources.
We are extremely grateful to all participants of the UK Biobank and the UK Biobank team. This research was conducted using the UK Biobank Resource under Application No. 37721.
A previous version of this article was published as a preprint on PsyArxiv: https://doi.org/10.31234/osf.io/m8zv4 and deposited on MPG.PuRe, the publication repository of the Max Planck Society (https://hdl.handle.net/21.11116/0000-000B-F3B5-6).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.04.008.
Supplementary Material
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulos G.S., Meyers B.S., Young R.C., Campbell S., Silbersweig D., Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 3.Sneed J.R., Culang-Reinlieb M.E. The vascular depression hypothesis: An update. Am J Geriatr Psychiatry. 2011;19:99–103. doi: 10.1097/jgp.0b013e318202fc8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizenstein H.J., Baskys A., Boldrini M., Butters M.A., Diniz B.S., Jaiswal M.K., et al. Vascular depression consensus report – A critical update. BMC Med. 2016;14:161. doi: 10.1186/s12916-016-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart R., Prince M., Mann A., Richards M., Brayne C. Stroke, vascular risk factors and depression: Cross-sectional study in a UK Caribbean-born population. Br J Psychiatry. 2001;178:23–28. doi: 10.1192/bjp.178.1.23. [DOI] [PubMed] [Google Scholar]
- 6.Almeida O.P. Vascular depression: Myth or reality? Int Psychogeriatr. 2008;20:645–652. doi: 10.1017/S1041610207006473. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.M., Stewart R., Kim S.W., Yang S.J., Shin I.S., Yoon J.S. Vascular risk factors and incident late-life depression in a Korean population. Br J Psychiatry. 2006;189:26–30. doi: 10.1192/bjp.bp.105.015032. [DOI] [PubMed] [Google Scholar]
- 8.Schaare H.L., Blöchl M., Kumral D., Uhlig M., Lemcke L., Valk S.L., et al. Associations between mental health, blood pressure and the development of hypertension. Nat Commun. 2023;14:1953. doi: 10.1038/s41467-023-37579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong N.M., Meoni L.A., Carlson M.C., Xue Q.L., Bandeen-Roche K., Gallo J.J., Gross A.L. Cardiovascular risk factors and risk of incident depression throughout adulthood among men: The Johns Hopkins Precursors Study. J Affect Disord. 2017;214:60–66. doi: 10.1016/j.jad.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valkanova V., Ebmeier K.P. Vascular risk factors and depression in later life: A systematic review and meta-analysis. Biol Psychiatry. 2013;73:406–413. doi: 10.1016/j.biopsych.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Almeida O.P., Flicker L., Norman P., Hankey G.J., Vasikaran S., van Bockxmeer F.M., Jamrozik K. Association of cardiovascular risk factors and disease with depression in later life. Am J Geriatr Psychiatry. 2007;15:506–513. doi: 10.1097/01.JGP.0000246869.49892.77. [DOI] [PubMed] [Google Scholar]
- 12.Kivimäki M., Shipley M.J., Allan C.L., Sexton C.E., Jokela M., Virtanen M., et al. Vascular risk status as a predictor of later-life depressive symptoms: A cohort study. Biol Psychiatry. 2012;72:324–330. doi: 10.1016/j.biopsych.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blöchl M., Schaare H.L., Kunzmann U., Nestler S. The age-dependent association between vascular risk factors and depressed mood. J Gerontol B Psychol Sci Soc Sci. 2022;77:284–294. doi: 10.1093/geronb/gbab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Agostino R.B., Vasan R.S., Pencina M.J., Wolf P.A., Cobain M., Massaro J.M., Kannel W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 15.Taylor W.D., Aizenstein H.J., Alexopoulos G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blöchl M., Schaare H.L., Kumral D., Gaebler M., Nestler S., Villringer A. Vascular risk factors, white matter microstructure, and depressive symptoms: A longitudinal analysis in the UK Biobank [published online Apr 5] Psychol Med. 2023 doi: 10.1017/S0033291723000697. [DOI] [PubMed] [Google Scholar]
- 17.Shao M., Lin X., Jiang D., Tian H., Xu Y., Wang L., et al. Depression and cardiovascular disease: Shared molecular mechanisms and clinical implications. Psychiatry Res. 2020;285:112802. doi: 10.1016/j.psychres.2020.112802. [DOI] [PubMed] [Google Scholar]
- 18.Mattina G.F., Van Lieshout R.J., Steiner M. Inflammation, depression and cardiovascular disease in women: The role of the immune system across critical reproductive events. Ther Adv Cardiovasc Dis. 2019;13 doi: 10.1177/1753944719851950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straub R.H., Schradin C. Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health. 2016;2016:37–51. doi: 10.1093/emph/eow001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netea M.G., Balkwill F., Chonchol M., Cominelli F., Donath M.Y., Giamarellos-Bourboulis E.J., et al. A guiding map for inflammation. Nat Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett J.M., Reeves G., Billman G.E., Sturmberg J.P. Inflammation–Nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front Med (Lausanne) 2018;5:316. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller A.H., Raison C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A., Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 27.Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalli A., Jovanova O., Hoogendijk W.J.G., Tiemeier H., Carvalho L.A. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacol (Berl) 2016;233:1669–1678. doi: 10.1007/s00213-015-3919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Au B., Smith K.J., Gariépy G., Schmitz N. The longitudinal associations between C-reactive protein and depressive symptoms: Evidence from the English Longitudinal Study of Ageing (ELSA) Int J Geriatr Psychiatry. 2015;30:976–984. doi: 10.1002/gps.4250. [DOI] [PubMed] [Google Scholar]
- 30.Frank P., Jokela M., Batty G.D., Cadar D., Steptoe A., Kivimäki M. Association between systemic inflammation and individual symptoms of depression: A pooled analysis of 15 population-based cohort studies. Am J Psychiatry. 2021;178:1107–1118. doi: 10.1176/appi.ajp.2021.20121776. [DOI] [PubMed] [Google Scholar]
- 31.Jokela M., Virtanen M., Batty G.D., Kivimäki M. Inflammation and specific symptoms of depression. JAMA Psychiatry. 2016;73:87–88. doi: 10.1001/jamapsychiatry.2015.1977. [DOI] [PubMed] [Google Scholar]
- 32.Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Eeden W.A., van Hemert A.M., Carlier I.V.E., Penninx B.W.J.H., Lamers F., Fried E.I., et al. Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Transl Psychiatry. 2020;10:235. doi: 10.1038/s41398-020-00920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriarity D.P., van Borkulo C., Alloy L.B. Inflammatory phenotype of depression symptom structure: A network perspective. Brain Behav Immun. 2021;93:35–42. doi: 10.1016/j.bbi.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milaneschi Y., Kappelmann N., Ye Z., Lamers F., Moser S., Jones P.B., et al. Association of inflammation with depression and anxiety: Evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. 2021;26:7393–7402. doi: 10.1038/s41380-021-01188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berk M., Williams L.J., Jacka F.N., O’Neil A., Pasco J.A., Moylan S., et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfaddagh A., Martin S.S., Leucker T.M., Michos E.D., Blaha M.J., Lowenstein C.J., et al. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am J Prev Cardiol. 2020;4:100130. doi: 10.1016/j.ajpc.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellulu M.S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. Obesity and inflammation: The linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.A., Vogiatzi G., Papaioannou S., et al. The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakhru A., Erlinger T.P. Smoking Cessation and cardiovascular disease risk factors: Results from the third national health and nutrition examination survey. PLoS Med. 2005;2:e160. doi: 10.1371/journal.pmed.0020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jukema R.A., Ahmed T.A.N., Tardif J.C. Does low-density lipoprotein cholesterol induce inflammation? If so, does it matter? Current insights and future perspectives for novel therapies. BMC Med. 2019;17:197. doi: 10.1186/s12916-019-1433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Eeden S., Leipsic J., Paul Man S.F., Sin D.D. The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med. 2012;186:11–16. doi: 10.1164/rccm.201203-0455PP. [DOI] [PubMed] [Google Scholar]
- 43.Pitharouli M.C., Hagenaars S.P., Glanville K.P., Coleman J.R.I., Hotopf M., Lewis C.M., Pariante C.M. Elevated C-reactive protein in patients with depression, independent of genetic, health, and psychosocial factors: Results from the UK Biobank. Am J Psychiatry. 2021;178:522–529. doi: 10.1176/appi.ajp.2020.20060947. [DOI] [PubMed] [Google Scholar]
- 44.Fried EI, von Stockert S, Haslbeck JMB, Lamers F, Schoevers RA, Penninx BWJH. Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychol Med 50:2682–2690. [DOI] [PubMed]
- 45.Köhler C.A., Freitas T.H., Maes M., de Andrade N.Q., Liu C.S., Fernandes B.S., et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 46.Daly M. The relationship of C-reactive protein to obesity-related depressive symptoms: A longitudinal study. Obesity (Silver Spring) 2013;21:248–250. doi: 10.1002/oby.20051. [DOI] [PubMed] [Google Scholar]
- 47.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 48.Pan A., Sun Q., Okereke O.I., Rexrode K.M., Hu F.B. Depression and risk of stroke morbidity and mortality: A meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valeri L., Vanderweele T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loh W.W., Ren D. Adjusting for baseline measurements of the mediators and outcome as a first step toward eliminating confounding biases in mediation analysis [published online Feb 7] Perspect Psychol Sci. 2023 doi: 10.1177/17456916221134573. [DOI] [PubMed] [Google Scholar]
- 51.de Miranda Azevedo Rde M, Roest AM, Hoen PW, de Jonge P Cognitive/affective and somatic/affective symptoms of depression in patients with heart disease and their association with cardiovascular prognosis: A meta-analysis. Psychol Med. 2014;44:2689–2703. doi: 10.1017/S0033291714000063. [DOI] [PubMed] [Google Scholar]
- 52.Bycroft C., Freeman C., Petkova D., Band G., Elliott L.T., Sharp K., et al. The UK biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriarity D.P., Horn S.R., Kautz M.M., Haslbeck J.M.B., Alloy L.B. How handling extreme C-reactive protein (CRP) values and regularization influences CRP and depression criteria associations in network analyses. Brain Behav Immun. 2021;91:393–403. doi: 10.1016/j.bbi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mac Giollabhui N., Ellman L.M., Coe C.L., Byrne M.L., Abramson L.Y., Alloy L.B. To exclude or not to exclude: Considerations and recommendations for C-reactive protein values higher than 10 mg/L. Brain Behav Immun. 2020;87:898–900. doi: 10.1016/j.bbi.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stamler J., Stamler R., Neaton J.D., Wentworth D., Daviglus M.L., Garside D., et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: Findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 56.Muntner P., Shimbo D., Carey R.M., Charleston J.B., Gaillard T., Misra S., et al. Measurement of blood pressure in humans: A scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.UK Biobank (2011): Blood sample collection, processing and transport. Available at: https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/Bloodsample.pdf
- 59.UK Biobank (2019): Biomarker assay quality procedures: Approaches used to minimise systematic and random errors (and the wider epidemiological implications). Available at: https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/biomarker_issues.pdf
- 60.UK Biobank (2019): Companion document to accompany serum biomarker data. Available at: https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/serum_biochemistry.pdf
- 61.Iob E., Kirschbaum C., Steptoe A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol Psychiatry. 2020;25:1130–1140. doi: 10.1038/s41380-019-0501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moriarity D.P. A primer on common analytic concerns in psychoneuroimmunology: Alternatives and paths forward. Brain Behav Immun. 2022;102:338–340. doi: 10.1016/j.bbi.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Townsend P. Deprivation. J Soc Pol. 1987;16:125–146. [Google Scholar]
- 65.Morris R., Carstairs V. Which deprivation? A comparison of selected deprivation indexes. J Public Health Med. 1991;13:318–326. [PubMed] [Google Scholar]
- 66.MacKinnon D.P. Taylor & Francis Group/Lawrence Erlbaum Associates; New York: 2008. Introduction to Statistical Mediation Analysis. [Google Scholar]
- 67.VanderWeele T.J. Mediation analysis: A practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 68.Rosseel Y. lavaan: An R package for structural equation modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 69.Enders C.K. The performance of the Full Information Maximum Likelihood estimator in multiple regression models with missing data. Educ Psychol Meas. 2001;61:713–740. [Google Scholar]
- 70.Graham J.W. Missing data analysis: Making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 71.Alwin D.F., Hauser R.M. The decomposition of effects in path analysis. Am Sociol Rev. 1975;40:37–47. [Google Scholar]
- 72.Preacher K.J., Kelley K. Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 73.Fritz M.S., Kenny D.A., MacKinnon D.P. The combined effects of measurement error and omitting confounders in the single-mediator model. Multivariate Behav Res. 2016;51:681–697. doi: 10.1080/00273171.2016.1224154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chirinos D.A., Murdock K.W., LeRoy A.S., Fagundes C. Depressive symptom profiles, cardio-metabolic risk and inflammation: Results from the MIDUS study. Psychoneuroendocrinology. 2017;82:17–25. doi: 10.1016/j.psyneuen.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaplin A.B., Daniels N.F., Ples D., Anderson R.Z., Gregory-Jones A., Jones P.B., Khandaker G.M. Longitudinal association between cardiovascular risk factors and depression in young people: A systematic review and meta-analysis of cohort studies. Psychol Med. 2023;53:1049–1059. doi: 10.1017/S0033291721002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: Double trouble. Neuron. 2020;107:234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kop W.J., Stein P.K., Tracy R.P., Barzilay J.I., Schulz R., Gottdiener J.S. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom Med. 2010;72:626–635. doi: 10.1097/PSY.0b013e3181eadd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davidson K.W., Schwartz J.E., Kirkland S.A., Mostofsky E., Fink D., Guernsey D., Shimbo D. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study) Am J Cardiol. 2009;103:755–761. doi: 10.1016/j.amjcard.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiles S.A., Baker A.L., de Malmanche T., McEvoy M., Boyle M., Attia J. The role of inflammatory markers in explaining the association between depression and cardiovascular hospitalisations. J Behav Med. 2015;38:609–619. doi: 10.1007/s10865-015-9637-2. [DOI] [PubMed] [Google Scholar]
- 80.Surtees P.G., Wainwright N.W.J., Boekholdt S.M., Luben R.N., Wareham N.J., Khaw K.T. Major depression, C-reactive protein, and incident ischemic heart disease in healthy men and women. Psychosom Med. 2008;70:850–855. doi: 10.1097/PSY.0b013e318183acd5. [DOI] [PubMed] [Google Scholar]
- 81.VanderWeele T.J., Tchetgen E.J. Mediation analysis with time varying exposures and mediators. J R Stat Soc Series B Stat Methodol. 2017;79:917–938. doi: 10.1111/rssb.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin S.H., Young J., Logan R., Tchetgen E.J., VanderWeele T.J. Parametric mediational g-formula approach to mediation analysis with time-varying exposures, mediators, and confounders. Epidemiology. 2017;28:266–274. doi: 10.1097/EDE.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mac Giollabhui N. Inflammation and depression: Research designs to better understand the mechanistic relationships between depression, inflammation, cognitive dysfunction, and their shared risk factors. Brain Behav Immun Health. 2021;15:100278. doi: 10.1016/j.bbih.2021.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khandaker G.M., Zuber V., Rees J.M., Carvalho L., Mason A.M., Foley C.N., et al. Shared mechanisms between coronary heart disease and depression: Findings from a large UK general population-based cohort [published correction appears in Mol Psychiatry 2021; 26:3659–3661] Mol Psychiatry. 2020;25:1477–1486. doi: 10.1038/s41380-019-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (Finger): A randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg A., Mangialasche F., Ngandu T., Solomon A., Kivipelto M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis. 2020;7:29–36. doi: 10.14283/jpad.2019.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ioannou M., Foiselle M., Mallet J., Stam E.L., Godin O., Dubertret C., et al. Towards precision medicine: What are the stratification hypotheses to identify homogeneous inflammatory subgroups. Eur Neuropsychopharmacol. 2021;45:108–121. doi: 10.1016/j.euroneuro.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Cramer A.O.J., Borsboom D., Aggen S.H., Kendler K.S. The pathoplasticity of dysphoric episodes: Differential impact of stressful life events on the pattern of depressive symptom inter-correlations. Psychol Med. 2012;42:957–965. doi: 10.1017/S003329171100211X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cramer A.O.J., Waldorp L.J., van der Maas H.L.J., Borsboom D. Comorbidity: A network perspective. Behav Brain Sci. 2010;33:137–150. doi: 10.1017/S0140525X09991567. discussion 150. [DOI] [PubMed] [Google Scholar]
- 90.Milaneschi Y., Lamers F., Berk M., Penninx B.W.J.H. Depression heterogeneity and its biological underpinnings: Toward immunometabolic depression. Biol Psychiatry. 2020;88:369–380. doi: 10.1016/j.biopsych.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 91.Fry A., Littlejohns T.J., Sudlow C., Doherty N., Adamska L., Sprosen T., et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manolio T.A. Using the data we have: Improving diversity in genomic research. Am J Hum Genet. 2019;105:233–236. doi: 10.1016/j.ajhg.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferrucci L., Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lind L., Sundström J., Ärnlöv J., Lampa E. Impact of aging on the strength of cardiovascular risk factors: A longitudinal study over 40 years. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaplin A.B., Smith N., Jones P.B., Khandaker G.M. Direction of association between cardiovascular risk and depressive symptoms during the first 18 years of life: A prospective birth cohort study. J Affect Disord. 2021;292:508–516. doi: 10.1016/j.jad.2021.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moriarity D.P., Mac Giollabhui N.M., Ellman L.M., Klugman J., Coe C.L., Abramson L.Y., Alloy L.B. Inflammatory proteins predict change in depressive symptoms in male and female adolescents. Clin Psychol Sci. 2019;7:754–767. doi: 10.1177/2167702619826586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.