This cohort study assesses the association between grade 3 or higher chemotherapy-related toxic effects and quality of life (QOL) and/or physical functioning or mortality at 6 months and 12 months among patients 70 years or older receiving chemotherapy.
Key Points
Question
What is the association between grade 3 or higher chemotherapy-related toxic effects and quality of life (QOL) and/or physical functioning at 6 months and 12 months in patients aged 70 years or older treated with chemotherapy?
Findings
In this multicenter cohort study that included 276 patients aged 70 years or older treated with chemotherapy, grade 3 or higher toxic effects were associated with a composite end point (decline in QOL and/or physical functioning or mortality) in patients with frailty. Grade 3 or higher toxic effects were not associated with the composite end point in patients without frailty.
Meaning
The findings suggest that, among patients with frailty, the occurrence of grade 3 or higher toxic effects was associated with a decline in QOL and/or physical functioning or mortality after 1 year, highlighting the importance of pretreatment frailty screening and individualized treatment adaptions.
Abstract
Importance
Although older patients are at increased risk of developing grade 3 or higher chemotherapy-related toxic effects, no studies, to our knowledge, have focused on the association between toxic effects and quality of life (QOL) and physical functioning.
Objective
To investigate the association between grade 3 or higher chemotherapy-related toxic effects and QOL and physical functioning over time in older patients.
Design, Setting, and Participants
In this prospective, multicenter cohort study, patients aged 70 years or older who were scheduled to receive chemotherapy with curative or palliative intent and a geriatric assessment were included. Patients were treated with chemotherapy between December 2015 and December 2021. Quality of life and physical functioning were analyzed at baseline and after 6 months and 12 months.
Exposures
Common Terminology Criteria for Adverse Events grade 3 or higher chemotherapy-related toxic effects.
Main Outcomes and Measures
The main outcome was a composite end point, defined as a decline in QOL and/or physical functioning or mortality at 6 months and 12 months after chemotherapy initiation. Associations between toxic effects and the composite end point were analyzed with multivariable logistic regression models.
Results
Of the 276 patients, the median age was 74 years (IQR, 72-77 years), 177 (64%) were male, 196 (71%) received chemotherapy with curative intent, and 157 (57%) had gastrointestinal cancers. Among the total patients, 145 (53%) had deficits in 2 or more of the 4 domains of the geriatric assessment and were classified as frail. Grade 3 or higher toxic effects were observed in 94 patients (65%) with frailty and 66 (50%) of those without frailty (P = .01). Decline in QOL and/or physical functioning or death was observed in 76% of patients with frailty and in 64% to 68% of those without frailty. Among patients with frailty, grade 3 or higher toxic effects were associated with the composite end point at 6 months (odds ratio [OR], 2.62; 95% CI, 1.14-6.05) but not at 12 months (OR, 1.09; 95% CI, 0.45-2.64) and were associated with mortality at 12 months (OR, 3.54; 95% CI, 1.50-8.33). Toxic effects were not associated with the composite end point in patients without frailty (6 months: OR, 0.76; 95% CI, 0.36-1.64; 12 months: OR, 1.06; 95% CI, 0.46-2.43).
Conclusions and Relevance
In this prospective cohort study of 276 patients aged 70 or older who were treated with chemotherapy, patients with frailty had more grade 3 or higher toxic effects than those without frailty, and the occurrence of toxic effects was associated with a decline in QOL and/or physical functioning or mortality after 1 year. Toxic effects were not associated with poor outcomes in patients without frailty. Pretreatment frailty screening and individualized treatment adaptions could prevent a treatment-related decline of remaining health.
Introduction
With the aging of the global population, the number of older patients with cancer will continue to rise in the coming years.1 Despite this demographic shift, current oncologic practices are still based on trials in which older patients are underrepresented,2 and relevant end points for older patients, such as quality of life (QOL) and functional independence,3,4 have often not been measured. As a result, data to guide anticancer treatment in older patients remain limited.
In daily practice, clinicians and patients weigh the expected benefit of chemotherapy against the risk of toxic effects. This is particularly true for older patients, who have an increased risk of developing toxic effects due to decreased bone marrow reserve, reduced renal and liver function, and multimorbidity,5,6,7 resulting in unplanned hospitalization and early treatment discontinuation. Predominantly older patients who are frail are at risk of these outcomes,8 since frailty, caused by a cumulative decline across multiple organ systems, may result in a decreased ability to tolerate chemotherapy.9
Chemotherapy-related toxic effects potentially may have a negative impact on QOL and physical functioning and may even threaten independence. Although studies have investigated the association of toxic effects with long-term QOL and physical functioning,10,11,12,13 none of these studies, to our knowledge, have specifically focused on the older population. Therefore, we conducted a cohort study to investigate the association between grade 3 or higher chemotherapy-related toxic effects and QOL and physical functioning at 6 months and 12 months in older patients with cancer.
Methods
Patients in this study were included in the prospective, ongoing Triage of Elderly Needing Treatment (TENT) cohort. The study was approved by the medical ethics committee of the Leiden University Medical Center and registered at the Dutch Trial Register (NL8107). Written informed consent was obtained from all patients. Extensive details of the TENT study were previously published.14,15 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.16
For this analysis, all patients aged 70 years or older with any malignant neoplasm who were scheduled to receive chemotherapy in 3 hospitals between December 2015 and December 2021 were selected. Patients who received chemotherapy with either curative or palliative intent within 90 days after inclusion and a baseline geriatric assessment (GA) were included. Patients who had already received 1 or more chemotherapy cycles before inclusion or were eventually treated in another hospital were not eligible. Concomitant radiotherapy, targeted therapy, immunotherapy, or surgery was allowed.
Before treatment initiation, participants underwent a geriatric frailty screening, consisting of the Geriatric 8 questionnaire17 and the Six-Item Cognitive Impairment Test.18 If one of the screening tools showed risk of frailty,14 participants underwent a comprehensive GA (CGA). If neither of the screening tools was positive, a GA was performed by telephone.
Functional status was assessed by the Katz Index of Independence in Activities of Daily Living (ADL)19 (scores range from 0 to 6, with higher scores indicating patient independence, with scores ≤2 indicating abnormal patient dependence). The Lawton Instrumental ADL (IADL)20 was used to assess independence (scores range from 0 to 5 for men and from 0 to 8 for women, with higher scores indicating high function and independence; the cutoff for abnormal dependence was ≤4 for men and ≤7 for women). Quality of life was assessed with the EuroQoL 5-Dimension 3-Level (EQ-5D-3L) questionnaire and visual analogue scale (EQ-VAS).21 We converted EQ-5D-3L scores into an index score,22 with a maximum score of 1 indicating the best health state. The Mini Nutritional Assessment23 was used to screen nutritional status (scores range from 0 to 14, with higher scores indicating normal nutritional status; the cutoff for risk of malnutrition was <12 points). The Patient Health Questionnaire-224 was used to screen for anxiety and depression (scores range from 0 to 6, with higher scores indicating likelihood of depressive disorder; the cutoff of ≥3 points indicates major depressive disorder is likely).24 Comorbidities were scored with the Charlson Comorbidity Index (CCI).25 Six and 12 months after treatment initiation, participants were contacted by telephone to obtain the ADL, IADL, EQ-5D-3L, and EQ-VAS questionnaires. Patients were followed up for 1 year or until death. Data on mortality were gathered from digital patient files or municipal registries.
Similar to a previous publication,15 we defined frailty according to the somatic, functional, psychological, and social domains. If 1 or more tests in a domain were scored as abnormal, the domain was considered abnormal. Participants who scored abnormally on 2 or more of 4 domains were classified as frail. Comorbidity (CCI ≥1), polypharmacy (≥5 medicines), and malnutrition (Mini Nutritional Assessment) made up the somatic domain. The functional domain included falls within the past 6 months, institutionalization, and functional dependency (ADL and IADL). The psychological domain included dementia, history of delirium, and cognitive impairments (Six-Item Cognitive Impairment Test). The social domain was considered abnormal when patients lived alone. To validate this definition, we also measured frailty with the 10-Item Frailty Index Based on a Comprehensive GA (FI-CGA-10),26 which has been validated in older patients with cancer27 (eMethods in Supplement 1).
Our primary goal was to assess whether grade 3 or higher toxic effects were associated with a decline in QOL and/or physical functioning. However, many participants died during the first year; thus, a decrease in QOL and physical functioning could no longer occur due to mortality. Arguably, mortality can be considered the ultimate form of loss of QOL and physical functioning. Therefore, we designed a composite end point in which an unfavorable outcome was defined as either a decline in QOL and/or physical functioning or mortality. A decline in QOL was assessed with the EQ-5D-3L or EQ-VAS. Scores were compared with baseline scores using the minimal clinically important difference (EQ-5D-3L ≥0.06 or EQ-VAS ≥7 points28). A decline in physical functioning was defined as a decline in ADL (≥1-point increase or new institutionalization) or IADL (≥1-point decrease).29 The occurrence of grades 3 to 5 toxic effects, scored with the Common Terminology Criteria for Adverse Events, version 530 up to 6 months after treatment initiation, was retrieved from the medical records. Secondary outcomes were dose reductions during treatment, early treatment withdrawal (inability to receive all planned cycles regardless of treatment duration), and unplanned hospitalizations within the first year (excluding scheduled admissions).
Statistical Analysis
In prespecified analyses, participants were stratified by frailty. We used descriptive statistics using Mann-Whitney tests and χ2 tests to compare characteristics of patients with frailty and without frailty. The correlation between the number of impaired geriatric domains (range, 0-4) and the FI-CGA-10 (robust, prefrail, or frail) was tested using a Pearson correlation. Treatment outcomes and composite end points were stratified for frailty status and the number of impaired frailty domains.
Associations between grade 3 or higher toxic effects and a composite end point (a decline in QOL and/or physical functioning or mortality) were analyzed with univariable and multivariable logistic regression models. We calculated odds ratios (ORs) and 95% CIs. In patients with frailty, we also studied the association between toxic effects and mortality alone at 12 months. Possible confounding factors were predefined by creating a directed acyclic graph31,32 (eFigure 1 in Supplement 1). Confounding variables included in the model were age, treatment intent, and hospital. An up-front dose reduction was identified as an ancestor of the exposure rather than as a confounder. We performed additional analyses in a full model in which we added surgery, radiotherapy, and polychemotherapy to the original model. To prevent loss of information, missing follow-up questionnaires of living patients (n = 16) were coded as decline. As a sensitivity analysis, we repeated the analyses with missing questionnaires coded as no decline. We performed another sensitivity analysis in which we stratified the end point for treatment intent to investigate the role of predefined, palliative intent on treatment outcomes in patients with frailty. All analyses were performed using SPSS, version 25 (IBM Corp), and P < .05 was considered statically significant.
Results
We included 276 older patients (eFigure 2 in Supplement 1); the median age was 74 years (IQR, 72-77 years); 99 patients (36%) were female, and 177 (64%) were male (Table 1). Of the total patients, 157 (57%) had gastrointestinal cancers, 196 (71%) received chemotherapy with curative intent, and 195 (71%) received polychemotherapy. Among all patients, 167 (61%) had a CCI of 1 or higher, and 145 patients (53%) had deficits in 2 or more of the 4 domains and were classified as frail. An impaired Geriatric 8 score was found in 68 patients (52%) without frailty and 113 patients (78%) with frailty. The number of impaired geriatric domains was strongly correlated with the FI-CGA-10 frailty index (Pearson correlation = 0.72).
Table 1. Baseline Characteristics.
| Variable | Participants, No. (%) | P valueb | ||
|---|---|---|---|---|
| Total (N = 276) | Without frailty (n = 131) | With frailty (n = 145)a | ||
| Sex | ||||
| Female | 99 (36) | 38 (29) | 61 (42) | .02 |
| Male | 177 (64) | 93 (71) | 86 (58) | |
| Age, median (IQR), y | 74 (72-77) | 74 (71-77) | 75 (72-78) | .28 |
| Tumor site | ||||
| Esophageal | 88 (32) | 48 (37) | 40 (28) | .09 |
| Colorectal | 43 (16) | 19 (15) | 24 (17) | |
| Gynecologic | 28 (10) | 12 (9) | 16 (11) | |
| Gastric cancer | 26 (9) | 17 (13) | 9 (6) | |
| Genitourinary | 24 (9) | 12 (9) | 12 (8) | |
| Lung | 22 (8) | 6 (5) | 16 (11) | |
| Otherc | 45 (16) | 17 (13) | 28 (19) | |
| Treatment intent | ||||
| Curative | 196 (71) | 104 (79) | 92 (63) | .004 |
| Palliative | 80 (29) | 27 (21) | 53 (37) | |
| Chemotherapy regimend | ||||
| Monotherapy | 81 (29) | 30 (23) | 51 (35) | .03 |
| Polytherapy | 195 (71) | 101 (77) | 94 (65) | |
| Starting dose | ||||
| Reduced up front | 53 (19) | 16 (12) | 37 (26) | .006 |
| Full dose | 183 (66) | 90 (69) | 9 (64) | |
| Unknown | 40 (15) | 25 (19) | 15 (10) | |
| No. of chemotherapy cycles, median (IQR) | 5 (3-6) | 5 (4-6) | 5 (3-6) | .50 |
| Surgery | 104 (38) | 61 (47) | 43 (30) | .004 |
| Concurrent radiotherapy | 145 (53) | 67 (51) | 78 (54) | .66 |
| Concurrent immunotherapy | 5 (2) | 1 (1) | 4 (3) | .22 |
| WHO statuse | ||||
| 0 | 83 (30) | 41 (31) | 42 (29) | .05 |
| 1 | 107 (39) | 47 (36) | 60 (41) | |
| ≥2 | 18 (7) | 4 (3) | 14 (10) | |
| Unknown | 68 (25) | 39 (30) | 29 (20) | |
| CCI comorbidityf | ||||
| 0 | 109 (40) | 68 (52) | 41 (28) | NA |
| 1 | 74 (27) | 28 (21) | 46 (32) | |
| 2 | 39 (14) | 13 (10) | 26 (18) | |
| ≥3 | 54 (20) | 22 (17) | 32 (22) | |
| Polypharmacyg | 165 (60) | 62 (47) | 103 (71) | NA |
| Home care | 22 (8) | 4 (3) | 18 (12) | NA |
| Delirium in past months | 7 (3) | 0 | 7 (5) | NA |
| Falls in last 6 mo | ||||
| No | 227 (82) | 128 (98) | 99 (68) | NA |
| Yes | 49 (18) | 3 (2) | 46 (32) | |
| Geriatric 8h | ||||
| Normal | 75 (27) | 50 (38) | 25 (17) | NA |
| Impaired | 181 (66) | 68 (52) | 113 (78) | |
| Unknown | 20 (7) | 13 (10) | 7 (5) | |
| 6CITh | ||||
| Normal | 239 (87) | 127 (97) | 112 (77) | NA |
| Impaired | 29 (11) | 1 (1) | 28 (19) | |
| Unknown | 8 (3) | 3 (2) | 5 (3) | |
| Mini Nutritional Assessmenth | ||||
| Well nourished | 103 (37) | 65 (50) | 38 (26) | NA |
| Risk of malnutrition | 169 (61) | 62 (47) | 107 (74) | |
| Unknown | 4 (1) | 4 (3) | 0 | |
| PHQ-2h | ||||
| Normal | 195 (71) | 86 (66) | 109 (75) | NA |
| Abnormal | 23 (8) | 6 (5) | 17 (12) | |
| Unknown | 58 (20) | 39 (30) | 19 (13) | |
| Activities of daily livingh | ||||
| Independent | 271 (99) | 130 (48) | 141 (52) | NA |
| Dependent | 3 (1) | 0 | 3 (1) | |
| Unknown | 2 (1) | 1 (1) | 1 (1) | |
| Instrumental activities of daily livingh | ||||
| Independent | 214 (78) | 126 (59) | 88 (41) | NA |
| Dependent | 56 (20) | 0 | 56 (39) | |
| Unknown | 6 (2) | 5 (4) | 1 (1) | |
Abbreviations: 6CIT, Six-Item Cognitive Impairment Test; CCI, Charlson Comorbidity Index; PHQ-2, Patient Health Questionnaire-2; WHO, World Health Organization.
Frailty was defined as having 2 or more impaired geriatric domains.
Not applicable (NA) indicates that all geriatric variables that were used to define patients with and without frailty were not tested for statistical differences between patients with and without frailty, since stratification for frailty was based on these variables.
Other tumor types included pancreatic cancer, breast cancer, liver cancer, anal cancer, lymphoma, myeloid leukemia, and head and neck cancer.
The most commonly prescribed chemotherapy regimens were carboplatin or paclitaxel (n = 99), a fluoropyrimidine combined with oxaliplatin (n = 48), fluoropyrimidine monotherapy (n = 36), other carboplatin-based chemotherapy (n = 26), cisplatin-based chemotherapy (n = 21), and docetaxel monotherapy (n = 18).
Indicates the patient’s general condition. Numbers range from 0 to 4, with higher numbers representing a poorer general condition.
Defined as CCI ≥1.
Defined as 5 or more medicines.
Functional status categories are described in the Methods section.
In total, 160 patients (58%) developed grades 3 or higher toxic effects, of whom 146 (91%) developed their first toxic effects within the first 2 months after chemotherapy initiation. Of patients with frailty, 94 (65%) developed grade 3 or higher toxic effects (Figure 1). Seventy patients (48%) had a dose reduction during treatment, 73 (50%) discontinued treatment early, and 87 (60%) were hospitalized in the first year, of whom 71 (82%) were hospitalized during chemotherapy treatment.
Figure 1. Incidence of Various Treatment Outcomes in All Patients by Frailty Status.
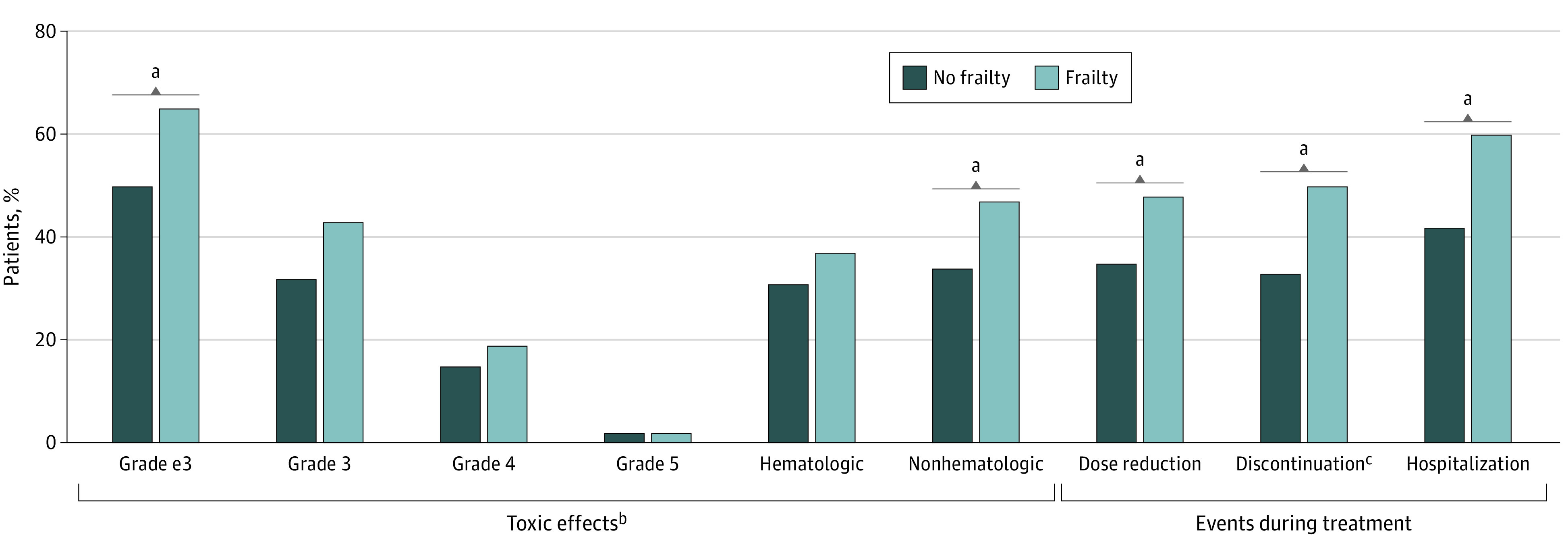
Frequencies of grades 3 to 5 toxic effects (P = .01), nonhematologic toxic effects (P = .03), dose reduction during treatment (P = .04), early treatment discontinuation (P = .009), and hospitalization (P = .003) were significantly different between patients with and without frailty, analyzed with a χ2 test.
aP < .05.
bThe worst grade of toxic effects was documented (grade e3). Of the patients who developed grades 3 to 5 toxic effects, 91% developed their first toxic effects within the first 2 months after chemotherapy initiation, and 9% developed their first toxic effects after 2 months.
cAmong patients with frailty, reasons for early treatment discontinuation were toxic effects (64%), tumor progression (29%), death to other cause (3%), clinical deterioration (3%), or other reasons (1%). Among patients without frailty, reasons for early treatment discontinuation were toxic effects (56%), tumor progression (28%), or clinical deterioration (16%).
Of patients without frailty, 66 (50%) developed grade 3 or higher chemotherapy-related toxic effects, 46 (35%) had a dose reduction during treatment, 43 (33%) discontinued treatment, and 55 (42%) were hospitalized in the first year, of whom 37 (67%) were hospitalized during chemotherapy treatment. Rates of grade 3 or higher toxic effects (65% vs 50%; P = .01), nonhematologic toxic effects (47% vs 34%; P = .03), dose reduction (48% vs 35%; P = .04), early discontinuation (50% vs 33%; P = .009), and hospitalization (60% vs 42%; P = .003) differed significantly between patients with frailty and those without (Figure 1).
Of patients with frailty, 30 (21%) reported a preserved QOL and physical functioning after 6 months and 110 (76%) had a poor outcome: 87 (60%) experienced a decline and 23 (16%) died (Figure 2A). After 12 months, 29 (20%) had a preserved QOL and physical functioning, while 110 (76%) had a poor outcome: 58 (40%) experienced a decline and 52 (36%) died. Of the patients without frailty, 43 (33%) had a preserved QOL and physical functioning after 6 months, while 84 (64%) had a poor outcome: 68 (52%) experienced a decline in QOL and/or physical functioning and 16 (12%) died (Figure 2B). After 12 months, 37 (28%) had a preserved QOL and physical functioning, while 89 (68%) had a poor outcome: 59 (45%) reported a decline in QOL and/or physical functioning, and 30 (23%) died. After stratifying results by the number of impaired frailty domains, the percentage of patients having a poor outcome at 6 months increased from 55% in patients without impaired domains to 74% in patients with 3 to 4 impaired domains (Figure 2C). Similarly, after 12 months, the percentage of patients with poor outcomes increased per number of impaired frailty domains (Figure 2D).
Figure 2. Composite End Points After 6 Months and 12 Months by Frailty Status and by the Absolute Number of Impaired Frailty Domains.
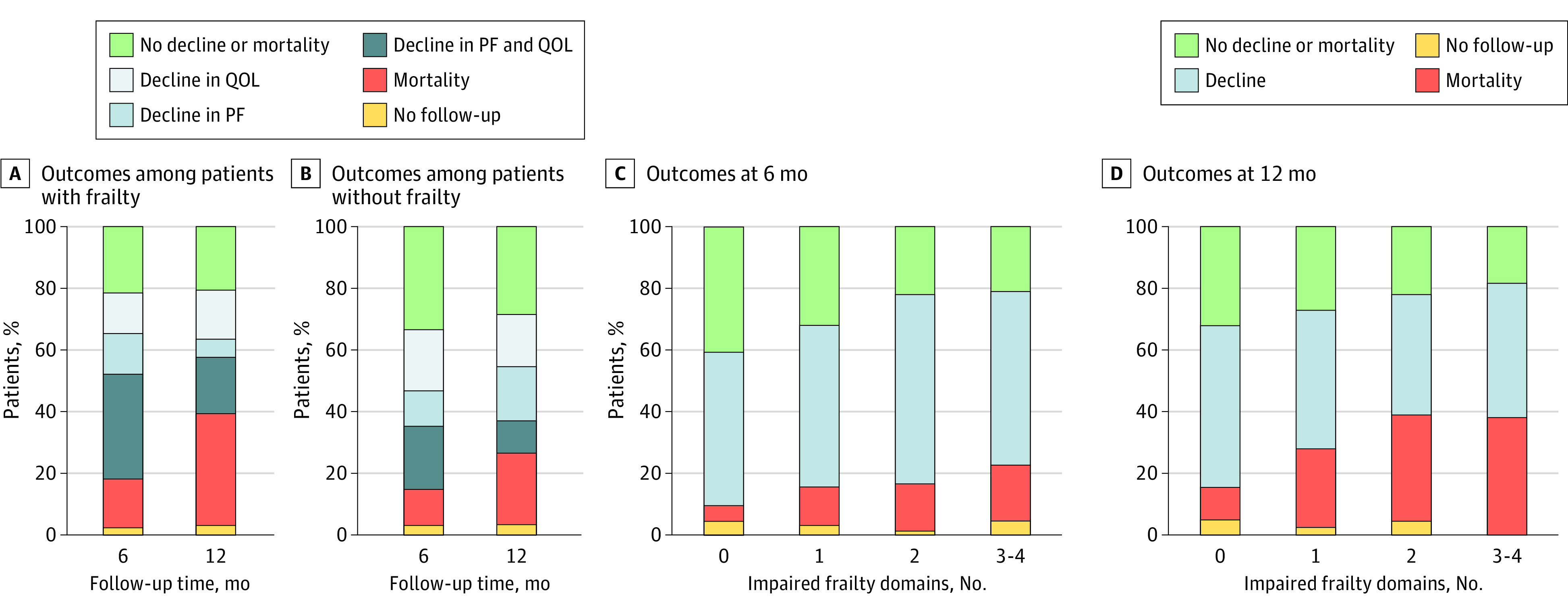
A, Among living patients with frailty at 6 months, 40% still received chemotherapy, and 60% either completed or discontinued treatment. Among living patients with frailty at 12 months, 17% still received chemotherapy, and 83% either completed or discontinued treatment. B, Among living patients without frailty at 6 months, 30% still received chemotherapy, and 70% either completed or discontinued chemotherapy. Among living patients without frailty at 12 months, 9% still received chemotherapy, and 91% completed or discontinued treatment. C and D, Frailty domains include somatic, functional, psychological, and social. Decline represents either a decline in quality of life (QOL) or a decline in physical functioning (PF). No follow-up consists of living patients without a completed follow-up questionnaire at the specified time point.
In all patients, composite end points at 6 months were comparable for individuals with and without toxic effects (Figure 3). Grade 3 or higher toxic effects were not associated with the composite end point at 6 months (OR, 1.47; 95% CI, 0.85-2.55) (Table 2). After 12 months, more patients with toxic effects died, but toxic effects were not associated with the composite end point (OR, 1.08; 95% CI, 0.60-1.94).
Figure 3. Composite End Points of Patients After 6 Months and 12 Months by Grade 3 or Higher Chemotherapy-Related Toxic Effects Status.
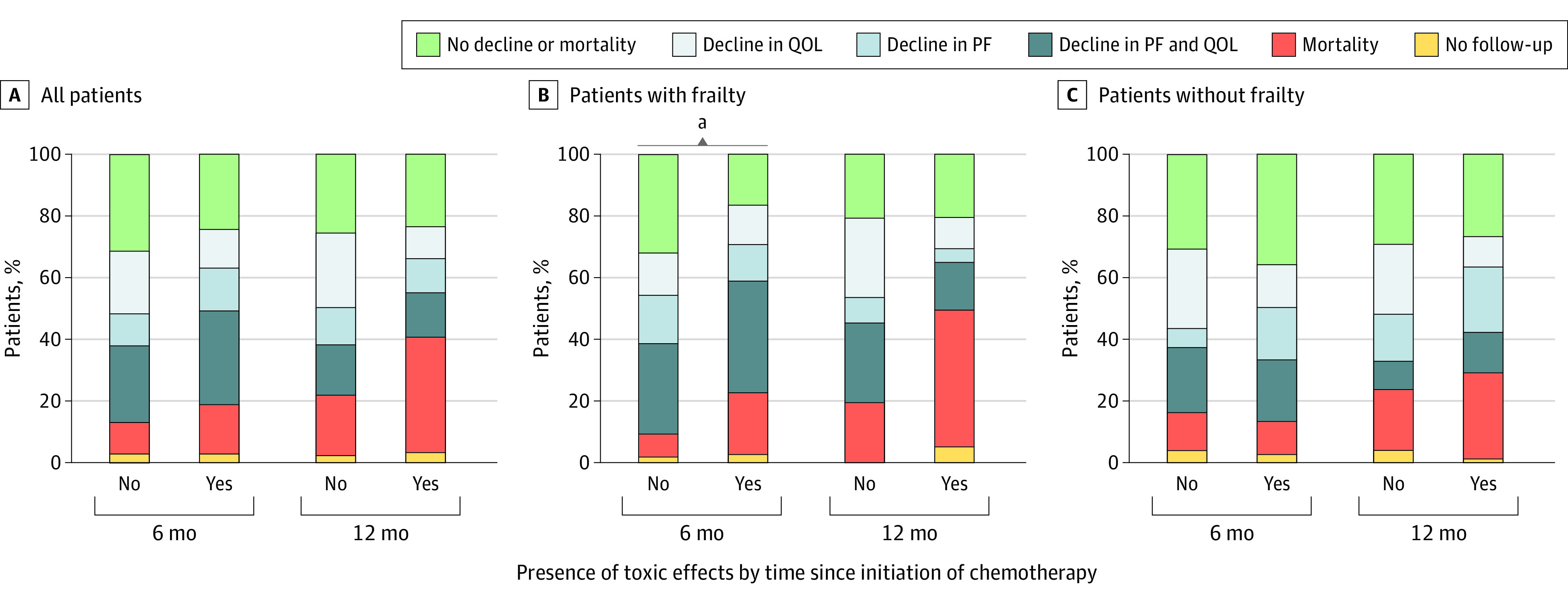
No follow-up consists of living patients without a completed follow-up questionnaire at the specified time point. PF indicates physical functioning; QOL, quality of life.
aP < .05, derived from the multivariable logistic regression models shown in Table 2.
Table 2. Association Between Grade 3 or Higher Toxic Effects and Unfavorable Outcomes at 6 Months and 12 Monthsa.
| Grade ≥3 toxic effects by time since initiation of chemotherapy | Univariable logistic regression | Multivariable logistic regressionb | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| All patients | ||||
| 6 mo | 1.42 (0.83-2.42) | .20 | 1.47 (0.85-2.55) | .17 |
| 12 mo | 1.08 (0.62-1.90) | .78 | 1.08 (0.60-1.94) | .80 |
| Patients with frailty | ||||
| 6 mo | 2.41 (1.07-5.41) | .03 | 2.62 (1.14-6.05) | .02 |
| 12 mo | 1.00 (0.42-2.37) | 1.00 | 1.09 (0.45-2.64) | .86 |
| Patients without frailty | ||||
| 6 mo | 0.79 (0.38-1.65) | .54 | 0.76 (0.36-1.64) | .49 |
| 12 mo | 1.05 (0.48-2.27) | .91 | 1.06 (0.46-2.43) | .90 |
Unfavorable outcomes include decline in quality of life and/or physical functioning or mortality.
Multivariable logistic regression models were adjusted for age, hospital type, and treatment intent. Toxic effects, treatment intent, and hospital were added to the model as binary variables and age was added as a continuous variable.
After stratification for frailty, the percentage of patients with frailty with preserved QOL and physical functioning after 6 months was lower in those with toxic effects (16%) compared with those without toxic effects (31%). Moreover, 19 patients (20%) among those with frailty and toxic effects died, whereas only 4 (8%) of those without toxic effects died. The multivariable logistic regression model showed that toxic effects were associated with an unfavorable outcome (OR, 2.62; 95% CI, 1.14-6.05) (Table 2 and eTable 1 in Supplement 1). Composite end points after 12 months also differed between those with and without toxic effects, as 42 (45%) of patients with toxic effects died compared with 10 (20%) without toxic effects. There was no association between toxic effects and an unfavorable outcome after 12 months in the multivariable analysis (OR, 1.09; 95% CI, 0.45-2.64). As these results might be biased by the high mortality rate of patients with toxic effects, we also analyzed the association between toxic effects and mortality and found a significant association between toxic effects and mortality after 12 months (OR 3.54; 95% CI, 1.50-8.33) (eTable 2 in Supplement 1).
In patients without frailty, composite end points after 6 months were similar for those with and without grade 3 or higher toxic effects. In a multivariable logistic regression, toxic effects were not associated with an unfavorable outcome after 6 months (OR, 0.76; 95% CI, 0.36-1.64) (Table 2 and eTable 3 in Supplement 1). After 12 months, composite end points were also similar for those who had toxic effects and those who did not. Grade 3 or higher toxic effects were not associated with an unfavorable outcome in a multivariable analysis (OR, 1.06; 95% CI, 0.46-2.43).
Using sensitivity analyses, we first investigated the association of predefined treatment intent with outcomes among patients with frailty (eFigure 3 in Supplement 1). High rates of a decline in QOL and/or physical functioning or mortality were seen both in those treated with palliative intent and in those treated with curative intent, illustrating that outcomes of patients with frailty were poor irrespective of treatment intent. Second, to assess whether the results of the logistic regression models were different if missing questionnaires were defined as no decline instead of decline, we performed a sensitivity analysis and found that results did not differ (eTable 4 in Supplement 1). Third, we added various treatment characteristics to the original model and found that, in the full model, toxic effects remained associated with an unfavorable outcome after 6 months in patients with frailty (eTable 5 in Supplement 1).
Discussion
This study is the first, to our knowledge, to investigate the association between grade 3 or higher chemotherapy-related toxic effects and a decline in QOL and/or physical functioning or mortality in older patients with cancer. Irrespective of frailty status and treatment intent, most patients had either a decline in QOL and/or physical functioning or died after 6 months and 12 months. Among patients with frailty, 65% experienced grade 3 or higher toxic effects, and grade 3 or higher toxic effects were associated with a decline in QOL and/or physical functioning or mortality. Grade 3 or higher toxic effects were not associated with unfavorable outcomes in older patients without frailty.
The percentages of patients with a decline in QOL and physical functioning in our study were slightly higher than in previous studies. Kenis et al33 investigated physical functioning 2 to 3 months after chemotherapy initiation in a prospective cohort (N = 439) and showed that functional decline occurred in one-third of older patients with various tumor types. Hoppe and colleagues34 found that 17% of patients reported a functional decline in a study with 364 older patients receiving first-line chemotherapy for various tumor types. Of our study’s participants, 157 (57%) had upper gastrointestinal cancers, which are generally tumor types with a poor prognosis. Additionally, 145 (53%) of our study’s participants lived with frailty and therefore may have had an increased risk of decreased QOL, irrespective of treatment.35 These factors may explain the higher percentages of a decline in QOL and physical functioning in our cohort.
Of patients with frailty, 65% developed grade 3 or higher toxic effects, 50% discontinued treatment early, and 60% were hospitalized, which aligns with previous literature.36,37,38 Not only did patients with frailty have an increased risk of toxic effects, but the occurrence of toxic effects was also associated with poor long-term outcomes. To date, few studies have investigated the association between toxic effects and long-term outcomes in older patients receiving chemotherapy. A prospective pilot study in older patients (N = 37) also found an association between grade 3 or higher chemotherapy toxic effects and physical functioning or mental health at the end of treatment.39 Preliminary secondary analyses from the Geriatric Assessment Intervention for Reducing Toxicity in Older Patients With Advanced Cancer trial demonstrated that older patients with patient-reported grade 3 or higher toxic effects were more likely to experience functional decline within 6 months as well.40 The association between toxic effects and long-term outcomes may be because toxic effects and hospitalizations may lead to accelerated functional decline. Additionally, the high rates of toxic effects in patients with frailty may result in early treatment discontinuation, possibly leading to disease progression and poor outcomes associated with the cancer itself. Last, those at risk of developing toxic effects may also be the patients with the worst baseline characteristics who are vulnerable to poor outcomes, irrespective of treatment.35 Decline after 12 months may be influenced by multiple factors, with disease progression possibly contributing more than toxic effects do.
In our study, the high rates of grade 3 or higher toxic effects, unplanned hospitalizations, and functional decline among patients with frailty, in both those treated with curative and palliative chemotherapy, imply that treatment goals are not met in the vast majority of this population. This raises the question of whether to use chemotherapy to treat older patients with frailty. However, patients with frailty who do not receive anticancer treatment may also decline. Moreover, chemotherapy may improve symptoms and thus may improve QOL. Personalized treatment with adapted treatment and intensified support trajectories should, however, be required, along with identification of patients at high risk of poor outcomes in whom chemotherapy may be contraindicated. Since an oncologist’s judgment in predicting toxic effects may be limited,41,42,43 pretreatment frailty screening, followed by a CGA, can be used to identify those at risk for poor treatment outcomes. A CGA is a multidimensional process to identify medical, social, and functional needs44 and is associated with chemotherapy-related toxic effects, functional decline, and mortality.34,37,45 By performing a pretreatment CGA, physicians may facilitate nononcologic interventions or individualize treatment plans, thereby likely reducing the risk of toxic effects and possibly improving QOL.46 Previous trials showed that a GA-based intervention could reduce chemotherapy-related toxic effects and early treatment discontinuation compared with usual care.47,48
Another possibility to improve treatment tolerability in a selected group of patients is to adapt treatment plans, such as performing up-front dose reduction or prescribing less-toxic chemotherapy regimens.49 Previous trials in older patients demonstrated that up-front dose-reduced chemotherapy led to fewer toxic effects and improved QOL, without compromising survival.50,51,52 Individualizing treatment plans based on frailty status might therefore be a promising solution to improve outcomes. Unfortunately, some patients with frailty do not benefit from chemotherapy. Although omitting treatment in these patients may lead to premature death, best supportive care can still be the preferred option. Taking into account aging populations, rising health care costs, and an anticipated shortage of health care workers worldwide,53 it will be important to identify those who may not benefit from chemotherapy in the coming years.
Strengths and Limitations
TENT is a unique, multicenter cohort that includes a large number of older patients with and without frailty. Recruiting older patients with frailty for clinical studies comes with many challenges, such as difficulties with traveling to the research site, other medical conditions, or lack of social support.54 By conducting follow-up questionnaires by telephone rather than in person, we kept the participation burden minimal, which might have resulted in a large number of patients with frailty willing to participate. Consequently, the outcomes of this study are relevant for older patients seen in daily practice and can be extrapolated to the general older population. Additional strengths are the high rates of completed follow-up, which may reduce bias, and relatively large sample size.
This study also has limitations. Although we made every effort to identify possible confounders and evaluated the robustness of our results in different models, results could still have been subject to unidentified or unmeasured confounders. Additionally, the OR tends to overestimate the risk estimate of having the outcome in the exposed group when the incidence of the outcome is high, so it should be interpreted with caution. Furthermore, even among patients without frailty, 68 (52%) had an impaired Geriatric 8 score, suggesting that vital older patients may be underrepresented in our study. It should also be noted that we measured only grades 3 to 5 toxic effects, while previous studies have shown that low-grade or patient-reported toxic effects may also influence treatment outcomes in older patients.55,56 In addition, while the findings showed that 91% of patients with toxic effects developed their first toxic effects within 2 months, the exact date of the registered toxic effect was unknown. Last, the modest sample size of the subgroup analyses may have resulted in wide CIs. While our study’s population was heterogeneous with various tumor types, it reflected a clinical setting’s practice and demonstrated that generalizability of positive outcomes observed in oncology trials is limited when applied to a more diverse, general patient population.57 To further support these findings, future studies should focus on specific tumor or treatment types.
Conclusions
In this cohort study, frailty was not only associated with grade 3 or higher chemotherapy-related toxic effects, but the occurrence of toxic effects among patients with frailty was also associated with a decline in QOL and/or physical functioning or mortality at 6 and 12 months. Grade 3 or higher chemotherapy-related toxic effects were not associated with poor outcomes in patients without frailty. Geriatric assessment–driven interventions and individualized treatment adaptions could prevent a treatment-related decline of remaining health.
eMethods.
eFigure 1. Directed Acyclic Graph (DAG)
eFigure 2. Flow Diagram of Included Patients
eFigure 3. Composite End Points After 6 and 12 Months in Patient With Frailty, Stratified by Treatment Intent
eTable 1. Association of Grade ≥3 Toxicity and Unfavorable Outcomes in Frail, Older Patients, Including Point Estimates of Confounding Factors
eTable 2. Association of Grade ≥3 Toxicity and Mortality After 12 Months in Frail, Older Patients
eTable 3. Association of Grade ≥3 Toxicity and Unfavorable Outcomes in Nonfrail, Older Patients, Including Point Estimates of Confounding Factors
eTable 4. Sensitivity Analysis: Association of Grade ≥3 Toxicity and Unfavorable Outcomes With Different Coding of Missing Questionnaires
eTable 5. Sensitivity Analysis: Association of Grade ≥3 Toxicity and Unfavorable Outcomes in the ‘Full Model’ With Both Confounders and Treatment Characteristics
eReferences
Data Sharing Statement
References
- 1.The Netherlands Comprehensive Cancer Organisation; the Netherlands Cancer Registry. Incidence by year, count. All cancer types. 2022, 2021. Accessed July 2021. https://nkr-cijfers.iknl.nl/viewer/incidentie-per-jaar?language=en&viewerId=eb5705e2-0165-48fa-abe4-39d0af719c44
- 2.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383-1389. doi: 10.1200/JCO.2003.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061-1066. doi: 10.1056/NEJMsa012528 [DOI] [PubMed] [Google Scholar]
- 4.Soto-Perez-de-Celis E, Li D, Sun C-L, et al. Patient-defined goals and preferences among older adults with cancer starting chemotherapy (CT). Abstract presented at: 2018 American Society of Clinical Oncology Annual Meeting I; May 20, 2018; Chicago, IL. Abstract 10009. [Google Scholar]
- 5.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi: 10.1200/JCO.2011.34.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feliu J, Espinosa E, Basterretxea L, et al. Prediction of unplanned hospitalizations in older patients treated with chemotherapy. Cancers (Basel). 2021;13(6):1437. doi: 10.3390/cancers13061437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishijima TF, Deal AM, Williams GR, Sanoff HK, Nyrop KA, Muss HB. Chemotherapy toxicity risk score for treatment decisions in older adults with advanced solid tumors. Oncologist. 2018;23(5):573-579. doi: 10.1634/theoncologist.2017-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce J, Swinson D, Cairns D, et al. ; GO2 Trial Investigators . Frailty and treatment outcome in advanced gastro-oesophageal cancer: an exploratory analysis of the GO2 trial. J Geriatr Oncol. 2022;13(3):287-293. doi: 10.1016/j.jgo.2021.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristensen A, Solheim TS, Fløtten Ø, Grønberg BH. Associations between hematologic toxicity and health-related quality of life during first-line chemotherapy in advanced non-small-cell lung cancer: a pooled analysis of two randomized trials. Acta Oncol. 2018;57(11):1574-1579. doi: 10.1080/0284186X.2018.1492151 [DOI] [PubMed] [Google Scholar]
- 11.Gomez D, Calderón C, Carmona-Bayonas A, et al. Impact of adjuvant therapy toxicity on quality of life and emotional symptoms in patients with colon cancer: a latent class analysis. Clin Transl Oncol. 2021;23(3):657-662. doi: 10.1007/s12094-020-02454-z [DOI] [PubMed] [Google Scholar]
- 12.Schuurhuizen CSEW, Braamse AMJ, Konings IRHM, et al. Does severe toxicity affect global quality of life in patients with metastatic colorectal cancer during palliative systemic treatment? a systematic review. Ann Oncol. 2017;28(3):478-486. doi: 10.1093/annonc/mdw617 [DOI] [PubMed] [Google Scholar]
- 13.Schuurhuizen CSEW, Marino P, Braamse AMJ, et al. Impact of patient- and clinician-reported cumulative toxicity on quality of life in patients with metastatic castration-naïve prostate cancer. J Natl Compr Canc Netw. 2018;16(12):1481-1488. doi: 10.6004/jnccn.2018.7069 [DOI] [PubMed] [Google Scholar]
- 14.van Holstein Y, van Deudekom FJ, Trompet S, et al. Design and rationale of a routine clinical care pathway and prospective cohort study in older patients needing intensive treatment. BMC Geriatr. 2021;21(1):29. doi: 10.1186/s12877-020-01975-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Holstein Y, Trompet S, van Deudekom FJ, et al. Geriatric assessment and treatment outcomes in a Dutch cohort of older patients with potentially curable esophageal cancer. Acta Oncol. 2022;61(4):459-467. doi: 10.1080/0284186X.2022.2036366 [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166-2172. doi: 10.1093/annonc/mdr587 [DOI] [PubMed] [Google Scholar]
- 18.Tuijl JP, Scholte EM, de Craen AJ, van der Mast RC. Screening for cognitive impairment in older general hospital patients: comparison of the Six-Item Cognitive Impairment Test with the Mini-Mental State Examination. Int J Geriatr Psychiatry. 2012;27(7):755-762. doi: 10.1002/gps.2776 [DOI] [PubMed] [Google Scholar]
- 19.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727. doi: 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- 20.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 21.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 22.Lamers LM, McDonnell J, Stalmeier PF, Krabbe PF, Busschbach JJ. The Dutch tariff: results and arguments for an effective design for national EQ-5D valuation studies. Health Econ. 2006;15(10):1121-1132. doi: 10.1002/hec.1124 [DOI] [PubMed] [Google Scholar]
- 23.Vellas B, Villars H, Abellan G, et al. Overview of the MNA—its history and challenges. J Nutr Health Aging. 2006;10(6):456-463. [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 26.Jones D, Song X, Mitnitski A, Rockwood K. Evaluation of a frailty index based on a comprehensive geriatric assessment in a population based study of elderly Canadians. Aging Clin Exp Res. 2005;17(6):465-471. doi: 10.1007/BF03327413 [DOI] [PubMed] [Google Scholar]
- 27.Nishijima TF, Shimokawa M, Esaki T, Morita M, Toh Y, Muss HBAA. 10-Item Frailty Index Based on a Comprehensive Geriatric Assessment (FI-CGA-10) in older adults with cancer: development and construct validation. Oncologist. 2021;26(10):e1751-e1760. doi: 10.1002/onco.13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suijker JJ, van Rijn M, Ter Riet G, Moll van Charante EP, de Rooij SE, Buurman BM. Minimal important change and minimal detectable change in activities of daily living in community-living older people. J Nutr Health Aging. 2017;21(2):165-172. doi: 10.1007/s12603-016-0797-8 [DOI] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. November 27, 2017. Accessed July 26, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 31.Suzuki E, Shinozaki T, Yamamoto E. Causal diagrams: pitfalls and tips. J Epidemiol. 2020;30(4):153-162. doi: 10.2188/jea.JE20190192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, Hernán MA. Results on differential and dependent measurement error of the exposure and the outcome using signed directed acyclic graphs. Am J Epidemiol. 2012;175(12):1303-1310. doi: 10.1093/aje/kwr458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenis C, Decoster L, Bastin J, et al. Functional decline in older patients with cancer receiving chemotherapy: a multicenter prospective study. J Geriatr Oncol. 2017;8(3):196-205. doi: 10.1016/j.jgo.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 34.Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol. 2013;31(31):3877-3882. doi: 10.1200/JCO.2012.47.7430 [DOI] [PubMed] [Google Scholar]
- 35.Kojima G, Iliffe S, Morris RW, et al. Frailty predicts trajectories of quality of life over time among British community-dwelling older people. Qual Life Res. 2016;25(7):1743-1750. doi: 10.1007/s11136-015-1213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciani A, Biganzoli L, Colloca G, et al. Estimating the risk of chemotherapy toxicity in older patients with cancer: the role of the Vulnerable Elders Survey-13 (VES-13). J Geriatr Oncol. 2015;6(4):272-279. doi: 10.1016/j.jgo.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 37.Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist. 2012;17(11):1439-1449. doi: 10.1634/theoncologist.2012-0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen HJ, Smith D, Sun CL, et al. ; Cancer and Aging Research Group . Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865-3872. doi: 10.1002/cncr.30269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, Cantor A, Meyer J, et al. Can older cancer patients tolerate chemotherapy? a prospective pilot study. Cancer. 2003;97(4):1107-1114. doi: 10.1002/cncr.11110 [DOI] [PubMed] [Google Scholar]
- 40.Culakova E, Mohile SG, Mohamed MR, et al. Impact of adverse events on independence in daily functioning of older adults with advanced cancer treated with systemic therapy. Abstract presented at: 2022 American Society of Clinical Oncology Quality Care Symposium; October 1, 2022; Chicago, IL. Abstract 269. [Google Scholar]
- 41.Alibhai SM, Aziz S, Manokumar T, Timilshina N, Breunis H. A comparison of the CARG tool, the VES-13, and oncologist judgment in predicting grade 3+ toxicities in men undergoing chemotherapy for metastatic prostate cancer. J Geriatr Oncol. 2017;8(1):31-36. doi: 10.1016/j.jgo.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 42.Moth EB, Kiely BE, Stefanic N, et al. Predicting chemotherapy toxicity in older adults: comparing the predictive value of the CARG Toxicity Score with oncologists’ estimates of toxicity based on clinical judgement. J Geriatr Oncol. 2019;10(2):202-209. doi: 10.1016/j.jgo.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 43.van Walree IC, Scheepers ERM, van den Bos F, van Huis-Tanja LH, Emmelot-Vonk MH, Hamaker ME. Clinical judgment versus geriatric assessment for frailty in older patients with cancer. J Geriatr Oncol. 2020;11(7):1138-1144. doi: 10.1016/j.jgo.2020.05.011 [DOI] [PubMed] [Google Scholar]
- 44.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi: 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16(11):1795-1800. doi: 10.1093/annonc/mdi368 [DOI] [PubMed] [Google Scholar]
- 46.Dale W, Klepin HD, Williams GR, et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO Guideline update. J Clin Oncol. 2023;41(26):4293-4312. doi: 10.1200/JCO.23.00933 [DOI] [PubMed] [Google Scholar]
- 47.Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904. doi: 10.1016/S0140-6736(21)01789-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Sun CL, Kim H, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158. doi: 10.1001/jamaoncol.2021.4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams GR, Outlaw D, Harvey RD, Lichtman SM, Zamboni WC, Giri S. Chemotherapy dosing in older adults with cancer: one size does NOT fit all. J Geriatr Oncol. 2023;14(1):101363. doi: 10.1016/j.jgo.2022.08.012 [DOI] [PubMed] [Google Scholar]
- 50.Hall PS, Swinson D, Cairns DA, et al. ; GO2 Trial Investigators . Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized trial. JAMA Oncol. 2021;7(6):869-877. doi: 10.1001/jamaoncol.2021.0848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winther SB, Liposits G, Skuladottir H, et al. Reduced-dose combination chemotherapy (S-1 plus oxaliplatin) versus full-dose monotherapy (S-1) in older vulnerable patients with metastatic colorectal cancer (NORDIC9): a randomised, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2019;4(5):376-388. doi: 10.1016/S2468-1253(19)30041-X [DOI] [PubMed] [Google Scholar]
- 52.Liposits G, Eshøj HR, Möller S, et al. Quality of life in vulnerable older patients with metastatic colorectal cancer receiving palliative chemotherapy—the randomized NORDIC9-study. Cancers (Basel). 2021;13(11):2604. doi: 10.3390/cancers13112604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boniol M, Kunjumen T, Nair TS, Siyam A, Campbell J, Diallo K. The global health workforce stock and distribution in 2020 and 2030: a threat to equity and ‘universal’ health coverage? BMJ Glob Health. 2022;7(6):e009316. doi: 10.1136/bmjgh-2022-009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassidy EL, Baird E, Sheikh JI. Recruitment and retention of elderly patients in clinical trials: issues and strategies. Am J Geriatr Psychiatry. 2001;9(2):136-140. doi: 10.1097/00019442-200105000-00005 [DOI] [PubMed] [Google Scholar]
- 55.Kalsi T, Babic-Illman G, Fields P, et al. The impact of low-grade toxicity in older people with cancer undergoing chemotherapy. Br J Cancer. 2014;111(12):2224-2228. doi: 10.1038/bjc.2014.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quinten C, Maringwa J, Gotay CC, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst. 2011;103(24):1851-1858. doi: 10.1093/jnci/djr485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baltussen JC, de Glas NA, Liefers GJ, et al. Time trends in treatment patterns and survival of older patients with synchronous metastatic colorectal cancer in the Netherlands: a population-based study. Int J Cancer. 2023;152(10):2043-2051. doi: 10.1002/ijc.34422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Directed Acyclic Graph (DAG)
eFigure 2. Flow Diagram of Included Patients
eFigure 3. Composite End Points After 6 and 12 Months in Patient With Frailty, Stratified by Treatment Intent
eTable 1. Association of Grade ≥3 Toxicity and Unfavorable Outcomes in Frail, Older Patients, Including Point Estimates of Confounding Factors
eTable 2. Association of Grade ≥3 Toxicity and Mortality After 12 Months in Frail, Older Patients
eTable 3. Association of Grade ≥3 Toxicity and Unfavorable Outcomes in Nonfrail, Older Patients, Including Point Estimates of Confounding Factors
eTable 4. Sensitivity Analysis: Association of Grade ≥3 Toxicity and Unfavorable Outcomes With Different Coding of Missing Questionnaires
eTable 5. Sensitivity Analysis: Association of Grade ≥3 Toxicity and Unfavorable Outcomes in the ‘Full Model’ With Both Confounders and Treatment Characteristics
eReferences
Data Sharing Statement


