Abstract
Background
In MAPT (Multidomain Alzheimer Preventive Trial), a cognitive effect of multidomain intervention (MI) was showed in non-demented subjects with positive amyloid PET. However, screening eligible patients for multidomain intervention by PET is difficult to generalize in real-world settings.
Methods
MAPT study was a 3-year, randomized, placebo-controlled trial followed by a 2-year observational and optional extension. All participants were non-demented and randomly assigned (1:1:1:1) to the MI plus omega 3, MI plus placebo, omega 3 alone, or placebo alone group. The objectives were to assess the cognitive effect of MAPT interventions (omega 3 supplementation, MI, combined intervention) in non-demented subjects according to amyloid blood status at 12, 36, and 60 months. In this subgroup analysis (n = 483), amyloid status was defined by plasma Aβ42/40 ratio (cutoff ≤ 0.0107). The primary outcome measure was the change in cognitive composite score after a 1, 3, and 5-year clinical follow-up.
Results
The intention-to-treat (ITT) population included 483 subjects (161 positive and 322 negative amyloid participants based on plasma Aβ42/40 ratio). In the positive amyloid ITT population, we showed a positive effect of MI plus omega 3 on the change in composite cognitive score in 12 (raw p = .0350, 0.01917, 95% CI = [0.0136 to 0.3699]) and 36 months (raw p = .0357, 0.2818, 95% CI = [0.0190 to 0.5446]). After correction of multiple comparisons and adjustments, these differences were not significant (adjusted p = .1144 and .0690). In the per-protocol positive amyloid group (n = 154), we observed a significant difference between the combined intervention and placebo groups at 12 (p = .0313, 0.2424, 0.0571 to 0.4276) and 36 months (p = .0195, 0.3747, 0.1055 to 0.6439) persisting after adjustment. In the ITT and per-protocol analyses, no cognitive effect was observed in the positive and negative amyloid group at 60-month visit.
Conclusions
These findings suggest a benefit of MI plus omega 3 in positive blood amyloid subjects. This promising trend needs to be confirmed before using blood biomarkers for screening in preventive trials.
Trial registration
ClinicalTrials.gov Identifier: NCT01513252.
Keywords: Clinical trial, Alzheimer’s disease, Amyloid blood biomarker, Prevention
Background
The MAPT (Multidomain Alzheimer Prevention Trial) study has tested cognitive effect of omega 3 polyunsaturated fatty acid supplementation (omega 3) and multidomain intervention (MI) in non-demented subjects with memory complaint [1]. In the total population of the MAPT study, MI and omega 3 had no significant effect on cognitive decline over 3 years [2]. Nevertheless, the FINGER (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) and MAPT studies showed concordant effects in subgroups of at-risk subjects. In FINGER, the cognitive beneficial effect of the MI was greater than that of the control intervention in APOE ε4 carriers but not in non-carriers [3]. In the ancillary amyloid MAPT study (MAPT-AV45), the MI effect was positive only in non-demented subjects with positive amyloid positron emission tomography (PET) [4]. These findings could suggest cognitive effect of a MI in early stage on the continuum of Alzheimer disease (AD). However, MAPT-AV45 and FINGER studies had several methodological limitations: (1) the long-term impact of MI was not evaluated after interruption of the interventional program to test durability, (2) the sub-group size of MAPT-AV45 was relatively low, and (3) APOE ε4 status used in FINGER to define at-risk subjects for cognitive decline cannot be considered as a diagnosis biomarker of AD. To date, amyloid level assessed by PET and cerebrospinal fluid (CSF) measures of Aβ isoforms are the most widely used amyloid biomarkers. Screening by amyloid PET is difficult to generalize in real-world settings given its cost and limited access. Blood-based biomarkers are less invasive and cost-effective options for identification of at-risk subjects eligible for these non-pharmacological interventions [5]. Recent improvements in technologies used to assess amyloid blood levels have shown promising results [6]. Several groups have showed that the blood Aβ42/40 ratio provides a sensitive and reliable measure of amyloid status, well correlated to CSF Aβ42/40, that can predict future brain amyloidosis (i.e., conversion to positive amyloid PET) [7–9]. These promising results suggest that plasma Aβ42/40 ratio could be used to detect amyloid plaques in individuals before cognitive symptoms onset. However, these markers still need to be validated in interventional studies for the selection of potential participants. In prevention trials, a blood Aβ42/40 test could be used as screening tool to identify at-risk subjects for AD and to facilitate pharmacological and non-pharmacological program discovery [10–12].
In a subgroup of the MAPT study, amyloid blood assays have been performed from the MAPT biobank to determinate amyloid status of the participants. These data are an opportunity to validate encouraging findings from MAPT-AV45 and to assess the possibility of such preventive trials based on blood biomarkers in the future. Moreover, two additional years of clinical observation were performed after completion of the MAPT interventional program to track durability of the intervention once discontinued. Therefore, we evaluated the long-term cognitive effect over a 36-month treatment period and at 60 months, 24 months after discontinuation of non-pharmacological intervention in the subgroup characterized by blood biomarkers.
Methods
Study design and participants
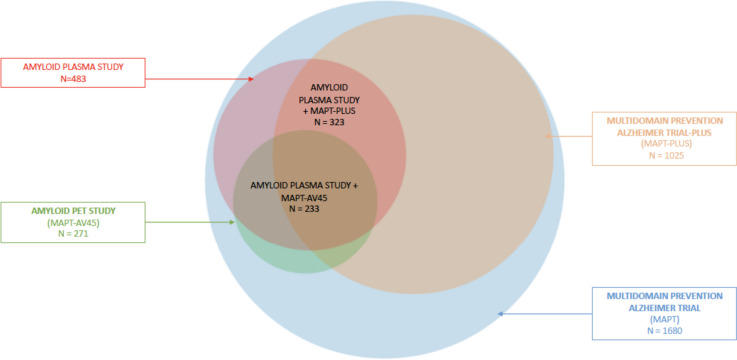
All subjects included in the present analysis were participants, from the MAPT and MAPT-PLUS studies, that were tested for amyloid blood biomarkers (Fig. 1). MAPT was a multicenter (13 memory centres in France and Monaco), randomized, placebo-controlled, 3-year trial whose objective was to assess effect of MI and omega 3 on cognitive performance. MAPT-PLUS was a 2-year observational and optional extension of MAPT after completion of the interventional program [1]. The objective of MAPT-PLUS was to evaluate the long-term cognitive effect of MAPT preventive strategies. This additional follow-up was systematically offered to MAPT participants during the end-of-study visit.
Fig. 1.
Place of the amyloid plasma analysis in relation to MAPT and ancillary studies. MAPT multidomain Alzheimer’s preventive trial
Based on MAPT inclusion criteria, subjects included in the present analysis were non-demented, 70 years old and over, and fulfilled one of the following three criteria: spontaneous memory complaint, limitation in one instrumental activity of daily living, or slow gait speed [2].
Randomization and masking
In MAPT, participants were randomly assigned (1:1:1:1) to the MI plus omega 3, MI plus placebo, omega 3 alone, or placebo alone group. A computer-generated randomization procedure was used with block sizes of eight and stratification by center. A clinical research assistant used a centralized interactive voice response system to identify which group to allocate the participant and which lot number to administer [2]. All participants and research staff including neuropsychologists were blinded to omega 3 or placebo assignment and to amyloid blood status.
Procedures
Participants took two capsules of either the placebo or omega 3 daily. The active supplement used was V0137, an oil mixture containing natural fish oil with a minimum of 65% docosahexaenoic acid (400 mg) and a maximum of 15% eicosapentaenoic acid (no more than 112.5 mg). As described previously, MI program consisted of 12 2-h group sessions focusing on three domains (cognitive stimulation, demonstrations of physical activity, and nutritional advice) and a preventive consultation for the management of cardiovascular risks at baseline, 12 and 24 months [2]. This interventional program lasted 3 years, and 2-year observational follow-up was added in MAPT-PLUS.
Clinical visits in MAPT and MAPT-PLUS were scheduled every 6 or 12 months to assess physical and functional conditions and adherence [1]. Cognitive assessment included the Free and Cued Selective Reminding Test (FCSRT) [13], the Controlled Oral Word Association Test and Category Naming Test (CNT) [14], the Digit Symbol Substitution Subtest of the Wechsler Adult Intelligence Scale–Revised [15], the Trail-Making Tests [16], the Mini-mental State Examination [17], and the Clinical Dementia Rating scale (CDR) [18]. Physical evaluation included the Short Physical Performance Battery (SPPB) [19] and Fried frailty criteria [20]. Autonomy in daily living activities was evaluated by the Alzheimer’s disease Cooperative Study-Activities of Daily Living Prevention Instrument (ADCS-ADL) [21]. One blood sample of 15 ml (10 ml in an EDTA vacutainer and a pair of × 2.5 ml in PAXgene RNA tubes) was collected yearly for the biobank. These samples were transferred directly at ambient temperature to the Cellular Biology and Cytology Laboratory at each site. The two PAXgene RNA tubes were frozen at −20° directly. The EDTA tube was centrifuged then aliquoted; the serum and the pellet were collected in two 5-ml dry tubes, then frozen at −20°. A biobank scientific committee has identified amyloid blood biomarkers as a research priority.
Plasma Aβ42/Aβ40 immunoprecipitation/mass spectrometry assay methods
Plasma samples of 0.46 ml were assessed to test plasma Aβ42 and Aβ40 levels by immunoprecipitation mass spectrometry as previously described [9, 22]. Aβ levels were analyzed and calculated by integrated peak area ratios to known concentrations of the internal standards using the Skyline software package [23].
Aβ42/Aβ40 cutoff (≤ 0.0107) has been defined, by Randall Bateman laboratory at Washington University School of Medicine in Saint-Louis, to discriminate as accurately as possible negative and positive amyloid participants in comparison to PET [24]. Indeed, many subjects included in the present analysis (n = 233) were participants from MAPT-AV45 with amyloid PET (Fig. 1). In the MAPT-AV45 study, the positivity threshold for amyloid PET was set at SUVr > 1.17 [4].
Adherence
For omega 3 supplementation and placebo, subjects were considered as adherent if they returned less than 25% of the prescribed capsules. For MI program, participants were considered as adherent if they attended at least 75% of the group sessions (if applicable) [2].
Primary outcome and objectives
The primary outcome measure was the change in cognitive composite score after a 1, 3, and 5-year follow-up. We used a composite of four measures, close to the PACC (Preclinical Alzheimer Cognitive Composite), well established to show sensitivity to decline in early stages of AD [25]. The MAPT cognitive composite score has been already described previously [2, 4, 26]. This cognitive composite score was calculated by combining FCSRT, CNT, Digit Symbol Substitution Subtest, and MMSE orientation scores.
The main objectives were to assess according to amyloid blood status: (1) the cognitive effect of MAPT interventions at 12 and 36 months and (2) the long-term impact at 60 months after 2-year interruption of these interventions.
Statistical analysis
Analysis was completed in the intention-to-treat (ITT, n = 483) and per-protocol (n = 457) populations. The ITT population consisted of all randomly assigned participants who completed a cognitive composite score at baseline and a minimum of one post-baseline visit. Per-protocol population excluded all major protocol violations at baseline and during follow-up [2]. Efficacy in subgroups according to amyloid blood status was assessed by post-hoc analysis.
We used the same statistical method as for the work carried out to determine the cognitive effect of MAPT interventions according to PET amyloid status [4]. Linear mixed-model repeated-measures analyses were used including baseline, 6, 12, 24, 36, 48, and 60-month follow-up data to assess between-group differences in the change in cognitive composite score from baseline to 12, 36, and 60 months. Time was used as a continuous variable, with a cubic trajectory, because the terms time2 and time3 were significant. For each linear mixed model, we included subject-specific random effects to consider the intra-subject correlation: a random intercept to consider the heterogeneity of the composite score at baseline and a random slope to consider the heterogeneity of the slopes between subjects. In the unadjusted linear mixed models, we included these fixed effects: intervention group by their amyloid blood status (8 categories), time, and interaction between group and time [4]. Then, to test the difference of the effect of the intervention between the negative and positive amyloid blood groups, we used the estimates of the interaction term parameters with the ESTIMATE command from the SAS MIXED procedure.
All the models were completed with and without adjustments for gender, age, educational level, CDR global score, and APOE ε4 genotype. All p values were presented before and after adjustment for multiple comparisons (using the Hochberg procedure) and the statistical significance was set at a P value < 0.05. All confidence intervals were two-sided with a 95% confidence level. All statistical analyses were achieved using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
Standard protocol approvals, registrations, and patient consents
The MAPT protocol is listed in a public-access clinical trial database (www.clinicaltrials.gov, no. NCT01513252). Written informed consent was given by all participants. A new informed consent form was signed by participants who volunteer for MAPT-PLUS during the end-of-study visit.
Data availability
The datasets generated and/or analyzed during this study are not publicly available. However, clinical data can be shared upon request following completion of the MAPT/DSA Data Access Application form (for further information contact the Data Sharing Alzheimer group: Info.u1027-dsa@inserm.fr).
Results
Enrollment and rates of study completion
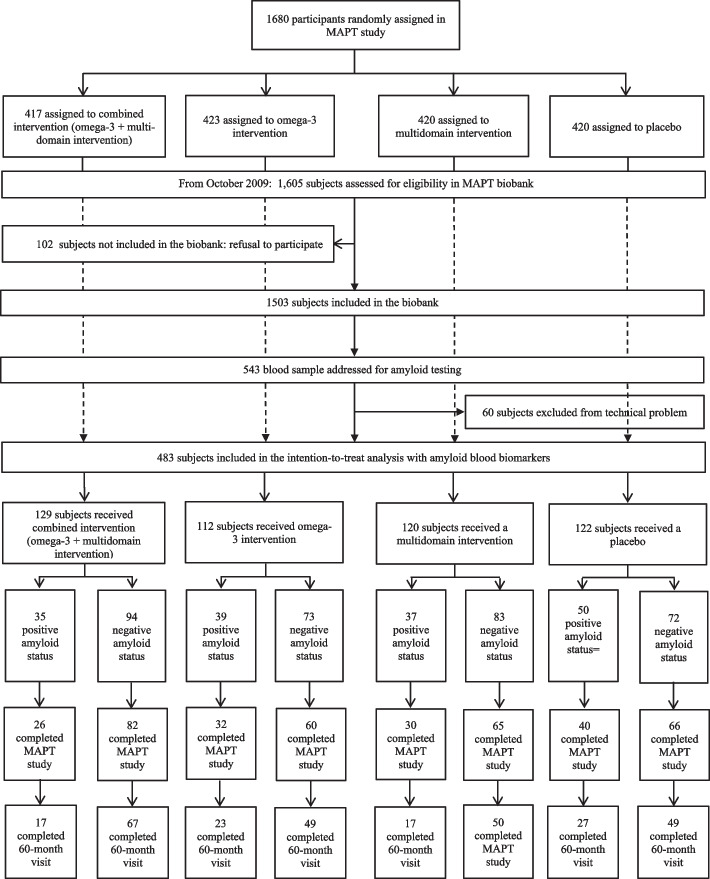
Among the 1680 participants in MAPT and 1503 in its biobank, 483 amyloid blood assays were performed for this analysis at 12 (448 subjects) and 24 months (35 subjects). These subjects (n = 483) were selected from MAPT biobank based on their participation in the MAPT-AV45 study and an available blood sample as close as possible to the baseline visit. Subjects were enrolled in the MAPT biobank from October 2009. The mean time interval between blood collection and baseline visit is 12.99 ± 3.15 months. From 483 subjects included in this analysis, 323 subjects had observational data at 48 months and 299 at 60 months in MAPT-PLUS. The flow chart of participants in this analysis is shown in Fig. 2.
Fig. 2.
Trial profile for the amyloid blood MAPT study. MAPT multidomain Alzheimer’s preventive trial, MI multidomain intervention
The ITT population included 161 positive and 322 negative amyloid subjects based on plasma Aβ42/Aβ40 ratio. In the ITT subgroup with positive amyloid blood status, 128 (79.5%) and 84 (52.2%) subjects completed respectively 36- and 60-month visits. In ITT subgroup with negative blood amyloid status, 273 (84.8%) and 215 (66.8%) subjects completed 36- and 60-month visits.
Baseline characteristics
Subjects who had amyloid blood assays (n = 483) were significantly older (on average 75.78 ± 4.55 vs. 75.15 ± 4.36 years, p = 0.0099), more frequently male (40.79 vs. 33.03%, p = 0.0026), APOE ε4 carriers (27.63 vs. 20.65%, p = 0.0.0047) and compliant to 3-year intervention (68.26 vs. 60.67%, p = 0.0045), had more frequently a CDR global score at 0.5 (47.00 vs. 40.08%, p = 0.0094), lower cognitive and functional performances respectively in composite cognitive (−0.10 ± 0.69 vs. 0.01 ± 0.67, p = 0.0017) and ADCS-ADL scores (39.13 ± 5.08 vs. 39.91 ± 4.66, p = 0.0035), than MAPT subjects not included in this analysis (n = 1196).
Baseline characteristics (clinical and blood-based biomarkers) of the ITT population are shown in Table 1. In the positive amyloid ITT population, the four groups are different in total SPPB (p = 0.0117) but not in the cognitive composite score (p = 0.4467, Table 1). In negative amyloid subjects, the four groups are different in plasma Aβ42/40 ratio (p = 0.0322) and DHA (p = 0.0310) but not in cognitive composite score (p = 0.6723, Table 1).
Table 1.
Baseline characteristics of the intention-to-treat ancillary amyloid blood MAPT study population (n = 483)
| ITT population (n = 483) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Negative amyloid blood status (n = 322) | Positive amyloid blood status (n = 161) | |||||||
| Omega-3 + MI (n = 94) | Omega-3 (n = 73) | MI (n = 83) | Placebo (n = 72) | Omega-3 + MI (n = 35) | Omega-3 (n = 39) | MI (n = 37) | Placebo (n = 50) | |
| Subject characteristics | ||||||||
| Male gender, N (%) | 40 (42.55) | 27 (36.99) | 26 (31.33) | 24 (33.33) | 20 (57.14) | 20 (51.28) | 18 (48.65) | 22 (44.00) |
| Age in years, mean (SD) | 75.97 (4.77) | 75.52 (4.64) | 75.08 (4.44) | 75.11 (3.96) | 76.69 (4.78) | 77.23 (4.45) | 75.30 (4.52) | 76.54 (4.69) |
| Education, N (%) | ||||||||
| No diploma or primary school certificate | 18 (19.35) | 20 (28.17) | 17 (20.99) | 21 (29.17) | 8 (22.86) | 14 (36.84) | 11 (29.73) | 12 (24.49) |
| Secondary education | 35 (37.63) | 19 (26.76) | 27 (33.33) | 16 (22.22) | 14 (40.00) | 15 (39.47) | 15 (40.54) | 17 (34.69) |
| High-school diploma | 16 (17.20) | 8 (11.27) | 17 (20.99) | 12 (16.67) | 4 (11.43) | 2 (5.26) | 2 (5.41) | 8 (16.33) |
| University level | 24 (25.81) | 24 (33.80) | 20 (24.69) | 23 (31.94) | 9 (25.71) | 7 (18.42) | 9 (24.32) | 12 (24.49) |
| APOE ε4 carrier, N (%) | 17 (20.24) | 9 (14.06) | 16 (21.33) | 19 (28.79) | 11 (34.38) | 12 (32.43) | 19 (55.88) | 18 (39.13) |
| DHA (μg/g RBC), mean (SD) | 30.08 (7.97) | 29.70 (9.43) | 32.84 (9.76) | 33.34 (10.48) | 28.63 (9.69) | 32.85 (10.20) | 31.24 (10.15) | 31.72 (9.43) |
| Plasma Aβ42/40, mean (SD) | 0.12 (0.02) | 0.12 (0.01) | 0.12 (0.01) | 0.12 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) | 0.10 (0.01) |
| Cognition | ||||||||
| Cognitive composite score, mean (SD) | 0.01 (0.66) | 0.09 (0.60) | 0.06 (0.68) | 0.13 (0.57) | -0.26 (0.90) | -0.10 (0.68) | -0.21 (0.82) | -0.02 (0.57) |
| MMSE total score/30, mean (SD) | 28.27 (1.58) | 28.18 (1.61) | 27.95 (1.76) | 28.21 (1.48) | 27.86 (1.54) | 27.72 (1.99) | 27.84 (1.50) | 27.50 (1.64) |
| MMSE orientation score/10, mean (SD) | 9.87 (0.42) | 9.88 (0.41) | 9.77 (0.55) | 9.86 (0.39) | 9.57 (0.74) | 9.74 (0.55) | 9.65 (0.68) | 9.82 (0.48) |
| CDR score, N (%) | ||||||||
| CDR = 0 | 63 (67.02) | 38 (52.05) | 40 (48.19) | 41 (56.94) | 16 (45.71) | 19 (48.72) | 19 (51.35) | 20 (40.00) |
| CDR = 0.5 | 31 (32.98) | 35 (47.95) | 43 (51.81) | 31 (43.06) | 19 (54.29) | 20 (51.28) | 18 (48.65) | 30 (60.00) |
| FCSRT scores, mean (SD) | ||||||||
| Free recall/48 | 26.87 (6.76) | 27.51 (6.04) | 27.24 (7.66) | 27.43 (6.22) | 25.20 (7.82) | 27.33 (6.78) | 24.41 (7.76) | 25.92 (6.64) |
| Total recall/48 | 44.38 (4.33) | 45.63 (3.27) | 44.80 (4.76) | 45.35 (3.41) | 43.91 (4.61) | 44.87 (4.37) | 44.57 (4.01) | 44.78 (4.46) |
| Delayed free recall/16 | 10.40 (2.83) | 10.74 (2.72) | 10.47 (3.11) | 10.65 (3.00) | 9.40 (3.94) | 10.21 (3.18) | 9.38 (3.62) | 10.04 (2.88) |
| Delayed total recall/16 | 15.19 (1.53) | 15.42 (1.18) | 15.29 (1.70) | 15.46 (1.06) | 14.74 (1.87) | 15.36 (1.09) | 14.92 (1.46) | 15.28 (1.34) |
| TMT A, mean (SD) | 49.87 (17.93) | 49.51 (22.23) | 46.82 (19.14) | 46.18 (17.73) | 48.14 (19.74) | 47.41 (13.44) | 51.11 (20.93) | 44.10 (10.56) |
| TMT B, mean (SD) | 128.86 (56.53) | 129.40 (72.96) | 114.72 (46.86) | 112.30 (39.45) | 139.94 (104.15) | 134.05 (52.82) | 131.53 (68.08) | 116.32 (40.36) |
| Code test score, mean (SD) | 36.29 (9.58) | 35.92 (10.20) | 37.81 (10.52) | 37.92 (9.32) | 35.40 (9.14) | 34.28 (9.58) | 35.19 (11.58) | 36.28 (7.86) |
| COWAT score, mean (SD) | 19.37 (6.22) | 19.34 (6.90) | 19.47 (6.75) | 20.15 (6.68) | 19.63 (7.50) | 18.72 (5.69) | 18.89 (6.11) | 18.76 (5.91) |
| CNT score, mean (SD) | 24.78 (7.19) | 25.95 (7.27) | 26.20 (7.93) | 26.15 (6.95) | 23.54 (8.40) | 24.31 (7.43) | 24.08 (7.05) | 25.16 (6.72) |
| Other measures | ||||||||
| ADCS-ADL PI /45; mean (SD) | 39.16 (4.63) | 39.03 (5.26) | 39.17 (5.06) | 38.54 (6.05) | 38.68 (4.35) | 39.49 (5.27) | 39.51 (5.42) | 39.72 (4.35) |
| GDS, mean (SD) | 2.92 (2.41) | 3.08 (2.86) | 3.57 (2.94) | 3.01 (2.88) | 2.57 (1.97) | 3.23 (2.56) | 2.81 (2.15) | 3.08 (2.33) |
| SPPB, mean (SD) | 10.50 (1.53) | 10.65 (1.72) | 10.28 (1.74) | 10.59 (1.55) | 10.60 (1.63) | 10.23 (1.31) | 11.11 (1.37) | 10.43 (1.65) |
| 3-year adherence ≥ 75%, N (%) | 51 (54.26) | 58 (84.06) | 40 (48.19) | 59 (89.39) | 19 (55.88) | 30 (93.75) | 19 (52.78) | 38 (82.61) |
ADCS-ADL Alzheimer’s Disease Cooperative Study–activities of daily living, CDR Clinical Dementia Rating score, CNT Category Naming Test, COWAT Controlled Oral Word Association Test, DHA docosahexaenoic acid, FCSRT Free and Cued Selective Reminding Test, GDS Geriatric Depression Scale, MMSE Mini-Mental State Examination, SPPB Short Physical Performance Battery, TMT Trail Making Test
Cognitive impact of MAPT interventions at 12-, 36-, and 60-month visits
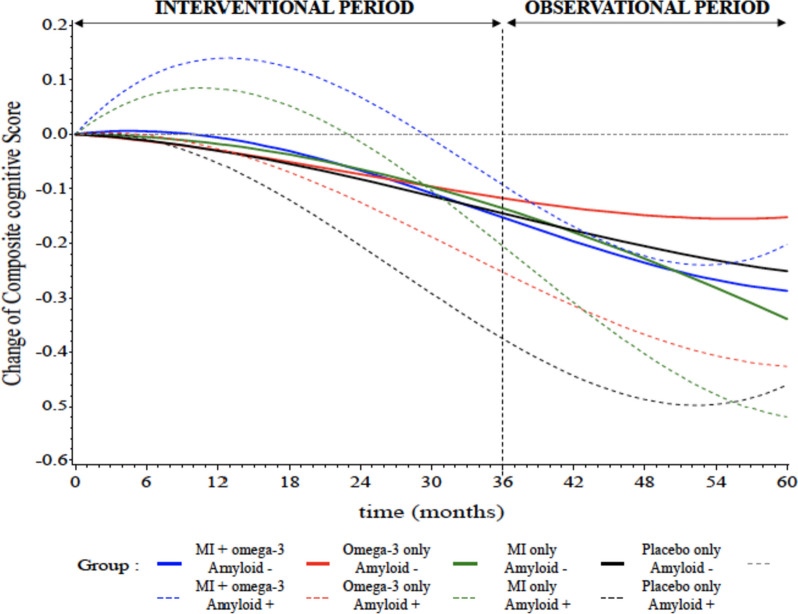
The main results are presented in Fig. 3 and Tables 2, 3, and 4.
Fig. 3.
Mean change from baseline in composite cognitive score over 60 months (intention-to-treat population, n = 483). MI multidomain intervention, Amyloid+ positive amyloid status, Amyloid − negative amyloid status
Table 2.
Estimated mean difference in 1- and 3-year change from baseline on composite Z score for the three intervention groups compared to the placebo group
| Groups | Estimated mean change from baseline (95% CI) | Estimated mean between-group difference in change from baseline (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||||
| vs. placebo | Raw P | Hochberg P | vs. placebo | Raw P | Hochberg P | ||
| 1-year ITT MAPT analysis (n = 483) | |||||||
| Positive plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | 0.1392 (0.0018; 0.2765) | 0.1917 (0.0136; 0.3699) | 0.0350 | 0.1049 | 0.1891 (0.0104; 0.3679) | 0.0381 | 0.1144 |
| Omega 3 alone | -0.0267 (-0.1555; 0.1022) | 0.0259 (-0.1458; 0.1976) | 0.7669 | 0.7669 | 0.0074 (-0.1676; 0.1824) | 0.9338 | 0.9338 |
| Multidomain plus placebo | 0.0835 (-0.0490; 0.2160) | 0.1361 (-0.0383; 0.3106) | 0.1260 | 0.2519 | 0.1113 (-0.0649; 0.2874) | 0.2153 | 0.4306 |
| Placebo | -0.0526 (-0.1661; 0.0609) | - | - | - | - | - | - |
| Negative plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.0059 (-0.0884; 0.0765) | 0.0241 (-0.1007; 0.1488) | 0.7050 | 0.9950 | 0.0199 (-0.1066; 0.1463) | 0.7578 | 0.9306 |
| Omega 3 alone | -0.0296 (-0.1240; 0.0648) | 0.0004 (-0.1325; 0.1334) | 0.9950 | 0.9950 | 0.0135 (-0.1584; 0.1879) | 0.8445 | 0.9306 |
| Multidomain plus placebo | -0.0176 (-0.1058; 0.0707) | 0.0124 (-0.1162; 0.1411) | 0.8494 | 0.9950 | 0.0058 (-0.1244; 0.1360) | 0.9306 | 0.9306 |
| Placebo | -0.0300 (-0.1236; 0.0636) | - | - | - | - | - | - |
| 1-year per-protocol MAPT analysis (n = 457) | |||||||
| Positive plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | 0.1880 (0.0440; 0.3319) | 0.2435 (0.0582; 0.4287) | 0.0101 | 0.0302 | 0.2424 (0.0571; 0.4276) | 0.0104 | 0.0313 |
| Omega 3 alone | -0.0264 (-0.1564; 0.1036) | 0.0291 (-0.1455; 0.2037) | 0.7437 | 0.7437 | 0.0070 (-0.1705; 0.1845) | 0.9383 | 0.9383 |
| Multidomain plus placebo | 0.0846 (-0.0527; 0.2220) | 0.1402 (-0.0400; 0.3203) | 0.1270 | 0.2540 | 0.1132 (-0.0684; 0.2949) | 0.2214 | 0.4428 |
| Placebo | -0.0555 (-0.1721; 0.0611) | - | - | - | - | - | - |
| Negative plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.0022 (-0.0894; 0.0850) | 0.0288 (-0.1002; 0.1578) | 0.6611 | 0.8971 | 0.0229 (-0.1073; 0.1532) | 0.7298 | 0.9902 |
| Omega 3 alone | -0.0399 (-0.1363; 0.0565) | -0.0089 (-0.1443; 0.1265) | 0.8971 | 0.8971 | -0.0009 (-0.1375; 0.1358) | 0.9902 | 0.9902 |
| Multidomain plus placebo | -0.0115 (-0.1046; 0.0816) | 0.0195 (-0.1136; 0.1526) | 0.7735 | 0.8971 | 0.0178 (-0.1165; 0.1522) | 0.7947 | 0.9902 |
| Placebo | -0.0310 (0.1261; 0.0641) | - | - | - | - | - | - |
| 3-year ITT MAPT analysis (n = 483) | |||||||
| Positive plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.0931 (-0.2959; 0.1098) | 0.2818 (0.0190; 0.5446) | 0.0357 | 0.1071 | 0.3030 (0.0420; 0.5640) | 0.0230 | 0.0690 |
| Omega 3 alone | -0.2530 (-0.4443; -0.0616) | 0.1219 (-0.1322; 0.3760) | 0.3461 | 0.3461 | 0.1257 (-0.1305; 0.3820) | 0.3353 | 0.3353 |
| Multidomain plus placebo | -0.2051 (-0.3972; -0.0130) | 0.1698 (-0.0849; 0.4244) | 0.1907 | 0.3461 | 0.1399 (-0.1148; 0.3946) | 0.2808 | 0.3353 |
| Placebo | -0.3749 (-0.5420; -0.2077) | - | - | - | - | - | - |
| Negative plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.1527 (-0.2729; -0.0325) | -0.0075 (-0.1890; 0.1740) | 0.9352 | 0.9352 | -0.0037 (-0.1861; 0.1787) | 0.9684 | 0.9684 |
| Omega 3 alone | -0.1173 (-0.2550; 0.0203) | 0.0278 (-0.1657; 0.2213) | 0.7775 | 0.9352 | 0.0194 (-0.1748; 0.2136) | 0.8443 | 0.9684 |
| Multidomain plus placebo | -0.1356 (-0.2663; -0.0049) | 0.0096 (-0.1791; 0.1982) | 0.9206 | 0.9352 | 0.0180 (-0.1710; 0.2071) | 0.8512 | 0.9684 |
| Placebo | -0.1452 (-0.2812; -0.0091) | - | - | - | - | - | - |
| 3-year per-protocol MAPT analysis (n = 457) | |||||||
| Positive plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.0298 (-0.2415; 0.1820) | 0.3490 (0.0769; 0.6210) | 0.0121 | 0.0362 | 0.3747 (0.1055; 0.6439) | 0.0065 | 0.0195 |
| Omega 3 alone | -0.2534 (-0.4461; -0.0607) | 0.1253 (-0.1322; 0.3828) | 0.3392 | 0.3392 | 0.1254 (-0.1334; 0.3842) | 0.3413 | 0.3413 |
| Multidomain plus placebo | -0.2158 (-0.4142; -0.0173) | 0.1630 (-0.0988; 0.4248) | 0.2217 | 0.3392 | 0.1297 (-0.1317; 0.3911) | 0.3299 | 0.3413 |
| Placebo | -0.3787 (-0.5495; -0.2080) | - | - | - | - | - | - |
| Negative plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.1452 (-0.2725; -0.0179) | 0.0047 (-0.1830; 0.1924) | 0.9605 | 0.9605 | 0.0051 (-0.1827; 0.1930) | 0.9572 | 0.9572 |
| Omega 3 alone | -0.1284 (-0.2683; 0.0116) | 0.0216 (-0.1750; 0.2181) | 0.8294 | 0.9605 | 0.0068 (-0.1897; 0.2033) | 0.9457 | 0.9572 |
| Multidomain plus placebo | -0.1321 (-0.2694; 0.0051) | 0.0178 (-0.1768; 0.2123) | 0.8576 | 0.9605 | 0.0289 (-0.1656; 0.2234) | 0.7703 | 0.9572 |
| Placebo | -0.1499 (-0.2878; -0.0120) | - | - | - | - | - | - |
ITT intention-to-treat, MAPT Multidomain Alzheimer Prevention Trial
aAnalysis adjusted for age, sex, level of education, APOE ε4 genotype, and clinical dementia rating global score
Table 3.
Estimated mean difference in 5-year change from baseline on composite Z score for the three intervention groups compared to the placebo group
| Groups | Estimated mean change from baseline (95% CI) | Estimated mean between-group difference in change from baseline (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||||
| vs. placebo | Raw P | Hochberg P | vs. placebo | Raw P | Hochberg P | ||
| 5-year ITT MAPT-PLUS analysis (n = 483) | |||||||
| Positive plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.2023 (-0.4824; 0.0778) | 0.2575 (-0.1004; 0.6154) | 0.1579 | 0.4737 | 0.2501 (-0.1071; 0.6073) | 0.1691 | 0.5074 |
| Omega 3 alone | -0.4255 (-0.6705; -0.1804) | 0.0343 (-0.2969; 0.3656) | 0.8385 | 0.8385 | -0.0288 (-0.3665; 0.3089) | 0.8666 | 0.8666 |
| Multidomain plus placebo | -0.5209 (-0.7967; -0.2451) | -0.0611 (-0.4157; 0.2935) | 0.7348 | 0.8385 | -0.1582 (-0.5153; 0.1988) | 0.3838 | 0.7676 |
| Placebo | -0.4598 (-0.6827; -0.2369) | - | - | - | - | - | - |
| Negative plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.2870 (-0.4388; -0.1351) | -0.0358 (-0.2674; 0.1959) | 0.7614 | 0.7614 | -0.0237 (-0.2569; 0.2095) | 0.8415 | 0.8415 |
| Omega 3 alone | -0.1524 (-0.3287; 0.0239) | 0.0988 (-0.1495; 0.3471) | 0.4339 | 0.7614 | 0.1008 (-0.1489; 0.3506) | 0.4271 | 0.8415 |
| Multidomain plus placebo | -0.3384 (-0.5079; -0.1690) | -0.0872 (-0.3307; 0.1563) | 0.4812 | 0.7614 | -0.1107 (-0.3563; 0.1348) | 0.3753 | 0.8415 |
| Placebo | -0.2512 (-0.4261; -0.0763) | - | - | - | - | - | - |
| 5-year per-protocol MAPT-PLUS analysis (n = 457) | |||||||
| Positive plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.1315 (-0.4197; 0.1567) | 0.3233 (-0.0453; 0.6919) | 0.0853 | 0.2560 | 0.3202 (-0.0475; 0.6880) | 0.0876 | 0.2628 |
| Omega 3 alone | -0.4254 (-0.6737; -0.1771) | 0.0293 (-0.3090; 0.3677) | 0.8645 | 0.8645 | -0.0423 (-0.3874; 0.3028) | 0.8095 | 0.8095 |
| Multidomain plus placebo | -0.5349 (-0.8177; -0.2522) | -0.0802 (-0.4445; 0.2842) | 0.6652 | 0.8645 | -0.1807 (-0.5482; 0.1868) | 0.3340 | 0.6679 |
| Placebo | -0.4548 (-0.6846; -0.2250) | - | - | - | - | - | - |
| Negative plasma Aβ42/40 | |||||||
| Multidomain plus omega 3 | -0.2920 (-0.4524; -0.1315) | -0.0378 (-0.2773; 0.2016) | 0.7559 | 0.7559 | -0.0277 (-0.2686; 0.2132) | 0.8209 | 0.8209 |
| Omega 3 alone | -0.1613 (-0.3418; 0.0192) | 0.0929 (-0.1605; 0.3462) | 0.4710 | 0.7559 | 0.0825 (-0.1719; 0.3370) | 0.5234 | 0.8209 |
| Multidomain plus placebo | -0.3388 (-0.5183; -0.1593) | -0.0847 (-0.3373; 0.1679) | 0.5098 | 0.7559 | -0.1017 (-0.3570; 0.1536) | 0.4334 | 0.8209 |
| Placebo | -0.2541 (-0.4319; -0.0764) | - | - | - | - | - | - |
ITT Intention-to-treat, MAPT Multidomain Alzheimer Prevention Trial
aAnalysis adjusted for age, sex, level of education, APOE ε4 genotype, and clinical dementia rating global score
Table 4.
Estimated mean difference between positive and negative participants in 1-, 3-, and 5-year change from baseline on composite Z score for each intervention group compared to the control group
| Groups | Estimated difference between positive and negative subjects for each intervention group (95% CI) | P value | ||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| 1-year ITT MAPT analysis (n = 483) | ||||
| Multidomain plus omega 3 | 0.1677 (-0.0498; 0.3852) | 0.1693 (-0.0490; 0.3875) | 0.1305 | 0.1282 |
| Omega 3 alone | 0.0255 (-0.1916; 0.2427) | -0.0060 (-0.2277; 0.2156) | 0.8176 | 0.9573 |
| Multidomain plus placebo | 0.1237 (-0.0931; 0.3404) | 0.1055 (-0.1145; 0.3255) | 0.2629 | 0.3467 |
| 1-year per-protocol MAPT analysis (n = 457) | ||||
| Multidomain plus omega 3 | 0.2147 (-0.0110; 0.4404) | 0.2194 (-0.0061; 0.4450) | 0.0623 | 0.0565 |
| Omega 3 alone | 0.0380 (-0.1830; 0.2590) | 0.0079 (-0.2170; 0.2327) | 0.7356 | 0.9453 |
| Multidomain plus placebo | 0.1207 (-0.1033; 0.3446) | 0.0954 (-0.1311; 0.3219) | 0.2904 | 0.4084 |
| 3-year ITT MAPT analysis (n = 483) | ||||
| Multidomain plus omega 3 | 0.2893 (-0.0301; 0.6087) | 0.3067 (-0.0110; 0.6244) | 0.0757 | 0.0584 |
| Omega 3 alone | 0.0941 (-0.2253; 0.4135) | 0.1063 (-0.2169; 0.4295) | 0.5628 | 0.5181 |
| Multidomain plus placebo | 0.1602 (-0.1567; 0.4771) | 0.1218 (-0.1969; 0.4406) | 0.3209 | 0.4528 |
| 3-year per-protocol MAPT analysis (n = 457) | ||||
| Multidomain plus omega 3 | 0.3442 (0.0137; 0.6747) | 0.3695 (0.0424; 0.6967) | 0.0413 | 0.0269 |
| Omega 3 alone | 0.1038 (-0.2201; 0.4277) | 0.1186 (-0.2078; 0.4450) | 0.5291 | 0.4754 |
| Multidomain plus placebo | 0.1452 (-0.1810; 0.4714) | 0.1008 (-0.2261; 0.4277) | 0.3819 | 0.5447 |
| 5-year ITT MAPT-PLUS analysis (n = 483) | ||||
| Multidomain plus omega 3 | 0.2932 (-0.1331; 0.7196) | 0.2738 (-0.1520; 0.6997) | 0.1768 | 0.2065 |
| Omega 3 alone | -0.0645 (-0.4785; 0.3495) | -0.1297 (-0.5534; 0.2940) | 0.7592 | 0.5471 |
| Multidomain plus placebo | 0.0261 (-0.4040; 0.4563) | -0.0475 (-0.4828; 0.3878) | 0.9049 | 0.8300 |
| 5-year per-protocol MAPT-PLUS analysis (n = 457) | ||||
| Multidomain plus omega 3 | 0.3611 (-0.0784; 0.8006) | 0.3479 (-0.0903; 0.7861) | 0.1069 | 0.1191 |
| Omega 3 alone | -0.0635 (-0.4862; 0.3591) | -0.1248 (-0.5571; 0.3075) | 0.7674 | 0.5701 |
| Multidomain plus placebo | 0.0045 (-0.4389; 0.4478) | -0.0790 (-0.5277; 0.3697) | 0.9841 | 0.7292 |
ITT Intention-to-treat, MAPT Multidomain Alzheimer Prevention Trial
aAnalysis adjusted for age, sex, level of education, APOE ε4 genotype, and clinical dementia rating global score
Positive amyloid group
In the positive amyloid ITT population (n = 161), we observed a positive effect of combined interventions (MI plus omega 3) on the change in composite cognitive score in 12 (raw p = 0.0350, 0.01917, 95% CI = [0.0136 to 0.3699]) and 36 months (raw p = 0.0357, 0.2818, 95% CI = [0.0190 to 0.5446]). After correction of multiple comparisons and adjustments, these differences were not significant (adjusted p = 0.1144 and 0.0690). In the per-protocol population (n = 154), we showed a significant cognitive effect at 12 (adjusted p = 0.0313, 0.2424, 95% CI = [0.0571 to 0.4276]) and 36 months (adjusted p = 0.0195, 0.3747, 95% CI = [0.1055 to 0.6439]) in favor of MI plus omega 3 group that persisted after adjustments and correction of multiple comparisons (Table 2). To assess if the interventional effect was durable after 2-year interruption of the interventional program, we tested at 60 months. In both ITT and per-protocol populations, we did not observe a remaining effect at 60 months between the three interventional (MI plus omega 3, omega 3 alone, MI alone) and placebo groups (Table 3).
Negative amyloid group
In the ITT and per-protocol populations (respectively n = 322 and n = 303), no cognitive difference was observed on cognitive composite score change at 12, 36, and 60 months for any of the three interventional groups in comparison to placebo group.
Comparison of cognitive impact between negative and positive amyloid subjects
In the ITT population, we showed a non-significant trend in the impact of the MI plus omega 3 on the cognitive composite score at 12 and 36 months for the positive amyloid group in comparison to the negative amyloid group (respectively adjusted p = 0.1282/0.0584, 0.1693/0.3067, 95% CI = [−0.0490 to 0.3875]/[−0.0110 to 0.6244]). This difference was significant in the per-protocol population at 36-month visit (adjusted p = 0.0269, 0.3695, 95% CI = [0.0424 to 0.6967]). There was no difference for the three interventional groups on cognitive composite score between the positive and negative amyloid groups at 60-month visit (Table 4).
Discussion
This work suggests a significant benefit of combined interventions at 1 and 3 years only in the amyloid positive group. These effects were significant both in magnitude and statistically in the per protocol population. These findings indicate that future prevention trials could target amyloid positive non-demented individuals for interventions utilizing multi-domains. We have demonstrated the utility of a blood-based biomarker to determine amyloid status of individuals likely to respond to intervention. This could enable future prevention trials to have more rapid screening and to enroll many more positive amyloid participants. The blood-based biomarker also enables prevention trials in regions without access to amyloid PET or CSF analyses. We failed to reach significantly different cognitive effect of a prevention program in non-demented subjects according to amyloid blood status at 5 years, after 2 years off treatment, demonstrating that the intervention effect is not durable after 2-year discontinuation.
Previously, in MAPT-AV45, we showed a cognitive impact of MI at 36 months in subjects with a positive amyloid PET and an association between MI and amyloid burden (lower in participants receiving MI) [4, 27]. Our findings confirm the potential cognitive benefit of non-pharmacological prevention strategies as MI in subjects with early AD. One of the main goals of prevention and precision medicine in AD is to deliver diagnosis and prevention “tailored” to the biological characteristics of cognitive unimpaired individuals [28]. Amyloid PET is proposed to be part of precision medicine [29] but blood-based biomarkers are potentially more cost-efficient and accessible tools in real-world settings and thus could be promising screening exams in a prevention and precision strategy.
Strengths
The strengths of our ancillary study were the long duration of interventional and observational periods. The implementation of an observational period after completion of interventional program allowed to assess long-term cognitive effect and its potential durability. In our knowledge, this work is the first analysis—to date—that assessed cognitive effect of a non-pharmacological intervention considering amyloid status defined by blood-based biomarkers.
Limitations
Our study has several limitations. First, the sample size is limited given that 483 subjects were divided into 8 groups. Second, amyloid blood biomarkers were not performed at the baseline visit, but in 12 (n = 448) and 24 months (n = 35). As in MAPT-AV45 study, we hypothesized that amyloid status does not change during follow-up and the risk of amyloid status misclassification is relatively low marginal in the present analysis [4]. Third, the sensitivity and specificity of the plasma amyloid cutoff (≤ 0.0107) were 43.3% and 79.4% respectively with an area under curve (AUC) of 0.634 in comparison to amyloid PET. This AUC is relatively poor and potentially related to the time interval between blood test and amyloid PET scan. Kappa coefficient was 0.2365 (95% CI = 0.1126–0.3605) between amyloid blood ratio and amyloid PET. Most blood biomarkers were performed at 1-year visit while PET scans were performed all along the MAPT follow-up. Also, it is known that amyloid blood tests become positive about 5 years before amyloid PET scans [9], and this could account for some discrepancy. Another limitation in using blood biomarkers is that the difference in amyloid ratio between positive and negative groups is relatively small (10–15%) potentially due to dilution of Aß from central nervous system to peripheral compartment. Thus, inter-assay variability and accuracy of the measurement may significantly contribute to decrease in AUC. Participants were not blinded to MI. It is possible some of difference between the MI plus omega 3 and placebo was attributable to the fact that participants knew whether or not the MI was given [4, 27]. It is also noted that the analysis of subjects according to amyloid blood status was not pre-specified in the statistical analysis plan and was only exploratory.
Conclusions
Considering the mentioned limitations, these results show a consistent pattern in favor of a MI effect in positive amyloid subjects. A new model of services in dementia prevention may need to be developed and to update the health offer with more efficient access to blood AD biomarkers and prevention program as MI. Blood biomarkers could offer opportunities to screen non-demented subject in future prevention programs and also detect brain amyloidosis in subjects with memory complaint in primary care [28]. Other blood tests could be evaluated to select subjects eligible for prevention programs. Subjects with a positive ptau blood test have also the potential to respond to prevention programs such as MI. These promising results need to be confirmed in others prevention studies prior their use in prevention trials and general practice [30, 31]. Using blood biomarkers as a tool for cognitive interventions may be valuable and this work may help open that door for future trials.
Acknowledgements
MAPT Study Group
Principal investigator: Bruno Vellas (Toulouse); coordination: Sophie Guyonnet; project leader: Isabelle Carrié; CRA: Lauréane Brigitte; investigators: Catherine Faisant, Françoise Lala, Julien Delrieu, Hélène Villars; psychologists: Emeline Combrouze, Carole Badufle, Audrey Zueras; methodology, statistical analysis, and data management: Sandrine Andrieu, Christelle Cantet, Christophe Morin; multidomain group: Gabor Abellan Van Kan, Charlotte Dupuy, Yves Rolland (physical and nutritional components), Céline Caillaud, Pierre-Jean Ousset (cognitive component), Françoise Lala (preventive consultation). The cognitive component was designed in collaboration with Sherry Willis from the University of Seattle, and Sylvie Belleville, Brigitte Gilbert, and Francine Fontaine from the University of Montreal.
Co-investigators in associated centers: Jean-François Dartigues, Isabelle Marcet, Fleur Delva, Alexandra Foubert, Sandrine Cerda (Bordeaux); Marie-Noëlle-Cuffi, Corinne Costes (Castres); Olivier Rouaud, Patrick Manckoundia, Valérie Quipourt, Sophie Marilier, Evelyne Franon (Dijon); Lawrence Bories, Marie-Laure Pader, Marie-France Basset, Bruno Lapoujade, Valérie Faure, Michael Li Yung Tong, Christine Malick-Loiseau, Evelyne Cazaban-Campistron (Foix); Françoise Desclaux, Colette Blatge (Lavaur); Thierry Dantoine, Cécile Laubarie-Mouret, Isabelle Saulnier, Jean-Pierre Clément, Marie-Agnès Picat, Laurence Bernard-Bourzeix, Stéphanie Willebois, Iléana Désormais, Noëlle Cardinaud (Limoges); Marc Bonnefoy, Pierre Livet, Pascale Rebaudet, Claire Gédéon, Catherine Burdet, Flavien Terracol (Lyon), Alain Pesce, Stéphanie Roth, Sylvie Chaillou, Sandrine Louchart (Monaco); Kristel Sudres, Nicolas Lebrun, Nadège Barro-Belaygues (Montauban); Jacques Touchon, Karim Bennys, Audrey Gabelle, Aurélia Romano, Lynda Touati, Cécilia Marelli, Cécile Pays (Montpellier); Philippe Robert, Franck Le Duff, Claire Gervais, Sébastien Gonfrier (Nice); Yannick Gasnier and Serge Bordes, Danièle Begorre, Christian Carpuat, Khaled Khales, Jean-François Lefebvre, Samira Misbah El Idrissi, Pierre Skolil, Jean-Pierre Salles (Tarbes).
MRI group: Carole Dufouil (Bordeaux), Stéphane Lehéricy, Marie Chupin, Jean-François Mangin, Ali Bouhayia (Paris); Michèle Allard (Bordeaux); Frédéric Ricolfi (Dijon); Dominique Dubois (Foix); Marie Paule Bonceour Martel (Limoges); François Cotton (Lyon); Alain Bonafé (Montpellier); Stéphane Chanalet (Nice); Françoise Hugon (Tarbes); Fabrice Bonneville, Christophe Cognard, François Chollet (Toulouse).
PET scans group: Pierre Payoux, Thierry Voisin, Julien Delrieu, Sophie Peiffer, Anne Hitzel, (Toulouse); Michèle Allard (Bordeaux); Michel Zanca (Montpellier); Jacques Monteil (Limoges); Jacques Darcourt (Nice).
Medico-economics group: Laurent Molinier, Hélène Derumeaux, Nadège Costa (Toulouse).
Biological sample collection: Bertrand Perret, Claire Vinel, Sylvie Caspar-Bauguil (Toulouse).
Safety management: Pascale Olivier-Abbal.
DSA group: Sandrine Andrieu, Christelle Cantet, Nicola Coley.
Abbreviations
- AD
Alzheimer disease
- ADCS-ADL
Alzheimer’s disease Cooperative Study-Activities of Daily Living Prevention Instrument
- AUC
Area under curve
- CDR
Clinical Dementia Rating scale
- CNT
Category Naming Test
- CSF
Cerebrospinal fluid
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FCSRT
Free and Cued Selective Reminding Test
- FINGER
Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
- ITT
Intention-to-treat
- MI
Multidomain intervention
- MAPT
Multidomain Alzheimer Prevention Trial
- PACC
Preclinical Alzheimer Cognitive Composite
- PET
Positron emission tomography
- SPPB
Short Physical Performance Battery
Authors’ contributions
JD: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data. BV: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data. SG: Major role in the acquisition of data; Analysis or interpretation of data. CC: Study concept or design; Analysis or interpretation of data, statistical analyses. Statistical Analyses: Christelle Cantet, MS (Toulouse University) performed the statistical analyses of this manuscript. VO: Major role in the acquisition of data; Analysis or interpretation of data. YL: Major role in the acquisition of data; Analysis or interpretation of data. JB: Major role in the acquisition of data; Analysis or interpretation of data. RB: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Analysis or interpretation of data. SA: Drafting/revision of the manuscript for content, including medical writing for content; Major role in the acquisition of data; Study concept or design; Analysis or interpretation of data.
Funding
The MAPT study was supported by grants from the Gérontopôle of Toulouse, the French Ministry of Health (PHRC 2008, 2009), the Pierre Fabre Research Institute (manufacturer of the polyunsaturated fatty acid supplement), Exonhit Therapeutics, and Avid Radiopharmaceuticals. The blood amyloid-beta measures were supported by departmental funds (RJB) and the NIH National Institute on Aging grants R56AG061900 and RF1AG061900 (R.J. Bateman, PI), The promotion of this study was supported by the University Hospital Center of Toulouse. The data sharing activity was supported by the Association Monegasque pour la Recherche sur la maladie d’Alzheimer (AMPA) and the INSERM-University of Toulouse III UMR 1027 Research Unit. We are indebted to the investigators from the University Hospital of Toulouse, Hôpital de Tarbes, Hôpital de Foix, Hôpital de Castres, the University Hospital of Limoges, the University Hospital of Bordeaux, Hôpital de Lavaur, the University Hospital of Montpellier, Hôpital de Montauban, and the University Hospital of Nice for their participation in this study.
The funders had no role in study design; data collection, analysis, or interpretation; or writing of the article.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available. However, clinical and blood biomarkers data can be shared by request via « Application for Access to the MAPT Database» (for further information contact of the Data Sharing Alzheimer group: Info.u1027-dsa@inserm.fr).
Declarations
Ethics approval and consent to participate
MAPT study protocol was approved by the French Ethics Committee in Toulouse (CPP SOOM II) and AFSSAPS (national agency for the safety of drugs and health products). All MAPT participants gave written informed consent at baseline visit. Participants in MAPT-PLUS gave separate written consent for clinical follow-up.
Consent for publication
Not applicable.
Competing interests
JD has received payment/honoraria from Biogen (presentation for Biogen in 2021); and has participated on a Data Safety Monitoring Board or Advisory Board for French board for Roche in 2020–2022. SA has received grants from Europe, Ipsen, and France Alzheimer’s, served as a consultant for Ipsen, Pierre Fabre, Lilly, Nestlé, Sanofi, and Servier, and received non-financial support from Biogen, Nutrition Santé, Pfizer, and Icon, and other forms of support from the AMPA Association. BV receives grants from Pierre Fabre, Avid, Exonhit, AbbVie, Lilly, Lundbeck, MSD, Otsuka, Regeneron, Sanofi, Roche, AstraZeneca, LPG Systems, Nestlé, and Alzheon, and personal fees from Lilly, Lundbeck, MSD, Otsuka, Roche, Sanofi, Biogen, Nestlé, Transition Therapeutics, and Takeda. Washington University and Randall Bateman have equity ownership interest in C2N Diagnostics and receive income based on technology (blood plasma assay) licensed by Washington University to C2N Diagnostics. RJB receives income from C2N Diagnostics for serving on the scientific advisory board. Washington University, with RJB as co-inventor, has submitted the US nonprovisional patent application “Plasma Based Methods for Determining A-Beta Amyloidosis.” RJB has received honoraria as a speaker/consultant/advisory board member from Amgen, AC Immune, Eisai, Hoffman-LaRoche, and Janssen; and reimbursement of travel expenses from AC Immune, Hoffman-La Roche and Janssen. All the other authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Julien Delrieu, Email: delrieu.j@chu-toulouse.fr.
on behalf of MAPT/DSA group:
Isabelle Carrié, Lauréane Brigitte, Catherine Faisant, Françoise Lala, Hélène Villars, Emeline Combrouze, Carole Badufle, Audrey Zueras, Christophe Morin, Gabor Abellan Van Kan, Charlotte Dupuy, Yves Rolland, Céline Caillaud, Pierre-Jean Ousset, Sherry Willis, Sylvie Belleville, Brigitte Gilbert, Francine Fontaine, Jean-François Dartigues, Isabelle Marcet, Fleur Delva, Alexandra Foubert, Sandrine Cerda, Marie-Noëlle-Cuffi, Corinne Costes, Olivier Rouaud, Patrick Manckoundia, Valérie Quipourt, Sophie Marilier, Evelyne Franon, Lawrence Bories, Marie-Laure Pader, Marie-France Basset, Bruno Lapoujade, Valérie Faure, Michael Li Yung Tong, Christine Malick-Loiseau, Evelyne Cazaban-Campistron, Françoise Desclaux, Colette Blatge, Thierry Dantoine, Cécile Laubarie-Mouret, Isabelle Saulnier, Jean-Pierre Clément, Marie-Agnès Picat, Laurence Bernard-Bourzeix, Stéphanie Willebois, Iléana Désormais, Noëlle Cardinaud, Marc Bonnefoy, Pierre Livet, Pascale Rebaudet, Claire Gédéon, Catherine Burdet, Flavien Terracol, Alain Pesce, Stéphanie Roth, Sylvie Chaillou, Sandrine Louchart, Kristel Sudres, Nicolas Lebrun, Nadège Barro-Belaygues, Jacques Touchon, Karim Bennys, Audrey Gabelle, Aurélia Romano, Lynda Touati, Cécilia Marelli, Cécile Pays, Philippe Robert, Franck Le Duff, Claire Gervais, Sébastien Gonfrier, Yannick Gasnier, Serge Bordes, Danièle Begorre, Christian Carpuat, Khaled Khales, Jean-François Lefebvre, Samira Misbah El Idrissi, Pierre Skolil, Jean-Pierre Salles, and Nicola Coley
References
- 1.Vellas B, Carrie I, Gillette-Guyonnet S, Touchon J, Dantoine T, Dartigues JF, et al. MAPT study: a multidomain approach for preventing Alzheimer’s disease: design and baseline data. J Prev Alzheimers Dis. 2014;1(1):13–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 3.Solomon A, Turunen H, Ngandu T, Peltonen M, Levälahti E, Helisalmi S, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75(4):462–470. doi: 10.1001/jamaneurol.2017.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delrieu J, Payoux P, Carrié I, Cantet C, Weiner M, Vellas B, et al. Multidomain intervention and/or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimers Dement. 2019;15(11):1392–1401. doi: 10.1016/j.jalz.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284(6):643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 6.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841–849. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JC, Han SH, Cho HJ, Byun MS, Yi D, Choe YM, et al. Chemically treated plasma Aβ is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Ther. 2017;9(1):20. doi: 10.1186/s13195-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergallo A, Mégret L, Lista S, Cavedo E, Zetterberg H, Blennow K, et al. Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimers Dement. 2019;15(6):764–775. doi: 10.1016/j.jalz.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647–e1659. doi: 10.1212/WNL.0000000000008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 2018;84(5):648–658. doi: 10.1002/ana.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Rojas I, Romero J, Rodríguez-Gomez O, Pesini P, Sanabria A, Pérez-Cordon A, et al. Correlations between plasma and PET beta-amyloid levels in individuals with subjective cognitive decline: the Fundació ACE Healthy Brain Initiative (FACEHBI) Alzheimers Res Ther. 2018;10(1):119. doi: 10.1186/s13195-018-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bateman RJ, Blennow K, Doody R, Hendrix S, Lovestone S, Salloway S, et al. Plasma biomarkers of AD emerging as essential tools for drug development: an EU/US CTAD Task Force report. J Prev Alzheimers Dis. 2019;6(3):169–173. doi: 10.14283/jpad.2019.21. [DOI] [PubMed] [Google Scholar]
- 13.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900–903. doi: 10.1212/WNL.38.6.900. [DOI] [PubMed] [Google Scholar]
- 14.Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg. 1990;90(4):207–17. [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler adult intelligence scale—revised, Psychological Corp. 1981.
- 16.Reitan R. Validity of the Trail Making Test as an indicator of brain damage. Percept Mot Skills. 1958;8:271–6. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–231. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 21.Galasko D, Bennett DA, Sano M, Marson D, Kaye J, Edland SD, et al. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006;20(4 Suppl 3):S152–169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- 22.He L, de Souto Barreto P, Giudici KV, Aggarwal G, Nguyen AD, Morley JE, et al. Cross-sectional and longitudinal associations between plasma neurodegenerative biomarkers and physical performance among community-dwelling older adults. J Gerontol A. 2020. Available from: https://academic.oup.com/biomedgerontology/advance-article/doi/10.1093/gerona/glaa284/5981337. Cited 2020 Dec 2. [DOI] [PubMed]
- 23.Pino LK, Searle BC, Bollinger JG, Nunn B, MacLean B, MacCoss MJ. The Skyline ecosystem: informatics for quantitative mass spectrometry proteomics. Mass Spectrom Rev. 2020;39(3):229–244. doi: 10.1002/mas.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raffin J, Rolland Y, Aggarwal G, Nguyen AD, Morley JE, Li Y, et al. Associations between physical activity, blood-based biomarkers of neurodegeneration, and cognition in healthy older adults: the MAPT study. J Gerontol A Biol Sci Med Sci. 2021;76(8):1382–1390. doi: 10.1093/gerona/glab094. [DOI] [PubMed] [Google Scholar]
- 25.Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71(8):961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhetri JK, de Souto BP, Cantet C, Cesari M, Coley N, Andrieu S, et al. Trajectory of the MAPT-PACC-preclinical Alzheimer cognitive composite in the placebo group of a randomized control trial: results from the MAPT study: lessons for further trials. J Prev Alzheimers Dis. 2018;5(1):31–35. doi: 10.14283/jpad.2017.21. [DOI] [PubMed] [Google Scholar]
- 27.Hooper C, Coley N, De Souto BP, Payoux P, Salabert AS, Andrieu S, et al. Cortical β-amyloid in older adults is associated with multidomain interventions with and without omega 3 polyunsaturated fatty acid supplementation. J Prev Alzheimers Dis. 2020;7(2):128–134. doi: 10.14283/jpad.2020.4. [DOI] [PubMed] [Google Scholar]
- 28.Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018;136(6):821–853. doi: 10.1007/s00401-018-1932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampel H, O’Bryant SE, Durrleman S, Younesi E, Rojkova K, Escott-Price V, et al. A Precision Medicine Initiative for Alzheimer’s disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric. 2017;20(2):107–118. doi: 10.1080/13697137.2017.1287866. [DOI] [PubMed] [Google Scholar]
- 30.Richard E, Van den Heuvel E, Moll van Charante EP, Achthoven L, Vermeulen M, Bindels PJ, et al. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord. 2009;23(3):198–204. doi: 10.1097/WAD.0b013e31819783a4. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from FINGER to World-Wide FINGERS. J Prev Alzheimers Dis. 2020;7(1):29–36. doi: 10.14283/jpad.2019.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during this study are not publicly available. However, clinical data can be shared upon request following completion of the MAPT/DSA Data Access Application form (for further information contact the Data Sharing Alzheimer group: Info.u1027-dsa@inserm.fr).
The datasets generated and/or analyzed during the current study are not publicly available. However, clinical and blood biomarkers data can be shared by request via « Application for Access to the MAPT Database» (for further information contact of the Data Sharing Alzheimer group: Info.u1027-dsa@inserm.fr).