Abstract

Extensive research has used dimethylarsinic acid (DMA) in urine as a marker of arsenic methylation. The premise is that humans methylate inorganic arsenicals to monomethylarsonic acid (MMA) and DMA and excrete these arsenic species into the urine. However, DMA in urine not only comes from the methylation of inorganic arsenic but also could be a result of metabolism of other arsenic species, such as arsenosugars and arsenolipids. Most environmental health and epidemiological studies of arsenic methylation might have overlooked confounding factors that contribute to DMA in urine. Here we critically evaluate reported studies that used methylation indexes, concentration ratios of methylated arsenicals, or the percentage of DMA in urine as markers of arsenic methylation efficiency. Dietary intake of arsenosugars potentially confounds the calculation and interpretation of the arsenic methylation efficiencies. Many studies have not considered incidental dietary intake of arsenosugars, arsenolipids, and other organic arsenic species. Future studies should consider the dietary intake of diverse arsenic species and their potential effect on the urinary concentrations of DMA.
Keywords: arsenic exposure, arsenosugars, arsenolipids, confounding, dimethylarsinic acid, metabolism, seafood consumption, speciation, urine analysis
Introduction
Inorganic arsenic has been consistently ranked the first on the contaminants priority list of the Agency of Toxic Substances and Diseases Registry (ATSDR), including the most recent 2022 report.1 Chronic exposure to inorganic arsenic is known to increase risks of skin lesions, cardiovascular diseases, diabetes, and many types of cancer, such as cancers of the bladder, lungs, and skin.1−3 Severity of health effect strongly varies between individuals, which could be related to interindividual differences in arsenic metabolism.4−8
Inorganic arsenate (iAsV) and arsenite (iAsIII) can be transformed in humans through a series of reduction and oxidative-methylation reactions, producing monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA).9−13 Efficiency of arsenic methylation can be estimated using concentrations of individual arsenic species (DMA, MMA, iAsV, and iAsIII) in urine. Concentration ratios and percentages of methylated arsenic species are often calculated from the urinary arsenic speciation data. These methylation efficiency parameters are then used to explore relationships between arsenic metabolism and the health outcomes of arsenic exposure.14−17 Arsenic methylation efficiency parameters have also been used to assess how arsenic metabolism is affected by lifestyle and other factors.18−21 The interpretation and conclusion of these relationships rely on the accurate determination of methylation efficiency parameters. Although proper estimation of exposure to inorganic arsenic has received much attention in recent decades, uncertainties and confounding related to assessing methylation efficiency have rarely been discussed.
In this Perspective, we first provide a brief overview of inorganic arsenic metabolism, approaches of methylation efficiency calculations, and potential exposure to various arsenic species (Supporting Information Figure S1). Our main goal is to highlight importance of considering exposure to complex organic arsenicals and potential effect of such exposure on the accuracy of methylation efficiency estimations.
Metabolism of Inorganic Arsenic
Mechanisms of arsenic biotransformation in the human body are not fully understood7 although the discovery and identification of the arsenic +3 methyltransferase (As3MT) enzyme have significantly advanced the field.22,23 Current understanding of inorganic arsenic (iAs) metabolism is also based on the arsenic metabolites detected in human urine, arsenic metabolites produced by various organisms, such as bacteria, fungi, cell lines, and animal species.8,13,24,25 Structural elucidation of trivalent arsenic complex with As3MT has also contributed to the understanding of inorganic arsenic methylation.26
The first model of arsenic metabolism was proposed by Frederick Challenger based on the observation of trimethylarsine (TMA) as a metabolite of iAs in fungi.9 According to the Challenger pathway, pentavalent iAsV is first reduced to trivalent arsenic iAsIII. The trivalent arsenic species accepts a positively charged methyl group (CH3+) from a methyl donor, forming pentavalent monomethylarsonic acid (MMAV). The methyl donor was later established as S-adenosyl methionine (SAM). MMAV also goes through the reduction and oxidative methylation cycle to form dimethylarsinic acid (DMAV). Further reduction of DMAV and the addition of a third methyl group yields trimethylarsine oxide (TMAO), which then is reduced to trimethylarsine (TMA) (Figure 1a).
Figure 1.
Simplified schemes showing the stepwise methylation of inorganic arsenic species to monomethyl, dimethyl, and trimethyl arsenicals. AsIII(GS)3 denotes arsenic glutathione conjugates; GSH denotes glutathione; SAM denotes S-adenosyl methionine; AS3MT denotes Arsenic (+3 oxidation state) Methyl Transferase. *Reduction in scheme c is by cysteine residues of the As3MT enzyme.
Because trivalent arsenicals have high affinities to thiols, they are likely bound to −SH groups of glutathione or proteins present in the cell cytosol.27,28 Two alternative arsenic methylation pathways that were proposed by Hayakawa et al.29 and Naranmandura et al.30 account for arsenic interaction with thiols within the cell.29,30 These proposals suggest that glutathione (GSH) conjugates and protein-bound arsenic are the intermediates in the arsenic methylation reactions. However, these proposed methylation pathways rely on attack of the CH3– anion on the AsIII lone pair electrons. CH3– is a very strong base and it is unlikely to leave a positive center on SAM to react with AsIII. Hence, these proposed pathways have been considered not chemically plausible.10,31,32
Arsenic glutathione conjugates [As(GS)3] were shown to have higher binding affinity than arsenite to the As3MT binding sites.28 Dheeman et al.28 proposed that As(GS)3 acted as a substrate for enzymatic methylation and that AsIII was bound to As3MT through three cysteine residues, which was later supported by the structural elucidation.26 According to this mechanism, the majority of MMAIII remains bound to As3MT after methylation is completed and the cysteine residues in the binding sites serve as electron donors to reduce positively charged methylated intermediates back to trivalent state. According to this model, DMAIII is the major product of methylation, which then can be nonenzymatically oxidized to DMAV.
In 2014, Cullen proposed a modified Challenger’s pathway that accounted for arsenic interaction with proteins in the cell (Figure 1b).10 Formation of thiolated arsenic metabolites, along with oxo-forms of arsenic metabolites, was also considered as a possibility.10 A detailed integrated metabolic pathway that accounts for methylation, thiolation, and hydrolysis of arsenic metabolites was proposed by Fan et al. (Figure 1c).31
Despite a variety of interpretations, all of the proposed mechanisms have a common goal to explain the excretion of arsenic metabolites MMAV, DMAV, MMAIII, and DMAIII in human urine. Relevant reviews also integrated the formation of thiolated arsenic compounds dimethyldithiolarsenate (DMDTAV), dimethylmonothiolarsenate (DMMTAV), and monomethylmonothiolarsenate (MMMTAV) into the classic arsenic methylation pathways.30,33,34
Inorganic arsenicals and their metabolites have a wide range of toxicities. It is important to account for the toxicity of the arsenic metabolites and intermediates to understand the role of arsenic exposure in disease development. Toxicities of the arsenic species have been evaluated in a number of human cell lines, such as hepatocytes,35,36 urothelial cells,36 epidermal cells,37 and neurons.38 Inorganic arsenic metabolites and their intermediates displayed a wide range of toxicities. Pentavalent methylarsenicals MMAV and DMAV have lower toxicities than iAs. However, the trivalent methyl arsenic species MMAIII and DMAIII are more toxic than iAs. The IC50 of various arsenic species in human bladder cell line (T24) ranged from 13 μM for DMAIII to greater than 1 mM for MMAV and DMAV.39 Similarly, 13 μM of either MMAIII or DMAIII induced cytotoxicity in human hepatocytes.35,36 Among the inorganic and methyl arsenic species tested in human neuron cells, MMAIII was most cytotoxic.38 In endothelial cells, MMAIII and DMAIII displayed significant toxicity at concentrations as low as 2.0 and 0.74 μM, respectively.40 Much research has also focused on the toxicity of thiolated arsenic species.41,42 DMMTAV had particularly high toxicity, similar to that of methylated trivalent arsenic species, whereas the toxicity of DMDTAV was much lower, similar to that of MMAV and DMAV.33,39 Based on the available data, relative toxicities of arsenic compounds are generally in the following order: MMAIII, DMAIII, and DMMTAV > iAsIII ≫ iAsV > MMMTAV > MMAV, DMAV, and DMDTAV.39
Detailed understanding of the mechanism of formation, intra- and intercellular mobility of these arsenic species is critical to a better understanding of arsenic toxicity. Current studies often rely on arsenic metabolites as a fingerprint of arsenic transformation in the body, particularly in attempts to establish relationships between arsenic metabolism and health outcomes. Some of the studies also examined the effects of diet, lifestyle, genetics, and social factors on the metabolism of arsenic. These studies are commonly done by using urinary concentrations of arsenic metabolites to estimate the efficiency of arsenic methylation. Therefore, it is important to understand potential confounding factors that influence the estimation of arsenic methylation efficiency, particularly when there is concurrent exposure to various arsenic species.
Calculation of Methylation Efficiency
Researchers have commonly calculated methylation efficiency based on urinary concentrations of inorganic arsenicals (iAs) and their metabolites. Methylation efficiency is generally expressed as percentages of total urinary As (or the sum of iAs + MMA + DMA) represented by an individual metabolite, ratios of concentrations of individual metabolites, and methylation indexes (Table 1). The Primary Methylation Index (PMI) is used to describe the efficiency of the first methylation step from iAs to MMA. The Secondary Methylation Index (SMI) describes the second methylation step from MMA to DMA (Figure 2).
Table 1. Calculation of the Methylation Efficiency Parameters.
| Parameter | Calculationa | Reference |
|---|---|---|
| Percentages of Individual Metabolite |  |
(16,43−45) |
| Methylation Indexes | 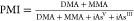 |
(18,20,43) |
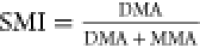 |
||
| Methylation Ratios |
b
|
(14,16,18,43,45−47) |
b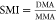
|
||
| Results of Principal Component Analysis | PC1 – Principal Component 1 | (17,19,21) |
| PC2 – Principal Component 2 |
Ind. Metabolite denotes Individual Metabolite. PMI denotes Primary Methylation Index. SMI denotes Secondary Methylation Index. PC denotes Principal Component.
Note that some studies refer to metabolite ratios as PMI and SMI. Therefore, it is important to pay close attention to the way the PMI and SMI were calculated when interpreting their values.
Figure 2.

Scheme illustrating the Primary Methylation Index (PMI) and Secondary Methylation Index (SMI).
Percentages of individual metabolites are calculated by dividing the concentration of each metabolite with the total concentration of inorganic arsenicals and their metabolites (sum of iAsIII, iAsV, MMA, and DMA concentrations). In contrast, there are several ways in which PMI and SMI are expressed (Table 1). While earlier work mainly expressed PMI and SMI as ratios of more methylated to less methylated metabolites, recent work often used proportions of the methylated metabolites. Yamauchi and Takata43 and Shen et al.18 used both approaches.18,43 Some studies also used inverted ratio: less methylated to more methylated metabolites. Therefore, methods of calculations need to be considered for the appropriate interpretation of reported results.
Several recent studies also applied principal component (PC) analysis, by which highly correlated iAs%, MMA%, and DMA% were transformed into two principal components PC1 and PC2. PC1 described the second methylation step, from MMA to DMA, an alternative to SMI and overall methylation efficiency. PC2 was thought to explain the first methylation step, from iAs to MMA, alternative to PMI.17,19,21
The calculations of arsenic methylation efficiency discussed above rely on the main assumption that inorganic arsenic metabolism is the only source of urinary MMA and DMA. The assumption implies that individuals are exposed mainly to iAs and that exposure to other forms of arsenic is negligible. This assumption may be valid only in cases where the population is exposed to arsenic primarily through contaminated drinking water where the main species of arsenic are iAsIII and iAsV.48,49 However, it may not be valid when there is substantial exposure to other forms of arsenic through the ingestion of seafood, mushrooms, and some forms of diet supplements. Exposure to organoarsenicals, such as arsenosugars, can also result in urinary excretion of DMA as a result of arsenosugar metabolism in humans. This would cause potential confounding in the calculation and interpretation of the methylation efficiencies. Because not necessarily all of the DMA in urine comes solely from iAs metabolism, we need to be careful with attributing DMA to the methylation of inorganic arsenic. Analysis of the percentages of metabolites and methylation indexes must be interpreted with caution. To assess potential confounding of methylation efficiency, we need to evaluate the likelihood of direct exposure to MMA and DMA and the amount of MMA and DMA formed because of exposure to other organic arsenicals.
Other assumptions include negligible post methylation modifications for oxo-forms of methylated arsenic species and similar retention of arsenic metabolites in human body. In methylation efficiency calculations, we rely on urinary concentrations of oxo-forms of DMA and MMA as the main forms of dimethylated and monomethylated arsenic species. Thiolated arsenic species are not included in the calculations.
Exposure to Methylated Arsenicals and Other Organoarsenicals
Data relevant to the exposure and metabolism of methylated arsenicals and other organic arsenic species are limited. Some organic arsenic species detected in foods, such as arsenobetaine (AsB), arsenocholine (AsC), trimethylarsine oxide (TMAO), and tetramethylarsonium, are very stable. These highly methylated arsenic species are not metabolized in humans and therefore are not expected to alter the proportions of inorganic arsenicals and their metabolites. In vitro studies have shown that AsB can be metabolized to DMA, dimethylarsenoylacetate (DMAA), and TMAO by human gut bacteria after about 7 days.50 However, the retention time of AsB in the gut is much shorter. Studies with ingestion of radiolabeled AsB suggest that AsB is dispersed throughout soft tissues within 24 h and that over 90% of ingested AsB is excreted after 7 days since the ingestion.51 Therefore, significant metabolism of AsB in humans is not expected.52 In contrast, ingestion of MMA, DMA, arsenosugars, and arsenolipids has the potential to alter the ratios of inorganic and methyl arsenicals and confound the interpretation of arsenic methylation efficiency.
MMA and DMA
Speciation studies suggest that MMA and DMA are rarely ingested. MMA and DMA are commonly assumed to be the results of iAs metabolism. Early studies established that MMA, if ingested, is metabolized to DMA.25 However, exposure to MMA from the environment is not common: only trace amounts of MMA were reported in rice and fish. It was estimated that intake of MMA by the Japanese population was on average 2.3 μg/day with the range of <0.18–6.5 μg/day.53
In general, direct exposure to DMA is not common.54 Small quantities of DMA were reported mainly in rice, rice products (e.g., rice crackers and cereal), and some fish.44,55,56 Relative concentrations of DMA vary substantially depending on the type of rice and the environment where rice was grown.57−60 DMA is primarily excreted through urine unchanged.25 Therefore, substantial consumption of rice with high DMA concentrations has a potential to alter arsenic metabolic profiles and affect the methylation ratios.61 Estimated DMA intake was reported to be 4.57 μg/day (range <1.8–49.9 μg/day) in the Japanese diet.53 Based on available data, exposure to low doses of MMA and DMA is not likely to confound calculations of arsenic methylation efficiency when exposure to inorganic arsenic is at higher levels. More caution in the evaluation of methylation efficiency and potential confounding from MMA and DMA ingestion would be required if overall exposure to arsenic is low. Reports of thiolated methylated arsenicals in rice also draw attention to the potential ingestion of these arsenic species.41,42
Arsenosugars and Arsenolipids
Larger amounts of urinary DMA may be excreted due to exposure to arsenosugars. A detailed summary of data available about metabolism and exposure to these organic arsenicals is available in the review by Taylor et al.52 Therefore, we focus here only on aspects relevant for the evaluation of potential confounding.
Intake of arsenosugars by the Japanese population has been estimated to be at 1 mg/day.52 However, lack of speciation data limits our ability to comprehensively predict the magnitude of exposure.62 Exposure to arsenosugars might occur from the consumption of such seafoods as seaweeds, clams, mussels, and oysters.63−66 Seaweeds have the highest reported total arsenic concentrations and the highest percentage of arsenosugars among all seafoods.52,66 Total arsenic concentrations and the relative proportion of arsenosugars vary substantially depending on seaweed species, sections of the thallus, harvesting seasons, and locations of growth.67,68 Crustaceans and mollusks contain both AsB and arsenosugars as the main arsenic species. The relative concentrations of arsenosugars in crustaceans and mollusks are higher than in finfish.52,66 Recent market basket surveys of arsenic species in seafood conducted in USA suggest high variabilities of total arsenosugar concentrations and the specific arsenosugar species in clams and crabs.65 AsB is generally reported as a major arsenic species in marine mussels,69 but arsenosugars have been reported to be the predominant arsenic species in freshwater mussels, comprising up to 95% of extractable arsenic.70
Exposure to arsenolipids is mainly associated with fish oils and fatty fish tissues.52 Lower concentrations of arsenolipids were also detected in some seaweeds.71 Average daily intake of arsenolipids for the Japanese population was estimated as 2100, 4200, and 4900 ng As/person/day for arsenic-containing fatty acids (AsFAs), arsenic-containing hydrocarbons (AsHCs), and arsenosugar phospholipids (AsSugPLs), respectively.72
Multiple studies showed that DMAV was the main urinary metabolite of arsenosugars and arsenolipids (Table 2).63,73,74 Several unique urinary metabolites of arsenosugars, e.g., thio- and oxo-forms of dimethylarsenoylethanol (DMAE) and dimethylarsenoylacetate (DMAA), have been observed.52,75 However, differences between the metabolism of different types of arsenosugars are not yet established. In the study by Taylor et al., urinary arsenic metabolites were similar after ingestion of nori and kombu seaweed.76 AsSug482 was the major arsenic species in nori, and AsSug392 was the main arsenosugar in kombu (see arsenosugar names in Supporting Information Figure S1). Similarly, intra- and interindividual variability for arsenosugars metabolism is largely unknown. Studies with ingestion of a pure arsenosugar were conducted using AsSug328 only. The results suggest drastic interindividual variability in metabolism of arsenosugars, in terms of both the amount of arsenic excreted and the identity of urinary metabolites. Limited data suggest that patterns of arsenosugar metabolism may stay consistent for individuals over time.77 The mechanism of arsenosugar metabolism in humans has not been established. Highly acidic conditions do not break down arsenosugars to DMA.63In vitro evaluation of arsenosugars transformation under conditions similar to those in the gastrointestinal tract (GIT) did not lead to the formation of the arsenosugar metabolites observed in urine; however, major transformation of oxo- to thio-arsenosugars was observed.78−80 Available research suggests that arsenosugars are biotransformed with the assistance of microbiome in the GIT and/or enzymes;63,78−80 however, the biochemical pathway(s) is(are) not known. Degradation of arsenosugars to DMA, DMAE, MMA, and iAs was observed in experiments simulating marine microenvironments. These studies highlight the importance of representative microbiome population, their complexity, and their role in breaking down arsenosugars.81−84 Large intra- and interindividual variability of the microbiome78 might explain the observation that some people excrete arsenosugars unchanged while others break down arsenosugars efficiently.77 An in vivo study of feeding mice with arsenosugars suggests that the pattern of arsenic metabolites and efficiency of arsenic excretion may be strongly influenced by the gut microbiome in mice, the food matrix, and the type of arsenosugars ingested.85 Mice with the gut microbiome disturbed by an antibiotic showed a much lower ability to transform arsenosugars and excrete arsenic species. Interestingly, the ingested AsSug482 from seaweed nori was metabolized to AsB, while AsSug392 ingested with kelp was excreted unchanged. Studies of differences in microbiomes of individuals that biotransform arsenosugars differently could provide further insight into specific microorganisms in the GIT assisting the breakdown of arsenosugars.
Table 2. Reported Urinary Arsenic Metabolites Following the Ingestion of Arsenosugars.
| Ingestion | Arsenic species in food (% of total arsenic) | Urinary Arsenic Metabolites | Participants | Ref |
|---|---|---|---|---|
| Arsenosugar-Containing Foods | ||||
| kelp (brown seaweed) | AsSug X | DMA | 2 volunteers | (63) |
| AsSug XI | iAsV | |||
| 3 Unknown As species | ||||
| nori (red seaweed) | AsSug X | DMA | 9 volunteers | |
| 6 Unknown As species | ||||
| yakinori | AsSug X | DMA | 4 volunteers | (64) |
| AsSug XI | 4 Unknown As species | |||
| nori (red seaweed) | AsSug X (92.5%)f | DMA | 1 volunteer | |
| DMA, iAsV (7.5%)f | 5 Unknown As species | |||
| wakame (brown seaweed) | AsSug328 (88.8%) | DMA (58.1%)a | 5 volunteers | (86) |
| AsSug482 (Traces) | 5 Unknown As species | |||
| Unknown As species (Traces) | ||||
| wakame (brown seaweed) | DMA (0.6%) | DMA (8.6%)b | 11 volunteers | (76) |
| AsSug482 (0.5%) | Thio-DMAA (4.2%)b | |||
| AsSug328 (0.3%) | Thio-DMAE (2.8%)b | |||
| AsSug482, AsSug328 (1.7%)b | ||||
| Thio-DMA (0.2%)b | ||||
| kombu (brown seaweed) | AsSug392(47.9%) | DMA (5.3%)b | 11 volunteers | |
| AsSug482 (14.7%) | Thio-DMAA (1.5%)b | |||
| AsSug328 (3.4%) | Thio-DMAE (0.9%)b | |||
| DMA (0.7%) | AsSug392, AsSug482, AsSug328 (0.7%)b | |||
| Thio-DMA (0.1%)b | ||||
| nori (red seaweed) | AsSug482 (60.7%) | DMA (13.6%)b | 11 volunteers | |
| DMA (2.8%) | Thio-DMAA (6.0%)b | |||
| AsSug328 (2.2%) | Thio-DMAE (4.2%)b | |||
| AsSug482, AsSug328 (1.6%)b | ||||
| Thio-DMA (0.6%)b | ||||
| Pure Arsenosugars | ||||
| AsSug328 (>99.5%) | DMA (67%)c | 1 volunteer | (87) | |
| DMA (<0.5%)e | Unknown As species A4 (20%)c | |||
| DMAE (5%)c | ||||
| Unknown As species A3 (5%)c | ||||
| TMAO (0.5%)c | ||||
| Traces of at least 8 Unknown As species (2.5%)c | ||||
| AsSug328 | DMA (51%)d | 1 volunteer | (75) | |
| Thio-DMAA (19%)d | ||||
| Thio-DMAE (10%)d | ||||
| Unknown As species A5 (10%)d | ||||
| Oxo-DMAE/Oxo-analogue of A5 (4%)d | ||||
| Oxo-DMAA (2%)d | ||||
| AsSug328 (1%)d | ||||
| Thio-AsSug328 and (possibly) thio-DMA (Traces)d | ||||
| Traces of Unknown As species 3%d | ||||
| AsSug328 | DMA (40–46%) | 4 volunteers | (77) | |
| Thio-DMAA (15–19%) | high excretors | |||
| Thio-DMAE (5–9%) | >85% As excreted | |||
| Thio-Unknown As species (3–8%). | ||||
| DMA | 2 volunteers | |||
| Thio-AsSug328 | low excretors | |||
| Oxo-AsSug328 | 4% and 15% As excreted | |||
% of total arsenic excreted over 5 days.
mean % of total arsenic consumed.
% of total arsenic excreted over 69 h.
% of total arsenic excreted over 48 h.
Impurity assigned based on retention time match.
Based on sum of arsenic species concentrations.
Data on the metabolism of arsenolipids are even more limited. Oxo-/thiodimethylarsenopropanoic (DMAP) acid appears to be a potential unique metabolite of arsenolipids.73,74 More research on the metabolism of arsenolipids is required to draw any specific conclusions.
Our current knowledge of arsenic speciation in urine following ingestion of arsenosugars and arsenolipids indicates that exposure to these arsenic species can increase urinary DMA concentrations and produce several other arsenic metabolites. However, there are very limited data on the amount of DMA produced as a metabolite of arsenosugars relative to the DMA formed as a metabolite of iAs methylation. Nonetheless, existing reports suggest that confounding might be a potential issue and that it could lead to overestimations of iAs methylation efficiency. Useful information related to potential confounding can be drawn from controlled feeding studies and studies that tested statistical associations between DMA concentrations and seafood intake (Table 2).
Controlled feeding studies revealed changes in relative concentrations of arsenic metabolites after specific changes in diet. Studies investigating metabolism of arsenosugars and arsenolipids reported increased urinary DMA concentrations after ingestion of arsenosugars or arsenolipids in pure form or in foods (Table 2). Ma and Le observed about 4.5 times increase of urinary DMA concentration from <20 μg/L in urine collected before ingestion to 90 μg/L in urine collected 26.5 h after the ingestion of 10 g yakinori seaweed.64 Hata et al.86 observed significant increases in the excretion rate of DMA at 24 h after ingestion of wakame seaweed: 2.14 μg As/h vs 0.38 μg As/h. A total of 176.3 μg/L of arsenic (DMA proportion was over 50%) was excreted over 5 days after the ingestion of 300 g of wakame seaweed.86 A study on 16 volunteers in Korea with total arsenic 6.98 mg/person/day through seafood consumption found significant increases of total urinary arsenic with primary increase of DMA. Average DMA% changed from 86% before seafood intake to 95% in urine collected 48 h after the end of six-day seafood intake. These studies suggest that the relative proportions of metabolites might be strongly altered because of seafood consumption. Importantly, the increased DMA% arising from arsenosugar ingestion might mislead researchers to interpret that the arsenic methylation efficiency of the individual was exceptionally high.88
Associations between DMA concentrations and seafood intake were also explored based on data of the National Health and Nutrition Examination Survey (NHANES) and Multiethnic Study of Atherosclerosis 2000–2002 (MESA) studies. Navas-Acien et al.54 found 1.8 times higher urinary DMA concentrations in participants who reported seafood intake within last 24 h in comparison to those who did not report seafood intake (median DMA 6.0 vs 3.5 μg/L while the median for AsB concentrations was 10.2 vs 0.9 μg/L).54 Although the absolute difference in median DMA concentrations was small, the DMA concentration was almost twice as high in the population with reported seafood intake. This increase is significant because the methylation efficiency is expressed as the ratio or proportion of methylated arsenic species. Therefore, effects of seafood consumption cannot be disregarded, especially where the exposure to organic arsenicals from seafood is much larger than exposure to inorganic arsenic.
Navas-Acien et al.54 analyzed the data of the urinary arsenic metabolites of the representative US population sample including 4276 participants in the NHANES database. The NHANES 24 h dietary recall was used to estimate overall seafood intake of the participants as well as consumption of specific types of seafood: fish, shellfish, mollusks, and sushi/seaweed. The authors could not evaluate relationships of seafood consumption with other iAs metabolites, namely, MMA, iAsIII, and iAsV, because these metabolites could not be detected in most of the urine samples.54 Jones et al.44 evaluated the results of arsenic metabolites in urine samples from the MESA study, which included 310 participants, and from 1175 participants in the NHANES data set. Most of the participants in both NHANES and MESA data sets had MMA and AsV below detection limits. Low MMA and iAs detection rates limited study to only examining the DMA relationship with AsB and seafood consumption. They could not investigate the possible relationships of MMA and iAs with seafood intake. Although a common limitation was self-reported seafood intake, this study also measured the n-3 polyunsaturated fatty acids as a marker to increase confidence of seafood intake evaluation.44
Figure 3 shows mean iAs%, MMA%, and DMA% in urine reported for populations in Bangladesh,15 USA,14 South Korea,89 and Taiwan, China.90 The amounts of fish and seafood consumption per capita is lower in the United States (22 kg per capita per year in 2020) and Bangladesh (26 kg) than in China (40 kg) and South Korea (54 kg) (https://ourworldindata.org/grapher/fish-and-seafood-consumption-per-capita). In general, higher percentages of DMA are found in the urine of populations that consume more seafood. This observation at the population level is consistent with our understanding of the potential contribution of DMA as a metabolite of arsenosugars due to the ingestion of seaweed, clams, mussels, and oysters.
Figure 3.

Comparison of mean percentages of iAs, MMA, and DMA in urine reported for populations in the USA,14 Bangladesh,15 South Korea,89 and Taiwan, China.90 The numbers on top of each bar are mean percentages of the corresponding arsenic species.
Reducing Potential Confounding Caused by Ingestion of Organoarsenicals
To minimize confounding, multiple studies considered three main approaches to assess or minimize seafood intake: (1) refrain from seafood ingestion 3–5 days before urine collection; (2) self-recall food frequency questionnaires (FFQ), which in some cases were also supplemented with polyunsaturated acid detection; and (3) measure urinary AsB as a biomarker of seafood consumption.
Seafood-Free Diet
Seafood-free diet prior to urine sample collection is a common approach to reduce potential confounding from seafood consumption. Given that most of metabolites of arsenosugars were eliminated within 9–60 h,52,64 a seafood-free diet for 3 days (72 h) prior to urine collection should be sufficient to eliminate arsenosugars ingestion and its potential bias of “additional” DMA metabolite. However, the calculation of methylation efficiency might be more susceptible to confounding in comparison to total exposure to iAs because the confounding is primarily due to increased DMA concentration. Ma and Le64 reported change of total arsenic in urine over a period of 4 days after yakinori seaweed ingestion: while the most increase of arsenic excretion was between 20 and 40 h, urinary arsenic concentrations remained about two times higher than the baseline levels (in 3 of 4 volunteers) at the end of day 4.64 Considering that DMA is a major metabolite of arsenosugars, its confounding effect on methylation efficiency might remain longer than 72 h. The significance of its relative contribution would depend on the total exposure to all arsenic species.
Food Frequency Questionnaires (FFQs)
When refraining people from eating seafood for 3 days is challenging to implement in large scale studies, the food frequency questionnaire theoretically can provide information on seafood consumption prior to collection of urine samples. However, the food frequency questionnaire approach has a number of limitations, including bias/errors in self-reported diet, coverage of only a short period, and only qualitative information. Often food frequency questionnaires include 24 h food consumption, while seafood ingestion was reported to cause increased DMA and total arsenic in urine for several days after consumption.54
AsB as a Biomarker of Seafood Consumption
Along with approaches mentioned above, AsB has been measured to indicate potential seafood ingestion.21,44,53 For the analysis of arsenic methylation data, these studies excluded urine samples that had high AsB concentrations. The study of Navas-Acien et al.54 suggested that the association between DMA and seafood intake was no longer present when AsB was below the limit of detection.54 However, the disadvantage of excluding AsB-containing samples are potential nonrandom exclusion and reduced sample size.44 Moreover, there is no specific threshold of AsB concentrations in urine as a marker of confounding. Therefore, excluding samples with detected AsB is challenging to apply in studies with high detection rates of AsB.
Alternatively, Jones et al.44 used urinary AsB to “calibrate” or normalize urinary iAs, MMA, and DMA concentrations. Calibrated values had much lower association with the seafood intake, while the association with rice intake remained. The authors concluded that normalization against AsB was useful to estimate exposure to iAs and DMA from foods and water in populations with low-to-moderate iAs exposure but could not be used in populations with high seafood consumption.44
Although AsB in urine is a biomarker of seafood ingestion, it is not a reliable biomarker of exposure to other organic arsenicals such as arsenosugars and arsenolipids. The use of AsB as a marker is primarily based on the assumption of the concurrent presence of AsB and arsenosugars in some seafoods. But the simultaneous presence of AsB and other organoarsenicals is not consistent.91−93 Luvonga et al.93 report high AsB and no detectable arsenosugars in shrimp and salmon samples, while spirulina and kelp powder samples had high concentrations of arsenosugars but no detectable AsB.93 In this study, the concurrent presence of AsB and arsenosugars was observed only for Geoduck clams. Moreover, seaweeds contain primarily arsenosugars but not AsB.63,94 Consistent with the report of Ma and Le,64 a recent study by Lee et al.67 showed that consumption of mussels was associated with urinary iAs, DMA, and AsB concentrations, while seaweed consumptions was only associated with urinary iAs and DMA, but not with AsB.67 Hence, exposure to high levels of AsB does not necessarily mean exposure to large amounts of arsenosugars or arsenolipids. Despite AsB being commonly used as a biomarker of seafood consumption, its absence does not eliminate the possibility of ingestion of arsenosugars and other organic arsenicals. Conversely, the presence of AsB in urine does not necessarily mean exposure to arsenosugars and arsenolipids. There is no study that clearly shows that AsB is a reliable biomarker of exposure to complex organic arsenicals. Moreover, research on determining the quantity of arsenosugars and arsenolipids ingested is very limited.52
Another issue is the differences in the elimination kinetics among the different arsenic species. AsB is rapidly excreted unchanged,63 while arsenosugars and arsenolipids are metabolized.73,74,77,87,95 The resulting metabolites of arsenosugars and arsenolipids are excreted into the urine for a longer period, extending after AsB is already eliminated.
Current approaches for accessing exposure to complex organic arsenicals are indirect in nature. Prevention of potential confounding relies on (1) elimination/assessment of seafood exposure and (2) assumption of concurrent presence of AsB and arsenosugars/arsenolipids in some seafoods. Direct methods of identifying exposure to arsenosugars or arsenolipids are not yet available.
Potential Direct Methods of Exposure Assessment to Complex Organic Arsenicals
Direct methods of the exposure assessment could use measurement of urinary metabolites uniquely formed during metabolism of arsenosugars or arsenolipids.52,96 Recent studies suggest that apart from DMA being the main metabolite of arsenosugars and arsenolipids, several new arsenic metabolites can be observed in urine, such as DMAE and DMAA from the metabolism of arsenosugars and DMAP from the metabolism of arsenolipids.73−75,77 Including identification of unique metabolites of arsenosugars and arsenolipids, along with metabolites of inorganic arsenic and AsB, could provide more specific information about types of arsenic compounds individual was exposed to and hence more accurate interpretation of arsenic metabolic profiles.
Practical applications of such an approach are limited largely due to poor understanding of the metabolism of arsenosugars and arsenolipids, the absence of commercially available standards of newly detected unique metabolites, and difficulties of identifying new, “unknown” arsenicals at low concentrations. Observing “unknown” arsenic species in urine besides routinely monitored arsenic species (iAsV, iAsIII, MMA, DMA, AsB) may warn us about potential exposure to arsenosugars and/or arsenolipids. The presence of several unknown arsenic species in the CRM18 reference material, which was created by pooling urine samples of Japanese males, may reflect the fact of a high intake of arsenosugar-containing seafood (such as seaweed) by the Japanese population. Some of the unknowns in this reference material urine have been identified.97−99 Further research will be useful to assess any associations of these “unidentified” arsenic species with the ingestion of arsenosugars and arsenolipids.
Concluding Remarks and Future Perspectives
Studies of arsenic exposure and metabolism are critical to a better understanding of the diverse health effects of arsenic species. For the general public under the most common environmental exposure scenarios, water and food are the primary sources of arsenic intake. While inorganic arsenicals (arsenate and arsenite) are the dominant arsenic species in drinking water, a wide variety of arsenic species can be present in food. Inorganic arsenate and arsenite are commonly metabolized in humans to produce monomethyl and dimethyl arsenicals, which are readily excreted into urine. Therefore, determination of inorganic arsenic species and monomethyl and dimethyl arsenicals provides a basis for the assessment of arsenic methylation efficiency. Concentrations, ratios, or proportions of DMA, MMA, and iAs in urine are usually used to calculate arsenic methylation efficiency values.
Ingestion of arsenosugars and arsenolipids from dietary sources can also produce dimethylarsenic species, which are excreted into urine. In its chemical nature, the DMA produced by the biomethylation of inorganic arsenic species is identical to the DMA produced from the metabolism of arsenosugars and arsenolipids. Therefore, the intake of arsenosugars and arsenolipids, sometimes unknowingly, contributes to increases in the urinary concentration of DMA and can cause confounding effects on the calculation and interpretation of arsenic methylation efficiency.
Arsenosugars are abundant in seaweed, clams, mussels, and oysters. Seaweeds, such as kelp and nori, contain arsenosugars as the dominant arsenic species, whereas clams, mussels, and oysters contain arsenobetaine and arsenosugars. Fish oil contains various arsenolipids, although their concentrations are usually lower than those of arsenosugars present in seaweed and bivalves. There is a knowledge gap in the occurrence and concentrations of arsenosugars and arsenolipids in food of terrestrial origin.
In addition to seafood, some “health food” supplements, such as fish protein and seaweed powders, may also contain arsenosugars and arsenolipids. With increased seafood access in many parts of the world, increasing uses of food supplements, and the limited knowledge about organoarsenicals in terrestrial foods, there is an increase in the potential risk of unintentional exposure to arsenosugars and arsenolipids. The unknown exposure to organoarsenicals could be overlooked in the “seafood-free diet” approach and the “food frequency questionnaire” approach. Hence, urinary DMA concentrations could be a result of not only inorganic arsenic methylation but also unknown ingestion of organoarsenicals, which could result in a biased interpretation of arsenic methylation efficiency. Future studies may focus on several aspects to improve the estimation of arsenic methylation efficiency:
-
1.
Assessment of using AsB to indicate potential confounding of seafood ingestion
It is important to understand the limitations of using AsB as a marker of seafood consumption. Relative concentrations of AsB and arsenosugars vary with the types of seafood. Some seafood, such as crabs and lobsters, contains predominantly AsB. Seaweed contains primarily arsenosugars, and bivalves often contain both AsB and arsenosugars. Studies of the methylation efficiency should assess the potential confounding from the ingestion of various types of seafoods that contain arsenosugars and arsenobetaine. Investigation of whether AsB is a reliable biomarker of exposure to complex organic arsenicals is necessary. Relations between exposure to complex organoarsenic compounds and unknown, unique arsenic metabolites need to be evaluated. Understanding of these relations could help to determine whether the unique metabolites of organoarsenicals could serve as markers to indicate the coexposure to arsenosugars, arsenolipids, and other organoarsenicals.
-
2.
Metabolism of arsenosugars and arsenolipids
To establish alternative biomarkers, researchers could investigate arsenic metabolites unique to the metabolism of arsenosugars and arsenolipids. This aspect of research requires further investigation into the sources and magnitude of exposure, metabolism of arsenosugars and arsenolipids, unique metabolites, efficiency and kinetics of urinary excretion, and intra- and interindividual variability.
-
3.
Understanding the role of thiolation in inorganic arsenic metabolism
Understanding the extent of thiolation and the range of thio- and oxomethylated arsenic species formed as a result of inorganic arsenic metabolism would be valuable in understanding the role of thiolation in post methylation processes. Evaluation of parameters associated with thiolation would require the determination of thio- and oxomethylated species included in the targeted arsenic species.
-
4.
Addressing Analytical Challenges
Proper monitoring of a diverse range of arsenic species relies on capabilities of analytical methods for arsenic speciation. There are a number of challenges associated with characterizing a wide range of arsenic metabolites.
Methods for the synthesis of methylated and thiolated arsenic species were summarized by Cullen et al.100 However, many of these arsenic species are not commercially available as individual standards. There is no standard reference material (SRM) with certified concentrations of these arsenic species, although several thioarsenic species have been detected in the urine reference material CRM18. Standards for arsenosugars and arsenolipids or their unique metabolites are not commercially available. In the absence of arsenosugar standards, researchers have extracted arsenosugars from hijiki seaweed and used the extract as a reference material for arsenosugar analysis.103
The trivalent arsenicals and some thiolated arsenicals have low stability. The ability to determine these unstable arsenic species requires appropriate sample collection, preservation, and storage conditions, sample preparation techniques, and methods of analysis.13,95,99,101,102 Strong retention of thiolated arsenic species on some anion exchange columns due to their interaction with the polymeric backbone of the column packing materials could lead to their loss on the column. Hydrogen peroxide has been used to convert the thiolated form to the oxo-form of arsenic species. This treatment also oxidizes trivalent to pentavalent arsenic species. Such sample preparation allows for detection of total methylated arsenicals. However, this approach of sample treatment could result in a loss of the original arsenic speciation information.
Another challenge is a lack of well-established techniques to distinguish new metabolites of organic arsenic species from commonly targeted arsenic species. DMAE and TMAO likely coelute with AsC and AsB when anion exchange chromatography is used. Methods for arsenic speciation in urine have mainly been designed to measure targeted arsenic species. Additional “unknown arsenicals” may be observed as unexpected chromatographic peaks. Potential metabolites of arsenosugars and arsenolipids may also coelute with the more frequently encountered organic arsenic species.
Methods that combine molecular and elemental information have been instrumental in the identification of diverse arsenic species without the availability of standards available. Liquid chromatography separation followed by simultaneous detection with both electrospray ionization mass spectrometry (ESIMS) and inductively coupled plasma mass spectrometry (ICPMS) provides complementary information for the identification and quantification of arsenic species. Unknown arsenic species at very low concentrations are still challenging to identify.104 Further research is needed to improve the efficiency of electrospray ionization of arsenic species present in complex sample matrixes, enhance resolution of separation and sensitivity of detection, develop new techniques, and refine existing techniques for arsenic speciation analysis.
Acknowledgments
This work was supported by Alberta Health, Alberta Innovates, the Natural Sciences and Engineering Research Council of Canada, and the Canadian Institutes of Health Research.
Biography

X. Chris Le is Distinguished University Professor and Director of the Analytical and Environmental Toxicology Division in the Faculty of Medicine and Dentistry at the University of Alberta (Canada). He held the inaugural Canada Research Chair in Bioanalytical Technology and Environmental Health for 17 years. He is an elected Fellow of the Royal Society of Canada (Academy of Science). Pictured here with co-authors Tetiana Davydiuk (PhD student), Jeffrey Tao (PhD student), and Xiufen Lu (Research Associate), Dr. Le’s team develops analytical techniques to enable studies of environmental health. The team’s research on arsenic speciation in biological and environmental systems focuses on human exposure to and metabolism of arsenic species, arsenic binding to proteins, and the effect of arsenic on DNA repair. Another area of Dr. Le’s research deals with bioanalytical chemistry, focusing on the development of DNA-protein binding assays, isothermal and signal amplification techniques, and CRISPR technology for highly sensitive and specific determination of biomolecules and for studies of molecular interactions. From left to right: Tetiana Davydiuk, Jeffrey Tao, Xiufen Lu, and X. Chris Le
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/envhealth.3c00090.
Examples of arsenic species, abbreviations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/ (accessed 2023-08-05).
- Kuo C.-C.; Moon K. A.; Wang S.-L.; Silbergeld E.; Navas-Acien A. The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ. Health Perspect. 2017, 125 (8), 0870001 10.1289/EHP577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas M. F.; Anderson B.; Ahsan H.; Aposhian H. V.; Graziano J. H.; Thompson C.; Suk W. A. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect. 2013, 121 (3), 295–302. 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgens E. E.; Drobna Z.; He B.; Le X. C.; Styblo M.; Rogers J.; Thomas D. J. Biological and Behavioral Factors Modify Urinary Arsenic Metabolic Profiles in a U.S. Population. Environ. Health 2016, 15 (1), 62. 10.1186/s12940-016-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull E. A.; Ahsan H.; Edwards J.; Longnecker M. P.; Navas-Acien A.; Pi J.; Silbergeld E. K.; Styblo M.; Tseng C.-H.; Thayer K. A.; Loomis D. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ. Health Perspect. 2012, 120 (12), 1658–1670. 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C.; Yuan Y.; Kalman D.; Rey O. A.; Skibola C. F.; Dauphine D.; Basu A.; Porter K. E.; Hubbard A.; Bates M. N.; Smith M. T.; Smith A. H. Individual Differences in Arsenic Metabolism and Lung Cancer in a Case-Control Study in Cordoba, Argentina. Toxicol. Appl. Pharmacol. 2010, 247 (2), 138–145. 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stýblo M.; Venkatratnam A.; Fry R. C.; Thomas D. J. Origins, Fate, and Actions of Methylated Trivalent Metabolites of Inorganic Arsenic: Progress and Prospects. Arch. Toxicol. 2021, 95 (5), 1547–1572. 10.1007/s00204-021-03028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M.; Concha G. Role of Metabolism in Arsenic Toxicity. Pharmacol. Toxicol. 2001, 89 (1), 1–5. 10.1111/j.1600-0773.2001.890101.x. [DOI] [PubMed] [Google Scholar]
- Challenger F. Biological Methylation. Chem. Rev. 1945, 36 (3), 315–361. 10.1021/cr60115a003. [DOI] [Google Scholar]
- Cullen W. R. Chemical Mechanism of Arsenic Biomethylation. Chem. Res. Toxicol. 2014, 27 (4), 457–461. 10.1021/tx400441h. [DOI] [PubMed] [Google Scholar]
- Cullen W. R.; McBride B. C.; Reglinski J. The Reduction of Trimethylarsine Oxide to Trimethylarsine by Thiols: A Mechanistic Model for the Biological Reduction of Arsenicals. J. Inorg. Biochem. 1984, 21 (1), 45–60. 10.1016/0162-0134(84)85038-2. [DOI] [Google Scholar]
- Drobna Z.; Naranmandura H.; Kubachka K. M.; Edwards B. C.; Herbin-Davis K.; Styblo M.; Le X. C.; Creed J. T.; Maeda N.; Hughes M. F.; Thomas D. J. Disruption of the Arsenic (+3 Oxidation State) Methyltransferase Gene in the Mouse Alters the Phenotype for Methylation of Arsenic and Affects Distribution and Retention of Orally Administered Arsenate. Chem. Res. Toxicol. 2009, 22 (10), 1713–1720. 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le X. C.; Ma M.; Lu X.; Cullen W. R.; Vasken Aposhian H.; Zheng B. Determination of Monomethylarsonous Acid, a Key Arsenic Methylation Intermediate, in Human Urine. Environ. Health Perspect. 2000, 108 (11), 1015–1018. 10.1289/ehp.001081015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C.; Smith-Gagen J.; Angermann J. Arsenic Methylation Capacity and Metabolic Syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES). Int. J. Environ. Res. Public Health 2018, 15 (1), 168. 10.3390/ijerph15010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Wu F.; Liu M.; Parvez F.; Slavkovich V.; Eunus M.; Ahmed A.; Argos M.; Islam T.; Rakibuz-Zaman M.; Hasan R.; Sarwar G.; Levy D.; Graziano J.; Ahsan H. A Prospective Study of Arsenic Exposure, Arsenic Methylation Capacity, and Risk of Cardiovascular Disease in Bangladesh. Environ. Health Perspect. 2013, 121 (7), 832–838. 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizam S.; Kato M.; Yatsuya H.; Khalequzzaman Md.; Ohnuma S.; Naito H.; Nakajima T. Differences in Urinary Arsenic Metabolites between Diabetic and Non-Diabetic Subjects in Bangladesh. Int. J. Environ. Res. Public Health 2013, 10 (3), 1006–1019. 10.3390/ijerph10031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Blasco R.; Murcia M.; Lozano M.; Sarzo B.; Esplugues A.; Riutort-Mayol G.; Vioque J.; Lertxundi N.; Santa Marina L.; Lertxundi A.; Irizar A.; Braeuer S.; Ballester F.; Llop S. Prenatal Arsenic Exposure, Arsenic Methylation Efficiency, and Neuropsychological Development among Preschool Children in a Spanish Birth Cohort. Environ. Res. 2022, 207, 112208 10.1016/j.envres.2021.112208. [DOI] [PubMed] [Google Scholar]
- Shen H.; Niu Q.; Xu M.; Rui D.; Xu S.; Feng G.; Ding Y.; Li S.; Jing M. Factors Affecting Arsenic Methylation in Arsenic-Exposed Humans: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2016, 13 (2), 205. 10.3390/ijerph13020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. J.; Argos M.; Tong L.; Li J.; Rakibuz-Zaman M.; Islam Md. T.; Slavkovich V.; Ahmed A.; Navas-Acien A.; Parvez F.; Chen Y.; Gamble M. V.; Graziano J. H.; Pierce B. L.; Ahsan H. Determinants and Consequences of Arsenic Metabolism Efficiency among 4,794 Individuals: Demographics, Lifestyle, Genetics, and Toxicity. Cancer Epidemiol. Biomarkers Prev. 2016, 25 (2), 381–390. 10.1158/1055-9965.EPI-15-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C.; Yang L.; Yu J.; Li H.; Wei B.; Guo Z.; Xia Y.; Wu K. Changes in Urinary Arsenic Species and Methylation Capacity in Original Arsenic Exposure Cohort after Water Quality Improvement. Environ. Geochem. Health 2020, 42 (9), 2841–2851. 10.1007/s10653-020-00523-4. [DOI] [PubMed] [Google Scholar]
- Soler-Blasco R.; Murcia M.; Lozano M.; Sarzo B.; Esplugues A.; Vioque J.; Lertxundi N.; Marina L. S.; Lertxundi A.; Irizar A.; Braeuer S.; Goesler W.; Ballester F.; Llop S. Urinary Arsenic Species and Methylation Efficiency during Pregnancy: Concentrations and Associated Factors in Spanish Pregnant Women. Environ. Res. 2021, 196, 110889 10.1016/j.envres.2021.110889. [DOI] [PubMed] [Google Scholar]
- Lin S.; Shi Q.; Nix F. B.; Styblo M.; Beck M. A.; Herbin-Davis K. M.; Hall L. L.; Simeonsson J. B.; Thomas D. J. A Novel S-Adenosyl-L-Methionine:Arsenic(III) Methyltransferase from Rat Liver Cytosol. J. Biol. Chem. 2002, 277 (13), 10795–10803. 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- Thomas D. J.; Li J.; Waters S. B.; Xing W.; Adair B. M.; Drobna Z.; Devesa V.; Stybloà M. Arsenic (+3 Oxidation State) Methyltransferase and the Methylation of Arsenicals. Exp. Biol. Med. 2007, 232 (1), 3–13. [PMC free article] [PubMed] [Google Scholar]
- Aposhian H. V.; Zheng B.; Aposhian M. M.; Le X. C.; Cebrian M. E.; Cullen W.; Zakharyan R. A.; Ma M.; Dart R. C.; Cheng Z.; Andrewes P.; Yip L.; O’Malley G. F.; Maiorino R. M.; Van Voorhies W.; Healy S. M.; Titcomb A. DMPS-Arsenic Challenge Test. II. Modulation of Arsenic Species, Including Monomethylarsonous Acid (MMA(III)), Excreted in Human Urine. Toxicol. Appl. Pharmacol. 2000, 165 (1), 74–83. 10.1006/taap.2000.8922. [DOI] [PubMed] [Google Scholar]
- Buchet J. P.; Lauwerys R.; Roels H. Comparison of the Urinary Excretion of Arsenic Metabolites After a Single Oral Dose of Sodium Arsenite, Monomethylarsonate, or Dimethylarsinate in Man. Int. Arch. Occup. Environ. Health 1981, 48 (1), 71–79. 10.1007/BF00405933. [DOI] [PubMed] [Google Scholar]
- Packianathan C.; Kandavelu P.; Rosen B. P. The Structure of an As(III) S-Adenosylmethionine Methyltransferase with 3-Coordinately Bound As(III) Depicts the First Step in Catalysis. Biochemistry 2018, 57 (28), 4083–4092. 10.1021/acs.biochem.8b00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S.; Li X.-F.; Cullen W. R.; Weinfeld M.; Le X. C. Arsenic Binding to Proteins. Chem. Rev. 2013, 113 (10), 7769–7792. 10.1021/cr300015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheeman D. S.; Packianathan C.; Pillai J. K.; Rosen B. P. Pathway of Human AS3MT Arsenic Methylation. Chem. Res. Toxicol. 2014, 27 (11), 1979–1989. 10.1021/tx500313k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T.; Kobayashi Y.; Cui X.; Hirano S. A New Metabolic Pathway of Arsenite: Arsenic-Glutathione Complexes Are Substrates for Human Arsenic Methyltransferase Cyt19. Arch. Toxicol. 2005, 79 (4), 183–191. 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- Naranmandura H.; Suzuki N.; Suzuki K. T. Trivalent Arsenicals Are Bound to Proteins during Reductive Methylation. Chem. Res. Toxicol. 2006, 19 (8), 1010–1018. 10.1021/tx060053f. [DOI] [PubMed] [Google Scholar]
- Fan C.; Liu G.; Long Y.; Rosen B.; Cai Y. Thiolation in Arsenic Metabolism: A Chemical Perspective. Metallomics 2018, 10 (10), 1368–1382. 10.1039/C8MT00231B. [DOI] [PubMed] [Google Scholar]
- Yamauchi H.; Agusa T.. Arsenic Metabolism and Toxicity in Humans and Animals: Racial and Species Differences. Current Topics in Environmental Health and Preventive Medicine Arsenic Contamination in Asia Biological Effects and Preventive Measures; Springer Nature: Singapore, 2019; pp 13–29, 10.1007/978-981-13-2565-6_1. [DOI] [Google Scholar]
- Naranmandura H.; Carew M. W.; Xu S.; Lee J.; Leslie E. M.; Weinfeld M.; Le X. C. Comparative Toxicity of Arsenic Metabolites in Human Bladder Cancer EJ-1 Cells. Chem. Res. Toxicol. 2011, 24 (9), 1586–1596. 10.1021/tx200291p. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Liu G.; Cai Y. Thiolated Arsenicals in Arsenic Metabolism: Occurrence, Formation, and Biological Implications. J. Environ. Sci. 2016, 49, 59–73. 10.1016/j.jes.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Petrick J. S.; Ayala-Fierro F.; Cullen W. R.; Carter D. E.; Vasken Aposhian H. Monomethylarsonous Acid (MMA(III)) Is More Toxic than Arsenite in Chang Human Hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163 (2), 203–207. 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Dopp E.; von Recklinghausen U.; Hartmann L. M.; Stueckradt I.; Pollok I.; Rabieh S.; Hao L.; Nussler A.; Katier C.; Hirner A. V.; Rettenmeier A. W. Subcellular Distribution of Inorganic and Methylated Arsenic Compounds in Human Urothelial Cells and Human Hepatocytes. Drug Metab. Dispos. 2008, 36 (5), 971–979. 10.1124/dmd.107.019034. [DOI] [PubMed] [Google Scholar]
- Naranmandura H.; Ibata K.; Suzuki K. T. Toxicity of Dimethylmonothioarsinic Acid toward Human Epidermoid Carcinoma A431 Cells. Chem. Res. Toxicol. 2007, 20 (8), 1120–1125. 10.1021/tx700103y. [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Sakurai K.; Shinde R.; Rodriguez M.; Rosen B. P.; El-Hage N. Comparative Cytotoxicity of Inorganic Arsenite and Methylarsenite in Human Brain Cells. ACS Chem. Neurosci. 2020, 11 (5), 743–751. 10.1021/acschemneuro.9b00653. [DOI] [PubMed] [Google Scholar]
- Moe B.; Peng H.; Lu X.; Chen B.; Chen L. W. L.; Gabos S.; Li X.-F.; Le X. C. Comparative Cytotoxicity of Fourteen Trivalent and Pentavalent Arsenic Species Determined Using Real-Time Cell Sensing. J. Environ. Sci. 2016, 49, 113–124. 10.1016/j.jes.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Dodmane P. R.; Arnold L. L.; Pennington K. L.; Singh R. K.; Cardoso A. P. F.; Cohen S. M. Effect of Trivalent Arsenicals on Cell Proliferation in Mouse and Human Microvascular Endothelial Cells. Toxicol. Rep. 2015, 2, 833–837. 10.1016/j.toxrep.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planer-Friedrich B.; Kerl C. F.; Colina Blanco A. E.; Clemens S. Dimethylated Thioarsenates: A Potentially Dangerous Blind Spot in Current Worldwide Regulatory Limits for Arsenic in Rice. J. Agric. Food Chem. 2022, 70 (31), 9610–9618. 10.1021/acs.jafc.2c02425. [DOI] [PubMed] [Google Scholar]
- Dai J.; Tang Z.; Gao A.-X.; Planer-Friedrich B.; Kopittke P. M.; Zhao F.-J.; Wang P. Widespread Occurrence of the Highly Toxic Dimethylated Monothioarsenate (DMMTA) in Rice Globally. Environ. Sci. Technol. 2022, 56 (6), 3575–3586. 10.1021/acs.est.1c08394. [DOI] [PubMed] [Google Scholar]
- Yamauchi H.; Takata A. Arsenic Metabolism Differs between Child and Adult Patients during Acute Arsenic Poisoning. Toxicol. Appl. Pharmacol. 2021, 410, 115352 10.1016/j.taap.2020.115352. [DOI] [PubMed] [Google Scholar]
- Jones M. R.; Tellez-Plaza M.; Vaidya D.; Grau M.; Francesconi K. A.; Goessler W.; Guallar E.; Post W. S.; Kaufman J. D.; Navas-Acien A. Estimation of Inorganic Arsenic Exposure in Populations with Frequent Seafood Intake: Evidence from MESA and NHANES. Am. J. Epidemiol. 2016, 184 (8), 590–602. 10.1093/aje/kww097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C. H.; Lin C.-Y.; Lai C.-H.; Jeng H. A. Arsenic Exposure and Methylation Efficiency in Relation to Oxidative Stress in Semiconductor Workers. Atmosphere 2020, 11 (5), 464. 10.3390/atmos11050464. [DOI] [Google Scholar]
- Guo M.; Lv J.; Chen X.; Wu M.; Zhao Q.; Hai X. Arsenic Trioxide Therapy During Pregnancy: ATO and Its Metabolites in Maternal Blood and Amniotic Fluid of Acute Promyelocytic Leukemia Patients. Front. Oncol. 2022, 12, 887026 10.3389/fonc.2022.887026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.; Du M.; Yang J.; Guo G.; Wang L.; Yan Y.; Li X.; Lei M.; Chen T. Changes in Arsenic Accumulation and Metabolic Capacity after Environmental Management Measures in Mining Area. Sci. Total Environ. 2023, 855, 158652 10.1016/j.scitotenv.2022.158652. [DOI] [PubMed] [Google Scholar]
- Komorowicz I.; Barałkiewicz D. Determination of Total Arsenic and Arsenic Species in Drinking Water, Surface Water, Wastewater, and Snow from Wielkopolska, Kujawy-Pomerania, and Lower Silesia Provinces, Poland. Environ. Monit. Assess 2016, 188 (9), 504. 10.1007/s10661-016-5477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal J. S.; Zheng Q.; Le X. C. Arsenic in Drinking Water—Recent Examples and Updates from Southeast Asia. Curr. Opin. Environ. Sci. Health 2019, 7, 126–135. 10.1016/j.coesh.2019.01.004. [DOI] [Google Scholar]
- Harrington C. F.; Brima E. I.; Jenkins R. O. Biotransformation of Arsenobetaine by Microorganisms from the Human Gastrointestinal Tract. Chem. Speciat. Bioavailab. 2008, 20 (3), 173–180. 10.3184/095422908X347278. [DOI] [Google Scholar]
- Brown R. M.; Newton D.; Pickford C. J.; Sherlock J. C. Human Metabolism of Arsenobetaine Ingested with Fish. Hum. Exp. Toxicol. 1990, 9 (1), 41–46. 10.1177/096032719000900109. [DOI] [PubMed] [Google Scholar]
- Taylor V.; Goodale B.; Raab A.; Schwerdtle T.; Reimer K.; Conklin S.; Karagas M. R.; Francesconi K. A. Human Exposure to Organic Arsenic Species from Seafood. Sci. Total Environ. 2017, 580, 266–282. 10.1016/j.scitotenv.2016.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga J.; Narukawa T. Dietary Intake and Urinary Excretion of Methylated Arsenicals of Japanese Adults Consuming Marine Foods and Rice. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38 (4), 622–629. 10.1080/19440049.2021.1877836. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A.; Francesconi K. A.; Silbergeld E. K.; Guallar E. Seafood Intake and Urine Concentrations of Total Arsenic, Dimethylarsinate and Arsenobetaine in the US Population. Environ. Res. 2011, 111 (1), 110–118. 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. N.; Price A. H.; Raab A.; Hossain S. A.; Feldmann J.; Meharg A. A. Variation in Arsenic Speciation and Concentration in Paddy Rice Related to Dietary Exposure. Environ. Sci. Technol. 2005, 39 (15), 5531–5540. 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- DeCastro B. R.; Caldwell K. L.; Jones R. L.; Blount B. C.; Pan Y.; Ward C.; Mortensen M. E. Dietary Sources of Methylated Arsenic Species in Urine of the United States Population, NHANES 2003–2010. PLoS One 2014, 9 (9), e108098 10.1371/journal.pone.0108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F.-J.; Zhu Y.-G.; Meharg A. A. Methylated Arsenic Species in Rice: Geographical Variation, Origin, and Uptake Mechanisms. Environ. Sci. Technol. 2013, 47 (9), 3957–3966. 10.1021/es304295n. [DOI] [PubMed] [Google Scholar]
- Kim J.-Y.; Kim W.-I.; Kunhikrishnan A.; Kang D.-W.; Kim D.-H.; Lee Y.-J.; Kim Y.-J.; Kim C.-T. Determination of Arsenic Species in Rice Grains Using HPLC-ICP-MS. Food Sci. Biotechnol. 2013, 22 (6), 1509–1513. 10.1007/s10068-013-0245-z. [DOI] [Google Scholar]
- Zavala Y. J.; Gerads R.; Gürleyük H.; Duxbury J. M. Arsenic in Rice: II. Arsenic Speciation in USA Grain and Implications for Human Health. Environ. Sci. Technol. 2008, 42 (10), 3861–3866. 10.1021/es702748q. [DOI] [PubMed] [Google Scholar]
- Limmer M. A.; Wise P.; Dykes G. E.; Seyfferth A. L. Silicon Decreases Dimethylarsinic Acid Concentration in Rice Grain and Mitigates Straighthead Disorder. Environ. Sci. Technol. 2018, 52 (8), 4809–4816. 10.1021/acs.est.8b00300. [DOI] [PubMed] [Google Scholar]
- Cascio C.; Raab A.; Jenkins R. O.; Feldmann J.; Meharg A. A.; Haris P. I. The Impact of a Rice Based Diet on Urinary Arsenic. J. Environ. Monit. 2011, 13 (2), 257–265. 10.1039/C0EM00482K. [DOI] [PubMed] [Google Scholar]
- Thomas D. J.; Bradham K. Role of Complex Organic Arsenicals in Food in Aggregate Exposure to Arsenic. J. Environ. Sci. 2016, 49, 86–96. 10.1016/j.jes.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Le X.-C.; Cullen W. R.; Reimer K. J. Human Urinary Arsenic Excretion after One-Time Ingestion of Seaweed, Crab, and Shrimp. Clin. Chem. 1994, 40 (4), 617–624. 10.1093/clinchem/40.4.617. [DOI] [PubMed] [Google Scholar]
- Ma M.; Le X. C. Effect of Arsenosugar Ingestion on Urinary Arsenic Speciation. Clin. Chem. 1998, 44 (3), 539–550. 10.1093/clinchem/44.3.539. [DOI] [PubMed] [Google Scholar]
- Wolle M. M.; Stadig S.; Conklin S. D. Market Basket Survey of Arsenic Species in the Top Ten Most Consumed Seafoods in the United States. J. Agric. Food Chem. 2019, 67 (29), 8253–8267. 10.1021/acs.jafc.9b02314. [DOI] [PubMed] [Google Scholar]
- Feldmann J.; Krupp E. M. Critical Review or Scientific Opinion Paper: Arsenosugars - a Class of Benign Arsenic Species or Justification for Developing Partly Speciated Arsenic Fractionation in Foodstuffs?. Anal. Bioanal. Chem. 2011, 399 (5), 1735–1741. 10.1007/s00216-010-4303-6. [DOI] [PubMed] [Google Scholar]
- Lee S.-G.; Kang I.; Seo M.-N.; Lee J.-E.; Eom S.-Y.; Hwang M.-S.; Park K. S.; Choi B.-S.; Kwon H.-J.; Hong Y.-S.; Kim H.; Park J.-D. Exposure Levels and Contributing Factors of Various Arsenic Species and Their Health Effects on Korean Adults. Arch. Environ. Contam. Toxicol. 2022, 82 (3), 391–402. 10.1007/s00244-022-00913-y. [DOI] [PubMed] [Google Scholar]
- Sim R.; Feldmann J.; Stengel D. B.; Pétursdóttir Á. H. Temporal and Intra-Thallus Variation in Arsenic Species in the Brown Macroalga Laminaria Digitata. Environ. Chem. 2023, 20 (1), 55–65. 10.1071/EN22123. [DOI] [Google Scholar]
- Li W.; Wei C.; Zhang C.; Van Hulle M.; Cornelis R.; Zhang X. A Survey of Arsenic Species in Chinese Seafood. Food Chem. Toxicol. 2003, 41 (8), 1103–1110. 10.1016/S0278-6915(03)00063-2. [DOI] [PubMed] [Google Scholar]
- Soeroes C.; Goessler W.; Francesconi K. A.; Schmeisser E.; Raml R.; Kienzl N.; Kahn M.; Fodor P.; Kuehnelt D. Thio Arsenosugars in Freshwater Mussels from the Danube in Hungary. J. Environ. Monit. 2005, 7 (7), 688–692. 10.1039/b503897a. [DOI] [PubMed] [Google Scholar]
- Pétursdóttir Á. H.; Blagden J.; Gunnarsson K.; Raab A.; Stengel D. B.; Feldmann J.; Gunnlaugsdóttir H. Arsenolipids Are Not Uniformly Distributed within Two Brown Macroalgal Species Saccharina Latissima and Alaria Esculenta. Anal. Bioanal. Chem. 2019, 411 (19), 4973–4985. 10.1007/s00216-019-01907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M. H. A.; Xiong C.; Glabonjat R. A.; Francesconi K. A.; Oguri T.; Yoshinaga J. Estimation of Daily Intake of Arsenolipids in Japan Based on a Market Basket Survey. Food Chem. Toxicol. 2018, 118, 245–251. 10.1016/j.fct.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Schmeisser E.; Goessler W.; Francesconi K. A. Human Metabolism of Arsenolipids Present in Cod Liver. Anal. Bioanal. Chem. 2006, 385 (2), 367–376. 10.1007/s00216-006-0401-x. [DOI] [PubMed] [Google Scholar]
- Schmeisser E.; Rumpler A.; Kollroser M.; Rechberger G.; Goessler W.; Francesconi K. A. Arsenic Fatty Acids Are Human Urinary Metabolites of Arsenolipids Present in Cod Liver. Angew. Chem., Int. Ed. 2006, 45 (1), 150–154. 10.1002/anie.200502706. [DOI] [PubMed] [Google Scholar]
- Raml R.; Goessler W.; Traar P.; Ochi T.; Francesconi K. A. Novel Thioarsenic Metabolites in Human Urine after Ingestion of an Arsenosugar, 2′,3′-Dihydroxypropyl 5-Deoxy-5-Dimethylarsinoyl- β-D-Riboside. Chem. Res. Toxicol. 2005, 18 (9), 1444–1450. 10.1021/tx050111h. [DOI] [PubMed] [Google Scholar]
- Taylor V. F.; Li Z.; Sayarath V.; Palys T. J.; Morse K. R.; Scholz-Bright R. A.; Karagas M. R. Distinct Arsenic Metabolites Following Seaweed Consumption in Humans. Sci. Rep. 2017, 7 (1), 3920. 10.1038/s41598-017-03883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raml R.; Raber G.; Rumpler A.; Bauernhofer T.; Goessler W.; Francesconi K. A. Individual Variability in the Human Metabolism of an Arsenic-Containing Carbohydrate, 2′,3′-Dihydroxypropyl 5-Deoxy-5-Dimethylarsinoyl- β-D-Riboside, a Naturally Occurring Arsenical in Seafood. Chem. Res. Toxicol. 2009, 22 (9), 1534–1540. 10.1021/tx900158h. [DOI] [PubMed] [Google Scholar]
- Calatayud M.; Xiong C.; Du Laing G.; Raber G.; Francesconi K.; van de Wiele T. Salivary and Gut Microbiomes Play a Significant Role in in Vitro Oral Bioaccessibility, Biotransformation, and Intestinal Absorption of Arsenic from Food. Environ. Sci. Technol. 2018, 52 (24), 14422–14435. 10.1021/acs.est.8b04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A.; Hasegawa M.; Yamauchi T.; Otomo Y.; Miura M.; Yamanaka K.; Yamano Y.; Fujitani N.; Endo G. Metabolism of 3-[5′-Deoxy-5′-(Dimethylarsinoyl)-β-Ribofuranosyloxy]-2-Hydroxypropylene Glycol in an Artificial Digestive System. Heliyon 2019, 5 (7), e02079 10.1016/j.heliyon.2019.e02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez-Capilla T.; Beshai M.; Maher W.; Kelly T.; Foster S. Bioaccessibility and Degradation of Naturally Occurring Arsenic Species from Food in the Human Gastrointestinal Tract. Food Chem. 2016, 212, 189–197. 10.1016/j.foodchem.2016.05.163. [DOI] [PubMed] [Google Scholar]
- Pengprecha P.; Wilson M.; Raab A.; Feldmann J. Biodegradation of Arsenosugars in Marine Sediment. Appl. Organomet. Chem. 2005, 19 (7), 819–826. 10.1002/aoc.579. [DOI] [Google Scholar]
- Duncan E. G.; Maher W. A.; Foster S. D. The Formation and Fate of Organoarsenic Species in Marine Ecosystems: Do Existing Experimental Approaches Appropriately Simulate Ecosystem Complexity?. Environ. Chem. 2015, 12 (2), 149–162. 10.1071/EN14124. [DOI] [Google Scholar]
- Duncan E. G.; Maher W. A.; Foster S. D.; Krikowa F.; Mikac K. M. The Degradation of Arsenoribosides from Ecklonia Radiata Tissues Decomposed in Natural and Microbially Manipulated Microcosms. Environ. Chem. 2014, 11 (3), 289–300. 10.1071/EN13155. [DOI] [Google Scholar]
- Duncan E. G.; Maher W. A.; Foster S. D.; Mikac K. M.; Krikowa F.; Florance A. Arsenoriboside Degradation in Marine Systems: The Use of Bacteria Culture Incubation Experiments as Model Systems. Chemosphere 2014, 95, 635–638. 10.1016/j.chemosphere.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Xue X.-M.; Wang H.-Y.; Yu X.-W.; Hu S.; Huang L.-J.; Yang H.-C.; Gong L.; Yang K.; Li H.-B.; Zhu Y.-G. Gut Microbiota Control the Bioavailability and Metabolism of Organoarsenicals of Seaweeds in Mice after Oral Ingestion. Environ. Sci. Technol. 2023, 57 (23), 8588–8597. 10.1021/acs.est.2c09167. [DOI] [PubMed] [Google Scholar]
- Hata A.; Yamanaka K.; Endo G.; Yamano Y.; Haba R.; Fujitani N.; Endo Y.. Arsenic Metabolites in Humans after Ingestion of Wakame Seaweed. Arsenic metabolites in humans after ingestion of wakame seaweed; E3S Web of Conferences; EDP Sciences, 2013; Vol. 1, p 26006. 10.1051/e3sconf/201301260. [DOI] [Google Scholar]
- Francesconi K. A.; Tanggaard R.; McKenzie C. J.; Goessler W. Arsenic Metabolites in Human Urine after Ingestion of an Arsenosugar. Drug Monit. Toxicol. 2002, 48 (1), 92–101. 10.1093/clinchem/48.1.92. [DOI] [PubMed] [Google Scholar]
- Choi B.-S.; Choi S.-J.; Kim D.-W.; Huang M.; Kim N.-Y.; Park K.-S.; Kim C.-Y.; Lee H.-M.; Yum Y.-N.; Han E.-S.; Kang T.-S.; Yu I.-J.; Park J.-D. Effects of Repeated Seafood Consumption on Urinary Excretion of Arsenic Species by Volunteers. Arch. Environ. Contam. Toxicol. 2010, 58 (1), 222–229. 10.1007/s00244-009-9333-8. [DOI] [PubMed] [Google Scholar]
- Choi J. W.; Song Y. C.; Cheong N.-Y.; Lee K.; Kim S.; Lee K.-M.; Ji K.; Shin M.-Y.; Kim S. Concentrations of Blood and Urinary Arsenic Species and Their Characteristics in General Korean Population. Environ. Res. 2022, 214 (Pt 2), 113846 10.1016/j.envres.2022.113846. [DOI] [PubMed] [Google Scholar]
- Huang Y.-K.; Huang Y.-L.; Hsueh Y.-M.; Wang J. T.-J.; Yang M.-H.; Chen C.-J. Changes in Urinary Arsenic Methylation Profiles in a 15-Year Interval after Cessation of Arsenic Ingestion in Southwest Taiwan. Environ. Health Perspect. 2009, 117 (12), 1860–1866. 10.1289/ehp.0900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Mirandes T.; Ruiz-Chancho M. J.; Barbero M.; Rubio R.; López-Sánchez J. F. Measurement of Arsenic Compounds in Littoral Zone Algae from the Western Mediterranean Sea. Occurrence of Arsenobetaine. Chemosphere 2010, 81 (7), 867–875. 10.1016/j.chemosphere.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Taylor V. F.; Jackson B. P. Concentrations and Speciation of Arsenic in New England Seaweed Species Harvested for Food and Agriculture. Chemosphere 2016, 163, 6–13. 10.1016/j.chemosphere.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luvonga C.; Rimmer C. A.; Yu L. L.; Lee S. B. Determination of Total Arsenic and Hydrophilic Arsenic Species in Seafood. J. Food Compos. Anal. 2021, 96, 103729 10.1016/j.jfca.2020.103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almela C.; Laparra J. M.; Vélez D.; Barberá R.; Farré R.; Montoro R. Arsenosugars in Raw and Cooked Edible Seaweed: Characterization and Bioaccessibility. J. Agric. Food Chem. 2005, 53 (18), 7344–7351. 10.1021/jf050503u. [DOI] [PubMed] [Google Scholar]
- Raml R.; Rumpler A.; Goessler W.; Vahter M.; Li L.; Ochi T.; Francesconi K. A. Thio-Dimethylarsinate Is a Common Metabolite in Urine Samples from Arsenic-Exposed Women in Bangladesh. Toxicol. Appl. Pharmacol. 2007, 222 (3), 374–380. 10.1016/j.taap.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Le X. C.; Ma M.; W-M Lai V.. Exposure to Arsenosugars from Seafood Ingestion and Speciation of Urinary Arsenic Metabolites. Proceedings of the Third International Conference on Arsenic Exposure and Health; Elsevier Science: San Diego, CA, 1999; pp 69–79.
- Yoshinaga J.; Chatterjee A.; Shibata Y.; Morita M.; Edmonds J. S. Human Urine Certified Reference Material for Arsenic Speciation. Drug Monit. Toxicol. 2000, 46 (11), 1781–1786. 10.1093/clinchem/46.11.1781. [DOI] [PubMed] [Google Scholar]
- Sloth J. J.; Larsen E. H.; Julshanm K. Selective Arsenic Speciation Analysis of Human Urine Reference Materials Using Gradient Elution Ion-Exchange HPLC-ICP-MS. J. Anal. At. Spectrom. 2004, 19 (8), 973–978. 10.1039/b402994a. [DOI] [Google Scholar]
- Raml R.; Goessler W.; Francesconi K. A. Improved Chromatographic Separation of Thio-Arsenic Compounds by Reversed-Phase High Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. A 2006, 1128 (1–2), 164–170. 10.1016/j.chroma.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Cullen W. R.; Liu Q.; Lu X.; McKnight-Whitford A.; Peng H.; Popowich A.; Yan X.; Zhang Q.; Fricke M.; Sun H.; Le X. C. Methylated and Thiolated Arsenic Species for Environmental and Health Research — A Review on Synthesis and Characterization. J. Environ. Sci. 2016, 49, 7–27. 10.1016/j.jes.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Reid M. S.; Hoy K. S.; Schofield J. R.M.; Uppal J. S.; Lin Y.; Lu X.; Peng H.; Le X. C. Arsenic Speciation Analysis: A Review with an Emphasis on Chromatographic Separations. Trends Anal. Chem. 2020, 123 (5), 115770. 10.1016/j.trac.2019.115770. [DOI] [Google Scholar]
- Gong Z.; Lu X.; Cullen W. R.; Chris Le X. Unstable Trivalent Arsenic Metabolites, Monomethylarsonous Acid and Dimethylarsinous Acid. J. Anal. At. Spectrom. 2001, 16 (12), 1409–1419. 10.1039/b105834g. [DOI] [Google Scholar]
- Narukawa T.; Raber G.; Itoh N.; Inagaki K. A New Candidate Reference Material for Inorganic Arsenic and Arsenosugars in Hijiki Seaweed: First Results from an Interlaboratory Study. Anal. Sci. 2020, 36 (2), 233–239. 10.2116/analsci.19P306. [DOI] [PubMed] [Google Scholar]
- Leese E.; Clench M.; Morton J.; Gardiner P. H. E.; Carolan V. A. The Investigation of Unexpected Arsenic Compounds Observed in Routine Biological Monitoring Urinary Speciation Analysis. Toxics 2017, 5 (2), 12. 10.3390/toxics5020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



