Abstract
Background
The association between sleep-related disorders and inflammation has been demonstrated in previous studies. The systemic immune-inflammation index (SII) is a novel inflammatory index based on leukocytes, but its relationship with sleep-related disorder is unclear. We aimed to investigate the relationship between sleep-related disorder and SII in a nationally representative nonhospitalized sample.
Methods
Data were obtained from the 2005–2008 National Health and Nutrition Examination Survey (NHANES). Exposure variables included self-reported sleep-related disorders, such as sleep duration, sleep problems, high risk of OSA, and daytime sleepiness. SII and other traditional markers of inflammation were considered as outcome variables, including platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR). Multiple linear regression models were employed to examine the correlation between sleep-related disorders and inflammatory markers. Subgroup interactions were analyzed using likelihood ratio tests, and nonlinear relationships were explored by fitting restricted cubic splines.
Results
A total of 8,505 participants were enrolled in this study. Overall, sleep-related disorders were found to have a stronger association with SII compared to the PLR and NLR. The results of multiple linear regression analysis revealed that participants who experienced sleep problems (β: 21.421; 95% CI 1.484, 41.358), had symptoms of OSA (β: 23.088; 95% CI 0.441, 45.735), and reported daytime sleepiness (β: 30.320; 95% CI 5.851, 54.789) exhibited a positive association with higher SII. For the analysis of other inflammatory markers, we only found that daytime sleepiness was associated with increased NLR levels (β: 0.081; 95% CI 0.002, 0.159).
Conclusion
Sleep problems, symptoms of OSA, and daytime sleepiness were found to have a positive association with the SII in US adults. However, further prospective studies are necessary to establish whether there is a causal relationship between these factors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-023-05286-7.
Keywords: Systemic immune-inflammation index, Sleep-related disorder, Obstructive sleep apnoea, Daytime sleepiness, Sleep problems, Sleep duration, NHANES
Introduction
Sleep is an indispensable physiological function, and everyone spends one-third of their lifetime asleep. With the increasing pace of life and social pressure, an increasing number of people are afflicted with sleep disorders. For example, less than half of Americans sleep as long as recommended by the National Sleep Foundation [1], and sleep disorders affect approximately one-third of the adult population [2]. The Global Prevalence and Burden Assessment of Obstructive Sleep Apnea (OSA) states that an estimated 936 million adults worldwide have mild to severe OSA, with the United States ranking second among those affected [3]. A growing body of research indicates that sleep disorders can exert a significant impact on various facets of human health, potentially giving rise to a range of physical ailments stemming from psychological factors [4]. Moreover, these complex psychosomatic disorders are associated with significant economic and social burdens, indicating that they are likely to become a growing global concern in the coming years.
Changes in sleep and circadian rhythms can dynamically adjust the immune system through physiological processes such as immune cell redistribution and inflammatory factor production [5]. Sleep disorders are associated with most diseases and are thought to disrupt physiological processes that regulate the immune system, resulting in abnormally increased inflammatory responses that drive disease progression [6, 7]. In addition, the relationships among the above processes are considered bidirectional [8]. The results of a meta-analysis indicated that sleep disorders and long sleep duration are associated with an increase in systemic inflammatory markers such as C-reactive protein [9]. In addition, a cross-sectional NHANES-based study also consistently observed that blunted rest-activity circadian rhythms were associated with increased leukocyte inflammatory indices derived from routine blood tests [10]. At the same time, Endogenous inflammation and pathogen infection disrupt normal sleep patterns by transmitting cytokines across the blood–brain barrier to the central nervous system through neuromodulation and humoral mediators [5]. For example, the production of inflammatory cytokines by TLR4-stimulated monocytes at bedtime has been found to predict the sleep efficiency and duration of slow-wave sleep in patients with rheumatoid arthritis [11]. Therefore, it is imperative to clarify the effects of changes in sleep behavior and the risk of sleep disorders on systemic inflammatory status. This will help to establish the connection between sleep and other inflammatory diseases, and enable the monitoring of healthy sleep patterns to enhance overall health.
The systemic immune-inflammation index (SII) is an inflammatory marker first proposed by Hu et al. that can be obtained based on blood leukocyte parameters [12]. The SII combines the combined manifestations of three types of inflammatory cells, including platelets, neutrophils and lymphocytes, which can reflect the systemic immune response and inflammatory level while having inexpensive, stable and effective characteristics [13]. Increasing evidence suggests that SII, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are markers of psychiatric disease severity derived from peripheral blood cell counts [14, 15]. Investigations into the psychopathological status of survivors of COVID-19 have revealed that long-standing systemic inflammatory status can be reflected by inflammatory parameters such as SII and is closely related to anxiety and somatization and sleep disorders [16, 17]. However, another study only reported higher SII levels in long-COVID patients, but no significant association was found with psychopathological items such as sleep disorders [18]. In a recent retrospective cohort study, Topuz et al. first reported that the SII was strongly associated with OSA severity and performed better than the NLR and PLR [19]. As far as we know, the study of sleep and SII is still in its infancy, and relationship between the sleep duration and sleep disorders and SII has not been clarified.
Inspired by the background of previous studies, the aim of our study was to explore the relationship between sleep-related disorder and SII through a nationally representative complex sampling sample from the National Health and Nutrition Examination Survey (NHANES) with performance of additional analyses of the traditional inflammatory indicators NLR and PLR.
Materials and methods
Study population
The NHANES provides information about the health and nutritional status of noninstitutionalized civilians in the United States, based on a large nationwide cross-sectional survey. NHANES uses a complex, multistage probability sampling approach to obtain a representative sample of individual composition to investigate the overall national population. In this study, we integrated data from the 2005–2006 and 2007–2008 surveys because these two cycles contained the most comprehensive sleep disorder questionnaires available to date. In a standard procedure, participants completed home interviews and subsequently underwent physical examinations and biochemical sample collections at a mobile examination center (MEC). The NHANES survey project was approved by the National Center for Health Statistics Institutional Review Board and Ethics Review Board, and all participants provided written informed consent. This study was drafted according to the Strengthening Reporting of Observational Studies in Epidemiology (STROBE) Guidelines for Reporting Cross-sectional Studies [20].
Exclusion criteria for participants we analyzed were (i) age < 20 years, (ii) missing or incomplete data on complete blood count and sleep questionnaires, (iii) having cancer, and (iv) being pregnant. In 2005–2008, 2 survey cycles were completed, and a total of 20,497 individuals were first enrolled; 8,505 participants were included in our final analysis after exclusion of subjects aged < 20 years (9,583), missing complete blood count (1,085) and sleep questionnaire data (116), having cancer (864), and being pregnant (344) (Fig. 1).
Fig. 1.
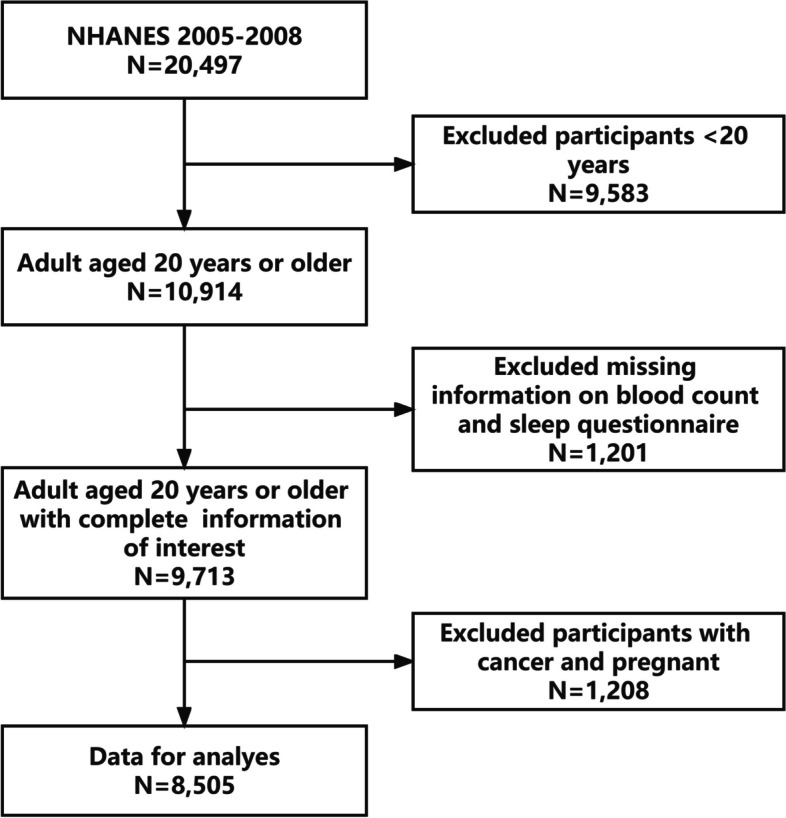
Flowchart of the participants selection from NHANES 2005–2008. NHANES, National Health and Nutrition Examination Survey
Definition of the sleep-related disorder
Questions on sleep are new to NHANES in 2005–2008. Adapted from the Sleep Habits Questionnaire for the Sleep Heart Health Study, this section contains questions about sleep habits and disorders [21]. We conducted a study on sleep-related disorders and examined several exposure variables, including sleep duration, sleep problems, high risk of OSA, and daytime sleepiness.
Sleep duration was analyzed separately as categorical outcome variables. Sleep duration < 7 h/night: insufficient sleep, 7–9 h/night: normal sleep, or > 9 h/night: excessive sleep [22]. Having sleep problems [23] was defined as having self-reported frequency greater than or equal to 5 times per month for the following questions: “How often do you wake up during night/have trouble falling asleep/wake up too early in morning?”; or affirmative answers to the following questions: “Have you ever been told by a doctor or other health professional that you have a sleep disorder or trouble sleeping?”. Daytime sleepiness [23] was also defined according to self-report and was considered to be present if the response frequency was more than or equal to 5 times per month when answering the following questions: “How often did you feel excessively sleepy/feel unrested during the day, no matter how much sleep you get?”.
High-risk for OSA was defined according to the STOP screening questionnaire for OSA [24]. This questionnaire defined high-risk for OSA as having two or more of the four symptoms recommended by the American Academy of Sleep Medicine for OSA screening, including hypertension, snoring, cessation of breathing, and daytime sleepiness [25]. To achieve this classification, snoring and cessation of breathing are obtained by answering “5 or more nights/week” to any of the following questions from the NHANES Sleep Disorders Questionnaire: “How often did you stop breathing, gasp, or snore while you slept in the past year?”. In addition to the previously described method, we directly classified participants who were definitively diagnosed with sleep apnea by a physician as being at high-risk for OSA.
Definition of the systemic immune-inflammation index
Lymphocyte, neutrophil and platelet counts were obtained by performing a complete blood count on blood specimens with a Beckman Coulter automated blood analyzer in an MEC and were expressed as × 103 cells/µL. According to previous studies [12, 26], SII was defined as follows: SII = Platelet count*Neutrophil count/Lymphocyte count. To more fully assess the association between sleep-related disorders and SII, we further assessed PLR and NLR, which are commonly used blood inflammatory markers in clinical studies. In this study, SII, PLR and NLR were designed as outcome variables.
Covariates
According to previous studies [23, 27], we included covariates in the analysis given the confounding of study results by other factors. We included the following covariates based on NHANES interview and examination, laboratory and questionnaire data: age, sex, race, education level, poverty-income ratio (PIR), body mass index (BMI), smoking status, alcohol consumption status, recreational activities, hypertension, diabetes, cardiovascular disease (CVD) and taking immunosuppressants. Socioeconomic factors were assessed and collected during the home interview. Based on the original survey records, PIR was categorized into three groups: < 1.3, 1.3 – 3.5, and > 3.5. It was observed that lower PIR was linked to higher levels of poverty. Behavioral factors were obtained through self-reports. A never smoker is an individual who has never smoked more than 100 cigarettes in their lifetime. Former smokers are defined as those who have smoked more than 100 cigarettes in their lifetime but have since quit smoking. Current smokers are individuals who have smoked at least 100 cigarettes in their lifetime and continue to smoke on some days or every day. Alcohol consumption was categorized as: Never drinkers: had < 12 drinks in lifetime. Former drinkers: had ≥ 12 drinks in 1 year and did not drink last year, or did not drink last year but drank ≥ 12 drinks in lifetime. Current mild-moderate drinkers: ≤ 1 drink per day for women or ≤ 2 drinks per day for men on average over the past year. Current heavier drinkers: > 1 drink per day for women or > 2 drinks per day for men on average over the past year. Recreational activity was defined based on individuals' self-reported levels of activity over a period of 30 days. BMI was measured at an MEC using standardized protocols and categorized into two groups: ≥ 30 (obese) and < 30 (non-obese). Participants who self-reported a medical diagnosis of heart failure, angina, coronary heart disease, heart attack, or stroke, as confirmed by a physician, were categorized as having CVD. The definition of hypertension is a diagnosis made by a healthcare professional, based on an average blood pressure reading of ≥ 130/80 mmHg or the use of hypertension medications. Diabetes was defined as a diagnosis made by a physician or other healthcare professional, glycated hemoglobin (%) greater than 6.5, random blood glucose (mmol/L) equal to or greater than 11.1, or use of diabetes medication or insulin. Obtaining information about the participant's use of immunosuppressants was done through a home interview conducted by the staff. They identified the participant's prescription medication sheet and kit information. Detailed definitions of covariates and remaining information are provided in Supplementary Table 1.
Statistical analysis
Descriptive statistics were calculated to describe the characteristics of the participants according to the classification of SII quartiles. Continuous variables are presented as the weighted mean ± standard deviation (SD), while categorical variables are presented as weighted percentages (95% confidence interval, 95% CI) and were compared using one-way ANOVA and the Rao-Scott chi-square test, respectively. Multiple linear regression was employed to assess the relationship between various inflammatory parameters as dependent variables and sleep-related disorders as independent variables. Beta coefficients and 95% CIs were calculated. Model 1 was crude (not adjusted). Model 2 was adjusted for age, sex, and race. Model 3 was adjusted for age, sex, race, education level, PIR, BMI, smoking status, alcohol consumption status, recreational activities, hypertension, diabetes, CVD and taking immunosuppressants. At the same time, subgroup analyses within fully adjusted models were performed stratified by age (< 60/ ≥ 60 years), sex (male/female), and race (non-Hispanic white/non-Hispanic black/other), and multiplicative interactions were assessed using likelihood ratio tests. Restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles were used to explore the nonlinear relationships of sleep exposure variables and inflammatory indicator outcomes in the fully adjusted model.
Because it is a complex sampling design for the national population, we considered 2-year MEC weights (WTMEC2YR), sampling units (SDMVPSU) and strata (SDMVSTRA) in all analyses [28]. In this case, new 4-year weights could be calculated by dividing the 2-year weights by two.
We used the MissForest package [29] to impute missing values for covariates, whether continuous or categorical. The number and percentage of missing covariate data are shown in Supplementary Table 2. Sensitivity analyses were performed as follows: (1) included only participants with complete data and excluded those with missing covariates; (2) excluded individuals who had recently experienced symptoms of infection, such as head or chest colds, gastric or intestinal diseases accompanied by vomiting or diarrhea, influenza, pneumonia, or ear infections; and (3) Furthermore, we adjusted for OSA symptoms when examining the relationship between daytime sleepiness and inflammatory markers. Statistical analysis was conducted using R software (version 4.1.3; https://www.R-project.org) with a complex sampling module. All statistical analysis P values were from two-sided tests, and the results were deemed statistically significant at P values less than 0.05.
Results
Participant characteristics
A total of 8,505 samples were included in this study, weighted to represent 180 million U.S. noninstitutionalized adult populations. The baseline participant characteristics are listed in Table 1.The mean age of the population surveyed was 45.74 ± 0.39 years, while 50.50% (95% CI: 46.08%, 54.91%) were female and 70.13% (95% CI: 60.31%, 79.96%) were non-Hispanic White. For the distribution of sleep duration of participants, 37.48% (95% CI: 34.51%, 40.45%) slept less than 7 h, and 2.17% (95% CI: 1.70%, 2.63%) slept more than 9 h. Moreover, For sleep-related disorder as defined in this study, sleep problems, high-risk for OSA and daytime sleepiness accounted for 41.40% (95% CI: 37.20%, 45.60%), 43.16% (95% CI: 38.97%, 47.34%) and 29.13 (95% CI: 26.27%, 32.00%) of the population, respectively.
Table 1.
General characteristics of included participants (n = 8,505) according to systemic immune-inflammation index quartiles in the NHANES 2005–2008
| Characters | Overall (n = 8,505) | Q1 (n = 2,127) (13.750, 364.219) |
Q2 (n = 2,124) (364.219, 506.647) |
Q3 (n = 2,127) (506.647, 708.923) |
Q4 (n = 2,127) (708.923, 5137.500) |
P-value |
|---|---|---|---|---|---|---|
| Age, year | 45.74 ± 0.39 | 44.69 ± 0.57 | 45.72 ± 0.41 | 45.88 ± 0.44 | 46.48 ± 0.66 | 0.031 |
| Sex | < 0.0001 | |||||
| Male | 49.50(45.43–53.58) | 58.03(55.96–60.10) | 49.63(46.76–52.50) | 50.40(48.30–52.50) | 41.39(39.35–43.42) | |
| Female | 50.50(46.08–54.91) | 41.97(39.90–44.04) | 50.37(47.50–53.24) | 49.60(47.50–51.70) | 58.61(56.58–60.65) | |
| Race | < 0.0001 | |||||
| Mexican American | 8.62( 6.96–10.29) | 8.76(6.88–10.64) | 8.40(6.52–10.28) | 8.94(6.78–11.11) | 8.40(6.17–10.63) | |
| Non-Hispanic Black | 11.15( 8.98–13.32) | 19.82(15.28–24.36) | 10.05( 7.35–12.74) | 8.63( 6.40–10.85) | 7.51( 5.49–9.52) | |
| Non-Hispanic White | 70.13(60.31–79.96) | 60.42(54.42–66.41) | 71.16(66.59–75.72) | 72.44(68.17–76.71) | 74.96(70.39–79.52) | |
| Other Hispanic | 4.33( 2.90–5.76) | 4.27(2.83–5.70) | 4.60(2.76–6.43) | 4.49(2.84–6.14) | 3.96(2.50–5.41) | |
| Other race | 5.76( 4.69–6.84) | 6.74(4.78–8.69) | 5.80(4.15–7.44) | 5.50(3.84–7.16) | 5.18(3.64–6.72) | |
| Education | 0.352 | |||||
| Less than hight school | 18.86(16.39–21.32) | 19.85(17.13–22.56) | 18.74(16.22–21.26) | 18.66(15.79–21.54) | 18.34(15.44–21.24) | |
| Hight school | 24.98(22.20–27.76) | 22.77(19.49–26.04) | 24.30(22.15–26.45) | 25.39(22.90–27.87) | 27.07(25.12–29.02) | |
| Above hight school | 56.17(50.81–61.52) | 57.39(53.74–61.03) | 56.96(53.71–60.21) | 55.95(51.74–60.16) | 54.59(50.75–58.44) | |
| Poverty-income ratio | 0.016 | |||||
| < 1.3 | 17.97(16.06–19.89) | 19.12(16.58–21.65) | 15.96(14.28–17.65) | 17.15(14.73–19.57) | 19.78(17.24–22.31) | |
| 1.3–3.5 | 38.34(35.03–41.65) | 40.24(36.59–43.89) | 37.37(34.35–40.38) | 37.01(33.65–40.37) | 41.21(37.21–45.21) | |
| > 3.5 | 43.69(38.03–49.35) | 40.64(36.11–45.17) | 46.67(43.14–50.20) | 45.84(41.06–50.62) | 41.21(37.21–45.21) | |
| BMI (kg/m2) | < 0.0001 | |||||
| < 30 | 65.95(60.79–71.11) | 72.39(69.81–74.97) | 66.89(64.16–69.61) | 63.74(61.22–66.27) | 61.87(58.57–65.18) | |
| ≥ 30 | 34.05(30.22–37.87) | 27.61(25.03–30.19) | 33.11(30.39–35.84) | 36.26(33.73–38.78) | 38.13(34.82–41.43) | |
| Smoking status | 0.045 | |||||
| Now | 24.03(21.22–26.83) | 21.64(19.03–24.26) | 21.70(18.85–24.56) | 25.24(22.74–27.75) | 27.06(23.90–30.21) | |
| Former | 23.57(21.23–25.91) | 24.50(22.13–26.86) | 23.78(21.65–25.91) | 23.15(20.86–25.44) | 23.01(20.77–25.26) | |
| Never | 52.40(47.82–56.98) | 53.86(51.31–56.41) | 54.52(51.75–57.29) | 51.61(48.34–54.88) | 49.93(45.69–54.17) | |
| Alcohol consumption status | 0.472 | |||||
| Never | 11.90(10.39–13.40) | 12.85(11.05–14.65) | 11.54( 9.12–13.96) | 11.87( 9.76–13.98) | 11.48( 9.74–13.21) | |
| Former | 16.55(14.08–19.02) | 16.25(14.30–18.21) | 16.95(14.34–19.56) | 16.72(14.26–19.18) | 16.24(14.16–18.33) | |
| Mild-Moderate | 33.18(29.79–36.56) | 33.50(30.71–36.30) | 34.28(31.48–37.07) | 33.78(30.94–36.61) | 31.25(28.46–34.05) | |
| Heavy | 38.38(34.64–42.11) | 37.39(34.19–40.60) | 37.24(34.02–40.46) | 37.63(34.64–40.63) | 41.03(38.32–43.73) | |
| Recreational activitie | < 0.0001 | |||||
| Vigorous | 31.45(28.19–34.70) | 36.95(33.10–40.80) | 33.15(30.07–36.23) | 30.27(27.78–32.76) | 26.37(23.10–29.65) | |
| Moderate | 28.03(25.11–30.95) | 26.16(23.62–28.69) | 28.93(26.47–31.39) | 28.72(25.65–31.79) | 28.05(25.51–30.59) | |
| Inactive | 40.52(35.49–45.55) | 36.89(32.90–40.88) | 37.92(34.09–41.74) | 41.01(37.74–44.28) | 45.58(40.99–50.16) | |
| CVD | 7.70( 6.55–8.85) | 8.19(7.06–9.32) | 6.25(5.15–7.35) | 7.26(5.75–8.76) | 9.14(7.60–10.69) | 0.011 |
| Hypertension | 46.70(42.31–51.10) | 44.11(40.71–47.52) | 44.78(41.99–47.56) | 48.31(45.69–50.93) | 49.12(46.74–51.51) | 0.028 |
| Diabetes | 9.95( 8.79–11.11) | 10.95(9.23–12.66) | 8.55(7.26–9.84) | 9.89(8.63–11.16) | 10.53(8.74–12.33) | 0.098 |
| Taking immunosuppressants | 0.71( 0.47–0.95) | 0.86(0.29–1.43) | 0.44(0.08–0.80) | 0.39(0.07–0.72) | 1.17(0.55–1.78) | 0.079 |
| Sleep duration | 0.301 | |||||
| < 7 h/night | 37.48(34.51–40.45) | 39.85(36.30–43.40) | 36.92(33.85–39.98) | 36.07(33.47–38.67) | 37.45(34.76–40.14) | |
| 7–9 h/night | 60.35(54.37–66.33) | 58.35(54.95–61.75) | 61.15(58.02–64.29) | 61.54(58.75–64.32) | 60.08(57.48–62.67) | |
| > 9 h/night | 2.17( 1.70–2.63) | 1.80(1.10–2.51) | 1.93(1.34–2.52) | 2.39(1.62–3.16) | 2.48(1.88–3.07) | |
| Sleep problems | 41.40(37.20–45.60) | 37.77(35.06–40.47) | 40.47(37.92–43.01) | 40.23(38.12–42.33) | 46.51(43.94–49.09) | < 0.001 |
| OSA symptoms | 43.16(38.97–47.34) | 38.83(36.70–40.97) | 40.62(37.77–43.47) | 45.32(43.01–47.63) | 47.05(44.28–49.82) | < 0.0001 |
| Daytime sleepiness | 29.13(26.27–32.00) | 26.48(24.29–28.68) | 27.44(25.21–29.67) | 28.09(26.02–30.16) | 34.01(31.42–36.60) | < 0.0001 |
Abbreviations: BMI Body mass index, CVD Cardiovascular Disease, OSA Obstructive sleep apnea
Continuous variables are presented as the weighted mean ± standard deviation, and were compared using the weighted one-way ANOVA test
Categorical variables are presented as weighted percentages (95% confidence interval), and were compared using the weighted Rao-Scott chi-square test
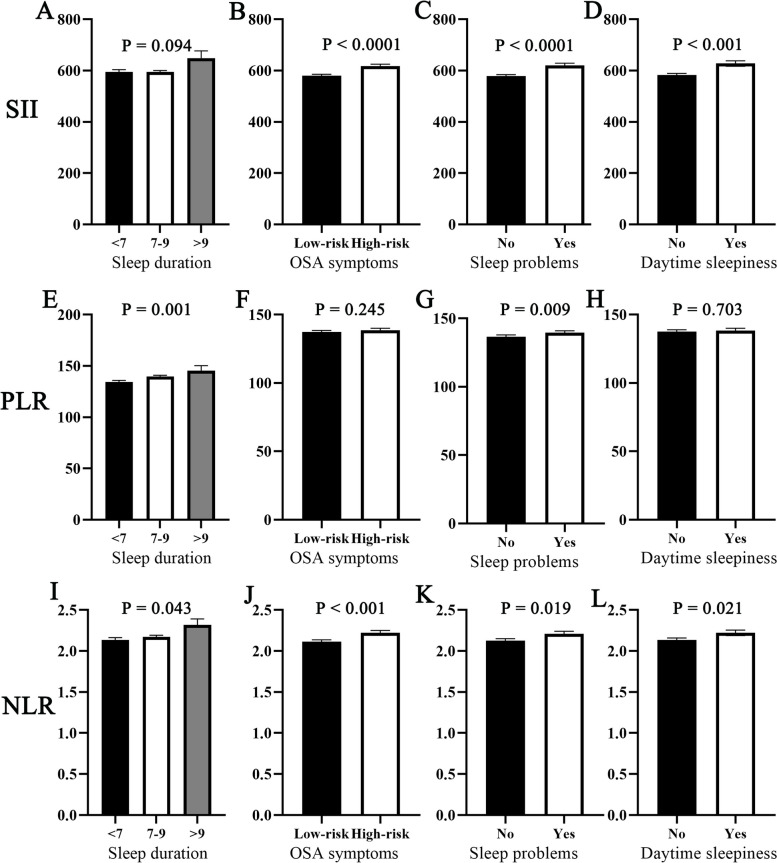
According to the SII quartiles, we observed differences in age, sex, race, PIR, BMI, smoking status, recreational activities, hypertension, CVD, sleep problems, high-risk for OSA and daytime sleepiness. It is crucial to highlight that the percentage of individuals living in poverty has risen within both the highest and lowest SII categories. At the same time, Fig. 2 demonstrates differences in SII, PLR and NLR inflammatory markers among different sleep-related exposure status groups. We found that the SII differed among the sleep problem, high-risk for OSA, and daytime sleepiness groups. We obtained similar results in NLR but differed from the former in sleep duration. Interestingly, the results of PLR were unique and differed only between the sleep duration and sleep problem groups.
Fig. 2.
Differences in SII, PLR and NLR inflammatory markers among different sleep-related disorder exposures. A SII and sleep duration; (B) SII and OSA symptoms; (C) SII and sleep problems; (D) SII and daytime sleepiness; (E) PLR and sleep duration; (F) PLR and OSA symptoms; (G) PLR and sleep problems; (H) PLR and daytime sleepiness; (I) NLR and sleep duration; (J) NLR and OSA symptoms; (K) NLR and sleep problems; (L)NLR and daytime sleepiness. P value was calculated by weighted T-test or one-way ANOVA test. SII, systemic immune-inflammation index; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio. OSA, obstructive sleep apnea
Association between sleep duration and SII
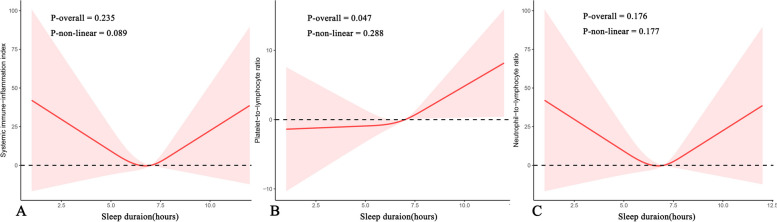
Table 2 presents the relationships between the sleep duration classification and SII and the other inflammatory markers. Compared to normal sleep duration, no association was found between both insufficient sleep and excessive sleep and SII. In further analyses of PLR and NLR, linear regression results revealed a negative association between insufficient sleep and PLR, and a positive association between excessive sleep and NLR. However, it is important to note that these associations disappeared in fully adjusted models. By applying a cubic spline curve, we attempted to explore a potential U-shaped association between sleep duration and SII and NLR. However, after conducting significance testing, it was determined that there is no significant relationship between the two variables. In addition, it was found that sleep duration had a linear association with PLR. The Fig. 3 illustrates the specific results.
Table 2.
Weighted linear regression coefficients (β) and 95% confidence intervals for the association between sleep duration and SII and inflammatory markers: The United States, 2005 to 2008
| Exposure | Outcome | Categories | Model 1a β (95% CI), P- value |
Model 2b β (95% CI), P- value |
Model 3c β (95% CI), P- value |
|---|---|---|---|---|---|
| Sleep duration | SII | 7–9 h/night | Reference | Reference | Reference |
| < 7 h/night |
0.038(-16.479, 16.555) P = 0.996 |
12.165(-4.594, 28.925) P = 0.147 |
1.229(-17.940, 20.399) P = 0.884 |
||
| > 9 h/night |
54.748(-0.861,110.357) P = 0.053 |
52.542(-0.858,105.941) P = 0.053 |
35.656(-29.280,100.592) P = 0.235 |
||
| PLR | 7–9 h/night | Reference | Reference | Reference | |
| < 7 h/night |
-5.311(-8.397,-2.224) P = 0.001 |
-4.848(-8.074,-1.622) P = 0.005 |
-3.227(-6.918, 0.465) P = 0.078 |
||
| > 9 h/night |
5.694(-3.956,15.344) P = 0.237 |
3.817(-6.155,13.788) P = 0.437 |
5.426(-6.092, 16.944) P = 0.302 |
||
| NLR | 7–9 h/night | Reference | Reference | Reference | |
| < 7 h/night |
-0.038(-0.085,0.010) P = 0.115 |
0.003(-0.045,0.050) P = 0.913 |
-0.024(-0.077, 0.029) P = 0.321 |
||
| > 9 h/night |
0.145(0.003,0.288) P = 0.046 |
0.161(0.028,0.295) P = 0.020 |
0.089(-0.072, 0.251) P = 0.232 |
Abbreviation: CI Confidence interval, SII Systemic immune-inflammation index, PLR Platelet-to-lymphocyte ratio, NLR Neutrophil-to-lymphocyte ratio
Bold fonts indicate P value < 0.05
aModel 1: Unadjusted
bModel 2: Adjusted for age, sex, and race
cModel 3: Adjusted for age, sex, race, education level, PIR, BMI, smoking status, alcohol consumption status, recreational activities, hypertension, diabetes, CVD and taking immunosuppressants
Fig. 3.
Nonlinear relationship between sleep duration and leukocyte inflammatory markers by restricted cubic spline fitting. A sleep duration and systemic immune-inflammation index; (B) sleep duration and platelet-to-lymphocyte ratio; (C) sleep duration and neutrophil-to-lymphocyte ratio. Adjusted for age, sex, race, education level, PIR, BMI, smoking status, alcohol consumption status, recreational activities, hypertension, diabetes, CVD and taking immunosuppressants
Association between sleep-related disorder and SII
The relationships between SII, PLR and NLR and sleep-related disorder were further analyzed by linear regression (Table 3). Overall, sleep-related disorders were found to be more closely associated with SII than the PLR and NLR. Participants had a high prevalence of sleep problems (β: 21.421; 95% CI 1.484, 41.358), OSA symptoms (β: 23.088; 95% CI 0.441, 45.735), and daytime sleepiness (β: 30.320; 95% CI 5.851, 54.789) showed a positive association with higher SII in fully adjusted linear models. In additional analyses of other inflammatory measures, we did not find any association between sleep problems, OSA symptoms, daytime sleepiness, and PLR in this study. However, it is worth mentioning that we did observe higher NLR in participants with daytime sleepiness (β: 0.081; 95% CI 0.002, 0.159).
Table 3.
Weighted linear regression coefficients (β) and 95% confidence intervals for the association between sleep-related disorder and SII and inflammatory markers: The United States, 2005 to 2008
| Exposure | Outcome | Categories | Model 1a β (95% CI), P- value |
Model 2b β (95% CI), P- value |
Model 3c β (95% CI), P- value |
|---|---|---|---|---|---|
| Sleep problems | SII | No | Reference | Reference | Reference |
| Yes |
42.557(24.671,60.444) P < 0.0001 |
30.607(12.271, 48.942) P = 0.002 |
21.421(1.484, 41.358) P = 0.038 |
||
| PLR | No | Reference | Reference | Reference | |
| Yes |
2.967(0.801,5.134) P = 0.009 |
0.545(-1.620, 2.709) P = 0.608 |
1.776(-0.485, 4.038) P = 0.108 |
||
| NLR | No | Reference | Reference | Reference | |
| Yes |
0.083(0.015,0.151) P = 0.019 |
0.050(-0.018,0.118) P = 0.143 |
0.027(-0.047, 0.102) P = 0.421 |
||
| OSA symptoms | SII | No | Reference | Reference | Reference |
| Yes |
36.329(20.009,52.650) P < 0.0001 |
38.260(20.259, 56.261) P < 0.001 |
23.088(0.441, 45.735) P = 0.046 |
||
| PLR | No | Reference | Reference | Reference | |
| Yes |
1.115(-1.208,3.439) P = 0.335 |
0.168(-2.129, 2.464) P = 0.881 |
0.427(-1.894, 2.747) P = 0.707 |
||
| NLR | No | Reference | Reference | Reference | |
| Yes |
0.105(0.047,0.163) P < 0.001 |
0.054(-0.002,0.111) P = 0.058 |
0.033(-0.050, 0.116) P = 0.382 |
||
| Daytime sleepiness | SII | No | Reference | Reference | Reference |
| Yes |
45.143(21.344,68.943) P < 0.001 |
39.479(17.142, 61.816) P = 0.001 |
30.320(5.851, 54.789) P = 0.021 |
||
| PLR | No | Reference | Reference | Reference | |
| Yes |
0.643(-2.772,4.058) P = 0.703 |
0.016(-3.183, 3.216) P = 0.992 |
1.258(-2.300, 4.816) P = 0.439 |
||
| NLR | No | Reference | Reference | Reference | |
| Yes |
0.086(0.014,0.157) P = 0.021 |
0.107(0.035,0.178) P = 0.005 |
0.081(0.002, 0.159) P = 0.046 |
Abbreviation: CI, Confidence interval, SII Systemic immune-inflammation index, PLR Platelet-to-lymphocyte ratio, NLR Neutrophil-to-lymphocyte ratio, OSA Obstructive sleep apnea
Bold fonts indicate P value < 0.05
aModel 1: Unadjusted
bModel 2: Adjusted for age, sex, and race
cModel 3: Adjusted for age, sex, race, education level, PIR, BMI, smoking status, alcohol consumption status, recreational activities, hypertension, diabetes, CVD and taking immunosuppressants
Subgroup and sensitivity analyses
Supplementary Tables 3 and 4 presents the results of our subgroup analyses. The likelihood ratio test for the interactions among the remaining results was not statistically significant, and the main analysis results can be considered stable. Supplementary Tables 5, 6, and 7 display the outcomes of our sensitivity analyses, which consistently yielded similar results when we excluded participants with missing covariates and recent infections. Recognizing that OSA could potentially influence the relationship between inflammation and daytime sleepiness, we conducted additional adjustments, and the findings remained consistent with the primary analysis. In the fully adjusted model, a positive association between daytime sleepiness and higher SII levels in participants was observed (β: 30.320; 95% CI 5.851, 54.789). This association remained significant even after further adjustment for OSA symptoms (β: 26.365; 95% CI 1.801, 50.930), indicating that the results of the model were robust.
Discussion
To our knowledge, this is the first cross-sectional study to demonstrate that the sleep-related disorders are strongly associated with SII in a nationally representative sample. In this study of 8,505 survey participants, we discovered that sleep problems, a high risk of OSA, and daytime sleepiness were all linked to higher levels of SII, which persisted after adjusting for numerous confounding covariates. Associations were similar across subgroups of subjects defined by age, sex, and race/ethnicity. At the same time, comparable correlations were shown in sensitivity analyses excluded participants with missing covariates and recent infections. Overall, sleep-related disorders were found to have a stronger association with SII compared to PLR and NLR. Additionally, in our analysis of other inflammatory factors, we only observed a positive association between daytime sleepiness and NLR.
To date, the literature on sleep and SII is sparse and confined to special study populations, thus the results of our study complements the literature well. A retrospective cohort study conducted by Topuz's team found a positive association between the SII and OSA prevalence, which increased with disease severity. In addition, that association was stronger than that of the association with NLR and PLR [19]. At the same time, in a prospective study of psychopathology and neurocognitive impairment in COVID-19 survivors, baseline SII levels predicted depressive symptoms such as insomnia and anxiety at the 3-month follow-up [17]. Another study also supports the notion that the systemic inflammatory response, as reflected by the baseline SII, is positively correlated with depression and anxiety scores at follow-up [16]. Recent studies provide additional evidence to support the aforementioned conclusion that inflammatory markers may act as a mediator in the potential influence of sleep disruption on depressive symptoms [30]. A study based on NHANES data examined the relationship between inflammatory markers, sleep disorders, and lifestyle habits. The findings revealed a strong association between leukocyte inflammatory markers, such as SII, and sleep disorders. Additionally, the study found that these inflammatory markers mediated the link between sedentary behavior and sleep disorders [31]. Our findings are consistent with those previously reported in the literature, where subjects with OSA and sleep problems were more likely to have elevated SII levels; however, the results of our study were obtained in nonhospitalized patients and were thus more representative.
The interaction of inflammation and sleep has become an established fact and has been confirmed by increasing research. The inflammatory cytokines interleukin (IL) and tumor necrosis factor (TNF) have been shown to play an important role in mediating inflammation and sleep, have well-established sleep regulatory functions [32, 33] and are dysregulated in sleep-related diseases such as sleep deprivation, circadian misalignment [34] and OSA [35]. Sleep is mediated by the central nervous system to regulate mental psychology and it dynamically regulates the immune system through the production and redistribution of inflammatory cytokines, with the main effectors including the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system [5]. Experimental sleep deprivation alters the circadian rhythm of IL-6 secretion [36], resulting in decreased IL-6 secretion at night and, conversely, increased daytime and correspondingly dysregulated TNF secretion [37]. In a meta-analysis of 72 studies, long sleep duration and sleep disturbance, but not extremely short sleep, were associated with increased C-reactive protein and IL-6 levels [9]. In addition, treatment of sleep disorders such as insomnia can reverse the levels of inflammatory markers and reduce IL-6 and TNF levels to alleviate systemic inflammatory responses [38]. Taken together, the results of these studies show that disturbed sleep may cause damage to host health by overactivating the inflammatory response [39]. At the same time, current perspectives suggest that the relationship between sleep and inflammation works both ways, and disruptions in the body's immune balance may also affect the quality of sleep. An animal study revealed that mice infected with the influenza virus exhibited an increase in non-rapid eye movement sleep, while experiencing a decrease in rapid eye movement sleep. However, the precise role of inflammation in this process remains uncertain [40]. In a sepsis model characterized by significant systemic inflammation, sleep in rats exhibited a fragmented pattern, which was found to be influenced by elevated mRNA and protein levels of cytokines, including IL-1β, IL-6, and TNF-α, in the nervous system [41]. The SII, as a simple and available indicator of the systemic inflammatory response, could play a crucial role in sleep monitoring.
In this study, we are presenting a novel finding that demonstrates a positive correlation between daytime sleepiness and SII. Since daytime sleepiness is prevalent among individuals with OSA [42], we conducted sensitivity analyses to account for this confounding factor by adjusting for OSA symptoms. Interestingly, the results of these analyses indicate that the positive association between daytime sleepiness and SII remains significant. At the same time, it has also been suggested that daytime sleepiness depends on sleep quality and timing and may also be associated with a variety of neuropsychological and cardiopulmonary diseases [43]. A cross-sectional study noted that patients with OSA and excessive daytime sleepiness exhibited elevated levels of high-sensitivity C-reactive protein in people without metabolic syndrome [44]. Another study also showed that objective daytime sleepiness was associated with increased IL-6 levels and decreased cortisol levels and that the disease phenotype of this inflammatory manifestation was associated with cardiometabolic morbidity and mortality [45]. Previous studies have demonstrated a U-shaped relationship between sleep duration and both inflammatory markers and mortality [46, 47]. Similar patterns were observed in this study, but statistically no significant association was found between sleep duration and inflammatory markers such as SII in this study. This finding therefore needs to be interpreted with caution.
The association of SII with sleep-related disorder outcomes carries important public health implications. In the context of the era of COVID-19, we face long-term coexistence with the novel coronavirus. Evidence thus far suggests that up to 50% to 70% of patients with new coronavirus pneumonia are expected to experience severe sleep disorders [48], possibly due to adverse psychological distress caused by the fear of the disease itself among the public affected by social media reports [49]. A single-center retrospective study of the impact of sleep quality on lymphopenia recovery and clinical outcomes in hospitalized patients with COVID-19 found that decreased absolute lymphocyte counts and increased NLR were more pronounced in patients with poor sleep quality [50]. Although our findings are derived from a nonhospitalized national population, it is undeniable that SII, as an indicator that can effectively reflect systemic immune inflammation, will give some inspiration for inflammation and sleep monitoring to subsequent new coronavirus-infected people.
Our study has some distinct strengths. This study represents a national-level population with a sufficiently large sample size. Then, we included a large number of covariates to control for confounding, and the definition of the outcome of sleep-related disorder was consistent with previous studies. However, there are some limitations that need to be noted. The cross-sectional study design does not provide the ability to make causal inferences, and prospective studies are needed to further explore these results. In addition, our combined data are older, but this is also an unavoidable problem, as complete sleep questionnaire data were only recorded in 2005–2008. Furthermore, the data used in this study were extracted from only one hematology test, and no repeat serial testing was performed. Meanwhile, the sleep-related disorders used in this study were based on self-reported data from participants, rather than objective measures like polysomnography or actigraphy. Further research is needed to determine if objective measures of sleep are also linked to SII. Additionally, future studies could explore sleep health as a multidimensional composite analysis, rather than focusing solely on one aspect of sleep health.
Conclusion
In this study, we discovered that sleep-related disorders had a stronger correlation with SII compared to PLR and NLR. Specifically, we found that sleep problems, a high risk of OSA, and daytime sleepiness were positively linked to SII. Furthermore, our analysis of other inflammatory markers revealed that daytime sleepiness was only associated with NLR. To validate our results, further comprehensive and detailed prospective studies are required.
Supplementary Information
Additional file 1: Supplementary Table 1. Definition and Details of Covariates. Supplementary Table 2. The numbers and percentages of missing covariate data. Supplementary Table 3. Subgroup analysis for the association of sleep duration with SII. Supplementary Table 4. Subgroup analysis for the association of sleep-related disorder with SII. Supplementary Table 5. Weighted linear regression coefficients (β) and 95% confidence intervals for the association between sleep-related disorder and SII and inflammatory markers: The United States, 2005 to 2008 (exclude participants with missing covariates). Supplementary Table 6. Weighted linear regression coefficients (β) and 95% confidence intervals for the association between sleep-related disorder and SII and inflammatory markers: The United States, 2005 to 2008 (exclude participants with recent infection). Supplementary Table 7. Weighted linear regression coefficients (β) and 95% confidence intervals for the association between daytime sleepiness and SII and inflammatory markers: The United States, 2005 to 2008 (additional adjustment for OSA symptoms).
Acknowledgements
We thank all the staff of the National Health and Nutrition Examination Survey program for their efforts in data collection.
Authors’ contributions
KK, DD and AA contributed to the conception and design, acquisition, analysis, interpretation of the data, and drafting of the manuscript or critical revision for important intellectual content. XZ-L, JD-L completed the software analysis and data visualization. PF-L, MA and GY collected and organized data. XM and YT-M contributed to the conception and design and reviewing of the manuscript or critical revision for important intellectual content. All authors approved the final version, and agree to be accountable for all aspects of the work.
Funding
This research was supported by State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia Fund (SKL-HIDCA-2021–3) and Key research projects in Xinjiang Uygur Autonomous Region (2022B03022).
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Helsinki declaration and its later amendments or comparable ethical standards. All information from the NHANES is available and free for public, so the agreement of the medical ethics committee board was not necessary. The studies involving human participants were approved by the National Center for Health Statistics Research Ethics Review Board, and informed consent was obtained from all participants in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kaisaierjiang Kadier, Diliyaer Dilixiati and Aikeliyaer Ainiwaer contributed equally to this work and share first authorship.
Contributor Information
Xiang Ma, Email: maxiangxj@yeah.net.
Yitong Ma, Email: myt_xj@sina.com.
References
- 1.Mireku MO, Rodriguez A. Sleep duration and waking activities in relation to the national sleep foundation’s recommendations: an analysis of US population sleep patterns from 2015 to 2017. Int J Environ Res Public Health. 2021;18:6154. doi: 10.3390/ijerph18116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leger D, Poursain B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. 2005;21:1785–1792. doi: 10.1185/030079905X65637. [DOI] [PubMed] [Google Scholar]
- 3.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira D, Elfering A. Social stressors at work, sleep quality and psychosomatic health complaints–a longitudinal ambulatory field study. Stress Health. 2014;30:43–52. doi: 10.1002/smi.2494. [DOI] [PubMed] [Google Scholar]
- 5.Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19:702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152:435–444. doi: 10.1016/j.chest.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zielinski MR, Systrom DM, Rose NR. Fatigue, sleep, and autoimmune and related disorders. Front Immunol. 2019;10:1827. doi: 10.3389/fimmu.2019.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99:1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Su S, McCall WV, Wang X. Blunted rest-activity rhythm is associated with increased white blood-cell-based inflammatory markers in adults: an analysis from NHANES 2011–2014. Chronobiol Int. 2022;39:895–902. doi: 10.1080/07420528.2022.2048663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjurström MF, Olmstead R, Irwin MR. Reciprocal relationship between sleep macrostructure and evening and morning cellular inflammation in rheumatoid arthritis. Psychosom Med. 2017;79:24–33. doi: 10.1097/PSY.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Zhang Q, Wang R, Ji H, Chen Y, Quan X, et al. Systemic immune-inflammatory index predicts clinical outcomes for elderly patients with acute myocardial infarction receiving percutaneous coronary intervention. Med Sci Monit. 2019;25:9690–9701. doi: 10.12659/MSM.919802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:229–236. doi: 10.1016/j.pnpbp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Bai X, Ren R, Tan L, Zhang Y, Lan H, et al. Association between depression or anxiety symptoms and immune-inflammatory characteristics in in-patients with tuberculosis: A cross-sectional study. Front Psychiatry. 2022;13:985823. doi: 10.3389/fpsyt.2022.985823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazza MG, Palladini M, De Lorenzo R, Magnaghi C, Poletti S, Furlan R, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemente I, Sinatti G, Cirella A, Santini SJ, Balsano C. Alteration of inflammatory parameters and psychological post-traumatic syndrome in long-COVID patients. Int J Environ Res Public Health. 2022;19:7103. doi: 10.3390/ijerph19127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topuz MF, Ture N, Akdag G, Arik O, Gulhan PY. The importance of systemic immune-inflammation index in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2022;279:5033–5038. doi: 10.1007/s00405-021-07227-0. [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 21.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 22.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Scinicariello F, Buser MC, Feroe AG, Attanasio R. Antimony and sleep-related disorders: NHANES 2005–2008. Environ Res. 2017;156:247–252. doi: 10.1016/j.envres.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22:405–411. doi: 10.1097/ACO.0b013e32832a96e2. [DOI] [PubMed] [Google Scholar]
- 25.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. doi: 10.5664/jcsm.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin Z, Li H, Wang L, Geng J, Yang Q, Su B, et al. Systemic Immune-Inflammation Index Is Associated With Increased Urinary Albumin Excretion: A Population-Based Study. Front Immunol. 2022;13:863640. doi: 10.3389/fimmu.2022.863640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Peng B, Liu J, Liu Z, Xia Y, Geng B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2007–2018. Front Immunol. 2022;13:975400. doi: 10.3389/fimmu.2022.975400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- 29.Stekhoven DJ, Bühlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 30.Yin J, Gong R, Zhang M, Ding L, Shen T, Cai Y, et al. Associations between sleep disturbance, inflammatory markers and depressive symptoms: Mediation analyses in a large NHANES community sample. Prog Neuropsychopharmacol Biol Psychiatry. 2023;126:110786. doi: 10.1016/j.pnpbp.2023.110786. [DOI] [PubMed] [Google Scholar]
- 31.You Y, Chen Y, Fang W, Li X, Wang R, Liu J,, et al. The association between sedentary behavior, exercise, and sleep disturbance: A mediation analysis of inflammatory biomarkers. Front Immunol (2023) 13:1080782. Published 2023 Jan 13. doi:10.3389/fimmu.2022.1080782 [DOI] [PMC free article] [PubMed]
- 32.Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) (2011) 3:632–42. 10.2741/s176 [DOI] [PMC free article] [PubMed]
- 33.Rockstrom MD, Chen L, Taishi P, Nguyen JT, Gibbons CM, Veasey SC, et al. Tumor necrosis factor alpha in sleep regulation. Sleep Med Rev. 2018;40:69–78. doi: 10.1016/j.smrv.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright KP, Jr, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y, Song Y, Ning P, Zhang L, Wu S, Quan J, et al. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: a meta-analysis update. BMC Pulm Med. 2020;20:215. doi: 10.1186/s12890-020-01253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 37.Dimitrov S, Besedovsky L, Born J, Lange T. Differential acute effects of sleep on spontaneous and stimulated production of tumor necrosis factor in men. Brain Behav Immun. 2015;47:201–210. doi: 10.1016/j.bbi.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial. Biol Psychiatry. 2015;78:721–729. doi: 10.1016/j.biopsych.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang J, Sanborn CK, Renegar KB, Majde JA, Krueger JM. Influenza viral infections enhance sleep in mice. Proc Soc Exp Biol Med. 1995;210:242–252. doi: 10.3181/00379727-210-43945. [DOI] [PubMed] [Google Scholar]
- 41.Granger JI, Ratti PL, Datta SC, Raymond RM, Opp MR. Sepsis-induced morbidity in mice: effects on body temperature, body weight, cage activity, social behavior and cytokines in brain. Psychoneuroendocrinology. 2013;38:1047–1057. doi: 10.1016/j.psyneuen.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D, Zhang Z, Li H, Ding K. Excessive daytime sleepiness in depression and obstructive sleep apnea: more than just an overlapping symptom. Front Psychiatry. 2021;12:710435. doi: 10.3389/fpsyt.2021.710435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slater G, Steier J. Excessive daytime sleepiness in sleep disorders. J Thorac Dis. 2012;4:608–616. doi: 10.3978/j.issn.2072-1439.2012.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andaku DK, D’Almeida V, Carneiro G, Hix S, Tufik S, Togeiro SM. Sleepiness, inflammation and oxidative stress markers in middle-aged males with obstructive sleep apnea without metabolic syndrome: a cross-sectional study. Respir Res. 2015;16:3. doi: 10.1186/s12931-015-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Vgontzas AN, Fernandez-Mendoza J, Kritikou I, Basta M, Pejovic S, et al. Objective, but Not Subjective, Sleepiness is Associated With Inflammation in Sleep Apnea. Sleep (2017) 40:zsw033. doi: 10.1093/sleep/zsw033 [DOI] [PMC free article] [PubMed]
- 46.Hall MH, Smagula SF, Boudreau RM, Ayonayon HN, Goldman SE, Harris TB, et al. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the Health. Aging and Body Composition Study. Sleep. 2015;38:189–195. doi: 10.5665/sleep.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin J, Jin X, Shan Z, Li S, Huang H, Li P, et al. Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc. 2017;6:e005947. Published 2017 Sep 9. 10.1161/JAHA.117.005947 [DOI] [PMC free article] [PubMed]
- 48.Pataka A, Kotoulas S, Sakka E, Katsaounou P, Pappa S. Sleep dysfunction in COVID-19 patients: prevalence, risk Factors, mechanisms, and management. J Pers Med. 2021;11:1203. doi: 10.3390/jpm11111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin CY, Broström A, Griffiths MD, Pakpour AH. Investigating mediated effects of fear of COVID-19 and COVID-19 misunderstanding in the association between problematic social media use, psychological distress, and insomnia. Internet Interv. 2020;21:100345. doi: 10.1016/j.invent.2020.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Xu D, Xie B, Zhang Y, Huang H, Liu H, et al. Poor-sleep is associated with slow recovery from lymphopenia and an increased need for ICU care in hospitalized patients with COVID-19: a retrospective cohort study. Brain Behav Immun. 2020;88:50–58. doi: 10.1016/j.bbi.2020.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Definition and Details of Covariates. Supplementary Table 2. The numbers and percentages of missing covariate data. Supplementary Table 3. Subgroup analysis for the association of sleep duration with SII. Supplementary Table 4. Subgroup analysis for the association of sleep-related disorder with SII. Supplementary Table 5. Weighted linear regression coefficients (β) and 95% confidence intervals for the association between sleep-related disorder and SII and inflammatory markers: The United States, 2005 to 2008 (exclude participants with missing covariates). Supplementary Table 6. Weighted linear regression coefficients (β) and 95% confidence intervals for the association between sleep-related disorder and SII and inflammatory markers: The United States, 2005 to 2008 (exclude participants with recent infection). Supplementary Table 7. Weighted linear regression coefficients (β) and 95% confidence intervals for the association between daytime sleepiness and SII and inflammatory markers: The United States, 2005 to 2008 (additional adjustment for OSA symptoms).
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/.