Abstract
Background
Posttraumatic stress disorder (PTSD) is a mental health condition triggered by exposure to traumatic events in an individual’s life. Patients with PTSD are also at a higher risk for comorbidities. However, it is not well understood how PTSD affects human health and/or promotes the risk for comorbidities. Nevertheless, patients with PTSD harbor a proinflammatory milieu and dysbiotic gut microbiota. Gut barrier integrity helps to maintain normal gut homeostasis and its dysregulation promotes gut dysbiosis and inflammation.
Methods
We used a mouse model of repeated social defeat stress (RSDS), a preclinical model of PTSD. Behavioral studies, metagenomics analysis of the microbiome, gut permeability assay (on mouse colon, using an Ussing chamber), immunoblotting, and immunohistochemical analyses were performed. Polarized intestinal epithelial cells and 3-dimensional crypt cultures were used for mechanistic analysis.
Results
The RSDS mice harbor a heightened proinflammatory gut environment and microbiota dysbiosis. The RSDS mice further showed significant dysregulation of gut barrier functions, including transepithelial electrical resistance, mucin homeostasis, and antimicrobial responses. RSDS mice also showed a specific increase in intestinal expression of claudin-2, a tight junction protein, and epinephrine, a stress-induced neurotransmitter. Treating intestinal epithelial cells or 3-dimensional cultured crypts with norepinephrine or intestinal luminal contents (fecal contents) upregulated claudin-2 expression and inhibited transepithelial electrical resistance.
Conclusions
Traumatic stress induces dysregulation of gut barrier functions, which may underlie the observed gut microbiota changes and proinflammatory gut milieu, all of which may have an interdependent effect on the health and increased risk of comorbidities in patients with PTSD.
Keywords: Claudin, Gut dysbiosis, Gut permeability, Inflammation, Social defeat
Posttraumatic stress disorder (PTSD) is a psychological and behavioral disorder characterized by changes in arousal and activity and alteration in cognition and moods because of traumatic events in an individual’s life (1, 2, 3). Although generally thought to be a health condition associated primarily with active-duty military personnel and veterans exposed to war-associated trauma, PTSD is widespread, including in the civilian population (4). Published subject reviews and meta-analysis studies have emphasized that people with PTSD are also at a higher risk of developing other diseases than people without PTSD (5, 6, 7, 8). However, the underlying mechanism by which PTSD predisposes to poor health and/or comorbidities remains unknown, though a possible association with subclinical levels of the proinflammatory environment has been suggested (1,9,10).
Recent evidence has demonstrated a critical role for the gut-brain axis in regulating mammalian gut homeostasis and inflammation, which can have bidirectional effects, i.e., brain to gut (efferent) and gut to brain (afferent) (11). However, the mechanistic understanding of gut-brain communication is not well understood, though a role for the immune system and gut microbiota has been suggested (12, 13, 14). Along this line, recent studies have emphasized the causal involvement of the gut microbiota in the regulation of inflammatory diseases via the microbiota-gut-brain axis (15, 16, 17, 18). A similar role of the gut microbiome is suggested in PTSD and neurological diseases (19, 20, 21). Also, elevated levels of proinflammatory cytokines, including interleukin 6 (IL-6), tumor necrosis factor α, IL-1, and interferon gamma, and metabolic and oxidative stress markers have been reported in patients with PTSD and neurological disorders, which may be regulated via the gut-brain axis (1,22). Based on the longitudinal studies to establish a causal relationship between PTSD and inflammation, few have emphasized that inflammation before trauma increases the risk of PTSD, whereas some have emphasized the role of PTSD in promoting inflammation. Thus a bidirectional relationship between PTSD and inflammation is plausible (23, 24, 25, 26). However, a detailed mechanistic understanding of PTSD and gut homeostatic regulation, in which gut barrier integrity is of pivotal significance, is lacking.
The gut barrier helps maintain the normal gut-brain axis communication. The tight junction (TJ), the most apical cell-cell adhesion, plays a key role in maintaining the epithelial barrier integrity and homeostasis (27, 28, 29). Accordingly, dysregulation of the TJ proteins leads to dysfunction of the barrier integrity, diarrhea, and irregular movements of luminal microbes and metabolites across the mucosa, promoting inflammation and disease pathology (29, 30, 31). The claudin family of proteins forms the backbone of the TJ (28,32, 33, 34). The studies aimed at understanding the gut barrier dysfunction during intestinal inflammatory conditions using genetically manipulated mice have validated a critical role of claudin proteins in the dynamic regulation of gut homeostasis and microbiota colonization (35, 36, 37). However, potential interdependence of the claudin-mediated regulation of gut homeostasis and microbiota dysbiosis is not clearly understood. In addition to the TJs, goblet cells are involved in maintaining the integrity of the gut barrier because they are responsible for the synthesis and secretion of mucins (38). Changes in the number of goblet cells or mucus secretion are linked with the gut barrier dysregulation and microbial dysbiosis (39,40). The antimicrobial functions of the gut epithelium (Paneth cells) further help in maintenance of the intestinal barrier (41). Dysregulated secretion of antimicrobial peptides promotes inflammatory gut pathology (42). However, how posttraumatic stress affects gut homeostasis, especially barrier functions and integrity, has not been experimentally evaluated in detail.
In the current study, using the mouse model of repeated social defeat stress (RSDS), a preclinical murine model that potentially recapitulates PTSD-like behavior in humans, we demonstrate that traumatic stress alters the gut barrier functions and gut microbiota. Accompanying changes in the proinflammatory signaling pathways suggest a possible causal connection between the gut barrier dysfunction and microbiota dysbiosis in promoting stress-associated dysregulation of gut homeostasis and thus increasing the risk of inflammatory disorders and comorbidities.
Methods and Materials
The details of materials and methods are provided in the Supplement.
Statistical Analyses
We used a total of 16–18 mice/group (control and RSDS groups) for the behavioral studies. For subsequent biochemical analyses, we used 4–6 mice/group that were chosen randomly. All the experimental values are expressed as mean ± SEM. To compare the 2 groups, we used Student’s t test. For more than 2 groups, we used a one-way analysis of variance followed by Tukey’s multiple comparison test. We used GraphPad Prism 9 software (https://www.graphpad.com/features) for statistical analyses. A p value < .05 was considered statistically significant. For the microbiota-related analysis, the comparison between the 2 specific groups, control and test, Welch’s t test was applied. To calculate the effect size and confidence intervals, the differences in mean proportion effect size measure and Welch’s confidence intervals were used. To determine the false discovery rate, the Benjamini-Hochberg multiple test correction method was used in all the comparisons. A statistical difference of at least p < .05 was used to select the significant features within a group of profiles. The alpha and beta diversity, relative frequency, and box plots were plotted using R package.
Results
RSDS Induces PTSD-like Behaviors
The key behavioral characteristics of posttraumatic stress include severe avoidance of situations that can bring back memories of the trauma, anxiety, or depression (https://www.nimh.nih.gov/health/topics/post-traumatic-stress-disorder-ptsd). Thus, to model PTSD-like symptoms, we used a mouse model of RSDS, as illustrated in Figure 1A. Anxiety-like behaviors and locomotor activities were monitored using an elevated zero maze with closed and open arms. The animals subjected to RSDS showed reduced locomotor activity as the total distance moved was lower than that of the control mice (Figure 1B). The mice subjected to RSDS also spent less time in the open arm of the elevated zero maze as compared with the nonstressed mice (Figure 1C). We also evaluated the depression-like and antisocial behavior in these mice using a social interaction test. The mice experiencing RSDS spent less time in the social interaction area and more time in isolation as compared with the control mice, as measured by the social interaction ratio and corner zone ratio (Figure 1D, E). Overall, these behavioral patterns suggested that the mice subjected to RSDS had behaviors similar to those observed in PTSD.
Figure 1.
RSDS induces posttraumatic stress disorder–like behavior: anxiety-like and depression-like behavior parameters were determined by the elevated zero maze and social interaction test, respectively. (A) Experimental design and timeline for creating an RSDS mouse model. (B) Distance moved (n = 16–18). (C) Time spent in the open field (n = 16–18). (D) Social interaction ratio (n = 16–18). (E) Corner zone ratio (n = 16–18). RSDS, repeated social defeat stress.
RSDS Induces Gut Dysbiosis
PTSD has an association with gut dysbiosis (43). Thus, to ascertain whether mice subjected to RSDS also develop gut dysbiosis, we examined the gut microbiota. We performed 16S ribosomal RNA shotgun metagenomic analysis using the stool (DNA) from control and RSDS mice. Heatmap analysis of the sequencing results revealed the differential richness of the bacterial species in the control and RSDS mice (Figure 2A). Further analysis of alpha diversity measured by the Shannon and Simpson diversity index also showed significant changes in the microbial diversity of RSDS versus control mice. (Figure 2B). We also calculated beta diversity using the Bray-Curtis distance measurement to understand the level of species overlap in the groups. Beta diversity is considered high when there is less overlap of species between groups and vice-versa. We found 30.5% species richness of the control mice samples on axis 1 and 14.8% species richness of the stressed mice samples on axis 2 (Figure 2C), suggesting that there was greater diversity and a small overlap between the control and stressed groups. Further analysis revealed an increased abundance of Firmicutes and a reduced abundance of Bacteroidetes at phylum level in the stressed mice, suggesting an altered Firmicutes:Bacteroidetes ratio (Figure 2D). Next, the linear discrimination analysis scores showed significantly enriched bacteria within both stressed and control mice groups at the family level. Specifically, we found an increased abundance of Lachnospiraceae in control mice, whereas stressed mice were dominated by Muribaculaceae family members, which included Parabacteroides sp. YL27 and Muribaculum bacterium (Figure 2E). Furthermore, species enrichment (phylogenetic relations) was determined by constructing a dendrogram using the linear discrimination analysis effect size, which suggests the phylogenetic distribution of bacteria that are significantly enriched at species level in the control and stressed mice (Figure 2F). Overall, these findings suggest that repeated traumatic stress affects intestinal microbiota abundance, leading to gut dysbiosis.
Figure 2.
Repeated social stress induces gut dysbiosis: 16S ribosomal RNA genomic analysis was performed on fecal DNA isolated from control and stressed mice. (A) Heat map of bacterial presence in fecal samples from control and RSDS mice. (B) Shannon and Simpson alpha diversity. (C) Bray-Curtis beta diversity. (D) Relative abundance of bacterial microbiota at the phylum level. (E) LDA effect size analysis of differential microbiota abundance between control and stressed mice groups, represented by histogram of LDA scores. The LDA score is used as an abscissa, higher scores represent greater differences. (F) Cladogram plot of differential species abundance in the gut microbiota of control and stressed groups. n = 5 mice/group. LDA, linear discriminant analysis; RSDS, repeated social defeat stress.
RSDS Induces Subclinical Levels of Intestinal Inflammation
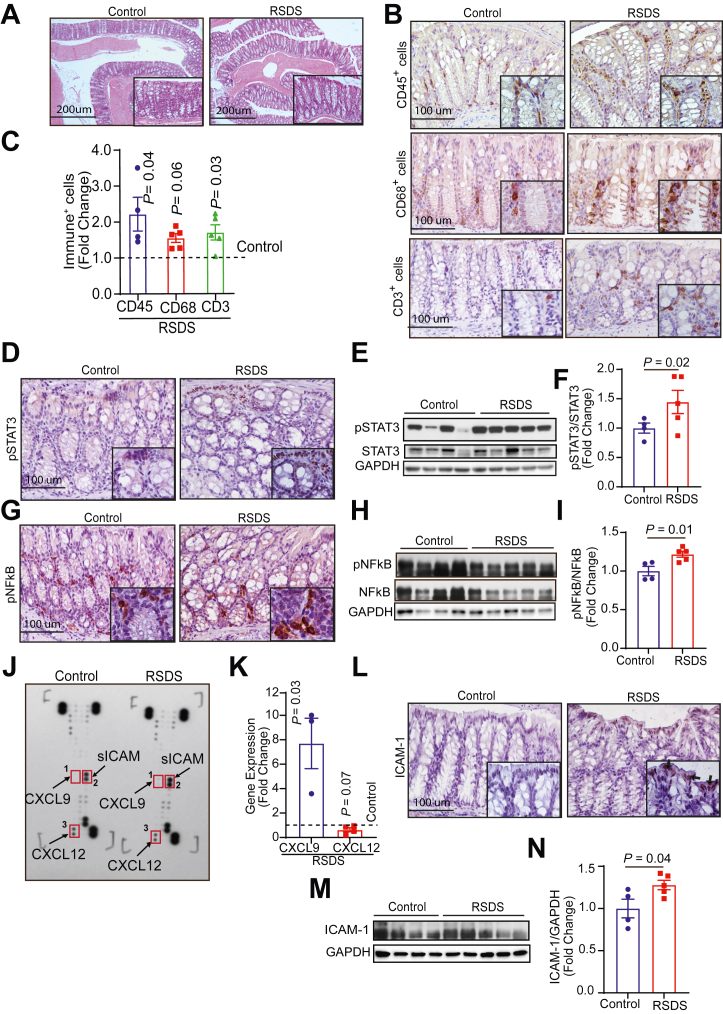
PTSD has been linked to changes in mucosal homeostasis and inflammation (44,45). However, the direct impact of PTSD on gut homeostasis and inflammation is not experimentally well established. Interestingly, the blinded evaluations of the hematoxylin and eosin staining of the colon and ileum from control and stressed mice did not demonstrate clinically relevant changes in intestinal inflammation and/or epithelial injury (Figures 3A and 4A). Therefore, we further examined whether subtle changes in the gut inflammatory milieu, not visible in the hematoxylin and eosin evaluation, existed. In this regard, we examined potential increase in mucosal infiltration by immune cells. The quantitative analysis of the specific immunoreactivity showed significant increases in the mucosal infiltration of CD45+ leukocytes, CD68+ macrophages, and CD3e+ T cells in both colon (Figure 3B, C) and ileum (Figure 4B, C) of stressed mice as compared to that in colon and ileum of control mice.
Figure 3.
Repeated social stress induces proinflammatory responses in the colon. Histological changes, immune cell filtration, and inflammatory signaling were determined in the colons of the control and RSDS mice. (A) Hematoxylin and eosin staining of control and stressed colon sections. (B, C) Immunohistochemical staining and quantification of CD45+ infiltrating leukocytes, CD68+ macrophages, and CD3+ T cells in the stressed and controlled colon. (D) Immunostaining of pSTAT3. (E, F) Western blot image and analysis of pSTAT3/STAT3 expression. (G) Immunostaining of pNF-κB. (H, I) Western blot image and analysis of pNF-κB/NF-κB expression. (J) Cytokine array blot image. (K) Relative gene expression analysis of the chemokines CXCL9 (n = 3) and CXCL12 (n = 4) in the stressed colon compared with that of control. (L) Immunohistochemical staining of ICAM-1 in the colon section. (M, N) Western blot image and analysis of ICAM-1. n = 4–5 mice/group unless otherwise stated. ICAM-1, intercellular adhesion molecule 1; pNF-κB, phosphorylated nuclear factor-κB; pSTAT3, phosphorylated signal transducer and activator of transcription 3; RSDS, repeated social defeat stress.
Figure 4.
Repeated social stress induces proinflammatory responses in the ileum. Histological changes, immune cells filtration, and inflammatory signaling were determined in the ileum of the control and RSDS mice. (A) Hematoxylin and eosin staining of control and stressed ileum sections. (B, C) Immunohistochemical staining and quantification of CD45+ infiltrating leukocytes, CD68+ macrophages, and CD3+ T cells in control and stressed ileum sections. (D) Immunostaining of pSTAT3. (E, F) Western blot image and analysis of pSTAT3/STAT3 expression (n = 6). (G) Immunostaining of pNF-κB. (H, I) Western blot image and analysis of pNF-κB/NF-κB expression. (J) Relative gene expression analysis of the chemokines CXCL9 and CXCL12 in the stressed ileum compared with control. (K) Immunohistochemical staining of ICAM-1 in control and stressed ileum. (L, M) Western blot image and analysis of ICAM-1 in control and stressed ileum. n = 4–5 mice/group unless otherwise stated. ICAM-1, intercellular adhesion molecule 1; pNF-κB, phosphorylated nuclear factor-κB; pSTAT3, phosphorylated signal transducer and activator of transcription 3; RSDS, repeated social defeat stress.
In light of these changes, we further examined the status of key proinflammatory signaling pathways like phosphorylated nuclear factor-κB (NF-κB) (Ser 536) and phosphorylated Stat3 (Tyr 705). The immunostaining and immunoblot analyses demonstrated an increase in the expression of phosphorylated Stat3 in both the colon and the ileum of the RSDS mice as compared to that in colon and ileum of the control mice (Figures 3D–F and 4D–F). Expression of phosphorylated NF-κB was also significantly upregulated in both the colon and ileum (Figures 3G–I and 4G–I) of the RSDS mice. Interestingly, immunolocalization demonstrated phosphorylated Stat3 changes predominantly in the epithelial cells (Figures 3D and 4D), while phosphorylated NF-κB (anti-P65) changes were predominantly observed in the immune cells (Figures 3G and 4G). Further analysis of the cytokines/chemokines using a commercial dot-blot array showed specific changes in the expression of CXCL9, CXCL12, and ICAM-1 (intercellular adhesion molecule 1) (Figure 3J). Subsequent analysis using reverse transcription quantitative polymerase chain reaction validated an increased expression of Cxcl9 but not Cxcl12 in the colon and ileum of RSDS mice (Figures 3K and 4J). Immunoblotting and immunostaining also validated an increased expression of soluble ICAM-1 in both colon and ileum of the RSDS mice (Figures 3L–N and 4K–M). Overall, these findings suggest that RSDS induces marked dysregulation of gut immune homeostasis.
RSDS Modulates Mucin/Goblet Cell Homeostasis and Antimicrobial Response
Gut barrier integrity is critical in regulating intestinal homeostasis, in which goblet cell-derived mucins provide the first physical barrier between the intestinal milieu and luminal contents, including the gut microbiota. Therefore, we determined whether intestinal mucin levels are altered in RSDS mice compared with control mice. Possible changes in the goblet cell number and size and mucins were examined. Remarkably, hematoxylin and eosin and Alcian blue staining (acidic mucins) showed that the goblet cells in the colon of the RSDS mice were enlarged and increased in number as compared to those in that of the control mice (Figure 5A, B). Periodic acid–Schiff staining (neutral mucins) did not differ between the stressed and control mice colon (data not shown). Neither periodic acid–Schiff nor Alcian blue staining in the ileum differed significantly between the RSDS and control mice (data not shown). In light of these findings, we further examined whether antimicrobial responses of the intestinal epithelial cells, yet another component of the gut barrier, were altered in RSDS mice. We examined the status of Reg proteins, defensin, and lysozyme. The quantitative reverse transcriptase polymerase chain reaction analysis showed a significant increase in the expression of Reg-3β in the colon of RSDS mice compared with that of control mice (Figure 5C); however, Reg-3γ and defensins levels were not significantly different (Figure 5C). In the ileum, Reg-3β and α-defensin were significantly higher in the RSDS mice (vs. control mice) while Reg-3γ remained unaltered (Figure 5D). Immunoblot analysis and immunostaining further showed a significant increase in lysozyme expression in the ileum of stressed mice versus that of control mice (Figure 5E–G). Overall, these findings suggest that traumatic stress markedly affects intestinal barrier properties, including antimicrobial responses.
Figure 5.
Repeated social stress alters the goblet cell homeostasis and antimicrobial responses in the host intestine. Goblet cell number and mucus status were determined by staining mucopolysaccharides and acidic mucins. (A, B) Alcian blue staining and quantification of secreted mucins in the colon sections of control and stressed mice. (C) Quantitative polymerase chain reaction analysis of the antimicrobial peptides Reg3β, Reg3γ, and α-defensin in the control and stressed colon. (D) Quantitative polymerase chain reaction analysis of antimicrobial peptides Reg3β, Reg3γ, and α-defensin in the ileum. (E, F) Western blot image and analysis of lysozyme in the ileum. (G) Immunohistochemical staining of lysozyme in control and stressed ileum. n = 4–5 mice/group. RSDS, repeated social defeat stress.
Repeated Traumatic Stress Causes Dysregulation of Gut Barrier Integrity and Promotes Claudin-2 Expression
Increased gut permeability has been associated with gut dysbiosis and inflammation in human neurological disease (46,47); however, experimental evidence is not well established. Therefore, we examined whether the TJ functions are also perturbed in RSDS mice. Ussing chamber–based analysis was performed to determine the gut barrier function, including transepithelial electrical resistance (TEER), conductance, and gut permeability (4 kDa fluorescein isothiocyanate-dextran). Data analysis revealed that the TEER was significantly downregulated in the colon of mice subjected to RSDS versus that of control mice, while transepithelial conductance was significantly higher (Figure 6A, B). Further determination of the TJ permeability using 4 kDa fluorescein isothiocyanate-dextran as a probe showed an increased trend in RSDS mice but the changes were not significant (Figure 6C). Taken together, the above data suggest specific dysregulation of the pore, but not the leak pathway, in the gut of RSDS mice.
Figure 6.
Repeated social stress induces dysregulation of the gut barrier functions and induces the expression of claudin-2. The functional parameters of tight junction integrity in the colons from control and stressed mice were determined using an Ussing chamber. Expression of the tight junction barrier proteins was analyzed by Western blot and immunostaining. (A) Transepithelial resistance. (B) Conductance. (C) Permeability for fluorescein isothiocyanate-dextran (4 kDa). (D, E) Representative Western blot image and analysis of claudin-2 expression in the control and stressed colon (n = 5). (F) Immunofluorescence staining of colonic claudin-2 expression. (G) Western blot image of claudin-1, -3, and -7, occludin, and ZO-1 in the control and stressed colon. n = 4–5 mice/group. RSDS, repeated social defeat stress.
Based on the above data, we further determined the effect of traumatic stress on the expression of major TJ proteins, including claudin proteins. Immunoblot analysis was performed using the total colon lysates, which demonstrated a significant increase in the expression of claudin-2 in the RSDS mice (Figure 6D, E). Immunofluorescence staining supported the findings of the immunoblot analysis (Figure 6F). This increase in claudin-2 expression was specific because we did not see major changes in the expression of other TJ proteins in the same lysates (Figure 6G). Overall, these findings suggest that traumatic stress induces barrier dysfunction and specifically alters claudin-2 expression.
Stress-Induced Neurohormone Induces Claudin-2 Expression to Dysregulate TJ Integrity
Traumatic stress has been linked to sympathetic nerve activation and release of neurotransmitters, including epinephrine and norepinephrine (NE) (48). In this regard, from immunostaining and immunoblot analyses using the colon lysates from control and stressed mice, we found that expression of tyrosine hydroxylase, a precursor enzyme for catecholamine (epinephrine, NE, and dopamine) synthesis, was significantly upregulated in RSDS mice compared with control mice (Figure 7A–C). Subsequent liquid chromatography/mass spectrometry-based analysis of the serum and colons from the control and RSDS mice further showed a significant increase in epinephrine expression in RSDS mice (Figure 7I, J). Based on this finding, we postulated a role for the stress-induced neurohormones in dysregulating gut barrier function. To test, we treated polarized Caco-2 cells with NE (10 μM) and determined the effects on TEER and claudin-2 expression. As shown in Figure 7D, NE treatment caused a significant decrease in the TEER compared with vehicle-treated cells. Immunoblot analysis further showed a significant and specific upregulation of claudin-2 expression in the NE-treated cells as compared with the vehicle control (Figure 7E, F). To further validate our in vitro finding, we treated ex vivo 3-dimensionally–cultured colonic crypts with vehicle or NE (10 μM) and determined the expression of claudin-2 protein. We found a significant and specific increase in the expression of claudin-2 in NE-treated crypts compared with vehicle-treated crypts (Figure 7G, H); however, expression of other claudin proteins did not change significantly (data not shown). To further validate the relevance of the above outcome to in vivo conditions, we treated Caco-2 cells and 3-dimensional crypt culture using fecal contents as the source of the intestinal luminal contents. As shown in Figure 7K–O, exposure to the fecal contents from RSDS versus control mice upregulated claudin-2 expression in both Caco-2 cells and colon crypt culture and inhibited the TEER. Overall, these results suggest that during RSDS, stress neurohormones dysregulate the gut barrier function by specifically dysregulating claudin-2 expression.
Figure 7.
Stress-induced neurotransmitter dysregulates barrier function in intestinal epithelial cells. (A) Immunostaining of TH in the colon sections of control and stressed mice. (B, C) Western blot image and analysis of TH in the colon of control and stressed mice. (D) Transepithelial electrical resistance was measured in Caco-2 cells after 24 hours of treatment with NE (10 μM). (E, F) Western blot image and analysis of claudin-2 in Caco-2 cells treated with 10 μM NE for 24 hours. (G, H) Protein expression analysis of claudin-2 in a colon crypt treated with 10 μM NE for 24 hours. Epinephrine levels in the serum (I) and colon lysates (J) of RSDS vs. control mice. (K, L) Immunoblotting using colon crypt lysates treated with fecal contents and Caco-2 cells (M, N). (O) Transepithelial electrical resistance in Caco-2 cells treated with the fecal contents. N = 4–5 mice/group. For in vitro study, 3–4 samples were used from independent experiments. NE, norepinephrine; RSDS, repeated social defeat stress; TH, tyrosine hydroxylase.
Discussion
The current statistics suggest that 6 out of every 100 people in the United States are expected to have PTSD at some point in their lives (49). Recent studies have helped to develop a better understanding of this disorder, especially the symptoms and associated comorbidities. However, how PTSD affects human health and increases the risk of other diseases is not clear, though a causal role of the dysregulated gut-brain axis has been implied. Outcomes from the current study support such an assumption and demonstrate that repeated psychological stress significantly alters gut homeostasis, potentially by altering the gut barrier functions. The key findings further suggest an interdependence between the dysregulated gut barrier, gut dysbiosis, and immune cell infiltration in creating a proinflammatory gut milieu and, thus, heightened susceptibility to inflammatory diseases. Mechanistically, we show that psychological stress modifies the gut barrier function by dysregulating the expression of the TJ protein claudin-2 and thus modulating the TJ pore pathway. Our observation that NE induces claudin-2 expression and inhibits TEER in the intestinal epithelial cells (Caco-2 cells and colon crypt) suggests that the stress hormone–mediated barrier dysfunction may promote the proinflammatory gut milieu, causing the gut microbiota changes.
An increasing number of studies have now demonstrated the significance of gut dysbiosis in regulating neurologic disorders (50, 51, 52, 53, 54). A causal association between gut dysbiosis and depression has also been reported (55,56). In this regard, we found that repeated psychological stress does induce gut dysbiosis, suggesting dysregulation of the microbiota-gut-brain axis. Specifically, we found a change in the ratio of the Bacteroidetes and Firmicutes. Firmicutes are anaerobic gram-positive bacteria involved in short-chain fatty acid production by promoting fermentation of dietary fibers. Bacteroidetes are facultative anaerobic gram-negative bacteria involved in the breakdown of organic molecules and energy production. Both phyla represent the dominant groups of bacteria in the human gut. A higher Firmicutes and lower Bacteroidetes presence suggests microbial imbalance that may be related with increased caloric extraction from food, accumulation of fat, insulin sensitivity impairment, obesity, and inflammation, traits that are linked with PTSD (57). The ratio of Firmicutes:Bacteroidetes is reported to be an important player in the maintenance of gut homeostasis. A change in the Firmicutes:Bacteroidetes ratio characterizes individuals with obesity and those with inflammatory gut diseases (58). We also found an increased abundance of Lachnospiraceae, an anaerobic butyrate producer residing in the intestine. Short-chain fatty acids including butyrate regulate intestinal inflammation and gut barrier integrity (59). However, the reduced abundance of the Muribaculaceae family members, a prevalent component of the gut microbiome in control mice compared with stressed mice suggests the possible dysbiotic environment. Muribaculum intestinale YL27 sp. is an anaerobic, mesophilic, gram-negative bacterium isolated from cecal contents of C57BL/6J mice. On the other hand, uncultured bacteria are uncharacterized most dominant (>90%) bacteria in the gut and identified only from 16S ribosomal DNA sequences. Disturbance of these bacteria promotes dysregulation of the fermentation of dietary fibers and cholesterol metabolism (60,61). Taken together, changes in the gut microbiota under conditions of chronic psychological stress may indicate the possible dysbiotic environment, inflammatory milieu, and disturbed microbiota-gut-brain axis.
Furthermore, gut microbiota contribute significantly to the development and function of the immune system (62, 63, 64, 65). Accordingly, recent studies using models of RSDS have shown significant changes in peripheral inflammation and increased levels of circulating cytokines and chemokines, including IL-6, IL-22, and IL-17 (66). Both IL-6 and IL-22 play critical roles in the regulation of mucosal epithelial and immune homeostasis, including antimicrobial responses (67). Our findings that RSDS mice harbor dysbiotic gut microbiota provide a possible explanation for the proinflammatory signaling under conditions of traumatic stress. Furthermore, our findings that repeated traumatic stress also induces significant change in the mucosal infiltration of immune cells, including macrophages and T cells as well as soluble ICAM-1 and CXCL9, suggest dysregulated mucosal immune homeostasis at the cellular level. Notably, ICAM-1 modulates inflammatory responses by promoting the infiltration of immune cells, while CXCL-9 has been reported to promote age-related inflammation (68,69). The gut epithelial-derived CXCL-9 has also been reported in the prevention of microbial overgrowth during infection (70,71). Our findings, in which Stat-3 and NF-κB signaling is modulated in the RSDS mice, further support a widespread dysregulation of the mucosal immunity and susceptibility to inflammation in response to RSDS at the molecular level. Notably, NF-κB promotes ICAM-1 activation to promote inflammation (72), indicating the activation of inflammatory signaling network during psychological trauma. Our additional findings showing altered antimicrobial response in RSDS mice further strengthens the concept that stress has a profound impact on the gut epithelial, immune, and microbiota homeostasis. Overall, our data suggest a potential integration between gut dysbiosis and an inflammatory gut milieu.
In addition to inflammation, gut dysbiosis has been associated with gut permeability (73,74). Notably, dysregulation of gut barrier integrity is considered central to the proinflammatory gut environment and inflammatory diseases (75,76). Of note, dysregulation of the pore pathways (ion and solutes transport) of the gut barrier can modulate the gut luminal environment and, thus, microbiota colonization (30). Along with gut barrier proteins, the role of mucins (first line of defense) in maintaining the normal gut microbiota has been described (77,78). Our finding that RSDS significantly inhibits TEER while promoting claudin-2 expression supports the postulation that posttraumatic stress dysregulates gut barrier function to promote the proinflammatory gut environment. It is worthy of noting that intestinal claudin-2 expression increases in response to inflammatory signaling, including IL-6, IL-17, and IL-22, and promotes gut permeability for ions and solutes and secretory diarrhea (30,76,79). Diarrhea is an established stress response. Our findings that significantly increased expression of tyrosine hydroxylase and epinephrine in RSDS mice gut and that NE treatment or fecal extract exposure dysregulated barrier function and claudin-2 expression in intestinal epithelial cells further signify the role of claudin-2 as an immediate early response protein to traumatic stress. As per a recent study, T-lymphocyte–derived NE precursor enzyme tyrosine hydroxylase regulates T helper 17 cells–derived induction of IL-22 and peripheral inflammation in the RSDS model (80). Taken together, our current findings suggest a possible interaction between the peripheral and mucosal homeostasis in the regulation of stress-induced inflammatory responses. However, further mechanistic studies are warranted to determine whether neurotransmitters and IL-22 modulate the gut barrier independently or cooperatively.
It is worth noting that despite the dysregulation of the gut barrier function, microbiota abundance, and proinflammatory signaling, the RSDS mice did not show clinical signs of intestinal inflammation and/or injury. This implies that clinical evaluation of the broader clinical markers of gut inflammation may not suffice for the prognosis of increased health risk and that more detailed analysis of proinflammatory signaling is required. Our findings that acidic mucins and antimicrobial responses were also altered in the gut of RSDS mice further imply that the gut in individuals with PTSD is possibly constantly adjusting to the proinflammatory environment, and repeated exposure to such conditions may ultimately lead to the broader changes in gut permeability, “the leak pathway,” and, thus, increased risk of poor health outcomes. Overall, the findings from our current study support the role of the microbiota-gut-brain axis in traumatic stress–associated health concerns and imply that traumatic stress induces gut barrier dysregulation, promoting disease conditions and comorbidities. We believe that the outcome of our current study provides the first of its kind data for not only barrier dysregulation in the specific capacity of claudin-2 upregulation as an immediate response to psychological stress but also its therapeutic significance in treating such conditions. However, further studies are needed to delineate the role of claudin-2 in microbiota-gut-brain axis regulation via control of dysbiosis and inflammation and the development of gut-associated diseases, including inflammatory bowel disease and colon cancer, using claudin-2 knockout mice, which is part of our ongoing study.
Limitations of the Study and Future Directions
Despite our data that RSDS induces gut barrier dysregulation, microbiota dysbiosis, and proinflammatory gut milieu, additional studies are needed to support causal interdependence. The key limitations of this study and future goals are as follows:
-
1)
Determination of the baseline gut microbiota colonization and stress hormones in control and RSDS mice and accompanying changes in the gut barrier functions to provide a better understanding of the causal integration between the gut-microbiota-brain axis.
-
2)
Transplantation of gut microbiota from control and RSDS mice into germ-free mice would help to determine the causal role of the gut microbiota in associated behavioral changes.
-
3)
Determination of the molecular mechanisms of stress-hormone regulation of claudin-2 expression, including the causal role of the inflammatory cytokines (IL-6, IL-22, etc.).
Acknowledgments and Disclosures
This work was supported in part by funds from a Veterans Affairs-merit award (Grant No. BX002761 [to ABS]) and National Institutes of Health R01 grant funding (Grant Nos. DK124095 [to ABS] and HL158521 [to AJC]).
We thank the University of Nebraska Medical Center Sequencing core facility for 16S DNA sequencing. The University of Nebraska DNA Sequencing Core receives partial support from the National Institute for General Medical Science Institutional Development Award Program Networks of Biomedical Research Excellence grant (Grant No. P20GM103427-19) and the Fred & Pamela Buffett Cancer Center Support grant (Grant No. P30 CA036727).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.03.005.
Supplementary Material
References
- 1.Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., et al. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 2.Vahia V.N. Diagnostic and statistical manual of mental disorders 5: A quick glance. Indian J Psychiatry. 2013;55:220–223. doi: 10.4103/0019-5545.117131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association, DSM-5 Task Force . 5th ed. American Psychiatric Association Publishing, Inc; Washington, DC: 2002. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™. [Google Scholar]
- 4.Gaylord K.M., Holcomb J.B., Zolezzi M.E. A comparison of posttraumatic stress disorder between combat casualties and civilians treated at a military burn center. J Trauma. 2009;66(suppl):S191–S195. doi: 10.1097/TA.0b013e31819d9c21. [DOI] [PubMed] [Google Scholar]
- 5.Edmondson D., von Känel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4:320–329. doi: 10.1016/S2215-0366(16)30377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmarais P., Weidman D., Wassef A., Bruneau M.A., Friedland J., Bajsarowicz P., et al. The interplay between post-traumatic stress disorder and dementia: A systematic review. Am J Geriatr Psychiatry. 2020;28:48–60. doi: 10.1016/j.jagp.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Taft T.H., Quinton S., Jedel S., Simons M., Mutlu E.A., Hanauer S.B. Posttraumatic stress in patients with inflammatory bowel disease: Prevalence and relationships to patient-reported outcomes. Inflamm Bowel Dis. 2022;28:710–719. doi: 10.1093/ibd/izab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donovan A., Cohen B.E., Seal K.H., Bertenthal D., Margaretten M., Nishimi K., Neylan T.C. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donovan A., Ahmadian A.J., Neylan T.C., Pacult M.A., Edmondson D., Cohen B.E. Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain Behav Immun. 2017;60:198–205. doi: 10.1016/j.bbi.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donovan A., Chao L.L., Paulson J., Samuelson K.W., Shigenaga J.K., Grunfeld C., et al. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology. 2015;51:557–566. doi: 10.1016/j.psyneuen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agirman G., Yu K.B., Hsiao E.Y. Signaling inflammation across the gut-brain axis. Science. 2021;374:1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 12.Gabanyi I., Lepousez G., Wheeler R., Vieites-Prado A., Nissant A., Chevalier G., et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science. 2022;376 doi: 10.1126/science.abj3986. [DOI] [PubMed] [Google Scholar]
- 13.Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 14.Chu C., Murdock M.H., Jing D., Won T.H., Chung H., Kressel A.M., et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574:543–548. doi: 10.1038/s41586-019-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu C., Lee S.K., Zhang D., Frenette P.S. The gut microbiome regulates psychological-stress-induced inflammation. Immunity. 2020;53:417–428.e4. doi: 10.1016/j.immuni.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Chen W.D., Wang Y.D. The relationship between gut microbiota and inflammatory diseases: The role of macrophages. Front Microbiol. 2020;11:1065. doi: 10.3389/fmicb.2020.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolis K.G., Cryan J.F., Mayer E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology. 2021;160:1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cryan J.F., O’Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 20.Simpson C.A., Diaz-Arteche C., Eliby D., Schwartz O.S., Simmons J.G., Cowan C.S.M. The gut microbiota in anxiety and depression – A systematic review. Clin Psychol Rev. 2021;83 doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- 21.MacKay M., Yang B.H., Dursun S.M., Baker G.B. The gut-brain axis and the microbiome in anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder [published online Feb 22] Curr Neuropharmacol. 2023 doi: 10.2174/1570159X21666230222092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik S., Singh R., Arora G., Dangol A., Goyal S. Biomarkers of major depressive disorder: Knowing is half the battle. Clin Psychopharmacol Neurosci. 2021;19:12–25. doi: 10.9758/cpn.2021.19.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng Q.X., Soh A.Y.S., Loke W., Venkatanarayanan N., Lim D.Y., Yeo W.S. Systematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol. 2019;34:68–73. doi: 10.1111/jgh.14446. [DOI] [PubMed] [Google Scholar]
- 24.Cámara R.J.A., Gander M.L., Begré S., von Känel R., Swiss Inflammatory Bowel Disease Cohort Study Group Post-traumatic stress in Crohn’s disease and its association with disease activity. Frontline Gastroenterol. 2011;2:2–9. doi: 10.1136/fg.2010.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumner J.A., Nishimi K.M., Koenen K.C., Roberts A.L., Kubzansky L.D. Posttraumatic stress disorder and inflammation: Untangling issues of bidirectionality. Biol Psychiatry. 2020;87:885–897. doi: 10.1016/j.biopsych.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahraini N.H., Breshears R.E., Hernández T.D., Schneider A.L., Forster J.E., Brenner L.A. Traumatic brain injury and posttraumatic stress disorder. Psychiatr Clin North Am. 2014;37:55–75. doi: 10.1016/j.psc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Landau D. Epithelial paracellular proteins in health and disease. Curr Opin Nephrol Hypertens. 2006;15:425–429. doi: 10.1097/01.mnh.0000232883.43093.76. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Hernandez V., Quiros M., Nusrat A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397:66–79. doi: 10.1111/nyas.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32:256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Tsai P.Y., Zhang B., He W.Q., Zha J.M., Odenwald M.A., Singh G., et al. IL-22 upregulates epithelial Claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe. 2017;21:671–681.e4. doi: 10.1016/j.chom.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doney E., Cadoret A., Dion-Albert L., Lebel M., Menard C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur J Neurosci. 2022;55:2851–2894. doi: 10.1111/ejn.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhat A.A., Uppada S., Achkar I.W., Hashem S., Yadav S.K., Shanmugakonar M., et al. Tight junction proteins and signaling pathways in cancer and inflammation: A functional crosstalk. Front Physiol. 2018;9:1942. doi: 10.3389/fphys.2018.01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colegio O.R., Van Itallie C.M.V., McCrea H.J., Rahner C., Anderson J.M. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 34.Krug S.M., Günzel D., Conrad M.P., Lee I.F., Amasheh S., Fromm M., Yu A.S. Charge-selective claudin channels. Ann N Y Acad Sci. 2012;1257:20–28. doi: 10.1111/j.1749-6632.2012.06555.x. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad R., Chaturvedi R., Olivares-Villagómez D., Habib T., Asim M., Shivesh P., et al. Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol. 2014;7:1340–1353. doi: 10.1038/mi.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Y., Wang K., Xu C., Hao M., Li H., Ding L. Intestinal claudin-7 deficiency impacts the intestinal microbiota in mice with colitis. BMC Gastroenterol. 2022;22:24. doi: 10.1186/s12876-022-02100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmad R., Kumar B., Chen Z., Chen X., Müller D., Lele S.M., et al. Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy by hyperactivating Wnt/β-catenin signaling. Oncogene. 2017;36:6592–6604. doi: 10.1038/onc.2017.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S., Yu M. Role of goblet cells in intestinal barrier and mucosal immunity. J Inflamm Res. 2021;14:3171–3183. doi: 10.2147/JIR.S318327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morampudi V., Dalwadi U., Bhinder G., Sham H.P., Gill S.K., Chan J., et al. The goblet cell-derived mediator RELM-β drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016;9:1218–1233. doi: 10.1038/mi.2015.140. [DOI] [PubMed] [Google Scholar]
- 41.Lueschow S.R., McElroy S.J. The Paneth cell: The curator and defender of the immature small intestine. Front Immunol. 2020;11:587. doi: 10.3389/fimmu.2020.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adolph T.E., Tomczak M.F., Niederreiter L., Ko H.J., Böck J., Martinez-Naves E., et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao F., Guo R., Ma Q., Li Y., Wang W., Fan Y., et al. Stressful events induce long-term gut microbiota dysbiosis and associated post-traumatic stress symptoms in healthcare workers fighting against COVID-19. J Affect Disord. 2022;303:187–195. doi: 10.1016/j.jad.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hori H., Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. 2019;73:143–153. doi: 10.1111/pcn.12820. [DOI] [PubMed] [Google Scholar]
- 45.Fields C.T., Chassaing B., de Vries G.J. Gut barrier dysfunction and Type 2 immunity: Implications for compulsive behavior. Med Hypotheses. 2022;161 doi: 10.1016/j.mehy.2022.110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stadlbauer V., Engertsberger L., Komarova I., Feldbacher N., Leber B., Pichler G., et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020;20:248. doi: 10.1186/s12877-020-01644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanuytsel T., van Wanrooy S., Vanheel H., Vanormelingen C., Verschueren S., Houben E., et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 48.Kolacz J., Kovacic K.K., Porges S.W. Traumatic stress and the autonomic brain-gut connection in development: Polyvagal Theory as an integrative framework for psychosocial and gastrointestinal pathology. Dev Psychobiol. 2019;61:796–809. doi: 10.1002/dev.21852. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Veterans Affairs PTSD: National Center for PTSD. 2022. https://www.ptsd.va.gov/ Available at: Accessed December 30, 2022.
- 50.Bajaj J.S., Sikaroodi M., Fagan A., Heuman D., Gilles H., Gavis E.A., et al. Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2019;317:G661–G669. doi: 10.1152/ajpgi.00194.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Q., Luo Y., Ray Chaudhuri K., Reynolds R., Tan E.K., Pettersson S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain. 2021;144:2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L., Meng J., Ban Y., Jalodia R., Chupikova I., Fernandez I., et al. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci USA. 2019;116:13523–13532. doi: 10.1073/pnas.1901182116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenner L.A., Stearns-Yoder K.A., Hoffberg A.S., Penzenik M.E., Starosta A.J., Hernández T.D., et al. Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain Behav Immun. 2017;65:57–67. doi: 10.1016/j.bbi.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Moya-Pérez A., Perez-Villalba A., Benítez-Páez A., Campillo I., Sanz Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun. 2017;65:43–56. doi: 10.1016/j.bbi.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Capuco A., Urits I., Hasoon J., Chun R., Gerald B., Wang J.K., et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37:1328–1346. doi: 10.1007/s12325-020-01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morais L.H., Schreiber H.L., Mazmanian S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 57.Kubzansky L.D., Bordelois P., Jun H.J., Roberts A.L., Cerda M., Bluestone N., Koenen K.C. The weight of traumatic stress: A prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry. 2014;71:44–51. doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stojanov S., Berlec A., Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bajaj J.S., Khoruts A. In: Comprehensive Pharmacology. Kenakin T., editor. Elsevier; Oxford: 2022. 5.14: Microbial therapeutics in liver disease; pp. 271–285. [Google Scholar]
- 60.Chijiiwa R., Hosokawa M., Kogawa M., Nishikawa Y., Ide K., Sakanashi C., et al. Single-cell genomics of uncultured bacteria reveals dietary fiber responders in the mouse gut microbiota. Microbiome. 2020;8:5. doi: 10.1186/s40168-019-0779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kenny D.J., Plichta D.R., Shungin D., Koppel N., Hall A.B., Fu B., et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe. 2020;28:245–257.e6. doi: 10.1016/j.chom.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H.J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weng M., Walker W.A. The role of gut microbiota in programming the immune phenotype. J Dev Orig Health Dis. 2013;4:203–214. doi: 10.1017/S2040174412000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sethi V., Kurtom S., Tarique M., Lavania S., Malchiodi Z., Hellmund L., et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology. 2018;155:33–37.e6. doi: 10.1053/j.gastro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elkhatib S.K., Moshfegh C.M., Watson G.F., Case A.J. Peripheral inflammation is strongly linked to elevated zero maze behavior in repeated social defeat stress. Brain Behav Immun. 2020;90:279–285. doi: 10.1016/j.bbi.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neufert C., Pickert G., Zheng Y., Wittkopf N., Warntjen M., Nikolaev A., et al. Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle. 2010;9:652–655. doi: 10.4161/cc.9.4.10615. [DOI] [PubMed] [Google Scholar]
- 68.Iruela-Arispe M.L. An inflammatory clock for healthy aging. Nat Aging. 2021;1:574–575. doi: 10.1038/s43587-021-00085-9. [DOI] [PubMed] [Google Scholar]
- 69.Bui T.M., Wiesolek H.L., Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020;108:787–799. doi: 10.1002/JLB.2MR0220-549R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei J., Zhang C., Gao Y., Li Y., Zhang Q., Qi H., et al. Gut epithelial-derived CXCL9 maintains gut homeostasis through preventing overgrown E. coli. J Crohns Colitis. 2022;16:963–977. doi: 10.1093/ecco-jcc/jjab234. [DOI] [PubMed] [Google Scholar]
- 71.Reid-Yu S.A., Tuinema B.R., Small C.N., Xing L., Coombes B.K. CXCL9 contributes to antimicrobial protection of the gut during Citrobacter rodentium infection independent of chemokine-receptor signaling. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leclercq S., Matamoros S., Cani P.D., Neyrinck A.M., Jamar F., Stärkel P., et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harbison J.E., Roth-Schulze A.J., Giles L.C., Tran C.D., Ngui K.M., Penno M.A., et al. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatr Diabetes. 2019;20:574–583. doi: 10.1111/pedi.12865. [DOI] [PubMed] [Google Scholar]
- 75.Ahmad R., Sorrell M.F., Batra S.K., Dhawan P., Singh A.B. Gut permeability and mucosal inflammation: Bad, good or context dependent. Mucosal Immunol. 2017;10:307–317. doi: 10.1038/mi.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luettig J., Rosenthal R., Barmeyer C., Schulzke J.D. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers. 2015;3 doi: 10.4161/21688370.2014.977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paone P., Cani P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut. 2020;69:2232–2243. doi: 10.1136/gutjnl-2020-322260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tailford L.E., Crost E.H., Kavanaugh D., Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet. 2015;6:81. doi: 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki T., Yoshinaga N., Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elkhatib S.K., Moshfegh C.M., Watson G.F., Case A.J. T-lymphocyte tyrosine hydroxylase regulates TH17 T-lymphocytes during repeated social defeat stress. Brain Behav Immun. 2022;104:18–28. doi: 10.1016/j.bbi.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.