Abstract
Background
The structure of negative symptoms of schizophrenia is still a matter of controversy. Although a two-dimensional model (comprising the expressive deficit dimension and the motivation and pleasure dimension) has gained a large consensus, it has been questioned by recent investigations.
Aims
To investigate the latent structure of negative symptoms and its stability over time in people with schizophrenia using network analysis.
Method
Negative symptoms were assessed in 612 people with schizophrenia using the Brief Negative Symptom Scale (BNSS) at baseline and at 4-year follow-up. A network invariance analysis was conducted to investigate changes in the network structure and strength of connections between the two time points.
Results
The network analysis carried out at baseline and follow-up, supported by community detection analysis, indicated that the BNSS's items aggregate to form four or five distinct domains (avolition/asociality, anhedonia, blunted affect and alogia). The network invariance test indicated that the network structure remained unchanged over time (network invariance test score 0.13; P = 0.169), although its overall strength decreased (6.28 at baseline, 5.79 at follow-up; global strength invariance test score 0.48; P = 0.016).
Conclusions
The results lend support to a four- or five-factor model of negative symptoms and indicate overall stability over time. These data have implications for the study of pathophysiological mechanisms and the development of targeted treatments for negative symptoms.
Keywords: Schizophrenia, negative symptoms, Brief Negative Symptom Scale, network analysis, structure stability
Negative symptoms are a fundamental aspect of schizophrenia, closely linked to poor quality of life and ineffective response to treatment.1–12 Despite their significance, they are still an unmet need in schizophrenia care, burdening patients, families and healthcare systems.2,13–17 Negative symptoms can be either primary or secondary manifestations of the disease; in the latter scenario, they can be subsequent to positive and depressive symptoms, or extrapyramidal side effects.2,6 The conceptualisation of negative symptoms proposed in the early 1900s included two aspects: the reduction of emotional expression and the loss of motivation.18 Indeed, Eugen Bleuler reported that people with schizophrenia had expressionless faces, were apathetic and lacked the desire to act on their own initiative or at the request of another.19 Emil Kraepelin described the presence of emotional apathy and a decline in volitional control in the same population.20 Contemporary understanding, stemming from the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative developed by the National Institute of Mental Health (NIMH), posits five domains: blunted affect, alogia, avolition, asociality and anhedonia.21 Second-generation rating scales, such as the Brief Negative Symptom Scale (BNSS)22 and the Clinical Assessment Interview for Negative Symptoms (CAINS),23 were developed according to the NIMH-MATRICS consensus statement21 to provide an accurate assessment of negative symptoms in their quantitative (frequency, duration and intensity) and qualitative aspects (such as differentiation between anticipatory and consummatory aspects of anhedonia or differentiation between behavioural and experiential aspects).22,23
The evidence base for the two- and five-factor models
The negative symptom structure has been widely investigated and exploratory factor analytic studies supported a two-factor model comprising a motivation and pleasure dimension (MAP, including avolition, asociality and anhedonia) and an expressive deficit dimension (EXP, including blunted affect and alogia).4,24–26 This model is consistent with the observation that different behavioural features, neurophysiological bases as well as clinical and social outcomes are associated with the two dimensions.2,6,27–36
However, evidence from recent multicentre studies utilising confirmatory factor analysis (CFA) has questioned the adequacy of this two-factor model.37–43 These studies suggested that a five-factor model or a hierarchical model better fit the data, irrespective of assessment scale, sample nationality/language or stage of illness.37,38,40,42,43 These findings indicate that conceptualising negative symptoms in relation to the MAP and EXP dimensions may not capture the complexity of the construct, and support a more complex view of negative symptoms, aligned with the five NIMH-MATRICS consensus domains.38,39,44–46
Latent structure of negative symptoms
To address the latent structure of negative symptoms has very strong pragmatic implications. For instance, exploratory factor analysis studies supporting the two-factor model have influenced researchers and pharmaceutical companies, resulting in the drafting of clinical and pharmacological research protocols.30,44,45,47 However, the two-factor model may prevent the identification of pathophysiological mechanisms or therapeutic effects that are unique to one of the five domains. Further studies are required, as there is some preliminary evidence showing distinct pathophysiological correlates of individual negative symptom domains.48
A recent network approach to psychopathology conceptualises disorders as systems of interconnected symptoms.49,50 Preliminary studies have used this approach to investigate the structure of negative symptoms across different diagnoses and in terms of treatment response.51–53 However, the longitudinal stability of this structure remains largely unexamined, especially with second-generation assessment tools aligned with the current conceptualisation.54–57
To address this gap, the primary aim of the present study was to delve deeper into the structure of negative symptoms over time, utilising network analysis. This study seeks to investigate the temporal stability of the negative symptom network over a 4-year period in a representative sample of individuals diagnosed with schizophrenia. By doing so, we aimed to enhance our understanding of the interplay and evolution of negative symptoms, ultimately contributing to the development of more targeted and effective treatment strategies.
Method
Participants
This observational prospective study was carried out as part of the Italian Network for Research on Psychoses.58–61
Participants in the study were community-dwelling individuals with schizophrenia who had been stabilised on antipsychotic medications for at least 3 months before enrolment and were seen consecutively at the out-patient clinics of 24 Italian university psychiatric clinics and/or mental health departments.
Participants were recruited between 1 March 2012 and 30 September 2013. All patients recruited by participating centres at baseline were asked to participate in the follow-up study carried out 4 years after the baseline assessment.
Inclusion criteria were a diagnosis of schizophrenia according to DSM-IV, confirmed with the Structured Clinical Interview for DSM-IV, Patient Version (SCID-I/P), and an age between 18 and 65 years. Given that the SCID includes both mandatory questions that correspond to DSM-IV operational criteria and a diagnostic algorithm, the diagnosis of schizophrenia is assigned when the following criteria are met: ‘The disturbance is not attributable to the physiological effects of a substance (e.g., a drug of abuse, a medication) or another medical condition’.62 In doing so physicians, as good clinical practice requires, are supported in the differential diagnoses by investigations (electrocardiograms, blood and urine samples, computed tomography/magnetic resonance imaging, electroencephalograms) as needed.
Exclusion criteria were: (a) history of head injury with loss of consciousness in the 4 years between baseline and follow-up; (b) progressive cognitive decline possibly caused by dementia or other neurological illness diagnosed in the past 4 years; (c) history of alcohol and/or substance misuse in the past 6 months; (d) current pregnancy or nursing; (e) inability to give informed consent; and (f) treatment modifications and/or hospital admission due to symptom exacerbation in the previous 3 months.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the Ethics Committee ‘Comitato Etico Università degli Studi della Campania ‘Luigi Vanvitelli’ – Azienda Ospedaliera Universitaria ‘Luigi Vanvitelli’ – AORN ‘Ospedali dei Colli’’ on 9 February 2012 (Protocol number 73, baseline study) and on 9 October 2015 (Protocol number 1382, follow-up study). After receiving a comprehensive explanation of the study procedures and goals, each participant gave written informed consent to participate.
Clinical assessment
Evaluation of negative symptoms was conducted using the Italian version of the Brief Negative Symptom Scale (BNSS).22,63 The scale consists of 13 items organised into six subscales (five negative symptom subscales: anhedonia, asociality, avolition, blunted affect and alogia; and a control subscale: lack of distress). All the items are rated on a 7-point (0–6) scale, thus ranging from absent (0) to moderate (3) to extremely severe (6) symptoms. According to the current conceptualisation of negative symptoms and similar research, BNSS item 4 (‘lack of normal distress’) was left out of the statistical analysis as it is not a negative symptom.1,40
The assessment of positive symptoms and disorganisation was conducted using the Positive and Negative Syndrome Scale (PANSS).64 In accordance with Wallwork and colleagues65 the positive dimension was determined through the sum of the scores on the following PANSS items: delusions (P1), hallucinatory behaviour (P3), grandiosity (P5) and unusual thought (G9). The disorganisation dimension was determined by adding the scores on the following PANSS items: conceptual disorganisation (P2), difficulty in abstract thinking (N5) and poor attention (G11). Depressive symptoms were assessed using the Calgary Depression Scale for Schizophrenia (CDSS).66 Last, the St. Hans Rating Scale (SHRS) was used to evaluate extrapyramidal symptoms.67 These clinical evaluations were conducted both at baseline and at the 4-year follow-up visit.
Statistical analysis
Network analysis was carried out on BNSS items at baseline and follow-up. Starting from a network built on partial correlations, where the association between each pair of nodes was controlled for the influence of all the other nodes, an adaptive least absolute shrinkage and selection operator (LASSO) network was obtained by assigning penalties to partial correlations between variables to make small correlations shrink to 0. A tuning parameter of 0.5 was used to control for the sparsity of the network. Because the study variables were not normally distributed, a non-paranormal transformation was applied to the data. The network graphical representation, in which variables are shown as nodes and their correlations are depicted as edges, was based on the Fruchterman–Reingold algorithm, which places strongly associated nodes at the centre of the graph and weakly associated ones at the periphery. To further facilitate readability, only correlations of 0.05 or more were included in the network diagram. The centrality indices of betweenness, closeness and strength were used to quantify the importance of each node in the adaptive LASSO network. The betweenness of a node equals the number of times that it lies on the shortest path length between any two other nodes. Closeness indicates how easy it is to reach all other nodes from the node of interest and is computed as the inverse of the weighted sum of distances of a given node from all other nodes in the network. Nodes with high betweenness are those that facilitate connections in the network, whereas nodes with high closeness affect the other nodes more quickly or are more affected by the other nodes. Last, the node strength is the sum of the correlations of one node to all other nodes. For each index, higher values reflect higher centrality in the network, but high strength may also derive from very strong correlations between peripheral nodes belonging to the same domain. Centrality plots were created to represent these indices. The robustness of the network solution was assessed by estimating the accuracy of edge weights and the stability of centrality indices using bootstrap analysis.68
We used R, version 3.3.3 for Windows (R Foundation for Statistical Computing) to perform the network analysis; specifically, the package ‘qgraph’ was used to obtain the network and centrality indices, and ‘bootnet’ to evaluate the network stability. We investigated whether the BNSS network structure differed between baseline and follow-up by means of the network comparison test using the R package ‘NetworkComparisonTest’.69
The network structure invariance test investigates differences in the overall structure of the network. The difference between network structures is measured as the deviation in absolute weighted sum scores of the connections.70 This permutation-based test randomly reclassifies individuals from the networks repeatedly and then computes the differences between the subnetworks. The resulting distribution under the null hypothesis, assuming that networks are equal, is used to test the observed difference of the subnetworks.69,71 We used the option for dependent samples of the network comparison test to test temporal stability. The global strength invariance test was used to investigate whether the overall level of connectivity was equal across networks. When this test was significant, post hoc analyses were carried out to determine which specific edges differed between networks using Bonferroni–Holm correction for multiple comparisons. Overall connectivity was computed as the weighted absolute sum of all edges in the network.72,73 The significance level of the network comparison tests was set at P < 0.05. Community detection analysis was conducted using the function cluster_spinglass of the R package ‘igraph’. The spinglass algorithm was chosen because it handles networks with negative weights, which were present in our data. To account for potential variability in the results based on the initial seed, the analysis was performed 10 000 times, thereby allowing calculation of the frequency of different community structures identified at baseline and follow-up. This rigorous approach allowed for a more comprehensive understanding of the stability of the network community structures and provided a robust estimate of the reproducibility of the findings across multiple iterations.
Results
Out of 921 individuals enrolled at baseline, 618 provided follow-up data and 612 with complete baseline and follow-up BNSS data were included in the analyses (422 men (69%) and 190 women (31%); mean age at follow-up 45.1 years (s.d. = 11.5)). The detail demographic characteristics of the sample and the ongoing treatment are given in Supplementary Table 1, available at https://doi.org/10.1192/bjo.2023.541.
The clinical characteristics are shown in Table 1: 59.5% of participants showed mild to moderate severity of negative symptoms (BNSS total score <36), 77.6% absent to mild positive symptom severity (PANSS positive dimension score <12); 59.2% PANSS disorganisation dimension score <9; 63.5% showed low levels of depression (CDSS total score <4); and 93.3% no or mild Parkinsonism (SHRS Parkinsonism score <1).
Table 1.
Clinical characteristics of the sample
| Baseline, mean (s.d.) | Follow-up, mean (s.d.) | |
|---|---|---|
| BNSS total score | 33.3 (16.3) | 30.5 (16.3) |
| PANSS positive | 9.7 (4.6) | 8.4 (4.2)) |
| PANSS disorganisation | 8.6 (3.8) | 8.1 (3.6) |
| CDSS | 3.9 (4) | 3.25 (3.7) |
| SHRS Parkinsonism | 0.87 (0.334) | 0.85 (1.2) |
BNSS, the Brief Negative Symptom Scale; PANSS, Positive and Negative Syndrome Scale; CDSS, Calgary Depression Scale for Schizophrenia; SHRS, St. Hans Rating Scale for extrapyramidal symptoms.
The means and standard deviations of BNSS items are reported in Table 2.
Table 2.
Mean and standard deviation of Brief Negative Symptom Scale (BNSS) items at baseline and follow-upa
| BNSS item | Participants, n | Baseline | Follow-up | ||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | ||
| 1 | 612 | 2.86 | 1.54 | 2.54 | 1.57 |
| 2 | 612 | 2.96 | 1.57 | 2.55 | 1.56 |
| 3 | 612 | 2.86 | 1.58 | 2.61 | 1.62 |
| 5 | 612 | 3.28 | 1.57 | 2.88 | 1.60 |
| 6 | 612 | 3.01 | 1.59 | 2.76 | 1.58 |
| 7 | 612 | 2.87 | 1.61 | 2.59 | 1.61 |
| 8 | 612 | 2.82 | 1.61 | 2.59 | 1.57 |
| 9 | 612 | 2.71 | 1.67 | 2.61 | 1.59 |
| 10 | 612 | 2.64 | 1.77 | 2.53 | 1.66 |
| 11 | 612 | 2.69 | 1.79 | 2.54 | 1.65 |
| 12 | 612 | 2.21 | 1.72 | 2.06 | 1.69 |
| 13 | 612 | 2.42 | 1.79 | 2.27 | 1.73 |
BNSS items: 1, intensity of pleasure during activities; 2, frequency of pleasurable activities; 3, intensity of expected pleasure from future activities; 5, asociality behaviour; 6, asociality internal experience; 7, avolition behaviour; 8, avolition internal experience; 9, facial expression; 10, vocal expression; 11, expressive gestures; 12, quantity of speech; 13, spontaneous elaboration.
All item scores decreased significantly (P < 0.001) from baseline to follow-up.
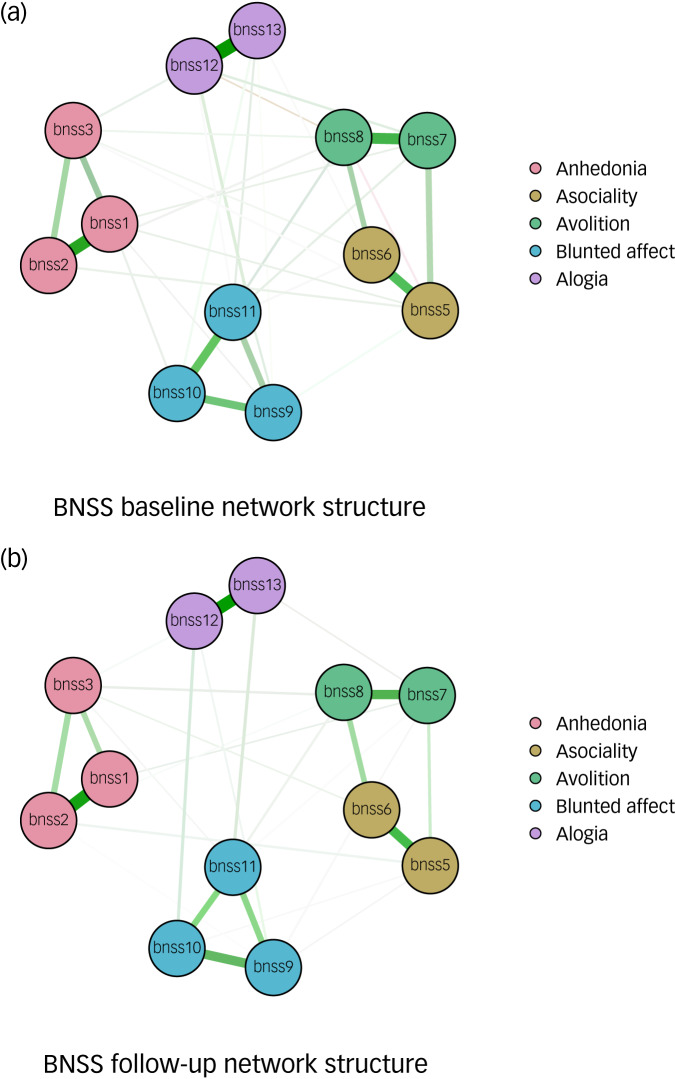
Figure 1 shows the baseline and follow-up networks of BNSS symptoms. Bootstrap analysis revealed that the edge weights were accurate (had small confidence intervals) at baseline and follow-up. In addition, the edge weights were relatively stable until 50% of nodes were removed (Supplementary Figs S1 and S2).
Fig. 1.
Network structures for Brief Negative Symptom Scale (BNSS) symptoms at (a) baseline and (b) follow-up.
Node colours reflect the five domains defined by the NIMH-MATRICS consensus conference and validated by Strauss et al.40 BNSS items: 1, intensity of pleasure during activities; 2, frequency of pleasurable activities; 3, intensity of expected pleasure from future activities; 5, asociality behavior; 6, asociality internal experience; 7, avolition behaviour; 8, avolition internal experience; 9, facial expression; 10, vocal expression; 11, expressive gestures; 12, quantity of speech; 13, spontaneous elaboration. The five negative symptom domains identified by the network analysis are: anhedonia, BNSS items 1–3; asociality, 5–6; avolition, 7–8; blunted affect, 9–11; alogia, 12–13. Item 4, measuring the lack of normal distress, was excluded from the analyses, consistent with Strauss et al.40
The result of the network invariance test indicated that the network structure was unchanged over time (network invariance test score 0.13, P = 0.169), whereas the global strength decreased significantly over time (6.28 at baseline and 5.79 at follow-up; global strength invariance test score 0.48, P = 0.016), suggesting that the level of connectivity was reduced at follow-up and at least one edge changed over time. Specifically, we found that six edges changed significantly over time (bnss5–bnss10, P = 0.021; bnss7–bnss12, P = 0.013; bnss10–bnss12, P = 0.004; bnss3–bnss13, P = 0.001; bnss6–bnss13, P = 0.013; bnss7–bnss13, P = 0.003).
The community analysis provided support for four or five domains of the BNSS: anhedonia, asociality, avolition, blunted affect and alogia. Specifically, although anhedonia, blunted affect and alogia communities remained stable at baseline and follow-up, the asociality and avolition items were located in two separate communities in 33.6% of the iterations at baseline and in 63.9% of the iterations at follow-up. Conversely, these items were grouped together into a single domain in 66.1% of the iterations at baseline and 36.1% at follow-up (Supplementary Figs S3 and S4).
Discussion
The current study used network analysis, a complex and innovative mathematical technique, to investigate the structure of negative symptoms and its stability over time in a sample of people with schizophrenia evaluated at baseline and at 4-year follow-up.
Our findings indicated that the 12 items of the BNSS (with the item ‘lack of normal distress’ being excluded from the analysis, according to the current conceptualisation of negative symptoms and similar research),2,40 through strong intra-domain connections, were structured in distinct domains. This network structure was unchanged between baseline and follow-up, whereas its global strength decreased significantly, thus suggesting that the influence of certain items on others diminished over the 4-year period, although the underlying structure of connections remained constant. Furthermore, performing a community analysis, we found that anhedonia, blunted affect and alogia communities remained stable at the two time points, whereas avolition and asociality were located in the same domain at baseline, but they constituted two different domains at follow-up. Therefore, overall, our results indicated a four- or five-factor model of negative symptoms at baseline and a five-factor model at follow-up.
The five-factor versus the hierarchical model
Our results, despite minor variations, are in line with those presented by Strauss and colleagues.51 Although Strauss et al included the BNSS item ‘lack of normal distress’ in their analyses, we chose to exclude this item since it is unclear whether this aspect belongs to the current negative symptom construct or whether it is part of other psychopathological constructs.2
Overall, our results partially support the five-domain solution identified in 2005 by the NIMH-MATRICS consensus statement.51 These results concur with those of recent confirmatory factor analysis (CFA) studies, despite modest variations in sample sizes and statistical analyses used.37–43 These previous investigations showed that the five-factor (five individual negative symptoms: avolition, anhedonia, asociality, blunted affect and alogia) and also the hierarchical model (MAP and EXP as second-order dimensions; five factors as first-order dimensions) offered a great fit, whereas one- and two-factor models performed poorly. Interestingly, the good fit for the hierarchical model should not be interpreted as further support for the two-factor model but rather as a confirmation of the five-factor model, since in the hierarchical model, MAP and EXP are considered second-order dimensions. Therefore, the model of negative symptoms that best accounts for the latent structure of these symptoms is the five-domain one, since all negative symptom ratings in the hierarchical model are directly influenced by primary dimensions.40
The results of this network analysis are to a large extent consistent with a four- or five-domain conceptualisation of negative symptoms.
The longitudinal network structure stability
The network analysis adds to previous CFA findings on interconnections between negative symptoms. It is of great importance to underline that our negative symptom network structure, in line with previous findings,51 indicated not only that items within the five domains cluster together, but also that they have minimal interactions with one another, suggesting a stronger reciprocal influence of the items within each domain and a lower association between items of different domains.
Furthermore, the results of our study indicate that the negative symptom structure derived from a second-generation, culturally unbiased and largely validated instrument such as the BNSS is longitudinally stable.
Despite the latest efforts to describe the latent structure of negative symptoms, much less attention has been focused on invariance of their longitudinal structure. The distinction between stable and unstable symptom clusters may aid in the improvement of diagnostic limits, the prediction of outcome and the identification of specific symptoms that might be prognostically significant. As regards negative symptom stability over time, various studies have investigated their long-term course, reporting controversial findings (e.g. relative stability over time but also reversibility or fluctuation in symptoms over time).74–77 However, these studies used the PANSS or the Scale for the Assessment of Negative Symptoms (SANS) and, although they investigated the course of negative symptoms over time, they did not evaluate the stability of the negative symptom structure. To our knowledge, only two studies were carried out with the aim of investigating negative symptom structure stability over time, using a network analysis55 or a CFA.56 In the study of Levine & Leucht,55 negative symptoms were assessed using the SANS in people with chronic schizophrenia and predominant negative symptoms. The authors used a network analysis and found preliminary evidence for a negative symptom severity network consisting of four dimensions (affect, poor responsiveness, lack of interest and apathy/inattentiveness), with these results being replicable at baseline and follow-up (60 days).55 These results are heavily influenced by the inadequate assessment instrument, which includes cognitive deficits among negative symptoms, does not distinguish anhedonia and asociality and is based only on behaviour, with poor evaluation of anhedonia and avolition. An antipsychotic-naive first-episode schizophrenia sample was used in the study by Kagan and colleagues to explore the longitudinal invariance of the negative symptom dimension using CFA on PANSS scores at baseline and at 10-week follow-up.56 In their study design, the authors examined the longitudinal invariance of the unidimensional and bidimensional models of negative symptoms and found that the unidimensional one had a good fit at baseline and acceptable fit at 10-week follow-up.56 However, comparisons between these two studies and our research are not possible in terms of methodology,56 assessment instruments used55,56 and population included (individuals with chronic schizophrenia and individuals with first-episode psychosis).56
Implications
Overall, the findings of the present study could have implications for clinical practice. First, considering the results of the previous exploratory factor analyses, DSM-5 based the description of negative symptoms on the two dimensions ‘MAP’ and ‘EXP’, with consequent risk of inaccurate diagnoses that do not capture the complexity of the construct of negative symptoms.27 Considering current findings, future versions of the DSM might take each of the five domains into account separately. Second, the analysis of the nodes’ centrality and the density of intra- and interdomain relationships may provide valuable information from a therapeutic perspective. An effective treatment targeting densely connected networks could in fact be more effective in inducing a global improvement in negative symptoms, compared with an effective treatment targeting weakly connected domains.40 The findings of the study by Strauss and colleagues53 demonstrated that roluperidone78 improved negative symptoms by reducing the level of centrality of avolition, thus supporting the idea that a global improvement of negative symptoms requires decoupling the influence of motivational processes from other domains of negative symptoms.53 Last, correct characterisation of the negative symptom structure and its longitudinal evaluation can allow the identification of pathophysiological mechanisms of the different domains and improving the design of pharmacological/rehabilitative treatment trials, which would be precluded from studying the correlates of the two factors on which the attention of research has been concentrated in recent years.
Our study emphasises the multidimensional nature of negative symptoms. This research underscores the need for continued exploration in this area, to refine psychopathological classifications and develop effective treatment strategies for schizophrenia.
Limitations
Certain limitations of this study should be taken into account. For instance, participants were predominantly male, which may limit the generalisability of our results. However, we have to note that a higher severity of negative symptoms has been previously reported in males with schizophrenia compared with females.79 In addition, positive symptoms, extrapyramidal side-effects and depression are possible sources of secondary negative symptoms, as reported in the introductory section. Therefore, these factors might account for some influences on the presented results. However, our sample comprised clinically stable individuals with schizophrenia, with absent to mild positive and disorganisation symptom severity (PANSS positive dimension mean score <12; PANSS disorganisation dimension mean score <9), low mean level of depression (CDSS total score <4) and Parkinsonism (SHRS Parkinsonism score <1), far below the threshold of clinical significance, thus limiting possible sources of secondary negative symptoms.
Supporting information
Rucci et al. supplementary material
Acknowledgements
The following members of the Italian Network for Research on Psychoses participated in this study: Giuseppe Piegari, Eleonora Merlotti, Francesco Brando (University of Campania ‘Luigi Vanvitelli’, Naples); Marco Papalino, Vitalba Calia, Raffaella Romano (University of Bari); Stefano Barlati, Giacomo Deste, Paolo Valsecchi (University of Brescia); Federica Pinna, Alice Lai, Silvia Lostia Di Santa Sofia (University of Cagliari); Maria Salvina Signorelli, Laura Fusar Poli, Teresa Surace (University of Catania); Giovanni Martinotti, Chiara Montemitro, Silvia Fatricelli (University of Chieti); Mario Altamura, Eleonora Angelini, Antonella Elia (University of Foggia); Pietro Calcagno, Martino Belvedere Murri, Simone Cattedra (University of Genoa); Francesca Pacitti, Rodolfo Rossi, Valentina Socci, Laura Giusti, Anna Salza, Silvia Mammarella (University of L'Aquila); Andrea de Bartolomeis (University of Naples Federico II); Angela Favaro, Enrico Collantoni, Paolo Meneguzzo (University of Padua); Matteo Tonna, Paolo Ossola, Maria Lidia Gerra (University of Parma); Carla Gramaglia, Valeria Binda, Eleonora Gambaro (University of Eastern Piedmont, Novara); Claudia Carmassi, Barbara Carpita, Ivan Mirko Cremone (University of Pisa); Giulio Corrivetti, Giammarco Cascino, Gianfranco del Buono (Department of Mental Health, Salerno); Roberto Brugnoli, Anna Comparelli, Valentina Corigliano, Antonio Buzzanca, Nicoletta Gerardi, Marianna Frascarelli (Sapienza University of Rome); Andrea Fagiolini, Arianna Goracci, Simone Bolognesi (University of Siena); Alberto Siracusano, Giorgio Di Lorenzo, Michele Ribolsi (Tor Vergata University of Rome); Cristiana Montemagni, Cecilia Riccardi, Elisa Del Favero (University of Turin).
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2023.541.
Data availability
The data that support the findings of this study are available from the corresponding author, G.M.G., on reasonable request.
Author contributions
All authors contributed to the conceptualisation and investigation of the study. F.S. and P.R. were responsible for the methodology, data curation and formal analysis. G.M.G. and E.C. wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was funded by the Italian Ministry of Education, the Italian Society of Psychopathology (SOPSI) and the Italian Society of Biological Psychiatry (SIPB). These organisations had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Declaration of interest
The authors declare no conflict of interest. S.G. and G.M.G. are members of the BJPsych Open editorial board and did not take part in the review or decision-making process of this paper.
References
- 1.Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry 2018; 5: 664–77. [DOI] [PubMed] [Google Scholar]
- 2.Galderisi S, Mucci A, Dollfus S, Nordentoft M, Falkai P, Kaiser S, et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur Psychiatry 2021; 64(1): e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat 2020; 16: 519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 2017; 16: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maj M, van Os J, De Hert M, Gaebel W, Galderisi S, Green MF, et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021; 20: 4–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordano GM, Caporusso E, Pezzella P, Galderisi S. Updated perspectives on the clinical significance of negative symptoms in patients with schizophrenia. Expert Rev Neurother 2022; 22: 541–55. [DOI] [PubMed] [Google Scholar]
- 7.Harvey PD, Strassnig M. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry 2012; 11: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. Pathways to functional outcome in subjects with schizophrenia living in the community and their unaffected first-degree relatives. Schizophr Res 2016; 175: 154–60. [DOI] [PubMed] [Google Scholar]
- 9.Ostuzzi G, Bertolini F, Tedeschi F, Vita G, Brambilla P, Del Fabro L, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry 2022; 21: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leichsenring F, Steinert C, Rabung S, Ioannidis JPA. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry 2022; 21: 133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliani L, Giordano GM, Bucci P, Pezzella P, Brando F, Galderisi S. Improving knowledge on pathways to functional outcome in schizophrenia: main results from the Italian network for research on psychoses. Front Psychiatry 2021; 12: 791117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCutcheon RA, Pillinger T, Efthimiou O, Maslej M, Mulsant BH, Young AH, et al. Reappraising the variability of effects of antipsychotic medication in schizophrenia: a meta-analysis. World Psychiatry 2022; 21: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galderisi S, Kaiser S, Bitter I, Nordentoft M, Mucci A, Sabé M, et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur Psychiatry 2021; 64(1): e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter WT. Primary psychosis: more to know, much more to do. World Psychiatry 2021; 20: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusar-Poli P, Estradé A, Stanghellini G, Venables J, Onwumere J, Messas G, et al. The lived experience of psychosis: a bottom-up review co-written by experts by experience and academics. World Psychiatry 2022; 21: 168–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Killaspy H, Harvey C, Brasier C, Brophy L, Ennals P, Fletcher J, et al. Community-based social interventions for people with severe mental illness: a systematic review and narrative synthesis of recent evidence. World Psychiatry 2022; 21: 96–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siskind D, Yung A. After the acute crisis – engaging people with psychosis in rehabilitation-oriented care. World Psychiatry 2022; 21: 246–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dollfus S, Lyne J. Negative symptoms: history of the concept and their position in diagnosis of schizophrenia. Schizophr Res 2017; 186: 3–7. [DOI] [PubMed] [Google Scholar]
- 19.Bleuer E. Dementia Praecox or the Group of Schizophrenias. NY International Universities Press, 1950. [Google Scholar]
- 20.Kraepelin E. Dementia praecox and paraphrenia. In Textbook of Psychiatry 8th ed (ed Barclay ES): 47–9. E & S Livingston, 1919. [Google Scholar]
- 21.Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 2006; 32: 214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull 2011; 37: 300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry 2013; 170: 165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull 2006; 32: 238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaebel W, Falkai P, Hasan A. The revised German evidence- and consensus-based schizophrenia guideline. World Psychiatry 2020; 19: 117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peralta V, Gil-Berrozpe GJ, Sánchez-Torres A, Cuesta MJ. Clinical relevance of general and specific dimensions in bifactor models of psychotic disorders. World Psychiatry 2021; 20: 306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Gaebel W, Maj M, Stein DJ, Kogan CS, Saunders JB, et al. An organization- and category-level comparison of diagnostic requirements for mental disorders in ICD-11 and DSM-5. World Psychiatry 2021; 20: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krueger RF, Hobbs KA, Conway CC, Dick DM, Dretsch MN, Eaton NR, et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): II. Externalizing superspectrum. World Psychiatry 2021; 20: 171–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahey BB, Moore TM, Kaczkurkin AN, Zald DH. Hierarchical models of psychopathology: empirical support, implications, and remaining issues. World Psychiatry 2021; 20: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson D, Levin-Aspenson HF, Waszczuk MA, Conway CC, Dalgleish T, Dretsch MN, et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry 2022; 21: 26–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventura J. Computer-based virtual reality assessment of functional capacity in primary psychosis. World Psychiatry 2022; 21: 464–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lysaker PH, Hasson-Ohayon I. Metacognition in psychosis: a renewed path to understanding of core disturbances and recovery-oriented treatment. World Psychiatry 2021; 20: 359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moritz S, Silverstein SM, Dietrichkeit M, Gallinat J. Neurocognitive deficits in schizophrenia are likely to be less severe and less related to the disorder than previously thought. World Psychiatry 2020; 19: 254–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klingberg T, Judd N, Sauce B. Assessing the impact of environmental factors on the adolescent brain: the importance of regional analyses and genetic controls. World Psychiatry 2022; 21: 146–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sass L. Subjectivity, psychosis and the science of psychiatry. World Psychiatry 2022; 21: 165–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumas G. From inter-brain connectivity to inter-personal psychiatry. World Psychiatry 2022; 21: 214–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed AO, Kirkpatrick B, Galderisi S, Mucci A, Rossi A, Bertolino A, et al. Cross-cultural validation of the 5-factor structure of negative symptoms in schizophrenia. Schizophr Bull 2019; 45: 305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang WC, Strauss GP, Ahmed AO, Wong SCY, Chan JKN, Lee EHM, et al. The latent structure of negative symptoms in individuals with attenuated psychosis syndrome and early psychosis: support for the 5 consensus domains. Schizophr Bull 2021; 47: 386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauss GP, Ahmed AO, Young JW, Kirkpatrick B. Reconsidering the latent structure of negative symptoms in schizophrenia: a review of evidence supporting the 5 consensus domains. Schizophr Bull 2019; 45: 725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strauss GP, Nuñez A, Ahmed AO, Barchard KA, Granholm E, Kirkpatrick B, et al. The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry 2018; 75: 1271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mucci A, Vignapiano A, Bitter I, Austin SF, Delouche C, Dollfus S, et al. A large European, multicenter, multinational validation study of the brief negative symptom scale. Eur Neuropsychopharmacol 2019; 29 947–59. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed AO, Kirkpatrick B, Granholm E, Rowland LM, Barker PB, Gold JM, et al. Two factors, five factors, or both? external validation studies of negative symptom dimensions in schizophrenia. Schizophr Bull 2022; 48: 620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li SB, Liu C, Zhang JB, Wang LL, Hu HX, Chu MY, et al. Revisiting the latent structure of negative symptoms in schizophrenia: evidence from two second-generation clinical assessments. Schizophr Res 2022; 248: 131–9. [DOI] [PubMed] [Google Scholar]
- 44.Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol 2014; 24: 725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messinger JW, Trémeau F, Antonius D, Mendelsohn E, Prudent V, Stanford AD, et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev 2011; 31: 161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull 2017; 43: 712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marder SR, Kirkpatrick B. Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol 2014; 24: 737–43. [DOI] [PubMed] [Google Scholar]
- 48.Shaffer JJ, Peterson MJ, McMahon MA, Bizzell J, Calhoun V, van Erp TG, et al. Neural correlates of schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol Neuropsychiatry 2015; 1: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borsboom D. A network theory of mental disorders. World Psychiatry 2017; 16: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epskamp S, Isvoranu AM. New trends in network modeling of psychopathology. World Psychiatry 2022; 21: 463–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strauss GP, Esfahlani FZ, Galderisi S, Mucci A, Rossi A, Bucci P, et al. Network analysis reveals the latent structure of negative symptoms in schizophrenia. Schizophr Bull 2019; 45: 1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strauss GP, Esfahlani FZ, Kirkpatrick B, Allen DN, Gold JM, Visser KF, et al. Network analysis reveals which negative symptom domains are most central in schizophrenia vs bipolar disorder. Schizophr Bull 2019; 45: 1319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauss GP, Zamani Esfahlani F, Sayama H, Kirkpatrick B, Opler MG, Saoud JB, et al. Network analysis indicates that avolition is the most central domain for the successful treatment of negative symptoms: evidence from the roluperidone randomized clinical trial. Schizophr Bull 2020; 46: 964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esfahlani FZ, Sayama H, Visser KF, Strauss GP. Sensitivity of the Positive and Negative Syndrome Scale (PANSS) in detecting treatment effects via network analysis. Innov Clin Neurosci 2017; 14: 59–67. [PMC free article] [PubMed] [Google Scholar]
- 55.Levine SZ, Leucht S. Identifying a system of predominant negative symptoms: network analysis of three randomized clinical trials. Schizophr Res 2016; 178: 17–22. [DOI] [PubMed] [Google Scholar]
- 56.Kagan S, Cogo-Moreira H, Barbosa MG, Cavalcante D, Shinji A, Noto M, et al. Longitudinal invariance of the Positive and Negative Syndrome Scale negative dimension in antipsychotic naïve first-episode schizophrenia. Early Interv Psychiatry 2022; 16: 581–6. [DOI] [PubMed] [Google Scholar]
- 57.Griffiths SL, Leighton SP, Mallikarjun PK, Blake G, Everard L, Jones PB, et al. Structure and stability of symptoms in first episode psychosis: a longitudinal network approach. Trans Psychiatry 2021; 11: 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 2014; 13: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galderisi S, Rucci P, Kirkpatrick B, Mucci A, Gibertoni D, Rocca P, et al. Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: a network analysis. JAMA Psychiatry 2018; 75: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galderisi S, Rucci P, Mucci A, Rossi A, Rocca P, Bertolino A, et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World psychiatry 2020; 19: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mucci A, Galderisi S, Gibertoni D, Rossi A, Rocca P, Bertolino A, et al. Factors associated with real-life functioning in persons with schizophrenia in a 4-year follow-up study of the Italian network for research on psychoses. JAMA Psychiatry 2021; 78: 550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version: Administration Booklet. American Psychiatric Association Publishing, 1996. [Google Scholar]
- 63.Mucci A, Galderisi S, Merlotti E, Rossi A, Rocca P, Bucci P, et al. The Brief Negative Symptom Scale (BNSS): independent validation in a large sample of Italian patients with schizophrenia. Eur Psychiatry 2015; 30: 641–7. [DOI] [PubMed] [Google Scholar]
- 64.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13: 261–76. [DOI] [PubMed] [Google Scholar]
- 65.Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res 2012; 137(1–3): 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Addington J, Shah H, Liu L, Addington D. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophr Res 2014; 153: 64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerlach J, Korsgaard S, Clemmesen P, Lauersen AM, Magelund G, Noring U, et al. The St. Hans Rating Scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr Scand 1993; 87(4): 244–52. [DOI] [PubMed] [Google Scholar]
- 68.Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods 2018; 50: 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Borkulo CD, van Bork R, Boschloo L, Kossakowski JJ, Tio P, Schoevers RA, et al. Comparing network structures on three aspects: a permutation test. Psychol Methods [Epub ahead of print] 11 Apr 2022. Available from: 10.1037/met0000476. [DOI] [PubMed] [Google Scholar]
- 70.Borsboom D, Haslbeck JMB, Robinaugh DJ. Systems-based approaches to mental disorders are the only game in town. World Psychiatry 2022; 21: 420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kossakowski JJ, Epskamp S, Kieffer JM, van Borkulo CD, Rhemtulla M, Borsboom D. The application of a network approach to health-related quality of life (HRQoL): introducing a new method for assessing HRQoL in healthy adults and cancer patients. Qual Life Res 2016; 25: 781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: generalizing degree and shortest paths. Soc Netw 2010; 32: 245–51. [Google Scholar]
- 73.Fried EI, Eidhof MB, Palic S, Costantini G, Huisman-van Dijk HM, Bockting CLH, et al. Replicability and generalizability of posttraumatic stress disorder (PTSD) networks: a cross-cultural multisite study of PTSD symptoms in four trauma patient samples. Clin Psychol Sci 2018; 6: 335–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Austin SF, Mors O, Budtz-Jørgensen E, Secher RG, Hjorthøj CR, Bertelsen M, et al. Long-term trajectories of positive and negative symptoms in first episode psychosis: a 10year follow-up study in the OPUS cohort. Schizophr Res 2015; 168: 84–91. [DOI] [PubMed] [Google Scholar]
- 75.Kulhara P, Chandiramani K. Positive and negative subtypes of schizophrenia. a follow-up study from India. Schizophr Res 1990; 3: 107–16. [DOI] [PubMed] [Google Scholar]
- 76.Lindenmayer JP, Kay SR, Friedman C. Negative and positive schizophrenic syndromes after the acute phase: a prospective follow-up. Compr Psychiatry 1986; 27: 276–86. [DOI] [PubMed] [Google Scholar]
- 77.Thara R, Henrietta M, Joseph A, Rajkumar S, Eaton WW. Ten-year course of schizophrenia – the Madras longitudinal study. Acta Psychiatr Scand 1994; 90: 329–36. [DOI] [PubMed] [Google Scholar]
- 78.Davidson M, Saoud J, Staner C, Noel N, Werner S, Luthringer E, et al. Efficacy and safety of roluperidone for the treatment of negative symptoms of schizophrenia. Schizophr Bull 2022; 48: 609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giordano GM, Bucci P, Mucci A, Pezzella P, Galderisi S. Gender differences in clinical and psychosocial features among persons with schizophrenia: a mini review. Front Psychiatry 2021; 12: 789179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rucci et al. supplementary material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, G.M.G., on reasonable request.