Abstract

The selective catalytic oxidation of NH3 (NH3–SCO) to N2 is an important reaction for the treatment of diesel engine exhaust. Co3O4 has the highest activity among non-noble metals but suffers from N2O release. Such N2O emissions have recently been regulated due to having a 300× higher greenhouse gas effect than CO2. Here, we design CuO-supported Co3O4 as a cascade catalyst for the selective oxidation of NH3 to N2. The NH3–SCO reaction on CuO–Co3O4 follows a de-N2O pathway. Co3O4 activates gaseous oxygen to form N2O. The high redox property of the CuO–Co3O4 interface promotes the breaking of the N–O bond in N2O to form N2. The addition of CuO–Co3O4 to the Pt–Al2O3 catalyst reduces the full NH3 conversion temperature by 50 K and improves the N2 selectivity by 20%. These findings provide a promising strategy for reducing N2O emissions and will contribute to the rational design and development of non-noble metal catalysts.
Keywords: ammonia-selective oxidation, CuO/Co3O4 catalyst, N2O decomposition, metal−support interaction, O2 activation
1. Introduction
Excess ammonia (NH3) is needed for selective catalytic reduction (NH3–SCR) of harmful nitrogen oxides (NOx) from diesel vehicle exhaust and power plants.1−3 The slip of unreacted NH3 can be eliminated by selective catalytic oxidation of NH3 (NH3–SCO) to nitrogen.4 The ideal catalysts for the NH3–SCO reaction downstream of NH3–SCR should completely convert NH3 to N2 and H2O without the reformation of N-containing pollutants. Co3O4 is a promising catalyst with comparable activity to replace noble metals.5−8 However, Co3O4 will lead to the release of nitrous oxide (N2O), which has a greenhouse gas effect 300× higher than that of CO2.9,10 In particular, N2O emission in the transportation sector is significant, with 56 mg N2O/km generated by on-road vehicles.11 Without a global mitigation strategy, N2O emissions are projected to be nearly double by 2050.12 Therefore, N2O emissions from diesel engines have been recently regulated and are included in the upcoming EU7 standard. Considering this, the high N2O release rate from Co3O4 makes it unsuitable for NH3–SCO.
NH3–SCR and NH3–SCO catalytic reactions have been extensively studied for decades, but limited attention has been paid to the side reactions of N2O formation.13−17 Noble metals typically have high NH3–SCO activity, but significant N2O release at high temperatures cannot be avoided.18−22 Gang et al.23 found that oxygen dissociation is the rate-controlling step of NH3–SCO on a Ag-powder catalyst. The selectivity for N2, N2O, and NO mainly depends on the surface oxygen coverage and reaction temperature. The nitrogen selectivity increases with the adsorption of NOx and N2Ox species, as the oxidation of NH3 is inhibited due to the reduced concentration of surface-active O. These results are consistent with studies on Ir-based24 and Au-based25 catalysts. Karatok et al.26 reported that disordered surface atomic O can selectively catalyze N–H bond cleavage, yielding mainly N2 products, while the O/Ag(111) surface with higher oxygen coverage shows higher selectivity for N2O than N2. Therefore, it has been pointed out that blocking oxygen dissociation sites on noble metals can effectively improve N2 selectivity, but this also leads to a loss of activity.6 Transition metal oxides, such as CuO,27−32 Fe2O3,33,34 and V2O5,35 have been reported to have high N2 selectivity but lack sufficient activity for practical use. Despite all these efforts, there remains an insufficient understanding of the fundamental methods for achieving high low-temperature activity with less N2O emission for NH3–SCO.
In this study, we designed a cascade catalyst that uses CuO to help remove the as-formed N2O from Co3O4. Such a 3d metal oxide system showed similar performance to Pt–Al2O3, which is usually applied to practical use.36 CuO–Co3O4 cascade catalysts were investigated for NH3–SCO using in situ X-ray adsorption fine structure (XAFS) combined with a near ambient pressure (NAP) near edge X-ray absorption fine structure (NEXAFS). Bulk Co3O4 has an excellent ability to activate gaseous O2 on the surface, leading to outstanding NH3 conversion and N2O formation. The enhanced redox capacity of the CuO–Co3O4 interface leads to the efficient breakage of the N–O bond in N2O at a high temperature, forming N2 and reactive O. The reactive O will then react with NH3 to generate N2. We call this reaction pathway of decomposing N2O at high temperatures in NH3–SCO the decomposition of N2O (de-N2O) mechanism. The N2O formation rate and N2O dissociation rate are related to the concentration of surface Co3O4 and the CuO–Co3O4 interface, respectively. 90 wt % CuO–10 wt % Co3O4 not only has comparable catalytic performance to the Pt–Al2O3 catalyst but also can assist the Pt–Al2O3 catalyst in reducing N2O emissions from 20% to 3% at 723 K.
2. Experimental Section
2.1. Catalyst Preparation
The copper-based catalysts were prepared by coprecipitation using copper nitrate trihydrate (Cu(NO3)2·3H2O), cobalt nitrate hexahydrate (Co(NO3)2·6H2O), and ammonium hydroxide solution (28.0–30.0% NH3 basis) as starting materials. In a typical procedure, 2 g of nitrate precursor Cu(NO3)2·3H2O and the corresponding mass of Co(NO3)2·6H2O were dissolved in 15 mL of deionized water. Subsequently, the mixture was stirred for 10 min, and then NH3·H2O was added dropwise until the pH reached 9 under continuous stirring. After aging for 2 h, the sample was filtered and washed with deionized water, then dried at 60 °C overnight. The as-prepared sample was calcined at 550 °C in a muffle furnace for 4 h with a heating rate of 5 °C·min–1. Finally, the sample was slowly cooled to room temperature in the muffle furnace. The obtained solid was finely ground and sieved below 250 μm grain size.
By changing the loading of Cu, catalysts with different Cu aggregation states were prepared by the above method. For comparison, pure CuO and Co3O4 were also prepared using the above method.
The 1 wt % Pt–Al2O3 was prepared by the incipient wetness impregnation method. The H2PtCl6·(H2O)6 was dissolved in ethanol and then added into γ-Al2O3. γ-Al2O3 was purchased from Johnson Matthey. The sample was then dried at 60 °C overnight. Finally, the dried sample was calcined in air at 550 °C for 4 h at a heating rate of 5 °C min–1.
2.2. H2 Activation
The catalyst was placed in a fixed-bed flow reactor. At room temperature, 5% H2 balanced in He was introduced, and then the temperature was raised to 573 K with a total flow rate of 100 mL·min–1. After 30 min, 5% H2 balanced in He was switched to He, and the sample was slowly cooled to room temperature.
2.3. Ex Situ Characterizations
X-ray diffraction (XRD) measurements were performed on a StadiP diffractometer from STOE with a Mo source (Kα = 0.7093165 Å). The operating voltage and current are 40 kV and 30 mA, respectively. With a resolution of 0.015° for each step, the signals of 2θ in the range of 2°–40° were collected.
Scanning transmission electron microscope images (STEM) of the samples were obtained on a probe-corrected (CEOS) JEM ARM 200CF electron microscope (JEOL, Japan) at the electron Physical Science Imaging Centre (ePSIC). The sample was prepared by sprinkling dry catalyst powder on 400-mesh gold grids with a lacey carbon film. Using a 20 μm probe-forming aperture, the beam current was 33 pA with a 15.3 mrad probe convergence semiangle at 200 keV operation voltage. The samples were loaded onto Au grids by sprinkling a small amount of dry sample powder.
The energy dispersive X-ray spectrum (EDX) of the sample was obtained using the JEM ARM 200CF instrument equipped with a large solid angle dual EDX detector. The data were collected in STEM Illumination mode at 200 kV and corrected using special drift correction. Au TEM grids are used to avoid any Cu EDX signals from the grid. Each EDX spectrum image is 100 × 100 pixels in size, with 0.05 s exposure time per pixel.
Temperature-programmed desorption of N2O (N2O-TPD) was performed in a fixed-bed flow reactor. Prior to the experiment, the catalyst sample was pretreated in a He flow (50 mL·min–1) at 400 °C for 1 h. After this, the sample was cooled to 50 °C in He flow. The sample was exposed to 1% N2O (balance He) gas for 1 h at 50 °C, followed by flushing with He. After these treatments, N2O-TPD was carried out in a flow of He (80 mL·min–1) with a heating rate of 10 °C min–1.
X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) of the Co K edge (7.709 keV) and Cu K edge (8.979 keV) were carried out in B18 in Diamond Light Source (UK) and BL01B1 in Spring8 (Japan). Samples with 1 wt % CuO loading were directly pressed into pellets for the fluorescence measurement of the Cu K edge. Other samples were diluted with boron nitride and pressed into a pellet with a diameter of 0.8 cm for the transmission measurement. CoO, Co3O4, CuO, and Cu2O standards were diluted with boron nitride and pressed into pellets for the transmission measurement. Co foil and Cu foil standards were used for energy shift calibration. For the EXAFS evaluation, at least three spectra were merged to improve the signal quality. XAFS data were processed using the Demeter software package (including Athena and Artemis).37 Athena software was used for the XANES analysis. Artemis software was used to fit the k2-weighted EXAFS data in real space with 3.0 Å–1 < k < 12.0 Å–1 and 1.0 Å < R < 3.3 Å. The calculated amplitude reduction factor S02 from the EXAFS analysis of Cu foil was 0.878, which was used as a fixed parameter for EXAFS fitting. The calculated amplitude reduction factor S02 from the EXAFS analysis of Co foil was 0.786, which was used as a fixed parameter for EXAFS fitting. The coordination number and bond length were calculated based on the reported structure from the Crystal open database: Cu (no. 9013014), CuO (no. 1011148), Co (no. 9011618), and Co3O4 (no. 1538531).
2.4. In Situ near-Edge X-ray Absorption Fine Structure (NEXAFS) Spectroscopy
In situ NEXAFS experiments for Co3O4 were performed at the ISISS beamline of BESSY II in Berlin (Germany). The X-ray is sourced from a bending magnet (D41) and a plane grating monochromator (PGM) with an energy range from 80 to 2000 eV (soft X-ray range) and a flux of 6 × 1010 photons s–1 with a 0.1 A ring current using a 111 μm slit and an 80 μm × 200 μm beam spot size. The reaction products were monitored online using an electron impact mass spectrometer (“PRISMA”, PFEIFFER VACUUM GmbH, Asslar, Germany)) connected directly to the main experimental chamber by a leak valve. The pressure in the specimen chamber was precisely controlled (UHV or 0.1–1 mbar) by simultaneous operation of several mass flow controllers for reactive gases and a PID-controlled throttle valve for pumping gas out. 100 mg samples of catalysts were pressed into pellets with a diameter of 6 mm. Sample pellets (6 mm diameter) were heated uniformly from an IR laser mounted on the rear part of the sample holder. Temperature control was realized by two K-type thermocouples. NEXAFS spectra at Co L edge (765–805 eV), O K edge (510–560 eV) and N K edge (390–420 eV) were measured in either the total electron yield (TEY) mode or the Auger electron yield (AEY) mode.
2.5. In Situ X-ray Absorption Fine Structure (XAFS) Experiments
In situ XAFS experiments for Co3O4 and 90 wt % CuO–Co3O4 (90CuCo) were performed at BL01B1 (Spring8, Japan). In situ XAFS of both catalysts was performed in a modified heating XAS cell (ASPF-20–03, Kyowa Vacuum). In the experiment, both catalysts were pressed into pellets with boron nitride (diameter 10 mm, thickness ca. 1 mm) and measured under various gas conditions at room temperature, 573 K, and 673 K. For Co3O4, around 5 mg of sample was mixed with 95 mg of boron nitride for measuring the Co K edge. For 90CuCo, around 5 mg of the sample was mixed with 95 mg boron nitride when measuring the Cu K edge, while 50 mg sample was mixed with boron nitride when measuring the Co K edge. The temperature was monitored by a K-type thermocouple and regulated by a PID controller. At 573 and 673 K, the catalysts were reduced by NH3 (5000 ppm of NH3 balanced in He) or NH3 + NO (5000 ppm of NH3 and 5000 ppm of NO balanced in He) and oxidized by NH3 + O2 (5000 ppm of NH3 and 5% O2 balanced in He), NH3 + NO + O2 (5000 ppm of NH3, 5000 ppm of NO, and 5% O2 balanced in He) or NO + O2 (5000 ppm of NO and 5% O2 balanced in He) with a total gas flow of 100 mL·min–1. The concentration of the respective gas was kept the same with balancing He while changing the composition of the gas flow. The outlet gases were sampled continuously with a quadrupole mass (Q-Mass) spectrometer. XANES spectra of each gas composition were recorded between 7400 and 8910 eV for the Co K edge and 8660–10170 eV for the Cu K edge in transmission mode with Si(111) crystal monochromator. The spectra processing and linear combination fitting (LCF) analysis were also processed with Athena, and the EXAFS fitting was processed using Artemis.37
2.6. In Situ Diffusion Reflection Infrared Fourier Transform Spectroscopy (DRIFTS)
In situ diffusion reflection infrared fourier transform spectroscopy (DRIFTS) was carried out on a Thermo Scientific NiCOLET iS50 spectrometer. Before testing, the powder sample was placed in the sample chamber. The detailed test programs could be divided into three parts as follows:
-
(1)
The sample was heated under Ar flow from room temperature (RT) to 400 °C with a ramping rate of 20 °C·min–1. The background profiles were collected at RT, 200 °C, 300 °C, and 400 °C, respectively. After that, the sample was cooled under a pure O2 flow.
-
(2)
At RT, the gas flow (5% NH3, 50% O2, 45% Ar, 50 mL·min–1) was introduced into the reaction cell. Under this predetermined atmosphere, the sample was heated from RT to 400 °C (20 °C min–1). During the heating process, the in situ DRIFTS spectra were detected at RT, 200 °C, 300 °C, and 400 °C, respectively.
-
(3)
At 400 °C, the in situ DRIFTS spectra were collected with a consecutive switch of predetermined gases and Ar. Followed by program (2), Ar flow was used to purge the sample cell for 5 min. After that, a mixed gas (100 mL·min–1) of 2.5% NH3, 2.5% N2O, 25% O2, and 70% Ar was switched to the sample room, and the signal was detected. After the purge of Ar, another mixed gas (3.3% N2O, 33.3% O2% and 63.4% Ar) with a flow rate of 75 mL·min–1 was introduced, followed by signal collection. Then, a switch of Ar, 5% N2O/Ar (50 mL·min–1), Ar, and mixed gas (3.3% NH3, 3.3% N2O, and 93.4% Ar, 75 mL·min–1) was carried out. Under the predetermined reaction gases, the in situ DRIFTS spectra were recorded .
2.7. Catalytic Performance Measurement
The selective catalytic oxidation of ammonia was evaluated in a fixed-bed flow system using a stainless-steel flow reactor (i.d. = 9.75 mm, 300 mm length). The composition and flow rate of the inlet gas mixture were set by the mass flow controller. A typical reaction gas composition was 5000 ppm of NH3, 5 vol % O2, and balance He. The flow rate of the mixed gas was 100 mL·min–1. Typically, 50 mg of catalyst powder diluted with 100 mg SiC (400 mesh) was placed in the reaction tube, and the product is detected with the quadrupole mass spectrometer (MS) quantitative gas analyzer (Hiden Analytical, UK). The Hiden QGA can be used to determine how overlapping species may be separated using soft ionization techniques, which allows one to selectively ionize different gases by setting the ionization energy for a particular mass. The reaction was studied in the temperature range of 423–723 K. After reaching a steady state at each reaction temperature, the reaction was maintained for at least 30 min to measure the MS signals of reactants (NH3 and O2) and products (N2, N2O, NO, and NO2).
2.8. DFT Calculations
The DFT+U calculations were utilized through the calculation software QuantumATK.38 The exchange-correction interactions were described by the projector augmented wave (PAW) pseudopotentials with the Perdew–Burke–Ernzerhof (PBE) functional.39 The values of U were carefully chosen according to the relevant paper, which is 4 eV for Co atoms.40 The Brillouin zone was sampled using a 9 × 9 × 9 Monkhorst–Pack k-point mesh and 520 eV cutoff energy for the primitive cell lattice optimization, while 1 × 1 × 1 k-points are employed for subsequent adsorption calculations. During the optimization, the convergence criteria were set to 0.03 eV/A and 10–5 eV for force and energy, respectively. We also chose the (100) surfaces of Co3O4 owing to their lower surface energy and greater stability.41
The adsorption energy per molecule was calculated as follows:42
in which Esubstrate+adsorbate is the total energy of the whole system, which includes the absorbate and the substrate structure. Esubstrate and Eadsorbate are the energies of substrate structure and adsorbed gas molecule, respectively. According to the equation, the negative adsorption energy indicates the existence of adsorption, while the positive one means no evident adsorption interactions.
3. Results and Discussion
Co3O4, which is composed of mixed valence Co2+ and Co3+ with a spinel structure, has been extensively studied for the oxidation of CO and the combustion of CH4.43,44 We hypothesize that the presence of Co(II) and its redox with Co(III) can activate gaseous O2, which is the main reason for the fast oxidation of NH3. Density functional theory (DFT) calculations support this hypothesis, showing the weakened O–O bond and the increased bond length of the oxygen molecule on the Co3O4 (100) surface (Figure 1a) with an adsorption energy of −5.27 eV (Table S1). In comparison, the calculated results over the CuO (001) surface show physical O2 adsorption with −0.13 eV adsorption energy.45
Figure 1.

Co3O4, CuO and 1 wt % Pt/Al2O3 catalysts. (a) DFT calculation of O2 over Co3O4 (100), see Figure S4 for details. The DFT calculations of NO and NH3 adsorption are shown in Figures S5 and S6. (b) O K edge NEXAFS spectra of Co3O4 under UHV and 0.3 mbar of O2 at 298 K. (c) NH3 conversion as the function of temperature for Co3O4, Pt/Al2O3, and CuO catalysts. (d–f) Selectivity of N2, N2O, NO, and NO2 as the function of temperature for (d) Co3O4, (e) Pt/Al2O3, and (f) CuO catalysts. Reaction conditions are as follows: mcat = 50 mg, 5000 ppm of NH3, 5% O2 balanced in He, gas flow of 100 mL·min–1, weight hourly space velocity (WHSV) = 600 mLNH3·h–1·g–1.
Such an O2 activation is observed in the O K edge near edge X-ray absorption fine structure (NEXAFS). After oxygen was introduced, the peak of gaseous oxygen in the O K edge (Figure S1, 530.6 eV) was not observed on the surface of Co3O4, while a peak at 530.1 eV was observed (Figure 1b). Compared with ultrahigh vacuum (UHV) and NH3 conditions, this peak only appears under O2 conditions (Figures S2 and S3). Such a peak shift (0.5 eV) toward low photon energy indicates the formation of more reactive O2δ- species on the surface, in which the 2pπ* peak shifts to lower energy.46,47 Surface superoxo- or peroxo-like O2 species are active in the oxidation of CO48 and hydrocarbons49 (e.g., toluene), and such reactive oxygen species are usually formed by O2 molecules on O vacancies50 on the surface of transition metal oxides.51,52 For Co3O4, the O vacancy near the Co2+ species can activate O2, taking electrons from Co2+ to form O2δ-, whereas Co2+ is oxidized to Co3+.53 The NH3–O2δ- complex has been reported as a key intermediate in the oxidation of NH3.54 The catalytic activity of NH3–SCO at low temperatures was also found to scale proportionally with the concentration of Cu(II) superoxo species.55 Both Co3O4 and CuO catalysts prepared by precipitation were evaluated for NH3 oxidation in the temperature window of 423–723 K (Figure 1c–f) and compared with 1 wt % Pt–Al2O3. Co3O4 is a very active material for oxidation reactions and is more active than Pt–Al2O3 in NH3 oxidation, with 100% conversion at 573 K (Figure 1c). Both Co3O4 and Pt–Al2O3 show a seagull shape of selectivity trend, with high N2 selectivity below 500 K and above 623 K, which is less discussed in the literature. In the mid-temperature range between 500 and 623 K, the major byproduct is N2O (Figure 1d and e). In comparison, CuO can still maintain a high N2 selectivity in this region (Figure 1f), though with a lower conversion. These results suggest that O2δ- on the surface of Co3O4 is the active site for NH3 conversion and N2O formation.
To understand the observed catalytic trends for Co3O4 catalysts, we considered the imide mechanism of low-temperature N2O formation.56,57 Adsorbed NH3 would first react with the active oxygen species to form NHx* (eqs 1 and 2). The NH* is subsequently oxidized to form the nitroxyl species (HNO*) (eq 3). N2 can be formed in the reaction between NH* and HNO* (eq 4). N2O is commonly accepted to be formed in the reaction of HNO* (eq 5). The product distribution is therefore determined by the relative concentration of NHx* and HNO* species on the catalyst surface. At low temperatures, the HNO* density on the surface is relatively low, leading to high selectivity toward N2. Increasing the temperature, more NH3 is oxidized to HNO*, resulting in N2O formation. This explains the trend of N2 toward N2O between 400 and 573 K.
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
However, the imide mechanism cannot explain the shift from N2O to N2 above 573 K. Here, we propose a new mechanism for NH3–SCO, which is the decomposition of N2O (de-N2O) with the help of NH3 to form N2. In this mechanism, N2O first dissociates to N2 and O* (eq 6). After that, O* can react with absorbed NHx* to form N2 (eq 7). The breaking of N–O bonds in N2O is usually carried out at high temperatures. The removal of O* by NH3 can further promote the dissociation of N2O. As a result, the selectivity changes from N2O to N2 at high temperatures.
| 6 |
| 7 |
Co3O4 generates N2O between 500 and 623 K, whereas CuO maintains high N2 selectivity. We hypothesize that the addition of CuO to Co3O4 will convert the newly formed N2O into N2 following eqs 6 and 7.
To prove this, we prepared a range of CuO–Co3O4 catalysts via the coprecipitation method with 1, 25, 90, and 99 wt % CuO loadings (denoted as 1CuCo, 25CuCo, 90CuCo and 99CuCo, respectively). Co3O4 nanoparticles are obtained by the precipitation method with an average size of 50 nm (Figure S7). Coprecipitating with 1 wt % Cu does not change the morphology of the catalysts (Figure S8). Co3O4(111) facets are observed in the bright field-scanning transmission electron microscopy images (BF-STEM) for 1CuCo (Figure S9a). The presence of Cu in 1CuCo is further confirmed in the energy dispersive X-ray spectrum (EDX), showing Cu Kα emission at 8.05 keV (Figure S10). Cu species are uniformly dispersed on Co3O4, as shown in the homogeneous Cu map compared to that of Co (Figure S9b). XRD patterns further confirm the crystallinity of CuO and Co3O4 (Figure S11). The diffraction features of CuO can be observed with CuO loadings above 25 wt %, and the diffraction characteristics of Co3O4 are relatively weak when the CuO loading is 90%.
The oxidation states and coordination environments for 1CuCo, 25CuCo, and 90CuCo catalysts were examined by X-ray absorption near edge spectroscopy (XANES) and extended X-ray absorption fine structure (EXAFS). Cu in these three catalysts has a similar peak shape and white line position to those of the CuO standard, showing the Cu(II) configuration. The obvious separation between 1s → 4pz and 1s → 4pxy is observed in 25CuCo and 90CuCo, which is due to the elongated octahedral coordination of Cu in CuO.58,59 In comparison, XANES spectra show no obvious separation between 1s → 4pz and 1s → 4pxy for 1CuCo (Figure S9c,d), suggesting a reduced Jahn–Teller effect. We speculate that Cu(II) replaces the position of tetrahedral-coordinated Co(II) in Co3O4 in 1CuCo, thus showing similar XANES spectra compared to CuCo2O4.60 For 25CuCo, the coordination number of Cu–Cu (1) is similar to that of the CuO standard, while the Cu–Cu (2) scattering is much lower than that of 90CuCo and the CuO standard (Figure S9e, fitting results in Figure S12 and Table S2), indicating the formation of a small CuO cluster that lacks long-range Cu–Cu order. Co in these three catalysts is in the form of Co3O4, as shown in the Co K edge XANES spectra (Figure S9g and h). The coordination number of Co–Co (2) of 90CuCo is less than that of Co3O4 (Figure S9f, fitting results in Figure S13 and Table S3) and is consistent with the XRD results, showing that Co3O4 clusters are formed over CuO. Therefore, highly dispersed Cu over Co3O4 (1CuCo), CuO cluster over Co3O4 (25CuCo), and Co3O4 cluster over CuO (90CuCo) were synthesized by changing the Cu loading.
We then evaluated the reduction of N2O by NH3 for the CuO–Co3O4 catalysts. CuO cannot convert N2O to N2 below 723 K. Co3O4 can convert N2O to N2, but only at 623 K or above (Figure S14). This also agrees with the trend of N2O selectivity in NH3 oxidation (Figure 1d). The addition of CuO to Co3O4 created two metal surfaces for the reaction. With 1 wt % CuO on Co3O4 (1CuCo), the N2O conversion temperature is reduced by 50 K. The 90 wt % CuO with Co3O4 (90CuCo) further reduced the required temperature by another 50 K, so that the N2O conversion can be achieved from 500 K onward. At 523 K, the N2O conversion at 90CuCo is nine-times higher than that of 1CuCo (Figure 2a), with the highest NH3 conversion (Figure 2b).
Figure 2.

The catalytic performance in N2O reduction by NH3. (a) Conversion of N2O for CuO–Co3O4 catalysts at 523 K. (b) Conversion of NH3 for CuO–Co3O4 catalysts at 523 K. Reaction conditions are as follows: 120 mg of catalyst, 5000 ppm of NH3, 5000 ppm of N2O balanced in He, and gas flow of 80 mL·min–1. (c) Conversion of N2O for pure CuO, 1 wt % Pt–Al2O3, and 90 wt % CuO–Co3O4 catalysts at 723 K. (d) Conversion of NH3 for pure CuO, 1 wt % Pt–Al2O3, and 90 wt % CuO–Co3O4 catalysts at 473 K. Reaction conditions are as follows: 120 mg of catalyst, 3000 ppm of NH3, 4500 ppm of N2O, 5% O2 balanced in He, and gas flow of 100 mL·min–1. Negative N2O conversion suggested the formation of N2O.
The N2O decomposition reaction was reported to be notably hindered by the presence of O2.12 Introducing O2, 90CuCo can still effectively convert N2O at 723 K (Figure S15), showing 5× and 14 × N2O conversion than 1 wt % Pt/Al2O3 and CuO (Figure 2c), respectively. With the promotion of N2O, 90CuCo has higher NH3 conversion than 1 wt % Pt/Al2O3 (Figure 2d and Figure S15). Without NH3, 90CuCo has lower N2O conversion than Co3O4 and 1CuCo (Figure S16), suggesting the important role of NH3 in the decomposition of N2O. To further derive the important role of NH3 in N2O decomposition, we compared N2O conversion for 90CuCo with the presence and absence of NH3. To prevent NH3 from being oxidized to N2O, we did not introduce O2. We found that NH3 is essential for the conversion of N2O at low temperatures (below 723 K). In the absence of NH3, N2O is essentially undecomposable at low temperatures (Figure S17). In contrast, more than 50% of N2O decomposes at 573 K in the presence of NH3.
The introduction of NH3 can significantly facilitate the decomposition of N2O in the absence of O2 (NH3 + N2O conditions). At the same time, active O formed from N2O decomposition also contributes to the consumption of NH3. 90CuCo exhibits worse NH3 oxidation activity than Pt/Al2O3 without the introduction of N2O (NH3 + O2 conditions) at 523 K. After the introduction of N2O (NH3 + N2O + O2 conditions), 90CuCo obtained a higher NH3 conversion than Pt/Al2O3 (Figure S15). This shows that N2O significantly facilitates the conversion of NH3 for the 90CuCo catalyst, which reflects the reaction between NH3 and N2O. The possible reason for this is that the reactive O from N2O decomposition contributes to the conversion of NH3, whereas Pt/Al2O3 is less capable of N2O decomposition. The results show that 90CuCo is a very active catalyst for the breakage of the N–O bond of N2O with the help of NH3, which will be promising in eliminating both NH3 and N2O emissions.
To validate that the decrease in N2O emissions resulted from its further decomposition, we performed an in situ DRIFTS experiment from room temperature to 400 °C under NH3 + O2 (Figure 3a). Strong bands at 1625 cm–1 are observed for 90CuCo from room temperature to 400 °C under NH3 + O2, which are assigned to the asymmetric bending vibrations of coordinated NH3.61 The bands at 1316 and 1415 cm–1 under room temperature can be ascribed to NH3 adsorbed on Lewis acid sites21 and the asymmetric bending vibrations of N–H bonds62 in NH4+, respectively. The band at 1282 cm–1 observed at 200 °C was attributed to monodentate nitrates.63 No adsorption signals for N2O were observed at both room temperature and 200 °C. Increasing the temperature to 300 °C, four adsorption peaks can be observed at 2237, 2213, 1302, and 1271 cm–1 in Figure 3, which were confirmed to be the stretching frequencies of N–N and N–O bonds in N2O.64 At 400 °C, the N2O signal can still be observed, suggesting that large amounts of N2O can still be produced and adsorbed on the catalyst surface.
Figure 3.
In situ DRIFTS investigations. DRIFT spectra of adsorbed species on the 90CuCo under (a) NH3 + O2 from room temperature to 400 °C and (b) different gas conditions (NH3 + O2, NH3 + N2O, NH3 + N2O + O2, and N2O + O2) at 400 °C.
For 90CuCo, the formation of monodentate nitrates at 200 °C suggests that N2 formation under low temperatures for 90CuCo follows the i-SCR mechanism. However, the nitrate species are less detectable under high temperatures (Figure 3a), indicating a different mechanism for N2 formation (see the detailed discussion in Text S1). Although there are other possible reaction paths to generate N2, we consider the decomposition of N2O to N2 to be the main reaction path at high temperatures.
To confirm the role of NH3 and O2 for the decomposition of N2O, we performed in situ DRFITS at 400 °C under different gas conditions (NH3 + O2, NH3 + N2O, NH3 + N2O + O2, and N2O + O2, Figure 3b). The intensity of the N2O bands of NH3 + N2O + O2 was stronger than that of N2O + NH3. These results suggest that O2 inhibits the reduction of N2O by NH3. The possible reasons are (1) O2 can oxidize NH3 to N2O and therefore NH3 cannot be a selective reductant and (2) the competitive adsorption of O2 into the active sites. We found that the intensity of the N2O band of NH3 + N2O + O2 was significantly weaker than that of N2O + O2 (Figure 3b). The weaker intensity of the N2O signals could potentially be attributed to various factors, including competitive adsorption. However, NH3 introduced at high temperatures can also react with oxygen to form additional N2O on the surface. These results reflect the importance of NH3 for reducing the surface coverage of N2O. In situ DRIFTS further supports our speculation that NH3 is oxidized to N2O at high temperatures (above 300 °C) and the as-formed N2O is decomposed to produce N2.
The CuO–Co3O4 catalysts were then evaluated for NH3 oxidation in the temperature window of 423–723 K (Figure 4) and compared with 1 wt % Pt–Al2O3. 1CuCo has a reactivity profile similar to that of Co3O4 (Figure 4a). With the increase of the Cu loading, the NH3 conversion decreases due to the reduced amount of Co on the surface. 90CuCo has a slightly lower NH3 conversion rate than Pt/Al2O3 (Figure 4b) but higher N2 selectivity at 723 K (71% vs 57%, Figure 4c).
Figure 4.
Catalytic performance in NH3-SCO for CuO–Co3O4. (a) Yields of N2, N2O, NO, and NO2 at 523 and 623 K. (b) NH3 conversion as a function of temperature. (c) Selectivity of N2, N2O, NO, and NO2 as the function of temperature for CuO–Co3O4 catalysts. (d) NH3 conversion and product selectivity as the function of temperature for the 90 wt % CuO–Co3O4 catalyst. Reaction conditions are as follows: (a–c) Tbed = 423–723 K, mcat = 50 mg, 5000 ppm of NH3, 5% O2 balanced in He, gas flow of 100 mL·min–1, WHSV = 600 mLNH3·h–1·g–1; (d) Tbed = 393–773 K, mcat = 50 mg, 1000 ppm of NH3, 5% O2 balanced in He, gas flow of 100 mL·min–1, and WHSV = 120 mLNH3·h–1·g–1.
Under realistic NH3 slip conditions (1000 ppm of NH3), NH3 can be completely converted by 90CuCo at 493 K (Figure 4d). More importantly, 90CuCo has higher N2 selectivity and similar selectivity trends under a low NH3 concentration, providing a wide operation window from 553 to 773 K, with the N2 yield exceeding 90% (Table S4). Such a wide operation window exceeds those of most catalysts reported in the literature (Figure S18). 90CuCo shows excellent catalytic stability without any noticeable drop during the 100 h reaction (Figure S19). This suggests the potential of replacing expensive Pt in the industrial NH3 slip process. In order to evaluate the performance of the catalyst under more realistic NH3 slip conditions, we conducted measurements on the 90CuCo catalyst at a low NH3 concentration (1000 ppm) with a significant amount of H2O (5%). It was observed that, in the presence of 5% H2O, the activity of the 90CuCo catalyst experienced a slight decrease while maintaining a selectivity of approximately 90% at elevated temperatures (Figure S20). This shows the potential of 90CuCo in practical applications.
With 1 wt % Co3O4 loaded on CuO, the activity of 99CuCo is slightly higher than that of pure CuO, suggesting the importance of Co3O4 in O2 activation at low temperatures. We have successfully tested our hypothesis that the bifunctional Co3O4 and CuO surface with the desired ratio at 90CuCo converts the as-formed N2O into N2 throughout the testing temperatures without losing too much NH3 oxidation activity.
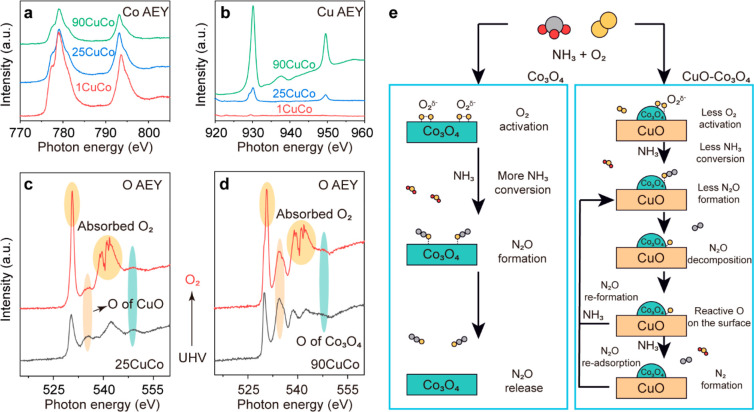
NAP-NEXAFS experiments were carried out to provide surface chemical details of 1CuCo, 25CuCo, and 90CuCo. 1CuCo has the highest amount of Co species on the surface with a weak Cu signal. 25CuCo has a higher Cu feature than 1CuCo, confirming the presence of CuO clusters on the surface. 90CuCo shows the majority of CuO on the surface (Figure 5a and b). With different surface metal ratios, lattice O of CuO and Co3O4 with different intensities can be detected over 25CuCo and 90CuCo. Unlike the pure Co3O4 (Figure 1b), surface physically adsorbed O2 can be observed over 25CuCo and 90CuCo (Figure 5c and d), showing a weaker activation of the O2, which leads to decreased NH3 conversion for 90CuCo (Figure 4b). For Co3O4, active surface O species lead to the rapid formation of N2O, which directly desorbs at low temperatures. For CuO–Co3O4, the as-formed N2O tends to decompose to N2 and O* on the surface. O* can then be removed by unreacted NH3, forming N2. After the surface of O* is scavenged, N2O can continue to decompose on the surface again (Figure 5e).
Figure 5.
NEXAFS study of CuO–Co3O4 catalysts. (a) Co L edge NEXAFS spectra of CuO–Co3O4 catalysts under UHV at 298 K. (b) Cu L edge NEXAFS spectra of CuO–Co3O4 catalysts under UHV at 298 K. (c) the O K edge NEXAFS spectra of 25 wt % CuO–Co3O4 under UHV or O2 (0.3 mbar) at 298 K. (d) O K edge NEXAFS spectra of 90 wt %CuO–Co3O4 under UHV or O2 (0.3 mbar) at 298 K. (e) Simplified reaction pathways for cobalt-catalyzed selective oxidation of ammonia.
To understand the transition of products from N2O to N2 at high temperatures for Co3O4, the Co L edge and the O K edge were studied under different NH3/O2 ratios. The adsorbed active molecular oxygen can provide oxygen for the oxidation of NH3, but the substrate lattice can also do this. In the latter case, the task of gas-phase O2 is to restore the catalyst by refilling the oxygen vacancies. This is a so-called Mars–van-Krevelen (MvK) mechanism, which has been reported as a method of CO oxidation on Co3O4.44,65 In addition to oxygen, N2O can also be considered as an O-donor molecule because of its low N2-oxygen affinity of 40 kcal/mol.66 Therefore, surface oxygen vacancies have been discussed as active sites for the decomposition of N2O,67 increasing the chemisorption of N2O on the catalyst surface.68 To examine the surface O vacancies during the reaction, the NH3 and O2 mixtures at different ratios are introduced to Co3O4 at 573 and 673 K (for the spectra and the corresponding mass spectrometry (MS) signals, see Figures S21–S23). Co(III) to Co(II) transition starts under NH3/O2 = 1:9 at 573 K, indicating the formation of stable surface O vacancies at the Co3O4 surface under low temperature despite the excess of oxygen. Changing from NH3 to O2, Co(II) can be oxidized to Co(III) at NH3/O2 = 1:1 at 673 K, suggesting that O vacancies are more likely to be refilled at high temperatures. This explains that N2O starts to decompose only on the surface of Co3O4 at high temperatures. CuO–Co3O4 catalysts were found to be more active for N2O decomposition (Figure 2a). Hence, the promotion of CuO in CuO–Co3O4 catalysts was followed by in situ XAFS investigations.
The cleavage of the N–O bond of N2O requires the catalyst to directly extract the O in the N2O molecule, which means that the redox of the catalyst is involved. Electron donation from Co(II) to N2O results in the formation of Co(III) species, while Co(III) can be reduced back to Co(II) by NH3. Pure CuO cannot effectively decompose N2O, but the addition of CuO into Co3O4 significantly increases the N2O decomposition ability. Therefore, both the redox of Co and Cu are crucial for the removal of N2O, which were studied by in situ XAFS at Co K and Cu K edges of Co3O4 and 90CuCo (for the spectra, see Figures S24–S29). The linear combination fitting (LCF) is used to quantify the oxidized and reduced Co/Cu species. At 573 K, bulk CuO can be partially reduced under NH3 (Figure S28), while bulk Co3O4 in Co3O4 and 90CuCo cannot be reduced (Figures S24 and S25), indicating the better reducibility of CuO. Increasing temperature to 673 K, the Cu shows a dramatic reduction to Cu2O from O2 to NH3 (Figure S29), whereas 20% of Co3O4 is reduced to CoO (Figure S27). Further reduction is observed under NO + NH3 conditions, with metallic Cu and more CoO formation. Co in bulk Co3O4 has similar reduction behavior compared to 90CuCo. Changing from reduction conditions to NH3 + O2 conditions, both Co and Cu are in the oxidized form at 573 K and are only slightly reduced at 673 K, while the Co(II) in Co3O4 remains stable (Figure 6a). The change of oxidation states of Cu consists well with the change of oxidation states of Co, suggesting the formation of Co(II)–Vo–Cu(I), which can dissociate the N–O bond (eq 8).
| 8 |
| 9 |
Figure 6.
In situ XAFS study of Co3O4 and 90CuCo. (a) Co K edge XANES spectra of Co3O4 and 90CuCo under NH3 + NO and NH3 + O2 conditions at 673 K. (b) Time-resolved Cu profile when changing from oxidative to reductive conditions and back to oxidative conditions for 90CuCo at 673 K. (c) Time-resolved Co profile when changing from oxidative to reductive conditions and back to oxidative conditions for Co3O4 at 673 K. (d) Time-resolved Co profile when changing from oxidative to reductive conditions and back to oxidative conditions for 90CuCo at 673 K.
The Co and Cu reduction/oxidation rates can be determined with time-resolved XAFS. The samples were diluted with boron nitride to ensure that the amount of Co was similar for both samples when measuring the Co K edge. The spectra were recorded every 150 s while the catalytic activity was simultaneously monitored by an online mass spectrometer connected to the outlet of in situ tube (Figures S30 and S31). The LCF analysis of Co K edge XANES under reduction conditions shows a trend similar to those of Co3O4 and 90CuCo samples regarding the fast reduction of CuO (Figure 6b). In the oxidation process, only 4% CoO is oxidized to Co3O4 for pure Co3O4 in 300 s (Figure 6c). In comparison, the Co in 90CuCo has a faster oxidation rate, with oxidation of 31% CoO to Co3O4 in the same time (Figure 6d). The higher Cu reduction/oxidation rate suggests that Cu acts as the catalyst or promoter for Co(II) oxidation, which is then important for N2O removal. As a result, the interface of CuO–Co3O4 is the active site for the dissociation of N2O (eq 8 and 9).
To summarize the in situ studies and reaction profiles, the activation of gaseous O2 over the Co3O4 surface is the key to the NH3 conversion and N2O formation. The interface of CuO–Co3O4 has an enhanced redox ability, promoting the dissociation of N2O at high temperatures. The reactive O produced by N2O cleavage in turn contributes to the oxidation of NH3. Controlling the ratio of CuO and Co3O4 on the surface can modulate the rates of N2O formation and N2O removal, thus regulating product selectivity. In this case, 90CuCo is found to be optimal.
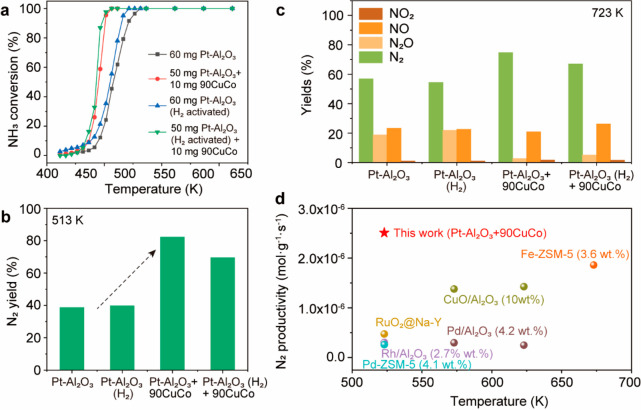
In light of the excellent N2O elimination ability of CuO–Co3O4 catalysts, we hypothesize that adding 90CuCo into the 1 wt % Pt–Al2O3 catalyst will inhibit the release of N2O and improve N2 selectivity at high temperatures. To prove it, the catalytic performance of 60 mg of Pt–Al2O3, 60 mg of Pt–Al2O3 (H2-activated), and 50 mg of Pt–Al2O3 + 10 mg of 90CuCo catalysts were compared (for 5000 ppm of NH3–SCO). The Pt–Al2O3 and 90CuCo were physically mixed. After the 50 mg of Pt/Al2O3 and 10 mg of 90CuCo were weighed, the two powders were ground in a mortar and pestle for a few minutes until 90CuCo was uniformly dispersed. The addition of just 10 mg of 90CuCo shifted the conversion to the low-temperature region by 50 K (Figure 7a). H2 activation slightly improves the activity of Pt–Al2O3, while the activity is still below that of Pt–Al2O3 + 90CuCo. With the addition of 90CuCo, complete NH3 conversion is achieved at 513 K, resulting in an increase in the N2 yield of approximately 40% (Figure 7b). At 723 K, 75% N2 selectivity is achieved after adding 90CuCo, which is much higher than that of pure Pt–Al2O3 (55%) and H2-activated Pt–Al2O3 (53%). The NO and NO2 yields are similar after adding 90CuCo, while the release of N2O is significantly suppressed (Figure 7c). N2O-TPD demonstrated that 90CuCo adsorbs N2O more readily than Pt/Al2O3 (Figure S32). Therefore, N2O released from the Pt/Al2O3 surface can be adsorbed and decomposed by 90CuCo. These results suggest that 90CuCo can enhance the low-temperature activity and help to remove N2O at high temperatures. Comparing the performance of the Pt–Al2O3 + 90CuCo with literatures,69−72 we achieved at least five times higher N2 productivity than other noble metal systems at 523 K (Figure 7d). Herein, the presented CuO–Co3O4 system provides a new strategy for increasing activity with the inhibited release of N2O in the selective NH3 oxidation.
Figure 7.
Catalysis performance of Pt–Al2O3 + 90CuCo catalysts. (a) NH3 conversion as the function of temperature. (b) N2 yield for 60 mg of 1 wt % Pt–Al2O3, 60 mg of 1 wt % Pt–Al2O3 (H2-activation), 50 mg of 1 wt % Pt–Al2O3 + 10 mg of 90CuCo, and 50 mg of 1 wt % Pt–Al2O3 (H2-activation) + 10 mg of 90CuCo catalysts at 513 K. (c) Yields of N2, N2O, NO, and NO2 for 60 mg of 1 wt % Pt–Al2O3, 50 mg of 1 wt % Pt–Al2O3 + 10 mg of 90CuCo, and 50 mg of 1 wt % Pt–Al2O3 (H2-activation) + 10 mg of 90CuCo catalysts at 723 K. (d) Comparison of N2 productivity for 50 mg of 1 wt % Pt–Al2O3 + 10 mg of 90CuCo with other catalysts. Reaction conditions are as follows: Tbed = 423–723 K, mcat = 60 mg, 5000 ppm of NH3, 5% O2 balanced in He, gas flow of 100 mL·min–1, and WHSV = 500 mLNH3·h–1·g–1.
4. Conclusions
Our work studied the rarely reported N2–N2O–N2 formation as a function of temperature in the selective ammonia oxidation reaction. In general, the selectivity of N2 decreases with increasing temperature, forming byproducts N2O or NO. To mitigate the NOx formation, CuO–Co3O4 catalysts can convert the as-formed N2O to N2 due to the formation of the Co(II)–VO–Cu(I) intermediate species, as proven in the in situ XAFS/NEXAFS studies. The O2 activation ability of Co3O4 is the key to the formation of N2O, while the redox of CuO–Co3O4 interfaces promotes the dissociation of the N–O bond of N2O, forming N2 and active O species. Here, we proposed a de-N2O mechanism for N2 formation over such a bifunctional surface. The rate of N2O generation and decomposition can be controlled by adjusting the ratio of surface CuO and Co3O4, thereby changing the product selectivity. 90 wt % CuO–Co3O4 has similar N2O generation and conversion rates. A 120 K operation window (N2 yield >90%) from 553 to 673 K is achieved, which is wider than most of the literature. This ability to remove N2O at high temperatures can be further exploited to reduce the N2O emissions from noble metal catalysts. Adding 90 wt % CuO–Co3O4 catalyst to Pt–Al2O3 can effectively improve the low-temperature activity and reduce the selectivity of N2O by 17% at high temperatures. This work develops a solution to address N2O release and provides a fundamental understanding toward the formation and removal of N2O for selective oxidation reactions in the chemical industry.
Acknowledgments
We acknowledge Diamond Light Source beamtime (MG23759, MG24450, and SI29094). We thank the Energy Materials Block Allocation Group SP14239. We acknowledge SPring-8 for the XAFS experiments conducted under the proposal 2021A1695. We acknowledge Helmholz-Zentrum Berlin for the beamtime in BESSY II (201-09217-ST). We are particularly grateful to Dr. Michael Haevecker for his help with the NAP-NEXAFS experi-ment in BESSY. The authors would like to thank the Research Complex for access and support to these facilities and equip-ment. X.G. would like to thank the China Scholarship Council (CSC) for the Ph.D. funding.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.3c02392.
NAP-NEXAFS spectra, DFT calculation results, HAADF-STEM images, XRD, Cu K edge and Co K edge XANES spectra. k3-weighted EXAFS oscillations, EXAFS fit parameters, reaction profiles, NH3–SCO performances of catalysts in the literature, and in situ XAFS spectra (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
The project is funded by EPSRC (EP/P02467X/1 and EP/S018204/2), Royal Society (RG160661, IES\R3\170097, IES\R1\191035 and IEC\R3\193038), and RS-JSPS funding.
The authors declare no competing financial interest.
Supplementary Material
References
- Becher J.; Sanchez D. F.; Doronkin D. E.; Zengel D.; Meira D. M.; Pascarelli S.; Grunwaldt J.-D.; Sheppard T. L. Chemical gradients in automotive Cu-SSZ-13 catalysts for NOx removal revealed by operando X-ray spectrotomography. Nat. Catal. 2021, 4 (1), 46–53. 10.1038/s41929-020-00552-3. [DOI] [Google Scholar]
- Marberger A.; Petrov A. W.; Steiger P.; Elsener M.; Krocher O.; Nachtegaal M.; Ferri D. Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1 (3), 221–227. 10.1038/s41929-018-0032-6. [DOI] [Google Scholar]
- Hu W.; Selleri T.; Gramigni F.; Fenes E.; Rout K. R.; Liu S.; Nova I.; Chen D.; Gao X.; Tronconi E. On the Redox Mechanism of Low-Temperature NH3-SCR over Cu-CHA: A Combined Experimental and Theoretical Study of the Reduction Half Cycle. Angew. Chem., Int. Ed. 2021, 60 (13), 7197–7204. 10.1002/anie.202014926. [DOI] [PubMed] [Google Scholar]
- Jablonska M.; Beale A. M.; Nocun M.; Palkovits R. Ag-Cu based catalysts for the selective ammonia oxidation into nitrogen and water vapour. Appl. Catal., B 2018, 232, 275–287. 10.1016/j.apcatb.2018.03.029. [DOI] [Google Scholar]
- Fung W. K.; Ledwaba L.; Modiba N.; Claeys M.; van Steen E. Choosing a suitable support for Co3O4 as an NH3 oxidation catalyst. Catal. Sci. Technol. 2013, 3 (8), 1905–1909. 10.1039/c3cy00041a. [DOI] [Google Scholar]
- Gao F. Y.; Liu Y. Y.; Sani Z.; Tang X. L.; Yi H. H.; Zhao S. Z.; Yu Q. J.; Zhou Y. S. Advances in selective catalytic oxidation of ammonia (NH3-SCO) to dinitrogen in excess oxygen: A review on typical catalysts, catalytic performances and reaction mechanisms. Journal of Environmental Chemical Engineering 2021, 9 (1), 104575 10.1016/j.jece.2020.104575. [DOI] [Google Scholar]
- Fung W. K.; Claeys M.; van Steen E. Effective Utilization of the Catalytically Active Phase: NH3 Oxidation Over Unsupported and Supported Co3O4. Catal. Lett. 2012, 142 (4), 445–451. 10.1007/s10562-012-0790-8. [DOI] [Google Scholar]
- Petryk J.; Kolakowska E. Cobalt oxide catalysts for ammonia oxidation activated with cerium and lanthanum. Appl. Catal., B 2000, 24 (2), 121–128. 10.1016/S0926-3373(99)00099-5. [DOI] [Google Scholar]
- Zabilskiy M.; Djinovic P.; Erjavec B.; Drazic G.; Pintar A. Small CuO clusters on CeO2 nanospheres as active species for catalytic N2O decomposition. Appl. Catal., B 2015, 163, 113–122. 10.1016/j.apcatb.2014.07.057. [DOI] [Google Scholar]
- Arakawa H.; Aresta M.; Armor J. N.; Barteau M. A.; Beckman E. J.; Bell A. T.; Bercaw J. E.; Creutz C.; Dinjus E.; Dixon D. A.; Domen K.; DuBois D. L.; Eckert J.; Fujita E.; Gibson D. H.; Goddard W. A.; Goodman D. W.; Keller J.; Kubas G. J.; Kung H. H.; Lyons J. E.; Manzer L. E.; Marks T. J.; Morokuma K.; Nicholas K. M.; Periana R.; Que L.; Rostrup-Nielson J.; Sachtler W. M. H.; Schmidt L. D.; Sen A.; Somorjai G. A.; Stair P. C.; Stults B. R.; Tumas W. Catalysis research of relevance to carbon management: Progress, challenges, and opportunities. Chem. Rev. 2001, 101 (4), 953–996. 10.1021/cr000018s. [DOI] [PubMed] [Google Scholar]
- Becker K. H.; Lorzer J. C.; Kurtenbach R.; Wiesen P.; Jensen T. E.; Wallington T. J. Nitrous oxide (N2O) emissions from vehicles. Environ. Sci. Technol. 1999, 33 (22), 4134–4139. 10.1021/es9903330. [DOI] [Google Scholar]
- Konsolakis M. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015, 5 (11), 6397–6421. 10.1021/acscatal.5b01605. [DOI] [Google Scholar]
- Marberger A.; Ferri D.; Elsener M.; Kröcher O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium-Based Catalysts. Angew. Chem., Int. Ed. 2016, 55 (39), 11989–11994. 10.1002/anie.201605397. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Lai J.-K.; Wachs I. E. Formation of N2O greenhouse gas during SCR of NO with NH3 by supported vanadium oxide catalysts. Applied Catalysis B: Environmental 2018, 224, 836–840. 10.1016/j.apcatb.2017.11.029. [DOI] [Google Scholar]
- Yates M.; Martín J. A.; Martín-Luengo M. Á.; Suárez S.; Blanco J. N2O formation in the ammonia oxidation and in the SCR process with V2O5-WO3 catalysts. Catal. Today 2005, 107–108, 120–125. 10.1016/j.cattod.2005.07.015. [DOI] [Google Scholar]
- Pérez-Ramírez J.; Kondratenko E. V. Evidences of the origin of N2O in the high-temperature NH3 oxidation over Pt–Rh gauze. Chem. Commun. 2004, (4), 376–377. 10.1039/B312685D. [DOI] [PubMed] [Google Scholar]
- Wang D.; Yao Q.; Mou C.; Hui S.; Niu Y. New insight into N2O formation from NH3 oxidation over MnOx/TiO2 catalyst. Fuel 2019, 254, 115719 10.1016/j.fuel.2019.115719. [DOI] [Google Scholar]
- Dann E. K.; Gibson E. K.; Blackmore R. H.; Catlow C. R. A.; Collier P.; Chutia A.; Erden T. E.; Hardacre C.; Kroner A.; Nachtegaal M.; Raj A.; Rogers S. M.; Taylor S. F. R.; Thompson P.; Tierney G. F.; Zeinalipour-Yazdi C. D.; Goguet A.; Wells P. P. Structural selectivity of supported Pd nanoparticles for catalytic NH3 oxidation resolved using combined operando spectroscopy. Nat. Catal. 2019, 2 (2), 157–163. 10.1038/s41929-018-0213-3. [DOI] [Google Scholar]
- Svintsitskiy D. A.; Kibis L. S.; Stadnichenko A. I.; Slavinskaya E. M.; Romanenko A. V.; Fedorova E. A.; Stonkus O. A.; Doronkin D. E.; Marchuk V.; Zimina A.; Casapu M.; Grunwaldt J. D.; Boronin A. I. Insight into the Nature of Active Species of Pt/Al2O3 Catalysts for low Temperature NH3 Oxidation. ChemCatChem. 2020, 12 (3), 867–880. 10.1002/cctc.201901719. [DOI] [Google Scholar]
- Sobczyk D. P.; de Jong A. M.; Hensen E. J. M.; van Santen R. A. Activation of ammonia dissociation by oxygen on platinum sponge studied with positron emission profiling. J. Catal. 2003, 219 (1), 156–166. 10.1016/S0021-9517(03)00191-X. [DOI] [Google Scholar]
- An Q.; Xu G.; Liu J.; Wang Y.; Yu Y.; He H. Designing a Bifunctional Pt/Cu-SSZ-13 Catalyst for Ammonia-Selective Catalytic Oxidation with Superior Selectivity. ACS Catal. 2023, 13 (10), 6851–6861. 10.1021/acscatal.3c01322. [DOI] [Google Scholar]
- Liu Y.; Liu Z.; Wang C.; Xu J.; Ai J.; Liu X.; Zhang A.; Zhao Y.; Du C.; Shan B. Unraveling the Lattice O Assisted Internal Selective Catalytic Reduction Mechanism on High N2 Selectivity of CuOx/PtCu Catalysts in NH3-SCO. ACS Catal. 2023, 13 (11), 7178–7188. 10.1021/acscatal.3c00314. [DOI] [Google Scholar]
- Gang L.; Anderson B. G.; van Grondelle J.; van Santen R. A. Intermediate species and reaction pathways for the oxidation of ammonia on powdered catalysts. J. Catal. 2001, 199 (1), 107–114. 10.1006/jcat.2000.3154. [DOI] [Google Scholar]
- Chen W. H.; Shen Q. T.; Bartynski R. A. Selective Oxidation of Ammonia by Co-adsorbed Oxygen on Iridium Surfaces: Formation of N2O. Catal. Lett. 2015, 145 (3), 757–761. 10.1007/s10562-014-1468-1. [DOI] [Google Scholar]
- Gong J. L.; Ojifinni R. A.; Kim T. S.; White J. M.; Mullins C. B. Selective catalytic oxidation of ammonia to nitrogen on atomic oxygen precovered Au(111). J. Am. Chem. Soc. 2006, 128 (28), 9012–9013. 10.1021/ja062624w. [DOI] [PubMed] [Google Scholar]
- Karatok M.; Vovk E. I.; Koc A. V.; Ozensoy E. Selective Catalytic Ammonia Oxidation to Nitrogen by Atomic Oxygen Species on Ag(111). J. Phys. Chem. C 2017, 121 (41), 22985–22994. 10.1021/acs.jpcc.7b08291. [DOI] [Google Scholar]
- Wang H. M.; Zhang Q. L.; Zhang T. X.; Wang J. F.; Wei G. C.; Liu M.; Ning P. Structural tuning and NH3-SCO performance optimization of CuO-Fe2O3 catalysts by impact of thermal treatment. Appl. Surf. Sci. 2019, 485, 81–91. 10.1016/j.apsusc.2019.04.196. [DOI] [Google Scholar]
- Jablonska M.; Palkovits R. Copper based catalysts for the selective ammonia oxidation into nitrogen and water vapour-Recent trends and open challenges. Appl. Catal., B 2016, 181, 332–351. 10.1016/j.apcatb.2015.07.017. [DOI] [Google Scholar]
- Wang Z.; Qu Z. P.; Quan X.; Li Z.; Wang H.; Fan R. Selective catalytic oxidation of ammonia to nitrogen over CuO-CeO2 mixed oxides prepared by surfactant-templated method. Appl. Catal., B 2013, 134, 153–166. 10.1016/j.apcatb.2013.01.029. [DOI] [Google Scholar]
- Kusar H. M. J.; Ersson A. G.; Vosecky M.; Jaras S. G. Selective catalytic oxidation of NH3 to N-2 for catalytic combustion of low heating value gas under lean/rich conditions. Appl. Catal., B 2005, 58 (1–2), 25–32. 10.1016/j.apcatb.2004.02.020. [DOI] [Google Scholar]
- Chmielarz L.; Kustrowski P.; Rafalska-Lasocha A.; Dziembaj R. Selective oxidation of ammonia to nitrogen on transition metal containing mixed metal oxides. Appl. Catal., B 2005, 58 (3–4), 235–244. 10.1016/j.apcatb.2004.12.009. [DOI] [Google Scholar]
- Sun H.; Wang H.; Qu Z. Construction of CuO/CeO2 Catalysts via the Ceria Shape Effect for Selective Catalytic Oxidation of Ammonia. ACS Catal. 2023, 13 (2), 1077–1088. 10.1021/acscatal.2c05168. [DOI] [Google Scholar]
- Zhang Q. L.; Wang H. M.; Ning P.; Song Z. X.; Liu X.; Duan Y. K. In situ DRIFTS studies on CuO-Fe2O3 catalysts for low temperature selective catalytic oxidation of ammonia to nitrogen. Appl. Surf. Sci. 2017, 419, 733–743. 10.1016/j.apsusc.2017.05.056. [DOI] [Google Scholar]
- Long R. Q.; Yang R. T. Selective catalytic oxidation of ammonia to nitrogen over Fe2O3-TiO2 prepared with a sol-gel method. J. Catal. 2002, 207 (2), 158–165. 10.1006/jcat.2002.3545. [DOI] [Google Scholar]
- Lee S. M.; Hong S. C. Promotional effect of vanadium on the selective catalytic oxidation of NH3 to N-2 over Ce/V/TiO2 catalyst. Appl. Catal., B 2015, 163, 30–39. 10.1016/j.apcatb.2014.07.043. [DOI] [Google Scholar]
- Slavinskaya E. M.; Kibis L. S.; Stonkus O. A.; Svintsitskiy D. A.; Stadnichenko A. I.; Fedorova E. A.; Romanenko A. V.; Marchuk V.; Doronkin D. E.; Boronin A. I. The Effects of Platinum Dispersion and Pt State on Catalytic Properties of Pt/Al2O3 in NH3 Oxidation. ChemCatChem. 2021, 13 (1), 313–327. 10.1002/cctc.202001320. [DOI] [Google Scholar]
- Ravel B.; Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrot. Radiat. 2005, 12, 537–541. 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- Smidstrup S.; Markussen T.; Vancraeyveld P.; Wellendorff J.; Schneider J.; Gunst T.; Verstichel B.; Stradi D.; Khomyakov P. A.; Vej-Hansen U. G.; Lee M. E.; Chill S. T.; Rasmussen F.; Penazzi G.; Corsetti F.; Ojanpera A.; Jensen K.; Palsgaard M. L. N.; Martinez U.; Blom A.; Brandbyge M.; Stokbro K. QuantumATK: an integrated platform of electronic and atomic-scale modelling tools. J. Phys.: Condens. Matter 2020, 32 (1), 015901. 10.1088/1361-648X/ab4007. [DOI] [PubMed] [Google Scholar]
- Blöchl P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50 (24), 17953–17979. 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- Niu H.; Wang X.; Shao C.; Liu Y.; Zhang Z.; Guo Y. Revealing the oxygen reduction reaction activity origin of single atoms supported on g-C3N4 monolayers: a first-principles study. J. Mater. Chem. A 2020, 8 (14), 6555–6563. 10.1039/D0TA00794C. [DOI] [Google Scholar]
- Kaptagay G. A.; Inerbaev T. M.; Mastrikov Y. A.; Kotomin E. A.; Akilbekov A. T. Water interaction with perfect and fluorine-doped Co3O4 (100) surface. Solid State Ion. 2015, 277, 77–82. 10.1016/j.ssi.2015.03.012. [DOI] [Google Scholar]
- Kaewmaraya T.; Ngamwongwan L.; Moontragoon P.; Karton A.; Hussain T. Drastic Improvement in Gas-Sensing Characteristics of Phosphorene Nanosheets under Vacancy Defects and Elemental Functionalization. J. Phys. Chem. C 2018, 122 (35), 20186–20193. 10.1021/acs.jpcc.8b06803. [DOI] [Google Scholar]
- Hu L.; Peng Q.; Li Y. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130 (48), 16136–16137. 10.1021/ja806400e. [DOI] [PubMed] [Google Scholar]
- Xie X.; Li Y.; Liu Z.-Q.; Haruta M.; Shen W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458 (7239), 746–749. 10.1038/nature07877. [DOI] [PubMed] [Google Scholar]
- Guan X.; Han R.; Asakura H.; Wang Z.; Xu S.; Wang B.; Kang L.; Liu Y.; Marlow S.; Tanaka T.; Guo Y.; Wang F. R. Designing Reactive Bridging O2– at the Atomic Cu–O–Fe Site for Selective NH3 Oxidation. ACS Catal. 2022, 12, 15207–15217. 10.1021/acscatal.2c04863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frati F.; Hunault M. O. J. Y.; de Groot F. M. F. Oxygen K-edge X-ray Absorption Spectra. Chem. Rev. 2020, 120 (9), 4056–4110. 10.1021/acs.chemrev.9b00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckman M. W.; Chen J.; Qiu S. L.; Kuiper P.; Strongin M.; Dunlap B. I. Interpreting the near edges of ${\mathrm{O}}_{2}$ and ${\mathrm{O}}_{2}∧{\mathrm{\ensuremath{-}}}$ in alkali-metal superoxides. Phys. Rev. Lett. 1991, 67 (18), 2533–2536. 10.1103/PhysRevLett.67.2533. [DOI] [PubMed] [Google Scholar]
- Guzman J.; Carrettin S.; Corma A. Spectroscopic Evidence for the Supply of Reactive Oxygen during CO Oxidation Catalyzed by Gold Supported on Nanocrystalline CeO2. J. Am. Chem. Soc. 2005, 127 (10), 3286–3287. 10.1021/ja043752s. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhao S.; Bi F.; Chen J.; Li Y.; Cui L.; Xu J.; Zhang X. Oxygen-vacancy-induced O2 activation and electron-hole migration enhance photothermal catalytic toluene oxidation. Cell Reports Physical Science 2022, 3 (8), 101011 10.1016/j.xcrp.2022.101011. [DOI] [Google Scholar]
- Sun X.; Luo X.; Zhang X.; Xie J.; Jin S.; Wang H.; Zheng X.; Wu X.; Xie Y. Enhanced Superoxide Generation on Defective Surfaces for Selective Photooxidation. J. Am. Chem. Soc. 2019, 141 (9), 3797–3801. 10.1021/jacs.8b13051. [DOI] [PubMed] [Google Scholar]
- Xie S.; Liu Y.; Deng J.; Yang J.; Zhao X.; Han Z.; Zhang K.; Dai H. Insights into the active sites of ordered mesoporous cobalt oxide catalysts for the total oxidation of o-xylene. J. Catal. 2017, 352, 282–292. 10.1016/j.jcat.2017.05.016. [DOI] [Google Scholar]
- Li Y.-F.; Aschauer U.; Chen J.; Selloni A. Adsorption and Reactions of O2 on Anatase TiO2. Acc. Chem. Res. 2014, 47 (11), 3361–3368. 10.1021/ar400312t. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Peng Y.; Naschitzki M.; Gewinner S.; Schöllkopf W.; Kuhlenbeck H.; Pentcheva R.; Roldan Cuenya B. Surface oxygen Vacancies on Reduced Co3O4(100): Superoxide Formation and Ultra-Low-Temperature CO Oxidation. Angew. Chem., Int. Ed. 2021, 60 (30), 16514–16520. 10.1002/anie.202103359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley A. F.; Davies P. R.; Roberts M. W.; Yan S. Oxygen states present at a Ag(111) surface in the presence of ammonia: evidence for a NH3–O2δ− complex. Chem. Commun. 1998, (1), 35–36. 10.1039/a707124h. [DOI] [Google Scholar]
- Han F.; Yuan M. Q.; Mine S.; Sun H.; Chen H. J.; Toyao T.; Matsuoka M.; Zhu K. K.; Zhang J. L.; Wang W. C.; Xue T. Formation of Highly Active Superoxide Sites on CuO Nanoclusters Encapsulated in SAPO-34 for Catalytic Selective Ammonia Oxidation. ACS Catal. 2019, 9 (11), 10398–10408. 10.1021/acscatal.9b02975. [DOI] [Google Scholar]
- Zawadzki J. THE MECHANISM OF AMMONIA OXIDATION AND CERTAIN ANALOGOUS REACTIONS. Discuss. Faraday Soc. 1950, 8, 140–152. 10.1039/df9500800140. [DOI] [Google Scholar]
- Otto K.; Shelef M.; Kummer J. T. STUDIES OF SURFACE REACTIONS OF NITRIC OXIDE BY N-15 ISOTOPE LABELING. 1. REACTION BETWEEN NITRIC OXIDE AND AMMONIA OVER SUPPORTED PLATINUM AT 200–250 DEGREES. J. Phys. Chem. 1970, 74 (13), 2690–2698. 10.1021/j100707a017. [DOI] [Google Scholar]
- Gaur A.; Klysubun W.; Nair N. N.; Shrivastava B. D.; Prasad J.; Srivastava K. XAFS study of copper(II) complexes with square planar and square pyramidal coordination geometries. J. Mol. Struct. 2016, 1118, 212–218. 10.1016/j.molstruc.2016.04.008. [DOI] [Google Scholar]
- Kang L.; Wang B.; Bing Q.; Zalibera M.; Büchel R.; Xu R.; Wang Q.; Liu Y.; Gianolio D.; Tang C. C.; Gibson E. K.; Danaie M.; Allen C.; Wu K.; Marlow S.; Sun L.-d.; He Q.; Guan S.; Savitsky A.; Velasco-Vélez J. J.; Callison J.; Kay C. W. M.; Pratsinis S. E.; Lubitz W.; Liu J.-y.; Wang F. R. Adsorption and activation of molecular oxygen over atomic copper(I/II) site on ceria. Nat. Commun. 2020, 11, 4008 10.1038/s41467-020-17852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. H.; Chen H.; Yang J.; Ma Y. Y.; Zhang Q. C.; Kou Z. K.; Ding X. Y.; Pang Y. J.; Zhang L.; Gu Q. L.; Yan C. L.; Wang J. CuCo2S4 Nanosheets@N-Doped Carbon Nanofibers by Sulfurization at Room Temperature as Bifunctional Electrocatalysts in Flexible Quasi-Solid-State Zn-Air Batteries. Adv. Sci. 2019, 6 (17), 1900628 10.1002/advs.201900628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G.; Liu Z.; Zhu Z.; Liu Q.; Ge J.; Huang Z. Simultaneous removal of SO2 and NOx from flue gas using a CuO/Al2O3 catalyst sorbent: II. Promotion of SCR activity by SO2 at high temperatures. J. Catal. 2004, 224 (1), 42–49. 10.1016/j.jcat.2004.02.016. [DOI] [Google Scholar]
- Liu J.; Shi X.; Shan Y.; Yan Z.; Shan W.; Yu Y.; He H. Hydrothermal Stability of CeO2–WO3–ZrO2 Mixed Oxides for Selective Catalytic Reduction of NOx by NH3. Environ. Sci. Technol. 2018, 52 (20), 11769–11777. 10.1021/acs.est.8b03732. [DOI] [PubMed] [Google Scholar]
- Chen L.; Si Z.; Wu X.; Weng D. DRIFT Study of CuO–CeO2–TiO2Mixed Oxides for NOx Reduction with NH3 at Low Temperatures. ACS Appl. Mater. Interfaces 2014, 6 (11), 8134–8145. 10.1021/am5004969. [DOI] [PubMed] [Google Scholar]
- Baek J. H.; Lee S. M.; Park J. H.; Jeong J. M.; Hwang R. H.; Ko C. H.; Jeon S. G.; Choi T. H.; Yi K. B. Effects of steam introduction on deactivation of Fe-BEA catalyst in NH3-SCR of N2O and NO. Journal of Industrial and Engineering Chemistry 2017, 48, 194–201. 10.1016/j.jiec.2017.01.002. [DOI] [Google Scholar]
- Lukashuk L.; Yigit N.; Rameshan R.; Kolar E.; Teschner D.; Hävecker M.; Knop-Gericke A.; Schlögl R.; Föttinger K.; Rupprechter G. Operando Insights into CO Oxidation on Cobalt Oxide Catalysts by NAP-XPS, FTIR, and XRD. ACS Catal. 2018, 8 (9), 8630–8641. 10.1021/acscatal.8b01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins R. A.; Mohammed K.; Sullivan D. A. Pressure and Density Series Equations of State for Steam as Derived from the Haar–Gallagher–Kell Formulation. J. Phys. Chem. Ref. Data 1988, 17 (1), 1–8. 10.1063/1.555819. [DOI] [Google Scholar]
- Franken T.; Palkovits R. Investigation of potassium doped mixed spinels CuxCo3–xO4 as catalysts for an efficient N2O decomposition in real reaction conditions. Applied Catalysis B: Environmental 2015, 176–177, 298–305. 10.1016/j.apcatb.2015.04.002. [DOI] [Google Scholar]
- Richards N.; Carter J. H.; Parker L. A.; Pattisson S.; Hewes D. G.; Morgan D. J.; Davies T. E.; Dummer N. F.; Golunski S.; Hutchings G. J. Lowering the Operating Temperature of Perovskite Catalysts for N2O Decomposition through Control of Preparation Methods. ACS Catal. 2020, 10 (10), 5430–5442. 10.1021/acscatal.0c00698. [DOI] [Google Scholar]
- Heylen S.; Delcour N.; Kirschhock C. E. A.; Martens J. A. Selective Catalytic Oxidation of Ammonia into Dinitrogen over a Zeolite-Supported Ruthenium Dioxide Catalyst. ChemCatChem. 2012, 4 (8), 1162–1166. 10.1002/cctc.201100489. [DOI] [Google Scholar]
- Li Y. J.; Armor J. N. Selective NH3 oxidation to N-2 in a wet stream. Appl. Catal., B 1997, 13 (2), 131–139. 10.1016/S0926-3373(96)00098-7. [DOI] [Google Scholar]
- Gang L.; Anderson B. G.; van Grondelle J.; van Santen R. A.; van Gennip W. J. H.; Niemantsverdriet J. W.; Kooyman P. J.; Knoester A.; Brongersma H. H. Alumina-supported Cu-Ag catalysts for ammonia oxidation to nitrogen at low temperature. J. Catal. 2002, 206 (1), 60–70. 10.1006/jcat.2001.3470. [DOI] [Google Scholar]
- Qi G. S.; Gatt J. E.; Yang R. T. Selective catalytic oxidation (SCO) of ammonia to nitrogen over Fe-exchanged zeolites prepared by sublimation of FeCl3. J. Catal. 2004, 226 (1), 120–128. 10.1016/j.jcat.2004.05.023. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.