Abstract
Background: Vitamin B12, or cobalamin (Cbl), is absorbed in the intestine and transported to the cells bound to transcobalamin (TC). We hypothesize that cyanocobalamin (CNCbl) is absorbed unchanged, thereby allowing measurement of the complex of CNCbl bound to TC (TC–CNCbl) to be used for studying the absorption of the vitamin.
Methods: TC was immunoprecipitated from serum samples obtained from healthy donors at baseline and at 24 h after oral administration of three 9-μg CNCbl doses over 1 day. Cbl was released by treatment with subtilisin Carlsberg. The different forms of Cbl were isolated by HPLC and subsequently quantified with an ELISA-based Cbl assay.
Results: At baseline, the median TC–CNCbl concentration was 1 pmol/L (range, 0–10 pmol/L); the intraindividual variation (SD) was 1.6 pmol/L (n = 31). After CNCbl administration, the TC–CNCbl concentration increased significantly (P = 0.0003, paired t-test), whereas no major changes were observed in any of the other Cbl forms bound to TC (n = 10). Only a moderate additional increase in TC–CNCbl was observed with prolonged (5 days) CNCbl administration (n = 10). We designed an absorption test based on measuring TC–CNCbl at baseline and 24 h after CNCbl intake and established a reference interval for the increase in TC–CNCbl (n = 78). The median absolute increase was 23 pmol/L (range, 6–64 pmol/L), and the relative increase was >3-fold.
Conclusions: Our data demonstrate that CNCbl is absorbed unchanged and accumulates on circulating TC. We suggest that measuring TC–CNCbl will improve the assessment of vitamin B12 absorption.
Cobalamin (Cbl),1 or vitamin B12, is a nutrient necessary for normal DNA synthesis, red blood cell production, and maintenance of the nervous system. Cbl deficiency is caused either by malabsorption or low dietary intake of Cbl. Cbl uptake from food involves the binding of intrinsic factor to dietary Cbl in the intestine (1). After binding of intrinsic factor–Cbl to the receptor complex cubam, which is localized in the apical pole of the ileal epithelium, the cubam–intrinsic factor–Cbl complex is internalized via endocytosis (2). In the circulation, Cbl is bound to 2 transport proteins, transcobalamin (TC) and haptocorrin. TC transports Cbl to the cells of the body, whereas the function of haptocorrin remains to be clarified. Only about 20% of the circulating Cbl is bound to TC (holoTC) and thus available for cellular uptake. Therefore, plasma holoTC has been suggested as a more sensitive marker for Cbl status than total plasma Cbl (3)(4)(5)(6).
For decades, the Schilling test has been used to measure Cbl absorption in humans for identifying patients with an acquired inability to absorb Cbl (7). This procedure has become increasingly unacceptable, however, because it uses radioactively labeled Cbl (8). Moreover, radiolabeled Cbl has become less available. Recently, a new approach, the CobaSorb test, has been introduced for the study of Cbl uptake. The CobaSorb assay is based on measuring holoTC before and after intake of a test dose of Cbl (9)(10)(11). Although the test seems to demonstrate excellent sensitivity, showing no increase in holoTC in patients unable to absorb Cbl (12), the test’s specificity depends on the baseline holoTC concentration. Obviously, the specificity of the test would be improved if it could selectively measure the newly absorbed vitamin. We speculated that such measurement was possible, provided that cyanocobalamin (CNCbl) is absorbed unmodified. In individuals on a typical diet, CNCbl accounts for only a minor part of the Cbl in the circulation. The major forms are hydroxocobalamin or aquocobalamin (OHCbl) and the 2 coenzyme forms of Cbl, 5′-deoxyadenosylcobalamin (AdoCbl) and methylcobalamin (MeCbl) (1)(13).
The aim of the present study was to document whether CNCbl is absorbed unmodified and if this is the case, to explore the use of measuring TC-bound CNCbl (TC–CNCbl) for assessing vitamin B12 absorption.
Experimental Design and Methods
study participants and samples
Serum samples were obtained from 3 previously characterized populations, all consisting of healthy donors. Except for the samples in cohort A, all samples were collected without any protection from light. Cohort A samples were collected into foil-wrapped test tubes, and all subsequent handling steps were performed in dim red light until the sample was applied to the HPLC instrument.
Cohort A consisted of 4 females and 10 males between 18 and 49 years of age. These individuals were randomly chosen from a population that received oral supplements of 9 μg CNCbl at 0, 5.5, and 11 h after collection of the baseline sample (9). Ten light-protected pairs of serum samples obtained at baseline and at 24 h after intake of the first CNCbl dose were analyzed for all forms of Cbl on TC.
Cohort B consisted of 9 males and 22 females between 25 and 57 years of age (11). We analyzed samples obtained on 2 consecutive days before vitamin B12 supplementation.
Cohort C consisted of 78 individuals, 40 males and 38 females between 21 and 81 years of age (10). These individuals received oral supplements of three 9-μg CNCbl doses at 0, 6, and 12 h after collection of the baseline sample; they continued to take 3 vitamin B12 doses per day for 5 days. Blood samples were collected at baseline (day 0) and on days 1, 2, 3, 4, and 7. We analyzed the samples collected at baseline and at 24 h for all individuals in this cohort. In addition, we evaluated all samples obtained from 10 randomly chosen individuals. HoloTC was previously measured in all of the samples from cohort C (10).
enzymatic extraction of cbl from tc
All handling of serum samples and material obtained from serum was done in 2-mL polypropylene tubes (Sarstedt) or 200-μL conical vials (MicroLab). TC in the samples was immunoprecipitated by incubating 200 μL serum with 25 μL magnetic microspheres covered with monoclonal antibodies to TC (kindly donated by Axis-Shield, Dundee, Scotland, UK). We treated the immunoprecipitated TC for 2 h at 37 °C with 100 μL of subtilisin Carlsberg from Bacillus lichenformis (EC 3.4.21.14; Sigma-Aldrich) in 0.1 mol/L Tris-HCl, pH 7.4 (Sigma-Aldrich), as previously described (14).
hplc of released cbl
The Cbl forms were separated by HPLC, as described by Jacobsen et al. (15). The HPLC apparatus (HP 1100; Agilent Technologies) was fitted with a precolumn (SecurityGuard cartridge C18, 4 × 3 mm; Phenomenex) followed by a reversed-phase column (Luna 3 μm C18, 150 mm × 4.6 mm; Phenomenex). Both columns were thermostated at 20 °C. A gradient of acetonitrile (J.T. Baker) in 0.05 mol/L phosphoric acid, pH 3, was applied 4 min after injection of 90 μL of sample or calibrator. Eluted fractions were collected, and the eluent in the fractions was lyophilized (HetoVac).
Standard solutions, calibrators, and samples.
Standard solutions containing 100 pmol/L OHCbl, CNCbl, AdoCbl, and MeCbl (all from Sigma-Aldrich) were separated with HPLC to determine the retention times of the different Cbls. Seven calibrators in the concentration interval of 0–436 pmol/L were prepared by diluting CNCbl with the subtilisin solution. One hundred microliters of calibrator or sample were transferred to light-protected vials (MicroLab), and 10 μL of an aqueous solution of 10-g/L acetaminophen (GlaxoSmithKline) was added as an internal standard before HPLC. For the standard solutions of the different Cbls, postcolumn fractions were collected every 30 s between 10 min and 22 min after sample injection. For the calibrators, only 1 fraction, corresponding to the retention time of CNCbl (15–16 min), was collected. For cohort A, fractions were collected every 30 s between 10 min and 22 min after sample injection. For cohorts B and C, only fractions corresponding to the retention time of CNCbl were collected.
quantification of cbl in postcolumn fractions
Recombinant unsaturated TC (350 pmol/L; kindly donated by Cobento Biotech, Aarhus, Denmark) in 0.1 mol/L phosphate buffer, pH 8, containing 1 g/L BSA (Sigma-Aldrich) was added to the lyophilized column fractions and incubated at room temperature for 1 h. The amount of the resulting holoTC was measured with an ELISA (16). Quantification of Cbl in the postcolumn fractions was based on the ratio of the absorbance signal from the ELISA to the acetaminophen peak areas, as determined with the chromatography software. The 7 calibrators containing CNCbl were used to construct calibration curves.
method validation and statistical analysis
Method imprecision was evaluated with duplicate estimates of a high (180 pmol/L) and a low (15 pmol/L) concentration of TC–CNCbl in 10 analytical runs performed over a period of 2 months. Calculations of intraassay imprecision and total imprecision were based on the recommendations of Krouwer and Rabinowitz (17). Statistical tests were performed with Prism software (version 4.0; GraphPad Software).
Results
The method we have developed combines TC immunoprecipitation, enzymatic release of Cbl, HPLC separation, and measurement of Cbl in the postcolumn fractions. The method had an intraassay (total) imprecision of 7%–9% (11)(12)(13)(14), as judged from 10 analytical runs of duplicate serum samples with high and low TC–CNCbl concentrations (i.e., 180 pmol/L and 15 pmol/L) measured on 10 days over a 2-month period.
We investigated the change in the Cbl pattern on TC in light-protected serum samples obtained from healthy individuals at baseline and 24 h after oral CNCbl administration (cohort A; Fig. 1A ). The mean (SD) increase in OHCbl, CNCbl, AdoCbl, and MeCbl on TC was 4 (4) pmol/L, 32 (6) pmol/L, 2 (4) pmol/L, and 5 (3) pmol/L, respectively (Fig. 1B ). Paired t-tests for each of the Cbl forms at baseline and 24 h after oral CNCbl administration showed a highly significant increase in CNCbl concentration (P = 0.0003) and a marginal, although significant, increase in MeCbl (P = 0.03), whereas no changes were observed for OHCbl (P = 0.25) and AdoCbl (P = 0.37). These results suggest that a major fraction of CNCbl is transferred unmodified over the ileal epithelium and that measurement of TC–CNCbl could be a sensitive marker of Cbl absorption.
Figure 1.
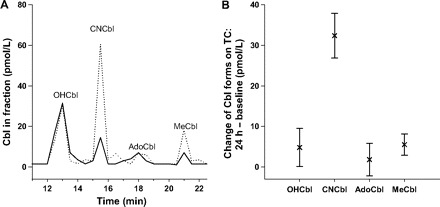
Forms of Cbl on TC before and 24 h after CNCbl intake.
Ten healthy donors were administered 3 oral doses of 9 μg CNCbl on a single day. Light-protected blood samples were withdrawn at baseline and 24 h after the first dose. Cbl on serum TC was isolated and separated by HPLC. (A), Chromatograms from 1 representative donor showing the baseline Cbl forms on TC (solid line) and the forms on TC 24 h after supplementation (dotted line). (B), The changes in OHCbl, CNCbl, AdoCbl, and MeCbl found on TC for all 10 donors at 24 h after supplementation. Data are presented as the mean and SD.
Furthermore, we examined the intraindividual variation of TC–CNCbl by measuring paired samples drawn on 2 separate days from individuals who were not given vitamin B12 supplements (n = 31, cohort B). The mean (SD) TC–CNCbl concentration was 1 (1.6) pmol/L (range, 0–10 pmol/L).
To explore the increase in TC–CNCbl amount with prolonged CNCbl administration, we analyzed samples taken before, during, and after 5 days of CNCbl administration (n = 10, randomly chosen from cohort C). The baseline samples contained 0–4 pmol/L TC–CNCbl (median, 1 pmol/L). After 1 day, the TC–CNCbl concentration had increased to 7–48 pmol/L (median, 26 pmol/L). Only small additional increases were observed on days 2, 3, and 4 (median, 9, 5, and −2.5 pmol/L, respectively). Two days after cessation of oral intake of CNCbl, the TC–CNCbl had returned almost to baseline concentrations (median, 5 pmol/L; range, 0–14 pmol/L) (Fig. 2 ).
Figure 2.
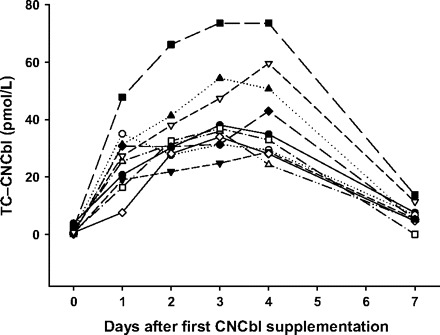
Accumulation of CNCbl on TC after intake of CNCbl.
Healthy donors (n = 10) were supplemented daily with 3 oral doses of 9 μg CNCbl for 5 days. TC–CNCbl was measured in serum from blood samples withdrawn at baseline (day 0) and on days 1, 2, 3, 4, and 7 after the first dose.
Our initial studies suggested that measuring TC–CNCbl before and 24 h after an oral CNCbl dose is more useful for assessing the ability to absorb CNCbl than the previously published method involving measuring holoTC before and after 2 days of CNCbl intake (10). To explore this possibility, we examined a third cohort (cohort C, n = 78). The median TC–CNCbl concentration at baseline and 24 h was 2 pmol/L (range, 0–14 pmol/L) and 24 pmol/L (range, 7–65 pmol/L), respectively. The median absolute increase in TC–CNCbl concentration was 23 pmol/L (range, 6–64 pmol/L), and the fractional increase was >3-fold (the increase is indefinite in samples with baseline CNCbl concentrations of 0 pmol/L) in all but 1 patient. This patient had a high baseline TC–CNCbl concentration (14 pmol/L), whereas all of the others had baseline concentrations between 0 and 7 pmol/L.
Discussion
We report that a major part of a test dose of CNCbl is absorbed from the intestinal cells without modification, and we propose that the capacity to absorb vitamin B12 can be evaluated by measuring TC–CNCbl before and after intake of a test dose of CNCbl. Our proposed test is an extension of the CobaSorb Cbl-absorption test, which is based on measuring holoTC before and after CNCbl intake (10). The sensitivity of the CobaSorb test has been judged excellent (12), a finding that supports the conclusion that no passive absorption occurs after administration of a high physiological dose (three 9-μg doses) of vitamin B12. In contrast, the specificity of the CobaSorb test depends on the baseline concentration of holoTC, and use of this test is not recommended if the baseline holoTC concentration is >75 pmol/L (10). Our new test, which we have named C-CobaSorb, has a markedly improved specificity, a conclusion reached by comparing the results obtained for 78 healthy individuals with the C-CobaSorb test (present study) and the CobaSorb test (10).
With the C-CobaSorb test, all but 1 of the studied individuals (baseline CNCbl-TC, 14 pmol/L) were classified as capable of absorbing vitamin B12 according to the following criteria: (a) an absolute TC–CNCbl increase of >6 pmol/L (>3 times the intraindividual variation of 1.6 pmol/L) and (b) a relative TC-CNCbl increase of >3-fold from the baseline sample to the 24-h sample. Until further experience has been accumulated, we suggest the test be used only if the baseline TC–CNCbl concentration is <10 pmol/L. With the CobaSorb test, only 69 of the individuals were classified as capable of absorbing vitamin B12, as judged by an absolute holoTC increase of >10 pmol/L and a relative increase of >22% (11). Of the 9 individuals that the CobaSorb test either could not classify as absorbing vitamin B12 (baseline holoTC >75 pmol/L; n = 8) or had classified as unable to absorb vitamin B12 (baseline holoTC, 68 pmol/L; n = 1), the C-CobaSorb assay classified all of these individuals as absorbers of vitamin B12 (Fig. 3 ). The results suggest that C-CobaSorb may prove a valuable complement to the previously introduced CobaSorb test.
Figure 3.
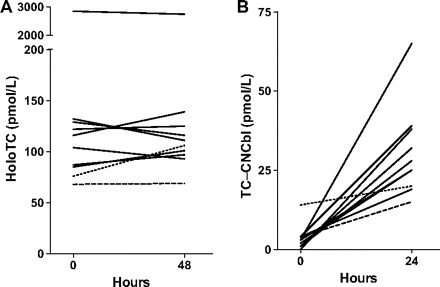
Absorption of vitamin B12(CNCbl), as judged by holoTC measurement (CobaSorb) or TC–CNCbl measurement (C-CobaSorb).
Donors (n = 78) were administered 3 oral doses of 9 μg CNCbl per day for 5 days. Blood samples were taken at baseline and at timed intervals after the start of supplementation and analyzed. Results for individuals judged able to absorb vitamin B12 (n = 68) with both methods are not indicated. Results for individuals that could not be classified with CobaSorb (n = 8) are indicated with a solid line. A dashed line indicates a donor judged by the CobaSorb assay to be unable to absorb vitamin B12; the dotted line indicates a donor that could not be classified with C-CobaSorb. (A), The CobaSorb test. Measurement of holoTC at baseline and 48 h after first test dose of vitamin B12. Data on the measurement of holoTC is obtained from Hvas et al. (10). (B), The C-CobaSorb test. Measurement of TC–CNCbl at baseline and 24 h after first test dose of vitamin B12.
Previous studies suggested that TC carries primarily OHCbl and to some degree AdoCbl (18). The results with our improved methods for extracting and quantifying circulating TC–Cbl support these findings and show that OHCbl accounts for a mean of 50% of the Cbl on TC at baseline. CNCbl intake produced a substantial increase in TC–CNCbl, and the concentration of TC–MeCbl showed a small but significant increase. This finding suggests that a minor fraction of the test dose of CNCbl is converted into the coenzyme MeCbl form after decyanation in the cytosol (19), whereas the major fraction remains unchanged on release from the intestinal cell and into the circulation.
In conclusion, we suggest that most of the CNCbl from oral supplements remains unchanged during intestinal absorption and that the resulting increase in circulating TC–CNCbl can be used as a sensitive marker for the absorption of vitamin B12.
Acknowledgments
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: The Lundbeck Foundation, the Novo-Nordisk Foundation, and The Danish Medical Research Council. Research with cohort A was conducted at the University of Florida, with blood collection and processing handled by Kristina von Castel-Roberts and grant support provided by the NIH (no. M01RR00082) and Shands General Clinical Research Center (no. 7591).
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Acknowledgments: We thank Jette Fisker Petersen at the Department of Clinical Biochemistry, Aarhus University Hospital, Denmark, for excellent technical assistance.
Footnotes
Nonstandard abbreviations: Cbl, cobalamin; TC, transcobalamin; CNCbl, cyanocobalamin; OHCbl, hydroxocobalamin; AdoCbl, 5′-deoxyadenosylcobalamin; MeCbl, methylcobalamin; TC–CNCbl, TC-bound CNCbl.
References
- 1.Nexo E. Cobalamin binding proteins. Kräutler B Arigoni D Golding BT eds. Vitamin B12 and B12-binding proteins 1998:p 461-475 Wiley-VCH Weinheim (Germany). . [Google Scholar]
- 2.Birn H, Verroust PJ, Nexo E, Hager H, Jacobsen C, Christensen EI, Moestrup SK. Characterization of an epithelial ∼460-kDa protein that facilitates endocytosis of intrinsic factor-vitamin B12 and binds receptor-associated protein. J Biol Chem 1997;272:26497-26504PubMed uses this format: “approximately 460-kDa.”. [DOI] [PubMed] [Google Scholar]
- 3.Herzlich B, Herbert V. Depletion of serum holotranscobalamin II. An early sign of negative vitamin B12 balance. Lab Invest 1988;58:332-337. [PubMed] [Google Scholar]
- 4.England JM, Down MC, Wise IJ, Linnell JC. The transport of endogenous vitamin B12 in normal human serum. Clin Sci Mol Med 1976;51:47-52. [DOI] [PubMed] [Google Scholar]
- 5.Hall CA. The carriers of native vitamin B12 in normal human serum. Clin Sci Mol Med 1977;53:453-457. [DOI] [PubMed] [Google Scholar]
- 6.Nexo E, Andersen J. Unsaturated and cobalamin saturated transcobalamin I and II in normal human plasma. Scand J Clin Lab Invest 1977;37:723-728. [DOI] [PubMed] [Google Scholar]
- 7.Chanarin I. The megaloblastic anemias 2nd ed. 1979:p 93-123 Blackwell Scientific Publications Oxford. . [Google Scholar]
- 8.Ward PC. Modern approaches to the investigation of vitamin B12 deficiency. Clin Lab Med 2002;22:435-445. [DOI] [PubMed] [Google Scholar]
- 9.von Castel-Roberts KM, Mørkbak AL, Nexo E, Edgemon CA, Maneval DR, Shuster JJ, et al. Holo-transcobalamin is an indicator of vitamin B-12 absorption in healthy adults with adequate vitamin B-12 status. Am J Clin Nutr 2007;85:1057-1061. [DOI] [PubMed] [Google Scholar]
- 10.Hvas AM, Mørkbak AL, Nexo E. Plasma holotranscobalamin compared with plasma cobalamins for assessment of vitamin B12 absorption; optimisation of a non-radioactive vitamin B12 absorption test (CobaSorb). Clin Chim Acta 2007;376:150-154. [DOI] [PubMed] [Google Scholar]
- 11.Bor MV, Nexo E, Hvas AM. Holo-transcobalamin concentration and transcobalamin saturation reflect recent vitamin B12 absorption better than does serum vitamin B12. Clin Chem 2004;50:1043-1049. [DOI] [PubMed] [Google Scholar]
- 12.Bor MV, Cetin M, Aytac S, Altay C, Nexo E. Nonradioactive vitamin B12 absorption test evaluated in controls and in patients with inherited malabsorption of vitamin B12. Clin Chem 2005;51:2151-2155. [DOI] [PubMed] [Google Scholar]
- 13.Gimsing P, Nexo E, Hippe E. Determination of cobalamins in biological material. II. The cobalamins in human plasma and erythrocytes after desalting on nonpolar adsorbent material, and separation by one-dimensional thin-layer chromatography. Anal Biochem 1983;129:296-304. [DOI] [PubMed] [Google Scholar]
- 14.Hardlei TF, Mørkbak AL, Nexo E. Enzymatic extraction of cobalamin from monoclonal antibody captured haptocorrin and transcobalamin. Clin Biochem 2007;40:1392-1397. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen DW, Green R, Quadros EV, Montejano YD. Rapid analysis of cobalamin coenzymes and related corrinoid analogs by high-performance liquid chromatography. Anal Biochem 1982;120:394-403. [DOI] [PubMed] [Google Scholar]
- 16.Hardlei TF, Nexo E. A new principle for measurement of cobalamin and corrinoids, used for studies of cobalamin analogs on serum haptocorrin. Clin Chem 2009;55:1002-1010. [DOI] [PubMed] [Google Scholar]
- 17.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem 1984;30:290-292. [PubMed] [Google Scholar]
- 18.Nexo E. Characterization of the cobalamins attached to transcobalamin I and transcobalamin II in human plasma. Scand J Haematol 1977;18:358-360. [PubMed] [Google Scholar]
- 19.Kim J, Gherasim C, Banerjee R. Decyanation of vitamin B12 by a trafficking chaperone. Proc Natl Acad Sci U S A 2008;105:14551-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]


