Abstract
Background
Despite advances in our understanding of the critical role of the microbiota in stroke patients, the oral microbiome has rarely been reported to be associated with stroke-associated pneumonia (SAP). We sought to profile the oral microbial composition of SAP patients and to determine whether microbiome temporal instability and special taxa are associated with pneumonia progression and functional outcomes.
Methods
This is a prospective, observational, single-center cohort study that examined patients with acute ischemic stroke (AIS) who were admitted within 24 h of experiencing a stroke event. The patients were divided into three groups based on the occurrence of pneumonia and the use of mechanical ventilation: nonpneumonia group, SAP group, and ventilator-associated pneumonia (VAP) group. We collected oral swabs at different time points post-admission and analyzed the microbiota using 16 S rRNA high-throughput sequencing. The microbiota was then compared among the three groups.
Results
In total, 104 nonpneumonia, 50 SAP and 10 VAP patients were included in the analysis. We found that SAP and VAP patients exhibited significant dynamic differences in the diversity and composition of the oral microbiota and that the magnitude of this dysbiosis and instability increased during hospitalization. Then, by controlling the potential effect of all latent confounding variables, we assessed the changes associated with pneumonia after stroke and explored patients with a lower abundance of Streptococcus were more likely to suffer from SAP. The logistic regression analysis revealed that an increase in specific taxa in the phylum Actinobacteriota was linked to a higher risk of poor outcomes. A model for SAP patients based on oral microbiota could accurately predict 30-day clinical outcomes after stroke onset.
Conclusions
We concluded that specific oral microbiota signatures could be used to predict illness development and clinical outcomes in SAP patients. We proposed the potential of the oral microbiota as a non-invasive diagnostic biomarker in the clinical management of SAP patients.
Clinical Trial registration
NCT04688138. Registered 29/12/2020, https://clinicaltrials.gov/ct2/show/NCT04688138.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-023-03057-8.
Keywords: Oral microbiota, Dysbiosis, Stroke-associated Pneumonia, Prognosis, Biomarker
Introduction
Stroke-associated pneumonia (SAP) is one of the most frequent complications of stroke [1–3]. Additionally, ventilator-associated pneumonia (VAP) is a common and serious clinical issue among critically ill stroke patients. Notably, AIS patients who have SAP or VAP tend to have poorer outcomes, including longer hospital stays, higher costs, increased discharge dependency, and a greater risk of death [2, 4, 5]. Despite this, there has been limited progress in the prevention of SAP. Therefore, we must urgently enhance our understanding of the unidentified factors that are associated with or even cause SAP.
Substantial growth in the body of research on the human gut microbiota and its potential role in promoting stroke has occurred during the past decade [6–8]. Gut microbiota dysbiosis has been reported to be associated with SAP [9, 10]. However, in recent years, there has been an increasing report on the disruption of the oral microbiome specifically, which has been found to promote stroke and complications following stroke [11–13]. The oral hygiene and cleanliness of stroke patients’ mouth and teeth may contribute to the development of SAP as highly diverse microbial communities [14], including anaerobes and indigenous oral bacteria, can result in pneumonia. In previous studies, SAP patients were found to exhibit oral microbiota dysbiosis [15–17], and the domination of certain oral pathogens has been identified as a risk factor for adverse outcomes in VAP [18–20]. However, there is limited information available regarding the changes that occur in the oral microbiota during stroke and whether the characteristics of the oral microbiome are linked to poor clinical outcomes among patients in stroke units.
Notably, some clinical trials have explored the impacts of heightened oral cleanliness care on decreasing SAP and VAP rate [14, 21–24]. However, there are still challenges and uncertainties surrounding the assessment and care of oral hygiene in stroke units. Researchers and clinicians do not agree on the best instrument or optimal timing for conducting oral assessments [21].
Therefore, oral microbial studies concerning SAP should aim to investigate potential oral microbial biomarkers and establish larger observational patient cohorts. In this prospective observational study, we investigated alterations in the composition of oral microbiota at various time points after admission and their association with SAP. The objective was to assess the progression and recovery of the microbial community shift after a stroke. Additionally, we aimed to determine the impact of oral microbial dysbiosis on patient outcomes and explore predictive variables using various features of the oral microbiota assessment.
Methods
Ethics statement and participants
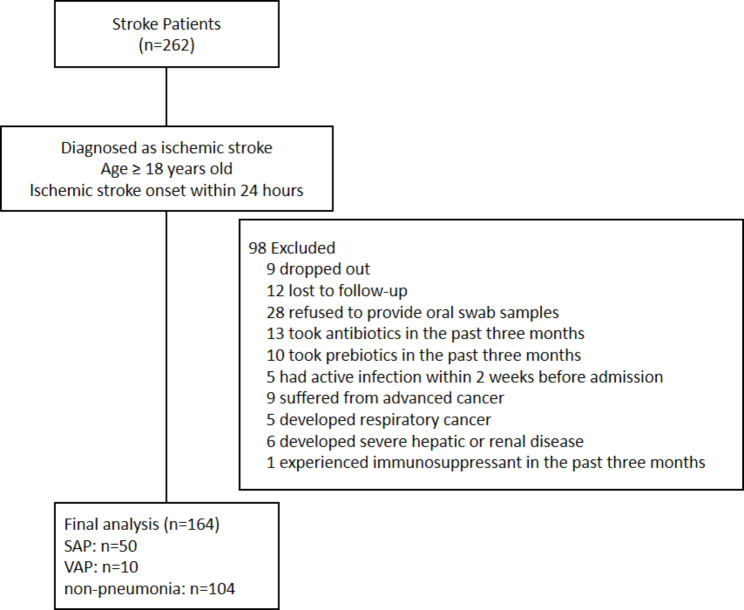
This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2020-169) and registered at http://clinicaltrials.gov (NCT04688138). Informed consent was obtained from all patients or their legal representatives. This research was conducted in accordance with the Declaration of Helsinki. Acute stroke patients were recruited from the Department of Neurology of Nanfang Hospital of Southern Medical University (Guangzhou, China) from December 2020 to September 2021. The inclusion criteria were as follows: (1) age > 18 years and (2) admission within 24 h of ischemic stroke onset. Exclusion criteria were as follows: (1) antibiotic, prebiotic, or probiotic use within three months before admission; (2) admission after 24 h of stroke onset; (3) active infection within the 2 weeks before admission; (4) history of oral and respiratory cancer or advanced cancer; (5) failure to offer oral swab samples during hospitalization; and (6) the use of an immunosuppressant or a history of systemic diseases like cirrhosis, renal failure, and hematologic conditions.
Clinical data and sample collection
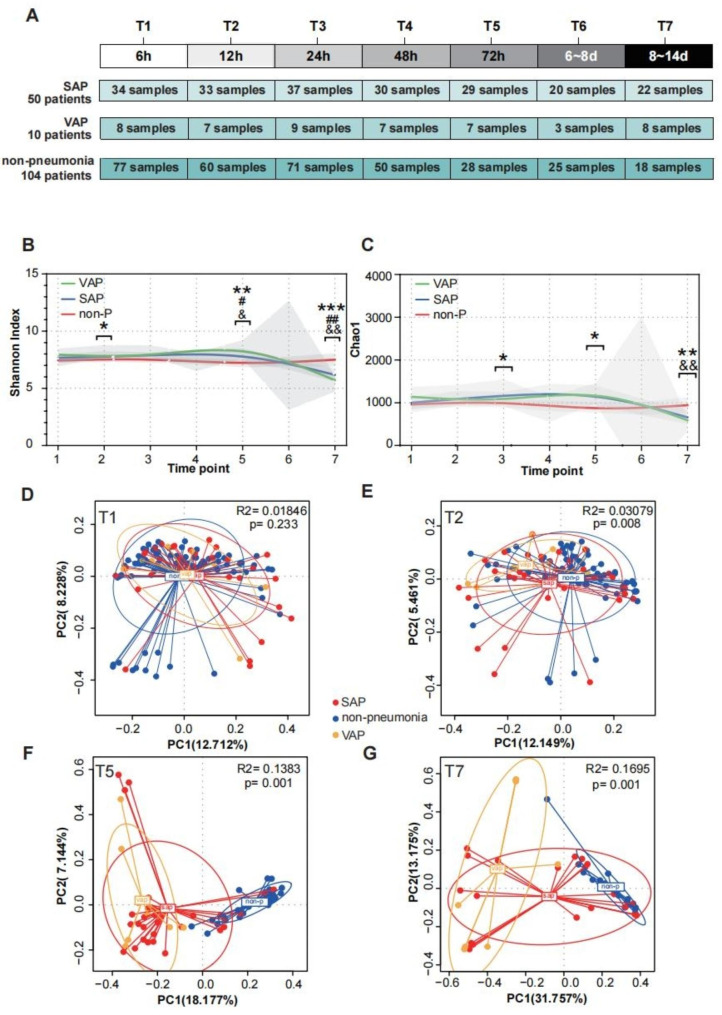
Clinical data and treatment records were collected by professional trained neurologists. As previously reported, the stroke risk factor assessment was carried out [10]. The Glasgow Coma Scale (GCS), Acute Physiology and Chronic Health Evaluation (APACHE-II), Sequential Organ Failure Assessment (SOFA) scores, modified Rankin Scale (mRS) and National Institutes of Health Stroke Scale (NIHSS) were recorded by specialized neurocritical care physicians at admission. Fasting blood samples were analyzed in the Clinical Laboratory of Nanfang Hospital, and routine blood parameters were estimated. Upon enrollment, we noninvasively collected oral biospecimens from the the buccal mucosa and gingiva using a sterile cotton swab, placed them in an Eppendorf tube with 1 mL phosphate-buffered saline and rapidly transported and stored them at -80 ℃ [25]. Specimen collection was performed at different time points after enrollment. However, the number of specimens at each time point has varied due to premature discharge, early transfer of patients, or early death. The quantity of specimens at each time point has been depicted in Fig. 2A.
Fig. 2.
Compositional differences in the oral microbiota of individuals among the three groups at different time points after stroke. (A) Trial profile of cohort. The α diversity (Shannon index) (B) and richness (Chao1 index) (C) at different time points after stroke. D, E, F, G PCoA plots based on Bray‒Curtis distances are drawn to display the dynamic differences among the three groups at T1, T2, T5 and T7. Each point represents the composition of the oral microbiota of one participant. P values comparing the different groups were calculated using Wilcoxon tests, *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001;*among three group,# SAP compared with non,& VAP compared with non,%SAP compared with VAP. SAP stroke-associated pneumonia; VAP ventilator-associated pneumonia; PCoA principal coordinate analysis
Definitions of SAP and VAP and functional outcomes
SAP was recorded as the improvement of lower respiratory infections during the initial 7 days after stroke onset [1, 2]. Furthermore, existing diagnostic criteria for VAP include that patients receive mechanical ventilation in the first 7 days after stroke [4, 26–30]. Additional diagnosis criteria in the Supplemental Material 1.
The clinical cohort’s stroke outcomes, including hospitalization duration, discharge NIHSS score, 30-day mortality, and mRS scores at 30 and 90 days, were examined through phone follow-up with patients or their caregivers or through medical records obtained during hospitalization or from outpatient clinics.
16 S rRNA sequencing and analysis
DNA was extracted using the Mabio® Bacterial DNA Extraction Mini Kit for oral swab samples. The concentration and purity were measured using a NanoDrop One (Thermo Fisher Scientific, MA, USA). Amplify different regions of 16 S rRNA (V3-V4) using specific primers (338 F and 806R) with a 12 bp barcode. Using the NEBNext® Ultra™ II DNA Library Prep Kit for Illumina® (New England Biolabs, MA, USA) to generate the sequencing libraries, and added index codes. The Qubit@ 2.0 Fluorometer (Thermo Fisher Scientific, MA, USA) was used to assess the quality of the library. Finally, the library was sequenced on the Illumina Nova6000 platform and a paired end reading of 250 bp was generated (Guangdong Magigene Biotechnology Co., Ltd. Guangzhou, China). Chimeric sequences were removed and sequencing noise was detected using the DADA2 method. With 99% similarity, the sequences were grouped into amplicon sequence variants (ASVs). Using the vsearch alignment method and the SILVA v132 database, the q2-feature-classifier plugin was used to classify the sequences according to their taxonomic information. Please refer to the supplementary material 1 for more information on the content of the 16 S rRNA sequencing analysis.
Statistical analyses
Data were analyzed using IBM’s SPSS 25.0 statistical software package. The study population was described by demographic and clinical variables. We used the mean (standard deviation) to express measurement data that conformed to a normal distribution, the median (interquartile range) to express measurement data that had a skewed distribution, and a percentage to express numerical data. The Kruskal–Wallis test and Mann–Whitney U test were also performed to determine statistical significance. Spearman correlation analysis was used for correlation tests. Univariate and multivariate logistic regression analyses were performed, and odds ratios (ORs) and 95% CIs were calculated to identify the risk factors for clinical outcomes. Furthermore, univariate and multivariate Cox proportional hazards models were used to evaluate the association between the abundance of specific taxa and SAP. Predictive performance for clinical outcomes was assessed by comparing receiver operator characteristic (ROC) curves.
Results
Demographic and clinical data
Between December 30, 2020, and September 15, 2021, we recruited 164 AIS patients admitted within 24 h after stroke events and divided them into three groups according to the presence of pneumonia and the use of ventilators. Figure 1 depicts the patient selection process’s flow diagram. Demographic and clinical data for the participants are introduced in Table 1. We found no significant difference among the three groups in terms of sex, history of stroke, or smoking or drinking habits. Patients in the SAP group were older than patients without pneumonia. Stroke severity was significantly higher in the SAP and VAP groups. Furthermore, the SAP and VAP groups suffered significantly more often from dysphagia and enteral nutrition than the nonpneumonia group. Regarding revascularization treatments for AIS and oral hygiene care, there was no significant difference among the three groups. The SAP and VAP groups showed a longer duration of ICU hospitalization, higher rates of 30-day mortality and more severe 90-day mRS score, indicating that these AIS patients who developed pneumonia during the hospital stay suffered from a poor stroke prognosis. For dental date, there were no significant differences in the percentage of caries, missing teeth, residual root, periodontitis or calculus between the three groups (Table 2).
Fig. 1.
Flow diagram of the patient selection process
Table 1.
Baseline characteristics of patients among the three groups
| nonpneumonia(n = 104) | SAP (n = 50) | VAP (n = 10) | P value | |
|---|---|---|---|---|
| Age (years) ± mean (SD) | 59.08 ± 10.65* | 68.46 ± 12.32 | 64.30 ± 15.64 | <0.001 |
| Sex (male), n (%) | 83 (79.8%) | 33 (66.0%) | 8 (80.0%) | 0.165 |
| Hypertension | 66 (63.5%) | 39 (78.0%) | 7 (70.0%) | 0.191 |
| Diabetes | 28 (26.9%) | 20 (40.0%) | 3 (30.0%) | 0.259 |
| Coronary Heart Disease | 10 (9.6%) | 6 (12.0%) | 2 (20.0%) | 0.582 |
| Atrial Fibrillation | 16 (15.4%)* | 16 (32.0%) | 1 (10.0%) | 0.039 |
| Hyperlipidemia | 35 (33.7%) | 8 (16.0%) | 2 (20.0%) | 0.061 |
| Previous Stroke, n (%) | 23 (22.1%) | 15 (30.0%) | 5 (50.0%) | 0.123 |
| Smoking status, n (%) | 64 (61.5%) | 26 (52.0%) | 6 (60.0%) | 0.529 |
| Drinking status, n (%) | 50 (44.2%) | 15 (30.0%) | 4 (40.0%) | 0.266 |
| NIHSS on Admission, median (IQR) | 6 (3,10)*# | 16 (8,23) | 21.5 (14.75-26) | <0.001 |
| mRS on Admission, median (IQR) | 4 (3,4)*# | 5 (4,5) | 5 (4.75,5) | <0.001 |
| GCS on Admission, median (IQR) | 15 (13,15)*# | 11.5 (7,14) | 8 (5.5,10.75) | <0.001 |
| Dysphagia, n (%) | 22 (21.2%)*# | 32 (64.0%)& | 10 (100%) | <0.001 |
| Enteral Nutrition, n (%) | 29 (27.9%)*# | 47 (94.0%) | 10 (100%) | <0.001 |
| MV before pneumonia, n (%) | 0 (0.0%) | 10 (100%) | ||
| MV after pneumonia, n (%) | 42 (84.0%) | 10 (100%) | ||
| Oral hygiene care, n (%) | 104 (100%) | 50 (100%) | 10 (100%) | 1 |
| Revascularization therapies, n (%) | 0.106 | |||
| Intravenous Thrombolysis | 16 (15.4%) | 1 (2.0%) | 0 (0.0%) | |
| Endovascular Thrombectom | 53 (51.0%) | 33 (66.0%) | 7 (70.0%) | |
| Bridging Therapy | 11 (10.6%) | 7 (14.0%) | 2 (20.0%) | |
| Drug Treatment | 24 (23.1%) | 9 (18.0%) | 1 (10.0%) | |
| Stroke subtype, n (%) | 0.569 | |||
| Large artery disease | 65 (62.5%) | 34 (68.0%) | 8 (80.0%) | |
| Cardioembolism | 21 (20.2%) | 11 (22.0%) | 1 (10.0%) | |
| Small vessel occlusion | 10 (9.6%) | 1 (2.0%) | 0 (0.0%) | |
| Others | 8 (7.7%) | 4 (8.0%) | 1 (10.0%) | |
| ICU length of stay, median (IQR) | 2 (1,3)*# | 7.5 (3,15) | 11 (5.75,17.5) | <0.001 |
| 30-day mRS, median (IQR) | 2 (1,3)*# | 5 (4,5) | 5 (4.75,6) | <0.001 |
| 90-day mRS, median (IQR) | 1 (0,2)*# | 5 (3,6) | 5 (4.75,6) | <0.001 |
| 30-day motality, n (%) | 4 (3.8%)*# | 7 (14.0%) | 3 (30.0%) | 0.005 |
SD standard deviation; IQR interquartile range; SAP stroke-associated pneumonia; VAP ventilator-associated pneumonia; GCS Glasgow Coma Scale; NIHSS National Institutes of Health Stroke Scale; mRS modified Rankin Scale; MV Mechanical ventilation. * SAP compared with nonpneumonia, # VAP compared with nonpneumonia, & SAP compared with VAP
Table 2.
Dental and oral health of patients among the three groups
| nonpneumonia(n = 104) | SAP (n = 50) | VAP (n = 10) | P value | |
|---|---|---|---|---|
| Serious caries, n (%) | 63(60.6%) | 26(52.0%) | 7(70.0%) | 0.449 |
| Serious periodontitis, n (%) | 53(51.0%) | 25(50.0%) | 6(60.0%) | 0.843 |
| Missing teeth, n (%) | 17(16.3%) | 7(14.0%) | 1(10.0%) | 0.831 |
| Residual root, n (%) | 27(26.0%) | 9(18.0%) | 1(10.0%) | 0.335 |
| Serious calculus, n (%) | 75(72.1%) | 32(66.0%) | 8(80.0%) | 0.591 |
Differences in the oral microbiota profile differentiate the SAP, VAP and nonpneumonia groups
To determine whether the oral microbiome structures differed significantly among the three groups of AIS patients (164 in total), we collected oral samples at various time intervals following admission. These intervals included 6 h (T1), 12 h (T2), 24 h (T3), 48 h (T4), 72 h (T5), 6 to 8 days (T6), and 8 to 14 days (T7) (Fig. 2A). The α-diversity metrics of the oral microbiota differed significantly over time after stroke (Fig. 2B, C). The microbial diversity and species richness in the SAP and VAP groups were significantly higher from T2 to T5 and then lower at T7 than those in the nonpneumonia group. The PCoA plots based on Bray-Curtis distances showed a temporal shift: there were significant differences in microbiome composition among the three groups at T2 that continued to T7 (Fig. 2D-G, Figure S1). The composition in the SAP and VAP groups showed considerable longitudinal dynamic changes. However, the nonpneumonia patients did not exhibit this characteristic change (Figure S2 A-C). Then, we analyzed the coefficient of variation (CV) of a longitudinal collection of α and β diversity values. We found significant differences in the stability values of the oral microbiota over time between the nonpneumonia and SAP groups. The SAP patients had a high CV, reflecting more variation. Nonpneumonia patients had relatively stable species diversity and community structure after stroke over time (Figure S2 D, E).
Dynamic characteristics of and trends in the oral microbiota after stroke among the three groups
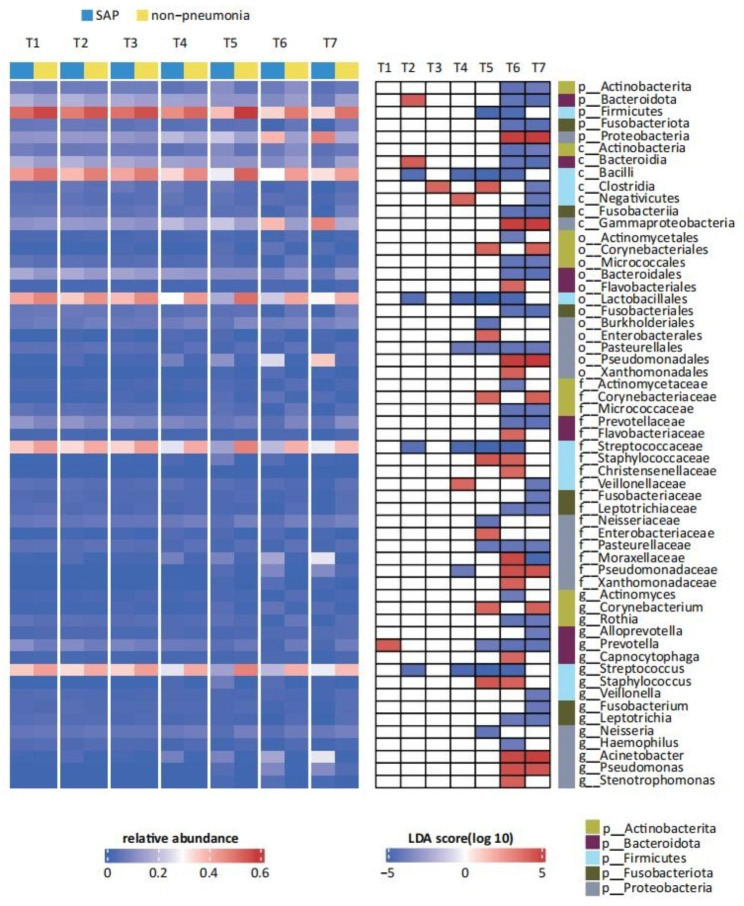
To further observe the dynamic changes of oral microbiota after stroke at the taxonomic level, we observed dysbiosis in the oral microbiota profile at the phylum and genus levels at different time points (Figure S3 A-C). We compared the oral microbiota composition at each time point in SAP patients with that at the same time points in the nonpneumonia group through LEfSe. We discovered that 16 genera were significantly altered in SAP participants. The relative abundance of taxa that differed between different time points revealed that the core oral microbes [31–33] in healthy individuals, including the genus Streptococcus, was significantly richer in the nonpneumonia group than in the SAP and Pre-SAP groups (Fig. 3, S6). The SAP group was characterized by the enrichment of several pathogens and opportunistic pathogens from T5 to T7, while some bacteria had different change trends over time. The relative abundance of the genera Acinetobacter and Corynebacterium increased at T5; the abundance of the genera Staphylococcus and Pseudomonas increased at T6 and T7, respectively (Fig. 3). Moreover, the VAP group showed higher levels of the phylum Proteobacteria, the class Gammaproteobacteria, the family Enterobacteriaceae and the genus Klebsiella than the nonpneumonia or SAP group from T4 to T7 (Figure S4, S5).
Fig. 3.
The LEfSe analysis shows that the representation of the various bacterial taxa changed over the time points between SAP and nonpneumonia. Only taxa with a statistically significant LDA score (log10) > 4 are shown. The heatmap on the left shows the relative abundance of the taxa, and the heatmap on the right shows the LDA scores. LEfSe Linear discriminant analysis effect size. p phylum, c class, o order, f family, g genus
Dynamic alterations in microbiome phenotype prediction for oral microbiota in SAP patients
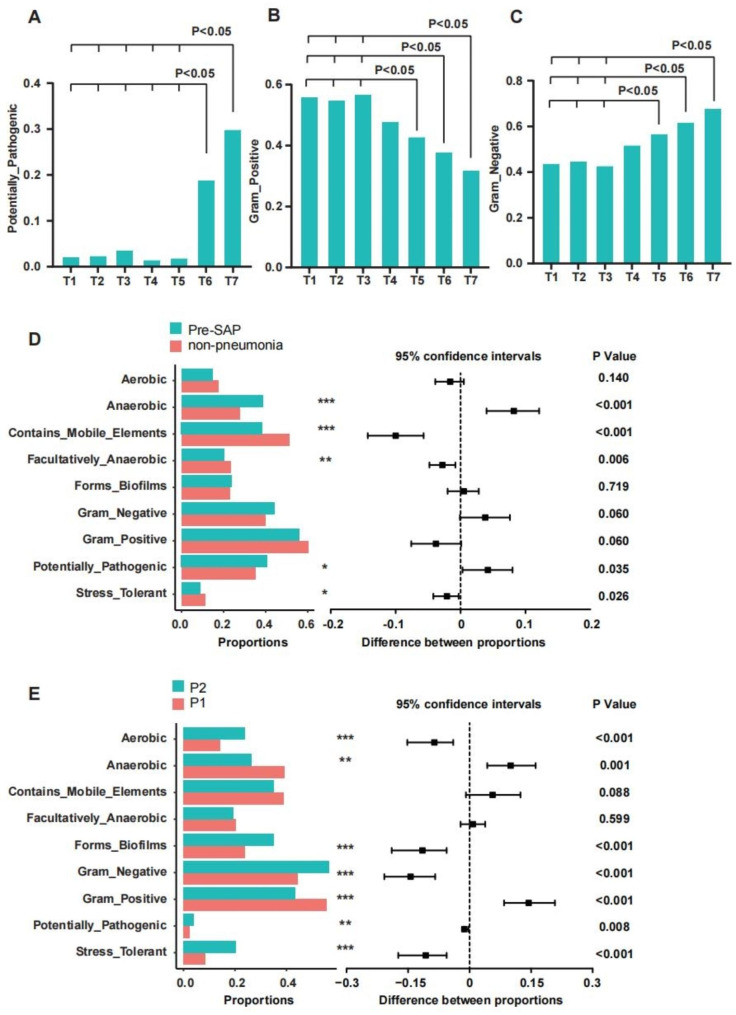
To predict microbiome phenotypes within complex microbiomes, we conducted the BugBase. First, when comparing patients with SAP and nonpneumonia, we observed a significant increase in potentially pathogenic bacteria and bacteria associated with biofilm formation in SAP patients (Figure S7). Then, in patients with SAP, we found a significantly higher presence of potentially pathogenic bacteria and bacteria tolerant to oxidative stress, especially between T6 and T7, highlighting the dynamic feature of the oral microbiota in SAP. Additionally, during the T5-T7 period, the representation of Gram-positive and Gram-negative bacteria exhibited contrasting trends (Fig. 4A-C,S8). We were concerned about the functional changes of oral microbiota before the onset of pneumonia, and we have found that potentially pathogenic bacteria and oxidative stress-tolerant bacteria have significantly enriched in SAP before pneumonia compared to nonpneumonia patients (Fig. 4D). Moreover, when comparing the oral microbiota before and after the occurrence of pneumonia, the results indicated an increase in aerobic bacteria, Gram-negative bacteria, potentially pathogenic bacteria, bacteria associated with biofilm formation, and oxidative stress-tolerant bacteria after the diagnosis of SAP (Fig. 4E).
Fig. 4.
Microbial phenotype prediction by BugBase. (A,B,C) Dynamic characteristics of oral microbiota in SAP. The connection of fold lines represents a significant difference between different time points. (D) The different microbial compositions between pre-SAP and nonpneumonia. Pre-SAP before diagnosis of SAP samples. (E) The different microbial compositions between P1 and P2. P1 before diagnosis of SAP samples; P2 after diagnosis of SAP samples
Microbial dynamic variation may be linked to pneumonia and different invasive and pharmacological medical treatments in SAP
The oral microbiota exhibited profound alterations in diversity and composition over time after stroke in the SAP groups, suggesting that the dynamics of the features of the oral microbiota can potentially respond to host development of pneumonia. Moreover, whether the composition of the oral microbiota is perturbed by invasive and pharmacological medical treatments was unknown.
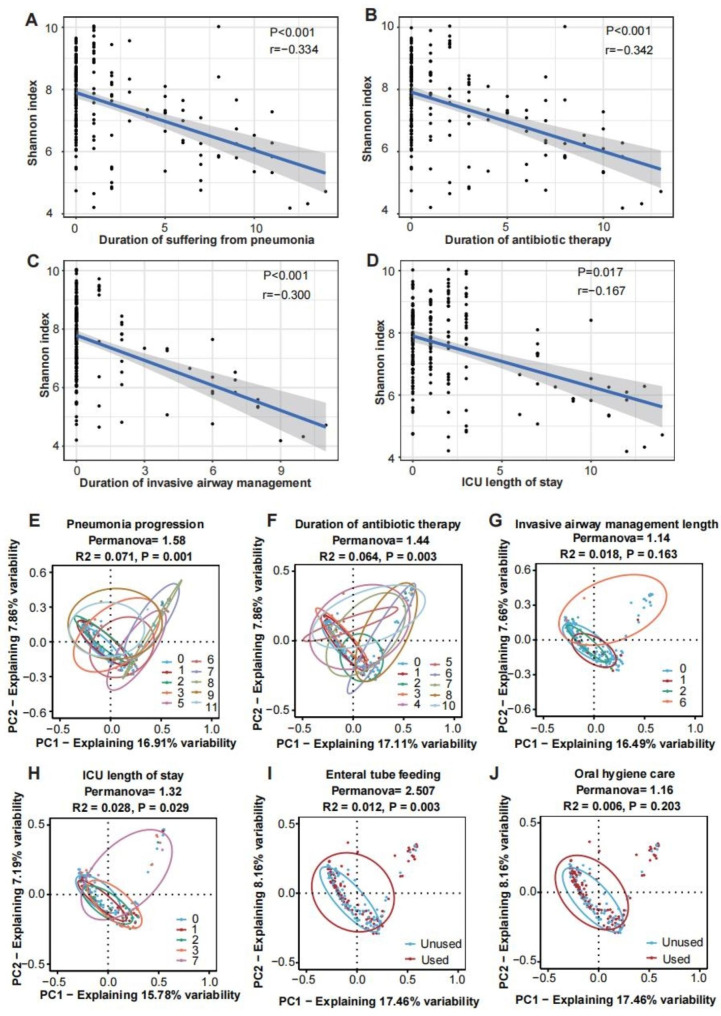
First, we found that the α-diversities of the samples from SAP groups, which included only samples collected more than once from a single individual, were contrarily related with the number of days patients suffered from pneumonia (r=-0.334, P < 0.001), antibiotic use (r=-0.342, P < 0.001), the use of invasive airway management (r=-0.300, P < 0.001) and ICU stay duration (r=-0.167, P = 0.017) (Fig. 5A-D, Figure S9).
Fig. 5.
Invasive and pharmacological medical treatments influence the oral microbiota after stroke. The duration of pneumonia (A), duration of antibiotic therapy (B), duration of invasive airway management (C) and ICU length of stay (D) were negatively associated with the Shannon index. PCoA results of multidimensional data are shown to display changes in microbial communities according to major variables in SAP: pneumonia progression (E), duration of antibiotic therapy (F), invasive airway management length of use (G), ICU length of stay (H), enteral tube feeding (I) and oral hygiene care (J). The x- and y-axes represent the two most informative PCs of the PCoA, and marginal boxplots describe the distribution of those values for the different groups. Color legends represent the respective variables under analysis. The number refers to the number of days in E, F, G, and H. The results of the PERMANOVA to compare dissimilarity indexes among samples are shown on top of plots accordingly. PCs principal coordinates; PERMANOVA permutation-based test
Permutational multivariate analysis of variance (PERMANOVA) indicated that pneumonia progression could drive significant changes in the microbial community structure (PERMANOVA = 1.58, p = 0.001) in the SAP group (Fig. 5E). However, we discovered that variables of medical treatments could modulate the microbiota as well. As a result, the duration of antibiotic therapy appeared to have a greater impact on the microbiota profiles than other parameters (PERMANOVA = 2.51, p = 0.003 and PERMANOVA = 1.32, p = 0.018 for enteral tube feeding and ICU length of stay, respectively), which was responsible for 6% of the variation in oral microbiota (PERMANOVA = 1.44, p = 0.003 for antibiotic therapy) (Fig. 5F, H, I). However, the invasive airway management duration (PERMANOVA = 1.14, p = 0.163) and oral hygiene care (PERMANOVA = 1.16, p = 0.203) may play a minor role in driving changes in composition in SAP patients (Fig. 5G, J).
Furthermore, through the mixed linear model (LMM), some specific features were identified, indicating that ASVs were strictly associated with each variable. After adjusting the model, the relative abundance of Fusobacteriota, Lactobacillales, Rothia, Prevotella and Streptococcus were negatively correlated with antibiotic use, and the relative abundance of Prevotella, Bacteroidota, Rothia, Veillonellaceae and Pseudomonas were negatively correlated with invasive airway management (Table S1).
Identification of the relative abundance of specific taxa associated with SAP
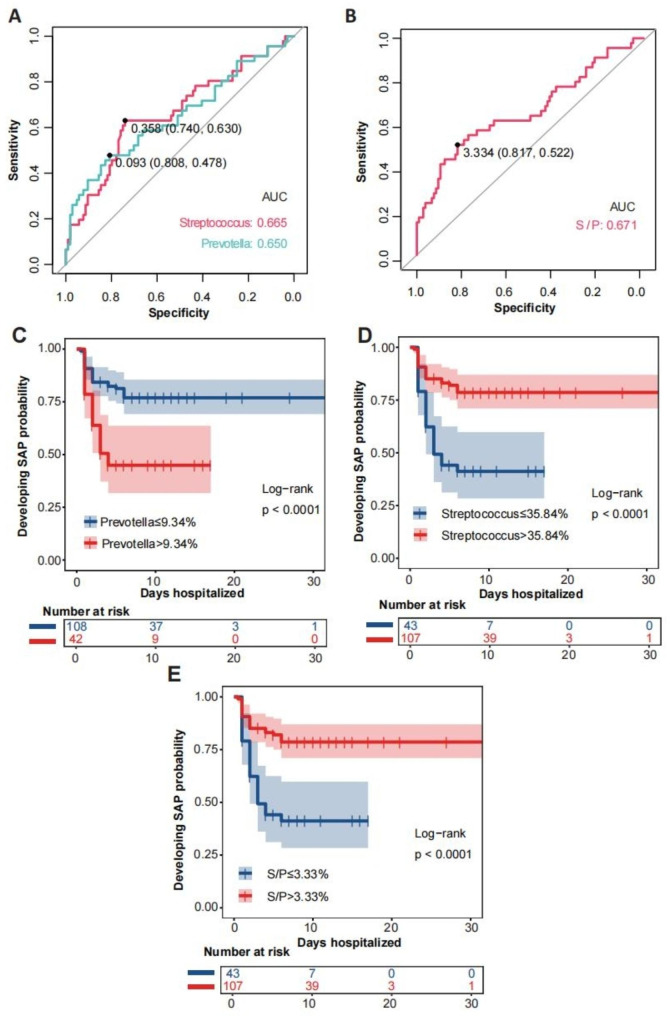
We evaluated 46 SAP with 114 longitudinal samples before diagnosis of SAP, as well as 104 nonpneumonia individuals, to determine whether any specific taxa were linked to the risk of developing pneumonia. By utilizing LMM analysis, we identified 11 ASVs significantly associated with SAP. After nonparametric testing, we finally determined that 5 ASVs were inversely correlated with SAP and were considered protective taxa, while there were 5 hazardous ASVs that were positively associated with SAP (Table S3). We sought to develop a simple predictive or hazardous index from these features. Using ROC curves to distinguish the SAP patients, we identified optimal thresholds of protective bacterial relative abundance (35.84% for Streptococcus) and hazardous bacterial relative abundance (9.34% for Prevotella) (Fig. 6A). Moreover, we determined thresholds of a Streptococcus/Prevotella (S/P) ratio < 3.33 that distinguished SAP patients from the rest of the cohort (Fig. 6B). Then, to further investigate the impact of the microbial index on SAP, proportions and time-to-event analyses were performed, we found that patients with lower protective bacteria relative abundance (Streptococcus < 35.84%) or S/P ratio(< 3.33)were more likely to suffer from SAP at 10 days after sampling (Fig. 6C-E). We then generated a multivariable Cox proportional hazards model, the log-transformed average of Streptococcus (aHR = 0.223, 95% CI 0.063–0.794, p = 0.021; aHR = 0.184, 95% CI 0.056–0.602, p = 0.005) was significantly associated with SAP (Table 3).
Fig. 6.
The relative abundance of specific taxa associated with SAP. ROC curve analyses for derivation of thresholds of protective bacterial relative abundance (Streptococcus)(A), hazardous bacterial relative abundance (Prevotella)(A) and Streptococcus/Prevotella (S/P) ratio(B). Kaplan‒Meier curves for developing SAP probability during hospitalization stratified by thresholds of protective bacterial relative abundance (Streptococcus)(D), hazardous bacterial relative abundance (Prevotella)(C) and Streptococcus/Prevotella (S/P) ratio(E). P values were derived from the log-rank test. ROC Receiver operator characteristic
Table 3.
Cox regression analysis of risk factors associated with SAP and the relative abundance of specific taxa
| Risk factor | Univariate analysis | Multivariate analysis* | Multivariate analysis# | |||||
|---|---|---|---|---|---|---|---|---|
| HR(95% CI) | P value | Model 1 aHR(95% CI) | P value | Model 2 aHR(95% CI) | P value | |||
| Protective bacteria | ||||||||
| p__Firmicutes | 0.016(0.002–0.160) | <0.001 | NA | NA | NA | NA | ||
| c__Bacilli | 0.090(0.028–0.290) | <0.001 | 0.190(0.042–0.864) | 0.032 | 0.139(0.035–0.552) | 0.005 | ||
| o__Lactobacillales | 0.095(0.031–0.292) | <0.001 | 0.183(0.044–0.766) | 0.020 | 0.154(0.043–0.557) | 0.004 | ||
| f__Streptococcaceae | 0.125(0.043–0.367) | <0.001 | 0.223(0.063–0.794) | 0.021 | 0.184(0.056–0.602) | 0.005 | ||
| g__Streptococcus | 0.125(0.043–0.367) | <0.001 | 0.223(0.063–0.794) | 0.021 | 0.184(0.056–0.602) | 0.005 | ||
| Hazardous bacteria | ||||||||
| p__Bacteroidota | 4.732(1.434–15.618) | 0.011 | NA | NA | NA | NA | ||
| c__Bacteroidia | 4.735(1.435–15.636) | 0.011 | NA | NA | NA | NA | ||
| o__Bacteroidales | 4.557(1.492–13.913) | 0.008 | NA | NA | NA | NA | ||
| f__Prevotellaceae | 4.147(1.559–11.032) | 0.004 | NA | NA | NA | NA | ||
| g__Prevotella | 4.508(1.781–11.778) | 0.002 | NA | NA | 3.224(1.147–9.063) | 0.026 | ||
| F/B | 0.254(0.107–0.601) | 0.002 | NA | NA | NA | NA | ||
| S/P | 0.345(0.202–0.590) | <0.001 | NA | NA | 0.424(0.231–0.776) | 0.005 | ||
*adjusted by age, initial NIHSS score, enteral nutrition, atrial fibrillation, dysphagia, and NLR(neutrophil to lymphocyte ratio)
#adjusted by A2DS2 score
F/B Firmicute/Bacteroidota (F/B) ratio; S/P Streptococcus/Prevotella (S/P) ratio
Associations between dysbiosis of the oral microbiota and clinical outcomes
SAP patients had a worse clinical prognosis after stroke. To investigate the potential association between oral microbiota and poor clinical prognosis, we evaluated 50 SAP patients and collected a total of 205 longitudinal samples (before diagnosis of SAP (P1) = 101 samples and after diagnosis of SAP (P2) = 104 samples). First, we attempted to identify a specific oral microbiota signature through LMM analysis associated with clinical outcomes and mortality by removing the potential effect of all mentioned latent confounding variables (Table S5).
Then, logistic regression was used to generate a potential predictive model of clinical outcomes in SAP patients based on changes associated with the abundance of ASVs calculated by LMM analysis. After adjusting for indicators of clinical indexs, the log-transformed average abundance of Actinobacteriota (P1), Actinobacteria (P1), Actinomycetales (P1) and Actinomycetaceae (P1) was significant in both the univariate and multivariate analyses, indicating that certain taxa in the phylum of the Actinobacteriota enrichment remained independent risk factors for 30-day functional poor outcomes (Table 4). Regarding 90-day functional outcomes, the log-transformed average abundance of Actinobacteriota (P1) was found to be significantly associated in the univariate and multivariate logistic regression analyses (Table S7).
Table 4.
Logistic regression analysis of risk factors associated with poor 30-day outcomes in SAP patients
| Risk factor | Univariate analysis | Multivariate analysis* | Multivariate analysis# | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR(95% CI) | P value | Model 1 OR(95% CI) | P value | Model 2 OR(95% CI) | P value | ||||
| P1 | |||||||||
| p__Actinobacteriota | 25.837(1.367-488.456) | 0.030 | 22.954(1.376-383.026) | 0.029 | 24.270(1.338-440.331) | 0.031 | |||
| c__Actinobacteria | 11.632(1.163-116.352) | 0.037 | 12.932(1.316-127.104) | 0.028 | 13.061(1.178-144.754) | 0.036 | |||
| o__Actinomycetales | 50.309(1.881-1345.855) | 0.019 | 22.372(1.075-465.609) | 0.045 | 63.506(1.643-2454.396) | 0.026 | |||
| f__Actinomycetaceae | 51.187(2.054-1275.877) | 0.016 | 23.662(1.190-470.657) | 0.038 | 62.317(1.721-2256.818) | 0.024 | |||
| g__Actinomyces | 5.162(1.049–25.411) | 0.044 | NA | NA | NA | NA | |||
| P2 | |||||||||
| p__Actinobacteriota | 9.773(1.230-77.633) | 0.031 | NA | NA | 119.502(1.283-11133.762) | 0.039 | |||
| c__Actinobacteria | 5.845(1.013–33.727) | 0.048 | NA | NA | NA | NA | |||
| Δ | |||||||||
| f__Actinomycetaceae | 0.148(0.023–0.967) | 0.046 | NA | NA | 0.043(0.002–0.865) | 0.040 | |||
| g__Actinomyces | 0.143(0.021–0.987) | 0.048 | NA | NA | 0.055(0.003–0.974) | 0.048 | |||
* Model 1 adjusted by SOFA, APACHE II, initial GCS score and length of ICU stay
# Model 2 adjusted by age, initial NIHSS score, enteral nutrition, atrial fibrillation, dysphagia, and NLR (neutrophil to lymphocyte ratio)
P1 = log-transformed average (before diagnosis of SAP samples); P2 = log-transformed average (after diagnosis of SAP samples); Δ = P2-P1.
To assess the potential use of the oral microbiota as a biomarker for early prediction of 30-day functional outcomes, ROC curve analysis was performed. We found that the predictive performance could be improved significantly by combining the clinical index model with the associated taxa in the phylum of the Actinobacteriota identified in logistic regression analysis (Fig. 7A, B).
Fig. 7.
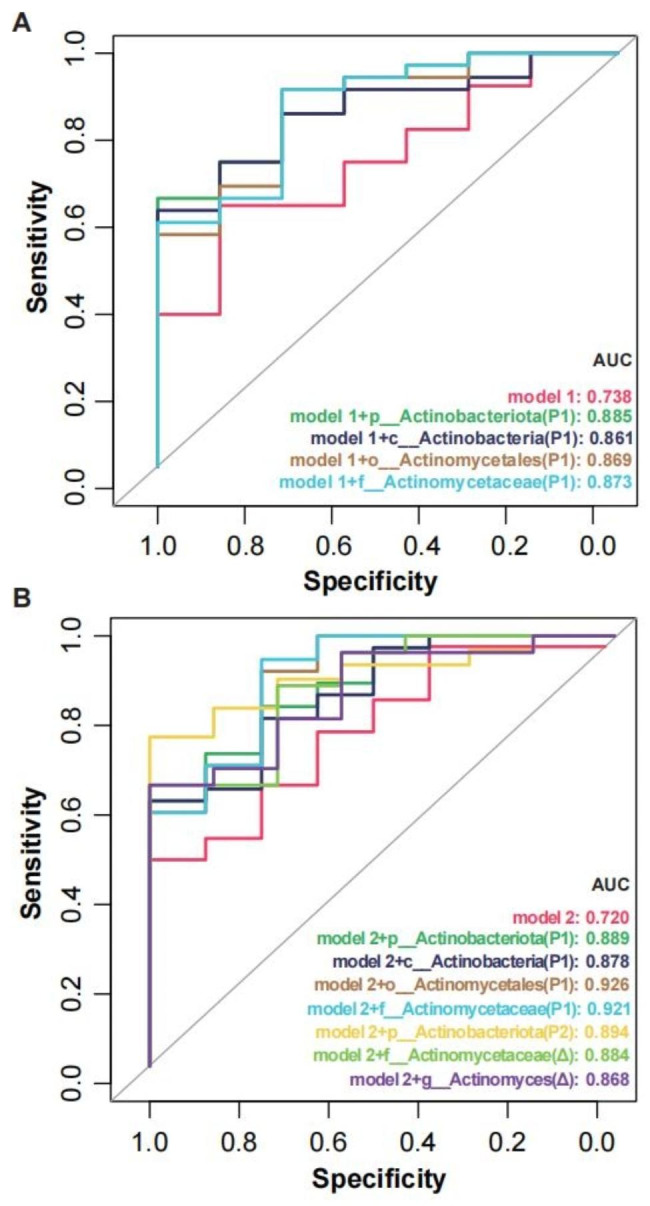
Predictive models based on microbiota and clinical index for early distinction of 30-day poor outcomes. Poor (mRS ≥ 3) and good (mRS<3) outcomes at the 30-day follow-up were defined in patients. The candidate variables of microbiota with an adjusted p value of < 0.05 in the multivariate logistic regression model. Model 1 (A) included age, initial NIHSS score, enteral nutrition, atrial fibrillation, dysphagia, and NLR. Model 2 (B) included indicators of critical illness (GCS, APACHE-II and SOFA scores and the length of ICU stay). mRS modified Rankin Scale; NIHSS National Institutes of Health Stroke Scale; NLR neutrophil to lymphocyte ratio; GCS Glasgow Coma Scale; APACHE-II Acute Physiology and Chronic Health Evaluation; SOFA Sequential Organ Failure Assessment scores
Discussion
In this prospective observational study of AIS patients, it was observed that patients with SAP and VAP experienced longer hospital stays and had more unfavorable outcomes. Additionally, the comparative taxonomic profiles of the SAP and VAP groups demonstrated a significant microbial shift at various timepoints following stroke. Furthermore, the oral microbial composition during the early acute stage in these groups differed from that of nonpneumonia patients. To predict SAP at an early stage, we conducted a study on the relationship between pathogenic bacteria and adverse outcomes, as well as the protective properties of bacteria derived from the oral cavity. Our analysis using Cox regression revealed that Streptococcus exhibited a protective effect against SAP. These findings provide valuable insights into the oral microbiota ecology in stroke patients and pivotal predictive factors in microbial profiles. Additionally, logistic regression analyses showed that enrichment of certain taxa in the phylum Actinobacteriota was an independent risk factor for poor 30-day clinical outcomes in the SAP group.
The oral microbiota is the second most diverse microbial community that colonizes the human body, following the intestinal microbiota [34]. The role of these microbes in maintaining oral and systemic health is crucial. When the oral microbiota becomes dysbiotic, it can impact the development of various diseases. Therefore, this community has been suggested as a potential source of valuable disease biomarkers [35–43]. Existing evidence clearly indicates that poor oral health and dysbiosis of the oral microbiota contribute to healthcare-associated infections and impact the deterioration of clinical outcomes [18, 19, 44, 45]. In recent times, substantial evidence has emerged suggesting that respiratory tract dysbiosis could be a crucial factor contributing to variations in systemic inflammatory responses [46] and clinical outcomes at the patient level during critical illness. Furthermore, it has been found that this dysbiosis can be altered [20, 47–49]. Consistent with the findings of prior studies [16, 17], we have confirmed that the microbiome in patients with SAP/VAP significantly differs from that in nonpneumonia AIS participants. This distinction could potentially have implications for clinical outcomes. To confirm the relationship between this microbiota imbalance and the onset of pneumonia, we have discovered that, apart from the significant impact pneumonia has on the microbiota of stroke patients, various interventions such as antibiotic treatment, invasive airway manipulation, oral hygiene maintenance, and time spent in the ICU can also influence the microbial composition. Therefore, it is probable that this dysbiosis occurs due to the stress caused by factors related to illness, invasive treatments, and pharmacological interventions.
Our findings that protective bacterial features, such as Streptococcus abundance and the S/P ratio, were significantly associated with SAP indicated that stroke patients with a lower relative abundance of protective bacteria were more likely to suffer from SAP. Streptococcus [50], Neisseria, Prevotella [51], Rothia, Haemophilus, and Fusobacterium were recently described to form the core oral microbiota in healthy individuals [31–33]. In line with previous reports [16, 17], Streptococcus were considered normal residents of the oral cavity in stroke patients. Participants who had a high prevalence of Streptococcus in their oral cavity tended to possess a more varied microbiota and a lower occurrence of systemic infections, indicating a potential protective effect [50, 52–54]. For example, Streptococcus salivarius is believed to function as a probiotic by producing bacteriocins that hinder the growth of other bacteria and support a balanced oral environment [55–57]. On the other hand, Streptococcus pneumoniae [58, 59] and Streptococcus pseudopneumoniae [60] appear to be respiratory tract colonizers with the potential to become pathogenic. Our findings provide evidence that maintaining a healthy balance in the core oral microbiota is crucial in reducing the risk of pneumonia and other infections, rather than the presence or absence of any single species or group of bacteria. However, due to the limited precision of the 16 S rRNA gene tag and the relatively small sample size, the results of our analysis are insufficient to identify specific protective species within the Streptococcus group. Nevertheless, the link between oral Streptococcus symbiosis and pneumonia prognosis could help to identify potential targets for predicting SAP in the future.
Moreover, our results showed that the increases in the abundance of Actinobacteriota, Actinomycetales, Actinomycetaceae and Actinomyces were associated with poor outcomes within 30 days in the SAP group. Actinomyces are colonizers of oral cavities in humans and are involved in the development of respiratory tract infections, which has been well documented [61, 62]. Notably, Actinomyces are recognized as significant sources of antibiotic resistance [63, 64], which may be attributed to the presence of patients with infectious diseases and frequent exacerbations, making them important microbial reservoirs. As a result, long-term antibiotic regimens were recommended to minimize bacterial burden and inflammation. However, the utilization of this strategy creates an environment that promotes antimicrobial resistance due to selective pressure [65, 66], which is a major global concern and serious threat to public health [67]. In addition, disruption of the oral microbiome may affect central nervous system (CNS) functions [54, 68–70]. Actinomyces has been identified in recent research as being connected to brain dysfunction or infection [71–73], and oral exposure to Actinomyces meyeri has led to an increase in the production of β-amyloid 42 protein and macrophage infiltration in mouse brains [74]. As a result, we believe that Actinomyces is a distinct type of oral bacterium with a correlation to both the functioning of the central nervous system and clinical outcomes.
As the oral cavity is hypothesized to play key roles in the progression of SAP or VAP, the restoration of commensal “healthy microbes” or the eradication of pathogens used to be considered beneficial effects in patients. Microbiome associated intervention therapy includes antibiotic treatment, probiotic administration [75–77] and oral hygiene care [14, 22–24, 78]. However, our results indicated that antibiotic therapy was inversely correlated with the healthy core oral microbiota (including Streptococcus, Prevotella, Rothia and Fusobacterium). This finding is consistent with previous research [17]. Although antibiotics play a vital role in managing stroke-related infections, there are still challenges and uncertainties. The existing guidelines do not recommend the use of prophylactic antibiotic treatment (PAT) before confirming a diagnosis of SAP [79]. The effectiveness of PAT in preventing infection and enhancing prognosis is still a contentious topic, and currently, no beneficial effects of antibiotics in preventing SAP have been identified.
It has been proven that oral diseases are closely linked to the occurrence of pneumonia [80, 81]. However, in our study, there was no difference in oral health among the three groups. On one hand, this may be attributed to the small number of patients included. On the other hand, it could also be due to a lack of professional dental and oral scoring.
The study’s strengths lie in its longitudinal prospective design, speedy participant recruitment within 24 h post-stroke, and meticulous microbial sampling. Nonetheless, there were several limitations to the study that should not be overlooked. Firstly, our findings were restricted to 16 S rRNA gene sequencing, resulting in the inability to analyze viability and virulence factors or attain species-level resolution. Secondly, as it was a single-center study without a control group, external validation was lacking. However, comparing healthy community-dwellers to stroke patients receiving equivalent nursing care was not feasible or comprehensive, resulting in the use of stroke patients without pneumonia as an internal control group due to their similar neurological risk profiles. Thirdly, professional oral cavity assessments and scores were not conducted due to the urgency of treatment for AIS patients admitted within 24 h of stroke onset. Fourthly, the sampling method for oral microbiota is oral swabs, which may be different from previous studies. AIS patients will receive thrombolysis therapy and antiplatelet therapy after admission, considering that collecting dental plaques is an invasive procedure that may cause periodontal bleeding. Moreover, individuals with AIS often experience dysphagia and may be placed on ventilators, which further complicates the task of collecting saliva samples. Therefore, in our study, we opted to gather samples from the buccal mucosa and gingiva instead. Therefore, we ultimately collected samples from buccal mucosa and gingiva. Lastly, the unequal distribution of patients between the groups and the small number of VAP patients were additional limitations. All patients meeting the inclusion criteria were included in the study across a 9-month period, but there were fewer patients with more severe strokes who were intubated during the first seven days after stroke onset in the stroke unit.
Conclusions
To the best of our knowledge, this prospective observational study is the largest longitudinal cohort study of AIS patients to analyze the oral microbiota using 16 S rRNA gene sequencing. The aim of this study is to describe the oral microbial characteristics before or after pneumonia in the context of AIS. Then, by controlling the potential effect of all latent confounding variables, we assessed the changes associated with pneumonia after stroke and explored the abundances of specific bacteria that were important risk factors for pneumonia and 30-day clinical outcomes in SAP patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all of the study participants and the clinical and research staff from Nanfang Hospital and Zhujiang Hospital for their contributions to this study.
Authors’ contributions
Study design J.Y. and H.Z.; collection of samples S.C. and Y.D.; sample processing and sequencing J.L., X.L., Y.R. and S.C.; bioinformatic analyses Y.R., J.L.,S.W.,J.Z.,X.Z. and Q.W.; manuscript writing Y.R.,X.L. and J.L.; manuscript revision J.Y.,Y.H. and H.Z.All authors read and approved the final manuscript.
Funding
J.Y. was supported by the National Natural Science Foundation of China (NSFC82171317) and the Guangdong Natural Science Foundation (2022A1515010477), and H.Z. was supported by the Guangzhou Key Research Program on Brain Science (202206060001). Funding agencies did not play a role in study design, data collection, analysis and interpretation, and manuscript writing.
Data Availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA004928) that are publicly accessible at https://bigd.big.ac.cn/gsa-human/browse/HRA004928.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2020-169) and registered at http://clinicaltrials.gov (NCT04688138). Informed consent was obtained from all patients or their legal representatives. This research was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yueran Ren, Jingru Liang, and Xiao Li are contributed equally to this work.
Contributor Information
Hongwei Zhou, Email: biodegradation@gmail.com.
Jia Yin, Email: yinj@smu.edu.cn.
References
- 1.Hannawi Y, Hannawi B, Rao CP, Suarez JI, Bershad EM. Stroke-associated Pneumonia: major advances and obstacles. Cerebrovasc Dis. 2013;35(5):430–43. doi: 10.1159/000350199. [DOI] [PubMed] [Google Scholar]
- 2.Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, Di Napoli M, Kalra L, Langhorne P, Montaner J, et al. Diagnosis of Stroke-Associated Pneumonia: recommendations from the Pneumonia in Stroke Consensus Group. Stroke. 2015;46(8):2335–40. doi: 10.1161/STROKEAHA.115.009617. [DOI] [PubMed] [Google Scholar]
- 3.Kishore AK, Vail A, Bray BD, Chamorro A, Napoli MD, Kalra L, Langhorne P, Montaner J, Roffe C, Rudd AG, et al. Clinical risk scores for predicting stroke-associated Pneumonia: a systematic review. Eur Stroke J. 2016;1(2):76–84. doi: 10.1177/2396987316651759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metersky ML, Kalil AC. Management of Ventilator-Associated Pneumonia: guidelines. Clin Chest Med. 2018;39(4):797–808. doi: 10.1016/j.ccm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Wu VKS, Fong C, Walters AM, Lele AV. Prevalence, clinical characteristics, and outcomes related to Ventilator-Associated events in neurocritically Ill patients. Neurocrit Care. 2020;33(2):499–507. doi: 10.1007/s12028-019-00910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, et al. Commensal microbiota affects ischemic Stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22(5):516–23. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179–94. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 8.Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, You C, Tian X, Di H, Tang W et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut 2021. [DOI] [PubMed]
- 9.Luo J, Chen Y, Tang G, Li Z, Yang X, Shang X, Huang T, Huang G, Wang L, Han Y, et al. Gut microbiota composition reflects Disease progression, severity and outcome, and dysfunctional immune responses in patients with hypertensive intracerebral Hemorrhage. Front Immunol. 2022;13:869846. doi: 10.3389/fimmu.2022.869846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia GH, Zhang MS, Wu QH, Wang HD, Zhou HW, He Y, Yin J. Dysbiosis of gut microbiota is an Independent risk factor of Stroke-Associated Pneumonia: a Chinese pilot study. Front Cell Infect Microbiol. 2021;11:715475. doi: 10.3389/fcimb.2021.715475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonelli A, Lumngwena EN, Ntusi NAB. The oral microbiome in the pathophysiology of Cardiovascular Disease. Nat Rev Cardiol 2023. [DOI] [PubMed]
- 12.Bryan NS, Tribble G, Angelov N. Oral microbiome and nitric oxide: the Missing Link in the management of blood pressure. Curr Hypertens Rep. 2017;19(4):33. doi: 10.1007/s11906-017-0725-2. [DOI] [PubMed] [Google Scholar]
- 13.Chen YL, Bai L, Dilimulati D, Shao S, Qiu C, Liu T, Xu S, Bai XB, Du LJ, Zhou LJ, et al. Periodontitis Salivary Microbiota aggravates ischemic Stroke through IL-17A. Front Neurosci. 2022;16:876582. doi: 10.3389/fnins.2022.876582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan D, Zhang J, Wang X, Chen S, Wang Y. Intensified oral Hygiene Care in Stroke-Associated Pneumonia: a pilot single-blind randomized controlled trial. Inquiry. 2020;57:46958020968777. doi: 10.1177/0046958020968777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millns B, Gosney M, Jack CI, Martin MV, Wright AE. Acute Stroke predisposes to oral gram-negative bacilli -- a cause of aspiration Pneumonia? Gerontology. 2003;49(3):173–6. doi: 10.1159/000069171. [DOI] [PubMed] [Google Scholar]
- 16.Boaden E, Lyons M, Singhrao SK, Dickinson H, Leathley M, Lightbody CE, McLoughlin A, Khan Z, Crean S, Smith C, et al. Oral flora in acute Stroke patients: a prospective exploratory observational study. Gerodontology. 2017;34(3):343–56. doi: 10.1111/ger.12271. [DOI] [PubMed] [Google Scholar]
- 17.Cieplik F, Wiedenhofer AM, Pietsch V, Hiller KA, Hiergeist A, Wagner A, Baldaranov D, Linker RA, Jantsch J, Buchalla W, et al. Oral health, oral microbiota, and incidence of Stroke-Associated Pneumonia-A prospective observational study. Front Neurol. 2020;11:528056. doi: 10.3389/fneur.2020.528056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emonet S, Lazarevic V, Leemann Refondini C, Gaia N, Leo S, Girard M, Nocquet Boyer V, Wozniak H, Despres L, Renzi G, et al. Identification of respiratory microbiota markers in ventilator-associated Pneumonia. Intensive Care Med. 2019;45(8):1082–92. doi: 10.1007/s00134-019-05660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommerstein R, Merz TM, Berger S, Kraemer JG, Marschall J, Hilty M. Patterns in the longitudinal oropharyngeal microbiome evolution related to ventilator-associated Pneumonia. Antimicrob Resist Infect Control. 2019;8:81. doi: 10.1186/s13756-019-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitsios GD, Yang H, Yang L, Qin S, Fitch A, Wang XH, Fair K, Evankovich J, Bain W, Shah F, et al. Respiratory tract dysbiosis is Associated with worse outcomes in mechanically ventilated patients. Am J Respir Crit Care Med. 2020;202(12):1666–77. doi: 10.1164/rccm.201912-2441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prendergast V, Hinkle JL. Oral Care Assessment Tools and interventions after Stroke. Stroke. 2018;49(4):e153–6. doi: 10.1161/STROKEAHA.117.017045. [DOI] [PubMed] [Google Scholar]
- 22.Horne M, McCracken G, Walls A, Tyrrell PJ, Smith CJ. Organisation, practice and experiences of mouth hygiene in Stroke unit care: a mixed-methods study. J Clin Nurs. 2015;24(5–6):728–38. doi: 10.1111/jocn.12665. [DOI] [PubMed] [Google Scholar]
- 23.Brady MC, Stott DJ, Weir CJ, Chalmers C, Sweeney P, Barr J, Pollock A, Bowers N, Gray H, Bain BJ, et al. A pragmatic, multi-centered, stepped wedge, cluster randomized controlled trial pilot of the clinical and cost effectiveness of a complex Stroke oral healthCare intervention pLan evaluation II (SOCLE II) compared with usual oral healthcare in Stroke wards. Int J Stroke. 2020;15(3):318–23. doi: 10.1177/1747493019871824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuon FF, Gavrilko O, Almeida S, Sumi ER, Alberto T, Rocha JL, Rosa EA. Prospective, randomised, controlled study evaluating early modification of oral microbiota following admission to the intensive care unit and oral hygiene with chlorhexidine. J Glob Antimicrob Resist. 2017;8:159–63. doi: 10.1016/j.jgar.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Santigli E, Koller M, Klug B. Oral Biofilm Sampling for Microbiome Analysis in Healthy Children. J Vis Exp 2017(130). [DOI] [PMC free article] [PubMed]
- 26.American Thoracic S. Infectious Diseases Society of a: guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated Pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 27.Cilloniz C, Torres A, Niederman MS. Management of Pneumonia in critically ill patients. BMJ. 2021;375:e065871. doi: 10.1136/bmj-2021-065871. [DOI] [PubMed] [Google Scholar]
- 28.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratala J, et al. Management of adults with hospital-acquired and ventilator-associated Pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spalding MC, Cripps MW, Minshall CT. Ventilator-Associated Pneumonia: New definitions. Crit Care Clin. 2017;33(2):277–92. doi: 10.1016/j.ccc.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired Pneumonia and ventilator-associated Pneumonia: guidelines for the management of hospital-acquired Pneumonia (HAP)/ventilator-associated Pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana Del Torax (ALAT). Eur Respir J 2017, 50(3). [DOI] [PubMed]
- 31.Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy core microbiome of oral microbial communities. BMC Microbiol. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nearing JT, DeClercq V, Van Limbergen J, Langille MGI. Assessing the variation within the oral microbiome of healthy adults. mSphere 2020, 5(5). [DOI] [PMC free article] [PubMed]
- 33.Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuganbaev T, Yoshida K, Honda K. The effects of oral microbiota on health. Sci (New York NY) 2022;376(6596):934–6. doi: 10.1126/science.abn1890. [DOI] [PubMed] [Google Scholar]
- 35.Campbell K. Oral microbiome findings challenge dentistry dogma. Nature 2021. [DOI] [PubMed]
- 36.Larson PJ, Zhou W, Santiago A, Driscoll S, Fleming E, Voigt AY, Chun OK, Grady JJ, Kuchel GA, Robison JT, et al. Associations of the skin, oral and gut microbiome with aging, frailty and Infection risk reservoirs in older adults. Nat Aging. 2022;2(10):941–55. doi: 10.1038/s43587-022-00287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouzid F, Gtif I, Alfadhli S, Charfeddine S, Ghorbel W, Abdelhedi R, Benmarzoug R, Abid L, Bouayed Abdelmoula N, Elloumi I et al. A potential oral microbiome signature associated with coronary artery Disease in Tunisia. Biosci Rep 2022, 42(7). [DOI] [PMC free article] [PubMed]
- 38.Zhang K, Zhou X, Qin J, Zhang W, Pan Y, Wang H, Lin J, Liu L, Jia Y. Dynamic change in oral microbiota of children with cleft lip and palate after alveolar bone grafting. Cleft Palate Craniofac J. 2022;59(11):1352–60. doi: 10.1177/10556656211044396. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, He P, Zhou M, Li S, Zhang J, Tao X, Wang A, Wu X. Variations in oral microbiome and its predictive functions between tumorous and healthy individuals. J Med Microbiol 2022, 71(8). [DOI] [PubMed]
- 40.Fak F, Tremaroli V, Bergstrom G, Backhed F. Oral microbiota in patients with Atherosclerosis. Atherosclerosis. 2015;243(2):573–8. doi: 10.1016/j.atherosclerosis.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 41.Desvarieux Ms, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Sacco RL, Papapanou PN. Periodontal Microbiota and Carotid Intima-Media thickness. Circulation. 2005;111(5):576–82. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desvarieux M, Demmer RT, Jacobs DR, Jr, Rundek T, Boden-Albala B, Sacco RL, Papapanou PN. Periodontal bacteria and Hypertension: the oral Infections and vascular Disease epidemiology study (INVEST) J Hypertens. 2010;28(7):1413–21. doi: 10.1097/HJH.0b013e328338cd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flemer B, Warren RD, Barrett MP, Cisek K, Das A, Jeffery IB, Hurley E, O’Riordain M, Shanahan F, O’Toole PW. The oral microbiota in Colorectal cancer is distinctive and predictive. Gut. 2018;67(8):1454–63. doi: 10.1136/gutjnl-2017-314814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sachdev M, Ready D, Brealey D, Ryu J, Bercades G, Nagle J, Borja-Boluda S, Agudo E, Petrie A, Suvan J, et al. Changes in dental plaque following hospitalisation in a critical care unit: an observational study. Crit Care. 2013;17(5):R189. doi: 10.1186/cc12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Wu K, Sun T, Chen B, Yi Y, Ren R, Xie L, Xiao K. Effect of invasive mechanical ventilation on the diversity of the pulmonary microbiota. Crit Care. 2022;26(1):252. doi: 10.1186/s13054-022-04126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, et al. The intermucosal connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell. 2020;182(2):447–62. e414. [DOI] [PMC free article] [PubMed]
- 47.Xu R, Lu R, Zhang T, Wu Q, Cai W, Han X, Wan Z, Jin X, Zhang Z, Zhang C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun Biol. 2021;4(1):240. doi: 10.1038/s42003-021-01796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Koff EM, Man WH, van Houten MA, Jansen NJG, Arp K, Hasrat R, Sanders EAM, Bogaert D. The respiratory microbiota during and following mechanical ventilation for Respiratory Infections in children. Eur Respir J 2021, 57(4). [DOI] [PMC free article] [PubMed]
- 49.Lamarche D, Johnstone J, Zytaruk N, Clarke F, Hand L, Loukov D, Szamosi JC, Rossi L, Schenck LP, Verschoor CP, et al. Microbial dysbiosis and mortality during mechanical ventilation: a prospective observational study. Respir Res. 2018;19(1):245. doi: 10.1186/s12931-018-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abranches J, Zeng L, Kajfasz JK, Palmer SR, Chakraborty B, Wen ZT, Richards VP, Brady LJ, Lemos JA. Biology of oral streptococci. Microbiol Spectr 2018, 6(5). [DOI] [PMC free article] [PubMed]
- 51.Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585–99. doi: 10.1038/s41579-021-00559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santagati M, Scillato M, Patane F, Aiello C, Stefani S. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol Med Microbiol. 2012;65(1):23–31. doi: 10.1111/j.1574-695X.2012.00928.x. [DOI] [PubMed] [Google Scholar]
- 53.Hosoki S, Saito S, Tonomura S, Ishiyama H, Yoshimoto T, Ikeda S, Ikenouchi H, Yamamoto Y, Hattori Y, Miwa K, et al. Oral carriage of Streptococcus mutans harboring the cnm gene relates to an increased incidence of cerebral microbleeds. Stroke. 2020;51(12):3632–9. doi: 10.1161/STROKEAHA.120.029607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosoki S, Hattori Y, Saito S, Takegami M, Tonomura S, Yamamoto Y, Ikeda S, Hosomi N, Oishi N, Morita Y, et al. Risk Assessment of Cnm-positive Streptococcus mutans in Stroke survivors (RAMESSES): protocol for a Multicenter prospective cohort study. Front Neurol. 2022;13:816147. doi: 10.3389/fneur.2022.816147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacDonald KW, Chanyi RM, Macklaim JM, Cadieux PA, Reid G, Burton JP. Streptococcus salivarius inhibits immune activation by periodontal Disease pathogens. BMC Oral Health. 2021;21(1):245. doi: 10.1186/s12903-021-01606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Li J, Zhang H, Zheng X, Wang J, Jia X, Peng X, Xie Q, Zou J, Zheng L, et al. Probiotic Streptococcus salivarius K12 alleviates Radiation-Induced oral mucositis in mice. Front Immunol. 2021;12:684824. doi: 10.3389/fimmu.2021.684824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zupancic K, Kriksic V, Kovacevic I, Kovacevic D. Influence of oral probiotic Streptococcus salivarius K12 on ear and Oral Cavity Health in humans: systematic review. Probiotics and Antimicrobial Proteins. 2017;9(2):102–10. doi: 10.1007/s12602-017-9261-2. [DOI] [PubMed] [Google Scholar]
- 58.Inomata M, Xu S, Chandra P, Meydani SN, Takemura G, Philips JA, Leong JM. Macrophage LC3-associated phagocytosis is an immune defense against Streptococcus pneumoniae that diminishes with host aging. Proc Natl Acad Sci U S A. 2020;117(52):33561–9. doi: 10.1073/pnas.2015368117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novick S, Shagan M, Blau K, Lifshitz S, Givon-Lavi N, Grossman N, Bodner L, Dagan R, Mizrachi Nebenzahl Y. Adhesion and invasion of Streptococcus pneumoniae to primary and secondary respiratory epithelial cells. Mol Med Rep. 2017;15(1):65–74. doi: 10.3892/mmr.2016.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsumoto A, Tabata A, Ohkura K, Oda H, Kodama C, Ohkuni H, Takao A, Kikuchi K, Tomoyasu T, Nagamune H. Molecular characteristics of an adhesion molecule containing cholesterol-dependent cytolysin-motif produced by mitis group Streptococci. Microbiol Immunol. 2021;65(2):61–75. doi: 10.1111/1348-0421.12868. [DOI] [PubMed] [Google Scholar]
- 61.Hall V. Actinomyces–gathering evidence of human colonization and Infection. Anaerobe. 2008;14(1):1–7. doi: 10.1016/j.anaerobe.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Lu H, Qian G, Ren Z, Zhang C, Zhang H, Xu W, Ye P, Yang Y, Li L. Alterations of Bacteroides sp., Neisseria sp., Actinomyces sp., and Streptococcus sp. populations in the oropharyngeal microbiome are associated with liver Cirrhosis and Pneumonia. BMC Infect Dis. 2015;15:239. doi: 10.1186/s12879-015-0977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mac Aogain M, Lau KJX, Cai Z, Kumar Narayana J, Purbojati RW, Drautz-Moses DI, Gaultier NE, Jaggi TK, Tiew PY, Ong TH, et al. Metagenomics reveals a core Macrolide Resistome related to Microbiota in Chronic Respiratory Disease. Am J Respir Crit Care Med. 2020;202(3):433–47. doi: 10.1164/rccm.201911-2202OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auer DL, Mao X, Anderson AC, Muehler D, Wittmer A, von Ohle C, Wolff D, Frese C, Hiller KA, Maisch T et al. Phenotypic Adaptation to Antiseptics and Effects on Biofilm Formation Capacity and Antibiotic Resistance in Clinical Isolates of Early Colonizers in Dental Plaque. Antibiotics (Basel) 2022, 11(5). [DOI] [PMC free article] [PubMed]
- 65.Ferrer M, Mendez-Garcia C, Rojo D, Barbas C, Moya A. Antibiotic use and microbiome function. Biochem Pharmacol. 2017;134:114–26. doi: 10.1016/j.bcp.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Al-Ahmad A, Ameen H, Pelz K, Karygianni L, Wittmer A, Anderson AC, Spitzmuller B, Hellwig E. Antibiotic resistance and capacity for biofilm formation of different bacteria isolated from endodontic Infections associated with root-filled teeth. J Endod. 2014;40(2):223–30. doi: 10.1016/j.joen.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 67.Garcia ER, Vergara A, Aziz F, Narvaez S, Cuesta G, Hernandez M, Toapanta D, Marco F, Fernandez J, Soriano A, et al. Changes in the gut microbiota and risk of colonization by multidrug-resistant bacteria, Infection, and death in critical care patients. Clin Microbiol Infect. 2022;28(7):975–82. doi: 10.1016/j.cmi.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Scassellati C, Marizzoni M, Cattane N, Lopizzo N, Mombelli E, Riva MA, Cattaneo A. The Complex Molecular Picture of gut and oral microbiota-brain-depression system: what we know and what we need to know. Front Psychiatry. 2021;12:722335. doi: 10.3389/fpsyt.2021.722335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martínez M, Postolache TT, García-Bueno B, Leza JC, Figuero E, Lowry CA, Malan-Müller S. The role of the oral microbiota related to Periodontal Diseases in anxiety, Mood and Trauma- and stress-related disorders. Front Psychiatry. 2021;12:814177. doi: 10.3389/fpsyt.2021.814177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borsa L, Dubois M, Sacco G, Lupi L. Analysis the Link between Periodontal Diseases and Alzheimer’s Disease: A Systematic Review. Int J Environ Res Public Health 2021, 18(17). [DOI] [PMC free article] [PubMed]
- 71.Könönen E, Wade WG. Actinomyces and related organisms in human Infections. Clin Microbiol Rev. 2015;28(2):419–42. doi: 10.1128/CMR.00100-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smego RA., Jr Actinomycosis of the central nervous system. Rev Infect Dis. 1987;9(5):855–65. doi: 10.1093/clinids/9.5.855. [DOI] [PubMed] [Google Scholar]
- 73.Clarridge JE 3rd, Zhang Q. Genotypic diversity of clinical Actinomyces species: phenotype, source, and Disease correlation among genospecies. J Clin Microbiol. 2002;40(9):3442–8. [DOI] [PMC free article] [PubMed]
- 74.Luo Z, Fitting S, Robinson C, Benitez A, Li M, Wu Y, Fu X, Amato D, Ning W, Funderburg N, et al. Chronic cannabis smoking-enriched oral pathobiont drives behavioral changes, macrophage infiltration, and increases beta-amyloid protein production in the brain. EBioMedicine. 2021;74:103701. doi: 10.1016/j.ebiom.2021.103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klarin B, Adolfsson A, Torstensson A, Larsson A. Can probiotics be an alternative to chlorhexidine for oral care in the mechanically ventilated patient? A multicentre, prospective, randomised controlled open trial. Crit Care. 2018;22(1):272. doi: 10.1186/s13054-018-2209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimizu K, Yamada T, Ogura H, Mohri T, Kiguchi T, Fujimi S, Asahara T, Yamada T, Ojima M, Ikeda M, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated Pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22(1):239. doi: 10.1186/s13054-018-2167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cook DJ, Johnstone J, Marshall JC, Lauzier F, Thabane L, Mehta S, Dodek PM, McIntyre L, Pagliarello J, Henderson W, et al. Probiotics: Prevention of severe Pneumonia and endotracheal colonization Trial-PROSPECT: a pilot trial. Trials. 2016;17:377. doi: 10.1186/s13063-016-1495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani M. The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated Pneumonia in adults’ intensive care units. J Crit Care. 2017;40:318–22. doi: 10.1016/j.jcrc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 79.Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, Patel A, Rebollo-Mesa I. Prophylactic antibiotics after acute Stroke for reducing Pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet (London England) 2015;386(10006):1835–44. doi: 10.1016/S0140-6736(15)00126-9. [DOI] [PubMed] [Google Scholar]
- 80.Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary Infections. Oral Dis. 2007;13(6):508–12. doi: 10.1111/j.1601-0825.2007.1410a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pace CC, McCullough GH. The association between oral microorgansims and aspiration Pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–22. doi: 10.1007/s00455-010-9298-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA004928) that are publicly accessible at https://bigd.big.ac.cn/gsa-human/browse/HRA004928.