Abstract
The pharmacokinetic interaction between indinavir and ritonavir was evaluated in five groups of healthy adult volunteers to explore the potential for twice-daily (b.i.d.) dosing of this combination. All subjects received 800 mg of indinavir every 8 h (q8h) on day 2. In addition, subjects in group I received one dose of 800 mg of indinavir on day 1 and 800 mg of indinavir q8h on day 17. Subjects in Groups II and IV each received one dose of 600 mg of indinavir on days 1 and 17, and subjects in groups III and V each received one dose of 400 mg of indinavir on days 1 and 17. During days 3 to 17, ritonavir placebo or ritonavir at 200, 300, 300, or 400 mg q12h was given to groups I, II, III, IV, and V, respectively. Ritonavir at steady state probably inhibited the cytochrome P-450 3A metabolism of indinavir and substantially increased plasma indinavir concentrations, with the area under the plasma concentration-time curve (AUC) increasing up to 475% and the peak concentration in serum (Cmax) increasing up to 110%. The Cmax/trough concentration ratio decreased from 50 in standard q8h regimens to less than 14 when indinavir was administered with ritonavir. For a constant indinavir dose, an increase in the ritonavir dose yielded similar indinavir AUCs, Cmaxs, and concentrations at 12 h (C12s). For a constant ritonavir dose, an increase in the indinavir dose resulted in approximately proportional increases in the indinavir AUC, less than proportional increases in Cmax, and slightly more than proportional increases in C12. Ritonavir reduced between-subject variability in the indinavir AUC and trough concentrations and did not affect indinavir renal clearance. With the altered pharmacokinetic profile, indinavir likely could be given as a b.i.d. combination regimen with ritonavir. This could potentially improve patient compliance and thereby reduce treatment failures.
Recent advances in the management of human immunodeficiency virus (HIV) infection have led to a new perception of the disease and new treatment paradigms (10). These advances include an improved understanding of the pathogenesis of HIV infection (20, 52), the development of reliable assay methodologies for the detection and quantification of viral load (48, 49), the availability of new, potent antiviral agents, and an appreciation that combination antiretroviral therapy reduces disease progression and mortality risks (7, 8, 16, 19, 37). In this context, indinavir, ritonavir, saquinavir, and nelfinavir are the promising new HIV protease inhibitors for the treatment of HIV infection. More potent and sustained suppression of viral replication and durable immunoreconstitution has been shown with the use of dual protease inhibitors or protease inhibitors in combination with nucleoside analogs compared with the suppression achieved with monotherapy (9, 13, 15, 37, 42, 43, 54, 55, 57, 60, 62).
Despite these encouraging advances, anti-HIV therapy remains suboptimal. The durability of the antiviral effect is transient in some individuals. Furthermore, effective combination therapies often demand complex regimens that may involve burdensome frequent dosing, side effects, food restrictions, and hydration requirements. These factors reduce the level of patient adherence to the treatment regimen and may ultimately lead to treatment failure.
Indinavir is a potent HIV protease inhibitor, with an in vitro 90% inhibitory concentration (IC90) (HIV-1IIIB) of approximately 0.1 μg/ml in a system containing 50% human serum and 10% fetal calf serum (1, 46, 47). Indinavir is rapidly metabolized by cytochrome P-450 3A isoenzymes (CYP3A), the major enzymes present in the liver and gastrointestinal tract, with an elimination half-life of 1.8 h (11, 45). The renal clearance (CLR) of indinavir is reported to be relatively constant over a wide range of concentrations (4, 63). When given as an 800-mg regimen three times a day (t.i.d.) under fasting conditions, the mean steady-state area under the concentration-time curve (AUC) of indinavir for HIV-infected patients over one dose interval was reported to be 18.8 μg · h/ml (37% coefficient of variation [CV]), and the maximum concentration of drug in serum (Cmax) and the minimum concentration in serum (Cmin) were reported to be 7.73 μg/ml (32% CV) and 0.154 μg/ml (71% CV), respectively (45). Meals with high fat and protein contents decreased the indinavir AUC and Cmax by 80 and 85%, respectively (45). To maintain therapeutic concentrations in plasma, indinavir is given at high doses on a strict schedule of once every 8 h (q8h) with rigid meal schedules.
Ritonavir has relatively high bioavailability and an elimination half-life of 3 to 5 h (1, 25, 27, 38). Although it is about 99% protein bound when it is given at a dosage of 600 mg twice daily (b.i.d.) concentrations in plasma in excess of the protein binding-adjusted IC90 (2.1 μg/ml) are usually maintained throughout the dosing interval (14). The bioavailability of ritonavir is minimally affected by food (1).
Ritonavir is a potent CYP3A inhibitor in vitro (34), with an estimated Ki of 0.085 μg/ml for indinavir in experiments with human liver microsomes (33a). In rats, the combined administration of ritonavir and indinavir (10 mg/kg of body weight for each drug) resulted in an eightfold increase in the indinavir AUC compared to that for indinavir given alone (26, 28). Recently, we described a clinically significant interaction between ritonavir and saquinavir (22). Probably due to the inhibition of the CYP3A metabolism of saquinavir by ritonavir, saquinavir AUC values were greatly enhanced (>50-fold) when saquinavir was coadministered with ritonavir; the combination regimen given b.i.d. with reduced doses of both drugs has been shown to suppress the level of viral RNA to below quantitation limits (<200 copies/ml) in 90% of the patients treated for 60 weeks (9). Coadministration with ritonavir is expected to significantly alter the pharmacokinetic profile of indinavir in patients. Since the extent of interaction was unknown, to avoid over- or underexposure to protease inhibitors in HIV-infected patients, this study was conducted with non-HIV-infected healthy volunteers.
In contrast to reverse transcriptase inhibitors, significant correlations between antiviral activity and plasma drug concentrations have been demonstrated for HIV protease inhibitors in various clinical studies (2, 6, 14, 17, 27, 59). Suboptimal concentrations in plasma are clearly associated with a rebound in viral titer, indicating the importance of maintaining high concentrations of protease inhibitors in plasma. For indinavir, although the average concentration in plasma from a standard 800-mg q8h regimen is well above the IC90, the mean Cmin is only slightly above the IC90. Furthermore, Cmin is highly variable between individuals, suggesting that some patients may have low antiviral coverage for several hours each day. Thus, if ritonavir inhibition of indinavir metabolism can increase the Cmin of indinavir and allow twice-daily administration, then the combination therapy is expected to reduce treatment failures associated with noncompliance or subtherapeutic lapses in Cmin.
MATERIALS AND METHODS
Study design.
This study evaluated the pharmacokinetics of indinavir in the absence and presence of ritonavir in five groups of non-HIV-infected healthy volunteers by a randomized, multiple-dose, open study design. On day 1, all subjects received an assigned single dose of indinavir (800 mg for group I, 600 mg for groups II and IV, and 400 mg for groups III and V). On day 2, all subjects received 800 mg of indinavir q8h. During days 3 to 17, subjects in groups I, II, III, IV, and V received ritonavir placebo or ritonavir at 200, 300, 300, or 400 mg every 12 h (q12h), respectively. On day 17, in addition to ritonavir doses, subjects in group I (control group) received 800 mg of indinavir q8h, while subjects in groups II and IV each received one dose of 600 mg of indinavir and subjects in groups III and V each received one dose of 400 mg of indinavir.
A total of 39 healthy subjects (8 in groups I, II, IV, and V and 7 in group III), including 14 females, were enrolled in and completed the study. Since a large interaction was expected, the sample size for the study was determined on the basis of the variability observed from previous interaction studies. All subjects were between the ages of 18 and 45 years, tobacco nonusers, and negative for recreational drug and alcohol as determined by screens with an enzyme multiplied immunoassay test kit. No statistically significant differences in demographic characteristics were found among the five treatment groups. Across the groups, the mean ± standard deviation age, body weight, height, and creatinine clearance were 29.0 ± 7.9 years, 72.0 ± 8.9 kg, 171.6 ± 9.4 cm, and 105 ± 18 ml/min, respectively. All subjects gave written informed consent to participate in this study.
Subjects were confined on study days 1, 2, and 17. Breakfast, morning snack, lunch, dinner, and evening snack were served at approximately 0630, 0930, 1230, 1700, and 2130 h, respectively. Indinavir doses were given at approximately 0700 h on day 1 for all groups and at 0700, 1500, and 2300 h on day 2 for all groups. On day 17, indinavir doses were given at approximately 0700, 1500, and 2300 h for group I and at 0700 h for groups II to V. Ritonavir doses were given at 0700 and 1900 h on day 17. The morning indinavir doses were given after a low-fat breakfast, which consisted of 370 kcal, with 5% of the calories from fat. The afternoon and evening indinavir doses were given between meals. The meals served were identical for days 1, 2, and 17. With the exception of breakfast, all meals and snacks served on days 1, 2, and 17 were regular meals containing approximately 30 to 35% fat. During days 4 and 15, subjects received ritonavir or ritonavir placebo doses at the study site after consuming breakfast and dinner at approximately 0600 and 1800 h, respectively, but were otherwise allowed to be outpatients. During the days that indinavir was given (days 1, 2, and 17), subjects were encouraged to drink at least 1.5 liters of fluid per day. All doses were given with 240 ml of water. Commercial formulations were used in the study. This study was approved by the Institutional Review Board of Victory Hospital, Waukegan, Ill.
Blood sampling and determination of ritonavir and indinavir concentrations.
Blood samples (5 ml on days 1 and 2 and 7 ml on day 17) for the determination of indinavir concentrations were collected on days 1, 2, and 17, and samples for the determination of ritonavir concentrations were collected on days 4, 5, 6, 7, 8, 10, 12, and 14 immediately before administration of the morning doses and on day 17 over a 40-h period. On day 1, samples were collected at 0 (predosing), 0.5, 1, 1.5, 2, 3, 4.5, 6, 8, 10, 12, and 18 h after administration of the morning dose on day 1. On day 2, samples were collected predosing and at 0.5, 1, 1.5, 2, 3, 4.5, 6, 8, 8.5, 9, 9.5, 10, 11, 12.5, 14, 16, 16.5, 17, 17.5, 18, 19, 20.5, 22, and 24 h after administration of the morning dose on day 2. On day 17, blood samples were collected predosing and at 0.5, 1, 1.5, 2, 3, 4.5, 6, 8, 8.5, 9, 9.5, 10, 11, 12.5, 14, 16, 16.5, 17, 17.5, 18, 19, 20.5, 22, 24, 26, 28, 32, 36, and 40 h after the administration of the morning dose for group I. For groups II to V, blood samples were collected predosing and at 0.5, 1, 1.5, 2, 3, 4.5, 6, 8, 10, 12, 12.5, 13, 13.5, 14, 15, 16.5, 18, 20, 22, 24, 28, 32, 36, and 40 h after administration of the morning dose. For the determination of concentrations of ritonavir and indinavir in plasma, a total of 440 ml of blood was collected for group I and 398 ml was collected for groups II to V. In addition, approximately 90 ml of blood was collected throughout the study for clinical laboratory tests. Urine samples (0 to 24 h) for the determination of indinavir concentrations were collected on days −1 (over a 12-h period, as a baseline), 1, 2, 17, and 18 for all groups.
The concentrations of ritonavir and indinavir in plasma were simultaneously measured on the basis of a validated reverse-phase high-performance liquid chromatography (HPLC) procedure with UV detection at 205 nm (44) and with a modified mobile phase. The assay precisions for indinavir and ritonavir were high, with CVs from replicate results ranging from 2.2 to 7.0% for indinavir and 2.9 to 6.7% for ritonavir. The lower limits of quantitation for the assay were 3.63 ng/ml for indinavir and 3.81 ng/ml for ritonavir with 1 ml of plasma.
In brief, plasma samples were supplemented with saquinavir prepared in methanol-water (1:1; vol/vol) as the internal standard. The samples were then alkalinized with 0.5 M Na2CO3 and extracted with hexane-ethyl acetate (1:1; vol/vol). The organic layer was evaporated to dryness and was redissolved in 300 μl of the reconstitution solution, a mixture of acetonitrile-methanol-aqueous tetramethylammonium perchlorate solution with trifluoroacetic acid, and washed with an aliquot of hexane. The aqueous layer was injected onto the HPLC system. Separation was achieved on a YMC ODS-AQ column (3-4-5; 50 by 4.6 mm; particle size, 3 μm) in conjunction with a YMC ODS-AQ cartridge (23 by 4.0 mm; particle size, 5 μm). The mobile phase for the separation was a mixture of acetonitrile, methanol, and an 0.05 M octanesulfonic acid solution containing 0.1% trifluoroacetic acid (37:5:58, by volume). The flow rate was maintained at 1.5 ml/min.
The extraction recoveries of indinavir and ritonavir at concentrations of 10.18, 101.8, and 1,018 ng/ml ranged from 85 to 111%. Standard curves were linear over the range of 3.63 to 3038 ng/ml for indinavir and 3.63 to 3,186 ng/ml for ritonavir. Samples with concentrations higher than the upper limit of the standard curve were diluted and reassayed. Mean correlation coefficients for the calibration curves for plasma and urine samples ranged between 0.9907 and 0.9999. Interassay CVs ranged from 4.5 to 6.8% for quality control samples for indinavir and from 4.7 to 6.3% for quality control samples for ritonavir for the following three pairs of ritonavir-indinavir concentrations: 20.24-20.36, 202.4-203.6, and 2,024-2,036 ng/ml.
Data analysis. (i) Noncompartmental analysis.
The pharmacokinetics of ritonavir and indinavir were characterized by standard noncompartmental methods (56). The Cmax, hour 8 concentration (C8), hour 12 concentration (C12) and the time to reach Cmax (Tmax) were obtained directly from the observed data. The AUC from hour 0 to infinity (AUC0–∞) was computed as AUCt + Ct/β, where t is the time of the last measurable concentration, Ct is the last measurable concentration, and β is the terminal elimination rate constant, which was computed from the last three measurable concentrations. AUC was calculated by using the linear trapezoidal rule. Apparent plasma clearance (CL/F) was computed as the quotient of daily dose and AUC0–∞ for indinavir. For ritonavir, the day 17 CL/F was computed as dose/AUC24, where AUC24 is the AUC over a 24-h period. CLR was computed as the quotient of the amount of unchanged indinavir recovered in urine over a time interval (Au,t) and AUCt. The day 17 CLR for indinavir was computed as Au,48/AUC48, where AUC48 is the AUC over a 48-h period. Indinavir plasma concentration-time profiles follow polyexponential decay; to evaluate the effect of ritonavir on the elimination of indinavir, the clinically relevant elimination rate constant (kτ) within one dose interval was computed by using the concentrations from hours 4.5 to 8 (k4.5–8).
(ii) Pharmacokinetic modeling.
To derive a greater appreciation of the factors affecting the interaction between ritonavir and indinavir, median ritonavir and indinavir concentration data were fitted with ADAPT II (written by David D’Argenio, University of Southern California, Los Angeles) by using the tenets of the well-stirred model of hepatic metabolism and quasiphysiologic parameterization that accounted for the first-pass metabolism of indinavir and CYP3A induction produced by ritonavir (56).
Assuming that a single dose of indinavir had little or no effect on the pharmacokinetics of ritonavir on day 17, ritonavir data for groups II to IV were simultaneously fitted first. The model is similar to the model described previously (25), with a few modifications. Exploratory analyses revealed that a zero-order input describes the characterization of the absorptive phase better than first-order input does, probably because ritonavir is poorly soluble at neutral pH, with absorption appearing to be dissolution rate limited. Thus, the rate was computed as dose/Tmax, with a lag time added as appropriate. Since ritonavir induces its own metabolism, the model estimated the extent of enzyme induction as a function of ritonavir dose. All four groups were fitted with a common value for the initial maximum rate of metabolism [Vmax(R),initial] and the Michaelis-Menten constant [Km(R)], where R represents ritonavir. The effect of CYP3A induction on Vmax as a function of time was modeled as Vmax(R),induced · [1 − exp(−kCYP3A) · t], where Vmax(R),induced is the maximum rate of metabolism due to induction at steady state and kCYP3A is the degradation rate constant of the CYP3A enzyme. Thus, at any time, the total maximal rate of metabolism is the sum of Vmax(R),initial and Vmax(R),induced · [1 − exp(−kCYP3A) · t]. Since groups III and IV received the same ritonavir dose, these two groups were modeled to have the same Vmax(R),induced. The volumes of distribution was estimated for each of the four groups.
After the extent of enzyme induction was estimated for each ritonavir dose level, the indinavir data for all five dose groups were fitted simultaneously, also with one set of Vmax(I),initial and Km(I), where I represents indinavir. The extent of enzyme induction by ritonavir was assumed to be the quotient of Vmax(R),induced and Vmax(R),initial since steady state was achieved on day 17. The intrinsic clearance for indinavir [CL(I)] in the absence of ritonavir was modeled as CL(I) = Vmax(I),initial/[Km(I) + CP(I)], where CP(I) is the observed indinavir concentrations and the fraction of the dose surviving first-pass metabolism was modeled as 1 − ER(I) = 1 − {[CL(I)/(Qh + CL(I)]}, where ER(I) is hepatic extraction ratio and Qh is hepatic plasma flow, which was assumed to be 50 liters/h (56). On day 17, the CL(I) in the presence of ritonavir was modeled as CL(I),induced = [Vmax(I),initial + Vmax(I),initial · Vmax(R),induced/Vmax(R),initial]/[Km(I) · (1 + CP(R)/Ki) + CP(I)], where CP(R) is the fitted ritonavir concentrations, and the fraction of the dose surviving first-pass metabolism was modeled as 1 − ER(I),induced = 1 − {CL(I),induced/[50 + CL(I)induced]}. A two-compartment model was assumed for indinavir, with CLR being assumed to be 9 liters/h for all groups. The two intercompartmental rate constants were highly correlated and weighting dependent. After inspection of residual errors, k21 (first-order rate constant for transfer of drug from compartment 2 to compartment 1) was fixed as 0.12 h−1, and k12 was estimated by curve fitting. Since indinavir also has limited aqueous solubility, the rate of absorption of indinavir was also computed as dose/Tmax, and the volume of distribution was estimated for each dose group. Since only predose ritonavir concentrations were obtained during days 4 to 16, to characterize the extent of induction, the day 17 plasma ritonavir concentrations were downweighted relative to the predose concentrations at a 1:5 ratio. For fitting of indinavir data, all datum points had a weight of unity. The final model was selected on the basis of R2 values, weighted sum of residuals, and percent CV for parameter estimates.
(iii) Statistical analysis.
For analyses other than paired t tests, ritonavir and indinavir concentration measurements (AUC, Cmax, C8, C12, Au,t, and CLR) were logarithmically transformed to provide approximately normal probability distributions and/or approximately equal variances across groups. With the exception of Tmax, paired t tests were performed on the differences between day 1 and day 17 indinavir pharmacokinetic parameters to assess the effect of ritonavir on the pharmacokinetics of indinavir. A signed rank test was performed on the changes of indinavir Tmax. To assess whether indinavir and ritonavir can potentially be coadministered as b.i.d. regimens, indinavir pharmacokinetic parameters between the combination regimens and the standard 800-mg q8h regimen on day 17 were compared by one-way analyses of covariance (ANCOVAs) with effects for group, body weight, an appropriate day 2 indinavir pharmacokinetic variable, and an interaction term of that variable with group. The appropriate day 2 indinavir pharmacokinetic covariate was selected on the basis of the fraction of variability accounted for by the model. Within this ANCOVA framework, all possible pairwise t tests were performed for testing whether group means were the same. In addition, since all subjects received indinavir at 800 mg q8h on day 2, paired t tests were also performed on the differences of indinavir Cmax, AUC (adjusted for the q12h regimen for day 17 for groups II to V), C12 (relative to C8), and CLR between day 17 and day 2 for each combination-dose group. To assess dose proportionality for indinavir in the presence of a fixed ritonavir dose, ANCOVAs were performed for groups III and IV, with effects for body weight, an appropriate day 2 indinavir pharmacokinetic variable, and its interaction term with indinavir dose level. In all statistical analyses, any P value less than or equal to 0.05 was considered statistically significant. Results with P values of >0.05 and ≤0.1 were considered a priori as marginally significant.
RESULTS
Indinavir pharmacokinetics. (i) Effect of ritonavir on indinavir pharmacokinetics.
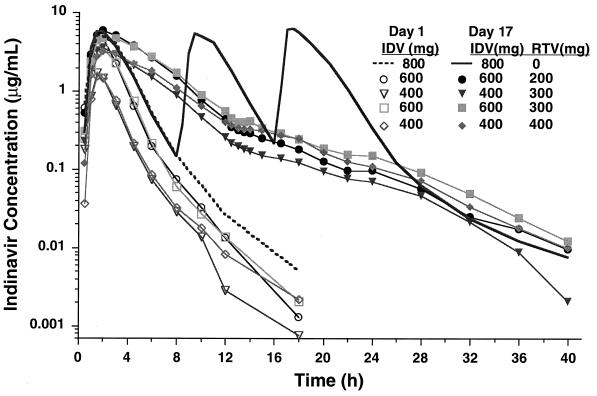
As shown in Table 1 and Fig. 1, ritonavir statistically significantly increased plasma indinavir concentrations compared to those achieved when indinavir was given alone. Across the four combination regimens, the increases in AUC, Cmax, and C8 ranged from 185 to 475%, 21 to 110%, and 11- to 33-fold, respectively. The kτ values (rate of disappearance from C4.5 to C8) decreased by 53 to 67%, with the corresponding half-life increasing from 1.2 ± 0.2 to 2.7 ± 0.1 h. In general, the effects were larger for the 400-mg indinavir dose (groups III and V) than for the 600-mg dose (groups II and IV). The slightly delayed Tmax (averaging 20 to 40 min later) when indinavir was given with ritonavir was only marginally significant (P = 0.063 for groups II and V) or was not statistically significant (P > 0.125 for groups III and IV). Also, with the exception of group V (from 12.0 to 8.1 liters/h; P = 0.030), indinavir CLR was not affected by ritonavir. The pharmacokinetic parameters for the control group (group I) determined for the morning dose on day 17 were not statistically significantly different from those determined on day 1.
TABLE 1.
Pharmacokinetic parameters for indinavir and ritonavir administered alone or in combinationa
| Study (time) and IDV dose (mg) or dosage | RTV dosage | Group | AUC (μg · h/ml)b | Cmax (μg/ml)c | C8 or C12 (μg/ml)d | Tmax (h)c | t1/2e | CL/F (liters/h) | CLR (liters/h) |
|---|---|---|---|---|---|---|---|---|---|
| Indinavir IDV alone (day 1) | |||||||||
| 800 | I | 17 ± 5 | 5.72 ± 1.62 | 0.15 ± 0.10 | 1.9 ± 0.5 | 1.0 | 55 ± 29 | 9.23 ± 2.75 | |
| 600 | II | 12 ± 3 | 4.95 ± 1.06 | 0.07 ± 0.03 | 1.8 ± 0.4 | 1.1 | 55 ± 14 | 9.53 ± 4.64 | |
| 400 | III | 4 ± 1 | 1.82 ± 0.80 | 0.03 ± 0.01 | 1.6 ± 0.2 | 1.3 | 113 ± 37 | 14.3 ± 7.35 | |
| 600 | IV | 10 ± 4 | 3.83 ± 1.31 | 0.06 ± 0.02 | 1.8 ± 0.6 | 1.0 | 70 ± 28 | 10.5 ± 2.94 | |
| 400 | V | 4 ± 2 | 1.66 ± 0.65 | 0.03 ± 0.02 | 1.8 ± 0.4 | 1.3 | 180 ± 241f | 12.0 ± 3.86 | |
| IDV alone (day 2) | |||||||||
| 800 q8h | I | 57 ± 16 | 7.14 ± 1.11 | 0.18 ± 0.10 | 2.0 ± 0.5 | 1.1 | 45 ± 14 | 8.57 ± 3.90 | |
| 800 q8h | II | 51 ± 13 | 6.86 ± 1.54 | 0.14 ± 0.05 | 1.8 ± 0.3 | 1.0 | 50 ± 10 | 9.38 ± 4.28 | |
| 800 q8h | III | 50 ± 15 | 5.97 ± 1.24 | 0.11 ± 0.05 | 1.7 ± 0.3 | 1.0 | 51 ± 12 | 9.17 ± 1.17 | |
| 800 q8h | IV | 54 ± 16 | 6.97 ± 2.34 | 0.13 ± 0.04 | 1.6 ± 0.4 | 1.0 | 51 ± 26 | 9.25 ± 3.49 | |
| 800 q8h | V | 49 ± 18 | 5.76 ± 1.52 | 0.13 ± 0.08 | 1.8 ± 0.5 | 1.1 | 58 ± 31 | 9.11 ± 1.97 | |
| IDV with placebo or RTV (day 17) | |||||||||
| 800 q8h | Placebo | I | 57 ± 15 | 6.25 ± 1.01 | 0.15 ± 0.08 | 1.7 ± 0.4 | 1.1 | 45 ± 13 | 9.59 ± 3.19 |
| 600 | 200 q12h | II | 33 ± 5 | 5.97 ± 1.12 | 0.43 ± 0.14 | 2.3 ± 0.5 | 2.7 | 18 ± 2 | 8.36 ± 3.29 |
| 400 | 300 q12h | III | 20 ± 3 | 3.72 ± 0.64 | 0.26 ± 0.08 | 1.9 ± 0.2 | 2.8 | 21 ± 3 | 9.92 ± 2.02 |
| 600 | 300 q12h | IV | 33 ± 8 | 5.06 ± 1.16 | 0.55 ± 0.26 | 2.3 ± 0.7 | 2.7 | 19 ± 5 | 9.14 ± 3.76 |
| 400 | 400 q12h | V | 22 ± 3 | 3.48 ± 0.52 | 0.40 ± 0.18 | 2.4 ± 0.7 | 2.7 | 18 ± 2 | 8.14 ± 2.16 |
| RTV with IDV (day 17) | |||||||||
| 600 | 200 q12h | II | 52 ± 12 | 5.06 ± 1.27 | 0.71 ± 0.18 | 2.8 ± 0.5 | 2.7 | 9 ± 2 | 0.24 ± 0.11 |
| 400 | 300 q12h | III | 78 ± 22 | 7.42 ± 1.16 | 0.89 ± 0.40 | 2.9 ± 0.4 | 2.5 | 9 ± 3 | 0.18 ± 0.07 |
| 600 | 300 q12h | IV | 81 ± 26 | 6.84 ± 2.45 | 1.27 ± 0.66 | 2.9 ± 0.8 | 3.1 | 9 ± 4 | 0.22 ± 0.06 |
| 400 | 400 q12h | V | 127 ± 33 | 8.43 ± 1.07 | 1.59 ± 0.82 | 2.9 ± 0.4 | 2.8 | 7 ± 2 | 0.26 ± 0.09 |
Abbreviations: IDV, indinavir; RTV, ritonavir; t1/2, half-life.
AUC24 for day 1 and AUC0–∞ for days 2 and 17.
After the first dose on days 2 and 17.
C8 for days 1 and 2 for indinavir, C12 for day 17 for indinavir and ritonavir, and day 17 C8 for indinavir were 1.55 ± 0.33, 0.89 ± 0.27, 1.70 ± 0.57, and 1.08 ± 0.34 μg/ml for groups II to V, respectively.
Harmonic means; estimated from hour 4.5 to hour 8 concentrations for indinavir and from hour 8 to hour 12 concentrations for ritonavir.
Excluding subject 120, the CL/F would be 95.3 ± 17.3 liters/h.
FIG. 1.
Mean plasma indinavir (IDV) concentration-time profiles after an administration of single dose of 400 mg (groups III and V), 600 mg (groups II and IV), or 800 mg (group I) alone or in combination with ritonavir (RTV) maintained at 200 mg (group II), 300 mg (groups III and IV), or 400 mg (group V) q12h for 2 weeks.
On the basis of ANCOVA of day 17 data, the regimen of indinavir at 400 mg plus ritonavir at 400 mg, if given q12h, would likely yield AUC values that are only marginally significantly lower (P = 0.053) than those achieved with the control regimen of indinavir at 800 mg q8h. The other three combination regimens likely would have AUC values statistically significantly different from that for the standard regimen (P = 0.003, 0.005, and 0.003 for groups II, III, and IV, respectively). The two 600-mg indinavir-containing regimens (groups II and IV) would likely yield a slightly higher q12h-adjusted AUC, while the regimen of indinavir at 400 mg plus ritonavir at 300 mg (group III) would likely yield a slightly lower AUC. The mean Cmax for group II was not statistically significantly different from that for the control group, while those for groups III, IV, and V were all statistically significantly lower than that for the control group (P < 0.05 for all comparisons). All four combination-dose groups had statistically significantly higher C12s compared to the C8s for the control group (P < 0.001 for all comparisons). The CLR for all four combination-dose groups was not statistically significantly different from that for the control group (group I), averaging 9.1 liters/h across the five dose groups. Body weight was shown to be a statistically significant explanatory variable for indinavir Cmax, AUC, and CLR in the analyses described above. There was no correlation between the creatinine clearance and CLR of indinavir given alone or with ritonavir, probably in part due to the small creatinine clearance range for those subjects with normal renal function.
The results of paired t tests comparing the pharmacokinetic parameters determined on day 17 and day 2 are generally consistent with those obtained from ANCOVA. For the control group (group I), the AUC24, Cmax, C8, and CLR values determined on day 2 and day 17 were not statistically significantly different, indicating the lack of any study day effects on the pharmacokinetics of indinavir.
(ii) Effect of ritonavir dose.
To assess whether an increase in the ritonavir dose had corresponding effects on plasma indinavir concentrations for a fixed indinavir dose, indinavir pharmacokinetics were compared between groups II and IV and between groups III and V in the framework of ANCOVA. The results showed that a 600-mg dose of indinavir yielded similar AUC and trough concentrations (Ctroughs) when it was administered with 200 or 300 mg of ritonavir q12h. However, the Cmax for group IV was marginally significantly lower than that for group II (P = 0.051). For the 400-mg indinavir doses, group V, which received the higher dose of ritonavir, yielded marginally significantly higher Ctroughs (P = 0.053) compared with those for group III but AUCs and Cmaxs similar with those for group III.
(iii) Dose proportionality of indinavir.
To assess whether plasma indinavir concentrations increased proportionally with indinavir dose for a fixed ritonavir dose, indinavir pharmacokinetics were compared between groups III and IV in the framework of ANCOVA. The results showed that compared to group III, group IV had statistically significantly higher AUCs, Cmaxs, and C12s (P = 0.0001, 0.006, and 0.002, respectively). The CLRs were similar between the two combination regimens. The estimates for AUC and C12 ratios for the two combination regimens (1.64 and 1.95, respectively) were slightly higher than the theoretical value of 1.5 (600 mg/400 mg = 1.5); however, the Cmax ratio (1.27) was slightly less than the theoretical value of 1.5.
Ritonavir pharmacokinetics.
The mean steady-state plasma ritonavir concentrations determined in this study were comparable to those of a previous 2-week study of ritonavir with healthy HIV-infected subjects (25). Predose ritonavir concentrations reached a maximum level after dosing for about 2 days; however, due to autoinduction, plasma ritonavir concentrations decreased with time, with stable concentrations reached by the end of 2 weeks.
Pharmacokinetic modeling results.
The observed data were well described by pharmacokinetic models that used the principles of the well-stirred model of hepatic metabolism, competitive inhibition, and autoinduction. The R2 values from the regression for indinavir ranged between 0.87 and 0.93, and those for ritonavir ranged between 0.89 and 0.96. The model estimated that the extents of induction were 107, 148, and 149% for ritonavir dosages of 200, 300, and 400 mg q12h, respectively. The half-life for CYP3A turnover was estimated to be 44.5 h, which is similar to that reported in the literature (36). The Vmax and Km for indinavir was estimated to be 86.3 mg/h and 1.11 μg/ml, respectively. The in vivo Ki was estimated to be 0.10 μg/ml, which agrees reasonably well with the estimated in vitro Ki of 0.085 μg/ml. The volume of distribution ranged from 37.9 to 40.1 liters for ritonavir for the four groups and ranged from 54 to 67 liters for indinavir. No apparent correlation was found between the volume of distribution and dose size for either drug. The errors associated with all parameter estimates were under 37%. However, it should be noted that the estimates presented above were based on the median data, which did not account for intersubject variability associated with both drugs.
Intersubject variability.
Intersubject variability in indinavir pharmacokinetic parameters was reduced when indinavir was coadministered with ritonavir. Across groups, the CVs for AUC24, Cmax, and C8 averaged 30, 21, and 50%, respectively, for the standard indinavir regimen of 800 mg q8h on day 2. When indinavir was given with ritonavir on day 17, the CVs averaged 16, 19, and 39% for AUC24, Cmax, and C12, respectively. For ritonavir, the CVs for day 17 AUC24, Cmax, and predose concentration were 28% ± 4%, 23% ± 10%, and 39% ± 11%, respectively.
Safety assessment.
All 39 subjects enrolled in the study completed the study. It should be noted that since the study design does not permit statistical analyses on whether the combination regimen had effects on the safety of either drug given alone, no such analysis was performed. However, it was possible to assess the dose-response relationship for ritonavir during the ritonavir-alone period (day 3 to day 16).
Overall, there were no changes of clinical relevance in vital signs recordings, physical examinations, or electrocardiograms during the conduct of the study. All adverse events observed were mild in nature. The most frequently occurring adverse events that were possibly or probably related to the study drugs were headache and asthenia during indinavir dosing alone and circumoral paresthesia, headache, and nausea after dosing with ritonavir alone or during dosing with indinavir and ritonavir in combination. Circumoral paresthesia was the only event that showed a statistically significant monotonic increasing trend with ritonavir dose during dosing with ritonavir alone. There was no clear correlation between the incidence of adverse events, including circumoral paresthesia, and Cmaxs or AUCs of either drug within the same treatment group.
The results of hematology analyses were generally unremarkable. One subject in group IV exhibited a low hematocrit (31.3%) and hemoglobin (10.7 g/dl) during days 10 through 16. This was attributed to repetitive blood drawing and resolved in the poststudy follow-up visits.
For abnormal clinical chemistry values, one subject had increased triglyceride levels (435 and 409 mg/dl on days 10 and 19, respectively) and cholesterol levels (292 mg/dl on day 19), one subject had an increased bilirubin level (2.5 mg/dl on days 10 and 12), and three subjects had increased gamma-glutamyl transferase levels (ranging from 106 to 201 IU/liter); all of these were attributed to ritonavir. Two subjects had increased bilirubin levels (1.8 and 2.3 mg/dl on day 3) that were attributed to indinavir. For triglycerides, the ritonavir placebo group (group I) had a statistically significantly lower mean level (90 mg/dl) than the groups receiving ritonavir at 300 mg (groups III and IV; 160 mg/dl) and the group receiving ritonavir at 400 mg (group V; 163 mg/dl) on day 16. Similar results were observed for cholesterol (175, 201, and 211 mg/dl for group I, groups III and IV, and group V, respectively).
DISCUSSION
Ritonavir induces its own metabolism. This study assessed the pharmacokinetics of indinavir in the presence of ritonavir under steady-state (induced) conditions, which are generally achieved after dosing for approximately 2 weeks. It should be noted that since the pharmacokinetics of indinavir alone were assessed under noninduced conditions, the actual magnitude of the inhibitory effect is underestimated in this study.
Indinavir is reported to be primarily eliminated by metabolism via CYP3A, with less than 20% of a dose being recovered as unchanged drug in the urine after oral dosing. The fecal recovery of unchanged indinavir after oral administration of a 400-mg dose was 19%, suggesting that the fraction of dose absorbed is at least 0.8 (45). The results of our study confirmed that the pharmacokinetics of indinavir are moderately nonlinear, with CL/F averaging 104 (excluding one subject who had a CL/F value of 774 liters/h), 63, and 55 liters/h for doses of 400, 600, and 800 mg, respectively. The CLR of indinavir averaged 9 liters/h after the administration of three 800-mg doses given q8h. Assuming that the fraction of the indinavir dose absorbed and reaching the portal circulation is 0.8 and that the CLR is 9 liters/h on the basis of well-stirred model of hepatic metabolism (56), the extents of first-pass hepatic metabolism, expressed as percent extraction = 100 · CLp/(CLp + Qh), where CLp is the metabolic clearance from plasma and Qh is the hepatic plasma flow rate at 50 liters/h, are computed to be 62, 50, and 47% for a 400-, 600-, or 800-mg dose given alone.
Consistent with the interaction effect observed in human liver microsomes and in rats, the results of this study showed that ritonavir increased the AUC of indinavir by 2.9- to 5.8-fold and the Cmax by 1.2- to 2.1-fold compared to those on day 1 for the indinavir-alone regimens. The net change in AUC could theoretically arise from two main effects: reduction in the systemic clearance and increase in the fraction of the dose systemically available, F. The F term has at least three components: fabs, the fraction of the dose absorbed; fgi, the fraction of the dose not metabolized or otherwise eliminated by gastrointestinal tissue; and fh, the fraction of the dose surviving first-pass hepatic metabolism.
Effect of ritonavir on F of indinavir.
Although the oral bioavailability of indinavir has been theorized to be limited by pH-dependent solubility (3, 4, 12, 40) and regiospecificity (35), the fraction of indinavir dose absorbed was approximately 0.8 (45). Therefore, the observed increases in indinavir AUC cannot be accounted for by an improvement in the fabs of indinavir.
For drugs with high intrinsic clearance, the gut wall may contribute substantially to the overall first-pass effect (30, 50). It is well known that CYP3A, the major isoform in the metabolism of ritonavir and indinavir, is present in intestinal tissue (31, 32, 41, 50, 61). However, assuming that the enterocyte CYP3A content is not greater than 10% of hepatocytes (50), and the maximal hepatic metabolic rate is 86 mg/h, as determined from the curve fitting described above, then Vmax,gi is approximately 9 mg/h. Assuming that indinavir is absorbed with zero-order kinetics over a 1.5-h period, then the metabolism in the intestinal epithelial cells represents less than 4% of the administered dose.
However, in considering potential changes in indinavir fgi by ritonavir, the role of glycoprotein P should be addressed. Glycoprotein P is a transmembrane protein associated with a phenotype of multidrug resistance (MDR1). It is capable of actively transporting drug out of intestinal, hepatic, and brain capillary endothelial cells. Recent reports have shown that HIV protease inhibitors are substrates of glycoprotein P and are capable of inhibiting, although not potently inhibiting, the transport of some known glycoprotein P substrates (21, 29, 39). Therefore, it is theoretically possible that ritonavir may increase the fgi of indinavir by inhibiting glycoprotein P transport.
In contrast to fgi, the fraction of the dose surviving first-pass hepatic metabolism (fh) can be estimated with a higher degree of certainty. When given with ritonavir, CL/F averaged approximately 19 liters/h for the four combinations. The fraction of indinavir dose surviving the first-pass hepatic metabolism when indinavir was given with ritonavir should be greater than 0.8, which is substantially higher than the day 1 fh under noninduced conditions. Therefore, part of the inhibitory effect of ritonavir should be due to the inhibition of the first-pass metabolism of indinavir.
Effect of ritonavir on the postabsorptive systemic indinavir clearance.
As can be seen from Fig. 1, the decline in plasma indinavir concentrations after Tmax for day 17 during coadministration with ritonavir was significantly slower compared to the decline when indinavir was given alone. The disappearance rate constant during hours 4.5 to 8 decreased about 50 to 63%, suggesting an effect (clearance ratio) of approximately two- to threefold on the basis of indinavir-alone data determined on day 1. Again, since the day 1 data were obtained under noninduced conditions, the comparison presented above probably also underestimates the postabsorptive inhibitory effect exerted by ritonavir.
In vitro-in vivo correlation in Ki.
The estimate of the in vivo Ki must be appreciated as being highly model dependent and is limited by factors that are indeterminate. For example, the model did not address the possibility of time delays in inhibition (hysteresis), the protein binding effects, and the potential for inhibitory effects from metabolism in intestinal epithelial cells or glycoprotein P transport. Despite these uncertainties, the estimated Ki value (0.10 μg/ml) agrees extremely well with the in vitro Ki (0.085 μg/ml).
Factors affecting the ritonavir-indinavir interaction. (i) Fraction of indinavir dose excreted in urine (fu).
Since the renal component of indinavir’s elimination clearance was not affected by ritonavir, the expected AUC ratio should be larger for subjects who metabolize a relatively large fraction of the dose, fm (56). Indeed, a positive relationship between AUC ratio and fm was observed (data not shown), where fm was estimated as the fraction of dose not excreted in the urine.
(ii) Indinavir dose.
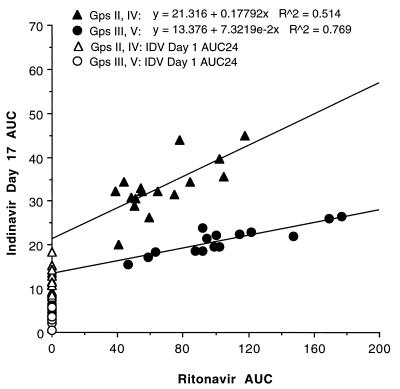
For a fixed ritonavir dose, an increase in the indinavir dose resulted in a relatively proportional increase in AUC, a more than proportional increase in C12, and a slightly less than proportional increase in Cmax. For a fixed indinavir dose (400 or 600 mg), an increase in the ritonavir dose did not have a proportional effect on the concentrations of indinavir in plasma. However, as shown in Fig. 2, there is a shallow but positive correlation between ritonavir AUCs and indinavir AUCs for a fixed indinavir dose. Indinavir metabolic clearance via CYP3A becomes less dominant with increasing ritonavir exposure, and the postabsorptive total clearance asymptotically approaches the non-CYP3A clearance, which is a composite of CLR and perhaps contributions from glucuronidation (3) (potentially induced) and other noninhibited isoforms. These factors may explain why indinavir AUCs for the two 600-mg and the two 400-mg regimens were relatively similar.
FIG. 2.
Relationship between day 17 ritonavir AUC and day 17 indinavir AUC for 400- and 600-mg indinavir doses (subjects in groups II and IV each received 600 mg of indinavir alone or with 200 or 300 mg ritonavir q12h, respectively; subjects in groups III and V each received 400 mg of indinavir alone or with 300 or 400 mg ritonavir q12h, respectively).
In this study, ritonavir significantly reduced the intersubject variability in indinavir pharmacokinetic parameters, particularly AUCs and Ctroughs. Since the indinavir AUC was negatively correlated with fm when it was given alone and since the inhibitory effect by ritonavir was larger for subjects with a higher fm, it is not unexpected that the intersubject variability in indinavir AUC and Ctrough was significantly reduced when indinavir was coadministered with ritonavir.
Ritonavir and indinavir combination as potential b.i.d. regimens.
Since treatment of HIV infection requires long-term therapy, the requirements of rigid restrictions on dosing and meal schedules in indinavir-containing regimens are expected to result in challenges for patient compliance. This study was conducted to evaluate if combination therapy with indinavir and ritonavir can be given as b.i.d. regimens and therefore improve upon the drawbacks of indinavir therapy. Since no significant accumulation was observed after the administration of multiple doses of indinavir (45), the single-dose data obtained in this study can be used to project multiple-dose exposures.
The results of this study showed that compared to the standard 800-mg q8h regimen, all four combination regimens, if given q12h, will likely have higher Ctroughs (C12 versus C8 of the standard q8h regimen), lower Cmax values, and similar CLRs. The lower Cmax values associated with the combination regimens are expected to have a minimal effect on antiviral activity, particularly for combination regimens that yield comparable indinavir AUCs, because the Ctroughs for combination regimens are expected to be at least 2.5-fold greater than the protein-binding corrected IC90 of indinavir. This study also showed that ritonavir significantly reduced the intersubject variability in indinavir AUCs and Ctroughs. In addition, the results of a recent study showed that the bioavailability of indinavir, when given together with ritonavir, was not affected by regular meals containing 32% of calories from fat (23). Thus, the combination regimens offer several significant practical benefits.
Ritonavir and indinavir combination in renal complications.
The CLR of indinavir, when given alone at 800 mg t.i.d., accounts for roughly 20% of the total clearance. Under such conditions, renal impairment is unlikely to have substantial effects on total clearance. However, in the presence of ritonavir, the CLR of indinavir approaches 50% of CL/F. Thus, in patients with substantial impairment in renal function, the possibility of a dosage reduction should be entertained.
Due to highly pH-dependent aqueous solubility, nephrolithiasis, which is associated with indinavir crystals, is reported in approximately 10% of patients treated with indinavir (5, 18, 33, 40, 45, 53, 58). The results of this study showed that ritonavir has no overall effect on the CLR of indinavir. Thus, for combination regimens that maintain the AUCs of the standard indinavir regimen, the total daily urinary excretion of unchanged indinavir should remain unchanged. It should be noted that the results of two recent clinical studies showed that in patients treated with 400 mg of ritonavir plus 400 mg indinavir b.i.d. combination regimen, no nephrolithiasis was reported. Since the combination regimen provides similar indinavir AUC, but substantially lower indinavir Cmax compared to that provided by the indinavir 800-mg t.i.d. regimen (55, 62), it is possible that the decreased indinavir Cmax in the combination regimen is responsible for the reduction in the incidence of nephrolithiasis.
Antiviral activities of combination regimens.
For protease inhibitor combination regimens, there are concerns about whether low drug concentrations (concentrations less than the IC90) in the combination regimen will favor the emergence of resistant viral strains. The results of our recent in vitro experiments with MT4 cells in the presence of 50% human serum and 10% fetal calf serum showed that there are additive or synergistic anti-HIV-1IIIB (wild type) effects between ritonavir and other protease inhibitors, particularly at concentrations near or below the IC50s (24, 46). It should be noted that the ongoing clinical studies show that regimens with dual protease inhibitors have potent antiviral effects (9, 13, 51, 55, 60, 62).
In conclusion, in addition to allowing the use of reduced doses of both drugs, use of the combination of ritonavir and indinavir can also offer the benefit of two potent HIV protease inhibitors. The preliminary results from use of the regimen of 400 mg of ritonavir and 400 mg of indinavir in combination b.i.d. in patients have shown that the regimen may be highly efficacious and well tolerated (55, 62).
ACKNOWLEDGMENT
We are grateful for the sharing of the in vitro Ki data by Gondi Kumar (Drug Metabolism, Abbott Laboratories).
REFERENCES
- 1.Abbott Laboratories. Norvir™ product information. North Chicago, Ill: Abbott Laboratories; 1998. [Google Scholar]
- 2.Acosta E P, Henry K, Weller D, Page L M, Bacon L, Rhame F, Gilson I, Rosenstein H, Schacker T, Fletcher C V. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Indinavir pharmacokinetics and relationships between exposures and antiviral effect, abstr. A-15; p. 3. [Google Scholar]
- 3.Balani S K, Woolf E J, Hoagland V L, Sturgill M G, Deutsch P J, Yeh K C, Lin J H. Disposition of indinavir, a potent HIV-1 protease inhibitor, after a oral dose in humans. Drug Metab Dispos. 1996;24:1389–1394. [PubMed] [Google Scholar]
- 4.Bjornsson T, Chiou R, Deutsch P, Haddix H, Hoagland V, Justice S, Nessly M, Pomerantz R, Saag M, Squires K, Teppler H, Waldman S, Woolf E, Yeh K C. Pharmacokinetics of indinavir. Pharm Res. 1996;13:S485. [Google Scholar]
- 5.Bruce R G, Munch L C, Hoven A D, Jerauld R S, Greenburg R, Porter W H, Rutter P W. Urolithiasis associated with the protease inhibitor indinavir. Urology. 1997;50:513–518. doi: 10.1016/S0090-4295(97)00399-3. [DOI] [PubMed] [Google Scholar]
- 6.Burger D M, Koopmans P P, Brinkman K, Keuter M, Dolmans W, Hoetelmans R M W, Meenhorst P L, Mulder J W, Wuis E W, Hekster Y A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Therapeutic drug monitoring of the HIV-protease inhibitor indinavir, abstr. A-19; p. 4. [Google Scholar]
- 7.CAESAR Coordinating Committee. Randomized trial of addition of lamivudine or lamivudine plus loviride to zidovudine-containing regimens for patients with HIV-1 infection: the CAESAR trial. Lancet. 1997;349:1413–1421. [PubMed] [Google Scholar]
- 8.Cameron D W, Heath-Choizzi M, Kravick S, Mills R, Potthoff A, Henry D, Leonard J M The Advanced HIV Ritonavir Study Group. Presented at the XI International Conference on AIDS. 1996. Prolongation of life and prevention of AIDS complications in advanced HIV immunodeficiency with RTV, update, abstr. Mo.B.411. [Google Scholar]
- 9.Cameron D W, Japour A, Mellors J, Farthing C, Cohen C, Markowitz M, Poretz D, Follansbee S, Ho D, Mahon D M, Berg J, Nieves J, Xu Y, Rode R, Salgo M, Leonard J, Sun E. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Antiretroviral safety & durability of ritonavir (RIT)-saquinavir (SQV) in protease inhibitor-naive patients in year two of follow-up, abstr. 388. [Google Scholar]
- 10.Carpenter C J, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1997: updated recommendations of the International AIDS Society-USA panel. JAMA. 1997;277:1962–1969. [PubMed] [Google Scholar]
- 11.Chiba M, Hensleigh M, Nishime J A, Balani S K, Lin J H. Role of cytochrome P450 3A4 in human metabolism of MK-639, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos. 1996;24:307–314. [PubMed] [Google Scholar]
- 12.Chiou R, Deutsch P, Carides A, Fu I, Kwei G, Hoagland V, Lambert G, Sturgill M, Yeh K C. Indinavir absorption following extended-release formulations in man and in the dog. Pharm Res. 1996;13:S498. [Google Scholar]
- 13.Cohen C, Siemon-Hryczk P, Pilson R, Holzknecht B, Phillips K, Karol C, Palleja S. Abstracts of the 12th World Congress on AIDS. 1998. Potent and convenient Fortovase (SQV) SGC BID regimens in combination with 2 nucleosides or nelfinavir (NFV) plus 1 nucleoside in HIV-1 infected patients, abstr. 12314. [Google Scholar]
- 14.Danner S A, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintade V, Aguado A G, de Lomas J G, Delgado R, Borleffs J C C, Hsu A, Valdes J M, Boucher C A B, Cooper D A. Safety, pharmacokinetics, and preliminary efficacy of RTV, an inhibitor of HIV-1 protease. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 15.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 16.Delta Coordinating Committee. Delta: a randomized double-blind trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Lancet. 1996;348:283–291. [PubMed] [Google Scholar]
- 17.Drusano G L, Sadler B M, Millard J, Symonds W T, Tisdale M, Rawls C, Bye A the 141W94 International Product Development Team. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Pharmacodynamics of 141W94 as determined by short term change in HIV RNA: influence of viral isolate baseline EC50, abstr. A-16; p. 4. [Google Scholar]
- 18.Gentle D L, Stoller M L, Jarrett T W, Ward J F, Geib K S, Wood A F. Protease inhibitor-induced urolithiasis. Urology. 1997;50:508–511. doi: 10.1016/S0090-4295(97)00401-9. [DOI] [PubMed] [Google Scholar]
- 19.Hammer S M, Katzenstein D A, Hughes M D, Gundacker H, Schooley R T, Haubrich R H, Henry W K, Lederman M M, Phair J P, Niu M, Hirsch M S, Merigan T C. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. N Engl J Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 20.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD-4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 21.Hoof T, Brandt T, Demmer A, Stellbrink H J. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Interaction of HIV protease inhibitors with MDR1—competitive binding and resistance modulation, abstr. I-111; p. 263. [Google Scholar]
- 22.Hsu, A., G. R. Granneman, G. Cao, L. Carothers, T. El-Shourbagy, P. Baroldi, K. Erdman, F. Brown, E. Sun, and J. M. Leonard. Pharmacokinetic interactions between two human immunodeficiency virus protease inhibitors, ritonavir and saquinavir. Clin. Pharmacol. Ther. 63:453–464. [DOI] [PubMed]
- 23.Hsu A, Granneman G R, Heath-Chiozzi M, Wong C, Japour A, Manning L, Brooks R, Sun E. Abstracts of the 12th World Congress on AIDS. 1998. Indinavir can be taken with regular meals when taken with ritonavir, abstr. 22361. [Google Scholar]
- 24.Hsu A, Granneman G R, Molla A, Vasavanonda S, Japour A, Kempf D, Sun E. Abstracts of the 12th World Congress on AIDS. 1998. Ritonavir-containing dual protease inhibitor regimens may have synergistic antiviral effects in patients—based on in vitro model, abstr. 22350. [Google Scholar]
- 25.Hsu A, Granneman G R, Witt G, Locks C, Denissen J, Molla A, Valdes J, Smith J, Erdman K, Lyons N, Niu P, Decourt J-P, Fourtillan J-B, Girault J, Leonard J M. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kempf D, Marsh K C, Denssen J, McDonald E, Vasavanonda S, Flentge C A, Green B E, Fino L, Park C H, Kong X-P, Wideburg N E, Saldivar A, Ruiz L, Kati W M, Sham H L, Robins T, Stewart K D, Hsu A, Plattner J J, Leonard J M, Norbeck D W. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci USA. 1995;92:2484–2488. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempf D J, Molla A, Sun E, Danner S, Boucher C, Leonard J. Abstracts of the 4th Conference on Retroviruses and Opportunistic Infections. 1997. The duration of viral suppression is predicted by the viral load during protease inhibitor therapy, abstr. 603. [Google Scholar]
- 28.Kempf D J, Marsh K C, Kumar G N, Rodrigues A D, Denissen J F, McDonald E, Kukulka M J, Hsu A, Granneman G R, Baroldi P A, Sun E, Pizzuti D, Plattner J J, Norbeck D W, Leonard J M. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim R B, Fromm M F, Wandel C, Leake B, Wood A J J, Roden D M, Wilkinson G R. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolars J C, Awni W M, Merion R M. First-pass metabolism of cyclosporin by the gut. Lancet. 1991;338:1488–1490. doi: 10.1016/0140-6736(91)92302-i. [DOI] [PubMed] [Google Scholar]
- 31.Kolars J C, Lown K S, Schmiedlin-Ren P, Ghosh M, Fang C, Wrighton S A, Merion R M, Watkins P B. CYP 3A gene expression in human gut epithelium. Pharmacokinetics. 1994;4:247–259. doi: 10.1097/00008571-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Kolars J C, Schmiedlin-Ren P, Schuetz J D, Fang C, Watkins P B. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp J B, Miller K D, Mican J A, Feuerstein I M, Vaughan E, Baker C, Pannell L K, Falloon J. Crystalluria and urinary tract abnormalities associated with indinavir. Ann Int Med. 1997;127:119–125. doi: 10.7326/0003-4819-127-2-199707150-00004. [DOI] [PubMed] [Google Scholar]
- 33a.Kumar, G. (Abbott Laboratories). Personal communication.
- 34.Kumar G N, Rodrigues A D, Buko A M, Denissen J F. Cytochrome P-450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. [PubMed] [Google Scholar]
- 35.Kwei G Y, Novak L B, Hettrick L A, Reiss E R, Ostovic D, Loper A E, Lui C Y, Higgins R J, Chen I W, Lin J H. Regiospecific intestinal absorption of the HIV protease inhibitor L-735,524 in beagle dog. Pharm Res. 1995;12:884–888. doi: 10.1023/a:1016269206048. [DOI] [PubMed] [Google Scholar]
- 36.Lai A A, Levy R H, Cutler R E. Time-course of interaction between carbamazepine and clonazepam in normal man. Clin Pharmacol Ther. 1979;24:316–323. doi: 10.1002/cpt1978243316. [DOI] [PubMed] [Google Scholar]
- 37.Lalezari J, Haubrich R, Burger H, Bettie D, Donatacci L, Salgo M P the NV 14256 Study Team. Abstracts of the XI International Conference on AIDS. 1996. Improved survival and decreased progression of HIV in patients treated with saquinavir (Invirase, SQV) plus Hivid (zalcitabine, ddC), abstr. LB.B.6033. [Google Scholar]
- 38.Lea A P, Faulds D. Ritonavir. Drugs. 1996;52:541–548. doi: 10.2165/00003495-199652040-00007. [DOI] [PubMed] [Google Scholar]
- 39.Lee C G L, Gottesman M M, Cardarelli C O, Ramachandra M, Jeang K T, Ambudkar S V, Pastan I, Dey S. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37:3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 40.Lin J H, Chen I W, Vastag K J, Ostovic D. pH-dependent oral absorption of L-735,524, a potent HIV protease inhibitor, in rats and dogs. Drug Metab Dispos. 1995;23:730–735. [PubMed] [Google Scholar]
- 41.Lown K S, Bailey D G, Fontana R J, Janardan K S K, Adair C H, Fortlage L A, Brown M B, Guo W, Watkins P B. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–2553. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markowitz M, Cao Y, Vesanen M, Talal A, Nixon D, Hurley A, O’Donovan R, Racz P, Tenner-Racz K, Ho D D. Abstracts of the 4th Conference on Retroviruses and Opportunistic Infections. 1997. Recent HIV infection treated with AZT, 3TC, and a potent protease inhibitor, abstr. LB8. [Google Scholar]
- 43.Markowitz M, Saag M, Powderly W, Hurley A M, Hsu A, Valdes J M, Henry D, Sattler F, LaMarca A, Leonard J M. Abstracts of the 3rd Conference on Retroviruses and Opportunistic Infections. 1995. Potent and sustained antiretroviral activity of indinavir in combination with zidovudine and lamivudine, abstr. LB7. [Google Scholar]
- 44.Marsh K C, Eiden E, McDonald E. Determination of ritonavir, a new HIV protease inhibitor, in biological samples using reversed-phase high-performance liquid chromatography. J Chromatogr B. 1997;704:307–313. doi: 10.1016/s0378-4347(97)00454-4. [DOI] [PubMed] [Google Scholar]
- 45.Merck & Co., Inc. Crixivan™ product information. Rahway, N.J: Merck and Co., Inc.; 1998. [Google Scholar]
- 46.Molla A, Chernyavskiy T, Vasavanonda S, Praestgaard J, Lin T, Sun E, Kihlbrenner W, Kempf D. Abstracts of the 12th World Congress on AIDS. 1998. Synergistic anti-HIV activity of ritonavir and other protease inhibitors in the presence of human serum, abstr. 12315. [Google Scholar]
- 47.Molla, A., G. R. Granneman, and E. Sun. Recent developments in HIV protease inhibitor therapy. Antivir. Res., in press. [DOI] [PubMed]
- 48.Mulder J, McKinney N, Christpherson C, Sninsky J, Greefield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S J, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, Kolberg J, Kokka R, Neuwald P, Urdea M S. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 50.Paine M F, Shen D D, Kunze K L, Perkins J D, Marsh C L, Barr D M, Gillies B S, Thummel K E. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 51.Pedersan C, Gerstoft J, Lunnogren J O, Mathiesen L, Kirk O, Nielsen H, Katzenstein T. Presented at the 12th World Congress on AIDS. 1998. Saquinavir/ritonavir may have better antiviral efficacy than either ritonavir or indinavir in HIV infected antiretroviral naive patients resistance. [Google Scholar]
- 52.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell lifespan, and viral generations. Science. 1995;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 53.Polyak B, Henley D B, Maudlin J, Postelnick M, Hirschtick R. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. The clinical incidence of nephrolithiasis in HIV-positive patients receiving indinavir, abstr. I-183; p. 277. [Google Scholar]
- 54.Rhone S A, Hogg R S, Yip B, Sherlock C, Conway B, O’Shaughnessy M V, Schechter M T, Montaner J S G. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. The antiviral effect ritonavir and saquinavir among HIV infected adults: preliminary results from a community based study, abstr. I-207; p. 282. [Google Scholar]
- 55.Rockstroh J. Internationaler Workshop. HIV-Therapie. Compliance und Wirksamkeit. 1998. Ritonavir plus indinavir—Vierfach-Kombinatherapie als firstline. [Google Scholar]
- 56.Rowland M, Tozer T N. Clinical pharmacokinetics: concepts and applications. 2nd ed. Philadelphia, Pa: Lea & Febiger; 1989. pp. 255–273. [Google Scholar]
- 57.Saah A, Riddler S, Havir D V, Squires K, Anderson R, Kerr B, Yeh K, Deutsch P. Presented at the 12th World Congress on AIDS. 1998. Coadministration of indinavir and nelfinavir; pharmacokinetics, tolerability, anti-viral activity, and preliminary viral resistance. [Google Scholar]
- 58.Sarcletti M, Petter A, Lhotta K, Konig P, Zangerle R. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Increased risk of indinavir nephrolithiases in women, abstr. 418. [Google Scholar]
- 59.Shapiro J M, Winters M A, Stewart F, Efron B, Norris J, Kozal M J, Merigan T C. The effect of high dose saquinavir on viral load and CD4+ T-cell counts in HIV infected patients. Ann Intern Med. 1996;124:1039–1050. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 60.Talal A, Cao Y, Hurley A, Schluger R, Fischer L, Salgo M, Smiley L, Keller A, Ho D D. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Saquinavir in combination with AZT/3TC and ritonavir: a convienient BID regimen, abstr. I-208; p. 282. [Google Scholar]
- 61.Watkins P B, Wrighton S A, Schuetz E G, Molowa D T, Guzelian P S. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Workman C, Musson R, Dyer W, Sullivan J. Presented at the 12th World Congress on AIDS. 1998. Novel double protease combinations-combining indinavir (IDV) with ritonavir (RTV): results from first study. [Google Scholar]
- 63.Yeh K C, Deutsch P J, Haddix H, Hesney M, Hoagland V, Ju W D, Justice S J, Osborne B, Sterrett A T, Sone J A, Woolf E, Waldman S. Single-dose pharmacokinetics of indinavir and the effect of food. Antimicrob Agents Chemother. 1998;42:332–338. doi: 10.1128/aac.42.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]