Abstract
Background
This study investigated the efficacy of chemoradiotherapy (CRT) followed by durvalumab as neoadjuvant therapy of locally advanced rectal cancer.
Patients and methods
The PANDORA trial is a prospective, phase II, open-label, single-arm, multicenter study aimed at evaluating the efficacy and safety of preoperative treatment with durvalumab (1500 mg every 4 weeks for three administrations) following long-course radiotherapy (RT) plus concomitant capecitabine (5040 cGy RT in 25-28 fractions over 5 weeks and capecitabine administered at 825 mg/m2 twice daily). The primary endpoint was the pathological complete response (pCR) rate; secondary endpoints were the proportion of clinical complete remissions and safety. The sample size was estimated assuming a null pCR proportion of 0.15 and an alternative pCR proportion of 0.30 (α = 0.05, power = 0.80). The proposed treatment could be considered promising if ≥13 pCRs were observed in 55 patients (EudraCT: 2018-004758-39; NCT04083365).
Results
Between November 2019 and August 2021, 60 patients were accrued, of which 55 were assessable for the study’s objectives. Two patients experienced disease progression during treatment. Nineteen out of 55 eligible patients achieved a pCR (34.5%, 95% confidence interval 22.2% to 48.6%). Regarding toxicity related to durvalumab, grade 3 adverse events (AEs) occurred in four patients (7.3%) (diarrhea, skin toxicity, transaminase increase, lipase increase, and pancolitis). Grade 4 toxicity was not observed. In 20 patients (36.4%), grade 1-2 AEs related to durvalumab were observed. The most common were endocrine toxicity (hyper/hypothyroidism), dermatologic toxicity (skin rash), and gastrointestinal toxicity (transaminase increase, nausea, diarrhea, constipation).
Conclusion
This study met its primary endpoint showing that CRT followed by durvalumab could increase pCR with a safe toxicity profile. This combination is a promising, feasible strategy worthy of further investigation.
Key words: durvalumab, neoadjuvant strategy, locally advanced rectal cancer, immunotherapy
Highlights
-
•
Neoadjuvant CRT followed by surgery ± adjuvant chemotherapy is the standard treatment of LARC.
-
•
However, a high percentage of patients will eventually develop a distant recurrence.
-
•
To improve patient outcomes, the use of consolidation durvalumab treatment after standard neoadjuvant CRT was investigated.
-
•
A neoadjuvant treatment with durvalumab following CRT showed promising activity and tolerability in the management of LARC.
Introduction
The cornerstone treatment of locally advanced rectal cancer (LARC) involves preoperative chemoradiotherapy (CRT) followed by surgery with total mesorectal excision (TME).1
Adjuvant treatment after preoperative CRT remains under debate for its poor compliance with therapy and its lack of impact on the patients’ overall survival (OS).2, 3, 4
Long-term analysis has shown that neoadjuvant chemoradiotherapy improves local control, leading to local recurrence rates of ∼5%-9%.1,5
Unfortunately, despite the multimodal therapeutic approach, a high percentage of patients (29%-39%) present a distant recurrence.6
The prognosis of patients undergoing neoadjuvant CRT is associated with the extent of post-treatment tumor regression and pathological complete response (pCR). Although it is not a validated surrogate endpoint of long-term outcomes, several data support that patients achieving pCR have a better prognosis.7, 8, 9, 10
To improve pCR and survival outcomes of LARC, preoperative treatment incorporating chemotherapy with CRT has recently been implemented as a consolidation or induction neoadjuvant chemotherapy.11, 12, 13
Total neoadjuvant therapy (TNT) showed superior rates of pCR compared with standard therapy (29.9% versus 14.9%). Furthermore, the TNT approach demonstrated a significant reduction of distance relapse improving survival outcome and this approach has now become one of the standard preoperative treatment.14
While the advantage of preoperative chemotherapy plus CRT is clear, the possible role of immunotherapy in association with CRT in the neoadjuvant treatment of LARC is still under investigation. As shown by previous studies, immunotherapy after CRT could offer a significant clinical benefit due to a synergism well-liked by the up-regulation of programmed death-ligand 1 (PD-L1) expression in cancer cells caused by radiotherapy (RT) or the ‘abscopal effect’.15,16
Through this mechanism, RT can induce tumor regression both in the primary site and in distant sites leading to the enhancement of local and systemic immune responses even in patients with proficient mismatch repair/microsatellite stable (pMMR/MSS) tumors.17
Based on this evidence, several trials have been developed to test the benefit of immunotherapy in the neoadjuvant treatment of LARC. Available data are promising and showed a manageable toxicity profile and an improvement of pCR, particularly for patients with high microsatellite instability/defective mismatch repair (dMMR/MSI-H).18, 19, 20
Nevertheless, the evidence is not strong and there are many differences in the therapeutic strategies and the timing of anti-programmed cell death protein 1 (PD-1) antibody administration in the trials published and ongoing.21
The PANDORA trial is an open-label, single-arm, phase II study designed to test if the addition of durvalumab after standard neoadjuvant CRT for the treatment of LARC may improve the pathological response rate. Moreover, the investigators explored the toxicity profile of this combination and carried out the analysis of PD-L1 expression by combined positive score (CPS) analysis, MSI status, and tumor mutational burden (TMB).
Patients and methods
Study design and participants
The PANDORA trial is a prospective, phase II, open-label, single-arm, non-profit multicenter study, conducted in seven Italian hospitals and coordinated by AUSL Romagna.
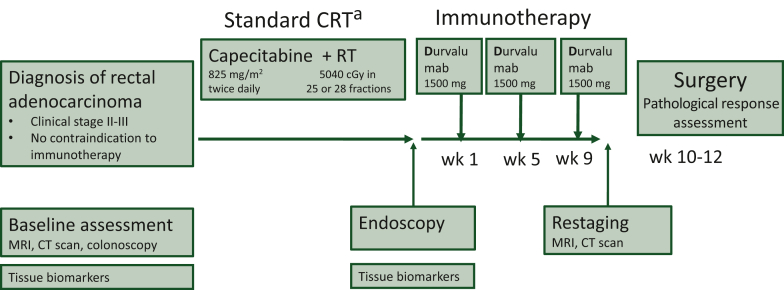
The trial design is provided in Figure 1.
Figure 1.
Study scheme.
CRT, chemoradiotherapy; CT, computed tomography; MRI, magnetic resonance imaging; RT, radiotherapy; wk, week.
aDuration of standard CRT: 5-5.5 weeks according to RT fractions.
A signed informed consent had to be obtained by each subject before study entry. Patients enrolled were at least 18 years old with a histological new diagnosis of adenocarcinoma of the rectum, clinical stage II or III (cT3/4, N0, M0 or cTx, N1-2, M0).
Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1 and to have adequate bone marrow, hepatic, and renal function. Exclusion criteria included comorbidities that contraindicate the use of immunotherapy, recurrent rectal cancer, metastatic disease, or other primary tumors within the previous 5 years. A full list of inclusion and exclusion criteria is available in the protocol. Due to the lack of knowledge on the possible negative impact of immunotherapy on the outcome of coronavirus disease 2019 (COVID-19) at the time of trial conduction, all patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during treatment with chemotherapy or durvalumab were withdrawn from study treatment and considered not assessable for the analysis.
At baseline, patients underwent a full colonoscopy and a pelvic magnetic resonance imaging (MRI) scan for local staging. A computed tomography (CT) scan of the chest, abdomen, and pelvis was required to exclude distant metastasis.
The study received approval from the Italian Medicines Agency (AIFA) on 21 May 2019, from the Romagna Ethics Committee (CEROM) on 12 June 2019, and from local ethics committees of all participating centers. It was also conducted in accordance with the 1964 Helsinki declaration, with Good Clinical Practice (GCP) guidelines, and with EQUATOR guidelines. The participants provided their written informed consent to participate in this study.
This trial is registered with EudraCT number 2019-004758-39 and ClinicalTrials.gov number NCT04083365. The full protocol is provided in Supplementary Appendix S1, available at https://doi.org/10.1016/j.esmoop.2023.101824.
Procedures
After careful staging, patients started a standard concomitant CRT. Chemotherapy consisted of capecitabine 825 mg/m2 twice daily for 5 weeks. Radiotherapy was given once a day, 5 days/week for a total of 5040 cGy.
At the end of CRT, patients underwent a new lesion biopsy by endoscopy, and 1 week after the end of CRT, patients were treated with a fixed dose of durvalumab 1500 mg every 4 weeks (q4w) for three administrations.
All adverse events (AEs) occurring up to 90 days from the last durvalumab administration were recorded. Toxicities considered related to durvalumab were recorded even after this window. All toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 5.0.
After neoadjuvant therapy, restaging with a CT of the chest, abdomen, and pelvis and an MRI scan were required. Clinical response was assessed by RECIST 1.1 guidelines.22
Surgery was mandatory and had to be carried out at 10-12 weeks after the end of CRT according to the principles of sharp mesorectal excision. Formalin-fixed paraffin-embedded (FFPE) tumor tissue sections were prepared for each patient, after surgical intervention. Samples were shipped to a central laboratory for assessment of pathological response and the surgical piece was analyzed both locally and centrally.
Specimens were evaluated with the recommendations of the Association of Directors of Anatomic and Surgical Pathology.23
Histopathologic assessment of tumor regression was carried out in compliance with the eighth edition of the American Joint Committee on Cancer (AJCC) staging system. Tumor regression grade (TRG), as described by Ryan et al., TRG 0 indicates no remaining viable cancer cells (complete response); TRG 1 indicates a single cell or small groups of cancer cells (moderate response); TRG 2 indicates residual cancer outgrown by fibrosis (minimal response); and TRG 3 indicates minimal, or no tumor death, with extensive residual cancer (poor response).24
Adjuvant chemotherapy for 6 months with mFOLFOX6 (oxaliplatin 85 mg/m2 given as an intravenous infusion, followed by leucovorin 400 mg/m2 given as a 2-h intravenous infusion, followed by fluorouracil 400 mg/m2 given as an intravenous bolus and then as continuous intravenous infusion 2400 mg/m2 over 46 h every 14 days) or CAPOX (capecitabine 1000 mg/m2 orally twice daily on days 1-14, oxaliplatin 130 mg/m2 intravenously on day 1, and a chemotherapy-free interval between days 15 and 21) could be administered at the discretion of the treating physician after surgery. The patients then began a follow-up period of 5 years. The follow-up visits, including a complete physical exam, ECOG performance status, vital signs, and clinical laboratory tests for carcinoembryonic antigen, had to be conducted every 4 months for the first 2 years after surgery and then every 6 months for the next 3 years.
Imaging by thorax abdomen pelvis CT scan had to be carried out every 4 months for the first 2 years after surgery and then every 6 months for the next 3 years. Pancolonoscopy had to be carried out 1 year after surgery, then 3 years, and finally every 5 years in the presence of a lesion-free colon.
For the MSI and TMB evaluation, genomic DNA (gDNA) was isolated from five to eight unstained 5-μm-thick sections of FFPE bioptic chemo-naive tumor specimens. gDNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany), quantified using Qubit® dsDNA HS Assay on Qubit 3.0 fluorometer (Invitrogen, Carlsbad, CA) and diluted for the subsequent molecular analyses; 40 ng of gDNA was fragmented using the ME220 ultrasonicator (Covaris, Woburn, MA). Libraries were prepared using the TruSight Oncology 500 High-Throughput assay (Illumina Inc., San Diego, CA) reagents following the manufacturer’s instructions. Paired-end sequencing was carried out using the NovaSeq 6000 on the S2 flow cell. Data were analyzed using the Illumina TSO500 Local App software version 2.0.1.4 (filters: median insert size ≥75; percentage of target bases with coverage >100× ≥75%). The selected cut-off values were ≥10 mut/Mb to define high TMB and >20% of unstable microsatellite sites to define MSI, with a minimum number of usable MSI sites to define MSI status equal to 40.25,26
From all included patients, FFPE pretreatment biopsies were immunohistochemically stained for PD-L1 expression using the standardized 22C3 pharmDx assay on the Dako Link 48 platform (Dako, Carpinteria, CA), dilution 1 : 50. The CPS was defined as the number of positive tumor cells, lymphocytes, and macrophages, divided by the total number of viable tumor cells multiplied by 100.
Outcomes
The primary endpoint was the proportion of patients achieving pCR, defined as the absence of tumor cells in the surgical specimen, both at the primary tumor site and at regional lymph nodes, as surrogate endpoint of the efficacy of treatment, in patients with rectal cancer treated with standard neoadjuvant chemotherapy with capecitabine and radiation followed by durvalumab and surgery. pCR was confirmed by central pathological revision.
Secondary endpoints were the proportion of patients with at least one cycle of study treatment (durvalumab) experiencing AEs characterized by type, grade according to NCI CTCAE v.5.0, seriousness, the relationship with the study treatments, and the proportion of patients achieving clinical complete response (cCR). Other secondary endpoints were disease-free survival (DFS) defined as the time from surgical intervention to (local or distant) disease relapse or death from any cause, whichever comes first, and quality of life. These will be reported in detail elsewhere.
Statistical analysis
Simon’s optimal two-stage design was adopted for the present trial.27
The sample size was estimated assuming a null pCR proportion of 0.15 and an alternative pCR proportion of 0.30 (α = 0.05, power = 0.80). If more than four pCRs were observed in the first 19 assessable patients, 36 additional patients were to be accrued for a total of 55 assessable patients. The proposed treatment could be considered promising if ≥13 pCRs were observed in 55 patients. Assuming a 10% drop-out, a total of 60 patients were to be enrolled in this trial.
The primary endpoint was estimated by means of the proportion of patients having pCR and corresponding exact Clopper–Pearson two-sided 95% confidence intervals (CIs). A similar analysis was carried out for cCR. AEs were summarized by means of absolute frequencies and percentages, reported by type and grade and separately for the chemoradiotherapy and durvalumab windows, respectively. The above-mentioned analyses were carried out on patients receiving at least one dose of study treatment, that is, durvalumab. DFS was estimated by the Kaplan–Meier estimator whereas the reverse Kaplan–Meier method was used to calculate the median follow-up time; median values and the corresponding 95% CIs were reported in the text.
The association between categorical variables was tested by Pearson’s χ2 test or Fisher’s exact test, when appropriate, whereas that between a continuous variable and a categorical one was tested by means of Student’s t-test or analogous non-parametric Wilcoxon–Mann–Whitney test, when appropriate.
Overall, absolute frequencies and percentages or median, first (IQ) and third (IIIQ) quartiles and minimum and maximum values were used to summarize categorical and continuous variables, respectively.
All analyses were carried out using STATA 15.0 software (College Station, TX).
Results
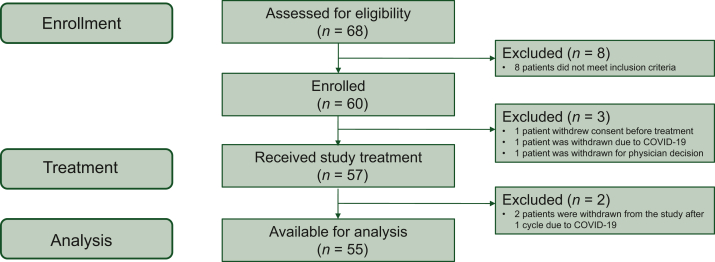
Between November 2019 and August 2021, 60 patients were enrolled in the trial (Figure 2). Three patients did not receive durvalumab (one withdrew consent, one for noncompliance, and one due to SARS-CoV-2 positivity); two patients received one cycle of durvalumab but were prematurely withdrawn from the study due to COVID-19 reasons (Figure 2).
Figure 2.
Study flow diagram.
COVID-19, coronavirus disease 2019.
Fifty-five patients were considered assessable for efficacy and safety analysis. Table 1 summarizes the demographics and tumor characteristics of the assessable 55 patients. Approximately 80% of the population presented node positive at MRI scan and 17.7% was extramural vascular invasion positive (EMVI+).
Table 1.
Baseline demographic and clinical characteristics (n = 55)
| n (%) | |
|---|---|
| Sex | |
| Female | 28 (50.9) |
| Male | 27 (49.1) |
| Age at enrollment (years) | |
| Median [IQ-IIIQ] | 64 [55-73] |
| Min-max | 35-84 |
| ECOG PS | |
| 0 | 47 (85.5) |
| 1 | 8 (14.6) |
| DRE result | |
| Palpable | 33 (73.3) |
| Not palpable | 12 (26.7) |
| Not assessed | 10 |
| MRF status | |
| Negative | 23 (53.5) |
| Positive | 20 (46.5) |
| Missing | 12 |
| LPLN | |
| Negative | 30 (61.2) |
| Positive | 19 (38.8) |
| Missing | 6 |
| Distance to anal verge (mm) | |
| Median [IQ-IIIQ] | 50 [30-80] |
| Min-max | 0-160 |
| Missing | 2 |
| MRI cT | |
| T2 | 3 (5.6) |
| T3 | 42 (77.8) |
| T3a | 4 |
| T3b | 15 |
| T3c | 4 |
| T3d | 6 |
| Not available | 13 |
| T4 | 9 (16.7) |
| Missing | 1 |
| MRI cN | |
| N+ | 43 (79.6) |
| N0 | 11 (20.4) |
| Missing | 1 |
| Tumor dimension (mm) | |
| Median [IQ-IIIQ] | 50 [35-60] |
| Min-max | 7-100 |
| EMVI | |
| Positive | 6 (17.7) |
| Negative | 28 (82.4) |
| Not assessed | 21 |
| CEA (μg/l) | |
| Median [IQ-IIIQ] | 3 [1.9-5.6] |
| Min-max | 0.7-42 |
Percentages may not equal 100 due to rounding.
CEA, carcinoembryonic antigen; cN, clinical node; cT, clinical tumor; DRE, digital rectal exploration; ECOG PS, Eastern Cooperative Oncology Group performance status; EMVI, extramural vascular invasion; IQ, first quartile; IIIQ, third quartile; LPLN, lateral pelvic lymph nodes; MRF, mesorectal fascia; MRI, magnetic resonance imaging.
All assessable patients received the planned chemoradiotherapy with capecitabine concomitant to RT. Fourteen (25.5%) patients required capecitabine dose modification (dose reduction or dose interruption) for toxicity. Three patients had a CRT interruption for toxicity, particularly one patient for grade 2 neutropenia and two patients for grade 3 diarrhea.
All 55 patients started durvalumab treatment after CRT; the median interval from the end of CRT to the first durvalumab infusion was 1 week (range 1-3 weeks, IQ-IIIQ 0.8-1.4 weeks). Fifty-two patients received all three planned infusions at the full dose, with no dose delay. Three patients stopped durvalumab after the second infusion for AEs: one patient with subclinical hypothyroidism at enrollment under pharmacologic control developed hypothyroidism grade 2 considered possibly related to durvalumab; one patient had a grade 3 transaminase increase considered possibly related to durvalumab; one patient had constipation with pain at defecation, hyporexia, and asthenia, considered not likely related to durvalumab, but in the opinion of the investigator, could not continue treatment.
At the end of neoadjuvant treatment, distant restaging was carried out by thoracic-abdomen CT scan and local restaging was assessed by pelvic MRI scan. The clinical response was evaluable for 54 patients; 1 patient (1.8%) was hospitalized for intestinal occlusion and underwent urgent surgery before an MRI scan. Three (5.5%) patients showed stable disease, 43 (78.1%) patients had a partial response, and 6 (10.9%) patients had a cCR. Two (3.6%) patients developed distant metastasis during treatment and were considered as non-responders in the pCR analysis.
The median time from the end of chemoradiotherapy to surgery was 12.7 weeks (IQ-IIIQ 11.6-15.0 weeks), while the median time from the last durvalumab infusion to surgery was 3 weeks (IQ-IIIQ 2.3-5.6 weeks). The delay was not due to treatment-related toxicity but rather due to organizational reasons, also partially due to the COVID-19 emergency.
All 55 patients were considered for primary endpoint evaluation. Of these, 53 patients underwent surgery with curative intent, 1 underwent surgery of the primitive tumor despite distant progression, and 1 did not undergo surgery due to progression. None of the patients died during or after surgery in the study group.
A local pathology evaluation was collected and a central revision of surgical specimens was carried out to confirm the local evaluation.
The pCR (ypT0N0) rate was 34.5% (95% CI 22.2% to 48.6%), therefore the study’s primary objective was achieved. A very good agreement between local assessment of response and central evaluation was observed with only one case classified as a complete responder by the local assessment and not by the centralized evaluation and one case classified as a complete responder by the centralized assessment and not by the local one. Fourteen (25.4%) patients had a near complete regression, while 15 (27.3%) patients presented a moderate regression and 5 (9.1%) had a minimal regression.
The median number of nodes examined was about 15 (IQ-IIIQ 4-34). Negative nodes (ypN0) were reported in 46 cases (85.2%). Eight patients had positive nodes, of which two cases were N2.
Fifty-three patients (98.1%) received a resection with negative margins (R0) and one patient (1.9%) presented positive margins (R1). Table 2 summarizes pathology findings.
Table 2.
Pathology findings according to central review (n = 55)
| n (%) | |
|---|---|
| Grading | |
| G2 | 27 (49.1) |
| G3 | 6 (10.9) |
| No tumor | 19 (34.5) |
| Not evaluable | 2 (3.6) |
| Not applicablea | 1 (1.8) |
| Pathological T stage | |
| ypT0 | 21 (38.2) |
| ypT1 | 4 (7.3) |
| ypT2 | 15 (27.3) |
| ypT3 | 13 (23.6) |
| ypT4 | 1 (1.8) |
| Not applicablea | 1 (1.8) |
| Pathological N stageb | |
| ypN0 | 46 (83.6) |
| ypN1 | 6 (10.9) |
| ypN2 | 2 (3.6) |
| Not applicablea | 1 (1.8) |
| Total resected nodesb | |
| Median [IQ-IIIQ] | 15.5 [11-19] |
| Min-max | 0-34 |
| Not applicablea | 1 |
| Distal and proximal marginsb | |
| R0 | 53 (96.4) |
| R1 | 1 (1.8) |
| Not applicablea | 1 (1.8) |
| Circumferential resection margins | |
| Positive (≤1 mm) | 2 (3.6) |
| Negative (>1 mm) | 52 (94.5) |
| Not applicablea | 1 (1.8) |
| Pathological complete response rate (ypT0N0) | |
| Yes | 19 (34.5) |
| No | 36 (65.5) |
| TRG | |
| 0 | 19 (34.5) |
| 1 | 14 (25.5) |
| 2 | 16 (29.1) |
| 3 | 5 (9.1) |
| Not applicablea | 1 (1.8) |
Percentages may not equal 100 due to rounding.
IQ, first quartile; IIIQ, third quartile; TRG, tumor regression grading.
One patient did not have surgery due to disease progression during the neoadjuvant treatment.
Parameters assessed by local review.
After surgery, 28 of 53 resected patients received standard adjuvant chemotherapy; of these 28, 7 patients had achieved a pCR after neoadjuvant therapy.
The actual median follow-up time is 22.2 months (95% CI 20.2-26.1 months) with a median DFS not reached (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.101824). Eight relapses (two patients had a local recurrence and six had a distance recurrence) and one death without relapse were observed so far. Detailed DFS analyses will be reported elsewhere, at the time a mature follow-up will be available.
Regarding toxicity, the most common treatment AEs of any grade during chemoradiotherapy were diarrhea (40.0%), asthenia (14.5%), nausea (14.5%), rectal/anal pain (14.5%), and lower urinary tract disorders (9.1%). About 18% of other toxicities were also observed, most frequently related to gastrointestinal toxicity (meteorism, gastric heartburn, proctitis, cramping pain, anal fissures, dyspepsia, mucorrhea). The grade 3 AEs were leukopenia (3.6%), neutropenia (3.6%), diarrhea (3.6%), skin toxicity (3.6%), anemia (1.8%), and hypokalemia (1.8%). No grade 4 and 5 AEs were observed.
Immune-related AEs were monitored from the start of the study treatment (durvalumab) until 90 days after the last dose was administered. The most common AEs were of grades 1-2 (asthenia 12.7%, lipase/amylase increase 9.1%, hypothyroidism 9.1%). Four patients reported grade 3 AEs: diarrhea, lipase/amylase increase, aspartate aminotransferase/alanine aminotransferase increase, and pancolitis. No grade 4 events related to durvalumab were observed. An overview of AEs is provided in Tables 3 and 4.
Table 3.
Adverse events (including SAEs) during chemo(radio)therapy: highest grade reported per patient
| Grade 1/2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Hematologic toxicity | |||
| Anemia | 2 (3.6) | 1 (1.8) | |
| Leukopenia | 2 (3.6) | ||
| Neutropenia | 3 (5.5) | 2 (3.6) | |
| Thrombocytopenia | 2 (3.6) | ||
| Gastrointestinal toxicity | |||
| Anorexia | 1 (1.8) | ||
| Diarrhea | 22 (40.0) | 2 (3.6) | |
| Nausea | 8 (14.5) | ||
| Vomiting | 1 (1.8) | ||
| Constipation | 4 (7.3) | ||
| Rectal tenesmus | 1 (1.8) | ||
| Rectal bleeding | 2 (3.6) | ||
| Rectal/anal pain | 8 (14.5) | ||
| General toxicity | |||
| Asthenia | 8 (14.5) | ||
| Cardiac toxicity | 1 (1.8) | ||
| Erythema | 3 (5.5) | ||
| Infection | 3 (5.5) | ||
| Liver hepatic toxicity | 1 (1.8) | ||
| Nervous system disorders | 1 (1.8) | ||
| Pain | 1 (1.8) | ||
| Pruritus | 1 (1.8) | ||
| Skin toxicity | 2 (3.6) | 2 (3.6) | |
| Stomatitis | 2 (3.6) | ||
| Lipase/amylase increase | 1 (1.8) | ||
| Lower urinary tract disorder | 5 (9.1) | ||
| AST/ALT increase | 1 (1.8) | ||
| Hypokalemia | 1 (1.8) | 1 (1.8) | |
| Other | 10 (18.2) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAE, serious adverse event.
Table 4.
Adverse events (including SAEs) from the start of study treatment (durvalumab) until 90 days after the last dose administered: highest grade reported per patient
| Grade 1/2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Gastrointestinal toxicity | |||
| Anorexia | 1 (1.8) | ||
| Diarrhea | 2 (3.6) | 1 (1.8) | |
| Mucorrhea | 1 (1.8) | ||
| Nausea | 1 (1.8) | ||
| Pancolitis | 1 (1.8) | ||
| General toxicity | |||
| Asthenia | 7 (12.7) | ||
| AST/ALT increase | 1 (1.8) | 1 (1.8) | |
| Cardiac toxicity | 1 (1.8) | ||
| Chest pain | 1 (1.8) | ||
| Dysgeusia | 1 (1.8) | ||
| Erythema | 1 (1.8) | ||
| Fever | 1 (1.8) | ||
| Hyperthyroidism | 1 (1.8) | ||
| Hypothyroidism | 5 (9.1) | ||
| Hot flushes | 1 (1.8) | ||
| Lipase/amylase increase | 5 (9.1) | 1 (1.8) | |
| Pneumonitis | 1 (1.8) | ||
| Pruritus | 1 (1.8) | ||
| Sarcoidosis-like reaction | 1 (1.8) | ||
| Skin toxicity | 2 (3.6) | ||
| Stomatitis | 1 (1.8) | ||
| Weight loss | 1 (1.8) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAE, serious adverse event.
Forty-nine cases for whom baseline bioptic samples were available underwent biological analysis to evaluate MSI status, CPS, and TMB at baseline. The MSI and CPS values were not available due to technical reasons for one and two patients, respectively. Two (4.2%) of 48 patients presented MSI-H, while in the rest of the cases microsatellite status was stable. Unexpectedly, the two patients with MSI-H did not achieve a pCR, but both had a moderate regression. About TMB analysis, 35.3% of patients achieving pCR present high TMB while the percentage of high TMB in the non-pCR population was 27.6%. By CPS analysis, it was detected that patients with pCR presented a median CPS of 20 while in other cases a median CPS of 10 was observed.
Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101824, shows the association between pCR and baseline clinical and molecular characteristics. None of the covariates had a statistically significant association with the primary endpoint.
Discussion
In this study, we found that standard chemoradiotherapy followed by consolidation durvalumab was associated with a promising rate of pCR. No grade 4 AEs were observed, and the majority of AEs reported during both CRT and durvalumab treatment were of grade 1-2.
Several phase II non-randomized trials investigated the feasibility and activity of immune checkpoint blockade in the neoadjuvant treatment of LARC, with relevant heterogeneity concerning the therapeutic strategies and the timing of anti-PD-L1 antibody administration.21
In the VOLTAGE-A trial, the anti-PD-1 agent nivolumab (five cycles) was administered as consolidation after long-course CRT, whereas in the AVANA phase II trial the anti-PD-L1 agent avelumab was given both concomitantly and sequentially to CRT. Both those studies showed that adding immunotherapy could improve the pCR rate. In the VOLTAGE-A trial, the pCR rate was 30% while in the AVANA trial it was 23%, suggesting how consolidation immunotherapy could be more effective than concomitant immunotherapy.20,28
Immunotherapy was also tested in the frame of TNT strategies. In the AVERECTAL trial as well as in the study of Lin and colleagues, immune checkpoint inhibitors (ICIs) (avelumab and camrelizumab, respectively) were administered with consolidation oxaliplatin-based chemotherapy after short-course RT. Despite the small sample size, the pCR rate was 37.5% in the AVERECTAL trial and 48.1% in the other one.18,19 However, in these trials, the association of ICI with systemic chemotherapy does not allow us to properly assess the added value of immunotherapy.
In contrast with those results, no improvement of either neoadjuvant rectal score or pCR was reported in the NRG-GI002 trial in which 185 patients were randomized to neoadjuvant FOLFOX for 4 months followed by CRT or the same induction regimen followed by CRT plus concomitant pembrolizumab.29
In our trial design, we selected to administer the anti-PD-L1 therapy as consolidation during the interval of 8-10 weeks after long-course CRT and before surgery, to take advantage of the immunostimulatory effects of CRT. Our data and the results of a similar study such as the VOLTAGE-A trial suggest that a sequential strategy could be more effective and worthy of future research.
As the preclinical data have shown, CRT could inhibit immunosuppressive cells, activate effector cells or increase immunogenicity and T-cell infiltration through several mechanisms, including exposure of neo-antigens, and increase PD-L1 expression on tumor cells and immune cells in the tumor microenvironment. The function and migration of effector CD8+ T cells are also facilitated by cytokine patterns triggered by RT. Nevertheless the mechanism of immunogenic role of CRT remains unclear, particularly the up-regulation of PD-L1 after RT exposure, which can be observed in experimental mouse tumor models, but it is not confirmed in exploratory analysis of some trials.16,17,30, 31, 32
High TMB and lymphocyte infiltration are crucial for the response to immunotherapy, and this could explain why dMMR/MSI-H cancers have an important benefit from immunotherapy treatment, but the challenge is to sensitize to immune therapies pMMR/MSS tumors, which constitute the majority of colorectal cancers and present a lower level of TMB and lymphocyte infiltration.33, 34, 35
In the exploratory analysis, we carried out the evaluation of key biological markers: MSI, TMB, and PD-L1 CPS. Unexpectedly, we did not observe any association between these biological markers and the pathological response, although this finding could be explained by the small trial sample size.
As previously described, dMMR/MSI-H is considered as a marker of response to immunotherapy in the treatment of LARC as demonstrated by Cercek and colleagues, where all the 12 dMMR/MSI-H rectal cancer patients treated with dostarlimab achieved a cCR without CRT or surgery.34,36
In our population, two patients presented MSI-H (6.7%), confirming the low percentage of dMMR/MSI-H early rectal cancer compared to dMMR/MSI-H early colon cancer which lines up to 15%.37 Interestingly, none of them achieved a pCR, Thus, in the small subgroup of patients with dMMR/MSI-H rectal cancers, future research should focus on the omission of chemotherapy and RT, or even surgery, whereas the strategies investigated by us and others should primarily focus on pMMR/MSS tumors.
The role of PD-L1 expression as a predictor for response to immunotherapy is controversial in rectal cancer.38
In the previously mentioned VOLTAGE-A study, a positive correlation between PD-L1 expression and neoadjuvant therapy plus nivolumab efficacy was observed.20
Similarly, Lin and colleagues observed a trend toward a better pCR in patients with PD-L1 expression, although the difference was not statistically significant.19
Our data showed that the majority of CPS values were from >1% to <50% and no correlation with the primary endpoint was found, not confirming the findings of previous studies.
Despite our encouraging results, the most important limitations of our research consist of the small sample size, and the single-arm design being more prone to selection bias and overestimation of the study outcomes. Another limitation is the length of the RT-to-surgery interval. In our population, the patients underwent surgery with a median of 12.7 weeks after the end of RT. This was partly due to the fact that our study was conducted during the COVID-19 pandemic. Although this delay could have increased the pCR rate, this topic is still under debate.39 Furthermore, as common in other similar trials, we selected pCR as the primary early endpoint despite the fact that it is not a validated surrogate measure of long-term endpoints and in some trials, pCRs do not lead to an effect on OS. Therefore, monitoring a longer follow-up of disease recurrences of the PANDORA trial will be important to support the efficacy of this strategy. The appropriate clinical endpoint in the field of preoperative treatment of LARC is actually under debate and further data are needed to show if improved pCR translates into improved survival outcomes or not.10,40
Finally, a potential limitation of our work is the administration of CRT to patients with high-risk features (e.g. cT4+, EMVI+), while today those patients would be eligible for a TNT approach. However, TNT was not a common practice at the time of PANDORA conception, design, and enrollment.
Certainly, future studies should investigate the association of immunotherapy and TNT for those patients.
Conclusions
In conclusion, the experimental neoadjuvant treatment with CRT followed by consolidation durvalumab achieved a promising pCR and was associated with a favorable toxicity profile. Considering that the activity results were independent of MMR status, this strategy is worth being investigated by randomized trials dedicated to patients with pMMR/MSS LARC.
Acknowledgements
We thank AstraZeneca and the IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) ‘Dino Amadori’ for the support.
Funding
This work was partially supported by AstraZeneca (no grant number). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Disclosure
FP received honoraria from Bayer, Servier, Pierre-Fabre, Lilly, MSD, BMS, Organon, Merck-Serono, Amgen, AstraZeneca and research grants from AstraZeneca, BMS, and Incyte. All other authors have declared no conflicts of interest.
Data sharing
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary data
Clinical study protocol.
References
- 1.Bosset J.F., Collette L., Calais G., et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 2.Glynne-Jones R., Counsell N., Quirke P., et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25(7):1356–1362. doi: 10.1093/annonc/mdu147. [DOI] [PubMed] [Google Scholar]
- 3.Breugom A.J., van Gijn W., Muller E.W., et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26(4):696–701. doi: 10.1093/annonc/mdu560. [DOI] [PubMed] [Google Scholar]
- 4.Hong Y.S., Kim S.Y., Lee J.S., et al. Oxaliplatin-based adjuvant chemotherapy for rectal cancer after preoperative chemoradiotherapy (ADORE): long-term results of a randomized controlled trial. J Clin Oncol. 2019;37(33):3111–3123. doi: 10.1200/JCO.19.00016. [DOI] [PubMed] [Google Scholar]
- 5.Braendengen M., Tveit K.M., Berglund A., et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26(22):3687–3694. doi: 10.1200/JCO.2007.15.3858. [DOI] [PubMed] [Google Scholar]
- 6.Sainato A., Cernusco Luna Nunzia V., Valentini V., et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT) Radiother Oncol. 2014;113(2):223–229. doi: 10.1016/j.radonc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Habr-Gama A., Perez R.O., Wynn G., Marks J., Kessler H., Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 8.Maas M., Nelemans P.J., Valentini V., et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 9.Park I.J., You Y.N., Agarwal A., et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(15):1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fokas E., Glynne-Jones R., Appelt A., et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol. 2020;21(5):e252–e264. doi: 10.1016/S1470-2045(20)30024-3. [DOI] [PubMed] [Google Scholar]
- 11.Bahadoer R.R., Dijkstra E.A., van Etten B., et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 12.Conroy T., Bosset J.F., Etienne P.L., et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–715. doi: 10.1016/S1470-2045(21)00079-6. [DOI] [PubMed] [Google Scholar]
- 13.Fokas E., Schlenska-Lange A., Polat B., et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol. 2022;8(1) doi: 10.1001/jamaoncol.2021.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasi A., Abbasi S., Handa S., et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonia S.J., Villegas A., Daniel D., et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 16.Reynders K., Illidge T., Siva S., Chang J.Y., De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krysko D.V., Garg A.D., Kaczmarek A., Krysko O., Agostinis P., Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 18.Shamseddine A., Zeidan Y.H., El Husseini Z., et al. Efficacy and safety-in analysis of short-course radiation followed by mFOLFOX-6 plus avelumab for locally advanced rectal adenocarcinoma. Radiat Oncol. 2020;15(1):233. doi: 10.1186/s13014-020-01673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Z., Cai M., Zhang P., et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer. 2021;9(11) doi: 10.1136/jitc-2021-003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bando H., Tsukada Y., Inamori K., et al. Preoperative chemoradiotherapy plus nivolumab before surgery in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. Clin Cancer Res. 2022;28(6):1136–1146. doi: 10.1158/1078-0432.CCR-21-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bando H., Tsukada Y., Ito M., Yoshino T. Novel immunological approaches in the treatment of locally advanced rectal cancer. Clin Colorectal Cancer. 2022;21(1):3–9. doi: 10.1016/j.clcc.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Stewart C.J., Hillery S., Plattell C. Protocol for the examination of specimens from patients with primary carcinomas of the colon and rectum. Arch Pathol Lab Med. 2009;133(9):1359–1361. doi: 10.5858/133.9.1359. [DOI] [PubMed] [Google Scholar]
- 24.Ryan R., Gibbons D., Hyland J.M., et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–146. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 25.Conroy J.M., Pabla S., Glenn S.T., et al. A scalable high-throughput targeted next-generation sequencing assay for comprehensive genomic profiling of solid tumors. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0260089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei B., Kang J., Kibukawa M., et al. Evaluation of the TruSight Oncology 500 assay for routine clinical testing of tumor mutational burden and clinical utility for predicting response to pembrolizumab. J Mol Diagn. 2022;24(6):600–608. doi: 10.1016/j.jmoldx.2022.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 28.Salvatore L., Bensi M., Corallo S., et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus avelumab (AVE) in patients (PTS) with locally advanced rectal cancer (LARC): the AVANA study. J Clin Oncol. 2021;39(suppl 15):3511. [Google Scholar]
- 29.Rahma O.E., Yothers G., Hong T.S., et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. 2021;7(8):1225–1230. doi: 10.1001/jamaoncol.2021.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinhuis K.M., Ros W., Kok M., Steeghs N., Beijnen J.H., Schellens J.H.M. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219–235. doi: 10.1093/annonc/mdy551. [DOI] [PubMed] [Google Scholar]
- 31.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 32.Levy A., Massard C., Soria J.C., Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: single centre subset analysis from a phase 1/2 trial. Eur J Cancer. 2016;68:156–162. doi: 10.1016/j.ejca.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cercek A., Lumish M., Sinopoli J., et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386(25):2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luchini C., Bibeau F., Ligtenberg M.J.L., et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30(8):1232–1243. doi: 10.1093/annonc/mdz116. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Wu T., Cai X., et al. Neoadjuvant immunotherapy for MSI-H/dMMR locally advanced colorectal cancer: new strategies and unveiled opportunities. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.795972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salem M.E., Weinberg B.A., Xiu J., et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8(49):86356–86368. doi: 10.18632/oncotarget.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexander P.G., McMillan D.C., Park J.H. A meta-analysis of CD274 (PD-L1) assessment and prognosis in colorectal cancer and its role in predicting response to anti-PD-1 therapy. Crit Rev Oncol Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103147. [DOI] [PubMed] [Google Scholar]
- 39.Guzmán Y., Ríos J., Paredes J., et al. Time interval between the end of neoadjuvant therapy and elective resection of locally advanced rectal cancer in the CRONOS study. JAMA Surg. 2023 doi: 10.1001/jamasurg.2023.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrelli F., Borgonovo K., Cabiddu M., Ghilardi M., Lonati V., Barni S. Pathologic complete response and disease-free survival are not surrogate endpoints for 5-year survival in rectal cancer: an analysis of 22 randomized trials. J Gastrointest Oncol. 2017;8(1):39–48. doi: 10.21037/jgo.2016.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical study protocol.