Abstract
Background
Neuro‐Behçet Syndrome (NBS) is a severe chronic inflammatory vascular disease involving the Central Nervous System (CNS), and it is an invalidating condition with disability and a huge impact on quality of life. Recommendations on treatments for NBS include the use of disease‐modifying therapies in general, although they are not supported by a systematic review of the evidence.
Objectives
To assess the benefit and harms of available treatments for NBS, including biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha.
Search methods
We searched the following databases up to 30 September 2014: Trials Specialised Register of The Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group, CENTRAL, MEDLINE, EMBASE, CINAHL, LILACS, ORPHANET, Clinicaltrials.gov and World Health Organization (WHO) International Clinical Trials Registry Portal.
Selection criteria
Randomised controlled trials (RCTs), controlled clinical trials (CCTs), prospective and retrospective controlled cohort studies were eligible to assess the benefit. Patients over 13 years of age with a diagnosis of NBS. For assessment of harms, open‐label extension (OLE), case‐control studies, population‐based registries, case‐series and case‐reports were additionally planned to be evaluated.
Data collection and analysis
Selection of studies, data extraction and assessment of risk of bias were planned to be carried out independently by two review authors. Standard methodological procedures expected by The Cochrane Collaboration were followed. We planned to perform standard pair‐wise meta‐analyses for RCTs, and meta‐analyses based on the adjusted estimates using the inverse‐variance weighted average method for non‐randomised studies (NRSs). We planned to present the main results of the review in a 'Summary of Findings' table using the GRADE approach.
Main results
No RCTs, CCTs or controlled cohort studies on the benefit of the treatments for NBS met the inclusion criteria of the review. Only one potentially eligible study was identified, but it did not report sufficient details on the patient characteristics. The author of this study did not provide additional data on request, and therefore it was excluded. Hence, no studies were included in the present review. Since no studies were included in the assessment of benefit, no further search was performed in order to collect data on harms.
Authors' conclusions
There is no evidence to support or refute the benefit of biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha for the treatment of patients with NBS. Thus, well‐designed multicentre RCTs are needed in order to inform and guide clinical practice.
Plain language summary
Modifying therapies such as biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha for Neuro‐Behçet's Syndrome
Neuro‐Behçet Syndrome (NBS) is an invalidating condition with a huge impact on quality of life. Recommendations on treatments for NBS include the use of disease‐modifying therapies such as biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha. To assess their benefit and harms, the review authors decided to perform a systematic review of the available treatments for NBS. No studies were found that met the inclusion criteria of this review, indicating that there is no evidence to support or refute the benefit of these treatments for patients with NBS. Thus, well‐conducted research is required before an evidence‐based recommendation can be supported.
Background
Description of the condition
Behçet's Syndrome (BS) is a chronic, relapsing, inflammatory vascular disease characterised by ulcers in the mouth and on the genitals, and inflammation in specific parts of the eye (uveitis) as well as arthritis (swollen, painful, stiff joints), skin problems, and involvement of the digestive tract, brain, and spinal cord. The typical histopathologic feature of BS is a vasculitis affecting veins and arteries of different sizes and presenting mainly with vein thrombosis and, to a lesser degree, arterial aneurysm or thrombosis. Onset most commonly occurs in adults, but paediatric cases have been reported. Both genders are affected equally, but the disease runs a more severe course in males (Yazici 2012). The disease course is characterised by exacerbations and remission ending in a total remission in at least 60% of patients at 20 years of follow‐up (Kural‐Seyahi 2003).
The disease is of unknown origin. There is no clear evidence showing the role of infections in the pathogenesis. A correlation between genetic predisposition and triggering extrinsic factors has been suggested, because more than 60% of BS patients are associated with HLA‐B 51 (Gül 2012; Kose 2012; Yazici 1980). Some clinical features show distinct geographical differences. The prevalence is high in Turkey (> 1/1000 people). Fewer cases of intestinal disease are reported in the Mediterranean area; eye disease causes considerable morbidity in Turkish patients (Kural‐Seyahi 2003; Tugal‐Tutkun 2004), but is rarely a severe problem among Italian (Salvarani 2007) or American patients (Calamia 2009). A positive skin pathergy test is less frequent among patients from northern Europe, America or Japan (Hatemi 2012; Yazici 2012).
The diagnostic criteria for BS were defined by the International Study Group (ISG) for Behçet's Disease in 1990, and include the presence of recurrent oral ulceration, with at least three episodes over 12 months, in addition to two of the following features: recurrent genital ulcers, eye lesions, skin lesions and a positive pathergy test (ISG 1990). An international team (from 27 countries) has recently proposed a revision of the ISG criteria (ICBD 2013), in which eye lesions, oral ulcers and genital ulcers are each assigned two points, while skin lesions, central nervous system (CNS) involvement and vascular manifestations are assigned one point each. The pathergy test, when used, was assigned one point. A patient scoring four or more points is classified as having BS. These new criteria have higher sensitivity over the ISG criteria, but considerably lower specificity (Yazici 2014).
When the disease involves the CNS it is defined as Neuro‐Behçet Syndrome (NBS). Sporadic neurological manifestations are frequent (> 20%), often occurring one to 10 years after initial symptoms, but in some cases neurological symptoms are the first manifestation of BS (Akman‐Demir 1999; Al‐Araji 2009). NBS is more frequent in men than women and it usually occurs at between 20 and 40 years of age ( Al‐Araji 2009; Dalvi 2012).
There are two main categories of NBS that should be considered separately: parenchymal and non‐parenchymal (Serdaroglu 1998). Parenchymal NBS includes the following four syndromes.
Brainstem involvement that includes ophthalmoparesis, cranial neuropathy, and cerebellar or pyramidal dysfunction.
Cerebral hemispheric involvement that presents with encephalopathy, hemiparesis, hemisensory loss, seizures, dysphasia, cognitive dysfunction and psychosis.
Spinal cord involvement that occurs with movement disorders, sensory dysfunctions, and, commonly, sphincter dysfunction.
Evidence for cerebral or spinal cord involvement in addition to the brainstem signs and symptoms.
Non‐parenchymal NBS occurs as cerebral venous thrombosis or intracranial and extracranial aneurysm (Al‐Araji 2009). Patients with non‐parenchymal NBS have a significantly better prognosis than those with parenchymal NBS (Siva 2001).
Around a third of NBS patients have single episodes, a third have repeated relapses with remission, and a third undergo a progressive disease course with accrual of multiple functional disability (Akman‐Demir 1999; Al‐Fahad 1999; Kidd 1999; Kural‐Seyahi 2003; Siva 2001). High cellular and/or protein content in the cerebrospinal fluid and parenchymal involvement of the brain, especially of the brainstem, are associated with a worse prognosis (Akman‐Demir 1999).
Description of the intervention
There is no cure for BS. Treatments focus on relieving the symptoms and preventing worsening or complications.
Treatments for BS include: biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha. Corticosteroids are prescribed for rapid suppression of inflammatory process during acute exacerbations, to reduce severe joint pain, skin sores, eye disease, or CNS symptoms. Long‐term use of corticosteroids in addition to immunosuppressants is also reported. However, long‐term corticosteroids may cause several side effects such as diabetes, osteoporosis, weight gain, infections, delayed wound healing, persistent heartburn, elevated blood pressure and mental disorders (Mat 2006). Immunosuppressants such as azathioprine, cyclophosphamide, chlorambucil, cyclosporine‐A, methotrexate, micophenolate, mitoxantrone, levamisole and tacrolimus are used for patients with BS to reduce inflammation, and prevent exacerbations and complications, but they also cause serious adverse events (Hatemi 2008).
In recent years, there has been considerable interest in evaluating the efficacy of biologics for BS. These compounds have been licensed for use in other conditions, e.g. rheumatoid arthritis, psoriasis, psoriatic arthritis and inflammatory bowel disease (Singh 2011). Biologics are a group of medications that suppress the immune system and reduce inflammation. Even though suppressing the immune system can increase the risk of infections, it also helps to stabilise an overactive immune system. The following biologics are used off‐label for patients with BS: anti‐CD20 (rituximab), anti‐interleukin (IL)‐1 (anakinra, canakinumab, gevokizumab, rilonacept), anti‐IL‐2 (daclizumab), anti‐IL‐6 (tocilizumab), and anti‐IL‐17 (secukinumab), and Tumor Necrosis Factor (TNF) inhibitors (adalimumab, etanercept, infliximab). Biologics are administered subcutaneously except for infliximab and rituximab, which are administered as intravenous infusions. Several adverse events such as tuberculosis reactivation with biologics are drug‐specific. However, some adverse events such as increased risk of infection are related to a general immunomodulator or immunosuppressive effect and are common to all biologics (Singh 2011).
How the intervention might work
Immunosuppressants are agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others act through activation of T‐cells or by inhibiting the activation of helper T‐cells, targeting immune mechanisms important in BS pathogenesis (Abbas 2001; Kose 2012).
Biologics are highly specific molecules targeting various immune cells that play a key role in local and systemic inflammation (Singh 2011). Anti‐TNF blockers include both soluble receptors that serve as decoy receptors competing with TNF‐receptors (etanercept) and monoclonal antibodies targeting the TNF‐receptors (adalimumab and infliximab). Rituximab is a monoclonal antibody against CD20, which is found primarily on B‐cells. Clinical and laboratory observations have suggested an important role of TNF‐mediated process in the pathogenesis of BS (Kose 2012). Increased levels of TNF, soluble TNF receptors, and TNF‐producing cells were found in the peripheral blood of patients with active disease. Among inflammatory cytokine‐related genes, TNF blockade reduced expression of IL‐1 receptor type 2, interferon γ receptors, IL‐6 receptors, and IL‐17 receptors (Keino 2011). It was found that infliximab is capable of interfering with gamma delta T cell function in BS characterised by dysregulated cell‐mediated immunity (Accardo‐Palumbo 2010). Arida and colleagues analysed published data on 369 patients treated with either adalimumab, etanercept or infliximab, and reported that the majority of patients showed improvement of their mucocutaneous manifestations (Arida 2011). Rituximab was found effective in retinal vasculitis and ocular manifestations in BS (Davatchi 2010). Rilonacept and canakinumab are human anti‐IL‐1β monoclonal antibodies, targeting a cytokine implicated in the pathogenesis of many inflammatory diseases. Reports from clinical trials suggest that rilonacept and canakinumab are well tolerated in patients with BS and no serious adverse effects were reported (Dubois 2011).
Why it is important to do this review
A Cochrane review, Saenz 1998, on general management of BS was published in 1998 but it did not consider NBS. NBS occurs in about one third of patients affected by BS and, together with gastrointestinal system and blood vessels involvement, seems to represent the main important prognostic factor of BS (Berlit 2010). A systematic review of all available studies is warranted to evaluate the benefit and harms of the treatments for patients with NBS.
Objectives
To assess the benefit and harms of biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha to improve health condition in patients with Neuro‐Behçet Syndrome (NBS).
Methods
Criteria for considering studies for this review
Types of studies
Benefit. Randomised controlled trials (RCTs), controlled clinical trials (CCTs), prospective and retrospective controlled cohort studies were eligible.
We classified a trial as a CCT if the author(s) did not state explicitly that the trial was randomised, but randomisation could not be ruled out. The classification of CCT was also applied to quasi‐randomised studies, where the method of allocation was reported in the primary study, but we did not judge it as strictly random (Higgins 2011).
We classified as a controlled cohort study a study in which a defined group of people (the cohort) was followed over time, to examine associations between different interventions received by patients and subsequent outcomes. A ‘prospective’ cohort study recruits participants before any intervention and follows them into the future. A ‘retrospective’ cohort study identifies subjects from past records describing the interventions received and follows them from the time of those records (Higgins 2011).
In order to be included, cohort studies had to report on a contemporary control group, and both groups needed to be described with sufficient detail to allow assessment of potential 'confounding by indication'. At least baseline characteristics, the treatment regimen and outcome data had to be reported separately for both groups.
Harms. RCTs, CCTs, open‐label extension (OLE), cohort and case‐control studies, population‐based registries, case‐series and case‐reports for each treatment for which benefit was assessed.
Types of participants
Patients over 13 years of age with a diagnosis of NBS (as first clinical manifestations of BS or as complication of BS), according to widely accepted diagnostic criteria, such as the International Study Group for Behcet's disease criteria (ISG 1990), or the International Criteria for Behçet's Disease (ICBD 2013), regardless of disease phase (first attack, recurrent or progressive NBS), patient gender and ethnicity, inpatient or outpatient setting.
Types of interventions
Any treatment compared with any other pharmacological treatment, placebo or no treatment.
Biologics: anti‐CD20 (rituximab), anti‐interleukin (IL)‐1 (anakinra, canakinumab, gevokizumab, rilonacept), anti‐IL‐2 (daclizumab), anti‐IL‐6 (tocilizumab), and anti‐IL‐17 (secukinumab), TNF inhibitors (adalimumab, etanercept, infliximab).
Colchicine.
Corticosteroids.
Immunosuppressants (azathioprine, cyclophosphamide, chlorambucil, cyclosporine‐A, methotrexate, micophenolate, mitoxantrone, levamisole, tacrolimus)
Interferon‐alpha.
Regimens were included irrespective of their duration and dose, as long as they were within therapeutic range.
Types of outcome measures
Primary outcomes
Benefit
Induction or maintenance of remission with disease activity considered as a dichotomous outcome, i.e. sustained‐remission rate and relapse‐free survival (RFS).
Change of patient‐reported outcomes (PROs, e.g. Short Form 36, Behcet disease quality of life (Gilworth 2004), or any other PRO validated measures as reported in primary studies).
Harms
Withdrawals due to serious adverse events (SAEs).
Proportion of patients with at least one of the following adverse events (AEs):
infections (pneumonia, fungal and opportunistic infections, tuberculosis reactivation);
cardiac disorders;
all cancers, including lymphomas and leukaemia;
mental disorders;
any other AE reported in the included studies.
Secondary outcomes
Time to remission.
Overall survival (OS).
Physical and cognitive disability measured with validated instruments, such as the Expanded Disability Status Scale (EDSS).
Disease activity, remission, relapse and disability measures could only be included if they had been assessed by validated instruments, as reported in primary studies. SAEs were defined according to the National Cancer Institute Common Terminology Criteria for AE (CTCAE 2003).
Search methods for identification of studies
No language restrictions were applied to the search.
Electronic searches
The Trials Search Co‐ordinator searched the following databases.
Trials Specialised Register of The Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group.
The Cochrane Central Register of Controlled Trials (CENTRAL) (2014, issue 9).
MEDLINE (PubMed) (1966 to 30 September 2014).
EMBASE (EMBASE.com) (1974 to 30 September 2014).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO host) (1981 to 30 September 2014).
Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (1982 to 30 September 2014).
ORPHANET (http://www.orpha.net/consor/cgi‐bin/index.php).
Clinical trials registries (http://clinicaltrials.gov/).
World Health Organization (WHO) International Clinical Trials Registry Portal (http://apps.who.int/trialsearch/).
The keywords used to search for studies for this review are listed in (Appendix 1).
The search terms were adapted to each database, where appropriate.
Searching other resources
Screening of reference lists of review articles and primary studies found.
Contacted authors and researchers active in this field for additional data when necessary.
Data collection and analysis
Selection of studies
Two review authors (FN, FG) independently identified each potentially‐relevant study obtained from the electronic and manual searches. The first screening was done by reviewing titles and abstracts. The full‐text copies of the selected articles were evaluated during the second screening. We recorded 'excluded studies' and the reasons for exclusion. Disagreements were discussed and resolved by consensus among review authors. We used EndNote (https://www.myendnoteweb.com/) to store references.
Data extraction and management
We planned that two review authors (FN, FG) would independently extract data from the included studies using a standardised Excel form. We intended to contact principal investigators of included studies, when necessary, to request additional data or confirmation of methodological aspects of the study. We planned to extract the following data from the included studies.
Study design and year of publication.
Participants (sample size of any treatment arm, study setting, demographic and clinical characteristics of participants such as age, gender, ethnicity, presence of other BS manifestations).
Details of the experimental and control interventions (type, duration, schedules and dose).
Outcomes previously defined (see Types of outcome measures section).
Disagreements were discussed and resolved by consensus among review authors.
Assessment of risk of bias in included studies
We planned to assess the risk of bias for each included study using The Cochrane Collaboration criteria (Higgins 2011). Any discrepancies were to be resolved through discussions among review authors.
1) Criteria for assessing risk of bias in RCTs and CCTs.
For RCTs and CCTs, these criteria included the following domains: random sequence generation, allocation concealment, blinding of personnel, patients and outcome assessors, incomplete outcome data, selective outcome reporting and other biases. The classification of each criterion included three categories: low, high or unclear risk of bias. To summarise the overall risk of bias for a study, we decided to classify RCTs as being at high risk of bias if at least one of the following domains was judged to be at high risk of bias: allocation concealment, blinding of outcome assessors, and incomplete outcome data defined as >15% lost to follow‐up. For CCTs, we decided to classify them as being at high risk of bias if at least one of the following domains was judged to be at high risk of bias: selection of patients, blinding of outcome assessors, and incomplete outcome data defined as >15% lost to follow‐up. RCTs and CCTs had to be classified as being at unclear risk of bias if they presented insufficient information or uncertainty over the potential for bias.
2) Criteria for assessing risk of bias in non‐randomised studies (NRSs).
We planned to assess the risk of bias of non‐randomised studies (NRSs) using the new Cochrane 'Risk of bias' tool for NRSs (Sterne 2013). This tool covers seven domains related to pre‐ and post‐intervention potential biases. Pre‐intervention biases include baseline confounding, selection of participants into the study, and measurement of intervention. Post‐intervention biases include departures from intended interventions, missing data, measurement of outcomes, and selection of the reported result.
We planned to consider the following potential confounding factors: age, gender, ethnicity, clinical phenotypes, disease phase and severity, activity state, treatment phase, and previous treatments.
Each domain‐level 'Risk of bias' judgment includes five categories: low (if the study is comparable to a well‐performed RCT), moderate (if the study is sound for a NRS but cannot be considered comparable to a well‐performed RCT), serious (if the study has some important problems), critical (if the study is too problematic to provide any useful evidence), and no information on which to base a judgment about risk of bias.
The overall risk of bias is judged at low (if all the domains are judged to be at low risk of bias), moderate (if all the domains are judged to be at low or moderate risk of bias), serious (if at least one domain is judged to be at serious risk of bias), critical (if at least one domain is judged to be at critical risk of bias), or no information if there is a lack of information in one or more key domains of bias.
3) Criteria for assessing quality of harm data.
We planned to assess risk of bias for adverse events (AEs) for each included study using the following criteria.
Did the researchers actively monitor for AEs (low risk of bias), or did they simply provide spontaneous reporting of AEs that arose (high risk of bias)?
Did the authors define SAEs according to an accepted international classification and report the number of SAEs?
Measures of treatment effect
We planned to use dichotomous, continuous, and survival data, and to calculate risk ratios (RRs) for dichotomous, means and standard deviations for continuous outcomes, and hazard ratios (HRs) for time to event data, with the corresponding 95% confidence intervals (CI).
Unit of analysis issues
The unit of analysis was the individual patient.
Dealing with missing data
For missing data, when possible we planned to contact the authors of studies to obtain data, otherwise we planned to perform any appropriate sensitivity analysis with different scenarios (i.e., likely, worst and best‐case scenarios).
Assessment of heterogeneity
We planned to assess clinical homogeneity among studies with respect to age, gender, ethnicity, clinical phenotype, disease phase and severity, activity state, type of immunosuppressant and biologic drugs, concomitant drug including steroids use, treatment phase, previous treatments, and length of follow‐up. We planned to assess statistical heterogeneity using the Chi2 test, I2 statistics (Higgins 2011) and by graphical presentations (forest plot) (Egger 1997).
Assessment of reporting biases
We planned to examine reporting bias as a part of the overall 'Risk of bias' assessment, by comparing outcomes stated in protocols to those reported in article, or by comparing outcomes listed in the methods section to those in results where protocols are not available or cannot be retrieved. If some indications of reporting bias were found, we planned to contact study authors for clarification. Funnel plots (Egger 1997) were planned to be drawn if at least 10 studies were included in the review, to assess the possibility of publication bias. Possible asymmetry of the funnel plot found either by inspection or statistical tests would be taken into account in interpreting the overall estimate of treatment effects.
Data synthesis
We planned to analyse data using Review Manager software (Review Manager 2014) (version 5.3.3), and report them as specified in Chapter 9 (Deeks 2011) and Chapter 13 (Reeves 2011) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) . For NRSs, if they were judged reasonably resistant to bias and relatively homogeneous in this respect, we planned to meta‐analyse adjusted estimates (i.e., analyses that attempt to control for the predefined confounders), using the inverse‐variance weighted average (Reeves 2011).
We planned to perform separate meta‐analyses according to the study design: RCTs, CCTs, prospective and retrospective cohorts. The main analyses were planned to be of biologics, colchicine, corticosteroids, immunosuppressant and interferon‐alpha compared to placebo and to each other.
We planned to compute pooled estimates using a fixed‐effect model, or a random‐effects model in case of heterogeneity (I2 > 50%).
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses based on the following.
Age.
Gender.
Ethnicity.
Clinical phenotypes (first attack of NBS, recurrent NBS, progressive NBS, NBS complicating BS).
Categories of NBS involvement (parenchymal and non‐parenchymal).
Type of immunosuppressant and biologic drug.
Concomitant drug including steroids use versus no concomitant therapy.
Treatment phase (therapy for induction or maintenance of remission).
Previous treatments with immunosuppressants, biologics or interferon‐alpha.
Duration of study (< 12 months versus ≥ 12 months).
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the robustness of our review results by repeating the analyses with the exclusion of studies classified as being at high risk of bias.
For missing data, we planned to perform any appropriate sensitivity analysis as mentioned above (see Dealing with missing data section).
'Summary of Findings' table
We planned to present the main results of the review in a 'Summary of Findings' table, as recommended by The Cochrane Collaboration (Schünemann 2011a). Using the GRADE approach (Schünemann 2011b), we defined that the Summary of Findings' table had to include an overall grading of the quality of evidence related to each of the following major outcomes.
Induction or maintenance of remission.
Change of PROs.
Withdrawals due to SAEs.
Results
Description of studies
Results of the search
Flow charts describe the results of the electronic search and article screening for evaluation of benefit (Figure 1, Figure 2). Searches returned a total of 7270 references. After a first screening by reviewing titles and abstracts, the full‐text copies of 11 articles were evaluated to assess for eligibility, but no studies met the inclusion criteria (Figure 1).
1.
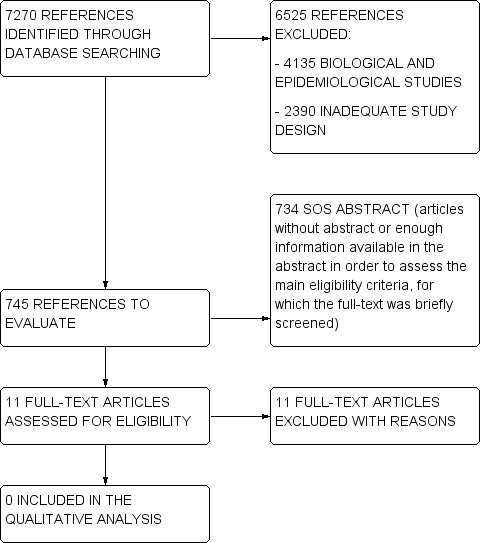
Flow diagram.
2.
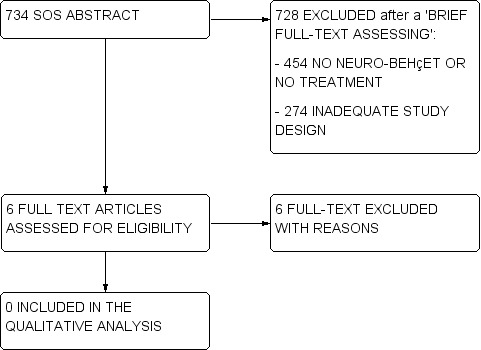
Flow diagram (2).
A total of 734 titles/abstracts did not provide enough information in order to verify the presence of at least the main eligibility criteria on study design, participants and treatment. For these studies (called 'SOS abstract') we performed a brief reading of the full‐text in order to define if they had to be excluded at the first screening (e.g., they did not include NBS patients, treatments of interest, or they had an inadequate study design as case‐series and registries), or if their full‐text had to be assessed for eligibility. Among these 734 SOS abstracts, the full‐text of six articles was assessed for eligibility, but no studies met the inclusion criteria (Figure 2).
Since no studies were included in the assessment of benefit, no further search was performed in order to collect data on harms.
Included studies
No studies were included in the present review, since no RCTs, CCTs or controlled cohort studies on the benefit of the treatments for NBS were found.
Excluded studies
Seventeen studies were excluded from the review (see Characteristics of excluded studies). Sixteen of these studies (Akman‐Demir 1996; Benamour 2006; Borhani Haghighi 2011; Desbois 2012; Dutra 2011; Essaadouni 2010; Farah 1998; Ideguchi 2010; Krupa 2011; Saadoun 2009; Serdaroğlu 1989; Tohmé 2009; Wechsler 1992; Whallett 1999; Yesilot 2007; Yesilot 2009) were excluded since they did not define a control group allowing to assess a causal relationship between intervention and outcome. Only one cohort study (Sbai 2003) defined a control group in order to examine associations between interventions and subsequent outcomes, but different intervention groups were not described with sufficient detail to allow assessment of potential bias due to confounding, and the corresponding data were not provided by the authors. Accordingly, this study (Sbai 2003) had the potential to meet the inclusion criteria: it was a retrospective cohort study on 109 consecutive NBS patients enrolled from 1968 to 2001 at the Pitié Salpetriere Hospital in Paris (France), in which treatment administration together with neurological attacks, disability and remissions were extracted from medical records. However, the authors merely reported that "all patients received colchicine after admission, if they had not before", "103 patients received corticosteroids and 6 did not", and "84 patients received immunomodulatory drugs (50 azathioprine, 57 cyclophosphamide, 12 chloraminophene, 6 methotrexate, 7 cyclosporine, 3 plasma exchange and 1 interferon)". They also reported odds ratios for the associations between treatments and attacks, disability and remissions, but how they managed potential 'confounding by indication', treatment duration, and treatment combinations were not provided.
Risk of bias in included studies
The searches retrieved no trials relevant to this review and thus no assessment of methodological quality was conducted.
Effects of interventions
No data were available for analysis.
Discussion
Summary of main results
No RCTs, CCTs or controlled cohort studies on the benefit of treatments for NBS could be included in this review. Only one potentially eligible study was identified, but since different intervention groups were not described with sufficient detail to allow assessment of potential confounding, and no additional data on treatments combinations were provided on request, this study was excluded (Sbai 2003). Hence, no benefit or harm assessment could be performed.
Overall completeness and applicability of evidence
In view of the lack of evidence available in the literature, well‐designed multicentre RCTs are needed that can inform and guide clinical practice.
Quality of the evidence
There is neither high or low level evidence to support or refute the benefit of biologics, colchicine, corticosteroids, immunosuppressants and interferon‐alpha for the treatment of patients with NBS.
Potential biases in the review process
Each attempt to limit bias in the review process was made by ensuring a comprehensive search of potentially eligible studies. The authors’ independent assessments of eligibility of studies for inclusion in this review minimised the potential for additional bias.
Agreements and disagreements with other studies or reviews
As already pointed out, there have been no controlled or comparative studies on NBS treatment, thus scanty and not reliable information on treatments for NBS (mostly parenchymal NBS) were provided by case‐series and case‐reports, only. In particular, a few case‐series studies reported that two‐thirds of patients with brainstem or cerebral lesions appeared to benefit from steroids (Akman‐Demir 1999; Al‐Fahad 1999; Kidd 1999; Siva 2001). Some publications indicated advantages for serious manifestations of NBS potentially related to several disease‐modifying therapies, such as azathioprine (Hatemi 2008), micophenolate (Shugaiv 2011), methotrexate (Hirohata 1998; Kikuchi 2003), chlorambucil (O'Duffy 1984), and cyclophosphamide (Ait Ben Haddou 2012). Other studies supported the use of infliximab (Fasano 2011; Giardina 2011; Pipitone 2008; Sarwar 2005), adalimumab (Leccese 2010; Olivieri 2011), etanercept (Alty 2007), tocilizumab (Shapiro 2012), and interferon‐alpha (Nichols 2001) as potential effective alternatives for NBS patients. On the contrary, cyclosporine has been linked with higher risk of NBS development (Akman‐Demir 2008; Kato 2001; Kotake 1999; Kötter 2006).
Our findings are in agreement with recent international consensus recommendations for diagnosis and management of NBS (Kalra 2014). In the aforementioned study, the authors did not find any controlled or comparative trial on the treatment of NBS. Hence, treatment recommendations arising from this international consensus were based on case‐reports, small series of patients or retrospective data from relatively wide, although not controlled, cohorts of patients. Accordingly, they concluded that NBS management recommendations include the use of disease‐modifying therapies in general, although future studies are needed to provide evidence‐based treatments.
Authors' conclusions
Implications for practice.
No eligible study was available for quantitative or qualitative analysis of treatment for patients with NBS, hence there is no evidence of specific therapy benefit in this condition. Treatments commonly used in this condition are also associated to side effects, some of which could be relevant, and thus treatment choice should be supported by high quality evidence of risk‐benefit profiles. Furthermore, as NBS is part of a multisystemic disease, involvement of other organs needs also to be considered in treatment choice.
Implications for research.
The review of different treatment options for patients with NBS highlights the need for RCTs, or at least controlled cohort studies with sufficient detail to allow assessment of potential biases, to evaluate the benefit of different therapies for this disease, as no eligible studies have been found to date. Future studies should provide reliable evidence to help and possibly drive clinical decision making, considering that NBS may be an invalidating condition with disability and huge impact on quality of life.
As NBS may be a chronic condition characterised by relapsing or progressive course, clinical trials should be long enough to establish drug effects on long‐term and correct management in the maintenance phase, and also to assess their safety. Furthermore, efficacy and safety outcomes should be established carefully. To date, only Neuro Behçet’s Disability Score has been created as specific tool to quantify NBS disability, although it still requires validation (Kalra 2014). Moreover, different phenotypes have been described in NBS and have been mainly divided into two main entities: parenchymal and non‐parenchymal, the former more frequent in adults and the latter in paediatric patients (Uluduz 2011). Of note, the two categories, probably mediated by two different pathogenic mechanisms, require different therapeutic approaches and have different prognosis, which is usually better in patients with non‐parenchymal NBS (Siva 2001). This heterogeneity should be taken into account in clinical trial designs, which should focus on single NBS phenotypes, although their low frequency may represent a limitation. Actually, most of the studies focused on parenchymal NBS and few data have been reported on the non‐parenchymal subtype. To this purpose, given that NBS is a rare condition, multicentre RCTs, in which National Disease Registries may help enrolment of patients, are strongly recommended. Any future RCT must be well‐designed, well‐conducted, and adequately delivered with subsequent reporting, including high‐quality descriptions of all aspects of methodology.
In addition, the definition and characterisation of prognostic factors for NBS may help clinicians to decide treatment schedules. Accordingly, for example, one third of patients affected by NBS experience only a single episode in their life, hence they could not require prolonged immunosuppressive treatment, however, since no clear evidence is available, additional data are needed. Some prognostic factors have been suggested by retrospective studies (Akman‐Demir 1999; Kidd 1999); however prospective natural history studies would provide more reliable prognostic factors. To this purpose, only one prospective study has been reported (Siva 2001), showing that cerebellar symptoms at onset and progressive disease course were negative prognostic factors. Hence, we suggest further prospective studies on wide cohorts of patients to investigate natural history of NBS.
Finally, clarification of NBS pathogenesis may also help targeting treatment potentially effective in this disease.
Acknowledgements
We thank Peter Tugwell and Richard Wormald for useful comments during the preparation of the manuscript.
We thank Andrea Fittipaldo, Trials Search Co‐ordinator of the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group, for her help and support in developing this review.
We thank Yoshifumi Tada (Department of Rheumatology, Faculty of Medicine, Saga University, Saga, Japan), and Corynne Marchal (Managing Editor ‐ Cochrane Lung Cancer Group, Besancon University Hospital ‐ University of Franche‐Comte, CHRU Besançon, France ‐ Service Pneumologie) for having read and extracted data from original articles.
Appendices
Appendix 1. Keywords
Behcet* OR “Triple‐Symptom Complex” OR “Triple Symptom Complex”
Filters: Humans; Adolescent: 13‐18 years; Adult: 19+ years.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akman‐Demir 1996 | No control group. |
| Benamour 2006 | No control group. |
| Borhani Haghighi 2011 | No control group. |
| Desbois 2012 | No control group. |
| Dutra 2011 | No control group. |
| Essaadouni 2010 | No control group. |
| Farah 1998 | No control group. |
| Ideguchi 2010 | No control group. |
| Krupa 2011 | No control group. |
| Saadoun 2009 | No control group. |
| Sbai 2003 | Control group was not described with sufficient detail to allow assessment of potential 'confounding by indication'. |
| Serdaroğlu 1989 | No control group. |
| Tohmé 2009 | No control group. |
| Wechsler 1992 | No control group. |
| Whallett 1999 | No control group. |
| Yesilot 2007 | No control group. |
| Yesilot 2009 | No control group. |
Differences between protocol and review
None.
Contributions of authors
Concept ‐ GF, IT
Title registration ‐ GF, IT, FN, FG
Protocol draft ‐ GF, IT, FN, FG
Protocol editing ‐ GF, IT
Data abstraction ‐ FN, FG
Data entry ‐ FN, FG
Drafting the review ‐ IT, LM
Editing and revising the review ‐ GF, IT, LM, GH, ADB, CM
Sources of support
Internal sources
-
Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta Milano, Italy.
Support the editorial base of "Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System Group"
External sources
-
Protocol submitted to the independent research programme of the Italian Medicines Agency (AIFA), Italy.
Financially support the reviewers
Declarations of interest
FN ‐ none
FG ‐ none
LM ‐ none
GH ‐ none
ADB ‐ none
CM ‐ none
GF ‐ none
IT ‐ none
New
References
References to studies excluded from this review
Akman‐Demir 1996 {published data only}
- Akman‐Demir G, Baykan‐Kurt B, Serdaroǧlu P, Gürvit H, Yurdakul S, Yazici H, et al. Seven‐year follow‐up of neurologic involvement in Behçet syndrome. Archives of Neurology 1996;53(7):691‐4. [DOI] [PubMed] [Google Scholar]
Benamour 2006 {published data only}
- Benamour S, Naji T, Alaoui FZ, El‐Kabli H, El‐Aidouni S. Neurological involvement in Behçet's disease, 154 cases from a cohort of 925 patients and review of the literature [Manifestations neurologiques de la maladie de Behçet]. Revue Neurologique 2006;162(11):1084‐90. [DOI] [PubMed] [Google Scholar]
Borhani Haghighi 2011 {published data only}
- Borhani Haghighi A, Sarhadi S, Farahangiz S. MRI findings of neuro‐Behcet's disease. Clinical Rheumatology 2011;30(6):765‐70. [DOI] [PubMed] [Google Scholar]
Desbois 2012 {published data only}
- Desbois AC, Wechsler B, Resche‐Rigon M, Piette JC, Huong Dle T, Amoura Z, et al. Immunosuppressants reduce venous thrombosis relapse in Behçet's disease. Arthritis and Rheumatism 2012;64(8):2753‐60. [DOI] [PubMed] [Google Scholar]
Dutra 2011 {published data only}
- Dutra LA, Braga‐Neto P, Pedroso JL, Guedes Bde V, Souza LT, Gonçalves CR, et al. Epilepsy and Behçet's disease: cortical and hippocampal involvement in Brazilian patients. Journal of the Neurological Sciences 2011;309(1‐2):1‐4. [DOI] [PubMed] [Google Scholar]
Essaadouni 2010 {published data only}
- Essaadouni L, Jaafari H, Abouzaid CH, Kissani N. Neurological involvement in Behçet's disease: evaluation of 67 patients [Les manifestations neurologiques de la maladie de Behçet: étude de 67 patients]. Revue Neurologique 2010;166(8‐9):727‐33. [DOI] [PubMed] [Google Scholar]
Farah 1998 {published data only}
- Farah S, Al‐Shubaili A, Montaser A, Hussein JM, Malaviya AN, Mukhtar M, et al. Behçet's syndrome: a report of 41 patients with emphasis on neurological manifestations. Journal of Neurology, Neurosurgery, and Psychiatry 1998;64(3):382‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ideguchi 2010 {published data only}
- Ideguchi H, Suda A, Takeno M, Kirino Y, Ihata A, Ueda A, et al. Neurological manifestations of Behçet's disease in Japan: a study of 54 patients. Journal of Neurology 2010;257(6):1012‐20. [DOI] [PubMed] [Google Scholar]
Krupa 2011 {published data only}
- Krupa B, Cimaz R, Ozen S, Fischbach M, Cochat P, Koné‐Paut I. Pediatric Behcet's disease and thromboses. Journal of Rheumatology 2011;38(2):387‐90. [DOI] [PubMed] [Google Scholar]
Saadoun 2009 {published data only}
- Saadoun D, Wechsler B, Resche‐Rigon M, Trad S, Thi Huong D, Sbai A, et al. Cerebral venous thrombosis in Behçet's disease. Arthritis and Rheumatism 2009;61(4):518‐26. [DOI] [PubMed] [Google Scholar]
Sbai 2003 {published data only}
- Sbai A, Wechsler B, Duhaut P, Du‐Boutin LT, Amoura Z, Cacoub P, et al. Neuro‐Behçet's disease (isolated cerebral thrombophlebitis excluded). Clinical pattern, prognostic factors, treatment and long term follow‐up. Advances in Experimental Medicine and Biology 2003;528:371‐6. [DOI] [PubMed] [Google Scholar]
Serdaroğlu 1989 {published data only}
- Serdaroğlu P, Yazici H, Ozdemir C, Yurdakul S, Bahar S, Aktin E. Neurologic involvement in Behçet's syndrome. A prospective study. Archives of Neurology 1989;46(3):265‐9. [DOI] [PubMed] [Google Scholar]
Tohmé 2009 {published data only}
- Tohmé A, Koussa S, Haddad‐Zébouni S, El‐Rassi B, Ghayad E. Neurological manifestations of Behcet's disease: 22 cases among 170 patients [Étude de 22 observations de neuroBehçet dans une série de 170 maladies de Behçet]. Presse Médicale 2009;38(5):701‐9. [DOI] [PubMed] [Google Scholar]
Wechsler 1992 {published data only}
- Wechsler B, Vidailhet M, Piette JC, Bousser MG, Dell Isola B, Blétry O, et al. Cerebral venous thrombosis in Behçet's disease: clinical study and long‐term follow‐up of 25 cases. Neurology 1992;42(3 Pt 1):614‐8. [DOI] [PubMed] [Google Scholar]
Whallett 1999 {published data only}
- Whallett AJ, Thurairajan G, Hamburger J, Palmer RG, Murray PI. Behçet's syndrome: a multidisciplinary approach to clinical care. QJM: monthly journal of the Association of Physicians 1999;92(12):727‐40. [DOI] [PubMed] [Google Scholar]
Yesilot 2007 {published data only}
- Yesilot N, Mutlu M, Gungor O, Baykal B, Serdaroglu P, Akman‐Demir G. Clinical characteristics and course of spinal cord involvement in Behçet's disease. European Journal of Neurology 2007;14(7):729‐37. [DOI] [PubMed] [Google Scholar]
Yesilot 2009 {published data only}
- Yesilot N, Bahar S, Yilmazer S, Mutlu M, Kurtuncu M, Tuncay R, et al. Cerebral venous thrombosis in Behçet's disease compared to those associated with other etiologies. Journal of Neurology 2009;256(7):1134‐42. [DOI] [PubMed] [Google Scholar]
Additional references
Abbas 2001
- Abbas AK, Lichtman AH. Basic Immunology: Functions and Disorders of the Immune System. Philadelphia: W. B. Saunders Co, 2001. [Google Scholar]
Accardo‐Palumbo 2010
- Accardo‐Palumbo A, Giardina AR, Ciccia F, Ferrante A, Principato A, Impastato R, et al. Phenotype and functional changes of Vγ9/Vδ2 T lymphocytes in Behcet’s disease and the effect of infliximab on Vγ9/Vδ2 T cell expansion, activation and cytotoxicity. Arthritis Research & Therapy 2010;12(3):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ait Ben Haddou 2012
- Ait Ben Haddou EH, Imounan F, Regragui W, Mouti O, Benchakroune N, Abouqal R, et al. Neurological manifestations of Behçet's disease: evaluation of 40 patients treated by cyclophosphamide. Revue Neurologique (Paris) 2012;168(4):344‐9. [DOI] [PubMed] [Google Scholar]
Akman‐Demir 1999
- Akman‐Demir G, Serdaroglu P, Tasci B. Clinical patterns of neurological involvement in Behçet’s disease: evaluation of 200 patients. Brain 1999;122:2171‐81. [DOI] [PubMed] [Google Scholar]
Akman‐Demir 2008
- Akman‐Demir G, Ayranci O, Kurtuncu M, Vanli EN, Mutlu M, Tugal‐Tutkun I. Cyclosporine for Behçet's uveitis: is it associated with an increased risk of neurological involvement?. Clinical and Experimental Rheumatology 2008;26(4 Suppl 50):S84‐90. [PubMed] [Google Scholar]
Al‐Araji 2009
- Al‐Araji A, Kidd DP. Neuro‐Behçet's disease: epidemiology, clinical characteristics, and management. Lancet Neurology 2009;8(2):192‐204. [DOI] [PubMed] [Google Scholar]
Al‐Fahad 1999
- Al‐Fahad S, Al‐Araji A. Neuro‐Behcet's disease in Iraq: a study of 40 patients. Journal of the Neurology Sciences 1999;170:105‐11. [DOI] [PubMed] [Google Scholar]
Alty 2007
- Alty JE, Monaghan TM, Bamford JM. A patient with neuro‐Behçet's disease is successfully treated with etanercept: further evidence for the value of TNFalpha blockade. Clinical Neurology and Neurosurgery 2007;109(3):279‐81. [DOI] [PubMed] [Google Scholar]
Arida 2011
- Arida A, Fragiadaki K, Giavri E, Sfikakis P. Anti‐TNF agents for Behcet’s disease: analysis of published data on 369 patients. Seminars in Arthritis and Rheumatism 2011;41(1):61‐70. [DOI] [PubMed] [Google Scholar]
Berlit 2010
- Berlit P. Diagnosis and treatment of cerebral vasculitis. Therapeutic Advances in Neurological Disorders 2010;3(1):29‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calamia 2009
- Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behçet's disease in the US: a population‐based study. Arthritis and Rheumatism 2009;61:600‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTCAE 2003
- Cancer Therapy Evaluation Program. Common terminology criteria for adverse event. www.ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3to2.pdf (accessed 4 March 2013).
Dalvi 2012
- Dalvi SR, Yildirim R, Yazici Y. Behcet's Syndrome. Drugs 2012;72(17):2223‐41. [DOI] [PubMed] [Google Scholar]
Davatchi 2010
- Davatchi F, Shams H, Rezaipoor M, Sadeghi‐Abdollahi B, Shahram F, Nadji A, et al. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single‐blind control study (pilot study). International Journal of Rheumatic Diseases 2010;13(3):246‐52. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dubois 2011
- Dubois EA, Rissmann R, Cohen AF. Rilonacept and canakinumab. British Journal of Clinical Pharmacology 2011;71(5):639‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fasano 2011
- Fasano A, D'Agostino M, Caldarola G, Feliciani C, Simone C. Infliximab monotherapy in neuro‐Behçet's disease: four year follow‐up in a long‐standing case resistant to conventional therapies. Journal of Neuroimmunology 2011;239(1‐2):105‐7. [DOI] [PubMed] [Google Scholar]
Giardina 2011
- Giardina A, Ferrante A, Ciccia F, Vadalà M, Giardina E, Triolo G. One year study of efficacy and safety of infliximab in the treatment of patients with ocular and neurological Behçet's disease refractory to standard immunosuppressive drugs. Rheumatology International 2011;31(1):33‐7. [DOI] [PubMed] [Google Scholar]
Gilworth 2004
- Gilworth G, Chamberlain MA, Bhakta B, Haskard D, Silman A, Tennant A. Development of the BD‐QoL: a quality of life measure specific to Behçet's disease. Journal of Rheumatology 2004;31(5):931‐7. [PubMed] [Google Scholar]
Gül 2012
- Gül A, Ohno S. HLA‐B*51 and Behçet Disease. Ocular Immunology and Inflammation 2012;20(1):37‐43. [DOI] [PubMed] [Google Scholar]
Hatemi 2008
- Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, et al. EULAR recommendations for the management of Behçet disease. Annals of the Rheumatic Diseases 2008;67:1656‐62. [DOI] [PubMed] [Google Scholar]
Hatemi 2012
- Hatemi G, Seyahi E, Fresko I, Hamuryudan V. Behçet's syndrome: a critical digest of the recent literature. Clinical Experimental Rheumatology 2012;30(3 Suppl 72):80‐9. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirohata 1998
- Hirohata S, Suda H, Hashimoto T. Low‐dose weekly methotrexate for progressive neuropsychiatric manifestations in Behcet's disease. Journal of the Neurological Sciences 1998;159(2):181‐5. [DOI] [PubMed] [Google Scholar]
ICBD 2013
- International Team for the Revision of the International Criteria for Behçet's Disease (ITR‐ICBD), Davatchi F, Assaad‐Khalil S, Calamia KT, Crook JE, Sadeghi‐Abdollahi B, et al. The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. Journal of the European Academy of Dermatology and Venereology 2014;28(3):338‐47. [DOI: 10.1111/jdv.12107.] [DOI] [PubMed] [Google Scholar]
ISG 1990
- International Study Group for Behcet's disease. Criteria for diagnosis of Behcet's disease. Lancet 1990;355:1078‐80. [PubMed] [Google Scholar]
Kalra 2014
- Kalra S, Silman A, Akman‐Demir G, Bohlega S, Borhani‐Haghighi A, Constantinescu CS, et al. Diagnosis and management of neuro‐Behçet's disease: international consensus recommendations. Journal of Neurology 2014;261(9):1662‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 2001
- Kato Y, Numaga J, Kato S, Kaburaki T, Kawashima H, Fujino Y. Central nervous system symptoms in a population of Behçet's disease patients with refractory uveitis treated with cyclosporine A. Clinical and Experimental Ophthalmology 2001;29(5):335‐6. [DOI] [PubMed] [Google Scholar]
Keino 2011
- Keino H, Watanabe T, Taki W, Okada AA. Effect of infliximab on gene expression profiling in Behcet’s disease. Investigative Ophthalmology and Visual Science 2011;52(10):7681‐6. [DOI] [PubMed] [Google Scholar]
Kidd 1999
- Kidd D, Steuer A, Denman AM, Rudge P. Neurological complications of Behcet's syndrome. Brain 1999;122:2183‐94. [DOI] [PubMed] [Google Scholar]
Kikuchi 2003
- Kikuchi H, Aramaki K, Hirohata S. Low dose MTX for progressive neuro‐Behçet's disease. A follow‐up study for 4 years. Advances in Experimental Medicine and Biology 2003;528:575‐8. [DOI] [PubMed] [Google Scholar]
Kose 2012
- Kose O. Development of immunopathogenesis strategies to treat Behcet’s disease. Pathology Research International 2012 Apr 3 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Kotake 1999
- Kotake S, Higashi K, Yoshikawa K, Sasamoto Y, Okamoto T, Matsuda H. Central nervous system symptoms in patients with Behçet disease receiving cyclosporine therapy. Ophthalmology 1999;106(3):586‐9. [DOI] [PubMed] [Google Scholar]
Kural‐Seyahi 2003
- Kural‐Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, et al. The long‐term mortality and morbidity of Behçet syndrome: a 2‐decade outcome survey of 387 patients followed at a dedicated center. Medicine 2003;82(1):60‐76. [DOI] [PubMed] [Google Scholar]
Kötter 2006
- Kötter I, Günaydin I, Batra M, Vonthein R, Stübiger N, Fierlbeck G, et al. CNS involvement occurs more frequently in patients with Behçet's disease under cyclosporin A (CSA) than under other medications‐‐results of a retrospective analysis of 117 cases. Clinical Rheumatology 2006;25(4):482‐6. [DOI] [PubMed] [Google Scholar]
Leccese 2010
- Leccese P, D'Angelo S, Angela P, Coniglio G, Olivieri I. Switching to adalimumab is effective in a case of neuro‐Behcet's disease refractory to infliximab. Clinical and Experimental Rheumatology 2010;28(4 Suppl 60):S102. [PubMed] [Google Scholar]
Mat 2006
- Mat C, Yurdakul S, Uysal S, Gogus F, Ozyazgan, Y, Uysal O, et al. A double‐blind trial of depot corticosteroids in Behcet’s syndrome. Rheumatology (Oxford, England) 2006;45:348‐52. [DOI] [PubMed] [Google Scholar]
Nichols 2001
- Nichols JC, Ince A, Akduman L, Mann ES. Interferon‐alpha 2a treatment of neuro‐Behcet disease. Journal of Neuro‐opthalmology 2001;21(2):109‐11. [DOI] [PubMed] [Google Scholar]
O'Duffy 1984
- O'Duffy JD, Robertson DM, Goldstein NP. Chlorambucil in the treatment of uveitis and meningoencephalitis of Behçet's disease. American Journal of Medicine 1984;76(1):75‐84. [DOI] [PubMed] [Google Scholar]
Olivieri 2011
- Olivieri I, Leccese P, D'Angelo S, Padula A, Nigro A, Palazzi C, er al. Efficacy of adalimumab in patients with Behçet's disease unsuccessfully treated with infliximab. Clinical and Experimental Rheumatology 2011;29(4 Suppl 67):S54‐7. [PubMed] [Google Scholar]
Pipitone 2008
- Pipitone N, Olivieri I, Padula A, D'angelo S, Nigro A, Zuccoli G, Boiardi L, Salvarani C. Infliximab for the treatment of Neuro‐Behçet's disease: a cases eries and review of the literature. Arthritis and Rheumatism 2008;59(2):285‐90. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saenz 1998
- Saenz A, Ausejo M, Shea B, Wells GA, Welch V, Tugwell P. Pharmacotherapy for Behcet's syndrome. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD001084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salvarani 2007
- Salvarani C, Pipitone N, Catanoso MG, Cimino L, Tumiati B, Macchioni P, et al. Epidemiology and clinical course of Behçet's disease in the Reggio Emilia area of northern Italy: a seventeen‐year population‐based study. Arthritis and Rheumatism 2007;57:171‐8. [DOI] [PubMed] [Google Scholar]
Sarwar 2005
- Sarwar H, McGrath H Jr, Espinoza LR. Successful treatment of long‐standing neuro‐Behçet's disease with infliximab. Journal of Rheumatology 2005;32(1):181‐3. [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2011. [Google Scholar]
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2011. [Google Scholar]
Serdaroglu 1998
- Serdaroglu P. Behçet’s disease and the nervous system. Journal of Neurology 1998;245:197‐205. [DOI] [PubMed] [Google Scholar]
Shapiro 2012
- Shapiro LS, Farrell J, Borhani Haghighi A. Tocilizumab treatment for neuro‐Behcet's disease, the first report. Clinical Neurology and Neurosurgery 2012;114(3):297‐8. [DOI] [PubMed] [Google Scholar]
Shugaiv 2011
- Shugaiv E, Tüzün E, Mutlu M, Kiyat‐Atamer A, Kurtuncu M, Akman‐Demir G. Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro‐Behçet's disease with parenchymal involvement: presentation of four cases. Clinical and Experimental Rheumatology 2011;29(4 Suppl 67):S64‐7. [PubMed] [Google Scholar]
Singh 2011
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, MacDonald JK, et al. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008794] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siva 2001
- Siva A, Kantarci OH, Saip S, Altintas A, Hamuryudan V, Islak C, et al. Behçet’s disease: diagnostic and prognostic aspects of neurological involvement. Journal of Neurology 2001;248:95‐103. [DOI] [PubMed] [Google Scholar]
Sterne 2013
- Sterne J, Higgins J, Reeves B. Extending the risk of bias tool to allow for assessment of non‐randomised studies, cluster‐randomised trials and cross‐over trials: a Cochrane methods innovation fund project. 21st Cochrane Colloquium, Quebec 19‐23 September 2013. Available from www.cochrane.org/features/2013‐cochrane‐colloquium.
Tugal‐Tutkun 2004
- Tugal‐Tutkun I, Onal S, Altan‐Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behçet disease: an analysis of 880 patients. American Journal of Ophthalmology 2004;138:373‐80. [DOI] [PubMed] [Google Scholar]
Uluduz 2011
- Uluduz D, Kürtüncü M, Yapıcı Z, Seyahi E, Kasapçopur Ö, Özdoğan H, et al. Clinical characteristics of pediatric‐onset neuro‐Behçet disease. Neurology 2011;77(21):1900‐5. [DOI] [PubMed] [Google Scholar]
Yazici 1980
- Yazici H, Tuzun Y, Pazarli H, Yalcin B, Yurdakul S, Muftuoglu A. The combined use of HLA‐B5 and the pathergy test as diagnostic markers of Behçet's disease in Turkey. Journal of Rheumatology 1980;7(2):206‐10. [PubMed] [Google Scholar]
Yazici 2012
- Yazici H, Ugurlu S, Seyahi E. Behçet syndrome: is it one condition?. Clinical Reviews in Allergy and Immunology 2012;43(3):275‐80. [DOI] [PubMed] [Google Scholar]
Yazici 2014
- Yazici H, Yazici Y. Criteria for Behçet's disease with reflections on all disease criteria. Journal of Autoimmunity 2014;48‐49:104‐7. [DOI] [PubMed] [Google Scholar]


