Abstract
The prokineticins (PKs) were discovered approximately 20 years ago as small peptides inducing gut contractility. Today, they are established as angiogenic, anorectic, and proinflammatory cytokines, chemokines, hormones, and neuropeptides involved in variety of physiologic and pathophysiological pathways. Their altered expression or mutations implicated in several diseases make them a potential biomarker. Their G-protein coupled receptors, PKR1 and PKR2, have divergent roles that can be therapeutic target for treatment of cardiovascular, metabolic, and neural diseases as well as pain and cancer. This article reviews and summarizes our current knowledge of PK family functions from development of heart and brain to regulation of homeostasis in health and diseases. Finally, the review summarizes the established roles of the endogenous peptides, synthetic peptides and the selective ligands of PKR1 and PKR2, and nonpeptide orthostatic and allosteric modulator of the receptors in preclinical disease models. The present review emphasizes the ambiguous aspects and gaps in our knowledge of functions of PKR ligands and elucidates future perspectives for PK research.
Significance Statement
This review provides an in-depth view of the prokineticin family and PK receptors that can be active without their endogenous ligand and exhibits “constitutive” activity in diseases. Their non- peptide ligands display promising effects in several preclinical disease models. PKs can be the diagnostic biomarker of several diseases. A thorough understanding of the role of prokineticin family and their receptor types in health and diseases is critical to develop novel therapeutic strategies with safety concerns.
I. Prokineticin and Prokineticin Receptors
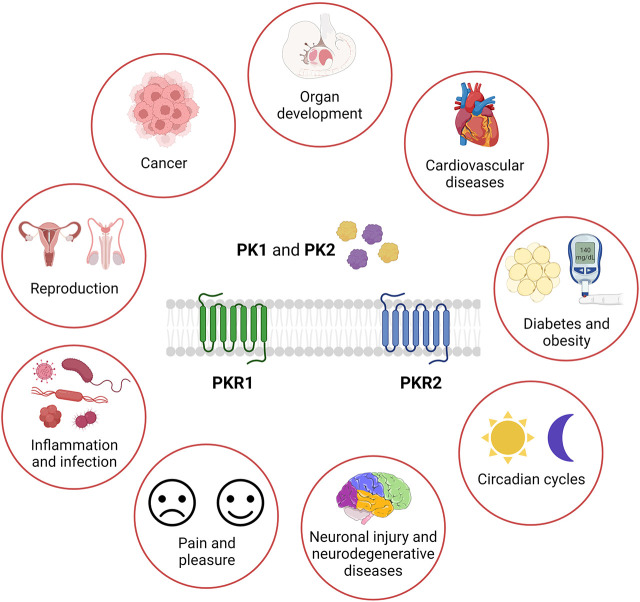
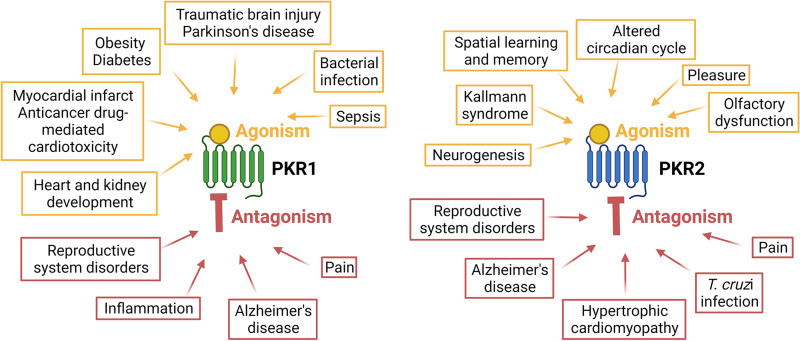
The history of the prokineticin family began in 1999 with the isolation of the mamba intestinal toxin-1 from the venom of the black mamba (Schweitz et al., 1999) and an 8 kDa protein from the skin secretions of the frog Bombina variegata (Bv8) (Mollay et al., 1999). These small proteins were named prokineticins (PKs) due to their ability to stimulate gastrointestinal smooth muscle contraction (Li et al., 2001). PKs exist in 2 forms: prokineticin 1 (PK1) and prokineticin 2 (PK2). PK1 was initially designated endocrine gland-specific vascular endothelial growth factor (EG-VEGF) because of its functional similarities to VEGF (LeCouter et al., 2001, 2003a; LeCouter and Ferrara, 2003). PKs bind to two closely related G protein-coupled receptors (GPCRs), prokineticin receptor 1 and 2 (PKR1 and PKR2, respectively) (Lin et al., 2002a; Masuda et al., 2002; Soga et al., 2002). Prokineticins and their receptors are broadly distributed in mammalian organs and tissues (Cheng et al., 2006; Ngan et al. 2007) where they are involved in numerous physiologic processes. The dysregulation of the PK system in several pathologic conditions as described in the following sections suggests that pharmacological antagonism or agonism of PKRs could be a potential and novel approach for the treatment of various diseases.
A. Prokineticins: Structure, Distribution, and Regulation
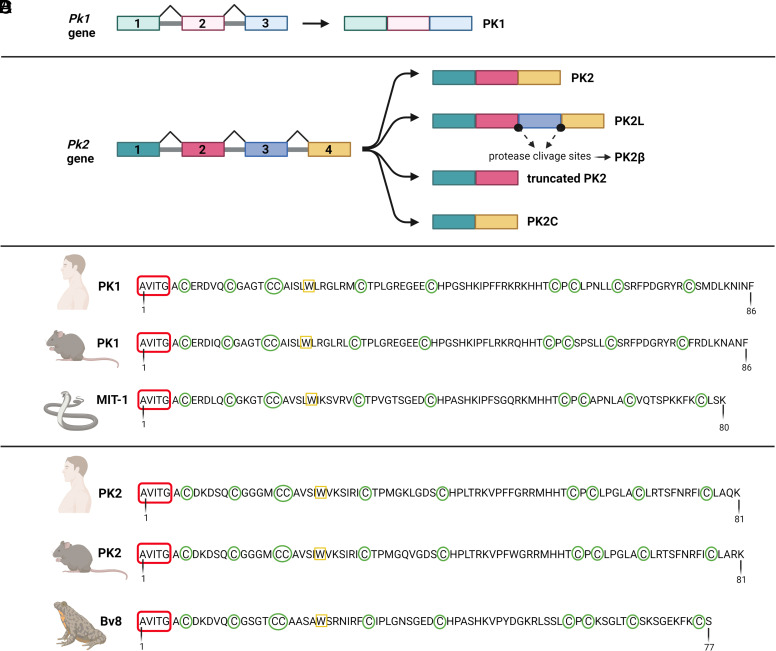
The genomic structure and chromosomal localization of human and murine PKs are determined. The pk1 gene, located on human chromosome 1p13.1 and murine chromosome 3, consists of 3 exons (Kaser et al., 2003): the first encodes 19 residues of the signal peptide and an alanine, valine, isoleucine, threonine, glycine (AVITG) sequence, the second 42 residues, and the third the last 39 residues (LeCouter et al., 2003a) (Fig. 1A). To date, only one PK1 transcript (86 amino acids) has been identified without any alternative splicing variants. In contrast, the pk2 gene is located on human chromosome 3p21.1 and murine chromosome 6 and consists in 4 exons (Jilek et al., 2000). Its transcription can result in four different splicing- variants due to the presence of the additional exon (exon 3) (Jilek et al., 2000): the canonical PK2 (81 amino acids) encoded by exons 1, 2, and 4 (LeCouter et al., 2003b); the inactive long form of PK2 (PK2L – 102 amino acids) encoded by all four exons and activated in vivo by proteolytic cleavage (PK2β) (Chen et al., 2005); the truncated PK2 (72 amino acids) encoded by exons 1 and 2 and containing part of introns 2 and 3′ (Jilek et al., 2000); and the PK2C (65 amino acids) encoded by exons 1 and 4 (Lattanzi et al., 2022) (Fig. 1B).
Fig. 1.
Schematical representation of prokineticin systems. (A, B) Prokineticin genes and their splicing isoforms. (C, D) Amino acid sequence of mammalian prokineticins, mamba intestinal toxin 1 and Bv8. Created with BioRender.com.
The amino acid sequence analysis of PK1 and PK2 revealed a high percentage of sequence conservation across the species (Fig. 1, C and D). They share characteristic, highly conserved structural motifs (Li et al., 2001; Lin et al., 2002a). The AVITG sequence at the amino-terminus (NH2)-terminus, the tryptophan at position 24 (Trp24), and the COOH-terminal domain are critical for receptor binding and activation (Kaser et al., 2003; Bullock et al., 2004). Substitutions (Bullock et al., 2004; Dodé and Rondard, 2013), insertions (Bullock et al., 2004), or deletions (Negri et al., 2005) in these portions result in products with antagonistic activity, with reduced receptor affinity and therefore lower efficacy, or without any biologic functionality (Bullock et al., 2004; Lattanzi et al., 2012).
Moreover, the presence of charged residues inside, hydrophobic residues on the surface, and ten evenly distributed Cys forming 5-disulphide bridges gives these molecules a compact structure noted as colipase folding, with NH2- and COOH-ends on the surface (Boisbouvier et al., 1998; Kaser et al., 2003; Morales et al., 2010).
PKs are ubiquitously expressed thoughout the mammalian species. In general, the expression profile of PKs is dynamic. Regulators of PKs have been found at both transcriptional and post-transcriptional levels. At the transcriptional stage, the expression of PK1 and PK2 is modulated by hypoxia via the hypoxia-induced factor one binding site in their promoter regions (LeCouter et al., 2001, 2003a). Hormones such as steroids, follicle-stimulating hormone, and human chorionic gonadotropin (Brouillet et al., 2012a), high insulin concentration (Ujvari et al., 2018), and peroxisome proliferator-activated receptor gamma (Garnier et al., 2015) regulate PK1 in the reproductive tract. Instead, proneural basic helix-loop-helix factors (Zhang et al., 2007), circadian rhythm genes (Cheng et al., 2005), homeobox transcription factors, and granulocyte colony-stimulating factor regulate PK2 expression (Hoffmann et al., 2006). At the post-transcriptional level, the major prokineticin regulators are microRNAs (Su et al., 2017; Meng et al., 2019; Wang et al., 2019).
B. Prokineticin Receptors: Structure, Distribution, Regulation, and Signaling
Prokineticin receptors were originally called as GPR73a and GPR73b, orphan receptors. Molecular cloning and characterization of prokineticin receptors (PKRs) were independently performed by three different research groups in 2002 (Lin et al., 2002a; Masuda et al., 2002; Soga et al., 2002). PKRs consist of seven transmembrane (TM) segments connected by alternative extracellular and intracellular loops (ECL and ICL, respectively). Due to the presence of two Cys forming a disulphide bond between ECL2 and ECL3, they belong to the “rhodopsin-like” or class A GPCRs, as the chemokine and opioid receptors (Vincenzi et al., 2022). Although PKRs are widely distributed and commonly co-expressed in various organs and tissues, analysis of expression pattern revealed that PKR1 is mainly localized in peripheral tissues such as the gastrointestinal tract, adipose tissue, endocrine glands, reproductive organs, lungs, heart, and hematopoietic cells, whereas PKR2 is mainly found in the central nervous system (CNS) (Masuda et al., 2002).
Human and mouse PKRs are 80% identical. PKR1 and PKR2 have approximatively 85% amino acid homology, with most differences localized in the NH2-terminus (Lin et al., 2002a). The genes encoding PKRs are organized in three exons and located on two different chromosomes: the pkr1 gene on human 2p.13.3 and mouse chromosome 6; the pkr2 gene on human 20p13 and mouse chromosome 2 (Parker et al., 2000; Lin et al., 2002a). Pkr2 gene is in a region with high mutational propensity. Indeed, numerous pkr2 pathogenic rare mutations and polymorphisms have been associated with various pathologies, including Kallmann syndrome, Hirschsprung syndrome, idiopathic pregnancy loss, precocious puberty, mood disorders, methamphetamine abuse and other diseases as documented in the supplemental data (Supplemental Table 1). In addition, a splicing variant of PKR2 containing only the last three TM domains (TM4-7) has been identified as a functional receptor that can form heterodimers with PKR2 and can be upregulated in pathologic states (Lattanzi et al., 2019a).
PKRs are activated by nanomolar concentrations of PK1 and PK2. PK2 has a higher affinity than PK1 for PKRs and slightly higher affinity for PKR1 than PKR2 (Lin et al., 2002a; Soga et al., 2002). PK2β acts as a PKR1-selective ligand (Chen et al., 2005; Lattanzi et al., 2018), whereas PK2C binds both PKR1 and PKR2 (Lattanzi et al., 2022).
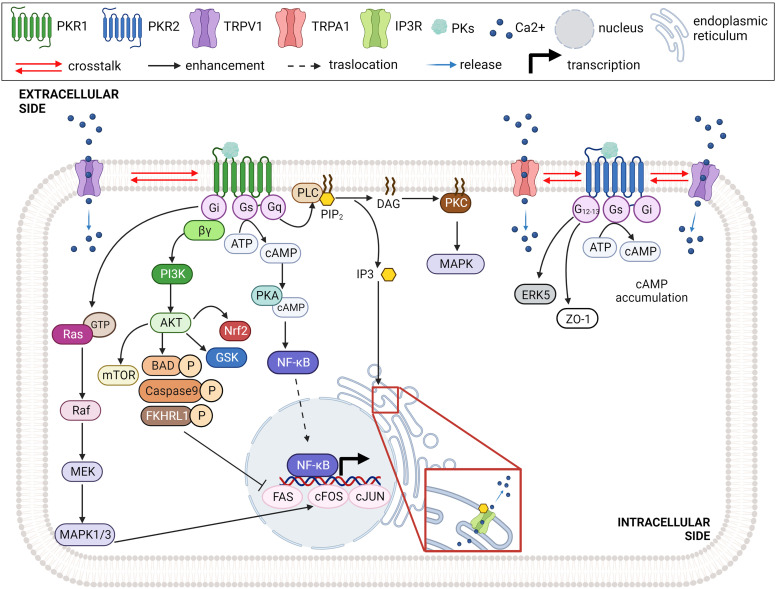
All these endogenous prokineticins were first recognized by NH2-end and ECL2 (extracellular site 1) of the PKRs. The insertion of the peptide sequence AVITG of prokineticins into an orthosteric TM-binding site (site 2) of receptors leads to the conformational changes required for activation of signal transduction. The orthosteric TM-binding pocket also accommodates small non-peptide antagonists of PKRs and is extremely conserved in PKR1 and PKR2 (Levit et al., 2011). Allosteric small-molecule binding site is located among TMs 3, 4, 5, 6, and 7 and accommodates PKR1 agonist that interacts with Arg144, Asn141, Gly219, and Phe300 within the PKR1 binding pocket (Gasser et al., 2015). Interestingly, the only different residue in the ECL2 (Val207 in PKR1 corresponding to Phe198 in PKR2) could be important for designing subtype-specific ligands and targeting PKR-mediated different pathologic conditions (Levit et al., 2011). Intracellular loops (ICL2 and ICL3) of PKRs are essential for interaction with G proteins and mutations at these sites can reduce G-coupling, leading to loss of function (Peng et al., 2011). Activated PKRs trigger multiple intracellular signaling pathways via the coupling of several heterotrimeric G proteins (Fig. 2), such as Gq/11, Gi/o and Gs or selectively binding to β-arrestin 2 (Casella and Ambrosio, 2021). The genetic inactivation of PKRs in mice displayed developmental defects and organ dysfunction as summarized in Table 1.
Fig. 2.
Intracellular signaling pathway triggered by PK/PKR binding. PKR1 exert its biologic activity by activating Gi, Gs, Gq signaling pathway, whereas PKR2 uses G12/13, Gs and Gi pathways dependent on cell type, expression levels, and pathologic condition. Created with BioRender.com.
TABLE 1.
The genetically inactivation of PKRs in mice displayed developmental defects and organ dysfunction
| Diseases | In vivo model | Reference | |
|---|---|---|---|
| Genetically manipulated mice | |||
| Abnormal organ development | Epicardial specific PKR1(−/−) | Embryonic lethality due to impaired heart development | (Arora et al., 2016a) |
| Nephron specific PKR1(−/−) | Impaired nephrogenesis and glomerulogenesis | (Arora et al., 2016b) | |
| PKR1(−/−) | Dilated cardiomyopathy and vascular rarefaction, macrophage infiltration, lipotoxicity, fibrosis in heart | (Boulberdaa et al., 2011) | |
| PKR2(−/−) | OB hypoplasia, severe atrophy of the reproductive system, including the testis, ovary, uterus, vagina, and mammary glands, defective migration and differentiation of neuronal progenitors | (Matsumoto et al., 2006; Prosser et al., 2007) | |
| PK2(−/−) | Small OB, and the accumulation of neuronal progenitors in the RMS, disrupted GnRH neuron migration, hypogonadotropic hypogonadism |
(Ng et al., 2005; Pitteloud et al., 2007) | |
| PKR2LacZ/+ and PK2EGFP mice PKR2(−/−) PK2(−/−) |
Tangential and radial migration defects of neuroblasts in the SVZ-RMS-OB resulting in loss of ∼75% of GABAergic interneurons in the OB | (Wen et al., 2019) | |
| Cardiovascular diseases (CVDs) | Endothelial specific-PKR1(−/−) | Dilated cardiomyopathy and vascular rarefaction | (Dormishian et al., 2013) |
| Cardiac fibroblast progenitor-specific PKR1(−/−) | Vascular rarefaction and development of Epicardial adipose tissue | (Qureshi et al., 2017) | |
| TG-PKR2 (PKR2 overexpression in cardiomyocytes) | Hypertrophic cardiomyopathy with endotheliopathy | (Urayama et al., 2009) | |
| TG-PKR1 (PKR1 overexpression in cardiomyocytes) | Neovasculogenesis, activation of epicardial progenitor cells | (Urayama et al., 2008) | |
| Diabetes | Endothelial specific-PKR1(−/−) | Lipodystrophy, Insulin resistance | (Dormishian et al., 2013) |
| PKR1(−/−) (40 weeks old) | Obesity and diabetes | (Szatkowski et al., 2013) | |
| Obesity | PKR1(−/−) and PKR2(−/−) | PK2 via PKR1 reduces food intake and body weight in a mouse model of human obesity. | (Beale et al., 2013) |
| PKR1(−/−) (40 weeks old) | Obesity and diabetes, Adipogenesis, infiltration of macrophage into fat tissue |
(Szatkowski et al., 2013) | |
| PK2(−/−) | Absence of the fasting-induced arousal, and d less energy expenditure, torpor after fasting | (Zhou et al., 2012) | |
| PKR2(−/−) | Hypothalamic regulation of energy balance, fasting induced hypothermia and torpor | (Jethwa et al., 2008) | |
| Adipocyte specific-PKR1(−/−) | Obesity, accumulation of fat tissue, increase adipogenesis | (Szatkowski et al., 2013) | |
| Circadian cycle alteration | PK2(−/−) PKR2(−/−) |
Significantly reduced rhythmicity for sleep-wake cycle, body temperature, as well as the expression of peripheral clock genes, precision in timing the onset of nocturnal locomotor activity | (Li et al., 2006; Hu et al., 2007; Prosser et al., 2007; Jethwa et al., 2008) |
| TG-PK2 (PK2 overexpression) | Reduced oscillation of PK2 mRNA levels in the SCN and decreased amplitude of behavioral rhythm | (Li et al., 2018) | |
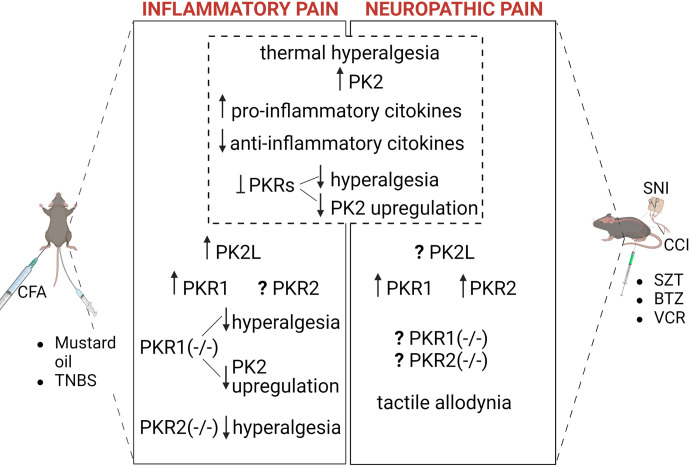
| Pain | PK2(−/−) PKR1(−/−) |
Attenuated thermal and noxious chemical stimuli-mediated nociception in -PK2(−/−) Impaired nociception and inflammatory pain sensation in PKR1(−/−) |
(Martucci et al., 2006; Negri et al., 2006a; Franchi et al., 2008) |
| PKR2(−/−) | Reduced nociceptive sensitivity to the noxious cold temperature of 4°C and hot temperatures of 46°C and 48°C in the workingrange | (Maftei et al., 2020) | |
| PKR1(−/−) and PKR2(−/−) | Less inflammation-induced hyperalgesia | (Giannini et al., 2009) | |
| PK2(−/−) | Strong reduction in nociception induced by thermal and chemical stimuli, capsaicin, but no difference in inflammatory response to capsaicin | (Hu et al., 2006) | |
| Inflammation and infection | PKR1(−/−) PK2(−/−) |
Loss of macrophage migration, proinflammatory phenotype, (T-helper1 cytokines (IL-2, IL-1beta) in PKR1(−/−) Low survival rate of sepsis in PK2(−/−) mice |
(Martucci et al., 2006; Franchi et al., 2008; Yu et al., 2022) |
The activation of PKRs and the interaction with Gq protein mediate the intracellular mobilization of calcium (Ca2+) through the activity of phospholipase-C and the formation of inositol triphosphate (Lin et al., 2002a; Masuda et al., 2002). Moreover, PK2 can induce translocation of protein kinase C (PKC)-ε to the plasma membrane in neurons via Gq (Vellani et al., 2006). Activation of PKRs/Gq sensitizes transient receptor potential cation channel subfamily V member 1 (TRPV1) and enhances the hyperalgesia mediated by PK2 (Negri et al., 2006a; Vellani et al., 2006; Maftei et al., 2020). PKR2 can also couple with G12 in endothelial cells (ECs) and reduce the expression of tight junction proteins, such as zona occludens-1, leading to fenestrations of these cells and increased vascular permeability (Urayama et al., 2007). PKRs can also signal through Gi-coupling. For example, Lin et al. (Lin et al., 2002b) reported that pertussis toxin pre-treatment inhibits the PK1/PKR1-induced p44/p42 mitogen-activated protein kinase (MAPK) cascade, suggesting Gi protein involvement. PK1, via Gi, can also trigger phosphorylation of Akt through activation of the phosphatidylinositol 3-kinase leading to EC differentiation, proliferation, and migration (Lin et al., 2002b). The ability of PK2 to stimulate phosphorylation of p44/p42 MAPK in both PKR1- and PKR2-transfected cells has also been described (Lin et al., 2002a). Moreover, the activation of both PKRs induces cAMP accumulation via the Gs protein and regulates, for example, contractility (Chen et al., 2005; Nguyen et al., 2013).
C. Exogenous Prokineticin Receptor Ligands: Antagonists
Several inhibitors of PKRs have been patented by pharmaceutical companies, but, details of their design have not been published. The first hits for such series were likely found as results of screening large high-throughput screening libraries, which were then optimized to increase activity and improve their ADMETox properties.
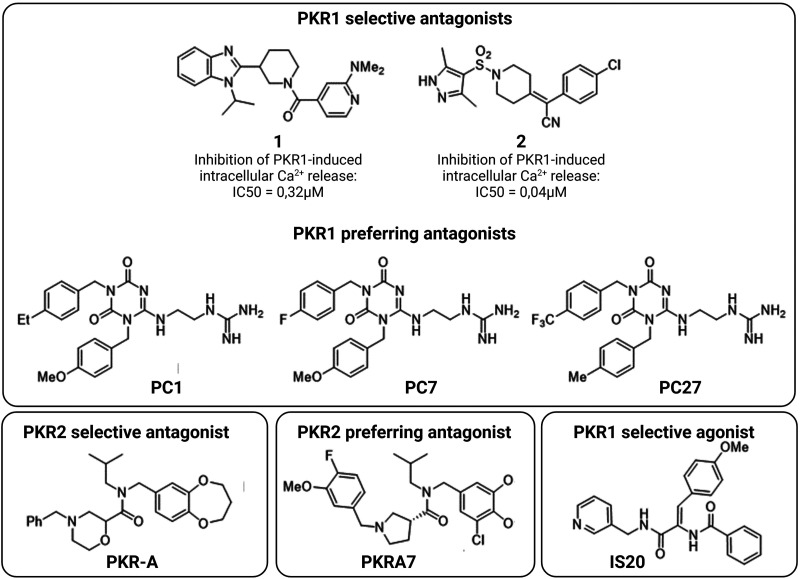
Scientists of Takeda Pharmaceutical Company identified two series of PKR1 antagonists, illustrated by compounds 1 (Goldby et al., 2015) and 2 (Mitchell and Teall, 2016) (Fig. 3), for the treatment of gastrointestinal, psychiatric, and neurologic disorders. Thompson et al. (Thompson and Melamed, 2007) of Merck & Co. patented a series of compounds, such as PKR-A, which acts as a PKR2 antagonist with IC50 < 10 μM. Cheng et al. showed that PKR-A inhibits PKR2-induced Ca2+ release with an IC50 of 48nM. In vivo, it reduces infarct volume and central inflammation while improving functional outcome in a rat model of cerebral ischemia (Cheng et al., 2012). Its analog PKRA7 non-selectively inhibits both PKR1 and PKR2 in low nanomolar range. In vivo, PKRA7 inhibits angiogenesis in gliomas and blocks myeloid cell infiltration in pancreatic cancer (Curtis et al., 2013).
Fig. 3.
Representative small molecules as PKR1 and PKR2 ligands. PC1, PC7, and PC27 are the PKR1 preferring antagonist. PKR-A and PKRA7 are PKR2 selective and preferring antagonist, respectively. IS20 is a PKR1 selective agonist. Created with BioRender.com.
Balboni et al. (Balboni et al., 2008) utilized patented nonpeptidic PKR antagonists deposited by Janssen and Merck as the basis for developing a series of triazinediones that selectively inhibit PKR1 and, to a lesser extent, PKR2. This group created homology models of the human PKR1 and PKR2 (universal protein codes: Q8TCW9 and Q8NFJ6, respectively) using the crystal structures of the human kappa opioid (Protein Data Bank code: 4DJH) and neurotensin-1 (Protein Data Bank code: 4GRV) receptors as templates. In this way, docking studies allowed the authors to design some triazinediones as new PKR1 antagonists, such as PC1 (Lattanzi et al., 2015a) (Fig. 3). PC1 was designed to mimic the N-terminal AVITG sequence, while the methoxybenzyl moiety acts as an isosterol of the indole of the Trp24. PC1 inhibits intracellular Ca2+ mobilization induced by both PKR1 and PKR2. In vivo, it alleviates various disorders triggered by overactivation of PK2 signaling, such as inflammatory (Giannini et al., 2009) and neuropathic pain (Maftei et al., 2014; Guida et al., 2015; Lattanzi et al., 2015a,b; Castelli et al., 2016; Moschetti et al., 2019a) and Alzheimer’s disease (Maftei et al., 2019). The analog PC7 shows enhanced analgesic effects and also attenuates experimental autoimmune encephalomyelitis and preeclampsia in mice (Abou-Hamdan et al., 2015; Reynaud et al., 2021). Further improvements led to PC27, which exhibits analgesic activity with an EC50 ten times lower than that of PC7 (Congiu et al., 2014).
1. Prokineticin Receptor Ligands: Agonists
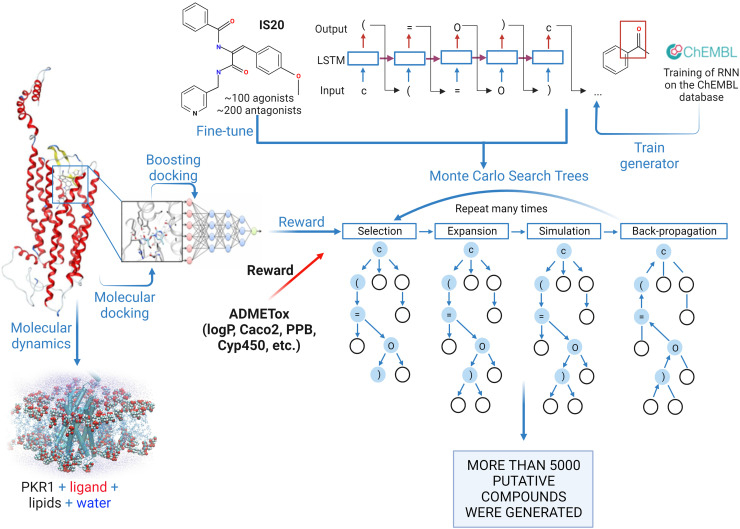
A similar approach of Balboni et al. (Balboni et al. 2008) was used to develop a series of dehydroamides as agonists of PKR1 (Gasser et al., 2015). PKR1 sequence alignment and protein folding using “CPHmodels” software tools were used to identify the best template crystal structure, which was the turkey β1 adrenergic receptor (Protein Data Bank ID: 2VT4). Whereas previous homology studies examined traditional structure-based design, a deep-learning method based on recurrent neural network and Monte Carlo Tree Search was used to generate a new set of putative PKR1 agonists (Karpov, 2022) (Fig. 4). These studies were based on recurrent neural networks, which were pre-trained on the ChEMBL database (Mendez et al., 2019) as described elsewhere (Xia et al., 2020). Among the putative hits, a series of dehydroamides, such as IS20 (Fig. 3), were found to act as selective, biased PKR1 agonists that bind the Arg144, Asn141, Gly219, and Phe300 in the allosteric pocket of PKR1 (Gasser et al., 2015). IS20 shows potent cardioprotective effects in mouse models of heart failure and doxorubicin (DOX)-induced cardiotoxicity (Gasser et al., 2015, 2019). It also shows potent neuroprotective effects in a model of Parkinson’s disease (PD) (Neal et al., 2018).
Fig. 4.
Illustration of the workflow used to generate new PKR1 agonists. These studies were based on recurrent neural networks, which were pre-trained on the ChEMBL database. A powerful machine learning algorithm have been developed which was the first trained on the SMILES canonicalization task using public ChEMBL molecules with the following fine-tuning on PKR1 data. ADMETox filters developed Transformer CNN (https://jcheminf.biomedcentral.com/articles/10.1186/s13321-020-00423-w) as well as molecular docking scores were used as reward to select compounds and fine-tune the generator. Molecular dynamic simulations were used to prioritize the generated compounds for experimental testing. Created with BioRender.com.
II. Role of the Prokineticins in Organ Development
Both PKR1 and PKR2 receptors are expressed in cardiovascular renal and neuronal tissues. PKR1 express in epicardial progenitor cells (EPDCs), coronary endothelial cells, cardiac fibroblasts, and cardiomyocytes (Urayama et al., 2007). PKR1 expression has been found in the glomerular epithelial cells (podocytes) and ECs, mesenchymal cells, more specifically in cap mesenchyme (nephron progenitors) during embryogenesis and tubular cells in the adult kidney (Arora et al., 2016b). PKR1 appears to be involved in heart and kidney development, while PKR2 is implicated in the development of olfactory bulb (OB).
A. Role of Prokineticin in Heart Development
Congenital heart defects are the principal birth defects that can lead to a variety of congenital diseases in adulthood. The epicardium, composed of heterogenous epithelial cells, covers the heart and plays a key role in cardiac development and regeneration. It has been implicated in potential repair strategies for the heart. The epicardium undergoes epicardial mesenchymal transformation to form EPDCs. EPDCs can differentiate into many cardiac cells (ECs, pericytes, smooth muscle cells) and play an important role in myocardium maturation. Previous studies have shown that epicardial-PKR1 signaling plays a role in cardiac development, and that a defect in epicardial signaling due to a deficiency of PKR1 in the epicardium leads to forms of congenital heart disease in the adult heart (Boulberdaa et al., 2011). More specifically, genetic inactivation of PKR1 specifically in the epicardium of mice, using epicardial specific such as Wilms tumor 1 (WT1 GFPcre) and Gata4cre mice, impairs epicardial mesenchymal transformation in the heart (Arora et al., 2016a). These mice showed inadequate development of coronary vascular networks and impaired interactions between EPDCs-cardiomyocytes, resulting in impaired cardiomyocyte proliferation and cardiac arrhythmia. All these abnormalities in cardiac developmenl lead to partial embryonic and postnatal mortality (Arora et al., 2016a) as seen in the total PKR1-knockout (PKR1(−/−)) (Boulberdaa et al., 2011). The impairment of the coronary vascular network in epicardial PKR1-deficient mice (PKR1−/−) is due to impaired differentiation of EPDCs into vasculogenic cell types. However, the reduced cardiomyocyte proliferation and contractile deficits result from abnormal release of epicardial paracrine factors (miRNAs) in the mutant hearts (unpublished observations). Lipid deposition in the mutant hearts is due to the lack of control of EPDCs’ differentiation into adipocytes as observed in vitro (Qureshi et al., 2018). Survival rates of adult mice were reduced by 80% after coronary ligation as a myocardial mouse model, suggesting that the mortality in these mice after myocardial infarction was due to congenital cardiac dysfunction.
B. Role of Prokineticin in Kidney Development
During kidney development, nephrogenesis and glomerulogenesis are important events. The mesenchymal cells form pretubular aggregates and undergo mesenchymal-epithelial transition (MET) to form nephrons during nephrogenesis. Nephrons are the filtering units of the kidney. However, glomerulogenesis occurs via angiogenesis and metanephric vasculogenesis through the differentiation of progenitor cells. The number of nephrons plays an important role in renal function, and any defect in nephron development can lead to hypertension problems later in life.
Studies in mice have shown that the PK2/PKR1 signaling pathway is essential for kidney development. Using two cre transgenic lines (Gata5 and WT1), ablation of PKR1 specifically in nephron progenitors caused partial embryonic and postnatal lethality because of a lack of MET in WT1+ renal mesenchymal cells. The defective MET leads to impaired proliferation and increased apoptosis in WT1+ renal mesenchymal cells, resulting in hypoplastic kidneys with premature glomeruli and necrotic nephrons. Moreover, PKR1 activation in cultured WT1+ embryonic kidney progenitor cells promote MET and accumulation of NFATc3 in the nucleus. Both events were alleviated by an NFATc3 inhibitor and siRNA for PKR1-mediated MET process in cultured WT1+ embryonic kidney cells, suggesting that PKR1 promotes the MET process via NFATc3 signaling. Similarly, PKR1(−/−) mice exhibited neonatal kidney disorders (Boulberdaa et al., 2011). Disruption of capillary angiogenesis and severe tubular defect were observed in endothelial-specific PKR1-knockout (ec-PKR1(−/−)) mice (Dormishian et al., 2013).
C. Prokineticin in Brain Development and Kallmann Syndrome
Neurogenesis begins when neuronal progenitor and stem cells begin differentiating division, resulting in the formation of neurons and glia in the cortical layers. Neurogenesis occurs in the embryonic to early postnatal stages. In the adult mammalian brain, neurogenesis also occurs in two neural niches, the subgranular layer of the dentate gyrus (DG) and the OB throughout life and after stroke during the recovery process. Indeed, PKR1 expression was observed in the granular and periglomerular layers of the OB. PKR2, on the other hand, is expressed in the subventricular zone (SVZ), the entire rostral migratory stream (RMS), and the ependymal and subependymal layers of the olfactory ventricle, where adult neurogenesis occurs (Cheng et al., 2006). In vertebrates, the hypothalamic-pituitary-gonadal axis controls reproduction through a pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. GnRH-expressing neurons are formed in the olfactory placode which directs their migration to their final destination, the hypothalamus. GnRH reaches the anterior pituitary, binds to GnRH receptor 1, and induces secretion of the two pituitary gonadotropins, luteinizing hormone and follicle-stimulating hormone, which ultimately stimulate steroid production and gametogenesis in both males and females (Plant, 2015).
Idiopathic hypogonadotropic hypogonadism (IHH) is a rare congenital disorder of the hypothalamic-pituitary-gonadal axis characterized by failure of gonadotropin secretion leading to delayed puberty and infertility. When congenital IHH is associated with anosmia, it is referred to as Kallmann syndrome (KS). Kallmann syndrome and IHH are genetically heterogeneous (Vezzoli et al., 2016). Like several GPCRs, PKR2 is also involved in GnRH neurons development and migration (Ng et al., 2005; Martin et al., 2011). Pkr2 and pk2 were identified as Kallmann genes by analyzing the phenotype of PKR2(−/−) or PK2(−/−) mice, which show many KS-like features. PKR2(−/−) or PK2(−/−) mice exhibit an OB that is reduced in size, has an altered architecture, and has an accumulation of neural progenitor cells (Ng et al., 2005), resulting in a reduced number of GnRH neurons in the hypothalamus. Lack of GnRH secretion is associated with low plasma levels of testosterone and follicle-stimulating hormone and impaired sexual development. Male PKR2(−/−) or PK2(−/−) mice show small testicular tubules with no lumen, Leydig cells with reduced interstitial space, and absent haploid spermatocytes and spermatids. Similarly, PKR2(−/−) or PK2(−/−) mice exhibit impaired estrous cycles due to the absence of mature follicles and corpora lutea (Matsumoto et al., 2006; Pitteloud et al., 2007).
The IHH phenotype is observed in mice only in the presence of homozygous pk2 mutations, whereas the KS phenotype also occurs in the presence of a heterozygous pk2 or pkr2 mutation, suggesting that additional mutations in disease-related genes may be involved in this case (Pitteloud et al., 2007; Cox et al., 2018). Pk2 and pkr2 mutations have also been observed in human patients with IHH and KS (Abreu et al., 2010). The severity of the phenotype of individuals with KS carrying pkr2 mutations in the heterozygous state is in most cases due to the synergistic effect of the pathogenic mutations. The pkr2 is involved in several digenic and trigenic associations such as PK2/PKR2, FGFR1/PKR2, PK2/GNRHR, and PKR2/CHD7/FEZF1 (Cole et al., 2008; Canto et al., 2009; Sarfati et al., 2010a,b; Méndez et al., 2015; Zhang et al., 2020).
Most Kallmann-pkr2 mutations localized in the highly conserved transmembrane domains alter the folding of receptor proteins retained by quality control system in the endoplasmic reticulum (Araki and Nagata, 2011). Some cell-permeable small antagonists/agonists have been described as molecules that can rescue the phenotype by interacting with intracellularly retained receptors to assist their folding and transport to the plasma membrane (Chen et al., 2014). Other mutations in the cytoplasmic domain of PKR2, which includes the first, second, and third ICL and the carboxyl tail, likely result in impaired G-protein coupling. Finally, pkr2-mutations in the extracellular domain impair ligand binding (Martin et al., 2011; Libri et al., 2014; Sbai et al., 2014).
The homozygous pkr2 founder mutation L173R is responsible for the infertility characteristic of KS. In contrast, patients with the heterozygous condition do not show infertility associated with GnRH deficiency, but exhibit selectively increased protection against Trypanosoma cruzi (T. cruzi) infection, which may explain the high occurrence of the heterozygous pkr2 L173R mutation in the human genome (Avbelj Stefanija et al., 2012; Lattanzi et al., 2021).
A paradoxical gain-of-function mutant of pkr2 was found in a patient with early puberty and designated TM1-5 because it lacks the last two transmembrane domains and the carboxyl-terminal tail. Analysis in cell culture showed that the TM1-5 mutant lacked signal transduction activity, but co-transfection of TM1-5 markedly increased ligand-induced signaling responses of cells expressing wild-type PKR2 (Sposini et al., 2015; Fukami et al., 2017). In 192 KS patients, Dodé et al. (Dodé et al., 2006) identified ten and four different point mutations in the genes encoding PKR2 and PK2, respectively. Interestingly, one patient carrying the p.R73C mutation in the pk2 gene suffered from severe sleep disturbance and marked obesity. Abreu et al. (Abreu et al., 2008) reported the case of three patients with KS who had mutations in the pkr2 gene (p.L173R or p.R268C mutations in the heterozygous state) and developed obesity without or with (in 1 of 3 patients) type 2 diabetes, chronic arterial hypertension, and hypertriglyceridemia. Recent studies have shown a loss of ∼75% of GABAergic interneurons in OB of both PK2 and PKR2 mutant mice, indicating that PK2/PKR2 signaling is an important player of survival and migration of GnRH neurons and is critical for tangential and radial migration of OB interneurons rather than affecting OB neurogenesis (Wen et al., 2019).
III. Role of the Prokineticins in Cardiovascular Diseases
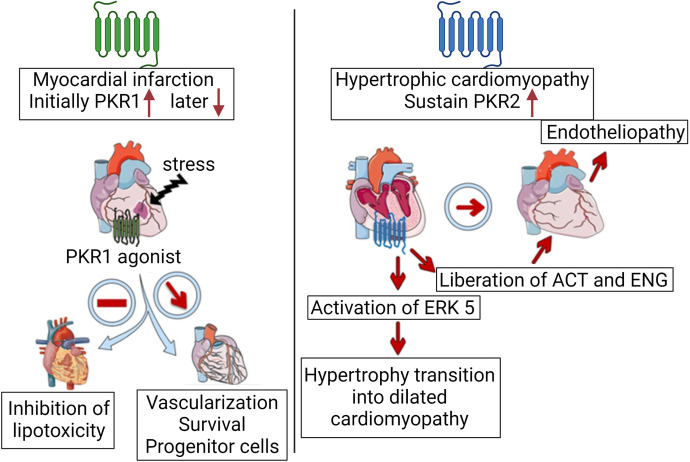
The expression and function of PKRs differ depending on cardiovascular pathology. For example, low expression of the PKR1 was found in human transplant hearts with heart failure (HF) compared with control transplant hearts (Urayama et al., 2007). PKR2 is highly expressed in human hypertrophic cardiomyopathy transplant heart samples. Elevated levels of PK2 have been detected in human abdominal aortic aneurysms (Choke et al., 2010). Both PKR1 and PKR2 are expressed in cardiomyocytes, epicardial progenitor cells, ECs, and fibroblasts. Indeed, the signaling pathways mediated by PKR1 and PKR2 act in opposite directions in cardiac cells (Nebigil, 2009; Désaubry et al., 2020) (Fig. 5). In mice, PK2/PKR1 signaling protects the heart from myocardial ischemia (Urayama et al., 2007), anticancer-mediated cardiotoxicity (Gasser et al., 2019), and diabetic-cardiomyopathy. PK2/PKR1 signaling activates proliferation, migration, and differentiation of epicardial progenitor cells (Urayama et al., 2008). However, PKR2 has been implicated in transition from hypertrophic cardiomyopathy to heart failure (Urayama et al., 2009). Expression of PK2 and its receptors increases after coronary ligation in mouse models of myocardial infarction (Nguyen et al., 2013). However, PKR1 expression rapidly decreases when apoptosis occurs, and PKR2 levels remain elevated (Urayama et al., 2007), suggesting that increased PK2/PKR1 expression reflects the initial compensatory remodeling, whereas increased PKR2 expression represents maladaptive remodeling in the injured heart. Targeting PKRs may be an important approach for the treatment of various types of cardiovascular diseases.
Fig. 5.
Role of PKRs in cardiac diseases. Increase in PKR1 and PKR2 levels together with PK2 has been observed right after the myocardial infarction in mice as a compensatory mechanism. However, PKR1 levels rapidly declines, and PKR1 gene therapy or PKR1 agonist activates survival pathway, induced capillary network formation for better perfusion, activates epicardial progenitor cells, and inhibits pathologic development of lipotoxicity. In the hypertrophic cardiomyopathy mice model, sustained PKR2 levels are involved in the transition of the hypertrophy to dilated cardiomyopathy via activating extracellular signal-regulated kinase 5 (ERK5) signaling. It also promotes liberation of activin (ACT) and endoglin (ENG) from hypertrophic cardiomyocytes acting as paracrine factors to promote endotheliopathy. Created with BioRender.com.
A. Prokineticin Receptor 1 Signaling in Myocardial Infarct-Induced Heart Failure
Myocardial infarction (MI) is the most common cause of HF leading to morbidity and mortality worldwide, despite the development of numerous therapeutic agents to treat HF. Expression of the entire PK system is increased three days after coronary ligation in a mouse model of MI (Nguyen et al., 2013). Only PKR1 levels declined rapidly after the compensatory remodeling phase of MI. Moreover, intracardiac pkr1 gene transfer after coronary ligation in a mouse model of MI reduces mortality and improves cardiovascular function by promoting angiogenesis and cardiomyocyte survival and increasing proliferation and mobilization of resident EPDCs toward the injured area (Urayama et al., 2009; Nguyen et al., 2013). Intraperitoneal administration of a PKR1 agonist, IS20, activates the AKT survival pathway in the heart. Moreover, IS20 treatment of mice with MI reduces mortality and improves cardiac function, which has a similar effect to intracardiac pkr1 gene transfer. Indeed, IS20 reduces apoptosis in cardiac cells, provokes proliferation of EPDCs, and increases capillary formation (Gasser et al., 2015).
B. Prokineticin Receptor 1 Signaling in Anticancer Drug-Mediated Heart Failure
Cardiotoxicity induced by anticancer drugs such as anthracyclines, targeted therapies, and immune checkpoint inhibitors causes ischemia, arrhythmia, hypertension, myocarditis, and cardiac dysfunction leading to HF. In mouse models of cardiotoxicity induced by the anthracycline doxorubicin (DOX), IS20 attenuates apoptosis and fibrosis and improves survival and cardiac function. Importantly, IS20 does not interfere with the antitumor effect of DOX in a mouse model of breast cancer (Gasser et al., 2019). In vitro activation or overexpression of PKR1 in cardiomyocytes activates AKT through phosphorylation to protect cardiomyocytes, EPDCs, and ECs from hypoxic insult (Guilini et al., 2010; Arora et al., 2016a) as well as DOX-mediated apoptosis (Gasser et al., 2019). DOX at high concentrations accumulates reactive oxygen species in cardiomyocytes, which are attenuated by IS20. The mechanism by which the detoxification pathway is activated is translocation of nuclear factor erythroid 2-related factor 2 (NRF2) to the nucleus, which increases expression of the detoxification gene.
In cardiac ECs, activation or overexpression of PKR1 induces ECs proliferation, migration, and branching to promote angiogenesis via Gα11-mediated regulation of both MAPK and AKT activity. Consistent with these findings, endothelial-specific PKR1(−/−) mice exhibit capillary rarefaction, apoptosis, and interstitial fibrosis, leading to cardiac and renal dysfunction. Indeed, these mice showed abnormal cardiac and renal insulin signaling, leading to ectopic lipid deposition (Dormishian et al., 2013).
C. Prokineticins/Prokineticin Receptor 2 Signaling in Development of Pathologic Hypertrophic Cardiomyopathy
Chronic overload of the heart caused by hypertension leads to pathologic hypertrophic growth of the myocardium and endotheliopathies such as vasoconstriction. It is not fully understood why there is a mismatch between the excessive energy demand of the myocardium during hypertrophic growth and angiogenesis in these pathologic events. Pathologic hypertrophy induced by transaortic constriction in mice increases the expression of PKR2 in their cardiomyocytes (Demir et al., 2021). Sustained activation of PKR2 activates the extracellular signal-regulated kinase 5 (ERK5) pathway to induce hypertrophy in cardiomyocytes. However, activation of the PKR2/Gα12/13/matrix metalloprotease pathway in cardiomyocytes leads to the release of activin A and soluble endoglin, which act as paracrine factors and induce endotheliopathies (vascular rarefaction) (Demir et al., 2021). Similarly, transgenic mice overexpressing PKR2 in their cardiomyocytes (TG-PKR2) showed hypertrophy and impaired endothelial integrity related to paracrine regulation (Urayama et al., 2009). Overall, pressure overload-mediated maintenance of PKR2 signaling in cardiomyocytes contributes to cardiac hypertrophy via autocrine signaling and to vascular rarefaction via cardiac cytokine-mediated communication between cardiomyocytes and ECs (Guilini et al., 2010; Alfaidy et al., 2019). In ECs cultured in vitro, activation of PKR2, unlike PKR1, does not induce angiogenesis but couples to Gα12-13-mediated degradation of zonula occludens one, a cell-cell adhesion molecule, at tight junctions and forms a fenestration of ECs. PKR1 expression is dominant in the physiologic state, whereas PKR2 becomes dominant upon pathologic stimuli (Guilini et al., 2010; Nebigil, 2016).
D. Prokineticin Receptor 1 Signaling Controls Fate of Adult Cardiac Transcription Factor 21-Positive Cardiac Fibroblast Progenitor Cells (Tcf21+CFP)
Transcription factor 21 (Tcf21) is expressed in mesenchymal tissues, including the epicardium, and plays a key role in cellular differentiation. Tcf 21+CFPs originate in the embryonic epicardium and continue to be expressed in quiescent adult CPFs with the promising potential for repairing injured heart. They localize to the epicardium, perivascular or interstitial areas, depending on the type of cardiac injury (Acharya et al., 2012). PK2 regulates the fate of adult tcf21+CFPs. It promotes vasculogenic transformation of tcf21+CFPs and inhibits their differentiation into adipocytes (Qureshi et al., 2017). Moreover, tcf21TMicre CFPs-restricted inducible knockout mice (tcf21-PKR1(−/−)) on a high-fat diet exhibit high levels of fat deposition in the pericardium, atrioventricular groove, and perivascular area, along with disrupted vascular networks, leading to impaired cardiac function (Qureshi et al., 2017).
PKR1 signaling also regulates EPDC activity in a paracrine manner. Overexpression of the pkr1 gene in transgenic mice heart does not cause structural and functional abnormalities in cardiomyocytes. In fact, increased PK2 production in their cardiomyocytes acts as a paracrine factor that promotes EPDC differentiation into endothelial and smooth muscle cells for neovascularization (Urayama et al., 2008).
IV. Role of the Prokineticins in Diabetes
Diabetes as a chronic metabolic disease can cause severe complications to the heart, eyes, kidneys, blood vessels and nerves due to high blood glucose levels. Type II diabetes, which is common in adults, is due to either insulin resistance or insufficient insulin production. The prokineticin/PKR1 pathway is involved in the transcapillary transport of insulin and protects various organs from the severe damage caused by diabetes. PKR1 agonists could therefore be potential agents for the treatment of co-morbidities of diabetes.
A. Prokineticin 2 Levels in Patients with Metabolic Syndrome
To date, two studies have been conducted in adult patients to investigate the association between PK2 and metabolic syndrome. Wang et al. (Wang et al., 2016) measured serum PK2 levels in 162 middle-aged and elderly Chinese patients with cardiovascular risk factors. They found a positive correlation with several cardiometabolic risk factors, including blood lipids, fasting plasma glucose, HbA1c, blood pressure, body mass index (BMI), and uric acid. However, multiple logistic regression analysis showed that PK2 was independently associated with metabolic syndrome.
Mortreux et al. (Mortreux et al., 2019) demonstrated that plasma PK2 was lower in participants with diabetes mellitus type 2 compared with nondiabetics in the D.E.S.I.R. cohort (Data from an Epidemiologic Study on the Insulin-Resistance syndrome), but this association disappeared after adjustment for BMI and/or caloric intake. In univariate regression studies, they showed that PK2 was significantly inversely associated with BMI, waist circumference, fasting blood glucose, HbA1c, and low-density lipoprotein cholesterol. In a multivariable model, BMI, energy intake, and plasma low-density lipoprotein cholesterol remained associated with PK2 levels.
B. Prokineticin Receptor 1 in Insulin Resistance
In blood vessels, the transcapillary passage of insulin from ECs to skeletal muscle is rate-limiting for the control of insulin-stimulated glucose uptake (Kubota et al., 2011). The impaired process of insulin delivery from ECs is a crucial step for the development of insulin resistance. Overexpression of PKR1 in ECs promotes not only angiogenesis but also transendothelial uptake of insulin, suggesting that PKR1 is a positive regulator of insulin uptake (Von Hunolstein and Nebigil, 2015). Interestingly, EC-specific PKR1 knockout mice (EC-PKR1(−/−)) show impaired capillary formation, low transcapillary insulin uptake, glucose and insulin sensitivity, resulting in polyphagia, polydipsia, and polyuria. EC-PKR1(−/−) mice have loss of adipose tissue with macrophage infiltration and fibrosis, resulting in severe lipodystrophy (Dormishian et al., 2013). This insulin resistance in EC-PKR1(−/−) could be rescued by pkr1 gene transfection with an adenovirus carrying PKR1 cDNA. The EC-PKR1(−/−) phenotype is reminiscent of peripheral insulin resistance, as human patients with type 2 diabetes exhibit impaired insulin secretion and endothelial dysfunction (Kolka and Bergman, 2013).
C. Prokineticin 2/Prokineticin Receptor 1 Pathway in Diabetes-Induced Cardiomyopathy
Diabetic cardiomyopathy is characterized by the structural, functional, and metabolic changes in the heart associated with diabetes, which leads to HF. Recently, studies in vitro and in vivo have shown that a drug used to treat type 2 diabetes, metformin, exerts a cardioprotective effect via the PK2/PKR1 pathway (Yang et al., 2020). Interestingly, the expression of PK2, PKR1, and PKR2 is reduced in diabetic mice. Accordingly, the low phosphorylated active form of Akt and glycogen synthase kinase-3 beta was attenuated by metformin treatment in these mice. Moreover, high glucose-mediated cardiomyocyte injury is protected by metformin or PK2 treatment, which can be reversed by a PKR1 antagonist (PC7) or an AKT inhibitor, indicating that metformin exerts a cardioprotective effect via activation of PKR1 pathway. Whether PKR1 agonists protect the heart from diabetes-associated cardiac remodeling remains to be investigated.
D. Role of Prokineticin Receptor 1 in Diabetes-Mediated Skeletal Muscle Dysfunction
Diabetes mellitus type 2 can also be caused by insulin resistance in skeletal muscle. The PKR1 pathway is involved in the regulation of metabolic function in murine myoblasts, satellite cells, and their differentiated myotubes via Gq-mediated phosphatidylinositol 3-kinase/AKT and MAPK/ERK signaling pathways. PKR1 promotes the translocation of glucose transporter 4 to the plasma membrane of skeletal muscle cells. In the model of palmitate-induced insulin resistance in myotubes, PKR1 increases insulin-stimulated glucose uptake and glucose transporter 4 translocation. Low levels of PKR1 were detected in obese mice induced to become obese by a high-fat diet and in human skeletal muscle cell-derived myotubes under conditions of insulin resistance. Taken together, these results suggest that PKR1 plays a key role in insulin sensitivity and may be a potential therapeutic target to improve skeletal muscle function in insulin resistance conditions (Mok et al., 2021).
E. Role of Prokineticin Receptor 1 in Diabetes-Mediated Renal, Neuronal, and Testicular Dysfunction
Diabetic nephropathy is characterized by severe glomerular and tubular damage, fibrosis, increased glomerular mesangial matrix, thickened basement membrane, exfoliated renal tubule brush border, and massive loss of podocytes. Proinflammatory and pro-fibrotic signaling pathways in glomerular and renal tubular cells lead to diabetic nephropathy. In db/db mice, low expression of PK2 and PKR1 is associated with low levels of phosphorylated Akt signaling. However, levels of PKR2 and phosphorylated ERK did not change significantly. A recent study showed that geniposide, a bioactive compound found in a variety of medicinal herbs such as Gardenia jasminoides, ameliorated renal damage in db/db mice with diabetic nephropathy, similar to WT1-PKR1(−/−) mice renal defects such as high glomerular and tubular injury, decreased WT1 in glomerular podocytes, and massive loss of podocytes (Arora et al., 2016b). The PK2/PKR1 pathway is involved in the protective effects of geniposide. Geniposide increases the expression levels of PK2, PKR1, and active Akt. It has been shown to be effective in the clinical treatment of diabetic nephropathy (Dai et al., 2022).
Matrine (Mat) is an active antidiabetic, cardioprotective, and neuroprotective component of Sophora flavescens ait root extracts. Mat administration increased protein expressions of PK2, PKR1, and PKR2 in the hippocampus, which decreased significantly in diabetic mice. In addition, Mat could also improve the diabetes-induced impairment of spatial learning and memory by alleviating endoplasmic reticulum stress and, in part, modulating PK2/PKR signaling (Zhang et al., 2022a).
More than 85% of male patients with diabetes develop testicular dysfunction. In streptozotocin-mediated mouse models of type 1 diabetes, reproductive capacity was found to be significantly impaired due to testicular dysfunction. This testicular dysfunction was ameliorated by metformin administration via the PK2/PKR pathway. The reduction of p-Akt and p-glycogen synthase kinase-3 beta in diabetes-induced testicular damage was normalized by metformin via the PK2/PKR1 pathway by attenuating apoptosis and inducing autophagy (Liu et al., 2019).
V. Prokineticins in Obesity and Visceral Adipose Tissue Growth
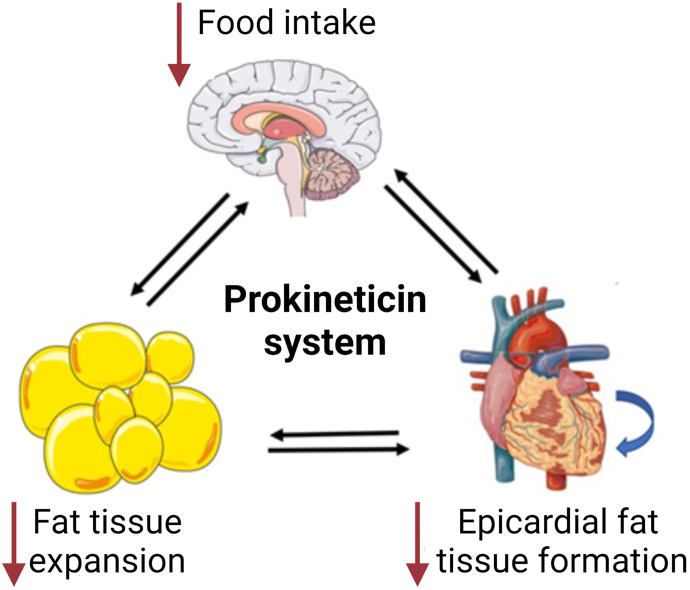
Obesity, defined as excessive adiposity, increases the risk of comorbidities such as heart disease, stroke, insulin resistance, diabetes, autoimmune diseases, and some cancers. The etiology of obesity is not fully understood, but there are several possible mechanisms that may be involved in the development of obesity, such as food intake and energy balance, pathologic enlargement of fat cells, inflammation of adipose tissue, insulin resistance, and epigenetic changes. Prokineticin signaling is involved in the control of food intake and energy expenditure in the CNS and suppresses the excessive development of adipose tissue. Thus, PKR1 agonists may be potential agents for the treatment of obesity (Fig. 6).
Fig. 6.
Effect of prokineticin system in food intake and fat tissue development. PK2 reduces food intake via PKR1 signaling and reduces adipose fat tissue expansion in whole body, including EAT formation in the heart through regulation of central, peripheral, or local pathways. Created with BioRender.com.
A. Prokineticin Levels in Obese Patients
Recently, Wang et al. (Wang et al., 2021) investigated the association between PK2 and childhood obesity. They demonstrated that children with obesity had significantly elevated fasting serum PK2 levels compared with age-matched healthy children. This positive correlation between serum PK2 levels and BMI was demonstrated in obese children with and without nonalcoholic fatty liver disease, which is a common and serious complication of childhood obesity. Although obesity is generally associated with a chronic low-level inflammatory state and numerous studies in rodents have demonstrated the role of PK2 in the inflammatory process, there was no correlation between PK2 and IL-6, tumor necrosis factor alpha, and white blood cell and neutrophil counts in this cohort study. Finally, circulating PK2 levels were found to correlate positively with homeostatic model assessment for insulin resistance in the entire cohort.
Interestingly, the obese children did not exhibit significant abnormalities in sleep and circadian rhythms, suggesting that high circulating PK2 levels are not mainly caused by a disturbed circadian rhythm.
These data may be related to another study in patients with a complete loss-of-function mutation in the pk2 gene (Balasubramanian et al., 2014). In these patients, no abnormalities were observed in circadian phase markers (e.g., melatonin, cortisol, and core body temperature), at the expense of their psychomotor vigilance performance. Although limited by small sample size, this study suggests that the activity of the central circadian pacemaker is intact despite the inactivity of PK2, suggesting that the regulation of circadian phenotype is not matched in humans and mice.
All these human studies on the relationship between PK2 and obesity lead to quite heterogeneous results and conclusions. The discrepancies may be related to the relatively small sample size of the cohorts, the different clinical characteristics of the different study cohorts (including age and sex distribution), the medical complications of the participants, the differences in the measurement of PK2 levels, etc. This implies that future studies with larger sample sizes detecting PK2 under different conditions and in different populations are needed. Either way, all these data suggest that PK2 or its receptors may be a therapeutic target for the treatment of obesity and related diseases.
B. Regulation of Prokineticin Signaling in Neuronal Basis of Obesity
The etiology of obesity is a complex process of dysregulation between food intake, energy expenditure, and energy stores which can be observed at both central and peripheral levels (Hill et al., 2012). In the CNS, the nucleus arcuate of the hypothalamus regulates food intake by balancing two neuronal circuits that secrete peptides with two opposite functions: an anorexigenic one triggered by pro-opiomelanocortin and the cocaine- and amphetamine-regulated transcript, and an orexigenic one triggered by neuropeptide Y (NPY) and the Aguti-related peptide. It is dysregulation in this system that increases or decreases food intake (Sohn, 2015). Food intake is affected not only by changes at the level of the hypothalamus, but also by changes at the level of adipose tissue (Würfel et al., 2022).
Negri et al. (Negri et al., 2004) were the first to study how injection of Bv8/PK2 into different brain regions can affect intake behaviors. They found that intracerebroventricular injection of Bv8/PK2 in rats reduced food intake but stimulated drinking. However, injection of Bv8/PK2 into the arcuate of the hypothalamus selectively suppressed eating but not drinking. In contrast, injection of Bv8/PK2 into the subfornical organ stimulated drinking but not eating.
Gardiner et al. (2010) have reported that in rodents, intracerebroventricular administration of Bv8/PK2 leads to an anorectic effect that is abolished by PK2 antibodies. PK2, even when administered to rodents via the peripheral route, leads to a regulation of food intake that acts via the brainstem and depends on activation of PKR1 (Gardiner et al., 2010; Beale et al., 2013). Indeed, PK2 administered intraperitoneally increases immunoreactivity in the dorsal motor vagus nucleus of the brainstem and induces anorexia in wild-type and PKR2(−/−) mice, but not in PKR1(−/−) or in wild-type mice treated with PC1, a PKR1-preferring antagonist (Negri et al., 2004; Beale et al., 2013).
Moreover, the PK2-mediated anorexic effect is partially dependent on arcuate of the hypothalamus activation of the melanocortin system. Indeed, PK2 induces c-fos immunoreactivity in pro-opiomelanocortin -expressing neurons. In hypothalamic explants, PK2 stimulates the release of alpha-melanocyte-stimulating hormone, a pro-opiomelanocortin-derived anorexigenic peptide that binds to the melanocortin-4 receptor and plays an important role in recognizing the balance between orexigenic Aguti-related peptide and anorexigenic melanocortin signaling and regulating feeding behavior. In contrast, simultaneous intracerebroventricular administration of PK2 with Aguti-related peptide, an orexigenic peptide, significantly reduced the anorectic effect of PK2 (Gardiner et al., 2010). The anorectic effect of intracerebroventricular administration of PK2-mediated anorectic effect is even more pronounced in melanocortin-4 receptor(−/−) mice (Chaly et al., 2016).
Melanocortin receptor accessory protein 2 (MRAP2), a regulator of energy homeostasis, enhances melanocortin-4 receptor signaling such that loss-of-function mutations in the Mrap2 gene are associated with obesity and hyperglycemia (Asai et al., 2013; Baron et al., 2019). MRAP2 and PKR1 co-localize in neurons. Moreover, MRAP2 inhibits the PKR1 cell surface trafficking via its C-terminal region (Rouault et al., 2017), acts as a suppressor of PKR1 signaling, and promotes food intake and weight gain, which can be reduced by activation of PKR1 (Chaly et al., 2016). MRAP2 C-terminal region also binds to the N-terminal region of PKR2, preventing glycosilation and transport on the cell surface (Verdinez and Sebag, 2021; Fullone et al., 2022a,b). In ex vivo hypothalamic explants, PK2 reduces MRAP2 expression. In adipocytes of PKR1(−/−) mice, which serve as models of obesity, MRAP2 expression is markedly increased (Fullone et al., 2022b).
Chronic administration of PK2 leads not only to a decrease in food intake but also to a decrease in body weight in lean and obese mice (Beale et al., 2013), in part due to the release of alpha-melanocyte-stimulating hormone via activation of signal transducer and activator of transcription 3 (STAT3) and ERK (Gardiner et al., 2010; Beale et al., 2013; Maftei et al., 2021). Conversely, PK2β does not induce STAT3 and ERK phosphorylation and, when injected intraperitoneally into mice, does not reduce food intake, likely because it cannot activate STAT3, a transcription factor whose dysregulation leads to obesity (Jiang et al., 2013a; Maftei et al., 2021).
C. Regulation of Energy Expenditure in Olfactory Bulb by Prokineticin System
The OBs help coordinate food selection and intake, and because they express high levels of PK2, which is involved in OB neurogenesis (Ng et al., 2005), they represent another important central area involved in food intake. PK2 injected into the OB of mice has an anorectic effect, whereas PK2 short hairpin RNA injected into the OB causes dysregulation of feeding behavior. In addition, OB shows a decrease in PK2L and PKR1 levels in fed mice compared with fasting mice. PK2L is cleaved into PK2β leading to the hypothesis that, at least at OB, this is the major PK2 isoform that regulates food intake by binding to PKR1 (Mortreux et al., 2019).
D. Role of Prokineticin 2 in Torpor and Temperature Regulation
The neurons of the paraventricular nucleus (PVN) receive a variety of inputs from different areas of the brain, integrate them, and transmit various outputs through different neurotransmitters and pathways involved in controlling stress, metabolism, growth, reproduction, immune system, and autonomic functions in the gastrointestinal, renal, and cardiovascular systems. The PVN also controls the central regulation of food intake and heat production. PK2 and PKR2 are expressed primarily in the PVN of the hypothalamus. Fasting increases PK2 expression in the PVN, which is decreased to undetectable baseline levels upon resumption of food intake (Zhou et al., 2012). PK2(−/−) mice respond to fasting with torpor, a compensatory mechanism for negative energy balance. This transient hypometabolic state is characterized by lower energy expenditure and weight loss in PK2(−/−) mice than in wild-type littermates. Moreover, in contrast to wild-type mice, no appreciable increase in arousal is observed throughout the fasting period. While daily ingestion of a limited amount of food during fasting rescued the body weight loss and hypothermic phenotype in wild-type mice, this was not the case in PK2(−/−) mice, which appeared unable to use food to compensate for body weight loss. It is suggested that increased expression of PK2 in the PVN during fasting may be a way to trigger physiologic responses, such as activation of the sympathetic nervous system, stimulation of locomotor activity, prolonged periods of wakefulness, and utilization of the body's energy stores. However, when PK2 expression is measured throughout the hypothalamus, it is not an increase but a decrease that is triggered by fasting (Gardiner et al., 2010).
Jethwa et al. (Jethwa et al., 2008) showed that targeted genetic disruption of PKR2- mediated signaling also predisposed mice to torpor when exposed to acute food deprivation, but also when maintained at room temperature (21–22°C) and ad libitum food. It was characterized by a marked decrease in body temperature, locomotor activity and respiratory quotient.
However, PKR2(−/−) mice showed comparable levels of hyperphagia to their control littermates after food deprivation, but their respiratory quotient tended to increase more slowly after refeeding, suggesting that the period of food deprivation had resulted in greater reliance on catabolism of fat reserves in the mutant mice. Surprisingly, food intake was reduced in the PK2R(−/−) mice compared with their control littermates which was unexpected given the known anorectic role of PK2, but their body weight and abdominal fat depots were similar to their intact littermates. The author suggested that loss of PKR2 signaling primarily leads to reduced food intake and likely also to an attenuation of circadian rhythms, so that compensatory strategies such as torpor entry are used to maintain energy expenditure.
E. Role of Prokineticin on Controlling Excessive Fat Formation, Insulin Resistance, and Epigenetic Regulation
The PK signaling suppresses the growth of visceral and subcutaneous adipose tissue. PK2 is released from adipose tissue and binds to PKR1, the receptor subtype expressed mainly in adipocytes (Soga et al., 2002), reducing adipose tissue growth (Szatkowski et al., 2013; Qureshi et al., 2017). Moreover, low PKR1 transcript has been found in obese patients visceral and subcutaneous tissues, demonstration of a key role of PKR1 in adipose tissue growth.
In vitro, PK2 suppresses mouse preadipocyte proliferation and their differentiation into adipocytes via PKR1 signaling, as observed in the human preadipocyte cell strain Simpson-Golabi-Behmel syndrome 10 days after the adipogenic stimuli. Accordingly, adipose tissue-specific PKR1(−/−) (ad-PKR1(−/−)) mice exhibit abnormally excessive accumulation of abdominal fat without altering in the glucose or insulin tolerance tests (Szatkowski et al., 2013). Both PKR1(−/−) and ad-PKR1(−/−) mice suffer from obesity as a result of adipocyte hyperplasia and macrophage infiltration in their adipose tissue. Interestingly, only PKR1(−/−), but not ad-PKR1(−/−) mice, exhibit insulin resistance and type 2 diabetes, suggesting that nonadipocyte incidents may contribute to the occurrence of the diabetes-like syndrome in PKR1(−/−) mice. Despite the increased body weight, PKR1(−/−) mice did not have increased food intake at 40 weeks and their body weight and visceral adipose tissue mass did not increase further on the high fat diet. The increase in fat mass in PKR1(−/−) on the normal diet is associated with chronic low-level inflammation and a marked increase in the expression of adipokines, which in turn cause a shift in the polarization of adipose tissue macrophages from an anti-inflammatory M2 to a pro-inflammatory M1 state (Mantovani et al., 2004). PK2 promotes the mouse M1 type of macrophage (an inflammatory phenotype) (Martucci et al., 2006) and attenuates the production of anti-inflammatory cytokines such as interleukin (IL)-10 and IL-4 in splenocytes (Franchi et al., 2008; Lattanzi and Miele, 2021). Whether PKR1 signaling promotes a switch from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype to preserve adipocyte function in obesity remains to be investigated.
One of the important events linking obesity and cardiovascular disease may be the excessive development of epicardial adipose tissue (EAT) between the myocardium and the visceral layer of the epicardium. EAT may differentiate into pericoronary EAT and infiltrate and surround the coronary arteries (coronary EAT), contributing to the development and progression of coronary artery disease and atrial fibrillation. Interestingly, human EPDCs derived from atrial appendages spontaneously undergo adipocyte differentiation to form EAT in the high-calorie intact model. However, the PK2/PKR1 pathway activates the demethylase lysine demethylase 6A, which suppresses peroxisome proliferator-activated receptor gamma expression and inhibits adipogenic signaling and epicardial progenitor cell differentiation into adipocytes (Qureshi et al., 2018). On the other hand, PK2/PKR1-mediated lysine demethylase 6A suppresses repressive marks on vascular gene promoters mediated by histone tri-methylation of lysine 27 on histone H3 protein. Activation of vascular and endothelial cell precursors leads to differentiation of epithelial cells into vascular smooth muscle and ECs, such that PK2/PKR1 signaling promotes vascular formation. Epigenetic changes in human EPDCs by PK2 and PKR1 orchestrate stem cell formation and differentiation of hEPDCs into vasculogenic and adipogenic cells.
VI. Role of Prokineticin System in Neuronal Injury and Neurodegenerative Diseases
Acute brain damage caused by trauma or stroke and chronic neurodegeneration are diseases that have different cellular and molecular mechanisms of initial cell death. However, they often share common outcomes, such as neuroinflammation and sequelae of varying degrees, depending on the extent of neuronal death. Although the complete picture from initial neuronal death to glial scar formation and other long-term responses has not been fully elucidated, several molecules, such as PK2, are recognized as relevant to these processes. Reactive oxygen species, hypoxia, and glutamate increase PK2 mRNA expression in mouse neurons, spinal cord, and astrocytes, but not in microglia (Cheng et al., 2012; Guida et al., 2015). PKR2 is mainly expressed in neurons, whereas PKR1 is mainly expressed in microglia. PK2 expression after traumatic brain injury (TBI) is more affected than PK1 expression in four brain regions: parietal neocortex, hippocampus, inferior parietal lobule, and neocortex of the posterior superior temporal gyrus (Mundim et al., 2019). PK2 and PKR1 also play important roles in several neurologic diseases, including TBI (Mundim et al., 2019; Bao et al., 2021) and PD (Neal et al., 2018), affecting both the central and peripheral nervous systems. PK2 and PKR2 are upregulated in neurologic diseases such as Alzheimer's disease (Lattanzi et al., 2019a) and ischemic stroke (Cheng et al., 2012). Preclinical models have shown that PK2 has a dual effect, acting as a neuroprotective molecule by stimulating neuroblast migration and modulating astrocyte activation, which can initiate a potential regeneration program (Ayari et al., 2010), or by increasing neuronal death through the accumulation of β-amyloid and contributing to neurotoxicity. Whether different PK2 receptors are involved in the beneficial or deleterious effects of PK2 in the CNS remains to be elucidated.
A. The Prokineticin System in Traumatic Brain Injury
TBI is the most common cause of death in trauma patients. It is characterized by persistent blood-brain barrier dysfunction and neuroinflammation that ultimately leads to cell death. In the adult mammalian brain, two neurogenic regions modulate physiologic functions: the subgranular zone of the dentate gyrus in the hippocampus and the SVZ, which produces neuroblasts that migrate to OBs via the RMS. Indeed, in TBI, proliferating cells mobilize from the SVZ into the injured cortex. However, the extent of the lack of change in RMS migration depends on the type of brain injury. In the mouse model with traumatic cortical injury, high expression of PK2 was detected in microglia. However, no PK2 level was found in reactive astrocytes, immature neurons, and leukocytes (Mundim et al., 2019), confirming that intact cortex does not express PK2. High levels of PK2 were found in astrocytes and neurons in the glutamate- (Cheng et al., 2012) and amyloid-beta-induced toxicity models (Severini et al., 2015), in which a PKR antagonist has beneficial effects (Caioli et al., 2017; Maftei et al., 2019). Indeed, PKR antagonism was detrimental in TBI because it inhibited SVT-derived neuroblast migration (Mundim et al., 2019). In the other study, migration of cells from neurospheres or SVZ occurred when neurospheres were co-cultured with PK2-expressing cells. These experiments demonstrated that PK2 plays a key role in the recruitment of SVZ cells to injured areas, which is an important compensatory restructuring in repair. Interestingly, PK2 upregulation in an injury model of zebrafish is associated with increased proliferation and migration of neuroprogenitors toward the injury site, indicating it may have a key role to induce a potential regenerative program (Ayari et al., 2010).
Another mechanism involved in neuroprotective pathway of PK2 is the inhibition of ferroptosis, a cell death program. Ferroptosis has been linked to the pathogenesis of various acute neuronal injuries, such as stroke and TBI (Xie et al., 2019) and chronic neurodegenerative diseases such as PD (Guiney et al., 2017) and Alzheimer's disease (AD) (Ayton et al., 2015).
Ferroptosis is characterized by the accumulation of phospholipid hydroperoxides (e.g., HOO- arachidonoyl-PE) catalyzed by iron-dependent mechanisms. A key enzyme in the biosynthesis of arachidonic acid-PE is the acyl-CoA synthetase long-chain family member 4 (Acsl4), which contributes to the performance of ferroptosis (Stockwell et al., 2017). PK2 has been shown to increase levels of Fbxo10, a ubiquitin ligase that binds to Acsl4 and promotes ubiquitination and degradation of Acsl4. PK2 inhibits ferroptosis in TBI, preventing mitochondrial dysfunction and protecting neurons from TBI (Bao et al., 2021).
B. The Prokineticin System in Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder caused by loss of nigrostriatal dopaminergic innervation and the appearance of Lewy bodies with aggregated α-synuclein.
As a disulfide-rich secretory peptide highly expressed in OB and the suprachiasmatic nucleus (SCN) (Ng et al., 2005), a new paradigm of compensatory protective function of PK2 signaling in response to neurotoxic stress has been documented in nigral dopaminergic neurons in experimental PD models (Gordon et al., 2016; Désaubry et al., 2020). PK2 expression in the adult mouse brain has been found to be either absent or sparse in the ventral midbrain, including the nigra (Cheng et al., 2006; Zhang et al., 2009), but high levels of PK2 expression have been found in surviving nigral dopaminergic neurons from brains, olfactory neurons (ON) and serum from patients with PD (Schirinzi et al., 2021, 2022) and in mouse models of PD (Gordon et al., 2016). Treatment with PK2 or overexpression of recombinant PK2 protects dopaminergic neurons from Parkinsonian neurotoxin-induced oxidative stress, mitochondrial dysfunction, and cell death, whereas antagonism of PKRs exacerbates dopaminergic degeneration in experimental PD (Gordon et al., 2016; Luo et al., 2019). Mechanistic studies revealed that activation of the survival signaling pathways ERK and AKT, as well as enhanced mitochondrial biogenesis, are involved in the potent neuroprotective effects mediated by PK2 (Schirinzi et al., 2021).
Recently, PK2 was shown to regulate a novel neuron-astrocyte signaling mechanism by promoting an alternative A2 protective phenotype in astrocytes (Neal et al., 2018). Astrocytes expressing high numbers of PKRs play an important role in postnatal neuroblast migration (Gengatharan et al., 2016). However, their dysfunction leads to neuronal death or neuronal dysfunction (Liddelow and Barres, 2017; Rivetti di Val Cervo et al., 2017). PK2 treatment or overexpression in primary astrocyte cultures and mouse brain increases the A2 phenotype of astrocytes, which reduces excitotoxicity and promotes long-term neuronal survival by modulating mitochondrial energy metabolism and antioxidant pathway and reducing inflammatory factors (Becerra-Calixto and Cardona-Gómez, 2017; Neal et al., 2018).
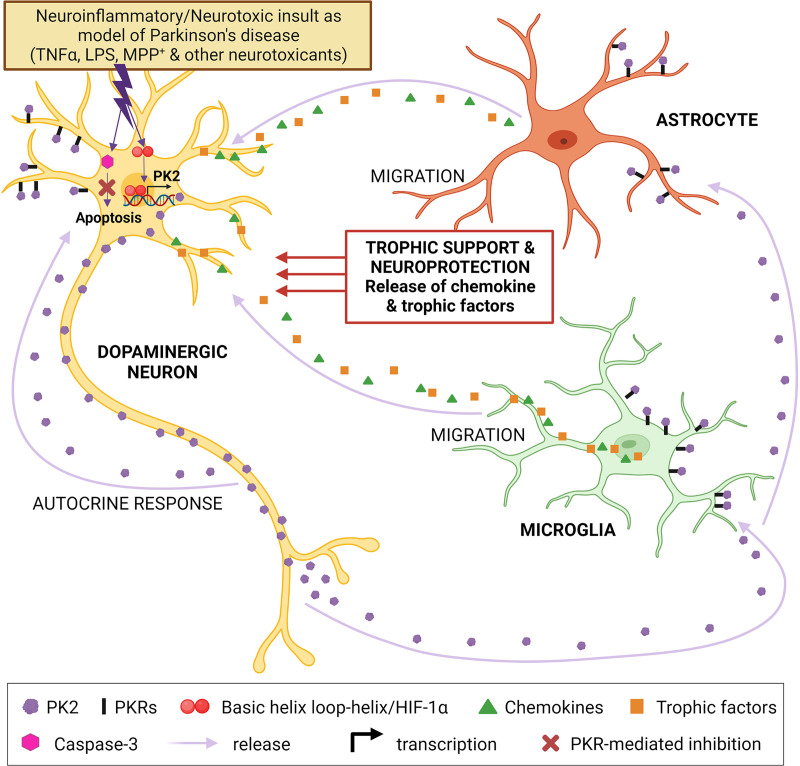
In primary astrocyte cultures, treatment with IS20, a small molecule PKR1 agonist, mimics the neuroprotective effect of PK2 and favors the A2 astrocyte phenotype over the pro-inflammatory A1 phenotype in neurodegenerative diseases such as PD (Neal et al., 2018). These findings collectively suggest that PK2 is upregulated and secreted during neurotoxic insults in dopaminergic neurons. The released PK2 subsequently activates astrocyte cells to promote a compensatory neuroprotective response against inflammatory stress by providing trophic support. PK2 also mediates survival of dopaminergic neurons via an autocrine effect on PKRs by activating the ERK and AKT signaling pathways and promoting mitochondrial biogenesis (Fig. 7).
Fig. 7.
A proposed model for PK2 to attenuate dopaminergic neurotoxicity and promote an alternative A2 protective phenotype in astrocytes in Parkinson disease. Basal PK2 expression is low or undetectable in the nigral region, but its expression is highly induced during the early stages of neurotoxic insult in dopaminergic neurons. This small regulatory peptide is then secreted from dopaminergic neurons and function as a neuroprotective signal to counteract inflammatory injury via binding and activation of PKRs on astrocyte cells. PK2 also mediates dopaminergic neuronal survival via an autocrine fashion acting on PKRs by activating the ERK and AKT signaling pathways and promoting mitochondrial biogenesis. Created with BioRender.com.
C. The Prokineticin System in Alzheimer’s Disease
Alzheimer’s disease (AD), the most common cause of dementia, is a neurodegenerative disease, characterized by the extracellular deposition of misfolded amyloid beta (Aβ) plaques and by intraneuronal tangles composed by the hyperphosphorylated tau protein (Karran et al., 2011).
Analysis of PK2 levels in human brain tissue samples collected postmortem from clinically well-documented and neuropathologically confirmed cases of AD revealed that PK2 expression levels in the hippocampus of AD patients are significantly higher than in cognitively normal controls. In addition, mean serum PK2 levels in AD patients are significantly higher than in controls, suggesting that blood PK2 is a potential biomarker for this pathology (Lattanzi et al., 2019b).
In a nontransgenic animal model of AD induced by intracerebroventricular administration of Aβ1-42, rats show consistent deficits in learning and long-term memory in parallel with a strong upregulation of the PK system in the cortex and hippocampus that occurs on the first day after Aβ infusion and persists for 35 days (Lattanzi et al., 2019b; Maftei et al., 2019). Notably, in the hippocampus, PK2 and PKR1 are overexpressed in both neurons and astrocytes, whereas PKR2 is overexpressed only in neurons (Maftei et al., 2019). Subchronic treatment with PC1 improves learning and memory performance of Aβ1-42-infused rats serving as control subjects by reducing overexpression of PK2/PKRs, decreasing Aβ1-42-induced activation of microglia and astrocytes, and restoring neurogenesis. In the hippocampus of Aβ1-42-infused rats, overexpression of the novel PKR2 splice variant, TM 4-7, occurs, and the expression ratio between TM4-7 and PKR2 increases with the progression of days after the Aβ1-42 insult (Lattanzi et al., 2019a).
The increase in PK2 was also observed in another AD animal model. In Tg2546 transgenic mice, PK2 levels are statistically higher than in controls at 6 and 20 months of age, a period when the disease is neuropathologically detectable. In the hippocampus of the same transgenic mice, PC1 prevents the reduction in long-term potentiation without altering basal synaptic transmission (Severini et al., 2015). Indeed, in vitro study has also demonstrated that Aβ1-42 in primary cortical cultures (CNs) increases PK2/PKR levels, and PC1, the PKR antagonist, protects CNs from Aβ1-42-induced neurotoxicity in a dose-dependent manner by reducing Aβ1-42-induced overexpression of PK2 (Severini et al., 2015). Moreover, incubation of CNs with Bv8 as well as with Aβ1-42 leads to an increase in neuronal α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) currents that is prevented by PC1, demonstrating the involvement of PK2 in altering glutamatergic transmission in this model (Caioli et al., 2017). PK2 acts as a mediator of brain damage and plays a crucial role in Aβ-induced neuronal death.
D. Role of Prokineticin 2 in Electroconvulsive Shock-Induced Memory Impairment
Although electroconvulsive shock (ECS) has become the most effective treatment of depression (McClintock et al., 2014), its role in learning and memory impairments has been controversial. Recently, Chen et al. (Chen et al., 2023) demonstrated that acute ECS promotes the activation of A1 type astrocytes and the release of pro-inflammatory factors associated with spatial learning and memory function in rats. Indeed, the recovery phase of learning and memory function after ECS was facilitated by an increase in A2 subtype astrocytes, which was associated with an increase in the level of PK2 in astrocytes. A PKR2 antagonist, PKRA7, inhibits PK2-mediated activation of A2 subtype astrocytes, and attenuates the enhancement of spatial learning and memory function by PK2 in rats. PKRA7 did not alter the antidepressant effect of ECS, but it further exacerbates the synaptic defects caused by ECS. Moreover, PKRA7 administration in rat hippocampus increases the expression of postsynaptic density protein-95 (PSD-95), indicating that PK2/PKR2 signaling promotes synaptic plasticity (Chen et al., 2023). This study indicates an acute compensatory role of PK2 in triggering synaptic plasticity, activating A2 astrocytes, and improving learning and memory function.
VII. Major Role of Prokineticin 2 in the Regulation of Circadian Cycles
The circadian clock is located in the SCN, in the hypothalamus (Moore, 1997; El Cheikh Hussein et al., 2019; Rijo-Ferreira and Takahashi, 2019) and is a hierarchical temporal network with complex autoregulatory transcriptional and translational feedback loops consisting of both activating and repressive pathways. The circadian clock, controlled by the daily light-dark cycle, sends synchronization signals to cell autonomous oscillators in tissues influencing physical, mental, and behavioral changes, such as appetite, body temperature, and sleep. Abnormal circadian rhythms have been linked to the development of obesity, diabetes, depression, bipolar disorder, seasonal affective disorder, and insomnia (Zhou and Cheng, 2005; Patke et al., 2020). PK2 acts as an output molecule of the SCN: projections of PK2-expressing neurons from the SCN transmit circadian output signals to distant hypothalamic and extrahypothalamic regions, including the ventral lateral septum, medial preoptic area, subparaventricular zone, paraventricular nucleus, dorsomedial hypothalamic nucleus, lateral hypothalamic area, and paraventricular thalamic nucleus (Cheng et al., 2002; Zhang et al., 2009; Morris et al., 2021).
In the SCN, PK2 is highly expressed during the day, whereas its expression is low or undetectable at night and is regulated by a heterodimer of CLOCK and BMAL1 by binding to the pk2 promoter. In CLOCK- and BMAL1(−/−) mice, PK2 expression is abolished, suggesting that PK2 is mainly controlled by the endogenous circadian clock. Light can also induce PK2 expression independently of the circadian oscillator (Li et al., 2006), suggesting that the PK2/PKR2 system can synchronize SCN inputs and outputs. PK2 also controls its own circadian transcription (Cheng et al., 2002, 2005; Matsumoto et al., 2006). Genetic inactivation of pk2 in mice attenuates glucocorticoid signaling and body temperature rhythms (Li et al., 2006). Misexpression of PK2 disrupts behavioral rhythms (Li et al., 2018). In addition, PK2(−/−) mice show a lack of precise timing of nocturnal locomotor activity (Prosser et al., 2007; Jethwa et al., 2008). Transgenic mice overexpressing PK2 show disrupted behavioral circadian rhythms and desynchronized molecular rhythms within the suprachiasmatic clock (Li et al., 2018). PKR2(−/−) mice exhibited phenotypes similar to PK2(−/−) mice (Prosser et al., 2007), indicating involvement of PKR2 in regulation of circadian and molecular rhythms.
A. Role of Prokineticin 2 in Sleep-Awakes and Neurobehavioral Neuronal Networks
In mammals, the duration and intensity of sleep are regulated by homeostatic processes, whereas the timing of sleep depends on circadian processes. PK2 expression in the SCN is rhythmic, preferentially absent at night and high during the day (Burton et al., 2015; Morris et al., 2021). PK2 suppresses GABAergic function to regulate circadian clock 2 (Per2). Antagonism or complete absence of PKR2 in mice reduces Per2 expression and favors GABAergic tone, reducing neuronal firing rate and output, weakening circadian coordination, and making the circadian system more susceptible to environmental perturbation (Li et al., 2006; Colwell, 2011). PK2- and PKR2-positive cells trigger circadian oscillations as pacemakers of the SCN and also tune their period and amplitude (Li et al., 2006). The firing rate of SCN neurons fluctuates in unison with the PK2 oscillation, likely due to the ability of PK2 to regulate the membrane translocation of TRPV2, an ion channel of the TRP family (Burton et al., 2015). Moreover, PK2(−/−) mice have increased rapid eye movement sleep during both the light and dark periods, although total sleep time decreases during the light period due to a reduction in non-rapid eye movement sleep time. These mice also have decreased arousal triggered by novel environments and increased alertness (Hu et al., 2007).
Despite the direct link between PK2/PKR2 and circadian cycle alteration in mice, the role of the PK2 pathway in human circadian rhythmicity remains to be elucidated. In two PK2(−/−) subjects, Balusabramanian et al. (Balasubramanian et al., 2014) reported no changes in circadian phase markers but impaired psychomotor vigilance task, suggesting that PK2 plays a role only in relaying circadian timing information to some neurobehavioral neuronal networks without affecting the function of the central circadian pacemaker.
B. Major Role of Prokineticin 2 in Mood Regulation and Stress
Disruption of circadian rhythms affects mood regulation and stress response, which is associated with mood disorders. Kishi et al. (Kishi et al., 2009) found a significant association between PKR2 and major depression and bipolar disorder in the Japanese population. Following this study, they also discovered a significant association between PKR2 and methamphetamine dependence, suggesting that pkr2 is altered as common circadian clock gene in mood disorders and drug dependence (Kishi et al., 2010). Otherwise, PK2 does not appear to play a role in the pathophysiology of methamphetamine addiction (Kishi et al., 2011). Intracerebroventricular infusion of PK2 enhances anxiety- and depression-like behaviors that are completely absent in PK2(−/−) mice. PK2(−/−) mice also showed a lack of adaptation to the new environment with low locomotor activity and arousal, no changes in body temperature and food intake. In addition, these mice lapse into torpor after fasting (Zhou et al., 2012). Similarly, PKR2(−/−) mice show spontaneous torpor that is enhanced by fasting (Jethwa et al., 2008).
VIII. The Prokineticin System in Pain and Pleasure
PK2 modulates nociceptive response and pain via binding to its receptors, PKR1 and PKR2. In several models of inflammatory and neuropathic pain, the development and persistence of pain are directly related to increased PK2 expression in the periphery and CNS. Blocking the PK system with receptor antagonists leads to analgesic effects and reduction in inflammation. On the other hand, a key role of PK2/PKR2 in pleasant touch may prime the PK signaling as a potential analgesic, since pleasant touch can result in pain relief, and provide emotional and psychologic support to alleviate social isolation and stress.
A. Nociceptive Pain
In rodents, local or systemic injections of very low doses of Bv8 lower the nociceptive threshold to thermal, mechanical, and chemical stimuli by activating both PKR1 and PKR2 in primary sensory neurons (Negri et al., 2002). Systemic Bv8-induced hyperalgesia shows a typical biphasic pattern consisting of a first phase due to direct activation of nociceptors and a second phase due to central sensitization by Bv8-induced increased expression of calcitonin gene-related peptide and substance P (De Felice et al., 2012).
The increased excitability of nociceptors is the result of a cooperative interaction between PKRs and TRP channels; in particular, PKR1 colocalizes with TRPV1 in small dorsal root ganglia (DRG) neurons, whereas PKR2 colocalizes mainly with transient receptor potential cation channel subfamily A member 1 (TRPA1) in medium/large DRG neurons (Negri et al., 2006b; Vellani et al., 2006; Maftei et al., 2020). This is a functional co-localization, because PKR1(−/−) mice show impaired nociception at temperatures between 46°C and 48°C, to capsaicin, and to protons (stimuli that activate TRPV1), whereas PKR2(−/−) mice show impaired nociception at cold temperatures and to mustard oil-induced inflammatory hyperalgesia (stimuli that activate transient receptor potential cation channel subfamily A member 1). The protein kinase C(PKC)-ε pathway is involved in this interaction between PKRs and TRP (Vellani et al., 2006). Also in skin preparations, Bv8 induces peripheral sensitization of nociceptors to heat and lowers their nociceptive threshold through TRPV1 activation (Hoffmann et al., 2016). Nevertheless, other mechanisms are also involved in Bv8/PK2-induced hyperalgesia: in rat DRG neurons, PK2 increases proton-gated channel activity and sensitizes ionotropic P2X receptors (Ren et al., 2015), and in rat primary sensory neurons, PK2 suppresses GABA-A-activated currents (Xiong et al., 2010).
B. Neuropathic Pain
Peripheral nerve injury induced by chronic constriction injury (CCI) and spared nerve injury (SNI) in mice results in thermal hyperalgesia and tactile allodynia. In the injured sciatic nerve, upregulation of PK2 occurs as early as 3 days after injury and then shifts toward the DRG and spinal cord, where it becomes significant at 10 days and persists up to 17 days after CCI and SNI (Maftei et al., 2014; Guida et al., 2015; Lattanzi et al., 2015b). In the injured sciatic nerve, PK2 is detectable in some axons, mainly in association with activated Schwann cells and infiltrating macrophages. PK2 levels are also markedly increased in DRG neurons and satellite cells and in activated astrocytes in the spinal cord, but not in microglia. Treatment with the PKR antagonists, PC1 or PC7, starting on the day of injury blocks the onset of thermal hyperalgesia and tactile allodynia, delays the recurrence of painful symptoms after interruption of treatment, and restores the balance between pro- and anti-inflammatory cytokines (Maftei et al., 2014; Guida et al., 2015; Lattanzi et al., 2015b).
Both diabetes and chemotherapy drugs are responsible for severe neuropathic pain symptoms. In a mouse model of diabetic neuropathy induced by streptozotocin administration and in two mouse models of chemotherapeutic neuropathy induced by bortezomib or vincristine injection, severe hyperalgesia and allodynia are observed in association with upregulation of PK2, PKRs, and proinflammatory cytokines in the sciatic nerve, DRG, and spinal cord. In these mice, subchronic administration of PC1 impairs pain and reduces PK2/PKRs and pro-inflammatory cytokines (Castelli et al., 2016; Moschetti et al., 2019a, 2019b). PC1 protects against known neuronal cell changes after chemotherapy (Livni et al., 2019): in DRG neurons from bortezomib- or vincristine-treated mice, PC1 impairs the reduction in neurite length and growth induced by both chemotherapeutic agents (Moschetti et al., 2020).
C. Inflammatory Pain
PK2 plays a central role in inflammatory pain. In an animal model of inflammatory pain induced by Freund's Complete Adjuvant (FCA) in the mouse paw, the development and duration of hyperalgesia in the inflamed paw is associated with PK2 and PK2L levels and is abolished by local or systemic injection of PKR antagonists such as A-24 (Lattanzi et al., 2012) or PC1 (Giannini et al., 2009). Significant PK2 overexpression by granulocytes is not only detectable locally in the paw but also systemically (Giannini et al., 2009). In rodents, inflammatory stimuli lead to an early and rapid increase in plasma levels of granulocyte colony-stimulating factor, which activates PK2 transcription in bone marrow-derived CD11b+/Gr1 cells (Shojaei et al., 2007), explaining the systemic increase in PK2 levels in splenic and paw granulocytes. PK2 released from inflamed tissue triggers macrophage recruitment and stimulates migration of proinflammatory macrophages. In vitro, PK2 activates the release of proinflammatory cytokines (IL-1β and IL-12) and inhibits anti-inflammatory cytokines (IL-10) from macrophages. Bv8 also alters the Th1/Th2 balance and promotes a Th1-like response. PKR1(−/−) mice show a significant decrease in inflammation-induced hyperalgesia and less upregulation of PK2 (Martucci et al., 2006; Franchi et al., 2008). PKR2(−/−) mice also show reduced inflammation-induced hyperalgesia, but deletion of the pkr2 gene does not affect inflammation-induced PK2 upregulation. These data suggest that both receptors are responsible for inflammatory pain, but only PKR1 is involved in regulating the increase in PK2 expression (Giannini et al., 2009). In two animal models of inflammatory visceral pain (intrarectal injection of TNBS and mustard oil), increased PK2 levels due to gastrointestinal inflammation also trigger visceral pain via direct activation of PKRs (Watson et al., 2012).
All these data suggest an underlying role of the PK system in inflammatory and neuropathic pain, at least in rodents (Fig. 8). Therefore, reducing the expression of PK2 or antagonizing PKRs may represent a promising target strategy for a new therapeutic approach.
Fig. 8.
Comparison of prokineticin system alterations in inflammatory and neuropathic pain. In inflammatory and neuropathic pain, the production of circulating factors shifts toward those with a pro- inflammatory phenotype. PK2 is highly upregulated and blocking its receptors inhibits hyperalgesia in both inflammatory and neuropathic pain. Interestingly, the largest gap in our knowledge is the role of PK2 splicing variants in neuropathic pain and the involvement of the PKR2 pathway in inflammatory pain. Created with BioRender.com.
D. Pleasure
Touch is one of the four modalities (touch, temperature, nociception-pain, and proprioception-body position) of somatic sensation distributed throughout the body. Touch as a tactile stimulus is perceived by mechanoreceptors and also provides the affiliative or emotional somatic pleasure (McGlone et al., 2014). Affective or pleasant touch such as stroking, caressing or hugging is important for the exchange of social information (Morris, 1946) and behavioral development (Bales et al., 2018). Any form of physical affection that evokes pleasant touch sensations (cuddling, hugging, hand holding, caressing, soothing pats, etc.) plays a critical role in early childhood development and provide feelings of security, care, and support at every stage of life. Pleasant touch provides emotional and psychologic support to alleviate social isolation and stress. Touch plays an important role in pain relief (Leknes and Tracey, 2008). In patients with chronic pain, gentle touch has been shown to lose its analgesic function and is perceived as abnormally low, contributing to pain-related disorders (Gossrau et al., 2021).
Recently, a study in mice has shown that PK2 encodes and transmits pleasant touch information from skin to brain via a hard-wired spinal pathway. PKR2 neurons are a unique population of spinal excitatory interneurons that are 85% positive to G Protein-Coupled Receptor 83 (GP83) and predominantly receive monosynaptic and polysynaptic C fiber inputs (Liu et al., 2022). Mice with ablation of spinal PKR2 neurons exhibited a profound loss of pleasant touch sensation (significant reduction of the heart rate or analgesic effect in response to gentle stroking) together with loss of sensitivity to acute thermal pain and itch transmission. However, they displayed no significant alteration in temperature pain or inflammatory pain induced by capsaicin. Mice with conditional knockout of pkr2 in small nociceptive sensory neurons of DRGs displayed normal pain and itch behaviors, but they failed to show significant reduction of the heart rate or analgesic effect in response to gentle stroking. DRG-PKR2(−/−) mice had deficit in social novelty recognition and the heightened stress/anxiety-like behaviors. Indeed, PK2 has been identified as a neuropeptide that encodes sensation of pleasant touch and transmits it to spinal PKR2 interneurons, which conveys a positive pleasant touch signal to the brain via gpr83 neurons (Liu et al., 2022). This finding on the role of PK2/PKR2 in pleasant touch identified PK2 as a potential analgesic factor, and represent a promising target for the treatment of certain type of pain.
IX. The Prokineticin Signaling in Inflammation and Infection
Dramatically elevated PK2 levels were found in the peripheral blood of patients with inflammatory diseases, such as multiple sclerosis (Abou-Hamdan et al., 2015) and psoriasis (He et al., 2016). However, patients with sepsis and sepsis shock were found to have a dramatic decrease in PK2 levels compared with healthy controls (Yu et al., 2022). PK2 levels were found to be higher in the synovial fluid of patients with rheumatoid arthritis (RA) than in the synovial fluid of patients with osteoarthritis (OA) and remain similar in the plasma of these patients. Based on these human studies, it is difficult to conclude whether PK2 represents a diagnostic marker for inflammation in general. However, recent studies have shown that PK2 plays an anti-inflammatory and inflammatory role in both chronic and acute inflammation. PK2 may act as an anti-inflammatory factor in critical cases such as sepsis, systemic inflammation, injury, intense exercise, and OA. In addition, the PK pathway is a critical mediator required in bacterial, protozoal, and viral infections. PK2 acts as a key player for recovery of odor loss in Covid-19 infection.
A. Prokineticin 2 as a Novel Immunomodulatory Factor in Diagnosis and Treatment of Sepsis
Sepsis is a highly lethal disease, and unfortunately, the exact causes of lethality remain poorly understood. Several studies suggest macrophage involvement in sepsis, which may alter the prognosis of the disease. PK2 levels were found to decrease dramatically in patients with sepsis and sepsis shock and to be associated with mortality (Yu et al., 2022). The authors concluded that PK2 may be a marker for diagnosing sepsis and septic shock and a marker for predicting mortality in adult patients with sepsis and septic shock. Accordingly, the role of PK2 in sepsis-related survival, bacterial burden, organ damage, and inflammation was investigated in an animal model with different sepsis models, such as cecal ligation and puncture–induced polymicrobial sepsis. In the mouse model of sepsis, PK2 levels were decreased as sepsis developed. In an in vivo model of polymicrobial sepsis induced by ligation and puncture of the cecum, administration of recombinant PK2 reduced organ damage and increased survival in both PK2(−/−) and wild-type mice. More specifically, PK2 significantly increased the phagocytic and bactericidal function of macrophages by activating STAT3. Depletion of macrophages attenuated the protective effect of PK2 against polymicrobial sepsis. Downregulation of PKR1, but not PKR2, with siRNA completely abolished the PK2-mediated enhancement of macrophage phagocytosis activity. Thus, the PK2/PKR1/STAT3 pathway was shown to promote bacterial clearance thereby playing a pivotal role in mitigating sepsis progression. It may not only represent a new target for sepsis therapy, but also an important marker for early diagnosis of sepsis, and a potential survival predictor.
B. Prokineticin 2 is Associated with the Pathogenesis of Collagen-Induced Arthritis in Mice
Rheumatoid arthritis (RA) is characterized by an increase in synovial cell proliferation and inflammatory cell infiltration in the synovial joints. Long-term inflammation in RA patients leads to cartilage and bone loss and causes joint deformities associated with chronic inflammation that affect quality of life. The hormones, neuropeptides sympathetic and sensory nerves connect the central nervous system (CNS) and peripheral tissues, resulting in morning stiffness and pain. Synovial cells play a key role in the chronic inflammatory tissue of rheumatoid arthritis.
PK2 levels were found to be significantly lower in synovial fluid than in plasma in patients with OA and RA. Moreover, plasma PK2 concentration correlated significantly with that in plasma in synovial fluid in OA patients but not in RA patients (Noda et al., 2021). In in vitro studies, they demonstrated that PK2 suppressed the secretion of IL-6 and MMP-3 from synovial fluid of patients in a concentration-dependent manner, which was abolished by the PKR1-preferential antagonist PC7. PKR1 was upregulated in the synovial tissue of OA patients. In addition, an anti-inflammatory effect of PK2/PKR1 signaling was mediated by inhibiting phosphorylation of NF-κB signaling. However, PK2 also suppressed IL-6 secretion from synovial fluid of RA patients without altering NF-κB phosphorylation and these effects were not abolished by PC7. The lower anti-inflammatory effect of PK2 in the synovial tissue of RA patient is due to the low expression of PKR1 and may be related to the chronicity of inflammation. This different regulation of PKR1 expression between RA-synovial fluid and OA-synovial fluid may contribute to the differential effect of PK2 in OS- versus RA-synovial fluid after exposure to inflammatory cytokines including IL-1β and tumor necrosis factor alpha. Indeed, injection of PK2 into healthy knee joints of mice resulted in increased inflammation due to mobilization of granulocytes but not macrophages into the synovial fluid. The inflammatory effect of PK2 can be observed in acute arthritis models, such as collagen antibody-induced arthritis (Noda et al., 2021).
The same authors have previously shown that PKR1 protein is expressed in infiltrating neutrophils, whereas PKR2 protein is present in macrophage-like mononuclear cells in the collagen-induced rheumatoid arthritis mouse model. In contrast to PKR1, pkr2 gene expression was higher in inflamed joints. PKRA7, an antagonist of both PKR1 and PKR2, significantly suppressed the severity of arthritis and inflammatory cytokines. However, the mechanism of how PKRA7 reduces arthritis has not been investigated in this mouse model (Ito et al., 2016). Therefore, PK2-mediated anti-inflammatory or inflammatory effects in arthritis are due to the effector cell type and receptor expression, acute or chronic phase of the disease, microenvironment in the presence of other pro-inflammation cytokines. This study should be verified by the effect of PKR1(−/−) or PKR2(−/−) or overexpression in tumor necrosis factor alpha-primed-OA- or RA-synovial fluids.
Recently, in the same arthritis model (collagen-induced arthritis type II), arthritic mice were shown to develop severe and prolonged hyperalgesia that was reversed by repeated administration of the PKRs antagonist PC1 (Impellizzeri et al., 2023). The anti-hyperalgesic effect of PC1 is due to its direct action on nociceptors and its ability to reduce the synthesis and release of PK2, since inflammation-induced PK2 upregulation is absent in mice lacking PKR1 (Giannini et al., 2009). In addition, PC1 is able to prevent the arthritis-induced increase in malondialdehyde in plasma, thereby reducing oxidative stress (Impellizzeri et al., 2023).
C. Role of Prokineticin in Infection
PK2 was recently identified as a novel modulator of bacterial pneumonia. Serum PK2 levels not only are lower in patients than in healthy controls, but also correlate with the severity of infection, being significantly lower in patients with severe pneumonia than in patients with simple pneumonia. In the mouse model of bacterial pneumonia induced by intratracheal instillation with Pseudomonas aeruginosa, PK2 levels in the lungs of infected mice are significantly lower than in that of control mice, confirming clinical findings and demonstrating a protective role of PK2 in the disease. This is due to the capacity of PK2 to increase the host's ability to eliminate bacteria by enhancing the chemotaxis, phagocytosis, and killing functions of macrophages. Because macrophages represent the most important cells required for the protective effect of PK2 in the host, this effect is lost when macrophages are depleted. In addition, PK2 increases the expression of NO and the activity of NOS in macrophages, which play a critical role in killing bacteria. These data, combined with the observation that administration of recombinant PK2 significantly alleviates lung injury and improves survival in mice, lead us to identify in PK2 a protective mediator factor and a potential adjuvant therapeutic for bacterial pneumonia (Tu et al., 2022).
Trypanosoma cruzi (T. cruzi), the etiological agent of Chagas disease, invades and resides in mammalian cells via a group of Gp85/trans-sialidase (TS). In particular, the specific LamG domain of Tc85, a group II TS, interacts with PKR2 and mediates parasite penetration and infiltration in mammalian cells (Lattanzi et al., 2021). Indeed, T. cruzi invasion into mammalian cells is inhibited by PKR2 interference or by treatment with anti-PKR2 antibodies or with a synthetic peptide (Khusal et al., 2015). The LamG domain of Tg85 not only binds to PKR2 but also activates it, demonstrating a physiologic role following T. cruzi infection of the nervous system by triggering STAT3 and ERK activation in mouse DRG (Lattanzi et al., 2021).
Olfactory dysfunction (OD), comprehending loss (anosmia) or reduction of the sense of smell, is one of the long-term effects of COVID-19. The expression of PK2 in olfactory neurons (ONs) of patients with post-COVID-19 OD has been significantly increased and correlated with the data obtained by odor tests to their residual odor, suggesting a contribution of PK2 in OD recovery (Schirinzi et al., 2023). Since efficient mitochondria in ONs are critical for proper olfactory signaling (Fluegge et al., 2012) and PK2 enhances mitochondrial bioenergetics (Neal et al., 2018), the authors hypothesize that PK2 may improve olfaction by stimulating mitochondrial activity or directly or counteracting the deleterious effects of chronic inflammation, which impairs mitochondrial activity exacerbating oxidative stress. The potential neuroprotective role of PK2 has recently been demonstrated in PD, where patients in the early stages of the disease have elevated levels of PK2 in ONs (Schirinzi et al., 2022). These data suggest the possibility that PK2 represents an alternative pharmacological target for the period after COVID-19. PK2 restores mitochondrial function and attenuates inflammation, thereby leading to recovery and improvement of odor.
X. Role of Prokineticin System in Reproductive System Functions and Disorders
PK1 and PK2 signaling have been reported to be associated with physiologic and pathologic aspects of the reproductive system (Ferrara et al., 2004; Brouillet et al., 2010, 2012a,b, 2013; Dunand et al., 2014; Alfaidy, 2016; Alfaidy et al., 2019). PK2 directly controls the migration of GnRH neurons, which affects the development of the female and male reproductive organs (Dodé et al., 2006; Dodé and Rondard, 2013), while PK1 mainly controls female reproductive functions, especially those associated with pregnancy. Several studies demonstrated that PK1 controls the menstrual cycle, ovulation, maintenance of the corpus luteum, blastocyst implantation, and its crosstalk with the myometrium that are essential processes for successful pregnancy (Hoffmann et al., 2006, 2007, 2016; Brouillet et al., 2010, 2012a,b, 2013; Dunand et al., 2014). In addition, PK1 and its receptors contribute to the placental development and the establishment of the feto-maternal circulation. Deregulations in PK1 and its receptors are associated with the pregnancy- induced hypertension, preeclampsia (PE), and fetal growth restriction (Hoffmann et al., 2006; 2007; Traboulsi et al., 2015). PK1 and its PKR2 are directly involved in gestational choriocarcinoma, and their targeting is proposed as alternative therapy for resistant patients to conventional therapies (Traboulsi et al., 2017). Importantly, PK1 can be potential biomarkers of pathologies in pregnancy or pregnancy-related disorders. Importantly, antagonization of the PKR signaling by PC7 and PKRA during pregnancy has been demonstrated in preclinical mice models to be safe, without any adverse effects on the growing embryos, which can be considered for treatment of pregnancy-related disorders to improve pregnancy outcome (Reynaud et al., 2021).
A. Prokineticin Function in Reproductive System
Although PK2 is undetectable in the female reproductive organs, expression of PK1 was found abundantly in the stroma of the ovaries, particularly in the cells involved in steroid production in the hilar region (Ferrara et al., 2004; Alfaidy et al., 2016, 2019). The main expression of PK1 was found in the granulosa of the primary and secondary cells controlled by the ovarian cycle and to a lesser extent in the tertiary follicle (Fraser et al., 2005; Alfaidy et al., 2016, 2019). However, the pk1 and pk2 genes are strongly expressed in mammalian testis (Ferrara et al., 2004). The PK1 is mainly present in Leydig cells, whereas PK2 is present in the testicular tubules in primary spermatocytes.
Circulating levels of PK1 are markedly increased during pregnancy and it is the only prokineticin expressed in the placenta (Hoffmann et al., 2006; Reynaud et al., 2021). The peak of PK1/PKR1 expression occurs in the first trimester of pregnancy (Hoffmann et al., 2006). PK1 plays a key role in placental development throughout pregnancy (Brouillet et al., 2012b) and contributes to the birth process (Dunand et al., 2014). PK1 promotes trophoblast cell adhesion to fibronectin and laminin matrices, thereby mediating fetal-maternal dialog during implantation (Brouillet et al., 2012b). It may regulate the expression of implantation-related genes (Haouzi et al., 2009; Alfaidy et al., 2016, 2019). During the first trimester of pregnancy, PK1 controls extravillous trophoblastic cell migration, invasion, and pseudovascular network formation (Brouillet et al., 2010). PK1 also acts on fetal ECs in the stroma, increasing their proliferation, migration, invasion, and branching (Brouillet et al., 2010).
In the myometrium, PK1 induces proinflammatory cascades and increases myometrial smooth muscle cell contractility (Catalano et al., 2010), promoting both preterm and term births (Gorowiec et al., 2011). In the uteroplacental unit, PK1 and its receptors have been shown to provide intrauterine quiescence during the last trimester of pregnancy (Dunand et al., 2014). PK1 has been associated with early pregnancy pathologies such as ectopic pregnancies (Shaw et al., 2010) and miscarriages (Salker et al., 2010). Recently, mutations in the pk1, pkr1, and pkr2 genes have been reported to be associated with recurrent miscarriage (Su et al., 2014), but some variants have a protective role in the early stages of pregnancy (Su et al., 2013). Thus, it is not yet known whether the increase in PK1 levels is a cause or consequence of ectopic pregnancy or miscarriage.
B. Prokineticin Signaling Linked to the Reproductive System Disorders Such as Gestational Hypertension, Preeclampsia and Choriocarcinoma
Pregnancy-induced hypertension leads to complications in 5–11% of all pregnancies (Burton, 2009). PE, a form of pregnancy-induced hypertension, is the most threatening pregnancy pathology as it brings serious consequences to both the mother and the fetus (Tjoa et al., 2004). Recent studies have shown that PE not only affects the maternal cardiovascular system during pregnancy, but may also have long-term adverse effects, including maternal cardiovascular disease, vascular dementia (RR=3.46), AD (RR=1.45), and unspecified dementia (RR=1.40). These adverse effects occur approximately 10–20 years after an PE episode (Basit et al., 2018). The incriminated organ in the pathology of PE is the placenta, the key organ that ensures the success of pregnancy.
Adverse factors released by the placenta in PE include PKs, particularly PK1, which is abundantly produced in the first trimester of human pregnancy, with a peak of expression just prior to the establishment of the feto-maternal circulation (Hoffmann et al., 2006; Brouillet et al., 2012a; Alfaidy, 2016; Alfaidy et al., 2016). PK1 is mainly expressed in the endocrine unit of the placenta (the syncytiotrophoblast layer) and its receptors, PKR1 and PKR2, are highly expressed in the trophoblasts and microvascular cells of the placenta (Hoffmann et al., 2006; Brouillet et al., 2010, 2012b; Bardin et al., 2015; Traboulsi et al., 2015). The expression of PK1 in the placenta has been reported to be upregulated by hypoxia and β-hCG (Brouillet et al., 2010, 2012b).
PK1 controls early invasion of extravillous trophoblast cells during the first trimester of pregnancy and increases proliferation, migration, and permeability of trophoblasts and ECs (Brouillet et al., 2010). Moreover, circulating PK1 levels in nonpregnant women are approximately 50 pg/ml and increase 5-fold (≈ 250 pg/ml) during the first trimester of pregnancy and even higher (400–500 pg/ml) in the context of PE (Hoffmann et al., 2007; Brouillet et al., 2013). These results suggest that sustained PK1 levels beyond the first trimester of pregnancy could cause the development of pregnancy pathologies, including PE.
Mimicking the increase in circulating PK1 levels beyond 11.5 days post coïtus (dpc) in gravid mice, similar to the levels observed beyond the first trimester of pregnancy in women with PE, caused the development of PE symptoms in mice (Sergent et al., 2016; Reynaud et al., 2018). These symptoms were manifested by i) a failure to establish normal feto-maternal circulation, ii) the development of hypoxic placenta, a hallmark of stressed placenta, iii) a decrease in the expression of key genes known to be involved in trophoblast differentiation toward an invasive phenotype, iv) an increase in local and circulating anti-angiogenic factors (i.e., soluble fms-like tyrosine kinase and soluble endoglin) that have been shown to have effects on maternal vasculature and renal function and are known to be elevated in PE (Burton, 2009; Brouillet et al., 2012b; Cotechini et al., 2014).
Importantly, the maintenance of PK1 in the first trimester causes stress to the placenta, whereas its increase in the third trimester is associated with a compensatory mechanism to ensure the continuation of the pregnancy (Hoffmann et al., 2007; Sergent et al., 2016). In the developed model, a significant increase in placental efficiency was observed in the PK1-treated group (at 18.5 dpc), suggesting an adaptation of the placenta in situ, which was supported by an increase in placental vascularization and proliferation (Hoffmann et al., 2007; Sergent et al., 2016). Recently, the use of antagonists for PKR1 and PKR2 (PC7, PKR-A) have been reported to reverse some of PK1 adverse effects during gestation, as they blocked excessive trophoblast proliferation, migration, and invasion (Nguyen et al., 2013; Severini et al., 2015; Landucci et al., 2016; Traboulsi et al., 2017; Reynaud et al., 2021). In addition, a recent study by Reynaud et al. (Reynaud et al., 2021) demonstrated that PC7 and PKRA that were tested independently or in combination in trophoblast cells and during early gestation in the gravid mouse did not cause any adverse pregnancy outcome, suggesting that their potential consideration to treat PE.
A group of pregnancy-related disorders that originated from trophoblastic cells is called gestational trophoblastic diseases, which include a rare, aggressive neoplasm called choriocarcinoma. The development of choriocarcinoma is associated with a previous gestational event (molar, physiologic, ectopic pregnancy, selective or spontaneous abortion) (Bruce and Sorosky, 2023). One study has shown that PK1 and its receptors may also be directly involved in the development of gestational choriocarcinoma (Traboulsi et al., 2017). Increased levels of PK1 were found in both placenta and bloodstream of gestational choriocarcinoma patients. Indeed, PK1 increased proliferation, migration, and invasion of the choriocarcinoma cell line (JEG3) in two-dimensional and three-dimensional tumor cell spheroid systems. The PKR2 antagonist (PKR-A) significantly reduced tumor development and progression and preserved pregnancy in animal models of gestational choriocarcinoma by JEG3 injection in the placenta (Traboulsi et al., 2017). Thus, the use of PKR2 antagonist can be a therapeutic option for the treatment of gestational choriocarcinoma.
XI. Prokineticin Signaling in Cancer
PK1 and PK2 can be involved in the processes of tumorigenesis by acting as angiogenic factors, involved in tumor growth, and as chemotactic factors for the recruitment of proinflammatory neutrophils. In this way, they can contribute to all steps of oncogenesis, such as cell proliferation, angiogenesis, and metastasis. Several reports have documented the role of PKs in many malignancies, including colorectal and prostate cancers and human tumors derived from steroidogenic cells in the adrenal cortex, testis, and ovaries, where they may be used in the future as biomarkers to aid in the early diagnosis and prognosis of cancer. Thus, better understanding the effects of PKs on cancer occurrence and progression will potentially enable the development of new anticancer drugs targeting PKRs.
PKs not only play an active role in physiologic angiogenesis of various body tissues but can also be involved in pathologic neoplastic angiogenesis, making them important components of tumor growth, progression, cellular plasticity, and metastasis. Several reports have demonstrated the pro-angiogenic role of PK1. Nevertheless, it is now well established that also PK2, which is strongly expressed in neutrophils and other myeloid cells after activation by granulocyte colony-stimulating factor through the STAT3 pathway (Qu et al., 2012), is associated with tumor angiogenesis and resistance to anti-VEGF drugs, as well as a number of inflammatory diseases (Shojaei et al., 2007; Itatani et al., 2020).
In 2001, LeCouter et al. (LeCouter et al., 2001) identified in EG-VEGF, also known as PK1, an endocrine gland growth factor with a unique activity and distinct expression pattern capable of inducing proliferation, migration, and fenestration of ECs only from steroidogenic endocrine glands, suggesting a highly specific, local mechanism to control angiogenesis. The angiogenic activity of PK1 in specific body organs links it not only to testicular carcinomas and neuroblastomas, but also to other cancers such as colorectal, pancreatic, and prostate cancers.
In testicular tumors, PK1/EG-VEGF is specifically expressed in testosterone-producing Leydig cell tumors, whereas other tumors are negative for this antigen (Samson et al., 2004). PK1, which physiologically mediates the growth and differentiation of enteric neural crest cells (NCCs) during development, is abnormally expressed in neuroblastomas and correlates with progression of tumor pathology (Ngan et al., 2007).
In colorectal carcinoma (CRC), PK1 has been suggested as a potential biomarker for poorer prognosis, invasion, and metastasis, which is significantly higher in cases with serous, lymphatic, or venous invasion and with lymph node, liver, or hematogenous metastasis (Nakazawa et al., 2015). In addition to PK1, PK2 has also been shown to be involved in CRC by both clinical and preclinical studies. The role of PK2 in the recurrence and progression of CRC in humans was illustrated by Yoshida et al. (Yoshida et al., 2018), who showed that approximately 50% of patients with primary CRC at various stages (I–III) had higher PK2 expression, which was absent in normal colorectal mucosa. Moreover, PK2 expression correlated with lymphatic invasion and lymph node metastasis in these patients at stages II and III and was inversely related to patient survival. Anti-VEGF antibodies (bevacizumab) are used clinically to treat CRC (Xie et al., 2020), but refractoriness or resistance to anti-VEGF is a limit to treatment efficacy. In a genetic model from CRC, Itatani et al. (Itatani et al., 2020) demonstrated a correlation between inflammation and loss of responsiveness to anti-VEGF. Namely, they showed that mice with aggressive tumor responded very well to anti-VEGF treatment, but when these mice were exposed to inflammation from chemically induced colitis, they became resistant to anti-VEGF antibody treatment. This is because these colitis mice had markedly elevated serum levels of granulocyte colony-stimulating factor, a major elicitor of PK2 in the CD11b+ Gr1+ neutrophil subpopulation that is increased in tumors and promotes their angiogenesis. These results suggest a therapy based on the combination of anti-VEGF and PK2 inhibitors for the treatment of CD11b+ Gr1+-associated tumor cells resistant to anti-VEGF. In vitro experiments have shown high PK2 mRNA expression in various CRC cell lines. In in vivo experiments in mice, subcutaneous implantation of CRC cell lines, constitutively expressing PK2, resulted in a marked increase in blood vessels and tumor mass. However, subcutaneous implantation of these cell lines in combination with a small interfering RNA for PK2 (small interfering RNA-PK2) resulted in suppression of angiogenesis and significant reduction in tumor mass (Kurebayashi et al., 2015).
Since human pancreatic cells have steroidogenic activity, PK1 has been found to be expressed not only in normal pancreas but also in pancreatic adenocarcinoma (PC), where it is upregulated in islet cells, mainly in males, in the exocrine part, mainly in females, and in the blood vessels (Morales et al., 2007, 2008). PC is characterized by poor vascularization and the presence of a large number of myeloid cells (Korc, 2007). Curtis et al. (Curtis et al., 2013) showed in a PC xenograft mouse model that injection of PKRA7 significantly reduced tumor mass due to a reduction in myeloid cell infiltration. Hasnis et al. (Hasnis et al., 2014) injected PC cells directly into the pancreas of mice and found increased expression of PK2 in the tumor, infiltrating granulocytes, and blood cells. Although antiangiogenic drug therapies have shown little effect in the treatment of PC (Kindler et al., 2010), PK2 antibody in combination with gemcitabine has been shown to significantly reduce primary tumor growth, angiogenesis, and metastasis in preclinical models (Hasnis et al., 2014).
Prostate cancer growth, invasion, and metastasis are associated with angiogenesis and treated with antiangiogenic therapies (Campbell, 1997). In most prostate carcinomas, evidence of a significant increase in PK1 expression in glandular epithelial cells, together with a high microvascular density, argues for the role of PK1 in angiogenesis and consequently in prostate carcinoma growth. Since prostate cancer progression is associated with an increase in PK1, this led to the identification of a potential prognostic marker for prostate cancer progression in PK1. In addition, PK2 and PKRs also show high expression in malignant cells, confirming PKs as a target for prostate cancer treatment (Pasquali et al., 2006).
The involvement of PK2 in breast cancer has been demonstrated in preclinical studies. Kowanetz et al. (Kowanetz et al., 2010) found that in a mouse model with different metastatic potential of breast cancer cells, PK2 expression increased in Ly6G+Ly6C+ myeloid cells of metastatic lung tumors. This metastatic lung tumor was reduced by treating mice with an anti-PK2 antibody. Recently, PK2 was shown to increase the proliferation of breast cancer cells in vitro (Sasaki et al., 2018). In a mouse model in which a breast cancer cell line was xenotransplanted into the mammary fat pad, 5-fluorouracil accelerated the development of lung metastases, which was due to massive migration of neutrophilic CD11b+Ly6G+ myeloid cells (also known as Ly6G+Ly6C+) into the lung. In these mice, high PK2 expression was detected in the Ly6G+Ly6C+ when neutrophil infiltration into the lungs became evident. Lung cancer is characterized by high PK2 levels associated with infiltrating neutrophils (Zhong et al., 2009). In a lung cancer mouse model obtained by subcutaneous implantation of a murine Lewis lung carcinoma cell line lacking PK2 expression, implantation of these Lewis lung carcinoma 1 cells into transgenic mice lacking granulocytes showed no increase in tumor size, suggesting that the source of PK2 is granulocytes (Jiang et al., 2013b).
Polyomavirus (MCPyV) or papillomavirus infection causes Merkel cell carcinoma, a rare and aggressive form of human skin cancer and oropharyngeal (Sihto et al., 2009) or cervical (Cohen et al., 2019) cancer. In Merkel cell carcinoma, high PK2 levels are associated with high numbers of tumor-infiltrating macrophages and the presence of Merkel cell polyomavirus DNA. PK1 is associated with an unfavorable prognosis, whereas high PK2 mRNA levels are associated with favorable survival, suggesting that PK2 may play a role in coordinating immune defenses against polyomaviruses in Merkel cell carcinoma patients and thus may influence their survival. Interestingly, high PK2 expression was found in the advanced stages (III and IV) of cervical cancer, which positively correlates with poor patient survival (Wu et al., 2020).
Hepatocellular carcinoma (HCC) is characterized by overexpression of angiogenic factors such as VEGF. PK2 has been shown to be constitutively expressed in normal livers and its levels are markedly reduced in HCC, where high levels of VEGF are found (Monnier and Samson, 2008). Indeed, the low PKR2 expression in HCC is related to the lower number of Kupffer cells (liver resident macrophages), which are the main source of PK2 (Liu et al., 2003). More importantly, PK2 levels do not correlate with the degree of angiogenesis in HCC. Consequently, antiangiogenic therapy with the anti-VEGF antibody bevacizumab has been shown to be highly beneficial for survival in patients with HCC (Llovet et al., 2022).
Further analysis of PKRs in tumor induction and cancer progression may allow the development of potential anticancer drugs and PKs as biomarkers for prognosis and diagnosis of cancer.
XII. Perspectives
In the last 20 years, studies on the prokineticin system have reported that prokineticins act as neuropeptides, cytokines, chemokines, and hormones, and their activity depends on various factors, including effector cell type or receptor expression profile and disease pathologies. Possible therapeutic use of targeting PKRs are summarized in Fig. 9. The expression levels of PK2 and their receptors at the levels of both mRNA and protein, are altered by different types of pathologic conditions, such as hypoxia, ischemia, oxidative stress, inflammation, glutamate release, Aβ accumulation, high glucose, dopaminergic neuron death, cardiac and neuronal injury, and obesity. Expression of PK1 is regulated by hCG, estradiol-17β, and progesterone, and it has a key role in the maintenance of pregnancy (Evans et al., 2008). PKs can be released from almost any tissue in response to injury as a compensatory mechanism. Depending on the amount released by pathologic insults (in the range of pmol or nmol), PK2 could activate a different type of receptor, PKR1 or PKR2, and sometimes different, even opposite, transduction pathways that initiate wound healing or trigger the deleterious stage of the disease. Therefore, it is not yet known whether the increase in PK levels is a cause or a consequence of these diseases and which receptor is involved in the underlying pathologies. The role of increased expression of PKs and PKRs and activation/inactivation of the PK2/PKRs pathway in some pathologies is still unclear.
Fig. 9.
Possible therapeutic use of PKR ligands. The studies on genetically inhibition of PKRs in mice and preclinical studies with PKR agonist or antagonist demonstrated that PKR1 and PKR2 can be targets for treatment of indicated diseases. Created with BioRender.com.
All these studies suggest that the PK family may be potential prognostic biomarkers (Table 2) or therapeutic targets for certain types of pathologies (Table 1). For example, based on the Allen Institute for Brain Science transcriptome dataset of brains study, PK2 expression is more affected than PK1 expression in four brain regions in brain injury: parietal neocortex, hippocampus, inferior parietal lobule, and neocortex from the posterior superior temporal gyrus. Further studies of PK2 expression are needed to evaluate its role in response to injury and other neurologic disorders and to investigate its potential as a biomarker of severity and outcome. In contrast, PK1 is an important pleiotropic factor in regulating female reproduction. Many studies have shown that PK1 or PK2 may also be positively/negatively associated with poor survival. However, due to small sample size, these data need to be confirmed in a large population and in other ethnicities. In clinical trials, the nature of chronic therapies and risk factors along with patients’ vascular complications need to be better documented. The mechanisms involved in controlling the expression of PKs and receptors, and the downstream signaling pathways are still not clear. However, it is clear that PK2 can be used as a biomarker for injury and as a target for drug development to treat many acute and chronic neuronal and peripheral diseases.
TABLE 2.
PKs as markers of diseases
| Prokineticin 1 (PK1) | |
|---|---|
| Disease | Reference |
| Colorectal cancer | (Tagai et al., 2021) |
| Gastrointestinal tumor | (Goi et al., 2013) |
| Human gliomas | (Xiao et al., 2017) |
| Implantation failure | (Karaer et al., 2020) |
| Lower-grade glioma | (Zhong et al., 2022) |
| Obstetric disorders | (Su et al., 2017) |
| Preeclampsia | (Wang et al., 2019) |
| Temporomandibular joint disorders | (Herr et al., 2011) |
| Prokineticin 2 (PK2) | |
| Disease | Reference |
| Alzheimer | (Lattanzi et al., 2019b) |
| Arthritis | (Noda et al., 2021) |
| Cervical cancer | (Wu et al., 2020) |
| CNS autoimmune demyelinating disease | (Abou-Hamdan et al., 2015) |
| Colorectal cancer | (Yoshida et al., 2018) |
| Diabetes | (Mortreux et al., 2019) |
| End stage cardiac failure | (Urayama et al., 2007) |
| Hepatocellular carcinoma | (Monnier et al., 2008) |
| Huntington's disease | (Mastrokolias et al., 2015) |
| Inflammation-induced pain | (Jacobson et al., 2011) |
| Klinefelter's syndrome | (Fiore et al., 2022) |
| Lower-grade glioma | (Zhong et al., 2022) |
| Lung metastasis | (Kowanetz et al., 2010) |
| Metabolic syndrome | (Wang et al., 2016) |
| Neuroendocrine neoplasms | (Puliani et al., 2022) |
| Obesity | (Wang et al., 2021) |
| Osteoporosis | (Zhang et al., 2022b) |
| Parkinson | (Schirinzi et al., 2021, 2022) |
| Pneumonia | (Tu et al., 2022) |
| Post-COVID olfactory recovery | (Schirinzi et al., 2023) |
| Pregnancy pathology | (Alfaidy et al., 2014) |
| Prostate cancer | (Zhong et al., 2009) |
| Septic choc Sepsis | (Yu et al., 2022) |
| Skin cancer | (Lauttia et al., 2014) |
Since PK1 and PK2 can activate both receptors, not only the identification of expression and localization of the PKR subtypes but also the generation of corresponding knockout mice may help to further clarify the functional target. Indeed, some studies have been performed using conventional knockout mice that exhibit many pathologies, such as in the PKR1(−/−) mice, raising the question of whether the dysfunctional phenotype is a consequence of another pathology. Tissue-specific knockout can help dissect target organs/tissues in the disease pathology. Further studies are also needed to clarify how receptor-dependent signaling may modulate PK2 responses by analyzing PKR1(−/−) or PKR2(−/−) mice in disease models such as neuropathies, toxicities, diabetes, and obesity. In addition, chemicals are often used as part of a research protocol to provoke pathology and establish a disease model in animals. These models should be verified with genetic disease models in rodents. Disease models from animals should also be verified using in vitro systems, such as human-derived 3D spheroids and organoids.
Mutations or dysfunctions of human PKs and their receptors are also associated with various diseases as summarized in Supplemental Table 1 in the supplemental data. Since some clinically observed mutations in mice should be verified by knockin mice, these may help to understand the specific pathways in the respective physiologic/pathologic functions. All naturally occurring mutations in the PK2 and PKR2 proteins described to date in human diseases and how this relates to mutational information may inform various antagonist molecules that are being developed. For example, PKR2 mutation imparts biased intracellular signaling deficits and they can be functionally rescued.
Nevertheless, targeting PKRs may provide a therapeutic approach for certain diseases. One prospect for the development of new powerful ligands may be related to the experimental resolution of the crystallographic structures of PKR1 and PKR2, which could dramatically improve the accuracy of structure-based methods. In the absence of that, an improvement of homology-based methods, which have already been successful in QSAR modeling could contribute new active lead compounds to treat diseases. Targeting PKRs can provoke some potential side effects. For example, in obesity or cardiovascular diseases targeting PKR1 may have beneficial effect but can increase the sensibility to pain. Further studies are also necessary for the development of more specific and bias ligands and to examine their effects on the efficacy of chronically used therapeutics in preclinical disease models and patient-derived organoids.
Acknowledgments
The authors thank P. Karpov for his contribution to the design of PKR1 agonists. Figures were created with BioRender.
Note Added in Proof: An incorrect last name for the seventh author was accidentally published in the Fast Forward version that appeared online September 14, 2023. The author line has now been corrected.
ABBREVIATIONS
- (−/−)
knockout
- Acsl4
acyl-CoA synthetase long-chain family member 4
- AD
Alzheimer's Disease
- AgRP
Aguti-related peptide
- AKT
protein kinase
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ATM
adipose tissue macrophage
- AVITG
alanine-valine-isoleucine-threonine glycine sequence
- Aβ
β amyloid
- Bmal-1
brain and muscle Arnt-like protein 1
- BMI
body mass index
- Bv8
Bombina variegata (8 kDa)
- Ca2+
calcium
- CCI
chronic constriction injury
- CFA
Freund’s complete adjuvant
- CFP
Cardiac fibroblast progenitor cells
- CNS
central nervous system
- COOH-
carboxy-terminus
- DOX
doxorubicin
- dpc
days post coitus
- DRG
dorsal root ganglia
- EAT
epicardial adipose tissue
- ECL
extracellular loop
- ECs
endothelial cells
- ECS
electroconvulsive shock
- EDPC
epicardial-derived progenitor cell
- EG-VEGF
endocrine gland-derived-vascular endothelial growth factor
- GnRH
gonadotropin releasing hormone
- GPCR
G protein-coupled receptor
- HCC
hepatocellular carcinoma
- HF
heart failure
- ICL
intracellular loop
- IHH
idiopathic hypogonadotropic hypogonadism
- IL
interleukin
- KS
Kallmann Syndrome
- MAPK
mitogen-activated protein kinase
- MAT
matrine
- MET
mesenchymal-epithelial transition
- MI
myocardial infarction
- MRAP2
melanocortin receptor accessory protein 2
- NH2-
amino-terminus
- NPY
Neuropeptide Y
- OB
olfactory bulb
- OD
olfactory dysfunction
- ONs
olfactory neurons
- OA
osteoarthritis
- PC
pancreatic adenocarcinoma
- PD
Parkinson’s Disease
- PE
preeclampsia
- PK
prokineticin
- PKR
mammalian prokineticin receptor
- PVN
paraventricular nucleus
- RA
rheumatoid arthritis
- RMS
rostral migratory stream
- SCN
suprachiasmatic nucleus
- SNI
spared nerve injury
- SVZ
subventricular zone
- T. cruzi
trypanosoma cruzi
- TBI
traumatic brain injury
- Tcf21
transcription factor 21 (or epicardin)
- Th
T helper
- TM
transmembrane
- TRPV 1
transient receptor potential cation channel subfamily V member 1
- TS
trans sialidase.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Vincenzi, Kremić, Jouve, Lattanzi, Miele, Benharouga, Alfaidy, Migrenne-Li, Kanthasamy, Porcionatto, Ferrara, Tetko, Désaubry, Nebigil.
Footnotes
C.G.N. endorses Fondation de France [Grant R023044MM]. N.F. acknowledges National Institutes of Health (NIH) National Eye Institute [Grant R01-EY031345] and A.G.K. endorses [Grant R01-EY031345], National Institute of Neurological Disorders and Stroke [Grants R01-NS078247 and R01-NS088206], National Institute of Environmental Health Sciences [Grant R01-ES026892], W. Eugene and Linda Lloyd Endowed Chair, UGA Johnny Isakson Chair and Georgia Research Alliance Eminent Scholar. C.G.N. and I.V.T. acknowledge ERA-CVD (https://era-cvd.eu) “Cardio-Oncology” [Grants ANR0005 and BMBF 01KL1710 respectively]. M.V. acknowledges Sapienza University of Rome grant “Progetti per Avvio alla Ricerca - Tipo 1” [AR12218162ED57D1].
No author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at pharmrev.aspetjournals.org.
This article has supplemental material available at pharmrev.aspetjournals.org.
References
- Abou-Hamdan M, Costanza M, Fontana E, Di Dario M, Musio S, Congiu C, Onnis V, Lattanzi R, Radaelli M, Martinelli V, Salvadori S, Negri L, Poliani PL, Farina C, Balboni G, Steinman L, Pedotti R (2015) Critical role for prokineticin 2 in CNS autoimmunity. Neurol Neuroimmunol Neuroinflamm 2:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu AP, Kaiser UB, Latronico AC (2010) The role of prokineticins in the pathogenesis of hypogonadotropic hypogonadism. Neuroendocrinology 91:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu AP, Trarbach EB, de Castro M, Frade Costa EM, Versiani B, Matias Baptista MT, Garmes HM, Mendonca BB, Latronico AC (2008) Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J Clin Endocrinol Metab 93:4113–4118. [DOI] [PubMed] [Google Scholar]
- Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, et al. (2012) The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139:2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaidy N (2016) Prokineticin1 and pregnancy. Ann Endocrinol (Paris) 77:101–104. [DOI] [PubMed] [Google Scholar]
- Alfaidy N, Baron C, Antoine Y, Reynaud D, Traboulsi W, Gueniffey A, Lamotte A, Melloul E, Dunand C, Villaret L, et al. (2019) Prokineticin 1 is a new biomarker of human oocyte competence: expression and hormonal regulation throughout late folliculogenesis. Biol Reprod 101:832–841. [DOI] [PubMed] [Google Scholar]
- Alfaidy N, Hoffmann P, Boufettal H, Samouh N, Aboussaouira T, Benharouga M, Feige J-J, Brouillet S (2014) The multiple roles of EG-VEGF/PROK1 in normal and pathological placental angiogenesis. BioMed Res Int 2014:451906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaidy N, Hoffmann P, Gillois P, Gueniffey A, Lebayle C, Garçin H, Thomas-Cadi C, Bessonnat J, Coutton C, Villaret L, et al. (2016) PROK1 Level in the Follicular Microenvironment: A New Noninvasive Predictive Biomarker of Embryo Implantation. J Clin Endocrinol Metab 101:435–444. [DOI] [PubMed] [Google Scholar]
- Araki K, Nagata K (2011) Protein folding and quality control in the ER. Cold Spring Harb Perspect Biol 3:a007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora H, Boulberdaa M, Qureshi R, Bitirim V, Gasser A, Messaddeq N, Dolle P, Nebigil CG (2016a) Prokineticin receptor-1 signaling promotes Epicardial to Mesenchymal Transition during heart development. Sci Rep 6:25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora H, Boulberdaa M, Qureshi R, Bitirim V, Messadeq N, Dolle P, Nebigil CG (2016b) Prokineticin receptor 1 is required for mesenchymal-epithelial transition in kidney development. FASEB J 30:2733–2740. [DOI] [PubMed] [Google Scholar]
- Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, et al. (2013) Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341:275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avbelj Stefanija M, Jeanpierre M, Sykiotis GP, Young J, Quinton R, Abreu AP, Plummer L, Au MG, Balasubramanian R, Dwyer AA, et al. (2012) An ancient founder mutation in PROKR2 impairs human reproduction. Hum Mol Genet 21:4314–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayari B, El Hachimi KH, Yanicostas C, Landoulsi A, Soussi-Yanicostas N (2010) Prokineticin 2 expression is associated with neural repair of injured adult zebrafish telencephalon. J Neurotrauma 27:959–972. [DOI] [PubMed] [Google Scholar]
- Ayton S, Faux NG, Bush AI; Alzheimer’s Disease Neuroimaging Initiative (2015) Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 6:6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R, Cohen DA, Klerman EB, Pignatelli D, Hall JE, Dwyer AA, Czeisler CA, Pitteloud N, Crowley WF (2014) Absence of central circadian pacemaker abnormalities in humans with loss of function mutation in prokineticin 2. J Clin Endocrinol Metab 99:E561–E566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni G, Lazzari I, Trapella C, Negri L, Lattanzi R, Giannini E, Nicotra A, Melchiorri P, Visentin S, Nuccio CD, et al. (2008) Triazine compounds as antagonists at Bv8-prokineticin receptors. J Med Chem 51:7635–7639. [DOI] [PubMed] [Google Scholar]
- Bales KL, Witczak LR, Simmons TC, Savidge LE, Rothwell ES, Rogers FD, Manning RA, Heise MJ, Englund M, Arias Del Razo R (2018) Social touch during development: Long-term effects on brain and behavior. Neurosci Biobehav Rev 95:202–219. [DOI] [PubMed] [Google Scholar]
- Bao Z, Liu Y, Chen B, Miao Z, Tu Y, Li C, Chao H, Ye Y, Xu X, Sun G, et al. (2021) Prokineticin-2 prevents neuronal cell deaths in a model of traumatic brain injury. Nat Commun 12:4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin N, Murthi P, Alfaidy N (2015) Normal and pathological placental angiogenesis. BioMed Res Int 2015:354359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M, Maillet J, Huyvaert M, Dechaume A, Boutry R, Loiselle H, Durand E, Toussaint B, Vaillant E, Philippe J, et al. (2019) Loss-of-function mutations in MRAP2 are pathogenic in hyperphagic obesity with hyperglycemia and hypertension. Nat Med 25:1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basit S, Wohlfahrt J, Boyd HA (2018) Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ 363:k4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale K, Gardiner JV, Bewick GA, Hostomska K, Patel NA, Hussain SS, Jayasena CN, Ebling FJ, Jethwa PH, Prosser HM, et al. (2013) Peripheral administration of prokineticin 2 potently reduces food intake and body weight in mice via the brainstem. Br J Pharmacol 168:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Calixto A, Cardona-Gómez GP (2017) The Role of Astrocytes in Neuroprotection after Brain Stroke: Potential in Cell Therapy. Front Mol Neurosci 10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisbouvier J, Albrand JP, Blackledge M, Jaquinod M, Schweitz H, Lazdunski M, Marion D (1998) A structural homologue of colipase in black mamba venom revealed by NMR floating disulphide bridge analysis. J Mol Biol 283:205–219. [DOI] [PubMed] [Google Scholar]
- Boulberdaa M, Turkeri G, Urayama K, Dormishian M, Szatkowski C, Zimmer L, Messaddeq N, Laugel V, Dollé P, Nebigil CG (2011) Genetic inactivation of prokineticin receptor-1 leads to heart and kidney disorders. Arterioscler Thromb Vasc Biol 31:842–850. [DOI] [PubMed] [Google Scholar]
- Brouillet S, Hoffmann P, Benharouga M, Salomon A, Schaal JP, Feige JJ, Alfaidy N (2010) Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells. Mol Biol Cell 21:2832–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet S, Hoffmann P, Chauvet S, Salomon A, Chamboredon S, Sergent F, Benharouga M, Feige JJ, Alfaidy N (2012a) Revisiting the role of hCG: new regulation of the angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci 69:1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet S, Hoffmann P, Feige JJ, Alfaidy N (2012b) EG-VEGF: a key endocrine factor in placental development. Trends Endocrinol Metab 23:501–508. [DOI] [PubMed] [Google Scholar]
- Brouillet S, Murthi P, Hoffmann P, Salomon A, Sergent F, De Mazancourt P, Dakouane-Giudicelli M, Dieudonné MN, Rozenberg P, Vaiman D, et al. (2013) EG-VEGF controls placental growth and survival in normal and pathological pregnancies: case of fetal growth restriction (FGR). Cell Mol Life Sci 70:511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S, Sorosky J (2023) Gestational Trophoblastic Disease, in StatPearls, StatPearls Publishing, Treasure Island, Florida. [PubMed] [Google Scholar]
- Bullock CM, Li JD, Zhou QY (2004) Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol 65:582–588. [DOI] [PubMed] [Google Scholar]
- Burton GJ (2009) Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KJ, Li X, Li JD, Hu WP, Zhou QY (2015) Rhythmic Trafficking of TRPV2 in the Suprachiasmatic Nucleus is Regulated by Prokineticin 2 Signaling. J Circadian Rhythms 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caioli S, Severini C, Ciotti T, Florenzano F, Pimpinella D, Petrocchi Passeri P, Balboni G, Polisca P, Lattanzi R, Nisticò R, et al. (2017) Prokineticin system modulation as a new target to counteract the amyloid beta toxicity induced by glutamatergic alterations in an in vitro model of Alzheimer’s disease. Neuropharmacology 116:82–97. [DOI] [PubMed] [Google Scholar]
- Campbell SC (1997) Advances in angiogenesis research: relevance to urological oncology. J Urol 158:1663–1674. [DOI] [PubMed] [Google Scholar]
- Canto P, Munguía P, Söderlund D, Castro JJ, Méndez JP (2009) Genetic analysis in patients with Kallmann syndrome: coexistence of mutations in prokineticin receptor 2 and KAL1. J Androl 30:41–45. [DOI] [PubMed] [Google Scholar]
- Casella I, Ambrosio C (2021) Prokineticin receptors interact unselectively with several G protein subtypes but bind selectively to β-arrestin 2. Cell Signal 83:110000. [DOI] [PubMed] [Google Scholar]
- Castelli M, Amodeo G, Negri L, Lattanzi R, Maftei D, Gotti C, Pistillo F, Onnis V, Congu C, Panerai AE, et al. (2016) Antagonism of the Prokineticin System Prevents and Reverses Allodynia and Inflammation in a Mouse Model of Diabetes. PLoS One 11:e0146259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano RD, Lannagan TR, Gorowiec M, Denison FC, Norman JE, Jabbour HN (2010) Prokineticins: novel mediators of inflammatory and contractile pathways at parturition? Mol Hum Reprod 16:311–319. [DOI] [PubMed] [Google Scholar]
- Chaly AL, Srisai D, Gardner EE, Sebag JA (2016) The Melanocortin Receptor Accessory Protein 2 promotes food intake through inhibition of the Prokineticin Receptor-1. eLife 5:e12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DN, Ma YT, Liu H, Zhou QY, Li JD (2014) Functional rescue of Kallmann syndrome-associated prokineticin receptor 2 (PKR2) mutants deficient in trafficking. J Biol Chem 289:15518–15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg T, Liu C (2005) Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Mol Pharmacol 67:2070–2076. [DOI] [PubMed] [Google Scholar]
- Chen L, Lv F, Min S, Yang Y, Liu D (2023) Roles of prokineticin 2 in electroconvulsive shock-induced memory impairment via regulation of phenotype polarization in astrocytes. Behav Brain Res 446:114350. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bittman EL, Hattar S, Zhou QY (2005) Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY (2002) Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417:405–410. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Lee AG, Culbertson C, Sun G, Talati RK, Manley NC, Li X, Zhao H, Lyons DM, Zhou Q-Y, et al. (2012) Prokineticin 2 is an endangering mediator of cerebral ischemic injury. Proc Natl Acad Sci USA 109:5475–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Leslie FM, Zhou QY (2006) Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol 498:796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choke E, Cockerill GW, Dawson J, Howe F, Wilson WR, Loftus IM, Thompson MM (2010) Vascular endothelial growth factor enhances angiotensin II-induced aneurysm formation in apolipoprotein E-deficient mice. J Vasc Surg 52:159–166.e1. [DOI] [PubMed] [Google Scholar]
- Cohen PA, Jhingran A, Oaknin A, Denny L (2019) Cervical cancer. Lancet 393:169–182. [DOI] [PubMed] [Google Scholar]
- Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, et al. (2008) Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab 93:3551–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS (2011) Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci 12:553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congiu C, Onnis V, Deplano A, Salvadori S, Marconi V, Maftei D, Negri L, Lattanzi R, Balboni G (2014) A new convenient synthetic method and preliminary pharmacological characterization of triazinediones as prokineticin receptor antagonists. Eur J Med Chem 81:334–340. [DOI] [PubMed] [Google Scholar]
- Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH (2014) Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med 211:165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Oliveira LMB, Plummer L, Corbin B, Gardella T, Balasubramanian R, Crowley WF (2018) Modeling mutant/wild-type interactions to ascertain pathogenicity of PROKR2 missense variants in patients with isolated GnRH deficiency. Hum Mol Genet 27:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis VF, Wang H, Yang P, McLendon RE, Li X, Zhou QY, Wang XF (2013) A PK2/Bv8/PROK2 antagonist suppresses tumorigenic processes by inhibiting angiogenesis in glioma and blocking myeloid cell infiltration in pancreatic cancer. PLoS One 8:e54916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SJ, Zhang QY, Lan Q, Chen Y, Zhang YZ, Huang Q (2022) [PK2/PKR1 signaling pathway participates in geniposide protection against diabetic nephropathy in mice]. Zhongguo Zhongyao Zazhi 47:1611–1617. [DOI] [PubMed] [Google Scholar]
- De Felice M, Melchiorri P, Ossipov MH, Vanderah TW, Porreca F, Negri L (2012) Mechanisms of Bv8-induced biphasic hyperalgesia: increased excitatory transmitter release and expression. Neurosci Lett 521:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir F, Urayama K, Audebrand A, Toprak-Semiz A, Steenman M, Kurose H, Nebigil CG (2021) Pressure Overload-Mediated Sustained PKR2 (Prokineticin-2 Receptor) Signaling in Cardiomyocytes Contributes to Cardiac Hypertrophy and Endotheliopathies. Hypertension 77:1559–1570. [DOI] [PubMed] [Google Scholar]
- Désaubry L, Kanthasamy AG, Nebigil CG (2020) Prokineticin signaling in heart-brain developmental axis: Therapeutic options for heart and brain injuries. Pharmacol Res 160:105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodé C, Rondard P (2013) PROK2/PROKR2 Signaling and Kallmann Syndrome. Front Endocrinol (Lausanne) 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodé C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, et al. (2006) Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormishian M, Turkeri G, Urayama K, Nguyen TL, Boulberdaa M, Messaddeq N, Renault G, Henrion D, Nebigil CG (2013) Prokineticin receptor-1 is a new regulator of endothelial insulin uptake and capillary formation to control insulin sensitivity and cardiovascular and kidney functions. J Am Heart Assoc 2:e000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunand C, Hoffmann P, Sapin V, Blanchon L, Salomon A, Sergent F, Benharouga M, Sabra S, Guibourdenche J, Lye SJ, et al. (2014) Endocrine gland-derived endothelial growth factor (EG-VEGF) is a potential novel regulator of human parturition. Biol Reprod 91:73. [DOI] [PubMed] [Google Scholar]
- El Cheikh Hussein L, Mollard P, Bonnefont X (2019) Molecular and Cellular Networks in The Suprachiasmatic Nuclei. Int J Mol Sci 20:2052 10.3390/ijms20082052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Morgan K, Critchley HO, Millar RP, Jabbour HN (2008) Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology 149:2877–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, LeCouter J, Lin R, Peale F (2004) EG-VEGF and Bv8: a novel family of tissue-restricted angiogenic factors. Biochim Biophys Acta 1654:69–78. [DOI] [PubMed] [Google Scholar]
- Fiore M, Tarani L, Radicioni A, Spaziani M, Ferraguti G, Putotto C, Gabanella F, Maftei D, Lattanzi R, Minni A, et al. (2022) Serum prokineticin-2 in prepubertal and adult Klinefelter individuals. Can J Physiol Pharmacol 100:151–157. [DOI] [PubMed] [Google Scholar]
- Fluegge D, Moeller LM, Cichy A, Gorin M, Weth A, Veitinger S, Cainarca S, Lohmer S, Corazza S, Neuhaus EM, et al. (2012) Mitochondrial Ca(2+) mobilization is a key element in olfactory signaling. Nat Neurosci 15:754–762. [DOI] [PubMed] [Google Scholar]
- Franchi S, Giannini E, Lattuada D, Lattanzi R, Tian H, Melchiorri P, Negri L, Panerai AE, Sacerdote P (2008) The prokineticin receptor agonist Bv8 decreases IL-10 and IL-4 production in mice splenocytes by activating prokineticin receptor-1. BMC Immunol 9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC (2005) Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab 90:427–434. [DOI] [PubMed] [Google Scholar]
- Fukami M, Suzuki E, Izumi Y, Torii T, Narumi S, Igarashi M, Miyado M, Katsumi M, Fujisawa Y, Nakabayashi K, et al. (2017) Paradoxical gain-of-function mutant of the G-protein-coupled receptor PROKR2 promotes early puberty. J Cell Mol Med 21:2623–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullone MR, Maftei D, Vincenzi M, Lattanzi R, Miele R (2022a) Arginine 125 Is an Essential Residue for the Function of MRAP2. Int J Mol Sci 23:9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullone MR, Maftei D, Vincenzi M, Lattanzi R, Miele R (2022b) Identification of Regions Involved in the Physical Interaction between Melanocortin Receptor Accessory Protein 2 and Prokineticin Receptor 2. Biomolecules 12:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JV, Bataveljic A, Patel NA, Bewick GA, Roy D, Campbell D, Greenwood HC, Murphy KG, Hameed S, Jethwa PH, et al. (2010) Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes 59:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier V, Traboulsi W, Salomon A, Brouillet S, Fournier T, Winkler C, Desvergne B, Hoffmann P, Zhou QY, Congiu C, et al. (2015) PPARγ controls pregnancy outcome through activation of EG-VEGF: new insights into the mechanism of placental development. Am J Physiol Endocrinol Metab 309:E357–E369. [DOI] [PubMed] [Google Scholar]
- Gasser A, Brogi S, Urayama K, Nishi T, Kurose H, Tafi A, Ribeiro N, Désaubry L, Nebigil CG (2015) Discovery and cardioprotective effects of the first non-Peptide agonists of the G protein-coupled prokineticin receptor-1. PLoS One 10:e0121027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser A, Chen YW, Audebrand A, Daglayan A, Charavin M, Escoubet B, Karpov P, Tetko I, Chan MWY, Cardinale D, et al. (2019) Prokineticin Receptor-1 Signaling Inhibits Dose- and Time-Dependent Anthracycline-Induced Cardiovascular Toxicity Via Myocardial and Vascular Protection. JACC CardioOncol 1:84–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengatharan A, Bammann RR, Saghatelyan A (2016) The Role of Astrocytes in the Generation, Migration, and Integration of New Neurons in the Adult Olfactory Bulb. Front Neurosci 10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini E, Lattanzi R, Nicotra A, Campese AF, Grazioli P, Screpanti I, Balboni G, Salvadori S, Sacerdote P, Negri L (2009) The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proc Natl Acad Sci USA 106:14646–14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goi T, Nakazawa T, Hirono Y, Yamaguchi A (2013) Prokineticin 1 expression in gastrointestinal tumors. Anticancer Res 33:5311–5315. [PubMed] [Google Scholar]
- Goldby A, Jenkins K, Teall M (2015) Preparation of piperidine derivatives for use in the treatment or prevention of psychiatric and neurological conditions, Takeda Cambridge Limited. [Google Scholar]
- Gordon R, Neal ML, Luo J, Langley MR, Harischandra DS, Panicker N, Charli A, Jin H, Anantharam V, Woodruff TM, et al. (2016) Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration. Nat Commun 7:12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorowiec MR, Catalano RD, Norman JE, Denison FC, Jabbour HN (2011) Prokineticin 1 induces inflammatory response in human myometrium: a potential role in initiating term and preterm parturition. Am J Pathol 179:2709–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossrau G, Klimova A, Lapp HS, Frost M, Peschel E, Weidner K, Koch T, Sabatowski R, Croy I (2021) C-tactile touch perception in patients with chronic pain disorders. Pain Rep 6:e941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guida F, Lattanzi R, Boccella S, Maftei D, Romano R, Marconi V, Balboni G, Salvadori S, Scafuro MA, de Novellis V, et al. (2015) PC1, a non-peptide PKR1-preferring antagonist, reduces pain behavior and spinal neuronal sensitization in neuropathic mice. Pharmacol Res 91:36–46. [DOI] [PubMed] [Google Scholar]
- Guilini C, Urayama K, Turkeri G, Dedeoglu DB, Kurose H, Messaddeq N, Nebigil CG (2010) Divergent roles of prokineticin receptors in the endothelial cells: angiogenesis and fenestration. Am J Physiol Heart Circ Physiol 298:H844–H852. [DOI] [PubMed] [Google Scholar]
- Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S (2017) Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem Int 104:34–48. [DOI] [PubMed] [Google Scholar]
- Haouzi D, Mahmoud K, Fourar M, Bendhaou K, Dechaud H, De Vos J, Rème T, Dewailly D, Hamamah S (2009) Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod 24:198–205. [DOI] [PubMed] [Google Scholar]
- Hasnis E, Alishekevitz D, Gingis-Veltski S, Bril R, Fremder E, Voloshin T, Raviv Z, Karban A, Shaked Y (2014) Anti-Bv8 antibody and metronomic gemcitabine improve pancreatic adenocarcinoma treatment outcome following weekly gemcitabine therapy. Neoplasia 16:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Shen C, Lu Q, Li J, Wei Y, He L, Bai R, Zheng J, Luan N, Zhang Z, et al. (2016) Prokineticin 2 Plays a Pivotal Role in Psoriasis. EBioMedicine 13:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr MM, Fries KM, Upton LG, Edsberg LE (2011) Potential biomarkers of temporomandibular joint disorders. J Oral Maxillofac Surg 69:41–47. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Peters JC (2012) Energy balance and obesity. Circulation 126:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Feige JJ, Alfaidy N (2006) Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology 147:1675–1684. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Feige JJ, Alfaidy N (2007) Placental expression of EG-VEGF and its receptors PKR1 (prokineticin receptor-1) and PKR2 throughout mouse gestation. Placenta 28:1049–1058. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Negri L, Maftei D, Lattanzi R, Reeh PW (2016) The prokineticin Bv8 sensitizes cutaneous terminals of female mice to heat. Eur J Pain 20:1326–1334. [DOI] [PubMed] [Google Scholar]
- Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY (2007) Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice. Sleep 30:247–256. [PMC free article] [PubMed] [Google Scholar]
- Hu WP, Zhang C, Li JD, Luo ZD, Amadesi S, Bunnett N, Zhou QY (2006) Impaired pain sensation in mice lacking prokineticin 2. Mol Pain 2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impellizzeri D, Maftei D, Severini C, Miele R, Balboni G, Siracusa R, Cordaro M, Di Paola R, Cuzzocrea S, Lattanzi R (2023) Blocking prokineticin receptors attenuates synovitis and joint destruction in collagen-induced arthritis. J Mol Med (Berl) 101:569–580. [DOI] [PubMed] [Google Scholar]
- Itatani Y, Yamamoto T, Zhong C, Molinolo AA, Ruppel J, Hegde P, Taketo MM, Ferrara N (2020) Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc Natl Acad Sci USA 117:21598–21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Noda K, Yoshida K, Otani K, Yoshiga M, Oto Y, Saito S, Kurosaka D (2016) Prokineticin 2 antagonist, PKRA7 suppresses arthritis in mice with collagen-induced arthritis. BMC Musculoskelet Disord 17:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson O, Weiss ID, Niu G, Balboni G, Congiu C, Onnis V, Kiesewetter DO, Lattanzi R, Salvadori S, Chen X (2011) Prokineticin receptor 1 antagonist PC-10 as a biomarker for imaging inflammatory pain. J Nucl Med 52:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jethwa PH, I’Anson H, Warner A, Prosser HM, Hastings MH, Maywood ES, Ebling FJ (2008) Loss of prokineticin receptor 2 signaling predisposes mice to torpor. Am J Physiol Regul Integr Comp Physiol 294:R1968–R1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Kim JH, Li F, Qu A, Gavrilova O, Shah YM, Gonzalez FJ (2013a) Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J Biol Chem 288:3844–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Kwak H, Tosato G (2013b) granulocyte infiltration and expression of the pro-angiogenic BV8 protein in experimental EL4 and lewis lung carcinoma tumors. Cureus 5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek A, Engel E, Beier D, Lepperdinger G (2000) Murine Bv8 gene maps near a synteny breakpoint of mouse chromosome 6 and human 3p21. Gene 256:189–195. [DOI] [PubMed] [Google Scholar]
- Karaer A, Tuncay G, Uysal O, Semerci Sevimli T, Sahin N, Karabulut U, Sariboyaci AE (2020) The role of prokineticins in recurrent implantation failure. J Gynecol Obstet Hum Reprod 49:101835. [DOI] [PubMed] [Google Scholar]
- Karpov PN, Koyuncu, H., Désaubry, L., Tetko, I. V. (2022) Design of PKR1 agonists new agents for treating cardiotoxicity.
- Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10:698–712. [DOI] [PubMed] [Google Scholar]
- Kaser A, Winklmayr M, Lepperdinger G, Kreil G (2003) The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep 4:469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusal KG, Tonelli RR, Mattos EC, Soares CO, Di Genova BM, Juliano MA, Urias U, Colli W, Alves MJ (2015) Prokineticin receptor identified by phage display is an entry receptor for Trypanosoma cruzi into mammalian cells. Parasitol Res 114:155–165. [DOI] [PubMed] [Google Scholar]
- Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, et al. (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28:3617–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Kitajima T, Tsunoka T, Okumura T, Ikeda M, Okochi T, Kinoshita Y, Kawashima K, Yamanouchi Y, Ozaki N, et al. (2009) Possible association of prokineticin 2 receptor gene (PROKR2) with mood disorders in the Japanese population. Neuromolecular Med 11:114–122. [DOI] [PubMed] [Google Scholar]
- Kishi T, Kitajima T, Tsunoka T, Okumura T, Kawashima K, Okochi T, Yamanouchi Y, Kinoshita Y, Ujike H, Inada T, et al. (2011) Lack of association between prokineticin 2 gene and Japanese methamphetamine dependence. Curr Neuropharmacol 9:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Kitajima T, Tsunoka T, Okumura T, Okochi T, Kawashima K, Inada T, Ujike H, Yamada M, Uchimura N, et al. (2010) PROKR2 is associated with methamphetamine dependence in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry 34:1033–1036. [DOI] [PubMed] [Google Scholar]
- Kolka CM, Bergman RN (2013) The endothelium in diabetes: its role in insulin access and diabetic complications. Rev Endocr Metab Disord 14:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korc M (2007) Pancreatic cancer-associated stroma production. Am J Surg 194(4, Suppl)S84–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al. (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA 107:21248–21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, et al. (2011) Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13:294–307. [DOI] [PubMed] [Google Scholar]
- Kurebayashi H, Goi T, Shimada M, Tagai N, Naruse T, Nakazawa T, Kimura Y, Hirono Y, Yamaguchi A (2015) Prokineticin 2 (PROK2) is an important factor for angiogenesis in colorectal cancer. Oncotarget 6:26242–26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landucci E, Lattanzi R, Gerace E, Scartabelli T, Balboni G, Negri L, Pellegrini-Giampietro DE (2016) Prokineticins are neuroprotective in models of cerebral ischemia and ischemic tolerance in vitro. Neuropharmacology 108:39–48. [DOI] [PubMed] [Google Scholar]
- Lattanzi R, Congiu C, Onnis V, Deplano A, Salvadori S, Marconi V, Maftei D, Francioso A, Ambrosio C, Casella I, et al. (2015a) Halogenated triazinediones behave as antagonists of pkr1: in vitro and in vivo pharmacological characterization. Int J Pharm Sci Res 6:1033–1042. [Google Scholar]
- Lattanzi R, Maftei D, Fullone MR, Miele R (2019a) Identification and characterization of Prokineticin receptor 2 splicing variant and its modulation in an animal model of Alzheimer’s disease. Neuropeptides 73:49–56. [DOI] [PubMed] [Google Scholar]
- Lattanzi R, Maftei D, Fullone MR, Miele R (2021) Trypanosoma cruzi trans-sialidase induces STAT3 and ERK activation by prokineticin receptor 2 binding. Cell Biochem Funct 39:326–334. [DOI] [PubMed] [Google Scholar]
- Lattanzi R, Maftei D, Marconi V, Florenzano F, Franchi S, Borsani E, Rodella LF, Balboni G, Salvadori S, Sacerdote P, et al. (2015b) Prokineticin 2 upregulation in the peripheral nervous system has a major role in triggering and maintaining neuropathic pain in the chronic constriction injury model. BioMed Res Int 2015:301292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi R, Maftei D, Negri L, Fusco I, Miele R (2018) PK2β ligand, a splice variant of prokineticin 2, is able to modulate and drive signaling through PKR1 receptor. Neuropeptides 71:32–42. [DOI] [PubMed] [Google Scholar]
- Lattanzi R, Maftei D, Petrella C, Pieri M, Sancesario G, Schirinzi T, Bernardini S, Barbato C, Ralli M, Greco A, et al. (2019b) Involvement of the Chemokine Prokineticin-2 (PROK2) in Alzheimer’s Disease: From Animal Models to the Human Pathology. Cells 8:1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi R, Maftei D, Vincenzi M, Fullone MR, Miele R (2022) Identification and Characterization of a New Splicing Variant of Prokineticin 2. Life (Basel) 12:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi R, Miele R (2021) Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines 9:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi R, Sacerdote P, Franchi S, Canestrelli M, Miele R, Barra D, Visentin S, DeNuccio C, Porreca F, De Felice M, et al. (2012) Pharmacological activity of a Bv8 analogue modified in position 24. Br J Pharmacol 166:950–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauttia S, Sihto H, Kavola H, Koljonen V, Böhling T, Joensuu H (2014) Prokineticins and Merkel cell polyomavirus infection in Merkel cell carcinoma. Br J Cancer 110:1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter J, Ferrara N (2003) EG-VEGF and Bv8. a novel family of tissue-selective mediators of angiogenesis, endothelial phenotype, and function. Trends Cardiovasc Med 13:276–282. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, et al. (2001) Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 412:877–884. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Lin R, Frantz G, Zhang Z, Hillan K, Ferrara N (2003a) Mouse endocrine gland-derived vascular endothelial growth factor: a distinct expression pattern from its human ortholog suggests different roles as a regulator of organ-specific angiogenesis. Endocrinology 144:2606–2616. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N (2003b) The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA 100:2685–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Tracey I (2008) A common neurobiology for pain and pleasure. Nat Rev Neurosci 9:314–320. [DOI] [PubMed] [Google Scholar]
- Levit A, Yarnitzky T, Wiener A, Meidan R, Niv MY (2011) Modeling of human prokineticin receptors: interactions with novel small-molecule binders and potential off-target drugs. PLoS One 6:e27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY (2006) Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci 26:11615–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY (2001) Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol 59:692–698. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang C, Zhou QY (2018) Overexpression of Prokineticin 2 in Transgenic Mice Leads to Reduced Circadian Behavioral Rhythmicity and Altered Molecular Rhythms in the Suprachiasmatic Clock. J Circadian Rhythms 16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri DV, Kleinau G, Vezzoli V, Busnelli M, Guizzardi F, Sinisi AA, Pincelli AI, Mancini A, Russo G, Beck-Peccoz P, et al. ; Italian Study Group on Idiopathic Central Hypogonadism (ICH) (2014) Germline prokineticin receptor 2 (PROKR2) variants associated with central hypogonadism cause differental modulation of distinct intracellular pathways. J Clin Endocrinol Metab 99:E458–E463. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Barres BA (2017) Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46:957–967. [DOI] [PubMed] [Google Scholar]
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY (2002a) Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem 277:19276–19280. [DOI] [PubMed] [Google Scholar]
- Lin R, LeCouter J, Kowalski J, Ferrara N (2002b) Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem 277:8724–8729. [DOI] [PubMed] [Google Scholar]
- Liu B, Qiao L, Liu K, Liu J, Piccinni-Ash TJ, Chen ZF (2022) Molecular and neural basis of pleasant touch sensation. Science 376:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, He X, Lei XZ, Zhao LS, Tang H, Liu L, Lei BJ (2003) Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J Gastroenterol 9:1946–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang Z, Kong D, Zhang Y, Yu W, Zha W (2019) Metformin Ameliorates Testicular Damage in Male Mice with Streptozotocin-Induced Type 1 Diabetes through the PK2/PKR Pathway. Oxid Med Cell Longev 2019:5681701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livni L, Lees JG, Barkl-Luke ME, Goldstein D, Moalem-Taylor G (2019) Dorsal root ganglion explants derived from chemotherapy-treated mice have reduced neurite outgrowth in culture. Neurosci Lett 694:14–19. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS (2022) Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 19:151–172. [DOI] [PubMed] [Google Scholar]
- Luo J, Padhi P, Jin H, Anantharam V, Zenitsky G, Wang Q, Willette AA, Kanthasamy A, Kanthasamy AG (2019) Utilization of the CRISPR-Cas9 Gene Editing System to Dissect Neuroinflammatory and Neuropharmacological Mechanisms in Parkinson’s Disease. J Neuroimmune Pharmacol 14:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maftei D, Lattanzi R, Vincenzi M, Squillace S, Fullone MR, Miele R (2021) The balance of concentration between Prokineticin 2β and Prokineticin 2 modulates the food intake by STAT3 signaling. BBA Adv 1:100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maftei D, Marconi V, Florenzano F, Giancotti LA, Castelli M, Moretti S, Borsani E, Rodella LF, Balboni G, Luongo L, et al. (2014) Controlling the activation of the Bv8/prokineticin system reduces neuroinflammation and abolishes thermal and tactile hyperalgesia in neuropathic animals. Br J Pharmacol 171:4850–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maftei D, Ratano P, Fusco I, Marconi V, Squillace S, Negri L, Severini C, Balboni G, Steardo L, Bronzuoli MR, et al. (2019) The prokineticin receptor antagonist PC1 rescues memory impairment induced by β amyloid administration through the modulation of prokineticin system. Neuropharmacology 158:107739. [DOI] [PubMed] [Google Scholar]
- Maftei D, Vellani V, Artico M, Giacomoni C, Severini C, Lattanzi R (2020) Abnormal Pain Sensation in Mice Lacking the Prokineticin Receptor PKR2: Interaction of PKR2 with Transient Receptor Potential TRPV1 and TRPA1. Neuroscience 427:16–28. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686. [DOI] [PubMed] [Google Scholar]
- Martin C, Balasubramanian R, Dwyer AA, Au MG, Sidis Y, Kaiser UB, Seminara SB, Pitteloud N, Zhou QY, Crowley WF Jr (2011) The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations. Endocr Rev 32:225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci C, Franchi S, Giannini E, Tian H, Melchiorri P, Negri L, Sacerdote P (2006) Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br J Pharmacol 147:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrokolias A, Ariyurek Y, Goeman JJ, van Duijn E, Roos RA, van der Mast RC, van Ommen GB, den Dunnen JT, ’t Hoen PA, van Roon-Mom WM (2015) Huntington’s disease biomarker progression profile identified by transcriptome sequencing in peripheral blood. Eur J Hum Genet 23:1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, et al. (2002) Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun 293:396–402. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. (2006) Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA 103:4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Choi J, Deng ZD, Appelbaum LG, Krystal AD, Lisanby SH (2014) Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J ECT 30:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone F, Wessberg J, Olausson H (2014) Discriminative and affective touch: sensing and feeling. Neuron 82:737–755. [DOI] [PubMed] [Google Scholar]
- Mendez D, Gaulton A, Bento AP, Chambers J, De Veij M, Félix E, Magariños MP, Mosquera JF, Mutowo P, Nowotka M, et al. (2019) ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res 47 (D1):D930–D940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez JP, Zenteno JC, Coronel A, Soriano-Ursúa MA, Valencia-Villalvazo EY, Soderlund D, Coral-Vázquez RM, Canto P (2015) Triallelic digenic mutation in the prokineticin 2 and GNRH receptor genes in two brothers with normosmic congenital hypogonadotropic hypogonadism. Endocr Res 40:166–171. [DOI] [PubMed] [Google Scholar]
- Meng L, Yang H, Jin C, Quan S (2019) miR-28-5p suppresses cell proliferation and weakens the progression of polycystic ovary syndrome by targeting prokineticin-1. Mol Med Rep 20:2468–2475. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Teall M Takeda Pharmaceutical Co., Ltd. (2016) Preparation of sulfonyl piperidine derivatives and their use for treating prokineticin mediated gastrointestinal disorders, Takeda Cambridge Limited. [Google Scholar]
- Mok J, Park TS, Kim S, Kim D, Choi CS, Park J (2021) Prokineticin receptor 1 ameliorates insulin resistance in skeletal muscle. FASEB J 35:e21179. [DOI] [PubMed] [Google Scholar]
- Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G (1999) Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol 374:189–196. [DOI] [PubMed] [Google Scholar]
- Monnier J, Piquet-Pellorce C, Feige JJ, Musso O, Clement B, Turlin B, Theret N, Samson M (2008) Prokineticin 2/Bv8 is expressed in Kupffer cells in liver and is down regulated in human hepatocellular carcinoma. World J Gastroenterol 14:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier J, Samson M (2008) Cytokine properties of prokineticins. FEBS J 275:4014–4021. [DOI] [PubMed] [Google Scholar]
- Moore RY (1997) Circadian rhythms: basic neurobiology and clinical applications. Annu Rev Med 48:253–266. [DOI] [PubMed] [Google Scholar]
- Morales A, Morimoto S, Díaz L, Robles G, Díaz-Sánchez V (2008) Endocrine gland-derived vascular endothelial growth factor in rat pancreas: genetic expression and testosterone regulation. J Endocrinol 197:309–314. [DOI] [PubMed] [Google Scholar]
- Morales A, Vilchis F, Chávez B, Chan C, Robles-Díaz G, Díaz-Sánchez V (2007) Expression and localization of endocrine gland-derived vascular endothelial growth factor (EG-VEGF) in human pancreas and pancreatic adenocarcinoma. J Steroid Biochem Mol Biol 107:37–41. [DOI] [PubMed] [Google Scholar]
- Morales RA, Daly NL, Vetter I, Mobli M, Napier IA, Craik DJ, Lewis RJ, Christie MJ, King GF, Alewood PF, et al. (2010) Chemical synthesis and structure of the prokineticin Bv8. ChemBioChem 11:1882–1888. [DOI] [PubMed] [Google Scholar]
- Morris C (1946) Signs, language and behavior, Prentice-Hall, Oxford, England. [Google Scholar]
- Morris EL, Patton AP, Chesham JE, Crisp A, Adamson A, Hastings MH (2021) Single-cell transcriptomics of suprachiasmatic nuclei reveal a Prokineticin-driven circadian network. EMBO J 40:e108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortreux M, Foppen E, Denis RG, Montaner M, Kassis N, Denom J, Vincent M, Fumeron F, Kujawski-Lafourcade M, Andréelli F, et al. (2019) New roles for prokineticin 2 in feeding behavior, insulin resistance and type 2 diabetes: Studies in mice and humans. Mol Metab 29:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschetti G, Amodeo G, Maftei D, Lattanzi R, Procacci P, Sartori P, Balboni G, Onnis V, Conte V, Panerai A, et al. (2019a) Targeting prokineticin system counteracts hypersensitivity, neuroinflammation, and tissue damage in a mouse model of bortezomib-induced peripheral neuropathy. J Neuroinflammation 16:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschetti G, Amodeo G, Paladini MS, Molteni R, Balboni G, Panerai A, Sacerdote P, Franchi S (2019b) Prokineticin 2 promotes and sustains neuroinflammation in vincristine treated mice: Focus on pain and emotional like behavior. Brain Behav Immun 82:422–431. [DOI] [PubMed] [Google Scholar]
- Moschetti G, Kalpachidou T, Amodeo G, Lattanzi R, Sacerdote P, Kress M, Franchi S (2020) Prokineticin Receptor Inhibition With PC1 Protects Mouse Primary Sensory Neurons From Neurotoxic Effects of Chemotherapeutic Drugs in vitro. Front Immunol 11:2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundim MV, Zamproni LN, Pinto AAS, Galindo LT, Xavier AM, Glezer I, Porcionatto M (2019) A new function for Prokineticin 2: Recruitment of SVZ-derived neuroblasts to the injured cortex in a mouse model of traumatic brain injury. Mol Cell Neurosci 94:1–10. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Goi T, Hirono Y, Yamaguchi A (2015) Prokineticin 1 protein expression is a useful new prognostic factor for human sporadic colorectal cancer. Ann Surg Oncol 22:1496–1503. [DOI] [PubMed] [Google Scholar]
- Neal M, Luo J, Harischandra DS, Gordon R, Sarkar S, Jin H, Anantharam V, Désaubry L, Kanthasamy A, Kanthasamy A (2018) Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes. Glia 66:2137–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebigil CG (2009) Prokineticin receptors in cardiovascular function: foe or friend? Trends Cardiovasc Med 19:55–60. [DOI] [PubMed] [Google Scholar]
- Nebigil CG (2016) Updates on Endothelial Functions of Proangiogenic Prokineticin. Hypertension 68:1091–1097. [DOI] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Colucci M, Margheriti F, Melchiorri P, Vellani V, Tian H, De Felice M, Porreca F (2006a) Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci 26:6716–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Colucci MA, Mignogna G, Barra D, Grohovaz F, Codazzi F, Kaiser A, Kreil G, et al. (2005) Biological activities of Bv8 analogues. Br J Pharmacol 146:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, De Felice M, Colucci A, Melchiorri P (2004) Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. Br J Pharmacol 142:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Melchiorri P (2006b) Modulators of pain: Bv8 and prokineticins. Curr Neuropharmacol 4:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, Melchiorri P (2002) Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol 137:1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY (2005) Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 308:1923–1927. [DOI] [PubMed] [Google Scholar]
- Ngan ES, Lee KY, Sit FY, Poon HC, Chan JK, Sham MH, Lui VC, Tam PK (2007) Prokineticin-1 modulates proliferation and differentiation of enteric neural crest cells. Biochim Biophys Acta 1773:536–545. [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Gasser A, Nebigil CG (2013) Role of Prokineticin Receptor-1 in Epicardial Progenitor Cells. J Dev Biol 1:20–31. [DOI] [PubMed] [Google Scholar]
- Noda K, Dufner B, Ito H, Yoshida K, Balboni G, Straub RH (2021) Differential inflammation-mediated function of prokineticin 2 in the synovial fibroblasts of patients with rheumatoid arthritis compared with osteoarthritis. Sci Rep 11:18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Liu M, Eyre HJ, Copeland NG, Gilbert DJ, Crawford J, Sutherland GR, Jenkins NA, Herzog H (2000) Y-receptor-like genes GPR72 and GPR73: molecular cloning, genomic organisation and assignment to human chromosome 11q21.1 and 2p14 and mouse chromosome 9 and 6. Biochim Biophys Acta 1491:369–375. [DOI] [PubMed] [Google Scholar]
- Pasquali D, Rossi V, Staibano S, De Rosa G, Chieffi P, Prezioso D, Mirone V, Mascolo M, Tramontano D, Bellastella A, et al. (2006) The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/prokineticin 1 and 2 and receptor expression in human prostate: Up-regulation of EG-VEGF/prokineticin 1 with malignancy. Endocrinology 147:4245–4251. [DOI] [PubMed] [Google Scholar]
- Patke A, Young MW, Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21:67–84. [DOI] [PubMed] [Google Scholar]
- Peng Z, Tang Y, Luo H, Jiang F, Yang J, Sun L, Li JD (2011) Disease-causing mutation in PKR2 receptor reveals a critical role of positive charges in the second intracellular loop for G-protein coupling and receptor trafficking. J Biol Chem 286:16615–16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, et al. (2007) Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 104:17447–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM (2015) 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. J Endocrinol 226:T41–T54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES (2007) Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc Natl Acad Sci USA 104:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliani G, Sesti F, Anastasi E, Verrico M, Tarsitano MG, Feola T, Campolo F, Di Gioia CRT, Venneri MA, Angeloni A, et al. ; Nettare Unit (2022) Angiogenic factors as prognostic markers in neuroendocrine neoplasms. Endocrine 76:208–217. [DOI] [PubMed] [Google Scholar]
- Qu X, Zhuang G, Yu L, Meng G, Ferrara N (2012) Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b+Gr1+ cells: key role of Stat3 signaling. J Biol Chem 287:19574–19584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi R, Kindo M, Arora H, Boulberdaa M, Steenman M, Nebigil CG (2017) Prokineticin receptor-1-dependent paracrine and autocrine pathways control cardiac tcf21+ fibroblast progenitor cell transformation into adipocytes and vascular cells. Sci Rep 7:12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi R, Kindo M, Boulberdaa M, von Hunolstein JJ, Steenman M, Nebigil CG (2018) A Prokineticin-Driven Epigenetic Switch Regulates Human Epicardial Cell Stemness and Fate. Stem Cells 36:1589–1602. [DOI] [PubMed] [Google Scholar]
- Ren C, Qiu CY, Gan X, Liu TT, Qu ZW, Rao Z, Hu WP (2015) Prokineticin 2 facilitates mechanical allodynia induced by α,β-methylene ATP in rats. Eur J Pharmacol 767:24–29. [DOI] [PubMed] [Google Scholar]
- Reynaud D, Sergent F, Abi Nahed R, Brouillet S, Benharouga M, Alfaidy N (2018) EG-VEGF Maintenance Over Early Gestation to Develop a Pregnancy-Induced Hypertensive Animal Model. Methods Mol Biol 1710:317–324. [DOI] [PubMed] [Google Scholar]
- Reynaud D, Sergent F, Abi Nahed R, Traboulsi W, Collet C, Marquette C, Hoffmann P, Balboni G, Zhou QY, Murthi P, et al. (2021) Evidence-Based View of Safety and Effectiveness of Prokineticin Receptors Antagonists during Pregnancy. Biomedicines 9:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijo-Ferreira F, Takahashi JS (2019) Genomics of circadian rhythms in health and disease. Genome Med 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivetti di Val Cervo P, Romanov RA, Spigolon G, Masini D, Martín-Montañez E, Toledo EM, La Manno G, Feyder M, Pifl C, Ng YH, et al. (2017) Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat Biotechnol 35:444–452. [DOI] [PubMed] [Google Scholar]
- Rouault AAJ, Lee AA, Sebag JA (2017) Regions of MRAP2 required for the inhibition of orexin and prokineticin receptor signaling. Biochim Biophys Acta Mol Cell Res 1864:2322–2329. [DOI] [PubMed] [Google Scholar]
- Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, et al. (2010) Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One 5:e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M, Peale FV Jr, Frantz G, Rioux-Leclercq N, Rajpert-De Meyts E, Ferrara N (2004) Human endocrine gland-derived vascular endothelial growth factor: expression early in development and in Leydig cell tumors suggests roles in normal and pathological testis angiogenesis. J Clin Endocrinol Metab 89:4078–4088. [DOI] [PubMed] [Google Scholar]
- Sarfati J, Dodé C, Young J (2010a) Kallmann syndrome caused by mutations in the PROK2 and PROKR2 genes: pathophysiology and genotype-phenotype correlations. Front Horm Res 39:121–132. [DOI] [PubMed] [Google Scholar]
- Sarfati J, Guiochon-Mantel A, Rondard P, Arnulf I, Garcia-Piñero A, Wolczynski S, Brailly-Tabard S, Bidet M, Ramos-Arroyo M, Mathieu M, et al. (2010b) A comparative phenotypic study of kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J Clin Endocrinol Metab 95:659–669. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Baba T, Muranaka H, Tanabe Y, Takahashi C, Matsugo S, Mukaida N (2018) Involvement of Prokineticin 2-expressing Neutrophil Infiltration in 5-Fluorouracil-induced Aggravation of Breast Cancer Metastasis to Lung. Mol Cancer Ther 17:1515–1525. [DOI] [PubMed] [Google Scholar]
- Sbai O, Monnier C, Dodé C, Pin JP, Hardelin JP, Rondard P (2014) Biased signaling through G-protein-coupled PROKR2 receptors harboring missense mutations. FASEB J 28:3734–3744. [DOI] [PubMed] [Google Scholar]
- Schirinzi T, Lattanzi R, Maftei D, Grillo P, Zenuni H, Boffa L, Albanese M, Simonetta C, Bovenzi R, Maurizi R, et al. (2023) Substance P and Prokineticin-2 are overexpressed in olfactory neurons and play differential roles in persons with persistent post-COVID-19 olfactory dysfunction. Brain Behav Immun 108:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirinzi T, Maftei D, Passali FM, Grillo P, Zenuni H, Mascioli D, Maurizi R, Loccisano L, Vincenzi M, Rinaldi AM, et al. (2022) Olfactory Neuron Prokineticin-2 as a Potential Target in Parkinson’s Disease. Ann Neurol 93:196–204. [DOI] [PubMed] [Google Scholar]
- Schirinzi T, Maftei D, Pieri M, Bernardini S, Mercuri NB, Lattanzi R, Severini C (2021) Increase of Prokineticin-2 in Serum of Patients with Parkinson’s Disease. Mov Disord 36:1031–1033. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Pacaud P, Diochot S, Moinier D, Lazdunski M (1999) MIT(1), a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett 461:183–188. [DOI] [PubMed] [Google Scholar]
- Sergent F, Hoffmann P, Brouillet S, Garnier V, Salomon A, Murthi P, Benharouga M, Feige JJ, Alfaidy N (2016) Sustained Endocrine Gland-Derived Vascular Endothelial Growth Factor Levels Beyond the First Trimester of Pregnancy Display Phenotypic and Functional Changes Associated With the Pathogenesis of Pregnancy-Induced Hypertension. Hypertension 68:148–156. [DOI] [PubMed] [Google Scholar]
- Severini C, Lattanzi R, Maftei D, Marconi V, Ciotti MT, Petrocchi Passeri P, Florenzano F, Del Duca E, Caioli S, Zona C, et al. (2015) Bv8/prokineticin 2 is involved in Aβ-induced neurotoxicity. Sci Rep 5:15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JL, Denison FC, Evans J, Durno K, Williams AR, Entrican G, Critchley HO, Jabbour HN, Horne AW (2010) Evidence of prokineticin dysregulation in fallopian tube from women with ectopic pregnancy. Fertil Steril 94:1601–1608.e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. (2007) Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450:825–831. [DOI] [PubMed] [Google Scholar]
- Sihto H, Kukko H, Koljonen V, Sankila R, Böhling T, Joensuu H (2009) Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst 101:938–945. [DOI] [PubMed] [Google Scholar]
- Soga T, Matsumoto Si, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K (2002) Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta 1579:173–179. [DOI] [PubMed] [Google Scholar]
- Sohn JW (2015) Network of hypothalamic neurons that control appetite. BMB Rep 48:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sposini S, Caltabiano G, Hanyaloglu AC, Miele R (2015) Identification of transmembrane domains that regulate spatial arrangements and activity of prokineticin receptor 2 dimers. Mol Cell Endocrinol 399:362–372. [DOI] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. (2017) Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M-T, Tsai P-Y, Tsai H-L, Chen Y-C, Kuo P-L (2017) miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors 43:210–219. [DOI] [PubMed] [Google Scholar]
- Su MT, Lin SH, Chen YC, Kuo PL (2014) Gene-gene interactions and risk of recurrent miscarriages in carriers of endocrine gland-derived vascular endothelial growth factor and prokineticin receptor polymorphisms. Fertil Steril 102:1071–1077.e3. [DOI] [PubMed] [Google Scholar]
- Su MT, Lin SH, Chen YC, Wu LW, Kuo PL (2013) Prokineticin receptor variants (PKR1-I379V and PKR2-V331M) are protective genotypes in human early pregnancy. Reproduction 146:63–73. [DOI] [PubMed] [Google Scholar]
- Szatkowski C, Vallet J, Dormishian M, Messaddeq N, Valet P, Boulberdaa M, Metzger D, Chambon P, Nebigil CG (2013) Prokineticin receptor 1 as a novel suppressor of preadipocyte proliferation and differentiation to control obesity. PLoS One 8:e81175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagai N, Goi T, Shimada M, Kurebayashi H (2021) Plasma Prokineticin 1, a prognostic biomarker in colorectal cancer patients with curative resection: a retrospective cohort study. World J Surg Oncol 19:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WJ, Melamed JY (2007) Preparation of morpholinecarboxamides as prokineticin 2 receptor antagonists, Merck & Co., Inc. [Google Scholar]
- Tjoa ML, Oudejans CB, van Vugt JM, Blankenstein MA, van Wijk IJ (2004) Markers for presymptomatic prediction of preeclampsia and intrauterine growth restriction. Hypertens Pregnancy 23:171–189. [DOI] [PubMed] [Google Scholar]
- Traboulsi W, Brouillet S, Sergent F, Boufettal H, Samouh N, Aboussaouira T, Hoffmann P, Feige JJ, Benharouga M, Alfaidy N (2015) Prokineticins in central and peripheral control of human reproduction. Horm Mol Biol Clin Investig 24:73–81. [DOI] [PubMed] [Google Scholar]
- Traboulsi W, Sergent F, Boufettal H, Brouillet S, Slim R, Hoffmann P, Benlahfid M, Zhou QY, Balboni G, Onnis V, et al. (2017) Antagonism of EG-VEGF Receptors as Targeted Therapy for Choriocarcinoma Progression In Vitro and In Vivo. Clin Cancer Res 23:7130–7140. [DOI] [PubMed] [Google Scholar]
- Tu Q, Yu X, Xie W, Luo Y, Tang H, Chen K, Ruan Y, Li Y, Zhou J, Yin Y, et al. (2022) Prokineticin 2 promotes macrophages-mediated antibacterial host defense against bacterial pneumonia. Int J Infect Dis 125:103–113. [DOI] [PubMed] [Google Scholar]
- Ujvari D, Jakson I, Oldmark C, Attarha S, Alkasalias T, Salamon D, Gidlöf S, Hirschberg AL (2018) Prokineticin 1 is up-regulated by insulin in decidualizing human endometrial stromal cells. J Cell Mol Med 22:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama K, Dedeoglu DB, Guilini C, Frantz S, Ertl G, Messaddeq N, Nebigil CG (2009) Transgenic myocardial overexpression of prokineticin receptor-2 (GPR73b) induces hypertrophy and capillary vessel leakage. Cardiovasc Res 81:28–37. [DOI] [PubMed] [Google Scholar]
- Urayama K, Guilini C, Messaddeq N, Hu K, Steenman M, Kurose H, Ert G, Nebigil CG (2007) The prokineticin receptor-1 (GPR73) promotes cardiomyocyte survival and angiogenesis. FASEB J 21:2980–2993. [DOI] [PubMed] [Google Scholar]
- Urayama K, Guilini C, Turkeri G, Takir S, Kurose H, Messaddeq N, Dierich A, Nebigil CG (2008) Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation. Arterioscler Thromb Vasc Biol 28:841–849. [DOI] [PubMed] [Google Scholar]
- Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, McNaughton PA (2006) Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci 26:5109–5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdinez JA, Sebag JA (2021) Role of N-Linked Glycosylation in PKR2 Trafficking and Signaling. Front Neurosci 15:730417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzoli V, Duminuco P, Bassi I, Guizzardi F, Persani L, Bonomi M (2016) The complex genetic basis of congenital hypogonadotropic hypogonadism. Minerva Endocrinol 41:223–239. [PubMed] [Google Scholar]
- Vincenzi M, Milella MS, D’Ottavio G, Caprioli D, Reverte I, Maftei D (2022) Targeting Chemokines and Chemokine GPCRs to Enhance Strong Opioid Efficacy in Neuropathic Pain. Life (Basel) 12:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hunolstein JJ, Nebigil CG (2015) Can prokineticin prevent obesity and insulin resistance? Curr Opin Endocrinol Diabetes Obes 22:367–373. [DOI] [PubMed] [Google Scholar]
- Wang CY, Tsai PY, Chen TY, Tsai HL, Kuo PL, Su MT (2019) Elevated miR-200a and miR-141 inhibit endocrine gland-derived vascular endothelial growth factor expression and ciliogenesis in preeclampsia. J Physiol 597:3069–3083. [DOI] [PubMed] [Google Scholar]
- Wang H, Jia Y, Yu X, Peng L, Mou C, Song Z, Chen D, Li X (2021) Circulating Prokineticin 2 Levels Are Increased in Children with Obesity and Correlated with Insulin Resistance. Int J Endocrinol 2021:6630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo X, Ma H, Lu L, Zhang R (2016) Prokineticin-2 is associated with metabolic syndrome in a middle-aged and elderly Chinese population. Lipids Health Dis 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RP, Lilley E, Panesar M, Bhalay G, Langridge S, Tian SS, McClenaghan C, Ropenga A, Zeng F, Nash MS (2012) Increased prokineticin 2 expression in gut inflammation: role in visceral pain and intestinal ion transport. Neurogastroenterol Motil 24:65–75, e12. [DOI] [PubMed] [Google Scholar]
- Wen Y, Zhang Z, Li Z, Liu G, Tao G, Song X, Xu Z, Shang Z, Guo T, Su Z, et al. (2019) The PROK2/PROKR2 signaling pathway is required for the migration of most olfactory bulb interneurons. J Comp Neurol 527:2931–2947. [DOI] [PubMed] [Google Scholar]
- Wu MH, Wu PR, Hsieh YH, Lin CL, Liu CJ, Ying TH (2020) Silencing PROK2 Inhibits Invasion of Human Cervical Cancer Cells by Targeting MMP15 Expression. Int J Mol Sci 21:6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würfel M, Breitfeld J, Gebhard C, Scholz M, Baber R, Riedel-Heller SG, Blüher M, Stumvoll M, Kovacs P, Tönjes A (2022) Interplay between adipose tissue secreted proteins, eating behavior and obesity. Eur J Nutr 61:885–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Karpov P, Popowicz G, Tetko IV (2020) Focused Library Generator: case of Mdmx inhibitors. J Comput Aided Mol Des 34:769–782. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tan L, Li D, Wang L, Xiao X, Meng G, Wu Z, Zhang J (2017) Clinical and prognostic significance of prokineticin 1 in human gliomas. Int J Clin Exp Pathol 10:7661–7669. [PMC free article] [PubMed] [Google Scholar]
- Xie BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, Jiang JY (2019) Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther 25:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YH, Chen YX, Fang JY (2020) Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong YC, Li XM, Wang XJ, Liu YQ, Qiu F, Wu D, Gan YB, Wang BH, Hu WP (2010) Prokineticin 2 suppresses GABA-activated current in rat primary sensory neurons. Neuropharmacology 59:589–594. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang M, Zhang Y, Cai F, Jiang B, Zha W, Yu W (2020) Metformin Ameliorates Diabetic Cardiomyopathy by Activating the PK2/PKR Pathway. Front Physiol 11:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Goi T, Kurebayashi H, Morikawa M, Hirono Y, Katayama K (2018) Prokineticin 2 expression as a novel prognostic biomarker for human colorectal cancer. Oncotarget 9:30079–30091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Chen J, Tang H, Tu Q, Li Y, Yuan X, Zhang X, Cao J, Molloy DP, Yin Y, et al. (2022) Identifying Prokineticin2 as a Novel Immunomodulatory Factor in Diagnosis and Treatment of Sepsis. Crit Care Med 50:674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ng KL, Li JD, He F, Anderson DJ, Sun YE, Zhou QY (2007) Prokineticin 2 is a target gene of proneural basic helix-loop-helix factors for olfactory bulb neurogenesis. J Biol Chem 282:6917–6921. [DOI] [PubMed] [Google Scholar]
- Zhang C, Truong KK, Zhou QY (2009) Efferent projections of prokineticin 2 expressing neurons in the mouse suprachiasmatic nucleus. PLoS One 4:e7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, He HH, Janjua MU, Wang F, Yang YB, Mo ZH, Liu J, Jin P (2020) Identification of two novel mutations in three Chinese families with Kallmann syndrome using whole exome sequencing. Andrologia 52:e13594. [DOI] [PubMed] [Google Scholar]
- Zhang R, Liao W, Wu K, Hua L, Wu M, Li C, Cai F (2022a) Matrine alleviates spatial learning and memory impairment in diabetic mice by inhibiting endoplasmic reticulum stress and through modulation of PK2/PKRs pathway. Neurochem Int 154:105289. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Hu N, Wang A (2022b) Alzheimer’s disease-associated inflammatory pathways might contribute to osteoporosis through the interaction between PROK2 and CSF3. Front Neurol 13:990779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Qu X, Tan M, Meng YG, Ferrara N (2009) Characterization and regulation of bv8 in human blood cells. Clin Cancer Res 15:2675–2684. [DOI] [PubMed] [Google Scholar]
- Zhong J, Xiang D, Ma X (2022) Prokineticins as a Prognostic Biomarker for Low-Grade Gliomas: A Study Based on The Cancer Genome Atlas Data. BioMed Res Int 2022:2309339. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhou QY, Cheng MY (2005) Prokineticin 2 and circadian clock output. FEBS J 272:5703–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Li J-D, Hu W-P, Cheng MY, Zhou Q-Y (2012) Prokineticin 2 is involved in the thermoregulation and energy expenditure. Regul Pept 179:84–90. [DOI] [PubMed] [Google Scholar]