The prevalence of obesity and type 2 diabetes are on the rise throughout the world. Obesity and the associated systemic insulin resistance are known to cause dysregulation of key functions in multiple peripheral organs, which collectively lead to many types of devastating complications of diabetes. Mounting evidence has demonstrated that obesity and diabetes also have a profound impact on the central nervous system. Thus, both obesity and diabetes increase the risks for cognitive decline and emotional disorders, such as depression and anxiety. The molecular mechanisms underlying these strong epidemiological links, however, are still not fully understood.
SEE CORRESPONDING ARTICLE ON PAGE 651
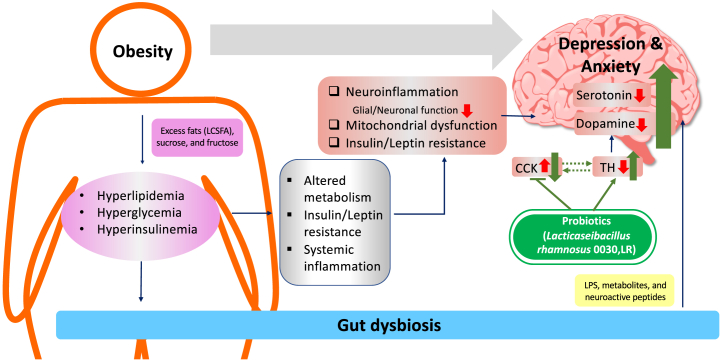
The obesity- and diabetes-induced emotional disorders are likely multifactorial (Figure 1) (1). The prolonged consumption of modern diets, which are high in fat and sugar, is a significant contributor. Excess fats from the diet, especially long-chain saturated fatty acids such as palmitate, induce inflammation both systemically and in the brain. High levels of sucrose and fructose disturb normal brain glucose metabolism and induce inflammation in the brain. Notably, chronic neuroinflammation in brain regions relevant to emotion control, such as the ventral hippocampus, nucleus accumbens, prefrontal cortex, anterior cingulate cortex, and amygdala, is highly associated with depression and anxiety and has been shown to contribute to the etiology of both conditions. In addition, dysregulation of homeostatic hormone signaling plays critical roles in the development of anxiety and depression. Thus, insulin resistance and leptin resistance in the brain impair mitochondrial homeostasis, alter brain energy metabolism, and impair key neuronal and glial cell functions in brain regions related to emotional control (2).
Figure 1.
Interactions between obesity, gut dysbiosis, and emotional disorders. An unhealthy diet containing excess fats and sugar, together with a sedentary lifestyle, causes obesity accompanied by hyperlipidemia, hyperglycemia, and hyperinsulinemia. All these contribute to systemic alterations in energy metabolism, insulin and leptin resistance, and inflammation. In addition to systemic effects, obesity impacts brain functions at multiple levels. Thus, neuroinflammation increases and insulin/leptin sensitivity decreases in the brain in obesity. Mitochondrial homeostasis is also severely disturbed. Collectively, these changes in the brain in obesity directly or indirectly lead to impaired serotonin and dopamine signaling, which is critical for emotional control. Gut dysbiosis caused by obesity also contributes to obesity-associated depression and anxiety. The remodeling of gut microbiota by probiotics such as Lactobacillus rhamnosus is sufficient to reverse depressive-like behavior in obesity. Mechanistically, L rhamnosus treatment suppresses the expression of cholecystokinin (CCK) and increases the expression of tyrosine hydroxylase (TH) in the nucleus accumbens, both of which may contribute to improved dopamine signaling and depressive-like behavior. LCSFA, long-chain saturated fatty acid; LPS, lipopolysaccharide.
A growing body of evidence has shown that gut dysbiosis is another key mediator in obesity- and diabetes-induced emotional disorders. Changes in microbiota composition are evident in subjects who are obese as well as in diet-induced obese mouse models. Importantly, transplanting gut microbiota of obese mice to germ-free lean mice is sufficient to induce not only obesity and the associated metabolic phenotypes but also depressive-like behavior (3). Microbiota can affect brain function via direct actions of microbial-derived lipopolysaccharide and metabolites. Gut microbiota and its metabolites are also poised to dynamically interact with enteroendocrine L cells in the gut to regulate the release of neuroactive peptides into circulation, many of which have been shown to play critical roles in metabolism, cognition, and emotion (Figure 1) (4).
Given the role of gut dysbiosis in the etiology of emotional disorders, is gut microbiota remodeling an effective approach to treat or alleviate obesity- and diabetes-induced emotional disorders? In the current issue of Biological Psychiatry: Global Open Science, Schell et al. (5) provide new evidence showing the effectiveness of probiotics in attenuating depressive-like behavior in diet-induced obese mice. In the study, both male and female mice fed with a 45% high-fat diet displayed metabolic deterioration, accompanied by increased anxiety and depressive-like behavior when compared with control mice fed with a semisynthetic low-fat diet. To assess the effects of probiotics on systemic metabolism and emotional behaviors in obesity, high-fat diet–fed mice were administered Lacticaseibacillus rhamnosus 0030 (LR) by daily oral gavage. While LR application for up to 7 weeks showed minor effects on body weight and systemic metabolism in both male and female obese mice, LR significantly reduced time of immobility during the forced swimming test in obese males. These findings are striking, as 1) they show that probiotic LR can reverse the depressive-like behavior even without a normalization of systemic metabolism and insulin resistance, and 2) the beneficial effect appears more profound in obese male mice.
To explore the mechanism by which LR administration modifies behavior in high-fat diet–fed male mice, Schell et al. (5) used an unbiased multiomics approach. Thus, high-fat diet feeding clearly shifted cecal microbiota composition, whereas LR treatment showed a moderate effect on microbiota diversity. Nevertheless, LR was sufficient to revert depressive-like behavior in male obese mice in the absence of a robust microbiota remodeling. One of the major mechanisms by which gut microbiome affect remote organs is the release of gut microbiota–derived metabolites, which travel through circulation into the brain. Schell et al. compared metabolome in plasma and cerebrospinal fluid of lean, obese, and obese + LR-treated males. Surprisingly, dietary- and LR-induced metabolomic changes were not overlapping between plasma and cerebrospinal fluid samples. These data suggest a different regulation of metabolism in the brain compared with metabolism in the periphery. In addition, many changes of the gut microbiota–derived metabolites may be masked by the overwhelming abundance of other tissue-derived metabolites in the plasma and cerebrospinal fluid samples. Alternatively, the gut-derived peptides secondary to microbiome changes may be the key factors driving the behavioral modulation. For example, gut-derived peptide YY regulates neural activity within the corticolimbic and hypothalamic brain regions to modulate feeding behavior (6).
The work by Schell et al. (5) is one of the recent examples showing that the gut microbiome affects neural functions. While identification of the gut microbiome–derived molecules responsible for the LR-mediated antidepressant effect is challenging in this study, Schell et al. propose cholecystokinin (Cck) as a potential downstream effector in the brain (Figure 1). Thus, transcriptomic analyses of the nucleus accumbens core (NAcc) from lean, obese, and obese + LR-treated male mice demonstrated an increase in Cck expression in obese mice, which was significantly reduced after LR treatment. Importantly, the messenger RNA levels of Cck in the NAcc were negatively associated with tyrosine hydroxylase (Th), which is the rate-limiting enzyme in dopamine biosynthesis. These data strongly suggest an interaction between CCK and dopamine in the reward circuit. Since its first discovery in the gastrointestinal system, CCK has become a well-known peptide that is heavily involved in digestion. In the brain, CCK is expressed in a select population of neurons, mostly GABAergic (gamma-aminobutyric acidergic) interneurons. CCK+ interneurons, together with parvalbumin+ interneurons, compose the family of basket interneurons and provide perisomatic GABAergic inhibition to modulate the activity and plasticity of neural circuits related to memory, emotion, and many other functions. Interestingly, CCK is also expressed in dopaminergic neurons in the ventral tegmental area (7), which project to the NAcc and control reward. Further, a subset of glutamatergic neurons in the amygdala projecting to the NAcc are CCK+ (8). The major contributor for the alterations in Cck expression following a high-fat diet and LR intervention remains unclear. Identification of the main neuronal populations responsible for the Cck changes will be essential to map out the neural circuit involved in LR-mediated antidepressant effects.
Similar to many other neuropeptides, CCK modulates many neural circuits, particularly the dopaminergic reward circuit. Elevation of CCK concentration in the brain coincides with increased anxiety and depressive-like behavior in mice (9). How CCK action affects dopamine (DA) signaling in the NAcc, however, is complex. While CCK potentiates DA release in the caudal NAcc, it suppresses stimulated DA release in the rostral NAcc (10). These opposing effects of CCK in different subregions of the NAcc are likely due to the heterogeneous nature of the CCK+ DA neurons and the distinct cellular functions of CCK1 versus CCK2 receptors on downstream neurons. The data from Schell et al. (5) showing the negative correlation of Cck expression with tyrosine hydroxylase provides new insight into how CCK affects the DA-dependent reward circuit. It opens a new avenue to further explore the underlying mechanisms of CCK–DA interaction. Does increased CCKergic signaling in the NAcc cause the reduced production of DA by suppressing tyrosine hydroxylase? Do increases in CCK levels reduce the innervation of DA neurons to the NAcc? Conversely, is it plausible to speculate that decreased tyrosine hydroxylase expression causes the up-regulation of CCK in diet-induced obese mice? In addition, it is important to identify the key gut microbiota–derived molecules responsible for the regulation of CCK in the brain, given the high therapeutic potential of these candidate molecules.
Acknowledgments and Disclosures
This work was supported by National Institute of Mental Health Grant No. R01MH125903 (to WC).
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Fulton S., Décarie-Spain L., Fioramonti X., Guiard B., Nakajima S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab. 2022;33:18–35. doi: 10.1016/j.tem.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Cai W., Hoover B., Kahn C.R. Insulin action in the brain: Cell types, circuits, and diseases. Trends Neurosci. 2022;45:384–400. doi: 10.1016/j.tins.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto M., Herzog C., Pacheco J.A., Fujisaka S., Bullock K., Clish C.B., et al. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry. 2018;23:2287–2301. doi: 10.1038/s41380-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemmensen C., Muller T.D., Woods S.C., Berthoud H.R., Seeley R.J., Tschop M.H. Gut-brain cross-talk in metabolic control. Cell. 2017;168:758–774. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schell M., Wardelmann K., Hauffe R., Rath M., Chopra S., Kleinridders A. Lactobacillus rhamnosus sex-specifically attenuates depressive-like behavior and mitigates metabolic consequences in obesity. Biol Psychiatry Glob Open Sci. 2023;3:651–662. doi: 10.1016/j.bpsgos.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batterham R.L., ffytche D.H., Rosenthal J.M., Zelaya F.O., Barker G.J., Withers D.J., Williams S.C.R. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 7.Schalling M., Friberg K., Seroogy K., Riederer P., Bird E., Schiffmann S.N., et al. Analysis of expression of cholecystokinin in dopamine cells in the ventral mesencephalon of several species and in humans with schizophrenia. Proc Natl Acad Sci U S A. 1990;87:8427–8431. doi: 10.1073/pnas.87.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen C.J., Zheng D., Li K.X., Yang J.M., Pan H.Q., Yu X.D., et al. Cannabinoid CB(1) receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25:337–349. doi: 10.1038/s41591-018-0299-9. [DOI] [PubMed] [Google Scholar]
- 9.Becker C., Zeau B., Rivat C., Blugeot A., Hamon M., Benoliel J.J. Repeated social defeat–induced depression-like behavioral and biological alterations in rats: Involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- 10.Rotzinger S., Vaccarino F.J. Cholecystokinin receptor subtypes: Role in the modulation of anxiety-related and reward-related behaviours in animal models. J Psychiatry Neurosci. 2003;28:171–181. [PMC free article] [PubMed] [Google Scholar]