Abstract
Alzheimer's disease (AD) is the most prevalent neurodegenerative disease, affecting approximately 6.5 million older adults in the United States. Development of AD treatment has primarily centered on developing pharmaceuticals that target amyloid-β (Aβ) plaques in the brain, a hallmark pathological biomarker that precedes symptomatic AD. Though recent clinical trials of novel drugs that target Aβ have demonstrated promising preliminary data, these pharmaceuticals have a poor history of developing into AD treatments, leading to hypotheses that other therapeutic targets may be more suitable for AD prevention and treatment. Impaired brain energy metabolism is another pathological hallmark that precedes the onset of AD that may provide a target for intervention. The brain creatine (Cr) system plays a crucial role in maintaining bioenergetic flux and is disrupted in AD. Recent studies using AD mouse models have shown that supplementing with Cr improves brain bioenergetics, as well as AD biomarkers and cognition. Despite these promising findings, no human trials have investigated the potential benefits of Cr supplementation in AD. This narrative review discusses the link between Cr and AD and the potential for Cr supplementation as a treatment for AD.
Keywords: Alzheimer’s disease, creatine, brain, bioenergetics, mitochondria
Introduction
Adults aged 65+ represent the fastest-growing population and the most affected age segment for neurodegenerative diseases [1]. The most common neurodegenerative disease is Alzheimer’s disease (AD), which currently affects about 6.5 million Americans and, by 2050, is expected to exceed 15 million [2]. This growth, combined with the lack of efficacious pharmacological treatments [3], constitutes a severe future risk to economic and social stability in the United States. Thus, there is a dire need for [1] effective preventive strategies that reduce AD risk and [2] therapeutic interventions that ameliorate symptoms for those diagnosed with AD. For decades, the focus of the AD drug development pipeline for treatment and prevention has primarily targeted the amyloid-β (Aβ) plaques that accumulate as a hallmark pathological change in the progression toward symptomatic AD. Although novel Aβ antibody therapies have shown promise in recent clinical trials [4, 5], Aβ-centric approaches have had a poor track record of treatment development, leading to hypotheses that other AD pathology targets may be more appropriate for prevention and treatment [6].
One such target is brain energy metabolism, termed “brain bioenergetics.” Along with the classic hallmark pathological signs of AD, Aβ plaques and tau tangles, impaired brain metabolism is also observed before and after the onset of symptomatic AD [7]. Mitochondrial dysfunction and impaired glucose utilization have been consistently cited as contributing to AD progression [8], and it is hypothesized that bioenergetic decline is an essential upstream contributor to AD development [7]. Indeed, adenosine triphosphate (ATP), the body’s primary energy-providing molecule, is decreased early in hippocampal and cortical neurons in AD pathogenesis [9]. Cellular levels of ATP are a critical factor in cellular survival [10], and reduced brain ATP is associated with altered Aβ processing [11] and cerebral accumulation of Aβ plaques [9]. Creatine (Cr) is an organic acid [12] important for maintaining ATP and energy homeostasis in organs with high energy flux, such as the brain [13]. Evidence suggests the brain Cr system is perturbed in AD [14] and represents a potential bioenergetic target for AD therapies.
Creatine monohydrate (CrM) is an oral nutritional supplement that safely and reliably increases intramuscular Cr levels and is commonly used as an ergogenic aid for sports and exercise [15]. Recent evidence suggests CrM supplementation may improve cognition in younger and older adults [13], and a few small-sample studies suggest brain Cr may increase after supplementation [[16], [17], [18], [19], [20], [21], [22], [23]]. Dolan et al. [24] provide a detailed and up-to-date review of the studies that have investigated brain Cr and phosphorylated Cr (PCr) response to varying CrM interventions and demographics. However, it remains unclear how permissible the blood-brain barrier (BBB) is to peripheral Cr [25]. Emerging and promising evidence from AD rodent models suggest CrM supplementation may improve mitochondrial function and be neuroprotective [[26], [27], [28]]. Thus, CrM may be a feasible supplement for AD risk prevention and symptom treatment.
No clinical trials have investigated CrM supplementation as a potential treatment in individuals with AD. Therefore, the purpose of this review is to discuss the potential link between Cr and AD, existing evidence for CrM in AD animal models, potential beneficial mechanisms of CrM supplementation in AD, and the need for CrM clinical trials to investigate the potential benefit of CrM supplementation in treating humans with symptomatic and prodromal AD.
Brain Cr and AD
The Cr system is integral in supporting both peripheral and brain energy requirements. Its role may be especially vital in the brain as it is a highly metabolic organ, demanding approximately 20% of total body energy [29], and can locally synthesize Cr from its precursors [30]. In a 2-step process, nearly half of the body’s Cr is synthesized from endogenous arginine, glycine, and s-adenosyl methionine (SAM) [12]. The first step involves the formation of guanidinoacetate (GAA) by glycine amidinotransferase, mitochondrial (GATM) activity, which is then used to synthesize Cr via guanidinoacetate N-methyltransferase (GAMT) activity. Cr can also access the brain from the periphery through the BBB via creatine transporter 1 (CRT1), though the rate of uptake and optimal peripheral concentration to facilitate increased uptake in the brain is not well understood [25]. Deficiency of either enzyme (GATM or GAMT) or CRT1 results in a cognitive disorder known as cerebral creatine deficiency syndrome (CCDS) [31].
To meet rapid energy demand and maintain energy homeostasis, Cr serves as the primary chaperone for transporting energy-producing phosphate groups generated by mitochondrial and cytosolic metabolism in muscle and brain cells [32, 33]. ATP generated by the tricarboxylic acid cycle and the ETC in mitochondria donate phosphate to Cr via mitochondrial Cr kinase (mtCK) activity to form PCr that then diffuses from the mitochondria into the cytosol. Likewise, brain-specific Cr kinase (BB-CK) [14] in the cytosol phosphorylates Cr to form a cytosolic pool of PCr. PCr is an important storage form of high energy phosphates that can be rapidly donated to adenosine diphosphate (ADP) to form ATP energy molecules to meet energy demand throughout the cell [34].
In individuals with AD, there is a recognized dysfunction in the brain's Cr system [35]. This dysfunction manifests in various ways. For instance, magnetic resonance spectroscopy imaging (MRSI) studies have revealed decreased levels of PCr in the brains of individuals with AD [36], and in the later stages of AD, there is a significant reduction in the levels of BB-CK [37]. Furthermore, nondemented older adults with at least one apolipoprotein E epsilon 4 (APOE4) allele, the strongest genetic risk factor for late-onset AD, had lower brain creatine levels than noncarriers and lower Cr levels were correlated with worse cognitive test performance [38]. This suggests Cr system impairments may be involved in early disease pathogenesis, preceding onset of AD symptoms.
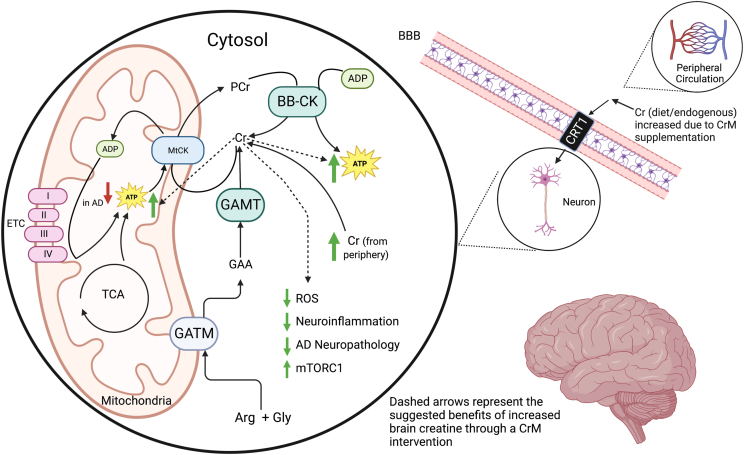
Decreased brain Cr levels may be explained by brain hypometabolism and acute cognitive stress. In times of hypometabolism, brain PCr stores may be able to donate a phosphate to regenerate ATP as a compensatory mechanism, but this may only be effective for a limited time before PCr is depleted, eventually leading to decreased brain Cr stores. In other conditions where brain metabolism is impaired, such as in Down syndrome [[39], [40], [41], [42]], where individuals are at high risk of developing AD at a young age [43, 44], and in schizophrenia [45, 46], it is similarly thought that PCr stores may be able to compensate for brain hypometabolism until PCr is diminished, resulting in decreased total brain Cr concentration. It remains unclear whether changes in the brain Cr system in AD are a downstream result of impaired energy metabolism or if dysfunction in the brain Cr system contributes to impaired brain energy metabolism and disease pathology. Nevertheless, brain Cr could be an important bioenergetic target for AD. Figure 1 illustrates transport, synthesis, bioenergetic roles, and potential mechanisms related to AD for Cr in the brain.
FIGURE 1.
Creatine physiology in the brain and the proposed benefit of CrM in AD.
Cr in peripheral circulation is transported through the BBB via a Cr transporter, which may increase due to CrM supplementation. This Cr transporter is also expressed by neurons to allow Cr transport into the cell. Neurons also endogenously produce creatine from the amino acids Arg and Gly via a two-step process. The first step is the formation of GAA by GATM, which then forms Cr by GAMT activity. Cr helps maintain energy flux as a chaperone for energy-producing phosphate groups. ATP generated by the ETC and the TCA cycle donate phosphate to Cr via mtCK to form PCr. PCr is an important storage form of high energy phosphate that can meet energy demand throughout the cell. In the AD brain, ATP levels are decreased. Increased Cr levels in the brain may signal mitochondria to upregulate aerobic respiration by replenishing free Cr chaperones for the Cr Phosphate shuttle. Increased Cr levels in the brain may have several benefits: free Cr may have antioxidant properties and may be able to sequester ROS, decrease neuroinflammation, increase mTORC1 signaling, and decrease AD neuropathologies. This figure was created with BioRender (www.biorender.com).
Cr: creatine; CrM: creatine monohydrate; BBB: blood-brain barrier; CRT1: creatine transporter 1; Arg: arginine; Gly: glycine; GATM: glycine amidinotransferase, mitochondrial; GAA: guanidinoacetate; GAMT: guanidinoacetate N-methyltransferase; MtCK: mitochondrial Cr kinase; PCr: phosphorylated creatine; BB-CK: brain-specific Cr kinase; ATP: adenosine triphosphate; ADP: adenosine triphosphate; ETC: electron transport chain; TCA: tricarboxylic acid cycle; ROS: reactive oxygen species; mTORC1: mammalian target of rapamycin complex 1. BioRender.com
Animal models suggest Cr supplementation may be beneficial in AD
Evidence suggests there are potential benefits of CrM supplementation in various populations [[47], [48], [49], [50], [51]]. Though no reported trials have examined the effects of CrM supplementation in individuals with AD, CrM supplementation may benefit dysfunction of the brain Cr kinase system seen in humans with AD [52]. Two studies of CrM supplementation in rodent AD and mild cognitive impairment (MCI) models support this concept.
Snow et al. [27] demonstrated that between 8 and 9 wk of CrM supplementation in 7-mo old 3xTg mouse model, a mouse model of AD that expresses Aβ plaques and tau neurofibrillary tangles [53], improved several AD-related outcome measures. Mice in the experimental group consumed ad libitum unpurified diet containing 3% CrM by weight, whereas the control group’s unpurified diet was unmodified. After 8 wk of their respective diets, all females had impaired Morris Water Maze (MWM) escape times, yet CrM supplemented females escaped more quickly and demonstrated improved spatial learning compared with control females. Males in both groups had similar MWM performance, with neither exhibiting impaired escape latency. Relative to control, CrM-fed females had increased hippocampal expression of proteins related to synaptic plasticity along with increased high-molecular weight Aβ oligomers and lower expression of low-weight Aβ oligomers, although amyloid precursor protein (APP) was unchanged. The investigators speculate that these sex differences were not likely due to sex differences in Cr metabolism, but likely due to sex differences in 3xTg mice. For instance, female 3xTg mice demonstrated worse initial cognitive impairment and learning ability than male mice at the time of study. Additionally, CrM supplementation in both males and females improved hippocampal mitochondrial function respiration. These data suggest a possible role for CrM in supporting cognition and beneficially altering amyloidogenic processing in AD.
Mao et al. [54] investigated the effects of 6 wk of Cr supplementation (in unspecified supplemental form) on cognition and the mammalian target of rapamycin complex 1 (mTORC1) in an MCI rat model. Seven-wk-old female Wistar rats were allocated to 3 different groups: one group was injected with vehicle, whereas 2 groups were injected with lipopolysaccharide (LPS) to induce an MCI-like condition where one received Cr and the other received placebo. The Cr group received a loading dose of 1.542 g Cr/kg/d for the first wk and a maintenance dose of 0.385 g Cr/kg/d for the subsequent weeks. Compared with LPS-injected control rats, Cr-supplemented rats completed the Barnes maze test more quickly and spent more time observing objects during the novel object recognition test, suggesting Cr supplementation diminished expected LPS-induced cognitive deficits. These mice also had upregulated mTORC1 and synaptic plasticity proteins in the dentate gyrus. In a second experiment from this study featuring rats that did not receive LPS injection, Cr-supplemented rats, similarly, had higher mTORC1 and synaptic protein expression in the dentate gyrus than the control mice. Unlike the LPS model, these rats did not have differing cognitive performance.
Evidence from these 2 studies in AD and MCI rodent models suggests CrM supplementation is potentially beneficial for cognition, bioenergetics, and AD-related biomarkers and may act in a sex-specific manner [27]. Clinical trials testing the effects of CrM on mechanisms and symptoms in AD are warranted and to determine if possible sex differences are directly related to sex or instead an advanced condition state.
Potential mechanisms for Cr in AD
The cause of AD is still not well understood, and many important etiological hypotheses attempt to explain its basis, including the leading hypothesis that Aβ initiates the disease cascade [55], as well as alternative hypotheses implicating impaired mitochondrial bioenergetics [7] or inflammation and oxidative stress [56]. CrM supplementation may be beneficial in AD as it is purported to influence each of the key components of these hypotheses as well as other potentially important mechanisms.
Decades prior to the development of histological and clinical manifestations of AD, brain mitochondrial bioenergetics and glucose metabolism are reduced [57]. Specifically, impairments have been found in the electron transport chain (ETC) complexes, including complex I [58], which is responsible for controlling a significant portion of mitochondrial respiration in synaptic mitochondria [59]. Evidence suggests mitochondrial respiration is partially regulated by the PCr/Cr ratio in human skeletal muscle [60], which could also be true in the brain. In mice, oral Cr supplementation increases mitochondrial respiration in the hippocampus. Specifically, Cr supplementation has been demonstrated to enhance the function of ETC complex I in healthy mice [26] and both ETC complexes I and III in mouse models of AD [27]. This suggests supplementing with CrM may signal mitochondria to upregulate respiration by increasing free Cr chaperones for the Cr Phosphate shuttle in the brain.
Brain insulin resistance is increasingly implicated in AD and may partially explain impaired brain glucose uptake in AD [61]. Therapies targeting brain insulin resistance have had mixed success [62, 63], yet this concept remains a reasonable bioenergetic target. In vitro and animal studies suggest Cr supplementation may improve peripheral insulin sensitivity [64, 65], though human trials have not affirmed these findings [66, 67]. Cr supplementation increases insulin-dependent GLUT-4 activity in muscle [68], a glucose transporter also expressed in the neurons of select brain regions [69], which facilitates increased glucose uptake [67]. Therefore, it is possible that Cr supplementation may enhance brain glucose uptake to provide key substrates for energy metabolism in the AD brain.
Oxidative stress is also implicated in the development and progression of AD [70, 71]. Oxidative stress is caused by excessive production of reactive oxygen species (ROS) due to aberrant mitochondrial and cytosolic energy metabolism [72, 73]. Free Cr may have direct effects as an antioxidant as it has been shown to have ROS scavenging properties [74, 75]. Evidence suggests free Cr antioxidant activity may be particularly important for protecting mitochondria from ROS damage, which are susceptible to oxidative stress that can exacerbate mitochondrial impairment [76]. Furthermore, the BB-CK is a prime target of oxidative damage by free radicals and is perturbed in AD [77, 78]. Cr supplementation has been shown to attenuate the deleterious effects of oxidative stress on the CK isoforms [76, 79]; therefore, Cr supplementation may protect CK from oxidative damage in the brain, which is observed in cognitive decline [77].
Furthermore, CrM supplementation may be protective of neuroinflammation in AD. Aberrations to transcription factor nuclear factor kappa-b (NF-kB) have been found in AD, which may stimulate transcription of proinflammatory cytokines [80]. Although focus on NF-kB has been primarily on cancer and inflammation, it plays integral roles in memory and synaptic function [80]. NFk-B has been shown to target neurons and negatively affect APP and Aβ aggregation in AD [80]. Cr supplementation in rodents has been shown to decrease levels of NF-kB in the brain and is associated with enhanced learning, memory, and hippocampal mitochondrial function [26]. Cr supplementation has also been shown to augment synaptic proteins implicated in memory formation and learning with concurrent enhanced hippocampal-associated learning, memory, and mitochondrial function in a rodent model [81].
CrM supplementation may benefit patients with AD by modulating classical AD pathologies. Brain Aβ accumulation and bioenergetic impairment are cited as 2 of the first AD pathology changes [82] that are believed to cyclically influence each other [83]. Downstream of these initial changes, neurofibrillary tangles of phosphorylated tau (pTau) aggregate followed by frank neurodegeneration and onset of AD symptoms [82]. One preclinical study demonstrated that cultured hippocampal neurons treated with Cr were protected from neurotoxic effects from adding Aβ and glutamate to the cell cultures [28]. These effects coincided with a large increase in phosphorylation of Cr to form PCr and the ratio of PCr/ATP compared with the control, suggesting protection was conferred by increased neuronal energy potential and reserve. In female 3xTg mice, Cr supplementation altered Aβ processing by upregulating expression of high-molecular weight species and downregulating the low-molecular weight 12kDa mOC87 Aβ oligomer, the only Aβ species where higher concentration was correlated with worse cognitive impairment in this study [27]. In both sexes, 3xTg mice with higher Cr hippocampal concentration had a lower concentration of pTau/Tau [27]. These data suggest the potential bioenergetic effects of CrM in AD may influence processing and phosphorylation of the hallmark proteins, A-beta and tau.
The upregulation of mTORC1 signaling seen in mice represents another pathway Cr supplementation may provide benefit in AD [54, 84]. mTORC1 is an anabolic pathway that responds to feeding and stimulates protein synthesis [85]. Aside from its role in muscle hypertrophy, mTORC1 may play a role in memory formation [54]. For example, a rat study showed it has a role in regulating long-term potentiation in the hippocampus [86], a process that connects changes in synaptic strength to changes in long-term behavior. However, it is hypothesized perpetual activation of mTORC1 may negatively impact the aging brain [87]. Mice studies suggest chronically active mTORC1 may upregulate Aβ accumulation and down-regulate autophagy, which both play a role in cognitive impairment in AD [87, 88]. Therefore, it may be prudent to selectively regulate mTORC1 in different phases of life. The aforementioned study by Mao et al. [54] supports the beneficial effects of Cr supplementation mediated through mTORC1 on the aging rodent brain.
CrM supplementation may have peripheral benefits outside of the brain. Specifically, Cr has the potential to enhance muscle protein synthesis by triggering signaling pathways activated through the osmotic effect of Cr in muscle cells [89]. In addition, Cr may promote muscle hypertrophy through various cellular mechanisms, including the inhibition of myostatin [90] and the activation of insulin-like growth factor/mTOR [54, 91]. Thus, CrM may be beneficial in AD because loss of lean body mass, primarily skeletal muscle, has been cross-sectionally associated with AD [92]. However, prospective studies show that muscle function and strength are better predictors for reducing risk of developing AD compared with maintenance of skeletal muscle mass volume [[93], [94], [95], [96]]. Cr supplementation has been shown to increase muscle strength, lean body mass, and muscle function in diverse populations, although data are less conclusive in interventions without concomitant exercise [[97], [98], [99], [100], [101], [102]]. Although speculative, Cr supplementation may help prevent and improve symptoms of AD through its effects on skeletal mass, muscle volume, and function [99, [103], [104], [105], [106], [107]], which warrants future investigation.
Taken together, it is reasonable to hypothesize that Cr supplementation may offer benefits to humans in prevention as well as the early stages of AD through both brain bioenergetic and peripheral mechanisms. These mechanisms in brain health are summarized in Table 1.
TABLE 1.
Putative roles for creatine in brain health
| Putative role for creatine in brain health | References |
|---|---|
| Phosphate carrier | Wyss et al. [12] |
| Hemmer et al. [14] | |
| Bonvento et al. [32] | |
| Bessman et al. [33] | |
| ROS scavenging properties | Tarafdar et al. [73] |
| Lawler et al. [74] | |
| Sestil et al. [75] | |
| Sestill et al. [76] | |
| Stachowiak et al. [79] | |
| Improved energy metabolism | Snow et al. [26] |
| Snow et al. [27] | |
| Brewer et al. [28] | |
| Snow et al. [80] | |
| Protection against inflammation | Snow et al. [26] |
| Snow et al. [80] | |
| Modulation of AD neuropathology | Snow et al. [26] |
| Snow et al. [27] | |
| Brewer et al. [28] | |
| mTORC1 signaling | Mao et al. [54] |
| Pazini et al. [84] |
AD, Alzheimer's Disease; ROS, reactive oxygen species.
Opportunities for Cr supplementation trials in humans
CrM supplementation trials in humans with AD are needed to glean essential knowledge regarding the utility of CrM as potential adjuvant therapy in AD. The mechanisms mentioned above about Cr in AD have not been tested in humans.
Currently, our group is conducting a single-arm proof-of-principle trial (NCT05383833) to determine if individuals with AD (n=20) can adhere to a 20 g/d CrM intervention for 8 wk. Our trial will generate preliminary data for key questions in AD, including change in the brain Cr levels, mitochondrial function, cognition, and muscle function and morphology to inform future, large-scale clinical trials. Our trial is a single-arm pilot trial; thus, all future results should be cautiously interpreted as preliminary, but our trial may serve as important justification for future investigation. Although this is a first step in the investigation of CrM for AD, other trials with larger sample size, a placebo control group, and an array of measures that interrogate different AD-related mechanisms and function will be necessary to determine efficacy and optimal dose in this population. For instance, since the ability of supplemental CrM to raise brain Cr levels still needs to be elucidated [13, 16, 17], future trials will be tasked with establishing optimal CrM dosing and interval required to effectively change brain Cr levels. Because the goal of AD therapies is to improve function and quality of life in individuals with AD, it will take large sample trials with an intervention interval of 1 y or longer to investigate whether CrM supplementation is beneficial for cognition and symptoms in AD.
Because bioenergetic decline is a progressive process that occurs early in AD's pathology physiology [7, 108], trials are also warranted to ascertain if CrM supplementation is valuable for AD prevention. In prodromal AD, there is a minor decrease in brain bioenergetics, but the brain may compensate until a threshold is exceeded, whereafter, a significant bioenergetic decline ensues, and AD symptoms are present [108]. Thus, a CrM intervention may be best suited early in AD pathophysiology to help delay the progression from preclinical or prodromal AD to symptomatic AD. Taken together, there is a strong rationale for Cr pilot trials in AD and those at high risk for AD.
Conclusions
It is imperative to identify effective, early treatments for AD. Cr is an important bioenergetic molecule, and the Cr system is shown to be dysfunctional in the brain of individuals with AD. Therefore, Cr may serve as a potential target for prevention and therapy and CrM supplementation may be beneficial in AD. To date, only rodent studies have investigated the use of CrM as a treatment for AD. Thus, clinical trials investigating the effects of CrM on cognition and CrM’s mechanisms in humans with AD as well as its potential as a strategy to prevent cognitive decline in those with normal cognition, are needed. There is much to be learned about CrM intervention and brain health in different life and disease phases.
Author contributions
The authors’ responsibilities were as follows—ANS and MKT wrote the first draft of the manuscript. All authors provided manuscript revisions and approved the final manuscript.
Conflicts of interest
The authors report no conflicts of interest.
Funding
This research was supported by the Alzheimer's Association (AARG-22-924314) and the National Institutes of Health (K01 AG065487 and P30 AG072973). Additional support was provided by the University of Kansas Medical Center Department of Dietetics and Nutrition.
Data availability
Not applicable. No data were analyzed.
References
- 1.Symens AaT E. American Community Survey Reports; 2019. The Older Population in Rural America: 2012-2016.https://www.census.gov/library/publications/2019/acs/acs-41.html [Google Scholar]
- 2.SaO C. Rural Health Information; 2015. Demographic Changes and Aging Population.https://www.ruralhealthinfo.org/toolkits/aging/1/demographics [Google Scholar]
- 3.Becker R.E., Greig N.H., Giacobini E. Why do so many drugs for Alzheimer's disease fail in development? Time for new methods and new practices? J. Alzheimers Dis. 2008;15(2):303–325. doi: 10.3233/jad-2008-15213. https://doi: 10.3233/jad-2008-15213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reardon S. Alzheimer's drug donanemab: what promising trial means for treatments. Nature. 2023;617(7960):232–323. doi: 10.1038/d41586-023-01537-5. https://doi:10.1038/d41586-023-01537-5 [DOI] [PubMed] [Google Scholar]
- 5.van Dyck C.H., Swanson C.J., Aisen P., Bateman R.J., Chen C., Gee M., et al. Lecanemab in early Alzheimer's disease. N. Engl. J. Med. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. https://doi:10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 6.Taylor M.K., Swerdlow R.H., Sullivan D.K. Dietary neuroketotherapeutics for Alzheimer’s disease: an evidence update and the potential role for diet quality. Nutrients. 2019;11(8):1910. doi: 10.3390/nu11081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow R.H., Khan S.M. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med. Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 8.Flannery P.J., Trushina E. Mitochondrial dynamics and transport in Alzheimer's disease. Mol. Cell. Neurosci. 2019;98:109–120. doi: 10.1016/j.mcn.2019.06.009. https://doi:10.1016/j.mcn.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mark R.J., Pang Z., Geddes J.W., Uchida K., Mattson M.P. Amyloid β-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J. Neurosci. 1997;17(3):1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. https://doi:10.1523/jneurosci.17-03-01046.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicotera P., Leist M., Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol. Lett. 1998;102:139–142. doi: 10.1016/s0378-4274(98)00298-7. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins H.M., Swerdlow R.H. Amyloid precursor protein processing and bioenergetics. Brain Res. Bull. 2017;133:71–79. doi: 10.1016/j.brainresbull.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiological reviews. 2000 doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 13.Roschel H., Gualano B., Ostojic S.M., Rawson E.S. creatine supplementation and brain health. Nutrients. 2021;13(2):586. doi: 10.3390/nu13020586. https://doi:10.3390/nu13020586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmer W., Wallimann T. Functional aspects of creatine kinase in brain. Dev. Neurosci. 1993;15(3–5):249–260. doi: 10.1159/000111342. https://doi:10.1159/000111342 [DOI] [PubMed] [Google Scholar]
- 15.Kreider R.B., Kalman D.S., Antonio J., Ziegenfuss T.N., Wildman R., Collins R., et al. International society of sports nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017;14:18. doi: 10.1186/s12970-017-0173-z. https:// doi:10.1186/s12970-017-0173-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dechent P., Pouwels P.J., Wilken B., Hanefeld F., Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. Am. J. Physiol. 1999;277(3):R698–704. doi: 10.1152/ajpregu.1999.277.3.R698. https://doi:10.1152/ajpregu.1999.277.3.R698 [DOI] [PubMed] [Google Scholar]
- 17.Lyoo I.K., Kong S.W., Sung S.M., Hirashima F., Parow A., Hennen J., et al. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003;123(2):87–100. doi: 10.1016/s0925-4927(03)00046-5. https://doi:10.1016/s0925-4927(03)00046-5 [DOI] [PubMed] [Google Scholar]
- 18.Pan J.W., Takahashi K. Cerebral energetic effects of creatine supplementation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(4):R1745–1750. doi: 10.1152/ajpregu.00717.2006. https://doi:10.1152/ajpregu.00717.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo D.G., Sung Y.H., Hellem T.L., Fiedler K.K., Shi X., Jeong E.K., et al. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011;135(1–3):354–361. doi: 10.1016/j.jad.2011.07.010. https://doi:10.1016/j.jad.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellem T.L., Sung Y.H., Shi X.F., Pett M.A., Latendresse G., Morgan J., et al. Creatine as a novel treatment for depression in females using methamphetamine: a pilot study. J. Dual. Diagn. 2015;11(3–4):189–202. doi: 10.1080/15504263.2015.1100471. https://doi:10.1080/15504263.2015.1100471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.E Turner C., Byblow W.D., Gant N. Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J. Neurosci. 2015;35(4):1773–1780. doi: 10.1523/JNEUROSCI.3113-14.2015. https://doi:10.1523/jneurosci.3113-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner C.E., Russell B.R., Gant N. Comparative quantification of dietary supplemented neural creatine concentrations with (1)H-MRS peak fitting and basis spectrum methods. Magn. Reson. Imaging. 2015;33(9):1163–1167. doi: 10.1016/j.mri.2015.06.018. https://doi:10.1016/j.mri.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 23.Kondo D.G., Forrest L.N., Shi X., Sung Y.H., Hellem T.L., Huber R.S., et al. Creatine target engagement with brain bioenergetics: a dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids. 2016;48(8):1941–1954. doi: 10.1007/s00726-016-2194-3. https://doi:10.1007/s00726-016-2194-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolan E., Gualano B., Rawson E.S. Beyond muscle: the effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019;19(1):1–14. doi: 10.1080/17461391.2018.1500644. https:// doi:10.1080/17461391.2018.1500644 [DOI] [PubMed] [Google Scholar]
- 25.Braissant O. Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. J. Inherit. Metab. Dis. 2012;35(4):655–664. doi: 10.1007/s10545-011-9433-2. https://doi:10.1007/s10545-011-9433-2 [DOI] [PubMed] [Google Scholar]
- 26.Snow W.M., Cadonic C., Cortes-Perez C., Roy Chowdhury S.K., Djordjevic J., Thomson E., et al. Chronic dietary creatine enhances hippocampal-dependent spatial memory, bioenergetics, and levels of plasticity-related proteins associated with NF-κB, Learn. Mem. 2018;25(2):54–66. doi: 10.1101/lm.046284.117. https://doi:10.1101/lm.046284.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snow W.M., Cadonic C., Cortes-Perez C., Adlimoghaddam A., Roy Chowdhury S.K., Thomson E., et al. Sex-specific effects of chronic creatine supplementation on hippocampal-mediated spatial cognition in the 3xTg mouse model of Alzheimer's disease. Nutrients. 2020;12(11) doi: 10.3390/nu12113589. https://doi:10.3390/nu12113589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer G.J., Wallimann T.W. Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J. Neurochem. 2000;74(5):1968–1978. doi: 10.1046/j.1471-4159.2000.0741968.x. https://doi:10.1046/j.1471-4159.2000.0741968.x [DOI] [PubMed] [Google Scholar]
- 29.Cunnane S.C., Trushina E., Morland C., Prigione A., Casadesus G., Andrews Z.B., et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020;19(9):609–633. doi: 10.1038/s41573-020-0072-x. https://doi:10.1038/s41573-020-0072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker S.A., Gajera C.R., Wawro A.M., Corces M.R., Montine T.J. GATM and GAMT synthesize creatine locally throughout the mammalian body and within oligodendrocytes of the brain. Brain Res. 2021;1770 doi: 10.1016/j.brainres.2021.147627. https://doi:10.1016/j.brainres.2021.147627 [DOI] [PubMed] [Google Scholar]
- 31.Farr C.V., El-Kasaby A., Freissmuth M., Sucic S. The creatine transporter unfolded: a knotty premise in the cerebral creatine deficiency syndrome. Front Synaptic. Neurosci. 2020;12 doi: 10.3389/fnsyn.2020.588954. https://doi:10.3389/fnsyn.2020.588954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonvento G., Valette J., Flament J., Mochel F., Brouillet E. Imaging and spectroscopic approaches to probe brain energy metabolism dysregulation in neurodegenerative diseases. J. Cereb. Blood Flow Metab. 2017;37(6):1927–1943. doi: 10.1177/0271678X17697989. https://doi:10.1177/0271678X17697989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bessman S.P., Carpenter C.L. The creatine-creatine phosphate energy shuttle. Annu. Rev. Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- 34.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107. https://doi:10.1152/physrev.2000.80.3.1107 [DOI] [PubMed] [Google Scholar]
- 35.Rackayova V., Cudalbu C., Pouwels P.J.W., Braissant O. Creatine in the central nervous system: from magnetic resonance spectroscopy to creatine deficiencies. Anal. Biochem. 2017;529:144–157. doi: 10.1016/j.ab.2016.11.007. https://doi:10.1016/j.ab.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 36.Pettegrew J.W., Panchalingam K., Klunk W.E., McClure R.J., Muenz L.R. Alterations of cerebral metabolism in probable Alzheimer's disease: a preliminary study. Neurobiol. Aging. 1994;15(1):117–132. doi: 10.1016/0197-4580(94)90152-X. [DOI] [PubMed] [Google Scholar]
- 37.AliMohammadi M., Eshraghian M., Zarindast M.-R., Aliaghaei A., Pishva H. Effects of creatine supplementation on learning, memory retrieval, and apoptosis in an experimental animal model of Alzheimer disease. Med. J. Islam. Repub. Iran. 2015;29:273. [PMC free article] [PubMed] [Google Scholar]
- 38.Laakso M.P., Hiltunen Y., Könönen M., Kivipelto M., Koivisto A., Hallikainen M., et al. Decreased brain creatine levels in elderly apolipoprotein E epsilon 4 carriers. J. Neural. Transm. (Vienna) 2003;110(3):267–275. doi: 10.1007/s00702-002-0783-7. https://doi:10.1007/s00702-002-0783-7 [DOI] [PubMed] [Google Scholar]
- 39.Labudova O., Cairns .N., Kitzmüller E., Lubec G. Impaired brain glucose metabolism in patients with Down syndrome. J. Neural. Transm. Suppl. 1999;57:247–256. doi: 10.1007/978-3-7091-6380-1_16. https://doi:10.1007/978-3-7091-6380-1_16 [DOI] [PubMed] [Google Scholar]
- 40.Shonk T., Ross B.D. Role of increased cerebral myo-inositol in the dementia of Down syndrome. Magn. Reson. Med. 1995;33(6):858–861. doi: 10.1002/mrm.1910330619. https://doi:10.1002/mrm.1910330619 [DOI] [PubMed] [Google Scholar]
- 41.Huang W., Alexander G.E., Daly E.M., Shetty H.U., Krasuski J.S., Rapoport S.I., et al. High brain myo-inositol levels in the predementia phase of Alzheimer's disease in adults with Down's syndrome: a 1H MRS study. Am. J. Psychiatry. 1999;156(12):1879–1886. doi: 10.1176/ajp.156.12.1879. https://doi: 10.1176/ajp.156.12.1879 [DOI] [PubMed] [Google Scholar]
- 42.Smith R.N., Agharkar A.S., Gonzales E.B. A review of creatine supplementation in age-related diseases: more than a supplement for athletes. F1000Res. 2014;3:222. doi: 10.12688/f1000research.5218.1. https://doi:10.12688/f1000research.5218.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballard C., Mobley W., Hardy J., Williams G., Corbett A. Dementia in Down's syndrome. Lancet Neurol. 2016;15(6):622–636. doi: 10.1016/S1474-4422(16)00063-6. https://doi:10.1016/s1474-4422(16)00063-6 [DOI] [PubMed] [Google Scholar]
- 44.Korbel J.O., Tirosh-Wagner T., Urban A.E., Chen X.N., Kasowski M., Dai L., et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc. Natl. Acad. Sci. U S A. 2009;106(29) doi: 10.1073/pnas.0813248106. https://doi:10.1073/pnas.0813248106 12031–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Shachar D., Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int. Rev. Neurobiol. 2004;59:273–296. doi: 10.1016/S0074-7742(04)59011-6. https://doi:10.1016/s0074-7742(04)59011-6 [DOI] [PubMed] [Google Scholar]
- 46.Ongür D., Prescot A.P., Jensen J.E., Cohen B.M., Renshaw P.F. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172(1):44–48. doi: 10.1016/j.pscychresns.2008.06.002. https://doi:10.1016/j.pscychresns.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMorris T., Mielcarz G., Harris R.C., Swain J.P., Howard A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol. Dev. Cogn. B. Aging Neuropsychol. Cogn. 2007;14(5):517–528. doi: 10.1080/13825580600788100. https://doi:10.1080/13825580600788100 [DOI] [PubMed] [Google Scholar]
- 48.Cook C.J., Crewther B.T., Kilduff L.P., Drawer S., Gaviglio C.M. Skill execution and sleep deprivation: effects of acute caffeine or creatine supplementation - a randomized placebo-controlled trial. J. Int. Soc. Sports Nutr. 2011;8:2. doi: 10.1186/1550-2783-8-2. https://doi:10.1186/1550-2783-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ling J., Kritikos M., Tiplady B. Cognitive effects of creatine ethyl ester supplementation. Behav. Pharmacol. 2009;20(8):673–679. doi: 10.1097/FBP.0b013e3283323c2a. https://doi:10.1097/FBP.0b013e3283323c2a [DOI] [PubMed] [Google Scholar]
- 50.Rae C., Digney A.L., McEwan S.R., Bates T.C. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proc. Biol. Sci. 2003;270(1529):2147–2150. doi: 10.1098/rspb.2003.2492. https://doi:10.1098/rspb.2003.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benton D., Donohoe R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br. J. Nutr. 2011;105(7):1100–1105. doi: 10.1017/S0007114510004733. https://doi:10.1017/s0007114510004733 [DOI] [PubMed] [Google Scholar]
- 52.David S., Shoemaker M., Haley B.E. Abnormal properties of creatine kinase in Alzheimer's disease brain: correlation of reduced enzyme activity and active site photolabeling with aberrant cytosol-membrane partitioning. Brain Res. Mol. Brain Res. 1998;54(2):276–287. doi: 10.1016/s0169-328x(97)00343-4. https://doi:10.1016/s0169-328x(97)00343-4 [DOI] [PubMed] [Google Scholar]
- 53.Virgili J., Lebbadi M., Tremblay C., St-Amour I., Pierrisnard C., Faucher-Genest A., et al. Characterization of a 3xTg-AD mouse model of Alzheimer's disease with the senescence accelerated mouse prone 8 (SAMP8) background. Synapse. 2018;72(4) doi: 10.1002/syn.22025. https://doi:10.1002/syn.22025 [DOI] [PubMed] [Google Scholar]
- 54.Mao X., Kelty T.J., Kerr N.R., Childs T.E., Roberts M.D., Booth F.W. Creatine supplementation upregulates mTORC1 signaling and markers of synaptic plasticity in the dentate gyrus while ameliorating LPS-induced cognitive impairment in female rats. Nutrients. 2021;13(8) doi: 10.3390/nu13082758. https://doi:10.3390/nu13082758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. https://doi:10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- 56.Decourt B., D'Souza G.X., Shi J., Ritter A., Suazo J., Sabbagh M.N. The cause of Alzheimer's disease: the theory of multipathology convergence to chronic neuronal stress. Aging Dis. 2022;13(1):37–60. doi: 10.14336/AD.2021.0529. https://doi:10.14336/AD.2021.0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao J., Brinton R.D. Targeting mitochondrial bioenergetics for Alzheimer's prevention and treatment. Curr. Pharm. Des. 2011;17(31):3474–3479. doi: 10.2174/138161211798072517. https://doi:10.2174/138161211798072517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker W.D., Jr., Parks J., Filley C.M., Kleinschmidt-DeMasters B.K. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994;44(6):1090–1096. doi: 10.1212/wnl.44.6.1090. https://doi:10.1212/wnl.44.6.1090 [DOI] [PubMed] [Google Scholar]
- 59.Davey G.P., Peuchen S., Clark J.B. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J. Biol. Chem. 1998;273(21):12753–12757. doi: 10.1074/jbc.273.21.12753. https://doi:10.1074/jbc.273.21.12753 [DOI] [PubMed] [Google Scholar]
- 60.Walsh B., Tonkonogi M., Soderlund K., Hultman E., Saks V., Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J. Physiol. 2001;537(Pt 3):971–978. doi: 10.1111/j.1469-7793.2001.00971.x. https://doi: 10.1111/j.1469-7793.2001.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kullmann S., Heni M., Hallschmid M., Fritsche A., Preissl H., Häring H.U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 2016;96(4):1169–1209. doi: 10.1152/physrev.00032.2015. https://doi:10.1152/physrev.00032.2015 [DOI] [PubMed] [Google Scholar]
- 62.Craft S., Asthana S., Newcomer J.W., Wilkinson C.W., Matos I.T., Baker L.D., et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch. Gen. Psychiatry. 1999;56(12):1135–1140. doi: 10.1001/archpsyc.56.12.1135. https://doi:10.1001/archpsyc.56.12.1135 [DOI] [PubMed] [Google Scholar]
- 63.Craft S., Raman R., Chow T.W., Rafii M.S., Sun C.K., Rissman R.A., et al. Safety, efficacy, and feasibility of intranasal Insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77(9):1099–1109. doi: 10.1001/jamaneurol.2020.1840. https://doi:10.1001/jamaneurol.2020.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rooney K., Bryson J., Phuyal J., Denyer G., Caterson I., Thompson C. Creatine supplementation alters insulin secretion and glucose homeostasis in vivo. Metabolism. 2002;51(4):518–522. doi: 10.1053/meta.2002.31330. https://doi:10.1053/meta.2002.31330 [DOI] [PubMed] [Google Scholar]
- 65.Alsever R.N., Georg R.H., Sussman K.E. Stimulation of insulin secretion by guanidinoacetic acid and other guanidine derivatives. Endocrinology. 1970;86(2):332–336. doi: 10.1210/endo-86-2-332. https://doi:10.1210/endo-86-2-332 [DOI] [PubMed] [Google Scholar]
- 66.Gualano B., Novaes R.B., Artioli G.G., Freire T.O., Coelho D.F., Scagliusi F.B., et al. Effects of creatine supplementation on glucose tolerance and insulin sensitivity in sedentary healthy males undergoing aerobic training. Amino Acids. 2008;34(2):245–250. doi: 10.1007/s00726-007-0508-1. https://doi:10.1007/s00726-007-0508-1 [DOI] [PubMed] [Google Scholar]
- 67.Gualano B., DE Salles Painneli V., Roschel H., Artioli G.G., Neves M., Jr., De Sá Pinto A.L., et al. Creatine in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Med. Sci. Sports Exerc. 2011;43(5):770–778. doi: 10.1249/MSS.0b013e3181fcee7d. https://doi:10.1249/MSS.0b013e3181fcee7d [DOI] [PubMed] [Google Scholar]
- 68.Ju J.S., Smith J.L., Oppelt P.J., Fisher J.S. Creatine feeding increases GLUT4 expression in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005;288(2):E347–352. doi: 10.1152/ajpendo.00238.2004. https://doi:10.1152/ajpendo.00238.2004 [DOI] [PubMed] [Google Scholar]
- 69.Koepsell H. Glucose transporters in brain in health and disease. Pflügers Arch. 2020;472(9):1299–1343. doi: 10.1007/s00424-020-02441-x. https://doi:10.1007/s00424-020-02441-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang W.-J., Zhang X., Chen W.-W. Role of oxidative stress in Alzheimer's disease. Biomed. Rep. 2016;4(5):519–522. doi: 10.3892/br.2016.630. https://doi:10.3892/br.2016.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nunomura A., Castellani R.J., Zhu X., Moreira P.I., Perry G., Smith M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropath. Exp. Neurol. 2006;65(7):631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. https://doi:10.1097/01.jnen.0000228136.58062.bf [DOI] [PubMed] [Google Scholar]
- 72.Wang X., Wang W., Li L., Perry G., Lee H.G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim. Biophys. Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. https://doi:10.1016/j.bbadis.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarafdar A., Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018;19(12):3824. doi: 10.3390/ijms19123824. https://doi:10.3390/ijms19123824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lawler J.M., Barnes W.S., Wu G., Song W., Demaree S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002;290(1):47–52. doi: 10.1006/bbrc.2001.6164. https://doi:10.1006/bbrc.2001.6164 [DOI] [PubMed] [Google Scholar]
- 75.Sestili P., Martinelli C., Bravi G., Piccoli G., Curci R., Battistelli M., et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic. Biol. Med. 2006;40(5):837–849. doi: 10.1016/j.freeradbiomed.2005.10.035. https://doi:10.1016/j.freeradbiomed.2005.10.035 [DOI] [PubMed] [Google Scholar]
- 76.Sestili P., Martinelli C., Colombo E., Barbieri E., Potenza L., Sartini S., et al. Creatine as an antioxidant. Amino Acids. 2011;40(5):1385–1396. doi: 10.1007/s00726-011-0875-5. [DOI] [PubMed] [Google Scholar]
- 77.Aksenov M., Aksenova M., Butterfield D.A., Markesbery W.R. Oxidative modification of creatine kinase BB in Alzheimer's disease brain. J. Neurochem. 2000;74(6):2520–2527. doi: 10.1046/j.1471-4159.2000.0742520.x. https://doi:10.1046/j.1471-4159.2000.0742520.x [DOI] [PubMed] [Google Scholar]
- 78.Aksenov M.Y., Aksenova M.V., Payne R.M., Smith C.D., Markesbery W.R., Carney J.M. The expression of creatine kinase isoenzymes in neocortex of patients with neurodegenerative disorders: Alzheimer's and Pick's disease. Exp. Neurol. 1997;146(2):458–465. doi: 10.1006/exnr.1997.6550. https://doi:10.1006/exnr.1997.6550 [DOI] [PubMed] [Google Scholar]
- 79.Stachowiak O., Dolder M., Wallimann T., Richter C. Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modification and inactivation. J. Biol. Chem. 1998;273(27):16694–16699. doi: 10.1074/jbc.273.27.16694. https://doi:10.1074/jbc.273.27.16694 [DOI] [PubMed] [Google Scholar]
- 80.Snow W.M., Albensi B.C. Neuronal gene targets of NF-κB and their dysregulation in Alzheimer's disease. Front. Mol. Neurosci. 2016;9:118. doi: 10.3389/fnmol.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Snow W.M., Stoesz B.M., Kelly D.M., Albensi B.C. Roles for NF-κB and gene targets of NF-κB in synaptic plasticity, memory, and navigation. Mol. Neurobiol. 2014;49(2):757–770. doi: 10.1007/s12035-013-8555-y. https://doi:10.1007/s12035-013-8555-y [DOI] [PubMed] [Google Scholar]
- 82.Sperling R., Johnson K. 2013. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria, Continuum (Minneap Minn) 19(2 Dementia) pp. 325–338.https://doi:10.1212/01.CON.0000429181.60095.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor M.K., Sullivan D.K., Keller J.E., Burns J.M., Swerdlow R.H. Potential for ketotherapies as amyloid-regulating treatment in individuals at risk for Alzheimer's disease. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.899612. https://doi: 10.3389/fnins.2022.899612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pazini F.L., Cunha M.P., Rosa J.M., Colla A.R., Lieberknecht V., Oliveira Á., et al. Creatine, similar to Ketamine, counteracts depressive-like behavior induced by Corticosterone via PI3K/Akt/mTOR pathway. Mol. Neurobiol. 2016;53(10):6818–6834. doi: 10.1007/s12035-015-9580-9. https://doi:10.1007/s12035-015-9580-9 [DOI] [PubMed] [Google Scholar]
- 85.Laplante M., Sabatini D.M. mTOR signaling at a glance. J. Cell. Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. https://doi:10.1242/jcs.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang S.J., Reis G., Kang H., Gingras A.C., Sonenberg N., Schuman E.M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. U S A. 2002;99(1):467–472. doi: 10.1073/pnas.012605299. https://doi:10.1073/pnas.012605299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hodges S.L., Reynolds C.D., Smith G.D., Jefferson T.S., Nolan S.O., Lugo J.N. Molecular interplay between hyperactive mammalian target of rapamycin signaling and Alzheimer's disease neuropathology in the NS-Pten knockout mouse model. Neuroreport. 2018;29(13):1109–1113. doi: 10.1097/WNR.0000000000001081. https://doi:10.1097/wnr.0000000000001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang F., Chu X., Yin M., Liu X., Yuan H., Niu Y., et al. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav. Brain. Res. 2014;264:82–90. doi: 10.1016/j.bbr.2014.02.005. https://doi:10.1016/j.bbr.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 89.Safdar A., Yardley N.J., Snow R., Melov S., Tarnopolsky M.A. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol. Genomics. 2008;32(2):219–228. doi: 10.1152/physiolgenomics.00157.2007. https://doi:10.1152/physiolgenomics.00157.2007 [DOI] [PubMed] [Google Scholar]
- 90.Saremi A., Gharakhanloo R., Sharghi S., Gharaati M.R., Larijani B., Omidfar K. Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol. Cell. Endocrinol. 2010;317(1–2):25–30. doi: 10.1016/j.mce.2009.12.019. https://doi:10.1016/j.mce.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 91.Burke D.G., Candow D.G., Chilibeck P.D., MacNeil L.G., Roy B.D., Tarnopolsky M.A., et al. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int. J. Sport. Nutr. Exerc. Metab. 2008;18(4):389–398. doi: 10.1123/ijsnem.18.4.389. https://doi:10.1123/ijsnem.18.4.389 [DOI] [PubMed] [Google Scholar]
- 92.Burns J.M., Johnson D.K., Watts A., Swerdlow R.H., Brooks W.M. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch. Neurol. 2010;67(4):428–433. doi: 10.1001/archneurol.2010.38. https://doi: 10.1001/archneurol.2010.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.S Beeri M., Leugrans S.E., Delbono O., Bennett D.A., Buchman A.S. Sarcopenia is associated with incident Alzheimer's dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 2021;69(7):1826–1835. doi: 10.1111/jgs.17206. https://doi:10.1111/jgs.17206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moon Y., Moon W.J., Kim J.O., Kwon K.J., Han S.H. Muscle strength is independently related to brain atrophy in patients with Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2019;47(4–6):306–314. doi: 10.1159/000500718. https://doi:10.1159/000500718 [DOI] [PubMed] [Google Scholar]
- 95.Moon Y., Choi Y.J., Kim J.O., Han S.H. Muscle profile and cognition in patients with Alzheimer's disease dementia. Neurol. Sci. 2018;39(11):1861–1866. doi: 10.1007/s10072-018-3505-0. https://doi:10.1007/s10072-018-3505-0 [DOI] [PubMed] [Google Scholar]
- 96.Boyle P.A., Buchman A.S., Wilson R.S., Leurgans S.E., Bennett D.A. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 2009;66(11):1339–1344. doi: 10.1001/archneurol.2009.240. https://doi:10.1001/archneurol.2009.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Candow D.G., Forbes S.C., Chilibeck P.D., Cornish S.M., Antonio J., Kreider R.B. Effectiveness of creatine supplementation on aging muscle and bone: focus on falls prevention and inflammation. J. Clin. Med. 2019;8(4) doi: 10.3390/jcm8040488. https://doi:10.3390/jcm8040488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dolan E., Artioli G.G., Pereira R.M.R., Gualano B. Muscular atrophy and sarcopenia in the elderly: is there a role for creatine supplementation? Biomolecules. 2019;9(11) doi: 10.3390/biom9110642. https://doi:10.3390/biom9110642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chilibeck P.D., Kaviani M., Candow D.G., Zello G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access J. Sports Med. 2017;8:213–226. doi: 10.2147/OAJSM.S123529. https://doi:10.2147/oajsm.S123529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stout J.R., Sue Graves B., Cramer J.T., Goldstein E.R., Costa P.B., Smith A.E., et al. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64 - 86 years) J. Nutr. Health Aging. 2007;11(6):459–464. [PubMed] [Google Scholar]
- 101.Rawson E.S., Wehnert M.L., Clarkson P.M. Effects of 30 days of creatine ingestion in older men. Eur. J. Appl. Physiol. Occup. Physiol. 1999;80(2):139–144. doi: 10.1007/s004210050570. https://doi:10.1007/s004210050570 [DOI] [PubMed] [Google Scholar]
- 102.Gotshalk L.A., Volek J.S., Staron R.S., Denegar C.R., Hagerman F.C., Kraemer W.J. Creatine supplementation improves muscular performance in older men. Med. Sci. Sports Exerc. 2002;34(3):537–543. doi: 10.1097/00005768-200203000-00023. https://doi:10.1097/00005768-200203000-00023 [DOI] [PubMed] [Google Scholar]
- 103.Aguiar A.F., Januário R.S., Junior R.P., Gerage A.M., Pina F.L., do Nascimento M.A., et al. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013;113(4):987–996. doi: 10.1007/s00421-012-2514-6. https://doi:10.1007/s00421-012-2514-6 [DOI] [PubMed] [Google Scholar]
- 104.Brose A., Parise G., Tarnopolsky M.A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2003;58(1):11–19. doi: 10.1093/gerona/58.1.b11. https://doi:10.1093/gerona/58.1.b11 [DOI] [PubMed] [Google Scholar]
- 105.Candow D.G., Little J.P., Chilibeck P.D., Abeysekara S., Zello G.A., Kazachkov M., et al. Low-dose creatine combined with protein during resistance training in older men. Med. Sci. Sports Exerc. 2008;40(9):1645–1652. doi: 10.1249/MSS.0b013e318176b310. https://doi:10.1249/MSS.0b013e318176b310 [DOI] [PubMed] [Google Scholar]
- 106.Candow D.G., Vogt E., Johannsmeyer S., Forbes S.C., Farthing J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 2015;40(7):689–694. doi: 10.1139/apnm-2014-0498. https://doi:10.1139/apnm-2014-0498 [DOI] [PubMed] [Google Scholar]
- 107.Chrusch M.J., Chilibeck P.D., Chad K.E., Davison K.S., Burke D.G. Creatine supplementation combined with resistance training in older men. Med. Sci. Sports Exerc. 2001;33(12):2111–2117. doi: 10.1097/00005768-200112000-00021. https://doi:10.1097/00005768-200112000-00021 [DOI] [PubMed] [Google Scholar]
- 108.Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochim. Biophys. Acta. 2014;1842(8):1219–1231. doi: 10.1016/j.bbadis.2013.09.010. https://doi:10.1016/j.bbadis.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. No data were analyzed.