Abstract
Concentrations of antibiotics below the MIC are able to modulate the expression of virulence-associated genes. In this study, the influence of subinhibitory doses of 31 antibiotics on the expression of the gene encoding the staphylococcal alpha-toxin (hla), a major virulence factor of Staphylococcus aureus, was investigated with a novel gene fusion protocol. The most striking observation was a strong induction of hla expression by subinhibitory concentrations of β-lactams and an almost complete inhibition of alpha-toxin expression by clindamycin. Whereas glycopeptide antibiotics had no effect, the macrolide erythromycin and several aminoglycosides reduced and fluoroquinolones slightly stimulated hla expression. Furthermore, Northern blot analysis of hla mRNA and Western blot (immunoblot) analysis of culture supernatants of both methicillin-sensitive and methicillin-resistant S. aureus strains revealed that methicillin-induced alpha-toxin expression is a common phenomenon of alpha-toxin-producing strains. Some methicillin-resistant S. aureus isolates produced up to 30-fold more alpha-toxin in the presence of 10 μg of methicillin per ml than in its absence. The results indicate that the novel gene fusion technique is a useful tool for studying the modulation of virulence gene expression by antibiotics. Moreover, the results suggest that the effects of certain antibiotics on virulence properties may be relevant for the management of S. aureus infections.
There is increasing evidence that subinhibitory concentrations of antibiotics interfere with processes of host-parasite interactions such as phagocytosis, adherence, and toxin production (24). Several virulence-associated determinants of the important human pathogen Staphylococcus aureus are affected in vitro by low levels of various antibiotics (5, 11, 12, 26, 34). Remarkably, subinhibitory concentrations of β-lactam antibiotics, which are the preferred agents in antistaphylococcal chemotherapy, induce the hemolytic activity of S. aureus strains, probably via increased alpha-toxin production (12, 18, 23, 39).
The pore-forming alpha-toxin (encoded by the hla gene) is a major virulence factor of S. aureus. Its role in pathogenesis has been demonstrated in several animal models with Hla-negative mutants (6, 28, 30, 31). Hla exhibits cytolytic, hemolytic, dermonecrotic, and lethal activities (2, 35). In addition, the generation of transmembrane pores triggers calcium-dependent and -independent secondary cellular reactions, such as eicosanoid production, release of cytokines, and apoptosis (3, 9, 36, 37). Many cell types, including erythrocytes, monocytes, lymphocytes, macrophages, epithelial cells, fibroblasts, and keratinocytes, are susceptible to the action of the toxin (2, 4, 40, 41).
Recently, it was shown that growth of S. aureus strains in the presence of the β-lactam nafcillin induces alpha-toxin expression and increases the lethal activity of broth filtrates in a murine model (16). These findings led to the speculation that β-lactam therapy may enhance the virulence of some S. aureus strains, in turn worsening the symptoms of serious S. aureus infections (16). On the other hand, subinhibitory concentrations of antibiotics which interfere with the protein synthesis machinery repressed the hemolytic activity of some S. aureus strains (18, 26). However, S. aureus produces four hemolysins, and most reports investigating the effect of antibiotics on hemolysis did not clearly show that the modulation of hemolytic activity by antibiotics was due to the action of alpha-toxin.
The aim of this study was a comprehensive characterization of specific effects of subinhibitory concentrations of antibiotics on the expression of the alpha-toxin gene of S. aureus by use of a recently described chromosomally located hla::lacZ gene fusion (29). In addition, the effects of β-lactams upon the transcription and translation of alpha-toxin were examined by Northern hybridization and immunoblotting analysis of both methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) isolates.
MATERIALS AND METHODS
Strains.
The bacterial strains used in this study are listed in Table 1. Wood 46-3 is a derivative of Wood 46 (13) carrying a transcriptional fusion between the promoter region of the alpha-toxin gene and the lacZ gene of Escherichia coli (29). Clinical isolates of S. aureus were recovered from patients in Mainz, Frankfurt am Main, and Würzburg, Germany. Each isolate was identified as a unique S. aureus strain by established methods (22). The classification of S. aureus strains as methicillin sensitive (MIC, ≤8 μg/ml) or methicillin resistant (MIC, ≥16 μg/ml) was carried out in accordance with the criteria of the National Committee for Clinical Laboratory Standards (27). TX71 is a constitutively β-galactosidase-producing S. xylosus strain carrying a chromosomal fusion between the vegII promoter of Bacillus subtilis and the S. xylosus β-galactosidase gene lacH (7).
TABLE 1.
Bacterial strains used
| Strain | Description | Source or reference |
|---|---|---|
| S. aureus | ||
| Wood 46 | High-level production of alpha-toxin | 13 |
| Wood 46-3 | Derivative of Wood 46 with hla::lacZ fusion | 29 |
| MA12 | MSSA, mucosal isolate | Nursing staff |
| MA13 | MSSA, wound infection | Clinical isolate |
| MA14 | MRSA, wound infection | Clinical isolate |
| MA15 | MRSA, wound infection | Clinical isolate |
| MA17 | MRSA, wound infection | Clinical isolate |
| MA19 | MSSA, wound infection | Clinical isolate |
| MA20 | MRSA, wound infection | Clinical isolate |
| MA23 | MSSA, wound infection | Clinical isolate |
| MA25 | MSSA, wound infection | Clinical isolate |
| MA31 | MRSA, sepsis | Clinical isolate |
| MA32 | MRSA, sepsis | Clinical isolate |
| W570 | MRSA, sepsis | Clinical isolate |
| W605 | MRSA, sepsis | Clinical isolate |
| W654 | MRSA, orthopedic implant-associated infection | Clinical isolate |
| W655 | MRSA, orthopedic implant-associated infection | Clinical isolate |
| W704 | MRSA, pneumonia | Clinical isolate |
| W724 | MRSA, osteomyelitis | Clinical isolate |
| W810 | MRSA, sepsis | Clinical isolate |
| W903 | MRSA, orthopedic implant-associated infection | Clinical isolate |
| S. xylosus TX71 | Constitutive β-galactosidase production | 7 |
Media.
For RNA extractions as well as for exoprotein analysis, S. aureus strains were cultured in brain heart infusion broth (Difco, Augsburg, Germany). For reporter gene studies, strain Wood 46-3 was cultivated in modified Luria-Bertani (LB) broth consisting of 1% peptone (Roth, Karlsruhe, Germany), 0.5% yeast extract (BRL, Eggenstein, Germany), 0.5% NaCl (Roth), and 0.1% K2HPO4 (E. Merck AG, Darmstadt, Germany).
Bacterial growth conditions.
S. aureus Wood 46-3 was cultivated following a 1:100 dilution of an overnight culture in 100-ml flasks that contained 20 ml of modified B broth. The cultivation was performed with a shaker at 180 rpm and 37°C. As alpha-toxin expression is growth phase dependent, with maximal expression in the late logarithmic to early stationary phases, the cultures were monitored with regard to β-galactosidase activity until the early stationary phase (19, 29). Following the cultivation of clinical isolates under the same conditions, cells were collected for RNA extraction and supernatants were collected for immunoblot analysis.
Antibiotics.
The antibiotics used in this study are listed in Table 2.
TABLE 2.
MICs of antibiotics for S. aureus Wood 46-3
| Antibiotic (source) | Abbreviation | MIC (μg/ml) |
|---|---|---|
| β-Lactams | ||
| Ampicillin (Sigma) | AMPC | 0.125 |
| Azlocillin (Sigma) | AZLC | 0.5 |
| Cloxacillin (Fluka, Deisenhofen, Germany) | CLOC | 0.25 |
| Flucloxacillin (SmithKline Beecham, Munich, Germany) | FLUC | 0.25 |
| Methicillin (Sigma) | METC | 1 |
| Nafcillin (Sigma) | NAFC | 0.25 |
| Oxacillin (Sigma) | OXAC | 0.25 |
| Penicillin G (Sigma) | PENG | 0.06 |
| Penicillin V (Sigma) | PENV | 0.06 |
| Piperacillin (Fluka) | PIPC | 0.5 |
| Cefazolin (Sigma) | CFAZ | 0.5 |
| Cefuroxime (Sigma) | CROX | 0.5 |
| Cefotaxime (Roussel Uclaf, Romainville, France) | CTAX | 0.5 |
| Ceftriaxone (Sigma) | CTRX | 1 |
| Cefoxitin (Merck Sharp & Dohme, Haar, Germany) | CFOX | 4 |
| Imipenem (MSD) (Merck Sharp & Dohme) | IMIP | 0.03 |
| Aztreonam (Bristol-Myers Squibb, Regensburg, Germany) | AZTR | >128 |
| Glycopeptides | ||
| Teicoplanin (Roussel Uclaf) | TPL | 1 |
| Vancomycin (Sigma) | VAN | 1 |
| Aminoglycosides | ||
| Gentamicin (Sigma) | GEN | 4 |
| Kanamycin (Sigma) | KAN | 16 |
| Netilmicin (Sigma) | NET | 2 |
| Streptomycin (Sigma) | STR | 16 |
| Tobramycin (Sigma) | TOB | 2 |
| Fluoroquinolones | ||
| Ciprofloxacin (Difco) | CIPX | 0.25 |
| Ofloxacin (Sigma) | OFLX | 0.5 |
| Macrolide: erythromycin (Sigma) | ERY | 1 |
| Other classes | ||
| Clindamycin (Sigma) | CLI | 0.25 |
| Rifampin (Sigma) | RIF | 0.008 |
| Tetracycline (Sigma) | TET | 0.5 |
| Trimethoprim (Sigma) | TMP | 0.25 |
β-Lactamase tests.
The β-lactamase production of the strains was determined by use of β-lactamase identification sticks (Oxoid, Wesel, Germany) with nitrocefin as the substrate.
β-Galactosidase assays.
β-Galactosidase assays were performed as described previously (29) with the Galacto Light Plus chemiluminescent reporter assay system (Tropix, Bedford, Mass.). β-Galactosidase activity was measured by use of an LB 9501 luminometer (Berthold, Wildbad, Germany) with a 300-μl automatic injector and a 5-s interval.
Extraction of RNA and DNA-RNA hybridization.
RNA was prepared with the RNeasy system (Qiagen, Hillen, Germany). After electrophoresis of samples with the same amount of total cellular RNA, as determined by measuring the A260, the gel was analyzed by Northern blot hybridization as described previously (1, 42). The probe, a 722-nucleotide intragenic ClaI fragment (13) from the hla gene, was labelled by use of an ECL kit (Amersham, Braunschweig, Germany), and hybridization was performed as described in the manufacturer’s instructions. The signals were quantified by densitometric scanning.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed as described by Laemmli (21) with discontinuous 12.5% acrylamide gels. For immunoblot analysis, proteins were transferred to nylon membranes by semidry electroblotting in a graphite chamber (20). Following blotting of the membranes, blocking was performed with 5% nonfat dry milk (Bio-Rad, Munich, Germany) in phosphate-buffered saline for 1 h. The filters were then incubated for 1 h with a polyclonal anti-alpha-toxin antibody in phosphate-buffered saline containing 0.05% Tween 20 (Sigma, Deisenhofer, Germany) followed by 0.5 h of incubation with horseradish peroxidase-conjugated anti-rabbit antiserum (DAKO, Hamburg, Germany) diluted 1:1,000. The blots were developed with ECL substrate (Amersham), and the signals were quantified by densitometric scanning.
Determination of the MICs.
The MICs were determined by the broth microdilution method (27), and the results are shown in Table 2.
Statistics.
Means ± standard deviations were calculated by the method described by Cavalli-Sforza (8). The values obtained with each antibiotic were compared to those obtained for the control without antibiotic by an unpaired t test. P values of ≤0.05 were judged significant.
RESULTS
Effects of β-lactams on hla::lacZ expression.
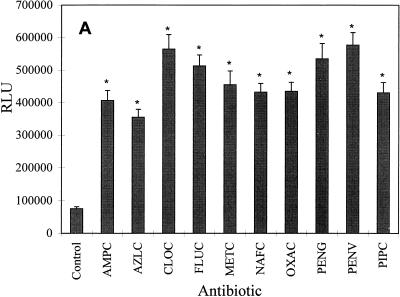
First, the expression of the hla::lacZ fusion in S. aureus Wood 46-3 was examined after growth with subinhibitory concentrations (one-fourth the MIC) of the following penicillin derivatives: ampicillin, azlocillin, cloxacillin, flucloxacillin, methicillin, nafcillin, oxacillin, penicillin G, penicillin V, and piperacillin. The highest level of β-galactosidase production, representing hla expression, was obtained after growth in the presence of penicillin V, cloxacillin, penicillin G, flucloxacillin, and methicillin (Fig. 1A). The β-galactosidase activity was elevated seven- to eightfold compared to that in a control culture grown without any antibiotic. The remaining penicillins induced hla::lacZ expression five- to sixfold (Fig. 1A).
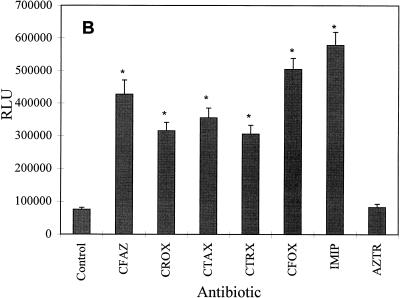
FIG. 1.
Influence of subinhibitory concentrations (one-fourth the MIC) of penicillins (A) and other β-lactam antibiotics (B) on β-galactosidase (LacZ) production of the fusion strain S. aureus Wood 46-3. Wood 46-3 cultures grown without and with antibiotics were monitored with regard to LacZ production until the early stationary phase. Maximal LacZ values are given as relative light units (RLU). Means ± standard deviations for five experiments are given. The values obtained with each antibiotic were compared to those obtained for the control without antibiotic by an unpaired t test: ∗, P ≤ 0.01; the other differences were not statistically significant (P > 0.05). The abbreviations are listed in Table 2.
To determine if β-lactam antibiotics other than penicillins also influence hla expression, β-galactosidase activity in strain Wood 46-3 was examined after growth with one-fourth the MIC of cephalosporins (cefazolin, cefuroxime, cefotaxime, ceftriaxone, and cefoxitin), the carbapenem imipenem, and the monobactam aztreonam. As shown in Fig. 1B, all of these antibiotics, except for aztreonam, induced β-galactosidase activity. The greatest extent of induction was obtained after growth with imipenem (eightfold) and cefoxitin (sevenfold). The other tested cephalosporins increased hla::lacZ expression four- to fivefold. In contrast, aztreonam had no effect on β-galactosidase production (Fig. 1B).
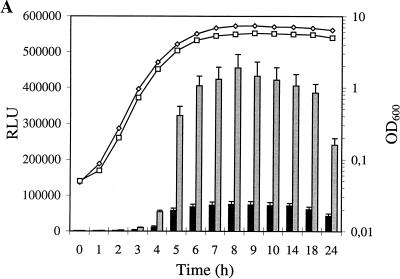
None of the β-lactams tested had any influence upon growth-phase-dependent induction of alpha-toxin expression. Although subinhibitory levels of β-lactams, except for aztreonam, slightly decreased the growth of Wood 46-3, the induction of alpha-toxin expression occurred during the late exponential phase of growth, and the maximal level of alpha-toxin expression was detected in the early stationary phase. A representative growth curve for strain Wood 46-3 grown in the presence of methicillin and the corresponding LacZ values are shown in Fig. 2A.
FIG. 2.
Kinetics of transcription of a chromosomal hla::lacZ fusion with growth in the presence of methicillin (A) and clindamycin (B) (drugs are indicated by gray bars and open squares) and a control without antibiotic (black bars and open diamonds). β-Galactosidase activity, indicated by bars, is expressed in relative light units (RLU). Means ± standard deviations for four experiments are given. Lines with squares and diamonds indicate representative growth, as measured by the optical density at 600 nm (OD600). Growth experiments were repeated four times.
Effects of glycopeptide antibiotics and aminoglycosides on hla::lacZ expression.
The influence of one-fourth the MIC of two glycopeptide antibiotics (vancomycin and teicoplanin) and five aminoglycosides (gentamicin, kanamycin, netilmicin, streptomycin, and tobramycin) on hla expression was investigated. The glycopeptide antibiotics did not significantly (P > 0.05) affect hla::lacZ expression (Fig. 3A). However, all of the aminoglycosides tested decreased hla::lacZ expression significantly (P ≤ 0.01): tobramycin, 54%; netilmicin, 46%; streptomycin, 40%; gentamicin, 34%; and kanamycin, 23% (Fig. 3A).
FIG. 3.
Influence of subinhibitory concentrations (one-fourth the MIC) of glycopeptides and aminoglycosides (A) and various antibiotics (B) on β-galactosidase (LacZ) production of the fusion strain S. aureus Wood 46-3. Wood 46-3 cultures grown without and with antibiotics were monitored with regard to LacZ production until the early stationary phase. Maximal LacZ values are given as relative light units (RLU). Means ± standard deviations for five experiments are given. The values obtained with each antibiotic were compared to those obtained for the control without antibiotic by an unpaired t test: ∗, P ≤ 0.01; ∗∗, P ≤ 0.05; the other differences were not statistically significant (P > 0.05). The abbreviations are listed in Table 2.
Effects of clindamycin, erythromycin, tetracycline, rifampin, fluoroquinolones, and trimethoprim on hla::lacZ expression.
Low concentrations of clindamycin strongly inhibited β-galactosidase activity (Fig. 3B). This antibiotic reduced hla::lacZ expression by 98% compared with that in a control culture. The repression occurred during the whole growth cycle (Fig. 2B). Further, erythromycin had a marked repressive effect on hla promoter activity. While rifampin and tetracycline slightly decreased the β-galactosidase production of the reporter strain, the fluoroquinolone ofloxacin and trimethoprim slightly increased hla::lacZ expression (P ≤ 0.05) (Fig. 3B).
Influence of methicillin on hla mRNA expression and alpha-toxin production of MSSA isolates.
The effect of β-lactam-induced alpha-toxin expression was further investigated with MSSA strains on both transcriptional and translational levels. First, total RNAs of six strains were analyzed by DNA-RNA hybridization and quantified by densitometric scanning after cultivation with one-fourth the MIC of methicillin (0.25 μg/ml). All strains produced higher hla mRNA levels after growth with than after growth without methicillin (Fig. 4). However, the increase was strain specific, ranging from 1.5- to 10-fold.
FIG. 4.
Northern blot analysis of total RNA from MSSA strains after growth without (−) and with (+) methicillin (0.25 μg/ml). RNA was prepared from cells after 8 h of growth in modified LB broth. Six micrograms of RNA was loaded into each well. After electrophoresis and blotting, the filter was probed for hla mRNA with a peroxidase-labelled 722-nt intragenic hla-specific ClaI fragment.
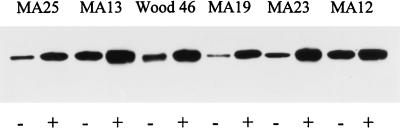
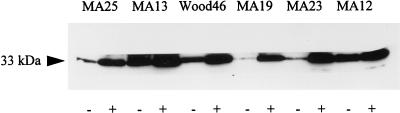
To determine whether the effect of methicillin on hla expression is also associated with an increase in alpha-toxin production, six MSSA strains were cultivated in the absence or presence of methicillin (0.25 μg/ml). The alpha-toxin concentration in supernatants was displayed by immunoblot analysis and quantified by densitometric scanning. Growth in the presence of methicillin elevated the alpha-toxin level of all strains tested (Fig. 5). Methicillin caused an average 4.5-fold increase in alpha-toxin production. However, while strain MA19 produced 12-fold more alpha-toxin after growth with than after growth without methicillin, the increase in strain MA12 was only 1.5-fold, indicating that strain-specific regulatory mechanisms which determine the extent of induction of alpha-toxin production by methicillin exist.
FIG. 5.
Immunoblot analysis of alpha-toxin production of MSSA strains after growth without (−) and with (+) methicillin (0.25 μg/ml). Culture supernatant samples were taken following 18 h of incubation, and 10 μl of each sample was loaded onto the gel.
Influence of methicillin on alpha-toxin production of MRSA isolates.
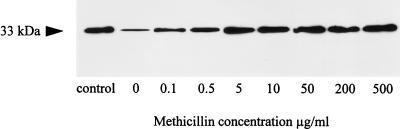
Methicillin increased the alpha-toxin production of MSSA strains. Therefore, we examined the effect of methicillin on methicillin-resistant strains. First, MRSA strain MA17 (MIC, 1 mg/ml) was grown with different concentrations of methicillin (0.1 to 500 μg/ml), and culture supernatants were analyzed by immunoblotting. Methicillin at 0.1 μg/ml elevated alpha-toxin production threefold, and growth in increasing concentrations of methicillin was accompanied by increasing alpha-toxin levels in culture supernatants (Fig. 6). However, the alpha-toxin levels reached a plateau at about 5 to 10 μg of methicillin per ml. Growth in higher methicillin concentrations did not stimulate alpha-toxin production further. Strain MA17 produced about 15-fold more alpha-toxin at maximal induction than the respective control culture grown without methicillin.
FIG. 6.
Immunoblot analysis of alpha-toxin production of MRSA strain MA17 after growth with different concentrations of methicillin. Culture supernatant samples were taken following 18 h of incubation, and 10 μl of each sample was loaded onto the gel. As a control, 1 μg of purified alpha-toxin (Sigma) was used in the control lane.
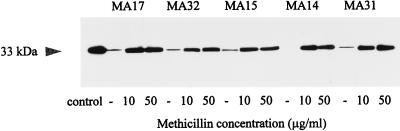
The effect of methicillin on the alpha-toxin production of four additional MRSA strains was analyzed by immunoblotting. Since strain MA17 produced maximal alpha-toxin levels at a methicillin concentration of about 10 μg/ml, these four strains were cultivated with 10 and 50 μg of methicillin per ml and, as a control, without the antibiotic added. As shown in Fig. 7, all strains tested produced dramatically more alpha-toxin in the presence of methicillin than in its absence (control cultures). Strain MA14 exhibited detectable alpha-toxin levels only in the presence of methicillin, and strain MA31 showed a 30-fold increase in alpha-toxin production in the presence of methicillin (quantified by densitometric scanning). In addition, nine additional strains were cultivated in 10 μg of methicillin per ml. These strains also produced more alpha-toxin in the presence of methicillin than in its absence (Table 3).
FIG. 7.
Immunoblot analysis of alpha-toxin production of MRSA strains after growth without (−) and with methicillin (10 and 50 μg/ml). Culture supernatant samples were taken following 18 h of incubation, and 10 μl of each sample was loaded onto the gel. As a control, 1 μg of purified alpha-toxin (Sigma) was used in the control lane.
TABLE 3.
Alpha-toxin produced by clinical MRSA strains after growth with or without methicillin
| Strain | Ratioa |
|---|---|
| MA20 | 6.2 |
| W570 | 21.5 |
| W605 | 12.0 |
| W654 | 4.4 |
| W655 | 4.0 |
| W704 | 18.4 |
| W724 | 2.5 |
| W810 | 9.1 |
| W903 | 2.4 |
The strains were cultivated either with 10 μg of methicillin per ml or without methicillin. Alpha-toxin levels were determined by immunoblotting and were quantified by densitometry. The ratio shows alpha-toxin produced in the presence of methicillin to that produced in the absence of methicillin.
DISCUSSION
The present study shows that subinhibitory concentrations of various antibiotics modulate the expression of the hla gene, encoding staphylococcal alpha-toxin (Hla). The main findings are that (i) β-lactam antibiotics of different classes strongly induce hla expression; (ii) clindamycin almost completely represses hla expression; and (iii) methicillin enhances Hla production of both MSSA and MRSA.
Numerous reports have described the effects of antibiotics below the MIC on bacterial cell functions, including alterations of virulence properties (14, 15, 17, 34). In S. aureus, exposure to subinhibitory concentrations of antimicrobial agents led to an increased expression of fibronectin-binding proteins by fluoroquinolones (5), an inhibition of toxic shock syndrome toxin 1 (TSST-1) production by clindamycin (32, 38), and an induction of hemolytic activity by β-lactams (12, 18, 23, 39). The last observation frequently has been ascribed to the increased production of alpha-toxin. However, S. aureus expresses four hemolysins, and the higher level of hemolytic activity after growth in the presence of β-lactams described in these reports was not entirely due to the action of alpha-toxin.
In this study, we used an S. aureus wild-type gene fusion between the hla determinant and the reporter gene lacZ (29). With this fusion, it was possible to determine specifically the influence of subinhibitory concentrations of various antibiotics of different classes on hla promoter activity. It was shown that all penicillins, cephalosporins, and carbapenems tested strongly induced hla expression. The highest induction rates were obtained with growth in the presence of penicillin V, penicillin G, cloxacillin, and imipenem, which all have strong activity for susceptible S. aureus strains. In contrast, aztreonam, a β-lactam of the monobactam group without antistaphylococcal activity, did not have an effect on hla expression. These results suggest that the induction of hla expression by β-lactams depends on a specific interaction of the agents with penicillin-binding proteins. As a consequence, such an interaction may induce signal transduction mechanisms, resulting in activation of the hla promoter. The details of the induction process, however, remain unknown. Another hypothesis is that there is cross talk between the β-lactamase regulatory system and the virulence regulation machinery in S. aureus. However, we found no link between the β-lactamase status of the strains and the induction of alpha-toxin expression by β-lactams. Both β-lactamase-positive strains (e.g., MA17, MA23, MA25, and MA31) and β-lactamase-negative strains (e.g., Wood 46, MA12, MA14, MA15, and MA19) showed increased alpha-toxin production after growth in the presence of subinhibitory concentrations of methicillin.
Further insights into the mechanisms underlying β-lactam-induced hla expression were provided by experiments with MRSA strains. Interestingly, methicillin resistance does not prevent the induction process, and methicillin concentrations far below the MIC (10−4) stimulated hla promoter activity. Furthermore, the increase in alpha-toxin production with growth in the presence of methicillin was concentration dependent, reaching a maximal level at about 10 μg of methicillin per ml. This finding indicates that the absolute antibiotic concentration rather than the ratio of the concentration to the MIC determines induction. The studies with MRSA strains also indicated that the induction of alpha-toxin expression by β-lactams is a specific process and cannot be explained simply by stress phenomena resulting from destabilization of the bacterial cell wall or the accumulation of cell wall precursors. This hypothesis is further supported by experiments with glycopeptide antibiotics, which interfered with peptidoglycan synthesis but did not alter hla::lacZ expression. Furthermore, strain-specific regulatory mechanisms determine the extent of the induction. One recent study provided evidence that the activation of hla transcription by subinhibitory levels of the β-lactam nafcillin cannot be explained by increased levels of the regulatory molecule RNA III (16). RNA III is the effector of the global regulatory locus agr (accessory gene regulator), which controls the expression of a number of virulence genes in S. aureus, including alpha-toxin (19). Our observations are consistent with these findings (data not shown).
In contrast, strong inhibition of hla expression was observed with growth in the presence of subinhibitory levels of clindamycin. It is noteworthy that low concentrations of clindamycin also inhibit the expression of TSST-1 (32, 38) and the exfoliative toxin (33). With respect to the beneficial effects of clindamycin on TSST-1 production, it has been recommended that clindamycin rather than β-lactams be used for the treatment of staphylococcal toxic shock syndrome (32, 38). Moreover, sublethal concentrations of clindamycin suppress the adhesion of S. aureus to bone surfaces of rabbits (25) and of S. epidermidis to vascular catheters (17) by as-yet-unknown mechanisms. Other protein synthesis inhibitors (erythromycin and aminoglycosides) tested also impaired alpha-toxin expression. However, inhibition by these agents was not as strong as that by clindamycin. Most of the antibiotics tested also slightly reduced the growth of the indicator strain, Wood 46-3, and it may be hypothesized that protein synthesis inhibitors in particular may influence toxin expression by slowing the growth rate. Growth effects may be somewhat involved in the decrease in hla expression by aminoglycosides. However, the strong inhibition of hla expression by clindamycin cannot be explained simply by a decrease in the growth rate, since very low clindamycin concentrations (below one-fourth the MIC), which did not influence growth, led to a strong inhibition of alpha-toxin expression (data not shown). In addition, clindamycin did not influence the β-galactosidase expression of constitutively β-galactosidase-producing S. xylosus TX71, whereas aminoglycosides slightly reduced β-galactosidase production in this strain (data not shown). Thus, clindamycin seems to affect toxin biosynthesis selectively without shutting off ribosomal protein biosynthesis completely.
The contribution of antibiotic-based alteration of alpha-toxin production to the pathogenesis of serious infections caused by S. aureus is difficult to evaluate. In the management of S. aureus infections, β-lactam antibiotics are the preferred class of drugs. However, we have shown that clinically achieved concentrations of β-lactams induce the production of a major staphylococcal virulence factor. Thus, S. aureus strains which do not respond to β-lactam therapy may show enhanced virulence potential, which in turn may lead to an unfavorable impact on the outcome of an infection. Further, the data support the value of high-dose regimens for the treatment of infections caused by MSSA strains to avoid a reduction of therapeutic levels of β-lactams below the MICs.
Since most staphylococcal diseases are multifactorial, involving the production of various virulence determinants, further studies, including animal models and clinical trials, are needed to elucidate the effects of antibiotics on the pathogenesis of serious S. aureus infections. Moreover, increasing problems with multiple-drug-resistant S. aureus strains demand a renewed effort to develop effective strategies against this pathogen. In addition to classic antimicrobial chemotherapy, a new approach could be the search for agents which suppress gene products associated with infection (10). With respect to this challenge, sensitive reporter gene-based techniques, such as that described in this report, are valuable screening tools and may help researchers find new effective compounds against molecular targets in S. aureus.
ACKNOWLEDGMENTS
We thank Sucharit Bhakdi (Mainz, Germany), Roland Brückner (Tübingen, Germany), and Michael Palmer (Mainz, Germany) for generous gifts of antisera, plasmid, and strains and Ute Hentschel for critical reading of the manuscript.
This work was supported by Hoechst Marion Roussel Deutschland GmbH, Frankfurt am Main, Germany, by a BMBF grant (AZ01KJ9608), and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D A, Seidman J G, Smith J A, Strahl K. Current protocols in molecular biology. Vol. 4. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 2.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1β associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S, Muhly M, Mannhardt U, Klapptek K, Müller-Eckhardt C, Roka L. Staphylococcal α-toxin promotes blood coagulation via attack on human platelets. J Exp Med. 1988;168:527–542. doi: 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisognano C, Vaudaux P E, Lew D P, Ng E Y W, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramley A J, Patel A H, O’Reilly M, Foster R, Foster T J. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–2494. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brückner, R. 1997. Personal communication.
- 8.Cavalli-Sforza L. Biometrie. Grundzüge biologisch-medizinischer Statistik. Jena, Germany: Fischer-Verlag; 1969. [Google Scholar]
- 9.Chen Y, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 10.Chopra I, Hodgson J, Metcalf B, Poste G. The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrob Agents Chemother. 1997;41:497–503. doi: 10.1128/aac.41.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doss S A, Tillotson G S, Amyes S G. Effect of subinhibitory concentrations of antibiotics on the virulence of Staphylococcus aureus. J Appl Bacteriol. 1993;75:123–128. doi: 10.1111/j.1365-2672.1993.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 12.Gemmel C G. Antibiotics and the expression of staphylococcal virulence. J Antimicrob Chemother. 1995;36:283–291. doi: 10.1093/jac/36.2.283. [DOI] [PubMed] [Google Scholar]
- 13.Gray S, Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus Wood 46. Infect Immun. 1984;46:615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacker J, Ott M, Hof H. Effects of low, subinhibitory concentrations of antibiotics on expression of virulence gene cluster of pathogenic Escherichia coli by using a wild-type gene fusion. Int J Antimicrob Agents. 1993;2:263–270. doi: 10.1016/0924-8579(93)90060-i. [DOI] [PubMed] [Google Scholar]
- 15.Hatano K, Nishino T. Morphological alterations of Staphylococcus aureus and Streptococcus pyogenes exposed to cefdinir, a new oral broad spectrum cephalosporin. Chemotherapy (Tokyo) 1994;40:73–79. doi: 10.1159/000239176. [DOI] [PubMed] [Google Scholar]
- 16.Kernodle D S, McGraw P A, Barg N L, Menzies B E, Voldari R K R, Harshman S. Growth of Staphylococcus aureus with nafcillin in vitro induces α-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis. 1995;172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 17.Khardouri N, Wong E, Nguyen H, Jeffery-Wiseman C, Wallin E, Tewari R P, Bodey G P. Effect of subinhibitory concentrations of clindamycin and trospectomycin on the adherence of Staphylococcus epidermidis in an in vitro model of vascular catheter colonization. J Infect Dis. 1991;164:108–113. doi: 10.1093/infdis/164.1.108. [DOI] [PubMed] [Google Scholar]
- 18.Kobayasi A, Barnett J A, Sanford J P. Effect of antibiotics on the in vitro production of alpha-hemolysin by Staphylococcus aureus. J Lab Clin Med. 1966;68:890. [Google Scholar]
- 19.Kornblum J, Kreiswirth B N, Projan S J, Ross H, Novick R P. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers Inc.; 1990. pp. 373–402. [Google Scholar]
- 20.Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Linhardt F, Ziebuhr W, Meyer P, Witte W, Hacker J. Pulse-field gel electrophoresis of genomic restriction fragments as a tool for the epidemiological analysis of Staphylococcus aureus and coagulase-negative staphylococci. FEMS Microbiol Lett. 1992;74:181–185. doi: 10.1016/0378-1097(92)90426-o. [DOI] [PubMed] [Google Scholar]
- 23.Lorian V. Effect of antibiotics on staphylococcal hemolysin production. Appl Microbiol. 1971;22:106. doi: 10.1128/am.22.1.106-109.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorian V, Gemmel G C. Effect of low antibiotic concentrations on bacteria: effects on ultrastructure, virulence, and susceptibility to immunodefenses. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: Williams & Wilkins; 1991. pp. 493–555. [Google Scholar]
- 25.Mayberry-Carson K J, Mayberry W R, Tober-Meyer B K, Costerton J W, Lambe D W. An electron microscopic study of the effect of clindamycin on adherence of Staphylococcus aureus to bone surfaces. Microbios. 1986;45:21–32. [PubMed] [Google Scholar]
- 26.Moneib N A, Shibl A M, elSais M A, elMasry E M. Macrolide-induced suppression of virulence factors produced by Staphylococcus aureus. J Chemother. 1993;5:289–292. doi: 10.1080/1120009x.1993.11739246. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 28.O’Callaghan R, Callegan M C, Moreau J M, Green L C, Foster T J, Hartford O M, Engel L S, Hill J M. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65:1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlsen K, Koller K-P, Hacker J. Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla::lacZ gene fusion. Infect Immun. 1997;65:3606–3614. doi: 10.1128/iai.65.9.3606-3614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Reilly M, Azavedo J C S, Kennedy S, Foster T J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986;1:125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 31.Patel A H, Nowlan P, Weavers E D, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlievert P M, Kelly J A. Clindamycin-induced suppression of toxic shock syndrome-associated exotoxin production. J Infect Dis. 1984;149:471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- 33.Shibl A M. Role of Staphylococcus aureus exfoliatin toxin in staphylococcal infections in mice. Chemotherapy (Basel) 1981;27:224–227. doi: 10.1159/000237982. [DOI] [PubMed] [Google Scholar]
- 34.Smith I M, Kong Y L. Enhanced virulence of Staphylococcus aureus exposed to trace amounts of nafcillin. In: Jeljaszewicz J, editor. Staphylococci and staphylococcal infections. Jena, Germany: Gustav Fischer; 1981. pp. 693–697. [Google Scholar]
- 35.Song L, Hobaugh M R, Shustak C, Cheley S, Bayley H, Gouaux J E. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 36.Suttorp N, Habben E. Effect of staphylococcal alpha-toxin on intracellular Ca2+ in polymorphonuclear leukocytes. Infect Immun. 1988;56:2228–2235. doi: 10.1128/iai.56.9.2228-2234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suttorp N, Seeger W, Dewein E, Bhakdi S, Roka L. Staphylococcal α-toxin stimulates synthesis of prostacyclin by cultured endothelial cells from pig pulmonary arteries. Am J Physiol. 1985;248:C127–C135. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- 38.van Langevelde P, van Dissel J T, Meurs C J C, Renz J, Groeneveld P H P. Combination of flucloxacillin and gentamicin inhibits toxic shock syndrome toxin 1 production by Staphylococcus aureus in both logarithmic and stationary phases of growth. Antimicrob Agents Chemother. 1997;41:1682–1685. doi: 10.1128/aac.41.8.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vymola F, Lochmann D. Effect of antibiotics on Staphylococcus aureus haemolysin production. J Hyg Epidemiol Microbiol Immunol. 1974;18:281–284. [PubMed] [Google Scholar]
- 40.Walev I, Martin E, Jonas D, Mohamadzadeh M, Müller-Klieser W, Kunz L, Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walev I, Palmer M, Martin E, Jonas D, Weller U, Höhn-Bentz H, Husmann M, Bhakdi S. Recovery of human fibroblasts from attack by pore-forming α-toxin of Staphylococcus aureus. Microb Pathog. 1994;17:187–201. doi: 10.1006/mpat.1994.1065. [DOI] [PubMed] [Google Scholar]
- 42.Ziebuhr W, Heilmann C, Götz F, Meyer P, Wilms K, Straube E, Hacker J. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect Immun. 1997;65:890–896. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]