Abstract
Introduction
Forty-four percent of lactating women in the United States consume beverages containing low calorie sweeteners (LCS), and the presence of LCS in the food supply has continued to increase in recent years. While LCS are approved by the United States Food and Drug Administration (FDA) and are believed to be safe for human consumption, intergenerational LCS transmission and the health impacts of early life LCS exposure are severely understudied.
Methods and analysis
In a tightly controlled, single site, prospective interventional study, mothers' plasma and breast milk, and infants’ plasma will be collected from 40 mother-infant dyads over the course of 72 h, with rich sampling following maternal ingestion of a LCS sweetened beverage containing sucralose and acesulfame potassium (ace-K). Concentration-time data will be used to build maternal and infant pharmacokinetic models for future simulations and analysis.
Conclusion
This study aims to measure LCS concentrations in breast milk, maternal plasma, and infant plasma, to gain insight into infant exposure and inform recommendations for LCS consumption during breastfeeding.
Keywords: Low calorie sweeteners, Sucralose, Acesulfame-potassium, Intergenerational transmission, Pharmacometrics
Highlights
-
•
A key strength of the study is the rich sampling and measurement of sucralose and ace-K in maternal breast milk and plasma under tightly controlled experimental conditions.
-
•
This study will be the first to directly measure infants' LCS exposure following maternal ingestion of a LCS-containing beverage.
-
•
This study will specifically measure sucralose and ace-K concentrations acknowledging the marked heterogeneity across different types of LCS.
-
•
This study will contribute to the first pharmacokinetic model of sucralose and ace-K in infants and will enhance the present understanding of intergenerational LCS transmission.
-
•
Due to the enrollment of exclusively breastfeeding mothers, it is difficult to estimate the volume of the milk consumed by the infant. This limits our ability to accurately determine the dose of sucralose and ace-K the infant ingested when modeling.
-
•
Mothers will self-report their daily dietary intake; therefore, some records may be incomplete or inaccurate.
1. Introduction
Low calorie sweeteners (LCS) such as sucralose and acesulfame-potassium (ace-K) provide sweetness without calories and are increasingly present in food and beverage products [1]. Since 2010, there has been a greater than 3-fold increase in consumer products containing LCS [[2], [3], [4]], and LCS are presently found in a variety of diet beverages and sweetener packets (e.g., Splenda™), as well as in a diverse range of products including sugar-free ice-cream, non-diet fruit drinks, high-fiber oatmeal, and reduced-calorie breads. Sucralose and ace-K are two commonly consumed LCS and are often used in combination in foods and beverages, due to their chemical and physical properties [2,5,6]. However, recommendations for or against maternal LCS consumption while breastfeeding are presently lacking.
Sucralose and acesulfame-potassium (ace-K) have distinct physicochemical and absorption, distribution, metabolism, and elimination (ADME) properties that influence their transfer from maternal plasma to breast milk and eventually to infant plasma. The lipophilicity of these compounds, represented by logP values of −1.5 for sucralose [7] and −0.3 for ace-K [8], reflects a relatively neutral balance between hydrophilicity and lipophilicity. This balance allows them to interact with both lipid and aqueous environments, facilitating their transfer into breast milk. The acid dissociation constant (pKa) of ace-K is 2 [8], which may affect its ionization state at physiological pH, further influencing its absorption and distribution pattern. Both sucralose and ace-K are not metabolized significantly in the human body [9]. Sucralose's absorption rate in humans is relatively low, with the majority of the ingested amount being excreted unchanged in feces, while a smaller portion appears unmetabolized in urine [10]. Ace-K, on the other hand, exhibits rapid absorption and is excreted largely in the urine [9]. The differences in excretion pathways between the two compounds highlight variations in their clearance processes. These specific physicochemical and pharmacokinetic characteristics directly impact the transfer of sucralose and ace-K from maternal plasma to breast milk, explaining their detectable presence following ingestion.
In a recent study using data collected by the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC), 44 % of lactating women in the United States reported consuming beverages containing LCS, and 15.3 % reported consuming LCS-containing beverages at least once a day [11,12]. Based on data from the National Health and Examination Survey (NHANES), there was an approximately 50 % increase in LCS consumption among pregnant women between 1999-2004 and 2007–2014, with 24 % reporting consumption of LCS on a given day in 2007–2014 [[13], [14], [15]]. While LCS, including sucralose and ace-K, are approved by the FDA as safe for human consumption below the acceptable daily intake (ADI), the impact of early life LCS exposure in humans is severely understudied [10,16]. This is concerning because fetuses and infants are exposed to LCS both in utero and via breast milk, yet little is known about whether this early life exposure may influence infants’ health.
Previous studies conducted in vitro and in rodent models have demonstrated that maternal LCS consumption may adversely impact infants’ weight and health [17]. When pregnant and/or lactating mice were exposed to sucralose and ace-K at doses consistent with amounts consumed in humans, the offspring showed downregulation of hepatic detoxification mechanisms and changes in bacterial metabolites [18]. In a separate murine study, ace-K exposure during pregnancy was found to be associated with maternal glucose intolerance, adipose tissue dysfunction, reduced fetal growth, and fetal hypoglycemia [19]. Sucralose was also found to increase expression of adipogenic genes and inhibit lipolysis when incubated with mesenchymal stem cells and pre-adipocytes [20]. Additionally, exposure to ace-K during lactation is reported to promote a preference for sweet taste in rodent offspring [21].
In a preliminary study of LCS pharmacokinetics in breast milk, concentrations of sucralose and ace-K were measured serially for 6 h following maternal ingestion of a single diet soda. As expected, both sucralose and ace-K were detected in breast milk as neither sucralose nor ace-K are metabolized [11,[22], [23], [24]]. However, LCS concentrations in plasma were not measured in this prior study, no biospecimens were collected from the infant, and peak concentrations of sucralose were not captured during the 6-h period of follow-up sampling. The purpose of this study is therefore to measure sucralose and ace-K in maternal breast milk and plasma at pre-specified, serial, time-points for 72 h, as well as in a sample of infants' plasma. The maternal plasma data can further inform infant plasma pharmacokinetics and combining all three measurements (maternal plasma, maternal breast milk, and infant plasma) will allow for the derivation of meaningful insights into the LCS profile from mother's consumption to infant elimination. Understanding infant exposure to LCS from maternal ingestion will lay the groundwork for future studies examining effects of LCS exposure during lactation on infants' weight, gut microbiota, taste preferences and/or cardiometabolic health and will inform critically needed recommendations for or against maternal LCS consumption while breastfeeding.
2. Methods and analysis
2.1. Study population
This is a single site, prospective intervention study. The study population will comprise 40 mother-infant dyads. Eligibility criteria include that mothers are within 6 months postpartum, ≥18 years of age, exclusively breastfeeding, and have an infant between 4 weeks and 6 months of age. Mother-infant dyads will be recruited from the GW Midwifery Service, a division within the GW School of Medicine and Health Sciences (GWSMHS), in-person and virtually, as well as through social media groups and community listservs.
2.2. Study beverage
Ocean Spray Diet Cranberry™ juice is the diet beverage that will be used in the study due to its palatability and relatively high (29.5 mg/L and 160.7 mg/L) ace-K and sucralose concentrations, respectively. This will allow for the consumption of a reasonably high amount of LCS in a volume most mothers can easily consume.
2.3. Sampling schedule
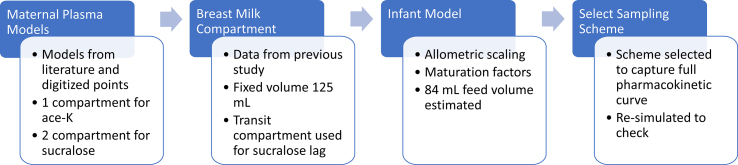
In order to select the recommended time points for sampling, pharmacokinetic simulations were performed for both sucralose and ace-K based on preliminary models (Fig. 1).
Fig. 1.
Sampling scheme development process.
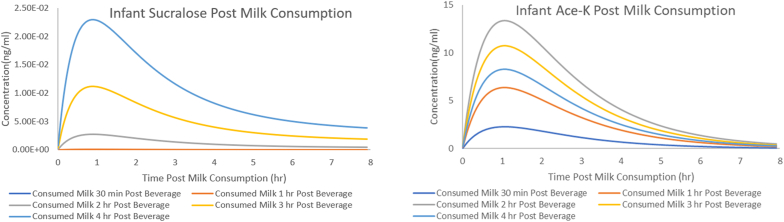
Models were built through several steps. First, maternal plasma models were constructed based on previous literature [25,26]. A one-compartment model with first order absorption was developed for ace-K using the reported parameter values and digitized data of 3 adult males [25]. A two-compartment model with first order absorption was developed for sucralose using the reported parameter values and fit to the digitized data [26]. The models were used to re-simulate the participants in the prior papers to ensure adequate performance. Next, maternal breast milk concentrations were considered. In both the sucralose and ace-K models, a breast milk compartment was added, and initial parameter values were estimated in accordance with methods used in previous literature [27]. Breast milk parameters were further adjusted using data from a prior study and a single pilot subject [22]. A pediatric model was then developed by allometrically scaling down the maternal model [28]. Simulations were generated for maternal plasma, maternal breast milk, and the infant plasma at 2 dose levels: the dose used in the prior breast milk study and at the proposed dose for the current trial [22]. For the simulations it was assumed that infants would consume 84 mL in a single feed based on the average milk consumption of 150 ml/kg/day and an average of 8 feeds [29]. Despite the utilization of 84 mL per feed in the simulations, any variability in actual consumption per feed is anticipated to primarily impact the concentration at key timepoints, rather than the timing of occurrences due to the assumed dose proportionality. Thus, such variability is not expected to influence the recommended timepoints but should be kept in mind when considering the concentrations expected in later stages. Several scenarios were tested with infants first consuming breast milk 30 min, 1 h, 2 h, 3 h, or 4 h post maternal dose. A sampling scheme for maternal plasma, maternal breast milk, and infant plasma was then selected to ensure that the full profile of both LCSs in maternal plasma, breast milk, and infant plasma is expected to be captured (Fig. 2, Fig. 3). When selecting timepoints for maternal plasma, additional care was taken to ensure that the full elimination phase of both LCS was adequately captured in our models such that extrapolation from maternal to infant plasma can be performed and the reliability of real-world exposure scenarios can be ensured. Ace-K breastmilk concentrations are expected to be negligible at 24 h, and sucralose breastmilk concentrations are anticipated to be in the terminal phase of elimination, as depicted in Fig. 2d and c, respectively. The adequacy of the 24-h timepoint for modeling the full sucralose breastmilk curve is thus inferred, and the absence of breastmilk samples beyond this timepoint is not considered to influence the infant dosing information and concentrations. The final selected sampling scheme (Fig. 4) was selected to be as minimally burdensome for the mothers and infants as possible without compromising the integrity and objectives of the study (Fig. 4). Models and simulations were performed using Pumas-AI version 2.0, Baltimore [30].
Fig. 2.
Model predicted concentrations and proposed sampling times. Fig. 2A is the Maternal Sucralose Plasma Predictions, Fig. 2B is the Maternal Ace-K Plasma Predictions, Fig. 2C Sucralose Breast Milk Predictions and Fig. 2D is the Ace-K Breast Milk Predictions. Green lines represent model predicted concentrations, blue squares represent sampling timepoints. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Fig. 3A is the Infant Sucralose Plasma and Fig. 3B is the Infant Ace-K Plasma profile. Each line represents a different infant feeding scenario.
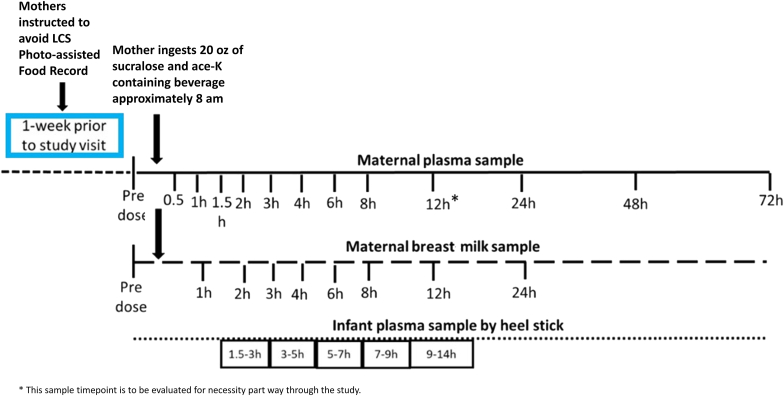
Fig. 4.
Study sampling protocol.
2.4. Study procedures
Mothers will be asked to avoid LCS and complete a 7 day online, photo-assisted food record during the week prior to an in-person study visit at Children's National Hospital, Clinical Research Unit (CRU) [31,32]. These photo assisted food records will be used to identify dietary sources of LCS intake to be accounted for during the analysis. During the visit, covariates that may impact sucralose and ace-K pharmacokinetics such as mothers' breastfeeding practices, maternal height, maternal weight, smoking status, alcohol intake, concomitant medications, and time since last feed will be collected. Infant anthropometric and birth data including length, weight, head circumference, birth order, sex, gestational age and the presence of any known health conditions will also be collected during the visit.
A pre-dose breast milk sample comprising the entire contents of the breast, will be collected from the mother to assess baseline sucralose and ace-K concentrations. The mother will then have an intravenous catheter (IV) placed, and a baseline plasma sample will be collected. After collection of the baseline breast milk and plasma samples, mothers will be asked to drink 20 ounces of Diet Ocean Spray cranberry juice, sweetened with sucralose and ace-k, within 10 min.
After ingestion of the diet beverage, mothers will remain at the CRU for 12 h and will provide a plasma and breast milk sample according to the sampling schedule (Fig. 2); following the successful completion of the first 8 dyads, the data will be analyzed to determine whether the 12-h maternal blood sample collection timepoint can be removed to allow for a shortened 8-h CRU stay and reduce participant burden. Mothers will then return to the CRU on the following 3 days to provide the 24-, 48-, and 72-h breast milk and blood samples. Mothers will continue avoiding LCS and recording their food/beverage intake using a photo-assisted food record to identify any potentially “hidden” sources of LCS until completion of the 72-h sample collection.
A blood sample will be collected from each infant via heel stick at one of the following pre-specified time intervals following maternal ingestion of the diet beverage: 1.5–3 h, 3–5 h, 5–7 h, 7–9 h, 9–14 h. The time of each feed, the length of feed and the exact time of sample collection will be recorded for each infant.
2.5. Sample analysis
Breast milk samples will be immediately frozen at −20 °C and transferred to a −80 °C freezer at the end of each visit for long-term storage. Blood samples will be centrifuged at 4 °C and the plasma stored at −80 °C until analysis.
All samples will be shipped to the Clinical Mass Spectrometry Laboratory at the National Institutes of Health for sucralose and ace-K analyses. Sucralose and ace-K concentrations in breast milk and plasma will be measured in triplicate using liquid chromatography-mass spectrometry (LC-MS), as detailed in prior publications.(1–4). Analyses will be performed with an Acquity I-Class UPLC (Waters Corp., Milford, MA, USA), coupled with a Q-Exactive MS (Thermo Scientific, Waltham, MA, USA), consistent with prior studies [33].
2.6. Sample size determination
This is the first study to gather information on LCS pharmacokinetics in infants; 40 mother-infant dyads were selected to ensure all useful information needed to characterize the pharmacokinetics of sucralose and ace-K is collected. The primary goal for this study is to precisely estimate key pharmacokinetic parameters in the maternal model and collect enough infant samples such that, in combination with the maternal model, the infant pharmacokinetics can be characterized. Following the methodologies described by Wang et al. [34], and assuming rich sampling in the mothers, the study was prospectively powered to precisely estimate the maternal geometric mean for clearance with a 95 % CI within 60 % and 140 % of for both sucralose and ace k with 90 % power. Areas under the concentration-time curve (AUCs) from the previous studies were used as surrogates for clearance and %CV was calculated, and both those and more extreme estimates for those variabilities (1.5x) were used in the power calculations. It was calculated that 30 participants would be needed, given the variability in breast milk sucralose and ace-K concentrations previously observed. Given that only one sample would be collected from each infant to minimize risk, and some infants will not have known dose volume information due to direct breastfeeding (as opposed to drinking pumped breast milk), an increase in sample size was deemed necessary. A sample size of forty participants was determined to allow characterization of the infants’ pharmacokinetic profiles of both LCS, while also allowing for consideration of potential confounders.
2.7. Data analysis plan
The maternal pharmacokinetic model will be constructed using maternal plasma and breast milk concentrations after a single oral dose. The LCS concentration-time data will first be analyzed in two steps for each LCS: graphical analysis and non-compartmental (NCA) analysis [35]. Graphical review of the data will include data qualification and summary analysis of patient specific prognostic factors. The milk-to-plasma ratio (M:P) will be calculated for sucralose and ace-K based on the maternal breast milk and infant plasma AUCs. The samples will then be analyzed by standard NCA in Pumas [30]. The individual NCA results will then be summarized and used as initial estimates in the development of the base model. The following individual pharmacokinetic parameters will be assessed: AUC (area under the curve), Cmax: maximum observed serum concentration, Tmax: time to reach Cmax, T1/2: terminal elimination half-life, Vd: Apparent volume of distribution, and CL/F: Apparent clearance. The time points used for calculating the terminal elimination will be determined by the software with uniform weighting when at least 3 decreasing points after Cmax were recorded. If an individual profile did not have at least 3 concentrations after Cmax that displayed a decreasing profile, then it will be excluded from the summary analysis.
The concentration-time data will then be used to develop a base structural model using the non-linear mixed effect approach in Pumas [30,[36], [37], [38]],. The model will be explored factoring in the previous literature data. Compartmental dynamics will be explored based on the best fit to the data, and the absorption model will be determined based on the characteristics of the compound and the available data. Plasma and breast milk data will be analyzed together in the population pharmacokinetic analyses. Once the base pharmacokinetic parameters have been estimated the data will be used to characterize the effects of participant-specific prognostic factors (e.g., weight, reported LCS consumption habits, dietary intake, maternal blood volume, age, sex, and race/ethnicity) to explain between subject variability in the pharmacokinetic parameters. Model selection will be guided by criteria such as the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and visual inspection of diagnostic plots.
The maternal pharmacokinetic model will then be scaled down to a pediatric pharmacokinetic model using principles of body-size-based allometry and maturation factors. As the mechanism of elimination of sucralose and ace-K between adults and infants can be expected to be the same, the estimates of the fundamental pharmacokinetic parameters (clearance and volume of distribution) will be adjusted accounting for organ maturation factors. Therefore, to estimate the maturation function, data from children younger than 2 years are essential. The typical maturation function connects maturation to the postmenstrual age (PMA) and is expressed as [39]:
| MAT = PMAH / (PMAH + PMA_50H) |
Even though both Sucralose and Ace-K are not metabolized, it is anticipated that this maturation factor will remain relevant due to the renal function component. Using the sparse infant data and informed by the scaled model, a base model of infant pharmacokinetics will be developed [40,41]. Additional infants' covariates, including infants’ age, weight, race, and sex will be evaluated in the combined pharmacokinetic model. The final population pharmacokinetic model for sucralose and ace-K in both mothers and infants will be evaluated and qualified using standard goodness of fit diagnostics and quantitative predictive check methods.
In many cases, the volume of milk consumed by the infants will not be captured; however, both the concentration of the LCS in the breast milk and the volume of milk consumed is necessary to determine the infant LCS dose for modeling. Several methods will be tested to address missing data. One method will be weighing the infant before and after each feed, and estimating the volume consumed by examining correlations between weight changes and volumes of breast milk consumed among infants who ingest pumped breast milk (among whom the volume of breast milk ingested can be measured). A second method is to use available literature on the average daily volume of milk consumed by infants by age and divide that volume across the number of feeds reported on the day of sampling [29]. Both methods will be compared the relative infant dose (RID) of sucralose and ace-K will be calculated per the FDA lactation guidance using both methods [42].
One limitation of this study is that the relationship between heel sticks and venous plasma concentrations for ace-K and sucralose in infants is not established, making this the first study to explore this link and leading to potential uncertainty in the results. While heel sticks are a preferred method due to their less invasive nature and have been validated for other drugs [[43], [44], [45]], the lack of previous research specifically on LCS may impact the accuracy of the correlations drawn between heel stick and venous plasma concentrations.
With the final linked maternal-infant models, alternative situations will be simulated using basic pharmacokinetic principles of superposition to determine expected concentrations of LCS in infants after repeated maternal consumption more similar to real world consumption.
3. Conclusion
LCS, such as sucralose and acesulfame-potassium (ace-K), are commonly consumed among pregnant and lactating women. Thus, infants are exposed to LCS both in utero and via breast milk; however, little is known about the potential impact of early life exposure to LCS on infants' health. This study will allow us to measure and model sucralose and ace-K concentrations in maternal breast milk and plasma, as well as in infants' plasma, to better understand infant exposure and inform recommendations for maternal LCS consumption while breastfeeding.
Patient and public involvement
We value the participation of potential subjects in our research on LCS in breastmilk. Throughout the recruitment process, we will gather feedback from potential participants to ensure that their comfort is prioritized during their visit. Additionally, a future aim involving this work is to use findings to help inform clinicians and the wider public to increase awareness and understanding of LCS in breastmilk.
Ethics and dissemination
Ethical approval for this study has been granted by the Institutional Review Board at the George Washington University (NCR213471) and the study was registered on ClinicalTrials.gov (NCT05379270) prior to beginning participant enrollment. Both sucralose and ace-K have undergone extensive safety evaluations and have been approved by the US Food and Drug Administration as food additives and safe from a toxicological perspective. Enrollment into this study will be limited to mothers who habitually consume LCS and have no known allergies or contraindications to LCS. The study's findings will be published in peer reviewed journal(s) and disseminated at national and international conferences.
Author contributions
MG, JV, JM, KA, and ACS conceptualized and designed the study. BL wrote the first draft of the manuscript. All authors contribute to revising and editing the manuscript and approve of the final version submitted.
Funding statement
This work was supported by R21HD105648 (PI: Sylvetsky) from the National Institute of Child Health and Development at the National Institutes of Health.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ACS has engaged in consulting work on behalf of Abbott. None of the other authors have any conflicts of interest to disclose.
Contributor Information
Mathangi Gopalakrishnan, Email: mgopalakrishnan@rx.umaryland.edu.
Allison C. Sylvetsky, Email: asylvets@email.gwu.edu.
References
- 1.Nutrition C for FS and A . FDA; 2020. Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States. [Google Scholar]
- 2.Sylvetsky A.C., Rother K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016;164:446–450. doi: 10.1016/j.physbeh.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sylvetsky A.C., Dietz W.H. Nutrient-content claims — guidance or cause for confusion? N. Engl. J. Med. 2014;371:195–198. doi: 10.1056/NEJMp1404899. [DOI] [PubMed] [Google Scholar]
- 4.Baker-Smith C.M., de Ferranti S.D., Cochran W.J., COMMITTEE ON NUTRITION SOG HEPATOLOGY, AND NUTRITION. Abrams S.A., Fuchs G.J., III, et al. The use of nonnutritive sweeteners in children. Pediatrics. 2019;144 doi: 10.1542/peds.2019-2765. [DOI] [PubMed] [Google Scholar]
- 5.Magnuson B.A., Carakostas M.C., Moore N.H., Poulos S.P., Renwick A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016;74:670–689. doi: 10.1093/nutrit/nuw032. [DOI] [PubMed] [Google Scholar]
- 6.The Market for High-Intensity Sweeteners Is Expected to Reach Nearly $1.9 Billion. 2017. https://www.bccresearch.com/pressroom/fod/market-high-intensity-sweeteners-expected-reach-nearly-$1.9-billion-2017 n.d. [Google Scholar]
- 7.PubChem. Sucralose n.d. https://pubchem.ncbi.nlm.nih.gov/compound/71485 (accessed August 15, 2023).
- 8.PubChem. Acesulfame n.d. https://pubchem.ncbi.nlm.nih.gov/compound/36573 (accessed August 15, 2023).
- 9.Pang M.D., Goossens G.H., Blaak E.E. The impact of artificial sweeteners on body weight Control and glucose homeostasis. Front. Nutr. 2021;7 doi: 10.3389/fnut.2020.598340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grotz V.L., Munro I.C. An overview of the safety of sucralose. Regul. Toxicol. Pharmacol. 2009;55:1–5. doi: 10.1016/j.yrtph.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Fein S.B., Labiner-Wolfe J., Shealy K.R., Li R., Chen J., Grummer-Strawn L.M. Infant feeding practices study II: study methods. Pediatrics. 2008;122:S28–S35. doi: 10.1542/peds.2008-1315c. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q., Murphy J., Smith E.R., Sylvetsky A.C. Diet beverage intake during lactation and associations with infant outcomes in the infant feeding practices study II. Nutrients. 2021;13:3154. doi: 10.3390/nu13093154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sylvetsky A.C., Figueroa J., Rother K.I., Goran M.I., Welsh J.A. Trends in low-calorie sweetener consumption among pregnant women in the United States. Curr. Dev. Nutr. 2019;3 doi: 10.1093/cdn/nzz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sylvetsky A.C., Jin Y., Clark E.J., Welsh J.A., Rother K.I., Talegawkar S.A. Consumption of low-calorie sweeteners among children and adults in the United States. J. Acad. Nutr. Diet. 2017;117:441–448. doi: 10.1016/j.jand.2016.11.004. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sylvetsky A.C., Welsh J.A., Brown R.J., Vos M.B. Low-calorie sweetener consumption is increasing in the United States. Am. J. Clin. Nutr. 2012;96:640–646. doi: 10.3945/ajcn.112.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts A. The safety and regulatory process for low calorie sweeteners in the United States. Physiol. Behav. 2016;164:439–444. doi: 10.1016/j.physbeh.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Archibald A., Dolinsky V., Azad M. Early-life exposure to non-nutritive sweeteners and the developmental origins of childhood obesity: global evidence from human and rodent studies. Nutrients. 2018;10:194. doi: 10.3390/nu10020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivier-Van Stichelen S., Rother K.I., Hanover J.A. Maternal exposure to non-nutritive sweeteners impacts progeny's metabolism and microbiome. Front. Microbiol. 2019;10:1360. doi: 10.3389/fmicb.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plows J.F., Morton-Jones J., Bridge-Comer P.E., Ponnampalam A., Stanley J.L., Vickers M.H., et al. Consumption of the artificial sweetener acesulfame potassium throughout pregnancy induces glucose intolerance and adipose tissue dysfunction in mice. J. Nutr. 2020;150:1773–1781. doi: 10.1093/jn/nxaa106. [DOI] [PubMed] [Google Scholar]
- 20.Simon B.R., Parlee S.D., Learman B.S., Mori H., Scheller E.L., Cawthorn W.P., et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J. Biol. Chem. 2013;288:32475–32489. doi: 10.1074/jbc.M113.514034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G.-H., Chen M.-L., Liu S.-S., Zhan Y.-H., Quan Y., Qin Y.-M., et al. Effects of mother's dietary exposure to acesulfame-K in pregnancy or lactation on the adult offspring's sweet preference. Chem. Senses. 2011;36:763–770. doi: 10.1093/chemse/bjr050. [DOI] [PubMed] [Google Scholar]
- 22.Rother K.I., Sylvetsky A.C., Walter P.J., Garraffo H.M., Fields D.A. Pharmacokinetics of sucralose and acesulfame-potassium in breast milk following ingestion of diet soda. J. Pediatr. Gastroenterol. Nutr. 2018;66:466–470. doi: 10.1097/MPG.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sylvetsky A.C., Bauman V., Blau J.E., Garraffo H.M., Walter P.J., Rother K.I. Plasma concentrations of sucralose in children and adults. Toxicol. Environ. Chem. 2017;99:535–542. doi: 10.1080/02772248.2016.1234754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sylvetsky A.C., Gardner A.L., Bauman V., Blau J.E., Garraffo H.M., Walter P.J., et al. Nonnutritive sweeteners in breast milk. J. Toxicol. Environ. Health. 2015;78:1029–1032. doi: 10.1080/15287394.2015.1053646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer DG, Kemper FH, editors. Acesulfame-K. New York: Marcel Dekker; 1991.
- 26.Roberts A., Renwick A.G., Sims J., Snodin D.J. Sucralose metabolism and pharmacokinetics in man. Food Chem. Toxicol. 2000;38:31–41. doi: 10.1016/S0278-6915(00)00026-0. [DOI] [PubMed] [Google Scholar]
- 27.Salman S., Sy S.K.B., Ilett K.F., Page-Sharp M., Paech M.J. Population pharmacokinetic modeling of tramadol and its O-desmethyl metabolite in plasma and breast milk. Eur. J. Clin. Pharmacol. 2011;67:899–908. doi: 10.1007/s00228-011-1023-6. [DOI] [PubMed] [Google Scholar]
- 28.Liu T., Ghafoori P., Gobburu J.V.S. Allometry is a reasonable choice in pediatric drug development: journal of clinical pharmacology. J. Clin. Pharmacol. 2017;57:469–475. doi: 10.1002/jcph.831. [DOI] [PubMed] [Google Scholar]
- 29.Yeung C.H.T., Fong S., Malik P.R.V., Edginton A.N. Quantifying breast milk intake by term and preterm infants for input into paediatric physiologically based pharmacokinetic models. Matern. Child Nutr. 2020;16 doi: 10.1111/mcn.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rackauckas C., Ma Y., Noack A., Dixit V., Mogensen P.K., Elrod C., et al. Accelerated predictive healthcare analytics with Pumas, A high performance pharmaceutical modeling and simulation platform. Pharmacol. Toxicol. 2020 doi: 10.1101/2020.11.28.402297. [DOI] [Google Scholar]
- 31.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sylvetsky A.C., Walter P.J., Garraffo H.M., Robien K., Rother K.I. Widespread sucralose exposure in a randomized clinical trial in healthy young adults. Am. J. Clin. Nutr. 2017;105:820–823. doi: 10.3945/ajcn.116.144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Jadhav P.R., Lala M., Gobburu J.V. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J. Clin. Pharmacol. 2012;52:1601–1606. doi: 10.1177/0091270011422812. [DOI] [PubMed] [Google Scholar]
- 35.Kalaria S.N., Armahizer M., McCarthy P., Badjatia N., Gobburu J.V., Gopalakrishnan M. A practice‐based, clinical pharmacokinetic study to inform levetiracetam dosing in critically ill patients undergoing continuous venovenous hemofiltration (PADRE‐01) Clin. Transl. Sci. 2020;13:950–959. doi: 10.1111/cts.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehrotra S., Gopalakrishnan M., Gobburu J., Greer J.M., Piekarz R., Karp J.E., et al. Population pharmacokinetics and site of action exposures of veliparib with topotecan plus carboplatin in patients with haematological malignancies: population pharmacokinetics of veliparib treatment in combination with topotecan plus carboplatin. Br. J. Clin. Pharmacol. 2017;83:1688–1700. doi: 10.1111/bcp.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalaria S.N., Gopalakrishnan M., Heil E.L. A population pharmacokinetics and pharmacodynamic approach to optimize tazobactam activity in critically ill patients. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.02093-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cacek A.T., Gobburu J.V.S., Gopalakrishnan M. Population pharmacokinetics of an intranasally administered combination of oxymetazoline and tetracaine in healthy volunteers: journal of clinical pharmacology. J. Clin. Pharmacol. 2017;57:247–254. doi: 10.1002/jcph.799. [DOI] [PubMed] [Google Scholar]
- 39.Bouazza N., Dokoumetzidis A., Knibbe C.A.J., de Wildt S.N., Ambery C., De Cock P.A., et al. General clinical and methodological considerations on the extrapolation of pharmacokinetics and optimization of study protocols for small molecules and monoclonal antibodies in children. Br. J. Clin. Pharmacol. 2022;88:4985–4996. doi: 10.1111/bcp.15571. [DOI] [PubMed] [Google Scholar]
- 40.Liu T., Lewis T., Gauda E., Gobburu J., Ivaturi V. Mechanistic population pharmacokinetics of morphine in neonates with abstinence syndrome after oral administration of diluted tincture of opium: mechanistic population pharmacokinetics of morphine. J. Clin. Pharmacol. 2016;56:1009–1018. doi: 10.1002/jcph.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booth B.P., Rahman A., Dagher R., Griebel D., Lennon S., Fuller D., et al. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J. Clin. Pharmacol. 2007;47:101–111. doi: 10.1177/0091270006295789. [DOI] [PubMed] [Google Scholar]
- 42.Research C for D.E. US Food Drug Adm; 2020. Clinical Lactation Studies: Considerations for Study Design.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-lactation-studies-considerations-study-design [Google Scholar]
- 43.De Rose D.U., Cairoli S., Dionisi M., Santisi A., Massenzi L., Goffredo B.M., et al. Therapeutic drug monitoring is a feasible tool to personalize drug administration in neonates using new techniques: an overview on the pharmacokinetics and pharmacodynamics in neonatal age. Int. J. Mol. Sci. 2020;21:5898. doi: 10.3390/ijms21165898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birnbaum A.K., Meador K.J., Karanam A., Brown C., May R.C., Gerard E.E., et al. Antiepileptic drug exposure in infants of breastfeeding mothers with epilepsy. JAMA Neurol. 2020;77:441–450. doi: 10.1001/jamaneurol.2019.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auriti C., Goffredo B.M., Ronchetti M.P., Piersigilli F., Cairoli S., Bersani I., et al. Validation of heel stick microsampling to optimize micafungin doses in neonates and young infants. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01199-18. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]