Abstract
Background
In older patients, comorbidities competed with cancer for mortality risk. We assessed the prognostic value of comorbidities in older patients with cancer.
Patients and methods
We analysed all patients >70 years of age with colorectal, breast, prostate, or lung cancer included in the prospective ELCAPA cohort. The Cumulative Illness Rating Scale-Geriatrics (CIRS-G) score was used to assess comorbidities. The primary endpoint was overall survival (OS) at 3, 12, and 36 months. The adjusted difference in the restricted mean survival time (RMST) was used to assess the strength of the relationship between comorbidities and survival.
Results
Of the 1551 patients included (median age 82 years; interquartile range 78-86 years), 502 (32%), 575 (38%), 283 (18%), and 191 (12%) had colorectal, breast, prostate, and lung cancer, respectively, and 50% had metastatic disease. Hypertension, kidney failure, and cognitive impairment were the most common comorbidities (67%, 38%, and 29% of the patients, respectively). A CIRS-G score >17, two or more severe comorbidities, more than seven comorbidities, heart failure, and cognitive impairment were independently associated with shorter OS. The greatest effect size was observed for CIRS-G >17 (versus CIRS-G <11): at 36 months, the adjusted differences in the RMST (95% confidence interval) were −6.0 months (−9.3 to −2.6 months) for colorectal cancer, −9.1 months (−13.2 to −4.9 months) for breast cancer, −8.3 months (−12.8 to −3.9 months) for prostate cancer, and −5.5 months (−9.9 to −1.1 months) for lung cancer (P < 0.05 for all).
Conclusions
Comorbidities’ type, number, and severity were independently associated with shorter OS. A 17-point cut-off over 56 for the total CIRS-G score could be considered in clinical practice.
Key words: geriatric assessment, comorbidities, CIRS-G, cancer
Highlights
-
•
Comorbidities’ type, number, and severity were independently associated with shorter OS in older patients with cancer.
-
•
CIRS-G score >17 was associated with decreased OS across all cancer subgroups colorectal, breast, prostate, and lung cancer.
-
•
Older cancer patients with a CIRS-G >17 had a 6- to 9-month reduction in overall 3-year survival, depending on tumour type.
Introduction
According to the World Health Organization, most developed countries define an older person as someone >65 years of age.1 Older adults are more likely than younger adults to have cancer and a worse prognosis. In 2020, 60% of the estimated new cancer diagnoses in Europe and 73% of the estimated cancer deaths occurred in people aged ≥65 years.2 Furthermore, the results of the EUROCARE-5 study showed that survival is worse in older patients.3
Comorbidities are acute or chronic diseases that co-exist with an index disease; they are common among older adults and in older cancer patients. In the United States, 40% of cancer patients have at least one comorbidity, and 15% have two or more.4 This accumulation of diseases is associated with greater morbidity and mortality rates in the general population5 and in cancer patients.6,7 However, there are very little published data on comorbidities’ prognostic value in older cancer patients. Also, available data differ regarding the study populations, comorbidities, and assessment tools. A recent literature review on the measurement of comorbidities in cancer patients identified 21 different approaches.8 One of the most well-known and widely used method is the Charlson score.9 The use of the Charlson score for cancer patients is limited because the majority of patients have a low or moderate Charlson score. For major cancers, four items of the Charlson score are excluded as they are related to the cancer primary site.10 The Cumulative Illness Rating Scale (CIRS) assesses the importance of comorbidities according to organs and systems. The CIRS rates comorbidities in 13 organs or systems according to severity. The information provided by the CIRS can be analysed as a total score, the number of affected organs, an average score by organs, or the number of severe comorbidities.10 The CIRS was developed in 1968 and modified in the 1990s for use in a geriatric population [the CIRS-Geriatric (CIRS-G)].11,12 This scale classifies the severity of comorbidities by the affected organ systems and assigns a score between 0 and 4 points per category according to severity. The American Society of Clinical Oncology has recommended the CIRS-G to assess comorbidities in older cancer patients.13
Despite the extended use of the CIRS-G, data regarding the prognostic value of comorbidities’ type, number, and severity are lacking. Therefore, the objectives of the present study were to evaluate the prevalence and the prognostic value of the type, number, and severity of comorbidities in older patients with colorectal, breast, prostate, or lung cancer.
Patients and methods
Design and patients
We analysed data from the Elderly Cancer Patients (ELCAPA) prospective, multicentre, open-cohort study (NCT02884375). Nineteen investigating centres in the Paris urban area of France recruited consecutive patients (i) aged >70 years, (ii) newly diagnosed with a solid tumour or haematological cancer, and (iii) referred for a multidimensional geriatric assessment (GA) before choice of a cancer treatment. Patients referred for GA were those for whom the oncologist (or surgeon, radiation therapist, or another physician) suspected a risk of frailty, either due to altered G8 score14 or based on clinical judgement. Verbal, informed consent was obtained from all study patients before inclusion. The study protocol was approved by the appropriate independent ethics committee (CPP Ile-de-France, Paris, France; reference: IORG0009918, approval code: N° SIRIPH2G: 12.00005.013216-MS06, approval date: 28 November 2012). For the present analysis, we selected ELCAPA cohort patients with colorectal, breast, prostate, or lung cancer between 2007 and 2017 (n = 1943).
Data collection
Baseline data were collected prospectively at the time of the initial GA. The GA was carried out by a senior geriatrician with expertise in oncology. The variables considered here were: age, sex, inpatient versus outpatient status at the time of inclusion, Eastern Cooperative Oncology Group performance status (ECOG-PS), tumour site, metastatic status (yes/no for distant metastasis), curative or palliative cancer treatment, G8 score, body mass index (BMI), the Mini Nutritional Assessment score, ≥10% weight loss in the previous 6 months (yes/no), a ‘timed up-and-go’ (TUG) test completion time >20 s (yes/no), a fall in the previous 6 months (yes/no), the Mini-Mental State Examination score (a value <24 out of 30 was considered to indicate cognitive impairment), the Activities of Daily Living (ADL) score,15 the Instrumental Activities of Daily Living (IADL) score,16 the family environment (marital status and the presence of a family caregiver or not), and the number of prescription medications taken daily.
Comorbidity
The CIRS-G score was used to measure the comorbidity burden at the time of the baseline comprehensive geriatric assessment (CGA). It was assigned by the physician conducting the CGA. The CIRS-G rates comorbidities in 14 organ systems on a 5-point scale ranging from 0 (no dysfunction) to 4 (extremely severe dysfunction); this gives a total score ranging from 0 (best) to 56 (worst). Severe comorbidity was defined by a score of 3 or 4 for each organ system concerned. The CIRS-G score was analysed according to the following approaches: total score, number of organ systems with a score ≥1, and organ systems with a score ≥3. A score ≥3 defined severe comorbidities. Other comorbidities were also recorded: ischaemic cardiopathy, heart failure, arrhythmia, hypertension, diabetes mellitus, obesity (BMI >30 kg/m2), chronic obstructive pulmonary disease (COPD), renal failure (Cockcroft creatinine clearance rate <60 ml/min), liver failure, depression, and cognitive impairment.
Outcomes
The primary outcome was overall survival (OS) at 3, 12, and 36 months. Follow-up time started at the baseline GA’s date and ended at the date of death or the date of the last follow-up, whichever came first. We chose the 3-month time point to assess the short-term prognostic value of potentially poorly controlled significant comorbidities, which could pose a life-threatening risk. The 12-month and 36-month time points were selected taking into account the median survival of the various selected cancers. The prevalence of comorbidities was calculated in each cancer subgroup as the percentage of patients who had a recorded diagnosis of the comorbidity in the initial GA and/or in the CIRS-G. For severe comorbidities, the prevalence was calculated as the frequency of organ systems with a score ≥3 in the CIRS-G.
Statistical analysis
Descriptive analysis
Continuous variables were quoted as the mean ± standard deviation (SD) when normally distributed or, if not, as the median [interquartile range (IQR)] or the median (range). Categorical data were quoted as the frequency (percentage). We compared baseline characteristics among tumour types using the chi-square test, analysis of variance, or the Kruskal–Wallis test, after Bonferroni correction for multiple testing.
Exposure ascertainment
Prognostic subgroups were identified using Classification and Regression Tree (CART) analysis with recursive partitioning of the OS data. The total CIRS-G score, the number of comorbidities, and the number of severe comorbidities defined subgroups.
Survival
The OS probability was analysed according to the Kaplan–Meier method. Mortality risks between groups were assessed using Cox proportional hazards (PH) regression models. The mean expected time to death was calculated as the restricted mean survival time (RMST). The RMST equates the area under the survival curve at specific time horizons and is independent of the PH hypothesis.17 The RMST intergroup difference was calculated for each prognostic group at 3, 12, and 36 months. Adjusted hazard ratios (HRs) and RMST were calculated using regression models adjusted for known prognostic factors in older patients with cancer: sex; age >80 years; metastatic status; curative treatment, palliative cancer treatment, or no cancer treatment; inpatient or outpatient status; abnormal TUG completion time; date of the baseline GA; and ≥10% weight loss in the previous 6 months.18 PH was verified using Schoenfeld residuals and Grambsch–Therneau tests. HRs were quoted with 95% confidence intervals (CIs).
Sensitivity analysis
We used a modified CIRS-G to analyse the OS in each cancer subgroup. For colorectal cancer, we removed the lower digestive system score; breast cancer, endocrine system score; prostate cancer, urinary system score; and lung cancer, the pulmonary system score. We carried out a CART analysis with recursive partitioning of the OS data to examine optimal thresholds by cancer subgroups. Finally, we evaluated the association of comorbidity parameters and OS for metastatic and non-metastatic patients in each cancer subgroup.
Missing data were not imputed. The threshold for statistical significance was set to P < 0.05. All tests were two-tailed, and statistical analyses were carried out with R software (version 4.0.3) or Stata software (version 13.1).
Results
Patients
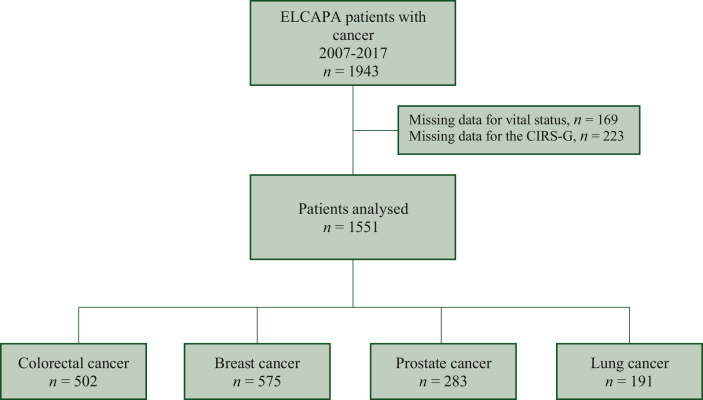
Of the 1943 patients included in the ELCAPA cohort between February 2007 and December 2017, 1551 had complete CIRS-G and follow-up data (Figure 1). The median (IQR) age was 82.2 years (77.8-86.1 years), 57% of the patients were women, 52% had a baseline ECOG-PS of 0 or 1, and 50% had metastatic disease. Of the 1551 patients included, 502 (32%) had colorectal, 575 (38%) had breast, 283 (18%) had prostate, and 191 (12%) had lung cancer. The mean (SD) CIRS-G score was 11.69 (5.22), and the mean (SD) number of comorbidities was 6.32 (2.40). Table 1 summarizes the characteristics of the patient (tumour site) subgroups. Curative cancer treatment was initiated for 54% of the included patients. Among the remaining patients, 33% underwent palliative treatments (chemotherapy, radiotherapy, or surgery), while 13% exclusively received palliative care. Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101831, provides an overview of the received treatments.
Figure 1.
Study flow diagram.
Table 1.
Description of the study population
| Colorectal cancer (n = 502) | Breast cancer (n = 575) | Prostate cancer (n = 283) | Lung cancer (n = 191) | P valuea | |
|---|---|---|---|---|---|
| Age, years (n = 1551) | 82.6 (78.4-86.6)d | 82.3 (78.1-86.2)e | 81.6 (76.4-85.1)d,e | 82.0 (78.0-85.3) | 0.002 |
| Median (IQR) | |||||
| Female sex, n (%) (n = 1551) | 247 (49.2)d,e | 567 (98.6)d,f | 0 | 62 (32.5)e,f | <0.001 |
| Metastases at inclusion, n (%) (n = 1403) | 229 (47.4)d,e,f | 180 (33.1)d,g,h | 176 (62.4)e,g,h | 113 (63.1)f,h,i | <0.001 |
| Inpatient at inclusion, n (%) (n = 1551) | 181 (36.1)d,e | 86 (14.6)d,f | 49 (17.3)e,g | 70 (36.6)f | <0.001 |
| ECOG-PS ≥ (%) (n = 1551) | 268 (53.3)d,e | 238 (41.4)d,f | 123 (43.5)e,g | 117 (61.3)f,g | <0.001 |
| G8 score ≤14, n % (n = 1551) | 91a,b | 81e,f,h | 72e,g,h | 93f,g | <0.001 |
| Impaired IADL, <7/8, n (%) (n = 1481) | 335 (62.6)d,e | 234 (38.7)d,f,g | 146 (50.4)e,f,h | 123 (63.8)g,h | <0.01 |
| Impaired ADL, <5/6, n (%) (n = 1551) | 119 (23.6) | 91 (15.7) | 55 (19.1) | 44 (23.0) | 0.07 |
| Smoking status (n = 1462) | <0.001 | ||||
| Never smoker, n (%) | 274 (58.1)d,e | 430 (82.7)d,e,f | 131 (49.1)f,h | 47 (24.9)e,g,h | |
| Current smoker, n (%) | 27 (5.7) | 26 (5.0) | 14 (5.2) | 22 (11.6) | |
| Former smoker, n (%) | 171 (36.2) | 64 (12.3) | 122 (45.7) | 120 (63.5) | |
| Number of medications taken daily (n = 1503) | |||||
| Mean (SD) | 5.85 (3.06) | 6.32 (3.47) | 6.36 (3.12) | 6.47 (3.22) | 0.04 |
| >5, n (%) | 235 (48.8) | 291 (53.3) | 160 (57.8) | 106 (57.6) | 0.3 |
| Fall in the previous 6 months, n (%) (n = 1536) | 135 (27.6) | 171 (30.3) | 82 (29.1) | 51 (27.6) | 0.79 |
| Abnormal TUG completion timeb, n (%) (n = 1286) | 131 (33.0)d,e | 151 (30.3)d | 55 (23.0)e | 45 (29.8) | 0.002 |
| Weight lossc, n (%) (n = 1530) | 247 (49.2)d,e | 148 (25.7)d | 82 (29.0)e,f | 56 (29.3)f | <0.001 |
| Albuminaemia, g/l, median (IQR) (n = 1266) | 33.50 (28.8-37.2)d,e | 38.40 (35.0-42.0)d,f | 38.00 (33.0-42.0)e,g | 34.00 (29.0-38.0)f,g | <0.001 |
| Serum C-reactive protein, mg/l, median (IQR) (n = 1145) | 15.10 (4.1-50.5)d,e,f | 4.00 (2.4-13.0)d,e,f,g | 4.00 (2.2-23.2)h | 23.90 (6.2-70.0)g,h | <0.001 |
| CIRS-G score, mean (SD) (n = 1551) | 12.40 (5.05)d | 10.56 (4.89)d,e,f | 12.07 (5.58)e | 12.64 (5.49)f | <0.001 |
| Number of comorbidities, mean (SD) (n = 1551) | 5.65 (2.34)d | 5.93 (2.28)d,e | 6.28 (2.44) | 6.59 (2.67)e | <0.001 |
| Number of severe comorbidities, mean (SD) (n = 1551) | 1.18 (1.27)d | 0.82 (1.13)d,e,f | 1.18 (1.18)e | 1.49 (1.25)f | <0.001 |
d,e,f,g,h,i A significant (P < 0.05) difference in pairwise analyses of cancer site subgroups in a chi-square test for categorical variables, an ANOVA for normally distributed continuous variables, or a Wilcoxon-Mann-Whitney test for non-normally distributed continuous variables, after Bonferroni correction.
In a chi-square test for categorical variables, an analysis of variance (ANOVA) for normally distributed continuous variables, and a Kruskal-Wallis test for non-normally distributed continuous variables.
Overall time >20 s or not feasible.
Time period not defined.
ADL, Activities of Daily Living; ECOG-PS, Eastern Cooperative Oncology Group performance status; IADL, Instrumental Activities of Daily Living; IQR, interquartile range; SD, standard deviation; TUG, timed up-and-go.
Pairwise comparisons of cancer subgroups
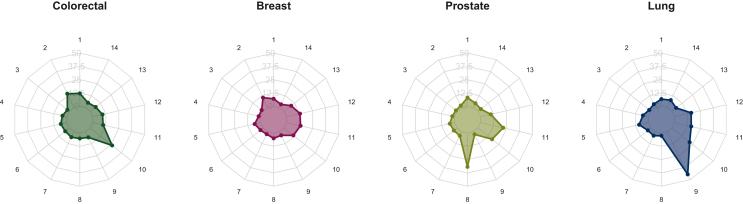
Compared with the patients in the other subgroups, prostate cancer patients were younger (P = 0.002), breast cancer patients were more likely to have non-metastatic cancer (P < 0.001), and patients with prostate or breast cancer were less likely to have been inpatients at baseline. Breast cancer patients had the lowest CIRS-G scores [mean (SD) value of 10.56 (4.89)] and the lowest number of severe comorbidities [mean value of 0.82 (1.13)]. Colorectal cancer patients had the lowest number of comorbidities [mean 5.65 (2.34)] (Table 1). The prevalences of severe comorbidities in the 14 organ systems for each subgroup are shown in Figure 2. Lung cancer patients were less likely to be obese (P < 0.001) and more likely to have respiratory failure (P < 0.001). Breast cancer patients were more likely to have ischaemic cardiopathy (P < 0.001) and less likely to have renal failure (P < 0.001). The prevalence of specific comorbidities for each subgroup is shown in Figure 3.
Figure 2.
Prevalence of severe comorbidities [proportion of Cumulative Illness Rating Scale-Geriatrics (CIRS-G) grades 3 or 4], by cancer site. 1, Heart; 2, lower digestive; 3, upper digestive; 4, liver; 5, hypertension; 6, kidney; 7, haematopoietic system; 8, genitourinary tract; 9, respiratory tract; 10, pancreas, endocrine, and metabolic diseases; 11, musculoskeletal/integument; 12, psychiatric disorders; 13, eyes, ears, nose, throat; 14, neurological diseases.
Figure 3.
Prevalence of comorbidities (axis: from 0% to 100%), by cancer site. 1, Hypertension; 2, heart failure; 3, ischaemic cardiopathy; 4, arrhythmia; 5, chronic obstructive pulmonary disease; 6, obesity; 7, diabetes mellitus; 8, kidney failure; 9, liver failure; 10, depressive syndrome; 11, cognitive impairment.
Survival
The median (range) follow-up time was 28 months (0-152 months). The 3-, 12-, and 36-month OS rates (95% CI) in the overall study population were, respectively, 86.8% (85.2% to 88.6%), 68.2% (65.9% to 70.5%), and 42.4% (39.8% to 45.2%). The all-cause mortality data by tumour site are summarized in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101831.
Using recursive partitioning analysis, the optimal thresholds to predict OS were 0-10, 11-14, 15-17, and 17-56 for the total CIRS-G score; 0-7 and >7 for the number of comorbidities; and 0, 1, and >1 for severe comorbidities.
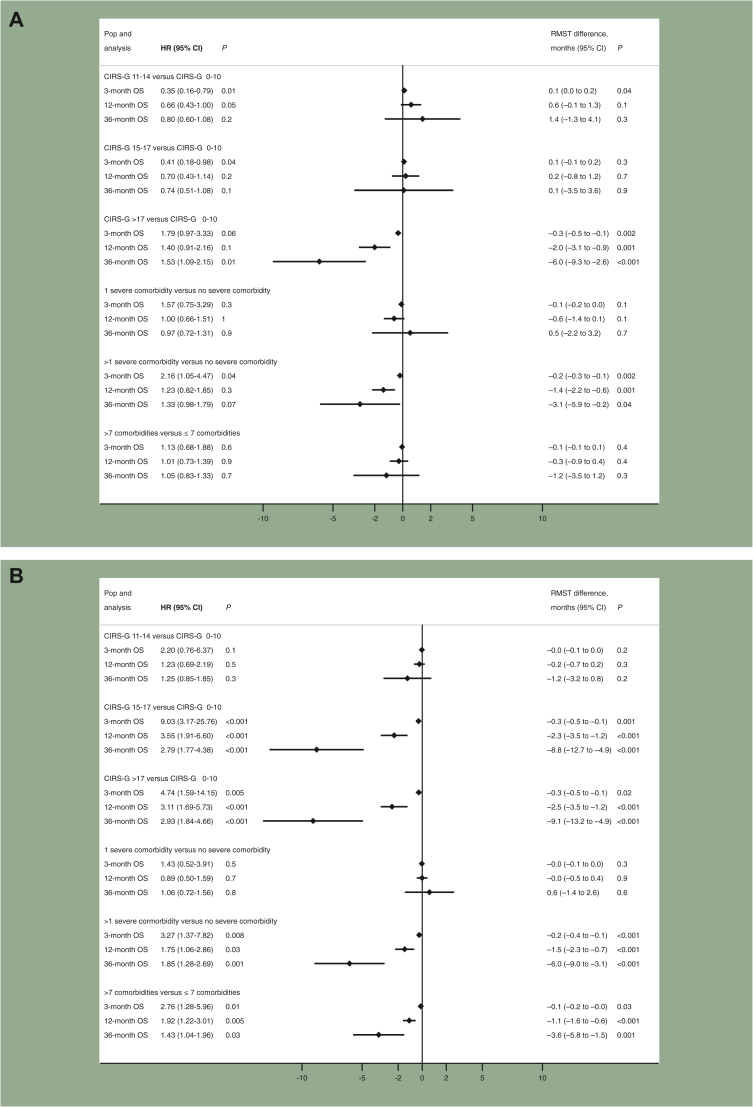
A CIRS-G score > 17 was associated with poorer survival among all tumour types at 3, 12, and 36 months. The largest effect sizes were observed at 36 months: in colorectal cancer patients, RMST difference = −6.0 months (−9.3 to −2.6 months), P < 0.001; in breast cancer patients, RMST difference = −9.1 months (−13.2 to −4.9 months), P < 0.001; in prostate cancer patients, RMST difference = −8.3 months (−12.8 to −3.9 months), P < 0.001; and in lung cancer patients, RMST difference = −5.5 months (−9.9 to −1.1 months), P = 0.01.
More than one severe comorbidity was associated with poorer survival at 36 months in colorectal, breast, and prostate cancer patients. Having more than seven comorbidities was associated with poorer survival at 12 and 36 months in breast and prostate cancer patients. Only a CIRS-G >17 was significantly associated with poorer survival in lung cancer patients at 3, 12, and 36 months. Detailed results are shown in Figure 4. The results of separate analyses for metastatic and non-metastatic cancer patients are presented in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.101831.
Figure 4.
Associations of comorbidities with OS at 3 months, 1 year, and 3 years. The difference in RMST is expressed in days. Not all HR estimations satisfied the proportional hazards assumptions. (A) Colorectal cancer. (B) Breast cancer. (C) Prostate cancer. (D) Lung cancer. The models were adjusted for sex, age >80 years, curative treatment, palliative treatment, or no cancer treatment, metastatic status, the baseline CGA as an inpatient or an outpatient, abnormal TUG completion time, the date of the baseline GA, and ≥10% weight loss. CGA, comprehensive geriatric assessment; CI, confidence interval; CIRS-G, Cumulative Illness Rating Scale for Geriatrics; GA, geriatric assessment; HR, hazard ratio; OS, overall survival; RMST, restricted mean survival time.
At 36 months, depression, cognitive impairment, arrhythmia, and liver failure were associated with poorer OS in colorectal cancer. Liver failure accounted for the larger effect [RMST difference = −11.0 months (−16.2 to −5.7 months)]. In breast cancer patients, cognitive impairment, respiratory failure, and heart failure were associated with lower survival. Heart failure had the larger effect [RMST difference = −7.0 months (−10.5 to −3.6 months)]. Depression, cognitive impairment, heart failure, and respiratory failure were associated with poorer 36-month OS in prostate cancer patients. The larger effect was observed for respiratory failure [RMST difference = −7.1 months (−12.2 to −2.0 months)]. In lung cancer, cognitive impairment was associated with poorer OS [RMST difference = −3.3 months (−6.6 to −0.04 months)]. Other results are shown in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.101831, and the results of the sensitivity analysis are given in Supplementary Table S5 and Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.101831.
Discussion
In our study cohort of 1551 older cancer patients, we observed that the total CIRS-G score and the number and severity of comorbidities were independent prognostic factors for OS. Specifically, a CIRS-G score >17 was associated with lower OS among all tumour types at 3, 12, and 36 months.
Within our cohort, half of the patients had more than six comorbidities, and 53% of patients had polymedication. This elevated comorbidity prevalence is primarily attributed to the advanced median age (82.2 years) of the population and the inclusion of patients referred for a GA. In contrast, Williams et al.19 reported that only 14% of older patients with cancer had five or more comorbidities. However, the median age at the time of the GA in William et al.’s study was only 72 years and data were obtained using patient-reported questionnaires. Edwards et al.4 observed 1 056 534 cancer patients aged ≥66 years from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked database. Here, 40% of patients had at least one comorbidity, and 15% had two or more comorbidities. The low comorbidity burden in Edwards et al.’s study could be explained by the limited quality and accuracy of data from medico-administrative databases.
The most common comorbidities observed in our study were hypertension (67%), renal failure (38%), cognitive impairment (29%), arrhythmia (27%), depression (26%), and diabetes mellitus (20%). These comorbidity profiles are similar to those described in studies20, 21, 22 based on hospital administrative data. Yancik et al.21 assessed comorbidity prevalence in 7600 older patients with cancer, as documented in the SEER database. Hypertension was the most prevalent condition, followed by heart conditions (13%-26%) and arthritis. COPD and diabetes were less prevalent. In a New Zealand study23 of 14 096 patients diagnosed with colon, rectal, breast, uterine, ovarian, liver, stomach, renal, or bladder cancer, the most common comorbidities were hypertension (from 8% to 21%, depending on the subgroup), cardiac conditions (2.1%-13.5%), and diabetes (2.3%-13.3%). Hypertension, COPD, and diabetes were the most prevalent comorbidities in a study of 331 655 patients in England.22 Overall, our study revealed two- to three-times higher frequency of key comorbidities compared with reported literature. This difference is probably attributed to our older population’s higher median age (82 years) with existing studies indicating increased comorbidity prevalence with age among older cancer patients.24 Furthermore, our prospective data collection method for comorbidities contributes to this variation. Our method is more exhaustive than the retrospective data collection from databases. Finally, patients in our study might be frailer than a non-selected population, given that all were referred for a GA.
Comorbidities can add complexity to cancer treatment by increasing the risk of adverse drug events and unplanned hospital admissions,25 leading to reduced completion rates of cancer treatment.5 Our study demonstrated that older cancer patients with a CIRS-G >17 had a 6- to 9-month reduction in overall 3-year survival, depending on tumour type. The difference in OS was most pronounced in breast cancer patients, with a 9-month decrease in overall 3-year survival for those with a CIRS-G >17. These patients with a CIRS-G >17 score accounted for 14% of the study population. The prognostic value of comorbidities was less pronounced in lung cancer, suggesting that several comorbidities might have less impact on cancers with lower survival rates.26,27 Another possible explanation could be that lung cancer patients received better management of their comorbidities, including consultations with organ specialists and earlier adaptation of comorbidity treatments.
Our study had several strengths. Firstly, all comorbidity and GA data were prospectively collected. Secondly, our cohort is one of the largest yet described in studies of comorbidities among older cancer patients. Thirdly, we studied four of the most prevalent cancers worldwide and in older adults.28,29 Lastly, our analysis took account of potential confounding factors (sex, age >80 years, metastatic status, baseline GA as an inpatient or an outpatient, abnormal TUG completion time, the date of the baseline GA, curative or palliative cancer treatment, and ≥10% weight loss in the previous 6 months) and included indicators of frailty (notably cognitive impairment, TUG, weight loss). This is in contrast to most literature studies, which relied on retrospective medico-administrative data and only adjusted for sex, age, and ethnicity.
Our study had some limitations. Firstly, it included older cancer patients referred for a GA, which might have introduced selection bias with more vulnerable patients. In our study, 83% of the included patients had a G8 score ≤14. For comparison, impaired G8 was 68.4% in the ONCODAGE study.30 Secondly, our primary endpoint was OS, and we did not have specific data on cancer-related mortality. While some studies show that comorbidities correlate with higher all-cause mortality rates rather than cancer-related mortality,31 others suggest an association between comorbidities and greater cancer-related mortality in colon, breast, or lung cancer patients.32, 33, 34, 35, 36 These studies, however, were not exclusive to the older population. Around 70% of cancer patient deaths are attributed to comorbidity,31 although more aggressive cancers may see higher cancer-related death rates.35 Lastly, our study did not describe the specific management of comorbidities, whether through consultation with an organ specialist, adjustment of comorbidity treatments, or any other interventions. The management of comorbidities may differ between organ specialists, geriatricians, and oncologists.
The CIRS-G score is sensitive to intra-individual variations and might be used in randomized clinical trials.37 However, some of the organ systems included in the CIRS-G may not always be relevant to the chosen outcome. Whether we need to consider all 14 CIRS-G systems when predicting mortality or treatment tolerance is unclear, which warrants further investigation. Furthermore, the different comorbidities may have a different impact on mortality or toxicity. However, the CIRS-G gives the same weight to all comorbidities. Further research into how specific comorbidities influence cancer outcomes is warranted.
The CIRS-G aims to provide comprehensive information on comorbidities in selected older patients with cancer in clinical practice. In contrast to the Charlson Index,9 another frequently used score, the CIRS-G allows for a precise study of comorbidity profiles, with an evaluation of the type, number, and severity of comorbidities. Therefore, the American Society of Clinical Oncology has recommended the CIRS-G to assess comorbidities in older cancer patients.13 Our study revealed that a total CIRS-G score exceeding 17 was associated with decreased OS across all four cancer subgroups and at all specified time points. This result suggests that this cut-off of 17 could be helpful in clinical practice for more accurately assessing the risk of mortality. Our results emphasize the importance of multidisciplinary management (by oncologists, geriatricians, and organ specialists) when treating older patients with cancer.
Conclusion
The type and number of comorbidities evaluated by the CIRS-G score influence survival prognosis in older patients with cancer. A total CIRS-G score >17 may help identify older patients with cancer at an increased risk of mortality.
Acknowledgements
The ELCAPA Study Group consists of geriatricians (Amelie Aregui, Melany Baronn, Mickael Bringuier, Eric Bouvard, Philippe Caillet, Gaelle Cosqueric, Lola Corsin, Tristan Cudennec, Anne Chahwakilian, Amina Djender, Eric Dupuydupin, Nargess Ebadi, Virginie Fossey-Diaz, Mathilde Gisselbrecht, Charlotte Goldstein, Beatrice Gonzalez, Marie Laurent, Julien Leguen, Madeleine Lefevre, Celine Lazarovici-Nagera, Emmanuelle Lorisson, Josephine Massias, Soraya Mebarki, Galdric Orvoen, Frederic Pamoukdjian, Anne-Laure Scain, Godelieve Rochette de Lempdes, Florence Rollot-Trad, Gwenaelle Varnier, Helene Vincent, Elena Paillaud, Agathe Raynaud-Simon), oncologists (Pascaline Boudou-Rouquette, Etienne Brain, Stephane Culine, Maxime Frelaut, Djamel Ghebriou, Joseph Gligorov, Stephane Henault Daniel Lopez-Trabada-Ataz, Olivier Mir, Christophe Tournigand), a digestive oncologist (Thomas Aparicio), a gynaecological oncologist (Cyril Touboul), a radiation oncologist (Jean-Leon Lagrange), nurses (Stephanie Benyahia, Sadia Bonhomme, Alzira Mota, Gwadlys Philocles, Corinne Ouakinine), epidemiologists (Etienne Audureau, Sylvie Bastuji-Garin, and Florence Canouï-Poitrine), a medical biologist (Marie-Anne Loriot), a pharmacist (Pierre-Andre Natella), a biostatistician (Claudia Martinez-Tapia), a clinical research physician (Nicoleta Reinald), clinical research nurses (Sandrine Rello, Melanie Lafage), data managers (Mylene Allain, Clelia Chambraud), and clinical research assistants (Aurelie Baudin, Margot Bobin, Johanna Canovas, Sabrina Chaoui, Lina Iratni, Sonia Garrigou, Sandrine Lacour, Helene Mabungu, Laure Morisset, Besma Saadaoui).
Funding
This work was supported by a grant from the French National Cancer Institute (Institut National du Cancer, INCa), Canceropôle Ile-de-France [grant number #RINC4], and Gerontopôle Ile-de-France (Gérond’if) (no grant number). The sponsor had no role in the design, methods, subject recruitment, data collections, analysis, and preparation of paper.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
Restrictions apply to the availability of these data. Data were obtained from the ELCAPA Study Group and individual data are available from the corresponding author with the permission of the ELCAPA Study Group investigators.
Supplementary data
References
- 1.World Health Organization . World Health Organization; 2015. World report on ageing and health.https://apps.who.int/iris/handle/10665/186463 Available at. [Google Scholar]
- 2.Dyba T., Randi G., Bray F., et al. The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308–347. doi: 10.1016/j.ejca.2021.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Angelis R., Sant M., Coleman M.P., et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15(1):23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 4.Edwards B.K., Noone A.M., Mariotto A.B., et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Søgaard M., Thomsen R.W., Bossen K.S., Sørensen H.T., Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(suppl 1):3–29. doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccirillo J.F., Feinstein A.R. Clinical symptoms and comorbidity: significance for the prognostic classification of cancer. Cancer. 1996;77(5):834–842. [PubMed] [Google Scholar]
- 7.Gross C.P., Guo Z., McAvay G.J., Allore H.G., Young M., Tinetti M.E. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2006;54(12):1898–1904. doi: 10.1111/j.1532-5415.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 8.Sarfati D. Review of methods used to measure comorbidity in cancer populations: no gold standard exists. J Clin Epidemiol. 2012;65(9):924–933. doi: 10.1016/j.jclinepi.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453–471. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 11.Parmelee P.A., Thuras P.D., Katz I.R., Lawton M.P. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 12.Salvi F., Miller M.D., Grilli A., et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56(10):1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 13.Mohile S.G., Dale W., Somerfield M.R., et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. doi: 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellera C.A., Rainfray M., Mathoulin-Pélissier S., et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 15.Katz S., Ford A.B., Moskowitz R.W., Jackson B.A., Jaffe M.W. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 16.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 17.Uno H., Claggett B., Tian L., et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–2385. doi: 10.1200/JCO.2014.55.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrat E., Paillaud E., Laurent M., et al. Predictors of 1-year mortality in a prospective cohort of elderly patients with cancer. J Gerontol A Biol Sci Med Sci. 2015;70(9):1148–1155. doi: 10.1093/gerona/glv025. [DOI] [PubMed] [Google Scholar]
- 19.Williams G.R., Deal A.M., Lund J.L., et al. Patient-reported comorbidity and survival in older adults with cancer. Oncologist. 2018;23(4):433–439. doi: 10.1634/theoncologist.2017-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarfati D., Gurney J., Lim B.T., et al. Identifying important comorbidity among cancer populations using administrative data: prevalence and impact on survival. Asia Pac J Clin Oncol. 2016;12(1):e47–e56. doi: 10.1111/ajco.12130. [DOI] [PubMed] [Google Scholar]
- 21.Yancik R., Havlik R.J., Wesley M.N., et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol. 1996;6(5):399–412. doi: 10.1016/s1047-2797(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 22.Fowler H., Belot A., Ellis L., et al. Comorbidity prevalence among cancer patients: a population-based cohort study of four cancers. BMC Cancer. 2020;20(1):2. doi: 10.1186/s12885-019-6472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarfati D., Tan L., Blakely T., Pearce N. Comorbidity among patients with colon cancer in New Zealand. N Z Med J. 2011;124(1338):76–88. [PubMed] [Google Scholar]
- 24.Wedding U., Roehrig B., Klippstein A., et al. Comorbidity in patients with cancer: prevalence and severity measured by cumulative illness rating scale. Crit Rev Oncol Hematol. 2007;61(3):269–276. doi: 10.1016/j.critrevonc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Williams G.R., Mackenzie A., Magnuson A., et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–257. doi: 10.1016/j.jgo.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccirillo J.F., Tierney R.M., Costas I., Grove L., Spitznagel E.L. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 27.Read W.L., Tierney R.M., Page N.C., et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22(15):3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 29.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 30.Soubeyran P., Bellera C., Goyard J., et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jørgensen T.L., Hallas J., Friis S., Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106(7):1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roxburgh C., McDonald A., Salmond J., et al. Adjuvant chemotherapy for resected colon cancer: comparison of the prognostic value of tumour and patient related factors. Int J Colorectal Dis. 2011;26(4):483–492. doi: 10.1007/s00384-010-1120-5. [DOI] [PubMed] [Google Scholar]
- 33.Sarfati D., Hill S., Blakely T., et al. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: a retrospective cohort study. BMC Cancer. 2009;9:116. doi: 10.1186/1471-2407-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riihimäki M., Thomsen H., Brandt A., Sundquist J., Hemminki K. Death causes in breast cancer patients. Ann Oncol. 2012;23(3):604–610. doi: 10.1093/annonc/mdr160. [DOI] [PubMed] [Google Scholar]
- 35.Kendal W.S. Dying with cancer: the influence of age, comorbidity, and cancer site. Cancer. 2008;112(6):1354–1362. doi: 10.1002/cncr.23315. [DOI] [PubMed] [Google Scholar]
- 36.van de Poll-Franse L.V., Haak H.R., Coebergh J.W.W., Janssen-Heijnen M.L.G., Lemmens V.E.P.P. Disease-specific mortality among stage I-III colorectal cancer patients with diabetes: a large population-based analysis. Diabetologia. 2012;55(8):2163–2172. doi: 10.1007/s00125-012-2555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Extermann M., Overcash J., Lyman G.H., Parr J., Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.