Abstract
Background
Anhedonia is hypothesized to be associated with blunted mesocorticolimbic dopamine (DA) functioning in samples with major depressive disorder. The purpose of this study was to examine linkages between striatal DA, reward circuitry functioning, anhedonia, and, in an exploratory fashion, self-reported stress, in a transdiagnostic anhedonic sample.
Methods
Participants with (n=25) and without (n=12) clinically impairing anhedonia completed a reward-processing task during simultaneous positron emission tomography and magnetic resonance (PET-MR) imaging with [11C]raclopride, a DA D2/D3 receptor antagonist that selectively binds to striatal DA receptors.
Results
Relative to controls, the anhedonia group exhibited decreased task-related DA release in the left putamen, caudate, and nucleus accumbens and right putamen and pallidum. There were no group differences in task-related brain activation (fMRI) during reward processing after correcting for multiple comparisons. General functional connectivity (GFC) findings revealed blunted fMRI connectivity between PET-derived striatal seeds and target regions in the anhedonia group. Associations were identified between anhedonia severity and the magnitude of task-related DA release to rewards in the left putamen, but not mesocorticolimbic GFC.
Conclusions
Results provide evidence for reduced striatal DA functioning during reward processing and blunted mesocorticolimbic network functional connectivity in a transdiagnostic sample with clinically significant anhedonia.
Keywords: anhedonia, dopamine, PET-MR, reward, mesolimbic, self-reported stress
Introduction
Anhedonia is characterized by impaired reward processing and blunted mesocorticolimbic dopamine (DA) system functioning (1–3). This ascending DA tract passes through reward learning (-meso), cognitive control (-cortico), and emotional (-limbic) hubs of the brain (4), and impairments in motivation and the anticipation of rewards are associated with alterations in striatal DA tone, DA release, and DA signaling (3,5–7). Associations between anhedonia and mesocorticolimbic DA system functioning have primarily been investigated in major depressive disorder (MDD) (8,9). While anhedonia is a core symptom of MDD, it is also a transdiagnostic symptom that is pervasive across numerous neuropsychiatric disorders (10). A putative neural mechanism of anhedonia is striatal hypoactivation, and anhedonia severity negatively correlates with ventral striatal activity during the anticipation of rewards in depressed populations (11–13). Anhedonia severity is also associated with altered intrinsic functional connectivity between striatal regions and areas of the prefrontal cortex (PFC) in adolescents (12) and adults (14,15). In a non-clinical adult sample, reduced nucleus accumbens response to reward was uniquely related to anhedonia severity, and not depressive or anxious symptoms (16). Together these findings demonstrate distinct patterns of mesocorticolimbic DA system activation and connectivity associated with anhedonia.
Simultaneous positron emission tomography and magnetic resonance (PET-MR) imaging using [11C]raclopride, a radioligand that allows for the quantification of DA D2/D3 receptor binding, has demonstrated that functional magnetic resonance imaging (fMRI) activation and functional connectivity in mesolimbic brain regions during reward anticipation correlate with ventral striatal DA release in MDD (17) and non-clinical (18) samples. Anhedonia is associated with altered DA functioning, including decreased striatal DA transporter availability in MDD (9) and increased striatal DA D2/D3 receptor availability in MDD (8), although no association between anhedonia and DA release capacity in MDD has been reported (19). This inconsistency may be explained, in part, by the diagnostic heterogeneity of MDD as opposed to sampling an anhedonic phenotype.
Additionally, alterations in DA signaling, and mesocorticolimbic DA system functioning more broadly, are linked to stress (3,7,20,21). Considerable animal research on this topic supports the idea that chronic, uncontrollable, and unpredictable stressors impact DA signaling (22) thereby contributing to the emergence of anhedonic-like behaviors, such as reduced sucrose preference or intake (23) and learned helplessness (24–26). Within human samples, stress is also associated with neural and behavioral deficits in reward processing, including reduced goal-directed behavior, blunted incentive motivation, impaired reward learning, and alterations in striatal activation and connectivity during anticipation and receipt of rewards (3,27–29). However, no research has examined associations between self-reported stress, anhedonia, and striatal dopamine functioning in a transdiagnostic anhedonic sample.
In the present study, we used simultaneous PET-MR imaging with the D2/D3 dopamine receptor antagonist [11C]raclopride in a transdiagnostic sample of adults with clinically impairing anhedonia to investigate relationships between anhedonia, striatal DA release, and mesocorticolimbic network functioning during reward processing. We hypothesized that the transdiagnostic anhedonia group would be characterized by decreased striatal task-related DA release to rewards, indexed by the non-displaceable binding potential () of [11C]raclopride, relative to a control group. We also hypothesized that striatal DA functioning would predict anhedonia severity. Next, we predicted that the anhedonia group would show decreased mesocorticolimbic network activation and connectivity during reward processing using fMRI. Finally, an exploratory aim was to examine associations between self-reported stress, anhedonia, and mesocorticolimbic DA system functioning. This aim was exploratory given that participants were not recruited based on stress exposure. We hypothesized that greater self-reported stress would be inversely associated with striatal DA release and mesocorticolimbic network fMRI activation and connectivity during reward processing.
Methods
Study Overview
The present study complements an ongoing NIMH-funded clinical trial (R61/R33 MH110027) investigating the effects of a novel psychosocial anhedonia treatment on neural responses to rewards and anhedonia symptoms (ClinicalTrials.gov Identifiers NCT02874534 and NCT04036136). Data from control participants, recruited as part of a separate study, have been reported previously (30). These companion studies met research standards for Institutional Review Board (IRB) approval at UNC-Chapel Hill and Duke University, and PET imaging protocols were approved by the UNC Radioactive Drug Research Committee. PET-MR imaging data acquisition occurred within four weeks of completing inclusion and exclusion assessment, and prior to randomization into psychotherapy treatment groups, for the companion study. Written informed consent was obtained prior to inclusion in the study.
Participants
Eligibility Criteria
Eligible participants in the ANH group were 18 to 50 years old, treatment-seeking for clinically significant anhedonia (i.e., Snaith-Hamilton Pleasure Scale (SHAPS) scores greater than or equal to 20 using the ordinal scoring of Franken and colleagues (31) and with Clinician’s Global Impression Scale Severity (CGI-S; (32) scores greater than or equal to 3, indicating clinical impairment). Eligible participants in the CON group had no present or past psychiatric diagnoses, as assessed by the Structured Clinical Interview for DSM-5 (SCID-5-RV) (33). Additional eligibility criteria are provided in Supplemental Materials IA.
Twenty-eight ANH participants and 23 CON participants completed PET-MR scans. Three ANH participants and 11 CON participants were excluded due to problems with the PET injection or scanner (4 CON participants), PET infusion (2 ANH participants), or technical errors at the scan (1 ANH and 7 CON participants). The final sample included 25 ANH participants and 12 CON participants.
Clinical Diagnostic & Symptom Measures
The SCID-5-RV was used to assess eligibility and for clinical characterization. (30) Only participants in the ANH group completed the following self-report measures assessing stress and anhedonia severity.
The Perceived Stress Scale (PSS-10) was the primary measure of self-reported stress. The PSS assesses self-reported unpredictable and uncontrollable stressors over the past month and contains 10 items (34). Total scores range from 0 to 40, whereby higher scores indicate greater perceived stress (35).
The posttraumatic stress disorder (PTSD) Checklist (PCL-5), the secondary measure of self-reported stress, was used to assess PTSD symptoms in the last month. The PCL-5 is a well-validated scale with 20 items (36). Total scores range from 0 to 80, whereby higher scores indicate greater severity of symptoms. The PCL-5 version used in the current study did not include the Criterion A component. Therefore, scores reflect general distress in relation to stressful life events rather than a Criterion A trauma (37).
The Snaith–Hamilton Pleasure Scale (SHAPS) was the primary measure of anhedonia. The SHAPS is a well-validated 14-item questionnaire that assesses hedonic capacity. Total scores range from 14 to 56, whereby higher scores indicate greater anhedonia severity in the present state (i.e., “the last few days”).
The 21-item Beck Depression Inventory (BDI-II) was administered to assess depression symptom severity. Total scores range from 0 to 63, whereby higher scores indicate greater depressive severity. The BDI-II Anhedonia Subscale was used as a secondary measure of anhedonia. This comprises four items from the BDI-II (i.e., loss of interest, loss of pleasure, loss of interest in sex, and loss of energy) (38). Whereas the SHAPS primarily assesses aspects of consummatory reward (39), the BDI-II anhedonia subscale captures aspects of both consummatory and anticipatory reward processing (38).
Neuroimaging Data
Simultaneous PET-MR scan protocol and pre-processing
Participants completed a 75-minute simultaneous PET-MR scan on a Siemens Biograph mMR scanner using a bolus+infusion protocol (Figure 1) with a planned of 105 min. List mode 3-D emission data were collected starting from bolus injection of [11C]raclopride (~1 min after scan start time; administered using a Medrad® Spectris Solaris® EP MR Injection System) and continuing over the 75-minute scan. [11C]Raclopride is a D2/D3 antagonist which selectively binds to striatal DA receptors (40). In the first portion of scan acquisition, participants completed two 8-minute resting-state scans and one 6-minute high resolution T1 scan (FOV = 256 mm, 111 mm resolution, TR = 2530ms, TE = 1.69ms, flip angle = 7 degrees), to allow time for tracer uptake. In the second portion, participants completed a monetary incentive delay task (MID), developed at McLean Hospital (by DGD and DAP) and modified for use in PET-MR studies (add Zurcher et al 2021 ref). Participants were provided instructions via an intercom (i.e., headphones) and informed when transitions took place between resting, structural, and functional fMRI sequences. Acquisition parameters were identical for the resting state and functional scans (echo planar imaging, FOV = 212 mm, 3.312 × 3.312 × 3.3 mm resolution, TR = 3000, TE = 30ms, flip angle = 90 degrees). The reward task used during scanning is described in Supplemental Materials IC and illustrated in Figure 2.
Figure 1. Timing of data collection, data modelling, and participant behavior during scanning.
Three task blocks were presented during which fMRI data were collected simultaneously with the PET acquisition.
Figure 2. PET-MR Monetary Incentive Delay (MID) Task.
Each trial consisted of a cue phase and an outcome phase. Trials were presented first in a neutral block that consisted of only neutral trials and then in two reward blocks that consisted of reward trials of varying magnitudes (small, medium, or large). The relationship between cue identity and outcome magnitude had to be learned by experience. Further details of the PET-MR Monetary Incentive Delay (MID) Task are provided in the Supplemental Materials IC.
Dynamic PET images were reconstructed from list mode data (reconstruction grid was 344×344 with 127 axial slices and a voxel size of 2.086mm × 2.086mm × 2.032mm) using the PseudoCT method (41), which uses the subject’s Dixon attenuation map and the T1 MPRAGE image to estimate a CT- equivalent attenuation map. Next, PET images underwent motion correction using the Realign procedure of SPM12. This method computes a rigid transformation for each time frame to align all to a common reference. As a quality-control measure, the motion-corrected frames were observed in cine mode to detect possible errors. In all but two cases, the motion correction was found to achieve good alignment (see Supplemental Materials IA). Structural MR images (T1 scans) were pre-processed using Freesurfer version 7.1.0. Functional MR images were pre-processed using FSL version 6.0. To control for excessive motion, we censored volumes that exceeded a framewise displacement threshold of 0.5mm (42). Lastly, functional connectivity data (i.e., resting-state and MID task runs) were preprocessed with the default preprocessing pipeline in the SPM12 CONN functional connectivity toolbox, version 19c (43).
PET Analysis
[11C]Raclopride is a D2/D3 receptor antagonist, and therefore competes with endogenous DA for receptors. Binding potential (), the ratio of selectively bound ligand to non-displaceable ligand in the tissue at equilibrium, was estimated from dynamic PET images for the baseline and reward phases of PET acquisition for each subject. It is worth noting that binding potential may relate to several factors including but not limited to 1) receptor density, 2) change in synaptic DA concentration resulting in increased occupancy (i.e., reduction in binding site availability), and 3) change in receptor state that influences raclopride Kd (e.g., conformational change, internalization, posttranslational modification etc.). was quantified using the simplified reference tissue model (SRTM) (44). Thus, each time-activity curve was fit with an SRTM model with four parameters: Baseline , Reward , , and . The baseline phase was defined from start of acquisition to start of the reward phase, including both the uptake phase and the neutral task block. Cerebellum was used as the reference region. More details on the two-phase model are found in Supplemental Materials IB.
The two-phase SRTM model was applied to time-activity curves from regional data (striatal regions of the AAL3 atlas) as well as to individual voxels of the dynamic PET sequences to create voxel maps of baseline and reward BPND. For groupwise analysis, individual subject maps were transformed to common MNI space using mappings derived from SPM12 and each subject’s T1 structural images.
Reward blocks encompass trials during which participants both anticipated and received rewards. Baseline and change in following reward task onset ( %) measures DA functioning during the non-reward state (i.e., baseline) and activation (reward task-related) states, respectively. This approach measures the extent to which endogenous DA displaces the radiotracer. A typical DA response to rewards in the striatum would be indicated by lower values during reward, relative to baseline, indicating that DA has increased and out-competed the tracer for binding sites (8). Accordingly, decreased is interpreted as increased task-related DA release.
To identify regions that showed between-group differences in from baseline to reward phases of the MID, for each subject, we estimated striatal DA functioning during uptake of the tracer and during each condition of the task. Here, we would expect negative values (Reward – Baseline) for controls if DA out-competes the tracer. A -score statistical map representing the difference between groups and conditions (ANH - CON; Reward – Baseline) was created from subject images by contrasting voxel-wise (Reward – Baseline) maps. Thus, z-scores compare for the ANH group to for the CON group in such a way that positive z-scores indicate that anhedonic participants demonstrate less response in the expected direction than control participants (i.e., for the ANH group is less negative than for the CON group). This -score statistical map was then thresholded at (uncorrected) and anatomically constrained to the bilateral caudate nucleus, putamen, pallidum, and nucleus accumbens using masks from the Harvard-Oxford probabilistic atlas. We estimated group differences only in the striatum, given that [11C]raclopride selectively binds to DA receptors in the striatum (40).
For each significant functionally-defined cluster that emerged from this contrast, condition-specific values were extracted per participant. To study the pattern of results in greater detail, these values were then compared by evaluating group (ANH, CON) × condition (reward, baseline) interactions via analyses of variance (ANOVAs). For a complete description of PET analyses see Supplemental Materials IB and (45).
fMRI Activation Analysis
To examine fMRI responses during reward anticipation, BOLD responses to reward cues of all magnitudes (small, medium, and large) vs. neutral cues were examined from cue onset to the end of the fixation period (i.e., the period between the cue and target onsets). To examine fMRI responses during reward processing, activation to successful vs. unsuccessful outcomes on reward trials of all magnitudes (small, medium, and large) were examined (i.e., the period between the start and end of feedback presentation; see Figure 2). A priori hypothesis testing was conducted using a region of interest (ROI) approach. ROI analyses examined regions implicated in reward anticipation (i.e., bilateral nucleus accumbens, caudate, and putamen) and reward outcome (i.e., medial prefrontal cortex and anterior cingulate cortex). Using FSL featquery, we calculated mean BOLD percent signal change in these ROIs for each contrast of interest. Separate from ROI analyses, exploratory whole-brain voxel-wise analyses were also conducted. See Supplemental Materials IF for additional details about fMRI activation analyses.
fMRI Connectivity Analysis
A general functional connectivity (GFC) approach examined whole-brain connectivity using striatal PET-derived seed regions that displayed significant differences in for the contrast ANH - CON; Reward - Baseline of the MID task. GFC, a method that combines resting-state and task fMRI data, offers better test-retest reliability and higher estimates of heritability than intrinsic connectivity estimates from the same amount of resting-state data alone (46). In the current study, the combination of two resting-state runs and three MID task blocks yielded approximately 45 minutes of fMRI data for connectivity analyses. This is critical given that >25 min of fMRI data are needed to reliably detect individual differences in connectivity (47). Voxel-wise whole-brain connectivity was evaluated using the CONN Toolbox’s seed-to-voxel analysis. Analyses corrected for multiple comparisons using a false-discovery rate (FDR) approach, at the familywise error (FWE) rate of .
Associations between PET-MR, Anhedonia, and Self-Reported Stress in the Anhedonia Group
To examine whether anhedonia severity and self-reported stress were associated with striatal DA function and mesocorticolimbic network functioning within the ANH group, we conducted statistical regression models in R, version 4.0.3 (48). Because only participants in the ANH group completed self-report measures assessing stress and anhedonia severity, analyses were limited to this group. PET-derived striatal and network functional connectivity values (i.e., fMRI-derived correlations between network regions with correlated BOLD signal change) were tested as individual predictors of anhedonia severity (i.e., SHAPS and BDI anhedonia subscale), in separate regressions. Additional regressions tested whether self-reported stress (i.e., PSS and PCL-5) predicted the magnitudes of these PET- and fMRI-derived variables. Corrections for multiple comparisons were made within each set of hypotheses (i.e., correcting across regression analyses that examined whether striatal DA release to rewards, or , predicted anhedonia severity).
Lastly, bivariate Pearson correlations between clinical and PET-MR variables of interest were explored. Corrections for multiple comparisons were made within each set of analyses (i.e., clinical variables with striatal values and clinical variables with network functional connectivity values) using the false-discovery rate (FDR) method (49).
Results
Participant Characteristics
Table 1 summarizes demographic information and descriptive statistics for the samples. Table 2 reports clinical characteristics for the ANH group; the self-report measures assessing stress and anhedonia severity were not collected in the CON group.
Table 1.
Sample Characteristics.
| Anhedonia Group (n=25) | Control Group (n=12) | Total Sample (n=37) | Group Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Variable | M | SD | Range | M | SD | Range | M | SD | Range | Test Statistic, p-value |
|
| ||||||||||
| Age (years) | 26.32 | 6.01 | 19–42 | 25.67 | 4.30 | 21–36 | 26.40 | 5.49 | I 19–42 | t(28.7) = −0.14, p=.887 |
|
| ||||||||||
| [11C] raclopride dose (mCi) | 13.27 | 1.28 | 9.88–15.74 | 11.73 | 2.13 | 8.10–15.01 | 12.77 | 1.74 | 8.10–15.74 | t(14.9) = 2.31, p=.036 |
| Variable | Count | Percent | Count | Percent | Count | Percent | Test Statistic, p-value |
|
| |||||||
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female | 15 | (60.0%) | 2 | (16.7%) | 17 | (45.9%) | χ2(1) = 6.13, p =.013 |
| Male | 10 | (40.0%) | 10 | (83.3%) | 20 | (54.1%) | |
|
| |||||||
| Race | |||||||
| White | 13 | (52.0%) | 8 | (66.7%) | 21 | (56.8%) | |
| Black / African American | 3 | (12.0%) | 2 | (16.7%) | 5 | (13.5%) | |
| Asian | 7 | (28.0%) | 1 | (8.3%) | 8 | (21.6%) | |
| American Indian / Alaska Native | 1 | (4.0%) | - | - | 1 | (2.7%) | |
| Other (Not Listed) | 2 | (8.0%) | - | - | 2 | (5.4%) | |
| Not Reported | - | - | 1 | (8.3%) | 1 | (2.7%) | |
|
| |||||||
| Ethnicity | |||||||
| Hispanic | 4 | (16.0%) | 2 | (16.7%) | 6 | (16.2%) | |
| Non-Hispanic | 21 | (84.0%) | 10 | (83.3%) | 31 | (83.8%) | |
Note – Participants were able to endorse one or more race categories.
Table 2.
Anhedonia Group Clinical Characteristics.
| Anhedonia Group (n=25) | |||
|---|---|---|---|
|
|
|||
| Variable | M | SD | Range |
|
| |||
| PSS | 20.84 | 3.64 | 13 – 27 |
| SHAPS | 36.64 | 4.37 | 30 – 45 |
| BDI-II Anhedonia Subscale | 5.04 | 2.03 | 2 – 9 |
|
| |||
| BDI-II Total | 20.20 | 9.12 | 3 – 41 |
|
| |||
| PCL-5 | 19.12 | 12.38 | 1 – 43 |
|
| |||
| Primary Diagnosis (SCID-5-RV) | |||
| No Current Diagnosis | 6 | (24%) | |
| Major Depressive Disorder (MDD) | 9 | (36%) | |
| Persistent Depressive Disorder (PDD) | 3 | (12%) | |
| Generalized Anxiety Disorder | 3 | (12%) | |
| Attention-Deficit Hyperactivity Disorder (ADHD) | 2 | (8%) | |
| Specific Phobia | 1 | (4%) | |
| Other Specified Anxiety Disorder | 1 | (4%) | |
PSS – Perceived Stress Scale; SHAPS – Snaith-Hamilton Pleasure Scale; BDI-II – Beck Depression Inventory-II; PCL-5 – Posttraumatic Stress Disorder Checklist; SCID-5-RV – Structured Clinical Interview for DSM-5.
ANH and CON groups did not differ in age (t(28.7) = −0.14, p = .887). There were significantly fewer females in the CON group (, p = .013). [11C]Raclopride dose differed between groups; for the ANH and CON groups, the average dose was 13.27 mCi (SD = 1.28) and 11.73 mCi (SD = 2.14), respectively (t(14.9) = 2.31, p = .036). Thus, analyses presented here controlled for sex and [11C]Raclopride dose.
ANH participants reported moderate levels of anhedonia, as assessed by the SHAPS, as well as moderate depressive symptoms, as assessed by the BDI-II (50). ANH participants’ PSS scores reflect moderate stress (51), and PCL-5 scores reflect mild stress (36). Five ANH participants had PCL-5 scores of 33 or greater, indicating clinically significant PTSD symptoms.
Within the ANH group, males reported significantly greater perceived stress on the PSS than females (t(22.7) = −2.73, p = .011). Anhedonia severity ratings did not differ by sex. In the ANH group, scores on the SHAPS and BDI-II anhedonia subscale were positively correlated (r = 0.65, p = .0005) and PSS and BDI-II anhedonia subscale scores were positively correlated (r = 0.47, p = .0179). Six ANH participants did not meet criteria for any current diagnoses; however, each had a CGI-S score of 3, indicating clinical impairment. See Supplemental Materials IIA for task reaction time and valence ratings analyses.
Striatal Dopaminergic Functioning
Group Differences in during the MID Task (Reward Condition relative to Baseline)
Striatal clusters in the left putamen, right putamen and pallidum, left caudate, and left nucleus accumbens (NAc), extending into the left putamen, demonstrated between-group differences in values (ANH - CON) for the Reward - Baseline contrast, F’s(1,20) > 7.38, p’s < .01. These analyses controlled for sex and [11C]raclopride dose, given that there were significantly fewer females in the CON group and [11C]raclopride dose was, on average, higher in the ANH group. See Table 3 for striatal cluster statistics. Figure 3 shows [11C]raclopride values for each participant by condition and group. Relative to CON participants, ANH participants showed higher [11C]raclopride during the reward condition relative to baseline (Figure 3). This finding indicates that, relative to CON participants, ANH participants exhibited reduced task-related DA release to rewards in the striatum. Results for exploratory PET analyses are reported in Supplemental Materials IIB–IIE.
Table 3.
Striatal Clusters demonstrating ANH - CON Group Differences in values (Reward - Baseline) at a threshold of z > 2.58 (uncorrected).
| Cluster Label | Cluster Size | Max Z value | Max X | Max Y | Max Z | Group x Condition Interaction p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Left putamen | 88 | 4.70 | −2 | 4 | 12 | .0006** |
| Right putamen/pallidum | 23 | 3.63 | 18 | 6 | −4 | .007** |
| Left caudate | 23 | 3.33 | −16 | 4 | 14 | .010* |
| Left NAc and putamen | 19 | 3.45 | −12 | 6 | −8 | .009* |
Contrast of ANH - CON; Reward - Baseline values. MNI Coordinates. For all clusters, the Group (ANH, CON) × Condition (Reward, Baseline) interaction effect on [11C]raclopride values were significant, controlling for sex and [11C]raclopride dose. This was expected given that ANOVA results are dependent on the cluster-defining contrast.
p-values <.05
<.01
<.001. NAc, Nucleus Accumbens. ANH, Anhedonia participants. CON, Control participants.
Figure 3.
[11C]Raclopride binding potential in functionally-defined striatal clusters demonstrating group differences for the contrast of (ANH - CON; Reward - Baseline). T-tests shown here (blue lines) are within-group comparisons of values (Reward - Baseline) and between-group comparisons of values (Baseline). In each of these four clusters, there was a significant group × condition interaction, Fs(1,20) > 7.38, ps <.010. This was expected given that ANOVA results are dependent on the cluster-defining contrast. The baseline phase depicted here encompasses the first 42 minutes of scanning, from start of acquisition to start of the reward phase, including both the uptake phase and the neutral task block.
fMRI Activation
Results of fMRI activation analyses are presented in Supplemental Materials IIF. ROI analyses showed a single between-group difference in right caudate activation, at an uncorrected threshold, which was not associated with clinical measures of anhedonia or self-reported stress.
fMRI Connectivity
PET-derived Seed-based General Functional Connectivity
Whole-brain GFC analysis revealed several significant group differences. PET-derived seeds demonstrated negative connectivity with subcortical and cortical regions in the ANH group, relative to the CON group. Target regions of these seeds included structures commonly implicated in reward processing, including bilateral caudate nucleus, putamen, and pallidum, as well as the medial prefrontal cortex. Associated regions in the anterior cingulate cortex and the thalamus were also identified as target regions. See Table 4 for connectivity statistics. Figure 4 illustrates group differences in GFC between the PET-derived seeds and their respective target regions.
Table 4.
Statistics for clusters demonstrating ANH - CON group difference in GFC seed-to-voxel analysis with PET-derived seeds.
| Seed | Cluster Size (voxels) | Effect size (b) | Size p-FWE | Peak p-FWE | Peak p-unc |
|---|---|---|---|---|---|
| Target Label (MNI Coordinates) | |||||
|
| |||||
| Left Putamen | |||||
|
| |||||
| Bilateral Striatum (−22, 0, 6) | 786 | −0.21 | .000 | .001 | .000 |
| Right Striatum (18, 6, 8) | 603 | −0.13 | .000 | .009 | .000 |
| Right Superior Frontal Gyrus (22, −4, 62) | 87 | −0.08 | .010 | .997 | .000 |
|
| |||||
| Right Putamen / Pallidum | |||||
|
| |||||
| Right Striatum (18, 8, −8) | 268 | −0.25 | .000 | .003 | .000 |
| Right Paracingulate Gyrus / Anterior Cingulate Gyrus (2, 36, 26) | 78 | −0.08 | .014 | .159 | .000 |
| Right Caudate (12, 6, 12) | 54 | −.09 | .085 | .975 | .000 |
|
| |||||
| Left Caudate | |||||
|
| |||||
| Bilateral Striatum / Left Thalamus (18, 18, −4) | 515 | −0.11 | .000 | .397 | .000 |
| Left Caudate (−16, 10, 20) | 324 | −0.27 | .000 | .001 | .000 |
| Left Striatum (−24, 2, −12) | 99 | −0.11 | .005 | .987 | .000 |
| Left Caudate / Thalamus (−10, −12, 16) | 69 | −0.09 | .035 | .148 | .000 |
|
| |||||
| Left Nucleus Accumbens and Putamen | |||||
|
| |||||
| Left Striatum (−14 6, −12) | 268 | −0.26 | .000 | .003 | .000 |
| Medial Frontal Cortex (−8, 50, −16) | 57 | −0.07 | .065 | .978 | .000 |
Effect sizes indicate average differences in connectivity between the two groups (ANH – CON) when controlling for age and sex. Size p-values indicate the significance of the size of the target cluster (voxels). Peak p-values indicate the significance of the signal of the target cluster, at its peak, or strongest point of connectivity. FWE, family-wise error. FDR, false-discovery rate. Unc, uncorrected. FWE and FDR are two common methods for correction of multiple comparisons.
Unc p-values have not been corrected for multiple comparisons. ANH, Anhedonia participants. CON, Control participants
Figure 4. Group differences in general functional connectivity of PET-derived seeds.
Seed-to-voxel analysis (ANH - CON) controlling for age and sex. Only negative connectivity values were found in the ANH group, represented in blue. PET striatal seeds are presented in radiologic view, so the left and right are reversed. ANH, Anhedonia participants. CON, Control participants.
Relations between Anhedonia and Mesocorticolimbic Network Functioning
Anhedonia and Task-Related DA Release in Functionally-defined Striatal Clusters (PET)
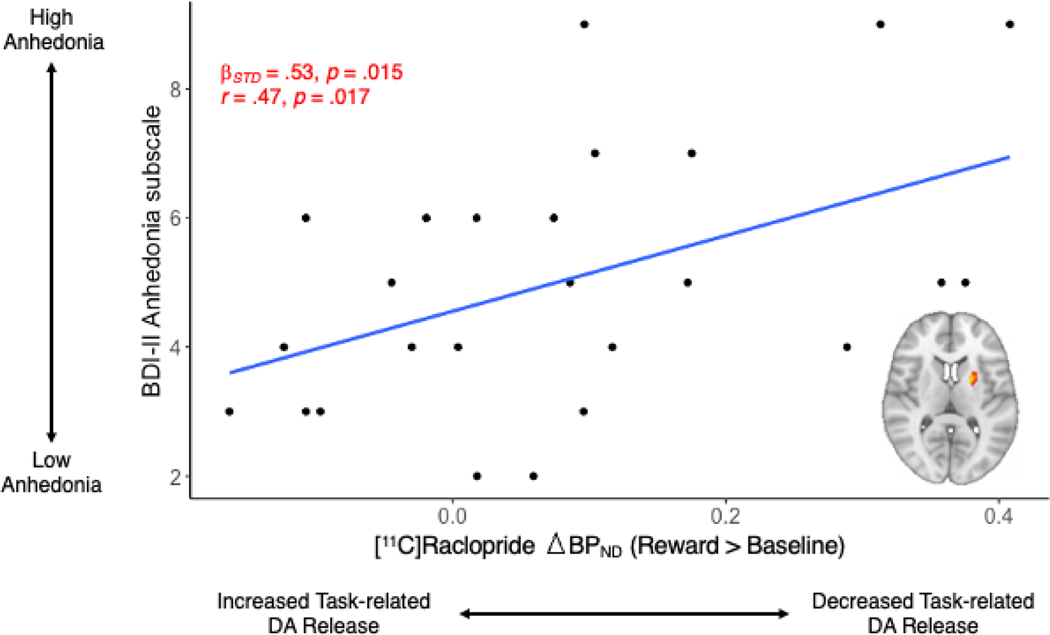
In the ANH group, we examined associations between values in the above striatal clusters that demonstrated group differences and anhedonia severity scores on the SHAPS and BDI-II anhedonia subscale. Reduced task-related DA release to rewards in the left putamen cluster was significantly associated with BDI-II anhedonia scores (, SE = 0.18, t = 2.57, p = .017, ) (Figure 5). BDI-II anhedonia subscale scores were not significantly associated with task-related DA release in the other three striatal clusters (p’s > .05). SHAPS scores were not significantly associated with task-related DA release in any of the four striatal clusters (p’s > .05). Results for exploratory analyses with anhedonia severity are reported in the Supplemental Materials IIG–IIH.
Figure 5. Task-related DA release to rewards in the functionally-defined left putamen striatal cluster predicts BDI-II anhedonia subscale scores for ANH participants (n=25).
In ANH participants, greater [11C]raclopride was associated with greater anhedonia severity on the BDI-II anhedonia subscale. Positive values represent decreased task-related DA release to rewards, relative to baseline. BDI-II, Beck Depression Inventory.
Anhedonia and Mesocorticolimbic Network Connectivity (PET-MR)
Neither the SHAPS nor BDI-II anhedonia subscale were significantly associated with PET-derived GFC strength for any region pairs (p’s > .05) (see Figure 6).
Figure 6. Pearson correlation matrix for variables of interest.
Pearson correlation values range from −1 to 1. Significant correlations (p <.05) are displayed in color; non-significant correlations (p >.05) are displayed in white. Correlations presented in the upper right triangles of the matrices are corrected for multiple comparisons, using the false-discovery rate (FDR) method. Correlations in the lower left triangles are uncorrected. L, left. R, right. GFC, general functional connectivity. Put, Putamen. Pall, Pallidum. NAc, Nucleus Accumbens. , binding potential non-displacement. SHAPS, Snaith-Hamilton Pleasure Scale. PCL-5, PTSD Checklist for DSM-5. BDI, Beck Depression Inventory. ANH, Anhedonia. CON, Control.
Relations between Self-Reported Stress and Mesocorticolimbic Network Functioning
Self-Reported Stress and Task-related DA Release to Rewards in Functionally-defined Striatal ROIs (PET)
Within the ANH group, analyses with the PSS and PCL-5 yielded no significant associations between self-reported stress and mesocorticolimbic task-related DA release to rewards in striatal clusters. Results for exploratory analyses with self-reported stress are reported in Supplemental Materials III–IIJ.
Self-Reported Stress and Mesocorticolimbic Network Connectivity (PET-MR)
Exploratory analyses with the PCL-5 yielded one significant association with GFC between the PET-derived right putamen and pallidum cluster and a target region in the paracingulate and anterior cingulate cortex; however, this association between PCL-5 scores and GFC was not significant after FDR-correction for multiple comparisons. Scores on the PSS were not significantly associated with mesocorticolimbic network connectivity.
Correlations between [11C]Raclopride Binding Potential, fMRI Network Connectivity, and Clinical Measures
Figure 6 summarizes bivariate Pearson correlations in the ANH group between primary and secondary clinical measures of stress and anhedonia, [11C]raclopride binding potential in striatal clusters demonstrating group differences, and general functional connectivity of these striatal clusters with their respective whole-brain target regions. As hypothesized, greater [11C]raclopride binding potential (i.e., reduced striatal DA release to rewards) in striatal clusters tended to be negatively associated with general functional connectivity values of these seeds and their target regions (see Figure 6, orange boxes in lower triangle). However, not all of these correlations remained after an FDR-correction for multiple comparisons (see Figure 6, upper right triangle).
Discussion
This investigation explored associations among anhedonia, striatal DA, and reward circuitry functioning in a transdiagnostic sample with clinically impairing anhedonia. Stress was also examined in an exploratory manner.
Striatal Dopamine and Anhedonia
Extending previous findings of decreased striatal DA release to rewards in MDD (8,17), we found reduced striatal DA release to rewards in ANH participants. Interestingly, in the ANH participant group alone, there were no regions that showed a significant change in from baseline to the reward condition of the MID, suggesting that participants with anhedonia demonstrated blunted DA response to rewards. Next, we found that relative to the CON group, ANH participants exhibited increased [11C]raclopride in the left and right dorsal striatum and left ventral striatum (Figure 3). Together, these findings represent the first report of reduced task-related DA release to rewards in a transdiagnostic sample with clinically impairing anhedonia.
Reduced striatal DA release to rewards in ANH participants may reflect impaired reward learning (1) although this was not seen behaviorally. The optimized MID task used here required learning which cues predicted differing reward magnitudes, enhancing the sensitivity of the task to positive prediction errors encoded by task-related DA release (4). Though we did not evaluate prediction errors per se, impaired modulation of behavior by rewards during a probabilistic reward task is characteristic of anhedonia in individuals with MDD (52,53). Although Hamilton and colleagues (2018) also reported lower availability of DA during baseline in an MDD sample, our findings are not consistent with this interpretation in the ANH group (17). That is, we did not find evidence that ANH participants were characterized by significantly lower baseline DA relative to CON participants (see Figure 3, for comparisons of baseline ). Supplemental results for baseline differences in raclopride binding potential during baseline, using an ROI approach, are presented in Supplemental Materials IIE.
Regarding associations between striatal and anhedonia, we found that increased in the left putamen, indicative of decreased task-related DA release, was positively associated with anhedonia severity on the BDI-II anhedonia subscale. Within functionally-defined striatal clusters, SHAPS scores were not significantly related to task-related DA reward signaling, which is consistent with at least one [11C]raclopride PET study in MDD (8). These contrasting results between the BDI-II anhedonia subscale and the SHAPS may be due to differences in the aspects of anhedonia that these two scales capture. Whereas the SHAPS primarily assesses aspects of consummatory reward capacity (i.e., pleasure) (39,54), the BDI-II anhedonia subscale captures aspects of both consummatory and anticipatory (i.e., motivation or interest) reward capacity (38,54,55). Nevertheless, our finding of an association between striatal and anhedonia on the BDI-II anhedonia subscale requires replication, particularly because the internal consistency of the BDI-II anhedonia subscale is not outstanding (). The internal consistency of the SHAPS has been shown to be higher than the BDI-II anhedonia subscale (56).
fMRI Activation during Reward Anticipation and Reward Outcome
We did not find evidence of altered mesocorticolimbic activation during reward anticipation or reward outcome phases in ANH participants after correcting for multiple comparisons (see Supplemental Materials IIF). The lack of fMRI activation differences may be attributable, in part, to inadequate power of the current study to detect smaller effects given the small sample. Prior fMRI research has shown hypo-responsivity of striatal regions during anticipatory (1,57,58) and consummatory processing (59–61) in psychiatric populations where anhedonia is a central feature. As stated above, the optimized MID task used here requires learning whereas the standard fMRI MID task does not, and this could contribute to differences relative to prior MID studies. Given that DA is important for learning, the current design is an improvement with reference to DA. The current study’s uncorrected fMRI activation results should be cautiously considered within the broader literature. Additionally, recent PET-MR investigations of striatal DA in MDD did not report group differences in fMRI activation during reward anticipation or reward outcomes (17,19).
Anhedonia and Mesocorticolimbic General Functional Connectivity
The present study also investigated functional connectivity seeded by regions exhibiting blunted striatal DA release to rewards (i.e., PET-derived seeds) using a whole-brain GFC approach. Compared to CON participants, ANH participants showed negative GFC between PET-derived seeds and several regions implicated in reward processing (i.e., bilateral caudate, putamen, and pallidum), as well as cognitive control (e.g., anterior cingulate gyrus) and control of attention (e.g., thalamus). These results are consistent with reports of altered functional cortico-striatal connectivity in MDD (12,62,63) and a previous [11C]raclopride PET-MR study of functional connectivity in MDD (17). In MDD, increased in the ventral striatum predicted decreased functional connectivity between PET-derived seeds and default-mode and salience network regions (17).
Impact of Stress on Anhedonia via Striatal Dopamine
Stress is believed to desensitize the mesocorticolimbic DA system and contribute to the emergence and maintenance of anhedonic behavior (3,27,64). We hypothesized that self-reported stress on the PSS would predict anhedonia severity and be associated with striatal DA release to rewards, illustrating one potential mechanism linking self-reported stress and anhedonia. This was an exploratory hypothesis, given that the current sample was not selected for their exposure to stress. Consistent with previous work (3,65), perceived stress and scores on the BDI-II anhedonia subscale were significantly correlated (r=.47). However, we did not find evidence for the contribution of self-reported stress on mesocorticolimbic DA system functioning. The PSS, our primary measure of self-reported stress, is a retrospective measure that assesses the extent to which stress is unpredictable and uncontrollable during the last month, but it does not assess different types and chronicity of stressors (66). It is possible that other scales that objectively assess stressful life conditions and situations may be better suited to illuminate the role of self-reported stress in DA function. Furthermore, the nucleus accumbens (NAc) is strongly implicated in stress regulation (20) and demonstrates a blunted response during reward consumption in patients with MDD (67). Here, group differences in dopaminergic response to rewards (Figure 3) highlighted one small cluster (size=19 voxels) located between the left NAc and left putamen. We may not have found evidence of a relation between self-reported stress and mesocorticolimbic DA system functioning because these clusters were primarily located outside of the NAc.
Limitations and Future Directions
This study has a number of limitations. First, the sample size, though comparable to many PET studies (68), was modest. As such, PET analyses are not corrected for multiple comparisons. Future work that attempts to replicate this study in larger samples should aim to use a more stringent method of statistical correction. Second, given that this is a cross-sectional study, we cannot determine causal relationships between reduced reward-related striatal DA release and anhedonia. Future research should investigate temporal relations between reduced reward-related striatal DA release and anhedonia. Third, striatal DA release may have reflected multiple reward processing components (e.g., novelty processing, associative learning). Future studies may implement alternative tasks to disentangle DA release that is solely related to rewards vs. other components activated by the MID task used here. Fourth, the current analyses estimated using a two-phase model, incorporating elements of both the neutral phase and uptake phase in the baseline. Future studies may consider the tradeoff between bias and variance in deciding how to estimate (69). Fifth, the ANH sample was not recruited based on severity of self-reported stress. Although the ANH sample demonstrated moderate levels of stress (see Table 2), the mean level of PSS scores was lower than typically reported in psychiatric samples and the variability of PSS scores was limited (70). Relatedly, the current study sought to examine how stress, broadly measured, relates to striatal reward responses. While we did not find any effects with respect to self-reported stress, exploring DA reward signaling during a PET-MR stress-related paradigm would be an area for future study. Sixth, while the current study did not find any dependence on total injected dose, it is possible that other unknown factors could differentially impact the dynamic uptake in the striatum and the reference region. Lastly, the length of the study presents a potential limitation on the precision of estimates. Given the approximately 20-minute half-life for [11C]Raclopride, the later time frames of the dynamic PET data have poorer signal-to-noise ratios. Because the reward task is applied late in the study protocol, the estimates for the reward phase are particularly sensitive to noise, particularly in the voxel-wise analysis. Future studies involving two scan visits could help to address this issue.
In summary, the present study is the first to investigate task-related striatal DA release to rewards in a transdiagnostic anhedonic sample. This study provides support for the association between blunted striatal DA functioning and transdiagnostic anhedonia. We found blunted general functional connectivity with PET-derived striatal seeds in anhedonia participants, but these group differences were not associated with anhedonia severity. We demonstrated that self-reported stress was strongly associated with anhedonia but was not associated with striatal DA. These findings provide support for the association between stress and anhedonia and highlight a potential molecular mechanism of impaired reward processing in anhedonia-related psychopathologies.
Supplementary Material
Highlights.
The purpose of this study was to examine linkages between anhedonia, neural responses to rewards using neuroimaging techniques, and in an exploratory fashion, self-reported stress.
We found that individuals with anhedonia who experience a loss of pleasure or motivation in daily activities demonstrate reduced neural responses during reward processing, when compared to healthy controls.
Acknowledgments:
This research was supported by R61/R33 MH110027 to GSD and MJS, R21 MH110933 to GSD and JMH, K23 MH113733 to ECW, and UL 1TR002489. DAP was partially supported by R37 MH068376 and R01 MH095809. DD was partially supported by R01 MH111676. The content is solely the responsibility of the author and does not necessarily represent the official views of NIH.
Disclosures:
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (formerly BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka, Sunovion, and Takeda; he has received honoraria from the Psychonomic Society (for editorial work) and from Alkermes; he has received research funding from the Brain and Behavior Research Foundation, the Dana Foundation, Millennium Pharmaceuticals, and NIMH; he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics, and Neuroscience Software. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
APPENDIX
Appendix A: Two-phase Kinetic Model
In the examination of changes to [11C] ) for the baseline and reward-task phases. After processing the dynamic PET images and applying the registered AAL3 atlas, time-activity curves (TACs) were extracted for atlas regions ( maps. The baseline phase was defined from start of the bolus injection up to the start of the reward task; therefore, the baseline phase includes effects of the uptake period and the neutral-task period (Figure A1). The reward phase was defined from start of the reward task to end of acquisition.
The SRTM model was applied with a two-phase component to account for the baseline (from injection time to start of reward task) and reward states:
| (A.1) |
| (A.2) |
| (A.3) |
| (A.4) |
Where
represents the start of the scan at time of bolus injection
and are the exponential system impulse responses in the reward and neutral states, respectively;
is a time-dependent discrete convolution of the reference time-activity curve (TAC) with the system response kernels accounting for the reward and neutral conditions;
represents the TAC of a given atlas region or voxel;
represents the TAC for the cerebellar reference region (measured from the cerebellar regions of the AAL3 atlas, excluding regions labeled as vermis);
is an estimated parameter of the SRTM model representing the ratio of kinetic transport rates from plasma to free-tracer tissue compartments in the TAC region studied and reference region;
is an estimated parameter of the SRTM model representing the kinetic transport rate from free-tracer tissue to plasma compartments in the TAC region studied;
and are estimated parameters representing the non-displaceable binding potential in each of the two phases, Baseline and Reward, respectively;
is the time at which the reward task is begun (usually 42 minutes into the study but varied for individual subjects based on the recorded task start time; the baseline binding potential is estimated from injection up to start of reward block).
For each TAC, the two-phase model was fitted with a custom MATLAB script applying a nonlinear least-squares fit to the model equations. Thus, for each subject and for each hypothesized atlas region or voxel, we obtained estimates of and as well as phase-independent parameters and . A composite region of cerebellum-labeled regions from the AAL3 atlas was used to compute the reference TAC.
Figure A1:
Example time-activity curves (TACs) and model fits for atlas regions in one ANH subject. Raw TAC data derived from the dynamic PET images are shown as individual points and model fits are shown as solid lines. The two phases of the kinetic model, baseline and reward, are indicated along with the periods for uptake and neutral task.
Footnotes
The other authors have no conflicts of interest or relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ (2020): Characterizing anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cognitive Affect Behav Neurosci 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge KC, Kringelbach ML (2008): Affective neuroscience of pleasure: reward in humansand animals. Psychopharmacology 199: 457–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzagalli DA (2014): Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. Clin Psychology 10: 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge KC, Robinson TE (2003): Parsing reward. Trends Neurosci 26: 507–513. [DOI] [PubMed] [Google Scholar]
- 5.Russo SJ, Nestler EJ (2013): The brain reward circuitry in mood disorders. Nature Reviews Neuroscience 14. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz W (2019): Recent advances in understanding the role of phasic dopamine activity.F1000research 8: F1000 Faculty Rev-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Der-Avakian A, Markou A (2012): The neurobiology of anhedonia and other reward-relateddeficits. Trends Neurosci 35: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peciña M, Sikora M, Avery ET, Heffernan J, Peciña S, Mickey BJ, Zubieta J-K (2017): Striatal dopamine D2/3 receptor-mediated neurotransmission in major depression: Implications for anhedonia, anxiety and treatment response. Eur Neuropsychopharm 27: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzagalli DA, Berretta S, Wooten D, Goer F, Pilobello KT, Kumar P, et al. (2019): Assessment of Striatal Dopamine Transporter Binding in Individuals With Major Depressive Disorder. Jama Psychiat 76: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husain M, Roiser JP (2018): Neuroscience of apathy and anhedonia: a transdiagnosticapproach. Nature Reviews Neuroscience 19: 470–484. [DOI] [PubMed] [Google Scholar]
- 11.Stringaris A, Belil PV-R, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, et al. (2015): The Brain’s Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. Am J Psychiat 172: 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, et al. (2013): Striatum-Based Circuitry of Adolescent Depression and Anhedonia. J Am Acad Child Adolesc Psychiatry 52: 628–641.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, et al. (2015): Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front Psychol 6: 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R, Wang Y, Chen X, Zhang Z, Xiao L, Zhou Y (2021): Anhedonia correlates with functional connectivity of the nucleus accumbens subregions in patients with major depressive disorder. Neuroimage Clin 30: 102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH (2015): Inflammationis associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatr 21: 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacker J, Dillon DG, Pizzagalli DA (2009): The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton JP, Sacchet MD, Hjørnevik T, Chin FT, Shen B, Kämpe R, et al. (2018): Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: a concurrent 11C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl Psychiat 8: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. (2008): Mesolimbic Functional Magnetic Resonance Imaging Activations during Reward Anticipation Correlate with Reward-Related Ventral Striatal Dopamine Release. J Neurosci 28: 14311–14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneier FR, Slifstein M, Whitton AE, Pizzagalli DA, Reinen J, McGrath PJ, et al. (2018): Dopamine Release in Antidepressant-Naive Major Depressive Disorder: A Multimodal [11C]-(+)-PHNO Positron Emission Tomography and Functional Magnetic Resonance Imaging Study. Biol Psychiat 84: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanton CH, Holmes AJ, Chang SWC, Joormann J (2018): From Stress to Anhedonia: Molecular Processes through Functional Circuits. Trends Neurosci 42: 23–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar P, Slavich GM, Berghorst LH, Treadway MT, Brooks NH, Dutra SJ, et al. (2015): Perceived Chronic Stress Exposure Modulates Reward-Related Medial Prefrontal Cortex Responses to Acute Stress in Depression. J Affect Disorders 180: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabib S, Puglisi-Allegra S (2012): The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev 36: 79–89. [DOI] [PubMed] [Google Scholar]
- 23.Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019): Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev 99: 101–116. [DOI] [PubMed] [Google Scholar]
- 24.Willner P, Muscat R, Papp M (1992): Chronic mild stress-induced anhedonia: A realisticanimal model of depression. Neurosci Biobehav Rev 16: 525–534. [DOI] [PubMed] [Google Scholar]
- 25.Riga D, Theijs JT, Vries TJD, Smit AB, Spijker S (2015): Social defeat-induced anhedonia: effects on operant sucrose-seeking behavior. Front Behav Neurosci 9: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 131: 391–404. [DOI] [PubMed] [Google Scholar]
- 27.Hollon NG, Burgeno LM, Phillips PEM (2015): Stress effects on the neural substrates of motivated behavior. Nat Neurosci 18: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, et al. (2012): Stress-induced changes in human decision-making are reversible. Transl Psychiat 2: e131–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson JL, Williams AV, Bangasser DA, Peña CJ (2021): Impact of Early Life Stress on Reward Circuit Function and Regulation. Frontiers Psychiatry 12: 744690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zürcher NR, Walsh EC, Phillips RD, Cernasov PM, Tseng C-EJ, Dharanikota A, et al. (2021): A simultaneous [11C]raclopride positron emission tomography and functional magnetic resonance imaging investigation of striatal dopamine binding in autism. Transl Psychiat 11: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franken IHA, Rassin E, Muris P (2007): The assessment of anhedonia in clinical and non-clinical populations: Further validation of the Snaith–Hamilton Pleasure Scale (SHAPS). J Affect Disorders 99: 83–89. [DOI] [PubMed] [Google Scholar]
- 32.Kadouri A, Corruble E, Falissard B (2007): The improved Clinical Global Impression Scale(iCGI): development and validation in depression. Bmc Psychiatry 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First M, Karg R, Williams J, Spitzer R (2015): Structured Clinical Interview for DSM-5Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association. [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R (1983): A Global Measure of Perceived Stress. J Health Soc Behav 24: 385. [PubMed] [Google Scholar]
- 35.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. (1994): Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiat 151: 1132–1136. [DOI] [PubMed] [Google Scholar]
- 36.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL (2015): The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress 28: 489–498. [DOI] [PubMed] [Google Scholar]
- 37.Gasperi M, Afari N, Goldberg J, Suri P, Panizzon MS (2021): Pain and Trauma: The Role of Criterion A Trauma and Stressful Life Events in the Pain and PTSD Relationship. J Pain 22: 1506–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizzagalli DA, Jahn AL, O’Shea JP (2005): Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biol Psychiat 57: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH (2016): Assessing anhedonia indepression: Potentials and pitfalls. Neurosci Biobehav Rev 65: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papenberg G, Jonasson L, Karalija N, Johansson J, Köhncke Y, Salami A, et al. (2019): Mapping the landscape of human dopamine D2/3 receptors with [11C]raclopride. Brain Struct Funct 224: 2871–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ladefoged CN, Law I, Anazodo U, Lawrence, Izquierdo-Garcia D, Catana C, et al. (2017): A multi-centre evaluation of eleven clinically feasible brain PET/MRI attenuation correction techniques using a large cohort of patients. Neuroimage 147: 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE (2014): Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum Brain Mapp 35: 1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitfield-Gabrieli S, Nieto-Castanon A (2012): Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity 2: 125–141. [DOI] [PubMed] [Google Scholar]
- 44.Lammertsma AA, Hume SP (1996): Simplified Reference Tissue Model for PET Receptor Studies. Neuroimage 4: 153–158. [DOI] [PubMed] [Google Scholar]
- 45.Sander CY, Hooker JM, Catana C, Rosen BR, Mandeville JB (2016): Imaging Agonist-Induced D2/D3 Receptor Desensitization and Internalization In Vivo with PET/fMRI. Neuropsychopharmacol 41: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott ML, Knodt AR, Cooke M, Kim MJ, Melzer TR, Keenan R, et al. (2019): General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage 189: 516–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D (2011): Reproducibility of Single-Subject Functional Connectivity Measurements. Am J Neuroradiol 32: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Team RC (2020): R: A language and environment for statistical computing. Retrieved from https://www.R-project.org/. [Google Scholar]
- 49.Benjamani Y, Hochberg Y (1995): A Practical and Powerful Approach to Multiple Testing: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society 57: 289–300. [Google Scholar]
- 50.Smarr KL, Keefer AL (2011): Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthrit Care Res 63: S454–S466. [DOI] [PubMed] [Google Scholar]
- 51.Cohen S, Janicki-Deverts D (2012): Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 20091. J Appl Soc Psychol 42: 1320–1334. [Google Scholar]
- 52.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, Boer P de, et al. (2013): Reduced Reward Learning Predicts Outcome in Major Depressive Disorder. Biol Psychiat 73: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA (2013): Blunted reward responsiveness in remitted depression. Journal of Psychiatric Research 47: 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P (1995): A Scalefor the Assessment of Hedonic Tone the Snaith–Hamilton Pleasure Scale. Brit J Psychiat 167: 99–103. [DOI] [PubMed] [Google Scholar]
- 55.Joiner TE, Brown JS, Metalsky GI (2003): A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiat Res 119: 243–250. [DOI] [PubMed] [Google Scholar]
- 56.Nakonezny PA, Carmody TJ, Morris DW, Kurian BT, Trivedi MH (2010): Psychometric evaluation of the Snaith–Hamilton pleasure scale in adult outpatients with major depressive disorder. Int Clin Psychopharm 25: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luijten M, Schellekens AF, Kühn S, Machielse MWJ, Sescousse G (2017): Disruption of Reward Processing in Addiction : An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. Jama Psychiat 74: 387. [DOI] [PubMed] [Google Scholar]
- 58.Leroy A, Amad A, D’Hondt F, Pins D, Jaafari N, Thomas P, Jardri R (2020): Reward anticipation in schizophrenia: A coordinate-based meta-analysis. Schizophr Res 218: 2–6. [DOI] [PubMed] [Google Scholar]
- 59.Nawijn L, Zuiden M van, Frijling JL, Koch SBJ, Veltman DJ, Olff M (2015): Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev 51: 189–204. [DOI] [PubMed] [Google Scholar]
- 60.Ng TH, Alloy LB, Smith DV (2019): Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiat 9: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang B, Lin P, Shi H, Öngür D, Auerbach RP, Wang X, et al. (2016): Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav 10: 920–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. (2017): Attenuation of Frontostriatal Connectivity During Reward Processing Predicts Response to Psychotherapy in Major Depressive Disorder. Neuropsychopharmacol 42: 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang L, Zhang A, Sun N, Liu P, Yang C, Li G, et al. (2018): Functional connectivity between the thalamus and the primary somatosensory cortex in major depressive disorder: a resting-state fMRI study. Bmc Psychiatry 18: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valenti O, Gill KM, Grace AA (2012): Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci 35: 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slavich GM, Irwin MR (2014): From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol Bull 140: 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slavich GM, Shields GS (2018): Assessing Lifetime Stress Exposure Using the Stress and Adversity Inventory for Adults (Adult STRAIN). Psychosom Med 80: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. (2009): Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals With Major Depressive Disorder. Am J Psychiat 166: 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baumgartner R, Joshi A, Feng D, Zanderigo F, Ogden RT (2018): Statistical evaluation oftest-retest studies in PET brain imaging. Ejnmmi Res 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levine MA, Mandeville JB, Calabro F, Izquierdo-Garcia D, Chonde DB, Chen KT, et al. (2022): Assessment of motion and model bias on the detection of dopamine response to behavioral challenge. J Cereb Blood Flow Metabolism 42: 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hewitt PL, Flett GL, Mosher SW (1992): The Perceived Stress Scale: Factor structure and relation to depression symptoms in a psychiatric sample. J Psychopathol Behav 14: 247–257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.