Abstract
Background
Newborn infants are more prone to seizures than older children and adults. The neuronal injury caused by seizures in neonates often results in long‐term neurodevelopmental sequelae. There are several options for anti‐seizure medications (ASMs) in neonates. However, the ideal choice of first‐, second‐ and third‐line ASM is still unclear. Further, many other aspects of seizure management such as whether ASMs should be initiated for only‐electrographic seizures and how long to continue the ASM once seizure control is achieved are elusive.
Objectives
1. To assess whether any ASM is more or less effective than an alternative ASM (both ASMs used as first‐, second‐ or third‐line treatment) in achieving seizure control and improving neurodevelopmental outcomes in neonates with seizures. We analysed EEG‐confirmed seizures and clinically‐diagnosed seizures separately.
2. To assess maintenance therapy with ASM versus no maintenance therapy after achieving seizure control. We analysed EEG‐confirmed seizures and clinically‐diagnosed seizures separately.
3. To assess treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates.
Search methods
We searched MEDLINE, Embase, CENTRAL, Epistemonikos and three databases in May 2022 and June 2023. These searches were not limited other than by study design to trials.
Selection criteria
We included randomised controlled trials (RCTs) that included neonates with EEG‐confirmed or clinically diagnosed seizures and compared (1) any ASM versus an alternative ASM, (2) maintenance therapy with ASM versus no maintenance therapy, and (3) treatment of clinical or EEG seizures versus treatment of clinical seizures alone.
Data collection and analysis
Two review authors assessed trial eligibility, risk of bias and independently extracted data. We analysed treatment effects in individual trials and reported risk ratio (RR) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence interval (CI). We used GRADE to assess the certainty of evidence.
Main results
We included 18 trials (1342 infants) in this review.
Phenobarbital versus levetiracetam as first‐line ASM in EEG‐confirmed neonatal seizures (one trial)
Phenobarbital is probably more effective than levetiracetam in achieving seizure control after first loading dose (RR 2.32, 95% CI 1.63 to 3.30; 106 participants; moderate‐certainty evidence), and after maximal loading dose (RR 2.83, 95% CI 1.78 to 4.50; 106 participants; moderate‐certainty evidence). However, we are uncertain about the effect of phenobarbital when compared to levetiracetam on mortality before discharge (RR 0.30, 95% CI 0.04 to 2.52; 106 participants; very low‐certainty evidence), requirement of mechanical ventilation (RR 1.21, 95% CI 0.76 to 1.91; 106 participants; very low‐certainty evidence), sedation/drowsiness (RR 1.74, 95% CI 0.68 to 4.44; 106 participants; very low‐certainty evidence) and epilepsy post‐discharge (RR 0.92, 95% CI 0.48 to 1.76; 106 participants; very low‐certainty evidence). The trial did not report on mortality or neurodevelopmental disability at 18 to 24 months.
Phenobarbital versus phenytoin as first‐line ASM in EEG‐confirmed neonatal seizures (one trial)
We are uncertain about the effect of phenobarbital versus phenytoin on achieving seizure control after maximal loading dose of ASM (RR 0.97, 95% CI 0.54 to 1.72; 59 participants; very low‐certainty evidence). The trial did not report on mortality or neurodevelopmental disability at 18 to 24 months.
Maintenance therapy with ASM versus no maintenance therapy in clinically diagnosed neonatal seizures (two trials)
We are uncertain about the effect of short‐term maintenance therapy with ASM versus no maintenance therapy during the hospital stay (but discontinued before discharge) on the risk of repeat seizures before hospital discharge (RR 0.76, 95% CI 0.56 to 1.01; 373 participants; very low‐certainty evidence). Maintenance therapy with ASM compared to no maintenance therapy may have little or no effect on mortality before discharge (RR 0.69, 95% CI 0.39 to 1.22; 373 participants; low‐certainty evidence), mortality at 18 to 24 months (RR 0.94, 95% CI 0.34 to 2.61; 111 participants; low‐certainty evidence), neurodevelopmental disability at 18 to 24 months (RR 0.89, 95% CI 0.13 to 6.12; 108 participants; low‐certainty evidence) and epilepsy post‐discharge (RR 3.18, 95% CI 0.69 to 14.72; 126 participants; low‐certainty evidence).
Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates (two trials)
Treatment of both clinical and electrographic seizures when compared to treating clinical seizures alone may have little or no effect on seizure burden during hospitalisation (MD ‐1871.16, 95% CI ‐4525.05 to 782.73; 68 participants; low‐certainty evidence), mortality before discharge (RR 0.59, 95% CI 0.28 to 1.27; 68 participants; low‐certainty evidence) and epilepsy post‐discharge (RR 0.75, 95% CI 0.12 to 4.73; 35 participants; low‐certainty evidence). The trials did not report on mortality or neurodevelopmental disability at 18 to 24 months.
We report data from the most important comparisons here; readers are directed to Results and Summary of Findings tables for all comparisons.
Authors' conclusions
Phenobarbital as a first‐line ASM is probably more effective than levetiracetam in achieving seizure control after the first loading dose and after the maximal loading dose of ASM (moderate‐certainty evidence). Phenobarbital + bumetanide may have little or no difference in achieving seizure control when compared to phenobarbital alone (low‐certainty evidence). Limited data and very low‐certainty evidence preclude us from drawing any reasonable conclusion on the effect of using one ASM versus another on other short‐ and long‐term outcomes.
In neonates who achieve seizure control after the first loading dose of phenobarbital, maintenance therapy compared to no maintenance ASM may have little or no effect on all‐cause mortality before discharge, mortality by 18 to 24 months, neurodevelopmental disability by 18 to 24 months and epilepsy post‐discharge (low‐certainty evidence).
In neonates with hypoxic‐ischaemic encephalopathy, treatment of both clinical and electrographic seizures when compared to treating clinical seizures alone may have little or no effect on seizure burden during hospitalisation, all‐cause mortality before discharge and epilepsy post‐discharge (low‐certainty evidence).
All findings of this review apply only to term and late preterm neonates.
We need well‐designed RCTs for each of the three objectives of this review to improve the precision of the results. These RCTs should use EEG to diagnose seizures and should be adequately powered to assess long‐term neurodevelopmental outcomes. We need separate RCTs evaluating the choice of ASM in preterm infants.
Keywords: Adolescent; Adult; Child; Humans; Infant; Infant, Newborn; Epilepsy; Epilepsy/drug therapy; Levetiracetam; Levetiracetam/therapeutic use; Phenobarbital; Phenobarbital/therapeutic use; Phenytoin; Phenytoin/therapeutic use; Seizures; Seizures/drug therapy
Plain language summary
Medication to treat fits in newborn babies
Review questions
What medication can be used effectively and safely to treat seizures in newborns?
How long should the medication for seizures be continued once started?
Should we treat seizures that are seen only on the EEG?
Note:
EEG is a test to analyse the electrical activity of the brain. It identifies seizure activity as well.
Phenobarbital and levetiracetam are anti‐seizure medications used in newborns.
'Maintenance treatment' refers to continuing the anti‐seizure medication at a smaller dose, once seizures are stopped with a larger dose of the medication.
Key messages
Phenobarbital is probably more effective than levetiracetam in achieving seizure control in newborns. However, we are uncertain about the effect of phenobarbital compared to levetiracetam on other outcomes.
Maintenance treatment with anti‐seizure medication during hospital stay and treating seizures only identified on EEG may or may not result in better outcomes in newborns.
Background
Newborns are more prone to develop seizures when compared to older children and adults. The brain damage caused by seizures in newborns is associated with cerebral palsy, intellectual disability, learning problems and a tendency to develop epilepsy in the future. There are only a few options for medications to treat seizures in newborns, and we do not know which is the ideal medication to use first, second or third. Similarly, whether to treat the seizures that are seen only on EEG and how long to continue the anti‐seizure medication is also not clear.
What did we want to find?
We looked for evidence from studies that assessed one medication versus another to treat seizures in newborns, studies that evaluated whether maintenance doses of anti‐seizure medication should be continued or not, and studies that assessed whether to treat seizures that were identified only on EEG.
What did we do?
We searched for studies that evaluated the effects of medications on treating seizures in newborns. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We identified 18 trials (including 1342 newborns).
Phenobarbital is probably more effective than levetiracetam in achieving seizure control in newborns. However, we are uncertain about the effect of phenobarbital on other outcomes such as death before discharge, requirement for invasive ventilation, sleepiness and epilepsy after discharge.
Maintenance therapy with anti‐seizure medication during hospital stay compared to no maintenance therapy may or may not result in better outcomes for newborns. Similarly, treating seizures only identified on EEG may or may not result in better outcomes.
What are the limitations of the evidence?
We are moderately confident that phenobarbital is better than levetiracetam in achieving seizure control. The confidence for the estimates of all other comparisons and outcomes is low to very low. More studies are needed to synthesise strong evidence on medications to treat seizures in newborns.
How up‐to‐date is this evidence?
Evidence is up‐to‐date as of June 2023.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures.
| Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures | ||||||
| Patient or population: neonates with EEG‐confirmed seizures Setting: Neonatal intensive care unit Intervention: phenobarbital as first‐line ASM Comparison: levetiracetam as first‐line ASM | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with levetiracetam as first‐line ASM | Risk with phenobarbital as first‐line ASM | |||||
| Proportion of infants who achieve seizure control after the first loading dose of ASM | 359 per 1000 | 834 per 1000 (586 to 1000) | RR 2.32 (1.63 to 3.30) | 106 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| Proportion of infants who achieve seizure control after the maximal loading dose of ASM | 283 per 1000 | 801 per 1000 (504 to 1000) | RR 2.83 (1.78 to 4.50) | 83 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| Mortality or neurodevelopment disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The trial did not report this outcome. |
| Mortality before hospital discharge | 78 per 1000 | 23 per 1000 (3 to 197) | RR 0.30 (0.04 to 2.52) | 106 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | |
| Requirement of mechanical ventilation | 375 per 1000 | 454 per 1000 (285 to 716) | RR 1.21 (0.76 to 1.91) | 106 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | |
| Proportion of infants who develop sedation or drowsiness | 109 per 1000 | 190 per 1000 (74 to 486) | RR 1.74 (0.68 to 4.44) | 106 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | |
| Proportion of infants who develop epilepsy post‐discharge | 481 per 1000 | 443 per 1000 (231 to 847) | RR 0.92 (0.48 to 1.76) | 45 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438900176882570386. | ||||||
a Downgraded by one level for serious imprecision due to small small size not meeting the 'Optimal Information Size' criteria b Downgraded by one level for indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well. c Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria
Summary of findings 2. Summary of findings table ‐ Phenobarbital versus levetiracetam as first‐line ASM for clinically diagnosed neonatal seizures.
| Phenobarbital versus levetiracetam as first‐line ASM for clinically diagnosed neonatal seizures | ||||||
| Patient or population: clinically diagnosed neonatal seizures Setting: Neonatal intensive care unit Intervention: phenobarbital as first‐line ASM Comparison: levetiracetam as first‐line ASM | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with levetiracetam as first‐line ASM | Risk with phenobarbital as first‐line ASM | |||||
| Proportion of infants who achieve seizure control after first loading dose of ASM | 443 per 1000 | 306 per 1000 (244 to 381) | RR 0.69 (0.55 to 0.86) | 286 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | |
| Proportion of infants who achieve seizure control after maximal loading dose of ASM | 777 per 1000 | 451 per 1000 (365 to 559) | RR 0.58 (0.47 to 0.72) | 260 (3 RCTs) | ⊕⊝⊝⊝ Very lowb,c | |
| Mortality or neurodevelopment disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in any included trial |
| Mortality before hospital discharge | 82 per 1000 | 116 per 1000 (67 to 200) | RR 1.41 (0.82 to 2.43) | 452 (6 RCTs) | ⊕⊕⊝⊝ Lowd,e | |
| Requirement of mechanical ventilation | 5 per 1000 | 11 per 1000 (3 to 49) | RR 2.20 (0.50 to 9.68) | 394 (5 RCTs) | ⊕⊝⊝⊝ Very lowd,f | |
| Proportion of infants who develop sedation or drowsiness | 54 per 1000 | 102 per 1000 (36 to 292) | RR 1.88 (0.66 to 5.37) | 180 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e,g | |
| Proportion of infants who develop epilepsy post discharge | 133 per 1000 | 67 per 1000 (7 to 659) | RR 0.50 (0.05 to 4.94) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowf,h | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_442643727142716799. | ||||||
a Downgraded by two levels for very serious risk of bias due to 'high risk of bias' in 2 trials and some concerns in the other trial b Downgraded by one level for serious imprecision due to small sample size not meeting the 'Optimal Information Size' criterion c Downgraded by two levels for very serious risk of bias due to high risk of bias in all included studies d Downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well e Downgraded by one level for serious imprecision due to low event rate not meeting the 'Optimal Information Size' criteria f Downgraded by two levels for very serious imprecision due to single digit event rate g Downgraded by one level for serious inconsistency due to substantial heterogeneity h Downgraded by two levels for very serious risk of bias due to high risk of bias in the only included study
Summary of findings 3. Summary of findings table ‐ Phenobarbital versus phenytoin as first‐line ASM for EEG‐confirmed neonatal seizures.
| Phenobarbital versus phenytoin as first‐line ASM for EEG‐confirmed neonatal seizures | ||||||
| Patient or population: neonates with EEG‐confirmed seizures Setting: Neonatal intensive care unit Intervention: phenobarbital as first‐line ASM Comparison: phenytoin as first‐line ASM | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with phenytoin as first‐line ASM | Risk with phenobarbital as first‐line ASM | |||||
| Proportion of infants who achieve seizure control after the first loading dose of ASM ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The trial did not report this outcome |
| Proportion of infants who achieve seizure control after the maximal loading dose of ASM | 448 per 1000 | 435 per 1000 (242 to 771) | RR 0.97 (0.54 to 1.72) | 59 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Mortality or neurodevelopmental disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The trial did not report this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438951560046041157. | ||||||
a Downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial b Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria
Summary of findings 4. Summary of findings table ‐ Phenobarbital versus phenytoin as first‐line ASM for clinically diagnosed neonatal seizures.
| Phenobarbital versus phenytoin as first‐line ASM for clinically diagnosed neonatal seizures | ||||||
| Patient or population: neonates with clinically diagnosed seizures Setting: Neonatal intensive care unit Intervention: phenobarbital as first‐line ASM Comparison: phenytoin as first‐line ASM | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with phenytoin as first‐line ASM | Risk with phenobarbital as first‐line ASM | |||||
| Proportion of infants who achieve seizure control after first loading dose of ASM | 356 per 1000 | 683 per 1000 (498 to 939) | RR 1.92 (1.40 to 2.64) | 179 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Proportion of infants who achieve seizure control after maximal loading dose ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Neither of the two included trials reported this outcome. |
| Mortality or neurodevelopmental disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Neither of the two included trials reported this outcome. |
| Mortality before hospital discharge | 211 per 1000 | 281 per 1000 (167 to 477) | RR 1.33 (0.79 to 2.26) | 179 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | |
| Requirement of mechanical ventilation | 0 per 1000 | 0 per 1000 (0 to 0) | RR 7.13 (0.38 to 134.78) | 109 (1 RCT) | ⊕⊝⊝⊝ Very lowd,f | |
| Proportion of infants who develop sedation or drowsiness | 0 per 1000 | 0 per 1000 (0 to 0) | RR 23.00 (1.41 to 375.77) | 70 (1 RCT) | ⊕⊝⊝⊝ Very lowd,f,g | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438949398863786458. | ||||||
a Downgraded by one level for serious risk of bias as the trial contributing > 50% weighting to the estimate has a high risk of overall bias b Downgraded by one level for serious inconsistency as there was considerable heterogeneity (I2 = 96%) c Downgraded by one level for serious inconsistency as there was substantial heterogeneity (I2=82%) d Downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well e Downgraded by one level for serious imprecision for sample size and event rate not meeting the 'Optimal Information Size' criteria f Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria g Downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial
Summary of findings 5. Summary of findings table ‐ Phenobarbital versus Lorazepam as first‐line ASM for clinically diagnosed neonatal seizures.
| Phenobarbital versus Lorazepam as first‐line ASM for clinically diagnosed neonatal seizures | ||||||
| Patient or population: Neonates with clinically diagnosed seizures Setting: Neonatal intensive care unit Intervention: Phenobarbital as first‐line ASM Comparison: Lorazepam as first‐line ASM | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Lorazepam as first‐line ASM | Risk with Phenobarbital as first‐line ASM | |||||
| Proportion of infants who achieve seizure control after the first loading dose of ASM | 889 per 1000 | 631 per 1000 (471 to 836) | RR 0.71 (0.53 to 0.94) | 71 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Proportion of infants who achieve seizure control after the maximal loading dose of ASM ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome |
| Mortality or neurodevelopmental disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome. |
| Mortality before hospital discharge | 194 per 1000 | 342 per 1000 (154 to 768) | RR 1.76 (0.79 to 3.95) | 71 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d | |
| Proportion of infants who develop sedation or drowsiness | 56 per 1000 | 314 per 1000 (75 to 1000) | RR 5.66 (1.35 to 23.71) | 71 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438956545731624502. | ||||||
a Downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial b Downgraded by one level for serious imprecision for sample size and event rate not meeting the 'Optimal Information Size' criteria c Downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well d Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria
Summary of findings 6. Summary of findings table ‐ Phenytoin versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures.
| Phenytoin versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures | ||||||
| Patient or population: neonates with clinically diagnosed seizures Setting: Neonatal intensive care unit Intervention: phenytoin as first‐line ASM Comparison: lorazepam as first‐line ASM | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lorazepam as first‐line ASM | Risk with phenytoin as first‐line ASM | |||||
| Proportion of infants who achieve seizure control after the first loading dose of ASM | 889 per 1000 | 684 per 1000 (533 to 880) | RR 0.77 (0.60 to 0.99) | 71 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Proportion of infants who achieve seizure control after the maximal loading dose of ASM ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome. |
| Mortality or neurodevelopmental disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome. |
| Mortality before hospital discharge | 194 per 1000 | 86 per 1000 (23 to 305) | RR 0.44 (0.12 to 1.57) | 71 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d | |
| Proportion of infants who develop sedation or drowsiness | 56 per 1000 | 12 per 1000 (1 to 229) | RR 0.21 (0.01 to 4.13) | 71 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438956906630512363. | ||||||
a Downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial b Downgraded by one level for serious imprecision for sample size and event rate not meeting the 'Optimal Information Size' criteria c Downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well. d Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria
Summary of findings 7. Summary of findings table ‐ Phenobarbital + bumetanide versus phenobarbital alone for EEG‐confirmed neonatal seizures.
| Phenobarbital + bumetanide versus phenobarbital alone for EEG‐confirmed neonatal seizures | ||||||
| Patient or population: neonates with EEG‐confirmed seizures Setting: Neonatal intensive care unit Intervention: phenobarbital + bumetanide Comparison: phenobarbital alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with phenobarbital alone | Risk with phenobarbital + bumetanide | |||||
| Proportion of infants who achieve seizure control after the first loading dose of ASM | 313 per 1000 | 297 per 1000 (116 to 750) | RR 0.95 (0.37 to 2.40) | 43 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| Proportion of infants who achieve seizure control after the maximal loading dose of the ASM ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome. |
| Mortality or neurodevelopmental disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome. |
| Mortality before hospital discharge | 188 per 1000 | 38 per 1000 (4 to 326) | RR 0.20 (0.02 to 1.74) | 43 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Cognitive impairment at 18‐24 months | 300 per 1000 | 159 per 1000 (39 to 645) | RR 0.53 (0.13 to 2.15) | 29 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Requirement of mechanical ventilation | Not pooled | Not pooled | Not pooled | (1 RCT) | ‐ | |
| Proportion of infants who develop epilepsy post‐discharge | 308 per 1000 | 348 per 1000 (132 to 914) | RR 1.13 (0.43 to 2.97) | 39 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438957230819281917. | ||||||
a Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria b Downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well
Summary of findings 8. Summary of findings table ‐ Lignocaine versus benzodiazepines as second‐line ASM for EEG‐confirmed neonatal seizures.
| Lignocaine versus benzodiazepines as second‐line ASM for EEG‐confirmed neonatal seizures | ||||||
| Patient or population: neonates with EEG‐confirmed seizures Setting: Neonatal intensive care unit Intervention: lignocaine as second‐line ASM Comparison: benzodiazepines as second‐line ASM | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with benzodiazepines as second‐line ASM | Risk with lignocaine as second‐line ASM | |||||
| Proportion of infants who achieve seizure control after first loading dose of ASM ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome. |
| Proportion of infants who achieve seizure control after maximal loading dose of ASM | 0 per 1000 | 0 per 1000 (0 to 0) | RR 8.17 (0.52 to 128.42) | 11 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Mortality or neurodevelopmental disability at 12 months | 1000 per 1000 | 1000 per 1000 (710 to 1000) | RR 1.00 (0.71 to 1.41) | 10 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Mortality before hospital discharge | 333 per 1000 | 400 per 1000 (83 to 1000) | RR 1.20 (0.25 to 5.71) | 11 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| Neurodevelopmental disability at 12 months | 600 per 1000 | 600 per 1000 (216 to 1000) | RR 1.00 (0.36 to 2.75) | 10 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438957465149804345. | ||||||
a Downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial b Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria c Downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well
Summary of findings 9. Summary of findings table ‐ Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures.
| Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures | ||||||
| Patient or population: neonates with clinically diagnosed seizures Setting: Neonatal intensive care unit Intervention: maintenance ASM after achieving seizure control Comparison: no maintenance ASM after achieving seizure control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no maintenance ASM after achieving seizure control | Risk with maintenance ASM after achieving seizure control | |||||
| Proportion of infants with repeat seizure before hospital discharge | 353 per 1000 | 268 per 1000 (198 to 356) | RR 0.76 (0.56 to 1.01) | 373 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | |
| Mortality or neurodevelopmental disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Neither of the two included studies reported this outcome. |
| Mortality before hospital discharge | 139 per 1000 | 96 per 1000 (54 to 170) | RR 0.69 (0.39 to 1.22) | 373 (2 RCTs) | ⊕⊕⊝⊝ Lowb | |
| Mortality at 18‐24 months | 121 per 1000 | 113 per 1000 (41 to 315) | RR 0.94 (0.34 to 2.61) | 111 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| Neurodevelopmental disability at 18‐24 months | 39 per 1000 | 35 per 1000 (5 to 240) | RR 0.89 (0.13 to 6.12) | 108 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| Proportion of infants who develop epilepsy post‐discharge | 33 per 1000 | 106 per 1000 (23 to 491) | RR 3.18 (0.69 to 14.72) | 126 (1 RCT) | ⊕⊕⊝⊝ Lowb | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438158727822576253. | ||||||
a Downgraded by one level for risk of bias due to 'some concerns' in the risk of bias in both the included studies b Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria
Summary of findings 10. Summary of findings table ‐ Treatment of clinical and electrographic seizures versus treatment of clinical seizures alone in neonates.
| Treatment of clinical and electrographic seizures versus treatment of clinical seizures alone in neonates | ||||||
| Patient or population: neonates Setting: Neonatal intensive care unit Intervention: treatment of clinical and electrographic seizures Comparison: treatment of clinical seizures alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with treatment of clinical seizures alone | Risk with treatment of clinical and electrographic seizures | |||||
| Seizure burden during hospitalisation | The mean seizure burden during hospitalisation was 0 | MD 1871.16 lower (4525.05 lower to 782.73 higher) | ‐ | 68 (2 RCTs) | ⊕⊕⊝⊝ Lowa | |
| Mortality or neurodevelopmental disability at 18 to 24 months' corrected age ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | The included trial did not report this outcome. |
| Mortality before hospital discharge | 345 per 1000 | 203 per 1000 (97 to 438) | RR 0.59 (0.28 to 1.27) | 68 (2 RCTs) | ⊕⊕⊝⊝ Lowa | |
| Proportion of infants who develop epilepsy post‐discharge | 133 per 1000 | 100 per 1000 (16 to 631) | RR 0.75 (0.12 to 4.73) | 35 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_438901735468111265. | ||||||
a Downgraded by two levels for very serious imprecision due to very low sample size and event rate not meeting the 'Optimal Information Size' criteria
Background
Description of the condition
The term 'seizure' is defined as a transient occurrence of signs or symptoms, due to abnormal excessive or synchronous neuronal activity in the brain (Fisher 2005). However, this definition does not include electrographic‐only seizures. The American Clinical Neurophysiology Society (ACNS) defines electrographic seizures in neonates based on electroencephalogram (EEG) as "a sudden, abnormal EEG event, defined by a repetitive and evolving pattern with a minimum 2 μV peak‐to‐peak voltage and duration of at least 10 seconds" (Pressler 2021; Tsuchida 2013). The incidence of neonatal seizures ranges from 1.5 to 5.5 per 1000 live births in term infants and 11 to 19 per 1000 live births in preterm infants (Buraniqi 2017; Lanska 1995; Ronen 1999; Saliba 1999; Vasudevan 2013). The clinical manifestations of neonatal seizures are motor (clonic, tonic, myoclonic, spasms or automatisms), non‐motor (autonomic or behavioural arrest) or a combination of both (sequential) (Pressler 2021).
Hypoxic‐ischaemic encephalopathy (HIE), a form of neonatal encephalopathy caused by perinatal asphyxia, is the most common cause of neonatal seizures. The other major causes are focal ischaemic lesions (stroke), intracranial haemorrhage, central nervous system (CNS) infections, CNS malformations, inborn errors of metabolism and genetic causes (Lanska 1995; Ronen 1999; Tekgul 2006). Though most neonatal seizures are acutely provoked (i.e. they are caused by an acute brain insult), 10% to 20% are the first manifestation of epilepsy (Shellhaas 2017).
A newborn infant's brain is more vulnerable to developing seizures compared to the brain of older children and adults. This is due to the imbalance between excitatory and inhibitory neurotransmitters; there is excessive excitatory glutamate activity and deficient inhibitory gamma‐aminobutyric acid (GABA) activity in the immature neonatal brain. Moreover, GABA exerts a paradoxical excitatory action in the neonatal brain due to delayed expression of potassium chloride co‐transporter 2 (KCC2) receptors, which result in high intracellular chloride concentration and depolarisation (Dzhala 2003; Dzhala 2005; Huttenlocher 1982; Khazipov 2004; Takashima 1980).
Neonatal seizures are diagnosed either clinically, or by recording the electrical activity of the brain using an EEG. Recent evidence suggests that clinical diagnosis of seizures is not reliable (Malone 2009; Pellegrin 2019; Soul 2019). It is now believed that all, or nearly all, seizures have an EEG correlate, while half of all seizures have no clinical correlate (Nash 2011). Continuous, video‐assisted recording of conventional electroencephalography (cEEG) is considered the gold standard for diagnosing and monitoring neonatal seizures (Clancy 1996; McCoy 2013; Wusthoff 2013). Amplitude‐integrated EEG (aEEG) is an alternative, though it may not detect all seizures due to the limited number of scalp electrodes and modification of signals (Glass 2013). Automated seizure detection using machine learning technology is increasingly used in neonatal intensive care units (NICUs) to improve the seizure detection rate (Pavel 2020). However, though EEG confirmation of seizures is considered essential, treatment of seizures based on clinical diagnosis does exist as a practice in many centres, especially in resource‐limited settings.
Seizures substantially increase the metabolic demand of the CNS (Younkin 1986). This results in a marked decline in brain high‐energy phosphates and glucose, causing neuronal injury by energy deprivation (Fujikawa 1988). In addition, the cardiorespiratory compromise and fluctuating arterial pressure during a seizure result in hypoxic and ischaemic injury to the brain, causing neuronal cell death (Clozel 1985; McDonald 1990). The neuronal injury caused by seizures often results in long‐term neurological sequelae such as cerebral palsy, cognitive impairment, learning disabilities and future epilepsy (Pisani 2012; Ronen 2007; Yildiz 2012).
Description of the intervention
Once the immediately correctable causes of neonatal seizures, such as hypoglycaemia and hypocalcaemia, are addressed, there are several options for anti‐seizure medications (ASMs). Phenobarbitone, phenytoin and levetiracetam are the commonly used ASMs in neonates (Slaughter 2013; Van Rooij 2013). Drugs such as lidocaine and midazolam are used as infusions for seizures that are refractory (difficult to control) (Abend 2011; Fürwentsches 2010; Slaughter 2013; Van Rooij 2013). Newer drugs, such as topiramate and bumetanide, have also been explored for the treatment of neonatal seizures (Glass 2011; Jensen 2009; Pressler 2015).
Anti‐seizure medications act through various mechanisms, the main ones being blockage of voltage‐gated ion channels, GABA‐mediated neuronal inhibition, and blockage of glutamatergic excitatory pathways. Barbiturates and benzodiazepines enhance GABA‐mediated inhibition by modulating the permeability of chloride channels. Vigabatrin potentiates GABA inhibition by blocking GABA transaminase, the GABA‐degrading enzyme. Gabapentin acts by enhancing GABA‐mediated inhibition and possibly also by inactivating sodium channels. It has been suggested that drugs that act through GABA may be less effective in neonatal seizures because of the paradoxical chloride response in GABA receptors, and the overall reduced GABA receptor expression in neonates (Dulac 2013; Jensen 2009). However, this has never been confirmed in humans.
Phenytoin, carbamazepine and lamotrigine cause blockage of voltage‐gated sodium channels and inhibit repetitive neuronal firing. Levetiracetam and brivaracetam act by binding to the synaptic vesicle protein, (SV2A) in the brain, resulting in modulation of synaptic neurotransmitter release (Abou‐Khalil 2008). Valproate acts by multiple mechanisms, such as blocking voltage‐gated sodium channels, interfering with glutamate‐mediated excitation, and increasing GABA concentration in the brain by influencing GABA synthesis and breakdown. Remacemide acts by blocking N‐methyl‐D‐aspartate (NMDA) receptors and voltage‐gated sodium channels. Topiramate acts on multiple sites, including GABA receptors, glutamate receptors, L‐type calcium receptors, and possibly voltage‐gated sodium channels (Brodie 1996; Gidal 1999; Meldrum 1996; Taylor 1995).
How the intervention might work
The aim of treating neonatal seizures with an ASM is to reduce seizure burden and stop progression to status epilepticus with the main aim of stopping seizures. This is assumed to reduce the risk of long‐term neurodevelopmental impairment (Wirrell 2005; Yager 2002). However, animal experiments indicate that they may cause neuronal apoptosis, and alter neurogenesis and neural cell migration in the developing brain (Bittigau 2002; Ikonomidou 2010). Further, many ASMs cause significant adverse effects. Phenobarbitone and benzodiazepines can cause respiratory depression and hypoventilation requiring ventilatory support; phenytoin can cause arrhythmias leading to circulatory disturbance; lidocaine can lead to hypotension requiring volume or inotropic support; valproate can cause hepatotoxicity; and other adverse effects of ASMs include nephrotoxicity and free‐radical injury (El‐Dib 2017; Yozawitz 2017).
Neonatal seizures are difficult to treat with conventional ASMs. This is due to the inadequate development of inhibitory systems and excessive activity of excitatory systems in the developing brain as discussed above, and the lack of novel targets on which these medications can act upon. Studies have shown that neonatal seizures were refractory to first‐line drugs in nearly 50% of cases and that an additional 30% failed to respond even when second‐line drugs were added (Boylan 2002; Boylan 2004). Studies on phenobarbitone and phenytoin have given conflicting evidence about the efficacy of one medication over the other (Painter 1999; Pathak 2013). Further, the risk of uncoupling (the persistence of electrographic seizures after the suppression of clinical seizures) is well documented with both phenobarbitone and phenytoin (Scher 1993; Scher 2003). This would increase the burden of unrecognised seizures in centres where continuous cEEG monitoring is not used.
Recently, drugs such as levetiracetam, topiramate and bumetanide are being investigated in research trials, with variable benefits. Though these drugs may have the advantage of not causing neuronal apoptosis, data regarding their efficacy, safety and optimal dosing are lacking (Cha 2002; Cleary 2013; Dzhala 2008; Kahle 2009; Kilicdag 2013; Kim 2007; Liu 2004; Liu 2012; Manthey 2005; McHugh 2018; Rao 2018; Sharpe 2020; Talos 2013).
Why it is important to do this review
There is no definitive evidence or guideline on the choice of first‐, second‐ and third‐line ASMs in neonates. Furthermore, it is not clear whether ASMs should be initiated for only electrographic seizures, only clinical seizures, or both electrographic and clinical seizures (Booth 2004; Boylan 2013; Slaughter 2013; Srinivasakumar 2015; Van Rooij 2010). Finally, it is unclear how long to continue the ASM for once it is initiated, that is, whether to continue maintenance doses once seizure control is achieved after the loading dose (Saxena 2016).
Given the benefits, as well as the potential harm of using ASMs for neonatal seizures, we have undertaken a Cochrane Review that identifies and appraises data from randomised controlled trials, to provide a synthesis of evidence regarding the efficacy and adverse effects of using ASMs in neonatal seizures and their influence on short‐, intermediate‐ and long‐term outcomes.
Objectives
To assess whether any anti‐seizure medication (ASM) is more or less effective than an alternative ASM (both ASMs used as first‐, second‐ or third‐line treatment) in achieving seizure control and improving neurodevelopmental outcomes in neonates with seizures. We analysed EEG‐confirmed seizures and clinically‐diagnosed seizures separately.
To assess maintenance therapy with ASM versus no maintenance therapy after achieving seizure control. We analysed EEG‐confirmed seizures and clinically‐diagnosed seizures separately.
To assess treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), both parallel‐design and cross‐over trials*, in this review. We did not identify any quasi‐ or cluster‐RCTs for inclusion in this review.
We included studies on any class of ASMs that are known to be used in neonatal seizures.
We excluded studies on the use of vitamins, medical gas or other interventions such as therapeutic hypothermia, which may have a role in seizure control in neonates. We also excluded trials with prophylactic use of ASMs to prevent neonatal seizures or to improve neurodevelopmental outcomes.
Types of participants
We included newborn infants of any gestational age, gender or ethnicity who were diagnosed with seizures. We included seizures due to any aetiology and treated with any ASM. We included seizures that were:
clinical with EEG confirmation (EEG‐confirmed clinical seizures or electro‐clinical seizures);
clinically diagnosed without EEG confirmation (clinically‐diagnosed seizures);
only electrographic without any clinical manifestation (electrographic‐only seizures).
Types of interventions
We compared:
any ASM versus an alternative ASM in EEG‐confirmed neonatal seizures and clinically‐diagnosed neonatal seizures (both ASMs used as first‐, second‐ or third‐line treatment);
maintenance therapy with ASM versus no maintenance therapy in EEG‐confirmed neonatal seizures and clinically‐diagnosed neonatal seizures;
treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone.
We excluded ASMs used for indications other than neonatal seizures, such as neonatal hyperbilirubinaemia, sedation, or anaesthesia. We analysed EEG‐confirmed seizures and clinically‐diagnosed seizures separately. This was because appropriate diagnosis of seizures is an essential prerequisite to test the efficacy of ASMs (accurate outcome measure). Therefore, trials that included only EEG‐confirmed seizures will provide more reliable data on the outcomes of treatment with ASMs. However, treatment of seizures based on clinical diagnosis is a common practice and could not be excluded, although it is recognised that clinical diagnosis is associated with a high risk of over and under‐diagnosis. Hence, we analysed both EEG‐confirmed seizures and clinically‐diagnosed seizures in separate comparisons.
Types of outcome measures
Primary outcomes
Proportion of infants who achieve seizure control after first or maximal loading dose of the given ASM;
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age. Neurodevelopmental disability was defined as one or more of the following: cerebral palsy on clinical examination; developmental delay more than two standard deviations (SDs) below population mean on a standardised test of development; blindness (visual acuity less than 6/60); deafness (any hearing impairment requiring amplification).
(*The outcomes are reported in different ways in the trials. We have mentioned the changes in the reported outcomes, if any, in theDifferences between protocol and review).
Secondary outcomes
Mortality before hospital discharge or at any time later;
Neurodevelopmental disability at 18 to 24 months' corrected age, defined as one or more of the following: cerebral palsy on clinical examination; developmental delay more than two SDs below population mean on a standardised test of development; blindness (visual acuity less than 6/60); deafness (any hearing impairment requiring amplification);
Proportion of infants who develop cognitive impairment at two years or more (defined as a cognitive score below 70 measured using a validated assessment tool);
Seizure burden (seizure hours per infant, or minutes per hour of monitoring) during hospitalisation;
-
Proportion of infants with one or more of the following adverse effects related to ASM(s) during hospitalisation:
Requirement for mechanical ventilation;
Sedation or drowsiness;
Arrhythmias causing circulatory disturbance;
Bradycardia;
Hypotension requiring volume or inotropic support;
Shock requiring volume or inotropic support;
Hepatotoxicity resulting in discontinuation of therapy;
Acute kidney injury (of any stage);
Any further individual adverse effects;
Proportion of infants with abnormal background pattern in EEG (as defined by the authors) during the ASM treatment and after stopping the ASM;
Duration of hospital stay (days);
Proportion of infants with persistent seizures or requiring ASM(s) at discharge (or both);
Proportion of infants discharged on gavage feeds;
Proportion of infants with abnormal neurological examination at discharge: as defined by trialists based on validated tools, or as hypotonia or muscle weakness;
Proportion of infants who develop epilepsy post‐discharge;
Time to establish full oral feeds (days);
Proportion of infants who required ≥ 3 ASMs.
Search methods for identification of studies
The Cochrane Neonatal Information Specialist, Chris Cooper, wrote and ran search strategies.
Electronic searches
We searched the following databases in May 2022 with an update search in June 2023. We searched without restrictions on language, publication year, publication type, or publication status.
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 6, 2023;
Ovid MEDLINE, MEDALL (1946 to 06 June 2023);
Ovid Embase (1980 to 2023 Week 22);
Epistemonikos (registry of systematic reviews) https://www.epistemonikos.org, 7 June 2023.
Search strategies are available in Appendix 1; Appendix 2.
Searching other resources
We identified trial registration records using CENTRAL and by independent searches of the following:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), 7 June 2023;
ICTRP‐‐World Health Organization International Clinical Trials Registry Platform (https://trialsearch.who.int/Default.aspx), 7 June 2023.
We screened the reference lists of included or related, or both, studies (e.g. in the subject area of our review but not eligible for inclusion), and related systematic reviews (e.g. reviews including the population or intervention examined in our review) for studies not identified by the database searches.
Data collection and analysis
Selection of studies
Search results were managed in Endnote. Duplicates were removed using both Endnote and Covidence. Titles and abstracts were assessed in two ways: using Cochrane's Screen4Me (S4M) system (https://community.cochrane.org/sites/default/files/uploads/S4M_Users_FAQs.pdf) and by author screening.
The S4M system includes three levels of assessment for identifying non‐RCT records. Of these three levels, we used two: Known Assessments and RCT Classifier (Marshall 2018; Noel‐Storr 2020; Thomas 2021). Records remaining after S4M classification were screened independently by two of four authors (TA, ST, VVR and HH). These same authors independently screened the full texts of studies remaining after title/abstract assessment. At any point during the screening process, disagreements were resolved by discussion or by another reviewer. Where a review author was involved in an included study, any decisions regarding inclusion were made by other authors.
We collated multiple reports of the same study so that the study, rather than the reference, was the unit of interest in the review. Information about studies is provided in the following tables: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification); and Characteristics of ongoing studies.
We reported the study selection process in sufficient detail to generate a PRISMA flow diagram (Liberati 2009; Moher 2009).
Data extraction and management
Two review authors (TA and ST) independently extracted, assessed, and coded all data for each study, using a form designed specifically for this review. We collected information regarding the method of randomisation, masking, intervention, stratification, and whether the trial was single‐ or multi‐centre for each included study. We noted information regarding trial participants, including gestational age, type of seizures, aetiology of seizures, and treatment details. We analysed the clinical outcomes noted above in the Types of outcome measures.
We described ongoing studies identified by our search (when available), detailing the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date. We reported such studies in the Characteristics of ongoing studies table.
We resolved any disagreements by discussion with a third review author (HH). Should any queries arise or, in cases for which additional data were required, we contacted study investigators/authors for clarification. We replaced any standard error of the mean by the corresponding standard deviation. One review author (TA) entered final data for each study into Review Manager web (RevMan Web 2023), which the other review author (ST) checked. All review authors reviewed the analysis, results and drafted the manuscript.
Assessment of risk of bias in included studies
The review authors (VVR and RP) independently assessed the risk of bias in all included trials using version 2 of the Cochrane Risk of bias tool (RoB 2) (Higgins 2019). We resolved any disagreements by discussion or by consulting a third author (TA).
We assessed the risk of bias for each study outcome using the following Cochrane RoB 2 criteria:
bias arising from the randomisation process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
For each domain, a series of signalling questions with answers (yes, probably yes, no information, probably no, or no) determined the risk of bias (low risk, some concerns, or high risk). We included relevant text alongside the judgements to provide supporting information for our decisions. We decided the overall risk of bias for an outcome by its performance in all the domains: the overall judgement was 'some concerns' if we assigned a judgement of 'some concerns' for one domain, and 'high risk' if we assigned a judgement of 'some concerns' for multiple domains or 'high risk' for one (or more) domains.
Measures of treatment effect
We performed the statistical analyses using Review Manager web (RevMan Web 2023). We summarised the data in a meta‐analysis if they were sufficiently homogeneous, both clinically and statistically. For dichotomous data, we presented results using risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we used the mean difference (MD) when outcomes were measured in the same way between trials.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials. We did not identify any cluster‐randomised trial for inclusion in our review.
Dealing with missing data
We requested additional data from the trialists if data on important outcomes were missing or were reported unclearly. We obtained additional data from the authors of five trials (Falsaperla 2019; Jindal 2021; Khan 2020; Sharpe 2020; Soul 2021).
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity amongst trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. We interpreted the degree of heterogeneity as follows:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity; and
75% to 100%, indicating considerable heterogeneity.
We explored the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments) if we identified substantial heterogeneity (i.e. an I2 value greater than 50%).
Assessment of reporting biases
We assessed reporting bias by comparing the studies' stated primary outcomes and secondary outcomes with the reported outcomes. Where study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias.
As we included fewer than 10 trials in all the meta‐analyses, we did not examine a funnel plot for possible publication bias.
Data synthesis
If we identified multiple studies that we considered to be sufficiently similar, we performed meta‐analysis using Review Manager web (RevMan Web 2023). We used a fixed‐effect model to combine data where it was reasonable to assume that studies were estimating the same underlying treatment effect. If we deemed meta‐analysis to be inappropriate, we analysed and interpreted individual trials separately.
Subgroup analysis and investigation of heterogeneity
We explored substantial statistical heterogeneity in the outcomes by visually inspecting the forest plots (Higgins 2020). Where statistical heterogeneity was significant, we interpreted the results of the meta‐analyses accordingly; and we downgraded the certainty of evidence in the summary of findings tables, according to the GRADE recommendations (see Summary of findings and assessment of the certainty of the evidence).
Where data were available, we planned to conduct subgroup analyses based on:
gestational age (term infants (born at 37 weeks' gestation or greater) versus preterm infants (born at less than 37 weeks' gestation));
aetiology of seizure (acquired or discrete CNS injury such as hypoxic‐ischaemic encephalopathy, intracranial haemorrhage, stroke or infections versus congenital disorders with ongoing epileptic potential such as metabolic disorders, brain malformations, channelopathies, or other genetic causes).
We did not perform any subgroup analysis as all the included trials were performed on term and late preterm infants, and data based on aetiology of seizures were not available.
Sensitivity analysis
Where we identified substantial heterogeneity, we planned to conduct sensitivity analysis to determine if the findings were affected by inclusion of only those trials considered to have used adequate methodology, i.e. those with a low risk of bias. We planned to report results of sensitivity analyses for primary outcomes only.
However, we did not perform any sensitivity analysis, as it was not required.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following outcomes for all comparisons:
proportion of infants who achieve seizure control;
mortality or neurodevelopmental disability at 18 to 24 months;
mortality (at any time);
neurodevelopmental disability at 18 to 24 months;
proportion of infants who develop cognitive impairment at three years or more;
proportion of infants who develop adverse effects of ASM;
proportion of infants who develop epilepsy post‐discharge.
(*The outcomes are reported in different ways in the trials. We have mentioned the changes in the reported outcomes, if any, in theDifferences between protocol and review.)
Two review authors (TA and FB) independently assessed the certainty of the evidence for each of the outcomes above. We resolved any disagreements by discussion with a third author (VVR). We considered evidence from RCTs as being high‐certainty, and downgraded the assessment by one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create 10 summary of findings tables to report the certainty of the evidence for the following comparisons:
Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures (Table 1);
Phenobarbital versus levetiracetam as first‐line ASM for clinically diagnosed neonatal seizures (Table 2);
Phenobarbital versus phenytoin as first‐line ASM for EEG‐confirmed neonatal seizures (Table 3);
Phenobarbital versus phenytoin as first‐line ASM for clinically diagnosed neonatal seizures (Table 4);
Phenobarbital versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures (Table 5);
Phenytoin versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures (Table 6);
Phenobarbital+bumetanide versus phenobarbital alone as first‐line ASM for EEG‐confirmed neonatal seizures (Table 7);
Lignocaine versus benzodiazepines as second‐line ASM for EEG‐confirmed neonatal seizures (Table 8);
Maintenance therapy with ASM versus no maintenance therapy after achieving seizure control for clinically diagnosed neonatal seizures (Table 9);
Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates (Table 10).
The GRADE approach results in an assessment of the certainty of a body of evidence in one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
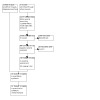
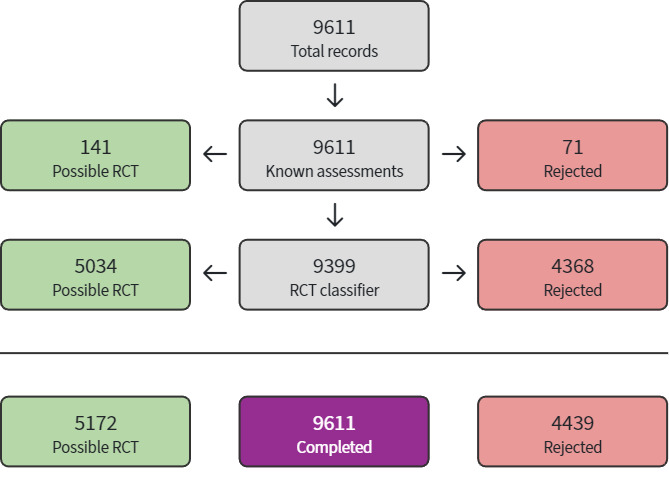
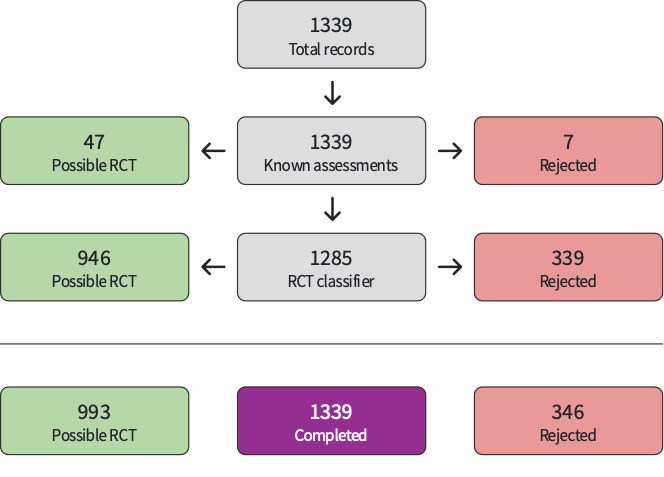
The study selection process is available in Figure 1. Searches identified 13,009 references. Of these, we processed 10,950 using Cochrane's Screen4Me (Figure 2; Figure 3). Screen4Me rejected 4475 references as non‐RCTs; of the remaining 8534 references, we removed 3166 duplicates, and screened 5368 references. We excluded 5300 based on title/abstract, and reviewed 68 full texts or trial registry records. We included 18 studies (Characteristics of included studies); excluded 30 (Characteristics of excluded studies); classified two as awaiting assessment (Characteristics of studies awaiting classification); and identified 23 ongoing studies (Characteristics of ongoing studies).
1.
Flow diagram
2.
Screen4Me 2022
3.
Screen4Me 2023
Comparison of one ASM versus another
We included 18 trials (1342 infants) in our analysis. See Characteristics of included studies.
Phenobarbital versus levetiracetam as first‐line ASM
Nine studies (Akeel 2022; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020; Perveen 2016; Prakash 2019; Sharpe 2020; Susnerwala 2022), compared phenobarbital versus levetiracetam as first‐line ASM. All nine studies had included term and late preterm neonates. While Sharpe 2020 utilised EEG to confirm seizures, the other eight studies used clinical diagnosis of seizures (Akeel 2022; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020; Perveen 2016; Prakash 2019; Susnerwala 2022). The aetiology of seizures included all causes except hypoglycaemia and hypocalcaemia in six studies (Akeel 2022; Ghaffar 2020; Gowda 2019; Khan 2020; Prakash 2019; Sharpe 2020); while Perveen 2016 included seizures due to any aetiology. The aetiologies were HIE, intracranial haemorrhage and meningitis in Falsaperla 2019. Susnerwala 2022 included seizures due to HIE alone. Seizure control was defined variably as seizure‐free for 24 hours in Akeel 2022; Ghaffar 2020; Gowda 2019, Sharpe 2020 and Susnerwala 2022; 48 hours in Khan 2020; five days in Prakash 2019 and one week in Falsaperla 2019. All nine studies have continued maintenance doses of ASM after achieving seizure control.
While Falsaperla 2019 excluded infants who required an additional ASM for seizure control, the other studies have included infants requiring further ASMs. In Ghaffar 2020; Khan 2020; Perveen 2016 and Sharpe 2020, second and third‐line ASMs were chosen as per the NICU protocol or at the discretion of the treating neonatologist. Akeel 2022; Gowda 2019; Prakash 2019 and Susnerwala 2022 are add‐on trials (strictly speaking, not cross‐over trials as phenobarbital has a long half‐life and there was no washout phase), where phenobarbital was used as the second‐line drug in the levetiracetam group and vice versa. For the outcomes on efficacy, i.e. 'seizure control after single dose ASM' and 'seizure control after maximum dose ASM', we considered only the monotherapy effect, that is, seizure control after the first‐line drug that was randomised. However, for all the other outcomes during further hospital stay, after discharge and for long‐term outcomes at 18 to 24 months, we analysed as per the randomisation, and we did not exclude infants who had received other drugs as second‐ or third‐ line ASMs. Further, we did not analyse cross‐over trials separately, because no study included washout periods due to ethical considerations (See Differences between protocol and review). Further, since we have only three or four drugs that can be used for neonatal seizures, we were of the view that all trials were essentially like cross‐over trials, as the authors would have used the comparator drug as a second‐ or third‐line ASM in the intervention group, and vice versa.
The dose of phenobarbital and levetiracetam also varied across the studies. While Falsaperla 2019; Perveen 2016 and Susnerwala 2022 used 20 mg/kg of phenobarbital, Akeel 2022 and Gowda 2019 used 30 mg/kg (20 mg/kg followed by 10 mg/kg); and Ghaffar 2020; Khan 2020; Prakash 2019 and Sharpe 2020 used 40 mg/kg (20 mg/kg followed by 2 doses of 10 mg/kg each). The maintenance dose used was 5 mg/kg/day in all studies. In the levetiracetam group, Falsaperla 2019 and Susnerwala 2022 used only a single loading dose of 20 mg/kg; Khan 2020 and Perveen 2016 used a single loading dose of 50 mg/kg and 60 mg/kg respectively; Prakash 2019 used an initial loading dose of 10 mg/kg and maximal loading dose of 15 mg/kg; Akeel 2022 used an initial loading dose of 20 mg/kg and maximal loading dose of 30 mg//kg; Ghaffar 2020 used an initial loading dose of 30 mg/kg and maximal loading dose of 40 mg/kg, Gowda 2019 used an initial loading dose of 20 mg/kg and maximal loading dose of 40 mg/kg, and Sharpe 2020 used an initial loading dose of 40 mg/kg and maximal loading dose of 60 mg/kg.
Phenobarbital versus phenytoin as first‐line ASM
Three studies (Painter 1999; Pathak 2013; Solanki 2015), compared phenobarbital versus phenytoin as first‐line ASM. All three studies included term and late preterm neonates. While Painter 1999 utilised EEG to confirm seizures, the two other studies (Pathak 2013; Solanki 2015), used clinical diagnosis of seizures. The aetiology of seizures included all causes except hypoglycaemia and hypocalcaemia in two studies (Pathak 2013; Solanki 2015), while Painter 1999 included seizures due to all causes. Seizure control was defined as stopping of seizures within 2.5 minutes of the loading dose in two studies (Painter 1999; Solanki 2015), while it was defined as seizure control soon after the loading dose in Pathak 2013. One study (Painter 1999), gave maintenance doses after the loading dose, while the other two studies (Pathak 2013; Solanki 2015), did not give maintenance doses of ASM.
Two studies (Painter 1999; Pathak 2013), were cross‐over trials where phenytoin was used as the second‐line ASM in the phenobarbital group and vice versa. In Solanki 2015, the choice of further ASMs was at the clinician's discretion. Both phenobarbital and phenytoin were used at a dose of 20 mg/kg for loading in Pathak 2013 and Solanki 2015, while Painter 1999 used the dose of ASM required to achieve a serum concentration of 2.5 mcg/mL.
Phenobarbital and phenytoin versus lorazepam as first‐line ASM
One study (Solanki 2015), compared phenobarbital and phenytoin versus lorazepam as first‐line ASM. The study included term and late preterm neonates, and used only clinical diagnosis of seizures. The aetiology of seizures included all causes except hypoglycaemia and hypocalcaemia. Seizure control was defined as stopping seizures within 2.5 minutes of the loading dose. The choice of further ASMs was at the clinician's discretion. Phenobarbital and phenytoin were used at a dose of 20 mg/kg, while lorazepam was used at a dose of 0.05 mg/kg for loading in the study. The study authors did not administer maintenance doses after the loading dose.
Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM
One study (Soul 2021), compared phenobarbital + bumetanide versus phenobarbital alone. The study included neonates born at ≥ 33 weeks' gestation who had EEG‐confirmed seizures. The aetiology of seizures included all causes except hypoglycaemia, hypocalcaemia, and inborn errors of metabolism. Neonates who had seizures despite 20 to < 40 mg/kg of phenobarbital were randomised to phenobarbital alone (5 to 10 mg/kg) or phenobarbital (5 to 10 mg/kg) and bumetanide (0.1 to 0.3 mg/kg). The choice of further ASMs was as per the unit protocol. The study primarily aimed to evaluate the pharmacokinetics and pharmacodynamics of bumetanide. Seizure control was a post hoc outcome.
Lidocaine versus benzodiazepines as second‐line ASM
One study (Boylan 2004), compared lidocaine versus benzodiazepines (midazolam or clonazepam) as second‐line ASM. The study included both term and preterm neonates who had EEG‐confirmed seizures. The aetiology of seizures included HIE, intracranial haemorrhage and meningitis. First‐line ASM was phenobarbital, given at 40 mg/kg maximal loading dose. Seizure control was defined as reduction in seizure burden by 80% in 12 hours. Lidocaine was given at a dose of 4 mg/kg over 20 minutes, followed by 2 mg/kg/h, and increased to 4 mg/kg/h if seizure control was not achieved. Midazolam was administered at a dose of 60 µg/kg loading followed by 150 µg/kg/h, and increased up to 300 µg/kg/h after 12 hours if seizure control was not achieved.
Maintenance therapy with ASM versus no maintenance therapy after achieving seizure control
Two trials and 373 infants (Jindal 2021; Saxena 2016), were included in the comparison of short‐term maintenance therapy with ASM versus no maintenance therapy for neonatal seizures during hospital stay. Both trials included neonates born at ≥ 34 weeks' gestation and had only clinically‐diagnosed seizures. The aetiologies of seizures were perinatal asphyxia, meningitis and intracranial haemorrhage in both trials, while Saxena 2016 also included seizures due to metabolic causes. Both trials included only those neonates who achieved seizure control after a single loading dose of 20 mg/kg of phenobarbital. Infants who required further doses of phenobarbital or other ASMs to achieve seizure control were excluded. The time of randomisation was 12 hours seizure‐free after 20 mg//kg phenobarbital in both trials. The duration of maintenance therapy with phenobarbital was five days in one trial (Saxena 2016), while it was until hospital discharge in the other trial (Jindal 2021).
Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates
Two trials and 68 infants (Srinivasakumar 2015; Van Rooji 2010), were included in the comparison of any ASM treatment versus no treatment for only‐electrographic seizures. Both trials were performed on neonates born at ≥ 35 weeks' gestation and both included only neonates with HIE. The ASMs used were phenobarbital, phenytoin and midazolam in Srinivasakumar 2015, while Van Rooji 2010 used phenobarbital, midazolam, lignocaine and clonazepam. The time of randomisation was before the onset of electrographic seizures in Srinivasakumar 2015, though the outcomes were reported only for those neonates who had electrographic seizures. The time of randomisation was after the onset of electrographic seizures in the Van Rooji 2010 study. While Srinivasakumar 2015 used continuous video EEG to diagnose seizures, Van Rooji 2010 used aEEG.
Excluded studies
We excluded 30 studies for the reasons described below. See Characteristics of excluded studies.
Studies without a comparator
Several studies described the effect of a single ASM without a comparator and therefore had to be excluded. Retrospective case series examined the effect of levetiracetam as first‐line ASM (Abend 2011; Han 2018; Kanmaz 2021), sodium valproate (Gal 1988), or lidocaine (Favié 2020; Weeke 2016). Uncontrolled cohort studies examined the effect of levetiracetam as first‐line medication (Sedighi 2016; Ramantani 2011), or lidocaine (Hellström‐Westas 1988). One study describing clinical, neuroimaging, and electrographic predictors of phenobarbital failure in newborns with hypoxic ischaemic encephalopathy and seizures also had to be excluded (Dwivedi 2019). Another study examined the effect of phenobarbital on EEG (Low 2016), without a comparator.
Comparison of one ASM versus another
Phenobarbital versus levetiracetam as first‐line ASM
The efficacy of phenobarbital versus levetiracetam was retrospectively compared by six studies, all of which had to be excluded because of the retrospective design (Liu 2020; Maitre 2013; Rao 2018; Thibault 2020; Verwoerd 2022; Wagner 2021). The authors of one study specifically addressed neurodevelopmental outcomes (Maitre 2013); another study focused on newborns undergoing cardiac surgery (Thibault 2020). One cross‐sectional study examined neurodevelopment of newborns with seizures following treatment with phenobarbital versus levetiracetam and also had to be excluded because of lack of randomisation (Arican 2020).
Phenobarbital versus other medications as first‐line ASM
One RCT comparing the effects of phenobarbital, phenytoin, clonazepam, and sodium valproate was excluded because data were only published as a conference abstract (Rochefort 1989).
Second and third‐line ASM
One uncontrolled cohort study compared the effects of phenytoin in newborns with clinical seizures not controlled by phenobarbital as second‐line ASM (Jawadekar 1992). This study also addressed the pharmacokinetics of phenobarbital in newborns. Another cohort study examined the effect of midazolam as third‐line ASM, without a comparator (Castro Conde 2005). Retrospective studies addressed possible effects of lidocaine or midazolam on newborns with seizures not controlled by phenobarbital (Shany 2007). Pharmacokinetics and effects of bumetanide in newborns with EEG‐confirmed seizures not responding to a loading dose of phenobarbital was examined in one study (Pressler 2015). A retrospective study examined the effect of lorazepam as third‐line ASM (Deshmukh 1986). The efficacy and safety of midazolam versus levetiracetam as third‐line ASM was investigated in one non‐randomised study, with no confirmation of seizures by EEG (Jayswal 2021). An uncontrolled cohort study examined the efficacy of oral levetiracetam as third‐line ASM in neonates with clinical seizures not responding to phenobarbital and phenytoin (Mollamohammadi 2018).
Maintenance therapy with ASM versus no maintenance therapy after achieving seizure control
Safety of early discontinuation of ASM after acute symptomatic neonatal seizures was retrospectively assessed in one study (Glass 2021). The study found no difference in neurodevelopment or epilepsy at age 24 months amongst children whose ASM was discontinued versus maintained at hospital discharge after resolution of acute symptomatic neonatal seizures. The study was excluded because of its retrospective nature.
Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates
A RCT examining neurodevelopment following treatment of electrographic‐only seizures versus clinical seizures (Hunt 2021) was excluded because seizure detection was based on aEEG alone and assessment of seizure burden was only initiated over 24 hours after birth. Thus, the study design impedes reaching conclusive results regarding the question examined.
Studies awaiting classification
There are two studies awaiting classification (Gyandeep 2023; Mohammadi 2023). They are awaiting classification for the following reason: we need additional data from the study authors to classify the studies and include in the appropriate meta‐analyses.
For further details, see Characteristics of studies awaiting classification.
Ongoing studies
There are 23 ongoing studies (ACTRN12622000470796; CTRI/2013/01/003310; CTRI/2013/04/003585; CTRI/2014/06/004659; CTRI/2015/06/005849; CTRI/2016/10/007412; CTRI/2018/04/013161; CTRI/2020/03/023961; CTRI/2021/02/031290; CTRI/2022/09/045658; CTRI/2023/02/049794; IRCT2014070318334N1; IRCT20160523028008N23; IRCT20190526043717N1; IRCT20200115046137N1; IRCT20200131046317N3; IRCT20200528047589N1; IRCT20220619055221N1; NCT01089504; NCT02550028; NCT03107507; NCT04320940; NCT05291455).
For further details, see Characteristics of ongoing studies.
Risk of bias in included studies
Amongst the 18 trials included in the review, the randomisation process domain had a low risk of bias for 12 trials (Akeel 2022; Gowda 2019; Jindal 2021; Pathak 2013; Perveen 2016; Prakash 2019; Saxena 2016; Sharpe 2020; Soul 2021; Srinivasakumar 2015; Susnerwala 2022; Van Rooji 2010). The domain had some concerns for four trials (Boylan 2004; Falsaperla 2019; Khan 2020; Ghaffar 2020), as there was no information on allocation concealment, but baseline characteristics did not show any difference between the two groups. The domain had high risk for two trials (Painter 1999; Solanki 2015), as there was no information on allocation concealment and baseline characteristics suggested a mismatch between the two groups despite randomisation.
The domain 'deviation from intended interventions' had a low risk of bias for all 18 trials, as one was a triple‐masked trial (Soul 2021) and, in the other 14 trials, although the personnel were aware of the intervention allocation, there seemed to be no deviations that arose outside the trial context. Also, all the patients were analysed as randomised in these trials.
The domain 'missing outcome data' also had a low risk of bias for all the trials, as the outcome data were reasonably complete for all randomised patients in 15 trials (Akeel 2022; Boylan 2004; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Jindal 2021; Khan 2020; Painter 1999; Pathak 2013; Perveen 2016; Prakash 2019; Solanki 2015; Soul 2021; Srinivasakumar 2015; Susnerwala 2022). In Sharpe 2020, although there were missing data, analysis methods that corrected for bias, such as sensitivity analyses showing that results were little changed under a range of plausible assumptions about the relationship between the missing value in the outcome and its true value, were performed. In Van Rooji 2010, although nine out of 42 patients randomised were excluded, the reasons for the same were stated, and they did not differ substantially between the groups, thus indicating that the results might not be biassed. In Saxena 2016, data were reasonably complete for all the included patients until hospital discharge. Though many of the enrolled participants were lost to follow‐up, it was balanced between the two groups. Hence, it seemed that the result was not biassed as reasons for the loss to follow‐up did not differ significantly between the groups.
For the domain 'measurement of outcome', objective outcomes were scored 'low risk' for all 18 trials. However, for subjective outcomes, only Soul 2021 had a low risk of bias as it was a triple‐masked trial. Other trials were scored 'some concerns' for subjective outcomes, as the assessors were aware of the intervention and there was a likelihood of assessment being influenced by the knowledge of the allocation group. For outcomes related to seizure assessment such as seizure control after a single loading dose of ASM, seizure control after maximal loading dose of ASM, and recurrence of seizures before hospital discharge, trials that used EEG/aEEG to diagnose seizures were scored low risk (Boylan 2004; Painter 1999; Sharpe 2020; Srinivasakumar 2015; Van Rooji 2010). Amongst the trials that used clinical diagnosis of seizures, seven trials (Falsaperla 2019; Ghaffar 2020; Gowda 2019; Jindal 2021; Khan 2020; Perveen 2016; Saxena 2016), were scored high risk, as there was no clear definition for different seizure types, how it was differentiated from non‐epileptic events and how it was assessed and by whom. Five trials (Akeel 2022; Pathak 2013; Prakash 2019; Solanki 2015; Susnerwala 2022), that have specified the details of the seizure definition used and who diagnosed the seizures, were scored 'some concerns'.
For the domain 'selection of reported results', 11 trials (Gowda 2019; Jindal 2021; Pathak 2013; Perveen 2016; Saxena 2016; Sharpe 2020; Solanki 2015; Srinivasakumar 2015; Susnerwala 2022; Van Rooji 2010), had a low risk of bias as these trials were analysed as per a priori registered protocol, while seven trials (Akeel 2022; Boylan 2004; Falsaperla 2019; Ghaffar 2020; Khan 2020; Painter 1999; Prakash 2019), had some concerns as the trial protocols were not available for assessment.
The overall risk of bias of the included trials was as follows: one trial had a low risk of overall bias for all the outcomes (Soul 2021). 12 trials had a low risk for objective outcomes and some concerns or high risk for subjective outcomes (Akeel 2022; Gowda 2019; Jindal 2021; Pathak 2013; Perveen 2016; Prakash 2019; Saxena 2016; Sharpe 2020; Srinivasakumar 2015; Susnerwala 2022; Van Rooji 2010). Five trials had a high risk of overall bias for all the outcomes (Boylan 2004; Falsaperla 2019; Khan 2020; Painter 1999; Solanki 2015).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Comparison 1: Comparison of one ASM versus another
Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures
Please see Table 1.
Primary outcomes
Proportion of infants who achieve seizure control after first loading dose of ASM
As the dosage regimen of ASM was variable across the studies, we defined the first loading dose of ASM as 20 mg/kg for phenobarbital and 20 to 40 mg/kg for levetiracetam. We defined (post hoc) a time limit of 24 to 48 hours from the time of ASM administration to evaluate seizure control.
Data from one trial (Sharpe 2020) showed that phenobarbital probably results in better seizure control after the first loading dose of ASM compared to levetiracetam in EEG‐confirmed neonatal seizures (RR 2.32, 95% CI 1.63 to 3.30; 106 participants; moderate‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the first loading dose of ASM
Proportion of infants who achieve seizure control after maximal loading dose of ASM
The maximum loading dose of ASM was defined as 30 to 40 mg/kg for phenobarbital and 40 to 60 mg/kg for levetiracetam.
Data from one trial (Sharpe 2020), showed that phenobarbital probably results in better seizure control after the first loading dose of ASM compared to levetiracetam in EEG‐confirmed neonatal seizures (RR 2.83, 95% CI 1.78 to 4.50; 83 participants; moderate‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 2: Proportion of infants who achieve seizure control after the the maximal loading dose of ASM
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
The trial did not report this outcome (Sharpe 2020).
Secondary outcomes
Mortality before hospital discharge
Based on the data from one trial (Sharpe 2020), we are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM on all‐cause mortality before hospital discharge (RR 0.30, 95% CI 0.04 to 2.52; 106 participants; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 3: Mortality before hospital discharge
Neurodevelopmental disability at 18 to 24 months' corrected age
This outcome was not reported in the included study.
Proportion of infants who develop cognitive impairment at two years or more
This outcome was not reported in the included study.
Seizure burden (seizure hours per infant, or minutes per hour of monitoring) during hospitalisation
This outcome was not reported in the included study.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement of mechanical ventilation
This outcome was reported in the one included trial (Sharpe 2020). We are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM for the requirement of mechanical ventilation (RR 1.21, 95% CI 0.76 to 1.91; 106 participants; very low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 4: Requirement for mechanical ventilation
Proportion of infants who develop sedation or drowsiness
Based on data from the included trial (Sharpe 2020), we are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM on the proportion of infants who develop sedation or drowsiness (RR 1.74, 95% CI 0.68 to 4.44; 106 participants; very low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 5: Proportion of infants who develop sedation or drowsiness
Arrhythmias causing circulatory disturbance
This outcome was not reported in the included study.
Bradycardia
Sharpe 2020 did not find a difference in the incidence of bradycardia between the two groups (RR 0.76, 95% CI 0.31 to 1.87; 106 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 6: Bradycardia
Hypotension requiring volume or inotropic support
The one included trial (Sharpe 2020) did not find a difference in hypotension between the phenobarbital and levetiracetam groups (RR 3.56, 95% CI 0.97 to 12.99; 106 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 7: Hypotension requiring volume or inotropes
Shock requiring volume or inotropes
The one included trial (Sharpe 2020) did not find a difference in the incidence of shock between the two groups (RR 1.98, 99% CI 0.76 to 5.15; 106 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 8: Shock requiring volume or inotropes
Hepatotoxicity resulting in discontinuation of therapy
This outcome was not reported in the included study.
Acute kidney injury (of any stage)
This outcome was not reported in the included study.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
This outcome was not reported in the included study.
Proportion of infants with an abnormal background pattern in EEG after ASM treatment
This outcome was not reported in the included study.
Duration of hospital stay (days)
This outcome was not reported in the included study.
Recurrence of seizures before hospital discharge
The one included trial (Sharpe 2020) did not find a difference in recurrence of seizures during hospital stay between the phenobarbital and levetiracetam groups (RR 1.33, 95% CI 0.52 to 3.40; 106 participants; Analysis 1.9).
1.9. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 9: Recurrence of seizure before hospital discharge
Proportion of infants with persistent seizures or requiring ASM(s) at discharge (or both)
This outcome was not reported in the included study.
Proportion of infants discharged on gavage feeds
This outcome was not reported in the included study.
Proportion of infants with abnormal neurological examination at discharge
This outcome was not reported in the included study.
Proportion of infants who develop epilepsy post‐discharge
Based on data from one trial (Sharpe 2020) we are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM on the proportion of infants who develop epilepsy post‐discharge (RR 0.92, 95% CI 0.48 to 1.76; 45 participants; very low‐certainty evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, Outcome 10: Proportion of infants with epilepsy post‐discharge
Phenobarbital versus levetiracetam as first‐line ASM for clinically diagnosed neonatal seizures
Please see Table 2.
Primary outcomes
Proportion of infants who achieve seizure control after the first loading dose of ASM
Three trials (Akeel 2022; Gowda 2019; Susnerwala 2022), have reported the outcome of seizure control until 24 to 48 hours after the first loading dose of ASM. We are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM on achieving seizure control after the first loading dose in clinically‐diagnosed seizures (RR 0.69, 95% CI 0.55 to 0.86; 286 participants; very low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the first loading dose of ASM
Proportion of infants who achieve seizure control after the maximal loading dose of ASM
Three trials (Ghaffar 2020; Gowda 2019; Khan 2020) reported this outcome. We are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM on achieving seizure control after maximal loading dose of ASM in clinically‐diagnosed seizures (RR 0.58, 95% CI 0.47 to 0.72; 260 participants; very low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 2: Proportion of infants who achieve seizure control after the maximal loading dose of ASM
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
This outcome was not reported in the eight included trials (Akeel 2022; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020; Perveen 2016; Prakash 2019; Susnerwala 2022).
Secondary outcomes
Mortality before hospital discharge
Six trials (Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020; Prakash 2019; Susnerwala 2022) reported this outcome. Use of phenobarbital versus levetiracetam as first‐line ASM may have little or no effect on all‐cause mortality before hospital discharge (RR 1.41, 95% CI 0.82 to 2.43; 452 participants; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 3: Mortality before hospital discharge
Neurodevelopmental disability at 18 to 24 months' corrected age
This outcome was not reported in the eight included trials.
Proportion of infants who develop cognitive impairment at two years or more
This outcome was not reported in the eight included trials.
Seizure burden (seizure hours per infant, or minutes per hour of monitoring) during hospitalisation
This outcome was not reported in the eight included trials.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
The requirement for mechanical ventilation in trial participants was reported in five trials (Akeel 2022; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020). We are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM on the need for mechanical ventilation (RR 2.20, 95% CI 0.50 to 9.68; 394 participants; very low‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 4: Requirement for mechanical ventilation
Proportion of infants who develop sedation or drowsiness
Meta‐analysis of two trials (Khan 2020; Prakash 2019) showed no difference in sedation/drowsiness between the two groups (RR 1.88, 95% CI 0.66 to 5.37; 180 participants; very low‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 5: Proportion of infants who develop sedation or drowsiness
Arrhythmias causing circulatory disturbance
This outcome was not reported in the eight included trials.
Bradycardia
Meta‐analysis of four trials (Akeel 2022; Falsaperla 2019; Gowda 2019; Khan 2020) showed no difference in the incidence of bradycardia between phenobarbital and levetiracetam groups (RR 6.00, 95% CI 0.74 to 48.97; 334 participants; Analysis 2.6).
2.6. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 6: Bradycardia
Hypotension requiring volume or inotropic support
Two trials (Falsaperla 2019; Khan 2020) reported this outcome. None of the infants in either study had hypotension requiring volume or inotropic support (Analysis 2.7).
2.7. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 7: Hypotension requiring volume or inotropes
Shock requiring volume or inotropes
Meta‐analysis of three trials (Falsaperla 2019; Khan 2020; Perveen 2016) showed no difference in the risk of shock between phenobarbital and levetiracetam groups (RR 0.67, 99% CI 0.30 to 1.51; 190 participants; Analysis 2.8).
2.8. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 8: Shock requiring volume or inotropes
Hepatotoxicity resulting in discontinuation of therapy
This outcome was not reported in the eight included trials.
Acute kidney injury (of any stage)
This outcome was not reported in the eight included trials.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
One trial (Falsaperla 2019), did not find a difference in the proportion of infants with an abnormal background pattern in EEG during ASM treatment between phenobarbital and levetiracetam groups (RR 1.00, 95% CI 0.88 to 1.13; 30 participants; Analysis 2.9).
2.9. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 9: Proportion of infants with an abnormal background pattern in EEG during ASM treatment
Proportion of infants with an abnormal background pattern in EEG after stopping ASM treatment
One trial (Falsaperla 2019), did not find a difference in the proportion of infants with an abnormal background pattern in EEG after the ASM treatment between phenobarbital and levetiracetam groups (RR 0.63, 95% CI 0.26 to 1.47; 30 participants; Analysis 2.10).
2.10. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 10: Proportion of infants with an abnormal background pattern in EEG after stopping ASM
Duration of hospital stay (days)
Meta‐analysis of two trials (Falsaperla 2019; Perveen 2016) showed an increase in the duration of hospital stay in the phenobarbital group compared to the levetiracetam group (MD 2.36 days, 95% CI 0.54 to 4.18; 90 participants; Analysis 2.11).
2.11. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 11: Duration of hospital stay
Recurrence of seizures before hospital discharge
Meta‐analysis of two trials (Falsaperla 2019; Khan 2020) showed no difference in recurrence of seizures during hospital stay between phenobarbital and levetiracetam groups (RR 1.67, 95% CI 0.42 to 6.60; 130 participants; Analysis 2.12).
2.12. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 12: Recurrence of seizure before hospital discharge
Proportion of infants with persistent seizures or requiring ASM(s) at discharge (or both)
One trial (Falsaperla 2019) did not find a difference in the proportion of infants with persistent seizures or requiring ASM at discharge (RR 0.50, 95% CI 0.05 to 4.94; 30 participants; Analysis 2.13).
2.13. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 13: Proportion of infants with persistent seizures and/or requiring ASM at discharge
Proportion of infants discharged on gavage feeds
Two trials (Falsaperla 2019; Khan 2020) reported this outcome. None of the babies in either group was discharged on gavage feeds (Analysis 2.14).
2.14. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 14: Proportion of infants discharged on gavage feeds
Proportion of infants with an abnormal neurological examination at discharge
Meta‐analysis of four trials (Falsaperla 2019; Khan 2020; Perveen 2016; Susnerwala 2022), showed no difference in the proportion of infants with an abnormal neurological examination at discharge between phenobarbital and levetiracetam groups (RR 0.80, 95% CI 0.51 to 1.24; 272 participants; Analysis 2.15).
2.15. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 15: Proportion of infants with an abnormal neurological examination at discharge
Proportion of infants who develop epilepsy post‐discharge
One trial (Falsaperla 2019) has reported this outcome. We are uncertain about the effect of using phenobarbital versus levetiracetam as first‐line ASM on achieving seizure control after the first loading dose in clinically‐diagnosed seizures (RR 0.50, 95% CI 0.05 to 4.9; 30 participants; very low‐certainty evidence; Analysis 2.16).
2.16. Analysis.

Comparison 2: Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 16: Proportion of infants who develop epilepsy post discharge
Phenobarbital versus phenytoin as first‐line ASM for EEG‐confirmed neonatal seizures
Please see Table 3.
Primary outcomes
Proportion of infants who achieve seizure control after first loading dose of ASM
The one included trial did not report this outcome (Painter 1999).
Proportion of infants who achieve seizure control after the maximal loading dose of ASM
One trial (Painter 1999) has reported this outcome. We are uncertain about the effect of using phenobarbital versus phenytoin as first‐line ASM on achieving seizure control after the maximal dose of ASM in EEG‐confirmed neonatal seizures (RR 0.97, 95% CI 0.54 to 1.72; 59 participants; very low‐certainty evidence).
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Secondary outcomes
Mortality before hospital discharge
The one included trial did not report this outcome.
Neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Proportion of infants who develop cognitive impairment at two years or more
The one included trial did not report this outcome.
Seizure burden during hospitalisation
The one included trial did not report this outcome.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
The one included trial did not report this outcome.
Proportion of infants who develop sedation or drowsiness
The one included trial did not report this outcome.
Arrhythmias causing circulatory disturbance
The one included trial (Painter 1999) reported this outcome. None of the babies in either group developed any arrythmia in this trial (Analysis 3.2).
3.2. Analysis.

Comparison 3: Phenobarbital versus phenytoin as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 2: Arrythmias causing circulatory disturbance
Bradycardia
The one included trial (Painter 1999) reported this outcome. None of the babies in either group developed bradycardia in this trial (Analysis 3.3).
3.3. Analysis.

Comparison 3: Phenobarbital versus phenytoin as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 3: Hypotension requiring volume or inotropes
Hypotension requiring volume or inotropic support
The one included trial (Painter 1999) reported this outcome. None of the babies in either group developed hypotension in this trial (Analysis 3.4).
3.4. Analysis.

Comparison 3: Phenobarbital versus phenytoin as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 4: Bradycardia
Shock requiring volume or inotropes
The one included trial did not report this outcome.
Hepatotoxicity resulting in discontinuation of therapy
The one included trial did not report this outcome.
Acute kidney injury (of any stage)
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG after stopping the ASM
The one included trial did not report this outcome.
Duration of hospital stay (days)
The one included trial did not report this outcome.
Proportion of infants with persistent seizures or requiring ASM(s) at discharge (or both)
The one included trial did not report this outcome.
Proportion of infants discharged on gavage feeds
The one included trial did not report this outcome.
Proportion of infants with abnormal neurological examination at discharge
The one included trial did not report this outcome.
Proportion of infants who develop epilepsy post‐discharge
The one included trial did not report this outcome.
Phenobarbital versus phenytoin as first‐line ASM for clinically diagnosed neonatal seizures
Please see Table 4.
Primary outcomes
Proportion of infants who achieve seizure control after the first loading dose of ASM
Both included trials (Pathak 2013; Solanki 2015) reported this outcome. Using phenobarbital may result in better seizure control after the first loading dose of ASM when compared to phenytoin in clinically diagnosed seizures (RR 1.92, 95% CI 1.40 to 2.64; 179 participants; low‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the first loading dose of ASM
Proportion of infants who achieve seizure control after the maximal loading dose of ASM
Neither of the two included trials reported this outcome.
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
Neither of the two included studies reported this outcome.
Secondary outcomes
Mortality before hospital discharge
Both included trials (Pathak 2013; Solanki 2015) reported this outcome. We are uncertain about the effect of using phenobarbital versus phenytoin as first‐line ASM on all‐cause mortality before hospital discharge (RR 1.33, 95% CI 0.79 to 2.26; 179 participants; very low‐certainty evidence; Analysis 4.2).
4.2. Analysis.

Comparison 4: Phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 2: Mortality before hospital discharge
Neurodevelopmental disability at 18 to 24 months' corrected age
Neither of the two included studies reported this outcome.
Proportion of infants who develop cognitive impairment at two years or more
Neither of the two included studies reported this outcome.
Seizure burden during hospitalisation
Neither of the two included studies reported this outcome.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
One trial (Pathak 2013) reported on the need for mechanical ventilation. We are uncertain about the effect of using phenobarbital versus phenytoin as first‐line ASM on the need for mechanical ventilation (RR 7.13, 95% CI 0.38 to 134.78; 109 participants; very low‐certainty evidence; Analysis 4.3).
4.3. Analysis.

Comparison 4: Phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 3: Requirement of mechanical ventilation
Proportion of infants with sedation or drowsiness
Based on data from one trial (Solanki 2015), we are uncertain about the effect of using phenobarbital versus phenytoin as first‐line ASM on the risk of sedation or drowsiness (RR 23.00, 95% CI 1.41 to 375.77; 70 participants; very low‐certainty evidence; Analysis 4.4).
4.4. Analysis.

Comparison 4: Phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 4: Proportion of infants who develop sedation or drowsiness
Arrhythmias causing circulatory disturbance
Neither of the two included studies reported this outcome.
Bradycardia
One trial (Pathak 2013), did not find a difference in the proportion of infants with bradycardia between the groups (RR 0.20, 95% CI 0.01 to 4.15; Analysis 4.5).
4.5. Analysis.

Comparison 4: Phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 5: Bradycardia
Hypotension requiring volume or inotropic support
Neither of the two included studies reported this outcome.
Shock requiring volume or inotropes
Neither of the two included studies reported this outcome.
Hepatotoxicity resulting in discontinuation of therapy
Neither of the two included studies reported this outcome.
Acute kidney injury (of any stage)
Neither of the two included studies reported this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
Neither of the two included studies reported this outcome.
Proportion of infants with an abnormal background pattern in EEG after stopping ASM
Neither of the two included studies reported this outcome.
Duration of hospital stay (days)
Neither of the two included studies reported this outcome.
Proportion of infants with persistent seizures or requiring ASM(s) at discharge (or both)
One trial (Solanki 2015) did not find a difference in the proportion of infants with persistent seizures or requiring ASM at discharge between the phenobarbital and phenytoin groups (RR 0.67, 95% CI 0.21 to 2.16; 70 participants; Analysis 4.6).
4.6. Analysis.

Comparison 4: Phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 6: Proportion of infants with persistent seizures and/or requiring ASM at discharge
Proportion of infants discharged on gavage feeds
Neither of the two included studies reported this outcome.
Proportion of infants with an abnormal neurological examination at discharge
Neither of the two included studies reported this outcome.
Proportion of infants who develop epilepsy post‐discharge
Neither of the two included studies reported this outcome.
Phenobarbital versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures
Please see Table 5.
Primary outcomes
Proportion of infants who achieve seizure control after the first loading dose of ASM
The one included trial (Solanki 2015) reported this outcome. We are uncertain about the effect of phenobarbital compared to lorazepam on seizure control after the first loading dose of ASM (RR 0.71, 95% CI 0.53 to 0.94; 71 participants; very low‐certainty evidence; Analysis 5.1).
5.1. Analysis.

Comparison 5: Phenobarbital versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the first loading dose of ASM
Proportion of infants who achieve seizure control after the maximal loading dose of ASM
The one included trial did not report this outcome (Solanki 2015).
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Secondary outcomes
Mortality before hospital discharge
The one included trial (Solanki 2015) reported this outcome. We are uncertain about the effect of phenobarbital compared to lorazepam on mortality before discharge (RR 1.76, 95% CI 0.79 to 3.95; 71 participants; very low‐certainty evidence; Analysis 5.2).
5.2. Analysis.

Comparison 5: Phenobarbital versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 2: Mortality before hospital discharge
Neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Proportion of infants who develop cognitive impairment at two years or more
The one included trial did not report this outcome.
Seizure burden during hospitalisation
The one included trial did not report this outcome.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
The one included trial did not report this outcome.
Proportion of infants who develop sedation or drowsiness
The one included trial (Solanki 2015) reported this outcome. We are uncertain about the effect of phenobarbital compared to lorazepam on sedation or drowsiness (RR 5.66, 95% CI 1.35 to 23.71; 71 participants; very low‐certainty evidence; Analysis 5.3).
5.3. Analysis.

Comparison 5: Phenobarbital versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 3: Proportion of infants who develop sedation or drowsiness
Arrhythmias causing circulatory disturbance
The one included trial did not report this outcome.
Bradycardia
The one included trial did not report this outcome.
Hypotension requiring volume or inotropic support
The one included trial did not report this outcome.
Shock requiring volume or inotropes
The one included trial did not report this outcome.
Hepatotoxicity resulting in discontinuation of therapy
The one included trial did not report this outcome.
Acute kidney injury (of any stage)
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG after stopping ASM
The one included trial did not report this outcome.
Duration of hospital stay (days)
The one included trial did not report this outcome.
Proportion of infants with persistent seizures or requiring ASM(s) at discharge
The one included trial (Solanki 2015) did not find a difference in the proportion of infants with persistent seizures or requiring ASM at discharge between the phenobarbital and lorazepam groups (RR 9.25, 95% CI 0.52 to 165.69; 71 participants; Analysis 5.4).
5.4. Analysis.

Comparison 5: Phenobarbital versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 4: Proportion of infants with persistent seizures and/or requiring ASM at discharge
Proportion of infants discharged on gavage feeds
The one included trial did not report this outcome.
Proportion of infants with an abnormal neurological examination at discharge
The one included trial did not report this outcome.
Proportion of infants who develop epilepsy post‐discharge
The one included trial did not report this outcome.
Phenytoin versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures
Please see Table 6.
Primary outcomes
Proportion of infants who achieve seizure control after the first loading dose of ASM
The one included trial (Solanki 2015) reported this outcome. We are uncertain about the effect of phenytoin compared to lorazepam on seizure control after the first loading dose of ASM (RR 0.77, 95% CI 0.60 to 0.99; 71 participants; very low‐certainty evidence; Analysis 6.1).
6.1. Analysis.

Comparison 6: Phenytoin versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the first loading dose of ASM
Proportion of infants who achieve seizure control after the maximal loading dose of ASM
The one included trial did not report this outcome.
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Secondary outcomes
Mortality before hospital discharge
The one included trial (Solanki 2015) reported this outcome. We are uncertain about the effect of phenytoin compared to lorazepam on mortality before discharge (RR 0.44, 95% CI 0.12 to 1.57; 71 participants; very low‐certainty evidence; Analysis 6.2).
6.2. Analysis.

Comparison 6: Phenytoin versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 2: Mortality before hospital discharge
Neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Proportion of infants who develop cognitive impairment at two years or more
The one included trial did not report this outcome.
Seizure burden during hospitalisation
The one included trial did not report this outcome.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
The one included trial did not report this outcome.
Proportion of infants who develop sedation or drowsiness
The one included trial (Solanki 2015) reported this outcome. We are uncertain about the effect of phenobarbital compared to lorazepam on sedation or drowsiness (RR 0.21, 95% CI 0.01 to 4.13; 71 participants; very low‐certainty evidence; Analysis 6.3).
6.3. Analysis.

Comparison 6: Phenytoin versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 3: Proportion of infants who develop sedation or drowsiness
Arrhythmias causing circulatory disturbance
The one included trial did not report this outcome.
Bradycardia
The one included trial did not report this outcome.
Hypotension requiring volume or inotropic support
The one included trial did not report this outcome.
Shock requiring volume or inotropes
The one included trial did not report this outcome.
Hepatotoxicity resulting in discontinuation of therapy
The one included trial did not report this outcome.
Acute kidney injury (of any stage)
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG after stopping the ASM
The one included trial did not report this outcome.
Duration of hospital stay (days)
The one included trial did not report this outcome.
Proportion of infants with persistent seizures or requiring ASM(s) at discharge
The one included trial (Solanki 2015) did not find a difference in the proportion of infants with persistent seizures or requiring ASM at discharge between the phenytoin and lorazepam groups (RR 13.36, 95% CI 0.78 to 228.6; 71 participants; Analysis 6.4).
6.4. Analysis.

Comparison 6: Phenytoin versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures, Outcome 4: Proportion of infants with persistent seizures and/or requiring ASM at discharge
Proportion of infants discharged on gavage feeds
The one included trial did not report this outcome.
Proportion of infants with an abnormal neurological examination at discharge
The one included trial did not report this outcome.
Proportion of infants who develop epilepsy post‐discharge
The one included trial did not report this outcome.
Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM for EEG‐confirmed neonatal seizures
Please see Table 7.
Primary outcomes
Proportion of infants who achieve seizure control after the first loading dose of ASM
The one included trial (Soul 2021) reported this outcome. Phenobarbital + bumetanide when compared to phenobarbital alone may have little or no effect on seizure control after the first loading dose of ASM (RR 0.95, 95% CI 0.37 to 2.40; 43 participants; low‐certainty evidence; Analysis 7.1).
7.1. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the first loading dose of ASM
Proportion of infants who achieve seizure control after the maximal loading dose of ASM
The one included trial did not report this outcome.
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Secondary outcomes
Mortality before hospital discharge
The one included trial (Soul 2021) reported this outcome. We are uncertain about the effect of phenobarbital + bumetanide when compared to phenobarbital alone on all‐cause mortality before hospital discharge (RR 0.20, 95% CI 0.02 to 1.74; 43 participants; very low‐certainty evidence; Analysis 7.2).
7.2. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 2: Mortality before hospital discharge
Neurodevelopmental disability at 18 to 24 months' corrected age
The one included trial did not report this outcome.
Proportion of infants who develop cognitive impairment at two years or more
The one included trial (Soul 2021) reported cognitive impairment at 18 to 24 months. We are uncertain about the effect of phenobarbital + bumetanide when compared to phenobarbital alone on all‐cause mortality before hospital discharge (RR 0.53, 95% CI 0.13 to 2.15; 43 participants; very low‐certainty evidence; Analysis 7.3).
7.3. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 3: Proportion of infants with cognitive impairment at 18‐24 months
Seizure burden during hospitalisation
The one included trial did not find a difference in seizure burden between the two groups (MD 1.90, 95% CI 0.52 to 3.28; 43 participants; Analysis 7.4).
7.4. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 4: Seizure burden during hospitalisation
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
In the one included trial (Soul 2021), none of the babies in either group required mechanical ventilation (Analysis 7.5).
7.5. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 5: Requirement for mechanical ventilation
Proportion of infants who develop sedation or drowsiness
The one included trial did not report this outcome.
Arrhythmias causing circulatory disturbance
The one included trial did not report this outcome.
Bradycardia
The one included trial did not report this outcome.
Hypotension requiring volume or inotropic support
In the one included trial (Soul 2021), none of the babies in either group developed hypotension (Analysis 7.6).
7.6. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 6: Hypotension requiring volume or inotropes
Shock requiring volume or inotropes
The one included trial did not report this outcome.
Hepatotoxicity resulting in discontinuation of therapy
The one included trial did not report this outcome.
Acute kidney injury (of any stage)
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
The one included trial (Soul 2021) found no difference in the proportion of infants with an abnormal background pattern in EEG during ASM treatment between phenobarbital + bumetanide and phenobarbital alone groups (RR 1.05, 95% CI 0.62 to 1.80; 43 participants; Analysis 7.7).
7.7. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 7: Proportion of infants with an abnormal background pattern in EEG during ASM treatment
Proportion of infants with an abnormal background pattern in EEG after stopping ASM
The one included trial did not report this outcome.
Duration of hospital stay (days)
The one included trial did not report this outcome.
Proportion of infants with persistent seizures or requiring ASM(s) at discharge
The one included trial did not report this outcome.
Proportion of infants discharged on gavage feeds
The one included trial did not report this outcome.
Proportion of infants with an abnormal neurological examination at discharge
The one included trial did not report this outcome.
Proportion of infants who developed epilepsy post‐discharge
The one included trial (Soul 2021) reported this outcome. We are uncertain about the effect of phenobarbital + bumetanide when compared to phenobarbital alone on the proportion of infants who developed epilepsy post‐discharge (RR 1.13, 95% CI 0.43 to 2.97; 39 participants; very low‐certainty evidence; Analysis 7.8).
7.8. Analysis.

Comparison 7: Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 8: Proportion of infants who develop epilepsy post discharge
Lignocaine versus benzodiazepines as second‐line ASM for EEG‐confirmed neonatal seizures
Please see Table 8.
Primary outcomes
Proportion of infants who achieve seizure control after the first loading dose of ASM
The one included trial did not report this outcome (Boylan 2004).
Proportion of infants who achieve seizure control after the maximal loading dose of ASM
The one included trial (Boylan 2004) reported this outcome. We are uncertain about the effect of lignocaine when compared to benzodiazepines as second‐line ASM on achieving seizure control after maximal loading dose of ASM (RR 8.17, 95% CI 0.52 to 128.42; 11 participants; very low‐certainty evidence; Analysis 8.1).
8.1. Analysis.

Comparison 8: Lignocaine versus benzodiazepine as second‐line ASM in EEG‐confirmed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the maximal loading dose of ASM
Mortality or neurodevelopmental disability at 12 months' corrected age
The one included trial (Boylan 2004) reported this outcome. We are uncertain about the effect of lignocaine when compared to benzodiazepines as second‐line ASM on mortality or neurodevelopmental disability at 18 to 24 months' corrected age (RR 1.00, 95% CI 0.71 to 1.41; 10 participants; very low‐certainty evidence; Analysis 8.2).
8.2. Analysis.

Comparison 8: Lignocaine versus benzodiazepine as second‐line ASM in EEG‐confirmed neonatal seizures, Outcome 2: Mortality or neurodevelopmental disability at 12 months' corrected age
Secondary outcomes
Mortality before hospital discharge
The one included trial (Boylan 2004) reported this outcome. We are uncertain about the effect of lignocaine when compared to benzodiazepines as second‐line ASM on all‐cause mortality before discharge (RR 1.20, 95% CI 0.25 to 5.71; 11 participants; very low‐certainty evidence; Analysis 8.3).
8.3. Analysis.

Comparison 8: Lignocaine versus benzodiazepine as second‐line ASM in EEG‐confirmed neonatal seizures, Outcome 3: Mortality before hospital discharge
Neurodevelopmental disability at 12 months' corrected age
The one included trial (Boylan 2004) reported this outcome. We are uncertain about the effect of lignocaine when compared to benzodiazepines as second‐line ASM on mortality or neurodevelopmental disability at 18 to 24 months' corrected age (RR 1.00, 95% CI 0.36 to 2.75; 10 participants; very low‐certainty evidence; Analysis 8.4).
8.4. Analysis.

Comparison 8: Lignocaine versus benzodiazepine as second‐line ASM in EEG‐confirmed neonatal seizures, Outcome 4: Neurodevelopmental disability at 12 months' corrected age
Proportion of infants who develop cognitive impairment at two years or more
The one included trial did not report this outcome.
Seizure burden during hospitalisation
The one included trial did not report this outcome.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement of mechanical ventilation
The one included trial did not report this outcome.
Proportion of infants who develop sedation or drowsiness
The one included trial did not report this outcome.
Arrhythmias causing circulatory disturbance
The one included trial did not report this outcome.
Bradycardia
The one included trial did not report this outcome.
Hypotension requiring volume or inotropic support
The one included trial did not report this outcome.
Shock requiring volume or inotropes
The one included trial did not report this outcome.
Hepatotoxicity resulting in discontinuation of therapy
The one included trial did not report this outcome.
Acute kidney injury (of any stage)
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
The one included trial did not report this outcome.
Proportion of infants with an abnormal background pattern in EEG after stopping ASM
The one included trial did not report this outcome.
Duration of hospital stay (days)
The one included trial did not report this outcome.
Proportion of infants with persistent seizures or requiring ASM(s) at discharge
The one included trial did not report this outcome.
Proportion of infants discharged on gavage feeds
The one included trial did not report this outcome.
Proportion of infants with an abnormal neurological examination at discharge
The one included trial did not report this outcome.
Proportion of infants who develop epilepsy post‐discharge
The one included trial did not report this outcome.
Comparison 2: Maintenance therapy with ASM versus no maintenance therapy after achieving seizure control for clinically diagnosed neonatal seizures
Please see Table 9.
Primary outcomes
Proportion of infants who achieve seizure control after a single or maximal dose of the given ASM
These outcomes are not relevant to this comparison.
Proportion of infants with repeat seizures before hospital discharge
The 'proportion of infants who achieve seizure control' was evaluated using the outcome 'proportion of infants who developed repeated seizures during hospitalisation'. Both included trials (Jindal 2021; Saxena 2016) reported this outcome. We are uncertain about the effect of maintenance therapy with ASM compared to no maintenance therapy after achieving seizure control on the incidence of recurrent seizures before hospital discharge (RR 0.76, 95% CI 0.56 to 1.01; 373 participants; very‐low certainty evidence; Analysis 9.1).
9.1. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 1: Proportion of infants with repeat seizure before hospital discharge
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
Neither of the two included studies reported this outcome.
Secondary outcomes
Mortality before hospital discharge
Both trials (Jindal 2021; Saxena 2016) reported this outcome. Maintenance therapy with ASM compared to no maintenance therapy may have little or no effect on all‐cause mortality before hospital discharge (RR 0.69, 95% CI 0.39 to 1.22; 373 participants; low‐certainty evidence; Analysis 9.2).
9.2. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 2: Mortality before hospital discharge
Mortality at 18 to 24 months
One trial (Saxena 2016) reported this outcome. Maintenance therapy with ASM compared to no maintenance therapy may have little or no effect on mortality at 18 to 24 months (RR 0.94, 95% CI 0.34 to 2.61; 111 participants; low‐certainty evidence; Analysis 9.3).
9.3. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 3: Mortality at 18 to 24 months
Neurodevelopmental disability at 18 to 24 months' corrected age
One trial (Saxena 2016) reported this outcome. Maintenance therapy with ASM compared to no maintenance therapy may have little or no effect on neurodevelopmental disability at 18 to 24 months (RR 0.89, 95% CI 0.13 to 6.12; 108 participants; low‐certainty evidence; Analysis 9.4).
9.4. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 4: Neurodevelopmental disability at 18 to 24 months' corrected age
Proportion of infants who develop cognitive impairment at two years or more
Neither of the two included studies reported this outcome.
Seizure burden during hospitalisation
Neither of the two included studies reported this outcome.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
Data from one trial (Jindal 2021) showed no difference in the requirement of mechanical ventilation between the two groups (RR 0.83, 95% CI 0.63 to 1.10; 221 participants; Analysis 9.5).
9.5. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 5: Requirement for mechanical ventilation
Proportion of infants with sedation or drowsiness
Neither of the two trials reported this outcome.
Arrhythmias causing circulatory disturbance
Neither of the two included studies reported this outcome.
Bradycardia
Neither of the two included studies reported this outcome.
Hypotension requiring volume or inotropic support
Neither of the two included studies reported this outcome.
Shock requiring volume or inotropes
Meta‐analysis of data from both trials (Jindal 2021; Saxena 2016) did not show a difference in the need for inotropes between the two groups (RR 0.84, 95% CI 0.67 to 1.07; 373 participants; Analysis 9.6).
9.6. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 6: Shock requiring volume or inotropes
Hepatotoxicity resulting in discontinuation of therapy
Neither of the two included studies reported this outcome.
Acute kidney injury (of any stage)
Neither of the two included studies reported this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
Neither of the two included studies reported this outcome.
Proportion of infants with an abnormal background pattern in EEG after achieving seizure control
Data from one trial (Saxena 2016) showed no difference in the proportion of infants with an abnormal background pattern in EEG after achieving seizure control between maintenance therapy with ASM and no maintenance therapy groups (RR 0.76, 95% CI 0.30 to 1.97; 118 participants; Analysis 9.7).
9.7. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 7: Abnormal background pattern in EEG after achieving seizure control
Duration of hospital stay (days)
Meta‐analysis of data from both trials (Jindal 2021; Saxena 2016) did not show a difference in the duration of hospital stay between maintenance therapy with ASM and no maintenance therapy groups (MD 0.13, 95% CI ‐0.44 to 0.70; 373 participants; Analysis 9.8).
9.8. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 8: Duration of hospital stay
Proportion of infants with persistent seizures or requiring ASM(s) at discharge
Data from one trial (Jindal 2021), showed no difference in the proportion of infants with persistent seizures or requiring ASM(s) at discharge between the two groups (RR 1.33, 95% CI 0.98 to 1.80; 221 participants; Analysis 9.9).
9.9. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 9: Proportion of infants with persistent seizures and/or requiring ASM at discharge
Proportion of infants discharged on gavage feeds
Neither of the two included studies reported this outcome.
Proportion of infants with an abnormal neurological examination at discharge
Meta‐analysis of data from both trials (Jindal 2021; Saxena 2016) did not show a difference in the proportion of infants with an abnormal neurological examination at discharge between the two groups (RR 0.88, 95% CI 0.62 to 1.26; 373 participants; Analysis 9.10).
9.10. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 10: Abnormal neurological examination at discharge
Proportion of infants who develop epilepsy post‐discharge
One trial (Saxena 2016) reported this outcome. Maintenance therapy with ASM compared to no maintenance therapy may have little or no effect on the proportion of infants who develop epilepsy post‐discharge (RR 3.18, 95% CI 0.69 to 14.72; 126 participants; low certainty evidence; Analysis 9.11).
9.11. Analysis.

Comparison 9: Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures, Outcome 11: Proportion of infants who develop epilepsy post‐discharge
Comparison 3: Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates
Please see Table 10.
Primary outcomes
Proportion of infants who achieve seizure control after the first or maximal dose of the given ASM
This outcome is not relevant to this comparison.
Seizure burden during hospitalisation
The 'proportion of infants who achieve seizure control' was evaluated using the outcome 'seizure burden'. (See Differences between protocol and review).
Both trials (Srinivasakumar 2015; Van Rooji 2010) reported this outcome. Treatment of both clinical and electrographic seizures when compared to treatment of clinical seizures alone may have little or no effect on all‐cause mortality before hospital discharge (MD ‐1871.16, 95% CI ‐4525.05 to 782.73; 68 participants; low‐certainty evidence; Analysis 10.1).
10.1. Analysis.

Comparison 10: Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates, Outcome 1: Seizure burden during hospitalisation
Mortality or neurodevelopmental disability at 18 to 24 months' corrected age
Neither of the two trials (Srinivasakumar 2015; Van Rooji 2010) that compared ASM treatment for only‐electrographic seizures versus no ASM treatment reported this outcome.
Secondary outcomes
Mortality before hospital discharge
Both trials (Srinivasakumar 2015; Van Rooji 2010) reported this outcome. Treatment of both clinical and electrographic seizures when compared to treatment of clinical seizures alone may have little or no effect on all‐cause mortality before hospital discharge (RR 0.59, 95% CI 0.28 to 1.27; 68 participants; low‐certainty evidence; Analysis 10.2).
10.2. Analysis.

Comparison 10: Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates, Outcome 2: Mortality before hospital discharge
Neurodevelopmental disability at 18 to 24 months corrected age
Neither of the two trials reported this outcome.
Proportion of infants who develop cognitive impairment at two years or more
Neither of the two trials reported this outcome.
Proportion of infants with one or more of the adverse effects related to ASM(s) during hospitalisation
Requirement for mechanical ventilation
Neither of the two trials reported this outcome.
Proportion of infants with sedation or drowsiness
Neither of the two trials reported this outcome.
Arrhythmias causing circulatory disturbance
Neither of the two trials reported this outcome.
Bradycardia
Neither of the two trials reported this outcome.
Hypotension requiring volume or inotropic support
Neither of the two trials reported this outcome.
Shock requiring volume or inotropes
Neither of the two trials reported this outcome.
Hepatotoxicity resulting in discontinuation of therapy
Neither of the two trials reported this outcome.
Acute kidney injury (of any stage)
Neither of the two trials reported this outcome.
Proportion of infants with an abnormal background pattern in EEG during ASM treatment
Neither of the two trials reported this outcome.
Proportion of infants with an abnormal background pattern in EEG after stopping the ASM
Neither of the two trials reported this outcome.
Duration of hospital stay (days)
Neither of the two trials reported this outcome.
Proportion of infants with persistent seizures or requiring ASM(s) at discharge
Neither of the two trials reported this outcome.
Proportion of infants discharged on gavage feeds
Neither of the two trials reported this outcome.
Proportion of infants with an abnormal neurological examination at discharge
Neither of the two trials reported this outcome.
Proportion of infants who develop epilepsy post‐discharge
One trial (Srinivasakumar 2015) reported this outcome. Treatment of both clinical and electrographic seizures when compared to treatment of clinical seizures alone may have little or no effect on the proportion of infants who developed epilepsy post‐discharge between the two groups (RR 0.75, 95% CI 0.12 to 4.73; 35 participants; low‐certainty evidence; Analysis 10.3).
10.3. Analysis.

Comparison 10: Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates, Outcome 3: Proportion of infants who develop epilepsy post‐discharge
Discussion
Summary of main results
We included a total of 18 trials (1342 infants) in this systematic review.
We included 14 trials for the comparison of one ASM versus an alternative ASM for the treatment of neonatal seizures (Akeel 2022; Boylan 2004; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020; Painter 1999; Pathak 2013; Perveen 2016; Prakash 2019; Sharpe 2020; Solanki 2015; Soul 2021; Susnerwala 2022). Amongst these, nine trials (Akeel 2022; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020; Perveen 2016; Prakash 2019; Sharpe 2020; Susnerwala 2022) compared phenobarbital versus levetiracetam as first‐line ASM; two trials (Painter 1999; Pathak 2013) compared phenobarbital versus phenytoin as first‐line ASM; one three‐armed trial (Solanki 2015) compared phenobarbital versus phenytoin versus lorazepam as first‐line ASM; one trial (Soul 2021) compared phenobarbital+bumetanide versus phenobarbital alone and one trial (Boylan 2004), compared lignocaine versus benzodiazepines as second‐line ASM.
One trial (Sharpe 2020) compared phenobarbital versus levetiracetam as first‐line ASM in EEG‐confirmed neonatal seizures. Phenobarbital is probably more effective than levetiracetam in achieving seizure control after the first loading dose (RR 2.32, 95% CI 1.63 to 3.30; 106 participants; moderate‐certainty evidence). Similarly, phenobarbital is probably more effective than levetiracetam in achieving seizure control after the maximal loading dose (RR 2.83, 95% CI 1.78 to 4.50; 106 participants; moderate‐certainty evidence). However, we are uncertain about the effect of phenobarbital when compared to levetiracetam on other outcomes such as mortality before hospital discharge (RR 0.30, 95% CI 0.04 to 2.52; 106 participants; very low‐certainty evidence); the requirement for mechanical ventilation (RR 1.21, 95% CI 0.76 to 1.91; 106 participants; very low‐certainty evidence); sedation or drowsiness (RR 1.74, 95% CI 0.68 to 4.44; 106 participants; very low‐certainty evidence); and proportion of infants with epilepsy post‐discharge (RR 0.92, 95% CI 0.48 to 1.76; 106 participants; very low‐certainty evidence). We did not find any data on the impact of phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed seizures on important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months or on cognitive impairment at two years or more.
Eight trials (Akeel 2022; Falsaperla 2019; Ghaffar 2020; Gowda 2019; Khan 2020; Perveen 2016; Prakash 2019; Susnerwala 2022) compared phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures. We are uncertain about the efficacy of phenobarbital versus levetiracetam in achieving seizure control after the first loading dose (RR 0.69, 95% CI 0.55 to 0.86; 286 participants; very low‐certainty evidence), and seizure control after the maximal loading dose (RR 0.58, 95% CI 0.47 to 0.72; 260 participants; very low‐certainty evidence). Use of phenobarbital versus levetiracetam as first‐line ASM may have little or no effect on all‐cause mortality before discharge (RR 1.41, 95% CI 0.82 to 2.43; 452 participants; low‐certainty evidence). We are also uncertain regarding the effect of phenobarbital versus levetiracetam as first‐line ASM on other important outcomes such as requirement for mechanical ventilation (RR 2.20, 95% CI 0.50 to 9.68; 394 participants; very low‐certainty evidence); sedation or drowsiness (RR 1.88, 95% CI 0.66 to 5.37; 180 participants; very low‐certainty evidence); and proportion of infants with epilepsy post‐discharge (RR 0.50, 95% CI 0.05 to 4.94; 30 participants; very low‐certainty evidence). There were no data on important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months or on cognitive impairment at two years or more.
One trial (Painter 1999) compared phenobarbital versus phenytoin as first‐line ASM in EEG‐confirmed neonatal seizures. We are uncertain about the effect of phenobarbital versus phenytoin on achieving seizure control after the maximal dose (RR 0.97, 95% CI 0.54 to 1.72; 59 participants; very low‐certainty evidence). We did not find any data on the impact of phenobarbital versus phenytoin as first‐line ASM for EEG‐confirmed seizures on seizure control after the maximal loading dose, mortality before hospital discharge, risk of various adverse effects due to ASM, proportion of infants who develop epilepsy post‐discharge and on important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months or on cognitive impairment at two years or more.
Two trials (Pathak 2013; Solanki 2015) compared phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures. Phenobarbital may be more effective than phenytoin in achieving seizure control after the first loading dose (RR 1.92, 95% CI 1.40 to 2.64; 179 participants; low‐certainty evidence). We are uncertain regarding the effect of phenobarbital when compared to phenytoin on other outcomes such as mortality before hospital discharge (RR 1.33, 95% CI 0.79 to 2.26; 179 participants; very low‐certainty evidence); requirement for mechanical ventilation (RR 7.13, 95% CI 0.38 to 134.78; 109 participants; very low‐certainty evidence); and sedation or drowsiness (RR 23.00, 95% CI 1.41 to 375.77; 70 participants; very low‐certainty evidence). We did not find any data on the impact of phenobarbital versus phenytoin as first‐line ASM for clinically diagnosed seizures on seizure control after the maximal loading dose of ASM and on important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months or on cognitive impairment at two years or more.
One trial (Solanki 2015) compared phenobarbital versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures. We are uncertain as to the effect of phenobarbital versus lorazepam on achieving seizure control after the first loading dose (RR 0.71, 95% CI 0.53 to 0.94; 71 participants; very low‐certainty evidence); mortality before hospital discharge (RR 1.76, 95% CI 0.79 to 3.95; 71 participants; very low‐certainty evidence); and sedation or drowsiness (RR 5.66, 95% CI 1.35 to 23.71; 71 participants; very low‐certainty evidence). We did not find any data on the impact of phenobarbital versus lorazepam as first‐line ASM for clinically diagnosed seizures on seizure control after the maximal loading dose of ASM, risk of various adverse effects due to ASM, proportion of infants who develop epilepsy post‐discharge and on important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months or cognitive impairment at two years or more.
One trial (Solanki 2015) compared phenytoin versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures. We are uncertain as to the effect of phenytoin versus lorazepam on achieving seizure control after the first loading dose (RR 0.77, 95% CI 0.60 to 0.99; 71 participants; very low‐certainty evidence); mortality before hospital discharge (RR 0.44, 95% CI 0.12 to 1.57; 71 participants; very low‐certainty evidence); and sedation or drowsiness (RR 0.21, 95% CI 0.01 to 4.13; 71 participants; very low‐certainty evidence). We did not find any data on the impact of phenytoin versus lorazepam as first‐line ASM for clinically diagnosed seizures on seizure control after the maximal loading dose of ASM, risk of various adverse effects due to ASM, proportion of infants who develop epilepsy post‐discharge and on important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months or cognitive impairment at two years or more.
One trial (Soul 2021) compared phenobarbital + bumetanide versus phenobarbital alone in EEG‐confirmed neonatal seizures. Phenobarbital + bumetanide when compared to phenobarbital alone may have little or no effect on seizure control after the first loading dose (RR 0.95, 95% CI 0.37 to 2.40; 43 participants; low‐certainty evidence). We are uncertain as to the effect of phenobarbital + bumetanide versus phenobarbital alone on mortality before hospital discharge (RR 0.20, 95% CI 0.02 to 1.74; 43 participants; very low‐certainty evidence); cognitive impairment at 18 to 24 months (RR 0.53, 95% CI 0.13 to 2.15; 43 participants; very low‐certainty evidence); and proportion of infants who develop epilepsy post‐discharge (RR 1.13, 95% CI 0.43 to 2.97; 43 participants; very low‐certainty evidence). We did not find any data on the effect of phenobarbital + bumetanide versus phenobarbital alone for EEG‐confirmed seizures on seizure control after the maximal loading dose of ASM, risk of various adverse effects due to ASM, and important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months.
One trial (Boylan 2004) compared lignocaine versus benzodiazepines as second‐line ASM in clinically diagnosed neonatal seizures. We are uncertain about the effect of lignocaine versus benzodiazepines in achieving seizure control after the maximal loading dose (RR 8.17, 95% CI 0.52 to 128.42; 11 participants; very low‐certainty evidence); mortality or neurodevelopmental disability at 12 months (RR 1.00, 95% CI 0.71 to 1.41; 10 participants; very low‐certainty evidence); all‐cause mortality before hospital discharge (RR 1.20, 95% CI 0.25 to 5.71; 11 participants; very low‐certainty evidence); and neurodevelopmental disability at 12 months (RR 1.00, 95% CI 0.36 to 2.75; 10 participants; very low‐certainty evidence). We did not find any data on the effect of lignocaine versus benzodiazepines as second‐line ASM on seizure control after the first loading dose of ASM, risk of various adverse effects due to ASM, and proportion of infants who develop epilepsy post‐discharge.
Two trials (Srinivasakumar 2015; Van Rooji 2010) compared the treatment of both clinical and electrographic seizures versus treating clinical seizures alone in neonates. Treatment of clinical and electrographic seizures when compared to treating clinical seizures alone may have little or no effect on seizure burden during hospitalisation (MD ‐1871.16, 95% CI ‐4525.05 to 782.73; 68 participants; low‐certainty evidence); mortality before hospital discharge (RR 0.59, 95% CI 0.28 to 1.27; 68 participants; low‐certainty evidence); and proportion of infants who develop epilepsy post‐discharge (RR 0.75, 95% CI 0.12 to 4.73; 35 participants; low‐certainty evidence). We found no data on the effect of treating clinical and electrographic seizures compared to treating clinical seizures alone on adverse effects due to ASM and other important long‐term outcomes such as mortality or neurodevelopmental disability at 18 to 24 months or cognitive impairment at two years or more.
Two trials (Jindal 2021; Saxena 2016) compared maintenance therapy with ASM versus no maintenance therapy after achieving seizure control in neonatal seizures. We are uncertain about the effect of maintenance therapy with ASM versus no maintenance therapy on the recurrence of seizures before hospital discharge (RR 0.76, 95% CI 0.56 to 1.01; 373 participants; very low‐certainty evidence). Maintenance therapy with ASM compared to no maintenance therapy may have little or no effect on mortality before hospital discharge (RR 0.69, 95% CI 0.39 to 1.22; 373 participants; low‐certainty evidence); mortality by 18 to 24 months (RR 0.94, 95% CI 0.34 to 2.61; 111 participants; low‐certainty evidence); neurodevelopmental disability by 18 to 24 months (RR 0.89, 95% CI 0.13 to 6.12; 108 participants; low‐certainty evidence);‐and proportion of infants with epilepsy post discharge (RR 3.18, 95% CI 0.69 to 14.72; 126 participants; low‐certainty evidence).
Overall completeness and applicability of evidence
Phenobarbital is probably more effective than levetiracetam as first‐line ASM in achieving seizure control in neonates with EEG‐confirmed seizures after both the first loading dose and the maximal loading dose of ASM (moderate‐certainty evidence). Phenobarbital may be more effective than phenytoin as first‐line ASM in achieving seizure control in clinically diagnosed seizures after the first loading dose of ASM (low‐certainty evidence). Phenobarbital + bumetanide may have little or no difference in achieving seizure control when compared to phenobarbital alone in EEG‐confirmed seizures (low‐certainty evidence). These results apply to term and late preterm neonates who have seizures due to any aetiology other than hypoglycaemia and hypocalcaemia. None of the included studies had recruited preterm neonates born at < 34 weeks' gestational age. Hence, the results cannot be used for this preterm population. For other comparisons of one ASM versus another to achieve seizure control, limited data and very low‐certainty evidence preclude us from drawing any reasonable conclusions.
We are uncertain as to the effect of one ASM versus another on other short‐term outcomes including mortality before hospital discharge. Most of the trials have included neonates who required other ASMs as well for seizure control. Hence, all the short‐ and long‐term outcomes other than seizure control would have been influenced by other ASMs as well. We did not analyse monotherapy and polytherapy separately due to the non‐availability of adequate data. Most trials did not provide data on long‐term outcomes such as mortality, neurodevelopmental disability or cognitive impairment. As the long‐term neurodevelopmental outcomes are the major determinant for the choice of ASM, the lack of data on long‐term outcomes is a major drawback in interpreting the results of this review.
It is well recognised that detection of neonatal seizures on a clinical observation basis alone is unreliable because most infants have only subtle clinical manifestations which are often missed in a clinical setting. In addition, clinical diagnosis has poor diagnostic accuracy as clinical behaviours are often misinterpreted as seizures (Murray 2008). In fact, one study revealed that even experts only correctly diagnosed 50% of events if relying on clinical observation only (Malone 2009). It is also known that many seizures are electrographic‐only or subclinical, particularly after treatment with some types of ASM which are known to induce uncoupling (Boylan 2002;Hahn 2004; Scher 2003). Electrographic seizures are also the most common seizure type in critical neonates as they are often on sedation, pain relief or muscle relaxation. In order for clinical trials to be meaningful and transferable, it is essential that outcome measures are well‐defined and can be measured accurately and precisely (Heneghan 2017). For the reasons outlined above, clinical diagnosis of seizures is neither. Thus, trials using clinical diagnosis should not be used for licencing ASM or to inform clinical guidelines or recommendations. Hence, the conclusions of this review are based on trials that used EEG‐confirmed seizures.
Treatment of both clinical and electrographic seizures, when compared with treating clinical seizures alone, may have little or no effect on mortality before hospital discharge, seizure burden during hospitalisation, and the proportion of infants who develop epilepsy post‐discharge (low‐certainty evidence). Since both the trials included only neonates with HIE, the results are applicable only to this subgroup. There were no data on long‐term mortality or neurodevelopmental outcomes.
Short‐term maintenance therapy with ASM after achieving seizure control when compared to no maintenance ASM may have little or no effect on mortality before hospital discharge, mortality by 18 to 24 months, neurodevelopmental disability by 18 to 24 months, and the proportion of infants with epilepsy post‐discharge (low‐certainty evidence). Both trials included only those neonates who achieved seizure control after the first loading dose of phenobarbital. Hence, the results do not apply to neonates who require more than one ASM for seizure control.
Quality of the evidence
For the comparison, phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures, the certainty of evidence was moderate for the outcomes: seizure control after first loading dose of ASM and seizure control after maximal loading dose of ASM (downgraded by one level for serious imprecision due to the small size not meeting the 'Optimal Information Size' criteria); and the certainty of evidence was very low for the outcomes: mortality before hospital discharge, requirement of mechanical ventilation, sedation or drowsiness, and proportion of infants with epilepsy post‐discharge (downgraded by one level for indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well and by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria).
For the comparison, phenobarbital versus levetiracetam as first‐line ASM for clinically diagnosed neonatal seizures, the certainty of evidence was very low for the outcomes: seizure control after first loading dose of ASM (downgraded by two levels for very serious risk of bias due to 'high risk of bias' in two trials and some concerns in the other trial and by one level for serious imprecision due to small sample size not meeting the 'Optimal Information Size' criterion); seizure control after maximal loading dose of ASM (downgraded by two levels for very serious risk of bias due to high risk of bias in all the three included trials and by one level for serious imprecision due to small sample size not meeting the 'Optimal Information Size' criterion); requirement of mechanical ventilation (downgraded by two levels for very serious imprecision due to a single digit event rate and by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well); sedation or drowsiness (downgraded by two levels for very serious risk of bias due to high risk of bias in all included trials, and by one level for serious inconsistency, serious imprecision and serious indirectness); and proportion of infants with epilepsy post‐discharge (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial and for very serious imprecision). The certainty of evidence was low for mortality before hospital discharge (downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well and by one level for serious imprecision due to a low event rate not meeting the 'Optimal Information Size' criteria).
For the comparison, phenobarbital versus phenytoin as first‐line ASM for EEG‐confirmed neonatal seizures, the certainty of evidence was very low for the outcome: seizure control after maximal loading dose of ASM (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial and by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria).
For the comparison, phenobarbital versus phenytoin as first‐line ASM for clinically diagnosed neonatal seizures, the certainty of evidence was low for the outcome: seizure control after the first loading dose of ASM (downgraded by one level for serious risk of bias as the trial contributing > 50% weighting to the estimate has a high risk of overall bias and by one level for serious inconsistency as there was considerable heterogeneity (I2 = 96%)). The certainty of evidence was very low for mortality before hospital discharge (downgraded by one level for serious inconsistency as there was substantial heterogeneity (I2 = 82%); by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well; and by one level for serious imprecision for sample size and event rate not meeting the 'Optimal Information Size' criteria); requirement for mechanical ventilation (downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well; and by two levels for very serious imprecision due to wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria); sedation or drowsiness (downgraded by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well; by two levels for very serious imprecision due to wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria; and by two levels for very serious risk of bias due to high risk of bias in the only included trial).
For the comparison, phenobarbital versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures, the certainty of evidence was very low for all the outcomes: seizure control after the first loading dose of ASM (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial and by one level for serious imprecision for sample size and event rate not meeting the 'Optimal Information Size' criteria); mortality before hospital discharge; and sedation or drowsiness (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial, by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs, as well and by two levels for very serious imprecision due to wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria).
For the comparison, phenytoin versus lorazepam as first‐line ASM for clinically diagnosed neonatal seizures, the certainty of evidence was very low for all the outcomes: seizure control after the first loading dose of ASM (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial and by one level for serious imprecision for sample size and event rate not meeting the 'Optimal Information Size' criteria); mortality before hospital discharge; and sedation or drowsiness (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial, by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs as well and by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria).
For the comparison, phenobarbital + bumetanide versus phenobarbital alone for EEG‐confirmed neonatal seizures, the certainty of evidence was low for the outcome: seizure control after the first loading dose of ASM (downgraded by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria); and very low for the outcomes: mortality before hospital discharge, cognitive impairment at 18 to 24 months and proportion of infants with epilepsy post‐discharge (downgraded by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria, and by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs, as well).
For the comparison, lignocaine versus benzodiazepines as second‐line ASM for EEG‐confirmed neonatal seizures, the certainty of evidence was very low for all the outcomes: seizure control after maximal loading dose of ASM (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial and by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria); mortality or neurodevelopmental disability at 12 months; all‐cause mortality before hospital discharge; and neurodevelopmental disability at 12 months (downgraded by two levels for very serious risk of bias due to high risk of bias in the only included trial and by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria, and by one level for serious indirectness of the intervention as the study population included neonates who required second‐ and third‐line ASMs, as well).
For the comparison, treating both clinical and electrographic seizures versus clinical seizures alone, the certainty of evidence was low for all the outcomes: mortality before hospital discharge, seizure burden during hospitalisation, and proportion of infants with epilepsy post‐discharge (downgraded by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria).
For the comparison, maintenance therapy with ASM after achieving seizure control versus no maintenance ASM, the certainty of evidence was very low for repeat seizures before hospital discharge (downgraded by one level for risk of bias due to some concerns in the risk of bias in both the included studies, and by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria); and low for other outcomes: mortality before hospital discharge, mortality at 18 to 24 months, neurodevelopmental disability at 18 to 24 months and proportion of infants who develop epilepsy post‐discharge (downgraded by two levels for very serious imprecision due to a wide confidence interval crossing the line of no difference, and sample size and event rate not meeting the 'Optimal Information Size' criteria).
Potential biases in the review process
We performed a comprehensive search of the medical literature to identify all RCTs evaluating the role of ASMs for neonates with seizures. However, although it is unlikely that we missed large relevant studies, it is still possible that we failed to identify small studies whose results have been published in abstract proceedings or in less accessible literature. We made every effort to contact the authors of any included study asking them to provide missing data. Furthermore, some authors of the present review were inevitably already familiar with most of the included studies.
Agreements and disagreements with other studies or reviews
Other systematic reviews have previously addressed the topic of treatment of neonatal seizures. Only the previous Cochrane Review (Booth 2004), and the International League Against Epilepsy/World Health Organization (ILAE/WHO) guidelines (WHO 2011) adopted a similar comprehensive approach as our review. Both these previous reviews included only studies on EEG‐confirmed seizures. They concluded that phenobarbital is the recommended ASM in neonates, but the certainty of evidence was very low. Such a discrepancy in certainty of evidence is due to the inclusion of the recently published NEOLEV2 study (Sharpe 2020) in our review. Other systematic reviews were limited to a literature search and, although they limited their search to EEG‐confirmed seizures, they did not synthesise results with meta‐analysis (Falsaperla 2021; Hellström‐Westas 2015; Slaughter 2013). One other systematic review used a different approach (network meta‐analysis) and thus is not easily comparable with ours (Xu 2021). Most of the other reviews evaluated single ASMs such as levetiracetam (Hooper 2021; McHugh 2018; Sharma 2022), phenobarbital (Kumar 2021), or levetiracetam versus phenobarbital (Qiao 2021), or they limited the review to a single aetiology such as stroke (Sortino 2022), and inborn errors of metabolism (Falsaperla 2021). All of these included only or mostly retrospective and uncontrolled studies. Thus, their evidence is of very low certainty and no strong recommendations can be based on this.
The present systematic review is the first to evaluate evidence on the duration of treatment. The ILAE/WHO guidelines (WHO 2011) recommended stopping ASM before discharge, but this was an expert opinion not based on data from the literature.
Authors' conclusions
Implications for practice.
Phenobarbital is probably more effective than levetiracetam in achieving seizure control after the first loading dose and after the maximal loading dose (moderate‐certainty evidence). Phenobarbital may be more effective than phenytoin in achieving seizure control after the first loading dose (low‐certainty evidence). However, as the latter finding is based on trials that utilised clinical diagnosis of seizures, this needs to be confirmed by a well‐powered RCT evaluating EEG‐confirmed seizures. Phenobarbital + bumetanide may have little or no difference in achieving seizure control when compared to phenobarbital alone (low‐certainty evidence). Limited data and very low‐certainty evidence preclude us from drawing any reasonable conclusion on the effect of using one ASM versus another on other short‐ and long‐term outcomes.
In neonates with HIE, treatment of both clinical and electrographic seizures when compared to treating clinical seizures alone may have little or no effect on mortality before hospital discharge, seizure burden during hospitalisation, and the proportion of infants who develop epilepsy post‐discharge (low‐certainty evidence).
In neonates who achieve seizure control after the first loading dose of phenobarbital, maintenance therapy with ASM when compared to no maintenance ASM may have little or no effect on mortality before hospital discharge, mortality by 18 to 24 months, neurodevelopmental disability by 18 to 24 months, and proportion of infants with epilepsy post‐discharge (low‐certainty evidence).
All findings of this review apply only to term and late preterm neonates.
We identified 23 studies that were registered as ongoing. However, most of these were either entered into the registry five to 10 years ago without follow‐up, or results were not published in spite of the apparently achieved sample size. We identified one study investigating treatment duration that may change the conclusions of this review (NCT04320940).
Implications for research.
We need well‐designed RCTs evaluating the effect of one ASM versus another to improve the precision of the results. These RCTs should use EEG to diagnose seizures, as clinical diagnosis of seizures is prone to errors and inaccurate. These studies should be adequately powered to assess the effect of ASMs on long‐term neurodevelopmental outcomes. As seizures are not uncommon in preterm neonates, we need separate RCTs evaluating the choice of ASM in this vulnerable population.
Similarly, the other two questions 'whether to treat only‐electrographic seizures with ASM or not' and 'whether to give routine maintenance therapy with ASM after achieving seizure control with loading doses of ASM' are very pertinent for the clinical management of neonates with seizures. We need further RCTs on these to evaluate the effect of ASM on short‐ and long‐term outcomes with more precision.
History
Protocol first published: Issue 3, 2022
Risk of bias
Risk of bias for analysis 1.1 Proportion of infants who achieve seizure control after the first loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method when compared to clinical diagnosis of seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 1.2 Proportion of infants who achieve seizure control after the the maximal loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method when compared to clinical diagnosis of seizures | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 1.3 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 1.4 Requirement for mechanical ventilation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method when compared to clinical diagnosis of seizures | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 1.5 Proportion of infants who develop sedation or drowsiness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performedThough there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. | Low risk of bias | Trial analysed as per a priori registered protocol. | Some concerns | Some concerns in one domain |
Risk of bias for analysis 1.6 Bradycardia.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method when compared to clinical diagnosis of seizures | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 1.7 Hypotension requiring volume or inotropes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 1.8 Shock requiring volume or inotropes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Some concerns | Some concerns in one domain |
Risk of bias for analysis 1.9 Recurrence of seizure before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method when compared to clinical diagnosis of seizures | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 1.10 Proportion of infants with epilepsy post‐discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Sharpe 2020 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though there was missing data analysis methods that correct for bias such as sensitivity analyses showing that results are little changed under a range of plausible assumptions about the relationship between missingness in the outcome and its true value was performed | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 2.1 Proportion of infants who achieve seizure control after the first loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Akeel 2022 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | Some concerns | Since clinical judgement was used to ascertain seizure control, and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Some concerns | Trial protocol not available. | High risk of bias | Some concerns in more than one domain. |
| Gowda 2019 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
| Susnerwala 2022 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | Some concerns | Since clinical judgement was used to ascertain seizure control, and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | Some concerns | Some concerns in one domain. |
Risk of bias for analysis 2.2 Proportion of infants who achieve seizure control after the maximal loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ghaffar 2020 | Some concerns | Allocation concealment not specified and not enough data provided for baseline characteristics evaluation | Some concerns | No information on blinding or if any deviations arouse outside the trial context | Low risk of bias | Data for all enrolled patients available | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Some concerns | No registered protocol accessible | High risk of bias | High risk in one domain |
| Gowda 2019 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Some concerns | A trial protocol is not available for assessment | High risk of bias | High risk in one domain |
Risk of bias for analysis 2.3 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Gowda 2019 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Perveen 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients at the time of discharge. Significant attrition at 3 and 6 months, though no outcomes assessed during this period was included in this analysis. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
| Prakash 2019 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group. | Some concerns | Trial protocol not available. | Some concerns | Some concerns in one domain. |
| Susnerwala 2022 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group. | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk in all the domains. |
Risk of bias for analysis 2.4 Requirement for mechanical ventilation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Akeel 2022 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group. | Some concerns | Trial protocol not available. | Some concerns | Some concerns in one domain. |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Gowda 2019 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Perveen 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients at the time of discharge. Significant attrition at 3 and 6 months, though no outcomes assessed during this period was included in this analysis. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 2.5 Proportion of infants who develop sedation or drowsiness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | High risk of bias in one domain |
| Prakash 2019 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | High risk of bias | Since the outcome is a subjective one and the assessors were aware of the intervention allocation, there is a likelihood of assessment being influenced by the knowledge of the allocation group. | Some concerns | Trial protocol not available. | High risk of bias | High risk of bias in one domain. |
Risk of bias for analysis 2.6 Bradycardia.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Akeel 2022 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group. | Some concerns | Trial protocol not available. | Some concerns | Some concerns in one domain. |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Gowda 2019 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 2.7 Hypotension requiring volume or inotropes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 2.8 Shock requiring volume or inotropes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Perveen 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients at the time of discharge. Significant attrition at 3 and 6 months, though no outcomes assessed during this period was included in this analysis. | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Some concerns | Some concerns in one domain |
Risk of bias for analysis 2.9 Proportion of infants with an abnormal background pattern in EEG during ASM treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 2.10 Proportion of infants with an abnormal background pattern in EEG after stopping ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 2.11 Duration of hospital stay.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Perveen 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients at the time of discharge. Significant attrition at 3 and 6 months, though no outcomes assessed during this period was included in this analysis. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 2.12 Recurrence of seizure before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Some concerns | A trial protocol is not available for assessment | High risk of bias | High risk in one domain |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Some concerns | A trial protocol is not available for assessment | High risk of bias | High risk in one domain |
Risk of bias for analysis 2.13 Proportion of infants with persistent seizures and/or requiring ASM at discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Some concerns | A trial protocol is not available for assessment | High risk of bias | High risk in one domain |
Risk of bias for analysis 2.14 Proportion of infants discharged on gavage feeds.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 2.15 Proportion of infants with an abnormal neurological examination at discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Khan 2020 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
| Perveen 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients at the time of discharge. Significant attrition at 3 and 6 months, though no outcomes assessed during this period was included in this analysis. | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Some concerns | Some concerns in one domain |
| Susnerwala 2022 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients. | High risk of bias | Since the outcome is a subjective one and the assessors were aware of the intervention allocation, there is a likelihood of assessment being influenced by the knowledge of the allocation group. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk of bias in one domain |
Risk of bias for analysis 2.16 Proportion of infants who develop epilepsy post discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Falsaperla 2019 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups | Low risk of bias | Single blinded study with the personnel aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group. | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 3.1 Proportion of infants who achieve seizure control after the maximal loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Painter 1999 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups, except in gender. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method. | Some concerns | Trial protocol not available | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 3.2 Arrythmias causing circulatory disturbance.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Painter 1999 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups, except in gender. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group, It was a double blinded RCT and hence the outcome assessors are blinded to allocation; EEG was used to diagnose abnormal activity | Some concerns | Trial protocol not available | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 3.3 Hypotension requiring volume or inotropes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Painter 1999 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups, except in gender. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group, It was a double blinded RCT and hence the outcome assessors are blinded to allocation; EEG was used to diagnose abnormal activity | Some concerns | Trial protocol not available | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 3.4 Bradycardia.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Painter 1999 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups, except in gender. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method. | Some concerns | Trial protocol not available | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 4.1 Proportion of infants who achieve seizure control after the first loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Pathak 2013 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Though EEG was performed, it was done after 48‐72 hours of clinical resolution of seizures. Since clinical judgement was used to ascertain seizure control, and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | Some concerns | Some concerns in one domain |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 4.2 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Pathak 2013 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one of the domains |
Risk of bias for analysis 4.3 Requirement of mechanical ventilation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Pathak 2013 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 4.4 Proportion of infants who develop sedation or drowsiness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one of the domains |
Risk of bias for analysis 4.5 Bradycardia.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Pathak 2013 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 4.6 Proportion of infants with persistent seizures and/or requiring ASM at discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 5.1 Proportion of infants who achieve seizure control after the first loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 5.2 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one of the domains |
Risk of bias for analysis 5.3 Proportion of infants who develop sedation or drowsiness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 5.4 Proportion of infants with persistent seizures and/or requiring ASM at discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 6.1 Proportion of infants who achieve seizure control after the first loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 6.2 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one of the domains |
Risk of bias for analysis 6.3 Proportion of infants who develop sedation or drowsiness.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 6.4 Proportion of infants with persistent seizures and/or requiring ASM at discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Solanki 2015 | High risk of bias | There is no information on allocation concealment and baseline characteristics suggest a mismatch between the two groups despite randomisation. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that the assessors were aware of the intervention, there is a likelihood of assessment being influenced by the knowledge of the allocation group. The authors have given details of the seizure definition used and who diagnosed the seizures. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 7.1 Proportion of infants who achieve seizure control after the first loading dose of ASM.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Triple‐masked trial | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 7.2 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Triple‐masked trial | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 7.3 Proportion of infants with cognitive impairment at 18‐24 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Triple‐masked trial | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 7.4 Seizure burden during hospitalisation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | aEEG was utilised to detect seizures which is a reliable method when compared to clinical diagnosis of seizures | Low risk of bias | Trial analysed as per a priori registered protocol. | Some concerns | Some concerns in one domain |
Risk of bias for analysis 7.5 Requirement for mechanical ventilation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Triple‐masked trial | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 7.6 Hypotension requiring volume or inotropes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Triple‐masked trial | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 7.7 Proportion of infants with an abnormal background pattern in EEG during ASM treatment.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Triple‐masked trial | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 7.8 Proportion of infants who develop epilepsy post discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Soul 2021 | Low risk of bias | Triple‐masked, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Triple‐masked trial | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 8.2 Mortality or neurodevelopmental disability at 12 months' corrected age.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Boylan 2004 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though there is no information on whether the allocation was blinded to participants or treating physicians, there were no significant deviations outside the trial context. Analysis was also as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 8.3 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Boylan 2004 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though there is no information on whether the allocation was blinded to participants or treating physicians, there were no significant deviations outside the trial context. Analysis was also as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 8.4 Neurodevelopmental disability at 12 months' corrected age.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Boylan 2004 | Some concerns | No information on allocation concealment, but baseline characteristics do not show any differences between the two groups. | Low risk of bias | Though there is no information on whether the allocation was blinded to participants or treating physicians, there were no significant deviations outside the trial context. Analysis was also as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Some concerns | A trial protocol is not available for assessment | High risk of bias | Some concerns in more than one domain |
Risk of bias for analysis 9.1 Proportion of infants with repeat seizure before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jindal 2021 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Diagnosis of seizures was based on clinical assessment and not EEG. Hence, there is a likelihood that assessment might have been influenced by knowledge of allocation. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Data reasonably complete for all the included patients till hospital discharge,Though many of the enrolled patients were lost to follow up, it was balanced between the two groups. Hence, it seems that the result is not biased as reasons for lost to follow up does not differ significantly between the groups. | High risk of bias | Diagnosis of seizures was based on clinical assessment and not EEG. Hence, there is a likelihood that assessment might have been influenced by knowledge of allocation. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 9.2 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jindal 2021 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Data reasonably complete for all the included patients till hospital discharge | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group, | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 9.3 Mortality at 18 to 24 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Though many of the enrolled patients were lost to follow up, it was balanced between the two groups. Hence, it seems that the result is not biased as reasons for lost to follow up does not differ significantly between the groups. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group, | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 9.4 Neurodevelopmental disability at 18 to 24 months' corrected age.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Though many of the enrolled patients were lost to follow up, it was balanced between the two groups. Hence, it seems that the result is not biased as reasons for lost to follow up does not differ significantly between the groups. | Low risk of bias | It was a double blinded RCT and hence the outcome assessors are blinded to allocation | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 9.5 Requirement for mechanical ventilation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jindal 2021 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 9.6 Shock requiring volume or inotropes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jindal 2021 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Data reasonably complete for all the included patients till hospital discharge, | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group, | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 9.7 Abnormal background pattern in EEG after achieving seizure control.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Data reasonably complete for all the included patients till hospital discharge,Though many of the enrolled patients were lost to follow up, it was balanced between the two groups. Hence, it seems that the result is not biased as reasons for lost to follow up does not differ significantly between the groups. | Low risk of bias | EEG was used to diagnose abnormal activity | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 9.8 Duration of hospital stay.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jindal 2021 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Data reasonably complete for all the included patients till hospital discharge,Though many of the enrolled patients were lost to follow up, it was balanced between the two groups. Hence, it seems that the result is not biased as reasons for lost to follow up does not differ significantly between the groups. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group, It was a double blinded RCT and hence the outcome assessors are blinded to allocation. | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk of bias across all domains |
Risk of bias for analysis 9.9 Proportion of infants with persistent seizures and/or requiring ASM at discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jindal 2021 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | High risk of bias | Diagnosis of seizures was based on clinical assessment and not EEG. Hence, there is a likelihood that assessment might have been influenced by knowledge of allocation. There is no clear definition for different seizure types, how it was differentiated from non‐epiletic events and how it was assesed and by whom. | Low risk of bias | Trial analysed as per a priori registered protocol. | High risk of bias | High risk in one domain |
Risk of bias for analysis 9.10 Abnormal neurological examination at discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jindal 2021 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Some concerns | Since the outcome is a subjective one and that there is no information on whether assessors were aware of the intervention allocation and there is a likelihood of assessment being influenced by the knowledge of the allocation group, a high risk was adjudged for this domain. | Low risk of bias | Low risk across all domains | Some concerns | Some concerns in one domain |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Data reasonably complete for all the included patients till hospital discharge | Low risk of bias | It was a double blinded RCT and hence the outcome assessors are blinded to allocation | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 9.11 Proportion of infants who develop epilepsy post‐discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Saxena 2016 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Double‐blinded study and neither the participants nor the treating physicians were aware of the allocation. | Low risk of bias | Though many of the enrolled patients were lost to follow up, it was balanced between the two groups. Hence, it seems that the result is not biased as reasons for lost to follow up does not differ significantly between the groups. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group, | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 10.1 Seizure burden during hospitalisation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Srinivasakumar 2015 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome (EEG used), it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
| Van Rooji 2010 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though 9 out of 42 patients randomised were excluded, the reasons for the same are stated and they do not differ substantially between the groups, thus indicating that the results might not be biased. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 10.2 Mortality before hospital discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Srinivasakumar 2015 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
| Van Rooji 2010 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Though 9 out of 42 patients randomised were excluded, the reasons for the same are stated and they do not differ substantially between the groups, thus indicating that the results might not be biased. | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Risk of bias for analysis 10.3 Proportion of infants who develop epilepsy post‐discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Srinivasakumar 2015 | Low risk of bias | Allocation concealed, sequence generation random and baseline characteristics does not reveal any imbalance between the two groups. | Low risk of bias | Though the personnel were aware of the intervention allocation, but there seems to be no deviations that arouse outside the trial context. Also all patients analysed as randomised. | Low risk of bias | Data reasonably complete for all the included patients | Low risk of bias | Being an objective outcome, it is unlikely that assessment of the outcome would be influenced by knowledge of allocation group | Low risk of bias | Trial analysed as per a priori registered protocol. | Low risk of bias | Low risk across all domains |
Acknowledgements
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
We would like to thank Cochrane Neonatal: Michelle Fiander and Jane Cracknell, Managing Editors; and Roger Soll and Bill McGuire, Co‐coordinating Editors, who provided editorial and administrative support. Michelle Fiander (Information Specialist), who designed the literature searches and search methods; and Chris Cooper (Information Specialist) who ran the searches.
We would like to thank the authors Raffaele Falsaperla, Tafazzal Hossain Khan, Ankush Jindal, Cynthia Sharpe and Janet Soul for providing additional data and details regarding their trials.
We thank the following peer reviewers:
Dr. Manigandan Chandrasekaran MD FRCPCH, Consultant Neonatologist, Liverpool Women's Hospital NHS Foundation Trust.
Balamurugan Palanisami, Liverpool Women's Hospital NHS Foundation Trust, UK.
We would also like to thank Anne Lethaby, Cochrane Central Production Service, for copy editing the review.
Appendices
Appendix 1. May 2022 Searches
| Resource | N |
| MEDLINE | 6863 (6702 trials 161 SR) |
| Embase | 2133 (1962 trials 171 SR) |
| CENTRAL | 1491 |
| Epistemonikos | 76 |
| ClincialTrials.gov | 594 |
| ICTRP | 112 |
| Other sources | 3 |
| Total | 11,272 |
Database: MEDLINE (MEDALL)
Host: Ovid
Data parameters: 1946 to May 13, 2022
Date of search: 16 May 2022
| # | Searches | Results |
| 1 | exp infant, newborn/ or Intensive Care, Neonatal/ or Intensive Care Units, Neonatal/ or Gestational Age/ | 702970 |
| 2 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 994641 |
| 3 | 1 or 2 [Neonatal search filter] | 1303636 |
| 4 | Anticonvulsants/ | 54692 |
| 5 | *Seizures/ | 36682 |
| 6 | (anticonvuls* or anti‐convuls* or antiepileptic* or anti‐epileptic* or antiseizur*or anti‐seizur* or (seizur* adj2 prevent*) or seizur*).ti,ab,kw,kf. | 164674 |
| 7 | 4 or 5 or 6 [terms for seizure] | 190968 |
| 8 | randomized controlled trial.pt. | 568423 |
| 9 | controlled clinical trial.pt. | 94868 |
| 10 | randomized.ti,ab. | 608631 |
| 11 | placebo.ti,ab. | 234348 |
| 12 | drug therapy.fs. | 2490206 |
| 13 | randomly.ti,ab. | 383116 |
| 14 | trial.ti,ab. | 696888 |
| 15 | groups.ti,ab. | 2378348 |
| 16 | 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 [Cochrane HSSS] | 5417493 |
| 17 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1040889 |
| 18 | (control* adj2 (group? or random* or trial? or study)).ti,ab,kw,kf. | 1036086 |
| 19 | 17 or 18 [additional trials terms] | 1611025 |
| 20 | 16 or 19 [combining HSSS or additional terms] | 5695751 |
| 21 | meta‐analysis/ or "systematic review"/ or network meta‐analysis/ | 273123 |
| 22 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kf,kw. | 268897 |
| 23 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kf,kw. | 34361 |
| 24 | (data synthes* or data extraction* or data abstraction*).ti,ab,kf,kw. | 35120 |
| 25 | (hand search* or handsearch*).ti,ab,kf,kw. | 10457 |
| 26 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kf,kw. | 31953 |
| 27 | meta‐analysis as topic/ or network meta‐analysis/ | 24965 |
| 28 | (meta analy* or metanaly* or meta regression* or metaregression*).ti,ab,kf,kw. | 236014 |
| 29 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 288285 |
| 30 | (cochrane or systematic review?).jw. | 19174 |
| 31 | 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 [CADTH SR filter] | 547208 |
| 32 | (2020* or 2021* or 2022*).dt,dp,ed,ep,yr. | 4376776 |
| 33 | 31 and 32 | 182970 |
| 34 | 3 and 7 and 20 [Neonates AND Seizures AND RCT filter] | 6702 |
| 35 | 3 and 7 and 33 [Neonates AND Seizures AND SRs date limited 2020‐current] | 161 |
Database: Embase
Host: Ovid
Data parameters: 1980 to 2022 Week 19
Date of search: 16 May 2022
| # | Searches | Results |
| 1 | newborn/ or prematurity/ or newborn intensive care/ or newborn care/ or gestational age/ | 701545 |
| 2 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1116223 |
| 3 | 1 or 2 [neonates filter] | 1353539 |
| 4 | *anticonvulsive agent/ | 23296 |
| 5 | *seizure/ | 40709 |
| 6 | (anticonvuls* or anti‐convuls* or antiepileptic* or anti‐epileptic* or antiseizur*or anti‐seizur* or (seizur* adj2 prevent*) or seizur*).ti,ab,kw,kf. | 238735 |
| 7 | 4 or 5 or 6 [terms for seizure] | 247955 |
| 8 | Randomized controlled trial/ or Controlled clinical study/ | 893379 |
| 9 | random$.ti,ab,kw. | 1776894 |
| 10 | Randomization/ | 93533 |
| 11 | placebo.ti,ab,kw. | 334789 |
| 12 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. | 248705 |
| 13 | double blind procedure/ | 191703 |
| 14 | (controlled adj7 (study or design or trial)).ti,ab,kw. | 402960 |
| 15 | parallel group$1.ti,ab. | 29261 |
| 16 | (crossover or cross over).ti,ab. | 113486 |
| 17 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. | 375586 |
| 18 | (open adj label).ti,ab. | 96587 |
| 19 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1454219 |
| 20 | (control* adj2 (group? or random*)).ti,ab,kw,kf. | 1177241 |
| 21 | 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 [trials filter] | 3030443 |
| 22 | meta‐analysis/ or "systematic review"/ or "meta analysis (topic)"/ | 498280 |
| 23 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kw. | 326015 |
| 24 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kw. | 48224 |
| 25 | (data synthes* or data extraction* or data abstraction*).ti,ab,kw. | 42804 |
| 26 | (hand search* or handsearch*).ti,ab,kw. | 12732 |
| 27 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kw. | 42052 |
| 28 | (meta analy* or metanaly* or meta regression* or metaregression*).ti,ab,kw. | 301073 |
| 29 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 362905 |
| 30 | (cochrane or systematic review?).jn,jx. | 30615 |
| 31 | (overview adj2 reviews).ti. | 108 |
| 32 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 | 776900 |
| 33 | (2020* or 2021* or 2022*).yr. | 4242025 |
| 34 | 32 and 33 [CADTH SR filter limited to the last 2 years] | 200764 |
| 35 | 3 and 7 and 34 [results of the SR search] | 171 |
| 36 | 3 and 7 and 21[results of the search for trials] | 1962 |
Database: Cochrane CENTRAL
Host: Wiley interface
Data parameters: Issue 5 of 12, May 2022
Date of search: 16 May 2022
ID Search Hits
#1 MeSH descriptor: [Infant, Newborn] explode all trees 17416
#2 MeSH descriptor: [Intensive Care, Neonatal] this term only 353
#3 MeSH descriptor: [Intensive Care Units, Neonatal] this term only 853
#4 MeSH descriptor: [Gestational Age] this term only 2760
#5 ("babe" or "babes" or baby* or "babies" or "gestational age?" or infant? or "infantile" or infancy or "low birth weight?" or "low birthweight?" or neonat* or "neo‐nat*" or newborn* or "new born?" or "newly born" or "premature" or "pre‐mature" or "pre‐matures" or prematures or prematurity or "pre‐maturity" or "preterm" or "preterms" or "pre term?" or "preemie" or "preemies" or "premies" or "premie" or "VLBW" or "VLBWI" or "VLBW‐I" or "VLBWs" or "LBW" or "LBWI" or "LBWs" or "ELBW" or "ELBWI" or "ELBWs" or "NICU" or "NICUs"):ti,ab,kw 96816
#6 #1 OR #2 OR #3 OR #4 OR #5 96816
#7 MeSH descriptor: [Anticonvulsants] this term only 2535
#8 MeSH descriptor: [Seizures] this term only 1035
#9 (anticonvuls* or anti‐convuls* or antiepileptic* or anti‐epileptic* or antiseizur*or anti‐seizur* or (seizur* NEAR/2 prevent*) or seizur*):ti,ab,kw 12276
#10 #7 or #8 or #9 12276
#11 #6 AND #10 1570
Database: Epistemonikos
Host: https://www.epistemonikos.org/en/
Date of search: 16 May 2022
((babe OR babes OR baby OR babies OR "gestational age" OR "gestational ages" OR infant OR infants OR infantile OR infancy OR "low birth weight" OR "low birthweight" OR neonate OR neonatal OR "neo nate" OR "neo natal" OR newbORn OR "new bORn" OR newborns OR "new borns" OR "newly bORn" OR premature OR "pre mature" OR "pre matures" OR prematures OR prematurity OR "pre maturity" OR preterm OR preterms OR "pre term" OR preemie OR preemies OR premies OR premie OR VLBW OR VLBWI OR "VLBW I" OR VLBWs OR LBW OR LBWI OR LBWs OR ELBW OR ELBWI OR ELBWs OR NICU OR NICUs) AND (anticonvulsant OR anticonvulsants OR "anti convulsant" OR "anti convulsants" OR antiepileptic OR antiepileptics OR "anti epileptic" OR antiseizure OR antiseizures OR "anti‐seizure" OR "anti seizures" OR seizure))
Resource: ClinicalTrials.gov
Host: https://clinicaltrials.gov/
Date of search: 12 April 2022
Searcher location: London, UK.
The search was run in expert search using the following search string. The results were downloaded and imported into EndNote.
((babe OR babes OR baby OR babies OR "gestational age" OR "gestational ages" OR infant OR infants OR infantile OR infancy OR "low birth weight" OR "low birthweight" OR neonate OR neonatal OR "neo nate" OR "neo natal" OR newbORn OR "new bORn" OR newborns OR "new borns" OR "newly bORn" OR premature OR "pre mature" OR "pre matures" OR prematures OR prematurity OR "pre maturity" OR preterm OR preterms OR "pre term" OR preemie OR preemies OR premies OR premie OR VLBW OR VLBWI OR "VLBW I" OR VLBWs OR LBW OR LBWI OR LBWs OR ELBW OR ELBWI OR ELBWs OR NICU OR NICUs) AND (anticonvulsant OR anticonvulsants OR "anti convulsant" OR "anti convulsants" OR antiepileptic OR antiepileptics OR "anti epileptic" OR antiseizure OR antiseizures OR "anti‐seizure" OR "anti seizures" OR seizure))
Resource: ICTRP
Host: https://trialsearch.who.int/
Date of search: 16 May 2022
Searcher location: London, UK.
The following search strings were run separately in the basic search box.
((babe OR babes OR baby OR babies OR "gestational age" OR "gestational ages" OR infant OR infants OR infantile OR infancy OR "low birth weight" OR "low birthweight" OR neonate OR neonatal OR "neo nate" OR "neo natal" OR newbORn OR "new bORn" OR newborns OR "new borns" OR "newly bORn" OR premature OR "pre mature" OR "pre matures" OR prematures OR prematurity OR "pre maturity" OR preterm OR preterms OR "pre term" OR preemie OR preemies OR premies OR premie OR VLBW OR VLBWI OR "VLBW I" OR VLBWs OR LBW OR LBWI OR LBWs OR ELBW OR ELBWI OR ELBWs OR NICU OR NICUs) AND (anticonvulsant OR anticonvulsants OR "anti convulsant" OR "anti convulsants" OR antiepileptic OR antiepileptics OR "anti epileptic" OR antiseizure OR antiseizures OR "anti‐seizure" OR "anti seizures" OR seizure))
Appendix 2. June 2023 Searches
| Resource | N |
| CENTRAL | 112 |
| MEDLINE | 603 (515 Trials 88 SR) |
| Embase | 283 (158 Trials 125 SR) |
| Epistemonikos | 0 |
| ClincialTrials.gov | 655 |
| ICTRP | 80 |
| Total | 1737 |
Database: Cochrane CENTRAL
Host: Wiley interface
Data parameters: Issue 6 of 12, June 2023
Date of search: 7 June 2023
ID Search Hits
#1 MeSH descriptor: [Infant, Newborn] explode all trees 20484
#2 MeSH descriptor: [Intensive Care, Neonatal] this term only 375
#3 MeSH descriptor: [Intensive Care Units, Neonatal] this term only 1025
#4 MeSH descriptor: [Gestational Age] this term only 3956
#5 ("babe" or "babes" or baby* or "babies" or "gestational age?" or infant? or "infantile" or infancy or "low birth weight?" or "low birthweight?" or neonat* or "neo‐nat*" or newborn* or "new born?" or "newly born" or "premature" or "pre‐mature" or "pre‐matures" or prematures or prematurity or "pre‐maturity" or "preterm" or "preterms" or "pre term?" or "preemie" or "preemies" or "premies" or "premie" or "VLBW" or "VLBWI" or "VLBW‐I" or "VLBWs" or "LBW" or "LBWI" or "LBWs" or "ELBW" or "ELBWI" or "ELBWs" or "NICU" or "NICUs"):ti,ab,kw 105658
#6 #1 OR #2 OR #3 OR #4 OR #5 105658
#7 MeSH descriptor: [Anticonvulsants] this term only 2972
#8 MeSH descriptor: [Seizures] this term only 1269
#9 (anticonvuls* or anti‐convuls* or antiepileptic* or anti‐epileptic* or antiseizur*or anti‐seizur* or (seizur* NEAR/2 prevent*) or seizur*):ti,ab,kw 13195
#10 #7 or #8 or #9 13195
#11 #6 AND #10 1720
Database: MEDLINE (MEDALL)
Host: Ovid
Data parameters: 1946 to June 06, 2023
Date of search: 7 June 2023
| # | Searches | Results |
| 1 | exp infant, newborn/ or Intensive Care, Neonatal/ or Intensive Care Units, Neonatal/ or Gestational Age/ | 721745 |
| 2 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1040979 |
| 3 | 1 or 2 | 1352125 |
| 4 | Anticonvulsants/ | 55873 |
| 5 | *Seizures/ | 36947 |
| 6 | (anticonvuls* or anti‐convuls* or antiepileptic* or anti‐epileptic* or antiseizur*or anti‐seizur* or (seizur* adj2 prevent*) or seizur*).ti,ab,kw,kf. | 173161 |
| 7 | 4 or 5 or 6 | 199675 |
| 8 | randomized controlled trial.pt. | 594019 |
| 9 | controlled clinical trial.pt. | 95326 |
| 10 | randomized.ti,ab. | 658117 |
| 11 | placebo.ti,ab. | 245248 |
| 12 | drug therapy.fs. | 2596416 |
| 13 | randomly.ti,ab. | 410650 |
| 14 | trial.ti,ab. | 754362 |
| 15 | groups.ti,ab. | 2553341 |
| 16 | 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 | 5741348 |
| 17 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1121180 |
| 18 | (control* adj2 (group? or random* or trial? or study)).ti,ab,kw,kf. | 1118054 |
| 19 | 17 or 18 | 1729481 |
| 20 | 16 or 19 | 6040695 |
| 21 | meta‐analysis/ or "systematic review"/ or network meta‐analysis/ | 313819 |
| 22 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kf,kw. | 317658 |
| 23 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kf,kw. | 38620 |
| 24 | (data synthes* or data extraction* or data abstraction*).ti,ab,kf,kw. | 40155 |
| 25 | (hand search* or handsearch*).ti,ab,kf,kw. | 11100 |
| 26 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kf,kw. | 35453 |
| 27 | meta‐analysis as topic/ or network meta‐analysis/ | 27037 |
| 28 | (meta analy* or metanaly* or meta regression* or metaregression*).ti,ab,kf,kw. | 273051 |
| 29 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 332990 |
| 30 | (cochrane or systematic review?).jw. | 20267 |
| 31 | 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 | 622595 |
| 32 | (2022* or 2023*).dt,dp,ed,ep,yr. | 2737024 |
| 33 | 3 and 7 and 20 and 32 | 515 |
| 34 | 3 and 7 and 31 and 32 | 88 |
Database: Embase
Host: Ovid
Data parameters: 1980 to 2023 Week 22
Date of search: 7 June 2023
| # | Searches | Results |
| 1 | newborn/ or prematurity/ or newborn intensive care/ or newborn care/ or gestational age/ | 750499 |
| 2 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1202220 |
| 3 | 1 or 2 | 1450047 |
| 4 | *anticonvulsive agent/ | 24042 |
| 5 | *seizure/ | 43417 |
| 6 | (anticonvuls* or anti‐convuls* or antiepileptic* or anti‐epileptic* or antiseizur*or anti‐seizur* or (seizur* adj2 prevent*) or seizur*).ti,ab,kw,kf. | 256824 |
| 7 | 4 or 5 or 6 | 266149 |
| 8 | Randomized controlled trial/ or Controlled clinical study/ | 975168 |
| 9 | random$.ti,ab,kw. | 1961250 |
| 10 | Randomization/ | 99003 |
| 11 | placebo.ti,ab,kw. | 360803 |
| 12 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. | 267132 |
| 13 | double blind procedure/ | 207714 |
| 14 | (controlled adj7 (study or design or trial)).ti,ab,kw. | 447636 |
| 15 | parallel group$1.ti,ab. | 32220 |
| 16 | (crossover or cross over).ti,ab. | 121838 |
| 17 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. | 411882 |
| 18 | (open adj label).ti,ab. | 109069 |
| 19 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1601646 |
| 20 | (control* adj2 (group? or random*)).ti,ab,kw,kf. | 1301207 |
| 21 | 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 3304495 |
| 22 | meta‐analysis/ or "systematic review"/ or "meta analysis (topic)"/ | 608593 |
| 23 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kw. | 399835 |
| 24 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kw. | 55865 |
| 25 | (data synthes* or data extraction* or data abstraction*).ti,ab,kw. | 50885 |
| 26 | (hand search* or handsearch*).ti,ab,kw. | 13742 |
| 27 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kw. | 47676 |
| 28 | (meta analy* or metanaly* or meta regression* or metaregression*).ti,ab,kw. | 360236 |
| 29 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 434934 |
| 30 | (cochrane or systematic review?).jn,jx. | 32153 |
| 31 | (overview adj2 reviews).ti. | 142 |
| 32 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 | 911864 |
| 33 | (2022* or 2023*).yr. | 2708204 |
| 34 | 3 and 7 and 21 and 33 | 158 |
| 35 | 3 and 7 and 32 and 33 | 125 |
Database: Epistemonikos
Host: https://www.epistemonikos.org/en/
Date of search: 7 June 2023
((babe OR babes OR baby OR babies OR "gestational age" OR "gestational ages" OR infant OR infants OR infantile OR infancy OR "low birth weight" OR "low birthweight" OR neonate OR neonatal OR "neo nate" OR "neo natal" OR newbORn OR "new bORn" OR newborns OR "new borns" OR "newly bORn" OR premature OR "pre mature" OR "pre matures" OR prematures OR prematurity OR "pre maturity" OR preterm OR preterms OR "pre term" OR preemie OR preemies OR premies OR premie OR VLBW OR VLBWI OR "VLBW I" OR VLBWs OR LBW OR LBWI OR LBWs OR ELBW OR ELBWI OR ELBWs OR NICU OR NICUs) AND (anticonvulsant OR anticonvulsants OR "anti convulsant" OR "anti convulsants" OR antiepileptic OR antiepileptics OR "anti epileptic" OR antiseizure OR antiseizures OR "anti‐seizure" OR "anti seizures" OR seizure))
Resource: ClinicalTrials.gov
Host: https://clinicaltrials.gov/
Date of search: 7 June 2023
Searcher location: London, UK.
The search was run in expert search using the following search string. The results were downloaded and imported into EndNote.
((babe OR babes OR baby OR babies OR "gestational age" OR "gestational ages" OR infant OR infants OR infantile OR infancy OR "low birth weight" OR "low birthweight" OR neonate OR neonatal OR "neo nate" OR "neo natal" OR newbORn OR "new bORn" OR newborns OR "new borns" OR "newly bORn" OR premature OR "pre mature" OR "pre matures" OR prematures OR prematurity OR "pre maturity" OR preterm OR preterms OR "pre term" OR preemie OR preemies OR premies OR premie OR VLBW OR VLBWI OR "VLBW I" OR VLBWs OR LBW OR LBWI OR LBWs OR ELBW OR ELBWI OR ELBWs OR NICU OR NICUs) AND (anticonvulsant OR anticonvulsants OR "anti convulsant" OR "anti convulsants" OR antiepileptic OR antiepileptics OR "anti epileptic" OR antiseizure OR antiseizures OR "anti‐seizure" OR "anti seizures" OR seizure))
Resource: ICTRP
Host: https://trialsearch.who.int/
Date of search: 7 June 2023
Searcher location: London, UK.
The following search strings were run separately in the basic search box.
((babe OR babes OR baby OR babies OR "gestational age" OR "gestational ages" OR infant OR infants OR infantile OR infancy OR "low birth weight" OR "low birthweight" OR neonate OR neonatal OR "neo nate" OR "neo natal" OR newbORn OR "new bORn" OR newborns OR "new borns" OR "newly bORn" OR premature OR "pre mature" OR "pre matures" OR prematures OR prematurity OR "pre maturity" OR preterm OR preterms OR "pre term" OR preemie OR preemies OR premies OR premie OR VLBW OR VLBWI OR "VLBW I" OR VLBWs OR LBW OR LBWI OR LBWs OR ELBW OR ELBWI OR ELBWs OR NICU OR NICUs) AND (anticonvulsant OR anticonvulsants OR "anti convulsant" OR "anti convulsants" OR antiepileptic OR antiepileptics OR "anti epileptic" OR antiseizure OR antiseizures OR "anti‐seizure" OR "anti seizures" OR seizure))
Data and analyses
Comparison 1. Phenobarbital versus levetiracetam as first‐line ASM for EEG‐confirmed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion of infants who achieve seizure control after the first loading dose of ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Proportion of infants who achieve seizure control after the the maximal loading dose of ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Mortality before hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Requirement for mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Proportion of infants who develop sedation or drowsiness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Bradycardia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7 Hypotension requiring volume or inotropes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8 Shock requiring volume or inotropes | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 1.9 Recurrence of seizure before hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Proportion of infants with epilepsy post‐discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Phenobarbital versus levetiracetam as first‐line ASM in clinically diagnosed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion of infants who achieve seizure control after the first loading dose of ASM | 3 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.55, 0.86] |
| 2.2 Proportion of infants who achieve seizure control after the maximal loading dose of ASM | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.47, 0.72] |
| 2.3 Mortality before hospital discharge | 6 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.82, 2.43] |
| 2.4 Requirement for mechanical ventilation | 5 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.50, 9.68] |
| 2.5 Proportion of infants who develop sedation or drowsiness | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.66, 5.37] |
| 2.6 Bradycardia | 4 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.00 [0.74, 48.97] |
| 2.7 Hypotension requiring volume or inotropes | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.8 Shock requiring volume or inotropes | 3 | 190 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.67 [0.30, 1.51] |
| 2.9 Proportion of infants with an abnormal background pattern in EEG during ASM treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10 Proportion of infants with an abnormal background pattern in EEG after stopping ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.11 Duration of hospital stay | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.36 [0.54, 4.18] |
| 2.12 Recurrence of seizure before hospital discharge | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.60] |
| 2.13 Proportion of infants with persistent seizures and/or requiring ASM at discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.14 Proportion of infants discharged on gavage feeds | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.15 Proportion of infants with an abnormal neurological examination at discharge | 4 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.24] |
| 2.16 Proportion of infants who develop epilepsy post discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Phenobarbital versus phenytoin as first‐line ASM in EEG‐confirmed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion of infants who achieve seizure control after the maximal loading dose of ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Arrythmias causing circulatory disturbance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Hypotension requiring volume or inotropes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 Bradycardia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3: Phenobarbital versus phenytoin as first‐line ASM in EEG‐confirmed neonatal seizures, Outcome 1: Proportion of infants who achieve seizure control after the maximal loading dose of ASM
Comparison 4. Phenobarbital versus phenytoin as first‐line ASM in clinically diagnosed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Proportion of infants who achieve seizure control after the first loading dose of ASM | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.40, 2.64] |
| 4.2 Mortality before hospital discharge | 2 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.26] |
| 4.3 Requirement of mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4 Proportion of infants who develop sedation or drowsiness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5 Bradycardia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.6 Proportion of infants with persistent seizures and/or requiring ASM at discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Phenobarbital versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Proportion of infants who achieve seizure control after the first loading dose of ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Mortality before hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.3 Proportion of infants who develop sedation or drowsiness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.4 Proportion of infants with persistent seizures and/or requiring ASM at discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Phenytoin versus lorazepam as first‐line ASM in clinically diagnosed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Proportion of infants who achieve seizure control after the first loading dose of ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 Mortality before hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.3 Proportion of infants who develop sedation or drowsiness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.4 Proportion of infants with persistent seizures and/or requiring ASM at discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Phenobarbital + bumetanide versus phenobarbital alone as first‐line ASM in EEG‐confirmed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Proportion of infants who achieve seizure control after the first loading dose of ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.2 Mortality before hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.3 Proportion of infants with cognitive impairment at 18‐24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4 Seizure burden during hospitalisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.5 Requirement for mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.6 Hypotension requiring volume or inotropes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.7 Proportion of infants with an abnormal background pattern in EEG during ASM treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.8 Proportion of infants who develop epilepsy post discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Lignocaine versus benzodiazepine as second‐line ASM in EEG‐confirmed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Proportion of infants who achieve seizure control after the maximal loading dose of ASM | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.2 Mortality or neurodevelopmental disability at 12 months' corrected age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.3 Mortality before hospital discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.4 Neurodevelopmental disability at 12 months' corrected age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Maintenance ASM versus no maintenance ASM after achieving seizure control in clinically diagnosed neonatal seizures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Proportion of infants with repeat seizure before hospital discharge | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.56, 1.01] |
| 9.2 Mortality before hospital discharge | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.39, 1.22] |
| 9.3 Mortality at 18 to 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.4 Neurodevelopmental disability at 18 to 24 months' corrected age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.5 Requirement for mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.6 Shock requiring volume or inotropes | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.67, 1.07] |
| 9.7 Abnormal background pattern in EEG after achieving seizure control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.8 Duration of hospital stay | 2 | 373 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.44, 0.70] |
| 9.9 Proportion of infants with persistent seizures and/or requiring ASM at discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.10 Abnormal neurological examination at discharge | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.26] |
| 9.11 Proportion of infants who develop epilepsy post‐discharge | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.69, 14.72] |
Comparison 10. Treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Seizure burden during hospitalisation | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐1871.16 [‐4525.05, 782.73] |
| 10.2 Mortality before hospital discharge | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.28, 1.27] |
| 10.3 Proportion of infants who develop epilepsy post‐discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akeel 2022.
| Study characteristics | |
| Methods | Prospective double‐blind randomised controlled trial |
| Participants | Neonates with seizures, diagnosis based on clinical examination. Both full‐term neonates as well as preterm included. Exclusion criteria: acute electrolyte disturbance, inborn error of metabolism, opioid withdrawal syndrome, ASM given prior to inclusion 136 neonates screened, 104 included The study was performed in a tertiary care centre in Benha, Egypt, between March 2020 and March 2022. |
| Interventions | Group A: Phenobarbital IV or orally, loading dose 20 mg/kg, second loading if not successful with 10 mg/kg. If successful, PB continued as maintenance (5 mg/kg*d). Add‐on of LEV if not successful after 40 min. Group B: Levetiracetam IV or orally, loading dose 20 mg/kg, second loading if not successful with 10 mg/kg. If successful, LEV continued as maintenance (20 mg/kg*d). Add‐on of PB if not successful after 40 min. |
| Outcomes | Primary outcome: clinical cessation of seizures within 20/40 min of IV drug application and seizure‐free for the following 24 hrs. Secondary outcome: adverse events |
| Notes | Demographic data and seizure aetiology show some differences between both groups (gestational diabetes mellitus, maternal hypertension, perinatal asphyxia). Seizure control (clinical impression) was better in the LEV group than in the PB group. Adverse events were more frequent in the PB group, including need for mechanical ventilation in 2/52. No information is given on EEG findings in participants. The authors reported no conflicting interests and no external funding for the research. |
Boylan 2004.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Neonates with seizures who failed to respond to first‐line phenobarbitone treatment (Quote:) "Neonates at high risk of developing seizures because of birth depression or cord blood acidosis, had abnormal movements suggesting seizures, or had meningitis. Neonates who had already received a single loading dose of phenobarbitone were not excluded from the study." Sample size was 27 neonates with EEG‐confirmed seizures, 5 were excluded because of protocol violations, 11 because they responded to phenobarbitone. 11 neonates were included in the analysis because they required second‐line treatments (3 clonazepam, 5 lignocaine, 3 midazolam). The study was performed in 2 neonatal intensive care units in London, UK. Information on study dates is not included in the publication. |
| Interventions | First‐line treatment (in all neonates, before randomisation): phenobarbitone in a dose of up to 40 mg/kg. (Quote:) "If this failed to abolish seizures or reduce the seizure burden by at least 80% within 12 hours of enrollment, the neonate was randomly assigned to receive midazolam or lignocaine as second‐line anticonvulsant therapy." Second‐line treatments: ‐ Midazolam bolus dose of 60 μg/kg followed by an infusion of 150 μg/kg/h, increased to either 300 μg/kg/h if midazolam failed to abolish or reduce seizure burden by at least 80% within 12 hours; ‐ Lignocaine bolus of 4 mg/kg over 20 minutes followed by an infusion of 2 mg/kg/h, increased to 4 mg/kg/h if midazolam failed to abolish or reduce seizure burden by at least 80% within 12 hours; Clonazepam was administered (quote:) "if the increased dose of either drug failed to improve the seizure burden within 48 hours of enrollment" or (quote:)"if parents were not willing for their child to be given a drug chosen randomly". |
| Outcomes | Primary endpoint: control of electrographic seizures, defined as (quote:) "complete absence of seizure activity on the EEG or a reduction of > 80% of pretreatment burden" Other endpoints: neurodevelopmental assessment evaluated with Amiel–Tison and Griffiths neurodevelopmental assessment at 1 year. |
| Notes | Response to treatment was assessed using continuous video‐EEG. (Quote:) "All neonates were monitored continuously for at least 24 hours after enrollment. If electrographic seizures were not detected during this time, recording was stopped. If seizures were present, monitoring was continued until seizure control was established or treatment was considered to have failed (at least 48 hours later)." Neonates receiving lignocaine continued to be given background midazolam at a dose of 30 to 60 μg/kg/h. Some neonates were also receiving continuous low‐dose morphine as analgesia (10 to 20 μg/kg/h). External funding sources or possible conflicts of interests were not mentioned in the publication. |
Falsaperla 2019.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote)"term neonates with seizures manifesting within the first 28 days of life." Exclusion criteria: (quote:)"Newborns with SE, GE, and seizures secondary to transient metabolic disorders, including hypoglycaemia and hypocalcaemia; neonates with a positive history for maternal drug ingestion; those who received more than one anticonvulsant medication; and those neonates in whom LEV was used as second‐line therapy" The study was performed at a single centre in Catania, Italy. Patients were recruited between February 2016 and February 2018. LEV group Number of patients: 15 Gestational age: 38.13 ± 1.24 Sex (F/M): 4/11 Prenatal anomalies: 40% APGAR score 1 min: 7 66 ± 1.29 APGAR score 5 min: 9 13 ± 1.12 Respiratory distress: 33.33% PB group Number of patients: 15 Gestational age: 38.33 ± 1.04 Sex (F/M): 8/7 Prenatal anomalies: 40% APGAR score 1 min: 8 66 ± 0 89 APGAR score 5 min: 9.03 ± 0.84 Respiratory distress: 40% |
| Interventions | Intravenous PB, initial dose of 20 mg/kg, followed by a maintenance dose of oral PB at 5 mg/kg; Intravenous LEV, initial dose of 20 mg/kg, followed by a maintenance dose of oral LEV at 20 mg/kg, with gradually increasing doses up to 40 mg/kg twice daily in case of nonresponse at initial doses. Therapy was maintained for one month after the seizures resolved. |
| Outcomes | Neurodevelopmental outcomes evaluated with HNNE at baseline and after 1 month of treatment. The assessment was made by trained neonatologists, who evaluated the following neurological items: (1) tone and posture, (2) tone patterns, (3) movements, (4) reflexes, (5) abnormal signs, and (6) orientation and behaviour. |
| Notes | External funding sources were not mentioned. All authors reported not having potential conflicts of interest to disclose. |
Ghaffar 2020.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "1. Age < 28 days; 2. Both genders; 3. Neonatal seizures as per operational definition for < 24 hours" Exclusion criteria: (quote:) "1. Who were already receiving anticonvulsants; 2. If seizures were due to correctable metabolic abnormalities (i.e. hypoglycaemia, hypocalcaemia, hypomagnesaemia, hyponatraemia); 3. Neonates with associated pulmonary, hepatic, renal, or cardiac dysfunction." PB group Number of patients: 30 Age (days, mean ± SD): 15.20 ± 5.62 14.90 ± 5.99 Male: 19 (63.3%) Duration of complaint (hours): 10.40 ± 4.83 Weight (kg): 4.056 ± 0.65 LEV group Number of patients: 30 Age (days, mean ± SD): 14.90 ± 5.99 Male: 19 (63.3%) Duration of complaint (hours): 11.433 ± 4.67 Weight (kg): 4.163 ± 0.64 The study was conducted at a single centre in Sialcot, Pakistan, from January 2019 to February 2020. |
| Interventions |
PB group: (Quote:) "Intravenous loading dose maximum 40 mg/kg (initial loading dose 20 mg/kg reloading with 10 mg/kg for further 2 times) and maintenance dose 5 mg/kg." Given in infusion form in dilution in 15 mL normal saline over 15 minutes. LEV group: (Quote:) "Intravenous loading maximum 40 mg/kg (initially with 30 mg/kg then reloading with 10 mg/kg) and maintenance dose 20 mg/kg/day." Given in infusion form in dilution in 15 mL normal saline over 15 minutes. If seizures reoccur with maximum loading dose, then the patient was switched to other drug. Patient was continuously monitored and observed for reoccurrence of seizures within 24 hours. |
| Outcomes | (Quote:)"Efficacy as per operational definition was noted after 24 hours by the researcher himself." No further details were provided. |
| Notes | External funding sources were not mentioned in the publication. The authors reported not having potential conflicts of interest to disclose. |
Gowda 2019.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (Quote:) "Outborn neonates (age 0‐28 days) with clinical seizures". (Quote:) "Neonatal seizures were clinically defined as
abnormal, stereotyped and paroxysmal dysfunction in the CNS, occurring within the first 28 days after birth in full‐term infants or before 48 weeks of gestational age in preterm infants." Exclusion criteria: (Quote:) "Neonates with hypoglycaemia, hypocalcaemia, hypomagnesaemia, those who received anticonvulsants prior to enrolment, and those with major congenital malformations e.g. congenital heart defects, neural tube malformations, diaphragmatic hernia, choanal atresia, oesophageal atresia, tracheo‐oesophageal fistula, omphalocele, gastroschisis, intestinal obstruction and imperforate anus". LEV group, 50 patients Age (days), mean (SD): 9.8 (8.50) Male, n (%): 28 (56) Mode of delivery, n (%) Vaginal: 35 (70) Caesarian: 15 (30) Gestation, n (%) Term: 40 (80), 42 (84) Preterm: 10 (20), 08 (16) Birth weight (kg), mean (SD): 2.56 (0.64) Aetiology of seizures, n (%) Hypoxic ischaemic encephalopathy: 20 (40) Neonatal sepsis/meningitis: 18 (36) Intracranial haemorrhage: 3 (6) Benign neonatal epilepsy syndrome: 2 (4) Malignant neonatal epilepsy syndrome: 1 (2) Cortical malformation: 1 (2) Inborn errors of metabolism: 1 (2) Unknown: 4 (8) PB group, 50 patients Age (days), mean (SD): 8 (8.33) Male, n (%): 28 (56) Mode of delivery, n (%) Vaginal: 36 (72) Caesarian: 14 (28) Gestation, n (%) Term: 42 (84) Preterm: 08 (16) Birth weight (kg), mean (SD): 2.73 (0.64) Aetiology of seizures, n (%) Hypoxic ischaemic encephalopathy: 24 (48) Neonatal sepsis/meningitis: 15 (30) Intracranial haemorrhage: 2 (4) Benign neonatal epilepsy syndrome: 1 (2) Malignant neonatal epilepsy syndrome: 1 (2) Cortical malformation: 1 (2) Inborn errors of metabolism: 2 (4) Unknown: 4 (8) The study was performed at a single neonatal intensive care unit in Bangalore, India, between November 2014 and April 2016. |
| Interventions | Intravenous LEV (20 mg/kg) at a rate of 1 mg/kg/min under cardiorespiratory monitoring. If seizures terminated, LEV was continued as maintenance at 20 mg/kg/day in 2 divided doses. If seizures continued, another loading dose of LEV (20 mg/kg) was injected, and if seizures still persisted, patient was switched over to PB. Intravenous PB (20 mg/kg) administered in the dose of 20 mg/kg diluted in 1:10 normal saline given intravenously slowly at the rate of 1 mg/kg/min under cardiorespiratory monitoring; if seizures were terminated, it was continued at 5 mg/kg/day in 2 divided doses as maintenance. Another loading dose of 10 mg/kg of PB was administered in neonates who failed to respond, and if seizures still persisted after 2 loading doses, patient was switched over to LEV. |
| Outcomes | Primary outcomes: (quote:) "proportion of patients achieving cessation of seizures following the first or second dose of the drug (PB or LEV), and those remaining seizure‐free for next 24 hours". (Quote:)"Termination of seizures was defined clinically if there were no abnormal movement/eyeball deviation/nystagmus, no change in heart rate, no change in respiration/saturation and autonomic dysfunction". Secondary outcomes: proportion of patients experiencing (quote:)"adverse events occurring within two hours of drug administration, including desaturation, reduced respiratory rate, increased ventilator support requirement, arrhythmias, blood pressure, or heart rate fluctuations by more than 10% compared to the previous 2 hours, or if vasopressors were initiated or increased". |
| Notes | The authors stated there was no external funding. The authors reported not having potential conflicts of interest to disclose. |
Jindal 2021.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: neonates 34 weeks of gestation to < 28 days of postnatal period admitted with neonatal seizure Exclusion criteria: neonates on 2 antiseizure medication; HIE stage III; metabolic cause (hypocalcaemia, hypoglycaemia); intracranial bleeding, brain infarct; major congenital malformation suspected storage disorders, IEM, chromosomal anomalies, and IUI; seizure recurrence within 12 hours of phenobarbitone loading; < 34 weeks of gestation; and > 28 days of postnatal life The study was performed in a single neonatal unit at a tertiary care hospital in India between January 2019 and December 2019. |
| Interventions | After a loading dose of PB (20 mg/kg), neonates who remained seizure‐free for at least 12 hours were enrolled. Group A: PB withdrawal group: (quote:)"phenobarbitone maintenance was stopped" (no further details reported) Group B: PB continued group: (quote:)"PB maintenance was continued until discharge and further continuation was decided based on clinician’s discretion." (no further details reported) PB withdrawal group (n = 112) Male, n (%): 73 (65.2) Age (days), mean (SD): 6.5 (1.8) Gestation (weeks), mean (SD): 37.1 (2.5) Birth weight (grams), mean (SD): 2536 (560) Antenatal comorbidities PIH, n (%): 3 (2.7) PROM, n (%): 13 (11.6) Foetal distress, n (%): 25 (22.3) Mode of delivery NVD, n (%): 75 (67) LSCS, n (%): 36 (32.1) Instrumentation, n (%): 1 (0.9) Resuscitation details Delayed cry at birth, n (%): 55 (49.1) Needed resuscitation, n (%): 53 (47.3) Postnatal neurological abnormality, n (%): 50 (44.6) Weight for age AGA, n (%): 69 (61.6) SGA, n (%): 42 (37.5) LGA, n (%): 1 (0.9) Anthropometry at admission Weight (grams), mean (SD): 2529 (566) Length (cm), mean (SD): 49.1 (2.9) Head circumference (cm), mean (SD): 31.5 (1.7) Abnormal neurological examination, n (%): 71 (63.4) Bulging anterior fontanelle, n (%): 4 (3.6) Tone Increased, n (%): 6 (5.4) Decreased, n (%): 48 (42.9) Abnormal posture, n (%): 36 (32.1) Deep tendon reflexes Exaggerated, n (%): 4 (3.6) Absent, n (%): 51 (45.5) Abnormal primitive neonatal reflexes, n (%): 64 (57.1) Abnormal pupillary reactions, n (%): 19 (17.0) Abnormal respiratory system, n (%): 24 (21.4) Abnormal cardiovascular system, n (%): 3 (2.7) Abnormal abdomen examination, n (%): 5 (4.5) Seizure onset (day of life), mean (SD): 4.0 (1.4) Frequency of seizures (episodes/day), mean (SD): 2.9 (1.6) Seizure semiology Subtle, n (%): 23 (20.5) Focal tonic, n (%): 26 (23.2) Focal clonic, n (%): 26 (23.2) Generalised tonic, n (%): 35 (31.2) Myoclonic, n (%): 2 (1.8) Autonomic changes, n (%): 9 (8) Status epilepticus, n (%): 11 (9.8) Phenobarbitone continued group (n = 109) Male, n (%): 75 (68.8) Age (days), mean (SD): 7.8 (2.1) Gestation (weeks), mean (SD): 37.1 (2.8) Birth weight (grams), mean (SD): 2527 (568) Antenatal comorbidities PIH, n (%): 10 (9.2) PROM, n (%): 9 (7.3) Foetal distress, n (%): 19 (17.4) Mode of delivery NVD, n (%): 81 (74.3) LSCS, n (%): 28 (25.7) Instrumentation, n (%): 0 (0) Resuscitation details Delayed cry at birth, n (%): 44 (40.4) Needed resuscitation, n (%): 43 (39.4) Postnatal neurological abnormality, n (%): 39 (35.8) Weight for age AGA, n (%): 71 (65.1) SGA, n (%): 37 (33.9) LGA, n (%): 1 (0.9) Anthropometry at admission Weight (grams), mean (SD): 2502 (568) Length (cm), mean (SD): 49.0 (2.9) Head circumference (cm), mean (SD): 31.4 (1.6) Abnormal neurological examination, n (%): 69 (63.3) Tone Increased, n (%): 11 (10.1) Decreased, n (%): 42 (38.5) Abnormal posture, n (%): 33 (30.3) Deep tendon reflexes Exaggerated, n (%): 4 (3.7) Absent, n (%): 46 (42.2) Abnormal primitive neonatal reflexes, n (%): 62 (56.9) Abnormal pupillary reactions, n (%): 15 (13.8) Abnormal respiratory system, n (%): 22 (20.2) Abnormal cardiovascular system, n (%): 4 (3.7) Abnormal abdomen examination, n (%): 2 (1.8) Seizure onset (day of life), mean (SD): 5.6 (1.9) Frequency of seizures (episodes/day), mean (SD): 3.1 (1.5) Seizure semiology Subtle, n (%): 16 (14.7) Focal tonic, n (%): 15 (13.8) Focal clonic, n (%): 35 (32.1) Generalised tonic, n (%): 39 (35.8) Myoclonic, n (%): 4 (3.7) Autonomic changes, n (%): 10 (9.2) Status epilepticus, n (%): 13 (11.9) |
| Outcomes | Primary outcome: seizure recurrence Secondary outcomes: (quote:) "time to reach full enteral feeds, duration of hospital stay, neurological status at discharge, and mortality." |
| Notes | The diagnosis of seizure was made on the basis of history and clinical observation and all types of clinical seizures were included. The authors declared there was no external funding. The authors declared they had no competing financial interests or personal relationships that could have appeared to influence the work. |
Khan 2020.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "premature newborns with a gestational age more than 34 weeks to less than 42 weeks and a birth weight of more than 2000 gm with neonatal seizures" Exclusion criteria: (quote:) "seizures caused by hypoglycaemia, hypocalcaemia or dyselectrolytaemia and sepsis." (Quote:) "Patients [who] had already received more than single loading doses of PB or medication with any other ASMs." LEV group, 50 patients Sex: male 30 (60.0); female 20 (40.0) Gestational age (weeks): Premature (< 37) 6 (12.0); full term (37‐42) 44 (88.0) Birth weight (gm): 2000‐< 2500 8 (16.0); 2500‐4000 42(84.0) Breathing status of neonates delivered outside hospital (n = 26): Within 1 minute 19 (79.2); breathing < 5 minutes 5 (20.8) PB group, 50 patients Sex: male 37 (74.0); female 13 (26.0) Gestational age (weeks): Premature (< 37) 5 (10.0); full term (37‐42) 45 (90.0) Birth weight (gm): 2000‐< 2500 9 (18.0); 2500‐4000 41(82.0) Breathing status of neonates delivered outside hospital (n = 14): Within 1 minute 31 (86.1); breathing < 5 minutes 5 (13.9) The study was performed at a single centre in Dhaka, Bangladesh. Patients were enrolled between July 2013 and June 2014. |
| Interventions | Intravenous LEV, loading dose of 50 mg/kg with 10 mg/kg/dose 8‐hourly (maintenance) Intravenous PB, loading dose of 20 mg/kg with 5 mg/kg/day 12‐hourly (maintenance) If seizures recurred, a second or third loading of PB were given as a dose of 10 mg/kg. |
| Outcomes | (Quote:) "Control of seizures and time required to control seizures" (Quote:)"Study end point was up to 48 hours but if seizure was not controlled within 48 hours it was labeled as treatment failure." |
| Notes | (Quote:) "Seizures were diagnosed clinically. No continuous EEG monitoring was performed at
time of diagnosis and enrolment." Information on external funding and possible conflicts of interests of the authors was not included in the manuscript. |
Painter 1999.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "neonates in the neonatal intensive care unit who were at risk for seizures because of reported abnormal movements" and (quote:) "with seizures that were confirmed by electroencephalography"; (quote:)"an Apgar score of less than 5 at five minutes with a base deficit of more than 10 mmol per litre; traumatic delivery; maternal exposure to nonprescription narcotic drugs, amphetamines, or barbiturates; or central nervous system infection or malformation." PB group, 30 patients Gestational age (weeks) ≤ 28: 4 (13) 29–32: 2 (7) 33–37: 5 (17) > 37: 19 (63) Male 14 (47); female 16 (53) Race White: 19 (63) Black: 10 (33) Asian: 1 (3) Primary cause of seizure Asphyxia, haemorrhage, or infarction: 4 (13) Central nervous system malformations: 2 (7) Central nervous system infection 2 (7) Undetermined 22 (73) PHT group, 29 patients Gestational age (wks) ≤ 28: 1 (3) 29–32: 3 (10) 33–37: 6 (21) > 37: 19 (66) Male 22 (76); female 7 (24) Race White: 18 (62) Black: 10 (34) Asian: 1 (3) Primary cause of seizure Asphyxia, haemorrhage, or infarction: 27 (93) Central nervous system malformations: 0 (0) Central nervous system infection 1 (3) Undetermined 1 (3) The study was conducted at a single centre in Pittsburgh, USA, between 1990 and 1995. |
| Interventions | Intravenous phenobarbital administered over a 5 to 15‐minute period once daily, with doses needed to achieve plasma concentrations of free drug of 25 μg per millilitre. If the target concentrations had not been achieved, an additional dose was administered, and the assessment process was repeated. Intravenous phenytoin administered over a 5 to 15‐minute period once daily, with doses needed to achieve plasma concentrations of free drug of 3 μg per millilitre. If the target concentrations had not been achieved, an additional dose was administered, and the assessment process was repeated. |
| Outcomes | Primary end point: (quote:) "complete control of seizures, as determined by electroencephalographic recording, during treatment with one drug or after the addition of the second drug." (Quote:)"Success", defined as a (quote:) "80 percent reduction in the severity of seizures (calculated as the mean severity per hour) in period 3, or period 5 for neonates receiving both drugs, as compared with the severity in period 1." |
| Notes | The study was supported by a grant (NS R01 26946‐01A2) from the National Institute of Neurological Disorders and Stroke, USA. No information on possible conflicts of interest of the authors was given in the publication. |
Pathak 2013.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "term or near term neonates (≥ 35 weeks of gestation) admitted with clinically apparent seizures not responding to treatment of hypoglycaemia, hypocalcaemia and other metabolic disorders. Clinical criteria for diagnosis of neonatal seizures were: (i) clonic movement which could be unifocal, multifocal or generalised (ii) tonic posturing with or without abnormal gaze (iii) subtle seizures and spontaneous paroxysmal, repetitive motor or autonomic phenomenon like lip‐smacking, chewing, paddling, cyclic movements or respiratory irregularities" Exclusion criteria: (quote:) "Seizures responding to correction of hypoglycaemia, hypocalcaemia or any other metabolic disorder, and babies with major congenital malformation or myoclonic jerks" PHT group, 55 patients Gestational age (wk), mean (SD): 38.6 (1.45) Weight (kg), mean (SD): 2.71 (0.4) Male sex: 39 (70.9) No of extramural deliveries: 30 (70.9) HIE stage 2: (n = 42) 21 (38.2) HIE stage 3: (n = 44) 26 (47.3) Cause of seizures Meningitis: (n = 18) 7 (12.7) Intracranial bleed: (n = 2) 1 (1.8) Kernicterus: (n = 4) 1 (1.8) Type of seizure Subtle: 27 (49) Tonic: 24 (43) Clonic: 6 (10.9) PB group, 54 patients Gestational age (wk), mean (SD): 38.09 (1.87) Weight (kg), mean (SD): 2.55 (0.5) Male sex: 40 (74.1) No of extramural deliveries: 36 (67.0) HIE stage 2: (n = 42) 21 (38.9) HIE stage 3: (n = 44) 18 (33.3) Cause of seizures Meningitis: (n = 18) 11 (20.4) Intracranial bleed: (n = 2) 1 (1.9) Kernicterus: (n = 4) 3 (5.6) Type of seizure Subtle: 24 (44) Tonic: 20 (37) Clonic: 8 (14) The study was conducted at a level II neonatal unit in Meerut, India from November 2008 to September 2009. |
| Interventions | Intravenous phenytoin, loading dose of 20 mg/kg administered over 30 minutes at a rate of 1 mg/kg/min. If seizure persisted, the babies were crossed over to intravenous phenobarbitone. Intravenous phenobarbitone, loading dose of 20 mg/kg administered over 30 minutes. If seizure persisted, the babies were crossed over to intravenous phenytoin. (Quote:)"If seizure persisted after two drugs, baby was reloaded with IV phenobarbitone at 10 mg/kg each to a maximum of 40 mg/kg and then a third‐line drug like midazolam was used IV at 0.1 mg/kg/dose." |
| Outcomes | Primary outcome: cessation of clinical seizure activity Secondary outcomes: (quote:) "(i) survival at discharge, (ii) neurodevelopment outcome at 3 months (Amiel‐Tieson method), (iii) time taken to control seizures, and (iv) EEG control of seizures." |
| Notes | According to the authors, there was no external funding. The authors reported not having potential conflicts of interest to disclose. |
Perveen 2016.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "babies of > 2 kg admitted in NICU within 48 hours of birth with neonatal seizures due to perinatal asphyxia with clinical features of HIE." "If seizures persisted even after correction of hypoglycaemia and hypocalcaemia, babies were randomised for intervention to either levetiracetam or phenobarbitone." Exclusion criteria: anticonvulsant prior to admission; serum creatinine greater than 2 mg/dL; major congenital malformations; refractory shock; need for assisted ventilation at admission. LEV group, 30 patients Gestational age (weeks), mean (SD): 38.29 (1.03) Weight (kg), mean (SD): 2.78 (0.33) Male: 19 (63.3) Intramural deliveries: 11 (36.7) > 0.05 HIE stage 2: 24 (80) HIE stage 3: 6 (20) Duration of hospital stay, mean (SD): (days) 7.7 (4.56) Need of boluses and inotropic support: 15 (50) Sepsis screen positive: 3/30 (10) ph < 7.0 at admission: 19/30 (63.3) Base deficit > 12: 20/30 (66.6) PB group, 30 patients Gestational age (weeks), mean (SD): 38.43 (1.10) Weight (kg), mean (SD): 2.90 (0.31) Male: 22 (73.3) Intramural deliveries: 13 (43.3) HIE stage 2: 25 (83.3) HIE stage 3: 5 (16.6) Duration of hospital stay (days), mean (SD): 8.9 (4.91) Need of boluses and inotropic support: 10 (30) Sepsis screen positive: 2/30 (6.6) ph < 7.0 at admission: 17/30 (56.6) Base deficit > 12: 18/30 (60) The study was performed at a single centre in Meerut, India, from July 2014 to December 2015. |
| Interventions | Intravenous LEV, loading dose of 60mg/kg diluted in 30 ml normal saline given slowly over 15 ‐ 20 minutes, under cardio respiratory system monitoring. If seizures were controlled, maintenance was continued (15mg/kg/day every 12 hr) for 5 days. If seizures persisted after the loading dose of LEV, babies crossed over to receive IV phenobarbitone, followed by maintenance (5 mg/kg/day every 12 hr) for 5 days. Intravenous PB, loading dose of 20mg/kg diluted in 1:10 of distilled water given slowly at the rate of 1mg/kg/min under strict cardiorespiratory monitoring. If seizures persisted, the babies were crossed over to treatment with IV levetiracetam. If seizures were controlled then they were kept on maintenance dose of both drugs. If seizures persisted despite crossover, the babies were treated as per unit policy. |
| Outcomes | Primary outcome: (quote:)"Clinical control of seizure activity ". (Quote:)"Seizures were considered to be controlled if the baby was seizures free 24 hrs after last seizures." Secondary outcomes: (quote:) "safety profile of levetiracetam, electrical seizures after control of clinical seizure, time taken to control seizures, and neurological examination till 6 months." |
| Notes | According to the others there was no external funding. The authors reported not having potential conflicts of interest to disclose. |
Prakash 2019.
| Study characteristics | |
| Methods | Randomised controlled trial, not blinded. A block randomisation model (blocks of 4) was used with sealed envelopes. The study was conducted at a tertiary care neonatal intensive care unit at Bihar, India, between April 2018 and September 2019. |
| Participants | 80 newborns with clinically apparent seizures, after acute metabolic disorders were ruled out. Inclusion was based on appearance of motor or autonomic phenomena suggestive of seizures. Exclusion criteria were prematurity, major congenital malformation, intubation at time of admission, newborns presenting with myoclonic jerks. Demographic data at baseline comparable |
| Interventions | Group A (n = 42) LEV loading 10 mg/kg IV, if seizures persisted additionally 5 mg/kg. Maintenance with dosage that had proved to control seizures. If seizures persisted, 'cross‐over' to PB. Group B (n = 38): PB loading 20 mg/kg IV, if seizures persisted, additional 10 mg in aliquots up to a maximum dosage of 40 mg/kg. Maintenance after 24 h. If seizures persisted, 'cross‐over' to LEV. Third‐line drug midazolam 0.2 mg/kg/dose followed by continuous infusion |
| Outcomes | Primary outcome variable: cessation of clinical seizure activity for 5 d Secondary outcome variables: time to control seizures, survival at discharge, short‐term adverse effects, neurodevelopmental outcome at 12 m, EEG control of seizures |
| Notes | Cessation of clinical seizures not different between groups. Adverse events (cardiorespiratory depression, sedation) in 3/42 in the LEV group vs 20/38 in the PB group. EEG only after clinical seizures were controlled, not different between groups. Neuromotor developmental delay, 'mental retardation' and comorbidities more frequent in PB group. |
Saxena 2016.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "term or near‐term neonates of ≥ 34 weeks of gestation up to 4 weeks postnatal age and weighing ≥ 2 kg. All types of clinical seizures were included in the study. The diagnosis of seizure was based on clinical observation only." Exclusion criteria: (quote:) "recurrence of seizures within 12 hrs of the loading dose of phenobarbitone, major congenital malformations, suspected storage disease (ruled out by metabolic screen), intrauterine infection (ruled out by serological screen) and suspected chromosomal abnormalities (based on facial dysmorphism and other phenotypic abnormalities)" Placebo group, 75 patients Weight (g), mean (SD): 2677 (448.7) Gestation (w), mean (SD): 37 (1.3) Male, n (%): 41 (54.7) Intramural delivery, n(%): 28 (37.3) Age at admission (h), median (IQR): 4 (0‐28) Onset of convulsion (h), median (IQR): 12 (5‐42.5) HIE (at admission) Stage I, n (%): 1 (1.3) Stage II 49, n (%): (65.3) Stage III 14, n (%): (18.7) Serum PB level(μg/mL) at 12 hours, mean (SD): 24.8 (23.4) Aetiology Birth asphyxia, n (%): 65 (86.7) Meningitis/sepsis, n (%): 6 (8) Metabolic, n (%): 2 (2.7) Intracranial haemorrhage, n (%): 2 (2.7) PB group, 77 patients Weight (g), mean (SD): 2742 (342.7) Gestation (w), mean (SD): 38 (1.4) Male, n(%): 50 (64.9) Intramural delivery, n (%): 27 (35.0) Age at admission (h), median (IQR): 3 (0‐16) Onset of convulsion (h), median (IQR): 12 (4‐24) HIE (at admission) Stage I, n (%): 4 (5.2) Stage II, n (%): 52 (68.8) Stage III, n (%): 9 (11.7) Serum PB level (μg/mL) at 12 hours, mean (SD): 20.2 (22.0) Aetiology Birth asphyxia, n (%): 69 (89.6) Meningitis/sepsis, n (%): 7 (9.1) Metabolic, n (%): 1 (1.3) Intracranial haemorrhage, n (%): 0 The study was conducted at a level II neonatal intensive care unit in India from September 2012 to September 2013. |
| Interventions | Initial correction of hypoglycaemia and hypocalcaemia, followed by load with intravenous PB at 20 mg/kg in 1:10 dilution with normal saline (NS) over a 15‐20‐minute period at a rate of 1 mg/kg/min. All responders (subjects who remained seizure‐free for a period of 12 hours after loading dose) were randomised into 2 groups. PB (200 mg/mL) was diluted 1:20 in NS (1 mL PB + 19 mL NS) to make its concentration 200 mg/20 mL or 10 mg/ mL. Maintenance dose was 2.5 mg/kg (of PB) which was equivalent to 0.25 mL/kg/dose of prepared solution every 12 hourly for 5 days. Placebo was 20 mL of normal saline kept in an identical syringe. Maintenance dose was equivalent to 0.25 mL/kg/dose of prepared solution every 12 hourly for 5 days. The study intervention stopped after 5 days of seizure‐free period. If a breakthrough seizure occurred, the baby was reloaded with 10 mg/kg of PB and put on open‐label maintenance of PB till discharge. |
| Outcomes | Seizure recurrence, mortality, need for inotropic support, time to reach full oral enteral nutrition, duration of hospital stay, neurodevelopment status, seizure recurrence and re‐hospitalisation up to 3 months of age. |
| Notes | The study was partially funded by 'Thesis/‐Research grant' of the Indian Council for Medical Research (ICMR). The authors reported not having potential conflicts of interest to disclose. |
Sharpe 2020.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: infants at risk of developing seizures or suspected of having seizures. Patients were term infants of a corrected gestational age between 36 and 44 weeks (< 2 weeks of age) with a weight of at least 2.2 kg. Exclusion criteria: (quote) "any previous ASMs (except short‐acting benzodiazepines administered for sedation > 24 hours before enrollment), if the serum creatinine level was > 1.6 mg/dL, or if seizures were due to correctable metabolic abnormalities (such as hypoglycaemia or hypocalcaemia). Patients in whom death was imminent were excluded. Patients in whom EEG monitoring could not be commenced before the need to treat definite clinical seizures were not recruited." LEV group, 64 patients HIE as seizure aetiology, n (%): 35 (55) Received hypothermia treatment, n (%): 24 (38) Male sex, n (%): 31 (48) Cord pH: n 31; Mean (SD): 7.07 (0.2) 5‐min Apgar score, n 64; mean (SD): 6.52 (3.01) Gestational age, n 64; mean (SD): wk 39.3 (1.3) Birth weight n 64; mean (SD): g 3342 (577) Pretreatment seizure severity n 52; mean (SD): min/h 12.3 (12.0) PB group, 42 patients HIE as seizure aetiology, n (%): 22 (52) Received hypothermia treatment, n (%): 18 (43) Male sex, n (%): 24 (57) Cord pH n 20, mean (SD): 7.15 (0.17) 5‐min Apgar score, n 40, mean (SD): 6.47 (2.4) Gestational age, n 42, mean (SD): wk 39.1 (1.3) Birth weight n 42, mean (SD): g 3317 (501) Pretreatment seizure severity, n 29, mean (SD): min/h 9.1 (9.3) The multicentre study was performed at hospitals in San Diego, USA; Oakland, USA, Auckland, New Zealand, and Loma Linda, USA. Patients were enrolled between March 2013 and October 2017. |
| Interventions | LEV: infusion over 15 minutes at 40 mg/kg, with an additional 15 minutes allowed for the medication to take effect. If electrographic seizures persisted or recurred 15 minutes after the first infusion was complete, an additional dose of the same treatment type was given. Patients who had received LEV at 40 mg/kg received an additional 20 mg/kg infusion over 15 minutes. If electrographic seizures persisted or recurred 15 minutes after the second infusion was complete, the patient was then treated with the alternate treatment. Patients given any LEV loading doses received maintenance LEV at 10 mg/kg per dose, given IV every 8 hours for 5 days. PB: infusion over 15 minutes at 20 mg/kg, with an additional 15 minutes allowed for the medication to take effect. If electrographic seizures persisted or recurred 15 minutes after the first infusion was complete, an additional dose of the same treatment type was given. Patients who had received LEV at 20 mg/kg received an additional 20 mg/kg infusion over 15 minutes. If electrographic seizures persisted or recurred 15 minutes after the second infusion was complete, the patient was then treated with the alternate treatment. Patients given any PB loading doses received maintenance PB at 1.5 mg/kg per dose, given IV every 8 hours for 5 days. |
| Outcomes | Primary outcome: rate of achieving and maintaining electrographic seizure freedom for 24 hours Secondary outcomes: seizure cessation for 48 hours; rate of achieving and maintaining seizure freedom for 1 hour; subanalyses of the primary outcome measure for subjects with hypoxic‐ischaemic encephalopathy (HIE) who underwent therapeutic hypothermia |
| Notes | Funding: quote: "The NEOLEV2 study was funded by the US Food and Drug Administration Orphan Products Division (1 RO1FD004147). The Research Electronic Data Capture database is supported by National Institutes of Health Cooperative Agreement UL1TR001442. The Persyst EEG software company worked closely with the authors on the NEOLEV2 study and provided their software to the researchers free of charge, but have had no input into this article. The CortiCare commercial EEG monitoring company worked closely with the authors on the NEOLEV2 study on a commercial basis. They have had no input into the writing of this article. The authors of this article discussed the use of the automated neonatal seizure detection algorithm created by the Persyst EEG software company, which is not yet US Food and Drug Administration–approved for commercial use. Funded by the National Institutes of Health (NIH)." The authors reported not having potential conflicts of interest to disclose. |
Solanki 2015.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "All neonates who clinically developed their first seizure before 28 days of life".
Exclusion criteria: (quote:) "Neonates already on ventilator support, or neonates with hypoglycaemia, hypocalcaemia and hypo/hypernatraemia, who responded to specific treatment (e.g. with glucose, calcium, etc.)". PB group Number of participants: 35 Gestation age (mean): 36.5 weeks Sex, %: male 71.4 (n = 25) Mean weight (kg): 2.4 Median age days: 1 Term/preterm (%): 77 (n = 27)/23 (n = 8) Religion Hindu/Muslim (%): 94 (n = 33)/6 (n = 2) Apgar score at 5 minutes (mean): 6.1 Perinatal asphyxia (%): 57 (n = 20) PHT group Number of participants: 35 Gestation age (mean): 36.6 weeks Sex, %: male 60 (n = 21) Mean weight (kg): 2.6 Median age days: 1 Term/preterm (%): 83 (n = 29)/17 (n = 6) Religion Hindu/Muslim (%): 91 (n = 32)/9 (n = 2) Apgar score at 5 minutes (mean): 4.9 Perinatal asphyxia (%): 85 (n = 28) Lorazepam group Number of participants: 36 Gestation age (mean): 35.6 weeks Sex, %: Male 63.9 (n = 23) Mean weight (kg): 2.3 Median age days: 1 Term/preterm (%): 70 (n = 25)/30 (n = 11) Religion Hindu/Muslim (%): 72 (n = 26)/28 (n = 10) Apgar score at 5 min (mean): 6.7 Perinatal asphyxia (%): 53 (n = 15) The study was conducted at a single neonatal intensive care unit in Bhavnagar, India, between August 2013 and July 2014. |
| Interventions | (Quote:)"The neonates were randomly assigned (single‐blinded) to different treatments according to a block design to ensure balanced treatment assignment." (no further details are provided). PB (20 mg/kg), lorazepam (0.05 mg/kg) or PHT (20 mg/kg) administered intravenously (IV) over a 5‐minute period. (Quote:) "If clinical seizures resumed after therapy had been discontinued, the attending physician decided whether to use another ASM. The heart rate and rhythm, mean blood pressure, and respiratory status were monitored continuously during treatment." No further details were provided. |
| Outcomes | (Quote:) "Complete control of seizures, within 2.5 min of starting a single dose ASM therapy, as determined by a physician". (Quote:) "Treatment was considered to have failed if the neonate had an episode of seizures lasting longer than 5 min or a total of 2.5 min of seizure activity within 5‐min period after a single dose". |
| Notes | There was no external funding. The authors reported not having potential conflicts of interest to disclose. |
Soul 2021.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "neonates at postmenstrual age 34 to 44 weeks if they had clinically suspected or EEG‐proven (i.e. confirmed) seizures, or were at high risk for developing seizures caused by hypoxic–ischaemic encephalopathy (HIE), focal stroke, ICH, acute meningoencephalitis, brain malformation, or a suspected/known genetic disorder." Exclusion criteria: (quote:) "neonates with seizures caused by transient metabolic abnormalities or inborn errors of metabolism; neonates who had received bumetanide, furosemide, phenytoin, or ≥ 40 mg/kg PB; neonates with total bilirubin > 15 mg/dL; and neonates treated with extracorporeal membrane oxygenation or at risk of imminent death" 0.1 mg/kg bumetanide, 7 patients Male sex, n (%): 3 (34) Gestational age at birth, wk, median (IQR): 39 (38, 40) Birth weight, kg, median (IQR): 3.4 (3.1‐3.8) Race Caucasian, n (%): 6 (86) Asian, n (%): 0 Unreported, n (%): 1 (14) Hispanic or Latino ethnicity, n (%): 1 (14) Seizure aetiology Hypoxic ischaemic encephalopathy, n (%): 3 (43) Stroke, n (%): 4 (57) Intracranial haemorrhage, n (%): 0 Other, n (%): 0 Therapeutic hypothermia, n (%): 3 (43) 0.2 mg/kg bumetanide, 15 patients Male sex, n (%): 10 (67) Gestational age at birth, wk, median (IQR): 40 (38‐41) Birth weight, kg, median (IQR): 3.4 (3.1‐ 3.8) Race Caucasian, n (%): 13 (87) Asian, n (%): 1 (7) Unreported, n (%): 1 (7) Hispanic or Latino ethnicity, n (%): 1 (7) Seizure aetiology Hypoxic ischaemic encephalopathy, n (%): 7 (47) Stroke, n (%): 3 (20) Intracranial haemorrhage, n (%): 2 (13) Other, n (%): 03 (20) Therapeutic hypothermia, n (%): 4 (27) 0.3 mg/kg bumetanide, 5 patients Male sex, n (%): 1 (20) Gestational age at birth, wk, median (IQR): 39 (39, 39) Birth weight, kg, median (IQR): 3.0 (2.9‐3.3) Race Caucasian, n (%): 4 (80) Asian, n (%): 0 Unreported, n (%): 1 (20) Hispanic or Latino ethnicity, n (%): 0 Seizure aetiology Hypoxic ischaemic encephalopathy, n (%): 4 (80) Stroke, n (%): 0 Intracranial haemorrhage, n (%): 1 (20) Other, n (%): 0 Therapeutic hypothermia, n (%): 3 (60) Control, 16 patients Male sex, n (%): 7(44) Gestational age at birth, wk, median (IQR): 39.5 (39, 41) Birth weight, kg, median (IQR): 3.3 (3.0‐3.5) Race Caucasian, n (%): 12 (75) Asian, n (%): 1 (6) Unreported, n (%): 3 (19) Hispanic or Latino ethnicity, n (%): 4 (25) Seizure aetiology Hypoxic ischaemic encephalopathy, n (%): 8 (50) Stroke, n (%): 0 Intracranial haemorrhage, n (%): 4 (25) Other, n (%): 4 (25) Therapeutic hypothermia, n (%): 5 (31) The study was conducted at 4 neonatal intensive care units in Boston, USA. Patients were enrolled from 2010 to 2017. |
| Interventions | Subjects were randomised if an EEG‐proven seizure (confirmed by a study paediatric neurophysiologist) occurred at least 30 minutes after a loading dose of ≥ 20 to < 40 mg/kg phenobarbital. (Quote:) "bumetanide doses of 0.1, 0.2, and 0.3 mg/kg in comparison to a control group (normal saline), given in conjunction with 5 to 10 mg/kg phenobarbital (bumetanide + phenobarbital vs saline + phenobarbital). The choice of 5 or 10 mg/kg phenobarbital was at the discretion of the treating physician; doses and levels were the same in the control and bumetanide groups". |
| Outcomes | Determination of the pharmacokinetics and safety of bumetanide as add‐on therapy to treat neonatal seizures An exploratory endpoint was the effect of bumetanide dose and exposure on seizure burden. |
| Notes | Funding: Quote: "The trial was funded by NIH National Institute of Neurological Disorders and Stroke grant 5R01 NS066929, and grants from the CURE foundation, Harvard Catalyst–Harvard Clinical and Translational Science Center, Charles H. Hood Foundation, Translational Research Program at Boston Children’s Hospital, and Mooney Family Initiative for Translation and Clinical Studies in Rare Diseases. Tufts Clinical and Translational Science Institute (CTSI), UL1 TR001064." The authors reported not having potential conflicts of interest to disclose. |
Srinivasakumar 2015.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "1. ≥ 36 weeks’ gestation at delivery; 2. Admitted to the NICU within the first 24 hours of life; and 3. Either fulfilled clinical criteria for moderate‐to‐severe HIE (Eunice Kennedy Shriver National Institute of Child Health and Human Development criteria) or had clinical seizures (suspected or confirmed)." Exclusion criteria: (quote:) "1. Neonates < 36 weeks’ gestation; 2. > 24 hours of age (to exclude nonHIE causes of seizures); 3. Infants with congenital anomalies of the central nervous system; 4. Moribund infants for whom no further aggressive treatment is planned; 5. Infants who demonstrated electrographic SE at the beginning of the cEEG study (initial 1 hour cEEG)". Treatment of electrographic seizures group Gestational age, mean ± SD: wk 38.3 ± 2 Birth weight, mean ± SD: g 3233 ± 585 Gender, boy:girl %: 60:40 5‐min Apgar Score: 4 Cord/first pH, mean ± SD: 7.05 ± 0.1 Inborn versus outborn, %: 40:60 Severity of HIE, moderate:severe: % 67:33 Abnormality on brain MRI: % 66 Therapeutic hypothermia: % 66 Age at start of cEEG monitoring, mean ± SD: h 12.5 ± 9.5 Electrographic SE: % 33 Duration of cEEG monitoring, mean ± SD: h 72.1 ± 37 Treatment of clinical seizures group Gestational age, mean ± SD: wk 38.5 ± 2 Birth weight, mean ± SD: g 3057 ± 602 Gender, boy:girl: % 60:40 5‐min Apgar Score: 4 Cord/first pH, mean ± SD: 7.08 ± 0.2 Inborn versus outborn: % 40:60 Severity of HIE, moderate:severe: % 70:30 Abnormality on brain MRI: % 85 Therapeutic hypothermia: % 65 Age at start of cEEG monitoring, mean ± SD: h 13.3 ± 10.1 Electrographic SE: % 20 Duration of cEEG monitoring, mean ± SD: h 69.5 ± 31 The study was conducted at a single centre in the USA from 2007 to 2011. |
| Interventions | Treatment of electrographic seizures alone versus treatment of clinical seizures Treatment of electrographic seizures Seizures were defined as (quote:) “rhythmic spike wave activity” lasting for > 10 seconds. Any EEG event, confirmed to be a seizure, with or without a clinical correlate lasting > 30 seconds, or more than 2 confirmed events detected by the algorithm in a 24‐hour period were thresholds to commence standardised ASM treatment. Treatment consisted of a stepwise approach: PB 20 mg/kg (first‐line); PB 20 mg/kg (second‐line, if seizures continued); fosphenytoin 20 mg/kg (third‐line, if seizures continued); if seizures continued: midazolam bolus 0.05 mg/kg followed by infusion at 0.15 mg/kg per hour for 24 hours, with decrease of dose to 0.1 mg/kg per hour for 24 hours and then to 0.05 mg/kg per hour for 24 hours before stopping. Treatment of clinical seizures Seizure diagnosis and treatment was based solely on clinical observation and was based on the following protocol: PB 20 mg/kg (first‐line); PB 20 mg/kg (second‐line, if seizures continued); fosphenytoin 20 mg/kg (third‐line, if seizures continued); if seizures continued: midazolam bolus 0.05 mg/kg followed by infusion at 0.15 mg/kg per hour for 24 hours, with decrease of dose to 0.1 mg/kg per hour for 24 hours and then to 0.05 mg/kg per hour for 24 hours before stopping. (Quote:) "Neonates who developed electrographic SE, detected by the study epileptologist, in this group were unblinded and treated as in the electrographic seizures group". |
| Outcomes | Primary outcome: seizure burden Other outcomes: neurodevelopmental development at 18 to 24 months evaluated using the BSID III |
| Notes | The study was funded by the Thrasher Foundation. The authors reported not having potential conflicts of interest to disclose. |
Susnerwala 2022.
| Study characteristics | |
| Methods | Randomised controlled trial, not blinded, 'pragmatic'. Randomisation via a computer‐generated random number. The study was conducted at a tertiary care neonatal intensive care unit in Aurangabad, India. Patients were recruited between January 2019 and April 2020. |
| Participants | Neonates with clinical seizures presenting before 48 hrs with movements considered abnormal and lasting longer than 30s. |
| Interventions | LEV group: LEV 20 mg/kg IV, if seizures controlled, maintenance with 10 mg/kg BID. If seizures not controlled within 20 min, add‐on of PB 20 mg/kg, followed by maintenance of 5 mg/kg*d PB group: PB 20 mg/kg IV, if seizures controlled, maintenance with 5 mg/kg*d. If seizures not controlled within 20 min, add‐on of LEV 20 mg/kg Third‐line PHT or midazolam for both groups |
| Outcomes | Primary outcome measure: clinical cessation of abnormal movements after loading for at least 24 hrs |
| Notes | 103 neonates screened, 82 randomised (44 LEV, 38 PB). Mean age at enrolment 4.8 hrs. Clonic seizures in 51.2%. Primary outcome achieved in the LEV group in 29/44 vs 13/38 in the PB group. Secondary seizure control after adding LEV in 22/25 in the PB group vs 14/15 in the LEV group. Children receiving therapeutic hypothermia and erythropoietin included No serious adverse events reported, but higher mortality in PB group (21% vs 9%) EEG not considered feasible No information on conflicts of interest and funding was included in the manuscript. |
Van Rooji 2010.
| Study characteristics | |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: (quote:) "gestational age of ≥ 37 weeks, admission to 1 of the NICUs 24 hours after birth, and diagnosis of HIE and neonatal seizures. HIE was defined on the basis of meeting ≥ 3 of the following criteria: (1) signs of intrauterine asphyxia (i.e. late decelerations on foetal electrocardiograms or meconium‐stained liquor), (2) arterial cord blood pH of < 7.10, (3) delayed onset of spontaneous respiration, (4) Apgar score of ≤ 5 at 5 minutes, or (5) multiorgan failure (elevated liver enzyme levels, reduced diuresis, and cardiovascular problems)." Exclusion criteria: (quote:) "presence of congenital or chromosomal abnormalities, maternal use of narcotics or sedatives, treatment with phenytoin before referral, and administration of muscle‐relaxing drugs." "Subclinical status epilepticus at the beginning of the aEEG registration". Treatment of both clinical seizures and subclinical seizure patterns (group A) Gestational age, mean SD: wk 39.5 ± 1.8 Birth weight, mean SD: g 3254 ± 701 Gender, n (%): male 8 (42); female: 11 (58) Outborn, n (%): 17 (90) Apgar score at 5 min of 5, n (%): 12 (67) Cord pH, mean (range) (group A, N = 12): 6.87 (6.67 to 7.00) Lactate level, mean (range), mmol/L (group A, N = 15): 14.1 (2.2 to 26) HIE, n (%) Grade II: 11 (58) Grade III: 8 (42) Mode of delivery, n (%) Vaginal: 3 (16) Ventouse extraction: 2 (10) Caesarean section, emergency: 14 (74) Meconium‐stained liquor, n (%): 9 (47) Mechanical ventilation, n (%): 15 (79) Blinding of the aEEG registration and treatment of clinical seizures only (group B) Gestational age, mean SD: wk 39.9 ± 1.3 Birth weight, mean SD: g 3416 ± 487 Gender, n (%): male 7 (50); female 7 (50) Outborn, n (%): 12 (86) Apgar score at 5 min of 5 n (%): 11 (79) Cord pH, mean (range) (group B, N = 11): 6.88 (6.64 to 7.30) Lactate level, mean (range), mmol/L (group B, N = 13): 9.3 (3.1 to 29.0) HIE, n (%) Grade II: 7 (50) Grade III: 7 (50) Mode of delivery, n (%) Vaginal: 4 (29) Ventouse extraction: 3 (21) Caesarean section, emergency: 7 (50) Meconium‐stained liquor, n (%): 7 (50) Mechanical ventilation, n (%): 13 (93) The multicentre study was conducted at eleven perinatal centres in the Netherlands and Belgium between November 2003 and April 2008. |
| Interventions | Treatment of both clinical seizures and subclinical seizure patterns (group A) versus blinding of the aEEG registration and treatment of clinical seizures only (group B) In both groups, the treatment consisted of the following protocol: First‐line: PB: 20 mg/kg, eventually another 10 mg/kg Second‐line: midazolam: loading dose of 0.05 mg/kg, followed by continuous infusion of 0.15 mg/kg per h, to maximum of 0.2 mg/kg per hour (when seizures have been stopped for 24 hours, tapered to 0.1 mg/kg per h and stopped after 48 hours) Third‐line: lidocaine: loading dose of 2 mg/kg, followed by continuous infusion of 6 mg/kg per h for 6 hours, then 4 mg/kg per h for 12 hours, and then 2 mg/kg per hour for 12 hours (always stopped after 36 hours) Fourth‐line: clonazepam: loading dose of 0.1 mg/kg, followed by continuous infusion of 0.1 to 0.5 mg/kg per day Fifth‐line: pyridoxine: 50 mg/kg Sixth‐line: further treatment on the basis of clinician’s decisions |
| Outcomes | Primary outcome: reduction of the total duration of seizures detected on aEEG Other outcomes: degree of brain injury seen on MRI scans. These were obtained 4 to 10 days after birth and retrospectively reviewed and scored by 2 investigators blinded to aEEG results. |
| Notes | Funding: Dr van Rooji was supported by the Dutch Epilepsy Foundation (grant NEF 3‐15). Interests: not mentioned in the publication |
aEEG: amplitude‐integrated electroencephalography; AGA: appropriate for gestational age; ASM: anti‐seizure medication; BSID: Bayley Scales of Infant Development; cEEG: continuous electroencephalography; CNS: central nervous system; EEG: electroencephalography; GE: genetic epilepsy; HIE: hypoxic‐ischaemic encephalopathy; HNNE: Hammersmitz Neurological Neonatal Neurological Examination; ICH: intracranial haemorrhage; IEM: inborn error of metabolism; IQR: inner‐quartile range; IUI: intrauterine infections; IV: intravenous; LEV: levetiracetam; LGA: large for gestational age; LSCS: lower segment caesarean section; MRI: magnetic resonance imaging; NICU: neonatal intensive care unit; NS: normal saline; NVD: normal vaginal delivery; PB: phenobarbitone; PIH: pregnancy‐induced hypertension; PHT: phenytoin; PROM: premature rupture of membranes; SD: standard deviation; SE: structural epilepsy; SGA: small for gestational age.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abend 2011 | Retrospective cohort study (on levetiracetam for neonatal seizures) |
| Arican 2020 | Cross‐sectional study (comparing the neurocognitive outcomes of neonates who were treated with levetiracetam or phenobarbitone) |
| Castro Conde 2005 | Prospective cohort study (evaluating midazolam as a third‐line drug) |
| Deshmukh 1986 | Case series (on 7 neonates treated with lorazepam as a third‐line drug) |
| Dwivedi 2019 | Retrospective cohort study (to examine the factors associated with failure of phenobarbitone as first‐line ASM in HIE) |
| Favié 2020 | Observational study (evaluating the pharmacokinetics of lignocaine in neonates) |
| Gal 1988 | Case series (of 6 neonates treated with valproic acid) |
| Glass 2021 | Prospective cohort study (comparing maintenance ASM versus no maintenance ASM on neurodevelopment and epilepsy at 24 months) |
| Han 2018 | Retrospective study (on levetiracetam as first‐line ASM) |
| Hellström‐Westas 1988 | Observational study (evaluating lignocaine in neonatal seizures) |
| Hu 2003 | This prospective open‐label study was designed to determine the efficacy and safety of continuous midazolam infusion in neonates with uncontrollable neonatal seizures. Patients whose seizures could not be controlled by diazepam, phenytoin or phenobarbital were enrolled. |
| Hunt 2021 | This RCT on treating both clinical and electrographic seizures versus treating clinical seizures alone has included neonates with and without electrographic seizures. Data on outcomes of only those neonates who had electrographic seizures were not available. |
| Jawadekar 1992 | Prospective observational study (evaluating phenobarbitone and phenytoin) |
| Jayswal 2021 | Prospective cohort study (comparing midazolam versus levetiracetam as third‐line ASM) |
| Kanmaz 2021 | Retrospective observational study (on levetiracetam as first‐line ASM) |
| Liu 2020 | Retrospective study (comparing phenobarbitone and levetiracetam as first‐line ASM) |
| Low 2016 | Prospective observational study (evaluating phenobarbitone for EEG‐confirmed seizures) |
| Maitre 2013 | Retrospective cohort study (comparing the effect of levetiracetam and phenobarbitone on neurodevelopmental outcomes) |
| Mollamohammadi 2018 | Single‐arm study evaluating levetiracetam as a third‐line drug |
| Pressler 2015 | This open‐label study without control group aimed to assess dose and feasibility of intravenous bumetanide as an add‐on to phenobarbital for treatment of neonatal seizures. |
| Ramantani 2011 | Prospective observational study (evaluating levetiracetam as first‐line ASM) |
| Rao 2018 | Retrospective cohort study (comparing levetiracetam and phenobarbitone as first‐line ASM in HIE) |
| Rochefort 1989 | Conference abstract. We did not have adequate information for risk of bias assessment and adequate data on outcomes. |
| Sedighi 2016 | Single‐arm study (evaluating levetiracetam as first‐line ASM) |
| Shany 2007 | Retrospective cohort study (comparing lignocaine and midazolam as second‐line ASM) |
| Thibault 2020 | Retrospective cohort study (comparing levetiracetam and phenobarbitone as first‐line ASM in seizures following neonatal cardiac surgery) |
| Verwoerd 2022 | Retrospective cohort study (comparing levetiracetam and phenobarbitone as first‐line ASM) |
| Wagner 2021 | Retrospective cohort study (comparing levetiracetam and phenobarbitone as first‐line ASM) |
| Weeke 2016 | Retrospective observational study (evaluating lignocaine in neonatal seizures) |
| Yamamoto 2007 | Retrospective cohort study (comparing lignocaine and midazolam in neonatal status epilepticus) |
ASM: anti‐seizure medication; EEG: electroencephalography; HIE: hypoxic‐ischaemic encephalopathy; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Gyandeep 2023.
| Methods | Randomised controlled trial |
| Participants | Preterm neonates born between 28 and 36 weeks' gestational age with clinical seizures |
| Interventions | Intervention 1 ‐ Phenobarbitone as first‐line ASM Intervention 2 ‐ Levetiracetam as first‐line ASM |
| Outcomes | Primary outcome ‐ cessation of clinical seizure and remaining seizure‐free for next 24 h Other outcome ‐ adverse events of ASMs such as apnoea, increase in respiratory support and hypotension |
| Notes | This is the only RCT including ASM in preterm neonates with seizures. The study is awaiting classification, as we need additional data from the study authors to classify the study and include in the appropriate meta‐analysis. |
Mohammadi 2023.
| Methods | Randomised controlled trial |
| Participants | Term neonates with seizures |
| Interventions | Intervention 1 ‐ Levetiracetam as second‐line ASM Intervention 2 ‐ Phenytoin as second‐line ASM |
| Outcomes | Cessation of seizures, adverse effects of the drug |
| Notes | Method of diagnosing seizures (clinical or EEG‐based) was not mentioned. Seizure control (timeline) was not defined. The study is awaiting classification, as we need additional data from the study authors to classify the study and include in the appropriate meta‐analysis. |
ASM: antiseizure medication; EEG: electroencephalogram
Characteristics of ongoing studies [ordered by study ID]
ACTRN12622000470796.
| Study name | EFFICACY AND SAFETY OF levetiracetam versus phenytoin for neonatal seizures. A randomized controlled trial |
| Methods | |
| Participants | Neonates up to 30 days of life presenting with seizures |
| Interventions | Levetiracetam loading dose 5‐10 mg/kg IV over 15 min, maintenance 10 mg/kg IV 12‐hourly |
| Outcomes | Clinical termination of seizures |
| Starting date | Not given |
| Contact information | drkamo50@gmail.com |
| Notes | No information on randomisation protocol; no information on phenytoin treatment protocol |
CTRI/2013/01/003310.
| Study name | Comparison of levetiracetam with phenobarbitone in neonatal seizures |
| Methods | RCT |
| Participants | Neonates with clinical seizures |
| Interventions | LEV 60 mg/kg vs PB 20‐30 mg/kg |
| Outcomes | Control of clinical seizures, adverse events, neurodevelopment |
| Starting date | 2012 |
| Contact information | anuamit7@rediffmail.com |
| Notes | Calculated sample size 80; no further entries; no entry in MEDLINE |
CTRI/2013/04/003585.
| Study name | Levetiracetam for management of seizures in newborn |
| Methods | Not specified |
| Participants | Neonates > 30 w and 1.5 kg with clinical or electrographic seizures |
| Interventions | LEV 20 mg/kg vs PB 20 mg/kg, maintenance |
| Outcomes | Not specified |
| Starting date | 2012 |
| Contact information | rabindranindia@yahoo.co.in |
| Notes | Calculated sample size 100; last entry 2013 |
CTRI/2014/06/004659.
| Study name | Levetiracetam vs phenobarbitone for the control of neonatal seizures: a double‐blind randomised controlled trial |
| Methods | RCT |
| Participants | Neonates (> 32 weeks) with clinical seizures |
| Interventions | LEV 20‐60 mg/kg, vs PB 20‐40 mg |
| Outcomes | Time until seizure control, mortality, mortality, neurodevelopment at 18 months, adverse events (not specified) |
| Starting date | 2014 |
| Contact information | skb.bmc@gmail.com |
| Notes | Expected sample size 300; entry last updated 2014 |
CTRI/2015/06/005849.
| Study name | Levetiracetam vs phenobarbitone in acute neonatal seizures |
| Methods | RCT |
| Participants | Neonates > 1000 g and > 28 weeks |
| Interventions | LEV 30‐40 mg/kg vs PB 20‐30 mg/kg |
| Outcomes | Recurrence of seizures, need for further ASM, adverse events, mortality, neurodevelopmental outcome |
| Starting date | 2013 |
| Contact information | drnikhilkulkarni83@gmail.com |
| Notes | Estimated sample size 32, apparently 38 achieved, completed, no results published |
CTRI/2016/10/007412.
| Study name | A clinical study to compare levetiracetam and phenobarbitone in newborns with birth asphyxia |
| Methods | RCT |
| Participants | Term neonates with HIE II/III, age < 24 hours |
| Interventions | PB 20 mg/kg, maintenance vs LEV 20‐60 mg/kg, maintenance |
| Outcomes | Seizure control with 1st‐line drug, need for further ASM, neonatal mortality, adverse events of LEV, duration of hospital stay, neurodevelopment |
| Starting date | 2016 |
| Contact information | sha.akht@gmail.com |
| Notes | Calculated sample size 60 |
CTRI/2018/04/013161.
| Study name | Levetiracetam used as first‐line anti‐epileptic versus phenobarbitone in neonatal convulsions |
| Methods | RCT, add‐on if required |
| Participants | Neonates with clinical seizures |
| Interventions | LEV 20‐40 mg/kg, maintenance vs PB 20‐30 mg/kg, maintenance |
| Outcomes | Cessation of seizures, recurrence at 24 hours, need for further ASM, adverse events, absence of seizures at 48 hours, adverse events |
| Starting date | 2014 |
| Contact information | zyee08@gmail.com |
| Notes | Sample size 100, achieved, reported that LEV more efficient than PB; no PubMed listing |
CTRI/2020/03/023961.
| Study name | A randomized controlled trial of levetiracetam vs phenobarbitone for treatment of neonatal seizures |
| Methods | RCT |
| Participants | Neonates (35‐42 weeks) with seizures |
| Interventions | PB 20‐30 mg/kg vs LEV 20‐30 mg |
| Outcomes | Seizure control within 1 hour, recurrence of seizures, adverse events (respiratory depression, heart rate fluctuation, duration of hospitalisation) |
| Starting date | 2020 |
| Contact information | manikant7@yahoo.com |
| Notes | Estimated sample size 90, EEG not specified |
CTRI/2021/02/031290.
| Study name | Comparison between phenobarbitone and levetiracetam as the initial anti convulsant in treating preterm neonatal seizures |
| Methods | RCT |
| Participants | Neonates < 37 weeks with clinical seizures |
| Interventions | PB 15 mg/kg vs LEV 40 mg/kg |
| Outcomes | Cessation of seizures at 24 hours, clinical response based on seizure aetiology at 1 month |
| Starting date | 2021 |
| Contact information | doc.sant@yahoo.co.in |
| Notes | Calculated sample size 106 |
CTRI/2022/09/045658.
| Study name | To compare the effect of two anticonvulsant drugs levetiracetam and phenobarbitone in neonates with seizures |
| Methods | Randomised controlled trial |
| Participants | Neonates with clinical seizures not controlled after correction of hypoglycaemia and hypocalcaemia |
| Interventions | Intervention1: Levetiracetam [LEV]: loading with LEV 20 mg/kg IV in neonates with seizures: if seizures stop, put on maintenance dose @ 20 mg/kg/day; if seizures continue, reload with LEV 20 mg/kg followed by maintenance dose of 40 mg/kg/day; if seizures still persist, switch over to phenobarbitone. Control Intervention1: Phenobarbitone [PB]: loading with PB 20 mg/kg IV in neonates with seizures: if seizures stop, put on maintenance dose @ 3 mg/kg/day; if seizures continue, reload with PB 10 mg/kg followed by maintenance dose of 5 mg/kg/day; if seizures still persist, switch over to levetiracetam |
| Outcomes | Cessation of seizures within 48 hours of administration of first or second loading dose of the drug [levetiracetam vs phenobarbitone], time point: 48 hours |
| Starting date | 2021 |
| Contact information | drmanisha99@yahoo.com |
| Notes | Clinical diagnosis of seizures and evaluation of treatment success |
CTRI/2023/02/049794.
| Study name | Study comparing efficacy of two drugs as first line drug in late preterm and term babies with neonatal seizure |
| Methods | Randomised controlled trial |
| Participants | Term and preterm neonates apparently presenting with seizures (clinical diagnosis) lasting 3 min or more |
| Interventions | Intervention 1: Levetiracetam: Injection of levetiracetam (40 mg/kg then 20 mg/kg) as 1st‐line drug in neonatal seizures given over 20 minutes Control intervention 1: Phenobarbitone: Injection of phenobarbitone (20 mg/kg then 20 mg/kg) loading dose is the standard 1st‐line drug for neonatal seizures given over 20 minutes |
| Outcomes | Termination of clinical seizures (seizure control in 60 minutes and no further seizure in 24 hours). Time point: Termination of clinical seizures (seizure control in 60 minutes and no further seizure in 24 hours) |
| Starting date | |
| Contact information | bhupendra.gupta@tatasteel.com |
| Notes | Clinical diagnosis of seizures and assessment of treatment success |
IRCT2014070318334N1.
| Study name | Study of levetiracetam effect in reduction of seizure frequency in neonates with seizure |
| Methods | Observational, uncontrolled |
| Participants | Neonates > 34 weeks and > 1999 g, clinical seizures |
| Interventions | Levetiracetam 10 mg/kg every 12 hours for 3 months |
| Outcomes | Seizure frequency at 4 weeks, seizure duration at 4 weeks, adverse events |
| Starting date | 2014 |
| Contact information | m.sedighi@kums.ac.ir |
| Notes | Calculated sample size 50; no further entries |
IRCT20160523028008N23.
| Study name | The effect of levetiracetam and phenobarbital on the control of neonatal seizures |
| Methods | Randomised controlled trial |
| Participants | Term neonates with seizures (clinical diagnosis) |
| Interventions | Intervention 1: Intervention group: Patients are treated with levetiracetam injection (500 mg/5 mL by Estragen Company, Switzerland) at a loading dose of 50 mg/kg and infusion rate of 2 mg/kg/min (within 10 cc of normal saline) under cardiorespiratory monitoring. If seizures continue with the first dose of levetiracetam, the drug is re‐loaded at a dose of 50 mg/kg at the same infusion rate (within 10 cc of normal saline). If the seizure does not stop or returns after 15 minutes, even after the second dose of medication, the treatment groups are changed. If the seizure does not stop or returns after 15 minutes after changing treatment groups, other anticonvulsant drugs are used. Intervention 2: Control group: Patients in the control group are treated with phenobarbital injection (200 mg/mL from Chemidarou company) at a loading dose of 20 mg/kg and at an infusion rate of 1 m/kg/min (within 10 cc of normal saline) under cardiorespiratory monitoring. If the seizure continues with the first dose, phenobarbital is re‐loaded by infusion at a dose of 20 mg per kg at the same rate as before. If the seizure does not stop or returns after 15 minutes, even after the second dose of medication, the treatment groups are changed. If the seizure does not stop or returns after 15 minutes after changing treatment groups, other anticonvulsant drugs are used. |
| Outcomes | Complete cessation of seizures for 24 hours after medication. Time point: in the first 24 hours after medication. Method of measurement: stopping seizure movements clinically (clinical assessment Number of doses received to stop seizures. Time point: in the first 24 hours after medication. Method of measurement: patient medical record |
| Starting date | |
| Contact information | naderfaraji59@gmail.com |
| Notes | Clinical diagnosis of seizures and assessment of treatment success |
IRCT20190526043717N1.
| Study name | Comparison of intravenous levetiracetam and phenobarbital for management of neonatal seizures |
| Methods | RCT, double‐blind |
| Participants | Neonates and infants > 37 weeks and > 2500 g up to 1 year of age with clinical seizures |
| Interventions | LEV 20‐40 mg/kg, maintenance vs PB 20‐30 mg/kg in 2 doses |
| Outcomes | Clinical seizures at 24 hours, recurrence of clinical seizures until 3 months after the intervention |
| Starting date | 2019 |
| Contact information | masoumeh‐hospital@muq.ac.ir |
| Notes | Calculated sample size 100; neonates and infants |
IRCT20200115046137N1.
| Study name | Comparison of the effects of phenobarbital, topiramate and levetiracetam in the treatment of neonatal seizures |
| Methods | RCT, single‐blind |
| Participants | Neonates with clinical seizures |
| Interventions | PB 5 mg in 2 doses, TPM 3 mg in 2 doses, LEV 20 mg in 2 doses |
| Outcomes | Seizures every month |
| Starting date | 2020 |
| Contact information | samiei.moh@gmail.com |
| Notes | Calculated sample size 60; no further entries |
IRCT20200131046317N3.
| Study name | Comparison of the effects of phenobarbital and levetiracetam on neonatal seizures after discharge |
| Methods | RCT, double‐blind |
| Participants | Term neonates with clinical seizures |
| Interventions | LEV 30 mg/kg*d maintenance for 3/6 months vs PB 5 mg/kg*d for 3/6 months |
| Outcomes | Growth at 3 and 6 months, recurrence of seizures at 3 and 6 months |
| Starting date | 2021 |
| Contact information | dr.nazanin_zand@yahoo.com |
| Notes | Calculated sample size 60; seizure diagnosis not specified |
IRCT20200528047589N1.
| Study name | Comparison of effects of phenobarbital and levetiracetam in the control of neonatal seizures |
| Methods | RCT, double‐blind |
| Participants | Neonates (> 33 weeks, > 2 kg), clinical seizures |
| Interventions | LEV 30‐50 mg/kg vs PB 20‐30 mg/kg |
| Outcomes | Cessation of clinical seizures, ASM continuation at discharge |
| Starting date | 2020 |
| Contact information | MaamouriGh@mums.ac.ir |
| Notes | Estimated sample size 74 |
IRCT20220619055221N1.
| Study name | Efficacy of levetiracetam compared to intravenous phenytoin in treatment of acute phase of neonatal seizure |
| Methods | Randomised controlled trial |
| Participants | Patients diagnosed with neonatal seizures |
| Interventions | Levetiracetam 20 mg/kg IV versus phenytoin 20 mg/kg IV |
| Outcomes | Seizure control and non‐recurrence within 24 hours |
| Starting date | 22/11/2022 |
| Contact information | parvanehbabaey@gmail.com |
| Notes | Recruitment complete |
NCT01089504.
| Study name | Prophylactic phenobarbital after neonatal seizures (PROPHENO) |
| Methods | RCT |
| Participants | Neonates (> 33 weeks), neonatal seizures (clinical or electrographic or electroclinical) |
| Interventions | PB 4‐5 mg/kg for 4 months vs placebo |
| Outcomes | Bayley at 18‐22 m, seizure recurrence |
| Starting date | 2016 |
| Contact information | ronnie_guillet@urmc.rochester.edu |
| Notes | Terminated in 2016 due to inadequate recruitment |
NCT02550028.
| Study name | Levetiracetam treatment of neonatal seizures |
| Methods | RCT |
| Participants | Term neonates (> 2500 g) with seizures confirmed by EEG |
| Interventions | LEV orally 50 mg/kg, maintenance 30 mg/kg*d vs PB IV 20‐40 mg/kg, maintenance 5 mg/kg*d |
| Outcomes | EEG (baseline) at 28 days, MRI, neurodevelopment, time to seizure control (days), adverse events |
| Starting date | 2015 |
| Contact information | zwhchfu@126.com |
| Notes | Calculated sample size 100, in last update 2021; no information if this was achieved |
NCT03107507.
| Study name | Efficacy of levetiracetam in control of neonatal seizures guided by an EEG |
| Methods | RCT |
| Participants | Term neonates with seizures (confirmed by aEEG) |
| Interventions | LEV oral 40‐50 mg/kg, maintenance vs PB IV 20‐40 mg/kg, maintenance |
| Outcomes | Number of seizures, hours to achieve seizure control, dose escalation data on LEV, aEEG accuracy, effect of LEV on aEEG background activity, short‐term outcome at 3 months |
| Starting date | 2017 |
| Contact information | yarasalah.shaheen@gmail.com |
| Notes | Estimated sample size not specified; no further entries |
NCT04320940.
| Study name | Efficacy and safety of intravenous phenobarbital in neonatal seizures |
| Methods | RCT, double‐blind |
| Participants | Neonates > 33 weeks with high probability of seizures, cEEG, seizures for at least 30 s/h |
| Interventions | PB 20/kg (if required plus 20) vs PB 40 mg/kg (if required plus 10) |
| Outcomes | No requirement of ASM after 1st dose PB at 24 hours; no requirement of ASM after 1st dose PB at 2 hours; no requirement of ASM after 2nd dose of PB; seizure burden |
| Starting date | 2020 |
| Contact information | rnoor@nemaresearch.net |
| Notes | Calculated sample size 490; very interesting study |
NCT05291455.
| Study name | Efficacy of lacosamide in neonatal status epilepticus: a randomised controlled study |
| Methods | RCT |
| Participants | Neonates with status epilepticus (not specified) |
| Interventions | LCM vs PB (doses not specified) |
| Outcomes | Cessation of seizures (not specified) |
| Starting date | 2022 |
| Contact information | abeersalamah84@yahoo.com |
| Notes | Estimated sample size not specified |
aEEG: amplitude‐integrated electroencephalography; ASM:antiseizure medication; cc: cubic centimetres; cEEG:continuous electroencephalography; EEG:electroencephalogram;; HIE:hypoxic‐ischaemic encephalopathy; IV:intravenous; LCM: lacosamide; LEV: levetiracetam; MRI:magnetic resonance imaging; PB: phenobarbitone; RCT: randomised controlled trial; s/h: ;TPM:topiramate; vs: versus
Differences between protocol and review
We made the following changes to the published protocol (Abiramalatha 2022).
For the comparison of 'one ASM versus another', we reported the outcomes of each comparison (on individual ASMs) in separate analyses and SoF tables.
The comparison 'any ASM treatment versus no ASM for clinically‐diagnosed or electrographic‐only seizures' was evaluated as 'treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone in neonates'.
We included cross‐over trials in this review. However, we did not analyse cross‐over trials separately, because no study included washout periods.
For the comparison 'one ASM versus another', the outcome 'proportion of infants who achieve seizure control after the first or maximal dose of ASM' was reported as two different outcomes: 'proportion of infants who achieve seizure control after the first loading dose of ASM' and 'proportion of infants who achieve seizure control after the maximal loading dose of ASM'.
The outcome 'mortality at any time' was reported as 'mortality before hospital discharge or at any time later'.
For the comparison 'lignocaine versus benzodiazepine as second‐line ASM in EEG‐confirmed neonatal seizures', long‐term mortality or neurodevelopmental disability and neurodevelopmental disability alone were assessed until 12 months in the only included trial (Boylan 2004).
For the comparison 'treatment of both clinical and electrographic seizures versus treatment of clinical seizures alone', the outcome 'proportion of infants who achieve seizure control' was reported as 'seizure burden during hospitalisation'.
For the comparison 'maintenance ASM versus no maintenance ASM after achieving seizure control in neonates with clinically diagnosed seizures', the outcome 'proportion of infants who achieve seizure control' was reported as 'proportion of infants with repeated seizures before hospital discharge'.
Though the time point of assessment for cognitive impairment was defined as three years or more in the protocol, the only trial (Soul 2021) that reported this outcome has reported cognitive impairment at 18 to 24 months.
-
The following are the changes in the outcome 'adverse effects related to ASM treatment during hospitalisation'.
We added other possible adverse effects of ASMs as an outcome: proportion of infants with sedation or drowsiness, bradycardia, shock requiring volume or inotropes.
The adverse effect 'respiratory depression or hypoventilation requiring any form of respiratory support' was reported as 'requirement for mechanical ventilation'.
'Hypotension' was reported as 'Hypotension requiring volume or inotropes'.
As the dosage regimen of phenobarbital and levetiracetam was variable across the studies, we defined the first loading dose of ASM as 20 mg/kg for phenobarbital and 20 to 40 mg/kg for levetiracetam. The maximal loading dose of ASM was defined as 30 to 40 mg/kg for phenobarbital and 40 to 60 mg/kg for levetiracetam. We defined (post hoc) a time limit of 24 to 48 hours from the time of ASM administration to evaluate seizure control.
In the SOF tables, for the outcome 'proportion of infants who develop adverse effects of ASM', we reported the two most relevant adverse effects: 'requirement for mechanical ventilation' and 'proportion of infants who develop sedation or drowsiness'.
Contributions of authors
All the authors (TA, ST, VVR, HH, FB and RP) contributed to the development and drafting of the review manuscript.
The authors TA, ST, VVR and HH reviewed the results of the search, independently in pairs of two, and selected studies for inclusion. We resolved any disagreements by discussion with RP.
TA and ST independently extracted data for each study. We resolved any disagreements by discussion with HH.
VVR and RP independently assessed the risk of bias for each study using RoB 2. We resolved any disagreements by discussion with TA. For Boylan 2004, ROB TA and VVR assessed the risk of bias.
FB and TA independently assessed the certainty of the evidence for important outcomes. We resolved any disagreements by discussion with VVR.
TA will be guarantor of the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
TA is an Associate editor with Cochrane Neonatal. However, she did not participate in the acceptance or editorial processes for this review.
ST is an Associate editor with Cochrane Neonatal. However, he did not participate in the acceptance or editorial processes for this review.
VVR declared that they have no conflict of interest.
RP reported that they have received the following: a contract payment to support a clinical trial from Union Chimique Belge; a payment to their employing institution UCL Institution for Child Health, London; a contract payment from Kephala (a company providing diagnostic expertise (EEG reporting), but which undertakes no drug development); honoraria from Natus for a lecture on EEG in neonatal epilepsy; and a payment from GW Pharmaceuticals for participation on an advisory board for a neuroprotective trial. They reported publication of opinions in the medical journal of Great Ormond Street Hospital, London, UK (review article on why we need new drugs for the treatment of epilepsy in infancy) and working as a Consultant in Clinical Neurophysiology at Great Ormond Street Hospital, London, UK (reporting neonatal EEG). RP is Chair of the neonatal task force at International League Against Epilepsy (ILAE). RP was chair of the neonatal working group of the International Neonatal Consortium (2017 to 2019). RP was also chair of the neonatal working group of the Brighton Collaboration.RP was involved in the trial included in the review Boylan 2004, funded by the UK National Lottery Community fund (investigator‐led). RP did not participate in assessing risk of bias, GRADE, or extracting data for this trial (see Contributions of authors). RP's research is supported by the National Institute of Health Research (NIHR), Biomedical Research Centre at Great Ormond Street Hospital (GOSH), Cambridge Biomedical Research Centre NIHR, and the James Bradfield Memorial Grant / Evelyn Trust.
FB is affiliated to the ILAE Standards and Best Practice Council. FB is an Editor for Cochrane Epilepsy.
HH declared that they are a member of the ILAE Pediatric Commission; co‐chair of the ILAE Neonatal Seizure Guideline Update group (co‐chair) ICNA Board Member, and Head of the ICNA Finance Committee. HH received a USD 500 travel grant for lecture by the American Epilepsy Society (AES) in 2022. However as he was unable to attend the American Epilepsy Congress, he donated the grant to AES.
New
References
References to studies included in this review
Akeel 2022 {published data only}
- Akeel NE, Suliman HA, Al-Shokary AH, Ibrahim AO, Kamal NM, Abdelgalil AA, et al. A comparative study of levetiracetam and phenobarbital for neonatal seizures as a first line treatment. Global Pediatric Health 2022;9:2333794X221143572. [DOI: 10.1177/2333794X221143572] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boylan 2004 {published data only}
- Boylan GB, Rennie JM, Chorley G, Pressler RM, Fox GF, Farrer K, et al. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology 2004;62(3):486-8. [DOI: 10.1212/01.wnl.0000106944.59990.e6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Falsaperla 2019 {published and unpublished data}
- Falsaperla R, Mauceri L, Pavone P, Barbagallo M, Vitaliti G, Ruggieri M, et al. Short-term neurodevelopmental outcome in term neonates treated with phenobarbital versus levetiracetam: a single-center experience. Behavioural Neurology 2019;2019:3683548. [DOI: 10.1155/2019/3683548] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghaffar 2020 {published data only}
- Ghaffar J, Riaz A, Uzair, Virk AO, Bhatti A. Comparative efficacy of intravenous levetiracetam vs phenobarbitone in neonatal seizures. Medical Forum 2020;31(7):25-8. [WEBSITE: medforum.pk/article/6-comparative-efficacy-of-intravenous-levetiracetam-vs-phenobarbitone-in-neonatal-seizures] [Google Scholar]
Gowda 2019 {published data only}
- Gowda VK, Romana A, Shivanna NH, Benakappa N, Benakappa A. Levetiracetam versus phenobarbitone in neonatal seizures — a randomized controlled trial. Indian Pediatrics 2019;56(8):643-6. [PMID: ] [PubMed] [Google Scholar]
Jindal 2021 {published and unpublished data}
- Jindal A, Angurana SK, Suthar R, Kumar P, Sundaram V. Effect of early withdrawal of phenobarbitone on the recurrence of neonatal seizures: an open-label randomized controlled trial. Epilepsy & Behavior 2021;117:107875. [DOI: 10.1016/j.yebeh.2021.107875] [PMID: ] [DOI] [PubMed] [Google Scholar]
Khan 2020 {published and unpublished data}
- Khan MT, Rahman MM, Banerjee M, Uddin MZ, Nahar N, Akhter M. Comparative efficacy of phenobarbitone versus levetiracetam in the initial treatment of neonatal seizure. Journal of Dhaka Medical College 2020;27(2):182-9. [DOI: 10.3329/jdmc.v27i2.45831] [URL: sciencegate.app/app/document/download#10.3329/jdmc.v27i2.45831] [DOI] [Google Scholar]
Painter 1999 {published data only}
- Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. New England Journal of Medicine 1999;341(7):485-9. [DOI: 10.1056/NEJM199908123410704] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatric Neurology 2003;28(4):277-80. [DOI: 10.1016/s0887-8994(02)00621-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pathak 2013 {published data only}
- Pathak G, Upadhyay A, Pathak U, Chawla D, Goel SP. Phenobarbitone versus phenytoin for treatment of neonatal seizures: an open-label randomized controlled trial. Indian Pediatrics 2013;50(8):753-7. [DOI: 10.1007/s13312-013-0218-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Perveen 2016 {published data only}
- Perveen S, Singh A, Upadhyay A, Singh N, Chauhan R. A randomized controlled trial on comparison of phenobarbitone and levetiracetam for the treatment of neonatal seizures: pilot study. International Journal of Research in Medical Sciences 2016;4(6):2073-8. [DOI: 10.18203/2320-6012.ijrms20161763] [URL: msjonline.org/index.php/ijrms/article/view/865/836] [DOI] [Google Scholar]
Prakash 2019 {published data only}
- Prakash A, Richa R, Sahni GS. Neonatal seizures – levetiracetam versus phenobarbital. Indian Journal of Child Health 2019;6(11):605-8. [DOI: 10.32677/IJCH.2019.v06.i11.008] [DOI] [Google Scholar]
Saxena 2016 {published data only}
- Saxena P, Singh A, Upadhyay A, Gupta P, Sharma S, Vishnubatla S. Effect of withholding phenobarbitone maintenance in neonatal seizures: a randomized controlled trial. Indian Pediatrics 2016;53(12):1069-73. [PMID: ] [PubMed] [Google Scholar]
Sharpe 2020 {published and unpublished data}
- Sharpe C, Reiner GE, Davis SL, Nespeca M, Gold JJ, Rasmussen M, et al, NEOLEV2 Investigators. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics 2020;145(6):e20193182. [DOI: 10.1542/peds.2019-3182] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solanki 2015 {published data only}
- Solanki DI, Gohil JR, Patel AP. Comparative efficacy of phenobarbital, phenytoin and lorazepam for the treatment of neonatal seizures: a randomized trial. Journal of Clinical Neonatololgy 2015;4(4):232-6. [DOI: ] [DOI] [Google Scholar]
Soul 2021 {published and unpublished data}
- Soul JS, Bergin AM, Stopp C, Hayes B, Singh A, Fortuno CR, et al, Boston Bumetanide Trial Group. A pilot randomized, controlled, double‐blind trial of bumetanide to treat neonatal seizures. Annals of Neurology 2021;89(2):327-40. [DOI: 10.1002/ana.25959] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasakumar 2015 {published data only}
- Srinivasakumar P, Zempel J, Trivedi S, Wallendorf M, Rao R, Smith B, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics 2015;136(5):e1302-9. [DOI: 10.1542/peds.2014-3777] [PMID: ] [DOI] [PubMed] [Google Scholar]
Susnerwala 2022 {published data only}
- Susnerwala S, Joshi A, Deshmukh L, Londhe A. Levetiracetam or phenobarbitone as a first-line anticonvulsant in asphyxiated term newborns? An open-label, single-center, randomized, controlled, pragmatic trial. Hospital Pediatrics 2022;12(7):647-52. [DOI: 10.1542/hpeds.2021-006415] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Rooji 2010 {published data only}
- Van Rooij LG, Toet MC, Van Huffelen AC, Groenendaal F, Laan W, Zecic A, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics 2010;125(2):e358-e66. [DOI: 10.1542/peds.2009-0136] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abend 2011 {published data only}
- Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. Journal of Child Neurology 2011;26(4):465-70. [DOI: 10.1177/0883073810384263] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arican 2020 {published data only}
- Arican P, Olgac Dundar N, Mete Atasever N, Akkaya Inal M, Gencpinar P, Cavusoglu D, et al. Comparison of the neurocognitive outcomes in term infants treated with levetiracetam and phenobarbital monotherapy for neonatal clinical seizures. Seizure 2020;80:71-4. [DOI: 10.1016/j.seizure.2020.06.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Castro Conde 2005 {published data only}
- Castro Conde JR, Hernández Borges AA, Doménech Martínez E, González Campo C, Perera Soler R. Midazolam in neonatal seizures with no response to phenobarbital. Neurology 2005;64(5):876-9. [DOI: 10.1212/01.WNL.0000152891.58694.71] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deshmukh 1986 {published data only}
- Deshmukh A, Wittert W, Schnitzler E, Mangurten HH. Lorazepam in the treatment of refractory neonatal seizures. A pilot study. American Journal of Diseases of Children 1986;140(10):1042-2. [DOI: 10.1001/archpedi.1986.02140240088032] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dwivedi 2019 {published data only}
- Dwivedi D, Lin N, Venkatesan C, Kline-Fath B, Holland K, Schapiro M. Clinical, neuroimaging, and electrographic predictors of phenobarbital failure in newborns with hypoxic ischemic encephalopathy and seizures. Journal of Child Neurology 2019;34(8):458-63. [DOI: 10.1177/0883073819838171] [PMID: ] [DOI] [PubMed] [Google Scholar]
Favié 2020 {published data only}
- Favié LM, Huitema AD, den Broek MP, Rademaker CM, Haan TR, Straaten HL, et al, PharmaCool study group*. Lidocaine as treatment for neonatal seizures: Evaluation of previously developed population pharmacokinetic models and dosing regimen. British Journal of Clinical Pharmacology 2020;86(1):75-84. [DOI: 10.1111/bcp.14136] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gal 1988 {published data only}
- Gal P, Oles KS, Gilman JT, Weaver R. Valproic acid efficacy, toxicity, and pharmacokinetics in neonates with intractable seizures. Neurology 1988;38(3):467-71. [DOI: 10.1212/wnl.38.3.467] [PMID: ] [DOI] [PubMed] [Google Scholar]
Glass 2021 {published data only}
- Glass HC, Soul JS, Chang T, Wusthoff CJ, Chu CJ, Massey SL, et al. Safety of early discontinuation of antiseizure medication after acute aymptomatic neonatal seizures. JAMA Neurology 2021;78(7):817-25. [DOI: 10.1001/jamaneurol.2021.1437] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2018 {published data only}
- Han JY, Moon CJ, Youn YA, Sung IK, Lee IG. Efficacy of levetiracetam for neonatal seizures in preterm infants. BMC Pediatrics 2018;18(1):131. [DOI: 10.1186/s12887-018-1103-1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hellström‐Westas 1988 {published data only}
- Hellström-Westas L, Westgren U, Rosén I, Svenningsen NW. Lidocaine for treatment of severe seizures in newborn infants.: I. Clinical effects and cerebral electrical activity monitoring. Acta Paediatrica 1988;77(1):79-84. [DOI: 10.1111/j.1651-2227.1988.tb10602.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hu 2003 {published data only}
- Hu KC, Chiu NC, Ho CS, Lee ST, Shen EY. Continuous midazolam infusion in the treatment of uncontrollable neonatal seizures. Acta Paediatrica Taiwanica 2003;44(5):279-81. [PMID: ] [PubMed] [Google Scholar]
Hunt 2021 {published data only}
- Hunt RW, Liley HG, Wagh D, Schembri R, Lee KJ, Shearman AD, et al, Newborn Electrographic Seizure Trial Investigators. Effect of treatment of clinical seizures vs electrographic seizures in full-term and near-term neonates: a randomized clinical trial. JAMA Network Open 2021;4(12):e2139604. [DOI: 10.1001/jamanetworkopen.2021.39604] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jawadekar 1992 {published data only}
- Jawadekar YM, Shah KN, Kshirsagar NA, Joshi MV, Pohujani SM. A study of phenobarbital and dilantin in neonatal seizures. Indian Journal of Pediatrics 1992;59(6):729-34. [DOI: 10.1007/BF02859409] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jayswal 2021 {published data only}
- Jayswal D, Roy UK, Ghosh T, Mandal P. Effectiveness and adverse drug reactions of levetiracetam and midazolam in refractory neonatal seizure: a cross‑sectional comparative study. Journal of Education and Health Promotion 2021;10:118. [DOI: 10.4103/jehp.jehp_937_20] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanmaz 2021 {published data only}
- Kanmaz S, Altun Köroğlu Ö, Terek D, Serin HM, Simsek E, Dokurel Cetin İ, et al. Efficacy of levetiracetam as first-line therapy for neonatal clinical seizures and neurodevelopmental outcome at 12 months of age. Acta Neurologica Belgica 2021;121(6):1495-503. [DOI: 10.1007/s13760-020-01366-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2020 {published data only}
- Liu BK, Jiang L, Li XJ, Hong SQ, Chen W, Hu Y. Efficacy and safety of levetiracetam in the off-label treatment of neonatal seizures. International Journal of Neuroscience 2020;130(4):336-42. [DOI: 10.1080/00207454.2019.1687469] [PMID: ] [DOI] [PubMed] [Google Scholar]
Low 2016 {published data only}
- Low E, Stevenson NJ, Mathieson SR, Livingstone V, Ryan AC, Rennie JM, et al. Short-term effects of phenobarbitone on electrographic seizures in neonates. Neonatology 2016;110(1):40-6. [DOI: 10.1159/000443782] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maitre 2013 {published data only}
- Maitre NL, Smolinsky C, Slaughter JC, Stark AR. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. Journal of Perinatology 2013;33(11):841-6. [DOI: 10.1038/jp.2013.116] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mollamohammadi 2018 {published data only}
- Mollamohammadi M, Amirhoseini ZS, Saadati A, Pirzadeh Z, Hassandvand Amouzadeh M. Oral levetiracetam as add-on therapy in refractory neonatal seizures. Iranian Journal of Child Neurology 2018;12(4):103-10. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Pressler 2015 {published data only}
- Pressler RM, Boylan GB, Marlow N, Blennow M, Chiron C, Cross JH, et al, NEonatal seizure treatment with Medication Off-patent (NEMO) consortium. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurology 2015;14(5):469-77. [DOI: 10.1016/S1474-4422(14)70303-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramantani 2011 {published data only}
- Ramantani G, Ikonomidou C, Walter B, Rating D, Dinger J. Levetiracetam: safety and efficacy in neonatal seizures. European Journal of Paediatric Neurology 2011;15(1):1-7. [DOI: 10.1016/j.ejpn.2010.10.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rao 2018 {published data only}
- Rao LM, Hussain SA, Zaki T, Cho A, Chanlaw T, Garg M, et al. A comparison of levetiracetam and phenobarbital for the treatment of neonatal seizures associated with hypoxic–ischemic encephalopathy. Epilepsy & Behavior 2018;88:212-7. [DOI: 10.1016/j.yebeh.2018.09.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rochefort 1989 {published data only}
- Rochefort MJ, Wilkinson AR. The safety and efficacy of alternative anticonvulsant regimes to control newborn seizures. Early Human Development 1989;19(3):218. [EMBASE: 10.1016/0378-3782(89)90090-X] [Google Scholar]
- Wilkinson AR, Rochefort MJ. Phenytoin reduces frequency and duration of neonatal seizures in the newborn: a randomised trial of four anticonvulsants. Pediatric Research 1989;26:522. [DOI: 10.1203/00006450-198911000-00138] [DOI] [Google Scholar]
Sedighi 2016 {published data only}
- Sedighi M, Asadi F, Moradian N, Vakiliamini M, Moradian M. Efficacy and safety of levetiracetam in the management of seizures in neonates. Neurosciences 2016;21(3):232-5. [DOI: 10.17712/nsj.2016.3.20150726] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shany 2007 {published data only}
- Shany E, Benzaqen O, Watemberg N. Comparison of continuous drip of midazolam or lidocaine in the treatment of intractable neonatal seizures. Journal of Child Neurology 2007;22(3):255-9. [DOI: 10.1177/0883073807299858] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thibault 2020 {published data only}
- Thibault C, Naim MY, Abend NS, Licht DJ, Gaynor JW, Xiao R, et al. A retrospective comparison of phenobarbital and levetiracetam for the treatment of seizures following cardiac surgery in neonates. Epilepsia 2020;61(4):627-35. [DOI: 10.1111/epi.16469] [PMID: ] [DOI] [PubMed] [Google Scholar]
Verwoerd 2022 {published data only}
- Verwoerd C, Limjoco J, Rajamanickam V, Knox A. Efficacy of levetiracetam and phenobarbital as first-line treatment for neonatal seizures. Journal of Child Neurology 2022;37(5):401-9. [DOI: 10.1177/08830738221086107] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wagner 2021 {published data only}
- Wagner CB, Kreimer AM, Carrillo NP, Autry E, Schadler A, Cook AM, et al. Levetiracetam compared to phenobarbital as a frst line therapy for neonatal seizures: an unexpected influence of benzodiazepines on seizure response. Journal of Pediatric Pharmacology and Therapeutics 2021;26(2):144-50. [DOI: 10.5863/1551-6776-26.2.144] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weeke 2016 {published data only}
- Weeke LC, Toet MC, Rooij LG, Groenendaal F, Boylan GB, Pressler RM, et al. Lidocaine response rate in aEEG-confirmed neonatal seizures: retrospective study of 413 full-term and preterm infants. Epilepsia 2016;57(2):233-42. [DOI: 10.1111/epi.13286] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yamamoto 2007 {published data only}
- Yamamoto H, Aihara M, Niijima S, Yamanouchi H. Treatments with midazolam and lidocaine for status epilepticus in neonates. Brain and Development 2007;29(9):559-64. [DOI: 10.1016/j.braindev.2007.02.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gyandeep 2023 {published data only}
- Gyandeep G, Behura SS, Sahu SK, Panda SK. Comparison between phenobarbitone and levetiracetam as the initial anticonvulsant in preterm neonatal seizures - a pilot randomized control trial in developing country setup. European Journal of Pediatrics 2023 May;182(5):2133-8. [DOI: 10.1007/s00431-023-04864-x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadi 2023 {published data only}
- Mohammadi M, Kadivar M, Sangsari R, Mirnia K, Saeedi M, Adhami P. Comparing the efficacy and safety of levetiracetam versus phenytoin for treating the acute phase of neonatal seizures. Iranian Journal of Child Neurology 2023;17(1):65-71. [DOI: 10.22037/ijcn.v17i1.36008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12622000470796 {published data only}
- ACTRN12622000470796. Efficacy and safety levetiracetam versus phenytoin for neonatal seizures. A randomized controlled trial [Efficacy and safety of levetricetam versus phenytoin for neonatal seizures: a randomized controlled trial]. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12622000470796 (first received 25 March 2022). [CENTRAL: CN-02408074]
CTRI/2013/01/003310 {published data only}Upadhyay
- CTRI/2013/01/003310. Comparison of levetiracetam with phenobarbitone in neonatal seizure. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2013/01/003310 (first received 21 January 2013). [CENTRAL: CN-01807519]
CTRI/2013/04/003585 {published data only}Rabindran
- CTRI/2013/04/003585. Levetiracetam for management of seizures in newborn. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2013/04/003585 (first received 26 April 2013). [CENTRAL: CN-01860078]
CTRI/2014/06/004659 {published data only}Bharadwaj
- CTRI/2014/06/004659. Does Levetiracetam reduce death/ control fits better than phenobarbitone in neonates. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2014/06/004659 (first received 9 June 2014). [CENTRAL: CN-01807076]
CTRI/2015/06/005849 {published data only}Kulkarni
- CTRI/2015/06/005849. To see if levetiracetam, a new seizure control medication is better over older medication phenobarbitone for immediate neonatal seizure control. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2015/06/005849 (first received 3 June 2015). [CENTRAL: CN-01884226]
CTRI/2016/10/007412 {published data only}Siddiqui
- CTRI/2016/10/007412. A clinical study to compare levetiracetam and phenobarbitone in newborns with birth asphyxia. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2016/10/007412 (first received 27 October 2016). [CENTRAL: CN-01819521]
CTRI/2018/04/013161 {published data only}Romana
- CTRI/2018/04/013161. Levetiracetam used as first line anti epileptic versus phenobarbitone in neonatal convulsions. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2018/04/013161 (first received 11 April 2018). [CENTRAL: CN-01903226]
CTRI/2020/03/023961 {published data only}Gandhi
- CTRI/2020/03/023961. Levetiracetam versus phenobarbitone for treatment of neonatal seizure. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2020/03/023961 (first received 13 March 2020). [CENTRAL: CN-02167335]
CTRI/2021/02/031290 {published data only}CTRI/2021/02/031290
- CTRI/2021/02/031290. Comparision between phenobarbitone and levetiracetam as the intial anti convulsant in treating preterm neonatal seizures. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/02/031290 (first received 15 February 2021). [CENTRAL: CN-02239413]
CTRI/2022/09/045658 {published data only}
- CTRI/2022/09/045658. To compare the effect of two anticonvulsant drugs levetiracetam and phenobarbitone in neonates with seizures [Efficacy of levetiracetam vs phenobarbitone in neonatal seizures: a randomized controlled trial ]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/09/045658 (first received 19 September 2022). [CENTRAL: CN-02473289]
CTRI/2023/02/049794 {published data only}
- CTRI/2023/02/049794. Study comparing efficacy of two drugs as first line drug in late preterm and term babies with neonatal seizure [Phenobarbitone versus levetiracetam as 1st line therapy for neonatal seizures- a randomized control trial]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/02/049794 (first received 16 February 2023). [CENTRAL: CN-02529443]
IRCT2014070318334N1 {published data only}Sidighi
- IRCT2014070318334N1. Study of levetiracetam effect in reduction of seizure frecuency in neonats with seizure. irct.ir/trial/16656 (first received 26 August 2014).
IRCT20160523028008N23 {published data only}
- IRCT20160523028008N23. The effect of levetiracetam and phenobarbital on the control of neonatal seizures [Comparison of efficacy and safety of levetiracetam and phenobarbital in controlling neonatal seizures]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20160523028008N23 (first received 13 June 2022). [CENTRAL: CN-02429576]
IRCT20190526043717N1 {published data only}Sadati
- IRCT20190526043717N1. Comparison of intravenous levetiracetam and phenobarbital in neonatal seizures. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20190526043717N1 (first received 31 May 2019). [CENTRAL: CN-01975695]
IRCT20200115046137N1 {published data only}Sadeghvand
- IRCT20200115046137N1. The effect of phenobarbital, topiramate, and levothiracetam on neonatal seizures. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200115046137N1 (first received 5 August 2020). [CENTRAL: CN-02187707]
IRCT20200131046317N3 {published data only}2021
- IRCT20200131046317N3. Comparison of the effects of phenobarbital and levetiracetam on neonatal seizure after discharge. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200131046317N3 (first received 25 August 2021). [CENTRAL: CN-02329536]
IRCT20200528047589N1 {published data only}Kalanimoghadam
- IRCT20200528047589N1. Comparison of effects of phenobarbital and levetiracetam in neonatal seizure control. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200528047589N1 (first received 8 September 2020). [CENTRAL: CN-02187848]
IRCT20220619055221N1 {published data only}
- IRCT20220619055221N1. Efficacy of levetiracetam compared to intravenous phenytoin in treatment of acute phase of neonatal seizure. irct.ir/trial/64286 (first received 09 November 2022).
NCT01089504 {published data only}Guillet
- NCT01089504. Prophylactic phenobarbital after neonatal seizures. clinicaltrials.gov/ct2/show/NCT01089504 (first received 18 March 2010). [CENTRAL: CN-01528653]
NCT02550028 {published data only}Zhou
- NCT02550028. Levetiracetam treatment of neonatal seizures. clinicaltrials.gov/ct2/show/NCT02550028 (first received 15 September 2015). [CENTRAL: CN-01580562]
NCT03107507 {published data only}Shaheen
- NCT03107507. Efficacy of levetiracetam in control of neonatal seizures guided by an EEG. clinicaltrials.gov/ct2/show/NCT03107507 (first received 11 April 2017). [CENTRAL: CN-01597110]
NCT04320940 {published data only}
- NCT04320940. Efficacy and safety of intravenous phenobarbital in neonatal seizures. clinicaltrials.gov/ct2/show/NCT04320940 (first received 25 March 2020). [CENTRAL: CN-02089336]
NCT05291455 {published data only}Salamah
- NCT05291455. Lacosamide in neonatal status epilepticus. clinicaltrials.gov/ct2/show/NCT05291455 (first received 22 March 2022). [CENTRAL: CN-02385527]
Additional references
Abend 2011
- Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. Journal of Child Neurology 2011;26(4):465-70. [DOI: 10.1177/0883073810384263] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abou‐Khalil 2008
- Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatric Disease and Treatment 2008;4(3):507-23. [DOI: 10.2147/ndt.s2937] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bittigau 2002
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proceedings of the National Academy of Sciences of the United States of America 2002;99(23):15089-94. [DOI: 10.1073/pnas.222550499] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Booth 2004
- Booth D, Evans DJ. Anticonvulsants for neonates with seizures. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD004218. [DOI: 10.1002/14651858.CD004218.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boylan 2002
- Boylan GB, Rennie JM, Pressler RM, Wilson G, Morton M, Binnie CD. Phenobarbitone, neonatal seizures, and video-EEG. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(3):F165-170. [DOI: 10.1136/fn.86.3.f165] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boylan 2004
- Boylan GB, Rennie JM, Chorley G, Pressler RM, Fox GF, Farrer K, et al. Second-line anticonvulsant treatment of neonatal seizures: a video-EEG monitoring study. Neurology 2004;62(3):486-8. [DOI: 10.1212/01.wnl.0000106944.59990.e6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boylan 2013
- Boylan GB, Stevenson NJ, Vanhatalo S. Monitoring neonatal seizures. Seminars in Fetal & Neonatal Medicine 2013;18(4):202-8. [DOI: 10.1016/j.siny.2013.04.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brodie 1996
- Brodie MJ, Dichter MA. Antiepileptic drugs. New England Journal of Medicine 1996;334(3):168-75. [DOI: 10.1056/NEJM199601183340308] [PMID: ] [DOI] [PubMed] [Google Scholar]
Buraniqi 2017
- Buraniqi E, Sansevere AJ, Kapur K, Bergin AM, Pearl PL, Loddenkemper T. Electrographic seizures in preterm neonates in the neonatal intensive care unit. Journal of Child Neurology 2017;32(10):880-5. [DOI: 10.1177/0883073817713918] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cha 2002
- Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Research 2002;51(3):217-32. [DOI: 10.1016/s0920-1211(02)00157-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clancy 1996
- Clancy RR. The contribution of EEG to the understanding of neonatal seizures. Epilepsia 1996;37(Suppl 1):S52-9. [DOI: 10.1111/j.1528-1157.1996.tb06022.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cleary 2013
- Cleary RT, Sun H, Huynh T, Manning SM, Li Y, Rotenberg A, et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PloS One 2013;8(3):e57148. [DOI: 10.1371/journal.pone.0057148] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clozel 1985
- Clozel M, Daval JL, Monin P, Dubruc C, Morselli PL, Vert P. Regional cerebral blood flow during bicuculline-induced seizures in the newborn piglet: effect of phenobarbital. Developmental Pharmacology and Therapeutics 1985;8(3):189-99. [DOI: 10.1159/000457036] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dulac 2013
- Dulac O, Milh M, Holmes GL. Brain maturation and epilepsy. Handbook of Clinical Neurology 2013;111:441-6. [DOI: 10.1016/B978-0-444-52891-9.00047-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dzhala 2003
- Dzhala VI, Staley KJ. Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus. Journal of Neuroscience: The Official Journal of the Society for Neuroscience 2003;23(5):1840-6. [DOI: 10.1523/JNEUROSCI.23-05-01840.2003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dzhala 2005
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nature Medicine 2005;11(11):1205-13. [DOI: 10.1038/nm1301] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dzhala 2008
- Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Annals of Neurology 2008;63(2):222-35. [DOI: 10.1002/ana.21229] [PMID: ] [DOI] [PubMed] [Google Scholar]
El‐Dib 2017
- El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Seminars in Fetal & Neonatal Medicine 2017;22(5):321-7. [DOI: 10.1016/j.siny.2017.07.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Falsaperla 2021
- Falsaperla R, Scalia B, Giugno A, Pavone P, Motta M, Caccamo M, et al. Treating the symptom or treating the disease in neonatal seizures: a systematic review of the literature. Italian Journal of Pediatrics 2021;47(1):85. [DOI: 10.1186/s13052-021-01027-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2005
- Fisher RS, Emde BW, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46(4):470-2. [DOI: 10.1111/j.0013-9580.2005.66104.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fujikawa 1988
- Fujikawa DG, Vannucci RC, Dwyer BE, Wasterlain CG. Generalized seizures deplete brain energy reserves in normoxemic newborn monkeys. Brain Research 1988;454(1-2):51-9. [DOI: 10.1016/0006-8993(88)90802-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fürwentsches 2010
- Fürwentsches A, Bussmann C, Ramantani G, Ebinger F, Philippi H, Pöschl J, et al. Levetiracetam in the treatment of neonatal seizures: a pilot study. Seizure 2010;19(3):185-9. [DOI: 10.1016/j.seizure.2010.01.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gidal 1999
- Gidal BE, Privitera MD, Sheth RD, Gilman JT. Vigabatrin: a novel therapy for seizure disorders. Annals of Pharmacotherapy 1999;33(12):1277-86. [DOI: 10.1345/aph.18376] [PMID: ] [DOI] [PubMed] [Google Scholar]
Glass 2011
- Glass HC, Poulin C, Shevell MI. Topiramate for the treatment of neonatal seizures. Pediatric Neurology 2011;44(6):439-42. [DOI: 10.1016/j.pediatrneurol.2011.01.006] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glass 2013
- Glass HC, Wusthoff CJ, Shellhaas RA. Amplitude-integrated electro-encephalography: the child neurologist's perspective. Journal of Child Neurology 2013;28(10):1342-50. [DOI: 10.1177/0883073813488663] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 3 May 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hahn 2004
- Hahn CD, Riviello JJ. Neonatal seizures and EEG: electroclinical dissociation and uncoupling. NeoReviews 2004;5(8):e350-e5. [DOI: 10.1542/neo.5-8-e350] [DOI] [Google Scholar]
Hellström‐Westas 2015
- Hellström-Westas L, Boylan G, Ågren J. Systematic review of neonatal seizure management strategies provides guidance on anti-epileptic treatment. Acta Paediatrica 2015;104(2):123-9. [DOI: 10.1111/apa.12812] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heneghan 2017
- Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017;18(1):122. [DOI: 10.1186/s13063-017-1870-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hooper 2021
- Hooper RG, Ramaswamy VV, Wahid RM, Satodia P, Bhulani A. Levetiracetam as the first-line treatment for neonatal seizures: a systematic review and meta-analysis. Developmental Medicine and Child Neurology 2021;63(11):1283-93. [DOI: 10.1111/dmcn.14943] [PMID: ] [DOI] [PubMed] [Google Scholar]
Huttenlocher 1982
- Huttenlocher PR, Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex--evidence for synapse elimination during normal development. Neuroscience Letters 1982;33(3):247-52. [DOI: 10.1016/0304-3940(82)90379-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ikonomidou 2010
- Ikonomidou C, Turski L. Antiepileptic drugs and brain development. Epilepsy Research 2010;88(1):11-22. [DOI: 10.1016/j.eplepsyres.2009.09.019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jensen 2009
- Jensen FE. Neonatal seizures: an update on mechanisms and management. Clinics in Perinatology 2009;36(4):881-900, vii. [DOI: 10.1016/j.clp.2009.08.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahle 2009
- Kahle KT, Barnett SM, Sassower KC, SJ. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na(+)-K(+)-2Cl(-) cotransporter NKCC1. Journal of Child Neurology 2009;24(5):572-6. [DOI: 10.1177/0883073809333526] [PMID: ] [DOI] [PubMed] [Google Scholar]
Khazipov 2004
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. European Journal of Neuroscience 2004;19(3):590-600. [DOI: 10.1111/j.0953-816x.2003.03152.xAbstract] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kilicdag 2013
- Kilicdag H, Daglıoglu K, Erdogan S, Guzel A, Sencar L, Polat S, et al. The effect of levetiracetam on neuronal apoptosis in neonatal rat model of hypoxic ischemic brain injury. Early Human Development 2013;89(5):355-60. [DOI: 10.1016/j.earlhumdev.2012.12.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim J-S, Kondratyev A, Tomita Y, Gale K. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia 2007;48(Suppl 5):19-26. [DOI: 10.1111/j.1528-1167.2007.01285.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2021
- Kumar J, Meena J, Yadav J, Saini L. Efficacy and safety of phenobarbitone as first-line treatment for neonatal seizure: a systematic review and meta-analysis. Journal of Tropical Pediatrics 2021;67(1):fmab008. [DOI: 10.1093/tropej/fmab008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lanska 1995
- Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ. A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology 1995;45(4):724-32. [DOI: 10.1212/wnl.45.4.724] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2004
- Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke 2004;35(6):1460-5. [DOI: 10.1161/01.STR.0000128029.50221.fa] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liu 2012
- Liu Y, Shangguan Y, Barks JD, Silverstein FS. Bumetanide augments the neuroprotective efficacy of phenobarbital plus hypothermia in a neonatal hypoxia-ischemia model. Pediatric Research 2012;71(5):559-65. [DOI: 10.1038/pr.2012.7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malone 2009
- Malone A, Ryan CA, Fitzgerald A, Burgoyne L, Connolly S, Boylan GB. Interobserver agreement in neonatal seizure identification. Epilepsia 2009;50(9):2097-101. [DOI: 10.1111/j.1528-1167.2009.02132.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manthey 2005
- Manthey D, Asimiadou S, Stefovska V, Kaindl AM, Fassbender J, Ikonomidou C, et al. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Experimental Neurology 2005;193(2):497-503. [DOI: 10.1016/j.expneurol.2005.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr A, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner's guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCoy 2013
- McCoy B, Hahn CD. Continuous EEG monitoring in the neonatal intensive care unit. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society 2013;30(2):106-14. [DOI: 10.1097/WNP.0b013e3182872919] [PMID: ] [DOI] [PubMed] [Google Scholar]
McDonald 1990
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Research. Brain Research Reviews 1990;15(1):41-70. [DOI: 10.1016/0165-0173(90)90011-c] [PMID: ] [DOI] [PubMed] [Google Scholar]
McHugh 2018
- McHugh DC, Lancaster S, Manganas LN. A systematic review of the efficacy of levetiracetam in neonatal seizures. Neuropediatrics 2018;49(1):12-7. [DOI: 10.1055/s-0037-1608653] [PMID: ] [DOI] [PubMed] [Google Scholar]
Meldrum 1996
- Meldrum BS. Update on the mechanism of action of antiepileptic drugs. Epilepsia 1996;37(Suppl 6):S4-11. [DOI: 10.1111/j.1528-1157.1996.tb06038.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murray 2008
- Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(3):F187-91. [DOI: 10.1136/adc.2005.086314] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nash 2011
- Nash KB, Bonifacio SL, Glass HC, Sullivan JE, Barkovich AJ, Ferriero DM, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology 2011;76(6):556-62. [DOI: 10.1212/WNL.0b013e31820af91a] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr A, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomized controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Painter 1999
- Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. New England Journal of Medicine 1999;341(7):485-9. [DOI: 10.1056/NEJM199908123410704] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pathak 2013
- Pathak G, Upadhyay A, Pathak U, Chawla D, Goel SP. Phenobarbitone versus phenytoin for treatment of neonatal seizures: an open-label randomized controlled trial. Indian Pediatrics 2013;50(8):753-7. [DOI: 10.1007/s13312-013-0218-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pavel 2020
- Pavel AM, Rennie JM, Vries LS, Blennow M, Foran A, Shah DK, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet. Child & Adolescent Health 2020;4(10):740-9. [DOI: 10.1016/S2352-4642(20)30239-X] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellegrin 2019
- Pellegrin S, Munoz FM, Padula M, Heath PT, Meller L, Top K, et al, Brighton Collaboration Neonatal Seizures Working Group. Neonatal seizures: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2019;37(52):7596-609. [DOI: 10.1016/j.vaccine.2019.05.031] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisani 2012
- Pisani F, Piccolo B, Cantalupo G, Copioli C, Fusco C, Pelosi A, et al. Neonatal seizures and postneonatal epilepsy: a 7-y follow-up study. Pediatric Research 2012;72(2):186-93. [DOI: 10.1038/pr.2012.66] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pressler 2015
- Pressler RM, Boylan GB, Marlow N, Blennow M, Chiron C, Cross JH, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet. Neurology 2015;14(5):469-77. [DOI: 10.1016/S1474-4422(14)70303-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pressler 2021
- Pressler RM, Cilio MR, Mizrahi EM, Moshé SL, Nunes ML, Plouin P, et al. The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia 2021;62(3):615-28. [DOI: 10.1111/epi.16815] [PMID: ] [DOI] [PubMed] [Google Scholar]
Qiao 2021
- Qiao MY, Cui HT, Zhao LZ, Miao JK, Chen QX. Efficacy and safety of levetiracetam vs. phenobarbital for neonatal seizures: a systematic review and meta-analysis. Frontiers in Neurology 2021;12:747745. [DOI: 10.3389/fneur.2021.747745] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2018
- Rao LM, Hussain SA, Zaki T, Cho A, Chanlaw T, Garg M, et al. A comparison of levetiracetam and phenobarbital for the treatment of neonatal seizures associated with hypoxic-ischemic encephalopathy. Epilepsy & Behavior 2018;88:212-7. [DOI: 10.1016/j.yebeh.2018.09.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan Web 2023 [Computer program]
- Review Manager Web (RevMan Web). Version 6.3.0. The Cochrane Collaboration, 2023. Available at revman.cochrane.org.
Ronen 1999
- Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. Journal of Pediatrics 1999;134(1):71-5. [DOI: 10.1016/s0022-3476(99)70374-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ronen 2007
- Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis inchildren with neonatal seizures: a population-based study. Neurology 2007;69(19):1816-22. [DOI: 10.1212/01.wnl.0000279335.85797.2c] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saliba 1999
- Saliba RM, Annegers JF, Waller DK, Tyson JE, Mizrahi EM. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. American Journal of Epidemiology 1999;150(7):763-9. [DOI: 10.1093/oxfordjournals.aje.a010079] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saxena 2016
- Saxena P, Singh A, Upadhyay A, Gupta P, Sharma S, Vishnubatla S. Effect of withholding phenobarbitone maintenance in neonatal seizures: a randomized controlled trial. Indian Pediatrics 2016;53(12):1069-73. [PMID: ] [PubMed] [Google Scholar]
Scher 1993
- Scher MS, Hamid MY, Steppe DA, Beggarly ME, Painter MJ. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia 1993;34(2):284-8. [DOI: 10.1111/j.1528-1157.1993.tb02412.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Scher 2003
- Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatric Neurology 2003;28(4):277-80. [DOI: 10.1016/s0887-8994(02)00621-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sharma 2022
- Sharma D, Hussain AM, Sharma SS. Efficacy of Levetiracetam in neonatal seizures: a systematic review. Journal of Maternal-Fetal & Neonatal Medicine 2022;35(20):3923-30. [DOI: 10.1080/14767058.2020.1844651] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharpe 2020
- Sharpe C, Reiner GE, Davis SL, Nespeca M, Gold JJ, Rasmussen M, et al, NEOLEV2 INVESTIGATORS. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics 2020;145(6):e20193182. [DOI: 10.1542/peds.2019-3182] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shellhaas 2017
- Shellhaas RA, Wusthoff CJ, Tsuchida TN, Glass HC, Chu CJ, Massey SL, et al, Neonatal Seizure Registry. Profile of neonatal epilepsies: characteristics of a prospective US cohort. Neurology 2017;89(9):893-9. [DOI: 10.1212/WNL.0000000000004284] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slaughter 2013
- Slaughter LA, Patel AD, Slaughter JL. Pharmacological treatment of neonatal seizures: a systematic review. Journal of Child Neurology 2013;28(3):351-64. [DOI: 10.1177/0883073812470734] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sortino 2022
- Sortino V, Praticò A, Marino S, Criscione R, Ruggieri M, Pisani F, et al. Efficacy of the anti-seizure medications in acute symptomatic neonatal seizures caused by stroke. A systematic review. Acta Bio-Medica 2022;93(6):e2022328. [DOI: 10.23750/abm.v93i6.13440] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soul 2019
- Soul JS, Pressler R, Allen M, Boylan G, Rabe H, Portman R, et al, International Neonatal Consortium. Recommendations for the design of therapeutic trials for neonatal seizures. Pediatric Research 2019;85(7):943-54. [DOI: 10.1038/s41390-018-0242-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srinivasakumar 2015
- Srinivasakumar P, Zempel J, Trivedi S, Wallendorf MI, Rao R, Smith B, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics 2015;136(5):e1302-9. [DOI: 10.1542/peds.2014-3777] [PMID: ] [DOI] [PubMed] [Google Scholar]
Takashima 1980
- Takashima S, Chan F, Becker LE, Armstrong DL. Morphology of the developing visual cortex of the human infant: a quantitative and qualitative Golgi study. Journal of Neuropathology and Experimental Neurology 1980;39(4):487-501. [DOI: 10.1097/00005072-198007000-00007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Talos 2013
- Talos DM, Chang M, Kosaras B, Fitzgerald E, Murphy A, Folkerth RD, et al. Antiepileptic effects of levetiracetam in a rodent neonatal seizure model. Pediatric Research 2013;73(1):24-30. [DOI: 10.1038/pr.2012.151] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 1995
- Taylor CP, Meldrum BS. Na+ channels as targets for neuroprotective drugs. Trends in Pharmacological Sciences 1995;16(9):309-16. [DOI: 10.1016/s0165-6147(00)89060-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tekgul 2006
- Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics 2006;117(4):1270-80. [DOI: 10.1542/peds.2005-1178] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2021
- Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for Cochrane reviews. Journal of Clinical Epidemiology 2021;133:140-51. [DOI: 10.1016/j.jclinepi.2020.11.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuchida 2013
- Tsuchida TN, Wusthoff CJ, Shellhaas RA, Hahn CD, Sullivan JE, Nguyen S, et al, American Clinical Neurophysiology Society Critical Care Monitoring Committee. American Clinical Neurophysiology Society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society. 2013;30(2):161-73. [DOI: 10.1097/WNP.0b013e3182872b24] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Rooij 2010
- Van Rooij LG, Toet MC, Van Huffelen AC, Groenendaal F, Laan W, Zecic A, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics 2010;125(2):e358-66. [DOI: 10.1542/peds.2009-0136] [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Rooij 2013
- Van Rooij LG, Hellström-Westas L, De Vries LS. Treatment of neonatal seizures. Seminars in Fetal & Neonatal Medicine 2013;18(4):209-15. [DOI: 10.1016/j.siny.2013.01.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vasudevan 2013
- Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Seminars in Fetal & Neonatal Medicine 2013;18(4):185-91. [DOI: 10.1016/j.siny.2013.05.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2011
- WHO. Guidelines on neonatal seizures. who.int/bitstream/handle/10665/77756/9789241548304_eng.pdf 2011. [AVAILABLE FROM: apps.who.int/iris/bitstream/handle/10665/77756/9789241548304_eng.pdf;sequence=1]
Wirrell 2005
- Wirrell EC. Neonatal seizures: to treat or not to treat? Seminars in Pediatric Neurology 2005;12(2):97-105. [DOI: 10.1016/j.spen.2005.03.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wusthoff 2013
- Wusthoff CJ. Diagnosing neonatal seizures and status epilepticus. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society 2013;30(2):115-21. [DOI: 10.1097/WNP.0b013e3182872932] [PMID: ] [DOI] [PubMed] [Google Scholar]
Xu 2021
- Xu ZE, Li WB, Qiao MY, Cui HT, Zhao LZ, Chen QX, et al. Comparative efficacy of anti-epileptic drugs for neonatal seizures: a network meta-analysis. Pediatrics and Neonatology 2021;62(6):598-605. [DOI: 10.1016/j.pedneo.2021.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yager 2002
- Yager JY, Armstrong EA, Miyashita H, Wirrell EC. Prolonged neonatal seizures exacerbate hypoxic-ischemic brain damage: correlation with cerebral energy metabolism and excitatory amino acid release. Developmental Neuroscience 2002;24(5):367-81. [DOI: 10.1159/000069049] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yildiz 2012
- Yildiz EP, Tatli B, Ekici B, Eraslan E, Aydinli N, Caliskan M, et al. Evaluation of etiologic and prognostic factors in neonatal convulsions. Pediatric Neurology 2012;47(3):186-92. [DOI: 10.1016/j.pediatrneurol.2012.05.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Younkin 1986
- Younkin DP, Delivoria-Papadopoulos M, Maris J, Donlon E, Clancy R, Chance B. Cerebral metabolic effects of neonatal seizures measured with in vivo 31P NMR spectroscopy. Annals of Neurology 1986;20(4):513-9. [DOI: 10.1002/ana.410200412] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yozawitz 2017
- Yozawitz E, Stacey A, Pressler RM. Pharmacotherapy for seizures in neonates with hypoxic ischemic encephalopathy. Pediatric Drugs 2017;19(6):553-67. [DOI: 10.1007/s40272-017-0250-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abiramalatha 2022
- Abiramalatha T, Thanigainathan S, Ramaswamy VV, Pressler R, Brigo F, Hartmann H. Anti-seizure medications for neonates with seizuresws. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD014967. [DOI: 10.1002/14651858.CD014967] [DOI] [PMC free article] [PubMed] [Google Scholar]


