Abstract

The rational design of light-responsive proteins and protein-based polymers requires both a photoswitch with suitable light-responsive properties and the ability to incorporate it at (multiple) defined positions in the protein chain. This Letter describes the evolution of high-performance aminoacyl-tRNA synthetases for recognizing a photoswitchable arylazopyrazole-bearing unnatural amino acid (AAP-uAA), which we then incorporated at multiple sites within elastin-like polypeptides (ELPs). The incorporation of AAP-uAA into ELPs yielded proteins capable of an isothermal, reversible, and robust light-mediated soluble-to-insoluble phase transition, which occurred faster (after only 1 min of light irradiation) and demonstrated a larger transition temperature difference (up to a 45 °C difference in the ELP transition temperature upon a cis to trans AAP isomerization) than similar azobenzene-containing ELPs. The evolved translation machinery can be used for the multisite incorporation of AAP at the polypeptide level; moreover, it constitutes a general methodology for designing light-responsive proteins and protein-based polymers with robust light-responsive behavior, made possible by the superior photoswitchable properties of AAP.
Keywords: genetic code expansion, elastin-like polypeptides, arylazopyrazole, light-responsive proteins
1. Introduction
The development of a versatile and robust technology to produce light-responsive proteins and protein-based polymers (PBPs) can facilitate the design of a myriad of new user-controlled biological agents. Although genetic fusion with natural or engineered light-responsive domains can be employed for such purposes, the relatively large fusion protein may interfere with the target protein’s function or limit the types of functionality that can be manipulated with light.1 Alternatively, photoswitchable small molecules can be chemically conjugated to a reactive amino acid side chain(s) or a peptide backbone.2−6 However, this methodology can be limited due to the efficiency of the conjugation chemistries, the functionalization of only accessible or unique sites, or the chemical synthesis of small peptides. In contrast, engineering the protein translation machinery for incorporating photoswitchable unnatural amino acids (uAAs) can enable their precise installation at multiple defined positions within the protein chain and hence the synthesis, evolution, and optimization of light-responsive protein and PBP behaviors.
Selection of the appropriate photoswitchable molecule is also paramount to attaining the desired light-responsive behavior. The most commonly used photoswitchable group is azobenzene (AB), where the basic molecule 1 (Figure 1A) undergoes trans to cis isomerization in response to UV light7,8 but the isomerization wavelength can be red-shifted by installing various substituents on the aromatic rings.3,9−11 Indeed, we12 and others1,13−15 have recently described the genetic incorporation of several azobenzene derivatives as uAAs in proteins and PBPs. Nevertheless, several other newly described photoswitchable molecules, in which one of the benzene rings is replaced with five-membered heterocycles, have demonstrated superior properties for bidirectional photocontrol, such as high photostationary state (PSS) compositions and thermal stability, resulting in more complete photoswitching and long-lived cis isomers, respectively. Specifically, it was demonstrated that arylazopyrazole 2 (Figure 1B) displays near complete photoswitching in both directions and excellent thermal stability of the cis isomer.16
Figure 1.
Illustration of (A) the azobenzene-uAA (AB) and azobenzene photoisomerization and (B) the arylazopyrazole-uAA (AAP) and arylazopyrazole photoisomerization. PSS compositions and half-lives were reported by ** ref (8) and * ref (16).
Here we describe the evolution of a high-performance orthogonal aminoacyl-tRNA synthetase (aaRS) capable of multisite genetic incorporation of an arylazopyrazole-bearing uAA (AAP). Further, we utilized the selected aaRS variants to genetically encode light-responsive PBPs based on elastin-like polypeptides (ELPs). ELPs are a family of artificial, stimulus-responsive, disordered PBPs comprising a repeating VPGXG motif (where X is permissive to both natural and unnatural amino acids) that undergo a reversible soluble-to-insoluble phase transition at their lower critical transition temperature (LCST)17−20. We previously reported that the multisite incorporation of AB-bearing uAAs into ELPs generated light-responsive behavior with up to a 12 °C difference in the LCST of the ELP upon cis to trans isomerization of AB. Here we characterize the photoswitchable properties of AAP-containing ELPs and show that AAP incorporation generates superior light-responsive properties compared with similar AB-decorated ELPs.
2. Results and Discussion
2.1. Evolving aaRSs for Multisite AAP Incorporation
We began by determining the ability of our previously evolved aaRSs,21 derived from the Methanocaldococcus jannaschii tyrosyl-tRNA synthetase (MjTyrRS),22 to incorporate AAP (10 instances per protein) in the Escherichia coli strain C321.ΔRF1,23 which lacks all the native TAG codons and their associated release factor (RF-1). To this end, we utilized our previously described ELP-based reporter protein ELP(10TAG)-GFP to evaluate the multisite incorporation of AAP in response to TAG codons (Table S1).24 A GFP fluorescence assay indicated that our previously described variants were not capable of multisite incorporation of AAP, even when expressed from a multicopy plasmid in C321.ΔRF1 (Figure S1).
To enable the multisite incorporation of an AAP-bearing uAA, we utilized a modified protein-evolution strategy that we previously developed12,25 to identify improved MjTyrRS mutants, which can efficiently charge an amber suppressor tRNA with AAP in C321.ΔRF1. Briefly, genomically integrated aaRS variants were subjected to 5–10 rounds of multiplex automated genome engineering (MAGE)-based diversification, followed by successive tolC-mediated negative–positive–negative selection cycles (ColE1-mediated negative selection or SDS-mediated positive selection). The first (negative) selection cycle was used to eliminate nonorthogonal variants generated in the diversification process, which, even if rare, would otherwise be enriched in the subsequent positive selection cycle; the second (positive) selection cycle was used to enrich the efficient aaRS variants; and the third (negative) selection cycle was used to eliminate “cheater” nonorthogonal clones generated in response to the stress applied in the positive selection step.
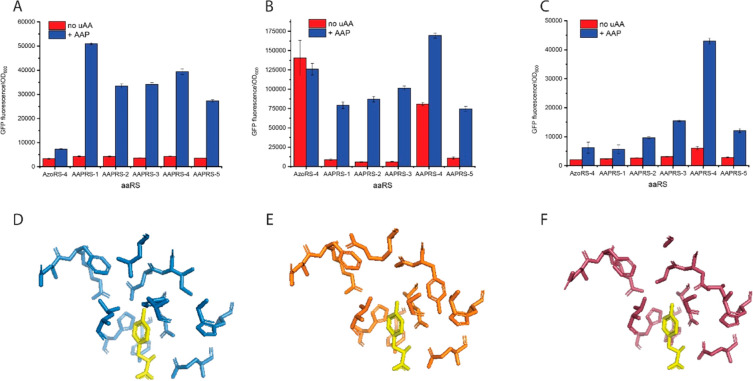
Given the similarity between AB and AAP, we chose to perform the MAGE-based diversification on the genomically integrated AzoRS-4 which was previously evolved for high-efficiency incorporation of AB.12 Following the diversification and selection steps, the production of GFP(2TAG) in the presence of AAP was used to evaluate the activity in genomically integrated individual clones. This analysis revealed several variants that, when expressed from a single chromosomal copy, were capable of improved GFP(2TAG) production compared with the parent enzyme (Figure 2A). After Sanger sequencing of all the evolved variants (Table 1), we evaluated them for multisite (2 or 10) incorporation of AAP by cotransforming C321.ΔRF1 with plasmids carrying the GFP(2TAG) or ELP(10TAG)-GFP reporter proteins and episomal versions of each evolved variant. As expected, the expression of the evolved aaRSs from multicopy plasmids increased their ability to incorporate multiple (2 or 10) instances of AAP. Notably, one of the evolved variants, designated AAPRS-4, increased the expression of ELP(10TAG)-GFP containing AAP by ∼7-fold compared with the progenitor AzoRS-4 (Figure 2B,C). Notably, when expressed from multicopy plasmids in the absence of AAP, AAPRS-4 exhibits relatively high incorporation of natural AAs compared with the other AAPRS variants. This property, which is typical of chromosomally evolved high-performance aaRS variants, results in relatively high levels of “background” expression of the ELP-GFP reporters in the absence of the uAA, although when present they accurately incorporate their cognate uAA.12,21,24,25 Such background expression can be reduced under conditions that highlight the differences in aaRS efficiencies, such as by increasing the number of TAG codons in the reporter protein or shortening the expression time, as the aaRSs incorporate the uAA more efficiently than the natural AAs. This point is illustrated by the fact that the relative protein production in the presence of the cognate uAA, compared with the absence of it, is lower for ELP(10TAG)-GFP than for GFP(2TAG) (Figure 2B,C) and by the expression kinetics of GFP(2TAG) and ELP(10TAG)-GFP over time (Figure S2). The kinetic data demonstrate that in the presence of AAP, the production of GFP(AAP×2) reaches 90% of the maximal value 5 h before the end point measurement (11 h postinduction). In contrast, protein production in the absence of any uAA at this time point is close to the background and is beginning to accumulate, reaching ∼30–45% of the signal obtained with AAP by the end point measurement.
Figure 2.
(A) Production of GFP(AAP×2) by AzoRS-4, which was the progenitor enzyme used to generate the library, and five evolved AAPRS variants, expressed from a single chromosomal copy. (B) Production of GFP(AAP×2) by AzoRS-4 and five evolved AAPRS variants, expressed from multicopy plasmids. (C) Production of ELP(AAP×10)-GFP fusion proteins containing 10 instances of the AAP-uAA by AzoRS-4 and five evolved AAPRS variants, expressed from multicopy plasmids. All proteins were expressed in the C321.ΔRF1 strain. The level of GFP fluorescence indicates the production of the GFP/ELP–GFP fusion and therefore the efficiency of uAA incorporation. n = 3; error bars indicate s.d. (D–F) Comparison of the AA-binding pocket of native MjTyrRS (D, blue) compared with AzoRS-4 (E, orange) and AAPRS-4 (F, purple). The structural models were generated (based on PDB ID 1J1U) using the PyMOL program (Schrödinger, LLC).
Table 1. Annotations of Specific Mutations in Evolved aaRS Variants Compared with the WT Methanocaldococcus jannaschii Tyrosyl-tRNA Synthetase (MjTyrRS) Sequence and the AzoRS-4 Sequence, Which Were Used to Generate the Library for Selection of AAPRSsa.

Mutations in evolved AAPRS variants compared with AzoRS-4 are indicated in red. In addition to the indicated mutations, all mutants also harbor the R257G and D286R mutations, which have been shown to improve tRNA binding.
Interestingly, compared with the progenitor AzoRS-4, all selected mutants harbored mutations in the same four amino acid sites, namely, amino acids 158, 159, 162, and 167. Modeling of the mutations found in MjTyrRS, AzoRS-4, and AAPRS-4 suggests that mutagenesis in the above-mentioned sites somewhat decreases the size of the uAA binding pocket compared to that formed in AzoRS-4 (Figure 2D–F). Not surprisingly, all but one of the variants selected for AAP incorporation generate very low amounts of the ELP(10TAG)-GFP reporter protein in the presence of AB (Figure S3), suggesting that mutually orthogonal aaRSs may be utilized to simultaneously encode several photoswitchable uAAs.
2.2. Genetically Encoding Phase-Separation Behavior by AAP Incorporation
To examine the effect of the photoisomerization of AAP on the phase-separation behavior of ELPs, we utilized our previously designed set of ELP variants to analyze the effect of AAP incorporation on the ELP LCST.12,21 The ELP pentapeptides are composed of glycine and alanine amino acids alternating in the X-guest residue position, with 2, 6, or 10 TAG codons (for the incorporation of AAP) distributed evenly along the ELP guest residue positions [termed ELP60(2TAG), ELP60(6TAG), and ELP60(10TAG), respectively; Table S1). We selected this set of hydrophilic ELPs as hosts for AAP incorporation since similarly to AB and other aromatic uAAs,21 the hydrophobic AAP molecule was expected to reduce the LCST when incorporated at multiple sites in the ELPs. Throughout this Letter, proteins expressed from the above-mentioned ELP genes are named according to the number and identity of the amino acids incorporated in the TAG codons. For example, ELP60(AAP×10) is the protein product of the ELP60(10TAG) gene, wherein the AAP-bearing uAA was incorporated in 10 encoded TAG codons.
We first produced the ELP60(AAP×10) protein in the C321.ΔRF1 strain using AAPRS-4. To determine protein yields, we purified small batches of ELP60(AAP×10). The protein yields were ∼4.3 mg L–1, compared with ∼7 mg L–1 for ELP60(Tyrosine×10), which is the same gene produced by the incorporation of tyrosine by the native MjTyrRS as previously reported.21 To determine the ability of a light-mediated isomerization of AAP to engender a difference in the LCST of the ELP (indicated as ΔLCSTcis/trans), we irradiated ELP60(AAP×2), ELP60(AAP×6), and ELP60(AAP×10) at 365 or 530 nm to induce isomerization to the cis (more hydrophilic) or trans (more hydrophobic) configuration, respectively. We confirmed that light irradiation indeed induced the isomerization of AAP within the ELP by examining the UV–vis spectra of ELP(AAP×2), ELP(AAP×6), and ELP(AAP×10) after irradiation with both wavelengths. Indeed, the characteristic peaks associated with the cis and trans isomers of AAP were clearly visible. Of note, the UV–vis spectra indicate near-complete cis to trans photoisomerization, as the spectra of dark-adapted and green-irradiated AAP and AAP-containing ELPs are nearly identical (Figure 3).
Figure 3.
UV–vis spectra of dark-adapted or irradiated (30 min) (A) AAP (250 μM), (B) ELP60(AAP×10), (C) ELP60(AAP×6), and (D) ELP60(AAP×2), each at 25 μM in water.
As expected, irradiation of AAP to produce the cis isomer generated ELPs with a higher LCST (more hydrophilic) than when AAP was irradiated to produce the trans isomer (more hydrophobic). AAP photoisomerization produced a ΔLCSTcis/trans of ∼5, 35, and 31 °C in ELP60(AAP×2), ELP60(AAP×6), and ELP60(AAP×10), respectively (Figure 4A–C). Of note, given the relative hydrophilicity of ELP60(AAP×2), we were not able to observe its LCST in DI water. Therefore, turbidity profiles for this protein were obtained in water supplemented with 2 M NaCl. To determine the effect of NaCl on ΔLCSTcis/trans, we also analyzed the light-dependent phase separation of ELP60(AAP×6) in water supplemented with 1 and 2 M NaCl. The results indicated a decrease in ΔLCSTcis/trans with increasing NaCl concentration (Figure S4). A similar extrapolation suggests that the ΔLCSTcis/trans value of ELP60(AAP×2) in water (without NaCl) may be as high as ∼15 °C.
Figure 4.
Turbidity profiles as a function of temperature and light irradiation of (A) ELP60(AAP×2), (B) ELP60(AAP×6), (C) ELP60(AAP×10), and (D) ELP60(Gly/AAP×10) (25 μM solutions in water, except for ELP60(AAP×2), which was supplemented with 2 M NaCl).
To evaluate the accuracy of AAP incorporation, we analyzed tryptic fragments of the MS-optimized reporter protein ELP60(10TAG)MS (Table S1) expressed with AAP (i.e., ELP60(AAP×10)MS) by liquid chromatography–tandem mass spectrometry (LC–MS/MS), which identifies and assesses the extent of natural amino acid misincorporation. The incorporation of AAP by AAPRS-4 was detected in ∼97% of the total ions (Table S3). Since MS-compatible ELPs bearing 2 or 6 AAP instances are too hydrophilic and cannot be purified, we also produced ELP60(AAP×2) using AAPRS-3, which has the lowest background of incorporation, and showed a similar ΔLCSTcis/trans to the same ELP produced with AAPRS-4 (Figure S5).
Surprisingly, although it was expected that the ΔLCSTcis/trans would increase with the number of AAP instances per protein (as was found in identical AB-containing ELPs12), we observed that the ΔLCSTcis/trans of ELP60(AAP×6) was slightly larger than that of ELP60(AAP×10). We hypothesized that the ΔLCSTcis/trans may also be affected (reduced) due to either the higher ELP hydrophobicity or increased proximity between neighboring AAP residues. To test this hypothesis, we produced another ELP (termed ELP60(Gly/10TAG)), in which glycine is encoded in the guest residue in all pentapeptides not containing AAP (Table S1). Since glycine is more hydrophilic than alanine, it is expected that the overall hydrophobicity of this ELP will be lower than that of ELP60(AAP×10) while maintaining the same number and positioning of the AAP residues. Analysis of the light-responsive behavior of ELP60(Gly/AAP×10) revealed that isomerization of AAP generated an ΔLCSTcis/trans of ∼45 °C, indicating that a higher ΔLCSTcis/trans is expected when the overall ELP hydrophobicity is decreased (Figure 4D).
We further characterized the irradiation time required for photoswitching of the AAP-uAA in solution and when incorporated in a protein. Under our experimental conditions, as little as 1 min or 2 s of irradiation with green (370 mW) or UV (1290 mW) light, respectively, was sufficient to generate the PSS (defined here as the spectrum observed after 30 min of irradiation) for both the uAA in solution and incorporated in all ELP proteins (Figures 5A,B, S6, and S7). Specifically, under our experimental conditions, AAP isomerizes slightly faster from the trans to the cis configuration and with a higher PSS than AB, as is demonstrated by the lower value of the peak absorbance of blue-irradiated AB compared with dark-adapted AB (Figure S6). Accordingly, ELP60(AAP×10) irradiated for either 30 or only 1 min with each wavelength exhibited a nearly identical ΔLCSTcis/trans of ∼31 °C (Figure 5C,D), whereas ELP60(AB×10) exhibited slower kinetics of isomerization and phase transition (Figure S8). We then determined the reversibility of the light-induced phase separation of ELP60(AAP×10) by measuring OD600 changes in the ELPs during 10 successive isothermal (38 °C) irradiation cycles (3 or 1 min irradiation time). The resulting measurements were nearly identical throughout all 10 successive cycles (Figure 5E), indicating that the effect of isomerization on the LCST was reproducible throughout multiple (and very short) irradiation cycles without decreasing efficiency. We also examined the effect of AAP incorporation on the self-assembly of this ELP family, motivated by an earlier finding that the incorporation of AB (as well as several other aromatic uAAs) engendered the self-assembly of ELP60(uAA×10) into thin two-dimensional sheets.12,21 An assessment of self-assembly was conducted by dynamic light scattering (DLS) analysis of a solution of each protein (25 μM in ddH2O) at a temperature below the LCST of all ELPs (10 °C). The DLS analysis indicated that unlike AB, AAP does not engender self-assembly in any of the ELPs, even when incorporated at 10 instances per protein (Figure S9). Interestingly, ELPs bearing cis- or trans-AAP isomers produced nearly identical CD spectra (7.5 μM at 10 °C; Figure S10), in contrast to the same AB-decorated ELPs.12 This may be related to the self-assembly propensity of AB-ELP, which may be different in the cis and trans configurations, in contrast to the lack of apparent self-assembly of AAP-ELPs.
Figure 5.
(A, B) UV–vis spectra of ELP60(AAP×10) (25 μM) irradiated with (A) UV or (B) green light for 1 s to 30 min. (C, D) ΔLCSTcis/trans of ELP60(AAP×10) as a function of irradiation time for (C) 30 min and (D) 1 min irradiation at each wavelength. (E) Reversibility of the light-mediated transition (600 nm) over 10 cycles of 3 or 1 min illumination of ELP60(AAP×10) (25 μM solutions in water at 38 °C).
The genetic encoding of AAP- and AB-bearing uAAs allows for the precise positioning of these photoswitchable groups within the protein sequence and thus the systematic examination and comparison of their performance in this context. From such a comparison it is clear that AAP provides superior photocontrol of the ELP phase transition in comparison with AB, as is evident from the significantly larger ΔLCSTcis/trans generated by AAP incorporation compared with AB incorporation (e.g., 35 vs 9 and 31 vs 12 °C for ELP60(6TAG) and ELP60(10TAG), respectively, containing either AAP or AB12). To examine whether this difference is entirely attributed to the higher PSS composition of AAP compared with AB, we irradiated AAP-containing ELPs with blue light (405 nm). As can be seen from the spectrum of the AAP molecule, irradiation with blue light reverts ∼60% of cis-AAP to trans-AAP, in contrast to irradiation with green light, which appears to completely restore the dark-adapted spectrum (Figure S11A). An examination of the ΔLCSTcis/trans generated by UV (cis) and blue (trans) light irradiation reveals a ΔLCSTcis/trans of ∼3.5, 18, and 22 °C in ELP60(AAP×2), ELP60(AAP×6), and ELP60(AAP×10), respectively (Figure S11B–D). Thus, it appears that even when AAP photoisomerization is at ∼60%, the ΔLCSTcis/trans generated by AAP is larger than that generated by AB.
This difference is likely attributed to other characteristics of AAP versus AB such as a larger difference in the hydrophilicities between the cis and trans isomers or the lack of self-assembly of AAP-bearing ELPs, which can affect the apparent LCST. The dipole moments of the isomers of AB and AAP have been computed to be 0 vs 4.3 for trans-AB and -AAP, respectively, and 3 vs 5.69 for cis-AB and -AAP, respectively.26,27 However, the dipole moment does not directly indicate the solubility of the compound, as was found for other arylazopyrazoles.28 While a previous study reported that the cis isomer of AB is 5–40 times more soluble than the trans isomer in water (20–50 μM and 0.65–2 mM for trans- and cis-AB, respectively),29 solubility data for AAP was not previously reported in the literature. While trying to obtain this information, we found significant self-assembly of both AAP isomers in water (Figure S12), despite the fact the AAP-containing ELPs appeared as monomers, likely due to a solubilization effect of the ELP on AAP. Unfortunately, high viscosity of a saturated cis-AAP solution, perhaps due to the self-assembly propensity of the AAP molecule, prohibited the determination of cis-AAP solubility. However, it was determined that the solubility of a similar arylazopyrazole, which had comparable calculated dipole moments to the AAP variant used in this study, was ≤ 1.51μM versus 48 mM for the trans versus cis isomers, indicating an ∼32000-fold difference in the solubility of the isomers.28 Yet another study described the high solubility of cis-AAP, although the exact solubility values were not reported.30
In summary, we provide the first orthogonal translation system for multisite genetic encoding of arylazopyrazole as a uAA. We demonstrate that encoding AAP in temperature-responsive ELPs can be utilized to engineer a light-responsive phase transition in these PBPs. The superior properties of AAP compared with AB also translate into improved light-responsive properties, i.e., a significantly larger ΔLCSTcis/trans in ELPs, suggesting that the genetic encoding of other heterocyclic azo compounds may be beneficial for the engineering of photoswitchable proteins and PBPs. Further, we demonstrate that the photoswitchable behavior appears to be affected by both the properties of the genetically encoded photoswitch and the ELP itself. By examining sequence-defined AAP-containing ELPs, we find that ΔLCSTcis/trans is affected by the number of AAPs incorporated in a single protein and the ELP hydrophilicity. In addition, the incorporation of AAP does not appear to engender the self-assembly of the single-block ELPs examined in this study, suggesting a reduced tendency for π–π interactions between AAP molecules compared with AB molecules. Such in-depth examination of the effect of the specific photoswitch properties on polymer behavior can only be attained by the precise positioning of multiple photoswitchable molecules withing the protein chain, enabling a comparison of the light-responsive behavior of such compositionally defined polymers. We note that during the review process of this manuscript, an additional study described the incorporation of AAP using a pyrrolysyl synthetase. While pyrrolysyl-based systems have the advantage of orthogonality in mammalian cells, the efficiencies of such systems are typically low. Accordingly, the above-mentioned study described the production of a GFP variant having a single site for AAP incorporation. In contrast, while MjTyrRS-based translation systems are not orthogonal in eukaryotic cells, they are able to achieve much higher incorporation efficiencies, as exemplified in this and previous studies.12,21,24,25 Thus, the pyrrolysyl- and MjTyrRS-based systems each offer distinct advantages, depending on the desired application. Looking forward, the aaRSs evolved in this work can also be used to incorporate multiple AAP instances at precise positions in the sequence of potentially any protein or PBP. This will facilitate the elucidation of additional sequence determinants for engineering light-responsive behavior as well as the engineering of improved light-responsive proteins and PBPs having near-complete photoswitching between isomer states.
3. Materials and Methods
Materials
(2S)-2-Amino-3-[4-[(E)-(1,3,5-trimethylpyrazol-4-yl)azo]phenyl]propanoic acid hydrochloride (the AAP uAA) was purchased from Chiroblock. Restriction endonucleases and ligation enzymes were purchased from New England Biolabs. DNA amplification was performed using the KAPA2G Fast HotStart ReadyMix or the KAPA HiFi PCR kit (Roche). Plasmid purification was conducted with Plasmid HiYield mini-prep (RBC Bioscience), and the PCR/restriction product was purified using a HiYield gel/PCR extraction kit (RBC Bioscience). Ligation was performed using the Quick Ligation Kit or with T4 DNA Ligase, both purchased from New England Biolabs. Ligation products were transformed into 5-alpha Competent E. coli (High Efficiency) or Stbl2 Competent E. coli (High Efficiency), purchased from New England Biolabs. SDS solution was purchased from Bio-Rad. Anhydrotetracycline hydrochloride was purchased from Sigma-Aldrich. C321.ΔA (Isaacs lab) was a gift from Farren Isaacs (Addgene plasmids #73581). Isomerization experiments were performed with 365 nm (UV) and 530 nm (green) LEDs (M365L3 and M530L3, Thorlabs).
Diversification and Selection of AzoRS Variants
aaRS libraries were generated by MAGE-based diversification of the previously isolated genomically integrated mutants AzoRS-4.12,22 Prior to MAGE cycling, cultures were established by inoculating the liquid medium with a single bacterial colony or by adding 30 μL of a confluent liquid culture (1:100 dilution) at 34 °C to mid logarithmic growth phase bacterial cells (OD600 = 0.6–0.7) in a shaking incubator. To induce the expression of the lambda-red recombination proteins, the cell cultures were shifted to 42 °C for 15 min and then immediately chilled on ice. Of these cultures, 1 mL of cells was centrifuged at 4 °C at 15000g for 30 s, the supernatant medium was removed, and the cells were resuspended in Milli-Q water. Then the cells were spun down, the supernatant was removed, and the washing procedure was repeated. After a final 30 s spin, the supernatant was removed, and MAGE oligos (5–6 μM in DNase-free water) were added to the cell pellet. The oligo/cell mixture was transferred to a prechilled 1 mm gap electroporation cuvette (Bio-Rad) and electroporated under the following conditions: 1.8 kV, 200 V, and 25 mF. LB medium (3 mL) was immediately added to the electroporated cells, which were then recovered from electroporation and grown at 34 °C for 3–3.5 h. Once the cells reached the mid log stage, they were used in additional MAGE cycles, subjected to negative and positive selection cycles, or frozen for further use.
After 5–10 rounds of diversification with MAGE and of negative (using varying concentrations of colicin E1 in the absence of AAP), positive (using varying concentrations of SDS in the presence of AAP), and a final negative selection, the improved AAPRS variants were screened by plating cells on LB plates supplemented with appropriate antibiotics, l-Arabinose (0.2%), anhydrotetracycline (60 ng mL–1), and AAP (0.25 mM). Colonies expressing high levels of GFP were selected and subjected to further GFP expression analysis by intact-cell fluorescence measurements in the presence or absence of AAP. The aaRS genes of the best-performing colonies were analyzed by Sanger sequencing.
Plasmid Construction
Plasmids bearing the OTS variants for AAP incorporation were constructed by inserting aaRS genes into a previously described plasmid (pEvol)24,31 harboring a p15A origin of replication and a chloramphenicol resistance marker. The evolved genomic aaRS genes were amplified by PCR from chromosomal templates. All variants were inserted sequentially by using flanking restriction sites BglII and SalI to obtain inducible expression under the control of the araBAD promoter and the rrnB terminator. The second constitutive copy of the aaRS that is typically found in the pEvol system was removed. Ligation was conducted with the Quick Ligation Kit (NEB), and the ligation products were transformed into NEB 5-alpha Competent E. coli (High Efficiency), later plated on LB-agar plates supplemented with chloramphenicol (25 μg mL–1), and analyzed by Sanger sequencing.
Analysis of GFP Expression by Intact-Cell Fluorescence Measurements
For 96-well plate-based assays, strains harboring chromosomally integrated orthogonal translation systems and GFP reporter plasmids were inoculated from frozen stocks and grown to confluence overnight. Cultures were then inoculated at a 1:50 dilution in LB medium supplemented with kanamycin (30 μg mL–1). For cells harboring the plasmid-based orthogonal translation system and GFP reporter proteins, the medium was also supplemented with chloramphenicol (25 μg mL–1). Cells were allowed to grow at 34 °C to an OD600 of 0.5–0.8 in a shaking plate incubator at 500 rpm (∼3 h). The expression of aaRS was then induced by adding arabinose (0.2%), GFP expression was induced by adding anhydrotetracycline (60 ng mL–1), and the uAA was added at a concentration of 0.25 mM. Following expression, the cells were centrifuged at 4000g for 5 min, the supernatant medium was removed, and the cells were resuspended in PBS. GFP fluorescence was measured on a Biotek spectrophotometric plate reader by using excitation and emission wavelengths of 485 and 528 nm, respectively. Fluorescence signals were normalized by dividing the fluorescence counts by the OD600 reading.
ELP Expression and Purification
Before batch expression, starter cultures (1:25 v/v of final expression volume) of 2xYT or LB medium, supplemented with kanamycin (30 μg mL–1) and chloramphenicol (25 μg mL–1), were inoculated with transformed cells from either a fresh agar plate or from stocks stored at −80 °C, incubated overnight at 34 °C while shaking at 220 rpm, and transferred to expression flasks containing 2xYT medium, antibiotics, arabinose (0.2%), and AAP (0.25 mM). For the expression of ELP60(10TAG), ELP60(6TAG), and ELP60(2TAG) by AAPRS-4, the C321.ΔRF1 strain,32 supplemented with AAP (0.25 mM) and arabinose (0.2%), was incubated at 34 °C for 4–5 h, and then protein expression was induced with isopropyl β-d-1-thiogalactopyranoside (IPTG) (1 mM). The cells were harvested 24 h after inoculation by centrifugation at 4000g for 30 min at 4 °C. The cell pellet was then resuspended by vortex in Milli-Q water (∼4 mL) and either stored at −80 °C or purified immediately. For purification, resuspended pellets were lysed by ultrasonic disruption (18 cycles of 10 s sonication, separated by 40 s intervals of rest). Poly(ethylenimine) was added (0.2 mL of a 10% solution) to each lysed suspension before centrifugation at 4000g for 15 min at 4 °C to separate cell debris from the soluble cell lysate. All ELP constructs were purified by a modified inverse transition cycling (ITC) protocol18 consisting of multiple “hot” and “cold” spins by using sodium chloride to trigger the phase transition. Before purification, the soluble cell lysate was incubated for 1–2 min at 42–55 °C to denature the native E. coli proteins. The cell lysate was then cooled on ice and centrifuged for 2 min at ∼14000 rpm, and the pellet was discarded. For “hot” spins, the ELP phase transition was triggered by adding sodium chloride to the cell lysate or to the product of a previous cycle of ITC at a final concentration of up to ∼5 M. The solutions were then centrifuged at ∼14000 rpm for 10 min, and the pellets were resuspended in Milli-Q water, after which a 2 min “cold” spin was performed without sodium chloride to remove denatured contaminant. Additional rounds of ITC were conducted as needed using a saturated solution of sodium chloride until sufficient purification was achieved. Purified proteins were visualized on SDS-PAGE (Figure S13).
Protein concentrations were calculated by measuring the OD280 of the purified protein according to the following extinction coefficients: ELP60(tyrosine×10), 16390; ELP60(AAP×10), 27039; ELP60(AAP×6), 16819.4; and ELP60(AAP×2), 6599.8, based on the experimentally determined extinction coefficient of AAP (2554.9 M cm–1).
Protein Digestion
ELP60(10TAG)MS was expressed by AAPRS-4 using the expression protocol indicated above. Then 100 pmol of purified ELP60(10TAG)MS was dissolved in 50 mM Tris-HCl, pH 8. Trypsin (0.1 μg/μL) and LysC (0.02 μg/μL), both dissolved in 50 mM acetic acid, were added to the protein to give an enzyme:protein mass ratio of 1:25. The reaction mixture was incubated at 37 °C and then quenched by adding 0.5% trifluoroacetic acid. All the peptides were desalted on a C18 column. Eluted peptide solutions were dried in a vacuum centrifuge operated at 42 °C. The dried peptides were then dissolved by vortexing in 10 μL of peptide solvent consisting of 2:3:7 v/v/v 70% formic acid/2-propanol/0.5% acetic acid. Shotgun proteomics analysis of peptides was performed by LC-MS/MS on an LTQ orbitrap XL mass spectrometer using the top five collision-induced dissociation (CID) method for peptide sequencing, with an activation time of 200 ms and a normalized CID collision energy of 35%. Survey full-scan spectra were collected over the m/z 200–2000 range.
Bioinformatics
Spectra from shotgun discovery experiments were matched with custom databases in Proteome Discoverer 1.4.0.288, the custom database for identification of ELP60(10TAG)MS protein sequences, each representing the incorporation of any one of the 20 natural amino acids for a given database entry at any of the TAG stop codons in the ELP protein. Variable custom modification for natural amino acids was specified to detect incorporation of AAP, with a composition of delta H7C4N3 on W, +97.064 Da. In all of the experiments, oxidation (M) was defined as a variable modification. The specified enzyme was trypsin, and only fully tryptic peptides were considered, while allowing up to two missed cleavages. The precursor mass tolerance was 12 ppm, and the fragment ion mass tolerance was 0.4 Da for all searches.
Phase Transition Analysis
To characterize the LCST of ELP variants, the OD600 of the ELP solution (in Milli-Q water, unless otherwise noted) was monitored as a function of temperature, with heating and cooling performed at a rate of 1 °C min–1 on a UV–vis spectrophotometer equipped with a multicell thermoelectric temperature controller (Thermo Scientific).
Photoisomerization Kinetics
To characterize time-dependent photoisomerization of AAP, AB, and AAP- and AB-bearing ELPs, the ODs of the respective molecule solutions in Milli-Q water were monitored as a function of irradiation time. Full spectra as well as peak values are reported for each measurement. Spectra of aggregated ELPs having a high background signal were discarded from the analysis.
DLS Analysis
ELP self-assembly was analyzed using a Zetasizer Nano ZS (Malvern Pananalytical). For each sample, 12–17 acquisitions (determined automatically by the instrument) were obtained at 10 °C. Populations comprising less than 1% of the total mass (by volume) were excluded from the analysis.
CD Analysis
The secondary structure of ELPs was studied using a J-715 spectropolarimeter (Jasco, Tokyo) equipped with a PTC-348WI temperature controller, using a 1 mm quartz cuvette instrument by scanning from 280 to 180 nm at 10 °C. Purified constructs were diluted to 7.5 μM in water. Data were considered for analysis whenever the Dynode voltage was below 800 V.
Acknowledgments
We thank Dr. Mark Karpasas from the Ilse Katz Institute for Nanoscale Science & Technology for the professional help with the mass spectrometry experiments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssynbio.3c00146.
Lists of protein and MAGE oligos used in this study, information on AAP incorporation kinetics and accuracy, isomerization kinetics of AAP molecule and AAP-containing ELPs, DLS and CD data, and SDS-PAGE gels of purified proteins (PDF)
Author Contributions
D.S.S carried out the experiments; D.H. assisted with mass spectrometry experiments; M.A. supervised the study and wrote the manuscript with feedback from all authors.
This work was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (Grant 756996), the Israel Science Foundation (Grant 939\21), and a Science Forefront Grant sponsored by the Ministry of Science & Technology, Israel (0002069). M.A. gratefully acknowledges support from the Avram and Stella Goldstein-Goren Fund and the Elaine S. and Alvin W. Wene Career Development Chair in Biotechnology Engineering.
The authors declare the following competing financial interest(s): M.A. and D.S.S. filed for a patent relating to the described technology.
Supplementary Material
References
- Hoppmann C.; et al. In Situ Formation of an Azo Bridge on Proteins Controllable by Visible Light. J. Am. Chem. Soc. 2015, 137 (35), 11218–21. 10.1021/jacs.5b06234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull K.; Morstein J.; Trauner D. In Vivo Photopharmacology. Chem. Rev. 2018, 118 (21), 10710–10747. 10.1021/acs.chemrev.8b00037. [DOI] [PubMed] [Google Scholar]
- Samanta S.; et al. Photoswitching azo compounds in vivo with red light. J. Am. Chem. Soc. 2013, 135 (26), 9777–84. 10.1021/ja402220t. [DOI] [PubMed] [Google Scholar]
- Albert L.; Vázquez O. Photoswitchable peptides for spatiotemporal control of biological functions. Chem. Commun. 2019, 55 (69), 10192–10213. 10.1039/C9CC03346G. [DOI] [PubMed] [Google Scholar]
- Yasuike N.; et al. Photoswitchable affinity reagents: Computational design and efficient red-light switching. ChemPhotoChem. 2019, 3 (6), 431–440. 10.1002/cptc.201900016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklock K. M.; et al. Computational Design of a Photocontrolled Cytosine Deaminase. J. Am. Chem. Soc. 2018, 140 (1), 14–17. 10.1021/jacs.7b08709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M. X.; et al. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48 (10), 2662–2670. 10.1021/acs.accounts.5b00270. [DOI] [PubMed] [Google Scholar]
- Bandara H. M. D.; Burdette S. C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41 (5), 1809–1825. 10.1039/C1CS15179G. [DOI] [PubMed] [Google Scholar]
- Dong M.; et al. Near-Infrared Photoswitching of Azobenzenes under Physiological Conditions. J. Am. Chem. Soc. 2017, 139 (38), 13483–13486. 10.1021/jacs.7b06471. [DOI] [PubMed] [Google Scholar]
- Bleger D.; et al. o-Fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-Way Isomerization with Visible Light. J. Am. Chem. Soc. 2012, 134 (51), 20597–20600. 10.1021/ja310323y. [DOI] [PubMed] [Google Scholar]
- Konrad D. B.; Frank J. A.; Trauner D. Synthesis of Redshifted Azobenzene Photoswitches by Late-Stage Functionalization. Chemistry 2016, 22 (13), 4364–4368. 10.1002/chem.201505061. [DOI] [PubMed] [Google Scholar]
- Israeli B.; Strugach D. S.; Gelkop S.; Weber S.; Gozlan D. S.; Amiram M. Genetically Encoding Light-Responsive Protein-Polymers Using Translation Machinery for the Multi-Site Incorporation of Photo-Switchable Unnatural Amino Acids. Adv. Funct. Mater. 2021, 31 (44), 2011276. 10.1002/adfm.202011276. [DOI] [Google Scholar]
- Bose M.; et al. The incorporation of a photoisomerizable amino acid into proteins in E. coli. J. Am. Chem. Soc. 2006, 128 (2), 388–9. 10.1021/ja055467u. [DOI] [PubMed] [Google Scholar]
- Luo J.; et al. Reversible and Tunable Photoswitching of Protein Function through Genetic Encoding of Azobenzene Amino Acids in Mammalian Cells. ChemBioChem 2018, 19 (20), 2178–2185. 10.1002/cbic.201800226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppmann C.; et al. Genetically encoding photoswitchable click amino acids in Escherichia coli and mammalian cells. Angew. Chem., Int. Ed. 2014, 53 (15), 3932–6. 10.1002/anie.201400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston C. E.; et al. Arylazopyrazoles: azoheteroarene photoswitches offering quantitative isomerization and long thermal half-lives. J. Am. Chem. Soc. 2014, 136 (34), 11878–81. 10.1021/ja505444d. [DOI] [PubMed] [Google Scholar]
- MacEwan S. R.; Chilkoti A. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers 2010, 94 (1), 60–77. 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- Hassouneh W.; Christensen T.; Chilkoti A. Elastin-like polypeptides as a purification tag for recombinant proteins. Curr. Protoc. Protein Sci. 2010, 10.1002/0471140864.ps0611s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico I. S.; et al. Lithographic patterning of photoreactive cell-adhesive proteins. J. Am. Chem. Soc. 2007, 129 (16), 4874–5. 10.1021/ja070200b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor R. E.; Tirrell D. A. Non-canonical amino acids in protein polymer design. Polym. Rev. 2007, 47 (1), 9–28. 10.1080/15583720601109552. [DOI] [Google Scholar]
- Gueta O.; et al. Tuning the Properties of Protein-Based Polymers Using High-Performance Orthogonal Translation Systems for the Incorporation of Aromatic Non-Canonical Amino Acids. Front. Bioeng. Biotechnol. 2022, 10, 913057. 10.3389/fbioe.2022.913057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M.; et al. The incorporation of a photoisomerizable amino acid into proteins in E. coli. J. Am. Chem. Soc. 2006, 128 (2), 388–389. 10.1021/ja055467u. [DOI] [PubMed] [Google Scholar]
- Kuru E.; et al. Release Factor Inhibiting Antimicrobial Peptides Improve Nonstandard Amino Acid Incorporation in Wild-type Bacterial Cells. ACS Chem. Biol. 2020, 15 (7), 1852–1861. 10.1021/acschembio.0c00055. [DOI] [PubMed] [Google Scholar]
- Amiram M.; et al. Evolution of translation machinery in recoded bacteria enables multi-site incorporation of nonstandard amino acids. Nat. Biotechnol. 2015, 33 (12), 1272–1279. 10.1038/nbt.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar D.; et al. Efficient Incorporation of Clickable Unnatural Amino Acids Enables Rapid and Biocompatible Labeling of Proteins in Vitro and in Bacteria. ChemBioChem 2021, 22 (8), 1379–1384. 10.1002/cbic.202000663. [DOI] [PubMed] [Google Scholar]
- Merino E.; Ribagorda M. Control over molecular motion using the cis-trans photoisomerization of the azo group. Beilstein J. Org. Chem. 2012, 8, 1071–1090. 10.3762/bjoc.8.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T. T.; Zhao Z. X.; Zhang H. X. A theoretical study on the thermal cis-trans isomerization of azoheteroarene photoswitches. New J. Chem. 2017, 41 (4), 1659–1669. 10.1039/C6NJ03095E. [DOI] [Google Scholar]
- Nagai Y.; et al. Light-Triggered, Non-Centrosymmetric Self-Assembly of Aqueous Arylazopyrazoles at the Air-Water Interface and Switching of Second-Harmonic Generation. Angew. Chem., Int. Ed. 2021, 60 (12), 6333–6338. 10.1002/anie.202013650. [DOI] [PubMed] [Google Scholar]
- Brown C.; et al. Differential azobenzene solubility increases equilibrium cis/trans ratio in water. J. Photochem. Photobiol., A 2017, 336, 140–145. 10.1016/j.jphotochem.2016.12.013. [DOI] [Google Scholar]
- Hanopolskyi A. I.; et al. Reversible switching of arylazopyrazole within a metal-organic cage. Beilstein J. Org. Chem. 2019, 15, 2398–2407. 10.3762/bjoc.15.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. S.; et al. An enhanced system for unnatural amino acid mutagenesis in E. coli. J. Mol. Biol. 2010, 395 (2), 361–74. 10.1016/j.jmb.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Lajoie M. J.; et al. Genomically recoded organisms expand biological functions. Science 2013, 342 (6156), 357–60. 10.1126/science.1241459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.