Abstract
For patients with localized pancreatic cancer with minimal vascular involvement, optimal survivability requires a multidisciplinary approach of surgical resection and systemic chemotherapy. FOLFIRINOX is a combination chemotherapy regimen that offers promising results in the perioperative and metastatic settings; however, it can cause significant adverse effects. Such toxicity can negatively impact some patients, resulting in chemotherapy discontinuation or surgical unsuitability. In an effort to reduce toxicities and optimize outcomes, this investigation explores the safety and feasibility of substituting liposomal irinotecan (nal-IRI) for nonliposomal irinotecan to improve tumor drug delivery and potentially reduce toxicity. This regimen, NALIRIFOX, has the potential to be both safer and more effective when administered in the preoperative setting.
Keywords: borderline resectable, chemotherapy, FOLFIRINOX, irinotecan, liposomal irinotecan, neoadjuvant, pancreatic adenocarcinoma, resectable, toxicity
Plain language summary
For patients with pancreatic cancer with little to no cancer near the blood vessels, the best life expectancy usually requires surgery and chemotherapy. FOLFIRINOX is a chemotherapy medicine that offers promising results for both patients getting surgery and for patients with widespread disease. However, it can cause harmful side effects. The side effects can be so bad that the chemotherapy has to be stopped or that surgery is no longer possible. In order to reduce the harmful side effects and improve outcomes, this investigation looks into the safety and practicality of using a different version of one of the medicines. The different version hopes to improve drug delivery and reduce harmful side effects. This regimen, NALIRIFOX, can be safer and more effective in patients awaiting surgery.
Clinical Trial Registration: UF-STO-PANC-004 (NCT03483038) (ClinicalTrials.gov)
Tweetable abstract
NALIRIFOX, a Phase II clinical trial for the treatment of resectable and borderline resectable pancreatic cancer. The regimen includes novel liposomal irinotecan in combination with 5-fluorouracil, leucovorin and oxaliplatin. Enrolling in multiple sites (NCT03483038).
The incidence of pancreatic cancer has doubled in the past two decades. In 2020, there were 495,000 cases of pancreatic cancer worldwide compared with 196,000 cases in 1990 [1,2]. Pancreatic cancer is projected to be the second leading cause of cancer deaths in the USA later this decade, with its mortality rate being very close to its incidence rate [1,3,4]. The overall 5-year survival rate ranges from 2 to 11%, in which higher survival rates are found in developed countries [1,3,5]. Long-term survival is only possible with complete resection of the cancer, with only a few patients being surgical candidates. Even then, outcomes are far from ideal, with 5-year survival in patients with resectable disease being 15–20% and the median survival 53.5 months [6,7].
Pancreatic cancer is thus typically classified clinically based upon its resectability, which is determined by the absence of distant metastases and characterization of the tumor vasculature involvement from CT or MRI [8,9]. A ‘resectable’ pancreatic cancer is commonly described as not having physical contact with the superior mesenteric artery, celiac axis or common hepatic artery. With regards to the venous system, a ‘resectable’ tumor has no contact with the superior mesenteric vein, portal vein or less than 180 degrees contact without vein contour irregularity [10,11].
Over the past several decades, ‘borderline resectable’ disease has been increasingly recognized as a stage in which operability is possible, but with increased risk for recurrence [6]. According to National Comprehensive Cancer Network guidelines, pancreatic tumors of the head and uncinate process are classified ‘borderline resectable’ if there is contact with the common hepatic artery without extension to the celiac axis or hepatic artery and abutment of the superior mesenteric artery of no greater than 180 degrees. ‘Borderline tumors’ of the pancreatic body or tail have contact with the celiac axis no greater than 180 degrees. For all areas of the pancreas, the definition allows for solid tumor contact with the superior mesenteric vein or portal vein greater than 180 degrees and no more than 180 degrees with contour irregularity of the vein to allow for safe and complete resection [10].
Beyond surgical potential for resectability, pancreatic cancer is mostly a systemic disease that requires chemotherapy in conjunction with surgery for optimal outcomes [11]. To date, the only treatment that offers a cure for some patients with pancreatic cancer is surgical resection followed by chemotherapy [12]. Adjuvant chemotherapy targets micrometastatic disease and has been shown to improve overall survival (OS) following surgery [13]. The CONKO-001 clinical trial demonstrated improved survival when the adjuvant gemcitabine was given after complete surgical resection versus surgery alone [13]. Despite these promising results, approximately 70% of patients still relapsed within 2 years. Multiagent chemotherapy, specifically FOLFIRINOX (5-fluorouracil [5-FU], oxaliplatin, leucovorin [LV] and irinotecan), is now a preferred systemic treatment option for those patients with adequate performance status [10,14,15]. Conroy et al. demonstrated FOLFIRINOX was superior to gemcitabine in the adjuvant setting, with a significantly improved OS (median survival 53.5 vs 35.5 months; hazards ratio [HR] 0.64; 95% CI: 0.48–0.86; p = 0.003) [7,14].
Owing to inherent challenges with delivering postoperative chemotherapy (e.g., adequate patient recovery), a neoadjuvant (preoperative) therapy is now favored by many [12,16]. Neoadjuvant chemotherapy offers the ability to target systemic disease earlier, optimizes patient selection for surgery and potentially downstages the tumor. Together, this should allow more patients to achieve complete surgical resection with negative margins, which is thus far the best predictor of good long-term outcomes [17]. National professional guidelines now support a neoadjuvant therapy approach with multiagent chemotherapy in patients with borderline resectable pancreatic cancer [18]. Since resectability of the cancer is determined by the characterization of tumor vasculature involvement, the duration of neoadjuvant chemotherapy for both resectable and borderline resectable is typically the same in order to treat possible micrometastases [17]. This protocol does not preclude an additional two cycles of chemotherapy postoperatively, nor does it preclude use of chemoradiotherapy preoperatively. The choice of 4 months of therapy was chosen as a compromise to ensure enough therapy had been delivered to get a clear safety signal. The use of neoadjuvant FOLFIRINOX chemotherapy has been evaluated in several nonrandomized studies with a promising safety profile, surgical outcomes and long-term results, but at the risk of significant toxicity [19–23].
Thus, the major challenge in treating patients with aggressive combination neoadjuvant therapy is toxicity, as patients ideally should receive full doses of therapy to ensure optimal downstaging and micrometastatic sterilization, while maintaining sufficient health to undergo successful surgical resection and recovery. Traditional FOLFIRINOX typically requires routine dose modifications, treatment delays secondary to toxicities and growth factor support. The FOLFIRINOX regimen contains the following agents and doses: irinotecan 180 mg/m2, oxaliplatin 85 mg/m2, LV 400 mg/m2 and bolus 5-FU 400 mg/m2 followed by continuous infusion of 5-FU 2400 mg/m2 (the latter over 48 h), all given intravenously every 2 weeks with growth factor support. In contrast, modified FOLFIRINOX (mFOLFIRINOX) involves a reduction of irinotecan from 180 mg/m2 to 150 mg/m2 and removal of the bolus [14]. In a patient-level meta-analysis of 13 studies, most commonly the 5-FU bolus was omitted, with two studies reporting a decrease of the 5-FU bolus dose by 25% [21]. Various doses for oxaliplatin were used ranging from 63.75 to 85 mg/m2. LV ranged from 0 to 400 mg/m2 and irinotecan ranged from 130 to 180 mg/m2 [24]. In the clinical trial by Conroy et al., 36% of patients in the modified FOLFIRINOX group were unable to complete all planned cycles due to discontinuation of therapy compared with 22% in the gemcitabine group [12]. Modified FOLFIRINOX demonstrates meaningful clinical downstaging [25]; however, its toxicities still limit the number of cycles and utility to a larger patient population [14]. While oxaliplatin also contributes to toxicity with thrombocytopenia and neuropathy, it less frequently requires treatment interruptions [26]. Irinotecan, however, is typically associated with adverse events (AE) of neutropenia, diarrhea and asthenia, which can impact treatment consistency [27].
Liposomal irinotecan combination with 5-FU/LV was US FDA-approved in October 2015 for use in patients with metastatic pancreatic adenocarcinoma whose disease has progressed after gemcitabine-based chemotherapy [28]. NALIRIFOX may have better tissue penetration of liposomal irinotecan given the nanoparticle formulation as a biologic rationale [29]. In the current study, irinotecan is encapsulated in liposomes in order to achieve a controlled and sustained release of drug to improve activity of this schedule-dependent drug. This drug form of the drug is brand named ONIVYDE. Increasing duration of exposure of tumor tissue to the drug allows a higher proportion of cells during the more sensitive S-phase of the cell cycle [30]. The preferentially local bioactivation should result in reduced exposure to potential sites of toxicity (e.g., less bone marrow suppression and peripheral neuropathy) and increased exposure to neighboring cancer cells within the tumor [30]. The improved pharmacokinetics and high intravascular drug retention in the liposomes may support site-specific drug delivery to pancreatic cancer, particularly in a preoperative setting when vascular disruption from surgery has not yet occurred [30].
The NALIRIFOX chemotherapy regimen was developed consisting of liposomal irinotecan (nal-IRI), 5-FU/LV and oxaliplatin, with early-phase data demonstrating manageable side effects for patients [31]. Wainberg et al. confirmed safe and effective dosing of NALIRIFOX with no unexpected safety outcomes. Table 1 outlines comparative dosing for NALIRIFOX, FOLFIRINOX and mFOLFIRINOX. All 32 patients that received the recommended dose experienced at least one treatment-emergent AE, with the most common AEs being grade ≥3 neutropenia (ten patients), any grade thromboembolic events (five patients), any grade febrile neutropenia (four patients) and grade ≥3 diarrhea (four patients). Expanding on these results, NAPOLI-3 was a randomized phase III clinical trial that compared the efficacy and safety of NALIRIFOX in the first-line setting for patients with metastatic pancreatic cancer, compared with gemcitabine and nab-paclitaxel (ClinicalTrials.gov identifier: NCT04083235) [32]. A total of 770 patients were randomized in a 1:1 ratio to NALIRIFOX on days 1 and 15 out of a 28-day cycle or nab-paclitaxel 125 mg/m2 plus gemcitabine 1000 mg/m2 on days 1, 8 and 15 of a 28-day cycle. The study met its primary end point of OS. At a median follow-up of 16.1 months, median OS was 11.1 months in the NALIRIFOX arm compared with 9.2 months in the nab-paclitaxel and gemcitabine arm (HR: 0.84; 95% CI: 0.71–0.99; p = 0.04); PFS was also significantly better (7.4 vs 5.6 months; HR: 0.70; 0.59–0.84; p = 0.0001). The safety profile of nal-IRI in the NAPOLI-3 trial was consistent with those observed in the previous phase I/II study. In conclusion, NAPOLI-3 showed that first-line NALIRIFOX demonstrated clinically meaningful and statistically significant improvement in OS and PFS compared with nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic cancer. Given these encouraging results in the metastatic setting, our study aims to answer the question of whether NALIRIFOX is a safe and feasible treatment in the neoadjuvant setting for patients with resectable or borderline resectable pancreatic cancer.
Table 1. . Comparison of multiagent chemotherapy regimens.
| Regimen | Irinotecan (mg/m2) | ONIVYDE® (mg/m2) | Oxaliplatin (mg/m2) | 5-Fluorouracil (mg/m2) bolus | 5-Fluorouracil (mg/m2) continuous infusion over 46 h | Leucovorin (mg/m2) |
|---|---|---|---|---|---|---|
| FOLFIRINOX | 180 | – | 85 | 400 | 2400 | 400 |
| mFOLFIRINOX | 150 | – | 85 | – | 2400 | 400 |
| NALIRIFOX | – | 50 | 60 | – | 2400 | 400 |
Each drug is intravenously administered every 2 weeks. Clinical follow-up 4–6 weeks after surgery and survival follow-up every 6 months for 5 years.
FOLFIRINOX: 5-Fluorouracil, oxaliplatin, leucovorin and irinotecan; mFOLFIRINOX: Modified FOLFIRINOX; NALIRIFOX: Liposomal irinotecan in combination with 5-fluorouracil/leucovorin and oxaliplatin.
Objectives
Primary
To establish the safety and feasibility of nal-IRI in combination with 5-FU/LV and oxaliplatin (NALIRIFOX) in the neoadjuvant setting of resectable or borderline resectable pancreatic adenocarcinoma. The primary end point that is being studied is the 30-day postoperative complication rate as measured by hospital readmission, death, second surgery or interventional procedure, or major complications extending hospital stay.
Secondary
To determine the treatment completion rate of therapy defined by completion of all intended cycles;
To determine the rate of complete surgical resection (R0) and other histopathologic downstaging;
To determine radiographic objective response rate (ORR) of the primary tumor as measured by Response Evaluation Criteria in Solid Tumours (RECIST) 1.1 criteria;
To determine the biochemical response rate and pattern of response as measured by serial serum CA19-9 levels;
To determine the patient-reported quality of life during treatment as measured by the NCI validated Functional Assessment of Cancer Therapy – General (FACT-G).
Exploratory
To collect tissues and blood for post-hoc secondary exploratory biomarker, circulating tumor cell (CTC), circulating tumor DNA (ctDNA), pharmacogenomic and tumor mutational profile assessments as well as potential patient-derived xenografts (PDX) development;
Biospecimens will be collected and subsequently examined for microbiota content and any associations with patient and tumor characteristics, treatment outcomes and change over time.
Methods
Study setting & treatment plan
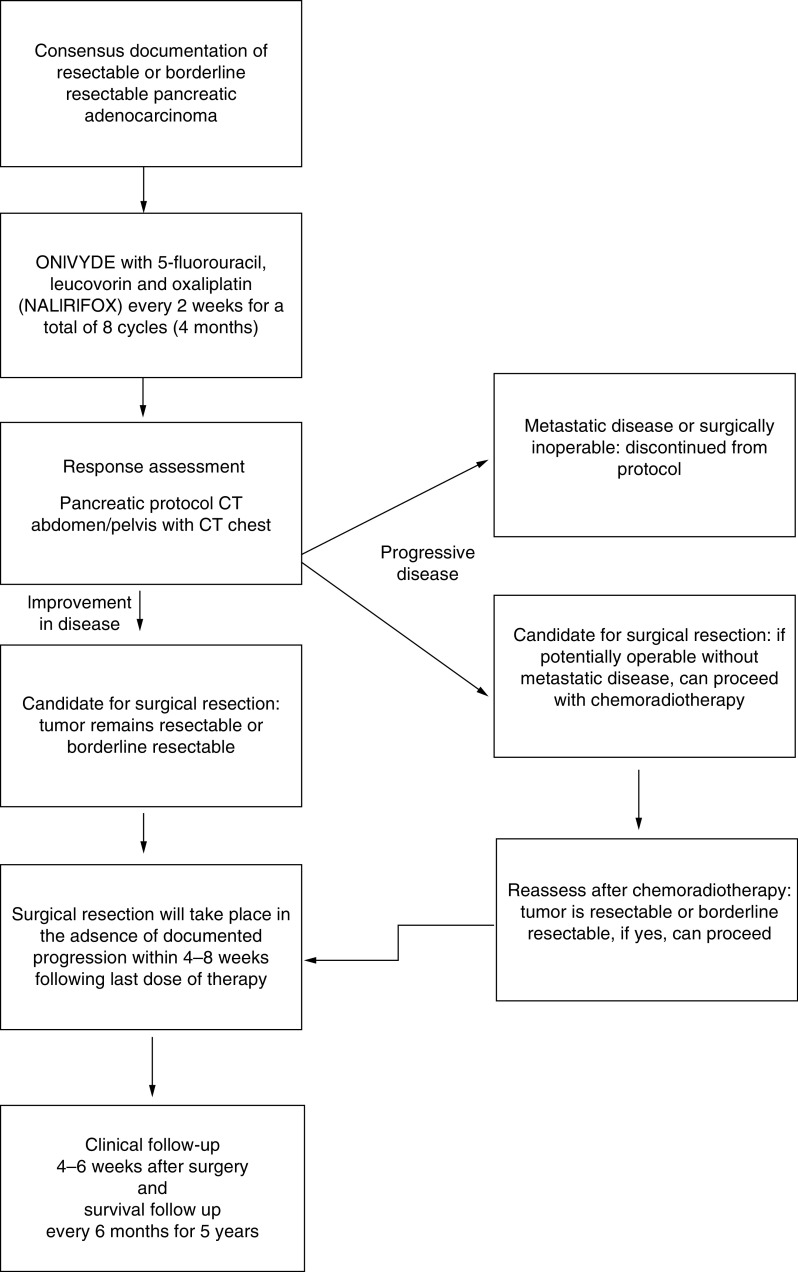
This is a prospective open-label, multicenter, single-arm pilot trial of neoadjuvant NALIRIFOX in patients with resectable or borderline resectable pancreatic adenocarcinoma. Written informed consent was obtained from all participants prior to enrollment in the trial. The study was conducted in compliance with the Declaration of Helsinki. Each subject will be administered NALIRIFOX every 2 weeks as described in Table 2 for a total of eight treatment cycles (4 months). A response assessment will follow the last cycle. This response assessment includes imaging with the preferred CT (MRI acceptable in certain situations; e.g., body scans) to assess measurability of the lesions. Surgical resection will take place in the absence of radiographic progression within 4–8 weeks following the last dose of therapy. Progression will be evaluated radiographs as per RECIST: Revised RECIST Guideline (version 1.1) [33]. Clinical evaluations are performed on day 1 of each cycle, within 4 weeks prior to planned surgery, and 4–6 weeks postoperatively. If a patient has disease progression during neoadjuvant therapy, they will be removed from the study protocol and deemed nonevaluable. Optional chemoradiotherapy is allowed following completion of NALIRIFOX and prior to surgery based upon multidisciplinary group recommendations of post-treatment radiographic concerns for potentially compromised surgical margins. There will be a clinic follow-up 4–6 weeks after surgery and a survival follow-up every 6 months for 5 years. The protocol schema is visualized in Figure 1.
Table 2. . NALIRIFOX agents and regimen used in this study (NCT03483038).
| Agent | Dose | Route/duration |
|---|---|---|
| ONIVYDE | 50 mg/m2 (starting dose is 36 mg/m2 [dose level -1] for subjects who are homozygous for UGT1A1*28) | iv. over 90 min |
| Oxaliplatin | 60 mg/m2 | iv. over 120 min |
| Leucovorin | 400 mg/m2 | iv. over 120 min |
| 5-Fluorouracil | 2400 mg/m2 | iv. continuous infusion over 46 h |
Treatment is administered every 14 days for 8 total cycles.
iv.: Intravenous; NALIRIFOX: Liposomal irinotecan in combination with 5-fluorouracil/leucovorin and oxaliplatin.
Figure 1. . Flowchart of protocol.
Eligibility criteria
Patients with a new clinical diagnosis of resectable or borderline resectable pancreatic adenocarcinoma confirmed by pathologic specimen and no evidence of metastatic disease are eligible for the study. Prior to treatment, medical history is obtained. Detailed information on inclusion and exclusion criteria are outlined in Table 3. The ultimate determination of whether a potential subject meets resectability criteria (resectable or borderline resectable as defined by the NCCN guidelines) by CT of the abdomen with intravenous (iv.) contrast (MRI abdomen if CT/contrast is contraindicated) assessment is discussed and verified by multidisciplinary tumor board consensus at the University of Florida (FL, USA) or an equivalent institutional body at a participating site. This consensus determination is required prior to any treatment being administered.
Table 3. . Inclusion and exclusion criteria of subjects.
| Inclusion criteria | Exclusion criteria |
|---|---|
| A. Written informed consent obtained from the subject and the ability for the subject to comply with all the study-related procedures | A. A medical history of prior anticancer treatment for pancreatic cancer |
| B. Both males and females ≥18 years of age | B. Locally advanced unresectable disease (see the criteria for resectability from inclusion criteria) or evidence of metastatic disease |
| C. A new clinical diagnosis of borderline resectable, or resectable, previously untreated pancreatic adenocarcinoma confirmed by pathologic specimen | C. Any other invasive malignancy within the past 3 years |
| D. No clinical evidence of metastatic disease, confirmed by CT of chest/abdomen and pelvis with iv. contrast (or MRI of pancreas with non-contrast chest CT, if iv. contrast is contraindicated) | D. Presence of any known contraindications to or hypersensitivities to the investigational products |
| E. Potentially resectable local disease, confirmed by CT of the abdomen with iv. contrast (MRI abdomen if CT/contrast is contraindicated) which radiographically confirmation of resectable or borderline resectable pancreatic cancer verified by documented consensus discussion of the GI Multidisciplinary Tumor Board and guided by the published expert consensus criteria for resectable or borderline resectable. The ultimate determination of whether a potential subject meets the criteria of ‘resectable or borderline resectable’ lies in the consensus of the UF GI Multidisciplinary Tumor Board, or equivalent body (for external sites) | E. Use of strong CYP3A4 inhibitors or inducers which cannot be discontinued prior to study entry |
| F. ECOG performance status score of 0 or 1 | F. A nonsurgical candidate |
| G. Treated biliary obstruction (if applicable) | G. Subject is unable to understand, provide consent or comply with study requirements, treatments or instructions in the opinion of the treating physician |
| H. Subjects with known or suspected Gilbert's disease must be formally tested for UGT1A1*28 with results available to study team prior to treatment initiation | H. Uncontrolled diarrhea, active infection, known interstitial lung disease or other medical condition that precludes safe administration of this combination therapy consistent with manufacturer recommendations |
| I. Adequate organ function; as defined by: i. Hematologic: ANC >1500 cells/μl without the use of hematopoietic growth factors; and platelet count >100,000 cells/μl; and hemoglobin >9 g/dl (blood transfusions are permitted for patients with hemoglobin levels below 9 g/dl) ii. Hepatic: Serum total bilirubin within 1.5 ULN for the institution, with a trend downwards (biliary drainage is allowed for biliary obstruction), AST and ALT ≤2.5× ULN iii. Renal: Serum creatinine ≤1.5× ULN iv. Cardiac: Normal ECG or ECG without any clinically significant findings as defined by the treating physician |
I. Unwilling/unable to comply with birth control requirements while on study |
| J. WOCBP must be using an adequate method of contraception to avoid pregnancy throughout the study and for at least 7 months after the last dose of study drug to minimize the risk of pregnancy. Prior to study enrollment, WOCBP must be advised of the importance of avoiding pregnancy during trial participation and the potential risk factors for an unintentional pregnancy. WOCBP include any woman who has experienced menarche and who has not undergone successful surgical sterilization (hysterectomy, bilateral tubal ligation or bilateral oophorectomy) or who is not post-menopausal. Postmenopause is defined as: • Amenorrhea that has lasted for ≥12 consecutive months without another cause; or • For women with irregular menstrual periods who are taking HRT, a documented serum FSH level of greater than 35 mIU/m |
J. Females or males of childbearing potential who are unwilling or unable to use an acceptable method to avoid pregnancy for the entire study period and for at least 7 months for females and 4 months for males after the last dose of study drug |
| K. Males with female partners of child-bearing potential must agree to use physician-approved contraceptive methods (e.g., abstinence, condoms, vasectomy) throughout the study and should avoid conceiving children for 4 months following the last dose of study drug | K. Females who are pregnant or breastfeeding |
| L. History of any other disease, metabolic dysfunction, physical examination finding, or clinical laboratory finding giving reasonable suspicion of a disease or condition that contraindicates the use of protocol therapy or that might affect the interpretation of the results of the study or that puts the subject at high risk for treatment complications, in the opinion of the treating physician | |
| M. Prisoners or subjects who are involuntarily incarcerated | |
| N. Subjects who are compulsorily detained for treatment of either a psychiatric or physical illness | |
| O. Subjects demonstrating an inability to comply with the study and/or follow-up procedures | |
| P. Known DPD deficiency |
ALT: Alanine transaminase; ANC: Absolute Neutrophil Count; AST: Aspartate transaminase; DPD: Dihydrypyrimidine; ECG: Electrocardiogram; ECOG: Eastern Cooperative Oncology Group; FSH: Follicle-stimulating hormone; GI: Gastrointestinal; HRT: Hormone-replacement therapy; iv.: Intravenous; ULN: Upper Limit Normal; UF: University of Florida; WOCBP: Women of childbearing potential.
Outcomes
The primary end point is a 30-day postoperative complication rate as measured by hospital readmission, death, second surgery or interventional procedure or major complications extending hospital stay. The primary end point of 30-day postoperative complication rate was chosen to determine whether the neoadjuvant therapy was safe for those who made it all the way through neoadjuvant therapy and on to surgery, which represents the full course of curative intent treatment. Although toxicity from neoadjuvant therapy could preclude patients from getting to surgery altogether and would thus underestimate any safety assessment, it is anticipated (from the data using mFOLFIRINOX) that the vast majority of patients who fail to achieve surgery will do so because of disease progression, not catastrophic treatment toxicity. However, data related to patients who fail to make it to surgery and the reason for it will be reported and included in the secondary end points of neoadjuvant treatment compliance and completion as well as the CONSORT diagram. The Common Terminology Criteria for Adverse Events (CTCAE) metrics for patients during neoadjuvant treatment will also be recorded and reported. As a single-arm feasibility study, the intention was to establish a composite end point for safety as the primary end point that represented the cumulative course of patients in their pursuit of curative intent therapy, which was inclusive of all the contributing variables (e.g., toxicity during therapy, patient performance status decline, candidacy for surgery, etc.) and report those important component variables in a descriptive manner.
Secondary end points include treatment completion rate, surgical resection rate, radiographic ORR, biochemical (serum CA19-9) response rate and patient quality of life.
Exploratory outcomes include analysis of CTCs, ctDNA, pharmacogenomic, tumor mutational profile and microbiota associated with patient and tumor characteristics, treatment outcomes and change over time.
Sample size & timeline
A total of 28 evaluable patients were planned. Evaluable patients are defined as those who receive at least one dose of NALIRIFOX treatment and complete definitive surgery. The total duration of active subject participation will be approximately 4 months of chemotherapy followed by surgery within 1–2 months of the last dose. Patients will then be followed in a survival clinic every 6 months for 5 years.
Data collection
Subjects who did not achieve definitive surgery (for any reason) or are lost to follow-up will be censored at the day of their last objective tumor assessment and will be unavailable for the primary end point. Subjects failing to achieve definitive surgery will be replaced, but all will support the secondary end points including feasibility, safety and correlative biologic analyses. Postoperative complications will be measured at the end of treatment (EOT) visit 4–6 weeks after surgery.
Secondary end points will be analyzed in a similarly descriptive manner. Treatment completion rate is defined by the administration of all eight intended cycles of neoadjuvant chemotherapy. The surgical complete resection rate will be determined from review of the pathology reports and inclusive of pathologic nodal status and tumor node metastasis staging (AJCC 8th Edition). Preoperative imaging will be compared to the baseline studies to assess radiographic response in those who have RECIST-measurable disease at baseline.
Microbiota with a dietary log is obtained at longitudinal time points including baseline, 2 months, completion of neoadjuvant chemotherapy and at EOT. Exploratory biomarkers and molecular assay results will be analyzed post-hoc.
Statistics
Sample size: for this prospective phase II pilot study, the sample size justification is based on the primary end point, the complication rate for evaluable patients who complete surgery. A sample size of 25 patients achieves approximately 80% to detect a reduction of 20% in the 30-day postoperative complication rate (from 30 to 10%), using a one-sided exact test with a significance level of 0.05. Assuming a 10% drop-out rate, we will accrue a total of 28 patients for this study. The study expects a 20-month accrual period with an additional follow-up of 7 months after the last patient is enrolled. Subjects failing to proceed to surgery will be replaced, but all will support the secondary analyses.
Data analysis plan: the primary analysis will use the modified intention-to-treat population consisting of subjects who are enrolled and received any dose of study medication. The modified intention-to-treat population will be included in summary tables of subject demographics and disease characteristics, and in analysis of efficacy. Subjects who did not progress to surgery (for any reason) or are lost to follow-up will be censored at the day of their last objective tumor assessment and will be unevaluable for the primary end point. Subjects failing to proceed to surgery will be replaced, but all will support the secondary end points including feasibility, safety and correlative biologic analyses. Postoperative complications will be measured at the EOT visit 4–6 weeks after surgery. Complications occurring within 30 days after surgery and contributing to the primary end point are defined as hospital readmission, death, second surgery or interventional procedure, or major complications extending hospital stay. The proportion and its exact 90% CI of 30-day postoperative complication rate will be calculated. Secondary, exploratory and safety data will be summarized in a descriptive manner and analyzed similar to the primary end point. All analyses will be conducted using SAS v9.4 (SAS institute, NC, USA).
The radiographic ORR is equal to the proportion of patients achieving a best overall response of partial or complete response (CR + PR), according to RECIST from the start of the treatment until disease progression/recurrence. Clinical benefit rate is equal to the ORR plus the proportion of patients attaining stable disease (CR + PR + SD). Patients with initially measurable disease who do not have a tumor response assessment for any reason are considered nonresponders and will be included in the denominator when calculating the response rate. Biochemical response rate is measured as the difference between baseline and maximal nadir of serum CA19-9 level. Proportion and exact 95% CI based on binomial distribution will be estimated for each of the secondary end points described above. Subjects will be characterized as those with normalization of CA19-9 during therapy, reduction without normalization or no reduction with assessment of the temporal nature of these changes and correlation to surgical resection rates.
Secondary and exploratory end points may be characterized between those with resectable versus borderline resectable disease at study entry, but without any planned formal analyses between these two subsets. Mean and 95% CI, assuming a normal distribution, median (range) will be estimated for quality of life parameters using the FACT-G validated patient-reported outcome instrument.
Descriptive statistics and correlative analysis will be reported. Continuous and categorical variables will be compared with Student's t-test and Chi-squared test. Multiple groups will be compared by ANOVA. Kaplan–Meier logistic regression will be performed to compare outcomes between the different microbiota groupings.
Data monitoring
University of Florida Health Cancer Center compliance monitors utilize on-site and/or remote monitoring periodically during the trial to independently determine whether sites are complying with the protocol. Source documents are reviewed for verification of agreement with data as submitted via the data collection system. The site investigator/institution guarantees access to source documents by the University of Florida Health Cancer Center or its designee and appropriate regulatory agencies.
Safety monitoring
Safety analyses are performed on all patients who receive any dose of study medication. Safety evaluations are performed based on the actual therapy received. The safety and tolerability of thestudy drug is determined by reported AEs, physical examinations, laboratory tests and electrocardiograms. All patients are assessed regularly for potential occurrence of AEs from the time that treatment starts until 30 days after the last dose of study therapy or 30 days postoperatively, whichever is longer.
Ethics
The study is conducted in compliance with the approved protocol. The protocol, any amendments, and the subject informed consent has received Institutional Review Board approval before initiation of the study. The Principal Investigator ensures that all persons assisting with the study are adequately informed about the protocol, any amendments to the protocol, the study treatments, and their study-related duties and functions. The Principal Investigator maintains a list of coinvestigators and other appropriately qualified persons to whom they have delegated significant study-related duties. Before being enrolled in this clinical trial, the subject must consent to participate after the nature, scope and possible consequences of the clinical study have been explained in a form understandable to them. A consenting response and signed informed consent are required to confirm agreement and clear communication.
An informed consent document that includes both information about the study and the consent form are prepared and given to the subject. This document contains all Good Clinical Practice and locally required regulatory elements. The document is in a language understandable to the subject and must specify the person who obtained informed consent.
All records identifying the subject are kept confidential and to the extent permitted by the applicable laws and/or regulations, will not be made publicly available.
Conclusion
The treatment for pancreatic adenocarcinoma is constantly evolving with improved outcomes needed. Neoadjuvant therapy is recommended for many potentially resectable patients, but the current approach of combination chemotherapy comes with significant toxicities. This study aims to assess the safety and feasibility of using NALIRIFOX, a modified drug regimen with a novel drug delivery mechanism, to optimize efficacy and ultimately improve outcomes. This clinical trial will also help establish a benchmark for radiographic response rate, pathologic downstaging and treatment compliance with this regimen. Taken together and supplemented with correlative biomarker and exploratory objectives, this study will support the continued development of this novel combination regimen in this disease. The next phase will be based upon the results of this phase II trial. If a favorable result occurs in regard to the primary and second end points, it will provide evidence that this is an acceptable regimen in the neoadjuvant setting.
Executive summary.
Pancreatic cancer overview
Pancreatic cancer is a systemic disease that requires chemotherapy, in addition to surgery, as part of curative treatment.
Treatment of pancreatic cancer
While FOLFIRINOX (5-fluorouracil [5-FU], oxaliplatin, leucovorin [LV] and irinotecan) is a well-established regimen for pancreatic cancer, it comes with notable toxicity, often resulting in patient selection, treatment delays and dose modifications.
Liposomal irinotecan (nal-IRI) is a novel approach to address the toxicities associated with irinotecan included in FOLFIRINOX. When nal-IRI is utilized, the regimen is referred to as NALIRIFOX.
NALIRIFOX: a novel regimen
NALIRIFOX has the potential to maximize drug delivery with reduced doses while maintaining efficacy.
This is a prospective open-label, multicenter, single-arm pilot trial of NALIRIFOX in the neoadjuvant setting of adults with resectable or borderline resectable pancreatic adenocarcinoma that is actively enrolling.
Acknowledgments
The authors would like to thank our patient partners and their caregivers who have participated in this clinical trial.
Footnotes
Author contributions
SC Rogers and TJ George are the principal investigators of this clinical trial. All co-authors have contributed to the development of this manuscript and approve of the final version.
Financial & competing interests disclosure
This work is supported by the Robert A. Winn Diversity in Clinical Trials Career Development Award and the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number 1U01DK108320. University of Florida Health Cancer Center Microbiome Biorepository supports the exploratory microbiota studies. Funding for NCT03483038 provided by Ipsen Pharmaceuticals and University of Florida Health Cancer Center. JC Fabregas: recipient of the Robert A Winn Diversity in Clinical Trials Career Development Award, funded by National Medical Fellowships and Bristol Myers Squibb Foundation. TJ George: Pfizer/Array, Tempus Labs and BillionToOne Consulting/Advisory Role. Kathryn Hitchcock: Research funding from the NIH, Merck & Co., Naveris. Paid writer for Medscape. B Ramnaraign: Ipsen Pharmaceuticals Advisory Board. SC Rogers: MedSphere Consultant, Natera Oncology Advisor, recipient of the Robert A Winn Diversity in Clinical Trials Career Development Award, funded by National Medical Fellowships and Bristol Myers Squibb Foundation. A Turk: Research funding from Loxo Oncology and Eli Lilly. Ipsen Pharmaceuticals had no input into the study design, analysis, or interpretation of results. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval and have followed the principles outlined by the University of Florida for all human or animal experimental investigations. Informed consent has been obtained on all human subjects participating in the clinical trial.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 18(7), 493–502 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 71(3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 22(44), 9694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74(11), 2913–2921 (2014). [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer Facts & Figures 2022. American Cancer Society, GA, USA: (2022). [Google Scholar]
- 6.Lopez NE. Borderline resectable pancreatic cancer: definitions and management. World J. Gastroenterol. 20(31), 10740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy T, Castan F, Lopez A et al. Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 8(11), 1571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong SB, Lee SS, Kim JH et al. Pancreatic cancer CT: prediction of resectability according to NCCN criteria. Radiology. 289(3), 710–718 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Jang JK, Byun JH, Kang JH et al. CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following FOLFIRINOX therapy. Eur. Radiol. 31(2), 813–823 (2021). [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer, Network, Inc, National Comprehensive Cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for pancreatic adenocarcinom (2022). www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- 11.He J. Management of borderline and locally advanced pancreatic cancer: where do we stand? World J. Gastroenterol. 20(9), 2255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 24(43), 4846–4861 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oettle H, Neuhaus P, Hochhaus A et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310(14), 1473 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Conroy T, Hammel P, Hebbar M et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379(25), 2395–2406 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364(19), 1817–1825 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Desai NV, Sliesoraitis S, Hughes SJ et al. Multidisciplinary neoadjuvant management for potentially curable pancreatic cancer. Cancer Med. 4(8), 1224–1239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Versteijne E, Suker M, Groothuis K et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 38(16), 1763–1773 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front. Oncol. 10, 245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen QP, Buettner S, Suker M et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. JNCI J. Natl Cancer Inst. 111(8), 782–794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Systemic review and meta-analysis that demonstrated promising results on overall survival and resection rate in patients treated with neoadjuvant Neoadjuvant 5-Fluorouracil, oxaliplatin, leucovorin and irinotecan (FOLFIRINOX).
- 20.Murphy JE, Wo JY, Ryan DP et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 4(7), 963 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Phase II clinical trial investigating the relationship between neoadjuvant chemotherapy and radiotherapy with margin negative resection rates. FOLFIRINOX demonstrated high rates of resection rates with prolonged median overall survival.
- 21.Suker M, Beumer BR, Sadot E et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 17(6), 801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Systemic review and meta-analysis that concluded patients with locally advanced pancreatic cancer treated with FOLFIRINOX had longer overall survival than patients treated with gemcitabine.
- 22.Ghaneh P, Palmer D, Cicconi S et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 8(2), 157–168 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Sohal D, Duong MT, Ahmad SA et al. SWOG S1505: Results of perioperative chemotherapy (peri-op CTx) with mfolfirinox versus gemcitabine/nab-paclitaxel (Gem/nabP) for resectable pancreatic ductal adenocarcinoma (PDA). J. Clin. Oncol. 38(Suppl. 15), 4504–4504 (2020). [Google Scholar]
- 24.Tong H, Fan Z, Liu B, Lu T. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: a systematic review and meta-analysis. Sci. Rep. 8(1), 8666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Jin C, Fu D-L, Warshaw AL. Modified FOLFIRINOX for resected pancreatic cancer: Opportunities and challenges. World J. Gastroenterol. 25(23), 2839–2845 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Explores clinical trials using a modified FOLFIRINOX regimen. The study concluded that the modified FOLFIRNOX regimen offers better overall survival when compared with adjuvant gemcitabine therapy.
- 26.Zhang B, Zhou F, Hong J et al. The role of FOLFIRINOX in metastatic pancreatic cancer: a meta-analysis. World J. Surg. Oncol. 19(1), 182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin. Pharmacokinet. 57(10), 1229–1254 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang-Gillam A, Hubner RA, Siveke JT et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer. 108, 78–87 (2019). [DOI] [PubMed] [Google Scholar]; • Phase III study using liposomal irinotecan plus 5-fluorouracil and leucovorin in patients with metastatic pancreatic cancer. The study concluded that overall survival was maintained when compared with gemcitabine-based therapy.
- 29.Nichetti F, Rota S, Gusmaroli E et al. NALIRIFOX vs FOLFIRINOX vs gemcitabine plus nab-paclitaxel as first-line treatment of advanced pancreatic cancer: An individual patient data pooled analysis of phase 3 registration trials. J. Clin. Oncol. 41(Suppl. 16), e16262–e16262 (2023). [Google Scholar]
- 30.Frampton JE. Liposomal irinotecan: a review in metastatic pancreatic adenocarcinoma. Drugs. 80(10), 1007–1018 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wainberg ZA, Bekaii-Saab T, Boland PM et al. First-line liposomal irinotecan with oxaliplatin, 5-fluorouracil and leucovorin (NALIRIFOX) in pancreatic ductal adenocarcinoma: a phase I/II study. Eur. J. Cancer 151, 14–24 (2021). [DOI] [PubMed] [Google Scholar]; •• Phase I/II study exploring the safety and efficacy of liposomal irinotecan by determining the maximum tolerated dose. The study concluded that the regimen was generally manageable and tolerable.
- 32.Wainberg ZA. NAPOLI-3: A randomized, open-label phase 3 study of liposomal irinotecan+5-fluorouracil/leucovorin+oxaliplatin (NALIRIFOX) versus nab-paclitaxel+gemcitabine in treatment-naïve patients with metastatic pancreatic ductal adenocarcinoma (mPDAC). J. Clin. Oncol. 41(Suppl. 4), LBA661–LBA661 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45(2), 228–247 (2009). [DOI] [PubMed] [Google Scholar]