Graphical abstract

Keywords: Ultrasonic assisted extraction, Extract, Bioactive components, Equipment, Combined technologies
Highlights
-
•
The research progress of UAE for bioactive components was overviewed.
-
•
The application of ultrasonic in extracting bioactive components had great potential.
-
•
Ultrasonic enhances extraction speed and the extracts with better quality and yield.
-
•
Ultrasonic ruptures cell walls, aiding content release and improving mass transfer.
Abstract
The increasing focus on health and well-being has sparked a rising interest in bioactive components in the food, pharmaceutical, and nutraceutical industries. These components are gaining popularity due to their potential benefits for overall health. The growing interest has resulted in a continuous rise in demand for bioactive components, leading to the exploration of both edible and non-edible sources to obtain these valuable substances. Traditional extraction methods like solvent extraction, distillation, and pressing have certain drawbacks, including lower extraction efficiency, reduced yield, and the use of significant amounts of solvents or resources. Furthermore, certain extraction methods necessitate high temperatures, which can adversely affect certain bioactive components. Consequently, researchers are exploring non-thermal technologies to develop environmentally friendly and efficient extraction methods.
Ultrasonic-assisted extraction (UAE) is recognized as an environmentally friendly and highly efficient extraction technology. The UAE has the potential to minimize or eliminate the need for organic solvents, thereby reducing its impact on the environment. Additionally, UAE has been found to significantly enhance the production of target bioactive components, making it an attractive method in the industry. The emergence of ultrasonic assisted extraction equipment (UAEE) has presented novel opportunities for research in chemistry, biology, pharmaceuticals, food, and other related fields. However, there is still a need for further investigation into the main components and working modes of UAEE, as current understanding in this area remains limited. Therefore, additional research and exploration are necessary to enhance our knowledge and optimize the application of UAEE. The core aim of this review is to gain a comprehensive understanding of the principles, benefits and impact on bioactive components of UAE, explore the different types of equipment used in this technique, examine the various working modes and control parameters employed in UAE, and provide a detailed overview of the blending of UAE with other emerging extraction technologies. In conclusion, the future development of UAEE is envisioned to focus on achieving increased efficiency, reduced costs, enhanced safety, and improved reliability. These key areas of advancement aim to optimize the performance and practicality of UAEE, making it a more efficient, cost-effective, and reliable extraction technology.
1. Introduction
Plant active components refer to a wide range of substances that constitute the plant body, including essential components like water, sugars, proteins, fats, and other compounds. These components also include secondary metabolites such as terpenes, flavonoids, alkaloids, steroids, lignin, and minerals. The majority of plant active components are primarily located within the vacuole of cell protoplasm. When a solvent enters the cell tissue, it dissolves these active components, which then transform into a leaching solution [1]. Bioactive components found in plants possess significant physiological activity and economic value [2], [3]. As a result, the extraction of these bioactive components from plants holds immense importance, particularly in industries such as bioengineering, pharmaceutical engineering, and food processing. Extraction serves as a pivotal unit in the production and processing of these industries. The extraction technology employed has a direct impact on the consumption of materials and energy during production, as well as the quality and quantity of the final product. Hence, selecting and optimizing the extraction process is crucial to achieving efficient and high-quality extraction of bioactive components from plants [4].
Traditional thermal extraction processes such as Soxhlet extraction, water distillation extraction, steam distillation extraction has been used to extract bioactive compounds from plants. However, traditional thermal extraction methods have drawbacks such as high energy consumption, loss of volatile components, difficulty in controlling extraction efficiency, disadvantage for thermosensitive substances, dependence on the use of large amounts of solvents, and long processing cycles. Thermal processing also causes changes in various physical and chemical parameters, and also reduces the activity of such biologically active substances. In recent years, there has been a surge in the development of emerging extraction technologies, such as ultrasonic assisted extraction (UAE), microwave assisted extraction (MAE), and supercritical fluid extraction (SFE). These cutting-edge techniques aim to address the limitations of traditional methods by offering advantages like reduced extraction time, lower solvent consumption, improved extraction rates, and enhanced extract quality. By efficiently, economically, and environmentally friendly extracting bioactive components from the complex matrix of plants, these innovative extraction technologies have revolutionized the field of extraction [5]. In recent years, green extraction has emerged as a prominent research direction in the field of extraction technology. It focuses on developing environmentally friendly solvent extraction methods that can effectively reduce consumption, minimize energy waste, mitigate environmental pollution, and simultaneously enhance extraction yield [6]. Ultrasonic technology plays a pivotal role in achieving the principles of green chemistry and extraction objectives [7]. UAE enables the efficient extraction of targeted bioactive components by leveraging its distinct cavitation effect. This innovative technique not only reduces the extraction time but also enhances the quality and yield of the extract. The UAE exhibits exceptional advantages when extracting bioactive components from plants. Complete extraction with higher purity of the final product can be achieved by ultrasonication in a few minutes with high reproducibility, thus reducing the use of organic solvents. By employing the UAE, researchers can achieve the dual goals of efficient extraction and sustainable practices in a more environmentally friendly manner [8], [9], [10], [11], [12]. Furthermore, research findings have demonstrated that when employing optimized UAE conditions, it is possible to preserve the biological characteristics of the extract. This is a significant advantage, as it ensures that the extract retains its desired biological activity and functionality, thereby enhancing its potential applications in various fields [13]. Consequently, when compared to traditional extraction technologies, UAE emerges as an economical and highly efficient extraction technique. Ultrasonic technique is a proven green bio-refining technology, primarily due to its ability to lower the solvent consumption costs, reduce operation time, lower energy consumption and produce higher yields. These advantages directly address the rising market demand for plant bioactive components. Most current studies mainly introduce the principles of UAE and the factors that affect the extraction results. While most existing studies primarily focus on elucidating the principles of UAE and factors influencing extraction outcomes, they collectively highlight the immense potential of UAE as a transformative extraction technology for meeting the evolving needs of industries reliant on plant-derived bioactive components. Nevertheless, despite the extensive research on the principles and influencing factors of UAE, the available information regarding the commonly used types, working principles, and working modes of UAE is still limited. As a result, this study aims to provide a comprehensive overview of the UAE, not only offering a detailed summary of its principles and influential factors but also presenting an in-depth introduction to the various types, working principles, and working modes of the UAE. It also provides research on the application of hyphenated technology which combines UAE with other processing technologies in the food industry.
2. Ultrasonic assisted extraction technology
Sound waves occur when there is a variation in stress, particle displacement, and particle velocity within an elastic medium. When the frequency of these waves reaches the ultrasonic range, they are referred to as ultrasonic waves. Unlike electromagnetic waves, ultrasonic waves are mechanical in nature and can propagate through solid, liquid, and gaseous mediums [14]. Ultrasonic technology has found application across a wide range of fields. It has proven to be valuable in diverse areas such as medical testing, non-destructive testing, distance measurement, welding, cleaning, and food technology, among others [15]. The applications of ultrasonic technology are typically categorized into two distinct groups: low-intensity ultrasonics and high-intensity ultrasonics [16]. Low intensity ultrasonic is distinguished by its high frequency ranging from 5 to 10 MHz, along with a relatively low power level of less than 1 W/cm2. It is non-destructive and is well-suited for the testing and characterization of various stuffs, hence low intensity ultrasonic is also known as diagnostic ultrasonic [14]. High intensity ultrasonic technology, often referred to as power ultrasonics, operates at low frequencies ranging from 20 to 100 kHz and substantial power levels ranging from 10 to 1000 W/cm2. Unlike low intensity ultrasonics, high intensity ultrasonics are characterized by their destructive capabilities. It is an effective method to accelerate the chemical reaction by cavitation. The implosion of cavitation bubbles releases energy that can lead to various effects such as extraction, crushing, and emulsification [17].
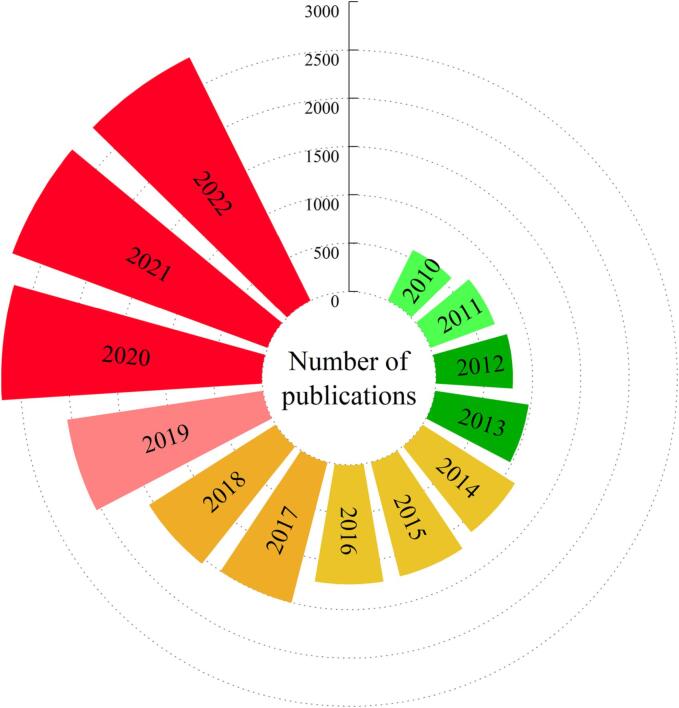
Fig. 1 shows the evolution of publications about UAE since 2010. On the whole, researchers' attention to UAE has increased dramatically over the past few decades.
Fig. 1.
Evolution of publications about UAE since 2010 (The search terms used for this chart were ultrasonic extraction or ultrasound extraction, and the database is WEB OF SCIENCE).
2.1. The principle of UAE
UAE belongs to power ultrasonic. Power ultrasonic is a branch of ultrasonology that focuses on the use of ultrasonic energy to process matter. UAE uses the thermal effect, the mechanical effect and the cavitation effect to extract bioactive components. During the action of ultrasonic, the cell wall is destroyed, while accelerating the release and diffusion of components in the cell [7]. In recent years, researchers have proposed several ultrasonic mechanisms of UAE. Several physical mechanisms, including fragmentation, erosion, the sonocapillary effect, sonoporation, local shear stress, and detexturation, have been identified and extensively discussed in numerous review articles [7], [18], [19].
The thermal effect of ultrasonic refers to the phenomenon where the vibration energy of ultrasonic is absorbed by the medium and converted into heat. Consequently, the heat of the medium increases accordingly. The calorific value is dependent on factors such as the properties of the medium, ultrasonic power, and exposure time [20]. The mechanical effect of ultrasonic refers to the phenomenon where the application of ultrasonic to a medium induces the vibration of particles within the medium in accordance with the mechanical wave. As a result, the motion of particles is enhanced, leading to an accelerated process of mass transfer [1]. Among the various ultrasonic effects, the cavitation effect is considered to be the most predominant [21]. Ultrasonic cavitation means that under the influence of the ultrasonic, the minuscule bubbles (cavitation nuclei) within the liquid undergo vibrations, expansion, and ongoing accumulation of energy from the acoustic field. And once the energy surpasses a specific threshold, the cavitation bubble collapses and closes sharply. There are generally two classifications of cavitation bubbles: transient cavitation bubble and stable cavitation bubble. The stable cavitation bubble has a relatively long life while the transient cavitation bubble exists for a very short time. When the cavitation bubble collapses sharply, it releases significant energy, creating a microjet that reaches speeds of about 200–700 m/s [166]. And this microjet possesses a substantial impact force. At the instant of sharp collapse, the cavitation bubble generates localized high temperature and high pressure (5000 K, 2000 atm), accompanied by an impressive cooling rate of up to 109 K/s [1]. During cavitation, intense hydrodynamic shear forces are present, which can result in the formation of free radicals and an increase in pressure and temperature [22]. Fig. 2 depicts the cavitation effect of ultrasonic.
Fig. 2.
The cavitation effect of ultrasonic.
The UAE applies the thermal effect, the mechanical effect and the cavitation effect produced by ultrasonic to the extraction process. As shown in Fig. 3, ultrasonic can enhance the penetration of solvents into cells, leading to improved mass transfer. Additionally, ultrasonic can disrupt cell walls, facilitating the release of cellular contents [23], [24], [25], [26]. Therefore, effective cell fragmentation and effective mass transfer are considered to be the two factors leading to the increase of production after employing UAE [27], [28]. In addition, of greater importance is the effect of ultrasound on changes in the intracellular matrix, as it has the potential to induce changes in the internal structure of the matrix [7]. While many laboratory scale studies attribute the increase in production to factors such as efficient cell fragmentation and enhanced mass transfer through UAE, another study presents a different perspective. This study suggests that the primary reason for the production increase after UAE is the enhanced solubility of substances, rather than the observed cell fragmentation [29]. The impact of UAE on different bioactive components varies when used in the extraction process. While most studies have reported an increase in the concentration of bioactive components due to ultrasonic treatment, there are also studies suggesting that ultrasonic cavitation can lead to the formation of free radicals and the subsequent reduction of bioactive components [30]. Thus, the effect of UAE on bioactive components can be both positive and potentially negative, depending on the specific conditions and parameters employed during the extraction process. As outlined in Chapter 3 of the article, achieving optimal extraction efficiency through UAE requires careful parameter optimization. The choice of extraction parameters significantly influences the extraction results. It is important to note that these different extraction parameters can also impact the physicochemical properties of bioactive components, further emphasizing the need for meticulous optimization to achieve desired outcomes.
Fig. 3.
The mechanism of ultrasonic assisted extraction. (a) indicates that ultrasonic will act on intact cell. (b) indicates cell rupture after ultrasonic treatment. (c) indicates that the bioactive components are released.
2.2. The effect of UAE on bioactive components
The UAE not only offers significant advantages in increasing the yield of bioactive components but also exerts certain favorable or unfavorable effects on the physicochemical properties of bioactivity. Using the three most common bioactive components (proteins, polyphenols, polysaccharides) as exemplars, the ensuing discussion will elucidate the influence of UAE on these bioactive components.
Protein is the most commonly encountered bioactive component. The structure of proteins can be categorized into four structural levels, which include primary, secondary, tertiary, and quaternary structures. The functionality of proteins is determined by its molecular structure. When subjected to high-intensity ultrasonic waves, proteins can undergo modifications that result in functional alterations. The disruptive effect of ultrasonic treatment on the interactions between protein molecules can lead to protein unfolding, thereby increasing the exposure of protein surfaces. This unfolding phenomenon induced by ultrasonic treatment has the potential to impact the functionality of proteins [31]. Different ultrasonic treatments conditions can change the secondary structure of protein to different extent. Specifically, low-frequency ultrasonic treatments (20, 28, and 35 kHz) have a more pronounced effect on the secondary structure of proteins compared to high-frequency ultrasonic treatments (40 and 50 kHz). This suggests that the choice of ultrasonic frequency significantly influences the extent of changes observed in the secondary structure of proteins [32]. Xue et al. [33] reported that ultrasonic pretreatment of rice protein can lead to spreading out of the α-helix region and the shaping of β-sheet. Similarly, Jin et al. [34] highlighted that subjecting corn prolamin to ultrasonic pretreatment resulted in noticeable changes in its secondary structure. Specifically, the ultrasonic treatment led to a reduction in the content of α-helix and an increase in the content of β-sheets within the prolamin. However, Huang et al. [35] observed the application of ultrasonic treatment for more than 10 min on soy protein isolate resulted in a significant increase in α-helix content, along with a noticeable decrease in the content of random coil structures. The disparity in these results could be attributed to variations in the inherent properties of different proteins and the specific methods employed for ultrasonic treatment. When using UAE for protein extraction, it is important to consider both the absolute sound pressure and the uniform distribution of the sound field. Similarly, ultrasonic power and time need to be taken into account. These considerations are crucial as they can potentially influence changes in protein structure, ultimately enhancing extraction efficiency.
Polyphenols are the collective term for all phenolic derivatives, mainly phenolic acids and flavonoids. UAE has demonstrated enhanced extraction efficiency for polyphenols compared to conventional methods, leading to higher yields of these valuable compounds. However, Zhang et al. [36] noted that gallic acid undergoes degradation when subjected to ultrasonic treatment. Similarly, catechin in tea can also be degraded under specific ultrasonic conditions. Furthermore, within a certain range, the degradation rate of catechin increases as the ultrasonic frequency and input power are elevated [37]. This might be due to under higher ultrasonic power, free hydroxyl radicals are generated, which form a reaction with phenolic compounds and disintegrate them. Thus, it is essential to evade the degradation of polyphenols triggered by higher power. Furthermore, in the case of polyphenol extraction using UAE, it is noteworthy that the optimal effectiveness is observed when utilizing a lower frequency, specifically below 40 kHz [13]. In parallel with the influence exerted by high ultrasound power, high-frequency ultrasound carries the potential to generate a significant quantity of free radicals, leading to the degradation of polyphenols and subsequent reduction in their biological activity. Moreover, due to the susceptibility of numerous phenolic compounds to hydrolysis and oxidation at elevated temperatures, it is imperative to carefully select an appropriate extraction temperature that ensures the maintenance of phenolic compound stability. Several studies have indicated that employing suitable ultrasonic conditions can not only enhance the content of phenolic compounds but also improve their antioxidant activity [38], [39]. On the other hand, the molecular weight of polyphenols may decrease under certain ultrasound conditions. This is because ultrasound can generate intense mechanical and shear forces, that can lead to molecular fracture and degradation. As a result, the average molecular weight of polyphenol molecules can be reduced, potentially improving their digestion, absorption, and utilization by organisms and increasing their bioavailability.
Polysaccharides are high molecular-weight compounds formed by a large number of monosaccharides through glycosidic bonds. Intense ultrasonic will excite loads of reactions within polysaccharides, for instance glycosylation, actualization, and oxidation [24]. Ultrasonic may reduce polysaccharide molecular weight. Kang et al. [40] discovered that the average molecular weight of Ganoderma lucidum polysaccharides extracted using hot water was higher when compared to those obtained through ultrasonic. In another investigation, a comparable outcome was observed, as the average molecular weight of starch exhibited a reduction from 3000KDa to 500KDa subsequent to undergoing ultrasonic treatment [41]. In addition, ultrasonic can enhance the bioactivities of polysaccharides. Xu et al. [42] conducted research to examine the impacts of ultrasonic on the characterization and bioactivities of the polysaccharide derived from blackcurrant fruits. It has been observed that ultrasonic treatment significantly enhances the antioxidant activity, including the hydroxyl and superoxide radicals scavenging, lipid peroxidation inhibition, and DNA damage protection activities. Additionally, it has also been found that ultrasonic treatment increases the α-amine and α-glossidase inhibition activities of polysaccharides. Specifically, higher power ultrasonic treatment at 600 W demonstrates superior effectiveness compared to lower power treatment at 400 W. However, another study showed that the antioxidant activity of Ganoderma lucidum polysaccharides, extracted using ultrasonic, exhibited a lower level compared to the traditional hot water extraction [40]. The researchers in this study proposed two explanations for these results. Firstly, they suggested that the antioxidant properties of polysaccharides may depend on the ratios of different monosaccharides present in their composition, as well as the specific linkages of their side chains. Secondly, they observed that the average molecular weight of Ganoderma lucidum polysaccharides treated with ultrasonic (465.65 KDa) was lower than the average molecular weight of Ganoderma lucidum polysaccharides extracted using hot water (703.45 KDa). This suggests that intense ultrasonic treatment can potentially cause damage to the primary structure of the polysaccharides, affecting factors such as the type of glycosyl units, substituents, and configuration of glycosidic bonds. On the other hand, the use of ultrasound can improve bioavailability. This is due to the ability of ultrasound can reduce the molecular weight of polysaccharides, which is beneficial for digestion, absorption, and utilization.
The aforementioned research findings collectively demonstrate that excessive ultrasound power, frequency, and duration can potentially lead to the loss or degradation of bioactive components. Therefore, it is imperative to make informed parameter selections based on specific circumstances to ensure maximal bioavailability during the UAE process.
2.3. The advantages of UAE
Fig. 4 illustrates the difference between the conventional extraction technique and the developed UAE. UAE offers distinct advantages, notably a significantly higher extraction rate and bioavailability rate [28]. The distinctive physical properties of ultrasonic waves enable them to effectively disrupt plant cell tissues, leading to the rupture or deformation of cell walls. This facilitates a more thorough extraction of the desired components, resulting in a significantly higher extraction rate compared to traditional methods. Moreover, the UAE offers the advantage of shorter extraction times while maintaining high extraction efficiency. Oprescu et al. [43] utilized UAE technology to extract rapeseed oil and compared its performance with the reflux method and impregnation method. The findings revealed that the UAE method achieved an extraction rate of 19.2 ± 0.04 within a remarkably short period of 5 min. The rate of increase then gradually slowed to reach an extraction rate of 21.36 ± 0.04 after 30 min. In comparison, the reflux extraction and impregnation methods yielded extraction rates of 21.32 ± 0.05 and 20.44 ± 0.05 respectively after 30 min. Although there is only a marginal disparity in the final extraction rates among the three methods, the advantage of the UAE method lies in its ability to approach oil saturation concentration in a significantly shorter timeframe. Furthermore, the UAE offers the advantage of low extraction temperatures. The optimal temperature range for UAE is typically set between 40 and 60° C, which proves beneficial for preserving the integrity of active components in materials. This is particularly important for compounds that are susceptible to degradation, hydrolysis, or oxidation at higher temperatures. Simultaneously, the utilization of lower extraction temperatures serves as an energy-saving approach, contributing to reduced energy consumption during the extraction process [44]. Fourthly, it is worth noting that water serves as the ideal medium for propagating ultrasonic waves [45], Therefore, when employing UAE, the process of water-based extraction is significantly enhanced without the need for additional solvents. In addition, when solvents are utilized, UAE offers the advantage of reducing the overall volume of solvents required for the extraction process. Bilgin et al. [46] obtained scarlet sage (Salvia Coccinea) extract using homogenizer assisted extraction and UAE respectively. The findings indicated that UAE shortened the extraction time and reduced the solvent consumption. Fifth, the extracted bioactive components has high purity [47], [48]. In the case of less impurity, separation and purification of effective components more easily achieved. Sixth, wide adaptability. The UAE method offers the advantage of being non-restricted by component polarity or molecular weight, making it suitable for extracting a wide range of plant types and various components [7]. Seventh, the UAE method is characterized by its simplicity and ease of operation [49]. Due to convenient equipment maintenance and low operating costs of the extraction process, the comprehensive economic benefits are significant. As a result, UAE technology is regarded as a green extraction technology. UAE is not limited to small scale extraction in laboratory settings but can also be successfully applied in pilot and industrial-scale operations.
Fig. 4.
Comparison between traditional extraction technique and developed UAE.
Indeed, the UAE process has some drawbacks. One prominent issue is the generation of approximately 65 dB of noise by the ultrasonic equipment during operation. To address this challenge, a feasible solution involves incorporating a sound shield, which increases the cost of the equipment. The second is the issue of UAE parameters need to be optimized. Several variables can impact the outcome of UAE. And the extraction parameters of different bioactive components on different substrates are not the same, so need to go through lots of experiments to optimize extraction parameters. Another challenge in the UAE process is the need to optimize the extraction parameters. Numerous factors can impact the effectiveness of UAE, and these parameters can vary for different bioactive components and substrates. Consequently, extensive experimentation is required to determine and fine-tune the optimal extraction parameters for each specific case. This iterative optimization process ensures that the UAE technique is tailored to achieve the best possible extraction outcomes based on the target bioactive components and substrate characteristics.
3. The factors affecting the extraction effect of UAE
Fig. 5 displays the factors that will affect the extraction effect when using UAE to extract bioactive components from plants, including physical parameters of ultrasonic equipment (such as power, frequency, etc.), characteristics of extraction solvent (such as viscosity, surface tension, etc.), and environmental parameters of extraction (such as temperature, pressure, etc.). To attain the optimum extraction efficiency, it is imperative to adjust these parameters. In the absence of optimized extraction conditions, UAE may result in low extraction efficiency, or even lower extraction efficiency than traditional extraction methods. Furthermore, inappropriate conditions may adversely affect the physicochemical properties and biological activities of bioactive components. For instance, high-frequency ultrasound can generate a large number of free radicals, disintegrate polyphenols, and diminish their biological activity [13]. Another study has proposed that ultrasonic conditions can affect the structure of proteins after UAE [50].
Fig. 5.
Factors affecting the extraction effect of UAE.
3.1. The physical parameters of UAE
3.1.1. Power of ultrasonic
Ultrasonic power in direct proportion to ultrasonic amplitude. Higher ultrasonic power generates a stronger shear force, which, in turn, enhances the yield and extraction efficiency of UAE [14]. Wang et al. [51] extracted curcumin species from turmeric with different ultrasonic power, it was observed that increasing the power from 100 W to 200 W led to an increase in yield. Also, Nipornram et al. [52] noticed a progressive rise in the yield of mandarin peel extract as the power level escalated from 30.34 to 59.36 W. However, pursuing a higher power may have negative effects. Ćurko et al. [53] observed a significant reduction of total polyphenols and total anthocyanidin in wine under high power. Because under high ultrasonic power, hydroxyl radicals are generated, which react with phenolic compounds and degrade them [13]. Similarly, Zhu et al. [54] revealed that there was a decrease in polysaccharide extraction from polygonum multiflorum when the ultrasonic power exceeded 140 W. Additionally, it was observed that the use of very high-intensity UAE or prolonged application of UAE can result in significant protein denaturation [22]. Hence, it is crucial to optimize the power parameters during the extraction experiment and carefully choose the suitable power level based on the specific characteristics of the sample.
3.1.2. Power density of ultrasonic
Power density, also known as ultrasonic intensity. Without losing the mass transfer efficiency, it can be represented by the following formula (1). Power density can better describe the intensity of ultrasonic compared to ultrasonic power.
| (1) |
where UI represents ultrasonic intensity (W/cm3), P denotes for ultrasonic power (W) and V stands for sample volume (cm3).
Generally, an increase in ultrasonic intensity (UI) results in a proportional increase in the acoustic chemical effect. This is due to the fact that higher UI leads to an increased number of cavitation bubbles. However, beyond a certain limit, excessive UI can lead to bubble collisions, which in turn reduce the cavitation effect. Furthermore, during the utilization of an ultrasonic probe, a significant accumulation of cavitation bubbles occurs on the surface of probe. This accumulation can impede the transfer of energy and subsequently diminish the efficiency of extraction [55]. Moreover, considering the properties of certain target molecules, the use of high-intensity ultrasonic power can cause the degradation of bioactive substances, ultimately leading to a decrease in extraction efficiency.
3.1.3. Frequency of ultrasonic
Ultrasonic with frequencies exceeding 20 kHz have demonstrated the ability to efficiently extract bioactive components from natural products [56]. Frequencies ranging from 20 kHz to 100 kHz are frequently employed. Frequency has a profound impact on the cavitation effect during extraction. It influences the physical and chemical consequences associated with the bursting of cavitation bubbles during the process. Specifically, frequencies ranging from 20 kHz to 100 kHz primarily govern the physical effects generated by the bursting of cavitation bubbles. On the other hand, frequencies within the range of 200 kHz to 500 kHz predominantly regulate the chemical effects resulting from cavitation bubbles during the extraction process [21]. Zhang et al. [30] used an oscilloscope and spectrum analyzer to observe the oscillation cycle of cavitation bubbles, as well as to analyze the intensity and distribution of the sound field. The research results show that various frequencies exhibit distinct oscillation cycles. As the ultrasonic frequency decreases, the oscillation cycle of cavitation bubbles increases, resulting in a larger bubble radius. Consequently, this leads to the generation of more powerful mechanical waves and a pronounced cavitation effect. There is also a connection between frequency and ultrasonic intensity. In order to attain the intended cavitation effect, the ultrasonic intensity should be amplified with rising frequency to conquer the cohesiveness in the mixture [57]. The choice of frequency range for UAE varies depending on the type of material being extracted. Based on numerous studies, it is generally recommended to use low frequencies (20–40 kHz) for extracting flexible materials such as vegetable matter and algae. On the other hand, rigid structures necessitate the use of higher frequencies (≤500 kHz) [13], [58]. Furthermore, there is a category of sound waves known as megasonic waves which operate within the frequency range of 400 kHz to 2 MHz. High frequency ultrasonic waves in this range as an innovative approach to enhance oil recovery and accelerate milk fat creaming [59]. Consequently, determining the ideal frequency for specific extractions necessitates conducting a significant number of optimization experiments and tests. This process is essential to identifying the most effective frequency tailored to each extract's unique characteristics.
3.1.4. Duty cycle of ultrasonic
The duty cycle is believed to have an indirect impact on the extraction effect by influencing the duration of ultrasonic exposure and the temperature. Kobus et al. [55] examined the effect of duty cycle on phenolic compounds. The study observed that the antioxidant activity of the extracts and the yield of chlorogenic acid, rutin, and total flavonoids were noticeably influenced by the duty cycle. Moreover, the study determined that the impact of the duty cycle on the yield was contingent upon the strength and time of the ultrasonic treatment.
3.1.5. Time of extraction
Extending the extraction time can enhance the extraction yield. However, it also carries the risk of degrading plant components due to prolonged exposure [60]. To accommodate longer ultrasonic processing times, it is advisable to utilize the pulse mode. This mode involves the generator periodically activating and deactivating the power of the ultrasonic probe. By intermittently switching the power, the pulse mode effectively prevents excessive buildup of reaction temperature during extended processing durations. Moreover, Kobus et al. [55] showed that the utilization of pulsed ultrasonic leads to energy savings ranging from 20 % to 51 % while achieving an increase in extraction efficiency. Yogesh et al. [61] used ultrasonic to enhance the extraction of catechins from the bark of Syzygium cumini. And they studied how varying ultrasonic times affected the extraction outcome. Experimental trials were conducted at temperatures of 30° C, 40° C, 50° C, and 60° C. The results showed that the extraction yield initially increased as the extraction time was prolonged to 15–20 min. However, beyond this point, longer extraction times led to a decrease in yield. Based on these findings, Yogesh et al. recommended keeping the processing time relatively short, typically between 5 and 15 min, depending on the specific component being extracted and the ultrasonic method employed [61].
3.1.6. The efficiency of the transducer
The efficiency of an ultrasonic transducer refers to the percentage of the output power to input power. In ultrasonic transducers, a portion of the input alternating current power is converted into electromagnetic losses, while the remaining energy is transformed into mechanical power through vibrations. A portion of the mechanical power is lost due to factors such as friction and sound absorption, while the remaining portion is emitted as radiation power to the sound transmission medium. Therefore, the efficiency of the transducer encompasses three distinct aspects and meanings: electromechanical efficiency, mechanical-acoustic efficiency, and electroacoustic efficiency. It is essential to enhance the efficiency of energy conversion in the transducer and reduce unnecessary energy losses. This entails selecting suitable manufacturing materials and considering the geometric shape of the transducer, both of which can impact the extraction effectiveness. Zea et al. [62] conducted a study to evaluate the acoustic properties of four transducers with varying geometries and assess their effectiveness in the extraction. These four transducers are the base model (T1), a larger cylindrical headmass (T2), a stepped circular section sonotrode (T3) and a multiplate configuration (T4). The result showed that the T4 exhibited the highest nominal power density. The T2 generated an evenly distributed ultrasonic field and a greater acoustic pressure. Therefore, the shape of the transducer affects the quantity and apportionment of energy transferred to the sample, consequently affecting the extraction effect.
3.1.7. The geometry of the reactor
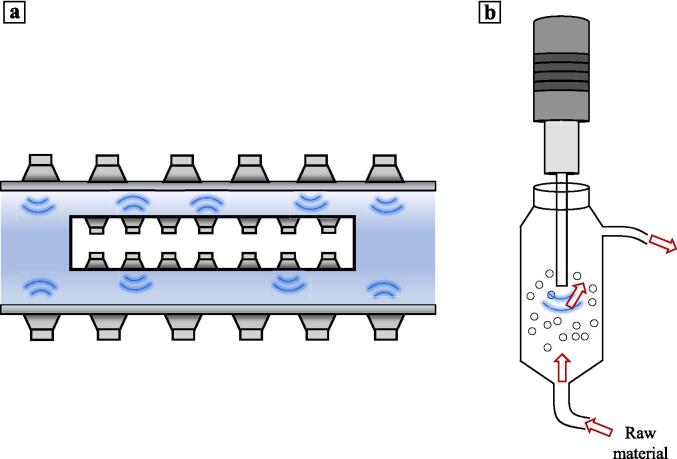
It is crucial to carefully consider the reactor's shape and size in order to reduce energy loss due to sound wave reflection at solid surfaces. Opting for a flat-bottomed reactor, such as a conical flask, can help minimize wave reflection. Additionally, the thickness of the reactor also influences wave reflection attenuation. Therefore, conducting calculations to determine the optimal shape and size of the reactor becomes necessary in order to reduce energy loss. Moreover, when designing larger ultrasonic reactors, it is important to incorporate multiple transducer arrays to ensure efficient coverage of the sound field. By strategically configuring the transducers, a more effective and comprehensive sound field can be achieved. Fig. 6a illustrates a tubular ultrasonic reactor, which achieves effective coverage of the sound field through multiple transducer arrays. Fig. 6b is a countercurrent ultrasonic device, that realizes the efficient utilization of sound field by changing the flow form of treated samples.
Fig. 6.
Tubular ultrasonic reactor (a) and Countercurrent ultrasonic (b).
The ultrasonic intensity decreases rapidly in both the radial and axial directions when using an ultrasonic probe. To ensure effective extraction, it is essential to maintain an effective spacing between the probe and the bottom of the container. It is also important to avoid any contact between the probe and the container to prevent damage. Consequently, factors such as the vertical position of the probe (that is, the depth at which the probe is submerged) and the horizontal arrangement of the probe all impact the efficiency of UAE. Consideration of these factors is crucial for optimizing the ultrasonic extraction technology. Son et al. [63] studied the effects of the depth at which the probe is submerged, power input and liquid height on the extraction results using an ultrasonic probe in a 500 mL container. The optimal extraction conditions they obtained was an immersion depth of 60 mm, setting input power at 75 %, and liquid height maintained at 70 mm.
3.2. The characteristics of medium
3.2.1. The type of solvent
The selection of solvent relies on the solubility and polarity of the extract. At the same time, the potential impact on UAE should also be considered. For instance, water is commonly used to extract acids, bases, sugars, amino acids and other polar substances. Ethanol is used to extract phenols, aldehydes, ketones, esters and other polar compounds. Chloroform is used to extract vegetable oil, spices, fatty acids and other fat-soluble substances. Ether is commonly used for extracting fat-soluble compounds as well. Benzene is used to extract phenol, phenylacetic acid, benzaldehyde and other aromatic compounds. When selecting an extraction solvent for bioactive compounds, various factors should be considered, including molecular affinity, mass transfer, environmental safety, human toxicity, and economic feasibility. Different solvents have varying properties, leading to different effects of ultrasonic cavitation under identical conditions. Firstly, the polarity and solubility of the target compound in the solvent can significantly impact the extraction efficiency. Secondly, the viscosity of the solvent plays a role, as more viscous solvents reduce the cavitation effect. Finally, the volatility of the solvent should be considered. If the extraction is conducted at higher temperatures for prolonged periods, volatile solvents may evaporate, leading to a potential reduction in extraction efficiency. Taking these factors into account is important when selecting an appropriate solvent for extraction to obtain optimal results. Bellumori et al. [64] had conducted such an experiment that extracting romanic and carnotic acids from rosemary leaves using UAE. The experimental results indicate that using ethanol as the solvent yields a high content of rosmarinic acid (6.8 % of the dried extract), while employing n-hexane as the solvent results in a high content of carnosic acids (13 % of the dried extract). Furthermore, it was found that the concentration of the solvent also affects the extraction results. Xu et al. [65] conducted a research investigation on the relationship between ethanol concentration and the total antioxidant activity of the extract. Specifically, the total antioxidant activity of the extract significantly increased as ethanol concentration raised from 10 % to 50 % (v/v). However, as ethanol concentration raised from 50 % to 90 %, a significant decrease was observed. These results highlight the critical role of ethanol concentration in influencing the antioxidant properties of the extract.
3.2.2. The property of substrate
Several parameters, such as substrate viscosity, surface tension, particle size, and pretreatment techniques, have an impact on the efficiency of UAE. Viscosity and surface tension, for instance, influence the threshold at which cavitation occurs. The motion impedance of the sample to the ultrasonic probe will increase with the stickiness of the sample on the rise. Consequently, it is recommended to use high intensities (or high amplitudes) to generate the required mechanical vibrations for inducing cavitation when dealing with high stickiness samples. Particle size reduction has been found to enhance extraction efficiency, leading to the grinding of samples in some studies. Grinding is just one type of pretreatment method, with others including chemical crushing, enzyme treatment, and degreasing. Numerous studies have demonstrated that extraction rates can be improved through appropriate preconditioning techniques [66]. Liu et al. [67] improved the sulforaphane extracted from broccoli seeds by using UAE through microwave pretreatment. Tzima et al. [68] examined the application of pulsed electric field (PEF) as a pretreatment for UAE to improve the extraction efficiency of phenolics from rosemary and thyme by-products. According to the findings, the utilization of PEF augmented (p < 0.05) the retrieval of phenolic compounds and antioxidant capacity in comparison to the standalone use of UAE. The content of phenolic compounds pretreated with PEF (297 mg GAE 100 g-1 FW) was 1.5 times higher than that treated with UAE alone (196 mg GAE 100 g-1 FW). In addition, the antioxidant capacity of phenolic compounds pretreated with PEF (593 mg TE 100 g-1 FW) was 1.3 times higher than that treated with UAE alone (445 mg TE 100 g-1 FW).
3.2.3. The solvent to material ratio
UAE can better blend solvents and materials, thereby reducing the amount of solvent added. Determining the optimal amount of solvent used in the extraction process holds economic importance. Typically, a larger volume of solvent yields higher extraction yields by enhancing the dissolution efficiency of the target compound. This can be attributed to the fact that an increased solvent-to-material ratio reduces the density of the mixture. As a result, the propagation speed of ultrasonic waves is enhanced while the attenuation of ultrasonic power is decreased. The reduction in mixture density enables more effective energy transfer, thereby improving extraction efficiency. Additionally, the decreased density of the mixture enhances the cavitation effect, further contributing to improved extraction. Understanding and optimizing the solvent-to-material ratio is therefore crucial for maximizing extraction efficiency and achieving higher yields. Maran et al. [69] found in their research that the production of anthocyanidin and polyphenols exhibited a progressive increase when the solid–liquid ratio gradually increased from 1:10 to 1:20 (g/ml). Setyaningsih et al. [70] also obtained the same result. The result showed that a solvent-to-material ratio of 5:1 (ml/mg) could yield more polyphenols than a solvent-to-material ratio of 2.5:1 (ml/mg) under UAE.
3.3. Parameters of environment
3.3.1. The temperature of extraction
The temperature of the sample solution is significant and should maintain at its optimum value. When the temperature is increased, the kinetic energy of the extracting fluid molecules is also increased. This heightened kinetic energy accelerates the diffusion rate of compounds, allowing for a more efficient extraction process. Moreover, the elevated temperature helps to break down the external chemical bonds present in the matrix, further enhancing the effectiveness of the ultrasonic field. However, excessively high temperatures can have detrimental effects on the integrity of the extracted material. While elevated temperatures can enhance extraction efficiency, there is a threshold beyond which the extracted material may be compromised. Wang et al. [164] conducted a study on the effect of temperature on the extraction of pea starch using UAE. According to their research results, as the temperature gradually increases from 30° C to 40° C, the starch production shows a gradual increase. However, beyond 40 °C, the yield decreases significantly. This phenomenon can be attributed to the potential damage to the starch structure caused by excessively high extraction temperatures. Liao et al. [165] conducted a study on the effect of temperature on the extraction of total phenolics and total anthocyanins using UAE. Their research findings indicate that increasing the extraction temperature increases the mass transfer rate of solutes. Therefore, as the temperature increases from 30° C to 45° C, there is an increase in the production of total phenolic and total anthocyanins. However, once the temperature exceeds 45° C, the extraction yield shows a decreasing trend. This can be attributed to the degradation of thermosensitive substances caused by excessively high temperatures. Similarly, prolonged exposure to ultrasonic waves may lead to damage to the ultrasonic transducer. Thus, finding the right balance between temperature and extraction time is crucial to ensuring optimal results without compromising the integrity of the extracted material or the functionality of the ultrasonic equipment. Tekin et al. [71] performed an extraction of essential oils from cloves, employing variables such as temperature, extraction time, and plant concentration. The findings indicated that extraction temperature was an important factor affecting extraction yield. The elevation in temperature leads to a rise in the vapor pressure of the solvent, which subsequently decreases the surface tension and viscosity of the liquid phase. This reduction in surface tension and viscosity enhances the occurrence of ultrasonic cavitation and mechanical decomposition of plant cells. Consequently, mass transfer between the plant material and the solvent is promoted. However, it is important to note that higher extraction temperatures can also result in a reduction of cavitation due to the presence of solvent vapors filling the cavitation voids. This causes the cavitation bubbles to collapse with less intensity. These insights highlight the complex relationship between temperature, ultrasonic cavitation, and the mass transfer processes during extraction, providing a clearer understanding of the underlying mechanisms involved. In conclusion, extraction must be carried out at a suitable temperature to improve the performance of UAE while protecting the structure and function of extract from being destroyed.
3.3.2. Air pressure
Air pressure has an impact on the cavitation bubbles' strength of collapse. Specifically, higher air pressure raises the cavitation threshold, making it necessary to employ a more intense level of ultrasonic energy to induce cavitation. The majority of UAE experiments are conducted under atmospheric pressure conditions, and these experiments have yielded satisfactory results [72]. This suggests that the prevailing atmospheric pressure is generally sufficient to generate the desired effects of ultrasonic cavitation. The use of atmospheric pressure allows for practical and effective implementation of UAE without requiring additional adjustments or equipment modifications.
In summary, a variety of factors can impact the effectiveness of the UAE. The effects of different factors on different substrates and different bioactive components vary greatly. Therefore, it is essential to understand how UAE parameters such as power, frequency, time, and temperature affect the extraction results and efficiency. By optimizing these extraction conditions, we can not only minimize material and solvent waste but also maximize extraction yield and preserve the biological properties of the extract. However, it's important to note that achieving high extraction yields should not be the sole objective of the extraction process. Sustainable practices, including the conservation of non-renewable resources and energy consumption, also need to be considered.
4. Ultrasonic assisted extraction equipment (UAEE)
UAEE is an equipment based on the principles of UAE that researchers continuously improve to effectively extract bioactive compounds from plants. Over the past few years, numerous research institutions and companies have been working on developing new UAEE to meet different application requirements. For example, a continuous flow ultrasonic crusher was developed based on the experimental ultrasonic crusher. The control circuit of this new equipment uses a microcomputer, which can control various functions at will. In addition, continuous flow processing can be performed on the sample, overcoming the disadvantage of ordinary models that cannot be processed continuously. In order to overcome the shortcomings of a single UAEE, a microwave light wave ultrasonic extraction instrument was also developed, which fully utilizes the cavitation effect of ultrasonic and the high-energy effect of light waves (microwaves) to carry out collaborative extraction under low temperature and atmospheric pressure conditions. These devices can effectively extract bioactive components, improve the concentration of bioactive components, improve efficiency on the premise of ensuring the quality, and bring good economic benefits for research and development in field of plant extraction and separation.
4.1. The main components of UAEE
An ultrasonic equipment setup typically consists of two main components: an ultrasonic generator and an ultrasonic oscillator. The term “ultrasonic oscillator” serves as a general term encompassing both ultrasonic transducer and ultrasonic horn.
The ultrasonic generator is an essential component of the equipment responsible for supplying alternating current power to the ultrasonic transducer. It plays a crucial role in generating the electrical power required to drive the transducer effectively [167]. Because of its role in providing power, it is often referred to as ultrasonic power.
The ultrasonic transducer is the core component of ultrasonic equipment [73]. The ultrasonic transducer is responsible for the conversion of energy, transforming the signals or energy from another source into the necessary ultrasonic signals or energy. There are numerous types of ultrasonic transducers available, each designed to fulfill specific requirements and perform particular functions [168], [169]. In terms of electroacoustic ultrasonic transducers, there are piezoelectric transducer, magnetostrictive transducer, electric transducer and electromagnetic transducer, etc. Piezoelectric ceramic ultrasonic transducer has become the most widely used ultrasonic transducer in modern ultrasonic instruments because of its own acoustic characteristics, such as high sound pressure amplitude and low power output [74]. The piezoelectric ceramic ultrasonic transducer is generally of the sandwich type, made up of a group of piezoelectric wafers forming the driving parts. These wafers are sandwiched between matching blocks of the same or different materials (commonly referred to as front and back cover plates), and a certain amount of prestress is applied.
The function of the ultrasonic horn is to magnify the amplitude produced by the ultrasonic transducer. The geometric shapes of the ultrasonic horn include uniform cylinder [75], flat [76], hemispherical [77], conical [78], etc. The magnification of the amplitude is determined by the configuration of the ultrasonic horn. The higher the amplitude provided by the ultrasonic horn, the more intense the ultrasonic treatment will be. The ultrasonic horn is usually made of titanium alloy, because titanium has heat and corrosion resistance properties in addition to low mass and high rigidity.
4.2. Two classifications of UAEE
At present, there are two main types of ultrasonic equipment used in the UAE: bath type ultrasonic equipment and probe type ultrasonic equipment [79]. And the ultrasonic bath (Fig. 7a) and ultrasonic probe (Fig. 7c) are the widely adopted ultrasonic equipment in numerous laboratories worldwide [14]. Among them, the bath type ultrasonic equipment mainly uses horn transducers, which are attached to the bottom of the bath (Fig. 7b) or attached to the four walls. The probe type ultrasonic equipment uses a long strip transducer that is inserted from the top of the target solution (Fig. 7d).
Fig. 7.
Ultrasonic bath (a,b) and Ultrasonic probe (c,d).
In addition, UAEE can be classified into direct extraction equipment and indirect extraction equipment. Direct ultrasonic and indirect ultrasonic are the two forms of UAE [170], [171]. In general, directly ultrasonic extraction can get a lot of extract, but processing the sample amount is not much. On the contrary, the sample size of indirect ultrasonic treatment is larger, but the extraction amount of target compounds is relatively small [1]. The corresponding UAEE of these two ultrasonic forms is represented by the ultrasonic probe and ultrasonic bath, respectively. The probe type ultrasonic equipment is direct ultrasonic extraction equipment, which is widely used in the ultrasonic processing of small volume samples. The ultrasonic bath is an indirect extraction, used to extract a large number of samples. Under the same conditions, the probe ultrasonic has stronger ultrasonic effect than ultrasonic bath [73]. Due to the ultrasound intensity is propagated solely at the tip of the horn [80], the horn is directly immersed in the solution for ultrasonic processing, which increases the contact area with the material and reduces the mass transfer resistance. However, During the procedure of utilizing probe ultrasonic treatment, the temperature of the sample will rise sharply. Also, due to the potential corrosion caused by chemical reactions between the ultrasonic probe material and the sample, the ultrasonic probe is likely to be damaged over time. Although the probe ultrasonic has a stronger ultrasonic effect than the ultrasonic bath, it cannot be understood that the probe ultrasonic is superior to the bath ultrasonic when choosing the ultrasonic mode. However, in terms of energy loss, probe ultrasonic is superior to ultrasonic bath [21]. Because when using an ultrasonic bath, the intensity will decrease as it traverses two media (water bath and sample container) prior to the sound waves reaching the sample. However, compared to probe ultrasonic, the advantage of an ultrasonic bath is that two consecutive experiments do not produce cross contamination. Table 1 shows the extraction experiments performed in recent years using ultrasonic probes and ultrasonic baths.
Table 1.
Extraction experiments using Ultrasonic probes and Ultrasonic baths.
| Equipment type |
Equipment diagram |
Extractive | Extraction conditions |
Extraction effect |
Ref. |
|---|---|---|---|---|---|
| Ultrasonic probe |
 |
Polyphenolic from Thymus serpyllum |
P = 750 W f = 20 kHz t = 15 min T = 25 °C A = 20,50,80 % S: ethanol |
Compared with maceration and heat-assisted technique, significantly higher yields of total flavonoids in the UAE | [81] |
| Phenolic from Achyrocline satureioides |
T = 25 °C Solute: Solvent ratio = 1: 40 |
UAE increased total phenolic compound content by 6.1 times and antioxidant activity by 3.4 times compared to conventional extraction. | [82] | ||
| Phenolic from Lime peel waste |
P = 750 W f = 20 kHz t = 4 min A = 38 % S: Ethanol |
UAE proved more effective in extracting total phenolics (54.4 mg GAE/g) with high antioxidant activity, saving 33 % of time compared to MAE. | [83] | ||
| Anthocyanins from Purple yam |
P = 750 W f = 20 kHz t = 10 min T = 60 °C A = 60 % S: Ethanol |
UAE was better than conventional extraction of soxhlet extraction and low-pressure solvent extraction | [84] | ||
| Phenolic from Mango peels |
t = 10 min T = 45 °C A = 30 % S: Ethanol |
The maximum extraction rate by UAE was 35.5 mg GAE/g. UAE is more advanced technique than conventional technique | [85] | ||
| Cannabinoids and Terpenes from Cannabis |
P = 450 W f = 20 kHz t = 60 min T = 30 °C A = 20–100 % S: Ethanol |
Compared to MAE, UAE increased crude oil yield by 14.39 %, cannabinoid concentration by 13.21–39.24 %, and total terpene extraction by 14.67 % | [86] | ||
| Phenolic from Raspberries Strawberries and Blackberries |
P = 700 W f = 20 kHz t = 45 min T ≤ 40 °C A = 50 % S: Ethanol |
Using methanol or ethanol as solvent is better than using water as solvent | [87] | ||
| Anthocyanin from Jaboticaba skin |
Pd = 150 W/L f = 20 kHz t = 10 min |
UAE was a time-saving extraction technology in comparison to agitated-bed and high hydrostatic pressure-assisted extraction. And UAE was mainly influenced by the extraction time | [88] | ||
| Oil from Rice bran |
P = 750 W f = 20 kHz t = 1 min T = 25 °C A = 40 % |
The yields obtained by solvent extraction and UAE were similar, but UAE significantly reduced the extraction time | [89] | ||
| Starch from Mango |
P = 480 W f = 20 kHz t = 20 min |
UAE significantly enhances starch properties, such as amylose content, water- and oil-holding capacity, solubility, and swelling power | [90] | ||
| Ultrasonic bath |
 |
Oil from Peanut seeds |
P = 300 W f = 40 kHz t = 60 min T = 30 °C S: Hexane |
Oxidation of oils extracted with UAE was slightly higher than that without UAE | [91] |
| Oil from Olea europaea L. | P = 165 W f = 40 kHz and 585 kHz t = 10 min |
A combination of low-frequency sonication and megasonic has more advantages than a double megasonic treatment | [92] | ||
| Oil from Crambe seed |
P = 165 W f = 25 kHz t = 90 min T = 60 °C S: Hexane |
UAE boosts the yield by 20 % compared to without UAE | [93] | ||
| Phenolic, Flavonoid, and Anthocyanin from Blueberry |
P = 64 W f = 35 kHz t = 30, 40, 60, 90 min T = 20,40,60 °C S: Water or Ethanol |
UAE is a preferred method for extracting bioactive phenolics from blueberry pomace | [94] | ||
| Polyphenolic from Blueberry pomace |
f = 35 kHz t = 5,10,15 min T = 20,40,80 °C S: Acidulated ethanol, Acidulated methanol |
UAE is inferior to pulsed electric field in improving polyphenol extraction from blueberries | [95] | ||
| Anthocyanins Phenolics from Jabuticaba peel |
Pd = 50 W/L f = 25 kHz t = 25 min |
UAE improves the yield of phenolic compounds obtained | [96] | ||
| Phenolics, Flavonoids, and Anthocyanins from Raspberry fruits |
f = 50 kHz t = 120 min T = 50 °C S: Acidulated methanol |
UAE maximizes the yield of total phenolics and flavonoids | [97] | ||
| Anthocyanin and Phenolic compound from Black jamun fruit | P = 100 W f = 40 kHz t = 150 min T = 70 °C |
Compared with microwave, UAE can obtain more phenolic compound and Anthocyanidin | [98] | ||
| Protein from Cherimoya seed |
P = 300 W f = 35 kHz t = 26.1 min T = 41.8 °C |
The combined UAE and alkaline extraction yielded 6 % more extraction and 12 % higher protein content than alkaline extraction alone | [99] | ||
| Pectin from Grape pomace |
P = 200 W f = 25 kHz t = 60 min |
Pulsed ultrasonic caused pectin breakdown, therefore lesser emulsifying properties | [100] |
Note: P: Power; Pd: Power density; f: Frequency; t: Time; T: Temperature; A: Amplitudes; S: Solvents.
4.3. Working modes of UAEE
To maximize the extraction effect of different plant active components, a variety of different ultrasonic modes have been developed, including single frequency ultrasonic and multi-frequency ultrasonic, continuous ultrasonic and pulse ultrasonic, fixed frequency ultrasonic and sweeping frequency ultrasonic. Among the multi-frequency ultrasonic modes, there are also dual-frequency multi-angle ultrasonic (Fig. 8a), multi-frequency variable power ultrasonic, dual-frequency (or multi-frequency) sequential ultrasonic (Fig. 9abc) and dual-frequency (or multi-frequency) simultaneous ultrasonic (Fig. 9def) and other modes. In addition, the liquid phase can not only be stationary, but can be forced to flow. According to the direction of forced liquid flow and the direction of ultrasonic action, it can be divided into countercurrent ultrasonic (Fig. 6b) and tangential flow ultrasonic (Fig. 8b).
Fig. 8.
Dual-frequency multi-angle ultrasonic (a) and Tangential flow ultrasonic (b).
Fig. 9.
Dual-frequency sequential ultrasonic (a,b,c) and Dual-frequency simultaneous ultrasonic (d,e,f).
Compared to single frequency ultrasonic, double frequency ultrasonic significantly enhance the rate of cavitation [101], [102], [103]. Due to its ability to generate a greater quantity of cavitation bubbles and induce “combined resonance”, double frequency ultrasonic produce significantly higher levels of sonochemical energy [104]. Xu et al. [105] studied the extraction of rose polyphenols under the influence of multi-frequency power ultrasonics. The results showed that the yield of rose polyphenols under single-frequency ultrasonic is 167.92 mg/g, and the yield of rose polyphenols under triple-frequency ultrasonic is 187.13 mg/g. Compared to single-frequency ultrasonic, multi-frequency ultrasonic exhibited a higher extraction rate, and concurrently, the hydrophone recorded a more robust sound signal. In addition, Xu et al. [106] also examined the impact of dual-frequency ultrasonic pretreatment on the vacuum freeze-drying of strawberry slices. The study showed that the drying time using dual-frequency ultrasonic was 6 h, while the drying time using single-frequency ultrasonic was 10 h. The dual-frequency ultrasonic significantly outperformed the single-frequency ultrasonic in terms of drying efficiency. The reason for the analysis is that the working frequency of dual frequency ultrasonic is higher, which overcomes the limitations of single frequency ultrasonic.
Dual-frequency multi-angle ultrasonic refers to the utilization of ultrasonic probes operating at two different frequencies, positioned at a specific angle for material treatment, as shown in Fig. 8a. Zhang et al. [30] examined the influence of different angles of dual-frequency ultrasonic (40 + 20 kHz 0°, 40 + 20 kHz 30°, 40 + 20 kHz 45°) on the physicochemical properties of raw soymilk and the structure of soybean protein, and compared them with conventional single-frequency ultrasonic (40 kHz, 20 kHz). The findings indicated that dual-frequency ultrasonic treatment, particularly at 40 + 20 kHz and 45-degree angle, significantly enhanced the soybean protein content (SPC) in raw soymilk compared to single-frequency treatment. The SPC of the 40 + 20 kHz 45° treatment was 20.01 % higher than that of the 40 kHz treatment and 12.05 % higher than that of the 20 kHz treatment. This difference can be attributed to the use of cavitation bubbles generated by high-frequency ultrasonic as the core for low-frequency ultrasonic, resulting in more intense cavitation in dual-frequency ultrasonic. The increased cavitation, in turn, induced significant shear forces, resulting in higher extraction of proteins. Moreover, the application of dual-frequency multi-angle ultrasonic treatment not only increased the SPC of raw soymilk, but also improved its physical and chemical properties, such as color, rheological properties, surface tension, emulsifying properties, and foaming properties. These improvements ultimately helped to improve the flavor of the raw soymilk.
Countercurrent ultrasound refers to force the direction of liquid phase flow to be opposite to the direction of ultrasound action. Specifically, it involves circulating the sample in a countercurrent manner through the ultrasonic field, enabling enhanced treatment efficacy, as shown in Fig. 6b. The operation of sweeping frequency (a ± b kHz) refers to a cyclic frequency sweep ranging from a - b to a + b kHz and then back from a + b to a − b kHz, following an isosceles triangle waveform with a consistent linear speed. Ma et al. [107] conducted a study to investigate the impact of single frequency countercurrent ultrasonic (SFCU) and sweeping frequency ultrasonic (SFU) on the ACE inhibitory activities of garlic powder. According to the findings, the ACE inhibitory activity of garlic powder after pretreatment with SFU and SFCU was 65.88 % and 67.78 %, respectively, while the untreated sample showed only 47.88 % ACE inhibitory activity. The application of SFU and SFCU pretreatments both led to significantly increased ACE inhibitory activity in garlic powder compared to untreated garlic. Furthermore, from an acoustic perspective, SFU has the ability to create a sound field that is more favorable for enhancing the cavitation effect [108].
Dual-frequency sequential ultrasonic (Fig. 9abc) refers to the alternating operation of two different ultrasound frequencies in a continuous and non-overlapping manner [109]. Dual-frequency simultaneous ultrasonic (Fig. 9def) refers to the simultaneous operation of two different ultrasonic frequencies. Xue et al. [110] had noted the significance of the ultrasonic working mode in protein extraction. They investigated the impact of ultrasonic treatment with multiple modes on the extraction of rice protein. The findings demonstrated that the sequential frequency ultrasonic treatment exhibited a higher protein extraction rate compared to simultaneous or single frequency ultrasonic treatment. Also, Xue et al. [31] examined the influence of ultrasonic frequency mode, power density, pretreatment time, and other parameters at low power density on the degree of hydrolysis (DH) of defatted wheat germ protein (DWGP) and the angiotensin-I-converting enzyme (ACE) inhibitory activity of DWGP hydrolysate. Under the dual-fixed frequency ultrasonic mode of 28/40 kHz, an ultrasonic power density of 60 W/L, a pretreatment time of 70 min, a temperature of 60 °C and a substrate concentration of 60 g/L, the DWGP hydrolysate exhibited the highest ACE inhibitory activity. Ding et al. [111] investigated the impact of ultrasonic pretreatment with various working modes, such as mono frequency ultrasonic (MFU), simultaneous dual frequency ultrasonic (SDFU), and alternate dual frequency ultrasonic (ADFU), utilizing energy-gather counter flow ultrasonic equipment. The results show that ADFU at 20/35 kHz exhibited the highest angiotensin-converting enzyme inhibitory activity.
The ultrasonic probe emits ultrasonic waves intermittently in the form of pulses, known as pulsed ultrasonic. For example, the meaning of a 5 s − 2 s pulse is that the ultrasonic is turned on for 5 s, turned off for 2 s, turned on for 5 s, and turned off for 2 s, in a cyclic manner. Pan et al. [112] used UAE to extract antioxidants from pomegranate peel in continuous and pulsed modes. This research unequivocally showcased the superior performance and effectiveness of pulse ultrasonic assisted extraction (PUAE) for producing antioxidants from the peel of pomegranate marc. From the point of view of industrial use, it is recommended to run the ultrasonic transducer in pulse mode. In this way, the service life of the transducer can be extended while the thermal effect of the extract is minimal. Pan et al. [113] extracted antioxidants from pomegranate peel by conventional extraction, continuous ultrasonic extraction and pulsed ultrasonic extraction, and studied the extraction yield and antioxidant activity. The findings from the experiment indicated that compared to conventional extraction, the amount of antioxidant extracted by pulsed ultrasonic extraction was increased by 22 % and the extraction time was shortened by 87 %. Similarly, a 24 % increase in antioxidant yield and a remarkable 90 % reduction in extraction time when utilizing continuous ultrasonic extraction at the same intensity level. However, pulsed ultrasonic extraction saves 50 % more energy compared to continuous ultrasonic extraction.
5. Application of UAEE
5.1. UAEE using generally recognized as safe (GRAS) solvents
The majority of studies have indicated that the utilization of organic solvents can enhance the efficiency of extraction. However, the cost of removing organic solvents after extraction is a significant limitation of employing organic solvents for extraction [114]. The application of ultrasonic technology in extraction has widened the range of available extraction solvents, enabling the substitution of toxic organic solvents with GRAS solvents in certain cases. Opting for GRAS solvents offers significant health and safety benefits, while also reducing extraction costs. A typical example is that Li et al. [115] extracted carotenoids from carrots using sunflower seed oil as solvent, combined with UAEE, and then compared it with the traditional extraction method using hexane as solvent. According to the findings, the highest yield of β-carotene (334.75 mg/L) using sunflower as a solvent was achieved by the UAEE within a short duration of 20 min. In contrast, the extraction using hexane as the solvent obtained a similar yield (321.35 mg/L) but required a longer extraction time of 60 min. Johanna et al. [114] used UAEE to extract caffeine from pills. The research investigated the efficacy of environmentally friendly solvents, namely ethyl acetate and water, in comparison to four conventional solvents (ethanol, methanol, acetone, and acetonitrile) for extracting caffeine from coffee pills. And they developed a solvent assessment method related to environmental capacity and sustainability, which is known as the GD. The study found that water achieved the highest GD rating for caffeine extraction. Moreover, its easy availability and low cost make it a viable alternative solvent for caffeine extraction. And they think that water could be an excellent alternative solvent in the extraction process. Table 2 shows the recent studies on UAEE using GRAS solvent as an extraction agent.
Table 2.
UAEE using GRAS solvent as extraction agent.
| Matrix | Extractive | Solvent | Benefit | Extraction conditions | Ref. |
|---|---|---|---|---|---|
| Camellia sinensis (green tea) leaves | Polyphenols Flavonoids |
Water | 1.Cheaper and easier to obtain; 2. More environmentally friendly; 3.Non-flammable, safer extraction; 4. More pollution-free to the environment; 5.Cost savings compared to organic solvents |
P = 500 W f = 20 kHz T = 77 °C A = 77 % |
[116] |
| Olive leaves | Oil | P = 2.8 kW f = 20 kHz A = 80 % |
[117] | ||
| Cinnamomum cassia bark | Essential oil | P = 300 W f = 25 kHz t = 35 min |
[118] | ||
| Leaf (Sinara Sko Rems L.) |
Phenolic Flavonoid |
P = 500 W f = 20 kHz t = 20.05 min T = 25 °C A = 65.02 % |
[119] | ||
| Coffee pills | Caffeine | P = 144–480 W f = 37 kHz t = 5–13 min |
[114] | ||
| Date palm | Phenolic Flavonoid |
f = 28, 40 kHz t = 20,40,60 min T = 30,45,60 °C |
[120] | ||
| Olive | Antioxidants (manly phenolic compounds and flavonoid) | Ethanol | 1. Volatile and easy to remove; 2.Easy to obtain; 3.General solubility; 4.By changing the concentration, the solvent polarity can be adjusted to meet the extraction needs of different solutes; 5.Can be recovered and reused by methods such as distillation, helping to reduce costs |
P = 400 W f = 24 kHz t = 15 min A = 70 % |
[121] |
| Hibiscus sabdariffa calyces | Anthocyanins | P = 296.6 W f = 20 kHz t = 26.1 min T = 30–35 °C |
[122] | ||
| Olive pomace | Hydroxytyrosol Maslinic acid Oleanolic acid |
f = 60 kHz t = 5 min T = 50 °C |
[123] | ||
| Pyrus communis ‘Starkrimson’ fruit peel | Cyanidin-3-O-galactoside | P = 162 W t = 11 min T = 71 °C |
[124] | ||
| Raphanus sativus L | Oil | P = 165 W f = 25 kHz t = 20,40,60 min T = 30,45,60 °C |
[125] | ||
| Cannabis | Cannabinoids Terpenes |
P = 450 W f = 20 kHz t = 30 min T = 50 °C |
[86] | ||
| Chinese propolis | Polyphenols | P = 135 W f = 22 kHz t = 20 min T = 40 °C |
[126] |
Note: P: Power; f: Frequency; t: Time; T: Temperature.
5.2. The use of UAEE in combination with other technologies
The extraction effect of using UAEE alone can be further improved. In order to enhance the extraction efficiency and improve the biological activity of the desired extract, UAEE can be combined with various emerging extraction techniques. Fig. 10 illustrates the microwave-assisted extraction (MAE) technologies, enzymatic assisted extraction (EAE) technologies, pulsed electric field-assisted extraction (PEFAE) technologies, and supercritical fluid extraction (SFE) technologies used in combination with UAEE.
Fig. 10.
The combination of UAEE and other extraction technologies.
SFE is an environmentally friendly process that utilizes supercritical fluids as solvents to extract compounds from solid substrates [127]. In the majority of SFE experiments, carbon dioxide is commonly employed as the preferred solvent. CO2 is non-toxic, non-flammable, cost-effective, and possesses a low critical temperature. It can be efficiently recycled during the extraction process and easily removed afterward. Additionally, CO2 exhibits high solubility for non-polar molecules, making it an ideal solvent in SFE [128]. The high diffusivity and low surface tension of SFE enable the solvent to better penetrate the sample. UAE can break the aggregation and agglomeration of particles. This facilitates the penetration of the supercritical fluid into the sample. So, the combination of UAE and SFE with excellent solubility can significantly improve extraction efficiency. SFE can selectively extract the required compounds by adjusting the pressure, temperature and ratio of supercritical fluid. So that, the combination of UAE and SFE can extract target components more quickly and accurately. By combining the high efficiency of UAE and the high selectivity of SFE towards target components, USFE can achieve more significant extraction results.
MAE accomplishes the goal of extraction by applying an electric field to the dielectric material. The ionic conduction and rotation of the dipoles contribute to the heating of the solvent-sample matrix. The particles are moved by the electric field generated by microwave energy, while the rotation of dipoles leads to the alternating displacement of polar molecules [129]. On the other hand, when high-energy microwaves penetrate the solvent and the cell wall of plants, they transfer energy to the cytoplasm, resulting in an increase in temperature and pressure within the cell. Once the pressure reaches a specific threshold, the cell wall undergoes swelling, leading to its rupture. As a consequence, the bioactive components present within the cell are released into the extract [130]. Microwave heating primarily affects molecules via ionic conduction and dipole rotation, making it selective and targeted depend on the material's dielectric constant. This enables specific components to be heated while leaving others relatively unaffected. When combined, the synergistic effect of microwave and ultrasonic provides high momentum and energy. This synergistic action leads to the rupture of plant cells and enhances the elution of active compounds into the extraction solvent. As a consequence, extraction processes benefit from shorter extraction times and lower solvent consumption. From another perspective, UAE and MAE are complementary. The mechanical oscillation and stirring of UAE can effectively compensate for the uniformity of microwave heating, and the microwave heating effect can compensate for the shortcomings of ultrasonic heating. Overall, the combination of UAE and MAE offers an efficient and effective approach for extraction, particularly for selectively targeting specific compounds while minimizing the impact on other components. The combination of ultrasonic assisted extraction and microwave-assisted extraction (UMAE) has been shown to be a highly efficient and cost-effective extraction technique. Wen et al. [131] examined the impact of different extraction methods, including conventional solvent extraction (CSE), UAE, MAE and UMAE, on the extraction efficiency of soluble dietary fiber (SDF) from coffee silver skin. The findings indicated that the yield of SDF from UMAE (42.7 ± 0.4 %) was 1.5, 1.9 and 1.2 times higher than that from CSE, UAE and MAE, respectively. Also, Wang et al. [132] used UMAE to extract flavonoid compounds from Eucommia ulmoides leaves. The findings indicated that the extraction yield of UMAE was 2.454 % higher than that of ultrasonic and microwave extraction.
It has been reported that UAE with enzymes can enhance the extraction efficiency of bioactive components, because the combination of UAE and MAE can accelerate the catalytic action of enzymes, increase the reaction rate between enzymes and substrates, and further improve extraction efficiency. The combination of these two techniques is called ultrasonic enzyme assisted extraction (UEAE). Wu et al. [133] studied the enzymatic extraction of polysaccharides from pumpkin with and without ultrasonic. Compared with EAE alone and UAE alone, ultrasonic and enzyme action together can increase the extraction efficiency of polysaccharides. Also, Tchabo et al. [134] observed a substantial improvement in the yield of UEAE compared with UAE alone and EAE alone. Therefore, the use of UEAE has great potential.
The principle of PEFAE is that an electric field is applied to the cell wall, causing the cell to decompose. The number and size of the generated holes are directly related to the applied electric field pulse and intensity. Pulse electric fields can change the permeability of cell membranes and promote the release of intracellular substances. Combined with the mechanism of ultrasonic, the extraction efficiency and yield are further accelerated. Manzoor et al. [135] studied the combined effects of PEFAE and UAE on the bioactive compounds in almond extract. The initial step involved the application of PEFAE to almond extract, followed by subsequent treatment with UAE. The combined treatment exhibits the highest values of total phenolics, total flavonoids, condensed tannins, anthocyanin contents and antioxidant activity in DPPH, reducing power and metal chelating activity surpassing other treatments. The findings support the notion that the combination of non-thermal technologies serves as a beneficial alternative technology in the food processing industry. Moreover, the combined processing of different technologies can produce safe, healthy, and high-quality products, which increases their market value. Table 3 summarizes recent studies on the use of UAEE in combination with other emerging extraction technologies.
Table 3.
The combination of UAEE and other emerging extraction technologies.
| Extraction technology |
Matrix | Extractive | Benefit | Extraction conditions | Ref. |
|---|---|---|---|---|---|
| USFE | Oregano | Antioxidants | After installing UAEE on SFE, the total phenolic content of the extract increased by 9–38 %, and the antioxidant capacity increased by 10–86 % | UAE: Pd = 150 W/L; f = 30 kHz; t = 60 min; T = 35 °C SFE: S: CO2 (1 kg/h) |
[62] |
| Scutellaria barbata |
Flavonoids | The yields of flavonoids of USFE were 24.64–27.74 % and 18.63–19.03 % higher than that of UAE and SFE, and the extraction time were 1.14 times and twice shorter | UAE: P = 185 W; f = 40 kHz SFE: S: CO2 (0.27–0.85 g/min) |
[136] | |
| Iberis amara seed |
Oil | The oil yield of USFE is 28 % higher than that of SFE. USFE improves the quality of oil, slightly improves the selectivity of monounsaturated fatty acids in the obtained oil, and improves the content of plant compounds and antioxidant activity of oil. | UAE: Pd = 2.5 W/mL; f = 25 kHz; t = 15 min; T = 60 ℃ SFE: S: CO2 (3 mL/min); t = 80 min |
[137] | |
| Ginger | Total phenols | Total phenols content (expressed as mg of GAE per 100 g) UAE: 1342.59 ± 56.75 SFE: 827.38 ± 6.58 USFE: 1455.37 ± 12.34 |
UAE: f = 40 kHz; t = 30 min; T = 25 ℃ SFE: S: CO2 |
[138] | |
| Iberis amara |
Flavonoids | The total flavonoids content of USFE is 18 % higher than that of SFE. USFE reduces extraction time, CO2 and energy consumption, while also increasing flavonoid concentrations and antioxidant activity | UAE: Pd = 0.34 W/mL; f = 40 kHz SFE: S: CO2 (0.5 g/min) |
[139] | |
|
Cosmos sulphureus seed |
Oil | Total phenolics content SFE: 95.79 mg GAE/kg oil USFE: 102.85 mg GAE/kg oil Oil yield SFE: 123.44 mg/g USFE: 194.69 mg/g |
UAE: P = 800 W; f = 40 kHz; t = 15 min SFE: S: CO2 (0.1–0.6 g/min) |
[173] | |
| Mustard seed | Sinapine | Sinapine content UAE: 5.531 mg/g SFE: 6.636 mg/g USFE: 7.194 mg/g |
UAE: P = 400 W; f = 24 kHz SFE: S: CO2 (60 g/min) |
[174] | |
| UMAE | Sweet potatoes |
Prebiotic oligosaccharides(PPOS4,PPOS5) | Yield of PPOS4 MAE:1.247 UAE:1.286 UMAE:1.472 Yield of PPOS5 MAE:5.026 UAE:4.824 UMAE:5.476 |
UAE: P = 300 W; f = 25 kHz; t = 100 s MAE: P = 200 W; f = 2450 MHz |
[140] |
| Potato pulp | Pectin | UMAE proved effective in enhancing potato pectin extraction efficiency, with a maximum yield of 22.86 % | UAE: P = 50 W; f = 40 kHz; t = 50 min; T = 93 °C MAE: P = 10–800 W; f = 2450 MHz |
[141] | |
| Brown Macroalgae | Fucose-sulphated polysaccharides; Soluble carbohydrates; Phenolic compounds |
UMAE outperformed UAE and MAE individually in terms of compound yields. However, the antioxidant properties of the extracts did not exhibit substantial enhancements with the use of UAE, MAE, or UMAE | UAE: P = 500 W; f = 20 kHz; t = 2, 5 min; A = 20, 50, 100 % MAE: P = 250,600,1000 W; f = 2450 MHz; t = 2, 5 min |
[142] | |
| Canola | Oil | UMAE exhibited a synergistic effect, resulting in improved oil yield compared to UAE | UAE: P = 500 W; f = 20 kHz MAE: P = 607 W; t = 5 min |
[143] | |
| Bark | Phenolic compounds | The optimum yield reached 15.72 %. UMAE doubles the extraction yield and reduces the extraction time 47 times. | UAE: P = 500 W; f = 20 kHz; A = 0–100 % MAE: P = 100–300 W; t = 30–120 s |
[144] | |
| Arabica coffee bean | Oil | UMAE combines ultrasonic cavitation and microwave irradiation, causing severe surface structure damage and facilitating oil extraction | UAE: P = 50 W; f = 40 kHz MAE: P = 350 W; t = 10 min; T = 70 °C |
[145] | |
| Solanum lycopersicum | Pectin | UAME gave highest dissolution rate and lower degradation rate compared to UAE. There was very less difference in the yield of MAE and UMAE extracted pectin, But the difference in esterification degree is 59.76 % and 73.33 %, respectively. | UAE: P = 450 W; f = 20 kHz MAE: P = 540 W; f = 2450 MHz; t = 4 min |
[146] | |
| Fresh coffee fruit | Oil | UMAE achieved the highest oil extraction yield (10.58 %), followed by MAE and UAE with yields of 9.34 % and 9.06 %, respectively | UAE: P = 50 W; f = 40 kHz MAE: P = 350 W; t = 10 min; T = 60 °C |
[147] | |
| Tomato | Pectin | Pectin yield (%) UAE: 28.25 ± 2.14bc MAE: 30.12 ± 0.12b UMAE: 34.06 ± 1.11a |
UAE: f = 37 kHz; t = 20 min; T = 80 °C MAE: P = 300 W; f = 2450 MHz; t = 10 min; T = 70–100 °C |
[148] | |
| Terminalia chebula fruit pulp | Phenolic compounds; Flavonoid |
Total phenolic compounds UAE: 305.77 mg GAE/g MAE: 301.91 mg GAE/g UMAE: 346.05 mg GAE/g Total flavonoid content UAE: 98.02 mg QE/g MAE: 95.68 mg QE/g UMAE: 116.93 mg QE/g Antioxidant capacity UAE: 89.51 % MAE: 90.49 % UMAE: 91.57 % |
UAE: P = 500 W; t = 20 min; A = 50 % MAE: P = 365 W; t = 5 min |
[172] | |
| UEAE | Sisal waste | Pectin | Pectin yield (%) EAE: 9.4 UAE: 11.9 UEAE: 31.1 |
UAE: P = 450 W; f = 20 kHz; t = 60 min Enzyme: Endo-glucanase |
[149] |
| Pomegranate seeds |
Oil | Ultrasonic usage increased yield of EAE by 18.4 % and reduced extraction time by 91.7 % | UAE: P = 130 W; f = 20 kHz; T = 55 °C Enzyme: Cellulase and Peclyve V |
[150] | |
|
Polygonum cuspidatum |
Resveratrol | UEAE exhibited a 43.6 % increase in resveratrol extraction yield compared to UAE | UAE: f = 53 kHz; t = 30 min; T = 25 °C Enzyme: Polydatin-β-glucosidase |
[151] | |
| Sesame bran | Protein; Phenolic |
Protein yield (%) EAE: 79.3 UAE: 59.8 UEAE: 87.9 The highest protein yield, total phenolic compound and antioxidant capacities were found in UEAE |
UAE: P = 836 W; f = 35 kHz; t = 98 min; T = 43 °C Enzyme: Viscozyme L and Alcalase |
[152] | |
| Date seed | Oil | Combination of UEAE and cooking pretreatment can improve the oil extraction efficiency and the quality of extracted oil | UAE: P = 1000 W; t = 60 min Enzyme: Cellulase enzyme |
[153] | |
| Raspberry wine residues |
Anthocyanins | Anthocyanins yield EAE: 0.75 mg/g UEAE: 0.85 mg/g The UEAE extracts exhibited superior antioxidant activities |
UAE: P = 290 W; t = 30 min; T = 44 °C Enzyme: Pectinase |
[154] | |
| Glutinous rice bran |
Soluble dietary fiber | Compared with the soluble dietary fiber obtained from hot water extraction, the whiteness, solubility, water holding capacity, and swelling properties of soluble dietary fiber extracted by UEAE improved significantly. | UAE: P = 50 W; f = 40 kHz; T = 50 °C Enzyme: Cellulase |
[155] | |
| UPEF | Almond | Phenolics; Flavonoids; Condense tannins; Anthocyanin contents |
Total phenolics (mg GAE/g dried extract) UAE: 18.38 ± 0.09c PEFAE: 19.22 ± 0.12b UPEF: 21.20 ± 0.06a Total flavonoids (mg CE/g dried extract) UAE: 8.41 ± 0.058c PEFAE: 8.60 ± 0.041b UPEF: 9.72 ± 0.051a Condense tannin content (mg CE/g dried extract) UAE: 1.05 ± 0.008c PEFAE: 1.18 ± 0.007b UPEF: 1.48 ± 0.013a Total anthocyanins (mg/L) UAE: 1.051 ± 0.03b PEFAE: 1.103 ± 0.04b UPEF: 1.283 ± 0.09a |
UAE: P = 200 W; f = 40 kHz; t = 20 min; T = 35 °C PEFAE: Pulse width = 500 μs; f = 1 kHz; Electric field = 18 kV·cm−1 |
[135] |
| Fresh rosemary and thyme by-products |
Phenolic compounds | UPEF enhanced phenolic and antioxidant recovery in rosemary and thyme by-products compared to UAE | UAE: P = 400 W; f = 24 kHz;A = 50 %; t = 4.16, 6.24, 12.48 min; T = 40 °C PEFAE: Pulse width = 30 μs; f = 10 Hz; Electric field = 1.1 kV·cm−1 |
[68] | |
| Pericarpium citri reticulatae | Flavonoids | Total flavonoid content UAE: 14.73 ± 0.98b mg/g PEFAE: 14.53 ± 1.23b mg/g UPEF: 15.59 ± 0.77a mg/g |
UAE: P = 180 W; f = 40 kHz; t = 30 min; T = 60 °C PEFAE: Pulse width = 50 μs; Electric field = 5 kV·cm−1 |
[156] | |
| Selenium-enriched tea | Polysaccharides | The combination of PEFAE and UAE showed promise in achieving higher extraction yields with a lower specific energy consumption rate | UAE: P = 400 W; t = 60 min PEFAE: Electric field = 10 kV·cm−1 |
[157] | |
| Tomato | Lycopene | Lycopene content UAE: 2.96 % PEFAE: 3.01 % UPEF: 3.56 % |
UAE: P = 100 W; A = 60 %, 80 %, 100 %; t = 20 min; T = 30 °C PEFAE: Electric field = 10 kV·cm−1 |
[175] | |
| Olive | Oil | Oil Yields UAE: 17.8 % UPEF: 18.1 % |
UAE: P = 600 W; f = 22 kHz PEFAE: Pulse width = 30 µs; f = 15 Hz; Electric field = 1.6 kV/cm−1 |
[176] |
Note: P: Power; Pd: Power density; f: Frequency; t: Time; T: Temperature; A: Amplitudes; S: Solvent.
5.3. Development of UAEE
In recent years, UAEE has experienced notable progress and advancements. The current status of UAEE reflects a growing emphasis on improving extraction efficiency, minimizing extraction time, and enhancing overall productivity. There is a clear emphasis on optimizing the UAEE process to achieve maximum results in terms of extraction yield and operational effectiveness. To achieve these goals, several innovative designs and technologies have emerged in the field. An example is the utilization of dual-frequency ultrasonic systems, which have been explored to generate enhanced levels of cavitation and sonochemical energy. Moreover, advancements in transducer design and configuration have resulted in improved uniformity and distribution of ultrasonic waves within the extraction medium. These developments contribute to higher extraction efficiency and more reliable outcomes in UAEE. Furthermore, the incorporation of automation and control systems has played a crucial role in enabling precise control over extraction parameters and optimizing process conditions in UAEE. These advancements have paved the way for the development of more efficient, reliable, and scalable extraction processes. As a result, ultrasonic technology is increasingly being applied across various industries, including pharmaceuticals, food processing, and natural product extraction. Nevertheless, there is a notable drawback to current UAEE technology, as it is not resistant to high temperatures. This limitation arises from the susceptibility of the ultrasonic oscillator to severe damage when exposed to prolonged high temperatures, significantly impacting the equipment's lifespan. Therefore, reducing the heat generated during processing has become one of the problems to be solved. However, researchers remain committed to continual research and development to overcome this obstacle and further improve the functionality and performance of UAEE. Table 4 shows the research status of UAEE in recent years.
Table 4.
Research status of UAEE.
| Extractive | Equipment parameters |
Diagram note |
Equipment advantages |
Equipment diagram |
Ref. |
|---|---|---|---|---|---|
| Paclitaxel from Taxus chinensis suspension cells |
P = 250 W f = 40 kHz (UC-10, Jeiotech, South Korea) P = 530 W f = 40 kHz (JAC-4020, KODO, South Korea) |
1. Mechanical stirrer; 2. Ultrasonic generator; 3. Biomass; 4. Ultrasonic; 5. Ultrasonic transducer; 6. Water; 7. Methanol |
This equipment applies mechanical stirring to the UAE process, making the liquid phase uniform and accelerating the extraction process |  |
[158] |
| Protein from Helianthus annuus L. (Sunflower) meal | P = 300 W f = 20, 28, 35, 40, 50 kHz (Designed by Meibo Biotechnology Co., Ltd) |
Dual frequencies ultrasonic equipment: 1. Ultrasonic transducer(20 kHz); 2. Ultrasonic transducer(40 kHz); 3. Sample outlet to sample vessel; 4. Water inlet; 5. Sample inlet; 6. Touch panel; 7. Water bath; 8. Sample; 9. Two pumps; 10. Ultrasonic generators; 11. Process chamber; 12. Water outlet to water bath; 13. Temperature sensor |
This equipment not only has a water temperature control system, but also has two ultrasonic probes, achieving constant temperature multi frequency ultrasonic |  |
[159] |
| Oil from Idesia polycarpa fruits |
P = 400, 600 W (Hongxianglong Inc., Beijing, China) |
Schematic of the ultrasonic circulating extraction equipment: 1. Agitator; 2. Heater; 3. Temperature sensor; 4. Inlet; 5. Outlet; 6. Ultrasonic generator; 7. Control panel |
This equipment is a dynamic UAEE that utilizes reflux-circulating controllers to achieve relative motion between the sample and the ultrasonic field | 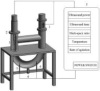 |
[160] |
| Essential oil from Iberis amara seeds |
P = 75 W f = 25 kHz The handheld ultrasonic cell pulverizer (Tenlin, TL-ST250) |
1. Condenser; 2. Ultrasonic cell pulverizer; 3. Seeds and water; 4. Heater; 5. Aqueous phase; 6. Essential oil |
This equipment is a handheld ultrasonic cell pulverizer, which achieves the combination of distillation and UAE use | 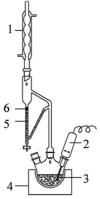 |
[161] |
| Lipid from Mortierella isabelline freeze-dried cells |
P = 400 W f = 24 kHz (Hielscher, Model UP 400S, Germany) |
1. High-intensity ultrasonic processor; 2. Soundproof box; 3. Biomass and solvent; 4. Water; 5. Jacketed reactor; 6. Water |
This equipment inserts ultrasonic probes into soundproof jacked reactor, achieving constant temperature and low noise extraction | 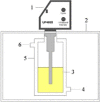 |
[162] |
| Polyphenols, Flavonoids from Camellia sinensis (green tea) leaves |
P = 500 W f = 20 kHz (Sonics & Materials Inc) |
1. Ultrasonic transducer; 2. Ultrasonic generator; 3. Thermometer; 4. Sample; 5. Oscillator; 6. Thermostat |
Combining thermostat and oscillator with UAE achieved temperature control and uniform stirring during extraction process | 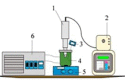 |
[116] |
| Essential oil from Cinnamomum cassia bark |
P = 300 W f = 25 kHz (JY92-IIN, Ningbo xinzhi biotechnology Co., Ltd., China) |
1. Ultrasonic transducer; 2. Sample; 3. Water outlet; 4. Condenser; 5. Water inlet; 6. Heater |
This equipment combines hydrodistillation and UAE, resulting in a 27 % increase in the cinnamon oil yield | 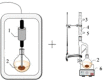 |
[118] |
| Anthocyanin from Strawberry | P = 120 W f = 62–64 kHz |
1. To computer; 2. Signal converter; 3. Stirrer; 4. Extraction tank; 5. Water outlet; 6. Generator and control unit; 7. Transducers; 8. Vibrating plate; 9. Water inlet |
This equipment achieves frequency search in different extraction tanks by connecting to a computer, and can achieve frequency band retrieval from 18 to 90 kHz. Ultimately, the optimal ultrasonic frequency band retrieved is 62 to 64 kHz | 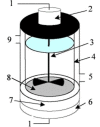 |
[163] |
Note: P: Power; f: Frequency.
6. Conclusions and future trends
This review provides a comprehensive overview of the principles, advantages, and applications of UAE, as well as the types and modes of UAEE based on the principles of UAE. The analysis reveals that the majority of studies in this field have utilized commercially available UAEE, primarily operating at frequencies of 20 kHz and 40 kHz. Interestingly, there is limited utilization of pilot-scale UAEE, indicating a gap in research investigations conducted at larger extraction scales. Therefore, there are still some technical challenges that need to be overcome, including issues arising from the expansion of equipment size during pilot-scale production, ensuring stable equipment operation, and verifying whether the sound field energy of the equipment is sufficient to meet the necessary requirements. To overcome these challenges, it is essential to conduct in-depth research and drive technological innovation. Additionally, there is significant potential for enhancing the work efficiency and extraction effectiveness of UAEE in order to meet the increasing demands of production. In further research on UAEE, specific attention should be given to the following areas to drive improvements and optimizations. By focusing on these key aspects, researchers can pave the way for advancements in the field of UAEE. Firstly, there is a need to enhance the design of UAEE, aiming to reduce costs and improve their usability, making them more accessible and suitable for enterprises of all sizes. Additionally, a comprehensive investigation into the interaction mechanism between ultrasonic waves and materials is essential in order to gain a deeper understanding of the UAE. Such research will provide precise insights and guidance for the application of UAEE in various industries. Furthermore, ultrasonic has the capacity to generate substantial heat, which profoundly influences the bioavailability, extraction efficiency, and extraction yield. Contemplating strategies to mitigate the heat generated during the UAE is thus an avenue for future development. Furthermore, because the UAEE is susceptible to serious damage when exposed to high temperatures for long periods of time, the service life of the equipment is affected. Therefore, reducing the heat generated during processing is also one of the unresolved problems. Finally, to further enhance equipment performance and control capabilities, it is crucial to explore innovative ultrasonic technologies. For instance, investing in efficient energy conversion systems and online monitoring technologies can lead to significant improvements. By exploring these areas, researchers can optimize the performance of UAEE, enabling more precise control over the process. This exploration of new ultrasonic technologies will ultimately result in improved equipment capabilities, providing valuable advancements for industrial applications.
The future development of UAEE will primarily emphasize enhancing efficiency, reducing costs, improving safety and reliability, and expanding its application range. It is essential to strengthen the widespread adoption of ultrasonic technology, enabling more enterprises to obtain its benefits. As a result of these actions, enterprises will experience enhanced economic benefits, while also making noteworthy contributions to the research, development, and application of bioactive component extraction. This collaborative endeavor will not only drive advancements in UAEE but also enable its seamless integration into various industries. By focusing on efficiency, cost reduction, safety, and reliability, the development of UAEE will open up new possibilities and create a solid foundation for its widespread adoption and utilization across industries. As technology continues to advance, the future of UAEE holds the promise of significant improvements in intelligence and automation. These advancements will enable UAEE to achieve a higher level of superiority, allowing for unified automatic operation that can effectively meet diverse application requirements. This means that UAEE will become more intelligent and adaptive, capable of optimizing operations to deliver optimal results in various industries. By embracing these advancements, UAEE will play a crucial role in revolutionizing the extraction process and addressing the evolving needs of different applications.
CRediT authorship contribution statement
Lipeng Shen: Writing – original draft, Writing – review & editing, Formal analysis. Shuixiu Pang: Formal analysis. Mingming Zhong: Formal analysis. Yufan Sun: Formal analysis. Abdul Qayum: Writing – review & editing, Formal analysis. Yuxuan Liu: Writing – review & editing, Formal analysis. Arif Rashid: Formal analysis. Baoguo Xu: Formal analysis. Qiufang Liang: Writing – review & editing. Haile Ma: Supervision. Xiaofeng Ren: Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to express their appreciation for the support obtained from the Natural Science Foundation of China (Grants No. 32372473&32072355); Youth Foundation of Jiangsu Provence (BK20220525); General Program of Jiangsu Education Department (22KJB550004); Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data availability
No data was used for the research described in the article.
References
- 1.Wen C.T., Zhang J.X., Zhang H.H., Dzah C.S., Zandile M., Duan Y.Q., Ma H.L., Luo X.P. Advances in ultrasound assisted extraction of bioactive compounds from cash crops - A review. Ultrason. Sonochem. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants-Basel. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen Y.M., Hao X.J. Natural product sciences: an integrative approach to the innovations of plant natural products. Sci. China Life Sci. 2020;63:1634–1650. doi: 10.1007/s11427-020-1799-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belwal T., Ezzat S.M., Rastrelli L., Bhatt I.D., Daglia M., Baldi A., Devkota H.P., Orhan I.E., Patra J.K., Das G., Anandharamakrishnan C., Gomez-Gomez L., Nabavi S.F., Nabavi S.M., Atanasov A.G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies, Trac-Trend. Anal. Chem. 2018;100:82–102. doi: 10.1016/j.trac.2017.12.018. [DOI] [Google Scholar]
- 5.Calinescu I., Vinatoru M., Ghimpeteanu D., Lavric V., Mason T.J. A new reactor for process intensification involving the simultaneous application of adjustable ultrasound and microwave radiation. Ultrason. Sonochem. 2021;77 doi: 10.1016/j.ultsonch.2021.105701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei Z.F., Wang X.Q., Peng X., Wang W., Zhao C.J., Zu Y.G., Fu Y.J. Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind. Crop Prod. 2015;63:175–181. doi: 10.1016/j.indcrop.2014.10.013. [DOI] [Google Scholar]
- 7.Chemat F., Rombaut N., Sicaire A.-G., Meullemiestre A., Fabiano-Tixier A.-S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review, Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Baiao Dias A.L., de Aguiar A.C., Rostagno M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrason. Sonochem. 2021;74:105584. doi: 10.1016/j.ultsonch.2021.105584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q.W., Lin L.G., Ye W.C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med.-Uk. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.F. Chemat, M. Abert Vian, A.-S. Fabiano-Tixier, M. Nutrizio, A. Rezek Jambrak, P.E.S. Munekata, J.M. Lorenzo, F.J. Barba, A. Binello, G. Cravotto, A review of sustainable and intensified techniques for extraction of food and natural products, Green Chem. 22 (2020) 2325-2353. https://doi.org/10.1039/c9gc03878g.
- 11.Kadam S.U., Tiwari B.K., O'Donnell C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agr. Food Chem. 2013;61:4667–4675. doi: 10.1021/jf400819p. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y., Zhang M., Fang Z. Efficient physical extraction of active constituents from edible fungi and their potential bioactivities: a review. Trends Food Sci. Tech. 2020;105:468–482. doi: 10.1016/j.tifs.2019.02.026. [DOI] [Google Scholar]
- 13.Dzah C.S., Duan Y., Zhang H., Wen C., Zhang J., Chen G., Ma H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020;35 doi: 10.1016/j.fbio.2020.100547. [DOI] [Google Scholar]
- 14.Carreira-Casais A., Otero P., Garcia-Perez P., Garcia-Oliveira P., Pereira A.G., Carpena M., Soria-Lopez A., Simal-Gandara J., Prieto M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Env. Res. Pub. He. 2021;18:9153. doi: 10.3390/ijerph18179153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y., Pan Y., Liu S. Power ultrasound and its applications: A state-of-the-art review. Ultrason. Sonochem. 2020;62 doi: 10.1016/j.ultsonch.2019.104722. [DOI] [PubMed] [Google Scholar]
- 16.Fu X., Belwal T., Cravotto G., Luo Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020;60 doi: 10.1016/j.ultsonch.2019.104726. [DOI] [PubMed] [Google Scholar]
- 17.Arvanitoyannis I.S., Kotsanopoulos K.V., Savva A.G. Use of ultrasounds in the food industry-Methods and effects on quality, safety, and organoleptic characteristics of foods: A review. Crit. Rev. Food Sci. 2017;57:109–128. doi: 10.1080/10408398.2013.860514. [DOI] [PubMed] [Google Scholar]
- 18.Khadhraoui B., Turk M., Fabiano-Tixier A.S., Petitcolas E., Robinet P., Imbert R., El Maataoui M., Chemat F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason. Sonochem. 2018;42:482–492. doi: 10.1016/j.ultsonch.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Vinatoru M., Mason T.J., Calinescu I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trac-Trend Anal. Chem. 2017;97:159–178. doi: 10.1016/j.trac.2017.09.002. [DOI] [Google Scholar]
- 20.Qiu L., Zhang M., Chitrakar B., Bhandari B. Application of power ultrasound in freezing and thawing Processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105230. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari B.K. Ultrasound: A clean, green extraction technology. Trac-Trend Anal. Chem. 2015;71:100–109. doi: 10.1016/j.trac.2015.04.013. [DOI] [Google Scholar]
- 22.A.B. Tomé Constantino, E.E. Garcia-Rojas, Modifications of physicochemical and functional properties of amaranth protein isolate (Amaranthus cruentus BRS Alegria) treated with high-intensity ultrasound, J Cereal Sci. 95 (2020) 103076. https://doi.org/10.1016/j.jcs.2020.103076.
- 23.Yang R.F., Geng L.L., Lu H.Q., Fan X.D. Ultrasound-synergized electrostatic field extraction of total flavonoids from Hemerocallis citrina baroni. Ultrason. Sonochem. 2017;34:571–579. doi: 10.1016/j.ultsonch.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues S., Fernandes F.A.N. In: Ultrasound: Advances for Food Processing and Preservation. Bermudez-Aguirre D., editor. Elsevier Inc.; New York: 2017. Extraction processes assisted by ultrasound; pp. 351–368. [Google Scholar]
- 25.Zhu Z., Guan Q., Koubaa M., Barba F.J., Roohinejad S., Cravotto G., Yang X., Li S., He J. HPLC-DAD-ESI-MS2 analytical profile of extracts obtained from purple sweet potato after green ultrasound-assisted extraction. Food Chem. 2017;215:391–400. doi: 10.1016/j.foodchem.2016.07.157. [DOI] [PubMed] [Google Scholar]
- 26.Giacometti J., Kovacevic D.B., Putnik P., Gabric D., Bilusic T., Kresic G., Stulic V., Barba F.J., Chemat F., Barbosa-Canovas G., Jambrak A.R. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018;113:245–262. doi: 10.1016/j.foodres.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Nn A. A review on the extraction methods use in medicinal plants, principle, strength and limitation. J. Appl. Res. Med. Aroma. 2015;4:1–6. doi: 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- 28.Mnayer D., Fabiano-Tixier A.-S., Petitcolas E., Ruiz K., Hamieh T., Chemat F. Extraction of green absolute from thyme using ultrasound and sunflower oil. Resour.-Effic. Technol. 2017;3:12–21. doi: 10.1016/j.reffit.2017.01.007. [DOI] [Google Scholar]
- 29.Preece K.E., Hooshyar N., Krijgsman A., Fryer P.J., Zuidam N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. 2017;113:94–101. doi: 10.1016/j.cep.2016.09.003. [DOI] [Google Scholar]
- 30.Zhang L., Wang X., Hu Y., Fakayode O.A., Ma H., Zhou C., Hu Z., Xia A., Li Q. Dual-frequency multi-angle ultrasonic processing technology and its real-time monitoring on physicochemical properties of raw soymilk and soybean protein. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X., Li Y., Li S., Oladejo A.O., Wang Y., Huang S., Zhou C., Wang Y., Mao L., Zhang Y., Ma H., Ye X. Effects of low power density multi-frequency ultrasound pretreatment on the enzymolysis and the structure characterization of defatted wheat germ protein. Ultrason. Sonochem. 2017;38:410–420. doi: 10.1016/j.ultsonch.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y., Ma H., Wang K., Azam S.M.R., Wang Y., Zhou J., Qu W. Ultrasound frequency effect on soybean protein: Acoustic field simulation, extraction rate and structure. LWT. 2021;145 doi: 10.1016/j.lwt.2021.111320. [DOI] [Google Scholar]
- 33.Yang X., Li Y., Li S., Oladejo A.O., Ruan S., Wang Y., Huang S., Ma H. Effects of ultrasound pretreatment with different frequencies and working modes on the enzymolysis and the structure characterization of rice protein. Ultrason. Sonochem. 2017;38:19–28. doi: 10.1016/j.ultsonch.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Jin J., Ma H., Wang B., Yagoub A.-E.-G.-A., Wang K., He R., Zhou C. Effects and mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason. Sonochem. 2016;30:44–51. doi: 10.1016/j.ultsonch.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Huang L., Ding X., Li Y., Ma H. The aggregation, structures and emulsifying properties of soybean protein isolate induced by ultrasound and acid. Food Chem. 2019;279:114–119. doi: 10.1016/j.foodchem.2018.11.147. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q.A., Shen H., Fan X.H., Shen Y., Wang X., Song Y. Changes of gallic acid mediated by ultrasound in a model extraction solution. Ultrason. Sonochem. 2015;22:149–154. doi: 10.1016/j.ultsonch.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y., Sun J., Xu D., Wang S., Yuan Y., Cao Y. Investigation of (+)-catechin stability under ultrasonic treatment and its degradation kinetic modeling. J. Food Process Eng. 2018;41:e12904. [Google Scholar]
- 38.A. Mehmood, M. Ishaq, L. Zhao, S. Yaqoob, B. Safdar, M. Nadeem, M. Munir, C. Wang, Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoria ternatea L.), Ultrason Sonochem. 51 (2019) 12-19. https://doi.org/10.1016/j.ultsonch.2018.10.013. [DOI] [PubMed]
- 39.Kwaw E., Ma Y., Tchabo W., Apaliya M.T., Sackey A.S., Wu M., Xiao L. Impact of ultrasonication and pulsed light treatments on phenolics concentration and antioxidant activities of lactic-acid-fermented mulberry juice. LWT. 2018;92:61–66. doi: 10.1016/j.lwt.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Kang Q., Chen S., Li S., Wang B., Liu X., Hao L., Lu J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2018;124:1137–1144. doi: 10.1016/j.ijbiomac.2018.11.215. [DOI] [PubMed] [Google Scholar]
- 41.Iida Y., Tuziuti T., Yasui K., Towata A., Kozuka T. Control of viscosity in starch and polysaccharide solutions with ultrasound after gelatinization. Innov. Food Sci. Emerg. 2008;9:140–146. doi: 10.1016/j.ifset.2007.03.029. [DOI] [Google Scholar]
- 42.Xu Y., Guo Y., Duan S., Wei H., Liu Y., Wang L., Huo X., Yang Y. Effects of ultrasound irradiation on the characterization and bioactivities of the polysaccharide from blackcurrant fruits. Ultrason. Sonochem. 2018;49:206–214. doi: 10.1016/j.ultsonch.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Oprescu E.E., Enascuta C.-E., Radu E., Ciltea-Udrescu M., Lavric V. Does the ultrasonic field improve the extraction productivity compared to classical methods-Maceration and reflux distillation? Chem. Eng. Process. 2022;179 doi: 10.1016/j.cep.2022.109082. [DOI] [Google Scholar]
- 44.A. Alupului, I. Calinescu, V. Lavric, Ultrasonic vs. Microwave Extraction Intensification of Active Principles from Medicinal Plants, Icheap-9: 9Th International Conference On Chemical And Process Engineering, PTS 1-3. 17 (2009) 1023-1028. https://doi.org/10.3303/CET0917171.
- 45.Panadare D.C., Gondaliya A., Rathod V.K. Comparative study of ultrasonic pretreatment and ultrasound assisted three phase partitioning for extraction of custard apple seed oil. Ultrason. Sonochem. 2020;61 doi: 10.1016/j.ultsonch.2019.104821. [DOI] [PubMed] [Google Scholar]
- 46.Bilgin M., Sahin S., Dramur M.U., Sevgili L.M. Obtaining scarlet sage (salvia coccinea) extract through homogenizer-and ultrasound-assisted extraction methods. Chem. Eng. Commun. 2013;200:1197–1209. doi: 10.1080/00986445.2012.742434. [DOI] [Google Scholar]
- 47.Spigno G., De Faveri D.M. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J. Food Eng. 2007;78:793–801. doi: 10.1016/j.jfoodeng.2005.11.020. [DOI] [Google Scholar]
- 48.Zhang L., Liu Z. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008;15:731–737. doi: 10.1016/j.ultsonch.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Lavilla I., Bendicho C. In: Water Extraction of Bioactive Compounds. González H.D., Muñoz M.J.G., editors. Elsevier Inc.; New York: 2017. Fundamentals of ultrasound-assisted extraction; pp. 291–316. [Google Scholar]
- 50.Zhu Z., Zhu W., Yi J., Liu N., Cao Y., Lu J., Decker E.A., McClements D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018;106:853–861. doi: 10.1016/j.foodres.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 51.Wang C., Yang H., Li J. Combination of microwave, ultrasonic, enzyme assisted method for curcumin species extraction from turmeric (Curcuma Longa L.) and evaluation of their antioxidant activity. eFood. 2021;2:73–80. doi: 10.2991/efood.k.210329.001. [DOI] [Google Scholar]
- 52.Nipornram S., Tochampa W., Rattanatraiwong P., Singanusong R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018;241:338–345. doi: 10.1016/j.foodchem.2017.08.114. [DOI] [PubMed] [Google Scholar]
- 53.Ćurko N., Kelšin K., Jambrak A.R., Tomašević M., Gracin L., Poturica V., Ružman E., Ganić K.K. The effect of high power ultrasound on phenolic composition, chromatic characteristics, and aroma compounds of red wines. Croatian J. Food Sci. Technol. 2017;9:136–144. doi: 10.17508/CJFST.2017.9.2.08. [DOI] [Google Scholar]
- 54.Zhu W., Xue X., Zhang Z. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2016;91:132–142. doi: 10.1016/j.ijbiomac.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 55.Kobus Z., Krzywicka M., Pecyna A., Buczaj A. Process efficiency and energy consumption during the ultrasound-assisted extraction of bioactive substances from hawthorn berries. Energies. 2021;14:7638. doi: 10.3390/en14227638. [DOI] [Google Scholar]
- 56.Yusoff I.M., Taher Z.M., Rahmat Z., Chua L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022;151 doi: 10.1016/j.foodres.2022.111268. [DOI] [PubMed] [Google Scholar]
- 57.Niazi S., Hashemabadi S.H., Noroozi S. Numerical simulation of operational parameters and sonoreactor configurations for the highest possibility of acoustic cavitation in crude oil. Chem. Eng. Commun. 2014;201:1340–1359. doi: 10.1080/00986445.2013.808999. [DOI] [Google Scholar]
- 58.Panda D., Manickam S. Cavitation technology-the future of greener extraction method: a review on the extraction of natural products and process intensification mechanism and perspectives. Appl. Sci.-Basel. 2019;9:766. doi: 10.3390/app9040766. [DOI] [Google Scholar]
- 59.Amarillo M., Perez N., Blasina F., Gambaro A., Leone A., Romaniello R., Xu X.Q., Juliano P. Impact of sound attenuation on ultrasound-driven yield improvements during olive oil extraction. Ultrason. Sonochem. 2019;53:142–151. doi: 10.1016/j.ultsonch.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 60.Ranjha M.M.A.N., Irfan S., Lorenzo J.M., Shafique B., Kanwal R., Pateiro M., Arshad R.N., Wang L., Nayik G.A., Roobab U., Aadil R.M. Sonication, a potential technique for extraction of phytoconstituents: a systematic review. Processes. 2021;9:1406. doi: 10.3390/pr9081406. [DOI] [Google Scholar]
- 61.Bhadange Y.A., Saharan V.K., Sonawane S.H., Boczkaj G. Intensification of catechin extraction from the bark of Syzygium cumini using ultrasonication: Optimization, characterization, degradation analysis and kinetic studies. Chem. Eng. Process. 2022;181 doi: 10.1016/j.cep.2022.109147. [DOI] [Google Scholar]
- 62.Santos-Zea L., Antunes-Ricardo M., Gutierrez-Uribe J.A., Garcia-Perez J.V., Benedito J. Effect of ultrasound transducer design on the acoustically-assisted supercritical fluid extraction of antioxidants from oregano. Ultrason. Sonochem. 2018;47:47–56. doi: 10.1016/j.ultsonch.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 63.Son Y., No Y., Kim J. Geometric and operational optimization of 20-kHz probe-type sonoreactor for enhancing sonochemical activity. Ultrason. Sonochem. 2020;65 doi: 10.1016/j.ultsonch.2020.105065. [DOI] [PubMed] [Google Scholar]
- 64.Bellumori M., Innocenti M., Binello A., Boffa L., Mulinacci N., Cravotto G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound-and microwave-assisted extraction procedures. C. R. Chim. 2016;19:699–706. doi: 10.1016/j.crci.2015.12.013. [DOI] [Google Scholar]
- 65.Xu D.P., Zhou Y., Zheng J., Li S., Li A.N., Li H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of jatropha integerrima by response surface methodology. Molecules. 2016;21:18. doi: 10.3390/molecules21010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ummat V., Sivagnanam S.P., Rajauria G., O'Donnell C., Tiwari B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci Tech. 2021;110:90–106. doi: 10.1016/j.tifs.2021.01.018. [DOI] [Google Scholar]
- 67.Liu Y., Zhang D., Li X., Xiao J., Guo L. Enhancement of ultrasound-assisted extraction of sulforaphane from broccoli seeds via the application of microwave pretreatment. Ultrason. Sonochem. 2022;87 doi: 10.1016/j.ultsonch.2022.106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tzima K., Brunton N.P., Lyng J.G., Frontuto D., Rai D.K. The effect of Pulsed Electric Field as a pre-treatment step in Ultrasound Assisted Extraction of phenolic compounds from fresh rosemary and thyme by-products. Innov. Food Sci. Emerg. 2021;69 doi: 10.1016/j.ifset.2021.102644. [DOI] [Google Scholar]
- 69.Maran J.P., Manikandan S., Nivetha C.V., Dinesh R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017;10:S1145–S1157. doi: 10.1016/j.arabjc.2013.02.007. [DOI] [Google Scholar]
- 70.Setyaningsih W., Saputro I.E., Carrera C.A., Palma M. Optimisation of an ultrasound-assisted extraction method for the simultaneous determination of phenolics in rice grains. Food Chem. 2019;288:221–227. doi: 10.1016/j.foodchem.2019.02.107. [DOI] [PubMed] [Google Scholar]
- 71.Tekin K., Akalin M.K., Seker M.G. Ultrasound bath-assisted extraction of essential oils from clove using central composite design. Ind. Crop Prod. 2015;77:954–960. doi: 10.1016/j.indcrop.2015.09.071. [DOI] [Google Scholar]
- 72.Santos H.M., Lodeiro C., Capelo-Martinez J.-L. In: Wiley-VCH Verlag GmbH & Co. Capelo-Martinez J.L., editor. KGaA; New Jersey: 2009. The power of ultrasound; pp. 1–16. [Google Scholar]
- 73.Hoo D.Y., Li Low Z., Low D.Y.S., Tang S.Y., Manickam S., Tan K.W., Ban Z.H. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H.-P., Kang W.S., Hong C.H., Lee G.-J., Choi G., Ryu J., Jo W. In: Advanced Ceramics for Energy Conversion and Storage. Guillon O., editor. Elsevier Ltd.; New York: 2020. 5-Piezoelectrics; pp. 157–206. [Google Scholar]
- 75.Jimenez-Morales K., Castaneda-Perez E., Herrera-Pool E., Ayora-Talavera T., Cuevas-Bernardino J.C., Garcia-Cruz U., Pech-Cohuo S.C., Pacheco N. Ultrasound-assisted extraction of phenolic compounds from different maturity stages and fruit parts of cordia dodecandra A. DC.: quantification and Identification by UPLC-DAD-ESI-MS/MS. Agriculture-Basel. 2022;12:2127. doi: 10.3390/agriculture12122127. [DOI] [Google Scholar]
- 76.Petkovsek M., Dular M. Cavitation dynamics in water at elevated temperatures and in liquid nitrogen at an ultrasonic horn tip. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104652. [DOI] [PubMed] [Google Scholar]
- 77.Tzanakis I., Khavari M., Titze M., Eskin D.G. Cavitation in thermoplastic melts: New insights into ultrasound-assisted fibre-impregnation. Compos Part B-Eng. 2022;229 doi: 10.1016/j.compositesb.2021.109480. [DOI] [Google Scholar]
- 78.Fang Y., Yamamoto T., Komarov S. Cavitation and acoustic streaming generated by different sonotrode tips. Ultrason. Sonochem. 2018;48:79–87. doi: 10.1016/j.ultsonch.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Lukic K., Brncic M., Curko N., Tomasevic M., Valinger D., Denoya G.I., Barba F.J., Ganic K.K. Effects of high power ultrasound treatments on the phenolic, chromatic and aroma composition of young and aged red wine. Ultrason. Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104725. [DOI] [PubMed] [Google Scholar]
- 80.Keven Silva E., Zabot G.L., Toledo Hijo A.A.C., Meireles M.A.A. In: Ultrasound: Advances for Food Processing and Preservation. Bermudez-Aguirre D., editor. Academic Press; New York: 2017. Encapsulation of bioactive compounds using ultrasonic technology; pp. 323–350. [Google Scholar]
- 81.Jovanovic A.A., Dordevic V.B., Zdunic G.M., Pljevljakusic D.S., Savikin K.P., Godevac D.M., Bugarski B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017;179:369–380. doi: 10.1016/j.seppur.2017.01.055. [DOI] [Google Scholar]
- 82.Goltz C., Avila S., Barbieri J.B., Igarashi-Mafra L., Mafra M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind. Crop Prod. 2018;115:227–234. doi: 10.1016/j.indcrop.2018.02.013. [DOI] [Google Scholar]
- 83.Rodsamran P., Sothornvit R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019;28:66–73. doi: 10.1016/j.fbio.2019.01.017. [DOI] [Google Scholar]
- 84.Ochoa S., Durango-Zuleta M.M., Osorio-Tobon J.F. Techno-economic evaluation of the extraction of anthocyanins from purple yam (Dioscorea alata) using ultrasound-assisted extraction and conventional extraction processes. Food Bioprod. Process. 2020;122:111–123. doi: 10.1016/j.fbp.2020.04.007. [DOI] [Google Scholar]
- 85.Kaur B., Panesar P.S., Anal A.K. Standardization of ultrasound assisted extraction for the recovery of phenolic compounds from mango peels. J. Food Sci. Tech. Mys. 2022;59:2813–2820. doi: 10.1007/s13197-021-05304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Addo P.W., Sagili S.U.K.R., Bilodeau S.E., Gladu-Gallant F.-A., MacKenzie D.A., Bates J., McRae G., MacPherson S., Paris M., Raghavan V., Orsat V., Lefsrud M. Microwave- and ultrasound-assisted extraction of cannabinoids and terpenes from cannabis using response surface methodology. Molecules. 2022;27:8803. doi: 10.3390/molecules27248803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hooper M., McNeilly A.D., Schaschke C., Wilkin J.D. Is high-frequency ultrasound a useful process to add value to out of specification strawberries, raspberries and blackberries industrially? Int. J. Food Sci. Tech. 2022;57:6540–6547. doi: 10.1111/ijfs.15994. [DOI] [Google Scholar]
- 88.Mattos G.N., de Araujo Santiago M.C.P., Doria Chaves A.C.S., Rosenthal A., Tonon R.V., Correa Cabral L.M. Anthocyanin extraction from Jaboticaba Skin (Myrciaria cauliflora Berg.) using conventional and non-conventional methods. Foods. 2022;11:885. doi: 10.3390/foods11060885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garofalo S.F., Demichelis F., Mancini G., Tommasi T., Fino D. Conventional and ultrasound-assisted extraction of rice bran oil with isopropanol as solvent. Sustain. Chem. Pharm. 2022;29 doi: 10.1016/j.scp.2022.100741. [DOI] [Google Scholar]
- 90.L. Mieles-Gomez, S.E. Quintana, L.A. Garcia-Zapateiro, Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties, Gels-Basel. 9 (2023) 136. https://doi.org/10.3390/gels9020136. [DOI] [PMC free article] [PubMed]
- 91.Zhang L., Zhou C., Wang B., Yagoub A.-E.-G.-A., Ma H., Zhang X., Wu M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017;37:106–113. doi: 10.1016/j.ultsonch.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 92.Juliano P., Bainczyk F., Swiergon P., Supriyatna M.I.M., Guillaume C., Ravetti L., Canamasas P., Cravotto G., Xu X.Q. Extraction of olive oil assisted by high-frequency ultrasound standing waves. Ultrason. Sonochem. 2017;38:104–114. doi: 10.1016/j.ultsonch.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 93.Tavares G.R., Massa T.B., Goncalves J.E., da Silva C., dos Santos W.D. Assessment of ultrasound-assisted extraction of crambe seed oil for biodiesel synthesis by in situ interesterification. Renew. Energ. 2017;111:659–665. doi: 10.1016/j.renene.2017.04.065. [DOI] [Google Scholar]
- 94.Bamba B.S.B., Shi J., Tranchant C.C., Xue S.J., Forney C.F., Lim L.-T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules. 2018;23:1685. doi: 10.3390/molecules23071685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loncaric A., Celeiro M., Jozinovic A., Jelinic J., Kovac T., Jokic S., Babic J., Moslavac T., Zavadlav S., Lores M. Green extraction methods for extraction of polyphenolic compounds from blueberry pomace. Foods. 2020;9:1521. doi: 10.3390/foods9111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernandes F.A.N., Fonteles T.V., Rodrigues S., de Brito E.S., Tiwari B.K. Ultrasound-assisted extraction of anthocyanins and phenolics from jabuticaba (Myrciaria cauliflora) peel: kinetics and mathematical modeling. J. Food Sci. Tech. Mys. 2020;57:2321–2328. doi: 10.1007/s13197-020-04270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krivokapic S., Vlaovic M., Vratnica B.D., Perovic A., Perovic S. Biowaste as a potential source of bioactive compounds-a case study of raspberry fruit pomace. Foods. 2021;10:706. doi: 10.3390/foods10040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma M., Dash K.K. Microwave and ultrasound assisted extraction of phytocompounds from black jamun pulp: Kinetic and thermodynamics characteristics. Innov. Food Sci. Emerg. 2022;75 doi: 10.1016/j.ifset.2021.102913. [DOI] [Google Scholar]
- 99.Orellana-Palacios J.C., Hadidi M., Yassamine Boudechiche M., Ortega M.L.S., Gonzalez-Serrano D.J., Moreno A., Kowalczewski P.L., Bordiga M., Khanegah A.M. Extraction optimization, functional and thermal properties of protein from cherimoya seed as an unexploited by-product. Foods. 2022;11:3694. doi: 10.3390/foods11223694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spinei M., Oroian M. Structural, functional and physicochemical properties of pectin from grape pomace as affected by different extraction techniques. Int. J. Biol. Macromol. 2023;224:739–753. doi: 10.1016/j.ijbiomac.2022.10.162. [DOI] [PubMed] [Google Scholar]
- 101.Liu H.L., Hsieh C.-M. Single-transducer dual-frequency ultrasound generation to enhance acoustic cavitation. Ultrason. Sonochem. 2009;16:431–438. doi: 10.1016/j.ultsonch.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y., Zhang Y., Li S. The secondary Bjerknes force between two gas bubbles under dual-frequency acoustic excitation. Ultrason. Sonochem. 2016;29:129–145. doi: 10.1016/j.ultsonch.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 103.Xu B., Azam S.M.R., Feng M., Wu B., Yan W., Zhou C., Ma H. Application of multi-frequency power ultrasound in selected food processing using large-scale reactors: A review. Ultrason. Sonochem. 2021;81 doi: 10.1016/j.ultsonch.2021.105855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wongwuttanasatian T., Jookjantra K. Effect of dual-frequency pulsed ultrasonic excitation and catalyst size for biodiesel production. Renew. Energ. 2020;152:1220–1226. doi: 10.1016/j.renene.2020.01.149. [DOI] [Google Scholar]
- 105.Xu B., Feng M., Tiliwa E.S., Yan W., Wei B., Zhou C., Ma H., Wang B., Chang L. Multi-frequency power ultrasound green extraction of polyphenols from Pingyin rose: Optimization using the response surface methodology and exploration of the underlying mechanism. LWT. 2022;156 doi: 10.1016/j.lwt.2021.113037. [DOI] [Google Scholar]
- 106.B. Xu, J. Chen, E. Sylvain Tiliwa, W. Yan, S.M. Roknul Azam, J. Yuan, B. Wei, C. Zhou, H. Ma, Effect of multi-mode dual-frequency ultrasound pretreatment on the vacuum freeze-drying process and quality attributes of the strawberry slices, Ultrason Sonochem. 78 (2021) 105714. https://doi.org/10.1016/j.ultsonch.2021.105714. [DOI] [PMC free article] [PubMed]
- 107.Ma H., Huang L., Peng L., Wang Z., Yang Q. Pretreatment of garlic powder using sweep frequency ultrasound and single frequency countercurrent ultrasound: Optimization and comparison for ACE inhibitory activities. Ultrason. Sonochem. 2015;23:109–115. doi: 10.1016/j.ultsonch.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 108.Qu W., Sehemu R.M., Feng Y., Shi S., Wang J., Ma H., Venkitasamy C. Sonochemical effect of flat sweep frequency and pulsed ultrasound (FSFP) treatment on stability of phenolic acids in a model system. Ultrason. Sonochem. 2017;39:707–715. doi: 10.1016/j.ultsonch.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 109.Jin J., Ma H., Wang K., Yagoub A.-E.-G.-A., Owusu J., Qu W., He R., Zhou C., Ye X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015;24:55–64. doi: 10.1016/j.ultsonch.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 110.Yang X., Li Y., Li S., Oladejo A.O., Wang Y., Huang S., Zhou C., Ye X., Ma H., Duan Y. Effects of ultrasound-assisted alpha-amylase degradation treatment with multiple modes on the extraction of rice protein. Ultrason. Sonochem. 2018;40:890–899. doi: 10.1016/j.ultsonch.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 111.Ding Q., Zhang T., Niu S., Cao F., Wu-Chen R.A., Luo L., Ma H. Impact of of ultrasound pretreatment on hydrolysate and digestion products of grape seed protein. Ultrason. Sonochem. 2018;42:704–713. doi: 10.1016/j.ultsonch.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 112.Pan Z.L., Qu W.J., Ma H.L., Atungulu G.G., McHugh T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011;18:1249–1257. doi: 10.1016/j.ultsonch.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Pan Z.L., Qu W.J., Ma H.L., Atungulu G.G., McHugh T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012;19:365–372. doi: 10.1016/j.ultsonch.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 114.J. Andrea Serna-Jimenez, L. Sofia Torres-Valenzuela, G. Mejia-Arango, Evaluation and comparison in caffeine extraction under green conditions: Solvent selection and ultrasound-assisted process, J Food Process Eng. 45 (2022) e14157. https://doi.org/10.1111/jfpe.14157.
- 115.Li Y., Fabiano-Tixier A.S., Tomao V., Cravotto G., Chemat F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013;20:12–18. doi: 10.1016/j.ultsonch.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 116.M.M. Maciel Bindes, M.H. Miranda Reis, V.L. Cardoso, D.C. Boffito, Ultrasound-assisted extraction of bioactive compounds from green tea leaves and clarification with natural coagulants (chitosan and Moringa oleifera seeds), Ultrason Sonochem. 51 (2019) 111-119. https://doi.org/10.1016/j.ultsonch.2018.10.014. [DOI] [PubMed]
- 117.Taticchi A., Selvaggini R., Esposto S., Sordini B., Veneziani G., Servili M. Physicochemical characterization of virgin olive oil obtained using an ultrasound-assisted extraction at an industrial scale: Influence of olive maturity index and malaxation time. Food Chem. 2019;289:7–15. doi: 10.1016/j.foodchem.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 118.Chen G., Sun F., Wang S., Wang W., Dong J., Gao F. Enhanced extraction of essential oil from Cinnamomum cassia bark by ultrasound assisted hydrodistillation. Chinese J. Chem. Eng. 2021;36:38–46. doi: 10.1016/j.cjche.2020.08.007. [DOI] [Google Scholar]
- 119.Turker I., Isleroglu H. Optimization of extraction conditions of bioactive compounds by ultrasonic-assisted extraction from artichoke wastes. Acta Chim. Slov. 2021;68:658–666. doi: 10.17344/acsi.2021.6679. [DOI] [PubMed] [Google Scholar]
- 120.Mohamed H., Al-Hajhoj M., Al-Saikhan M., Alqahtani N., Zayed M., Moawad M., Alsenaien W., Mohamed M.E. Green extraction of date palm fruits via ultrasonic-assisted approach: optimizations and antioxidant enrichments. Processes. 2022;10:1049. doi: 10.3390/pr10061049. [DOI] [Google Scholar]
- 121.J. Carlos Martinez-Patino, B. Gullon, I. Romero, E. Ruiz, M. Brncic, J.S. Zlabur, E. Castro, Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology, Ultrason Sonochem. 51 (2019) 487-495. https://doi.org/10.1016/j.ultsonch.2018.05.031. [DOI] [PubMed]
- 122.Pinela J., Prieto M.A., Pereira E., Jabeur I., Barreiro M.F., Barros L., Ferreira I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019;275:309–321. doi: 10.1016/j.foodchem.2018.09.118. [DOI] [PubMed] [Google Scholar]
- 123.Xie P., Huang L., Zhang C., Deng Y., Wang X., Cheng J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019;276:662–674. doi: 10.1016/j.foodchem.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 124.Belwal T., Huang H., Li L., Duan Z., Zhang X., Aalim H., Luo Z. Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from Pyrus communis 'Starkrimson' fruit peel. Food Chem. 2019;297 doi: 10.1016/j.foodchem.2019.124993. [DOI] [PubMed] [Google Scholar]
- 125.Stevanato N., da Silva C. Radish seed oil: Ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind. Crop Prod. 2019;132:283–291. doi: 10.1016/j.indcrop.2019.02.032. [DOI] [Google Scholar]
- 126.Peng S., Zhu M., Li S., Ma X., Hu F. Ultrasound-assisted extraction of polyphenols from Chinese propolis. Front Sustain Food s. 2023;7:1131959. doi: 10.3389/fsufs.2023.1131959. [DOI] [Google Scholar]
- 127.Tzima S., Georgiopoulou I., Louli V., Magoulas K. Recent advances in supercritical CO2 extraction of pigments, lipids and bioactive compounds from microalgae. Molecules. 2023;28:1410. doi: 10.3390/molecules28031410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uwineza P.A., Waśkiewicz A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules. 2020;25:3847. doi: 10.3390/molecules25173847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu W., Zhao Y., Yang Y., Zhang H., Ding C., Hu C., Zhou L., Zhang Z., Yuan S., Chen Y., Yuan M. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from Camptotheca acuminata fruits. Int. J. Biol. Macromol. 2019;133:127–136. doi: 10.1016/j.ijbiomac.2019.04.086. [DOI] [PubMed] [Google Scholar]
- 130.P.F.P. Chaves, P.d.A.S. Hocayen, J.L. Dallazen, M.F. de Paula Werner, M. Iacomini, R. Andreatini, L.M.C. Cordeiro, Chamomile tea: Source of a glucuronoxylan with antinociceptive, sedative and anxiolytic-like effects, Int J Biol Macromol. 164 (2020) 1675-1682. https://doi.org/10.1016/j.ijbiomac.2020.08.039. [DOI] [PubMed]
- 131.Wen L., Zhang Z., Zhao M., Senthamaraikannan R., Padamati R.B., Sun D.-W., Tiwari B.K. Green extraction of soluble dietary fibre from coffee silverskin: impact of ultrasound/microwave-assisted extraction. Int. J. Food Sci. Tech. 2020;55:2242–2250. doi: 10.1111/ijfs.14477. [DOI] [Google Scholar]
- 132.W. Xiang, M. Peng, Z.h. Wang, Q.l. Yang, S. Peng, Ultrasound-microwave assisted extraction of flavonoid compounds from Eucommia ulmoides leaves and an evaluation of their antioxidant and antibacterial activities, Arch Biol Sci. 72 (2020) 211-221. https://doi.org/10.2298/ABS191216015W.
- 133.Wu H., Zhu J., Diao W., Wang C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata) Carbohyd Polym. 2014;113:314–324. doi: 10.1016/j.carbpol.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 134.Tchabo W., Ma Y., Engmann F.N., Zhang H. Ultrasound-assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Ind. Crop Prod. 2015;63:214–225. doi: 10.1016/j.indcrop.2014.09.053. [DOI] [Google Scholar]
- 135.Manzoor M.F., Zeng X.A., Rahaman A., Siddeeg A., Aadil R.M., Ahmed Z., Li J., Niu D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Tech. 2019;56:2355–2364. doi: 10.1007/s13197-019-03627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Y.C. Yang, C.S. Wang, M.C. Wei, Kinetics and mass transfer considerations for an ultrasound-assisted supercritical CO2 procedure to produce extracts enriched in flavonoids from Scutellaria barbata, J. Co2 Util. 32 (2019) 219-231. https://doi.org/10.1016/j.jcou.2019.04.008.
- 137.Liu X., Ou H., Xiang Z., Gregersen H. Ultrasound pretreatment combined with supercritical CO2 extraction of Iberis amara seed oil. J. Appl. Res. Med. Aroma. 2020;18 doi: 10.1016/j.jarmap.2020.100265. [DOI] [Google Scholar]
- 138.Žitek T., Kučuk N., Postružnik V., Leitgeb M., Knez Ž., Primožič M., Marevci M.K. Synergistic effect of supercritical and ultrasound-assisted ginger (Zingiber officinale Roscoe) extracts. Plants. 2022;11:2872. doi: 10.3390/plants11212872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu X.Y., Ou H., Zuo J., Gregersen H. Supercritical CO2 extraction of total flavonoids from Iberis amara assisted by ultrasound. J. Supercrit. Fluid. 2022;184 doi: 10.1016/j.supflu.2022.105581. [DOI] [Google Scholar]
- 140.Z. Guo, B. Zhao, H. Li, S. Miao, B. Zheng, Optimization of ultrasound-microwave synergistic extraction of prebiotic oligosaccharides from sweet potatoes (Ipomoea batatas L.), Innov. Food Sci. Emerg. 54 (2019) 51-63. https://doi.org/10.1016/j.ifset.2019.03.009.
- 141.Yang J.S., Mu T.H., Ma M.M. Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chem. 2019;289:351–359. doi: 10.1016/j.foodchem.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 142.Garcia-Vaquero M., Ummat V., Tiwari B., Rajauria G. Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs. 2020;18:172. doi: 10.3390/md18030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sánchez R.J., Fernández M.B., Nolasco S.M. Canola oil with high antioxidant content obtained by combining emerging technologies: microwave, ultrasound, and a green solvent. Eur. J. Lipid Sci. Tech. 2019;121:1900152. doi: 10.1002/ejlt.201900152. [DOI] [Google Scholar]
- 144.Sillero L., Prado R., Labidi J. Simultaneous microwave-ultrasound assisted extraction of bioactive compounds from bark. Chem. Eng. Process. 2020;156 doi: 10.1016/j.cep.2020.108100. [DOI] [Google Scholar]
- 145.Chen Q., Dong W., Wei C., Hu R., Long Y. Combining integrated ultrasonic-microwave technique with ethanol to maximise extraction of green coffee oil from Arabica coffee beans. Ind. Crop Prod. 2020;151 doi: 10.1016/j.indcrop.2020.112405. [DOI] [Google Scholar]
- 146.Sengar A.S., Rawson A., Muthiah M., Kalakandan S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020;61 doi: 10.1016/j.ultsonch.2019.104812. [DOI] [PubMed] [Google Scholar]
- 147.Dong W., Chen Q., Wei C., Hu R., Long Y., Zong Y., Chu Z. Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lasunon P., Sengkhamparn N. Effect of ultrasound-assisted, microwave-assisted and ultrasound-microwave-assisted extraction on pectin extraction from industrial tomato waste. Molecules. 2022;27:1157. doi: 10.3390/molecules27041157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y., Wang Z., Hu D., Xiao K., Wu J.-Y. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018;79:189–196. doi: 10.1016/j.foodhyd.2017.11.051. [DOI] [Google Scholar]
- 150.Goula A.M., Papatheodorou A., Karasavva S., Kaderides K. Ultrasound-assisted aqueous enzymatic extraction of oil from pomegranate seeds. Waste Biomass Valori. 2018;9:1–11. doi: 10.1007/s12649-016-9740-9. [DOI] [Google Scholar]
- 151.Zhou L., Jiang B., Zhang T., Li S. Ultrasound-assisted aqueous two-phase extraction of resveratrol from the enzymatic hydrolysates of Polygonum cuspidatum. Food Biosci. 2019;31 doi: 10.1016/j.fbio.2019.100442. [DOI] [Google Scholar]
- 152.Görgüç A., Bircan C., Yılmaz F.M. Sesame bran as an unexploited by-product: effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019;283:637–645. doi: 10.1016/j.foodchem.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 153.S. Amigh, S. Taghian Dinani, Combination of ultrasound-assisted aqueous enzymatic extraction and cooking pretreatment for date seed oil recovery, Heat Mass Transfer. 56 (2020) 2345-2354. https://doi.org/10.1007/s00231-020-02865-2.
- 154.Xue H., Tan J., Li Q., Tang J., Cai X. Ultrasound-assisted enzymatic extraction of anthocyanins from raspberry wine residues: process optimization, isolation, purification, and bioactivity determination. Food Anal. Method. 2021;14:1369–1386. doi: 10.1007/s12161-021-01976-8. [DOI] [Google Scholar]
- 155.Chen H., He S., Sun H., Li Q., Gao K., Miao X., Xiang J., Wu X., Gao L., Zhang Y. A comparative study on extraction and physicochemical properties of soluble dietary fiber from glutinous rice bran using different methods. Separations. 2023;10:90. doi: 10.3390/separations10020090. [DOI] [Google Scholar]
- 156.J. Zhan, Z. Liang, J. Li, X.a. Zeng, G. Ou, C. Zhong, Pulsed electric field‐ultrasonic assisted extraction combined with macroporous resin for the preparation of flavonoids from Pericarpium Citri Reticulatae, J Food Process Pres. 46 (2022) e16823. https://doi.org/10.1111/jfpp.16823.
- 157.Gao W., Zhang N., Li S., Li S., Zhu S., Cong X., Cheng S., Barba F.J., Zhu Z. Polysaccharides in selenium-enriched tea: extraction performance under innovative technologies and antioxidant activities. Foods. 2022;11:2545. doi: 10.3390/foods11172545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yoo K.-W., Kim J.-H. Kinetics and mechanism of ultrasound-assisted extraction of paclitaxel from Taxus chinensis. Biotechnol. Bioproc e. 2018;23:532–540. doi: 10.1007/s12257-018-0190-z. [DOI] [Google Scholar]
- 159.Dabbour M., He R., Ma H., Musa A. Optimization of ultrasound assisted extraction of protein from sunflower meal and its physicochemical and functional properties. J. Food Process Eng. 2018;41:e12799. [Google Scholar]
- 160.Hou K., Yang X., Bao M., Chen F., Tian H., Yang L. Composition, characteristics and antioxidant activities of fruit oils from Idesia polycarpa using homogenate-circulating ultrasound-assisted aqueous enzymatic extraction. Ind. Crop Prod. 2018;117:205–215. doi: 10.1016/j.indcrop.2018.03.001. [DOI] [Google Scholar]
- 161.Liu X.Y., Ou H., Xiang Z.B., Gregersen H. Optimization, chemical constituents and bioactivity of essential oil from Iberis amara seeds extracted by ultrasound-assisted hydro-distillation compared to conventional techniques. J. Appl. Res. Med. Aroma. 2019;13 doi: 10.1016/j.jarmap.2019.100204. [DOI] [Google Scholar]
- 162.Sallet D., Souza P.O., Fischer L.T., Ugalde G., Zabot G.L., Mazutti M.A., Kuhn R.C. Ultrasound-assisted extraction of lipids from Mortierella isabellina. J. Food Eng. 2019;242:1–7. doi: 10.1016/j.jfoodeng.2018.08.015. [DOI] [Google Scholar]
- 163.Liao J., Xue H., Li J., Peng L. Effects of ultrasound frequency and process variables of modified ultrasound-assisted extraction on the extraction of anthocyanin from strawberry fruit. Food Sci. Technol. 2022;42:e20922. [Google Scholar]
- 164.Wang N., Shi N., Fei H., Liu Y., Zhang Y., Li Z., Ruan C., Zhang D. Physicochemical, structural, and digestive properties of pea starch obtained via ultrasonic-assisted alkali extraction. Ultrason. Sonochem. 2022;89 doi: 10.1016/j.ultsonch.2022.106136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liao J., Xue H., Li J. Extraction of phenolics and anthocyanins from purple eggplant peels by multi-frequency ultrasound: Effects of different extraction factors and optimization using uniform design. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tzanakis I., Eskin D.G., Georgoulas A., Fytanidis D.K. Incubation pit analysis and calculation of the hydrodynamic impact pressure from the implosion of an acoustic cavitation bubble. Ultrason. Sonochem. 2014;21:866–878. doi: 10.1016/j.ultsonch.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 167.Zhang K., Gao G., Zhao C., Wang Y., Wang Y., Li J. Review of the design of power ultrasonic generator for piezoelectric transducer. Ultrason. Sonochem. 2023;96 doi: 10.1016/j.ultsonch.2023.106438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zarezadeh M.R., Aboonajmi M., Ghasemi-Varnamkhasti M. Applications of ultrasound techniques in tandem with non-destructive approaches for the quality evaluation of edible oils. J. Food Sci. Technol. 2022;59:2940–2950. doi: 10.1007/s13197-022-05351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abdulstar A.R., Altemimi A.B., Al-Hilphy A.R. Exploring the power of thermosonication: a comprehensive review of its applications and impact in the food industry. Foods. 2023;12:1459. doi: 10.3390/foods12071459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Castellarin I., Coelho R.H., Zukowski E., Ponce N.M.A., Stortz C., Gerschenson L.N., Fissore E.N. Effect of ultrasonic pretreatments on the characteristics of pectin extracted from Salustiana orange cultivated in Argentina. J. Food Process Eng. 2023;46:e14229. [Google Scholar]
- 171.AlYammahi J., Rambabu K., Thanigaivelan A., Bharath G., Hasan S.W., Show P.L., Banat F. Advances of non-conventional green technologies for phyto-saccharides extraction: current status and future perspectives. Phytochem. Rev. 2023;22:1067–1088. doi: 10.1007/s11101-022-09831-2. [DOI] [Google Scholar]
- 172.Jha A.K., Sit N. Effect of ultrasound, microwave, and enzymatically pre-treated Terminalia chebula pulp on extraction of bioactive compounds using supercritical CO2. Sustain. Chem. Pharm. 2023;33 doi: 10.1016/j.scp.2023.101098. [DOI] [Google Scholar]
- 173.X. Liu, H. Ou, H. Gregersen, J. Zuo, Supercritical carbon dioxide extraction of Cosmos sulphureus seed oil with ultrasound assistance, J CO2 Util. 70 (2023) 102429. https://doi.org/10.1016/j.jcou.2023.102429.
- 174.Chadni M., Boussetta N., Guerin C., Lagalle F., Zoghlami A., Perré P., Allais F., Grimi N., Ioannou I. Improvement of sinapine extraction from mustard seed meal by application of emerging technologies. Foods. 2023;12:520. doi: 10.3390/foods12030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kumar S., Rawson A., Kumar A., Sunil C.K., Vignesh S., Venkatachalapathy N. Lycopene extraction from industrial tomato processing waste using emerging technologies, and its application in enriched beverage development. Int. J. Food Sci. Tech. 2023;58:2141–2150. doi: 10.1111/ijfs.16156. [DOI] [Google Scholar]
- 176.Grillo G., Boffa L., Gaudino E.C., Binello A., Rego D., Pereira M., Martínez M., Cravotto G. Combined ultrasound and pulsed electric fields in continuous-flow industrial olive-oil production. Foods. 2022;11:3419. doi: 10.3390/foods11213419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.