Abstract
Dioxins are a group of chemicals not only regarded as highly toxic trace environmental contaminants, but also considered typical contaminants in food. Dioxins spread across the ecosystem after factory manufacture, contaminate the soil and vegetation before either directly or indirectly entering the food chain through meat products, dairy products, and aquatic products. The compound in question poses a challenge for metabolic processes within the human body, due to its intricate mechanism for inducing diseases. Therefore, it presents a significant risk and is largely undisclosed. Dioxins are mainly exposed to humans by water, food, and air, as well as inducing organ failure and metabolic disorders through but not limited to the activation of aryl hydrocarbon receptors (AhR). As a notorious compound in the family of dioxins, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exhibits long-term toxic effects on diverse organs, which induces continuous metabolic disorders. This review discussed the mechanisms of TCDD-associated metabolic syndrome. The expression of the cytochrome P450 subfamily transfers TCDD into liver, promotes its accumulation in fat tissue, and affects cholesterol metabolism. This process also alters the glucose tolerance of the human organism, disrupting glucose metabolism. It can also elicit cardiovascular pathogenesis, exacerbate liver fibrosis and neuronal death. The long-term metabolic impact of this effect is found to be sex-related. This review summarized the toxicity of TCDD on the human metabolism system and discussed the plausible correlation between TCDD and five metabolic disorders, which helped offer novel insights for future research and therapeutic interventions for these ailments.
Keywords: Dioxin; 2,3,7,8-tetrachloro-p-dibenzodioxin (TCDD); Metabolic disorder; Aryl hydrocarbon receptor (AhR); Food contaminant
Graphical abstract
Highlights
-
•
TCDD enters human body through air, soil, water and food contamination.
-
•
The toxic effects of TCDD require the activation of AhR and cytochrome P450.
-
•
Both long-term and short-term exposure to TCDD induces toxic effects.
-
•
TCDD can cause damage to diverse tissues and induce metabolic disorders.
Abbreviations in this review❈
- Acaca
acetyl-CoA carboxylase α
- Acly
ATP citrate lyase
- AhR
aryl hydrocarbon receptors
- ALT
alanine transaminase
- AMPK
AMP-activated protein kinase
- ARNT
aryl hydrocarbon receptor nuclear translocator
- Bcl-2
B-cell lymphoma-2
- C/EBPα
CCAAT/enhancer-binding protein α
- CDDs
dibenzo-p-dioxins
- CLOCK
circadian locomotor output cycles kaput
- Con A
concanavalin A
- COX-2
cyclooxygenase-2
- cTn-T
cardiac troponin T
- CYP
cytochrome P450
- DLCs
dioxin-like compounds
- EP3
prostaglandin E receptor 3
- ESC
embryonic stem cell
- Fasn
fatty acid synthase
- FXR
farnesoid X receptor
- Gck
glucokinase
- Glut4
glucose transporter type 4
- GSK-3β
glycogen synthase kinase 3β
- GULT2
glucose transporter type 2
- Hmgcr
3-hydroxy-3-methylglutaryl-CoA reductase
- Hsp90
heat shock protein 90
- HUVEC
human umbilical vein endothelial cell
- IFN-γ
interferon-γ
- IL
interleukin
- KC
keratinocyte chemoattractant
- LTs
leukotrienes
- MAP
mitogen-activated protein
- MAPK
mitogen-activated protein kinase
- Me2
malate enzyme
- NF
neurofilament
- NFL
neurofilament light chain
- NF-κB
nuclear factor kappa B
- NGF
nerve growth factor
- Nkx 2.5
NK2 homeobox 5
- Nrf-2
nuclear factor-erythroid 2-related factor-2
- Ntcp
sodium taurocholate co-transporting polypeptide
- oatp2
Slc21a5
- OH-TCDD
1, 3, 7, 8-tetrachloro-2-hydroxy-dibenzo-p-dioxins
- OH-TriCDD
2, 3, 7-trichloro-8-hydroxy-dibenzo-p-dioxins
- PAF
platelet-activating factor
- PCDFs
dibenzofurans
- PGI2
prostaglandin I2
- PPARγ
peroxisome proliferator-activated receptor γ
- PRKAG1
5′-AMP-activated protein kinase subunit gamma-1
- REV-ERBα/β (Nr1d2)
nuclear receptor subfamily 1 group D member 2
- ROS
reactive oxygen species
- SGLT1
sodium–glucose co-transporter 1 glucose transporter
- SHP
short heterodimer partner
- Srebf1
sterol regulatory element binding factor 1
- SREBP
sterol regulatory element binding protein
- TCDD
2,3,7,8-tetrachloro-p-dibenzodioxin
- TEQs
toxic equivalents
- TNF-α
tumor necrosis factor α
- TxA2
Thromboxane A2
- vWF
von Willebrand factor
- Wnt/β-catenin
wingless-related integration site/β-catenin
- XAP2
hepatitis B virus X protein associated protein 2
- XRE
xenobiotic response element
- ❈
Ordered in alphabetical
1. Introduction
On February 21st, 2023, a train derailed while passing through the town of East Palestine, Ohio State in the United States. Among them, 10 compartments of derailed parts were loaded with toxic substances and 5 of them were vinyl chloride, a total of 100 thousand gallons. The main leak from the explosion was vinyl chloride, a chlorine-containing polymer that exhibited high toxicity and took a long time to degrate. It was burning continually for weeks with hard-dissipate smoke. To avoid explosions, the local government poked several holes in five carriages. It was burnt immediately after the liquid flowed out. Complete combustion of vinyl chloride produced dioxins, hydrogen chloride and other toxic gases, which simultaneously caused devastating collateral damage to neighbor creatures, and entered human body through contaminated water, foods, and sediments. The toxic chemicals are continuously doing harm to people and animals in the vicinity.
Dioxins are a group of polyhalogenated aromatic hydrocarbons compounds including chlorinated dibenzo-p-dioxins (CDDs), dibenzofurans (PCDFs) and the ‘dioxin-like’ biphenyls (PCBs), which have similar structure and properties (Kulkarni et al., 2008). The most notorious substance in CDDs, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is well-recognized as the compound that has the strongest harm to human body. Overall, human exposure to dioxins, including all family members, can be divided into two ways-nature sources (forest fire) and anthropogenic origins, include food sources and industrial wastes (Fig. 1.). Food sources, especially animal source food, including dairy products, meat, and fish (Ghimpeţeanu et al., 2014; Kulkarni et al., 2008). Dioxins are lipophilic persistent organic pollutants, which enter the environment by discharging into surface water and diffusing into the air. Dioxins can be stored in the bottom sediments of the ocean or absorbed by benthic organisms, they can also be adsorbed by plant and soil surfaces in terrestrial environments, and enter the food chain directly or indirectly (Pajurek et al., 2023). Occupational individuals are exposed to dioxins mainly by inhalation, skin contact, and ingestion of dust (González and Domingo, 2021). However, most other public are exposed to dioxins mainly through consuming contaminated high-fat diets as the toxins are hydrophobic substances, which can be easily enriched into the fat part of food. The common way that livestock is exposed to dioxins is through the consumption of contaminated feed (Pajurek et al., 2023). Citrus is one of the primary ingredients in dairy cattle feed. In 1997, dioxins were detected in dairy milk in Germany (Carvalhaes et al., 2002). Lime contaminated with dioxins was added to the citrus to reduce water content and increase pH, which further contaminated feed for dairy cows (Carvalhaes et al., 2002). Another accident happened in 2004 in Holland. Kaolinic clay, an alternative to salt, was used to select the good-quality potatoes. The contamination of clay by dioxins resulted in the appearance of dioxin residues in potato peel. As potato peel is a major composition in feeds for dairy cows, it caused the contamination in milk (Hoogenboom et al., 2015). For poultry and mammals, dioxins normally accumulate in their fat tissue and liver after consumption of dioxins-contaminated feed (Ghimpeţeanu et al., 2014). Dioxins released from the factory into the aquatic environment not only pollute the water source, but also accumulate in aquatic organisms. The content of dioxins in aquatic organisms such as fish is much higher than that in water (Kawamoto and Weber, 2021), which indicates the existence of biological accumulation in this process. For instance, dioxins have been found in the sediment - snail - Chinese mitten crab and sediment - Chinese mitten crab food chain. The total risk posed by dioxins in materials of interest can be estimated via toxic equivalents (TEQs) calculated using Toxic Equivalency Factors (Rao et al., 2022). Although there was no data on the TEQ value of TCDD reported currently, another member in CDDs, called PCDD/Fs, has been found to present with a significantly higher amount in crabs than that in water. Compared with the TEQ value of PCDD/Fs in the sampled water was 0.767 pg TEQ kg−1, the mean value of PCDD/Fs and its analogs in the sampled crabs was 4370 pg TEQ kg−1 ww (wet weight) (Rao et al., 2022).
Fig. 1.
Human exposure to TCDD is divided into industrial pollution and food sources, where dioxins accumulate in the food chain and cause damage to humans and animals.
The other exposure approach is industrial exposure mainly comes from the incineration of chlorinated wastes. A high level of dioxins is released during the process of plastic destruction and steelmaking using rolled iron oxide sheets. Chlorine and carbon-containing substances can also produce dioxins under the catalytic of metal ions, like paper mills. Wastewater and accidental leak from chemical plants are sources of dioxins production. Specifically, the median half-life of TCDD is 7.1 years (Pirkle et al., 1989), which keeps accumulating in the environment at a gradually increased level and is considered a big threat to public health.
Dioxins are absorbed by small intestine and streamed into blood, where they are carried to other internal organs and tissues. Most TCDDs are not absorbed at once, but are stored in the lipid tissue and liver. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) as a representative substance of dioxin-like compounds (DLCs) have been justified to produce a variety of hydroxylated products during toxin metabolism, including 2, 3, 7-trichloro-8-hydroxy-dibenzo-p-dioxins (OH-TriCDD) and 1, 3, 7, 8-tetrachloro-2-hydroxy-dibenzo-p-dioxins (OH-TCDD), which are detected in feces, serum and urine (Sorg et al., 2009).
Enterohepatic recirculation refers to the phenomenon that compounds are discharged into the intestine through bile being reabsorbed in the intestine, then returned to the liver. TCDD is mainly stored in lipid tissue and the liver, which prevents it from further metabolism, thus making it hard to eliminate from human body. It was mentioned that TCDD can induce higher expression of ileal bile acid transporter Slc10a2 (apical sodium dependent bile acid transporter) and organic solute transporter Slc51a (solute carrier family 51 subunit α), indicating that the process might promote the active bile acid reabsorption, thus prevent TCDD from being excreted by bile (Fader et al., 2017a, Fader et al., 2017b).
This review provides a comprehensive introduction to the metabolic concequences of TCDD. As a persistent organic environmental and food pollutant, TCDD has been classified and banned as a Category 1 carcinogen by International Agency for Research on Cancer (IARC) as early as 1997. People have been commenting on its human toxicity, environmental and food-resourced toxicity, and risk assessment since 1998 (Bertazzi et al., 1998). There have been many papers on TCDD risk assessment and morbidity studies. However, there has been a dearth of evaluations concentrating explicitly on the metabolic toxicity of dioxins over the last decade, especially review on the TCDD toxicity through food exposure. This review summarizes the damage and possible mechanisms caused by TCDD to human metabolic function at this stage by evaluating the food source, metabolism, and bio-logical toxicity of TCDD, thus opening up new ideas for the control the exposure of the pollutant and prevention of related diseases.
2. AhR and cytochrome P450
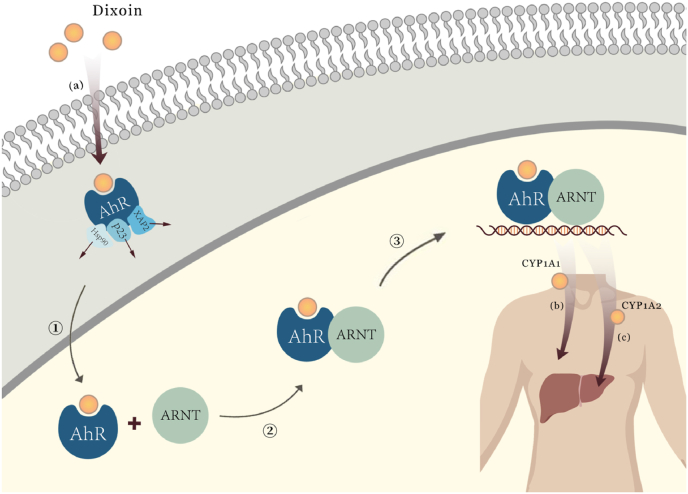
Dioxins, including TCDD, are ligands specifically binding to transcription factor AhR (Kulkarni et al., 2008). AhR is known as a key transcriptional regulated protein. Lipophilic AhRs are in the cytoplasm and can be activated by spreading freely across the cell membrane (Sorg, 2014; Sorg et al., 2009). AhR binds to heat shock protein 90 (Hsp90), cochaperone p23, and the hepatitis B virus X protein associated protein 2 (XAP2) as a complex in the absence of a ligand. After binding with dioxins, the complex dissociates and AhR transfers to the nucleus to form an AhR-ARNT heterodimer with its molecular partner aryl hydrocarbon receptor nuclear translocator (ARNT). Heterodimers bind to specific DNA sequences called xenobiotic response element (XRE) and become nuclear transcription factors. As shown in Fig. 2., AhR in this way directly or indirectly regulates the transcription of genes which are involved in cell growth and development, as well as the normal metabolic rhythms of the body (Mandal, 2005; Mimura and Fujii-Kuriyama, 2003; Sato et al., 2008). The long-term receptor-ligand binding pattern exhibits toxic effects on experimental animals, while the toxic effects were not observable in AhR-null mice, suggesting that AhR is required in this process (Fernandez-Salguero et al., 1996; Mimura et al., 1997). More mechanism studies suggested that AhR activated by dioxins induces gene expression of cytochrome P450 (CYP), which disrupts the hormone signaling pathway and further results in toxic effects on tissues (Kulkarni et al., 2008).
Fig. 2.
Signaling pathways in vivo following activation of AhR by TCDD
(a) Dioxin cross the cell membrane and bind to AhR. After binding, AhR was activated, while the ligands Hsp90, p23, XAP2 dissociates; (b) CYP1A1 was mainly expressed outside the liver; (c) CYP1A2 was expressed inside liver, mainly involved in the metabolism of aromatic amines and heterocyclic compounds. ①Activated AhR transported to nuclear after combination; ②Activated AhR bound with ARNT, formed an AhR-ARNT heterodimer; ③Heterodimer bound to target DNA and induced the expression of cytochrome P450.
CYP is a superfamily of hemoproteins richest in the liver. They are involved in complex physical activities and play a crucial role in drug interaction as well as xenobiotic metabolism, reflecting the close relationship between drugs and CYP and their pharmacokinetic effects on the human body (McDonnell and Dang, 2013). More research found that CYP plays key roles in the development of cardiovascular diseases, hepatic failure, neurodegeneration. As one of the main subfamilies of CYP, CYP1A metabolizes drugs and pollutants and mediates endogenous substance metabolism (Lu et al., 2020). Humans have two CYP1A subfamily members: CYP1A1 and CYP1A2, which mainly participate in dioxins metabolism, and subsequently cause divergent symptoms and reactions (Sato et al., 2008). CYP1A1 is mainly expressed outside the liver and prefers aromatic hydrocarbons. CYP1A2 is expressed in the liver, mainly involved in the metabolism of aromatic amines and heterocyclic compounds (Lu et al., 2020). CYP1A1 is considered a direct biomarker of AhR activation. In other words, the function of AhR in dioxins-induced gene expression is mainly reflected by transcriptional activation of CYP1A1 (Sato et al., 2008). CYP1A1 is involved in heterogenic metabolism, especially the biological transformation of dioxins, which contributes to the accumulation of dioxins in adipose tissue (Anzenbacher and Anzenbacherová, 2001; Molcan et al., 2017). Moreover, the overexpression of CYP1A1 might lead to the overproduction of reactive oxygen species (ROS) by attenuated the activity of mitochondrial membrane, thereby inducing the oxidation damage of mitochondrial (Mescher and Haarmann-Stemmann, 2018). However, further mechanism still needs to be investigated.
3. Toxic effects of TCDD and mechanisms
TCDD was discovered the toxicity as it induces metabolic disorders in all aspects of the body. The different exogenous damages and internal disorders induced or exacerbated by TCDD are due to its different effects on AhR and the downstream pathways activated by this lesion targeting different genes, cells, and organs. Specifically, disorders of glucose metabolism, excessive fat loss, cardiovascular disease, hepatobiliary injury, and neurodegenerative diseases are the main toxic outcomes induced by TCDD. Fig. 3 summarized the major pathways discussed in the current review, in order to present a more directly view of the mechanism of TCDD-induced metabolic toxicity. Relevant studies by peers were listed in Table 1.
Fig. 3.
The primary mechanism through which TCDD causes cardiovascular diseases, liver injury, neurodegenerative diseases, fat metabolism and glucose metabolism disorders.
Table 1.
Toxic effects of TCDD in development of various disorders.
| Toxicity effects | Models | Doses | Duration | Toxic effects | Suggested mechanisms | References |
|---|---|---|---|---|---|---|
| Glucose metabolism disorders | Non-pregnant female C57BL/6 mice (TCDD enhanced HFD-induced obesity) | 20 ng/kg/d | 12 weeks(2x/week) | Accelerated beta cell dysfunction | ↓MafA, Slc2a2, and Nkx6.1 | Hoyeck et al. (2020) |
| Male C57BL/6 mice Female C57BL/6 mice (TCDD enhanced HFD-induced obesity) |
20 ng/kg/d | 2x/week starting one week prior to pairing with male mice and lasting throughout mating, pregnancy and lactation | Accelerated onset of hyperglycemia; Impaired glucose-induced plasma insulin levels; Reduced islet size; Increased proinsulin accumulation; Accelerated the onset of glucose intolerance without altering insulin sensitivity; Accelerated beta cell dysfunction |
↑CYP1A1, ↓AhR |
||
| C57BL/6N wild-type mice Ahr-null male mice |
5, 50, or 500 ng/kg bw | 18 days | Disturbed insulin synthesis and secretion in the pancreas; Impaired glucose uptake and glycolysis in the liver |
↓Gck | Sato et al. (2008) | |
| Mouse 3T3-L1 cells | 0.1, 1, and 10 nM | 3 days | Reduced insulin-induced glucose uptake | AhR-independent | Hsu et al. (2010) | |
| Male C57BL/6J mice Male DBA/2J mice |
100 μg/kg bw | 10 days | Increased initial blood glucose lever; Increased the activity of sucrase and lactase |
AhR ↑SGLT1 |
Ishida et al. (2005) | |
| Male C57BL/6 mice | 20 μg/kg or 200 μg/kg | A single | Males: Lost beta cell mass, increased insulin sensitivity, lost weight and induced hypoglycemia; Females: Suppressed plasma insulin levels, induced glucose intolerance but had either minimal or no changes to islet composition, insulin sensitivity and body weight |
N/A | Hoyeck et al. (2020) | |
| Male C57BL/6 mice Female C57BL/6 mice |
20 μg/kg | A single | ||||
| Fat metabolism disorders | C57BL/6N wild-type mice Ahr-null male mice |
5, 50, or 500 ng/kg bw | 18 days | Reduced cholesterol synthesis; | ↓Hmgcr and other genes | Sato et al. (2008) |
| Suppressed the lipogenesis (low-dose TCDD) | Srebf1 | |||||
| Mouse 3T3-L1 cells | 0.1, 1, and 10 nM | 3 days | Inhibited the adipogenic differentiation | ↓AhR,↓PPARγ,↓C/EBPα,↓Glut4 | Hsu et al. (2010) | |
| Male Hartley guinea pigs | 1 μg/kg | 7 days 21 days |
Inhibited the adipocyte differentiation Lost body weight accompanied by a decrease in adipose tissue weight; |
↓SREBPs | Nishiumi et al. (2008) | |
| Accumulated lipid in the liver and induced fatty liver | ↓PPARα level | |||||
| Male Ldlr−/− mice | 1 μmol/kg dioxin-like polychlorinated biphenyl 126 (PCB126) | At weeks 2 and 4 | Increased lipid droplet formation within hepatocytes | ↑CD36, IL-1β, LPL, INSIG-1 ↓Fads1, Scd1, Gyk |
Petriello et al. (2017) | |
| Male Sprague–Dawley outbred CD rats | 0, 0.4 or 40 μg/kg bw | A single | Interfered cholesterol metabolism | ↓CYP7A1, FXR, SHP, Ntcp, oatp2 | Fletcher et al. (2005) | |
| Cardiovascular diseases | C57BL/6J mice | 5 μg/kg TCDD | 3 days (continuously) | Increased in blood pressure and atherogenic lipids; Exacerbated atherosclerosis |
AhR ↑monooxygenase ↑intracellular calcium in myocytes ↓decreasing contractility |
Dalton et al. (2001) |
| Apoe+/+/hyperlipidemic Apoe−/− | 150 ng/kg, 3 times/w 10 μL dimethyl sulfoxide (DMSO) |
7 weeks | ||||
| female Harlan Sprague-Dawley rats | 0, 3, 10, 22, 46, 100 ng/kg/d | 14, 31, 53 weeks | Increased risk of cardiomyopathy and chronic active arteritis (in mesentery and pancreas); Increased vascular permeability |
Cytosolic AhR activation ↑oxidative stress ↑ROS release |
Jokinen et al. (2003) | |
| female Sprague-Dawley rats (1/2 ovariectomy) | PCB126: First 2 times 64 μg/kg Follow: 32 μg/kg |
12 weeks | Increased myocardial weight; Reduced serum triglyceride levels; Increased HDL cholesterol levels |
N/A | Lind et al. (2004) | |
| EP3 knockout (EP3−/−) mice wild-type (WT) C57BL/6 | 10 μg TCDD/kg | 10 days | Induced endothelial injury (atherosclerosis); Triggered HUVECs apoptosis |
↑COX-2, PG, p38 ↓Bcl-2 |
Yu et al. (2017) | |
| Undifferentiated HepaRG cells | N/A | 48 + 24h | Triggered cell apoptosis; Upregulated TRAIL |
↑BMF, expression of constituents and/or transcriptional targets of signaling pathways | Svobodová et al. (2019) | |
| Liver injury | Male C57BL/6J mice | 10% low-fat (LFD)/45% high-fat (HFD) 5 μg/kg TCDD |
14 weeks | Triggered hepatic steatosis and hepatic fibrosis; | ↑Cd36, PPARg ↓MTTP, DGAT1, PKLR, Slc2a2/Glut2 |
Duval et al. (2017) |
| Male ApoE−/− mice | 15 μg/kg | 6 weeks/4 times | Triggered liver steatosis, liver necrosis and inflammatory stimuli | Activated Nrf2 ↑ALT, AST, CYP1A1 |
Shan et al. (2015) | |
| Male Sprague-Dawley rats | 8 μg/kg 15 mg/kg |
21 days | Inhibited hepatic antioxidant activity; Increased formation of MNHEPs |
Inactivated antioxidant enzymes ↑ROS, LPO |
Türkez et al. (2012) | |
| Male C57BL/6J | 0.3, 3, or 30 μg/kg TCDD | 4 days | Increased neutrophil chemokines (plasma concentration) | ↑ALT, IFNγ ↓KC |
Fullerton et al. (2013) | |
| Pregnant female C57BL/6J mice | 3 μg/kg | 14 days | Increased liver weight; Induced lipid peroxidation |
↑SOD, GSH-Px, CAT, GSH ↓MDA |
Ciftci et al. (2013) | |
| Neurodegenerative diseases | Pregnant Sprague-Dawley rats | 10−11 to 10−10 mol/L | 18 days | Increased NFL's mRNA and protein | ↑phosphorylation of NFL, ERK1/2, p38 | Chen et al., 2020a, Chen et al., 2020b |
| CGC from AhR−/− cell from AhR-null mice | 1–500 nM | N/A | Triggered cell loss; Induced DNA laddering |
AhR ↑CYP1A2, caspase-3 |
Sánchez-Martín et al. (2011) | |
| Female Sprague-Dawley mice | 0.5 LD50 25 μg/kg | 7 days | Triggered apoptosis of neuronal cell lines | Wnt/β-catenin pathway ↓pSer9-GSK-3β ↓β-catenin |
Xu et al. (2013) |
↑- increase; ↓- decrease; N/A, not available.
Acronyms: AhR, aryl hydrocarbon receptor; ALT, alanine transaminase; AST, aspartate aminotransferase; Bcl-2, B-cell lymphoma 2; BMF, Bcl-2 modifying factor; C/EBPα, CCAAT/enhancer-binding protein α; CAT, catalase; CD36, cluster of differentiation 36; CGC, cerebellar granule cell; COX-2, cyclooxygenase-2; CYP1A1,cytochrome P450 1A1; CYP1A2, cytochrome P450 1A2; CYP7A1, cytochrome P450 7A1; DGAT1, diacylglycerol acyltransferases; ERK1/2, extracellular signal-regulated kinase; Fads1, fatty acid desaturase 1; FXR, farnesoid X receptor; Gck, Glucokinase; Glut4, glucose transporter type 4; GSH, glutathione; GSH-Px, glutathione peroxidase; Gyk, glycerokinase; Hmgcr, 3-hydroxy-3-methylglutaryl-CoA reductase; HUVEC, human umbilical vein endothelial cells; IFNγ, interferon γ; INSIG-1, insulin induced gene 1; KC, keratinocyte chemoattractant; LPL, lipoprotein lipase; LPO, lactoperoxidase; MDA, malondialdehyde; MNHEP, hepatocyte micronuclei; MTTP, microsomal triglyceride transfer protein; NFL, neurofilament light; Nrf2, nuclear factor erythroid 2-related factor 2; Ntcp, sodium taurocholate co-transporting polypeptide; oatp2, Slc21a5; p38, mitogen-activated protein kinase; PG, prostaglandin; PKLR, kyruvate kinase L/R; PPARg, peroxisome proliferator-activated receptor g; PPARα, peroxisome proliferator-activated receptor α; PPARγ, peroxisome proliferator-activated receptor γ; pSer9-GSK-3β, phospho-glycogen synthase kinase-3β; ROS, reactive oxygen species; Scd1, stearoyl-CoA desaturase 1; SGLT1, sodium–glucose co-transporter 1; SHP, short heterodimer partner; Slc2a2/Glut2, solute carrier family 2 member 2/glucose transporter 2; SOD, superoxide dismutase; Srebf1, sterol regulatory element binding factor 1; SREBP, sterol regulatory element binding protein; TRAIL: tumor necrosis factor (TNF)-related apoptosis-inducing ligand; Wnt, wingless-related integration site.
3.1. Cardiovascular diseases
Based on existing articles, TCDD exacerbates the incidence of cardiovascular diseases through multiple pathways. For instance, Dalton and his colleagues (2001) found that CYP1A1 and COX-2 (cyclooxygenase-2) were upregulated under the induction of TCDD. The two proteins converted internal arachidonic acid into vasoactive eicosanoids by promoting the generation of vasodilation eicosanoid prostaglandin I2 (PGI2) and vascular contraction of eicosanoid Thromboxane A2 (TxA2) at the same time. All evidence above proves that TCDD can alter the status and distribution of arachidonic acid in blood vessels. Another relevant article indicates that leukotrienes (LTs), the metabolites of arachidonic acid, can induce the expression of adhesion and pro-inflammatory factors vWF (von Willebrand factor) and PAF (platelet-activating factor), and prove the incidence of atherosclerosis (Sonnweber et al., 2018). Meanwhile, through affecting the regular order of cyclin protein, TCDD induced plaque formation and the dysfunction of vascular smooth muscle cells (Dalton et al., 2001).
Human exposure to TCDD was also found to contribute to the formation of atherosclerotic plaque and the augment of intima-medical thickness. TCDD promoted the condensation and fragmentation of nuclei in human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner, which accelerates cell apoptosis (Yu et al., 2017). After the AhR-TCDD axis was activated, AhR entered the nucleus to dimerize with ARNT, bound to DNA and regulated target genes (Mohsenzadeh et al., 2018). Further research found that when the ARNT gene was disrupted, the upregulation of COX-2 mRNA and protein expression in HUVECs was abolished, indicating that TCDD could upregulate the expression of AhR/ARNT transcription complex by combining with AhR, thereby promoting the expression of COX-2 (Yu et al., 2017). On the other hand, reduced viabilities of HUVECs upon TCDD exposure can be restored by p38 MAPK inhibitor treatment. Similarly, TCDD can inhibit the transcription of anti-apoptotic protein B-cell lymphoma-2 (Bcl-2) to destroy cell integrity (Svobodová et al., 2019), which proved that EP3/p38 MAPK/Bcl-2 pathway was involved in the process of TCDD-triggered HUVECs apoptosis (Yu et al., 2017). Genetic analysis revealed that many AhR-bound genes participated in transcriptional regulation and cardiac differentiation. Brachyury (T) gene is essential for post-mesoderm formation and axial development (Chen et al., 2020a, Chen et al., 2020b). One study reported that TCDD treatment downregulated the expression of T gene in differentiated human embryonic stem cells (ESCs). When AhR was inhibited by AhR inhibitor CH22391, the reduced expression of T gene was significantly reversed. This finding indicates that the activation of AhR is important in TCDD-induced downregulation of T gene. To be specific, TCDD is not harmful to the maintenance and pluripotency of ESC. However, the expression of mesoderm markers T and GSC were declined. The conclusion was confirmed again that the inhibition of human ESC differentiation by TCDD was mainly presented in the mesodermal lineage, and peaked at 2 nM and 4 days after TCDD treatment (Fu et al., 2019). Moreover, DNA methylation can be affected by TCDD, by forming a unique pattern, the maturation of normal cardiomyocytes will be impacted significantly by the disruption of methylation. According to relative research, TCDD-induced DNA methylation was on PRKAG1 gene, one of the regulators of the AMP-activated protein kinase (AMPK) pathway, which also promoted the mutual regulation (reversely) between mitogen-activated protein kinase (MAPK) and AMPK pathways, aggravating the damage of TCDD to human ESCs (de Gannes et al., 2020; Yuan et al., 2020). In addition, TCDD inhibited the mRNA synthesis of α- and β-myosin heavy chain genes and the expression of transcription factor NK2 homeobox 5 (Nkx2.5), thereby inhibiting cardiomyocyte differentiation. Ten days after embryogenesis, TCDD was used to treat differentiated ES cells. It turned out that TCDD could completely block the expression of Nkx2.5. Similarly, the amount of cardiac troponin cTn-T (cardiac troponin T) expressed in differentiated ES cells treated with 10 pM TCDD was reduced to half of the original amount, and replaced with 100pM can almost completely inhibit the expression of cTn-T (Wang et al., 2010). Based on the above evidence, it can be determined that TCDD can significantly inhibit the beating phenotype in a dose-dependent manner and increase the risk of cardiovascular disease.
3.2. Liver injury
Liver fibrosis is normally the first stage of liver chronic diseases, which can develop into cirrhosis and hepatocellular carcinoma (Dhar et al., 2020). Groups of evidence justified that TCDD can deteriorate the quality of liver cells, affecting the normal function of liver by inducing hepatic steatosis (Duval et al., 2017; Türkez et al., 2012a, Türkez et al., 2012b). More research revealed that TCDD causes oxidative stress in the liver by suppressing the activation of antioxidant enzymes. Activated macrophages produce ROS, which induces an increase in LPO levels, leading to further liver injury (Ibrahim et al., 2022; Peng et al., 2022). Excessive ROS breaks the body's oxidation balance, intracellular polyunsaturated fatty acids, proteins and nucleic acids, resulting in liver necrosis and inflammatory stimuli (Shan et al., 2015; Türkez et al., 2012a, Türkez et al., 2012b).
In concanavalin A (Con A)-induced autoimmune hepatitis mouse model, the addition of TCDD contributed to the lesion of liver. Keratinocyte chemoattractant (KC) in serum increased by the administration of the combination of TCDD and Con A, which revealed that TCDD can upregulate inflammatory chemokines expression in liver to induce hepatic injury (Stefanovic et al., 2005). Subsequently, the serum KC level will increase, while stimulating fibrosis by secreting fibrogenic cytokines. (Liu et al., 2010; Tang et al., 2021). Another evidence proved that interleukin-12 (IL-12), secreted by Kupffer cell, enhanced the cytolytic activity of immune cells, such as natural killer cells and natural killer T cells, and the synergistic action of interferon-γ (IFN-γ) and tumor necrosis factor α (TNF-α) from Kupffer cell can kill liver cells furtherly. Compared with the control group, the expression of TNF-α increased 4 times more than in liver injury group (Fullerton et al., 2013; Liu et al., 2010). Besides, the combination treatment of TCDD and Con A increased the serum (IFN-γ) level and alanine transaminase (ALT) activity to induce immune damage. More mechanism studies reported that the enhanced secretion of IL-6, IL-1β, and TNF-α from immune cells may result in liver inflammation with liver fibrosis, and this process is AhR-required (Sulentic and Kaminski, 2011; Zhang et al., 2022). Hamp and Hamp2 are two genes that help to encode the hepcidin protein (Fader et al., 2017a, Fader et al., 2017b). One study reported that TCDD inhibited the expression of Hamp and Hamp2 in a dose-dependent manner in the liver. As hepcidin is a key marker for maintaining iron balance, the reduction of it led to non-transfer bound iron and iron deposits and further caused liver injury (Brissot et al., 2012). TCDD can also inhibit the expression of heme while promoting the increase of AhR, which not only provides a site for more TCDD binding, but also because free heme is needed to combine with heme to ensure the body's homeostasis. The reduction of heme can also damage the body's antioxidant defense. In addition, the apoptotic cell ratio of 10 μg/kg and 25 μg/kg TCDD administered for 1 week is directly proportional to the administration concentration as a whole, suggesting that hepatocyte apoptosis by TCDD is another factor to induce liver injury. Heme is the biological ligand of REV-ERBα/β (Nr1d1/Nr1d2). TCDD can trigger hepatotoxicity by activating REV-ERBα/β, inducing the increase of Hmox1 and inhibiting fatty acid synthesis and resolution (Fader et al., 2017a, Fader et al., 2017b). Meanwhile, Nr1d2, as a biological clock gene, which is related to circadian locomotor output cycles kaput (CLOCK) and aryl hydrocarbon receptor nuclear translocator-like protein: TCDD inhibits certain circadian-regulated genes to enhance hepatic steatosis in mice. Although the detailed mechanism of this pathway remains unclear, TCDD has been shown to cause increased hepatotoxicity and compromise liver integrity by disrupting homeostasis and circadian rhythm (Sato et al., 2020; Zhao et al., 2019).
3.3. Neurodegenerative diseases
It is proved that TCDD can impair the complete neuron system by modulating diverse pathways. Wnt/β-catenin is an important pathway that regulates cell proliferation and differentiation. Caspase-3 was used as a biomarker to justify that the excessive activation of glycogen synthase kinase 3β (GSK-3β) results in decreased levels of β-catenin. TCDD activated the expression of GSK-3β and inhibited the Wnt/β-catenin pathway, thus inducing the apoptosis of mice cortical neurons (Xu et al., 2013). According to more articles, TCDD triggers ROS generation and induces oxidative stress. It exhibits similar destructive effects in brain tissue, specifically exacerbates secondary brain damage, neurological deficits, and neuronal apoptosis (Sivandzade et al., 2019; Wan et al., 2014). Neurofilament (NF) is a neuron-specific component. Neurofilament light chain (NFL), as a subunit of NF, whose expression is adjusted by the Mitogen-activated protein (MAP) kinases pathway, can serve as a biomarker of neurodegeneration. Overexpression of NFL leads to abnormal accumulation of NF and increased frequency of motor neuron axonal degeneration (Yuan and Nixon, 2021). A quite small amount of TCDD (10−10 mol/L) will significantly elevate the expression level of NFL in neurons. At the same time, the synergistic action of TCDD and nerve growth factor (NGF) cause the phosphorylation of ERK1/2 and p38, thereby activating the transcription of NFL and dramatically increasing NFL mRNA level. Similarly, more experiments showed that the AhR antagonist CH223191 can block the abnormal expression of NFL mRNA induced by TCDD, indicating that AhR plays a critical role as an NFL regulator (Chen et al., 2020a, Chen et al., 2020b). Moreover, NF-κB and nuclear factor-erythroid 2-related factor-2 (Nrf2) interfere with each other. In brief, when inflammation is heightened, Nrf2 binds to the adapter protein Kelch-like ECH-associated protein 1, and its proteosomes are degraded after ubiquitination; but when it was trended up, NF-ĸB downregulated pro-inflammatory responses controlled by transcription. Therefore, it's clear that the effect of NF-κB and Nrf2 is antagonistic, but it is still unknown about its detailed mechanism (Sivandzade et al., 2019). Furthermore, the inhibitor of p38, SB203580 can decrease the TCDD effect towards the induction of NGF to NFL, which means p38 can be raised by the combination of TCDD and NGF (Chen et al., 2020a, Chen et al., 2020b). All these involved pathways indicate that neuron damage is quite sensitive to TCDD, even with a very small amount. Preventing chronic TCDD exposure is considered vital to protect brain from neurodegenerative diseases.
3.4. Fat metabolism disorders
Dioxins have been reported to affect lipid metabolism through multiple pathways. For example, low dose TCDD (5, 50 ng/kg bw) inhibited fatty acid synthesis and lipogenesis in an AhR-dependent manner through Srebf1 (sterol regulatory element binding factor 1)-mediated pathway (Sato et al., 2008). Srebf1 is a transcription factor that controls adipogenesis by upregulating sterol-regulated genes. TCDD also interfered with adipocyte differentiation by inhibiting peroxisome proliferator-activated receptor γ (PPARγ) and/or CCAAT/enhancer-binding protein α (C/EBPα) expression (Hsu et al., 2010).
The expression of the fatty acid synthase (Fasn) in TCDD-treated C57BL/6N mice was decreased. At the same time, ATP citrate lyase (Acly), malate enzyme (Me2) and acetyl-CoA carboxylase α (Acaca) involved in fatty acid synthesis were reduced in TCDD-treated mice. The above results showed that TCDD can inhibit fat generation. None of the genes has been found to be changed in TCDD-treated AhR-null mice, suggesting that AhR is required for TCDD inhibited fat accumulation. Moreover, genes responsible for cholesterol synthesis, such as 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr), have been found to be reduced after TCDD treatment (Sato et al., 2008). Consistently, another study reported that TCDD inhibited 3T3-L1 adipocyte differentiation via downregulating the expression of three adipogenic markers–i.e., PPARγ, C/EBPα, and Glut4 (glucose transporter type 4). As PPARγ and C/EBPα are key factors in adipogenesis, the inhibition of them by TCDD suppresses the downstream biomarkers in adipose differentiation (Hsu et al., 2010).
Wasting/depletion syndrome is an acute dioxin-induced disease characterized by excessive(unwanted) weight and fat loss. Nishiumi et al. (2008) conducted experiments on male guinea pigs and found that the depletion syndrome resulted in a decrease in adipose tissue weight and a two-to-three-fold increase of triacylglycerol, total cholesterol, and free fatty acids in plasma. Moreover, the levels of PPARγ, C/EBPα, and Glut4 in adipose tissue decreased, which is consistent with the findings above. The amount of SREBP decreased in adipose tissue. Acetyl-CoA carboxylase and cytoplasmic HMG-CoA synthase, which are regulated by SREBP during transcription, were also decreased. This change was also observed in the C57BL/6N mouse model mentioned above. Another finding suggests that TCDD downregulated the SREBP level. However, more mechanism studies on TCDD-induced depletion syndrome are highly required (Nishiumi et al., 2008). Moreover, the contents of CYP7A1, farnesoid X receptor (FXR), short heterodimer partner (SHP), Ntcp, and Slc21a5 (oatp2) were decreased in mice treated with single oral feeding, which affected cholesterol metabolism, bile acid synthesis and transport (Fletcher et al., 2005).
3.5. Glucose metabolism disorders
As previously mentioned in the article, the toxicity of dioxins is attributed to the induction of cytotoxicity through the activation of AhR and up-regulation of AhR target genes such as CYP1A1. Research conducted by Myriam P. Hoyeck et al. (2020) found that CYP1A1 is notably induced in the islets of pregnant female mice. They hypothesized that this may be due to increased islet blood flow resulting from elevated metabolic demands and the pancreas potentially being more susceptible to AhR-mediated dioxin signaling during the second trimester. Mice treated with TCDD exhibited accelerated glucose intolerance and severe hyperglycemia, which is typically caused by impaired insulin secretion. The experimental results demonstrate reduced plasma insulin levels, and a high-fat diet has been shown to have a tendency towards reducing plasma insulin levels compared with a typical diet. This study is the first to examine weight gain in mice exposed to low doses of TCDD during pregnancy and lactation, which is a noteworthy topic when compared with studies on acute high-dose TCDD exposure causing depletion syndrome. Another research study found that female mice exposed to long-term low-dose TCDD and an HFD did not gain weight, making researchers speculate that TCDD exposure during pregnancy was responsible for the weight gain observed. However, there is currently no experimental evidence to support this theory, and future studies should include groups of pregnant and non-pregnant mice exposed to similar conditions. In conclusion, female mice exposed to low doses of TCDD during pregnancy and lactation are susceptible to developing obesity and diabetes, particularly when induced by an HFD. Although gestational diabetes may not occur during pregnancy, it can result in metabolic disturbances for a prolonged period even after TCDD has been excreted (Hoyeck et al., 2020a, Hoyeck et al., 2020b).
To explore the hepatic toxicity of low-dose TCDD, one research group studied the changes of gene expression in mouse liver by administering low-dose TCDD. They found that TCDD affected glucose metabolism by reducing the expression of the glucokinase gene. Glucokinase (Gck) is an enzyme that regulates insulin synthesis and secretion in pancreas β-cells and regulates glucose uptake and glycolysis in liver, acting as a glucose sensor. Previous studies have also shown a close association between Gck and Type 2 diabetes (Everett et al., 2007), so environmental exposure to low doses of TCDD may increase the risk of diabetes (Sato et al., 2008). Another interesting study found that in the glucose tolerance test, the initial serum glucose level in AhR more-sensitive C57BL/6J mice treated by TCDD was significantly higher, compared with that in AhR less-responsive DBA/2J mice. Gastrointestinal cells absorb glucose through sodium–glucose co-transporter 1 glucose transporter (SGLT1). TCDD treatment increased the expression of the SGLT1 in C57BL/6J mice, in order to promote glucose uptake in the gut. However, the expression of SGLT1/GULT2 (glucose transporter type 2) in DBA/2J mice (AhR-less-sensitive model) treated with TCDD did not increase, indicating that TCDD affects the expression of intestinal glucose transporter gene through AhR-related mechanism (Ishida et al., 2005).
Subsequent studies have revealed that acute exposure to dioxin have diverse but long-term metabolic effects on male and female mice. In male mice treated with a single dose of 20 μg/kg TCDD, researchers observed sustained weight loss, decreased fasting blood glucose levels and fasting plasma insulin levels, as well as significantly increased insulin sensitivity. Meanwhile, female mice showed only a brief and slight decrease in body weight and fasting blood glucose levels during the first week of TCDD treatment, but experienced a significant decrease in plasma insulin levels at weeks 4 and 6. To investigate the sex-dependent effects of TCDD on metabolism, glucose tolerance and β cell function were evaluated. The male mice displayed symptoms of mild hypoglycemia and a significant increase in beta cell apoptosis, whereas the female mice experienced transient hyperglycemia but no impact on their islet cell population. According to that study, male mice can maintain glucose homeostasis through adjusting their insulin sensitivity, however, female mice were less able to adjust their insulin sensitivity which resulted in hyperglycemia and hyperinsulinemia, two typical disorders in diabetes. Studies on the link between exposure to pollutants and diabetes generally only focus on one sex, and the reasons behind the difference in response to TCDD exposure remain unclear. The authors suggested that both males and females should be investigated when researching the relationship between exposure to contaminants and diabetes, as the mechanism of action may be sex-specific. Additionally, women may be more vulnerable to metabolism-related effects compared with men. The researchers also discovered that 20 μg/kg of TCDD inhibited plasma insulin levels in mice beyond the expected retention time of TCDD in the pancreas, indicating a long-term and irreversible toxic effect on beta cell (Hoyeck et al., 2020a, Hoyeck et al., 2020b).
4. Conclusion and future work
TCDD exposure primarily occurs through contaminated food and polluted feed with its accumulation in animals at the top of the food chain. The current review suggested that TCDD exacerbates energy metabolism disorders mainly by interfering with glucose and fat metabolism. This exposure leads to severe hyperglycemia and glucose intolerance in mice, especially when combined with the high-fat diet, and contributes to cardiovascular diseases, hepatobiliary injury, and neurodegenerative diseases. Given its widespread presence due to industrial development, reducing and controlling TCDD in the environment is now critical. Dioxins are mainly stored in meat and adipose tissue, due to its accumulating toxicity to humans, more comprehensive standards and sensitive detection methods are required to control its daily exposure. The indirect way that contaminants enter the food chain is through contaminated animal feed, so it is also necessary to monitor the areas where crops are grown (soil, water), the environment in which livestock are raised. Additionally, future research could focus on natural agents like lipophilic dietary fat substitutes to reduce or eliminate dioxins toxicity and further investigate the specific mechanisms by which dioxins affect human metabolism to develop AhR targeted therapies.
CRediT authorship contribution statement
Jiuhe Gao: Project administration, Writing – original draft, Format editing, Figure illustration design, Data curation. Yuqing Xu: Project administration, Writing – original draft, Format editing, Figure illustration design, Data curation. Tian Zhong: Writing – review & editing. Xi Yu: Conceptualization, Writing – review & editing. Ling Wang: Writing – review & editing. Ying Xiao: Supervision, Writing – review & editing. Ye Peng: Project administration, Supervision, Writing – review & editing. Quancai Sun: Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no conflict of interest associated with this manuscript.
Acknowledgement
This work was supported by the Faculty Research Grants at Macau University of Science and Technology, Macao SAR, China [FRG-22-111-FMD].
Handling Editor: Dr. Yeonhwa Park
Contributor Information
Ye Peng, Email: pengye@must.edu.mo.
Quancai Sun, Email: sqctp@hotmail.com.
Data availability
No data was used for the research described in the article.
References
- Anzenbacher P., Anzenbacherová E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. CMLS. 2001:737–747. doi: 10.1007/PL00000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzi P.A., Bernucci I., Brambilla G., Consonni D., Pesatori A.C. Environmental health perspectives; 1998. The Seveso Studies on Early and Long-Term Effects of Dioxin Exposure: a Review; pp. 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissot P., Ropert M., Le Lan C., Loréal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim. Biophys. Acta Gen. Subj. 2012:403–410. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Carvalhaes G.K., Brooks P., Marques C.G., Azevedo J.A.T., Machado M.C.S., Azevedo G.C. Lime as the source of PCDD/F contamination in citrus pulp pellets from Brazil and status of the monitoring program. Chemosphere. 2002:1413–1416. doi: 10.1016/S0045-6535(01)00263-6. [DOI] [PubMed] [Google Scholar]
- Chen M., Wu Y., Zhang H., Li S., Zhou J., Shen J. The roles of embryonic transcription factor BRACHYURY in tumorigenesis and progression. Front. Oncol. 2020:961. doi: 10.3389/fonc.2020.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xie H.Q., Sha R., Xu T., Zhang S., Fu H., Xia Y., Liu Y., Xu L., Zhao B. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and up-regulation of neurofilament expression in neuronal cells: evaluation of AhR and MAPK pathways. Environ. Int. 2020 doi: 10.1016/j.envint.2019.105193. [DOI] [PubMed] [Google Scholar]
- Ciftci O., Vardi N., Ozdemir I. Effects of quercetin and chrysin on 2,3,7,8-tetrachlorodibenzo-p-dioxin induced hepatotoxicity in rats. Environ. Toxicol. 2013:146–154. doi: 10.1002/tox.20707. [DOI] [PubMed] [Google Scholar]
- Dalton T.P., Kerzee J.K., Wang B., Miller M., Dieter M.Z., Lorenz J.N., Shertzer H.G., Nebert D.W., Puga A. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc. Toxicol. 2001:285–298. doi: 10.1385/ct:1:4:285. 10.1385/CT:1:4:285. [DOI] [PubMed] [Google Scholar]
- de Gannes M., Ko C.-I., Zhang X., Biesiada J., Niu L., Koch S.E., Medvedovic M., Rubinstein J., Puga A. Dioxin disrupts dynamic DNA methylation patterns in genes that govern cardiomyocyte maturation. Toxicol. Sci. 2020:325–337. doi: 10.1093/toxsci/kfaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar D., Baglieri J., Kisseleva T., Brenner D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020:96–108. doi: 10.1177/1535370219898141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C., Teixeira-Clerc F., Leblanc A.F., Touch S., Emond C., Guerre-Millo M., Lotersztajn S., Barouki R., Aggerbeck M., Coumoul X. Chronic exposure to low doses of dioxin promotes liver Fibrosis development in the C57BL/6J diet-induced obesity mouse model. Environ. Health Perspect. 2017:428–436. doi: 10.1289/EHP316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett C.J., Frithsen I.L., Diaz V.A., Koopman R.J., Simpson W.M., Mainous A.G. Environmental Research; 2007. Association of a Polychlorinated Dibenzo-P-Dioxin, a Polychlorinated Biphenyl, and DDT with Diabetes in the 1999–2002 National Health and Nutrition Examination Survey; pp. 413–418. [DOI] [PubMed] [Google Scholar]
- Fader K.A., Nault R., Zhang C., Kumagai K., Harkema J.R., Zacharewski T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-elicited effects on bile acid homeostasis: alterations in biosynthesis, enterohepatic circulation, and microbial metabolism. Sci. Rep. 2017;7:5921. doi: 10.1038/s41598-017-05656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader K.A., Nault R., Kirby M.P., Markous G., Matthews J., Zacharewski T.R. Convergence of hepcidin deficiency, systemic iron overloading, heme accumulation, and REV-ERBα/β activation in aryl hydrocarbon receptor-elicited hepatotoxicity. Toxicol. Appl. Pharmacol. 2017:1–17. doi: 10.1016/j.taap.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero P.M., Hllbert D.M., Rudikoff S., Ward J.M., Gonzalez F.J. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 1996:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Fletcher N., Wahlström D., Lundberg R., Nilsson C.B., Nilsson K.C., Stockling K., Hellmold H., Håkansson H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: a microarray study. Toxicol. Appl. Pharmacol. 2005:1–24. doi: 10.1016/j.taap.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Fu H., Wang L., Wang J., Bennett B.D., Li J.-L., Zhao B., Hu G. Dioxin and AHR impairs mesoderm gene expression and cardiac differentiation in human embryonic stem cells. Sci. Total Environ. 2019:1038–1046. doi: 10.1016/j.scitotenv.2018.09.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton A.M., Roth R.A., Ganey P.E. Pretreatment with TCDD exacerbates liver injury from Concanavalin A: critical role for NK cells. Toxicol. Sci. 2013:72–85. doi: 10.1093/toxsci/kft174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimpeţeanu O.-M., Militaru M., Scippo M.L. Dioxins and polychlorinated biphenyls contamination in poultry liver related to food safety – a review. Food Control. 2014;47–53 doi: 10.1016/j.foodcont.2013.09.054. [DOI] [Google Scholar]
- González N., Domingo J.L. Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in food and human dietary intake: an update of the scientific literature. Food Chem. Toxicol. 2021;112585 doi: 10.1016/j.fct.2021.112585. [DOI] [PubMed] [Google Scholar]
- Hoogenboom R., Traag W., Fernandes A., Rose M. European developments following incidents with dioxins and PCBs in the food and feed chain. Food Control. 2015:670–683. doi: 10.1016/j.foodcont.2014.10.010. [DOI] [Google Scholar]
- Hoyeck M.P., Merhi R.C., Blair H.L., Spencer C.D., Payant M.A., Alfonso D.I.M., Zhang M., Matteo G., Chee M.J., Bruin J.E. Female mice exposed to low doses of dioxin during pregnancy and lactation have increased susceptibility to diet-induced obesity and diabetes. Mol. Metabol. 2020 doi: 10.1016/j.molmet.2020.101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyeck M.P., Blair H., Ibrahim M., Solanki S., Elsawy M., Prakash A., Rick K.R.C., Matteo G., O'Dwyer S., Bruin J.E. Long-term metabolic consequences of acute dioxin exposure differ between male and female mice. Sci. Rep. 2020;1448 doi: 10.1038/s41598-020-57973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.-F., Tsou T.-C., Chao H.-R., Kuo Y.-T., Tsai F.-Y., Yeh S.-C. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J. Hazard Mater. 2010:649–655. doi: 10.1016/j.jhazmat.2010.06.081. [DOI] [PubMed] [Google Scholar]
- Ibrahim I.M., Althagafy H.S., Abd-alhameed E.K., Al-Thubiani W.S., Hassanein E.H.M. Promising hepatoprotective effects of lycopene in different liver diseases. Life Sci. 2022;121131 doi: 10.1016/j.lfs.2022.121131. [DOI] [PubMed] [Google Scholar]
- Ishida T., Kan-o S., Mutoh J., Takeda S., Ishii Y., Hashiguchi I., Akamine A., Yamada H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced change in intestinal function and pathology: evidence for the involvement of arylhydrocarbon receptor-mediated alteration of glucose transportation. Toxicol. Appl. Pharmacol. 2005:89–97. doi: 10.1016/j.taap.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Jokinen M.P., Walker N.J., Brix A.F., Sells D.M., Haseman J.K., Nyska A. Increase in cardiovascular pathology in female sprague-dawley rats following chronic treatment with 2,3,7,8-Tetrachlorodibenzo-p-Dioxin and 3,3′,4,4′,5-pentachlorobiphenyl. Cardiovasc. Toxicol. 2003:299–310. doi: 10.1385/CT:3:4:299. 10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K., Weber R. Dioxin sources to the aquatic environment: Re-assessing dioxins in industrial processes and possible emissions to the aquatic. Emerging Contam. 2021:52–62. doi: 10.1016/j.emcon.2021.01.002. [DOI] [Google Scholar]
- Kulkarni P.S., Crespo J.G., Afonso C.A.M. Dioxins sources and current remediation technologies — a review. Environ. Int. 2008;139–153 doi: 10.1016/j.envint.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Lind P.M., Örberg J., Edlund U.-B., Sjöblom L., Lind L. The dioxin-like pollutant PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol. Lett. 2004:293–299. doi: 10.1016/j.toxlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Liu C., Tao Q., Sun M., Wu J.Z., Yang W., Jian P., Peng J., Hu Y., Liu C., Liu P. Laboratory Investigation; 2010. Kupffer Cells Are Associated with Apoptosis, Inflammation and Fibrotic Effects in Hepatic Fibrosis in Rats; pp. 1805–1816. [DOI] [PubMed] [Google Scholar]
- Lu J., Shang X., Zhong W., Xu Y., Shi R., Wang X. New insights of CYP1A in endogenous metabolism: a focus on single nucleotide polymorphisms and diseases. Acta Pharm. Sin. B. 2020:91–104. doi: 10.1016/j.apsb.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P.K. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J. Comp. Physiol. B. 2005:221–230. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- McDonnell A.M., Dang C.H. Basic review of the cytochrome p450 system. J. Adv. Pract. Oncol. 2013:263–268. doi: 10.6004/jadpro.2013.4.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M., Haarmann-Stemmann T. Modulation of CYP1A1 metabolism: from adverse health effects to chemoprevention and therapeutic options. Pharmacol. Therapeut. 2018:71–87. doi: 10.1016/j.pharmthera.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Mimura J., Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta Gen. Subj. 2003:263–268. doi: 10.1016/S0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- Mimura J., Yamashita K., Nakamura K., Morita M., Takagi T.N., Nakao K., Ema M., Sogawa K., Yasuda M., Katsuki M., Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Gene Cell. 1997:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Mohsenzadeh M.S., Zanjani B.R., Karimi G. Mechanisms of 2,3,7,8-tetrachlorodibenzo-p-dioxin- induced cardiovascular toxicity: an overview. Chem. Biol. Interact. 2018;1–6 doi: 10.1016/j.cbi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Molcan T., Swigonska S., Orlowska K., Myszczynski K., Nynca A., Sadowska A., Ruszkowska M., Jastrzebski J.P., Ciereszko R.E. Structural-functional adaptations of porcine CYP1A1 to metabolize polychlorinated dibenzo-p-dioxins. Chemosphere. 2017;205–216 doi: 10.1016/j.chemosphere.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Nishiumi S., Yabushita Y., Furuyashiki T., Fukuda I., Ashida H. Involvement of SREBPs in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced disruption of lipid metabolism in male Guinea pig. Toxicol. Appl. Pharmacol. 2008:281–289. doi: 10.1016/j.taap.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Pajurek M., Warenik-Bany M., Mikolajczyk S. Dioxin transfer simulation from feed to animal tissues and risk assessment. Chemosphere. 2023;137379 doi: 10.1016/j.chemosphere.2022.137379. [DOI] [PubMed] [Google Scholar]
- Peng Y., Gu T., Zhong T., Xiao Y., Sun Q. Endoplasmic reticulum stress in metabolic disorders: opposite roles of phytochemicals and food contaminants. Curr. Opin. Food Sci. 2022 doi: 10.1016/j.cofs.2022.100913. [DOI] [Google Scholar]
- Petriello M.C., Brandon J.A., Hoffman J., Wang C., Tripathi H., Abdel-Latif A., Ye X., Li X., Yang L., Lee E., Soman S., Barney J., Wahlang B., Hennig B., Morris A.J. Dioxin-like PCB 126 increases systemic inflammation and accelerates atherosclerosis in lean LDL receptor-deficient mice. Toxicol. Sci. 2017:548–558. doi: 10.1093/toxsci/kfx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkle J.L., Wolfe W.H., Patterson D.G., Needham L.L., Michalek J.E., Miner J.C., Peterson M.R., Phillips D.L. Estimates of the half‐life of 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin in Vietnam veterans of operation ranch hand. J. Toxicol. Environ. Health. 1989:165–171. doi: 10.1080/15287398909531288. [DOI] [PubMed] [Google Scholar]
- Rao Q., Wang X., Zhang Q., Hoogenboom R., Li H., Deng Z., Song W., Cheng L., Liu X., Guan S., Song W., Yao C., Chen S., Zhou J. New insights into the transfer and accumulation of dioxins and dioxin-like PCBs in the food web of farmed Chinese mitten crabs: a typical case from the Yangtze River area. J. Hazard Mater. 2022 doi: 10.1016/j.jhazmat.2022.129178. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Martín F.J., Fernández‐Salguero P.M., Merino J.M. Aryl hydrocarbon receptor‐dependent induction of apoptosis by 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin in cerebellar granule cells from mouse. J. Neurochem. 2011:153–162. doi: 10.1111/j.1471-4159.2011.07291.x. [DOI] [PubMed] [Google Scholar]
- Sato K., Meng F., Francis H., Wu N., Chen L., Kennedy L., Zhou T., Franchitto A., Onori P., Gaudio E., Glaser S., Alpini G. Melatonin and circadian rhythms in liver diseases: functional roles and potential therapies. J. Pineal Res. 2020 doi: 10.1111/jpi.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Shirakawa H., Tomita S., Ohsaki Y., Haketa K., Tooi O., Santo N., Tohkin M., Furukawa Y., Gonzalez F.J., Komai M. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol. Appl. Pharmacol. 2008:10–19. doi: 10.1016/j.taap.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Shan Q., Huang F., Wang J., Du Y. Effects of co-exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and polychlorinated biphenyls on nonalcoholic fatty liver disease in mice. Environ. Toxicol. 2015:1364–1374. doi: 10.1002/tox.22006. [DOI] [PubMed] [Google Scholar]
- Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;101059 doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnweber T., Pizzini A., Nairz M., Weiss G., Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018;3285 doi: 10.3390/ijms19113285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg O. AhR signalling and dioxin toxicity. Toxicol. Lett. 2014:225–233. doi: 10.1016/j.toxlet.2013.10.039. [DOI] [PubMed] [Google Scholar]
- Sorg O., Zennegg M., Schmid P., Fedosyuk R., Valikhnovskyi R., Gaide O., Kniazevych V., Saurat J.H. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) poisoning in Victor Yushchenko: identification and measurement of TCDD metabolites. Lancet. 2009;1179–1185 doi: 10.1016/S0140-6736(09)60912-0. [DOI] [PubMed] [Google Scholar]
- Stefanovic L., Brenner D.A., Stefanovic B. Direct hepatotoxic effect of KC chemokine in the liver without infiltration of neutrophils. Exp. Biol. Med. 2005:573–586. doi: 10.1177/153537020523000809. [DOI] [PubMed] [Google Scholar]
- Sulentic C.E.W., Kaminski N.E. The long winding road toward understanding the molecular mechanisms for B-cell suppression by 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2011:S171–S191. doi: 10.1093/toxsci/kfq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svobodová J., Procházková J., Kabátková M., Krkoška M., Šmerdová L., Líbalová H., Topinka J., Kléma J., Kozubík A., Machala M., Vondráček J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) disrupts control of cell proliferation and apoptosis in a human model of adult liver progenitors. Toxicol. Sci. 2019:368–384. doi: 10.1093/toxsci/kfz202. [DOI] [PubMed] [Google Scholar]
- Tang J., Yan Z., Feng Q., Yu L., Wang H. The roles of neutrophils in the pathogenesis of liver diseases. Front. Immunol. 2021;625472 doi: 10.3389/fimmu.2021.625472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkez H., Geyikoglu F., Yousef M.I. Modulatory effect of L-glutamine on 2,3,7,8 tetrachlorodibenzo-p-dioxin-induced liver injury in rats. Toxicol. Ind. Health. 2012:663–672. doi: 10.1177/0748233711420474. [DOI] [PubMed] [Google Scholar]
- Türkez H., Geyikolu F., Yousef M.I. Modulatory effect of l-glutamine on 2,3,7,8 tetrachlorodibenzo-p-dioxin-induced liver injury in rats. Toxicol. Ind. Health. 2012;663 doi: 10.1177/0748233711420474. [DOI] [PubMed] [Google Scholar]
- Wan C., Liu J., Nie X., Zhao J., Zhou S., Duan Z., Tang C., Liang L., Xu G. 2, 3, 7, 8-tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS One. 2014 doi: 10.1371/journal.pone.0089811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fan Y., Puga A. Dioxin exposure disrupts the differentiation of mouse embryonic stem cells into cardiomyocytes. Toxicol. Sci. 2010:225–237. doi: 10.1093/toxsci/kfq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Zhou Q., Wan C., Wang Y., Liu J., Li Y., Nie X., Cheng C., Chen G. 2,3,7,8-TCDD induces neurotoxicity and neuronal apoptosis in the rat brain cortex and PC12 cell line through the down-regulation of the Wnt/β-catenin signaling pathway. Neurotoxicology. 2013;63–73 doi: 10.1016/j.neuro.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Yu Y., Liu Q., Guo S., Zhang Q., Tang J., Liu G., Kong D., Li J., Yan S., Wang R., Wang P., Su X., Yu Y. 2, 3, 7, 8‐Tetrachlorodibenzo‐p‐dioxin promotes endothelial cell apoptosis through activation of EP3/p38MAPK/Bcl‐2 pathway. J. Cell Mol. Med. 2017:3540–3551. doi: 10.1111/jcmm.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A., Nixon R.A. Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Front. Neurosci. 2021 doi: 10.3389/fnins.2021.689938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Dong X., Yap J., Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020;113 doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xie H.Q., Li Y., Zhou M., Zhou Z., Wang R., Hahn M.E., Zhao B. The aryl hydrocarbon receptor: a predominant mediator for the toxicity of emerging dioxin-like compounds. J. Hazard Mater. 2022 doi: 10.1016/j.jhazmat.2021.128084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Zhao H., Deng J., Guo L., Wu B. Role of the CLOCK protein in liver detoxification. Br. J. Pharmacol. 2019:4639–4652. doi: 10.1111/bph.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.