Abstract
Background
Studies have shown a relationship between oestrogen and Alzheimer's disease. However, there is neither clear nor strong evidence on the use of oestrogen-only therapy in reducing the risk of Alzheimer's disease.
Aims
To assess the effects of oestrogen-only therapy on reducing the risk of Alzheimer's disease.
Method
Inclusion criteria was determined with the PICO framework. Outcome was cognitive function measured by neuropsychological tests and strict protocols. Exclusion criteria included non-Alzheimer's dementia, progesterone-only therapy and pre-menopausal women. Searches were conducted in nine electronic healthcare databases, last searched in July 2022. Quality assessments conducted on randomised controlled trials (RCTs) were performed with the GRADE assessment, and cohort studies and case–control studies were assessed with the Newcastle–Ottawa Scale. Extracted data were used to analyse participants, interventions and outcomes.
Results
Twenty-four studies satisfied the search criteria (four RCTs, nine cohort studies, 11 case–control studies). Fifteen studies showed positive associations for oestrogen-only therapy reducing the risk of Alzheimer's disease, and the remaining nine found no evidence of association.
Conclusions
Fifteen studies showed that oestrogen-only therapy effectively reduced the risk of Alzheimer's disease, whereas nine showed no correlation. Studies also investigated oestrogen-related variables such as length of oestrogen exposure, being an apolipoprotein E ε4 carrier and concomitant use of non-steroidal anti-inflammatory drugs, and their role in neuroprotection. This review was limited by the limited ranges of duration of oestrogen treatment and type of oestrogen-only therapy used. In conclusion, oestrogen-only therapy has potential for use in preventing Alzheimer's disease, although current evidence is inconclusive and requires further study.
Keywords: Dementias/neurodegenerative diseases, neuroendocrinology, cognitive neuroscience, out-patient treatment, clinical outcomes measures
Alzheimer's disease is a result of beta-amyloid deposition and neurofibrillary tangles, which cause loss of neurons and their synapses, and, ultimately, atrophy of the brain. Studies have shown a relationship between oestrogen and Alzheimer's disease.1 However, there is insufficient evidence to confidently prompt the use of oestrogen to reduce the risk of Alzheimer's disease.
Oestrogen, a reproductive and sex hormone, is produced by ovaries in women, as well as the adrenal glands and fat cells in small amounts. It plays a role in the formation of secondary sex characteristics during puberty, as well as for ovulation and thickening of the uterine wall in the menstrual cycle. Oestrogen also regulates cholesterol and glucose levels, synaptic plasticity in the brain 2 and other non-reproductive functions. In the menopause, ovaries produce less oestrogen, hence the use of hormone replacement therapy (HRT) to resupply oestrogen levels in post-menopausal women.
There is some evidence suggesting that oestrogen replacement therapy enhances spatial working memory3 and cognitive function4 in aged ovariectomised rhesus monkeys. A study using in vitro binding concluded that regulation of apolipoprotein E (APOE) induced by oestrogen increases the risk of Alzheimer's disease in women with an ε4 allele.1 Another study utilising cultured basal forebrain neurons demonstrated that select oestrogens play a neuroprotective role.5 However, a study using PDAPP mice showed that oestrogen does not affect the deposition of beta-amyloid in the brain, thus having no effect on amyloid deposition in Alzheimer's disease.6
Similarly, a randomised controlled trial (RCT) conducted in the USA highlighted the use of oestrogen replacement therapy in reducing beta-amyloid deposition.7 Additionally, findings from a cohort study suggest the duration of HRT use could be associated with the likelihood of Alzheimer's disease incidence in women.8 A case–control study revealed that HRT may prevent and delay the onset of Alzheimer's disease; moreover, findings showed that HRT might influence Alzheimer's disease risk when used during a critical window in early post-menopause.9
To date, it is unclear to what degree the association between oestrogen-only therapy has on the development of dementia, specifically Alzheimer's disease. Consequently, this systematic review is conducted to evaluate whether oestrogen replacement therapy has any significance in reducing the risk of Alzheimer's disease. In this paper, reducing risk is defined as including both prevention and delaying the onset of Alzheimer's disease.
Objective
This review aims to assess the effects of oestrogen replacement therapy on reducing the risk of Alzheimer's disease, and provide guidance on the future management of Alzheimer's disease with oestrogen replacement therapy. The hypothesis is that oestrogen replacement therapy is likely to reduce the risk of Alzheimer's disease in post-menopausal women.
Method
Details of the protocol for this systematic review were registered on PROSPERO (identifier CRD42022355335) and can be accessed at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42022355335. The following are not publicly available: template data collection forms, data extracted from included studies, data used for all analyses and analytic code.
Criteria for considering studies for this review
All inclusion criteria were decided with the PICO framework, which focuses on population, intervention, comparison and outcomes to determine the different clinical components of this review.
Types of studies
A mix of double-blinded placebo RCTs, cohort studies and case–control studies were reviewed. All double-blinded placebo RCTs in which oestrogen replacement therapy or HRT was administered to women for at least 2 weeks, and cognitive function was measured, were eligible for inclusion. All cohort studies in which oestrogen replacement therapy or HRT was recorded to have been given to women, and cognitive function was measured or diagnosis of Alzheimer's disease was made, were eligible for inclusion. All case–control studies in which oestrogen replacement therapy or HRT was recorded to be administered to women, and cognitive function was measured or diagnosis of Alzheimer's disease was made, were eligible for inclusion.
Types of participants
Participants in RCTs were required to be women of any age, be peri- or post-menopausal, have an intact uterus or have had a hysterectomy, and not have been diagnosed with Alzheimer's disease. Participants in cohort studies were required to be peri- or post-menopausal women (naturally or surgically induced), with an intact uterus or having undergone a hysterectomy, of any age, with suspected or diagnosed Alzheimer's disease. Participants in case–control studies were peri- or post-menopausal women with an intact uterus or having undergone a hysterectomy, of any age, with suspected or diagnosed Alzheimer's disease.
Types of interventions
Interventions containing oestrogens alone (oestrogen replacement therapy) or combined with a progestogen (HRT) at any dose, any dosing schedule and any mode of administration (oral, subdermal, transdermal or intravenous), were eligible for inclusion.
Types of outcome measures
The primary outcome of interest is cognitive function, as measured either by validated neuropsychological tests (some of the tests have multiple functions and may span other categories to the one in which they have been placed) or by strict protocols.
Tests used for overall global cognitive function were the Modified Mini-Mental State Examination (3MSE), Mini-Mental State Examination (MMSE), Cambridge Mental Disorders of the Elderly Examination (CAMDEX), Clinical Dementia Rating (CDR) Scale, Bi-factor Model for the Cognitive Baseline Data, Global Deterioration Scale and Alzheimer's Disease Assessment Scale (ADAS-Cog).
Cognitive impairment (defined and assessed by strict protocols) was defined according to the DSM-III, DSM-III-R and DSM-IV; the National Institute of Neurological and Communicative Disease and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria and the ICD-10.
Specific tests used for individual cognitive domains were categorised as verbal memory and language tests (measured with the California Verbal Learning Test (CVLT) and Wechsler Memory Scale (WMS)), visuospatial tests (measured with the Benton Visual Retention Test and the WMS) and executive function tests (measured with the WMS). WMS includes portions of both the WMS and WMS Revised: logical memory subtest, visual reproductions subtest, forward and backward visual memory spans, and forward and backward digit spans.
Magnetic resonance image (MRI) scans were obtained with a 1.5 T Siemens Magnetom Vision scanner with a standard radio frequency head coil. T1-weighted images with 1 mm isotropic resolution was acquired.
Outcomes for cohort and case–control studies included questionnaires, interviews and death certificates to collate additional information, including total lifetime oestrogen exposure (age of menarche, age of menopause, natural or surgical menopause, parity, lifetime breastfeeding history, HRT history, type of oestrogen preparation, dosage and oral contraceptive use), vascular pathology (history of myocardial infarction, hypertension and hypercholesterolaemia) and education level.
Search methods for identification of studies
This systematic review targeted articles published within the past 30 years (1992–2022), as we believe this time frame would adequately allow us to find the minimum number of RCTs (four) needed to increase the reliability of our study and provide the most relevant data for our evaluation.
Through the use of healthcare databases available to us, our selections were narrowed down to RCTs, cohort studies and case–control studies, as the three offered higher levels of evidence for our systematic review. Among the databases we have listed below, Ovid was used to access Medline and PsycINFO, and EBSCO was used to access EMBASE and CINAHL. Each source was last searched in July 2022.
Studies evaluated in this systematic review had an inclusion criteria of English language publication; the objective of reducing risk, delaying onset or prevention; and data involving peri-menopausal and post-menopausal women. Exclusion criteria include diagnosis of dementia other than Alzheimer's disease, progesterone-only therapy and pre-menopausal women.
The following are the list of healthcare databases and the general search terms used (full list in Supplementary Appendix 1 available at https://doi.org/10.1192/bjo.2023.579): PubMed, Science Direct, Scopus, BMJ, EMBASE (EBSCO), Web of Science, CINAHL (EBSCO), PsycINFO (Ovid) and The Cochrane Central Register of Controlled Trials; search terms ‘oestrogen therapy’ OR ‘estrogen therapy’ OR ‘hormone replacement therapy’ and ‘Alzheimer's disease’ OR ‘Alzheimer's dementia’.
Selection of studies and data collection
Studies were selected based on their abstracts. All reviewers read the abstracts (G.R.M.W., H.R., Q.Y.L. and E.J.A.L.) to screen for eligible studies in the review (RCTs, cohort studies and case–control studies). Further reading was done on each study, and any disparities in opinion were discussed and finalised among all reviewers. The selection of studies was made based on the reviewers’ definition of ‘reducing risk’, which includes ‘prevention’ and ‘delaying onset’. All reviewers performed data collection from included papers. Data were extracted on 2 September 2022.
Description of studies
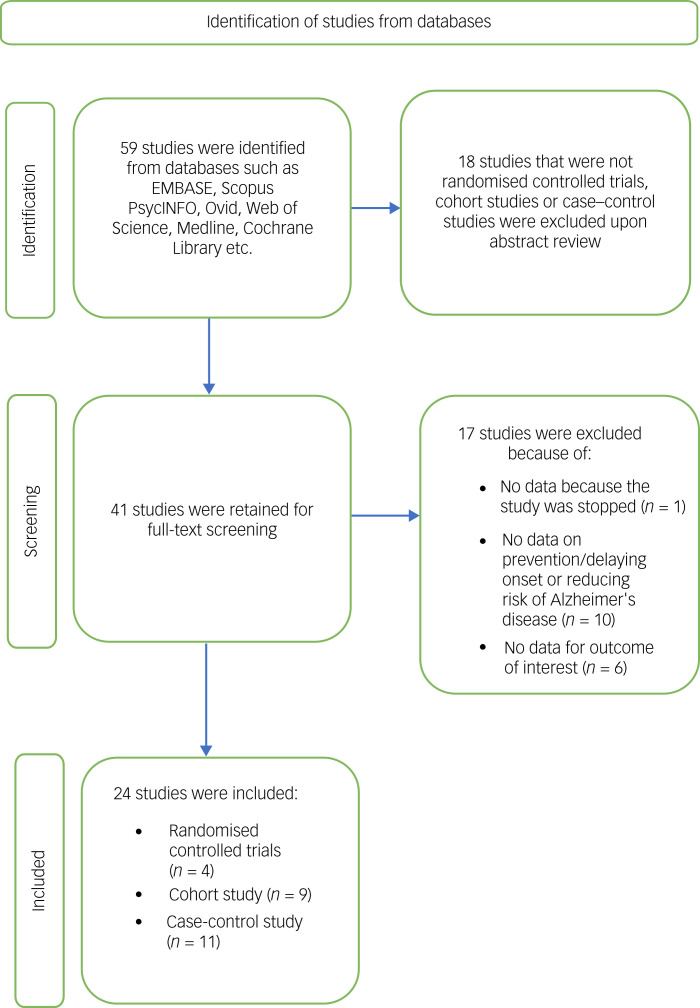
Fifty-nine studies were initially identified and discussed among the reviewers (Fig. 1). After exclusion of studies that were not RCTs, cohort studies or case–control studies, a total of 41 studies remained. A final discussion was held after a thorough reading of the remaining studies, and 24 studies were selected after matching the criteria set by the reviewers.
Fig. 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram of search process and identification of studies.
Of the 24 studies, there were four RCTs, nine cohort studies and 11 case–control studies. Exposure to oestrogen replacement therapy ranged from 16 weeks to 8 years. Twenty studies did not have data on treatment intervention duration because they utilised previous use of oestrogen as a measure for oestrogen replacement therapy.
Participants
Screening and selection
As this systematic review includes RCTs, cohort studies and case–control studies, the total number of participants differs by the type of study. The total number of participants in RCTs is on a smaller scale; for instance, 1187 or 4210 participants, whereas another had 4532 participants for their clinical trial11 (Table 1). In comparison, the largest number of participants was 4 696 633 in a nationwide study,12 and the remaining cohort studies ranged from 68813 to 50928 (Table 2). The sample size used in case–control studies also varied, ranging from 28014 to 230 58015 (Table 3). This trend is because of the aim, duration and nature of each study.
Table 1.
Summary of randomised controlled trials
| Study | Objective | Country | Sample size | Attrition | ITT analysis | Oestrogen intervention | Statistical results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Kantarci et al (2016)7 | Prevention of Alzheimer's disease only | USA | 118 | 50 (42.4%) | No | 0.45 mg/day CEE orally or 50 μg/day 17β-oestradiol transdermally for four years each | Transdermal groupa: odds ratio 0.04 [95% CI 0.004–0.44] Oral groupa: odds ratio 0.01 [95% CI 0.0006–0.23] |
Transdermal 17β-oestradiol was associated with reduced beta-amyloid deposition in APOE ε4 carriers |
| Henderson et al (2000)10 | Prevention and delaying onset of Alzheimer's disease | USA | 42 | 6 (14.3%) | No | 1.25 mg/day CEE orally for 16 weeks | Not applicable | No association between short-term oestrogen therapy and Alzheimer's disease |
| Shumaker et al (2003)11 | Prevention of Alzheimer's disease only | USA | 4532 | 0 (0%) | Yes | 0.625 mg/day CEE orally | Relative risk 1.97 (odds ratio 1.99 [95% CI 1.17–3.38]) | No association between short-term oestrogen therapy and Alzheimer's disease, but oestrogen plus progestin therapy increased risk of probable dementia |
| Shumaker et al (2004)22 | Prevention of Alzheimer's disease only | USA | 7479 | 0 (0%) | Yes | 0.625 mg/day CEE orally | Relative risk 1.36b (odds ratio 1.39 [95% CI 1.01–1.91]) | No association between oestrogen therapy and reducing risk of Alzheimer's disease. Increased risk of probable dementia in both oestrogen-only group and oestrogen plus progestin group |
ITT, intention to treat; CEE, conjugated equine oestrogen; APOE, apolipoprotein E.
Data to calculate relative risk was not available.
Calculated based on the treatment group utilising oestrogen therapy only.
Table 2.
Summary of cohort studies
| Study | Objective | Country | Type of HRT | Sample size | Attrition | Method | Statistical results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Zandi et al (2002)8 | Reducing risk of Alzheimer's disease | USA | Oral oestrogen preparation | 5092 | 1846 (36.3%) | Current/former user or never used via history | Relative risk 0.34 (odds ratio 0.32 [95% CI 0.20–0.51]) | Duration of HRT use is associated with decreasing Alzheimer's disease risk |
| Yoo et al (2020)12 | Reducing risk of Alzheimer's disease | Korea | Not collecteda | 4 696 633 | 0% | Survey | Relative risk 0.52 (odds ratio 0.51 [95% CI 0.51–0.52]) | Use of HRT is associated with reduced Alzheimer's disease risk |
| Paganini-Hill and Henderson (1994)13 | Reducing risk of Alzheimer's disease | USA | Oral oestrogen (1.25 mg) | 688 | 7 (1.02%) | Survey | Relative risk 0.75 (odds ratio 0.67 [95% CI 0.38–1.17]) | No significant findings that oestrogen replacement therapy may be useful for preventing and delaying onset of Alzheimer's disease |
| Tang et al (1996)16 | Reducing risk of Alzheimer's disease | USA | Oral CEE | 1124 | 0% | Current/former user or never used via history | Relative risk 0.40 (odds ratio 0.31 [95% CI 0.16–0.63]) | History of oestrogen use during post-menopausal period delays onset of Alzheimer's disease and lowers risk of disease |
| Lord et al (2008)17 | Prevention of Alzheimer's disease (neuroprotective effects of oestrogen replacement therapy) | Canada | CEE or oestradiol therapy (oral or transdermal) | 56 | 0% | Telephone interviews and questionnaires | Not applicable | Oestrogen replacement therapy is neuroprotective |
| Kawas et al (1997)23 | Reducing risk of Alzheimer's disease | USA | Oral or transdermal oestrogen | 514 | 42 (8%) | Current/former user or never used via history | Relative risk 0.46 (odds ratio 0.42 [95% CI 0.20–0.92]) | Reduced risk of Alzheimer's disease for women who had reported the use of oestrogen |
| Baldereschi et al (1998)24 | Prevention of Alzheimer's disease | Italy | Not collecteda | 2727 | 1159 (42.5%) | Survey | Relative risk 0.25 (odds ratio 0.24 [95% CI 0.07–0.77]) | Oestrogen replacement therapy is associated with a reduced prevalence of Alzheimer's disease in post-menopausal women |
| Imtiaz et alb (2017)27 | Reducing risk of Alzheimer's disease | Finland | Oestrogen or combination therapy | 8195 | 0% | Questionnaires | Relative risk 0.98c | Long-term oestrogen replacement therapy use reduces risk of Alzheimer's disease |
| Mortel and Meyer (1995)33 | Reducing risk of Alzheimer's disease | USA | Not collecteda. | 306 | Undisclosed | Medical records, questionnaires, interviews | Relative risk 0.67 (odds ratio 0.55 [95% CI 0.26–1.16]) | Oestrogen replacement therapy reduces risk of Alzheimer's disease |
HRT, hormone replacement therapy; CEE, conjugated equine oestrogen.
Unable to contact authors for further clarification.
Note that this study is a different study from the Imtiaz et al15 case–control study.
Data to calculate odds ratio was not available.
Table 3.
Summary of case studies
| Study | Objective | Country | Type of HRT | Sample size | Attrition | Method | Statistical Results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Henderson et al (2005)9 | Preventing and delaying onset of Alzheimer's disease | USA | Oestrogen (any form) | 971 | 0 (0%) | Current/former user or never used via history | Relative risk 0.64 (odds ratio 0.47 [95% CI 0.35–0.63]) | Oestrogen containing HRT may prevent and delay onset of Alzheimer's disease |
| Seshadri et al (2001)14 | Reducing risk of Alzheimer's disease | UK | Oral oestrogen with or without progestin or transdermal oestrogen | 280 | 0 (0%) | Medical records and medical history | Relative risk 1.18 (odds ratio 1.08 [95% CI 0.56–2.10]) | No reduction in risk of developing Alzheimer's disease in post-menopausal use of oestrogen replacement therapy |
| Imtiaz et ala (2017)15 | Reducing risk of developing Alzheimer's disease | Finland | Oral, transdermal or combination oestrogen only; progesterone only, combined or mixed | 230 580 | 0 (0%) | Medical records | Relative risk 1.05 (odds ratio 1.06 [95% CI 1.02–1.10]) | No association between HRT and risk of Alzheimer's disease |
| Brenner et al (1994)18 | Reducing risk of developing Alzheimer's disease | USA | Oral conjugated oestrogens, diethyistilbestrol tablets, ethinylestradiol tablets, vaginal conjugated oestrogens, dlenestrol vaginal cream, other vaginal oestrogens | 236 | 9 (3.8%) | Medical records and medical history | Relative risk 1.01 (odds ratio 1.10 [95% CI 0.6–1.8]) | No evidence that oestrogen replacement therapy has an effect on risk of Alzheimer's disease |
| Slooter et al (1999)19 | Prevention of Alzheimer's disease | The Netherlands | Not collectedb | 228 | Undisclosed | Information collected from the next of kin via interview | Relative risk 0.47 (odds ratio 0.34 [95% CI 0.12–0.94]) | Oestrogen use is beneficial to Alzheimer's disease with early onset |
| Kim et al (2021)20 | Reducing risk of Alzheimer's disease | USA | Oral or transdermal oestrogen | 379 352 | 1998 (0.53%) | Medical insurance records | Relative risk 0.43 (odds ratio 0.43 [95% CI 0.41–0.45]) | HRT is associated with reduced risk of developing Alzheimer's disease |
| Henderson et al (1994)21 | Reducing risk of Alzheimer's disease | USA | Oral oestrogen | 235 | 0 (0%) | Medical history, autopsy and post-mortem confirmation | Relative risk 0.58 (odds ratio 0.33 [95% CI 0.14–0.76]) | Post-menopausal oestrogen replacement therapy may decrease risk of Alzheimer's disease. Cognitive performance may be improved with oestrogen replacement therapy |
| Paganini-Hill and Henderson (1996)26 | Preventing and delaying onset of Alzheimer's disease | USA | Oral CEE (1.25 mg), oral plus injection and/or cream, injection and/or cream | 1446 | 0 (0%) | Questionnaire, medical history and records from death certificates | Relative risk 0.73 (odds ratio 0.65 [95% CI 0.49–0.88]) | Oestrogen replacement therapy may prevent or delay onset of Alzheimer's disease in post-menopausal women |
| Roberts et al (2006)27 | Reducing risk of Alzheimer's disease | USA | Oral or transdermal oestrogen | 528 | 38 (7.2%) | Medical records | Relative risk 1.04 (odds ratio 1.10 [95% CI 0.63–1.93]) | No association between oestrogen replacement therapy and Alzheimer's disease |
| Pourhadi et al (2021)28 | Reducing risk of developing Alzheimer's disease | Denmark | Vaginal oestrogen | 50 314 | Undisclosed | Medical records | Relative risk 0.93 (odds ratio 0.92 [95% CI 0.86–0.99]) | No association between vaginal oestrogen therapy with Alzheimer's disease |
| Waring et al (1999)29 | Reducing risk of Alzheimer's disease | USA | Oestrogen (any form) | 444 | Undisclosed | Medical records and autopsy reports | Relative risk 0.79 (odds ratio 0.65 [95% CI 0.40–1.06]) | Oestrogen replacement therapy reduces risk of Alzheimer's disease in post-menopausal women |
HRT, hormone replacement therapy; CEE, conjugated equine oestrogen.
Note that this study is a different study from the Imtiaz et al25 cohort study.
Unable to contact authors for further clarification.
All except one8 of the studies included in this review only involved women, specifically peri- or post-menopausal women, and each study stated its criteria. For example, participants with existing neurological or psychiatric illnesses,7,16–19 or other types of dementia,13 were excluded. Because of the role and interaction of oestrogen with other reproductive and cardiovascular factors, participants who had cancer within a specified time range,20 or had either an oophorectomy or hysterectomy,12,21 or stroke,16 were excluded. Since RCTs required administration of oestrogen replacement therapy, participants who used oestrogen or progesterone before the time of initiation, or had a previous diagnosis of dementia, were excluded from analysis;7,10 two studies even excluded participants with high risk of developing dementia at baseline.11,22
Confounding factors (age, mood and others)
Most studies included participants from a wide age range with a minimum age of 50–60 years (except for the study by Kim et al20), in an attempt to standardise age effects. A history of mental health illness is said to influence study results. Therefore, to standardise results, some studies excluded patients with psychiatric disorders or those receiving antipsychotic or antidepressant medications.17,18 Education and income level was mentioned in multiple studies, as it affects access to healthcare. One study17 specifically mentioned that all participants were controlled so that there was no obvious discrepancy in education level, depressive episodes, body mass index, exercise level, alcohol intake, smoking habits and income level, as these were identified to possibly influence results.
Interventions
A variety of methods were used in administration of oestrogen replacement therapy, dosage and types of oestrogen replacement therapy, as well as whether progesterone was added.
All RCTs used Premarin, a conjugated equine oestrogen (CEE), and a placebo. Two of the RCTs used a dosage of 0.625 mg per day of Premarin.11,22 One study10 used 1.25 mg per day of Premarin. One RCT7 used either 0.45 mg per day of Premarin or 50 μg per day of Climara (oestradiol). All four RCTs administered oestrogen via oral tablet, including one that utilised both transdermal patches and oral tablets. Of the four RCTs, all co-administered progesterone together with oestrogen replacement therapy. One study7 used 200 mg per day of Prometrium (micronized progesterone) orally for the first 12 days of each month in their trial. In one study,10 progesterone was given only if participants had an intact uterus.
Among the RCTs, one study7 did not list any adherence checks and three studies10,11,22 stated that they checked for participant adherence without stating the methods used.
Three cohort studies8,16,23 used medical histories and three studies12,13,24 used surveys to categorise participants with lifetime use of oestrogen therapy. Among these studies, four12,16,23,25 categorised participants based on the duration of oestrogen use.
Six case–control studies accessed participant information via medical records, three studies9,18,21 accessed information through medical interviews and only one study26 accessed information through a survey. One study19 collected information from the next of kin through structured interviews. Two studies2728 categorised their participants based on the initial age of initiation of oestradiol tablets. One study15 categorised their participants based on years of oestrogen use, progestogen use and combined HRT use. Five studies14,20,27,29,30 categorised their participants based on the previous duration of use of oestrogen. One study compared a group of patients with early-onset Alzheimer's disease and a group of patients with none of the symptoms of dementia.19
Quality assessment
The overall quality of evidence for all included RCTs were assessed with the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment (Supplementary Table 4). Quality of cohort studies and case–control studies were assessed with the Newcastle–Ottawa Scale, of which the studies included were classified as ‘good’ (n = 13) or ‘fair’ (n = 7) (Supplementary Table 5). Quality of evidence was assessed by all four reviewers.
Results
RCTs
Outcomes
Cognitive assessments
The RCTs assessed the cognitive functions of participants by using specific tests for individual cognitive domains. However, each trial used different cognitive tests with little overlap, thus making the pooling of data for analysis difficult. It was noted that distinct aspects of the same tests or different versions of the same test were used in different studies. Analysis was split for tests of verbal and visual memory, and for those that tested other aspects of cognitive function.
Two studies11,22 used the same global measure to assess cognitive function, the 3MSE. In addition, both studies analysed the incidence of mild cognitive impairment among participants and compared the treatment group with a placebo group.
Statistics
Some studies10,11,22 analysed baseline and post-treatment effects separately. Because of the increased number of comparisons, adjustment is required to reduce the incidence of chance findings.
Risk of bias in included studies
The RCTs included were all double-blinded and placebo-controlled.
Randomisation
Three of the studies provided details on their randomisation methods,10,11,22 whereas one study did not.7
Allocation concealment
One study was clear on their method of allocation concealment;10 however, the remaining three studies had inadequate description of their method for allocation concealment.7,11,22 There was also presumably suspected allocation to active treatment in one study.10
Attrition
All studies lost a variable number of participants throughout their trials. One study7 excluded 42.4% of participants from analysis. Most of the participants excluded from the analysis were from the CEE treatment group, with only 43.6% included.7 This was largely because of non-participation in their MRI and positron emission tomography scans. In two studies,11,22 the attrition was 0%, as the authors included all participants in the data analysis despite losing some participants because of incomplete data and other reasons.
Intention-to-treat analyses
Two studies11,22 described the use of intention-to-treat analyses performed. However, the remaining two studies had no information on any intention-to-treat analysis performed.7,10
Power calculation for sample size
Two studies11,22 undertook power calculations for their sample size based on the protocols of the Women's Health Initiative Memory Study (WHIMS),31 which estimates at least 80% power to detect a difference of 40%. The other studies did not disclose any information on performing power calculations for their trials.7,10
Effects of interventions
Test of overall global cognitive function
One study used the 3MSE to screen for global cognitive impairment and track changes in global cognitive function.11 The cut-off point for the study was a 3MSE score of ≤80 points for participants with ≤8 years of education and ≤88 points for participants with ≥9 years of education. The main effects for baseline 3MSE scores alone were statistically significant (P < 0.001). Results showed that the risk of developing probable dementia was 3.78 times (95% CI 1.91–7.50) greater for women with baseline 3MSE scores ranging from cut-off to 94 points; and 24.48 times (95% CI 13.19–46.75) greater for women with baseline 3MSE scores at or below cut-off compared with women with 3MSE scores ranging from 95 to 100.
In another study, the 3MSE was used similarly, and the cut-off point remained the same.15 Results showed that combining both the oestrogen-only and the oestrogen plus progestin trials showed an overall hazard ratio of 1.76 (95% CI 1.19–2.60; P = 0.005). However, after excluding participants with baseline 3MSE scores at or below the cut-off point, the hazard ratio was 1.77 (95% CI 0.74–4.23; P = 0.20) in the oestrogen-only trial.
One study10 used the ADAS-Cog scale and one study7 used a bi-factor model adapted from Dowling et al32 to measure outcomes for change in global cognitive function. One study showed no significant correlation between global cognitive scores and beta-amyloid deposition in the brain, measured by Pittsburgh compound-B standard unit value ratio.7 The remaining trial10 found no significant differences in participant groups with global cognitive function measures.
Mild cognitive impairment
One study found that the risk of being diagnosed with mild cognitive impairment was not statistically significant between the two groups (hazard ratio 1.07, 95% CI 0.74–1.55; P = 0.72).11 However, the risk of being diagnosed with mild cognitive impairment or probable dementia increased by 37% for women in the exposure group who were receiving oestrogen plus progestin (hazard ratio 1.37, 95% CI 0.99–1.89; P = 0.06).11
Another study showed that the risk of being diagnosed with mild cognitive impairment was 34% higher in the CEE group than in the placebo group (hazard ratio 1.34, 95% CI 0.95–1.89).22 Likewise, the risk was similar in the combined oestrogen and progestin trial (hazard ratio 1.25, 95% CI 0.97–1.60).22 Neither of the hazard ratios were statistically significant.
Specific tests for individual cognitive domains
Verbal memory and language tests
No association was noted in one study7 between oral CEE therapy and beta-amyloid deposition compared with placebo. However, after adjusting for age, education, time from menopause to randomisation and APOE ɛ4 status, the CVLT total score was lower in the oral CEE group compared with the placebo group (P = 0.03).
One study,10 showed overall insignificant results, with only delayed paragraph recall showing a positive result at 16 weeks for the CEE group.
Visuospatial tests
Two studies did not include details on visuospatial tests. One study tested visual memory with the Benton Visual Retention Test, but the results were unavailable.7 Another study10 showed a significant difference on the visual memory span forward test at week 16.
Speed tests, attention and manual dexterity, and semantic memory tests
Outcome measures for speed tests, attention tests and semantic memory tests showed no statistically significant differences in all of the studies. Some studies10,11,22 did not include specific details on the P-values of each test carried out.
Executive function
All studies performed outcome measures for executive function. In one study,10 a statistically significant difference was found in the Trail Making Test Part B, with a P-value of <0.05. In the same study, the backward digit span test found a near statistically significant difference, with a P-value of between 0.05 and 0.1. However, the authors did not disclose the exact value of the test. All other studies found no statistically significant difference in the other tests that measured executive function.
Cohort studies
Outcomes
Cognitive assessments
Most of the cohort studies utilised widely used cognitive assessment tools that mainly assessed global cognitive functions. For instance, the MMSE, CDR and Global Deterioration Scale were used in one study.12 Other than the MMSE, DSM-III-R and NINCDS-ARDA, one study24 used an extensive list of cognitive assessments, such as the CAMDEX and ICD-10 criteria for other dementia diseases. One study17 used MRI scans to assess integrity of the amygdala and hippocampus to investigate the neuroprotective effects of HRT.
One study13 that used a case–control methodology within a prospective cohort study conducted their study using death certificates to confirm the diagnosis of Alzheimer's disease, therefore no cognitive assessments were performed in the course of their study.
Statistics
Although most studies investigated various reproductive factors, some chose Cox proportional hazards regression analysis to accommodate multiple covariates,8,16,23,25 whereas others used multivariate regression analysis.13,24 Studies that performed age-specific comparisons utilised the combination of analysis of variance (ANOVA) for continuous variables and chi-squared for categorical variables;16,24 one study used the combination of ANOVA for continuous and Student's t-test for categorical variables.33 Other characteristics of those who did and those who did not use HRT were analysed with chi-squared tests for categorical variables and two-sample t-tests for continuous measures.8,25
Risk of bias in included studies
Attrition
One study had an attrition rate of 27.3%,24 mainly because of an inability to obtain a history of oestrogen therapy, drop-out rate and other unspecified reasons. The trial with the highest attrition rate of 42.4%25 reported lack of response, changes in address and death as the main causes of attrition. Some studies12,16,23 had comparatively lower rates of attrition of <10%. One study33 did not disclose the attrition rate in their study.
Because of the scale and duration of some studies, follow-ups could not be performed because of reasons such as participants passing away, missing data over the course of the study, refusal to participate in the study and inability to locate participants.8,12,13,24,25 This shows the possibility of bias, as the loss of participants to follow-up potentially affects the overall results. Furthermore, as most of the studies involved older women, attrition highlights the particular bias resulting from death and decline in the health of participants, which leads to potential bias of data toward those who are healthy enough to participate until the end of the study.
Test of overall global cognitive function
Two studies12,24 measured cognitive function with the MMSE. They had a cut-off score of 26 and 23/24 out of 30 to indicate dementia for each of the studies, respectively. Significantly more women who had never used HRT were diagnosed with Alzheimer's disease in these two studies. One study12 used the CDR to diagnose new-onset dementia after a median of 5.73 years from baseline. In contrast, another study showed that women using HRT had lower risks and delayed onset of dementia.24 Another study16 showed that the age at onset for Alzheimer's disease is significantly later in women who have used oestrogen replacement therapy compared with those who have not (log-rank test P < 0.01). It also showed that adjustments made for possible confounding factors, such as ethnic group, education and participation group, did not significantly affect the relative risk (relative risk 0.5, 95% CI 0.25–0.9; P = 0.02).
Five studies8,23–25,33 compared the results from different cognitive assessment tests to specific criteria such as the NINCDS-ADRDA criteria for probable Alzheimer's disease and DSM-III-R criteria for dementia syndrome. They all yielded positive results favouring the use of hormone therapy in delaying the onset and prevention of dementia, with one study23 highlighting the benefits of long-term HRT use.
Case–control studies
Outcomes
Cognitive assessments
One study29 measured Alzheimer's disease according to DSM-III criteria; however, not all of their patients received routine neuropsychological testing. Two studies15,27 measured Alzheimer's disease according to DSM-IV criteria. Two studies1415 used the NINCDS-ADRDA criteria to measure for probable Alzheimer's disease. Two studies18,21 made use of the MMSE as part of their neuropsychological testing to measure dementia severity. One study28 used the criteria for Alzheimer's disease set by ICD-10. Another study19 used NINCDS-ARDA criteria, the CDR and a short portable mental status questionnaire to assess any progressive decline of intellectual functions during the course of study.
Statistics
One study21 used a general linear modelling programme to control for potential confounding factors such as age and education, and used the extension chi-squared procedure to remove the controlled variables. Another trial9 evaluated confounding factors by comparing crude odd ratios with Mantel–Haenszel odds ratios. There was use of multivariable models and subsequent evaluation in indirect analysis to examine confounding variables in another study.27 Logistic regression models were also used with regards to matching in some studies,14,152029 whereas another study27 discounted it to reduce loss of statistical power caused by missing data. Finally, a study that included the APOE population conducted stratified analysis by using unconditional logistic regression to test for statistical interaction between oestrogen use and the APOE genotype.19
Risk of bias in included studies
Attrition
Two studies20,29 had no information on the attrition in their studies. Most studies had <10% attrition, three14,15,21 of which had an attrition rate of 0%. One study28 had an attrition rate of 78.1% because of censoring during follow-up, which occurred for a multitude of reasons including, but not limited to, emigration and missing information on socioeconomic confounders.
Test of overall global cognitive function
There was no significant overall benefit of oestrogen replacement therapy and HRT on Alzheimer's disease seen in the case–control studies, with only half of them showing positive results.9,20,21,26,29 The other trials all showed little to no association of oestrogen replacement therapy with Alzheimer's disease. One trial29 showed improved cognition for patients that used HRT for a longer duration, at least 6 months (odds ratio 0.42, 95% CI 0.18–0.96; P = 0.04). Another trial26 showed a decreased risk of Alzheimer's disease with both an increased dosage and duration of oestrogen replacement therapy (odds ratio 0.65, 95% CI 0.49–0.88; P = 0.01). One study19 used the CDR to screen new participants for dementia.
Discussion
Out of all of the studies analysed in this systematic review, some studies show positive associations in oestrogen replacement therapy reducing risk of Alzheimer's disease (n = 15), and the remaining studies found no evidence of such an association (n = 9). One RCT associated transdermal 17β-oestradiol with reducing the progression of Alzheimer's disease pathology in the population of APOE ɛ4 carriers,7 whereas three other studies showed no association between short-term oestrogen replacement therapy and Alzheimer's disease. Cohort and case–control studies had a similar number of positive associations. In conclusion, the evidence of oestrogen replacement therapy in reducing risk of Alzheimer's disease remains inconsistent; however, further details regarding oestrogen's role in Alzheimer's disease were discovered.
Some studies investigated oestrogen-related variables, such as age of menarche, date of most recent menstrual period and number of pregnancies, and found that risk for Alzheimer's disease increased with shorter oestrogen exposure.12,24 This principle seemed to be applied similarly with the time of HRT exposure. Longer use of oestrogen replacement therapy is associated with lower risk of developing Alzheimer's disease,9 whereas women who had never used oestrogen replacement therapy had 65% risk of the disease.13 One case study, with a mean treatment duration of 3.1 years, found that those who received oestrogen replacement therapy had a higher MMSE score of 16.6, compared with an MMSE score of 9.9 for those who did not receive oestrogen replacement therapy (P = 0.02).21 Further, despite the smaller effect, a cohort study found that women taking oestrogen replacement therapy had better MMSE scores (25.9 v. 24.2; P < 0.001).24 Duration of treatment and types of treatment are believed to heavily influence cognitive outcomes,34 both of which differed in both studies. The difference may also be attributable to women in the two trials differing in many factors at baseline, including cognitive function.
Two studies that evaluated the effects of oestrogen replacement therapy on five domains of cognitive function resulted in significant findings on ‘learning and memory’, whereas no evidence of significant effect was found in any other domains. One study concluded with no correlations between oestrogen replacement therapy and cognitive function measured with the CVLT,7 whereas another study found that verbal memory performances (measured via logical memory test) were better after 16 weeks of oestrogen replacement therapy.10 A possible explanation for this contrasting conclusion could be the use of different cognitive tests; for instance, a study investigated the differences between several neuropsychological assessments, including the CVLT and logical memory test, and discourages using the two interchangeably because there is a high chance of generating a discrepant score.35 Similarly, a meta-analysis comprising 24 papers revealed that most trials showed significant findings in ‘learning and memory’, suggesting that this domain is the most susceptible to the effects of oestrogen.36 It is believed that this is likely because of an interaction between oestrogen and the cholinergic neurotransmitter systems on the function of hippocampus and prefrontal cortex, affecting the main stage of memory processing.37
The APOE genotype is the most common genetic determinant of risk of Alzheimer's disease. Several studies included additional investigation of APOE ɛ4 carriers who have greater risk of Alzheimer's disease. One study16 suggested reduced risk of Alzheimer's disease in women heterozygous for ɛ4 with oestrogen replacement therapy use, whereas another study8 claimed that two ɛ4 alleles had a greater risk reduction, although the author was uncertain of this finding. This highlights the differences in mechanisms of cognitive decline and pathology in APOE ɛ4 carriers compared with non-carriers. According to Yaffe, oestrogen may play a role against carotid atherosclerosis by interacting with APOE ε4, as cardiovascular problems have been proven to result in Alzheimer's disease.38 This was similar to a hypothesis by Depypere et al, who additionally highlighted the importance of looking for the ‘window of opportunity’ to see a maximal effect of oestrogen replacement.39 Yaffe also proposed a possible modulation of acetylcholine in the brain, resulting in increased neuronal growth and synaptic reorganisation as a result of oestrogen's effect in regulating synaptic plasticity through stimulation of axonal sprouting and dendritic spine formation, which is evident in studies carried out on rodent tissues. This is also reiterated by Stone et al, who demonstrated that oestrogen causes changes in synaptic sprouting through an APOE-mediated mechanism in response to injury in mice.40 Stone et al also hypothesised that the increased risk of Alzheimer's disease in female APOE ε4 carriers could be a result of a combination of effect between oestrogen-deficient state and ability for neuronal reorganisation and choline acetyltransferase activity through the ε4 genotype. On the other hand, Depypere et al also proposed that APOE plays a role in increasing potential neuroprotective actions of oestrogen. This is thought to be a result of lipoprotein receptor-related protein, which is an APOE receptor, where expression after stimulation by oestrogen causes an increased amount of APOE produced by astrocytes. The produced APOE then stimulates an increase in lipids, which is necessary for cell membrane growth, ultimately resulting in neuronal growth. A trial conducted by Kantarci et al, using transdermal oestrogen on APOE carriers, found that oestrogen also plays a role in reducing amyloid plaque formation.7 This was further solidified by a study done on primary cultures derived from rodent and human neocortex.41
Future studies should explore the critical time window of HRT intervention, as some studies have suggested early menopause to be the optimal period for HRT to play an effective role in delaying dementia progression.9 It will be interesting to see whether this critical time period has any associations with different ethnicities or subgroups of patients who are APOE ɛ4 carriers or non-carriers.
Two studies investigated the concomitant use of non-steroidal anti-inflammatory drugs, one of which suggested a potential role in neuroprotection of Alzheimer's disease.8 In contrast, the other study had inconclusive results because of a lack of data.23 This variable has yet to justify a role in oestrogen replacement therapy and Alzheimer's disease. The proposed mechanism of how non-steroidal anti-inflammatory drugs decrease beta-amyloid 1-42 production is by allosteric modulation of gamma-secretase activity, the enzyme responsible for the formation of beta-amyloid.42 The potential concomitant use of non-steroidal anti-inflammatory drugs not only provides new understanding of the pathogenesis of Alzheimer's disease, but has also created opportunities to explore other potential preventive pharmacologic agents for reducing Alzheimer's disease risk.
A strength of this review is that it includes papers published recently,20 which suggests that this topic remains an ongoing debate. Also, only RCTs, cohort studies and case–control studies are included in our analysis, to provide more credible and quality analysis. Finally, a selection of oestrogen products, multiple routes of administration (oral, transdermal, vaginal, injections) and different durations of oestrogen replacement therapy were analysed in the papers reviewed.
A limitation of the studies used is the duration of oestrogen treatment. Trials conducted between 16 weeks and 12 months found no associations, and suggested that the trial period was unlikely to have significant results.10,11 From this, clinical trial periods between 12 months and 4 years are highly recommended, as none of the papers reviewed included this time frame. Furthermore, since most RCTs use the same type of oestrogen replacement therapy, dose and route in their trials, the data found may not be applicable to other scenarios or the general population. A combination of different routes, dosage or type of oestrogen replacement therapy explored in a single RCT might be helpful to uncover new findings.
As surveys or interviews are used to gather information in cohort and case–control studies, data collected are based on participants’ memories, which could be inaccurate19 and may be incomplete or insufficient.29 Similarly, for observational studies and retrospective analysis, some participants could have changed oestrogen type or administration route, which leads to less accurate results.8,16,20
Selection biases are bound to occur in case–control studies. For example, data for progestin use were unobtainable, therefore the effect between unopposed oestrogen and oestrogen plus progestin was not determined.9
Only studies that were published in English were included, leading to a language bias in our results and findings. Positive findings are also more likely to be published in English as it is the predominant language in research.37 Most studies are conducted in countries with high White populations, and this can introduce some ethnic bias as other ethnicities had little involvement in the studies.
Although many studies have taken place, the results remain inconsistent and insufficient to justify oestrogen replacement therapy's role in reducing risk of Alzheimer's disease. A major limitation to this systematic review is the few RCTs available that matched our inclusion criteria.
To conclude, our hypothesis stating ‘oestrogen replacement therapy is likely to reduce the risk of Alzheimer's disease in post-menopausal women’ is supported to a certain extent, although further studies are required. To support this theory, we recommend further research on longer oestrogen replacement therapy duration, because studies have yet to find an effective time period, except for a single study with a trial duration of 4 years.7 Another consideration could be possible interaction between oestrogen and other treatment modalities, as some studies found a potential role of non-steroidal anti-inflammatory drugs.8
In addition to new findings of oestrogen involvement in gene modulation, further investigation is encouraged with larger sample sizes, to further establish association of oestrogen replacement therapy with the presence of different numbers of ɛ4 alleles.16
In women who are predisposed to Alzheimer's disease, initiating a pathway of preventive oestrogen replacement therapy or HRT administration can be considered. Depending on whether the patient has had a hysterectomy, patients without a uterus should undertake APOE ɛ4 genetic testing. If the use of preventive measures are proven by future studies, the presence of ε4 alleles in the APOE ɛ4 genetic testing results should encourage immediate initiation of oestrogen replacement therapy. For patients with an intact uterus, clinical judgement is heavily required to determine whether benefits of APOE ɛ4 genetic testing and HRT administration outweigh the risks. However, more research is required in this area, and the conclusions we have drawn here are only from the studies in this paper, which we acknowledge are insufficient to fuel clinical decisions.
Supporting information
Wong et al. supplementary material
Acknowledgements
We would like to acknowledge Dr Christopher Haxton (Goldenhill Mental Health Resource Centre, Clydebank, Scotland) for the advice and guidance he provided throughout the study.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2023.579
Data availability
Data availability is not applicable to this article as no new data were created or analysed in this study.
Author contributions
G.W., E.L., Q.Y.L. and H.R. were all equally involved in writing the protocol, performing initial searches of databases, performing data extraction and quality assessment of included studies and writing up of the manuscript.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.
References
- 1.Lambert J, Coyle N, Lendon C. The allelic modulation of apolipoprotein E expression by oestrogen: potential relevance for Alzheimer's disease. J Med Genet 2004; 41: 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossetti MF, Cambiasso MJ, Holschbach MA, Cabrera R. Oestrogens and progestagens: synthesis and action in the brain. J Neuroendocrinol 2016; 28(7): 12402. [DOI] [PubMed] [Google Scholar]
- 3.Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging 2002; 23(4): 589–600. [DOI] [PubMed] [Google Scholar]
- 4.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci 2003; 23(13): 5708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (premarin®) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci 2006; 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green PS, Bales K, Paul S, Bu G. Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer's disease. Endocrinology 2005; 146(6): 2774–81. [DOI] [PubMed] [Google Scholar]
- 7.Kantarci K, Lowe VJ, Lesnick TG, Tosakulwong N, Bailey KR, Fields JA, et al. Early postmenopausal transdermal 17β-estradiol therapy and amyloid-β deposition. J Alzheimers Dis 2016; 53(2): 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zandi P, Carlson M, Plassman B, Welsh-Bohmer K, Mayer L, Steffens D, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County study. JAMA 2002; 288(17): 2123. [DOI] [PubMed] [Google Scholar]
- 9.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry 2005; 76(1): 103–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, et al. Estrogen for Alzheimer's disease in women: randomized, double-blind, placebo-controlled trial. Neurology 2000; 54(2): 295. [DOI] [PubMed] [Google Scholar]
- 11.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the women's health initiative memory study: a randomized controlled trial. JAMA 2003; 289(20): 2651–62. [DOI] [PubMed] [Google Scholar]
- 12.Yoo JE, Shin DW, Han K, Kim D, Won HS, Lee J, et al. Female reproductive factors and the risk of dementia: a nationwide cohort study. Eur J Neurol 2020; 27: 1448–58. [DOI] [PubMed] [Google Scholar]
- 13.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol 1994; 140(3): 256–61. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S, Zornberg GL, Derby LE, Myers MW, Jick H, Drachman DA. Postmenopausal estrogen replacement therapy and the risk of alzheimer disease. Arch Neurol 2001; 58(3): 435–40. [DOI] [PubMed] [Google Scholar]
- 15.Imtiaz B, Taipale H, Tanskanen A, Tiihonen M, Kivipelto M, Heikkinen A-M, et al. Risk of Alzheimer's disease among users of postmenopausal hormone therapy: a nationwide case-control study. Maturitas 2017; 98: 7–13. [DOI] [PubMed] [Google Scholar]
- 16.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 1996; 348: 429–32. [DOI] [PubMed] [Google Scholar]
- 17.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging 2008; 29: 95–101. [DOI] [PubMed] [Google Scholar]
- 18.Brenner DE, Kukull WA, Stergachis A, van Belle G, Bowen JD, McCormick WC, et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer's disease: a population-based case-control study. Am J Epidemiol 1994; 140(3): 262–67. [DOI] [PubMed] [Google Scholar]
- 19.Slooter AJ, Bronzova J, Witteman JC, Van Broeckhoven C, Hofman A, van Duijn MC. Estrogen use and early onset Alzheimer's disease: a population-based study. J Neurol Neurosurg Psychiatry 1999; 67: 779–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YJ, Soto M, Branigan GL, Rodgers K, Brinton RD. Association between menopausal hormone therapy and risk of neurodegenerative diseases: implications for precision hormone therapy. Alzheimers Dement 2021; 7(1): e12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women: comparisons between Alzheimer's disease cases and nondemented control subjects. Arch Neurol 1994; 51(9): 896–900. [DOI] [PubMed] [Google Scholar]
- 22.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: women's health initiative memory study. JAMA 2004; 291(24): 2947–58. [DOI] [PubMed] [Google Scholar]
- 23.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease. Neurology 1997; 48(6): 1517–21. [DOI] [PubMed] [Google Scholar]
- 24.Baldereschi M, Di Carlo A, Lepore V, Bracco L, Maggi S, Grigoletto F, et al. Estrogen-replacement therapy and Alzheimer's disease in the Italian longitudinal study on ageing. Neurology 1998; 50: 996–1002. [DOI] [PubMed] [Google Scholar]
- 25.Imtiaz B, Tuppurainen M, Rikkonen T, Kivipelto M, Soininen H, Kröger H, et al. Postmenopausal hormone therapy and Alzheimer disease. Neurology 2017; 88: 1062–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med 1996; 156(19): 2213–17. [PubMed] [Google Scholar]
- 27.Roberts RO, Cha RH, Knopman DS, Petersen RC, Rocca WA. Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis Assoc Disord 2006; 20(3): 141–6. [DOI] [PubMed] [Google Scholar]
- 28.Pourhadi N, Mørch LS, Holm EA, Torp-Pedersen CT, Meaidi A. Vaginal estrogen and association with dementia: a nationwide population-based study. Alzheimers Dement 2021; 18(4): 625–34. [DOI] [PubMed] [Google Scholar]
- 29.Waring SC, Rocca WA, Petersen RC, O'Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology 1999; 52(5): 965–70. [DOI] [PubMed] [Google Scholar]
- 30.Kim YJ, Soto M, Branigan GL, Rodgers K, Brinton RD. Association between menopausal hormone therapy and risk of neurodegenerative diseases: implications for precision hormone therapy. Alzheimers Dement 2021; 7(1): e12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, et al. The women's health initiative memory study (WHIMS). Control Clin Trials 1998; 19(6): 604–21. [DOI] [PubMed] [Google Scholar]
- 32.Dowling NM, Gleason CE, Manson JAE, Hodis HN, Miller VM, Brinton EA, et al. Characterization of vascular disease risk in postmenopausal women and its association with cognitive performance. PLoS One 2013; 8(7): e68741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortel KF, Meyer JS. Lack of postmenopausal estrogen replacement therapy and the risk of dementia. J Neuropsychiatry Clin Neurosci 1995; 7(3): 334–7. [DOI] [PubMed] [Google Scholar]
- 34.Ryan J, Carriere I, Scali J, Dartigues JF, Tzourio C, Poncet M, et al. Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C study. Neurology 2009; 73(21): 1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiruselvam I, Vogt EM, Hoelzle JB. The interchangeability of CVLT-II and WMS-IV verbal paired associates scores: a slightly different story. Arch Clin Neuropsychol 2015; 30(3): 248–55. [DOI] [PubMed] [Google Scholar]
- 36.Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev 2008; 1: CD003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maki P, Dumas J. Mechanisms of action of oestrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med 2009; 27(03): 250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe K. Estrogens, selective estrogen receptor modulators, and dementia: what is the evidence? Ann N Y Acad Sci 2006; 949(1): 215–22. [DOI] [PubMed] [Google Scholar]
- 39.Depypere H, Vierin A, Weyers S, Sieben A. Alzheimer's disease, apolipoprotein E and hormone replacement therapy. Maturitas 2016; 94: 98–105. [DOI] [PubMed] [Google Scholar]
- 40.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer's disease. J NEeUuRrOoSsci 1998; 18(9): 3180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med 1998; 4(4): 447–51. [DOI] [PubMed] [Google Scholar]
- 42.Imbimbo BP. The potential role of non-steroidal anti-inflammatory drugs in treating Alzheimer's disease. Expert Opin Invest Drugs 2004; 13(11): 1469–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wong et al. supplementary material
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analysed in this study.