Abstract
Leukocoria is an aberration of the eyeball that interferes with normal reflection. It shows up as a white or gray pupillary reflex rather than the bright red or orange pupil of the other eye. Leukocoria can be brought on by a variety of ocular pathologies, with retinoblastoma being the most common. We present the case of a 17-month-old guy who had unilateral leukocoria and whose orbital MRI was ordered on the basis of retinoblastoma suspicion. The results, however, were more suggestive of Coats disease than retinoblastoma. Telangiectasia and exudate, which frequently afflict males’ unilateral eyes, are the hallmarks of Coats’ illness. Depending on the stage of the disease, there are differences in its severity, course, and outlook. It is crucial to get therapy and a diagnosis for retinal problems as soon as possible. Although uncommon, there are still many people who are not familiar with Coats’ disease. This paper aims to describe imaging findings in Coats' disease.
Keywords: Childhood, Coats’ disease, Leukocoria, MRI, Ultrasound
Introduction
Leukocoria, which literally translates to “white pupil,” is the term for the clinical finding of a white pupillary reflex on examination. This discovery can be made through direct ophthalmoscopy by detecting an asymmetric red response, or it can be accidentally captured on flash photography. It comes about as a result of an aberration in the eyeball that obstructs the natural reflecting mechanism. Leukocoria can be caused by a variety of ocular conditions, but the most frequent one is retinoblastoma [1], which often presents as a yellow-white retinal mass that is frequently encircled by subretinal fluid, subretinal seeds, and vitreous seeds [2]. Diagnostic confusion exists, however, because similar findings can be connected to a wide range of juvenile fundus illnesses [2]. In this report, we describe the case of a 17-month-old male born full-term presented with unilateral leukocoria for whom an orbital MRI was requested on suspicion of retinoblastoma; the findings, however, pointed more toward Coats’ disease than retinoblastoma. Although uncommon, there are still many people who are not familiar with Coats’ disease of eye. This paper aims to describe imaging findings in Coats’ disease.
Case report
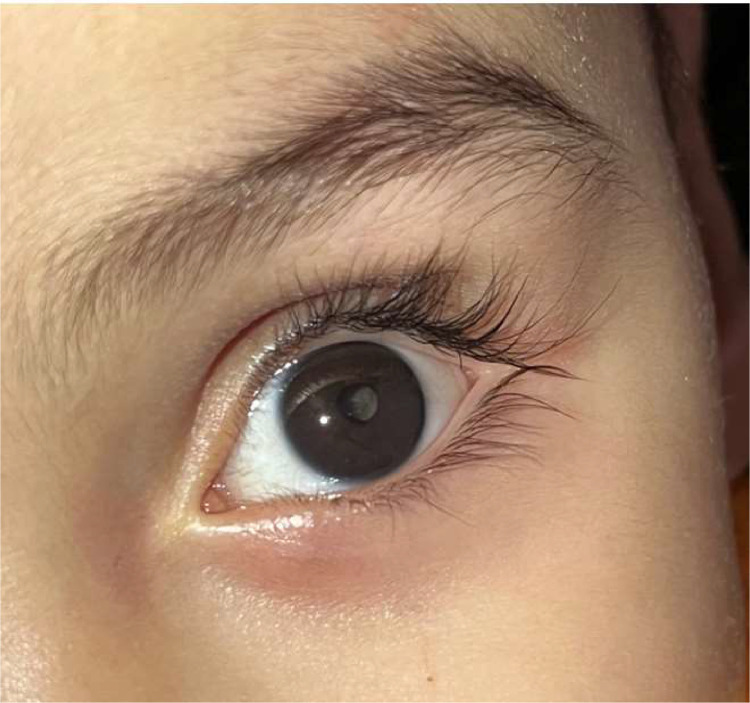
A 17-month-old male infant from a full-term pregnancy presents with leukocoria of the right eye (Fig. 1). No similar case in the family. The birth history of the patient revealed a non-consanguineous marriage. There were no complications during the intra-natal period to mother or child. The patient was full-term born of normal vaginal delivery, with notion of neonatal suffering for which the patient had to stay in an intensive care unit. An MRI scan showing leukodystrophy. Ophthalmological examination: Slit-lamp examination revealed anterior segment was normal in the left eye and leukocoria (amaurotic cat-eye reflex) in the right eye.
Fig. 1.
Clinical finding: leukocoria of the right eye.
An orbital ultrasound was performed (Fig. 2), showing retinal detachment with an echogenic appearance of the vitreous, with individualization of pure anechogenic cystic formations. No mass lesion or calcification was found. Orbital MRI (Fig. 3) was ordered to rule out the diagnosis of retinoblastoma, which revealed a right retinal detachment with subretinal fluid that is T1 hyperintense, T2 hypointense, and nonsuppressing on FLAIR, compared to normal vitreous with intravitreous macrocyst. Smaller right eye size compared to the left with spherical appearance of the lens, no ciliary body deformity, central stalk under the retinal detachment, or optic nerve atrophy. The diagnosis of coats’ disease of eye was made in the base of these findings. The patient was referred to the ophthalmology department for further management.
Fig. 2.
Ultrasonography showing an echogenic appearance of the vitreous, with individualization of pure anechogenic cystic formations.
Fig. 3.
Magnetic resonance, T2 FAT/SAT axial (A), T2-weighted axial (B), CISS axial (C), T1-weighted sagittal (D), FLAIR axial (E), and T1-weighted postenhancement axial (F) section images, showing a right retinal detachment with subretinal fluid that is T1 hyperintense, T2 hypointense, and nonsuppressing on FLAIR, compared to normal vitreous with intraretinal macrocyst. Smaller right eye size compared to the left, with spherical appearance of the lens, no ciliary body deformity, central stalk under the retinal detachment, or optic nerve atrophy. Noted leukodysrophy in the right et left temporal lobe.
Discussion
Exudative retinitis, commonly known as Coats disease, is a rare, sporadic condition that is not heritable and is characterized by idiopathic retinal vascular telangiectasias that may also be linked to exudative retinal detachments [3,4]. Initially characterized as a unilateral retinal vascular anomaly with exudation in young males by Scottish ophthalmologist George Coats in 1908 [3]. Later, Leber identified a different syndrome that was marked by a high number of retinal aneurysms and retinal degeneration [5]. Retinal telangiectasia, the underlying pathology that led to progressive exudation and retinal detachment, was the condition that Reese initially recognized as being similar to Coats disease and Leber miliary aneurysms in 1955 [4,6]. Its incidence is approximately 0.09 per 100,000 persons, and it primarily affects young males in 85% of cases [7]. Even though there have been a few reports of occurrences in adults [8,9], they are often less severe. Before the age of twenty, the majority of cases are identified [10]. More than 75% of cases of the illness are unilateral [11]. There have been no discovered geographic or ethnic relationships [11].
The specific cause of Caots’ disease, a nonhereditary condition, is still unknown. Coats syndrome has been linked to two distinct pathogenic pathways [12,13]: (1) Changes to the endothelial nature of the retinal vasculature lead to the breakdown of the blood-retinal barrier. Then, plasma leaks into the vessel wall, thickening it and giving rise to “sausage-like” shaped vessels; (2) damaged endothelium mixed with aberrant pericytes results in the bulging of the vessels and identifiable telangiectasia. The exudation of lipids from this pathogenic vasculature, which also causes retinal hypoxia, results in the formation of retinal cysts, retinal separation, and retinal thickening [12].
While adults typically appear with painless vision loss, children typically present with reduced vision, strabismus, nystagmus, and leukocoria [11,14].
The Shields classification [10] divides Coats’ disease into 5 stages: stage 1 is characterized by the presence of only retinal telangiectasias; stage 2 by the presence of telangiectasias and exudates (2A: extrafoveal exudates and 2B: foveal exudates); stage 3 by retinal detachment without glaucoma (3A: subtotal and 3B: total), with subtotal detachment distinguished by the presence (3A2) or the absence (3A1) of foveal involvement; stage 4 by total retinal detachment with glaucoma; and stage 5 by advanced end-stage disease [10,15].
Retinoblastoma is the predominant intraocular cancer in children with the highest prognosis risk of mortality if ignored, making it the most important differential diagnosis for this entity and one that demands careful consideration while being diagnosed. The most common cause of inappropriate enucleation, it has also been reported, is Coats disease misdiagnosed as retinoblastoma. Additionally, retinopathy of prematurity must be ruled out because it may be treated and has no effect on vision. As well as the unilateral disorders ocular toxocariasis and persistent fetal vasculature, it is crucial to include the more often occurring bilateral conditions such as familial exudative vitreoretinopathy, hemangioblastoma von Hippel, pars planitis, and incontinentia pigmenti [16].
Despite the fact that there are several methods available to diagnose Coats’ disease and, more significantly, to distinguish it from the entities listed above, the fundus examination remains the most reliable diagnostic tool. Due to the frequent yellow exudation and bulbous terminal shape, retinal telangiectasias, a common occurrence, are also known as “light bulb telangiectasias” [12].
The inferior and temporal quadrants are most typically affected. Intraretinal exudation is almost always present and may be substantial and separate from the telangiectasia. Additionally, MRI found its interest in the diagnosis of advanced Coats’ illness, while ultrasonography and CT scans are helpful in excluding other differentials, including retinoblastoma [[12], [17], [18], [19]].
Macular edema, disc retinal and iris neovascularization, capillary dropouts, early hypofluorescence in the areas of retinal exudation with late staining and late leakage from the abnormal vessels, and hyperfluorescence of the retinal telangiectasia in the venous phase are all revealed by fluorescein angiography [20]. The representation of subretinal/intraretinal fluid, central macular thickness, subfoveal choroidal thickness, cystoid macular edema, epiretinal membrane, vitreomacular traction, and choroidal thickness is made possible by optical coherence tomography (OCT) [20,12].
Ophthalmic B-scan ultrasonography is useful in clinical situations when posterior segment investigation is limited by media opacity or recalcitrant patients. Ultrasonography can determine the severity of Coats’ disease and the absence of a choroidal mass lesion. There may be a relatively immobile, serous retinal detachment adjacent to the optic nerve head, hyperreflective masses of exudate, or clear subretinal space without considerable choroidal thickening or vitreoretinal tension, among other common findings. A possible malignancy's presence can also be determined by calcification [11].
MRI is far superior to CT scan in ruling out retinoblastoma because the distinction between subretinal exudation and a solid mass is apparent on MRI [16]. However, MRI may be less effective in the early stages of Coats’ disease. Both T1- and T2-weighted MRI images of Coats’ disease exhibit hyperintense exudate [16]. Y-shaped retinal detachment, absence of calcifications, intraretinal macrocysts (degenerative changes that occur frequently as the duration of retinal detachment increases [12], absence of contrast enhancement within the solid lesion, and contrast enhancement outside the solid lesion were additional findings that strongly supported the diagnosis of coats syndrome [18,21]. Coats disease subfoveal enhancing nodules were recently discovered on MR imaging, despite a prevalence of 53% reported in ophthalmologic and histopathologic evaluations. Histopathologically, subfoveal nodules are composed primarily of lipid- and protein-rich material, and they develop into macular fibrosis over time, which is linked to less favorable visual results [21]. In individuals with Coats' disease, the presence of a subfoveal nodule at presentation is a risk factor for the development of macular fibrosis and a worse visual prognosis [8,14]. In our case, the MRI findings of retinal detachment with T1 hyperintense, T2 hypointense subretinal fluid, smaller eye size compared to contralateral, absence of calcifications, and intraretinal macrocysts are consistent with the typical presentation of Coats’ disease. There is typically no lens deformation.
A CT scan can also be helpful in ruling out retinoblastoma. In contrast to Coats’ disease, patients with retinoblastoma might present with solid tumors and calcifications that can be seen on a CT scan. It should be noted, nonetheless, that CT might not be helpful in identifying retinoblastoma instances without calcification. In addition, a submacular nodule may develop and become calcified in severe Coats' disease. When the condition is advanced, CT can also reveal the retinal detachment and the lipid exudate as a hyperdense region in the orbit [16].
The choice of treatment depends on the severity of the condition and the surgeon's preferences, and alternatives include laser photocoagulation, cryotherapy, and surgical correction of the retinal detachment. Laser photocoagulation is used to treat telangiectatic vessels in the early stages of the disorder. When considerable exudation or subretinal fluid hinder laser uptake, aberrant blood vessels are destroyed by cryotherapy. Cryotherapy may not be able to remove retinal vascular lesions when there is bullous subretinal fluid in advanced illness or when there is substantial retinal detachment. The visual prognosis for individuals with severe disease is poor, but if they are not treated, their condition may proceed to secondary angle closure or neovascular glaucoma, which would need enucleation [15,14].
Conclusion
Leukocoria from uncommon causes needs special consideration. The identification of leukocoria demands a thorough investigation into the etiology. With retinoblastoma, Coats disease continues to be a debatable condition that is frequently challenging to identify. Due to severe amblyopia, quick deterioration, and challenges with therapeutic care, the prognosis is frequently poor.
Authors’ contributions
All authors contributed to this work. All authors have read and approved the final version of the manuscript.
Patient consent
Written informed consent for publication was obtained from patient's parents.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Damasco CPT, Veronica C, Dire DJ. A child with Leukocoria. Pediatr Emerg Care. 2011;27(12):1170–1174. doi: 10.1097/pec.0b013e31823b0316. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Schoenberg E, Kocher K, Shukla SY, Kaliki S, Shields JA. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases: results based on age at presentation. Ophthalmology. 2013;120(2):311–316. doi: 10.1016/j.ophtha.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 3.Coats G. Forms of retinal diseases with massive exudation. R Lond Ophthalmol Hosp Rep. 1908;17:440–525. [Google Scholar]
- 4.Reese AB. Telangiectasis of the retina and Coats disease. Am J Ophthalmol. 1956;42:1–8. doi: 10.1016/0002-9394(56)90002-2. [DOI] [PubMed] [Google Scholar]
- 5.Leber T. Ueber eine durch Vorkommen multipler Miliaraneurysmen charakterisierteForm von Retinaldegeneration. Albrecht von Graefe's Arch Klin Ophthalmol. 1912;81:1–14. [Google Scholar]
- 6.Sen M, Shields CL, Honavar SG, Shields JA. Coats disease: an overview of classification, management and outcomes. Indian J Ophthalmol. 2019;67(6):763–771. doi: 10.4103/ijo.IJO_841_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: epidemiology and clinical features at diagnosis. Eye (Lond) 2010;24(12):1797–1801. doi: 10.1038/eye.2010.126. [DOI] [PubMed] [Google Scholar]
- 8.Andonegui J, Aranguren M, Berástegui L. Coats disease of adult onset. Arch Soc Esp Oftalmol. 2008;83:117–120. doi: 10.4321/s0365-66912008000200010. [DOI] [PubMed] [Google Scholar]
- 9.Wang KY, Cheng CK. A combination of intravitreal bevacizumab injection with tunable argon yellow laser photocoagulation as a treatment for adult-onset Coats’ disease. J Ocul Pharmacol Ther. 2011;27:525–530. doi: 10.1089/jop.2011.0088. [DOI] [PubMed] [Google Scholar]
- 10.Shields JA, Shields CL, Honavar SG, Demirci H, Cater J. Classification and management of Coats disease: the 2000 Proctor Lecture. Am J Ophthalmol. 2001;131(5):572–583. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]
- 11.Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG. Current management of Coats disease. Surv Ophthalmol. 2014;59(1):30–46. doi: 10.1016/j.survophthal.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Madan Kulkarni V, Sabnis M, Kailashbhai Kalaria H, Agarwal V. A rare case report of Coats’ disease. Trop J Ophthalmol Otolaryngol. 2020;5(7):194. [Google Scholar]
- 13.Fernandes BF, Odashiro AN, Maloney S, Zajdenweber ME, Lopes AG, Burnier MN. Clinical-histopathological correlation in a case of Coats' disease. Diagn Pathol. 2006;1(1):1–4. doi: 10.1186/1746-1596-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adriono GA, Nadhira AM, Mahfudz SR. Coats disease in adolescence and adulthood with preserved vision after laser photocoagulation monotherapy: two case reports. J Med Case Rep. 2022;16:287. doi: 10.1186/s13256-022-03474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong SS. Handbook of pediatric retinal OCT and the eye-brain connection. Elsevier; 2020. Chapter 31—Coats disease and Coats plus syndrome; pp. 149–153. [DOI] [Google Scholar]
- 16.Ghorbanian S, Jaulim A, Chatziralli IP. Diagnosis and treatment of Coats disease: a review of the literature. Ophthalmologica. 2012;227(4):175–182. doi: 10.1159/000336906. [DOI] [PubMed] [Google Scholar]
- 17.Galluzzi P, Venturi C, Cerase A, Vallone IM, Bracco S, Bardelli AM, et al. Coats disease: smaller volume of the affected Globe1. Radiology. 2001;221(1):64–69. doi: 10.1148/radiol.2211010017. [DOI] [PubMed] [Google Scholar]
- 18.Char DH. Coats’ syndrome: long term follow up. Br J Ophthalmol. 2000;84(1):37–39. doi: 10.1136/bjo.84.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields CL, Udyaver S, Dalvin LA, Lim LAS, Atalay HT, Khoo CTL, et al. Coats disease in 351 eyes- analysis of features and outcomes over 45 years (by decade) at a single center. Indian J Ophthalmol. 2019;67(6):772–783. doi: 10.4103/ijo.IJO_449_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen RW, de Bloeme CM, Brisse HJ, Galluzzi P, Cardoen L, Göricke S, et al. MR imaging features to differentiate retinoblastoma from coats’ disease and persistent fetal vasculature. Cancers (Basel) 2020;12(12):3592. doi: 10.3390/cancers12123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casella BP. Coats’ disease: a case study of a 4-year old boy. Retina. 2020;12(2):18. [Google Scholar]