Abstract
Oxycodone, a semisynthetic derivative of naturally occurring thebaine, an opioid alkaloid, has been available for more than 100 years. Although thebaine cannot be used therapeutically due to the occurrence of convulsions at higher doses, it has been converted to a number of other widely used compounds that include naloxone, naltrexone, buprenorphine, and oxycodone. Despite the early identification of oxycodone, it was not until the 1990s that clinical studies began to explore its analgesic efficacy. These studies were followed by the pursuit of several preclinical studies to examine the analgesic effects and abuse liability of oxycodone in laboratory animals and the subjective effects in human volunteers. For a number of years oxycodone was at the forefront of the opioid crisis, playing a significant role in contributing to opioid misuse and abuse, with suggestions that it led to transitioning to other opioids. Several concerns were expressed as early as the 1940s that oxycodone had significant abuse potential similar to heroin and morphine. Both animal and human abuse liability studies have confirmed, and in some cases amplified, these early warnings. Despite sharing a similar structure with morphine and pharmacological actions also mediated by the μ-opioid receptor, there are several differences in the pharmacology and neurobiology of oxycodone. The data that have emerged from the many efforts to analyze the pharmacological and molecular mechanism of oxycodone have generated considerable insight into its many actions, reviewed here, which, in turn, have provided new information on opioid receptor pharmacology.
Significance Statement
Oxycodone, a μ-opioid receptor agonist, was synthesized in 1916 and introduced into clinical use in Germany in 1917. It has been studied extensively as a therapeutic analgesic for acute and chronic neuropathic pain as an alternative to morphine. Oxycodone emerged as a drug with widespread abuse. This article brings together an integrated, detailed review of the pharmacology of oxycodone, preclinical and clinical studies of pain and abuse, and recent advances to identify potential opioid analgesics without abuse liability.
I. Introduction
A. Brief History of Early Opioid Pharmacology
Issues surrounding the effects and potential abuse liabilities of opioids have been known for more than 150 years. In a review titled “Morphine Addiction and Its Physiological Interpretation Based on Experimental Evidences,” Tatum et al. (1929) stated that the renowned French physiologist Claude Bernard was the first scientist to give a careful and complete description of the dose-related effects of morphine in dogs, with low doses leading to salivation, retching, and vomiting and higher doses producing analgesia, sedation, convulsions, and death (Bernard, 1864). Bernard also described the development of tolerance following repeated exposure to morphine. Subsequent studies some years later by Tatum et al. (1929) on “morphine poisoning” in the dog and rhesus monkey also described acute effects of morphine leading to convulsions and lethality and made the observation that if the dogs or monkeys were treated with sodium barbital and paraldehyde during the convulsions, they could “recover” the animals and stop the progression to respiratory mortality. This finding suggested that lethality is not related to direct depression of the respiratory center by morphine because the addition of a depressant (sodium barbital) should lower rather than raise the lethal dose of morphine. “The fatal outcome of morphine at this stage of its action in the monkey can be combatted by the use of certain depressants” (Tatum et al., 1929, p. 460). These early studies by Bernard, Tatum et al., and others on dogs, cats, rabbits, and monkeys, separated in time from a larger and more expansive experimental focus on the wide range of opioid pharmacology, provided the foundation for subsequent approaches to further investigate tolerance and cross-tolerance, dependence, abstinence, and withdrawal and respiratory depression, together with the analgesic and antinociceptive effects of opioids (see also Seevers, 1936; Deneau and Seevers, 1964). These studies also presaged countless developments that followed over the course of several decades that have vastly improved our understanding of opioid receptor diversity and pharmacology and reaffirmed the commitment to discover a safe and effective analgesic lacking abuse liability. The seemingly unrelenting opioid crisis has become part of this quest and oxycodone emblematic of the many unresolved challenges.
B. The Opioid Crisis
The current opioid crisis has its basis in several intersecting developments that have included inappropriate prescribing and marketing, diversion, illicit trafficking of less expensive opioids, and misuse for the treatment of acute and chronic noncancer pain. There were several early indications of a developing crisis. Okie (2010), in an article titled “A Flood of Opioids, a Rising Tide of Deaths,” provided evidence that deaths from unintentional overdoses in the United States had been rising steeply since the early 1990s, with the increase propelled by the rising number of overdoses of synthetic versions of opium. In 2020 an average of 44 people died each day from overdoses involving prescription opioids, totaling more than 16,000 deaths (https://www.cdc.gov/drugoverdose/deaths/prescription/maps.html). The staggering number of opioid-related deaths over the past two decades has come at an economic cost of more than $2.5 trillion between 2015 and 2018 and an estimated $700 billion to $1 trillion in 2018 alone (Kharasch et al., 2022), not to mention the toll and emotional burden on families and friends. COVID-19 also has had a significant impact on opioid use and misuse, overdose, and mortality, with opioid overdoses continuing to evolve since the onset of COVID-19 (Garcia et al., 2022). The Centers for Disease Control and Prevention published provisional data for the 12-month period ending in April 2021 stating that there were an estimated 100,306 drug overdose deaths in the United States, an increase in approximately 29% from the same period the year before (https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm).
Contributions to opioid use and misuse include a related epidemic—that of pain, which affects somewhere between 40 million and 100 million adults, with societal costs exceeding the combined costs of heart disease, cancer, and diabetes; it is also deeply rooted in the overprescription and overuse of oral opioids combined with “avaricious and illegal marketing of prescription oral opioids” (Seltzer, 2020; Kharasch et al., 2022). An important aspect of the patterns of opioid abuse is related to trends in the initiation of heroin use where, according to the National Survey on Drug Use and Health, the heroin incidence rate was 19 times higher among those individuals who reported prior nonmedical pain reliever use. This survey also indicated that four out of five heroin users report previous use of nonmedical prescription opioid pain relievers (Muhuri et al., 2013). There is evidence that the nonmedical use of prescription opioids in childhood and early adolescence is strongly associated with transitions to heroin use in adolescence and young adulthood (Cerdá et al., 2015).
Related to these statistics is the licit and illicit increase in oxycodone use over the past few decades. Oxycodone prescriptions for the treatment of pain for conditions other than cancer increased by 588% between 1998 and 2007 (Manchikanti, 2007; Kanouse and Compton, 2015). As would be expected, there has been a concurrent increase in adverse events, including overdose and death. The number of visits to emergency departments related to oral use of opioids increased from 59 to 121 per 100,000 between 2004 and 2008, with a 123% increase attributed to hydrocodone and 152% attributed to oxycodone (Webster et al., 2011).
Oxycodone has been a major factor in these multifaceted issues. Even though oxycodone has been available for clinical use for more than 100 years, and its clinical analgesic effects have been studied for some time, until relatively recently there has been very little work on its basic preclinical pharmacology. Although the abuse of other opioid drugs such as fentanyl has been the focus of research and societal concern more recently, this review is intended to organize and provide a comprehensive review of the experimental research involving the pharmacology of oxycodone. It reviews the key features of oxycodone, including its initial discovery, its basic and clinical pharmacology, clinical and preclinical analgesia, early concerns identifying its abuse liability, and studies directed toward arriving at a clearer understanding of its excessive abuse. Several studies of the human behavioral pharmacology of oxycodone that have been directed toward assessing its subjective effects in laboratory settings are covered in this review and represent important contributions, together with experiments using animal models of oxycodone self-administration to assess abuse liability and potential treatment approaches. Sex differences in the analgesic effects and abuse liability of oxycodone are also covered. The review concludes with studies probing available drugs for possible treatment approaches to oxycodone (i.e., “repurposing” or “repositioning”) and developments in the use of vaccines for opioid use disorders (OUDs), anticipating that these efforts will be beneficially applied to the misuse of other opioids. Finally, the review briefly focuses on recent developments in bitopic and biased opioid receptor modulators being pursued as alternative approaches to analgesics devoid of or with reduced abuse liabilities. It is hoped that this review of a pivotal drug spanning more than 100 years with a significant pharmacological and societal impact will be informative and might also be beneficial in the evaluation of new opioids that may be considered for therapeutic use in the future.
C. History of Oxycodone
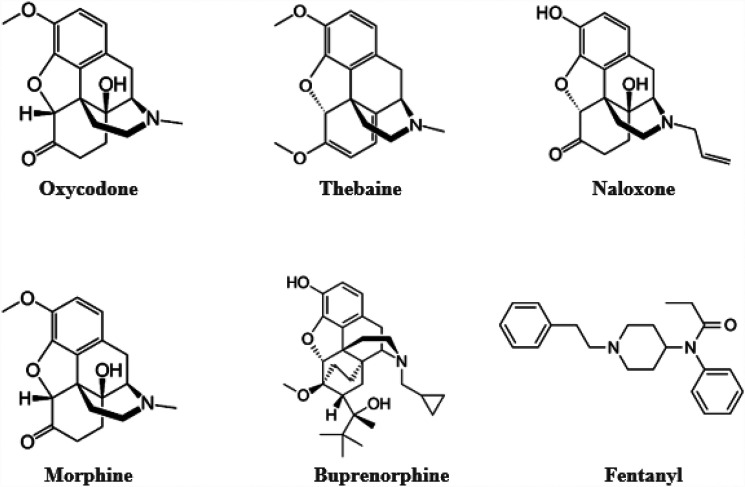
Oxycodone (Fig. 1), a semisynthetic derivative of the opioid alkaloid thebaine, is a μ-opioid receptor agonist synthesized in 1916 and introduced into clinical use in Germany in 1917 (Kalso, 2005). Although high doses of thebaine can produce convulsions and cannot be used therapeutically, it can be converted into a variety of opioids including not only oxycodone but also naloxone, buprenorphine, and oxymorphone (Olkkola et al., 2013). Ironically, the effort at the time oxycodone was initially synthesized was to discover a potent opioid analgesic devoid of the dependence and abuse liability surrounding heroin that was marketed at the time as an analgesic (Sneader, 2005). Despite the original effort to develop an opioid analgesic devoid of abuse liability and dependence, oxycodone in its various dosage forms shares the abuse liability of other μ-opioid agonists, and reports of its abuse and addiction potential occurred shortly following its introduction in 1918 (see Eddy et al., 1957 for a detailed overview of early clinical studies of oxycodone). Reports of early studies conducted in Germany cautioned that the use of oxycodone should be restricted to the lowest adequate dose and administered for the shortest possible time. Additional studies cited by Eddy et al. (1957) describe other reports that determined the addiction liability of oxycodone to be at least as great as that of morphine.
Fig. 1.
Structures of opioid receptor drugs.
Oxycodone became available for use in the United States in 1939, with formal approval by the Food and Drug Administration (FDA) in 1991. Initially, oxycodone was only available as a combination product that included either salicylates or acetaminophen. In 1995 oxycodone became available in a sustained-release formulation and, in 1996, as an immediate-release formulation and a single-entity product. The sustained-release formulation of oxycodone, OxyContin©, has been used for the treatment of moderate to severe acute and postoperative pain, neuropathic pain, and cancer pain (Kalso, 2005; Moradi et al., 2012). By 2001, oxycodone was the bestselling narcotic pain reliever in the United States, with 2008 sales in the United States reaching approximately $2.5 billion. Despite its beneficial clinical applications, oxycodone became one of the most widely used drugs of abuse in the country. Roughly between the years 1996 and 2016, the United States was responsible for approximately 73% of the world’s total consumption of oxycodone (Kinnunen et al., 2019). The illicit use of oxycodone has been reduced due to a number of restrictions on prescribing practices, heightened sensitivity to its widespread abuse and abuse potential, and the growth in the illicit use of other less costly and more readily available opioids, such as fentanyl.
D. Illicit Use and Abuse of Oxycodone
Although there is a perception that concerns surrounding oxycodone abuse emerged relatively recently, there were apprehensions as early as 1954 in France that the 3-methyl congener of oxymorphone, also known as 14- dihydrooxycodeinone or oxycodone (Murphree, 1962), “has proved to be particularly dangerous with regard to drug addiction … and that it seems to act more like heroin than like morphine” (Vaille and Stern, 1954). In the United States, the addiction potential of oxycodone was emphasized first in 1963 (Bloomquist, 1963) where the “habit forming potential” was said to approach that of morphine, prompting a revision of the detail literature to state that oxycodone “may be habit forming.” The revised warning was deemed unfortunate because oxycodone production increased in the United States from 9 kg in 1948–1950 to 569 kg in 1960, resulting in increased misuse and addiction of “numerous persons normally not associated with the illicit drug traffic.” Bloomquist (1963) also pointed out that oxycodone acquired the “unenviable status” of being the principal choice as a substitute for individuals physically dependent on heroin in California, describing several cases of individuals ages 15 to 85 with diverse occupations who developed severe oxycodone misuse, abuse, and dependence. Halpern and Bonica (1976) concluded that “we find the risk of addiction [to oxycodone] to be greater than that attributed to morphine … and do not recommend the use of oxycodone past the initial phases for the treatment of pain.” There were numerous additional concerns about the use and abuse of oxycodone that arose such that Sapienza (2003, p. 85) wrote in 2003 that “all data point to a serious problem with the diversion and abuse of oxycodone … and to a very serious problem with OxyContin.” An examination of theft data of controlled substances reported to the Drug Enforcement Administration showed that oxycodone thefts involving armed robberies and robberies of pharmacies in which suspects sought oxycodone or OxyContin occurred more than four times more frequently than those involving the next most frequently encountered substance (see also Young, 2001).
Approximately 60+ years later, following the initial warnings and concerns in the 1950s and 1960s, Remillard et al. (2019) published a manuscript titled “Oxycodone’s Unparalleled Addictive Potential: Is it Time for a Moratorium?” Remillard et al. conducted a survey of 86 study participants, all of whom were diagnosed with OUD or dependence. The study participants were stratified into two groups: one group who exclusively used non-heroin opioids enterally and a second group who injected opioids, primarily heroin. Based on the results of the survey, and on the known pharmacology of oxycodone, Remillard et al. concluded that “oxycodone possesses pharmacologic qualities that render it disproportionally liable to abuse and addiction” such that the risks outweigh the benefits. Oxycodone was rated the most desirable prescription opioid by 60% of the responders and by 75% of the drug-using peers. Remillard et al. (2019) also summarized the results of studies and surveys that provided evidence for oxycodone serving as the gateway drug to heroin. Other survey studies involving a much larger number of participants (896 in Katz et al., 2008; 1818 in Cicero et al., 2010) examined prescription opioid-dependent patients entering drug treatment programs. These studies, together with a larger study of 3,520 opioid-dependent patients (Cicero et al., 2013), uniformly concluded a higher use rate of oxycodone products (designated as “favorite” and “most desirable”) with surprising preferences for oxycodone even over fentanyl (Katz et al., 2008) and heroin (Cicero et al., 2010, 2013). Although acknowledging certain limitations to data collected by surveys and self-reports, Remillard et al. concluded that their review of the literature supported the conclusion that “oxycodone is the most addictive and thus [is an] abuse-liable prescription opioid,” a conclusion also echoed by Wightman et al. (2012) who reported that, based on extensive database searches (MEDLINE and EMBASE), oxycodone demonstrated an elevated abuse liability on the basis of its high likability scores and relative absence of negative subjective effects (see also Kibaly et al., 2021). This study also reported that patients with a history of drug misuse preferred oxycodone over other opioids, confirming results reported by Cicero et al. (2010), a conclusion supported further by several experimental laboratory studies of oxycodone in human heroin users, summarized later in this review, that have supported the results of these findings. The literature corroborated oxycodone’s place as the drug of choice for most prescription opioid abusers. Zacny and colleagues (Zacny et al., 2003; Zacny and Lichtor, 2008) published a series of studies, described in more detail later in this review, with non-drug-abusing individuals that compared the “likeability”/abuse liability of oxycodone to other opioids that included hydrocodone, methadone, and hydromorphone. The participants in these studies reported greater scores of subjective psychologic reward (e.g., “dreamy,” “elated,” “high,” “sedated [calm, tranquil]”, drug liking, and desiring it again) during estimated peak plasma oxycodone levels compared with the alternate opioids or to placebo. Interestingly, during trough levels of the drug, drug liking and desiring it again were notably lower for oxycodone compared with morphine or hydrocodone. Although many of the subjective and pain-relieving effects of oxycodone were similar to those of other μ-opioid agonists, oxycodone produced stronger and different psychopharmacological effects. Among human heroin-dependent individuals, oxycodone was considered to be the “Rolls Royce” of opioids (Comer et al., 2008).
E. Transition to Heroin
As mentioned previously, oxycodone has been viewed to be the most addictive prescription opioid and has been considered as a primary gateway to heroin use (Remillard et al., 2019). In addition to the abuse liability of oxycodone, there are a number of reports documenting a relationship between illicit oral use of oxycodone or OxyContin, leading to dependency, followed by the transition to the initiation of heroin abuse (Mars et al., 2014; Carlson et al., 2016). The Mars et al. study documented pathways to heroin injections in Philadelphia and San Francisco between 2010 and 2012. In both cities, the majority of young heroin injectors began their drug-use trajectories with opioid pills, usually with oxycodone and acetaminophen, oxycodone, or OxyContin before transitioning to heroin. Using the Ohio Substance Abuse Monitoring Network, Siegal et al. (2003) examined recently initiated heroin users where most of the subjects reported prior use of OxyContin before initiating heroin use. Although the sample was relatively small, subjects reported that they switched to heroin after developing tolerance to OxyContin, that heroin was more readily available and less expensive, and that they believed that they would never have tried heroin had they not developed an addiction to OxyContin. Cerdá et al. (2015) in a sample of 223,534 respondents to a National Survey on Drug Use and Health reported that nonmedical use of prescription opioids in childhood and early adolescence is strongly associated with transitions to heroin use in adolescence and young adulthood. Those initiating nonmedical use of opioids at ages 10 to 12 years had the highest risk of subsequently transitioning to heroin use, and the conclusions were independent of race/ethnicity or income.
These findings have prompted a number of studies comparing the effects of oxycodone exposure during adolescence and adulthood and to the identification of genes that may be involved in some of these effects (see Section IV.C on Gene and Protein Expression Studies). Data collected by Dart et al. (2015) over a decade between 2003 and 2013 have shown a relationship between the introduction of the reformulated release abuse-deterrent version of OxyContin in 2010 and the dramatic rise in the rate of heroin use over the next three years. This finding was also reported earlier by Cicero et al. (2012) for the three-year period from 2009 to 2012, where it was demonstrated that there was no evidence that OxyContin abusers ceased their drug abuse as the result of the abuse-deterrent formulation but rather shifted their drug of choice. Cicero et al. (2012) also point out that the newer formulation may actually have produced an unanticipated outcome, namely the shift to heroin, which may pose a much greater public health risk than OxyContin, suggesting that abuse-deterrent formulations may not be the “magic bullets” for solving the growing problem of opioid abuse.
F. Pain, Oxycodone, and Abuse
During the first decade of 2000, the rate of opioid use for the relief of pain increased greatly (Kolodny et al., 2015). Maruta and Swanson (1981) had pointed out problems with the use of oxycodone in patients with chronic pain, stating that their clinical observations indicated that patients taking oxycodone have greater difficulty tapering off the medication than do patients taking other analgesics. From 1999 to 2011, the rate of opioid pain reliever use in the United States increased substantially, with the consumption of oxycodone increasing by nearly 500% (Jones, 2013). During this same time, opioid-related overdose death rates nearly quadrupled (Chen et al., 2014). In the early 2000s a number of individuals noted the high consumption of opioids, particularly the controlled-release form of oxycodone (Cicero et al., 2005), along with an increase in oxycodone-related deaths in certain regions of the United States (Forrester, 2007; Baker and Jenkins, 2008), and in individuals seeking opioid detoxification from oxycodone (Sproule et al., 2009). In the Sproule et al. study, over the four-year period from 2000 to 2004, the number of admissions related to controlled-release oxycodone increased significantly from 3.8% to 55.4% of the total opioid admissions to the Centre for Addiction and Mental Health in Toronto, Ontario. The significant comorbid pain, psychiatric conditions, and other psychoactive substance use problems, coupled with the finding that prescriptions were an important source of opioids, all contributed to the rise in controlled-release oxycodone abuse. Despite these early indications of potential abuse and overdose mortality with oxycodone, most studies of oxycodone prior to 2000 were predominantly clinical in nature along with studies examining its pharmacokinetics and pharmacodynamics. Detailed studies of the preclinical pharmacology of oxycodone did not fully emerge until the second decade of 2000.
II. Basic Pharmacology of Oxycodone
A. Receptor Binding and Comparisons with Morphine
Although oxycodone and morphine share many pharmacological characteristics, with both being effective analgesics, oxycodone differs from morphine in a number of pharmacological, clinical, and physiologically relevant aspects that are described throughout subsequent sections of this review (Lemberg et al., 2006a,b, 2009; Nielsen et al., 2007; Olkkola et al., 2013; Kiyatkin, 2019). Pert and Snyder (1973) were the first to examine receptor binding affinities for morphine and oxycodone, using competition against [3H]naloxone, and reported ED50 (nM) values for morphine and oxycodone of 7 and 30,000 nM, respectively. Mu receptor binding of morphine and oxycodone were also examined by Chen et al. (1991) using [3H]DAMGO that, unlike naloxone, is highly specific for the μ-opioid receptor. In these experiments, the Ki (nM) for morphine was 1.2 and for oxycodone 47.4. Chen et al. (1991) also studied thebaine, from which oxycodone is derived, which had a Ki value of 636.2 nM. Generally, however, depending on the assay, the affinity of oxycodone for the μ-opioid receptor is between 5 to 40 times lower when compared with morphine (Chen et al., 1991; Lalovic et al., 2006; Olkkola et al., 2013). Studies comparing the receptor binding of oxycodone to other opioid receptors have demonstrated μ-opioid receptor specificity with lower Ki values for the δ-opioid receptor (958 ± 499) and κ-opioid receptor (677 ± 326); the Ki (nM) of oxycodone was 18 ± 4 (Monory et al., 1999). In comparison, morphine has been reported to bind to the μ-opioid receptor with an affinity of 1.8 nM, with an affinity of 90 nM for the δ site and 317 nM for the κ site (Robson et al., 1983). Some studies of oxycodone receptor binding have reported that the selectivity of μ over κ has been as much as 196 (Yoburn et al., 1995) compared with the selectivity ratio of 38 in the Monory et al. (1999) study. In general, and with some variation in the results that depend upon on the specific properties of the assays, oxycodone and morphine are relatively selective μ-opioid receptor agonists, both with lower affinities for the δ- and κ-opioid receptors, and with the potency of morphine higher than that of oxycodone at the μ-opioid receptor.
B. Role of Kappa and Delta Opioid Receptors in the Effects of Oxycodone
Despite the relatively low affinity of oxycodone at the κ- and δ-opioid receptors, there have been several experimental reports suggesting that the antinociceptive effects of oxycodone are mediated in part by these two other opioid receptors. Most of these suggestions appear to be related to the route of administration. For example, although both morphine and oxycodone produce potent antinociception when administered intramuscularly or intravenously, oxycodone and morphine differ in their effects when administered epidurally or intrathecally. Whereas oxycodone is not particularly effective when administered epidurally or intrathecally in humans, morphine has a powerful spinal analgesic effect (Pöyhiä and Kalso, 1992; Kalso, 2005)
An early study that examined the effects of intracerebroventricular administration of oxycodone reported that the antinociceptive effect of oxycodone, assessed by tail-flick latency to radiant heat, was blocked by the administration of naloxone, indicating that the analgesic effects were opioid mediated (Leow and Smith, 1994). However, because the reported affinity of oxycodone (Ki = 47.4 nM) for the μ-opioid receptors in the brain was significantly lower than that reported for morphine (Ki = 1.2 nM), Leow and Smith suggested that other opioid receptor subtypes may be involved in the antinociceptive effects of oxycodone. A subsequent study using more selective antagonists than naloxone reported that the analgesic effects of intracerebroventricular oxycodone were completely attenuated by the κ-selective opioid antagonist norbinaltorphimine (nor-BNI), whereas the selective μ- or δ-opioid receptor antagonists naloxonazine and naltrindole, respectively, were without effect (Ross and Smith, 1997). Importantly, nor-BNI did not prevent antinociception produced by intracerebroventricular morphine. This group of investigators also compared the onset of nociception produced by intracerebroventricular oxycodone and morphine and showed that the onset of nociception by oxycodone was approximately 5 to 7 minutes, whereas that of morphine was approximately 30 to 40 minutes (Leow and Smith, 1994; Ross and Smith, 1997). Additional studies comparing the antinociceptive onset of several κ-opioid agonists such as U68,593, U50, 488H, and bremazocine all produced a rapid onset similar to that of oxycodone, lending further support to the view that analgesia produced by intracerebroventricular oxycodone is mediated through interactions with the κ-opioid receptor. These investigators concluded that their findings support the concept that oxycodone and morphine produce antinociception through distinctly different opioid receptor populations and that oxycodone seems to act as a κ-opioid agonist with a relatively low affinity for the μ-opioid receptor. In contrast to this perspective, Lemberg et al. (2007) have stated unequivocally that oxycodone is a μ-opioid receptor agonist and not a κ-opioid receptor agonist, suggesting that the low intrathecal potency of oxycodone is related to its low efficacy and potency to stimulate intracellular G protein activation of the μ-opioid receptor in the spinal cord. Lemberg et al. (2007) conclude that the key to understanding these differences may lie in the complex pharmacology of the central nervous system (CNS) G protein receptors, a statement with foresight considering how the field of G protein-coupled opioid receptors has evolved over the past 15 years since (Wang et al., 2023).
The conclusion that the activity of oxycodone is mediated by actions at the κ-opioid receptor is tempered further by several other studies. Aceto et al. (2002) showed that the antinociceptive activity of oxycodone, administered subcutaneously in the tail-flick assay, was antagonized by β-Funaltrexamine (β-FNA), a μ-selective opioid receptor antagonist. However, this group did not find that oxycodone had κ-opioid properties since the κ-opioid receptor antagonist nor-BNI was ineffective against the antinociception produced by oxycodone, as was naltrindole, the δ-opioid receptor antagonist. Similar conclusions noting a lack of κ-opioid receptor activity were reported by Beardsley et al. (2004), showing that the selective μ-opioid antagonist β-FNA, but not the κ-opioid receptor antagonist nor-BNI or the δ-opioid receptor antagonist naltrindole, blocked the antinociceptive effects of oxycodone when administered subcutaneously in mice in the tail-flick test. The analgesic effects of oxycodone in squirrel monkeys were examined using the warm water tail withdrawal procedure (Withey et al., 2018). Oxycodone produced antinociceptive effects, as did heroin, buprenorphine, and methadone. When the antinociceptive and behaviorally disruptive effects of oxycodone and buprenorphine were characterized using Schild plots to calculate the apparent pA2 values for the antagonism by naltrexone, the results suggested that μ-opioid receptor mechanisms were likely mediating both the antinociceptive and behaviorally disruptive effects of these drugs. Several other studies have shown that withdrawal from morphine is not suppressed by κ-opioid receptor agonists, nor does morphine completely suppress signs of κ-opioid receptor mediated dependence (Gmerek and Woods, 1986; Fukagawa et al., 1989). Additionally, oxycodone has been shown to substitute for morphine, completely suppressing signs of morphine withdrawal in rhesus monkeys, a finding suggesting that oxycodone produces μ-opioid dependence and μ-opioid selectivity (Beardsley et al., 2004). Studies described later in this review using drug self-administration and drug discrimination procedures to assess abuse liability and subjective effects show that, in contrast to oxycodone, κ-opioid receptor agonists are not self-administered, nor do they substitute in drug discrimination studies when the training drug is a μ-opioid receptor agonist.
Just as there have been suggestions for an involvement of the kappa opioid receptor in mediating the actions of oxycodone, there have also been suggestions that the delta opioid receptor contributes to the analgesic effect produced by oxycodone (Yang et al., 2016). Using μ receptor knockout mice, Yang et al. found that high doses of oxycodone (40 mg/kg, s.c.) resulted in a small but significant antinociceptive effect as measured by the tail-flick response. The δ-opioid receptor antagonist naltrindole blocked this effect, suggesting a role for this receptor in mediating the antinociceptive effects of oxycodone. Further, administration of intracerebroventricular oxycodone to the μ-opioid receptor knockout mice produced comparable levels of antinociception to that found in wild type mice and these effects were also blocked by intracerebroventricular naltrindole. Yang et al. (2016) concluded that both mu and delta receptors contribute to the central antinociceptive effects of oxycodone. The authors recognized that these findings differed from those found in the Ross and Smith (1997) study that did not observe an antagonism of oxycodone’s analgesic effect when naltrindole was administered. Yang et al. (2016) comment that the reasons for the differences in the two studies may be due to different experimental conditions, drug doses, the species of animals, and the possible formation of mu/delta receptor complexes, all of which require further investigation. In a subsequent set of experiments, Yang and colleagues reported that naltrindole, administered intraperitoneally, did not affect the antinociceptive efficacy of subcutaneous oxycodone in the tail-flick test, nor did it block the respiratory depression produced by oxycodone. However, naltrindole did attenuate the tolerance and withdrawal induced by chronic oxycodone administration. In addition, using the conditioned place preference (CPP) method of assessing potential abuse liability of drugs, intraperitoneal naltrindole attenuated the development of preference for the oxycodone-related chamber and also attenuated reinstatement following a period of extinction. These effects were also obtained with the delta receptor antagonist ICI 154,129 administered intracerebroventricularly. Finally, the decrease in intestinal transit, or constipating effects of oxycodone, were also reduced by naltrindole (Yang et al., 2019). Yang et al. (2019) suggested that a combination of naltrindole and oxycodone may be a potent analgesic with reduced side effects of addiction liability and constipation. In keeping with the disparity in findings related to the antinociceptive and other effects of oxycodone, these findings by Yang et al. stand in contrast to those of Bossert et al. (2019) who, in a study of oxycodone self-administration and context-induced oxycodone reinstatement, found that naltrexone, a μ-opioid receptor antagonist, decreased reinstatement and oxycodone self-administration, but neither naltrindole, a δ−opioid receptor antagonist, nor LY2456302, a κ−opioid receptor antagonist, affected these two indices of oxycodone abuse liability. Bossert et al. concluded that μ-opioid receptors but not κ and δ receptors, are involved in oxycodone’s reinforcing effects and relapse.
A series of experiments that bears on the question of the relative role of μ-, κ- and δ−opioid receptors involvement in oxycodone’s pharmacological effects comes from research using blood oxygen level dependent (BOLD) imaging in awake wild-type and μ-opioid receptor knockout mice (Moore et al., 2016). Using this technology with the wild-type and the knockout mice provided an opportunity to evaluate the response to oxycodone, administered intraperitoneally, and to compare the BOLD signal change in 122 areas of the brain relevant to the different opioid receptors. Following the administration of 2.5 mg/kg oxycodone, BOLD activation was detected in 72 regions with the activation most prominent in areas of high μ-opioid receptor density. Oxycodone-induced positive BOLD activation was eliminated in most brain regions in the μ-opioid receptor knockout mice except in some regions where receptor expression was low or absent in the wild-type mice. Although most of the changes in BOLD by oxycodone indicate that the effects are mediated through the μ-opioid receptor, Moore et al. point out that “off target” effects of oxycodone in the knockout mice may suggest that those effects are mediated by κ- and δ-opioid receptors. While Moore et al. comment that the data from their study does not contest the findings by others (e.g., Ross and Smith, 1997) for a role of κ-opioid receptors in oxycodone’s pharmacological effects, they do point out that since there are no μ- and δ-opioid receptors in the cerebellum, there are κ-opioid receptors that are activated by oxycodone in the knockout mice, suggesting a possible interaction with the κ receptor and the conclusion that future studies using BOLD imaging should address the effects of oxycodone in κ-opioid receptor knockout mice.
Taken collectively, the majority of studies that have examined the role of the three opioid receptors in mediating the antinociceptive and other pharmacological effects of oxycodone provide strong support that the predominant pharmacological activity of parenterally administered oxycodone is related to its actions at μ-opioid receptors and that some of the ambiguity in the discrepant results involving κ- or δ-opioid receptors may be related to the route of administration, to the species, or to a significant role for the metabolites of oxycodone (Aceto et al., 2002; Lemberg et al., 2006a,b, 2007; see also Zacny and Gutierrez, 2003).
C. Respiratory Depression
Respiratory depression is a leading cause of death due to opioid overdose and continues to be a serious public health concern (Montandon, 2022). Early assessment of respiratory depression is undoubtedly one of the key criteria for assessing the safety of new analgesic compounds, particularly those that interact with μ-opioid receptors. Hill and Canals (2022) have provided a number of experimental considerations critical for the assessment of in vivo and in vitro opioids to evaluate their pharmacological activity and to address many of the issues surrounding the analysis of candidate opioids and their transition to further clinical development.
The leading cause of death related to opioid overdose is hypoxia caused by opioid-induced respiratory depression (White and Irvine, 1999). The μ-opioid receptor is expressed throughout the brainstem where μ-opioid receptor agonists reduce respiratory drive and the responsiveness of the respiratory centers to increased carbon dioxide (CO2), such that minute ventilation increases that would normally be triggered by hypercapnia are depressed. Webster et al. (2020) point out that there is no standard definition of respiratory depression and that, generally, it refers to a failure to maintain normal pulmonary exchange of CO2 and oxygen (O2). With respiratory depression there is an inadequate response to hypercapnia or hypoxia resulting in increased CO2 and/or decreased O2 blood levels (see Bateman et al., 2023 for a review on understanding and countering opioid-induced respiratory depression).
1. Human Studies
Several studies in humans have examined the effect of oxycodone on various measures of respiration. One of the earlier studies (Tarkkila, et al., 1997) compared the respiratory effects of intravenous tramadol and oxycodone in a placebo-controlled, double-blind study. Tramadol, an opioid with low affinity for the μ-opioid receptor, is also a serotonin and norepinephrine reuptake inhibitor. Whereas a tramadol dose of 0.6 mg/kg had no effect on respiratory depression that differed from placebo, oxycodone, given at a dose of 0.04 mg/kg, produced significant respiratory depression that was observed as an increase in the inspiratory-expiratory oxygen difference and in end-tidal CO2 concentrations as well as in respiratory rate. Comparable effects were also obtained in an exploratory study that compared equianalgesic doses of oxycodone with tapentadol, also a μ opioid receptor agonist and noradrenalin reuptake inhibitor, and found an advantage of tapentadol over oxycodone on respiratory depression (van der Schrier et al., 2017). Leino et al. (1999) studied time-course changes in breathing patterns and compared morphine (35.1 mg) with oxycodone (41.3 mg), given in incremental intravenous doses, by examining pulse oximetry and plethysmography to measure breathing patterns. Four of the planned oxycodone infusions had to be stopped because of respiratory depression as determined by pulse oximetry; none of the morphine infusions had to be terminated. The investigators suggested that the more profound changes with oxycodone were most likely due to dosing, where 1 mg of oxycodone is equivalent to 0.78 mg of morphine, not necessarily the different actions of the two drugs. Chang et al. (2010) added to the differences in the effects of morphine and oxycodone on respiration in a randomized double-blind, placebo-controlled study in patients undergoing elective surgery. Although patients receiving either morphine (0.1 mg/kg) or oxycodone (0.05, 0.1 or 0.2 mg/kg) intravenously demonstrated significant respiratory depression, as measured by changes in minute volume relative to placebo, the mean reduction from baseline was approximately 23% for the morphine group and 53% for the oxycodone group, with dose dependent increases up to 89% for oxycodone. All three doses of oxycodone produced statistically significant respiratory depression, and several patients in the oxycodone group, even at the lowest dose, required naloxone administration when, if at any point during the first 10 minutes after the study medication was administered, the respiratory rate decreased by ≥ 33% and/or the end-expiratory CO2 had risen by ≥ 1.5 kPa. The speed and extent of oxycodone-induced respiratory depression was greater for oxycodone than for an equivalent dose of morphine.
Webster et al. (2020) studied the effects of 30 and 60 mg of orally administered oxycodone in 19 men and women ages, 27 to 41years of age, who were recreational opioid users as determined by a naloxone challenge. Respiratory drive was assessed by measuring the ventilatory response to hypercapnia (excessive CO2 in the bloodstream caused by inadequate respiration) and by assessing the maximum decrease in minute ventilation after drug treatment. Compared with placebo, and with several doses of buprenorphine administered as a buccal film, the 60-mg dose of oxycodone produced a significant decrease in respiratory drive, whereas respiratory drive was not affected at any dose of buprenorphine. In a follow-up to this report, Webster et al. (2022) conducted a proof-of-concept study to evaluate whether it was possible to predict the relative risk of oxycodone’s potential to produce respiratory depression by measuring ventilatory response to hypercapnia. A focus of the study was to determine whether this method, incorporating end-tidal CO2 and minute ventilation, could serve to predict the relative effect of a drug on respiratory depression, a result that might have widespread utility. Using the 30-mg dose of oxycodone that did not produce respiratory depression in their previous study, Webster et al. (2022) found that this dose of oxycodone produced a significant reduction in minute volume and also reduced the slope of the ratio of minute volume to end tidal volume at the Cmax of oxycodone. The authors conclude that this method might have clinical utility and be advantageous in assessing drugs in development that are at risk for producing respiratory depression, for informing clinicians for improved decision-making, and for stratifying drugs on the basis of their relative effects on respiratory depression.
2. Animal Studies
An extensive study in male Sprague-Dawley rats examined the in vivo profile of several opioids (morphine; morphine-6-glucoronide; fentanyl; oxycodone; buprenorphine; [D-penicillamine2,5]-enkephalin, a selective δ-opioid receptor agonist; and the κ-opioid receptor agonist U69,593), all administered intracerebroventricularly for their effects on antinociception, constipation, and respiratory depression (Kuo et al., 2015). No two compounds had the same profile across these conditions, suggesting that the effects are regulated differentially. With regard to the effects of the different drugs on respiratory depression, the profile and potency rank of oxycodone were similar to that of fentanyl with a rapid onset and with peak effects at 15 minutes following administration of the drug. The administration of morphine, oxycodone, and fentanyl produced dose-dependent antinociception in the warm water tail withdrawal procedure and were full agonists. The results from these studies with morphine, oxycodone, fentanyl, and buprenorphine are summarized for antinociception, constipation, and respiratory depression in Table 1 for the ED50 values and in Table 2 for the rank order of potencies in each assay. Kuo et al. suggested that for constipation and respiratory depression, oxycodone appeared to be a partial agonist at the μ-opioid receptor based on the ceiling effects of the drug in these two assays. Kuo et al. concluded that the different pharmacokinetic profiles of these opioids suggest that it might be possible to discover potent analgesics with markedly improved adverse event profiles.
TABLE 1.
Mean ED50 values (with 95% CI) of four opioid agonists for producing antinociception, constipation, and respiratory depression in Sprague Dawley rats
| Opioid | Antinociception | Constipation | Respiratory Depression |
|---|---|---|---|
| Morphine | 52.2 (27.6-98.5) | 111.5 (111.4-111.7) | 88.5 (39.7-197.4) |
| Oxycodone | 287.9 (199.2-416.2) | 355.6 (335.4-377.1)a | ND |
| Fentanyl | 4.9 (1.53-15.7) | 9.9 (9.6-10.2) | 13.9 (10.0-19.3) |
| Buprenorphine | ∼20 (13.0-31.7)a | ∼7.5b | 12.3 (8.7-17.4) |
ND, not determinable.
Table adapted from Kuo et al. (2015).
aA ceiling effect and the ED50 was estimated using doses up to that which produced the maximal effect.
TABLE 2.
Potency rank order for opioids for producing antinociception, respiratory depression, and constipation following i.c.v. administration
| Opioid | Rank Order of Potency |
|---|---|
| Morphine | Antinociception > respiratory depression > constipation |
| Oxycodone | Antinociception > constipation > respiratory depression |
| Fentanyl | Antinociception > constipation > respiratory depression |
| Buprenorphine | Constipation > respiratory depression > antinociception |
Table adapted from Kuo et al. (2015).
Kiyatkin (2019) compared the effects of morphine, oxycodone, fentanyl, and heroin on respiratory depression and brain hypoxia in rats. Brain oxygen recordings were measured using oxygen sensors coupled with fixed-potential high speed amperometry surgically implanted in the nucleus accumbens (NAc) or into subcutaneous space in the medio-frontal area of the rat’s head, an area densely vascularized and an area with little or no metabolism. The latter measurements permit a surrogate for changes in systemic blood oxygen levels, a parameter directly related to respiratory activity. The animals were also prepared with intravenous catheters that permitted drug infusions. Heroin and fentanyl produced a rapid and strong dose-related decrease in NAc oxygen levels within the first 3 to 4 minutes following administration of the drug. Measurements of oxygen in the subcutaneous space showed significant decreases in respiratory depression that lasted much longer, up to 60 minutes, at the 100 μg/kg dose of heroin and for approximately 40 minutes for fentanyl at 40 μg/kg. In contrast to these effects with heroin and fentanyl, low to moderate doses of oxycodone that maintain drug self-administration (0.3 and 0.6 mg/kg) actually increased NAc oxygen levels, and, at the highest dose of 1.2 mg/kg, there was a short transient decrease followed by an increase in oxygen levels in the NAc. Based on these observations, oxycodone was approximately 6 times less potent than heroin and approximately 60- to 120-fold weaker than fentanyl in producing brain hypoxia. Morphine at 1.6 mg/kg also increased oxygen levels in the NAc, and, at 6.4 mg/kg, there was a protracted 2-hour decrease followed by a gradual rise to that which exceeded baseline at approximately 2.5 hours. When morphine was compared with oxycodone, the time to maximum decrease in oxygen and the duration of the decrease was substantially higher than that of oxycodone, as well as that of fentanyl and heroin. Clearly, there are temporal differences in NAc oxygen levels following morphine and oxycodone, suggesting that the increases in blood oxygen levels produced by oxycodone could be related to increased cerebral blood flow and vasodilation (Kiyatkin, 2019).
3. Polydrug Use and Respiratory Depression
Hill et al. (2018) demonstrated that tolerance developed to the respiratory depressant effects of prolonged oxycodone administration and that cross-tolerance also occurred to morphine. Of interest, and a concern surrounding polydrug use, tolerance to repeated administration of oxycodone was reversed by low-dose ethanol, pregabalin, and calphostin C, a brain-penetrant inhibitor of protein kinase C. In keeping with this finding, Gonek et al. (2017) had reported that the benzodiazepine diazepam reversed the development of antinociceptive tolerance, and, although the effects on respiratory depression were not studied, diazepam did reverse the tolerance to oxycodone on locomotor behavior. The respiratory depression produced by oxycodone was reversed by naloxone but not altered significantly by the δ receptor antagonist naltrindole or by the κ receptor antagonist nor-BNI, suggesting specific μ-opioid receptor mediated effects.
There has been heightened awareness of serious risks and deaths when combining opioids with benzodiazepines, with the FDA in 2016 issuing a strong boxed warning to the labeling of opioids and benzodiazepines (https://www.fda.gov/Drugs/DrugSafety/ucm518473.htm). The National Institute on Drug Abuse has reported that in 2020 the co-usage of opioids with benzodiazepines or antidepressants resulted in over 12,290 and 5,597 overdose deaths, respectively (https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates). In an effort to further >evaluate drug interactions of oxycodone with diazepam on respiratory depression, and to set the stage for a broader analysis of opioid–drug interactions, Xu et al. (2020) developed a rat model that measured increases in arterial partial pressure of oxygen and carbon dioxide (pCO2) to detect changes in respiratory depression. These measures are commonly used clinically to reflect respiratory function where drug-associated increases in resting arterial pCO2 suggest that the normal ventilatory response to compensate for increased CO2 is blunted. Studies were conducted with diazepam doses of 2, 20, and 200 mg/kg and with oxycodone doses of 6.75, 60, and 150 mg/kg, with all doses and dose combinations given orally. Oxycodone produced a dose-dependent decrease in arterial partial pressure of oxygen and an increase in arterial pCO2, both effects consistent with respiratory depression. Diazepam produced similar partial pressure changes only at the highest dose. When rats were coadministered 150 mg/kg of oxycodone, which produced significant respiratory depression, together with 20 mg/kg of diazepam, which had no effect on respiratory depression, decreases in arterial partial pressure of oxygen and increases in arterial pCO2 occurred that were consistent with an exacerbation of respiratory depression produced by oxycodone alone. Further, the potentiation of respiratory depression by diazepam and oxycodone was seen also in pharmacokinetic (PK)/pharmacodynamic (PD) analyses where the Cmax of oxycodone was 100% higher than that of animals administered oxycodone alone. This study could set a precedent and provide an experimental model to further pursue other psychotherapeutic drugs that, in combination with opioids, might interact in a similar manner as was obtained with diazepam and oxycodone. Although the Xu et al. (2020) study used a dose of oxycodone that alone produced respiratory depression, it would be important to have dose interactions with oxycodone and diazepam where the oxycodone dose does not produce respiratory depression and where there is tolerance following chronic administration to the effects of oxycodone on respiratory depression prior to administration of the other drugs.
A follow-up study by Xu et al. (2021) compared the effects of several psychotropic drugs in combination with oxycodone on respiratory depression, also in the Sprague-Dawley rat. These drugs cover a spectrum of conditions and mechanisms that are summarized in Table 3. As in their previous study, oxycodone was given at a dose of 150 mg/kg and administered orally with one dose of the other drugs. The selection of the dose that was given in combination with oxycodone was determined after evaluating blood concentrations of three doses of each drug and also using drug effects on pCO2 and pO2. The doses were selected based on the human dose equivalent according to the FDA conversion guidance, drug concentrations in previous studies with rats, and the recorded lethal dose of 50% of rats when this information was available. Thus, for each drug, the low dose was determined as the human dose equivalent, with the middle dose equivalent to the nontoxic literature concentrations and the high dose below the LD50 to avoid severe toxicity. The PD analysis of pCO2 and pO2 of each drug, together with the PK parameters, were used in determining dose and scheduling of the combination experiments. With the exception of topiramate, the middle dose was selected for all drugs given in combination with oxycodone. The low dose was used for topiramate due to the finding that it had no PD effect, whereas the middle and high doses decreased pCO2. Significant increases from baseline in resting arterial pCO2 occurred at all three doses with carisoprodol, duloxetine, and paroxetine. Paroxetine, administered alone, resulted in increases in pCO2, whereas none of the doses of quetiapine administered without oxycodone produced any change in pCO2 at any of the doses tested.
TABLE 3.
Drugs, drug classes, and clinical use for interaction studies with oxycodone
| Drug(s) | Class - Clinical Use |
|---|---|
| Clozapine, Quetiapine, Risperidone | Atypical Antipsychotics |
| Diazepam | Benzodiazepine - Anxiety |
| Zolpidem | Imidazopyridine - Insomnia |
| Trazodone | Serotonin receptor antagonist and reuptake inhibitor - Major Depressive Disorder |
| Carisoprodol – Cyclobenzaprine | GABA - Tricyclic - Serotonin 5-HT2 receptor antagonist - Skeletal muscle relaxants |
| Mirtazapine | Atypical Tricyclic Antidepressant |
| Topiramate | Blocks voltage-gated sodium channels & enhances GABA receptor activity - Anticonvulsant |
| Paroxetine – Duloxetine | Selective serotonin reuptake inhibitor - Serotonin & norepinephrine reuptake inhibitor - Depression |
| Ramelteon | Melatonin receptor antagonist - Insomnia |
| Suvorexant | Orexin receptor antagonist - Insomnia |
Table adapted from Xu et al. (2021); copyright CC-BY-NC-ND.
At clinically relevant exposures, paroxetine, trazodone, and quetiapine administered with oxycodone resulted in significant increases in resting arterial pCO2 that were above the effects of oxycodone alone. When coadministered with oxycodone, the increases in pCO2 for paroxetine and quetiapine were correlated with the increased Cmax and area under the curve exposure to oxycodone. These findings indicate that the interactions between opioids and non-opioid drugs can exacerbate respiratory depression and that these effects were mostly due to PK interactions that resulted in large increases in exposure to oxycodone.
An experimental focus on drug interactions with oxycodone also requires that these interactions are examined under conditions where oxycodone and other μ-opioid receptor agonists are administered chronically to more closely parallel typical usage in pain management and under conditions of OUDs. Although not focused on respiratory depression, Lawson et al. (2023) investigated oxycodone-benzodiazepine interactions following acute and chronic administration of oxycodone using ex vivo, in vivo, and in silico techniques. These studies examined the possible PD interactions between oxycodone and benzodiazepines when oxycodone was administered acutely and chronically for 15 days. Acute coadministration of oxycodone and the benzodiazepines diazepam and diclazepam to mice inhibited the metabolism of oxycodone, resulting in higher levels than those reached with oxycodone alone. When diclazepam was administered to mice that had been chronically treated with oxycodone for 15 days, the levels of oxymorphone, a toxic metabolite, were dramatically increased. In vitro studies conducted suggested that, whereas acute combinations of these drugs produce oxycodone accumulation, benzodiazepines administered following chronic oxycodone exposure produce metabolic interactions that inhibit oxycodone metabolism through CYP3A4, which is diverted toward CYP2D6. Thus, the overdoses and toxicity associated with oxycodone and benzodiazepine combinations are related to the usage patterns, i.e., whether the use of oxycodone is acute or more chronic. The early finding mentioned earlier in this review by Tatum et al. (1929) suggesting that an overdose of morphine during convulsions prevented the progression to respiratory arrest and mortality remains somewhat of an enigma considering the several studies that have been conducted since that initial observation.
D. Tolerance and Cross-Tolerance
In preclinical studies of tolerance and cross-tolerance, oxycodone has demonstrated many of the same effects observed with other μ-opioid receptor agonists. Tolerance developed to the antinociceptive effects of oxycodone in the complete Freund’s adjuvant (CFA) and chronic constriction injury (CCI) models of inflammatory and neuropathic pain, respectfully, following twice-daily administration of oxycodone for 7 days in Sprague-Dawley rats (Thorn et al., 2017). For those rats in the CFA condition, the administration of repeated oxycodone produced an approximate 16-fold rightward shift in the dose response curve, whereas for the CCI group, this shift was approximately 15-fold. Lilius et al. (2018) studied the development of tolerance to morphine (9.6 mg/d) or oxycodone (3.6 mg/d) administered for 6 days through subcutaneous minipumps to Sprague-Dawley rats. Tolerance developed to the antinociceptive effects of morphine and oxycodone using the hot plate. Acute administration of ketamine (10 mg/kg) and norketamine (30 mg/kg) attenuated the tolerance to both morphine and oxycodone, although the effect was of shorter duration in the oxycodone-treated animals. These investigators also found that ketamine and norketamine increased the brain concentrations of morphine but did not alter brain concentrations of oxycodone, suggesting that the differences may be due to the inhibition of morphine metabolism by ketamine and norketamine.
In a study that examined the role of βarrestin2 on opioid tolerance, Raehal and Bohn (2011) reported that although βarrestin2 knockout mice did not develop tolerance to the antinociceptive effects of chronic morphine in the hot place assay, tolerance did develop to chronic oxycodone, fentanyl, and methadone. These findings suggested that different μ-opioid agonists can produce different effects on antinociceptive responses mediated by opioid receptors in a βarrestin2-dependent manner. In a subsequent study from this laboratory, Schmid et al. (2017) evaluated morphine, oxycodone, and a biased μ-opioid receptor agonist, SR-17018, for tolerance development and for efficacy in the hot-plate assay. In cellular assays, SR-17018 preferentially stimulates GTPγS binding over the recruitment of βarrestin2, demonstrating pathway signaling bias. In the hot-plate assay, SR-17018 produced analgesia with potencies comparable to those of morphine but with less respiratory depression. SR-17018 did not produce tolerance to the antinociceptive effects, whereas morphine did. Pantouli et al. (2021) continued this line of investigation and examined the effects of acute and repeated dosing of morphine, oxycodone, and SR-17018 in several pain models that included the mouse warm water tail immersion assay, the formalin inflammatory pain model, and a chemotherapeutic-induced neuropathic pain model induced by paclitaxel. In the warm water tail immersion procedure, all three compounds produced tolerance when administered repeatedly. However, whereas tolerance did develop to oxycodone in the formalin model, tolerance did not develop to repeated administration of SR-17018 in the formalin or chemotherapeutic-induced neuropathic pain procedures. These findings suggest that it may be possible to develop biased μ-opioid receptor agonists that are devoid of some of the unwanted effects of opioid agonists. Clearly, an effective opioid analgesic lacking respiratory depression, tolerance, and abuse liability would be a significant advance in the pharmacological management of pain (see also Section X in this review for an elaboration of this view).
Other studies, conducted earlier, examined the possibility of differential profiles between oxycodone and morphine in their effects on tolerance and cross-tolerance (Nielsen et al., 2000). The Dark Agouti rat was used in these studies because this rat is genetically deficient in the CYP2D1 enzyme that catalyzes the O-demethylation of oxycodone to oxymorphone, a potent μ-opioid agonist (Cleary et al., 1994). This rat strain is therefore more appropriate to examine the potential relevance to humans because the O-demethylation of oxycodone to oxymorphone undergoes glucuronidation to oxymorphone-3-glucuronide and accounts for less than 5% of an oxycodone dose in humans (Pöyhiä et al., 1992). Rats were administered equi-antinociceptive doses of oxycodone (2.5 or 5.0 mg) or morphine (10 mg or 20 mg) intravenously over a 24- to 84- hour period to produce tolerance to the tail-flick response. Subsequently, when drug-naïve rats and rats that were tolerant to morphine-induced antinociceptive effects were administered bolus intracerebroventricular injections of oxycodone, the ED50 values of oxycodone on the tail-flick response in the drug-naïve and morphine-tolerant rats were comparable, suggesting an absence of cross-tolerance between supraspinally administered oxycodone and intravenous morphine. However, when intracerebroventricular morphine was administered to oxycodone-tolerant rats, there was a high degree of cross-tolerance. Similarly, there was no indication of cross-tolerance between morphine and oxycodone when intravenous doses of oxycodone were administered to morphine-tolerant rats. Following the administration of intracerebroventricular morphine, the dose-response curves of both oxycodone- and morphine-tolerant rats were shifted to the right of the naïve rats, indicating cross tolerance to intracerebroventricular morphine in rats made tolerant to intravenous oxycodone. Although the rightward shift in the dose-response curve for the oxycodone tolerant rats administered intravenous morphine was not as great as that of the morphine tolerant rats administered intravenous morphine, this result does suggest evidence of a degree of cross-tolerance to oxycodone in rats tolerant to morphine when the same route of administration is used to develop tolerance and assess cross-tolerance.
Thus, in summary, when rats developed tolerance to the antinociceptive effects of intravenous morphine, neither intracerebroventricular nor intravenous administration of oxycodone produced evidence of cross-tolerance. However, following the development of tolerance to oxycodone, there was cross-tolerance to morphine following both intracerebroventricular and, to a lesser extent, intravenous routes of morphine administration. Nielsen et al. (2000) posited the view that the asymmetric cross-tolerance between oxycodone and morphine suggested that, following chronic intravenous administration, oxycodone is metabolized to a μ-opioid agonist metabolite, which is then responsible for the substantial tolerance developed to intracerebroventricular morphine. However, following the development of tolerance to intravenous morphine, the administration of intracerebroventricular oxycodone, where metabolism is negligible, resulted in a lack of cross-tolerance. The explanation provided by Nielsen et al. (2000) does not appear to take into consideration that cross-tolerance to intravenous morphine was observed in rats tolerant to intravenous oxycodone, as evidenced by the rightward shift of the morphine dose-response curve in the oxycodone-tolerant rats away from the naïve animals. These authors conclude that after parenteral but not supraspinal administration, oxycodone is metabolized to a µ-opioid agonist metabolite, thereby accounting for the asymmetric and incomplete cross-tolerance between oxycodone and morphine. Finally, the authors conclude that these results support their view that the antinociceptive effects of oxycodone and morphine are mediated through different opioid receptor populations, a theme that occurs in a number of studies reviewed previously. However, it seems appropriate to conclude that there is cross-tolerance between morphine and oxycodone when the same route of administration is used.
E. Dependence and Withdrawal
Preclinical studies of physical dependence and withdrawal produced by oxycodone have, as in experiments on tolerance and cross-tolerance, demonstrated many of the same effects observed with other μ-opioid receptor agonists (Carper et al., 2021). An early study demonstrated that rhesus monkeys given increasing intragastric doses of oxycodone up to 80 mg/kg every 12 hours over a 20-day period showed signs of physical dependence that, when challenged with naloxone, precipitated withdrawal (Swain et al., 1977). A number of studies have examined oxycodone-induced dependence and withdrawal, mainly in mice, although in a study using rhesus monkeys, oxycodone produced a dose-dependent suppression of withdrawal signs following the discontinuation of morphine (Beardsley et al., 2004). In a series of studies that examined physical dependence of oxycodone, Enga et al. (2016) administered increasing doses of oxycodone from 9.0 to 33.0 mg/kg s.c. over nine days and then administered increasing doses of naloxone from 0.1 to 10.0 mg/kg s.c. Naloxone administration produced dose-dependent increases in several somatic signs of withdrawal that included paw tremors, jumps, and increases in body weight, similar to those seen with morphine in other studies. A second feature of this study included oral self-administration of oxycodone, developed using an operant conditioning procedure, that initially involved post-prandial consumption of water that was followed by switching water availability to increasing doses of oxycodone. The sequence of steps ended with a period whereby the post-prandial feature was discontinued but oxycodone remained available by responding under a fixed ratio four-response schedule of lever pressing. As the concentration of oxycodone was increased, the estimated consumption of oxycodone increased and opioid-like behavioral signs were observed that consisted of Straub tail and hyperlocomotion at the higher concentrations of oxycodone. When the prandial procedure was discontinued and oxycodone remained available, the mice continued to lever press to obtain oxycodone, suggesting that oxycodone was serving as a reinforcer and that the procedure could be used to develop dependence.
Carper et al. (2021) induced dependence on oxycodone using an incremental dose regimen of subcutaneous oxycodone for eight days, reaching a final dose of 33 mg/kg on day 9 that was followed by the administration of naloxone 1.0 mg/kg 6 hours after the final dose of oxycodone. Both precipitated and spontaneous withdrawal (no naloxone) resulted in jumping, paw tremors, and decreases in body weight, with these measures greater and more intense in the naloxone precipitated withdrawal animals at 6 hours, whereas more withdrawal signs were seen at 24 hours in those mice that underwent spontaneous withdrawal. These studies provide evidence that chronic administration of oxycodone produces tolerance, dependence, and withdrawal that is not distinctive from that of morphine. Following the suggestions obtained thus far with compounds possessing selective signaling properties, it may ultimately be possible to identify and develop efficacious μ-opioid receptor agonists that do not produce tolerance and that, therefore, should not produce dependence as well.
F. Pharmacodynamic and Pharmacokinetic Effects
As pointed out in an updated review of the clinical PK and PD of oxycodone by Kinnunen et al. (2019), although oxycodone has a lengthy history of clinical use, since most studies were conducted in the 1990s, it is without a detailed knowledge of its PK. A major difference between morphine and oxycodone recognized quite early is that oxycodone has much better oral bioavailability, with the bioavailability of oxycodone between 60% and 87%, whereas with morphine it is only 19% to 30% (Pöyhiä et al., 1993). Oxycodone is relatively well absorbed following oral administration, with approximately 40% of oxycodone bound to plasma proteins in vitro, results that are similar to those of morphine (Lemberg et al., 2009). The half-life of oxycodone administered intravenously is approximately 2 to 3 hours, whereas when administered intramuscularly it is approximately 5 hours and following oral administration is between 3 and 5 hours with the extended-release form roughly 8 hours (Umukoro et al., 2021). The volume of distribution at steady state was 2 to 5 L/kg in adults, which is also comparable to that of morphine (Olkkola et al., 2013). In healthy female volunteers, the clearance of oxycodone on a weight-adjusted basis was found to be 25% slower than in men (Kaiko et al., 1996).
Oxycodone is primarily metabolized via CYP3A4/3A5 and to a lesser extent via CYP2D6. Women metabolize oxycodone faster than men, and women also have higher metabolite levels when compared with men; exposure is greatly increased in the elderly, with patients over 70 years of age having a 50% to 80% higher exposure to oxycodone (Liukas et al., 2008; Umukoro et al., 2021). The predominant metabolic pathways in a variety of species, including humans, involve oxidation to oxymorphone and noroxycodone, conjugation to α−D-glucuronic acid, and conversion to 6-oxycodol. O-demethylation by CYPD6 leads to the formation of the main active metabolite, oxymorphone (Ishida et al., 1982; Cone et al., 1983). It appears that noroxycodone and noroxymorphone are not able to significantly affect the analgesic properties of oxycodone (Lemberg et al., 2006a, 2008). In clinical studies, when administered orally, intramuscularly, or intravenously, oxycodone produces pain relief similar to that of other μ-opioid receptor agonists (Pöyhiä et al., 1991, 1992). However, as mentioned previously, oxycodone and morphine differ in their effects when administered epidurally; whereas oxycodone is not particularly effective, morphine has a powerful spinal analgesic effect (Kalso, 2005). In humans, epidurally administered morphine has been shown to be 10 times more potent than oxycodone following abdominal surgery (Backlund et al., 1997). In rats, intrathecal administration of morphine has been shown to be approximately 14 times more potent than oxycodone, whereas with subcutaneous and intraperitoneal administration, oxycodone is 2 to 4 times more potent than morphine (Pöyhiä and Kalso, 1992a). The nature of these differences remains rather unclear, but it has been suggested that they are related to the effects of intrathecal oxycodone on κ-pioid receptors, a recurring theme that persists along with findings implicating the involvement of δ-opioid receptors (Ordóñez Gallego et al., 2007; Yang et al., 2016, 2019; Ruan et al., 2017; Bossert et al., 2019; Olson et al., 2019). Lemberg and colleagues (2006b) noted the discrepancy in clinical efficacy after systemic administration and the loss of potency after spinal administration, commenting that even after considerable clinical use, the pharmacology of oxycodone was poorly understood, requiring a better understanding of the pharmacokinetics of oxycodone and its metabolites.
A number of studies reviewed by Kalso (2005) summarized results conducted in healthy volunteers and those individuals with kidney or liver failure and also included PK drug-drug metabolism interactions. As oxycodone is metabolized in the liver by O-demethylation to form oxymorphone in a reaction catalyzed by the P450 2D6 enzyme, it is likely that PK interactions that block CYP2D6 are anticipated. Due to the fact that the active metabolite of oxycodone, oxymorphone, may contribute significantly to analgesia, it is expected that there would be a decrease in the efficacy of oxycodone in poor metabolizers and during coadministration of drugs that inhibit CYP2D6. A case report in fact did suggest that fluoxetine hydrochloride, a potent CYP2D6 inhibitor, increased the oxycodone requirement in a poor metabolizer (Otton et al., 1993).
Oxycodone and morphine have distinctly different metabolic pathways, and active metabolites may complicate the comparison (Nielsen et al., 2007). A series of studies mentioned earlier was conducted that drew starkly different conclusions about whether oxycodone produced its analgesic effects through the μ-opioid receptor or through the κ-opioid receptor (Ross and Smith, 1997; Lemberg et al., 2006a, 2007; Nielsen et al., 2007; Smith et al., 2007). Lemberg et al. (2009) compared oxycodone and its metabolite oxymorphone in a variety of analgesia models and found that in the tail-flick assay, both subcutaneous oxycodone and oxymorphone produced dose-dependent analgesia, whereas in the hot plate and mechanical models of nociception, oxymorphone was much more effective than oxycodone, with analgesia produced by oxymorphone lasting for a much longer duration. These effects were also found when the effects of oxycodone and oxymorphone were measured following intrathecal administration. Oxymorphone appears to be critically important in producing analgesia after systemic administration.
A major difference between oxycodone and morphine that might account for some of these differences could be in the passage of these opioids through the blood–brain barrier. The concentrations of oxycodone are threefold higher in the brain interstitial fluid compared with plasma, whereas the reverse is true with morphine (Kalso, 2007). Both drugs have similar logD values (are equally hydrophilic) but the higher concentration in the brain 3 times higher than in blood suggests the presence of an active influx transporter for oxycodone (Boström et al., 2006). Okura et al. (2008) have suggested that this may be accomplished by an organic cation transporter. The concentrations of the unbound drug in the target organ (brain) correlate more closely with the CNS drug effects (analgesia) than the plasma levels. For the same unbound concentration in blood, the concentrations of unbound oxycodone in brain are 6 times higher than those of morphine. This difference could explain the higher efficacy of oxycodone compared with morphine at similar plasma levels. (Kalso, 2007).
Hassan et al. (2007) reported that repeated administration of oxycodone for 6 days to male Sprague-Dawley rats at a dose that was antinociceptive in the hot-plate test (5.0 mg/kg i.p.) stimulated P-glycoprotein (P-gp) ATPase activity, increasing P-gp protein levels that significantly decreased the tissue distribution of the chemotherapeutic agent paclitaxel. These findings suggest that oxycodone is a P-gp substrate that, when administered repeatedly, may affect the pharmacokinetics and pharmacodynamics of other drugs that are also P-gp substrates. Additionally, the upregulation of P-gp induced by repeated administration of oxycodone may lead to the reduction of oxycodone levels in the CNS, resulting in the development of tolerance to the analgesic effects of oxycodone and to cross-tolerance to other μ-opioid receptor agonists such as morphine and methadone.
The PD properties of oxycodone and its metabolites have been reviewed by Olkkola et al. (2013) and Ruan et al. (2017). The PD effects of oxycodone are comparable to those of other opioid analgesics such as morphine and include pain relief, sedation, nausea, vomiting, and respiratory depression (Tarkkila et al., 1997; Chan et al., 2008). In addition to differences in oral bioavailability, oxycodone has been reported to produce less nausea compared with morphine when administered to cancer patients (Kalso and Vainio, 1990). The primary metabolite of oxycodone, nororoxycodone, is 4 times lower than that of oxycodone at the µ-opioid receptor and produces 4 to 6 times lower G-protein activation as measured in a GTPγ[35S] binding assay. Oxymorphone, the other primary oxidative metabolite, has an approximately 50-fold higher G-protein activation than that of oxycodone (Thompson et al., 2004; Lalovic et al., 2006). Lalovic et al. conclude that the metabolites of oxycodone do not contribute to the central effects due either to their low potency or low abundance in the circulation or as a result of their poor uptake into the brain.
Finally, although not specifically related to PD and PK effects, Lyu et al. (2022) have published data demonstrating that long-term developmental exposure to oxycodone in utero has a long-standing effect on the gut microbiome when microbiota are examined in adulthood. In this study female mice were treated daily with 5 mg of oxycodone for two weeks prior to breeding and then throughout gestation. Male and female offspring pups were examined using a variety of behavioral and metabolic tests, and fecal boli were collected and analyzed in adulthood. Several bacteria in females and males were elevated in mice exposed to oxycodone, though these elevations were not uniform across sexes. The bacterial changes were correlated with metabolic pathway alterations that could affect drug action throughout the lifespan. Although this may affect children born to mothers who have been using oxycodone or other opioids, further work is clearly needed and of importance.
III. Pharmacogenomics/Pharmacogenetics of Oxycodone
A. Genotype Variations in Humans and Responses to Oxycodone
Pharmacogenomics, sometimes also referred to as pharmacogenetics, is an important element of precision medicine and of pharmacology. The term “pharmacogenetics” is attributed to the German pharmacologist Friedrich Vogel who coined it in 1959. The use of the term followed the publication by Motulsky who wrote about how “drug reactions may be considered pertinent models for demonstrating the interaction of heredity and environment in the pathogenesis of disease” (see Pirmohamed, 2011). The benefits of developing an understanding of the genetic associations with drug dose, efficacy, and toxicity as a means of optimizing clinical care of patients are self-evident (Cascorbi and Tyndale, 2014; Sadée et al., 2023). In clinical oncology the presence of mutations in tumor tissue is critical in determining treatment approaches, and similar examples can be found in cardiovascular diseases and in psychiatry (Crettol et al., 2014). The role of pharmacogenomics has emerged as a significant area of interest to aid in the selection of the most appropriate opioid analgesic for palliative care for cancer patients where there is existing pharmacogenomic evidence to guide the prescribing of codeine and tramadol based on their relationship to CYP2D6 gene variants (Wong et al., 2022).
Research with oxycodone has increased due in part to its widespread use and abuse as well as interests in its unique analgesic actions. Individual responses to oxycodone have been shown to vary due to genetic differences. Umukoro et al. (2021) published a narrative literature review of the pharmacogenomics of oxycodone and have provided an excellent review of pharmacokinetics, pharmacodynamics, and genetic factors affecting the pharmacodynamics of oxycodone. In their review, Umukoro et al. conclude that there is conflicting evidence for a clinical effect of genetic polymorphisms but there is much stronger evidence linking polymorphic genetic enzymes CYP2D6 and CYP3A with therapeutic outcomes.
Samer et al. (2010a) evaluated the effects of the CYP2D6 genetic polymorphism and CYP2D6 and CYP3A on drug-drug interactions and on the pharmacodynamic effects (antinociception, pupil size, sedation, respiration, side effects) of oxycodone in healthy male volunteers. Both CYP2D6 genetic polymorphism and drug-drug interactions by CYP2D6 and CYP3A had major effects on the antinociceptive responses to oxycodone. CYPD2D6 activity was correlated with the assessment of experimental pain in ultra-rapid metabolizers of CYP2D6 who experienced greater analgesic effects, whereas the poor metabolizers had reduced CYP2D6 and showed no change in these measures. Several other differences between high and low metabolizers of CYP2D6 were reported that included greater sedation and respiratory depression when CYPD2D6 was high; ultra-metabolizers also reported mild to severe side effects whereas no toxicity was reported among poor metabolizers.
Inhibition of CYP2D6 with quinidine greatly reduced the analgesic effects of oxycodone so that the results in the pain test were no different than those of placebo. Further, CYP2D6 inhibition significantly increased exposure to oxycodone along with a decrease in oxymorphone and noroxymorphone, suggesting that oxycodone may not be responsible for the analgesic effects (see also Samer et al., 2010b). In the Samer et al. (2010b) study, CYP3A inhibition with ketoconazole produced significantly higher PD effects than those with placebo. The conclusion of this extensive set of experiments was that oxycodone has to be used with extreme caution in ultra-high metabolizers, especially when a CYP3A inhibitor is coprescribed. Finally, it was also suggested that for those deficient metabolizers for CYP2D6, analgesic activity may be reduced, and perhaps other alternative treatments should be provided. This emphasis was also reinforced in a subsequent study urging the development of personalized oxycodone dosing focused on determining the patient’s metabolic response through testing the CYP2D6 phenotype to improve the safety and efficacy of oxycodone (Linares et al., 2014).
Pharmacogenetic approaches have been incorporated into postoperative pain management in a prospective randomized study of pain medication following hip and knee arthroplasty (Hamilton et al., 2022). These investigators performed pharmacogenetic testing for genetic variants that included CYP2D6, CYP2C9, OPRM1, and CYP1A2. Pharmacogenetic testing of these patients prior to surgery allowed for the collection of information on pain and pain management following surgery. Genetic variants were found in a number of patients that influenced drug metabolism. It was concluded that when patient’s pharmacogenetics are identified and medications customized to their genetic profile, pain scores and opioid use are greatly reduced for 10 days following surgery.
A study conducted in Sweden that controlled for allelic frequency found a significant association between the 118G allele in the OPRM1 gene and heroin addiction (Bart et al., 2004). However, there are a number of reports, including a large meta-analysis of 16 case-control studies of opioid dependence in a total of 5169 subjects, that concluded there was a lack of association between the A118G allele and genotype frequencies and opioid dependence (Coller et al., 2009; see also Franke et al., 2001 for an earlier study with similar conclusions). Coller et al. did add a few qualifiers to their conclusions including the suggestion that there was significant heterogeneity between the studies and that although there was no evidence of a direct association with the risk of dependence, A118G may still have an influence on the pharmacological response to opioids.
Two studies by Zwisler et al. (2010, 2012) examined the possible association of OPRM1 and ABCB1 polymorphisms in response to experimental and postoperative pain and adverse effects in humans following treatment with oxycodone. The G allele of the A118G single nucleotide polymorphism (SNP) of the opioid receptor gene (OPRM1) has been shown to influence analgesia, respiratory depression, emesis, and adverse reactions produced by the active metabolite of morphine, morphine-6-glucuronide (Skarke et al., 2003; Romberg et al., 2005). The ABC1 gene encodes P-gp, the efflux transporter that influences drug transport in the intestine, kidneys, and blood-brain barrier, thereby altering the pharmacokinetics of some drugs. P-gp activity can be influenced by genetic variability of the C3435T and G2677T/A SNPs.
In their initial study Zwisler et al. (2010), examined the antinociceptive and possible adverse effects of SNPs in 33 healthy subjects exposed to experimental pain that included electrical nerve stimulation and the cold-pressor test. The variant G allele of the A118G SNP was associated with a reduced antinociceptive effect of oxycodone in the electrical nerve stimulation pain tolerance procedure (i.e., a lower increase in pain tolerance threshold) compared with placebo, but there was no effect on adverse drug reactions to oxycodone. Carriers of the variant T allele of the C3435T SNP had less adverse reactions (dizziness, nausea/vomiting, itching) to oxycodone than the wild-type carriers, whereas the carriers of the T allele of the G2677T/A SNP had a better antinociceptive response in the cold-pressor procedure following oxycodone than the wild-type carriers, a result that was accompanied with less severe adverse drug reactions.
A subsequent study that included a total of 268 patients undergoing various surgical procedures examined the possible association between the SNPs A118G in OPRM1 and C3435T and G2677T/A in ABC1 in the response to oxycodone in postoperative pain (Zwisler et al., 2012). In contrast to their prior study (Zwisler et al., 2010) there was no association between these SNPs and changes in the analgesic effects of oxycodone or in adverse drug reactions. The authors conclude that the contradictory findings may be related to the different types of pain that were studied in the two experiments (i.e., experimental vs. postoperative pain), to the fact that many of the patients were comedicated with P-gp inhibitors, and to the low consumption of oxycodone.
Jones et al. (2019) took a step toward attempting to predict individuals that might be susceptible to opioid use disorders by assessing genetic polymorphisms in an effort to determine whether those polymorphisms were associated with the subjective responses to oxycodone. The 36 volunteers (33 men and 3 women) for this study had previously used opioids as part of pain management exclusively for medical use. Several gene variations were examined, including the μ-opioid receptor ORPM1, the δ-opioid receptor OPRD1, the κ-opioid receptor (OPRK1 and the major dopamine-metabolizing enzyme, catechol-O-methyltransferase (COMT). A number of findings were noted with the small nuclear proteins encoding the μ-opioid and δ-opioid receptors influencing the subjective effects of oxycodone with the small nuclear protein in the δ-opioid receptor being the most robust predictor of opioid reward. This study, together with those of Samer et al. (2010a,b), Linares et al. (2014)” and Wong et al. (2022), point to the utility and the necessity of further research examining the pharmacogenetic and pharmacogenomics of oxycodone as well as other opioids. Further, considerations of ‘phenoconversion,” a condition where genotypic extensive metabolizers are converted into phenotypic poor metabolizers due to concomitant drug administration should be incorporated into these approaches to safely and effectively reduce pain and to allow for the stratification of individuals for effective pain management that are at lower risk to convert to abuse (Deodhar et al., 2021).
B. Gene and Protein Expression Studies in Animals
A number of studies have examined gene expression in animals following periods of exposure to oxycodone to develop a better understanding of the neurobiological mechanisms underlying oxycodone, particularly under conditions when it is self-administered. Zhang et al. (2014) provided access to intravenous oxycodone in male C57BL/6J mice using a nose-poking response for 14 days in daily extended (4-hour) or shortened (1-hour) experimental sessions and assessed the effects on striatal neurotransmitter receptor gene expression. Mice exposed to the 4-hour sessions escalated the amount of oxycodone that was self-administered and showed changes in a number of neurotransmitter receptor genes in the dorsal striatum, including the GABAA receptor subunit beta 2 (Gabrb2) as well as changes in cholinergic receptors, neuropeptide Y, 5-HT3, and the glycine receptor relative to saline controls. The investigators of this study point out that the mRNA of only one subunit of the GABAA receptor Gabrb2 showed a significant decrease in mice that had self-administered oxycodone and suggest that decreases in this mRNA may underlie the mechanism responsible for the increased intake of oxycodone during the extended periods of self-administration. In contrast to these changes, mice in the 1-hour condition did not escalate intake of oxycodone, nor did this group show changes in the expression of neurotransmitter genes. This study also incorporated a “yoked control” group that received saline, not oxycodone; this group did not show sustained responding throughout the 14-day period. It would be interesting to determine the effects of a “true” yoked control where the yoked animals received the same frequency of oxycodone deliveries but did not have to respond for oxycodone as was the case with the active oxycodone subjects.
A follow-up study by Zhang et al. (2017) examined whether oxycodone self-administration under the extended access procedure affects gene expression in the terminal areas of the nigrostriatal and mesolimbic dopaminergic pathways in mice. Several alterations in the expression levels of genes related to inflammation and immune functions were found, suggesting that the systems related to inflammation and immune genes undergo large changes during the chronic administration of oxycodone.
Zhang et al. (2015), studying the intravenous self-administration of oxycodone (14 consecutive days at 2 hours/day at 0.25 mg/kg/infusion) in adolescent and adult C57BL/6J mice measured gene expression in the hippocampus. Prior to self-administering oxycodone, it was shown that adolescent and adult control mice differed significantly in the expression of several genes that included those coding for mitogen-activated protein kinase (mapk1), calcium/calmodulin-dependent protein kinase II gamma subunit (Camkl2g), the glutamate receptor, ionotropic AMPA 2 (Gria2), and the metabotropic glutamate 5 receptor (Grm5). Self-administered oxycodone produced a significant alteration in a number of genes, particularly those involved in synaptic plasticity. For example, Pim1, a proviral integration site that belongs to the Ca2+/calmodulin-dependent protein kinase family and is known to attenuate apoptosis, was increased in both adult and adolescent mice. A second gene that was increased significantly in the self-administration animals compared with saline controls was thymoma viral proto-oncogene 1 (Akt1), also a serine-threonine protein kinase like Pim1, that is a key mediator of growth factor-induced neuronal survival. The Akt1 pathway plays an important role in cell proliferation, differentiation, and survival. One interpretation of these findings is that oxycodone may be inhibiting the process of neurogenesis in the hippocampus and these changes in gene expression may be activated to counteract or to compensate for these changes.
This group of investigators has also demonstrated that extended access to oxycodone self-administration produced alterations in the expression of several genes related to axon guidance gene families that include integrins, semaphorins, and ephrins (Yuferov et al., 2018). Yuferov et al. speculated that oxycodone-induced alterations in these genes produce neuroadaptations in axon-target connections and synaptogenesis that may play a role in the neurobiological adaptations that occur in OUDs. Some of these genes are also known to modulate glutamate transporter currents in astrocytes and to alter dendritic morphology and synaptic connectivity.
The question of whether exposure to opioids such as oxycodone during adolescence affects responses to opioids in adulthood and may be a factor in subsequently transitioning to heroin use (Cerdá et al., 2015) has been the focus of a number of investigators using animal models. Mayer-Blackwell et al. (2014) studied self-administration of oxycodone by adolescent (28-day-old) and adult (78-day-old) C57BL/6 mice to determine whether there was a differential expression of genes in the dorsal striatum. Adolescent mice self-administered significantly less oxycodone than adult mice over a 14-day period, and there were more gene changes in the adolescent mice than in the adult mice. Adolescent mice had lower monoamine oxidase A mRNA levels. In addition to these changes, there were significant differences between adolescent and adult mice with regard to the gene encoding neuropeptide Y (Npy5r) where the levels of this mRNA were lower in the adolescent mice than in the adults. One other difference that was found in this study was that gene expression of gastrin-releasing peptide receptor (Grpr) was increased in mice that had self-administered oxycodone as adolescence, but this was decreased in the adults that also self-administered oxycodone. Even though the adolescent mice self-administered lower amounts of oxycodone than the adult mice, there were a larger number of genes altered in the adolescent group than in the adults, suggesting that the adolescent brain is more sensitive to oxycodone, changes that may persist into adulthood.
Adolescent exposure to oxycodone and its impact on subsequent behavior has also been addressed by Sanchez et al. (2016) who studied both early exposure to oxycodone as well as gene expression changes together with other potential behavioral consequences. In this study, adolescent C57Bl/6 male mice (postnatal day 28) received oxycodone (3.0 mg/kg/d), delivered through an osmotic minipump for 28 days, and then underwent a 28-day period of withdrawal when they were adults (postnatal day 84). Adult mice (postnatal day 56) were treated identically to the adolescents, and both groups were subsequently tested with morphine in the CPP procedure, as well as in assays to assess sensitization, anxiety, and depressive behaviors. In addition, this group also examined the expression of genes related to reward that included dopamine D1 and the dopamine transporter. Exposure to oxycodone during adolescence significantly increased the response to morphine in the CPP procedure during adulthood and also reduced the expression of D1 in the NAc and transporter expression in the ventral tegmental area. Exposure to oxycodone as adults did not have any effect on morphine-induced CPP, and for both groups there were no differences in behavioral assays with the exception of a significant reduction in corticosterone to the stress induced in the forced swim test for those mice that received oxycodone during adolescence. Although there was a significant decrease in D1 mRNA expression in the NAc and a reduced expression of the dopamine transporter in the ventral tegmental area for those mice treated with oxycodone in adolescence, these changes, as the authors of the publication point out, may be confounded with the changes in the developing brain where expression levels of D1 decline with age. Based on these results, however, it was concluded that adolescent exposure to oxycodone produced alteration in the mesolimbic pathway associated with opioid abuse that may contribute to the subsequent sensitivity in adulthood to the effects of morphine and that these effects are long-lasting. This finding was followed by Carpenter et al. (2021) who, using procedures similar to those of Sanchez et al. (2016), demonstrated that oxycodone exposure during adolescence produced long-lasting epigenetic modifications at key genes related to dopamine transmission.
Blackwood et al. (2019a) also studied neurobiological consequences of withdrawal from oxycodone under escalated (9-hour) and nonescalated (3-hour) oxycodone self-administration conditions. After 20 days of self-administration, both groups were withdrawn from oxycodone and over a 31-day period were tested for cue-induced reinstatement. One of the main findings of this study was that the long-assess group could be further differentiated into rats with high levels of oxycodone intake and rats that responded for lower amounts of oxycodone. Rats responding for higher amounts of oxycodone showed an increase in the expression of hippocampal μ and κ receptors, whereas there were no changes with δ receptor expression in any of the short- or long-access animals. The authors speculated that large doses of oxycodone may produce changes in hippocampal-dependent learning and memory processes that could also trigger psychiatric disorders in individuals addicted to opioids.
The possible role of μ-opioid receptor variants in the effects of oxycodone was studied by Collins et al. (2020) using both CPP and oxycodone self-administration, both procedures that examine the reinforcing effects of drugs and potential abuse liability. The mice were developed based on the knowledge that, in humans, the μ-opioid receptor gene (OPRM1) contains a SNP, A118G, which has been associated with opioid addiction risk. Collins et al. compared the effects of oxycodone in A112G male and female mice that possess a functionally analogous SNP in the mouse μ-opioid receptor gene (Oprm1). These effects were compared with mice homozygous for the A112 (wild-type; AA) or the G112 (GG) allele. Although there was no effect of genotype or sex in the CPP procedure, both male and female GG mice self-administered significantly more oxycodone compared with the wild-type AA littermates. The results of these experiments suggest that the G allele contributes to increased oxycodone intake and may be a factor in OUDs.
Blackwood et al. (2020) used a model of extended oxycodone self-administration access where rats received response-contingent foot shock, a procedure that resulted in two groups of rats: one being a “shock-sensitive” group that reduced responding for oxycodone and the other a “shock-resistant” group that continued to lever press to receive oxycodone. Differences between these two groups were also seen in the expression of immediate early genes where the shock-resistant rats showed increases in the prefrontal cortex of egr3, suggesting that this gene may play a role in the persistence of taking oxycodone under adverse consequences.
More recently, Beierle et al. (2022) reported the identification of a candidate gene, Zhx2, that appears to be involved in sex-specific sensitivity to the reinforcing effects of oxycodone (see Section VI.C, Sex Differences in Abuse Liability).
C. Summary
Several pharmacogenetic studies in both animals and humans have demonstrated that polymorphisms of drug-metabolizing enzymes, transporters, and receptors can significantly contribute to their expression and in the response to drugs. As Sadée and Dai (2005) commented nearly 20 years ago, pharmacogenomics has emerged as the harbinger of personalized medicine. Corresponding advances in pharmacometabolomics and systems pharmacology will undoubtedly aid in helping to resolve some of the many challenges facing progress in these areas as many diseases are unquestionably complex and a large number of factors that include age, sex, nutrition, as well as epigenetic differences contribute to the variability in an individual’s phenotype and response to a drug (Beger et al., 2016; Danhof, 2016).
Advances in the study of genetics have provided significant opportunities to probe potential genetic contributions to the risk of developing substance use disorders (SUDs), to the occurrence of adverse effects, as well as to individual therapeutic responses to the opioid management of pain. A better understanding of specific genes and gene variants can shed insight into the pharmacogenetics of SUDs and can aid in the development of personalized medicine for these challenging conditions (Crist et al., 2019; Sadée et al., 2023). Although studies of gene variants hold significant promise in the study of the pharmacogenetics of OUDs, at the present time there are mixed and/or equivocal results likely based on the substantial variability between cohorts due to the lack of statistical power in individual studies, methodological differences, or other factors in the genetic background of individuals that add to the confounding of results. As Crist et al. (2019) suggest, OUD research will need to move beyond the more common variants to explore other sources of variation that include gene-environment effects, gene-gene interactions, and epigenetic factors.
IV. Pain and Analgesia—Clinical Studies
A. Cancer Pain
In clinical studies, when administered orally, intramuscularly, or intravenously, oxycodone produces pain relief similar to or, in some cases, more effectively than that of other μ-opioid receptor agonists (Kalso and Vainio, 1990; Kalso et al., 1991; Pöyhiä et al., 1991, 1992; Heiskanen and Kalso, 1997; review by Ordóñez Gallego et al., 2007). One of the earlier clinical studies compared the analgesic effects of intramuscular oxymorphone, a metabolite of oxycodone, with morphine in patients with chronic cancer pain (Beaver et al., 1977. Using the intensity and duration of analgesia as a measure of the total analgesic effect, intramuscular oxymorphone was 8.7 times as potent as morphine and 13 times as potent in terms of its peak effect. When the duration and intensity of analgesia was assessed following oral administration, oxymorphone was one-sixth as potent as the intramuscular form with the peak effect only 1/14th as potent. Side effects of equianalgesic doses were qualitatively and quantitatively similar for oral and intramuscular morphine and for intramuscular oxymorphone and morphine. Beaver and colleagues also compared the analgesic effects of oral and intramuscular codeine with oral and intramuscular oxycodone in cancer patients (Beaver et al., 1978a). A companion publication to this study compared the analgesic effects of intramuscular oxycodone with intramuscular morphine and codeine (Beaver et al., 1978b). When oral oxycodone was compared with intramuscular oxycodone, oxycodone retained at least half its analgesic activity when administered orally, compared with morphine where the oral to intramuscular ratio was one-sixth as potent.
Additional studies have reported that high doses of oxycodone or controlled-release forms can effectively relieve pain in patients suffering from cancer-related pain (Heiskanen and Kalso, 1997; Watson and Babul, 1998; Gimbel et al., 2003; Watson et al., 2003; Bercovitch and Adunsky, 2006; Schmidt-Hansen et al., 2017). Bruera et al. (1998) studied controlled-release oxycodone and morphine in patients with cancer-related pain who were permitted to use escape analgesics as needed for pain control. Pain was well controlled by both oxycodone and morphine, but patients who received oxycodone consumed significantly more escape doses, and the mean pain intensities were significantly greater when oxycodone was administered after morphine. A few of the patients receiving morphine in the Heiskanen and Kalso (1997) study showed a tendency to have nightmares as well as in the Kalso and Vainio (1990) study, which reported hallucinations and delirium that were attenuated when patients were switched to oxycodone (Maddocks et al., 1996). Less nausea and pruritus and fewer hallucinations have been reported with oxycodone compared with morphine (Ordóñez et al., 2007).
Ong (2008) studied the effects of controlled-release oxycodone in 67 patients with moderate to severe neuropathic pain. There were 35 patients with neuropathic pain unrelated to malignancy and 32 patients with pain secondary to malignancy. The patients with nonmalignant causes included postherpetic and trigeminal neuralgia and radiculopathy, whereas the patients with malignant neuropathic pain predominantly included lung and breast cancer but also colorectal and cervical cancer. Baseline pain using the Visual Analog Scale in the nonmalignant group ranged between 8 and 10 at the initiation of the study, and, after 2 to 4 weeks of 25 mg/d of oxycodone, pain scores decreased to 1 to 2 to 10 in 94% of the patients. The average dose of oxycodone for the subgroup with neuropathic pain secondary to malignancy was 40 mg/d; the baseline score was 10, which improved to 2 to 4 on the follow-up after 2 to 4 weeks of treatment. Though a relatively small nonrandomized study, the results suggest that controlled release of oxycodone may be effective in this population of patients.
In a systematic review of randomized controlled trials on the effectiveness of opioids for the treatment of cancer pain, Koyyalagunta et al. (2012) concluded that there was fair evidence for the efficacy of transdermal fentanyl but, overall, poor evidence for morphine, tramadol, oxycodone, methadone, and codeine. However, there were differences in the number of trials with morphine (six) compared with oxycodone (one) and transdermal fentanyl (four). The conclusions are also limited because the studies included cancer pain with different etiologies and of different types, making it rather difficult to draw definitive conclusions about the relative efficacy of these compounds for cancer pain. The authors concluded that there is no concrete evidence for the effectiveness and safety of opioids in chronic cancer patients. However, when focusing on a more homogeneous population, a randomized controlled study comparing controlled-release forms of oral morphine (30mg/d) or oxycodone (20 mg/d) in pancreatic cancer pain found no difference between these drugs in terms of efficacy or in the occurrence of adverse effects (Mercadante et al. 2010).
A more recent review of oxycodone for cancer-related pain was published by Schmidt-Hansen et al., (2017) in the Cochrane database where the comparison was with morphine. The analysis evaluated 42 studies with more than 4,485 participants that included 3,945 analyzed for efficacy and 4,176 for safety. Constipation and hallucinations occurred less frequently with controlled-release oxycodone than with controlled-release morphine, but, overall, there was very little difference between oxycodone and morphine in the management of pain related to cancer. This conclusion was similar to that of Guo et al. (2018) who, through a meta-analysis, compared oxycodone with morphine for the treatment of patients with moderate and advanced cancer pain and reported no differences in analgesic efficacy or tolerability for the two drugs. Although some of the studies included in their analysis did not directly compare morphine and oxycodone, the authors propose the conduct of prospective, randomized clinical trials to directly compare these two drugs and to do so while also evaluating the treatment effects based on gene polymorphism analyses to more effectively provide the best treatment.
Although there are differences in the pharmacokinetics and analgesic effects with oxycodone when compared with morphine, depending on the route of administration, there appears to be relatively little difference between these two analgesics in the treatment of cancer pain either in terms of efficacy or adverse effects.
B. Neuropathic Pain
Injury to peripheral nerves, including chemotherapy-induced neuropathies and other diseases such as diabetic neuropathy, often lead to abnormal neuropathic pain states that include hyperalgesia, allodynia, and spontaneous pain, which frequently remain long after the injury heals or the initiating conditions have resolved. Although opioid agonists remain the gold standard for the treatment of moderate to severe nonneuropathic pain, they have been shown to have reduced efficacy against neuropathic pain (Zochodne and Max, 2003; Martínez-Navarro et al., 2019), Alles and Smith (2018) in a review of the etiology and pharmacology of neuropathic pain have commented that the “various manifestations of neuropathic pain are notoriously resistant to the actions of opioids and, in contrast to the noted efficacy of opioids in nociceptive pain, there is not a comparable degree of efficacy for the treatment of neuropathic pain” (see also Yekkirala et al., 2017). Alles and Smith comment further that “any pain that is opioid resistant is likely neuropathic pain.” Although a few studies have reported that high doses of oxycodone or controlled-release forms can effectively relieve neuropathic pain induced by post-herpetic neuralgia or diabetic neuropathy (Watson and Babul, 1998; Gimbel et al., 2003; Watson et al. 2003), the evidence for oxycodone efficacy, as well as other strong opioids, is low for these particular indications (McNicol et al., 2013; Derry et al., 2016; Gaskell et al., 2016; Cooper et al., 2017; Els et al., 2017). Clinical studies that have reported significant efficacy may be biased due to small sample sizes, the manner in which dropouts were handled, or the results based on a relatively brief treatment duration. The general conclusion is that there is equivocal and insufficient evidence to conclude that opioid treatments, including oxycodone, are effective in the management of neuropathic pain and that the risks outweigh the benefits.
C. Surgical Procedures
In one of the first studies comparing oxycodone with morphine, Kalso et al. (1991) conducted a randomized double-blind study that compared intravenous oxycodone and morphine following major abdominal surgery and found that significantly less oxycodone was required to control postoperative pain compared with morphine. Additionally, the first stage of pain relief was achieved faster for oxycodone than for morphine (28 minutes compared vs. 46 minutes) and lasted longer (39 minutes vs. 27 minutes). Although this study suggested a favorable analgesic effect for oxycodone, the fact that identical doses of oxycodone and morphine were used may be a limiting aspect for drawing any definitive conclusions. A subsequent study of patients undergoing breast reconstruction or major back surgery, where intravenous patient-controlled analgesia was used along with bolus doses of morphine (45 ug/kg) and oxycodone (30 ug/kg), the same amount of morphine and oxycodone was consumed, with no difference in the quality of analgesia or in the incidence of side effects (Silvasti et al., (1998). Backlund et al. (1997) compared the effects of epidural and intravenous oxycodone for pain with epidural morphine following abdominal surgery; Yanagidate and Dohi (2004) conducted a similar comparison following gynecologic surgery. Epidural administration of oxycodone resulted in poor analgesia compared with morphine suggesting that most of the analgesia with oxycodone is the result of systemic absorption (Lemberg et al., 2009). Pain relief at rest immediately after surgery was somewhat higher with morphine, compared with pain scores with either intravenous or epidural oxycodone. At 14 hours after surgery, and when coughing, pain scores were significantly lower in the oxycodone groups compared with morphine but at 17 hours pain scores while coughing were significantly higher in the intravenous oxycodone group than in either of the two groups receiving epidural oxycodone or morphine. There were no differences in all groups in the incidences of nausea or pruritus. Overall, this study concluded that there were no significant advantages of epidural oxycodone over that of morphine for the doses that were studied and no significant advantages of epidural oxycodone over that of intravenous routes of administration. Similar conclusions were made by Cuvillon et al. (2021) who found that intravenous oxycodone did not significantly reduce opioid-related side effects following total hip arthroplasty compared with morphine within the first 24 hours post-surgery.
Finally, in a review of 26 clinical trials of several surgical procedures including spine surgery, knee arthroplasty, caesarean section, cardiac surgery, bunionectomy, breast surgery, and laparoscopic colorectal surgery, when compared with intravenous opioids, oral oxycodone produced superior analgesia, provided comparable or better pain control, and reduced the demand for rescue medication (Cheung et al., 2017). This study also reported that patients receiving oxycodone experienced fewer opioid-related side effects than those on other opioids and had similar occurrences of postoperative nausea and vomiting as patients on placebo. However, as Cheung et al. point out, it is important to acknowledge that there are a limited number of randomized double-blind studies in individual surgical procedures as well as the exploration of few dose-ranging comparisons that make it difficult to draw definitive conclusions about the efficacy and side effects of oxycodone compared with morphine.
D. Sex Differences in Pain and Analgesia
There has been growing recognition over the past several years that there are significant male-female differences in the perception and response to pain, as well as in responses to pain therapeutics. Women experience more severe pain and have more chronic pain that is longer lasting than in men (Unruh, 1996; Riley et al., 1998; Fillingim and Gear 2004). Moreover, the prevalence of several common pain conditions such as fibromyalgia, migraine, chronic tension-type headache, and interstitial cystitis is greater in women than in men (Edwards et al., 2003). Mogil (2020) has provided a very comprehensive review of qualitative sex differences in pain, concluding that the processing of pain is “robustly sex dependent.” Bartley and Fillingim (2013) conclude in their review of sex differences in pain that both epidemiologic and clinical studies “demonstrate convincingly” that women are at substantially higher risk for many common pain conditions, commenting that multiple biopsychosocial factors contribute to sex differences in pain and to the variability in many of the findings. These include sex hormones, endogenous opioid function, genetic factors, and gender roles, all of which require further research to elucidate the mechanisms contributing to the sex differences in the response to pain and to its pharmacological treatment.
Opioids are known to show marked interindividual differences with respect to analgesia and unwanted side effects. Although some of these differences may be due to pharmacogenetic factors, sex is known to contribute to the effects of opioids with regard to the potency of opioid analgesia and in the prevalence of side effects that occur following opioid administration. Sex differences occur across the different opioid receptor subtypes (Berkley, 1997; Kest et al., 2000; Fillingim, 2002; Fillingim and Gear, 2004) and occur under several conditions where opioids are used or abused. Subsequent sections of this review, for example, include an overview of sex and gender differences in both animal and human abuse liability studies.
Despite the differences between males and female animals in response to pain and opioids, human studies do not appear to indicate greater opioid analgesia among females. Direct comparisons of the role of sex in the effects of oxycodone, as well as in other opioid receptors, are difficult to summarize as there are multiple variables underlying the contributions to any experimental study. Often it is not stated whether and how many women were included, making cross-comparisons challenging and limiting definitive conclusions. In a review of oxycodone and its use in the management of pain, Riley et al. (2008) concluded that “gender … [has] been shown to have no significant effects on the analgesic efficacy of oxycodone.” One experimental study of 10 women and 10 men, all sporadic prescription opioid users, found that intranasal oxycodone significantly decreased pain in the cold-water pressor test (Lofwall et al., 2012). Subjective measures of opioid liking and the estimated street value of oxycodone were also recorded, and a number of differences between females and males emerged. Females were more sensitive to oxycodone than males, vomited more frequently, and also gave higher ratings of street value and other abuse-related measures that included “opiate desire” for oxycodone. The relative paucity of data from humans on potential differences between females and males suggests that future studies should include an equivalent number of both sexes.
V. Pain and Analgesia—Preclinical Studies
Oxycodone has been evaluated in a variety of preclinical models of pain. Generally, oxycodone has shown potent antinociceptive effects in multiple analgesia assays including the mouse paraphenylquinone writhing, hot-plate, and tail-flick tests (Beardsley et al., 2004). Several studies have compared the analgesic effects of oxycodone with those of morphine. One of the earliest studies in rats using the tail-flick and hot-plate procedures showed that in both tests the subcutaneous and intraperitoneal administration of oxycodone was 2 to 4 times more potent than that of morphine, whereas, following intrathecal administration, morphine was 14 times more potent than oxycodone (Pöyhiä and Kalso, 1992). The antinociceptive effects, induction of catalepsy, and loss of reflexes produced by both oxycodone and morphine were reversed by administration of naloxone, suggesting a µ-mediated basis for the effects of both drugs. However, Pöyhiä and Kalso were puzzled by the finding that oxycodone was more effective than morphine following subcutaneous and intraperitoneal administration and speculated that oxycodone might be a partial μ/κ-agonist since intracerebroventricular administration of some κ-agonists produced antinociception. As indicated elsewhere in this review, this has been the subject of a number of studies, the majority of which attest to oxycodone’s specific pharmacology mediated by μ-opioid receptors. In models of inflammatory pain, the potency of oxycodone was increased in CFA-induced arthritis in male but not in female rats (Cook and Nickerson, 2005). Oxycodone was also shown to be more potent than morphine in the formalin-induced inflammation model (Meert and Vermeirsch, 2005) and showed potent antihyperalgesic effects in carrageenan-induced inflammation in rats (Lemberg et al., 2008).
Meert and Vermeirsch (2005) compared several different opioids for their antinociceptive effects using the tail-withdrawal test for acute thermal nociception and the formalin test for chemically induced inflammatory pain, with pain assessed using the von Frey method for mechanical hypersensitivity. These investigators also used a drug discrimination method to evaluate the discriminative stimulus properties associated with fentanyl. The opioids that were included in this study were morphine, fentanyl, buprenorphine, codeine, hydrocodone, and oxycodone; all were administered subcutaneously. Most drugs produced a dose-related increase in tail-withdrawal latencies. The effects with buprenorphine, however, differed from those of the other drugs in that the maximal effect, achieved at 2.5 mg/kg was the peak analgesic dose with all other higher doses from 10 to 80 mg/kg resulting in decreases from the 2.5 mg/kg dose. The onset of analgesia was fastest for fentanyl, and the order of potency (ED50 values) in the tail withdrawal was fentanyl > buprenorphine > morphine and hydrocodone > oxycodone > codeine.
Following intraplantar injections of formalin, all drugs decreased the number of flinches during phase I (the first 10 minutes following the pretreatment time). With buprenorphine, however, there was an initial decrease in the number of flinches at the lower doses (0.01–0.16 mg/kg) that was followed by increases in the number of flinches that, at the highest dose of 40 mg/kg, resulted in more flinches than in the control animals. Similar results were obtained in phase II (subsequent 40 minutes) with the lower doses of buprenorphine producing effects comparable to those of fentanyl, but, again increases in flinches occurred as the doses of buprenorphine were increased. The order of potency for the drugs administered in the intraplantar portion of the study following fentanyl and buprenorphine was oxycodone > morphine > hydrocodone > codeine. The differences in the potency of oxycodone and morphine in the inflammatory and thermal pain procedures likely reflect differences in pain modalities and differential sensitivity to the opioids used in these experiments.
In the drug discrimination procedure, Meert and Vermeirsch (2005) found that all drugs substituted for the training drug, fentanyl (0.04 mg/kg s.c.), showing that all compounds shared the discriminative stimulus effects and the pharmacological mechanism(s) mediated by the μ-opioid receptor. The drug discrimination procedure is typically an additional measure of potential abuse liability; the other is drug self-administration. It is of some interest that for oxycodone, in contrast to all the other compounds with the exception of buprenorphine, the ED50 for responding to the fentanyl stimulus was lower than that for analgesia, suggesting that animals were responding to the subjective effects of oxycodone prior to the achievement of the analgesic dose, which may translate to potential abuse liability. Buprenorphine’s “ceiling” effect, with higher doses producing a decrease in analgesia, may imply a safety margin for adverse effects.
In summary, there were differences between μ-opioid receptor compounds concerning relative potency and maximal analgesic effect with the type of pain influencing the results. Morphine, fentanyl, hydrocodone, and codeine had their highest potency in the tail-withdrawal procedure that is an assessment of acute pain. The formalin assay is considered to measure tonic pain and inflammation, and, in this procedure, buprenorphine, and oxycodone were more potent than the other compounds. Across the two types of analgesic tests, fentanyl was the most potent, followed by buprenorphine, oxycodone, morphine, hydrocodone, and codeine. These orders of potency are in agreement with data from the treatment of pain in humans (Reisine and Pasternak, 1996).
A. Neuropathic Pain
As mentioned previously, opioids are not very effective in alleviating neuropathic pain in humans. A number of studies using a variety of animal models of neuropathic pain have shown mixed results. It has been recognized for some time that systemically administered morphine has relatively low antinociceptive efficacy in animal models of neuropathic pain (Ossipov et al., 1995; Przewlocki and Przewlocka, 2001; Obara et al., 2004; Rashid et al., 2004), findings that have been supported in controlled clinical trials, suggesting that morphine lacks potent analgesic activity in relieving neuropathic pain (Arnér and Meyerson, 1988; Cooper et al., 2017; Martínez-Navarro, et al., 2018; see also Section IV.B). In the Rashid et al. study, using partial sciatic nerve-injured mice as a model of neuropathic pain and the Hargreaves thermal test for the assessment of morphine analgesia, the dose-response curves for subcutaneous and intrathecal administration of morphine were shifted to the right of the sham-operated group, whereas the dose-response curves for intracerebroventricular administration of morphine were comparable to those of the sham-operated mice. These findings of lower analgesic potency of systemically administered morphine suggested that the reduced effectiveness of morphine analgesia in neuropathic pain may be related to the loss of peripheral analgesia due to the decreased μ-opioid receptor expression in the dorsal root ganglion.
In one of the first studies to compare oxycodone and morphine in rodent models of neuropathic pain, Nielsen at al., (2007) reported the potential involvement of the κ-opioid receptor in the CCI model of neuropathic pain. Using (CCI) of the sciatic nerve as well as the streptozotocin (STZ)-induced diabetes model, these investigators studied intrathecal administration of oxycodone and morphine in the CCI animals and subcutaneous administration in the STZ animals. Oxycodone at a dose of 35 nmol i.t. produced significant antinociception as measured by the paw-withdrawal response to mechanical stimulation in both the ipsilateral and contralateral hind paws of the CCI animals. These effects of oxycodone were blocked by intrathecal pretreatment with nor-BNI, again suggesting the involvement of κ-opioid receptors in the analgesic effect of oxycodone when administered intrathecally and confirming earlier reports concerning κ-opioid receptor involvement in oxycodone analgesia. Oxycodone did not produce antinociception in nonoperated or in sham-operated rats. These results differed from those found with morphine at the same 35-nmol dose administered intrathecally in that morphine-produced significant antinociception in both sham-operated CCI animals and in nonoperated animals. In contrast to the effects of nor-BNI and oxycodone, the effects with morphine were not attenuated by nor-BNI but were blocked by intrathecal naloxone. The implication of κ-opioid receptor involvement in these studies was also supported by binding studies that demonstrated higher affinity of oxycodone for κ-opioid receptors and relatively low affinity for μ-opioid receptors.
The STZ-diabetic rats in the Nielsen et al. (2007) report were studied over a 24-week period where there were differences in the efficacy and potency of morphine and oxycodone in the attenuation of the paw withdrawal responses. Increasing doses of morphine and oxycodone were administered over the 24-week period and ranged from 2.0 mg/kg to 14.2 mg/kg of morphine and from 0.9 mg/kg to 9.0 mg/kg of oxycodone. Whereas the efficacy of morphine was reduced over this time period, starting at approximately 3 weeks and showing the progressive development of morphine hyposensitivity, oxycodone retained full efficacy over the 24 weeks of the STZ study period. The effects of μ- or κ-opioid antagonists were not examined in the STZ diabetic model. Taken together, these studies suggest that oxycodone and morphine produce their antinociceptive effects through different opioid receptors.
Somewhat similar results with oxycodone and morphine were reported by Nozaki et al. (2005) who also studied STZ-diabetic mice. Previous studies by this investigator and colleagues suggested that diabetic mice were selectively hyporesponsive to the antinociceptive effects of μ-opioid receptor drugs, but the nondiabetic control group did experience significant nociception. These studies also reported that the κ-opioid receptor agonist U-50, 488H produced antinociceptive effects in both diabetic and nondiabetic STZ mice (e.g., Kamei et al., 1992; Suzuki et al., 2001). In the Nozaki et al. (2005) studies, 5.0 mg/kg s.c. oxycodone produced a robust antinociceptive response in both diabetic and nondiabetic mice, assessed using the latency of a tail-flick response, whereas 5.0 mg/kg s.c. morphine did not inhibit tail-flick latencies in diabetic mice but did produce a significant antinociceptive effect in nondiabetic mice. The antinociceptive effects of oxycodone were antagonized by the μ-opioid receptor antagonist β-flunaltrexamine in both diabetic and nondiabetic mice. The κ-opioid receptor antagonist nor-BNI significantly reduced the antinociceptive effects of oxycodone in nondiabetic mice but abolished the peak and persistent effects of oxycodone in diabetic mice. The authors suggest that the antinociceptive effects of oxycodone are mediated by the μ- and κ-opioid receptors in diabetic mice and nondiabetic mice but that κ-opioid receptors appear to be strongly involved in the antinociceptive effects of oxycodone in nondiabetic mice. It is interesting that the diabetic condition, modeled by STZ, influences the antinociceptive effects of oxycodone and appears to recruit or diminish the activity of different opioid receptors.
An extensive series of studies using mouse pain models focused on a comparison of the effects of oxycodone with morphine and fentanyl and also examined potential differences in the mechanism of oxycodone from other opioids (Narita et al., 2008; Minami et al., 2009; Nakamura et al., 2013, 2014; Kanbara et al., 2014; Takasu et al., 2015). Narita et al. compared the pharmacological profiles of morphine and oxycodone in mice using a spinal nerve ligation(SNL) model of neuropathic pain and an inflammatory pain procedure induced by CFA. These investigators also examined [3H]DAMGO binding of morphine and oxycodone to mouse brain (without the cerebellum) and found that oxycodone binding was approximately 10-fold lower than that of morphine. In the radiant tail-flick procedure, 3.0 mg/kg s.c. of oxycodone attenuated the antinociceptive response and that was antagonized by the μ receptor antagonist β-FNA but not by NTI or nor-BNI, δ and κ receptor antagonists, respectively. Narita et al. also reported that in the sciatic nerve-ligated mice, morphine significantly decreased the antinociceptive tail-flick response, whereas oxycodone produced “profound antinociception in” these animals. These investigators also found that intrathecal and intracerebroventricular; morphine or oxycodone produced maximal antinociceptive effects comparable to those of sham-operated animals. When the effects of subcutaneous morphine or oxycodone were studied in the sciatic nerve-ligated mice in a CPP procedure, neither drug produced a place preference in the neuropathic pain-like state whereas in the sham animals, both morphine and oxycodone produced a preference for the drug associated place. The failure to find a preference for the compartment where the SNL animals achieved antinociceptive relief from pain with either morphine or oxycodone is somewhat surprising as the alleviation of pain should be reinforcing but this finding remains as an outstanding issue to be addressed.
Minami et al. (2009) studied morphine, oxycodone, and fentanyl in an SNL model of neuropathic pain with oxycodone showing the greatest efficacy. Although morphine and fentanyl also reversed the decreased withdrawal threshold, the doses that reversed this measure were close to or at the same doses that significantly affected withdrawal thresholds in the sham-treated group. All three drugs produced an antinociceptive effect on thermal nociception using the tail-flick procedure as well as on measures of paw withdrawal as assessed using von Frey mechanical stimulation. These investigators concluded that the three opioids have different efficacies in these pain models and that the distinctive analgesic profile of oxycodone differs from those of fentanyl and morphine, suggesting that oxycodone may possess a distinctive pharmacological profile for some types of neuropathic pain that are currently not well managed by more traditional opioids, a conclusion also reported in a previous study by Lemberg et al. (2006b). Other support for the efficacy of oxycodone in the neuropathic SNL model mirrors the clinical reports of oxycodone efficacy in painful diabetic neuropathy and in postherpetic neuralgia (Watson and Babul, 1998; Watson et al., 2003).
Minami et al. (2009) also examined whether morphine, oxycodone, or fentanyl produce different efficacy profiles in a femur bone cancer pain induced by the injection of mouse osteolytic NCTC 2472 tumor cells. Although all three of the opioids reversed guarding behavior (the lifting time of the hind paw on the ipsilateral side during ambulation), only oxycodone and fentanyl significantly reversed limb-use abnormality; morphine, even at high doses (50 mg/kg) did not restore limb use to normal levels.
Although there has been speculation that oxycodone’s unique pharmacological effects could be mediated through the κ-opioid receptor (Nielsen et al., 2007), in a preliminary study cited by Minami et al. (2009), the effects of all three opioids in the femur bone cancer pain model were completely antagonized by the μ-opioid receptor antagonist β-FNA but not by the κ-opioid receptor antagonist nor-BNI. Oxycodone and morphine were also studied in a model of femur bone cancer pain (Nakamura et al., 2013). Activation or attenuation of oxycodone and morphine in pain-related brain regions (e.g., periaqueductal gray, mediodorsal thalamus) was assessed through [35S]-GTPγS binding. The effects of oxycodone and morphine were differentially modulated in this model. Activation of the μ-opioid receptor was attenuated by oxycodone in brain regions related to pain signaling and compared with morphine was attenuated less. When administered intracerebroventricularly, the overall potency of oxycodone was stronger than that of morphine. Nakamura et al. conclude that modulation of μ-opioid receptor in bone cancer pain is one of the mechanisms that confers the unique analgesic profile of oxycodone, thereby contributing to its analgesic efficacy and control of pain.
Using a relatively new model of thermal pain and operant responding in squirrel monkeys, Kangas and Bergman (2014) and Leonard and Kangas (2020) studied the effects of oxycodone. In this procedure, the chair-restrained monkeys were trained to pull a thermode that was attached to a chain suspended from above. A pull on the thermode that lasted 3 seconds produced the delivery of sweetened condensed milk. The temperature of the thermode was initially 38°C, which was approximately body temperature. The temperature of the thermode increased by 2°C on successive trial blocks until 20 seconds elapsed without a response. Thermal thresholds for the six squirrel monkeys were determined and were the primary measure of nociception and drug effects. A maximum of 60°C was established to preclude contact with the thermode that might result in tissue damage. Oxycodone (0.003–0.1 mg/kg i.p.), studied against this baseline of thermal nociception, produced dose-related increases in thermal thresholds at doses of 0.01 and 0.03 mg/kg, with the highest dose of oxycodone abolishing responding in five of the six monkeys.
In summary, when studied in a variety of procedures involving neuropathic pain in experimental animals, oxycodone has been demonstrated to be effective in producing an antinociceptive effect. These findings of several positive effects obtained in preclinical models, particularly with morphine, stand in contrast to the lack of efficacy in humans suffering from neuropathic pain. Translational deficiencies or shortcomings are difficult to understand and address. Efforts to address this issue require close collaboration and interaction between preclinical and clinical researchers as well as the continued quest to discover and develop new chemical entities and mechanisms of action.
B. Mechanistic Studies of Oxycodone
In light of the differences observed between morphine and oxycodone, a number of studies have attempted to identify possible neuropharmacological dissimilarities between these two drugs, with most of these studies focusing on analgesia. As pointed out elsewhere in this review, although both morphine and oxycodone are both potent analgesics, they have different analgesic profiles that are separate and distinct from the studies described earlier focusing on the possible involvement of κ- and δ−opioid receptors mediating some of the effects of oxycodone. Despite the lower agonist affinity of oxycodone at μ-opioid receptors compared with morphine (Lemberg et al., 2006b; Narita et al., 2008), these two drugs show equivalent analgesic effects when administered subcutaneously with oxycodone on occasion showing more potent analgesic effects than morphine (Narita et al., 2008). Some of the differences between the in vitro and in vivo profiling of oxycodone and morphine may be related to differences in blood-brain barrier transport between the two drugs where a sixfold difference was found in the concentration of oxycodone in the rat brain compared with that of morphine (Boström et al., 2008). The differences in transport through the blood-brain-barrier, the different effects in potency and activity of oxycodone when administered systemically, and the similar potency of these two drugs when administered intracerebroventricularly, suggest that the mechanisms underlying the supraspinal and systemic antinociceptive effects of morphine and oxycodone differ. Certain studies, described later, have been conducted in an effort to explore other pharmacological variables that might help to account for some of the differences between these two analgesics.
Nakamura et al. (2014) investigated possible mechanisms involved in the in vivo antinociceptive effects of oxycodone at supraspinal sites, examining whether inhibition of KIR3 channels, known to play a role in mediating the effects of morphine at the spinal level (Marker et al., 2002, 2004), might account for the effects of oxycodone at supraspinal sites. Antinociceptive effects in the tail-flick test were examined in C57BL/6 male mice following intracerebroventricular or intrathecal morphine and oxycodone. KIR3.1 siRNA knockdown mice were also studied. Both morphine and oxycodone, administered intracerebroventricularly. produced comparable effects, with similar ED50 and ED80 values and with a similar time course for onset of maximal antinociceptive effects. Following the intracerebroventricular administration of the KIR3 channel blocker tertiapin-Q, the antinociceptive effects of oxycodone were markedly attenuated, whereas the effects of morphine were not, suggesting a difference in the antinociceptive mechanisms of morphine and oxycodone at supraspinal sites with oxycodone’s effects mediated by tertiapin-Q. The oxycodone dose-response curve was shifted markedly to the right in a dose-dependent manner in the presence of tertiapin-Q, whereas the effects of morphine and tertiapin-Q showed only a small difference that was not dose related. When these effects were examined following intrathecal administration, tertiapin-Q produced marked shifts to the right for both oxycodone and morphine indicating that at spinal sites, the antinociceptive effects of both morphine and oxycodone involve a tertiapin-Q sensitive mechanism.
When this same approach was used to study chronic pain in a bone cancer model and neuropathic pain in an SNL mouse model, both oxycodone and morphine, subcutaneous, produced comparable analgesic effects assessed using mechanical stimulation paw withdrawal. When oxycodone and morphine were given together with tertiapin-Q, the antinociceptive effects of oxycodone were attenuated, whereas there was no effect with tertiapin-Q and morphine. These results provide very good evidence that, in addition to the differences in effects of oxycodone and morphine, depending on the route of administration, the effects of oxycodone in both acute and chronic pain are mediated by different signaling mechanisms with KIR3 channels playing an important role in the effects of oxycodone but not those of morphine.
Bone cancer pain was also studied by Takasu et al. (2015) who reported another difference between morphine and oxycodone in this model. KIR3.1channels are known to be activated following the binding of opioid agonists to μ-opioid receptors (Marker et al., 2004). Takasu et al. repeated the finding described earlier (Nakamura et al., 2014) with tertiapin-Q showing that KIR3.1 channels are critical for the supraspinal antinociceptive effects of oxycodone in the bone cancer pain model but not those of morphine. Takasu et al. went on to demonstrate in coronal slices from the bone cancer pain model that GABAergic synaptic transmission in the ventrolateral periaqueductal gray neurons was enhanced. Oxycodone reduced the inhibition of presynaptic GABA release, but morphine did not. Takasu et al. concluded that the enhanced GABAergic synaptic transmission at ventrolateral periaqueductal gray neurons in bone cancer pain is an important site of supraspinal antinociception by oxycodone mediated via KIR3.1 channel activation.
In addition to the importance of KIR3.1 channels contributing to the different effects of oxycodone and morphine, it has also been shown that the regulator of G-protein signaling RGS9-2, a brain specific splice variant of the RGS9 gene, modulates responses to oxycodone in both pain free states and in chronic neuropathic pain (Gaspari et al., 2017). Previous studies had shown that RGS9-2 is highly enriched in the Nac and dorsal striatum and is expressed at lower levels in the periaqueductal gray and spinal cord, regions known to be involved in the effects of morphine (Zachariou et al., (2003). In studies using morphine, RGS9-2 has been characterized as a “negative modulator” of μ-opioid receptor signal transduction as well as interacting with dopamine signaling pathways, regulating a variety of μ-opioid receptor mediated effects including analgesia, tolerance, and reward (Zachariou et al., 2003; Psifogeorgou et al., 2007, 2011; Traynor et al., 2009; Xie et al., 2012; Gaspari et al., 2014). In the Zachariou et al. (2003) study, acute administration of 15 mg/kg s.c. morphine increased levels of RGS9-2 approximately 50% in the Nac and spinal cord of C57BL/6 mice, whereas chronic morphine (6 days of subcutaneous administration of morphine via an implantation of 25 mg morphine pellets on days 1 and 3) decreased RGS9-2 levels, also by approximately 50% in these brain regions. Mice that have had RGS9 deleted compared with the wild-type mice showed enhanced behavioral responses to both acute and chronic morphine that included increases in analgesia in the tail-flick procedure, physical dependence, and withdrawal, as well as increases in reward across a broad dose range using CPP, findings that suggested that RGS9-2 is critical in regulating behavioral responses to opioids.
Based on subsequent studies with oxycodone, Gaspari et al. (2017) suggest RGS9-2 is a “positive modulator” of oxycodone reward in both pain-free states and in neuropathic pain. Acute administration of oxycodone did not affect the antinociceptive activity in mice lacking the RGS9-2 gene when tested using the hot plate. Additionally, RGS9-2 protected against the development of analgesic tolerance to oxycodone in models of both acute and chronic pain. The knockout mice were also less sensitive to the rewarding effects of oxycodone in the CPP procedure. Longer term treatment with oxycodone resulted in decreases in the antiallodynic effects in the spinal nerve injury model of neuropathic pain. Overall, these studies indicate that RGS9-2 plays an important role in the pharmacological effects of μ-receptor opioids, that it can act as a positive or a negative modulator of opioid action, and that although oxycodone and morphine produce comparable behavioral and pharmacological effects, RGS9-2 modulates the actions of oxycodone differently than that of morphine and does so through different mechanisms.
Further differences between morphine and oxycodone have also been reported by Vander Weele et al. (2014). Using fast-scan cyclic voltammetry and microdialysis coupled to HPLC-tandem mass spectrometry, these investigators measured rapid dopamine transmission along with changes in GABA, glutamate, monoamines, and their metabolites following intravenous delivery of either oxycodone or morphine. Both morphine and oxycodone increased the release of dopamine from the Nac, but the patterning of release was dramatically different. Oxycodone produced a robust and stable increase in dopamine concentration, whereas morphine produced a brief increase in dopamine that was coincident with a surge in GABA. These patterning and differential effects of oxycodone and morphine on dopamine and on other neurotransmitters in the brain may account for some of the differences in the subjective effects of these two drugs that warrant further investigation to include other opioids and different outcomes following longer term administration.
C. Sex Differences
Vacca et al. (2014) performed a comprehensive analysis of sex-related differences in pain perception and recovery from neuropathic pain in female and male CD1 mice. Neuropathic pain was induced using the CCI model and the mechanical threshold procedure to evaluate nociception. These investigators found that male mice showed a gradual decrease in CCI-induced allodynia that completely recovered 81 days after the nerve ligation procedure. Female mice, however, were still allodynic 121 days after the CCI surgery, demonstrating a slower regenerative process compared with males. Sex-related differences were also found using proteomic analyses of proteins associated with nerve regeneration. Vacca et al. point out that although sex differences in the response to pain in humans can be influenced by sociocultural and experiential factors, study of the neurobiological differences contributing to differences in pain sensation and recovery from neuropathic pain where these factors do not play a role may yield insight into novel mechanisms and new therapeutic approaches to treatment.
Studies that have examined the effects of μ-opioid receptor agonists on nociception in male and female rats have shown that male rats are more sensitive to the antinociceptive effects of morphine than female rats. The differences in antinociception appear to be independent of estrus cycle (Peckham et al., 2005) and to the particular strain of rat, including the Sprague-Dawley, F-344, and Lewis rats that have been studied in a variety of antinociceptive assays including warm-water tail withdrawal, the hot-plate assay, an d the abdominal constriction test using acetic acid injected intraperitoneally (Cicero et al., 1996, 1997; Bartok and Craft 1997; Cook et al., 2000; Kest et al., 2000; Peckham et al., 2005). The presence of a sex difference in antinociceptive responsiveness was also not related to drug potency, efficacy, or affinity, suggesting that sex differences in antinociception were related to differential opioid metabolism.
Peckham and Traynor (2006) examined whether differences in the structure-activity of compounds could account for whether μ-opioids would show a difference in antinociceptive responses between female and male rats. Sprague-Dawley rats were studied using the warm water tail withdrawal assay. Morphine, administered subcutaneously, was found to be more potent in males compared with females, with ED50 values of 2.17 and 6.08 mg/kg for males and females, respectively. There was no difference in the rank order of potency of the compounds across male and female rats for the different compounds that included (in order of potency) fentanyl, oxymorphone, hydromorphone, heroin, methadone, oxycodone, morphine, hydrocodone, and codeine. This study found no observable sex difference in the antinociceptive potency of oxycodone, heroin, methadone, or fentanyl, indicating that the difference between the male and female rats was specific to the compound.
Holtman and Wala (2006) studied the effects of oxycodone (0.25–4.0 mg/kg, i.p.) in male and female Sprague-Dawley rats using the tail-flick response to radiant heat. An enhanced sensitivity to noxious stimuli (hyperalgesia) was noted at low doses of oxycodone (0.25–1.0 mg/kg) at the later time points (90 to 120 minutes) in male but not female subjects. Female rats in this study had a greater antinociceptive response to oxycodone compared with male rats with the dose-response curves for the female rats shifted to the left of the males. The potency of oxycodone was approximately twofold greater in female that in male rats with ED50 values of 0.63 and 1.46 mg/kg, respectively. These results differ from those found with morphine that have shown male rats are more sensitive than females and have a greater antinociceptive effect, findings that differ from other studies, including that of Peckham and Traynor (2006), described earlier, where the ED50 for oxycodone was similar for males and females. Holtman and Wala speculate that their data with oxycodone appear to support work described previously suggesting that the antinociceptive effect of oxycodone is mediated to some degree by κ-opioid receptors and that sex differences in opioid antinociceptive effects may depend upon the receptor at which they act.
Chan et al. (2008) reported a number of significant sex-related differences in the pharmacokinetics and metabolism of oxycodone in Sprague-Dawley rats. The clearance of intravenous oxycodone was significantly higher in male than in female rats, but the systemic exposure to oxycodone was greater in female compared with male rats. Chan et al. (2008) suggested that the higher systemic exposure in female rats, compared with males, may account for the more potent effect of oxycodone in the Holtman and Wala (2006) studies. The oral bioavailability of oxycodone was low in both sexes at 1.2% and 5.0% of male and female rats, respectively, in contrast to the 60% to 87% bioavailability in humans following oral administration (Pöyhiä et al., 1992). The bioavailability of oxycodone following subcutaneous administration was found to be approximately 57% in male Sprague-Dawley rats. Chan et al. suggest that intestinal absorption of oxycodone is likely rapid and complete and that first-pass metabolism following oral administration is more extensive in the rat than in the human. These findings provide a cautionary note about the oral route of administration of oxycodone in rodent studies of antinociception, which may be a poor model of the human for studying PD effects.
Acknowledging that sex differences in opioid analgesia occur both in humans and rodents, Arguelles et al. (2021) examined sex differences and the role of the estrous cycle in analgesia. These investigators also examined sex and cycle differences in brain and plasma oxycodone levels along with metabolites. Females in diestrus achieved higher levels of analgesia, assessed using a thermal stimulus and tail-flick latency, compared with males and females in estrus. Microdialysis measures of oxycodone brain levels in females in diestrus correlated with analgesia whereas brain levels of oxymorphone or noroxycodone and plasma blood or metabolite levels did not. Increases in brain oxycodone levels were increased following the administration of the CYP2D inhibitor propranolol in males and females in estrus but did not affect females in diestrus. Arguelles et al. conclude that sex and estrous cycle influence oxycodone-induced analgesia and brain levels of oxycodone, likely through the regulation of CYD2D metabolism of oxycodone and, insofar as CYP2D6 is expressed in the human brain, sex and cycle stage may influence analgesia in humans.
In summary, not all μ-opioid receptor agonists show potency or sensitivity differences between male and female rats, nor do these sex differences necessarily apply to other opioid receptor drugs acting at κ- or δ-opioid receptors (Craft, 2003). There still seems to be a number of inconsistencies and ambiguities in the literature with regard to sex differences in pain and analgesia that warrant further study. Although the sex-specific data with morphine in rodents appears relatively clear with regard to morphine, as Peckham and Traynor (2006) point out, not all opioid analgesics are the same. Bartok and Craft (1997) made the point that methodological differences make contribute to the variability in findings and emphasize the importance of time- and dose-effect relationships when investigating the contribution of sex, particularly in studies of nociception. Recently, Gabel et al. (2023) suggested that some of the sex differences with morphine may be related to metabolism differences in the CNS. These differences may also play a role with oxycodone and may shed some light on the differences in analgesic efficacy of morphine versus oxycodone depending on the route of administration where the role of the κ-opioid receptor has been implicated.
VI. Psychopharmacology and Human Subjective Effects of Oxycodone
A. Early Studies
A few early clinical and experimental studies set the framework for subsequently examining in more detail the effects of opioids in human subjects. The predominant focus has been on evaluating the subjective effects of drugs using self-scoring questionnaires. Initial studies were conducted by Lasagna et al. (1955) at Harvard Medical School in concert with studies performed at the U.S. Public Health Service Hospital in in Lexington, Kentucky. Three groups of subjects were evaluated for their responses to amphetamine, pentobarbital, heroin, morphine, or placebo with all drugs administered subcutaneously with the exception of pentobarbital sodium, which was given intravenously. The three groups were normal healthy male subjects (n = 20, ages from 21–27), patients (n = 30, ages 45–87) with a chronic disease (malignancies, neurologic disorders), and “post addicts” (n = 30, ages not specified), who were “incurable addicts” with demonstrated “recidivist tendencies”; none of the subjects in this group had been more than a week without a narcotic, with some having used a narcotic as recently as 2 days prior to participation in the study. Although oxycodone was not studied, there were several striking findings that are directly relevant to the design and execution of studies with oxycodone that followed, as well as shedding interesting information on the different drugs that were used in this study. First, the subjective effects of the drugs differed across the groups that were studied. For the normal healthy volunteers, amphetamine generally was considered the most pleasant drug of the five received. This group typically responded to heroin and morphine with dysphoria, reporting that these drugs were predominantly unpleasant, although the effects appeared to be somewhat dose related; pentobarbital yielded a mixed response. The rank ordering of mood scores for the normal healthy volunteers ranked heroin and morphine as dysphoric, without any euphoria, whereas amphetamine was considered euphoric without any dysphoria. The responses of the patients to these drugs were generally mixed with many experiencing pain relief with heroin, morphine, and even amphetamine. The post addicts reported that all of the drugs produced euphoria (pentobarbital was not studied in this group), with morphine producing the highest level of euphoria, and, in contrast to the effects of opioids in normal volunteers and patients, the post addicts reported virtually no dysphoria.
Lasagna et al. (1955) made several cogent points that, still today, are frequently overlooked and reflect a failure to understand that abused drugs do not have uniform effects across individuals. For example, Lasagna et al. pointed out that there was a strong tendency to describe the CNS effects of a drug like morphine in oversimplified terms and with sweeping generalizations as if morphine “produced a certain set of effects that were evident in all persons at all times” (p. 1016). Further, they pointed out that the subjective effects of drugs can be dependent on the situation in which the drug is administered—i.e., the “context,” results that have been found with a variety of abused drugs studied in nonhuman primates (Barrett, 1985; Nader et al., 1992).
A number of studies have examined the effects of oxycodone in human subjects to examine the potential relative abuse liability of oxycodone compared with other opioids and to more fully assess oxycodone’s subjective effects. Some of these experimental studies have been conducted with non-drug-abusing volunteers, whereas others were done in drug-using volunteers (see next section). These studies have provided informative insights to aid in developing a better understanding of the subjective effects of oxycodone.
An early preliminary study was conducted at the Addiction Research Center in Lexington Kentucky, in six subjects with a history of opioid use but who were not physically dependent at the time the study was conducted (Martin et al., 1966). The subjects were administered single doses of oxycodone (25 or 50 mg s.c., and 15 or 30 mg i.v) or morphine (12.5 and 25 mg s.c.). Substitution studies with oxycodone and morphine were also conducted with eight opioid-dependent subjects to evaluate whether these two drugs could suppress signs of abstinence. Although preliminary, these experiments, which used a quantitative “attitude” questionnaire for evaluating opioid drugs (Fraser et al., 1961), demonstrated that oxycodone was slightly more potent than morphine in producing subjective effects (e.g., “liking,” “feel drug”); oxycodone also was effective in suppressing signs of abstinence (withdrawal signs and symptoms from morphine).
B. Studies in Non-Opioid-Abusing and Nondependent Opioid Users
A more detailed series of studies with larger numbers of subjects was initiated by Zacny and colleagues who characterized the subjective (psychopharmacological), psychomotor, and physiologic effects of oral oxycodone in non-drug-abusing volunteers (Zacny and Gutierrez, 2003, 2009; Zacny and Lichtor, 2008). These studies were also conducted to evaluate the role of alcohol drinking and sex, as well as to determine the effects of oxycodone on individuals with generalized anxiety disorder (GAD) (Zacny and Drum, 2010; Zacny et al., 2011). The examination of oxycodone in non-drug-abusing individuals was unique in light of the fact that many patients are administered oxycodone for its therapeutic effects, without prior experience with oxycodone, and a study in this population of “naïve” individuals could provide information on abuse liability that might lead to abuse.
Zacny and Gutierrez (2003) and Zacny and Lichtor (2008) examined the effects of oxycodone on psychomotor and cognitive performance, comparing the effects of oxycodone with those of morphine. Zacny and Gutierrez (2003) also included the benzodiazepine lorazepam, to validate measures of performance and impairment. In general, oxycodone produced a profile of psychopharmacological and physiologic effects that were consistent with those of other μ-opioid receptor agonists. Some effects were observed on the psychomotor and cognitive assessment with higher doses of oxycodone, but these did not approach the level of effects seen with lorazepam. On measures of euphoria, believed to be related to potential abuse liability, oxycodone produced an increase in this measure that was not observed with the 40-mg dose of morphine. However, after the end of the experimental session, oxycodone also produced unpleasant effects that included ratings of “feel bad,” along with headache and nausea, results that were also obtained with other μ-receptor opioid agonists in non-drug-abusing volunteers. Zacny and Lichtor (2008) concluded that an oxycodone dose of 20 mg had more abuse liability-related effects and fewer aversive effects than a morphine dose of 60 mg. Intravenous oxycodone was also studied alone and with naltrexone in recreational opioid users. The combination was found to produce high “drug liking scores” together with higher scores on using it again, along with reported “highs,” relative to the combination of oxycodone and naltrexone (Backonja et al., 2016).
Zacny and Gutierrez (2009) point out that, in the studies they performed, there were individual differences in the degree to which non-drug-abusing volunteers report liking or disliking the effects of opioids, making it difficult to make a general statement about the abuse liability of opioids in the population of subjects included in their studies. These subjects were physically healthy volunteers with a history of recreational drug use but without a history of substance use-related or psychiatric disorders. They acknowledge that a “worthwhile research endeavor would be to identify variables, either organismic or environmental, that modulate the abuse liability related effects of prescription opioids in this population as there may be risk factors for non-medical use.”
One of the first studies to examine this possibility was an investigation of the contribution of alcohol drinking and gender to the subjective and other effects of 10 and 20 mg of immediate-release oxycodone (Zacny and Drum, 2010). Light (n = 15, with 8 males and 7 females) and moderate (n = 8 males and 6 females) alcohol drinkers with some level of current recreational drug use were studied. There were differences in recreational drug use between the light and moderate drinkers with the moderate drinkers reporting a higher lifetime use of stimulants, marijuana, and hallucinogens than the light drinkers. Alcohol drinking levels prior to the study did not modulate the subjective, reinforcing, and abuse-liability-related effects of oxycodone, nor did those effects differ between male and female participants. Females reported larger and more dysphoric effects following the administration of oxycodone.
A further study attempting to assess whether there were other potential indicators of opioid use/abuse was directed toward the question of whether volunteers with GAD responded differently to oxycodone (Zacny et al., 2011). Epidemiologic studies have suggested that individuals with GAD and other psychopathologies are associated with an increased vulnerability to nonmedical prescription opioid use and are more likely to develop opioid dependence (Martins et al., 2009a,b). Zacny et al. (2011) refer to a number of studies suggesting that some opioid users report the use of opioids for tension and anxiety reduction, comments that are supported by Martins et al. (2009a,b) who reported that OxyContin use was associated with a higher level of mental health problems, including anxiety. More recently, Bruijnzeel et al. (2022) reported that oxycodone decreased anxiety-like behavior measured in the elevated plus-maze, with male Sprague-Dawley rats showing a greater anxiolytic-like effect than females.
The possibility that oxycodone may have different effects on individuals with GAD was examined experimentally to evaluate whether volunteers with GAD would report greater reinforcing effects and drug liking than those without GAD. However, despite the fact that the subjects with GAD had significantly higher scores than the control subjects on several measures of anxiety that included not only anxiety but also obsessive-compulsive measures, depression, psychoticism and on the overall Global Severity Index, there were no differences (other than in dysphoria, which was higher in the control subjects) in the response to 10 or 20 mg of oxycodone between those individuals with GAD and control subjects. Zacny et al. (2011) acknowledge that one of the fundamental central tenants of behavioral pharmacology is the importance of the context in which a drug is administered as a determinant of the response to a drug and suggest that one contextual factor that might have contributed to the results is that participants in the GAD group did not report feeling anxious during the experimental session any more than did the control subjects (see also Lasagna et al., 1955 and previous comments on the role of context in SUDs).
The effects of repeated administration of oxycodone on its subjective and analgesic effects were studied in 10 (7 men and 3 women) normal healthy volunteers with no reported history of drug dependence or current drug use (Cooper et al., 2012). This study examined two different dosing regimens to determine if tolerance developed to the analgesic, subjective, and physiologic effects of oxycodone. The participants, aged 21 to 55, had to have taken opioids at least twice previously for medical purposes but had no history of recreational use of opioids. The study consisted of two separate 5-day phases. During one phase, oxycodone was administered daily, whereas in the other phase, dosing was intermittent, occurring on days 1 and 5 with placebo administered on days 2 to 4. On the first and fifth day, all participants received cumulative oral doses of 0, 5, and 20 mg/70kg; on days 2 to 4 participants in the daily dosing phase received 15 mg, twice daily, also orally. Analgesia was assessed using the cold-water pressor test and subjective effects measured by the McGill Pain Questionnaire, a drug effects questionnaire, and a visual analog scale to assess a variety of mood and physiologic states. When oxycodone was administered daily, tolerance did not develop to the analgesic effects, although tolerance did develop to some of the participant’s ratings of positive subjective effects. Under the intermittent dosing schedule, both the analgesic and positive subjective effects were greater on day 5 compared with day 1 of the dosing schedule, suggesting that the schedule and the frequency of oxycodone administration can impact both the analgesic and subjective effects of oxycodone. Cooper et al. also point out that, though the data were obtained under limited conditions, tolerance may not develop to the analgesic effects when oxycodone is given under a relatively brief period of administration and the decline in positive subjective effects may be beneficial with regard to abuse liability. Finally, the increase in analgesic effects under the intermittent dosing regimen suggests that this might be a beneficial dosing regimen, with the caveat that it may also increase the subjective effects.
Stoops et al. (2010) compared the effects of intravenous oxycodone, hydrocodone, and morphine in recreational opioid users with histories of intravenous opioid use. Generally, there were no significant differences in the physiologic, subjective, and performance effects of these three drugs at any of the doses studied (5, 10, and 20 mg i.v.). The time course of the physiologic effects of the drugs, including respiratory changes and decreases in pupil diameter for oxycodone and morphine, were similar and typically lasted approximately 6 hours, whereas these effects with hydrocodone lasted only about 2 hours. The subjective effects (i.e., liking scores, good effects) dissipated quickly, within 30 minutes following dosing.
The subjective, reinforcing, and analgesic effects of oxycodone doses (10–60 mg/70 kg by mouth) were examined in opioid-dependent individuals with chronic, nonmalignant pain who were also maintained on sublingual buprenorphine and naloxone (Jones et al., 2011). Painful medical conditions included accident-related injuries, osteoarthritis or osteoporosis, scoliosis, and spinal stenosis, among other conditions, and the mean duration for using daily opioids was 43.6 months. The buprenorphine/naloxone combination did attenuate the pain symptoms. When given in addition to buprenorphine/naloxone, oxycodone also attenuated experimentally induced pain as well as clinical pain, with minimal aversive effects but with a number of positive subjective effects. Of interest, although oxycodone produced increases in measures such as “feeling high,” the magnitude of these effects was diminished relative to other studies (e.g., Zacny and Gutierrez, 2003; 3009). Oxycodone under these experimental conditions did not produce effects that are typically related to abuse liability such as “drug liking” and “would take again.” Jones et al. (2011) interpreted these results as suggesting that this population may not be taking chronic opioids for recreational purposes. Finally, oxycodone did not function as a reinforcer in this study, as measured by a choice procedure for either oxycodone or $20, suggesting that the buprenorphine/naloxone dosing regimen was responsible for the differences in results between prescription opioid-abusing pain patients and those using other opioids such as heroin. An additional conclusion of this study was that the ability of the buprenorphine/naloxone combination to reduce the subjective effects of oxycodone while maintaining analgesic efficacy suggests that sublingual buprenorphine may have utility as an opioid abuse treatment as well as a pain management tool with the caveat that it may be necessary to use additional opioids to address breakthrough pain.
C. Studies in Opioid Drug Abusing Volunteers—Pain, Comorbidities, and Drug History
Relatively few nonepidemiologic studies have been pursued that examine potential variables that contribute to the use and abuse of opioids. In addition to those studies described previously that evaluated variables in non-drug abusers that potentially contribute to opioid use and abuse, other studies have suggested that drug use history and pain may modulate the reinforcing and subjective effects of opioids. Several studies have demonstrated in both animals (Colpaert et al., 1982; Dib and Duclaux, 1982; Shaham et al., 1992, 1993; Shaham and Stewart, 1994) and humans that a number of variables including the presence or absence of pain, drug history, and stress can influence the subjective and reinforcing effects, as well as some of the other effects, such as respiration (Borgbjerg et al., 1996). Pharmacological and behavioral history have been shown in squirrel monkeys to dramatically alter the behavioral effects of a number of abused drugs, including morphine, chlordiazepoxide, and amphetamine (McKearney and Barrett, 1975, 1978; Barrett and Stanley, 1983; Glowa and Barrett, 1983; Barrett, 1992). At this point, little is known about the underlying mechanisms associated with these dramatic changes whereby the pharmacological and behavioral history dramatically modify the behavioral effects of these abused drugs.
Walsh et al. (2008) examined the relative abuse liability of oral oxycodone, hydrocodone, and hydromorphone in individuals that sporadically used prescription opioids recreationally. Of the 9 subjects studied (8 male and 1 female), there were no reported differences in the three drugs, including the subjective effects, with all three producing a profile of pharmacodynamic effects characteristic of μ-opioid receptor agonists.
The abuse liability or reinforcing effects, as well as the subjective physiologic and performance effects of the prescription opioids, oxycodone, fentanyl, buprenorphine, morphine, and heroin, were evaluated in eight heroin-dependent users maintained on orally administered morphine (Comer et al., 2008). Evaluation of the drugs was assessed following intravenous administration. A key finding of this extensive study was that the abuse liability of oxycodone appeared to be substantial. Oxycodone produced robust reinforcing effects, comparable to those of morphine and heroin, and also produced some of the most robust increases in positive subjective ratings that, unlike the other drugs studied, were without increases in ratings of “bad drug effects.” Comer et al. (2008) commented that heroin-dependent individuals reported oxycodone was the “Rolls Royce” of opioids because it produces a “smooth” high and that its pharmacological profile, coupled to the ready availability, may contribute to the high prevalence of abuse.
Several studies have demonstrated that patients experiencing postoperative pain readily administer opioids using patient-controlled self-administration. Higher pain levels, together with heightened anxiety and less social support, correlated with the amount of the opioid being self-administered (Gil et al., 1990; Hudcova et al., 2006). The presence of pain also has been shown to increase the intravenous self-administration of fentanyl in healthy non-drug-using volunteers undergoing experimentally induced pain induced by cold-water immersion of the forearms (Zacny et al., 1996). Experiments in rats conducted by Colpaert and colleagues (Colpaert et al., 1982, 2001) using an adjuvant arthritis model of chronic pain induced by inoculation with the arthritogenic Mycobacterium butyricum found that the oral intake of a fentanyl solution was higher in arthritic than in nonarthritic control rats. Several other studies have shown an increase in operant intravenous opioid self-administration and a sigma-1 antagonist following neuropathic pain induced by partial ligation of the sciatic nerve or by spinal nerve ligation (Martin et al., 2007; Martin and Ewan, 2008; Bura et al., 2013; Wade and Fairbanks, 2014). Some of these studies also reported modulation of the subjective (humans) or anhedonic (rats) effects associated with pain reduction through self-administration (Zacny et al., 1996; Bura et al., 2013).
Pain has been shown in several other studies to modulate the subjective and reinforcing effects of opioids in both normal human volunteers and in those with opioid abuse histories (Wolff et al., 1940; Zacny et al., 1996; Conley et al., 1997; Comer et al., 2010). Comer et al. (2010) evaluated oxycodone abuse liability in prescription opioid abusers as a function of pain and drug use history to determine if pain and a drug use history would alter the subjective effects of oxycodone. Two groups of healthy volunteers were studied, with one group (n = 9) that was abusing prescription opioids and a second group (n = 9) that had used prescription opioids medically but did not abuse them. Experimental pain was induced using the cold-water (4°C) immersion and the warm-water (37°C) procedure. The results were quite striking with oxycodone producing similar subjective effects in prescription drug abusers and non-drug users. The main difference between the two groups was that the non-drug-abusing subjects only self-administered oxycodone when in pain, whereas those subjects that were opioid abusers self-administered oxycodone regardless of the pain condition.
The relationship between the rate of oxycodone infusion on the subjective and reinforcing strength of oxycodone was studied in 12 heroin-dependent volunteers (Comer et al., 2009). Intravenous infusion rates over intervals that started at 2 minutes and ranged further from 15 to 90 minutes resulted in peak subjective and reinforcing effects of oxycodone at the shorter duration infusion; there were no differences in subjective ratings or liking between placebo and oxycodone over the 15- to 60-minute time period.
When Comer et al. (2013) compared choice behavior of opioid addicts maintained on sublingual buprenorphine, they found that when the choice was between morphine and oxycodone, the participants consistently preferred the high dose over the low dose of each drug compared with placebo. Under a different procedure where morphine and oxycodone were compared or money was an alternative, the participants chose money over both drugs. At the high doses of oxycodone and morphine, oxycodone was chosen more frequently than morphine.
D. Sex Differences in Abuse Liability of Oxycodone—Human Studies
There has been growing evidence that men and women differ with regard to their risks for substance abuse, but this view is not unequivocal as there are relatively few studies, sometimes small in numbers, and occasionally with inconsistent results. Becker and Hu (2008) and Becker et al. (2017) reviewed sex differences in drug abuse and concluded that sex differences are present during all phases in the progression from initiation, escalation, addiction, relapse, and withdrawal, but this view is predominantly based on rat models (see also Carroll et al., 2004). A series of four studies on “Sex Differences in Addict Careers” published in 1987 covered four stages related to heroin abuse that started with the initiation of heroin use, “becoming addicted,” “being addicted” (Hser et al., 1987a,b; Anglin et al., 1987b), with the last article in the series focused on treatment (Anglin et al., 1987a) These studies, conducted with more than 500 heroin-dependent people admitted to methadone maintenance treatment programs, permitted a systematic comparison between men and women with regard to antecedent behaviors leading to initial drug use and then proceeding through to addiction. Although these studies did not include oxycodone, they are, nevertheless, informative of the processes leading to SUDs and to potential differences between men and women in these different phases of OUDs.
Accumulated evidence has demonstrated the occurrence of several differences between male and females in the biologic response, causes and correlates of drug abuse, craving and relapse, along with residual long-term effects (Lynch et al., 2002; Nicolas et al., 2022). Although the majority of the focus has been on alcohol, nicotine, and cocaine, there are a growing number of studies recognizing the importance of including women in behavioral and clinical studies in light of the increasing SUDs involving women. The role of sex in OUDs in humans has been difficult to elucidate due to the challenges in attempting to isolate biologic effects from environmental context, the ambiguities surrounding historical factors leading to the development of OUDs, the role of genetics, and difficulties surrounding multidrug use. There are several other complications and difficulties in attempting to interpret the results of some studies comparing females and males due to inconsistent results, the lack of placebo controls, the heterogeneity of subjects and pain conditions and, typically the small numbers involved.
Sex differences in the initial exposure to abused drugs have been suggested to emerge due to differences in the initial likelihood of recreational exposure opportunities between males versus females, an emphasis that might be historical rather than current (Van Etten et al., 1999; Van Etten and Anthony, 2001). However, the outcome of this large study, conducted over a 15-year period with 131,226 U.S. residents that were recruited for the National Household Surveys on Drug Abuse, emphasizes the importance of environmental factors as drivers of initial exposure and continued involvement leading to SUDs, rather than biologic or genetic predispositions. Sex differences at an early stage of drug exposure may account for differences in later stages of drug involvement that ultimately lead to dependence. Van Etten et al. also found that, across marijuana, cocaine, hallucinogens, and heroin, the evidence demonstrated no difference between males and females in the probability of making a transition from the initial “exposure opportunity” to more continued use. Van Etten et al. acknowledge that this study was not a prospective study and provides appropriate caveats about self-reported data.
There is one study on the effects of oxycodone in healthy volunteers that showed sex differences in several subjective effects of oxycodone (Zacny and Drum, 2010). Although both men and women reported dysphoric effects and nausea from oxycodone, women reported effects that were of greater magnitude.
Although these studies are informative, the number of subjects in each of the reports was small, occasionally with just a single woman, precluding the likelihood of drawing of any definitive conclusions. These and other studies of laboratory-based abuse liability assessments are covered in a subsequent section of this review.
VII. Oxycodone Abuse Liability Studies in Animals
A. Drug Self-Administration
Studies examining the abuse liability of oxycodone in experimental animals have been numerous. Oxycodone is a robust reinforcer, capable of initiating and maintaining responding in all species in which it has been studied. The maintenance of responding by oxycodone has been used frequently to evaluate novel and repurposed therapeutics for the treatment of OUD that will decrease oxycodone-maintained responding but not affect responding maintained by other reinforcers such as food. These studies are summarized later in this review under “Treatment Approaches to Oxycodone Abuse.” Although the majority of studies examining oxycodone self-administration have used intravenous administration, an increasing number of studies have developed procedures using the oral route, which has been the usual form of oxycodone when used and abused (Enga et al., 2016; Jimenez et al., 2017; Fulenwider et al., 2020; Phillips et al., 2020; Zanni et al., 2020; Slivicki et al., 2023).
Oxycodone engendered relatively high rates of responding for intravenous infusions of oxycodone in rhesus monkeys with the rate of responding similar to those observed with alfentanil, a μ-opioid agonist also with high reinforcing efficacy (Woods et al., 2003). Leri and Burns (2005) studied oxycodone self-administration in male Sprague-Dawley rats including an examination of whether ultra-low-dose naltrexone would attenuate oxycodone self-administration as well as reinstatement. The combination of naltrexone and oxycodone enhanced oxycodone self-administration, suggesting a reduction in the reinforcing potency. The combination of oxycodone and naltrexone also reduced drug taking following a “priming” dose of oxycodone, with the coadministration also reducing the “breakpoint” under a progressive-ratio schedule of reinforcement (see later discussion). Similar results were also reported with CPP that was used to evaluate the reinforcing effects of oxycodone (Olmstead and Burns, 2005).
Wade et al. (2015) compared the self-administration of several opioid analgesics in rats using the extended access model and responding under a progressive-ratio schedule of reinforcement. Animals were trained to self-administer heroin, fentanyl, oxycodone, or buprenorphine under conditions where the response requirement to receive the drug progressively increased following the infusion of drug. The breakpoint measured the “reinforcing strength” or the motivational properties (Hodos, 1961; Richardson and Roberts, 1996) of each drug and was based on the last infusion taken in the 6-hour experimental session. Heroin, fentanyl, and oxycodone produced an initial escalation of responding followed by stable lever pressing, whereas buprenorphine did not. Comparable increases in the breakpoint were seen at the middle doses for each of the three drugs, again with the exception of buprenorphine. The progressive escalation of oxycodone-maintained responding has been observed in a variety of studies in both rats and mice (Zhang et al., 2014; Wade et al., 2015; Matzeu and Martin-Fardon, 2020; Nguyen et al., 2021).
Zhang et al. (2009) reported a differential sensitivity to striatal dopamine levels following the self-administration of oxycodone in adolescent and adult mice. An initial period of self-administration concluded that adult mice self-administered more oxycodone across the spectrum of doses than did adolescent mice. When mice were subsequently implanted with a guide cannula implanted into the striatum and doses of oxycodone were administered intraperitoneally, followed by in vivo microdialysis, it was found that adolescent mice self-administered a lower number of oxycodone infusions at the lowest dose of oxycodone but had increased levels of striatal dopamine, suggesting to these authors a differential sensitivity to the reinforcing and neurobiological effects of oxycodone in younger mice.
More recently, Samson et al. (2022) were interested in determining whether alterations in dopamine transmission in the mesolimbic pathway were related to abstinence from oxycodone. Female and male Long-Evans rats were trained initially to self-administer intravenous oxycodone in a session that was 6 hours long. Following acquisition, the rats were switched to an intermittent schedule for 10 days where access to oxycodone was limited to 5 minutes and was followed by a 25-minute period where drug access was not available. Access to oxycodone was then completely eliminated for either 1 or 14 days and, on these days, responding produced only the stimulus cue previously associated with oxycodone delivery. When tested on days 1 and 14, there was robust responding engendered by presentations of the stimulus associated with oxycodone, and this was sustained over the 14-day period of abstinence. Dopamine uptake was dramatically reduced during this time period, leading to changes and a dysfunction in dopamine transmission. Samson et al. suggest that these changes in dopamine neurotransmission and the sustained responding to the drug-associated cue may be viewed as an index of “craving” that is related to a compensatory response to a reduction in dopamine associated with opioid abstinence that, in turn, may contribute to drug-seeking behavior. Samson et al. did not note any sex differences in any of the experiments in that both females and males responded similarly across conditions.
B. Reinstatement and Craving
In addition to drug self-administration procedures and drug discrimination procedures (see Section VI.D) that are used frequently to evaluate the potential abuse liability of a drug, two other methods have been used with increasing frequency that broadly reflect key issues surrounding relapse. Relapse to drug use following a period of abstinence is of clinical importance and has been the focus of a number of preclinical studies. This procedure typically establishes responding and maintains access to the drug of interest for a period of time that is followed by discontinuation of the drug’s availability (extinction). When responding declines to low levels due to the absence of drug reinforcement, responding can typically be reinstated by the brief administration of the drug (a “priming” stimulus), by the presentation of a stimulus (“cue”) that was previously associated with drug delivery or by exposure to the context in which the drug has been administered previously (Venniro et al., 2016. The conditioned stimuli that have been paired with drug delivery have also been shown to maintain responding reinstated by foot shock or by a priming dose of oxycodone (Grella et al., 2011).
In the context of SUDs, craving has been defined as a persistent and intense desire to use a drug. Craving is listed in the International Classification of Diseases (ICD-11, 2018) as one of the six characteristics of psychoactive substance dependence and the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (American Psychiatric Association, 2013) has recommended that craving become the recommended standard for diagnosing SUDs. Recently, there has been increasing focus on the neurobiological correlates of craving related to OUDs Kakko et al., 2019; Lueptow et al., 2020).
Susceptibility to relapse is, among other variables, frequently believed to be related to craving, which has emerged as an important indicator of drug seeking and relapse. Gauld et al. (2023), following an extensive network analysis of opioids and other drugs, have concluded that craving is a potential central marker, connecting to the entire symptom network regardless of the specific substance, and can be used to aid and facilitate the understanding and treatment of SUDs.
An example of context-induced reinstatement using oxycodone was reported by Bossert et al. (2019) who trained rats to self-administer oxycodone in daily 6-hour sessions in one context (A); in a different context (B) responding was extinguished when oxycodone was not delivered. The two contexts also differed in terms of auditory, tactile, and visual cues. During extinction, the number lever-press responses declined, but when re-exposed to context A and B, responding increased in context A above that when the rats were exposed to context B. Bossert et al. (2019) were also able to show that the oxycodone’s effects were mediated by the μ-opioid receptor through administration of the antagonist naltrexone, but there was no clear evidence for a role of δ- or κ-opioid receptors in oxycodone self-administration.
Previous studies have reported that neurokinin 1 receptor (NK1R) is involved in SUDs and that antagonism of this receptor attenuates opioid self-administration (Ripley et al., 2002; Sandweiss et al., 2018; Schank, 2014). Fulenwider et al. (2020) studied the effects of NK1R antagonism on stress-induced reinstatement of oral oxycodone self-administration in male and female Long-Evans rats. Following the extinction of responding when water replaced oxycodone and using footshock to induce stress, Fulenwider et al. found lever pressing was increased. The administration of 15 mg/kg, i.p. of the NK1R antagonist L822429 significantly attenuated reinstatement of oxycodone self-administration in both male and female rats. More detailed studies with NK1R are given in Section VII of this review under Pharmacological Modulation of Oxycodone in Laboratory Animals.
Reinstatement of extinguished responding that had been maintained by oxycodone was attenuated by administration of buprenorphine and by a “biased” μ-opioid receptor agonist TRV130 (Bossert et al., 2020) reaffirming the utility of buprenorphine as a treatment option for OUDs and suggesting that biased μ-opioid receptor agonists may also be effective treatment options for relapse. A number of studies addressing relapse associated with drug craving have focused on neurobiological sequelae that include the role of certain brain regions (Altshuler et al., 2021, Fredriksson et al., 2023), glutamate receptors in the rat hippocampus (Salisbury et al., 2021), changes in mRNA expression of fibroblast growth factors, and immediate early genes (Blackwood et al., 2019b).
Reinstatement of oxycodone-maintained responding has been studied in male and female (N = 4/sex) squirrel monkeys (FB De Moura et al., preprint, DOI: https://doi: 10.1101/2023.01.12.523850FB). The study was focused on the availability of an alternative reinforcer (sweetened condensed milk) in its effect on oxycodone self-administration and reinstatement. The availability of milk decreased oxycodone self-administration and significantly attenuated oxycodone-primed responding in both male and female monkeys. Low milk concentrations were more effective in lowering self-administration in males whereas low concentrations of milk were more effective in decreasing priming reinstatement in the females. De Moura et al. suggest that treatment strategies that focus on the use of alternative reinforcers should be examined carefully for sex-specific effects.
Studies of potential abuse liabilities of compounds are increasingly incorporating reinstatement and relapse methods into experimental analyses in addition to drug self-administration. Accordingly, the inclusion of these methods permits a more global assessment of the initiation, maintenance, and cessation of drug-taking behavior, along with environmental factors that occasion relapse to drug taking. Treatment approaches of SUDs must take these different facets into consideration when evaluating potential pharmacological as well as behavioral interventions.
C. Impulsivity
A number of studies have demonstrated that several drugs of abuse produce impulsive behavior in both human and animal models (de Wit, 2009; Perry and Carroll, 2008). Impulsivity is believed to be a determinant as well as a consequence of drug use whereby impulsivity is responsible for the initial drug taking and that drug use itself further drives behaviors related to impulsivity, poor decision-making, lack of sensitivity to negative consequences, and risky behaviors (see also Poulton and Hester, 2020). The majority of research on impulsivity has focused on cocaine, amphetamine, methylphenidate, and alcohol and has used a variety of measures and methodologies, with a number of studies conducted with humans in addition to rodents (see Perry and Carroll, 2008; de Wit, 2009 for extensive summaries of drugs and procedures to assess impulsivity and Weafer et al., 2014 for studies on the translation of impulsivity findings in relation to substance use). Very few studies have focused on opioids, although Kieres et al. (2004) and Pitts and McKinney (2005) have examined the effects of morphine in rats on measures of impulsivity and found an increase in impulsivity across a range of doses. Similar findings with morphine were also obtained in rats (Pattij et al., 2009) and in rats dependent on morphine (Harvey-Lewis et al., 2012), with comparable results also reported in rhesus monkeys (Maguire et al., 2012).
Hunt et al. (2020) studied the effects of oxycodone on sensitivity to the magnitude of reinforcement in rats using a procedure that assessed choice between a large or small reinforcer. Under control (non-drug) conditions, all rats developed a reliable preference for the larger reinforcer. Administration of oxycodone produced a dose-related decrease in the preference for the larger reinforcer, indicating that oxycodone decreased the sensitivity to reinforcer magnitude and did so without affecting any of the other behavioral measures such as motor function that might affect responding. The decreased sensitivity to reinforcement magnitude with oxycodone and other μ-receptor opioid agonists may represent an important behavioral and pharmacological mechanism that underlies the relationship between risky choice and impulsivity.
Although very few studies have examined the effects of oxycodone on measures of impulsivity in humans, Zacny and de Wit (2009) examined the effects of orally administered oxycodone (5,10, and 20 mg) on several measures of impulsivity in 12 healthy volunteers and found no effects of oxycodone on any of the tasks that were examined. Although the authors of this study commented that there was considerable variability on all of the measures of impulsivity, and that some of the data were not usable, limiting sample size, they concluded that oxycodone in the doses used were unlikely to increase impulsive or risky behaviors in most patients.
D. Sex Differences in Abuse Liability of Oxycodone—Animal Studies
Although traditionally the focus of most substance use related research was conducted in male animals, this has changed considerably with the recognized need to use both male and female subjects in studies of SUDs. Most of the studies in animals have examined opioids other than oxycodone. However, sex differences in several dimensions, including antinociceptive activity, discriminative stimulus properties, dependence and abuse liability ascertained in drug self-administration studies, prompt a review of all these areas that are likely to expand as more attention is directed toward delineating sex and gender differences in SUDs, particularly when it comes to treatment options and future medications.
Although not investigating oxycodone but illustrative of the type of study examining the abuse liability of opioids in female and male rats, Cicero et al. (2003) found strong sex differences in intravenous self-administration of heroin and morphine, with female rats consuming significantly greater amounts of these drugs than males. In addition, the breakpoint, i.e., where the animals stop responding to increases in the response requirement to obtain the drug, was higher in females than in males. Although these results were similar to findings reported earlier with heroin (Lynch and Carroll, 1999), they differed from those reported by Stewart et al. (1996). Although the basis of the differences between the outcomes of these studies remains somewhat unclear, there were differences in the range of doses examined and other features, including schedule parameters, that could have contributed to the different outcomes. In any event, these findings reinforce the need to include both male and female subjects in the various procedures examining opioid pharmacology.
Several studies have examined the effects of various opioids in drug self-administration in an effort to determine whether there are differences in drug-seeking behavior between male and female rats and mice. Generally, despite some variations in procedures, drugs such as heroin, fentanyl, and morphine result in higher intake in female than in male rats (Bossert et al., 2022; D’Ottavio et al., 2023), suggesting that μ-opioid agonists are more reinforcing in females compared with males (see review by Craft (2008).
Studies that evaluated the effects of oxycodone self-administration in male and female rats have also been reported. For example, Mavrikaki et al. (2017) established oxycodone self-administration in male and female Sprague-Dawley rats and compared these results with a separate group of rats that were provided with sucrose pellets as a reinforcer to determine whether there were sex-related differences between drug and food reinforcers. When the response requirement was 1 to obtain food or an intravenous injection of 0.03 mg/kg, of oxycodone (fixed ratio or FR 1), males made more lever responses to obtain oxycodone than females. Under the same FR 1 response requirement, females responded more to obtain sucrose pellets than males, with this difference quite dramatic. However, when the schedule for the self-administration of 0.03 mg/kg of oxycodone was changed to FR2 and FR5, the sex-related differences disappeared. Overall, at the higher FR5 value, there was not a dramatic difference in the patterns or frequency of oxycodone self-administration between males and females. The nature of the differences depending on the different response requirements raises an important point about studies that examine only a single response requirement when, in fact the schedule of reinforcement may play an important role in the results. As drug seeking in humans typically involves multiple sequences of responses, not just a single response, more experiments should examine a range of schedule parameter values to explore the generality of the findings. The schedule under which a drug is available is as important as the dose, and both require careful consideration.
An operant oral oxycodone self-administration procedure (0.1 mg/ml) was used by Fulenwider et.al. (2020) to examine sex differences in adult male and female rats. Female rats consumed significantly more oxycodone than did males, and the self-administration of oxycodone was decreased in both sexes following repeated naloxone administration (10 mg/kg i.p.). Similar findings were reported by Zanni et al. (2020) where both male and female Long-Evans rats readily consumed oxycodone and preferred it to water in a two-bottle chronic, continuous access paradigm with water in one bottle and oxycodone in the other. All rats readily drank the oxycodone solution and escalated their intake over a 22-week period. Females in this study self-administered twice as much oxycodone by body weight than males, resulting in higher blood levels of oxycodone.
Using an extended access procedure where rats were given 12-hour experimental sessions, Kimbrough et al. (2020) evaluated intravenous oxycodone self-administration (150 μg/0.1 ml infusion) and withdrawal behaviors in female and male Wistar rats. Both male and female rats showed a rapid escalation of oxycodone self-administration with female rats self-administering significantly more oxycodone than males. Overall hourly rates of intake were significantly higher for females during the final 4 hours of the experimental session. Although there were no differences between male and female rats in plasma oxycodone levels, levels of oxycodone in the brain of males were significantly higher than those in female rats at 30 minutes. Following a 12-hour period of withdrawal, rats were tested for sensitivity to paw withdrawal stimulated by von Frey fibers that produced pronounced hyperalgesia, with no differences between male and female rats.
Nicolas et al. (2022) reviewed experiments using cue-, context-, and stress-induced reinstatement in animal models of opioid and psychostimulant craving in an effort to determine whether there was experimental support for sex difference in these two measures of reinstatement for assessing substance use. Overall, although there were a limited number of studies and none with oxycodone, there was little support for sex differences in cue-, context-, and stress-induced reinstatement of opioid seeking. In a study that did focus on oxycodone, Phillips et al. (2020) reported no differences between sexes in cue-induced reinstatement of oxycodone self-administration in mice.
Using a different procedure, Collins et al. (2016) also found no differences between male and female C57BL/6J mice in the CPP procedure with oxycodone doses of 1, 3, and 10 mg/kg, although at each of the doses, males spent more time in the compartment associated with oxycodone than did the females, but these differences were not statistically different. Locomotor activity in the open field was increased at all three doses in the females but only at the 3 and 10 mg/kg in the males. Plasma corticosterone levels were higher in females following the injection of oxycodone, and, in tests of analgesia, there was no difference in the time course but the total antinociceptive effect using the hotplate was larger in males compared with the females. Collins et al. concluded that their data suggest that male and female mice are “modestly different” in their response to oxycodone.
Acknowledging that the role of sex and gender in addiction has been difficult to elucidate, Ryan et al. (2018) studied oxycodone CPP in female and male Sprague-Dawley rats and examined possible changes in hippocampal opioid circuitry. Hippocampal neural circuitry of both sexes is known to be involved in associative memory formation and in encoding motivational incentives that may be important in the transition from initial drug use to drug abuse and dependence. Both female and male rats acquired CPP, but the development of oxycodone CPP produced several sex-dependent redistributions of opioid receptors in hippocampal circuits. Both μ-opioid and δ-opioid receptors redistributed differently in hippocampal circuits of males and females following the development of CPP. Among the many results is one in particular that stands out and may help to better explain the differences in the greater susceptibility of females to opioid abuse and in relapse. After oxycodone-induced CPP, μ-opioid and δ-opioid receptors redistributed in the hilar interneurons in females that, as a result, would enhance disinhibition of granule cells vis à vis two different circuits. According to Ryan et al., this redistribution could facilitate plasticity-associated learning associated with oxycodone, which might account for the greater female susceptibility to μ-opioid agonists.
Sex differences were found by Randesi et al. (2019) who studied neuroplasticity and stress-related gene expression and protein levels in the hippocampus of male and female Sprague-Dawley rats following oxycodone-induced CPP. Both male and female rats exhibited CPP following repeated exposure to 3.0 mg/kg oxycodone. Several differences emerged between male and female rats that included changes in plasticity genes, stress, and kinase markers in the circuitry of the hippocampus. For example, females showed hippocampal region-specific changes that included increases in the activity of regulated cytoskeletal-associated protein immunoreactivity and corticotropin releasing factor receptor. There were also decreases in neuropeptide Y gene expression in the medial hippocampus and in phosphorylated mitogen activated protein kinase. These changes contrast with those in males where, for example, brain derived-neurotrophic factor was increased as was Mapk1, relative to females. These differences suggest mechanisms by which oxycodone can interact differently within the hippocampal opioid systems of male and female rats to affect differences in plasticity related to learning processes and, presumably in the acquisition of drug use and dependence.
Chalangal et al. (2022) have provided an informative and comprehensive review on sex differences in the rodent hippocampal opioid system following stress and oxycodone. Using electron microscopic immunocytochemistry to investigate changes in the distribution of opioid peptides and receptors in specific hippocampal circuits following stress- and oxycodone-induced CPP, Chalangal et al. found a number of differences between male and female rats. A key finding in this report is that, as found in the Ryan et al. (2018) study, opioid peptides and their receptors are redistributed in hippocampal circuits in females such that they would conceivably enhance sensitivity to both endogenous and exogenous opioids. In addition, the authors suggest that chronic stress “primes” the opioid system in females, and this would promote opioid-associated learning, again a sex-specific response that may account for the known sex differences in opioid use and abuse and in the effects of stress since these changes are not seen in males and the absence of such changes in these parameters may reduce the capacity to support opioid-mediated learning.
Beierle et al. (2022) found robust sex-dependent substrain differences when comparing the effects of oxycodone in BALB/cj and BALB/cByj mice. In CPP, the female BALB/cj mice spent more time in the side associated with oxycodone than the BALB/cByj mice, showing enhanced sensitivity to oxycodone, whereas the male strains did not differ. The female BALB/cy mice also showed an increase in the concentration of the oxycodone metabolites noroxycodone and oxymorphone when compared with the BALB/cByj mice. Using quantitative trait locus mapping and whole brain proteomics, Beirele et al. identified a candidate gene Zhx2, a transcriptional factor that has an established role in protection against hepatocellular carcinoma (Li et al., 2022). This gene also appears to be involved in sex-specific sensitivity to oxycodone’s reinforcing effects that may be related to the brain concentrations of oxycodone in the specific substrain of mice.
Reinstatement of oxycodone self-administration has been studied by Guha et al. (2022) in an attempt to assess possible differences between male and female Sprague-Dawley rats. Following an 8-day training period of oxycodone self-administration, rats were exposed to 14 additional days of either short access (1 hour/d), longer access (6 hour/d), or saline. Cue-induced reinstatement was studied following a 14-day period of abstinence. During the reinstatement phase, lever pressing produced the light previously associated with drug delivery but there was no delivery of oxycodone. The magnitude of responding during the long-access procedure was higher than that under the short-access procedure, but there were no differences between male and female subjects. However, responding during the reinstatement phase was higher in females compared with males exposed to both the long- and short-access procedures.
Finally, although not specifically related to abuse liability, oxycodone has been shown to decrease anxiety-like behavior in the elevated plus-maze in both male and female rats (Bruijnzeel et al., 2022). Anxiety, as measured by the percentage of time spent in the open arms of the maze and the percentage of entries into the open arms, was decreased more with oxycodone in males than in females, suggesting a sex-related difference in the anxiolytic effects of oxycodone. This result may be related to the fact that vehicle-treated males made fewer entries into the open arms (a sign of “anxiety-like” behavior) than did females treated with vehicle, which would bear on the percentage changes. Similar differences between male and female rats were also obtained using an open field test where the females traveled greater distances than males while also showing more rearing responses.
Taken as a whole, the studies that examined whether there were differences between males and females in the effects specifically of oxycodone in models of substance use point to a number of differences with females generally responding at higher rates in self-administration experiments, showing changes in brain circuitry, primarily in the hippocampal region, along with sex differences in reinstatement. However, it is clear that there is a relative paucity of data on this topic and further systematic studies are warranted.
E. Drug Discrimination
Drugs can produce interoceptive stimuli when administered to laboratory animals and to humans. Drug discrimination has been used often to ask a number of questions about agonist-antagonist relationships, receptor-mediated effects, and generalization across drug classes and to provide additional information about abuse liability that adds to the information obtained in drug self-administration studies. The drug discrimination procedure establishes a discrimination, typically between a drug and saline, by providing differential reinforcement of responding, typically in a two-lever procedure where, following the administration of a drug, responses on one of the levers results in the delivery of food and, on separate days, following the administration of saline, responses on the alternate lever result in food presentation. Typically, training continues until the animal is discriminating the drug from saline at approximately 90% to 95% correct and this step is followed by completion of a dose-response curve with the training drug. Once the discrimination is established, it is then possible to substitute other drugs, including pharmacological antagonists to evaluate mechanistic studies with the training drug, to determine if those substitutions produce responding on the lever associated with the drug or on the lever associated with saline administration (reviews by Glennon et al., 1983, 1991; Craft et al., 1996; Colpaert, 1999; Craft, 2008; Swedberg, 2016). It is a significant feature of drug discrimination procedures that abused drugs from with the same neuropharmacological receptor-mediated mechanism will reliably substitute for the training drug, whereas drugs from a different pharmacological class will generate responding on the saline lever. Not only is there pharmacological specificity between different drug classes, e.g., opioids and psychostimulants, but there is also discriminative specificity within a drug class. Following the establishment of a discrimination with a μ-opioid receptor agonist responding will not generalize to δ- or κ-opioid receptor agonists.
These methods have been used to establish oxycodone-saline drug discrimination as a way of evaluating other opioid compounds that are mediated by different opioid receptors. In one of the first studies of this nature, Beardsley et al. (2004) trained male Long-Evans rats to discriminate saline from 0.3 mg/kg s.c. heroin. This training dose produced near 100% correct responding, whereas the water vehicle produced near 0% responding on the heroin lever. Substitution of oxycodone produced dose-dependent increases on the lever associated with heroin, reaching approximately 100% responding; the potencies of the two drugs were comparable. This study also reported that oxycodone substituted for heroin in self-administration studies, providing good agreement between these two behavioral procedures.
Morphine and oxycodone were also studied in C57/Bl/6 male and female mice trained to discriminate morphine from saline (Neelakantan et al., 2015), with oxycodone substituting completely for morphine at equipotent doses in both sexes. Walentiny et al. (2019) also trained C57Bl/6 mice to discriminate 1.3 mg/kg s.c. oxycodone from vehicle using a fixed-ratio 10 schedule of food presentation. Fentanyl and several fentanyl-related drugs were examined (ocfentanill, 3-furanyl fentanyl, crotonyflfentanyl, and valerlfentnyl), with all compounds completely substituting for oxycodone in a dose-related manner. Naltrexone pretreatment decreased oxycodone-like responding for fentanyl and all fentanyl-related compounds.
In another study with oxycodone established as a discriminative stimulus in mice, Walentiny et al. (2018) examined whether a nociception/orphanin FQ (NOP) agonist, Ro64-6198 could modify the discriminative stimulus effects of oxycodone. These investigators antagonized the effects of oxycodone with a naloxone pretreatment and also showed that heroin and morphine, but not the κ-opioid receptor agonist U50488, fully substituted for oxycodone. The NOP receptor agonist Ro64-6198 reduced oxycodone lever responding and shifted the oxycodone curve to the right, and the effects of Ro64-6198 were reversed following administration of the NOP receptor antagonist J-113397. Walentiny et al. (2018) suggests that the attenuation of oxycodone’s discriminative stimulus effects by NOP receptor agonists may be mediated by modulation of dopamine signaling since the endogenous NOP ligand nociceptin/orphan FQ reduces basal dopamine release and reverses morphine-induced increases in dopamine (Murphy et al., 1996; Murphy and Maidment, 1999; Di Giannuario, et al., 1999; Di Giannuario and Pieretti, 2000). Finally, since it is believed that the discriminative stimulus effects of drugs are mediated by interoceptive stimuli and the information derived from these studies have relevance for OUDs, attenuation of the effects of oxycodone and other μ-receptor opioids by NOP agonists suggests that this class of compounds may have utility in the treatment of OUDs. Finally, the absence of substitution with κ-opioid receptor agonist U50488 reaffirms that at least the discriminative stimulus effects of oxycodone do not involve κ-opioid-mediated activity.
In a slightly different approach, Withey et al. (2020) examined the effects of oxycodone on learning a discrimination in squirrel monkeys. Using a touchscreen-based visual discrimination procedure, the effects of oxycodone were examined during a period when the monkeys were self-administering oxycodone and when oxycodone was administered during chronic treatment. The effects of naltrexone precipitated withdrawal and cessation of oxycodone treatment were also studied in the context of the visual discrimination procedure. When the effects on the development or the performance of the visual discrimination were evaluated during the period when oxycodone was self-administered or when chronically treated, there was no impairment of the performance on the discrimination, but the discrimination was substantially impaired during withdrawal precipitated by naltrexone and discontinuation of oxycodone.
F. Imaging Studies
In addition to the BOLD study described earlier (Moore et al., 2016, Section II.B), there are a few studies that also have examined the effects of both acute and chronic exposure to oxycodone in experimental animals. Nasseef et al. (2019) used pharmacological magnetic resonance imaging in mice to examine whether oxycodone affects coordinated activities in brain networks (functional connectivity) typically associated with pain and substance use. Oxycodone administration produced a reduction in the communication between brain regions. Although widespread effects in the brains were observed, two regions associated with pain (periacqueductal gray) and reward centers (Nac), both rich in μ-opioid receptors, were the primary targets that underwent a marked reduction in normal functional connectivity. Oxycodone-induced alterations in these brain regions parallel their well-known behavioral involvement in pain and abuse. As with the Moore et al. study, there was no effect of oxycodone on the functional connectivity of these brain regions in the μ-opioid receptor knockout animals.
BOLD imaging was also used by Iriah et al. (2019) to study the response to acute exposure to oxycodone in male rats. In contrast to the Moore et al. (2016) study and the Nasseef et al. (2019) study, rats given 2.5 mg/kg of oxycodone intraperitoneally did not show effects on the BOLD signal in the Nac, suggesting that the first acute administration of oxycodone may not be rewarding. In an effort to image changes in the brain following repeated administration of oxycodone, the researchers had to move away from BOLD due to movement artifacts and employ manganese-enhanced magnetic resonance imaging. This effort involved injecting MNCl2 into the right lateral ventricle. Manganese is readily taken up by active neurons that accumulate the ion, and, once inside the neuron, it can move across active synapses to label integrated neural circuits and their functional connections. Oxycodone was administered twice daily for 4 days, with imaging occurring following the last dose. Under these conditions, the accumbens core and shell and ventral pallidum were activated, as were the forebrain limbic system, amygdala and hippocampus. The differences in brain activity following acute or chronic oxycodone administration are not surprising, and it would be of great interest to expand these studies to female rats to examine potential sex differences in these effects.
VIII. Pharmacological Modulation of Oxycodone in Laboratory Animals
The majority of studies in animals that have been directed at evaluating potential pharmacological treatments for OUDs have established responding maintained by intravenous heroin, fentanyl, morphine, or oxycodone. Although some efforts have been made to identify “pan-therapies” that also would include psychostimulant use as well as opioid use, this section focuses on experiments where oxycodone has served as the baseline drug either in self-administration experiments or when using CPP. There has been a major effort to identify novel or “repurposed” compounds that prevent drug-seeking behavior and relapse. Compounds described here are typically drugs that are being used therapeutically for other indications and would be repurposed if shown to be effective in these procedures.
A. Ultra-Low Dose Naltrexone
Leri and Burns (2005) followed up on a previous study (Olmstead and Burns, 2005) showing that low-dose naltrexone blocked the development of CPP in rats. Leri and Burns examined the effects of ultra-low-dose naltrexone on oxycodone self-administration and also included an assessment of whether naltrexone pretreatment would affect vulnerability to relapse that was precipitated by a priming injection of oxycodone, by foot-shock stress, or by a drug cue previously established by its association with oxycodone self-administration. Whereas the addition of naltrexone doses of 10 or 100 pg/kg/inf in combination with oxycodone (0.1 mg/kg/inf) did not alter the self-administration of oxycodone, a reduction to 1 pg/kg/inf of naloxone increased the number of oxycodone infusions thath the authors suggested indicated a reduction in the reinforcing effects of oxycodone. Following a period of extinction, where lever press responding had no consequence, responding decreased significantly. However, all three doses of naltrexone significantly reduced the reinstatement of responding that was produced by a priming dose of oxycodone (0.25 mg/kg s.c.), the presentation of the oxycodone-conditioned cue, or foot shock. Finally, the lowest dose of naltrexone also reduced the ‘breakpoint under a progressive-ratio schedule, with animals lowering the number of responses to produce oxycodone, suggesting that naltrexone decreased the reinforcing value of oxycodone. Taken together, these studies suggest that ultra-low-dose naltrexone decreases the reinforcing potency and the motivation to self-administer oxycodone and attenuates the vulnerability to relapse.
B. Kappa Opioids
As described throughout sections of this review, the possible involvement of the κ-opioid receptor has been woven inextricably into the pharmacology of oxycodone. The effects of the κ-opioid receptor agonist nalfurafine has been shown to reduce the reinforcing and respiratory depressant effects of oxycodone while augmenting the effects of oxycodone on thermal antinociception in Sprague-Dawley rats (Townsend et al., 2017). Oxycodone self-administration (56 μg/kg/inj) was conducted using a progressive-ratio schedule of reinforcement. Nalfurafine decreased the reinforcing effects of oxycodone under this self-administration procedure, and, by itself, nalfurafine did not maintain responding. Both oxycodone and nalfurafine produced dose-dependent antinociception and, when given in combination, were additive, with the mixtures not producing respiratory depression. These results suggested that appropriate doses of nalfurafine in combination with oxycodone could be effective in decreasing the abuse liability, retaining a nociceptive profile while also not impacting respiration.
Kappa opioid receptor agonists have been reported also to reduce oxycodone self-administration in rhesus monkeys (Zamarripa et al., 2020). A procedure was used by Zamarripa et al. whereby, on alternate days, either cocaine or oxycodone was self-administered under a progressive-ratio schedule. On days when oxycodone was self-administered, the effects of pretreatment with salvinorin A or nalfurafine, two κ-opioid receptor agonists, were examined. Both agonists reduced the number of oxycodone injections to saline self-administration levels, and the effects of nalfurafine were reversed by pretreatment with nor-BNI the κ-opioid receptor antagonist.
In effort to evaluate further the possible efficacy of κ-opioid receptor agonists to reduce oxycodone self-administration, Zamarripa et al. (2021) examined the effects of a novel κ-opioid receptor agonist, triazole 1.1, on responding of Sprague-Dawley rats maintained by intravenous delivery of 0.056 mg/kg/inj oxycodone. In addition to triazole 1.1, that had been reported to be devoid of typical κ-opioid receptor-mediated side effects, Zamarripa et al. also examined U50,488 and nalfurafine using a progressive-ratio schedule of reinforcement. All three compounds reduced oxycodone self-administration, with the order of potency nalfurafine > U50,588 > triazole 1.1.
C. Nociceptin/Orphanin FQ
Nociception/orphanin FQ is a 17-amino acid opioid-like peptide that binds with high affinity to the nociception/orphanin FQ receptor but has no affinity for μ, κ, or δ-opioid receptors. It has been studied with a wide range of drugs, including opioids, but also psychostimulants and alcohol. Early studies by Mogil et al. (1996) concluded that orphanin FQ is a “functional anti-opioid peptide” based on its modulation of various opioid actions that included morphine nociception. Rutten et al. (2010) studied the effects of a NOP receptor agonist, Ro65-6570, with a number of opioid and psychostimulant drugs. Ro65-6570 did not produce place preference, but when given in combination with the minimally effective dose of oxycodone or tilidine, a low potency opioid agonist, the minimal effective dose of these drugs was higher than when administered alone. These effects were reversed by pretreatment with the NOP receptor antagonist J-113397. Rutten et al. concluded that activation of NOP receptors effectively attenuates the rewarding effects of opioids since the NOP receptor agonist reduced the acquisition of place preference produced by the two opioids.
Several additional studies using CPP have reported that intracerebroventricular injections of nociception/orphanin FQ, the endogenous ligand of the opioid receptor-like 1 receptor, eliminated the CPP produced by morphine (Ciccocioppo et al., 2000), a finding that has been reproduced in several different experiments (see review by Ciccocioppo et al., 2020).
Kallupi et al. (2020) used an outbred, genetically diverse line of heterogeneous stock rats that were created at the National Institutes of Health by outbreeding eight inbred rat strains. These rats were characterized as having high or low addiction-like behaviors [high-anxiety (HA) and low-anxiety (LA) rats, respectively] that were determined after 3 weeks of chronic 12-hour access to oxycodone. Kallupi et al. derived a composite measure of addiction based on 1) escalation of intake under a fixed-ratio schedule, 2) maintenance of responding under a progressive-ratio schedule, and 3) withdrawal-induced analgesia. A cue-induced reinstatement procedure revealed that the HA rats exhibited significantly increased responding in response to the cue associated with drug delivery, whereas the LA rats did not. When recording spontaneous inhibitory postsynaptic currents in slices taken from the central nucleus of the amygdala, HA rats were found to have higher basal levels of GABA release than the LA rats and there was a significant difference in spontaneous inhibitory postsynaptic currents frequency between the HA and LA rats that was indicative of nociception-mediated modulation of GABA neurotransmission. Finally, using oxycodone-maintained self-administration, delivery of nociceptin through guide cannula implanted in the central nucleus of the amygdala selectively decreased oxycodone responding in the HA rats compared with the LA rats where there were no effects on responding. The authors of this study hypothesize that high levels of oxycodone intake in the HA rats may lead to a downregulation of the nociceptin system in the central nucleus of the amygdala, and, as a consequence, the upregulation of GABA neurotransmission in this region that promotes addiction-like behaviors. Dysregulation of nociception may be a critical step in the transition to OUDs.
D. Lorcaserin
The role of serotonin (5-HT) in substance use disorders has been noted for some time where the emphasis has been primarily on the examination of a possible role in psychostimulants including cocaine and nicotine. Neelakantan et al. (2017) studied the effect of the selective 5-HT2C receptor agonist lorcaserin in rats self-administering oxycodone and also examined its effects on vulnerability to relapse using extinction of oxycodone self-administration and forced abstinence where lever pressing during the “cue reactivity” phase produced a visual stimulus and sounds from the pump infusion that had been previously paired with the delivery of oxycodone. Responding of rats was maintained by 0.1 mg/kg/0.1 mL infusion of oxycodone. Lorcaserin (0.25–1.0 mg/kg s.c.) significantly reduced the self-administration of oxycodone at 1.0 mg/kg of lorcaserin; these effects were reversed with the administration of the 5-HT2C antagonist SB 242084. Following extinction-induced abstinence, lorcaserin decreased the reinstatement of responding to the cues that had been associated with oxycodone administration following extinction and following abstinence.
E. Orexin/Hypocretin
Orexin neurons are known to play a role in the modulation of behavior that is directed toward drugs of abuse (Harris et al., 2005; Aston-Jones et al., 2010; Mahler et al., 2012). Two G-protein coupled orexin receptors have been identified, orexin-1 and orexin-2, that appear to have different distributions in the brain and likely affect different physiologic functions (Aston-Jones et al., 2010). In rodents, it has been shown that approximately 50% of orexin neurons express μ-opioid receptors and that these receptors respond to chronic morphine administration and opioid-antagonist precipitated withdrawal (Georgescu et al., 2003). Moreover, orexin knockout mice develop attenuated dependence on morphine, and the precipitated antagonist withdrawal is less severe than that in the wild-type littermates. There are a number of studies of the relationship of orexin and opioids, particularly morphine, where administration of the orexin-1 antagonist SB 334867 reduced the expression of morphine in the CPP procedure and orexin knockout mice demonstrated a lack of preference in this procedure, although these effects were not subsequently replicated [see review by Mahler et al. (2012)].
Matzeu and Martin-Fardon (2020) studied an orexin-1 antagonist, SB334867, and an orexin-2 antagonist, TCSOX229, to evaluate their effects on oxycodone self-administration (0.15 mg/kg/0.1 ml i.v.) in rats and on two measures that examined conditioned reinstatement and resistance to extinction. SB334867 produced decreases in oxycodone maintained responding at 5, 10, and 30 mg/kg i.p. Following extinction, where responding had no scheduled consequences and responding declined to very low levels, the presentation of stimuli that previously accompanied oxycodone delivery reinstated responding on the lever associated with oxycodone; these effects of conditioned stimuli were also reduced by all SB334867 doses. In contrast to these effects, the orexin-2 agonist TCHOX229 did not modify oxycodone self-administration at any of the doses studied, which were identical to those of SB334867. Following reinstatement, the discriminative stimuli that were associated with oxycodone delivery produced a robust increase in responding that was decreased by SB334867 but not reversed by TCHOX229. These findings suggest that selective orexin-1 antagonists may be beneficial for the treatment of OUD.
F. Glucagon-Like Peptide
Glucagon-like peptide-1 (GLP-1), a G protein-coupled receptor, is a 30-amino acid peptide hormone produced in the intestinal epithelial endocrine L-cells of the small intestine by the differential processing of proglucagon. The primary actions of GLP-1 are to stimulate insulin secretion, although it also appears to be a regulator of appetite and food intake (Holst, 2007). GLP-1 is expressed in several brain regions, including the arcuate nucleus and other hypothalamic regions, as well as in the shell of the Nac where it is expressed on dopamine D1 and D2 expressing medium spiny neurons (Merchenthaler et al., 1999). Zhang et al. (2020) reported that systemic administration of the GLP-1 receptor agonist exendin-4, which mimics the activity of GLP-1 and promotes insulin secretion and functions in the control of glucose, penetrates the blood-brain barrier and binds GLP-1 receptors expressed on both dopamine D1 and D2 receptors on medium spiny neurons in the Nac shell. There have been suggestions for a role of GLP-1 in drugs of abuse with studies that have focused primarily on alcohol, cocaine, and nicotine (Brunchmann et al., 2019) including one study in humans with cocaine use disorder (Angarita et al., 2021). The suggestion that GLP-1 could play a potential role in OUDs is based on a study with male Sprague-Dawley rats by Zhang et al. (2020) who evaluated the effects of GLP-1 on oxycodone self-administration, reinstatement, and nociception using exendin-4. Oxycodone-maintained responding under a fixed-ratio 5 schedule (0.06 mg/kg/59 ml saline infused over a 5-second period), as well as responding under a progressive-ratio schedule, was significantly decreased by exendin-4 doses of 0.3 and 3.0 μg/kg i.p.; the breakpoint under the progressive-ratio schedule was also decreased significantly by these doses. Excendin-4 also attenuated cue-induced reinstatement but had no effect on operant responding maintained by sucrose, although food consumption was transiently decreased 1 and 3 hours post-experimental session. These studies suggest that the reductions in oxycodone-maintained responding by excendin-4, administered peripherally, did not result from a more generalized reduction in behaviors leading to other reinforcers. Further, when excendin-4 was administered directly into the shell of the NAc, responding maintained by oxycodone under both the fixed- and progressive-ratio schedules was decreased, as was cue-induced reinstatement. Administration of exendin-4 directly into the Nac or systemically did not affect ad libitum food or water intake. Although there was a reduction in the reinforcing effects of oxycodone by excendin-4, there was no effect of excendin-4 on the antinociceptive effects of oxycodone, measured using the warm-water withdrawal paradigm. Douton et al. (2021) have also reported that exendin-4 reduces cue-induced heroin administration as well as heroin-induced reinstatement.
In a follow-up to their initial study Zhang et al. (2021) examined the effects of exendin-4 attenuated fentanyl self-administration (2.5 μg/kg/infusion) in male Sprague-Dawley rats but did so at doses that also produced malaise-like effects. These investigators then examined the effects of a dual-acting compound, GEP44, that is a combined GLP-1R/neuropeptide Y2 agonist that reduced fentanyl self-administration and reinstatement but with fewer adverse effects compared with exendin-4 when administered alone. This result suggests that an approach targeting these two receptors may have therapeutic utility for the treatment of OUDs (see also Merkel et al. (2021).
Although the reports by Zhang et al. (2020, 2021) suggest a potential role for GLP-1 in the treatment of OUDs, it has been reported that there was no effect of systemic excendin-4 on mice self-administering remifentanil (Bornebusch et al., 2019). There were differences between these two reports that warrant further studies. For example, there may be a species difference related to PK factors, which are not well characterized in the mouse, and there are also differences in the details of some of the results other than possible differences between oxycodone and remifentanil. For example, mice failed to self-administer more remifentanil than saline under the progressive-ratio schedule. However, Bornebusch et al. did find that exendin-4 produced dose-dependent decreases in the oral consumption of alcohol. The findings that excendin-4 was efficacious in abuse liability studies with oxycodone in rats, did not affect oxycodone’s antinociceptive effects, and displays interesting interactions in the brain with other receptors, together with the results obtained with heroin, fentanyl, and the dual-acting GLP-1/neuropeptide Y2 suggest that further studies are essential to arrive at a clearer understanding of the potential use of GLP-1 and dual agents for therapeutic utility in OUDs (see also Klausen et al., 2022).
G. Cannabinoids
Following the acquisition of responding maintained by oxycodone (0.15 mg/kg/infusion), rats were exposed to Δ9-tetrahyrocannabinol (THC) either by vapor inhalation or by the intraperitoneal injection (Nguyen et al., 2019). Under extended access to oxycodone (8-hour sessions), as well as under a 1-hour access condition, both THC vapor inhalation and intraperitoneal administration of THC reduced oxycodone self-administration. In studies of antinociception using the warm-water bath withdrawal response, the combination of THC with oxycodone, both when THC was injected and when exposed to vaporized THC, significantly increased the latency of withdrawal. Specificity of the effects of THC were obtained with the administration of the CB1 antagonist SR 141716 ,which blocked the effects of THC inhalation. Nguyen et al. comment that their data demonstrate an additive effect of oxycodone and THC, suggesting that THC may enhance the therapeutic efficacy of opioids while reducing their abuse liability.
H. Biased G Protein-Based Mu Opioid Receptor Agonist TRV130
Biased signaling of μ-opioid receptor agonists has captured a great deal of attention following the reports that it might be possible to design compounds that preferentially activate the G protein signaling pathway, responsible for analgesic effects, while minimizing or eliminating activation of the β-arrestin pathway, which is responsible for the side effects that include respiratory depression and abuse liability. The first G-protein biased mu-opioid agonist TRV130 oliceridine (or Olinvyk) was advanced into clinical development and was approved by the FDA for intravenous short-term use to treat moderate to severe pain and when alternative treatments are inadequate. Preclinical studies in rats showed that TRV130 produced antinociceptive effects but was also self-administered and comparable to oxycodone in terms of potency and efficacy (Zamarripa et al., 2018). Other preclinical studies suggesting abuse liability have been reported (Altarifi et al., 2017; Schwienteck et al., 2019). Bossert et al. (2020) examined the effects of the G protein-biased μ-opioid receptor agonist TRV130 on relapse from oxycodone self-administration and on brain hypoxia that results from acute oxycodone-induced decreases in oxygen levels in the Nac. Using a context-induced reinstatement procedure, TRV130 modestly decreased reinstatement following extinction of responding maintained by oxycodone in male rats but had no effect on females, a finding that also occurred during reacquisition of oxycodone self-administration. When TRV130 was delivered for 14 days via an osmotic minipump, and these rats were then administered acute doses of oxycodone, when compared with control rats, TRV130 produced a protective effect against decreases in Nac oxygen levels. The authors suggest that TRV130 be considered for opioid maintenance treatment that could protect against relapse and severe respiratory depression. At this point, it appears that TRV130 shares many effects with oxycodone, including abuse liability, which limits its utility as a therapeutic.
I. Neurokinin-1 Receptor Antagonists
Speculation surrounding the potential utility of neurokinin-1 (NK1) receptor antagonists as potential agents for the treatment of OUDs has been available for some time following the publication of studies showing interactions between the peptide neurotransmitter substance P that activates the neurokinin-1 (NK1) receptor and its interaction with opioids (Gadd et al., 2003). Interest in NK1 and its relationship to opioid pharmacology likely rests in the interest in the NK1 system as a potential pain therapeutic and whether there may be a role in the modulation of analgesia (Schank, 2014). Administration of the NK1 receptor antagonist RP 67580 intracerebroventricularly decreased some of the withdrawal signs from morphine that were precipitated by naloxone (Maldonado et al., 1993). Mice lacking the NK1 receptor failed to show typical withdrawal signs when undergoing spontaneous withdrawal, and these mice did not develop conditioned place aversion in response to naloxone administration that precipitated withdrawal in morphine-dependent mice (Murtra et al., 2000). Murtra et al. also showed that the mice lacking the substance P receptor (NK1−/−) did not develop CPP following morphine administration; this finding was specific to morphine since neither cocaine nor food produced CPPs. Mutra et al. concluded that substance P plays an important role in mediating the reinforcing and motivational effects of opioids and may represent a new pharmacological approach to OUDs.
This general conclusion was also found in a study of self-administration of morphine in wild-type and NK1−/− mice (Ripley et al., 2002) where lever pressing to receive morphine was established and maintained in the wild-type mice but, in contrast, the NK1−/− mice responded at low rates on both the lever that produced morphine and the lever where responding had no scheduled consequences, never really acquiring and establishing morphine self-administration. These investigators also studied cocaine self-administration in the two groups of mice and found no differences in responding, suggesting that the effects observed with morphine are specific to opioids and that the NK1 receptor may be necessary for the development of opioid addiction but not other abused drugs. Ripley et al. concluded that their results “strongly support” a role for NK1 receptors in mediating the rewarding properties of opiates.
These effects whereby NK1 appears to play a role in some of the abuse-related aspects of opioids do not generalize to opioid-mediated analgesia as the analgesic actions of opioids are not attenuated in NK1−/− mice when tested using the hot-plate assay, tail pinch, or warm-water tail flick (De Felipe et al., 1998).
In summary, some of these results are difficult to interpret due to findings reporting that substance P (NK1) tachykinins administered alone and when injected intracerebroventricularly or intracerebrally can produce effects that resemble the opioid withdrawal syndrome, including wet dog shakes and rearing (Elliott and Iversen, 1986). Clearly, however, the suggestive nature of these results for OUDs appears to justify studies with human volunteers. Studies with humans and two NK1 antagonists, aprepitant (Walsh et al., 2013) and tradipitant (Coe et al., 2021), are summarized in this review in Section VII, Pharmacological Modulation of Oxycodone in Humans. It appears that only a single study of NK1 and oxycodone has been published, and this was summarized in the section on sex differences in reinstatement (Section VII.B, Fulenwider et al., 2020).
J. Dopamine D3 Receptor Compounds
The distribution of dopamine D3 receptors in the brain and preclinical evidence indicating that these receptors are intimately involved in drug reinforcement and addiction has prompted an effort to develop dopamine D3 receptor compounds for the treatment of SUDs (Heidbreder and Newman, 2010). Several studies with novel compounds developed by Newman and colleagues at the Molecular Targets and Medications Discovery Branch in the National Institute on Drug Abuse Intramural Research Program have demonstrated that dopamine D3 antagonists/partial agonists play a significant role in inhibiting oxycodone self-administration, facilitating extinction, and inhibiting reinstatement (Kumar et al., 2016; You et al., 2017, 2019; de Guglielmo et al., 2020). Two D3 antagonists/partial agonists, CAB2-015 and BAK4-54, produced dose-dependent decreases in oxycodone self-administration. When given as a pretreatment, both compounds facilitated extinction of oxycodone self-administration and inhibited reinstatement of responding to obtain oxycodone (You et al., 2017). In a study that examined oral sucrose self-administration, CAB2-015 decreased intake at doses that reduced oxycodone-maintained responding, whereas BAK4-54 did not.
A subsequent study by You et al. (2019) investigated VK4-116, a dopamine D3 receptor antagonist developed to avoid attributes of prior compounds in this class that include poor pharmaceutical properties or cardiotoxicity. Pretreatment with intraperitonea administration of VK4-116 blocked the acquisition of oxycodone self-administration, an effect that persisted for a number of sessions when the pretreatment with VK4-116 was discontinued, gradually reaching levels maintained by the control group of rats that did not receive VK4-116. Other studies with VK4-116 indicated that it facilitated decreases in responding during extinction, did not show any effect on sucrose consumption, and lowered the breakpoint under a progressive-ratio schedule, indicating an attenuation of the reinforcing effects of oxycodone. A hot-plate assay was used to assess the effects of oxycodone analgesia in combination with VK4-116. Addition of VK4-116 to the dose-response curve of oxycodone produced a significant leftward shift, indicating an enhancement of the analgesia produced by oxycodone. This group also demonstrated that VK4-116 is metabolically stable in rat, rhesus monkey, and human liver microsomes, an initial step in determining whether this compound addresses the liabilities of other D3 antagonists that would preclude preclinical development. Finally, these behavioral studies involving oxycodone self-administration and antinociception have been extended to monkeys and include the D3 receptor partial agonist VK4-40 (Woodlief et al., 2023).
Further studies with VK4-116 were conducted in the genetically diverse heterogeneous stock rats (de Guglielmo et al., 2020) where it was shown that both male and female rats progressively escalated oxycodone intake and there were no differences in oxycodone intake for either sex. Treatment with VK4-116 reduced oxycodone self-administration in both sexes. This study also discovered two subpopulations of rats designated as High and Low responders based on the level of oxycodone self-administration. VK4-116 selectively reduced self-administration of High responders and was also shown to reduce withdrawal-induced hyperalgesia and aggressive responses compiled using an irritability score.
Since the dopamine D3 receptor target appears to demonstrate potential utility for a wide range of SUDs, not just μ-opioid agonists substances but also psychostimulants such as cocaine, methamphetamine, and nicotine, it will be interesting to see how these different drug classes respond to drugs directed toward this receptor. The speculative notion of a pan therapeutic that could be used for diverse SUDs is appealing but remains to be established.
In summary, though a wide range of compounds with different receptor-mediated activity has been studied, none have yet progressed to a point where they can add to the treatment of OUDs that are currently employing methadone or buprenorphine. At this point, it is not clear whether single mechanism-based compounds will be effective or whether a polypharmacological approach may have better efficacy as treatment approaches. The approach of using biased μ-opioid receptor drugs that retain effective analgesia but are without abuse liability remains an open question, as do approaches that employ “bitopic” or “bifunctional” targets that, for example target two or more of the opioid receptors (see Section X, Future Directions) may be developed as analgesics, but whether these may add to the current approaches to drug treatment is unclear. Several of the compounds and mechanisms described in this section appear to be promising and worth further research (e.g., orexin/hypocretin, GLP-1, and dopamine D3 compounds) to determine whether the preclinical findings actually translate to clinical efficacy.
IX. Pharmacological Modulation of Oxycodone in Humans
A few laboratory-based studies have examined a small number of prescription drugs in an effort to determine whether there were any indications of potential therapeutic utility for the treatment of OUDs. In addition to the studies described in this section, new methodology to identify drugs for the treatment of OUDs have proposed drug repurposing approaches to aid in the identification of potential candidate drugs (Zhou et al., 2021). This approach, though still in its infancy, combines computational prediction, patient I-based clinical corroboration, and mechanisms of action analysis. Genetic and functional analyses have shown that candidate drugs identified by this method target multiple OUD-associated genes and pathways that include opioid signaling, G-protein activation, serotonin receptors, and GPCR signaling, thus providing a foundation and an incentive for further evaluation of candidate drugs to treat OUDs identified by this methodology. Additionally, Cao et al. (2023) have provided a review of potential targets and treatment strategies to develop medications for OUDs.
A. Buprenorphine/Naloxone
Roux et al. (2013) studied the effectiveness of different doses of sublingual buprenorphine/naloxone in 25 patients with chronic, nonmalignant pain who were abusing their prescription opioid medications. Subjects were able to self-administer oxycodone during the experimental phases of the study. Over the 7-week period of the inpatient study, the combination of buprenorphine/naloxone was effective in reducing pain and withdrawal symptoms as well as supplemental oxycodone use. Roux et al. concluded that the adequate management of pain and withdrawal in this population may reduce the preference for oxycodone.
B. Pioglitazone, a Peroxisome Proliferator-Activated Gamma Receptor Agonist
The peroxisome proliferator-activated gamma receptor agonist pioglitazone, a glial modulator, has been shown to attenuate the development of tolerance (de Guglielmo et al., 2014) and to reduce heroin self-administration under a fixed and a progressive ratio schedule (de Guglielmo et al., 2015). These findings, together with the expression of peroxisome proliferator-activated gamma receptors in the CNS, prompted a study by Jones et al. (2016) to examine the subjective effects of oxycodone, together with the influence of pioglitazone on the analgesic, cognitive, and physiologic effects when nondependent prescription opioid abusers were maintained on various doses of pioglitazone. Despite the positive findings with heroin and pioglitazone in laboratory animals, pioglitazone did not alter the subjective, cognitive, analgesic, or physiologic effects of oxycodone in the subject enrolled in this study.
C. Ibudilast
Ibudilast is a nonselective phosphodiesterase inhibitor that, in studies with animals, inhibits glial cell activation and may modify opioid-mediated effects such as analgesia and withdrawal (Hutchinson et al., 2009). The effects of ibudilast were studied in nontreatment-seeking opioid-dependent male volunteers who underwent detoxification from morphine as inpatients and were maintained on placebo for the duration of the study. The effects of ibudilast were examined with oxycodone (0, 15, and 30 mg/70kg) on analgesia, on subjective responses to oxycodone, and on craving (Metz et al., 2017). Heroin craving, assessed using a visual analog scale and a drug effects questionnaire, was high across the different oxycodone conditions with significantly less craving for heroin in the active ibudilast condition. Modest but statistically significant decreases occurred in drug liking at the 15-mg dose of oxycodone with the active dose of ibudilast. Metz et al. also studied the reinforcing effects of oxycodone with ibudilast and found that at the 15-mg dose of oxycodone, breakpoint values were significantly lower with ibudilast compared with the placebo control. There were significant differences in pain reductions with both the 15 and 30 mg of oxycodone in combination with ibudilast using the cold-pressor test and the McGill Pain Questionnaire to assess analgesia, but these results were not consistent across the different analgesia scales. Although the subjective ratings of pain were lower with ibudilast, the overall effects were relatively modest. The effects of ibudilast on craving and drug liking, together with the increased analgesic response to oxycodone, suggest that additional studies are warranted to characterize this compound more fully.
D. Cannabis
Cannabinoids and opioids have several common pharmacological properties, suggesting there may be synergistic interactions with μ-opioid receptor agonists such as oxycodone. Abrams et al. (2011) examined the interaction of inhaled vaporized cannabis with sustained-release formulations of oxycodone or morphine in 21 individuals (11 men and 10 women) with chronic pain. The types of pain included musculoskeletal, posttraumatic, peripheral neuropathy, cancer, and arthritic. Pharmacokinetic analyses showed no significant change in the area under the plasma concentration time curves for either morphine or oxycodone following cannabis exposure. Pain levels were assessed by participant ratings. Overall average pain scores for the combined oxycodone and morphine groups decreased significantly by an average of 27%. However, whereas this measure was significantly different for those in the morphine condition, individuals in the oxycodone group did not show a significant change in pain scores. Although the reasons for this difference was unclear, it may be related to the relatively small numbers of subjects or to the baseline differences in that the initial pain ratings for the group receiving oxycodone were higher than those for the morphine group. Despite this difference, Abrams et al. concluded that vaporized cannabis augmented the analgesic effects of opioids without altering plasma opioid levels, suggesting an opioid-sparing effect that might permit lower doses of opioids with fewer side effects but enhanced analgesic efficacy. Possible differences between male and female subjects were not noted.
Cooper et al. (2018) studied the effects of coadministering oxycodone and cannabis on analgesia and abuse liability in volunteers who currently smoked greater than or equal to three cannabis cigarettes at least three times a week for the 4 weeks before screening. Using the cold-pressor test, smoked cannabis produced analgesia when given in combination with an ineffective dose of oxycodone when neither of these drugs produced analgesia when administered alone. This finding also suggested an opioid-sparing effect whereby lower doses of oxycodone, for example, in combination with cannabis produce effective analgesia. This combination did not significantly affect cannabis abuse liability but did increase opioid-related positive subjective ratings.
E. Lorcaserin
As mentioned earlier in this review, lorcaserin has been shown to decrease oxycodone self-administration and to attenuate cue-induced reinstatement. Brandt et al. (2020) studied the effects of lorcaserin on oxycodone self-administration and assessed subjective responses in participants with OUD. Males with moderate to severe OUD were detoxified, then stabilized on lorcaserin or placebo. Intranasal oxycodone was examined on several parameters over a 2-week period. Lorcaserin did not alter oxycodone self-administration but had a trend toward increasing “wanting heroin” when oxycodone was available. Generally, although oxycodone increased participants’ ratings of oxycodone with “good drug effects” and “drug liking,” lorcaserin had minimal effects on oxycodone subjective effects and did not alter oxycodone self-administration. These results clearly differ from those reported by Neelakantan et al. (2017), and the authors suggest the need to examine a range of doses of both oxycodone and lorcaserin to more completely evaluate the utility of lorcaserin as a potential therapeutic.
F. Minocycline
A number of preclinical studies have suggested that the antibiotic minocycline interacts with the opioid system presumably by inhibiting opioid-induced glial cell activation resulting in an enhancement of the effects that include opioid-induced analgesia as well as reducing CPP following morphine administration (Hutchinson et al., 2008; Nazemi et al., 2012; Mei et al., 2013; Ghazvini et al., 2015). In a double-blind, within-subject outpatient study, Mogali et al. (2021) investigated whether the acute administration of oral minocycline would alter the subjective, physiologic, analgesic, and cognitive effects of oxycodone. In addition to placebo, a single dose of 40 mg oxycodone and two doses of minocycline, 100 and 200 mg, were studied. The physiologic effects of oxycodone on pupillary constriction were not affected by minocycline. Although there was a significant increase in expired CO2 in the oxycodone + minocycline 200 mg condition, the investigators acknowledged the variability in this measure and considered this result clinically insignificant. Neither dose of minocycline had any effect on pain induced by the cold-water pressor test or on the measures of cognition. However, the 200-mg dose of minocycline attenuated the positive subjective effects produced by oxycodone, a finding that led to the suggestion that minocycline may attenuate the abuse liability of μ-opioid receptor agonists and appears to be relatively safe in combination with oxycodone.
G. Neurokinin 1 Antagonists
The preclinical data summarized in Section VIII.I suggested a role for NK1 compounds interacting with morphine, data that is also supported using genetically modified mice lacking the NK1 receptor. The are some questions about the full extent of these interactions in tolerance and withdrawal, but the data has been sufficiently compelling to follow up on these suggestions using human subjects and with oxycodone. Walsh et al. (2013) studied the effects of the NK1 antagonist aprepitant on the response to oral and intranasal oxycodone. The subjects in this study were nondependent prescription opioid users. Both routes of administration of oxycodone produced significant dose-related effects that included subjective measures of abuse liability, respiratory depression, and miosis. Pretreatment with the highest dose of aprepitant (200 mg) produced significantly enhanced ratings of the subjective effects of the highest dose of oxycodone (40 mg/70kg) and doubled the estimated street value of the combination for both routes of administration, suggesting modulation of morphine’s effects by aprepitant’s action at the NK1 receptor. There were significant effects on end-tidal CO2 with the highest dose of oxycodone in combination with the highest dose of aprepitant, but there was no evidence of clinically significant respiratory depression, with all drug combinations tolerated safely.
A second study with a different NK1 antagonist, tradipitant, and oxycodone was also conducted (Coe et al., 2021) using participants who were recreational opioid users, except in this study only the intranasal route for oxycodone was evaluated. Again, as with the previous study, oxycodone produced a wide range of opioid effects, but these were not altered by coadministration of tradipitant. The authors of this manuscript concluded that, whereas the animal data provide meaningful interactions between NK1 antagonists and morphine, the human data with oxycodone do not support continued pursuit of NK1 antagonists for the treatment of OUDs.
Taken together, these two studies, the first showing an enhancement of the effects of oxycodone implicating abuse liability and the second showing an absence of support for a role of NK1 in oxycodone’s effects, raise a number of questions about the potential role of NK1 antagonists in modulating the effects of μ-opioid receptor agonists. The authors of the two studies acknowledge possible factors that might contribute to differences in the two studies and the lack of positive translation from preclinical models and conclude that, despite these differences, it is clear that there is an interaction between the opioid and the NK1 systems that is worthy of further exploration.
These experimental studies in humans provide useful approaches to the evaluation of the analgesic and subjective effects of drugs, along with efforts to examine potential drugs that may have clinical treatment efficacy. As such, these experiments are restricted somewhat to investigating early-stage compounds (e.g., phase I or II) that are safe and well tolerated or drugs that might potentially be repurposed if there is a degree of efficacy. Both approaches are essential and complementary in the experimental analysis of drugs in development or those readily available where there is some rationale for study.
X. Vaccines to Treat Opioid Use Disorders
A. General Introduction
The effort to develop vaccines for the treatment of SUDs has been underway for almost 50 years. Both active immunization (vaccination) and passive immunization (through the transfer of premade antibodies) approaches to treat SUDs are based on the PK principle of developing antidrug antibodies to bind drugs in the serum and extracellular fluids and prevent passage through the blood-brain barrier and into the CNS, thereby precluding the constellation of pharmacological events contributing to CNS receptor activation and psychoactive drug effects (Skolnick, 2015). Because vaccines have a long half-life, they should provide longer lasting protection compared with small molecule therapeutics targeted to antagonize the pharmacological effects of the substance of abuse. Immunopharmacotherapic approaches with a focus on OUDs have captured a great deal of interest and have been reviewed extensively over the past few years as the momentum to develop vaccine alternatives to small molecules has increased in light of the widespread issues related to the opioid epidemic (Pravetoni, 2016; Banks et al., 2018; Baehr and Pravetoni, 2019; Pravetoni and Comer, 2019; Baehr et al., 2020; Townsend and Banks, 2020; Hossain et al., 2022; Vasiliu et al., 2022; Martinez et al., 2023).
Drugs that are abused are small molecules (haptens) and are too small to stimulate an immune response. Drugs of abuse are made immunogenic by conjugation to a carrier protein [e.g., cholera toxin, keyhole limpet hemocyanin (KLH), or tetanus toxoid] mixed with an adjuvant such as alum to enhance the immunogenic response. There are a number of challenges unique to the development of opioid vaccines due to the availability of several different opioids, most of which have active metabolites, raising the question of whether a vaccine against one opioid would also apply to others. In addition, it would be important to have alternative options for opioid management of pain if someone is vaccinated against an opioid that would attenuate the analgesic effect of another opioid.
B. Historical Background
The initial impetus for pursuing vaccines for the treatment of OUDs was precipitated by a series of early methodological studies in the 1970s by Spector and colleagues reporting on the development of a quantitative and sensitive method for determining morphine in the serum by a radioimmunoassay (Spector and Parker, 1970; Spector, 1971; Spector et al., 1973). These studies raised the question of whether active or passive immunization of animals could modify the pharmacological and physiologic effects of opioids. Berkowitz and Spector (1972) subsequently reported immunization to morphine in rodents where the morphine immunogen selectively reduced morphine-induced analgesia in mice and also altered the concentration of morphine in plasma. This report was followed by a study demonstrating a reduction in intravenous heroin self-administration in rhesus monkeys following antibody activation with a vaccine consisting of a morphine-based hapten conjugated to bovine serum albumin (Bonese et al., 1974). Killian et al. (1978) subsequently examined the effects of passive immunization against morphine on heroin self-administration, also in rhesus monkeys and under a fixed-ratio 19 schedule of drug delivery. In contrast to the results shown by Bonese et al. following active immunization, heroin self-administration was increased in the study by Killian et al. Several reasons for the different results were suggested by Killian et al. that included lower levels of circulating antibodies in the passively immunized animals, the rate of decay in circulating antibody activity in the passive immunization monkeys that paralleled the return of heroin self-administration, differences in the experimental protocols (the intervals between initiating drug self-administration and immunization), and differences in antibody levels in the cerebral spinal fluid that were not found in one of the passively immunized animals but were found in the actively immunized monkeys.
Since these initial reports, multiple efforts over the past several years have been directed toward the development of vaccines against methamphetamine, cocaine, nicotine, and opioids. Pravetoni and Comer (2019) have provided a very thorough review of the mechanisms of action of OUD vaccines, together with their preclinical development, the clinical status for SUDs and OUDs, as well as manufacturing and regulatory requirements. Truong and Kosten (2022) have reviewed the current status of vaccines for a number of SUDs, pointing out that despite promising findings in animal models of SUDs, clinical trials with humans to date have been disappointing. For the most part, as Truong and Kosten point out, most of the vaccines have not achieved sufficient antibody levels, and, even when doing so, only a small percentage of antibodies may have had a high affinity for the antigen. The focus of the remaining section of this review covers vaccines directed toward oxycodone.
C. Recent Research and Development
Pravetoni et al. (2012) developed an opioid conjugate vaccine (Oxy(Gly)4-sKLH) that when injected into rats produced high antibody titers to oxycodone and its metabolite oxymorphone. Immunization increased the retention of oxycodone in serum and reduced oxycodone distribution in the brain while also reducing the effects of oxycodone in a thermal antinociception procedure. Lower affinities also were obtained with the related opioid agonists and antagonists that included methadone, buprenorphine, and naltrexone. The absence of cross-reactivity with these drugs suggested that continued use of these therapeutic agents would still be possible following the administration of the oxycodone vaccine. A follow-up to this study (Pravetoni et al. (2013) demonstrated that modifications of oxycodone at the C6 position conjugated to KLH (6 OXY(Gly–4 - KLH) produced effective immunogens for eliciting antibodies against oxycodone and hydrocodone. This immunogen was also effective in attenuating the distribution of oxycodone and hydrocodone into the brain and in blunting the analgesic effects of oxycodone and hydrocodone in mice and rats.
Pravetoni et al. (2014) also studied the oxycodone conjugate vaccine on oxycodone self-administration in rats. Vaccination reduced the proportion of rats acquiring oxycodone self-administration and also significantly reduced the number of infusions and total intake of oxycodone. Vaccine efficacy correlated with serum antibody titers and immunization with this conjugate vaccine reduced oxycodone-induced analgesia in a thermal nociception assay while also shifting the dose-response curve to the right for respiratory depression (Raleigh et al., 2017). Raleigh et al. (2018) showed that the dose of oxycodone and the route of administration can play a major role in determining the efficacy of the vaccine. Vaccination with the oxycodone conjugate was more effective when immunized rats were challenged with oxycodone administered subcutaneously rather than intravenously, and distribution of oxycodone in the brain was also greater following subcutaneously administered oxycodone. A subsequent study (Raleigh et al., 2021) reported that, in rats, vaccination with Oxy(Gly)4-secondKLHproduced sustained antibody titers that lasted over 5 months following the initial vaccination. Further, the vaccine did not interfere with fentanyl-induced nociception or the distribution of fentanyl to the brain, demonstrating in vivo selectivity and markedly altering the pharmacokinetics of oxycodone, increasing the half-life of oxycodone in serum in both male and female rats. While there were significant differences between male and female rats in the levels of oxycodone-specific antibody titers, there were no sex differences in other experiments. This oxycodone antibody also reduced the self-administration of oxycodone.
This group also demonstrated that it is possible to combine an oxycodone vaccine with a long-acting opioid receptor antagonist, naltrexone, to offer better protection against OUD and overdose (Raleigh et al., 2020). Over a range of oxycodone doses, the combination provided greater antinociceptive efficacy while also reducing respiratory depression.
Nguyen et al. (2018) examined the effects of an Oxy-Tetanus Toxoid vaccine that had previously shown efficacy attenuating the antinociceptive effects and oxycodone overdose in mice (Kimishima et al., 2017). Vaccination with Oxy-Tetanus Toxoid resulted in fewer rats acquiring stable self-administration of oxycodone, showing an effect on reinforcing efficacy. Although rats were less sensitive to the effects of oxycodone, there was no loss of sensitivity with heroin, demonstrating selectivity of the vaccine.
Altogether, these studies suggest that active immunization with Oxy(Gly)4-secondKLH results in long-lasting selective antibodies that effectively decrease the reinforcing effects of oxycodone while maintaining the efficacy of medications to treat OUD and overdose. Oxy(Gly)4-secondKLH has recently completed preclinical safety and toxicology studies with no indication of toxicological findings, while also showing that it is well tolerated and immunogenic and does not produce undesirable effects in rats (Hamid et al., 2022). At the time of this review, Oxy(Gly)4-secondKLH was in phase IA/1B clinical trials to evaluate safety, degree of antibody production, and efficacy (reduction of drug liking following the administration of an opioid) in participants with OUD (Clinical Trials. gov identifier NCT04458545, Phase 1A/1B Clinical Trials of Multivalent Opioid Vaccine Components).
Although there has been growing evidence over the past several years that vaccines may be beneficial addition to the pharmacological options to treat OUDs, the field has been hampered somewhat by the failure to translate preclinical findings with non-OUDs such as cocaine, nicotine, and methamphetamine into clinical efficacy (Ohia-Nwoko et al., 2016). The entry of oxycodone vaccines into clinical development based on several preclinical findings are encouraging, and the results of clinical trials are enthusiastically awaited.
XI. Future Directions: Opioid Analgesia Without Opioid-Related Side Effects?
The quest to discover and develop opioid analgesics that do not carry the untoward effects of current μ-opioid receptor agonists but that retain their potent analgesic efficacy has been the holy grail of this field of research, ongoing for nearly a century. Indeed, Deneau and Seevers (1964) wrote nearly 60 years ago that the “search for an analgesic devoid of morphine’s undesirable properties continues unabated” (p. 274), and the quest for the holy grail continues. Recall that oxycodone was initially synthesized as an attempt to develop a potent opioid analgesic devoid of the dependence and abuse liability surrounding heroin, which was marketed at the time as an analgesic. Several relatively recent approaches to the development of drugs that may succeed either as effective analgesics without abuse liability or as potential treatment medications have included opioids targeting multiple receptors (i.e., bitopic or bivalent dual multifunctional μ-κ, μ-δ-, or μ-NOP agonists, or μ-dopamine D3 dual partial agonists/antagonists), splice variants of the μ receptor, G-protein biased agonism with signaling directed toward separating the analgesic effects from the side effects and abuse liability, allosteric activation of the opioid receptor, heteromers of the μ receptor, and a focus on natural products (Corbett et al., 2006; Majumdar et al., 2011; Newman et al., 2012, 2020; Burford et al., 2013; Lane et al., 2013; Fujita et al., 2015; Schmid et al., 2017; Kenakin, 2019; Bonifazi et al., 2021; Chakraborty and Majumdar, 2021; de Melo Candeia et al., 2022; Varga et al., 2023). Together with the efforts underway for vaccine development, this collective activity and the diverse approaches being studied bodes well for the possibility of significant breakthroughs but always and necessarily viewed with the appropriate cautious optimism. These efforts, some of which are in the early stages, present an exciting breadth of approaches that represent and build upon several significant advances in pharmacology and in our understanding of G protein-coupled receptors and vaccines. This progress also demonstrates the integration of different disciplines into pharmacology that include computational approaches to drug design and development (Feng et al., 2015) that are required to target multiple opioid receptors and to aid in the design of suitable compounds. The publication of the structures of the entire human opioid family (Wang et al., 2023), together with biochemical results, provide a structural framework to aid and facilitate the design of opioid drugs that are devoid of unwanted side effects.
In conducting a comprehensive molecular pharmacology screening of several clinically relevant opioids, including oxycodone, against multiple potential targets such as monoamine transporters and sigma-1, Olson et al. (2019) identified a number of novel receptor interactions that might address or clarify some of the disparities in the effects of these drugs mentioned in previous sections of this review. These include the absence of cross-tolerance between some opioids and discrepancies between various in vitro and in vitro results. Among the findings and implications of this approach was the identification, based on in vitro radioligand binding and in vivo verification, that buprenorphine was activated by the monoamine transporters in vitro, and, in vivo, duloxetine, which had no effect administered separately, increased the antinociceptive response of buprenorphine in the tail-flick procedure. Hydrocodone and tapentadol were also shown to bind to sigma-1 target, which suggests that this binding may amplify signaling, thereby also enhancing the antinociceptive responses of these two drugs. This approach may also facilitate the search for repurposing drugs with potential treatment of OUDs and for developing more effective analgesics devoid of the current limitations.
The impediments are well known and not trivial. Many past compounds have fallen into the “valley of death,” a demise that is due in part to the significant challenges of identifying appropriate pharmacological targets and mechanisms with the goal of treating pain without abuse liability along with the translational difficulties of moving from preclinical to clinical assessment. However, the goal of identifying a nonaddicting opioid analgesic is an imperative objective. At the present time, it appears to be ever increasingly more achievable and should include comparable efforts to continue to identify potential medications to enhance the ability to treat OUDs.
XII. Conclusions
Lemberg et al. published an article in 2009 that was titled the “Pharmacology of Oxycodone: Does It Explain Why Oxycodone Has Become a Bestselling Opioid?” (Lemberg et al., 2009). Lemberg et al. concluded that “oxycodone is an effective analgesic, but its more liberal use has also increased iatrogenic addiction and individuals seeking detoxification from oxycodone” (p. 521). This article was followed approximately 4 years later by another article, “Does the Pharmacology of Oxycodone Justify Its Increasing Use as an Analgesic?” (Olkkola et al., 2013). Olkkola et al. echoed a portion of the comments of Lemberg et al., concluding that “our current understanding of the pharmacology of oxycodone does not explain the significant increase in its clinical use” (p. 212). Several studies summarized here point to the strong positive subjective effects in human volunteers that appear to have contributed directly to the use and abuse of oxycodone, quite apart from its clinical utility. For several years oxycodone played a dominant role in opioid misuse and overdose mortalities. Olkkola et al. comment on the many similarities between oxycodone and morphine but also acknowledge several properties that differ with oxycodone including a faster onset of action, good oral bioavailability, a longer duration of action, less suppression of the immune system, and lessened tendency to produce hallucinations. As treated in this review, and as pointed out by Olkkola et al., a number of questions still remain despite the intensive research efforts surrounding oxycodone. These include a poor understanding of why oxycodone is less effective than morphine following spinal administration than after intravenous administration, the role of κ-opioid receptors in the analgesic activity of oxycodone, along with how these two drugs might differ in second messenger signaling and immunologic effects.
Despite the lack of present clarity around these questions, oxycodone is unquestionably an effective analgesic. The intensive research over the past several decades has produced additional insights not only with regard to oxycodone but into μ-opioid behavioral and molecular neuropharmacology generally. Experimental studies that have probed other features of oxycodone, including gene expression, use in adolescence, and the longer term effects in adulthood, together with studies of its subjective effects in human volunteers and variables contributing to its abuse, have increased our understanding of this drug and have also provided opportunities to address other features of importance to opioid pharmacology more broadly. Studies with oxycodone and comparisons with other μ-opioid receptor compounds have demonstrated that, despite what appear to be similarities in their pharmacological effects, these similarities betray the multiple intriguing differences between μ-receptor opioids that can be exploited, hopefully, to arrive at the holy grail.
Abbreviations
- β-FNA
beta-Funaltrexamine
- BOLD
blood oxygen level dependent
- CCI
chronic constriction injury
- CFA
complete Freund’s adjuvant
- CNS
central nervous system
- CO2
carbon dioxide
- CPP
conditioned place preference
- FDA
Food and Drug Administration
- GAD
generalized anxiety disorder
- GLP1
glucagon-like peptide-1
- HA
high-anxiety
- KLH
keyhole limpet hemocyanin
- LA
low-anxiety
- NAc
nucleus accumbens
- NK1
neurokinin 1
- NK1R
neurokinin 1 receptor
- NOP
nociceptin/orphanin FQ
- nor-BNI
norbinaltorphimine
- O2
oxygen
- OUD
opioid use disorder
- pCO2
partial pressure of oxygen and carbon dioxide
- PD
pharmacodynamic
- P-gp
P-glycoprotein
- PK
pharmacokinetic
- SNL
spinal nerve ligation
- SNP
single nucleotide polymorphism
- STZ
streptozotocin
- SUD
substance use disorder
- THC
Δ9-tetrahyrocannabinol
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Barrett, Shekarabi, Inan.
Footnotes
Partial support for the preparation of this manuscript was provided by National Institutes of Health National Institute on Drug Abuse [Grant DA047700].
None of the authors declare any conflicts of interest.
References
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL (2011) Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 90:844–851. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Kipps BR, Harris LS, Bowman ER (2002) Updated studies on oxycodone, the active ingredient of the controlled-release formulation of oxycontin. Drug Alcohol Depend 66:S2. [Google Scholar]
- Alles SRA, Smith PA (2018) Etiology and pharmacology of neuropathic pain. Pharmacol Rev 70:315–347. [DOI] [PubMed] [Google Scholar]
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS (2017) Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler RD, Yang ES, Garcia KT, Davis IR, Olaniran A, Haile M, Razavi S, Li X (2021) Role of orbitofrontal cortex in incubation of oxycodone craving in male rats. Addict Biol 26:e12927. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013).Diagnostic and Statistical Manual of Mental Disorders, 5th ed., American Psychiatric Association, Washington, DC. [Google Scholar]
- Angarita GA, Matuskey D, Pittman B, Costeines JL, Potenza MN, Jastreboff AM, Schmidt HD, Malison RT (2021) Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend 221:108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin MD, Hser YI, Booth MW (1987a) Sex differences in addict careers. 4. Treatment. Am J Drug Alcohol Abuse 13:253–280. [DOI] [PubMed] [Google Scholar]
- Anglin MD, Hser Y-I, McGlothlin WH (1987b) Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse 13:59–71. [DOI] [PubMed] [Google Scholar]
- Arguelles N, Miksys S, Tyndale RF (2021) Sex and estrous cycle differences in analgesia and brain oxycodone levels. Mol Neurobiol 58:6540–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnér S, Meyerson BA (1988) Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain 33:11–23. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA (2010) Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res 1314:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund M, Lindgren L, Kajimoto Y, Rosenberg PH (1997) Comparison of epidural morphine and oxycodone for pain after abdominal surgery. J Clin Anesth 9:30–35. [DOI] [PubMed] [Google Scholar]
- Backonja M, Webster LR, Setnik B, Bass A, Sommerville KW, Matschke K, Malhotra BK, Wolfram G (2016) Intravenous abuse potential study of oxycodone alone or in combination with naltrexone in nondependent recreational opioid users. Am J Drug Alcohol Abuse 42:539–549. [DOI] [PubMed] [Google Scholar]
- Baehr C, Kelcher AH, Khaimraj A, Reed DE, Pandit SG, AuCoin D, Averick S, Pravetoni M (2020) Monoclonal antibodies counteract opioid-induced behavioral and toxic effects in mice and rats. J Pharmacol Exp Ther 375:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr C, Pravetoni M (2019) Vaccines to treat opioid use disorders and to reduce opioid overdoses. Neuropsychopharmacology 44:217–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DD, Jenkins AJ (2008) A comparison of methadone, oxycodone, and hydrocodone related deaths in northeast Ohio. J Anal Toxicol 32:165–171. [DOI] [PubMed] [Google Scholar]
- Banks ML, Olson ME, Janda KD (2018) Immunopharmacoherapies for treating opioid use disorder. Trends Pharmacol Sci 39:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley EJ, Fillingim RB (2013) Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JE (1985) Modification of the behavioral effects of drugs by environmental variables, in Behavioral Pharmacology: The Current Status (Seiden LS, Balster RL, eds) pp 7–22, Alan R. Liss, Inc., New York. [Google Scholar]
- Barrett JE (1992) Historical influences affecting the behavioral actions of abused drugs, in Neurobiological Approaches to Brain Behavior Interactions, Research Monograph 124 (Frascella J, Brown RM, eds) pp 161–172. National Institute on Drug Abuse, Bethesda, MD. [PubMed] [Google Scholar]
- Barrett JE, Stanley JA (1983) Prior behavioral experience can reverse the effects of morphine. Psychopharmacology (Berl) 81:107–110. [DOI] [PubMed] [Google Scholar]
- Bart G, Heilig M, LaForge KS, Pollak L, Leal SM, Ott J, Kreek MJ (2004) Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry 9:547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman JT, Saunders SE, Levitt ES (2023) Understanding and countering opioid-induced respiratory depression. Br J Pharmacol 180:813–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok RE, Craft RM (1997) Sex differences in opioid antinociception. J Pharmacol Exp Ther 282:769–778. [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS (2004) Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol 12:163–172. [DOI] [PubMed] [Google Scholar]
- Beaver WT, Wallenstein SL, Houde RW, Rogers A (1977) Comparisons of the analgesic effects of oral and intramuscular oxymorphone and of intramuscular oxymorphone and morphine in patients with cancer. J Clin Pharmacol 17:186–198. [DOI] [PubMed] [Google Scholar]
- Beaver WT, Wallenstein SL, Rogers A, Houde RW (1978a) Analgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and of oral with intramuscular oxycodone. J Pharmacol Exp Ther 207:92–100. [PubMed] [Google Scholar]
- Beaver WT, Wallenstein SL, Rogers A, Houde RW (1978b) Analgesic studies of codeine and oxycodone in patients with cancer. II. Comparisons of intramuscular oxycodone with intramuscular morphine and codeine. J Pharmacol Exp Ther 207:101–108. [PubMed] [Google Scholar]
- Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017) Sex differences, gender and addiction. J Neurosci Res 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierle JA, Yao EJ, Goldstein SI, Lynch WB, Scotellaro JL, Shah AA, Sena KD, Wong AL, Linnertz CL, Averin O, et al. (2022) Zhx2 is a candidate gene underlying oxymorphone metabolite brain concentration associated with state-dependent oxycodone reward. J Pharmacol Exp Ther 382:167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger RD, Dunn W, Schmidt MA, Gross SS, Kirwan JA, Cascante M, Brennan L, Wishart DS, Oresic M, Hankemeier T, et al. for Precision Medicine and Pharmacometabolomics Task Group. (2016) Metabolomics enables precision medicine: a white paper, community perspective. Metabolomics 12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovitch M, Adunsky A (2006) High dose controlled-release oxycodone in hospice care. J Pain Palliat Care Pharmacother 20:33–39. [PubMed] [Google Scholar]
- Berkley KJ (1997) Sex differences in pain. Behav Brain Sci 20:371–380, discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Berkowitz B, Spector S (1972) Evidence for active immunity to morphine in mice. Science 178:1290–1292. [DOI] [PubMed] [Google Scholar]
- Bernard C (1864) Experimental medicine. Experimental research on opium and its alkaloids. Compt Rend de Sc 59:406–415. [Google Scholar]
- Blackwood CA, Hoerle R, Leary M, Schroeder J, Job MO, McCoy MT, Ladenheim B, Jayanthi S, Cadet JL (2019a) Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence-induced incubation of drug seeking after escalated oxycodone self-administration. Mol Neurobiol 56:3603–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood CA, Leary M, Salisbury A, McCoy MT, Cadet JL (2019b) Escalated oxycodone self-administration causes differential striatal mRNA expression of FGFs and IEGs following abstinence-associated incubation of oxycodone craving. Neuroscience 415:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood CA, McCoy MT, Ladenheim B, Cadet JL (2020) Escalated oxycodone self-administration and punishment: differential expression of opioid receptors and immediate early genes in the rat dorsal striatum and prefrontal cortex. Front Neurosci 13:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist ER (1963) The addiction potential of oxycodone (Percodan). Calif Med 99:127–130. [PMC free article] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR (1974) Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 252:708–710. [DOI] [PubMed] [Google Scholar]
- Bonifazi A, Battiti FO, Sanchez J, Zaidi SA, Bow E, Makarova M, Cao J, Shaik AB, Sulima A, Rice KC, et al. (2021) Novel dual-target m-opioid receptor and dopamine D3 receptor ligands as potential nonaddictive pharmacotherapeutics for main management. J Med Chem 64:7778–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgbjerg MF, Nielsen K, Franks J (1996) Experimental pain stimulates respiration and attenuates morphine-induced respiratory depression: a controlled study in human volunteers. Pain 64:123–128. [DOI] [PubMed] [Google Scholar]
- Bornebusch AB, Fink-Jensen A, Wörtwein G, Seeley RG, Thomsen M (2019) Glucagon-like peptide-1 receptor agonist treatment does not reduce abuse-related effects of opioid drugs. eNeuro 6:443-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Hoots JK, Fredriksson I, Adhikary S, Zhang M, Venniro M, Shaham Y (2019) Role of mu, but not delta or kappa, opioid receptors in context-induced reinstatement of oxycodone seeking. Eur J Neurosci 50:2075–2085. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Kiyatkin EA, Korah H, Hoots JK, Afzal A, Perekopskiy D, Thomas S, Fredriksson I, Blough BE, Negus SS, et al. (2020) In a rat model of opioid maintenance, the G protein-biased mu opioid receptor agonist TRV130 decreases relapse to oxycodone seeking and taking and prevents oxycodone-induced brain hypoxia. Biol Psychiatry 88:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Townsend EA, Altidor LK-P, Fredriksson I, Shekara A, Husbands S, Sulima A, Rice KC, Banks ML, Shaham Y (2022) Sex differences in the effect of chronic delivery of the buprenorphine analogue BU08028 on heroin relapse and choice in a rat model of opioid maintenance. Br J Pharmacol 179:227–241. [DOI] [PubMed] [Google Scholar]
- Boström E, Hammarlund-Udenaes M, Simonsson US (2008) Blood-brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology 108:495–505. [DOI] [PubMed] [Google Scholar]
- Boström E, Simonsson US, Hammarlund-Udenaes M (2006) In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab Dispos 34:1624–1631. [DOI] [PubMed] [Google Scholar]
- Brandt L, Jones JD, Martinez S, Manubay JM, Mogali S, Ramey T, Levin FR, Comer SD (2020) Effects of lorcaserin on oxycodone self-administration and subjective responses in participants with opioid use disorder. Drug Alcohol Depend 208:107859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruera E, Belzile M, Pituskin E, Fainsinger R, Darke A, Harsanyi Z, Babul N, Ford I (1998) Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. J Clin Oncol 16:3222–3229. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Behnood-Rod A, Malphurs W, Chellian R, Caudle RM, Febo M, Setlow B, Neubert JK (2022) Oxycodone decreases anxiety-like behavior in the elevated plus-maze test in male and female rats. Behav Pharmacol 33:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunchmann A, Thomsen M, Fink-Jensen A (2019) The effect of glucagon-like peptide-1 (GLP-1) receptor agonists on substance use disorder (SUD)-related behavioural effects of drugs and alcohol: a systematic review. Physiol Behav 206:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bura AS, Guegan T, Zamanillo D, Vela JM, Maldonado R (2013) Operant self-administration of a sigma ligand improves nociceptive and emotional manifestations of neuropathic pain. Eur J Pain 17:832–843. [DOI] [PubMed] [Google Scholar]
- Burford NT, Clark MJ, Wehrman TS, Gerritz SW, Banks M, O’Connell J, Traynor JR, Alt A (2013) Discovery of positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor. Proc Natl Acad Sci USA 110:10830–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D-N, Li F, Wu N, Li J (2023) Insights into the mechanisms underlying opioid use disorder and potential treatment strategies. Br J Pharmacol 180:862–878. [DOI] [PubMed] [Google Scholar]
- Carlson RG, Nahhas RW, Martins SS, Daniulaityte R (2016) Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: a natural history study. Drug Alcohol Depend 160:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MD, Manners MT, Heller EA, Blendy JA (2021) Adolescent oxycodone exposure inhibits withdrawal-induced expression of genes associated with the dopamine transmission. Addict Biol 26:e12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper M, Contreras KM, Walentiny DM, Beardsley PM, Damaj MI (2021) Validation and characterization of oxycodone physical dependence in C57BL/6J mice. Eur J Pharmacol 903:174111. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP (2004) Sex and estrogen influence drug abuse. Trends Pharmacol Sci 25:273–279. [DOI] [PubMed] [Google Scholar]
- Cascorbi I, Tyndale R (2014) Progress in pharmacogenomics: bridging the gap from research to practice. Clin Pharmacol Ther 95:231–235. [DOI] [PubMed] [Google Scholar]
- Cerdá M, Santaella J, Marshall BDL, Kim JH, Martins SS (2015) Nonmedical prescription opioid use in childhood and early adolescence predicts transitions to heroin use in young adulthood: a national study. J Pediatr 167:605–12.e1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2021).
- Chakraborty S, Majumdar S (2021) Natural products for the treatment of pain: chemistry and pharmacology of salvinorin A, mitragynine, and collybolide. Biochemistry 60:1381–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalangal J, Mazid S, Windisch K, Milner TA (2022) Sex differences in the rodent hippocampal opioid system following stress and oxycodone associated learning processes. Pharmacol Biochem Behav 212:173294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD, Smith MT (2008) Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol 35:295–302. [DOI] [PubMed] [Google Scholar]
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V (2010) A comparison of the respiratory effects of oxycodone versus morphine: a randomized, double-blind, placebo-controlled investigation. Anaesthesia 65:1007–1012. [DOI] [PubMed] [Google Scholar]
- Chen LH, Hedegaard H, Warner M (2014) Drug-Poisoning Deaths Involving Opioid Analgesics: United States, 1999–2011. NCHS Data Brief No. 166. National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F (1991) Mu receptor binding of some commonly used opioids and their metabolites. Life Sci 48:2165–2171. [DOI] [PubMed] [Google Scholar]
- Cheung CW, Ching Wong SS, Qiu Q, Wang X (2017) Oral oxycodone for acute postoperative pain: a review of clinical trials. Pain Physician 20(2S):SE33–SE52. [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M (2000) Effect of nociptin /orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol 404:153–159. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, Weiss F (2020) NOP-related mechanisms in substance use disorders, in The Nocicepin/Orphanin FQ Peptide Receptor (Ko M-C, Calo G, eds) Handbook of Experimental Pharmacology Vol. 254, pp 187–212, Springer Nature, Cham, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER (2003) Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav 74:541–549. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Paradis A, Ortbal Z (2010) Determinants of fentanyl and other potent µ opioid agonist misuse in opioid-dependent individuals. Pharmacoepidemiol Drug Saf 19:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL (2012) Effect of abuse-deterrent formulation of OxyContin. N Engl J Med 367:187–189. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP (2013) Factors influencing the selection of hydrocodone and oxycodone as primary opioids in substance abusers seeking treatment in the United States. Pain 154:2639–2648. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Muñoz A (2005) Trends in abuse of oxycontin and other opioid analgesics in the United States: 2002-2004. J Pain 6:662–672. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER (1996) Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther 279:767–773. [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER (1997) Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther 282:939–944. [PubMed] [Google Scholar]
- Cleary J, Mikus G, Somogyi A, Bochner F (1994) The influence of pharmacogenetics on opioid analgesia: studies with codeine and oxycodone in the Sprague-Dawley/Dark Agouti rat model. J Pharmacol Exp Ther 271:1528–1534. [PubMed] [Google Scholar]
- Coe MA, Lofwall MR, Vessels V, Nuzzo PA, Walsh SL (2021) Evaluation of tradipitant, a selective NK1 antagonist, on response to oxycodone in humans. Psychopharmacology (Berl) 238:1857–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Beardsley J, Bignold J, Li Y, Merg F, Sullivan T, Cox TC, Somogyi AA (2009) Lack of association between the A118G polymorphism of the mu opioid receptor gene (OPRM1) and opioid dependence: a meta-analysis. Pharm Genomics Pers Med 2:9–19. [PMC free article] [PubMed] [Google Scholar]
- Collins D, Reed B, Zhang Y, Kreek MJ (2016) Sex differences in responsiveness to the prescription opioid oxycodone in mice. Pharmacol Biochem Behav 148:99–105. [DOI] [PubMed] [Google Scholar]
- Collins D, Zhang Y, Blendy J, Kreek MJ (2020) Murine model of OPRM1 A118G alters oxycodone self-administration and locomotor activation, but not conditioned place preference. Neuropharmacology 167:107864. [DOI] [PubMed] [Google Scholar]
- Colpaert FC (1999) Drug discrimination in neurobiology. Pharmacol Biochem Behav 64:337–345. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Meert T, De Witte P, Schmitt P (1982) Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sci 31:67–75. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W (2001) Opiate self-administration as a measure of chronic pain in the rat. Pain 91:33–45. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Sullivan MA, Vosburg SK, Saccone PA, Foltin RW (2009) Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J Opioid Manag 5:203–212. [DOI] [PubMed] [Google Scholar]
- Comer SD, Metz VE, Cooper ZD, Kowalczyk WJ, Jones JD, Sullivan MA, Manubay JM, Vosburg SK, Smith ME, Peyser D, et al. (2013) Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav Pharmacol 24:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J (2010) Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend 109:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ (2008) Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology 33:1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Darwin WD, Buchwald WF, Gorodetzky CW (1983) Oxymorphone metabolism and urinary excretion in human, rat, guinea pig, rabbit, and dog. Drug Metab Dispos 11:446–450. [PubMed] [Google Scholar]
- Conley KM, Toledano AY, Apfelbaum JL, Zacny JP (1997) Modulating effects of a cold water stimulus on opioid effects in volunteers. Psychopharmacology (Berl) 131:313–320. [DOI] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ (2000) Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology (Berl) 150:430–442. [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD (2005) Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund’s adjuvant-induced arthritic male and female rats. J Pharmacol Exp Ther 313:449–459. [DOI] [PubMed] [Google Scholar]
- Cooper TE, Chen J, Wiffen PJ, Derry S, Carr DB, Aldington D, Cole P, Moore RA (2017) Morphine for chronic neuropathic pain in adults. Cochrane Database Syst Rev 5:CD011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M (2018) Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology 43:2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Sullivan MA, Vosburg SK, Manubay JM, Haney M, Foltin RW, Evans SM, Kowalczyk WJ, Saccone PA, Comer SD (2012) Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behav Pharmacol 23:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett AD, Henderson G, McKnight AT, Paterson SJ (2006) 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol 147(Suppl 1):S153–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM (2003) Sex differences in opioid analgesia: “from mouse to man.” Clin J Pain 19:175–186. [DOI] [PubMed] [Google Scholar]
- Craft RM (2008) Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol 16:376–385. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kalivas PW, Stratmann JA (1996) Sex differences in discriminative stimulus effects of morphine in the rat. Behav Pharmacol 7:764–778. [PubMed] [Google Scholar]
- Crettol S, de Leon J, Hiemke C, Eap CB (2014) Pharmacogenomics in psychiatry: from therapeutic drug monitoring to genomic medicine. Clin Pharmacol Ther 95:254–257. [DOI] [PubMed] [Google Scholar]
- Crist RC, Reiner BC, Berrettini WH (2019) A review of opioid addiction genetics. Curr Opin Psychol 27:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillon P, Alonso S, L’Hermite J, Reubrecht V, Zoric L, Vialles N, Luc Faillie J, Kouyoumdjian P, Boisson C, Raux M, et al. (2021) Post-operative opioid-related adverse events with intravenous oxycodone compared to morphine: a randomized controlled trial. Acta Anaesthesiol Scand 65:40–46. [DOI] [PubMed] [Google Scholar]
- Danhof M (2016) Systems pharmacology—towards the modeling of network interactions. Eur J Pharm Sci 94:4–14. [DOI] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL (2015) Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 372:241–248. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJH, Laird JMA, Belmonte C, Cervero F, Hunt SP (1998) Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 392:394–397. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Stopponi S, Demopulos G, Gaitanaris G, Ciccocioppo R (2014) Analgesic tolerance to morphine is regulated by PPARγ. Br J Pharmacol 171:5407–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O (2020) Dopamine D3 receptor antagonism reverses the escalation of oxycodone self-administration and decreases withdrawal-induced hyperalgesia and irritability-like behavior in oxycodone-dependent heterogeneous stock rats. Front Behav Neurosci 13:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, Giordano A, Senzacqua M, Somaini L, Cippitelli A, et al. (2015) PPARγ activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology 40:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo Candeia GLO, Costa WK, de Oliveira AM, Napoleão TH, Guedes Paiva PM, Ferreira MRA, Lira Soares LA (2022) Anti-inflammatory, antinociceptive effects and involvement of opioid receptors in the antinociceptive activity of Eugenia uniflora leaves obtained with water, ethanol, and propylene glycol mixture. J Ethnopharmacol 296:115508. [DOI] [PubMed] [Google Scholar]
- de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneau GA, Seevers MH (1964) Pharmacological aspects of drug dependence. Adv Pharmacol 3:267–283. [DOI] [PubMed] [Google Scholar]
- Deodhar M, Turgeon J, Michaud V (2021) Contribution of CYP2D6 functional activity to oxycodone efficacy in pain management: genetic polymorphisms, phenoconversion, and tissue-selective metabolism. Pharmaceutics 13:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry S, Stannard C, Cole P, Wiffen PJ, Knaggs R, Aldington D, Moore RA (2016) Fentanyl for neuropathic pain in adults. Cochrane Database Syst Rev 10:CD011605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S (2000) Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides 21:1125–1130. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S, Catalani A, Loizzo A (1999) Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett 272:183–186. [DOI] [PubMed] [Google Scholar]
- Dib B, Duclaux R (1982) Intracerebroventricular self-injection of morphine in response to pain in the rat. Pain 13:395–406. [DOI] [PubMed] [Google Scholar]
- D’Ottavio G, Reverte I, Ragozzine D, Meringolo M, Milella MS, Boix F, Venniro M, Badiani A, Caprioli D (2023) Increased heroin intake and relapse vulnerability in intermittent relative to continuous self-administration: sex differences in rats. Br J Pharmacol 180:910–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douton JE, Augusto C, Stoltzfus B, Carkaci-Salli N, Vrana KE, Grigson PS (2021) Glucagon-like peptide-1 receptor agonist, exendin-4, reduces reinstatement of heroin-seeking behavior in rats. Behav Pharmacol 32:265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy NB, Halbach H, Braenden OJ (1957) Synthetic substances with morphine-like effect: clinical experience: potency, side-effects, addiction liability. Bull World Health Organ 17:569–863. [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Augustson E, Fillingim R (2003) Differential relationships between anxiety and treatment-associated pain reduction among male and female chronic pain patients. Clin J Pain 19:208–216. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Iversen SD (1986) Behavioural effects of tachykinins and related peptides. Brain Res 381:68–76. [DOI] [PubMed] [Google Scholar]
- Els C, Jackson TD, Hagtvedt R, Kunyk D, Sonnenberg B, Lappi VG, Straube S (2017) High-dose opioids for chronic non-cancer pain: an overview of Cochrane Reviews. Cochrane Database Syst Rev 10:CD012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enga RM, Jackson A, Damaj MI, Beardsley PM (2016) Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. Eur J Pharmacol 789:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Hu G, Ma S, Xie X-Q (2015) Computational advances for the development of allosteric modulators and bitopic ligands in G-protein-coupled receptors. AAPS J 17:1080–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB (2002) Sex differences in analgesic responses: evidence from experimental pain models. Eur J Anaesthesiol Suppl 26:16–24. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Gear RW (2004) Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain 8:413–425. [DOI] [PubMed] [Google Scholar]
- Forrester MB (2007) Oxycodone abuse in Texas, 1998-2004. J Toxicol Environ Health A 70:534–538. [DOI] [PubMed] [Google Scholar]
- Franke P, Wang T, Nöthen MM, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W (2001) Nonreplication of association between μ-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. Am J Med Genet 105:114–119. [PubMed] [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H (1961) Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs. (B) a short-term “direct” addiction test. J Pharmacol Exp Ther 133:371–387. [PubMed] [Google Scholar]
- Fredriksson I, Tsai PJ, Shekara A, Duan Y, Applebey SV, Minier-Toribio A, Batista A, Chow JJ, Altidor L, Barbier E, et al. (2023) Role of ventral subiculum neuronal ensembles in incubation of oxycodone craving after electric barrier-induced voluntary abstinence. Sci Adv 9:eadd8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita W, Gomes I, Devi LA (2015) Heteromers of μ-δ opioid receptors: new pharmacology and novel therapeutic possibilities. Br J Pharmacol 172:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa Y, Katz JL, Suzuki T (1989) Effects of a selective kappa-opioid agonist, U-50,488H, on morphine dependence in rats. Eur J Pharmacol 170:47–51. [DOI] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Hafeez H, Price ME, Baruffaldi F, Pravetoni M, Cheng K, Rice KC, Manvich DF, Schank JR (2020) Sex differences in oral oxycodone self-administration and stress-primed reinstatement in rats. Addict Biol 25:e12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel F, Hovhannisyan V, Andry V, Goumon Y (2023) Central metabolism as a potential origin of sex differences in morphine antinociception but not induction of antinociceptive tolerance in mice. Br J Pharmacol 180:843–861. [DOI] [PubMed] [Google Scholar]
- Gadd CA, Murtra P, De Felipe C, Hunt SP (2003) Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J Neurosci 23:8271–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GP, Stringfellow EJ, DiGennaro C, Poellinger N, Wood J, Wakeman S, Jalali MS (2022) Opioid overdose decedent characteristics during COVID-19. Ann Med 54:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell H, Derry S, Stannard C, Moore RA (2016) Oxycodone for neuropathic pain in adults. Cochrane Database Syst Rev 7:CD010692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari S, Cogliani V, Manouras L, Anderson EM, Mitsi V, Avrampou K, Carr FB, Zachariou V (2017) RGS9-2 modulates responses to oxycodone in pain-free and chronic pain states. Neuropsychopharmacology 42:1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari S, Papachatzaki MM, Koo JW, Carr FB, Tsimpanouli ME, Stergiou E, Bagot RC, Ferguson D, Mouzon E, Chakravarty S, et al. (2014) Nucleus accumbens-specific interventions in RGS9-2 activity modulate responses to morphine. Neuropsychopharmacology 39:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauld C, Baillet E, Micoulaud-Franchi J-A, Kervran C, Serre F, Auriacombe M (2023) The centrality of craving in network analysis of five substance use disorders. Drug Alcohol Depend 245:109828. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ (2003) Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23:3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini H, Rezayof A, Ghasemzadeh Z, Zarrindast MR (2015) μ-Opioid and N-methyl-D-aspartate receptors in the amygdala contribute to minocycline-induced potentiation of morphine analgesia in rats. Behav Pharmacol 26:383–392. [DOI] [PubMed] [Google Scholar]
- Gil KM, Ginsberg B, Muir M, Sykes D, Williams DA (1990) Patient-controlled analgesia in postoperative pain: the relation of psychological factors to pain and analgesic use. Clin J Pain 6:137–142. [DOI] [PubMed] [Google Scholar]
- Gimbel JS, Richards P, Portenoy RK (2003) Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology 60:927–934. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Jarbe TUC, Frankenheim J (1991) Drug discrimination: applications to drug abuse research. Natl Inst Drug Abuse Res Monogr Ser 116:1–408. [Google Scholar]
- Glennon RA, Rosecrans JA, Young R (1983) Drug-induced discrimination: a description of the paradigm and a review of its specific application to the study of hallucinogenic agents. Med Res Rev 3:289–340. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Barrett JE (1983) Drug history modifies the behavioral effects of pentobarbital. Science 220:333–335. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Woods JH (1986) Kappa receptor mediated opioid dependence in rhesus monkeys. Life Sci 39:987–992. [DOI] [PubMed] [Google Scholar]
- Gonek M, Akbarali HI, Henderson G, Dewey WL (2017) Reversal of oxycodone and hydrocodone tolerance by diazepam. Brain Res 1674:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella SL, Levy A, Campbell A, Djazayeri S, Allen CP, Goddard B, Leri F (2011) Oxycodone dose-dependently imparts conditioned reinforcing properties to discrete sensory stimuli in rats. Pharmacol Res 64:364–370. [DOI] [PubMed] [Google Scholar]
- Guha SK, Alonso-Caraballo Y, Driscoll GS, Babb JA, Neal M, Constantino NJ, Lintz T, Kinard E, Chartoff EH (2022) Ranking the contribution of behavioral measures comprising oxycodone self-administration to reinstatement of drug-seeking in male and female rats. Front Behav Neurosci 16:1035350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K-K, Deng C-Q, Lu G-J, Zhao G-L (2018) Comparison of analgesic effect of oxycodone and morphine on patients with moderate and advanced cancer pain: a meta-analysis. BMC Anesthesiol 18:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern IM, Bonica JJ (1976). Analgesics, in Drugs of Choice 1976–1977 (Modell W ed), pp 213, Mosby, St. Louis, MO. [Google Scholar]
- Hamid FA, Marker CL, Raleigh MD, Khaimraj A, Winston S, Pentel PR, Pravetoni M (2022) Pre-clinical safety and toxicology profile of a candidate vaccine to treat oxycodone use disorder. Vaccine 40:3244–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WG, Gargiulo JM, Reynolds TR, Parks NL (2022) Prospective randomized study using pharmacogenetics to customize postoperative pain medication following hip and knee arthroplasty. J Arthroplasty 37(6S):S76–S81. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Perdrizet J, Franklin KBJ (2012) The effect of morphine dependence on impulsive choice in rats. Psychopharmacology (Berl) 223:477–487. [DOI] [PubMed] [Google Scholar]
- Hassan HE, Myers AL, Lee IJ, Coop A, Eddington ND (2007) Oxycodone induces overexpression of P-glycoprotein (ABCB1) and affects paclitaxel’s tissue distribution in Sprague Dawley rats. J Pharm Sci 96:2494–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH (2010) Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci 1187:4–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiskanen T, Kalso E (1997) Controlled-release oxycodone and morphine in cancer related pain. Pain 73:37–45. [DOI] [PubMed] [Google Scholar]
- Hill R, Canals M (2022) Experimental considerations for the assessment of in vivo and in vitro opioid pharmacology. Pharmacol Ther 230:107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Dewey WL, Kelly E, Henderson G (2018) Oxycodone-induced tolerance to respiratory depression: reversal by ethanol, pregabalin and protein kinase C inhibition. Br J Pharmacol 175:2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134:943–944. [DOI] [PubMed] [Google Scholar]
- Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439. [DOI] [PubMed] [Google Scholar]
- Holtman JR Jr, Wala EP (2006) Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacol Biochem Behav 83:100–108. [DOI] [PubMed] [Google Scholar]
- Hossain MK, Davidson M, Kypreos E, Feehan J, Muir JA, Nurgali K, Apostolopoulos V (2022) Immunotherapies for the treatment of drug addiction. Vaccines (Basel) 10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser Y-I, Anglin MD, Booth MW (1987a) Sex differences in addict careers. 3. Addiction. Am J Drug Alcohol Abuse 13:231–251. [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Anglin MD, McGlothlin W (1987b) Sex differences in addict careers. 1. Initiation of use. Am J Drug Alcohol Abuse 13:33–57. [DOI] [PubMed] [Google Scholar]
- Hudcova J, McNicol E, Quah C, Lau J, Carr DB (2006) Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev 4:CD003348. [DOI] [PubMed] [Google Scholar]
- Hunt KH, Hughes CE, Pitts RC (2020) Effects of oxycodone on sensitivity to reinforcement magnitude: Implications for effects of opioids on impulsive and risky choice. Behav Pharmacol 31:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MRLewis, SSCoats BDSkyba DACrysdale NYBerkelhammer DLBrzeski ANorthcutt AVietz CMJudd CM, et al. (2009) Reduction in opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun 23:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, et al. (2008) Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun 22:1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICD-11 (2018) International Classification of Diseases 11th Revision. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Iriah SC, Trivedi M, Kenkel W, Grant SE, Moore K, Yee JR, Madularu D, Kulkarni P, Ferris CF (2019) Oxycodone exposure: a magnetic resonance imaging study in response to acute and chronic oxycodone treatment in rats. Neuroscience 398:88–101. [DOI] [PubMed] [Google Scholar]
- Ishida T, Oguri K, Yoshimura H (1982) Determination of oxycodone metabolites in urines and feces of several mammalian species. J Pharmacobiodyn 5:521–525. [DOI] [PubMed] [Google Scholar]
- Jimenez SM, Healy AF, Coelho MA, Brown CN, Kippin TE, Szumlinski KK (2017) Variability in prescription opioid intake and reinforcement amongst 129 substrains. Genes Brain Behav 16:709–724. [DOI] [PubMed] [Google Scholar]
- Jones CM(2013) Trends in the distribution of selected opioids by state, U.S., 1999–2001. National Meet Safe States Alliance; 2013 Jun 6; Baltimore, MD. [Google Scholar]
- Jones JD, Mumtaz M, Manubay JM, Mogali S, Sherwin E, Martinez S, Comer SD (2019) Assessing the contribution of opioid- and dopamine-related genetic polymorphisms to the abuse liability of oxycodone. Pharmacol Biochem Behav 186:172778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay JM, Mogali S, Metz VE, Ciccocioppo R, Comer SD (2016) The effects of pioglitazone, a PPARγ receptor agonist, on the abuse liability of oxycodone among nondependent opioid users. Physiol Behav 159:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay J, Vosburg SK, Comer SD (2011) The subjective, reinforcing, and analgesic effects of oxycodone in patients with chronic, non-malignant pain who are maintained on sublingual buprenorphine/naloxone. Neuropsychopharmacology 36:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD (1996) Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther 59:52–61. [DOI] [PubMed] [Google Scholar]
- Kakko J, Alho H, Baldacchino A, Molina R, Nava FA, Shaya G (2019) Craving in opioid use disorder: from neurobiology to clinical practice. Front Psychiatry 10:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Carrette LLG, Kononoff J, Solberg Woods LC, Palmer AA, Schweitzer P, George O, de Guglielmo G (2020) Nociceptin attenuates the escalation of oxycodone self-administration by normalizing CeA-GABA transmission in highly addicted rats. Proc Natl Acad Sci USA 117:2140–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalso E (2005) Oxycodone. J Pain Symptom Manage 29 (Suppl 5):S47–S56. [DOI] [PubMed] [Google Scholar]
- Kalso E (2007) How different is oxycodone from morphine? Pain 132:227–228. [DOI] [PubMed] [Google Scholar]
- Kalso E, Pöyhiä R, Onnela P, Linko K, Tigerstedt I, Tammisto T (1991) Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand 35:642–646. [DOI] [PubMed] [Google Scholar]
- Kalso E, Vainio A (1990) Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther 47:639–646. [DOI] [PubMed] [Google Scholar]
- Kamei J, Ohhashi Y, Aoki T, Kawasima N, Kasuya Y (1992) Streptozotocin-induced diabetes selectively alters the potency of analgesia produced by m-opioid agonists, but not by δ-and κ−opioid agonists. Brain Res 571:199–203. [DOI] [PubMed] [Google Scholar]
- Kanbara T, Nakamura A, Takasu K, Ogawa K, Shibasaki M, Mori T, Suzuki T, Hasegawa M, Sakaguchi G, Kanemasa T (2014) The contribution of Gi/o protein to opioid antinociception in an oxaliplatin-induced neuropathy rat model. J Pharmacol Sci 126:264–273. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J (2014) Operant nociception in nonhuman primates. Pain 155:1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanouse AB, Compton P (2015) The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. J Pain Palliat Care Pharmacother 29:102–114. [DOI] [PubMed] [Google Scholar]
- Katz N, Fernandez K, Chang A, Benoit C, Butler SF (2008) Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain 24:528–535. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2019) Biased receptor signaling in drug discovery. Pharmacol Rev 71:267–315. [DOI] [PubMed] [Google Scholar]
- Kest B, Sarton E, Dahan A (2000) Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology 93:539–547. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Clark JD, Adams JM (2022) Opioids and public health: the prescription opioid ecosystem and need for improved management. Anesthesiology 136:10–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibaly C, Alderete JA, Liu SH, Nasef HS, Law P-Y, Evans CJ, Cahill CM (2021) Oxycodone in the opioid epidemic: high “liking,” “wanting,” and abuse liability. Cell Mol Neurobiol 41:899–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB (2004) Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology (Berl) 173:167–174. [DOI] [PubMed] [Google Scholar]
- Killian A, Bonese K, Rothberg RM, Wainer BH, Schuster CR (1978) Effects of passive immunization against morphine on heroin self-administration. Pharmacol Biochem Behav 9:347–352. [DOI] [PubMed] [Google Scholar]
- Kimbrough A, Kononoff J, Simpson S, Kallupi M, Sedighim S, Palomino K, Conlisk D, Momper JD, de Guglielmo G, George O (2020) Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacology (Berl) 237:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimishima A, Wenthur CJ, Zhou B, Janda KD (2017) An advance in prescription opioid vaccines: overdose mortality reduction and extraordinary alteration of drug half-life. ACS Chem Biol 12:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen M, Piirainen P, Kokki H, Lammi P, Kokki M (2019) Updated clinical pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacokinet 58:705–725. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA (2019) Respiratory depression and brain hypoxia induced by opioid drugs: morphine, oxycodone, heroin, and fentanyl. Neuropharmacology 151:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen MK, Thomsen M, Wortwein G, Fink-Jensen A (2022) The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br J Pharmacol 179:625–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC (2015) The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health 36:559–574. [DOI] [PubMed] [Google Scholar]
- Koyyalagunta D, Bruera E, Solanki DR, Nouri KH, Burton AW, Toro MP, Bruel BM, Manchikanti L (2012) A systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician 15(3, Suppl):ES39–ES58. [PubMed] [Google Scholar]
- Kumar V, Bonifazi A, Ellenberger MP, Keck TM, Pommier E, Rais R, Slusher BS, Gardner E, You Z-B, Xi Z-X, et al. (2016) Highly selective dopamine D3 receptor (D3R) antagonists and partial agonists based on eticlopride and the D3R crystal structure: new leads for opioid dependence treatment. J Med Chem 59:7634–7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Wyse BD, Meutermans W, Smith MT (2015) In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile. Br J Pharmacol 172:532–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (2006) Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther 79:461–479. [DOI] [PubMed] [Google Scholar]
- Lane JR, Sexton PM, Christopoulos A (2013) Bridging the gap: bitopic ligands of G-protein-coupled receptors. Trends Pharmacol Sci 34:59–66. [DOI] [PubMed] [Google Scholar]
- Lasagna L, Von Felsinger JM, Beecher HK (1955) Drug-induced mood changes in man. I. Observations on healthy subjects, chronically ill patients, and postaddicts. J Am Med Assoc 157:1006–1020. [DOI] [PubMed] [Google Scholar]
- Lawson R, Čechová P, Zarrouk E, Javellaud J, Bazgier V, Otyepka M, Trouillas P, Picard N, Marquet P, Saint-Marcoux F, et al. (2023) Metabolic interactions of benzodiazepines with oxycodone ex vivo and toxicity depending on usage patterns in an animal model. Br J Pharmacol 180:829–842. [DOI] [PubMed] [Google Scholar]
- Leino K, Mildh L, Lertola K, Seppälä T, Kirvelä O (1999) Time course of changes in breathing pattern in morphine- and oxycodone-induced respiratory depression. Anaesthesia 54:835–840. [DOI] [PubMed] [Google Scholar]
- Lemberg KK, Heiskanen TE, Kontinien VK, Kalso EA (2009) Pharmacology of oxycodone: does it explain why oxycodone has become a bestselling strong opioid. Scand J Pain 1:S18–S23. [Google Scholar]
- Lemberg KK, Kontinen VK, Siiskonen AO, Viljakka KM, Yli-Kauhaluoma JT, Korpi ER, Kalso EA (2006a) Antinociception by spinal and systemic oxycodone: why does the route make a difference? In vitro and in vivo studies in rats. Anesthesiology 105:801–812. [DOI] [PubMed] [Google Scholar]
- Lemberg K, Kontinen VK, Viljakka K, Kylänlahti I, Yli-Kauhaluoma J, Kalso E (2006b) Morphine, oxycodone, methadone and its enantiomers in different models of nociception in the rat. Anesth Analg 102:1768–1774. [DOI] [PubMed] [Google Scholar]
- Lemberg K, Korpi ER, Siiskonen AO, Yli-Kauhaluoma JT, Kontinen VK, Vijakka KM, Kalso EA (2007) Oxycodone’s mechanism of action and potency differences after spinal and systemic routes of administration. Anesthesiol 106:1064–1065. [DOI] [PubMed] [Google Scholar]
- Lemberg KK, Siiskonen AO, Kontinen VK, Yli-Kauhaluoma JT, Kalso EA, Korpi ER, Kalso EA (2008) Pharmacological characterization of noroxymorphone as a new opioid for spinal analgesia. Anesth Analg 106:463–470. [DOI] [PubMed] [Google Scholar]
- Leonard MZ, Kangas BD (2020) Effects of oxycodone and diazepam alone and in combination on operant nociception. Behav Pharmacol 31:168–173. [DOI] [PubMed] [Google Scholar]
- Leow KP, Smith MT (1994) The antinociceptive potencies of oxycodone, noroxycodone and morphine after intracerebroventricular administration to rats. Life Sci 54:1229–1236. [DOI] [PubMed] [Google Scholar]
- Leri F, Burns LH (2005) Ultra-low-dose naltrexone reduces the rewarding potency of oxycodone and relapse vulnerability in rats. Pharmacol Biochem Behav 82:252–262. [DOI] [PubMed] [Google Scholar]
- Li N, Wu Z, Ma C (2022) ZHX2 in health and disease. Front Oncol 12:1038890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius T, Kangas E, Niemi M, Rauhala P, Kalso E (2018) Ketamine and norketamine attenuate oxycodone tolerance markedly less than that of morphine: from behaviour to drug availability. Br J Anaesth 120:818–826. [DOI] [PubMed] [Google Scholar]
- Linares OA, Daly D, Linares AD, Stefanovski D, Boston RC (2014) Personalized oxycodone dosing: using pharmacogenetic testing and clinical pharmacokinetics to reduce toxicity risk and increase effectiveness. Pain Med 15:791–806. [DOI] [PubMed] [Google Scholar]
- Liukas A, Kuusniemi K, Aantaa R, Virolainen P, Neuvonen M, Neuvonen PJ, Olkkola KT (2008) Plasma concentrations of oral oxycodone are greatly increased in the elderly. Clin Pharmacol Ther 84:462–467. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Nuzzo PA, Walsh SL (2012) Effects of cold pressor pain on the abuse liability of intranasal oxycodone in male and female prescription opioid abusers. Drug Alcohol Depend 123:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow LM, Shashkova EC, Miller MG, Evans CJ, Cahill CM (2020) Insights into the neurobiology of craving in opioid use disorder. Curr Anesthesiol Rep 10:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144:77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 164:121–137. [DOI] [PubMed] [Google Scholar]
- Lyu Z, Schmidt RR, Martin RE, Green MT, Kinkade JA, Mao J, Bivens NJ, Joshi T, Rosenfeld CS (2022) Long-term effects of developmental exposure to oxycodone on gut microiota and relationship to adult behaviors and metabolism. mSystems 7:e0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks I, Somogyi A, Abbott F, Hayball P, Parker D (1996) Attenuation of morphine-induced delirium in palliative care by substitution with infusion of oxycodone. J Pain Symptom Manage 12:182–189. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Li J-X, France CP (2012) Impulsivity and drugs of abuse: a juice-reinforced operant procedure for determining within-session delay discounting functions in rhesus monkeys. J Pharmacol Toxicol Methods 66:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G (2012) Multiple roles for orexin/hypocretin in addiction. Prog Brain Res 198:79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW (2011) Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA 108:19778–19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Girdlestone D, Roques BP (1993) RP 67580, a selective antagonist of neurokinin-1 receptors, modifies some of the naloxone-precipitated morphine withdrawal signs in rats. Neurosci Lett 156:135–140. [DOI] [PubMed] [Google Scholar]
- Manchikanti L (2007) National drug control policy and prescription drug abuse: facts and fallacies. Pain Physician 10:399–424. [PubMed] [Google Scholar]
- Marker CL, Cintora SC, Roman MI, Stoffel M, Wickman K (2002) Hyperalgesia and blunted morphine analgesia in G protein-gated potassium channel subunit knockout mice. Neuroreport 13:2509–2513. [DOI] [PubMed] [Google Scholar]
- Marker CL, Stoffel M, Wickman K (2004) Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci 24:2806–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D (2014) “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy 25:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Ewan E (2008) Chronic pain alters drug self-administration: implications for addiction and pain mechanisms. Exp Clin Psychopharmacol 16:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC (2007) Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology 106:312–322. [DOI] [PubMed] [Google Scholar]
- Martin WR, Gorodetzky CW, Kay DC, McClane TK, Jasinski DR (1966) Appendix 19. Activities of the Addiction Research Center during 1965, Dependence assessment studies of narcotic analgesics, in Committee on Problems of Drug Dependence, Minutes of the 28th meeting, National Academy of Sciences, National Research Council, Washington, DC, pp. 4658–4861. [Google Scholar]
- Martinez S, Harris H, Chao T, Luba R, Pravetoni M, Comer SD, Jones JD (2023) The potential role of opioid vaccines and monoclonal antibodies in the opioid overdose crisis. Expert Opin Investig Drugs 32:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins SS, Keyes KM, Storr CL, Zhu H, Chilcoat HD (2009b) Pathways between nonmedical opioid use/dependence and psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 103:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Navarro M, Maldonado R, Baños J-E (2019) Why mu-opioid agonists have less analgesic efficacy in neuropathic pain? Eur J Pain 23:435–454. [DOI] [PubMed] [Google Scholar]
- Martins SS, Storr CL, Zhu H, Chilcoat HD (2009a) Correlates of extramedical use of OxyContin versus other analgesic opioids among the US general population. Drug Alcohol Depend 99:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Swanson DW (1981) Problems with the use of oxycodone compound in patients with chronic pain. Pain 11:389–396. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Martin-Fardon R (2020) Targeting the orexin system for prescription opioid use disorder: orexin-1 receptor blockade prevents oxycodone taking and seeking in rats. Neuropharmacology 164:107906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E (2017) Oxycodone self-administration in male and female rats. Psychopharmacology (Berl) 234:977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Blackwell B, Schlussman SD, Butelman ER, Ho A, Ott J, Kreek MJ, Zhang Y (2014) Self administration of oxycodone by adolescent and adult mice affects striatal neurotransmitter receptor gene expression. Neuroscience 258:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearney JW, Barrett JE (1975) Punished behavior: increases in responding after d-amphetamine. Psychopharmacology (Berl) 41:23–26. [DOI] [PubMed] [Google Scholar]
- McKearney JW, Barrett JE (1978) Schedule-controlled behavior and the effects of drugs, in Contemporary Research in Behavioral Pharmacology (Blackman DE, Sanger DJ, eds) pp 1–68, Plenum Press, New York. [Google Scholar]
- McNicol ED, Midbari A, Eisenberg E (2013) Opioids for neuropathic pain. Cochrane Database Syst Rev 2013:CD006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meert TF, Vermeirsch HA (2005) A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol Biochem Behav 80:309–326. [DOI] [PubMed] [Google Scholar]
- Mei XP, Chen L, Wang W, Wu D, Wang LY, Zhang T, Zhang H, Xu LX, Li YQ (2013) Combination of tramadol with minocycline exerted synergistic effects on a rat model of nerve injury-induced neuropathic pain. Neurosignals 21:184–196. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Tirelli W, David F, Arcara C, Fulfaro F, Casuccio A, Gebbia V (2010) Morphine versus oxycodone in pancreatic cancer pain: a randomized controlled study. Clin J Pain 26:794–797. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P (1999) Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280. [DOI] [PubMed] [Google Scholar]
- Merkel R, Moreno A, Zhang Y, Herman R, Ben Nathan J, Zeb S, Rahematpura S, Stecyk K, Milliken BT, Hayes MR, et al. (2021) A novel approach to treating opioid use disorders: dual agonists of glucagon-like peptide-1 receptors and neuropeptide Y2 receptors. Neurosci Biobehav Rev 131:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz VE, Jones JD, Manubay J, Sullivan MA, Mogali S, Segoshi A, Madera G, Johnson KW, Comer SD (2017) Effects of ibudilast on the subjective, reinforcing, and analgesic effects of oxycodone in recently detoxified adults with opioid dependence. Neuropsychopharmacology 42:1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Hasegawa M, Ito H, Nakamura A, Tomii T, Matsumoto M, Orita S, Matsushima S, Miyoshi T, Masuno K, et al. (2009) Morphine, oxycodone, and fentanyl exhibit different analgesic profiles in mouse pain models. J Pharmacol Sci 111:60–72. [DOI] [PubMed] [Google Scholar]
- Mogali S, Askalsky P, Madera G, Jones JD, Comer SD (2021) Minocycline attenuates oxycodone-induced positive subjective responses in non-dependent, recreational opioid users. Pharmacol Biochem Behav 209:173241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2020) Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 21:353–365. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK (1996) Orphanin FQ is a functional anti-opioid peptide. Neuroscience 75:333–337. [DOI] [PubMed] [Google Scholar]
- Monory K, Greiner E, Sartania N, Sallai L, Pouille Y, Schmidhammer H, Hanoune J, Borsodi A (1999) Opioid binding profiles of new hydrazone, oxime, carbazone and semicarbazone derivatives of 14-alkoxymorphinans. Life Sci 64:2011–2020. [DOI] [PubMed] [Google Scholar]
- Montandon G (2022) The pathophysiology of opioid-induced respiratory depression. Handb Clin Neurol 188:339–355. [DOI] [PubMed] [Google Scholar]
- Moore K, Madularu D, Iriah S, Yee JR, Kulkarni P, Darcq E, Kieffer BL, Ferris CF (2016) BOLD imaging in awake wild-type and mu-opioid receptor knock-out mice reveals on-target activation maps in response to oxycodone. Front Neurosci 10:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M, Esmaeili S, Shoar S, Safari S (2012) Use of oxycodone in pain management. Anesth Pain Med 1:262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhuri PK, Gfroerer JC, Davies MC (2013) Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Review, SAMHSA, https://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm.
- Murphy NP, Maidment NT (1999) Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem 73:179–186. [DOI] [PubMed] [Google Scholar]
- Murtra P, Sheasby AM, Hunt SP, De Felipe C (2000) Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature 405:180–183. [DOI] [PubMed] [Google Scholar]
- Nader MA, Tatham TA, Barrett JE (1992) Behavioral and pharmacological determinants of drug abuse. Ann N Y Acad Sci 654:368–385. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Fujita M, Ono H, Hongo Y, Kanbara T, Ogawa K, Morioka Y, Nishiyori A, Shibasaki M, Mori T, et al. (2014) G protein-gated inwardly rectifying potassium (KIR3) channels play a primary role in the antinociceptive effect of oxycodone, but not morphine, at supraspinal sites. Br J Pharmacol 171:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Hasegawa M, Minami K, Kanbara T, Tomii T, Nishiyori A, Narita M, Suzuki T, Kato A (2013) Differential activation of the μ-opioid receptor by oxycodone and morphine in pain-related brain regions in a bone cancer pain model. Br J Pharmacol 168:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nakamura A, Ozaki M, Imai S, Miyoshi K, Suzuki M, Suzuki T (2008) Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: evidence for less sensitivity to morphine. Neuropsychopharmacology 33:1097–1112. [DOI] [PubMed] [Google Scholar]
- Nasseef MT, Singh JP, Ehrlich AT, McNicholas M, Park DW, Ma W, Kulkarni P, Kieffer BL, Darcq E (2019) Oxycodone-mediated activation of the mu opioid receptor reduces whole brain functional connectivity in mice. ACS Pharmacol Transl Sci 2:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazemi S, Manaheji H, Zaringhalam J, Sadeghi M, Haghparast A (2012) Post-injury repeated administrations of minocycline improve the antinociceptive effect of morphine in chronic constriction injury model of neuropathic pain in rat. Pharmacol Biochem Behav 102:520–525. [DOI] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA (2017) Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem Neurosci 8:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Ward SJ, Walker EA (2015) Discriminative stimulus effects of morphine and oxycodone in the absence and presence of acetic acid in male and female C57Bl/6 mice. Exp Clin Psychopharmacol 23:217–227. [DOI] [PubMed] [Google Scholar]
- Newman AH, Battiti FO, Bonifazi A (2020) 2016 Philip S. Portoghese Medicinal Chemistry Lectureship: designing bivalent or bitopic molecules for G-protein coupled receptors. The whole is greater than the sum of its parts. J Med Chem 63:1779–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P (2012) Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol 84:882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Hwang CS, Vandewater SA, Janda KD, Cole M, Taffe MA (2019) Δ9-tetrahydrocannabinol attenuates oxycodone self-administration under extended access conditions. Neuropharmacology 151:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Grant Y, Taffe MA (2021) Paradoxical changes in brain reward status during oxycodone self-administration in a novel test of the negative reinforcement hypothesis. Br J Pharmacol 178:3797–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Hwang CS, Grant Y, Janda KD, Taffe MA (2018) Prophylactic vaccination protects against the development of oxycodone self-administration. Neuropharmacology 138:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y (2022) Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharmacol Rev 74:119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT (2007) Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain 132:289–300. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Ross FB, Smith MT (2000) Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the Dark Agouti rat. J Pharmacol Exp Ther 295:91–99. [PubMed] [Google Scholar]
- Nozaki C, Saitoh A, Tamura N, Kamei J (2005) Antinociceptive effect of oxycodone in diabetic mice. Eur J Pharmacol 524:75–79. [DOI] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B (2004) Local peripheral effects of μ-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett 360:85–89. [DOI] [PubMed] [Google Scholar]
- Ohia-Nwoko O, Kosten TA, Haile CN (2016) Animal models and the development of vaccines to treat substance use disorders. Int Rev Neurobiol 126:263–291. [DOI] [PubMed] [Google Scholar]
- Okie S (2010) A flood of opioids, a rising tide of deaths. N Engl J Med 363:1981–1985. [DOI] [PubMed] [Google Scholar]
- Okura T, Hattori A, Takano Y, Sato T, Hammarlund-Udenaes M, Terasaki T, Deguchi Y (2008) Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug Metab Dispos 36:2005–2013. [DOI] [PubMed] [Google Scholar]
- Olkkola KT, Kontinen VK, Saari TI, Kalso EA (2013) Does the pharmacology of oxycodone justify its increasing use as an analgesic? Trends Pharmacol Sci 34:206–214. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Burns LH (2005) Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology (Berl) 181:576–581. [DOI] [PubMed] [Google Scholar]
- Olson KM, Duron DI, Womer D, Fell R, Streicher JM (2019) Comprehensive molecular pharmacology screening reveals potential new receptor interactions for clinically relevant opioids. PLoS One 14:e0217371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong EC (2008) Controlled-release oxycodone in the treatment of neuropathic pain of nonmalignant and malignant causes. Oncology 74(Suppl 1):72–75. [DOI] [PubMed] [Google Scholar]
- Ordóñez Gallego A, González Barón M, Espinosa Arranz E (2007) Oxycodone: a pharmacological and clinical review. Clin Transl Oncol 9:298–307. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F (1995) Inhibition by spinal morphine of the tail-flick response is attenuated in rats with nerve ligation injury. Neurosci Lett 199:83–86. [DOI] [PubMed] [Google Scholar]
- Otton SV, Wu D, Joffe RT, Cheung SW, Sellers EM (1993) Inhibition by fluoxetine of cytochrome P450 2D6 activity. Clin Pharmacol Ther 53:401–409. [DOI] [PubMed] [Google Scholar]
- Pantouli F, Grim TW, Schmid CL, Acevedo-Canabal A, Kennedy NM, Cameron MD, Bannister TD, Bohn LM (2021) Comparison of morphine, oxycodone and the biased MOR agonist SR-17018 for tolerance and efficacy in mouse models of pain. Neuropharmacology 185:108439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen MCW, Wiskerke J, Schoffelmeer ANM (2009) Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl) 205:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham EM, Barkley LM, Divin MF, Cicero TJ, Traynor JR (2005) Comparison of the antinociceptive effect of acute morphine in female and male Sprague-Dawley rats using the long-lasting mu-antagonist methocinnamox. Brain Res 1058:137–147. [DOI] [PubMed] [Google Scholar]
- Peckham EM, Traynor JR (2006) Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther 316:1195–1201. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME (2008) The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 200:1–26. [DOI] [PubMed] [Google Scholar]
- Pert CB, Snyder SH (1973) Properties of opiate-receptor binding in rat brain. Proc Natl Acad Sci USA 70:2243–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, McGovern DJ, Lee S, Ro K, Huynh DT, Elvig SK, Fegan KN, Root DH (2020) Oral prescription opioid-seeking behavior in male and female mice. Addict Biol 25:e12828. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M (2011) Pharmacogenetics: past, present and future. Drug Discov Today 16:852–861. [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP (2005) Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav 83:297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton A, Hester R (2020) Transition to substance use disorders: impulsivity for reward and learning from reward. Soc Cogn Affect Neurosci 15:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyhiä R, Kalso EA (1992) Antinociceptive effects and central nervous system depression caused by oxycodone and morphine in rats. Pharmacol Toxicol 70:125–130. [DOI] [PubMed] [Google Scholar]
- Pöyhiä R, Olkkola KT, Seppälä T, Kalso E (1991) The pharmacokinetics of oxycodone after intravenous injection in adults. Br J Clin Pharmacol 32:516–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyhiä R, Seppälä T, Olkkola KT, Kalso E (1992) The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol 33:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyhiä R, Vainio A, Kalso E (1993) A review of oxycodone’s clinical pharmacokinetics and pharmacodynamics. J Pain Symptom Manage 8:63–67. [DOI] [PubMed] [Google Scholar]
- Pravetoni M (2016) Biologics to treat substance use disorders: current status and new directions. Hum Vaccin Immunother 12:3005–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Comer SD (2019) Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 158:107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR (2012) An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther 341:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, Pentel PR (2013) Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J Med Chem 56:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG (2014) Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One 9:e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewłocki R, Przewłocka B (2001) Opioids in chronic pain. Eur J Pharmacol 429:79–91. [DOI] [PubMed] [Google Scholar]
- Psifogeorgou K, Papakosta P, Russo SJ, Neve RL, Kardassis D, Gold SJ, Zachariou V (2007) RGS9-2 is a negative modulator of mu-opioid receptor function. J Neurochem 103:617–625. [DOI] [PubMed] [Google Scholar]
- Psifogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, Gold SJ, Zachariou V (2011) A unique role of RGS9-2 in the striatum as a positive or negative regulator of opiate analgesia. J Neurosci 31:5617–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM (2011) The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Accetturo C, Pravetoni M (2020) Combining a candidate vaccine for opioid use disorders with extended-release naltrexone increases protection against oxycodone-induced behavioral effects and toxicity. J Pharmacol Exp Ther 374:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, King SJ, Baruffaldi F, Saykao A, Hamid FA, Winston S, LeSage MG, Pentel PR, Pravetoni M (2021) Pharmacological mechanisms underlying the efficacy of antibodies generated by a vaccine to treat oxycodone use disorder. Neuropharmacology 195:108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Laudenbach M, Baruffaldi F, Peterson SJ, Roslawski MJ, Birnbaum AK, Carroll FI, Runyon SP, Winston S, Pentel PR, Pravetoni M (2018) Opioid dose- and route-dependency of oxycodone and heroin vaccines in rats. J Pharmacol Exp Ther 365:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Peterson SJ, Laudenbach M, Baruffaldi F, Carroll FI, Comer SD, Navarro HA, Langston TL, Runyon SP, Winston S, et al. (2017) Safety and efficacy of an oxycodone vaccine: addressing some of the unique considerations posed by opioid abuse. PLoS One 12:e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randesi M, Contoreggi NH, Zhou Y, Rubin BR, Bellamy JR, Yu F, Gray JD, McEwen BS, Milner TA, Kreek MJ (2019) Sex differences in neuroplasticity- and stress related gene expression and protein levels in the rat hippocampus following oxycodone conditioned place preference. Neuroscience 410:274–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Toda K, Ueda H (2004) Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther 309:380–387. [DOI] [PubMed] [Google Scholar]
- Reisine T, Pasternak G (1996) Opioid analgesics and antagonists, in Goodman and Gilman’s the Pharmacological Basis of Therapeutics (Hardman JG, Limbird LE, eds) pp 521–556, McGraw-Hill, New York. [Google Scholar]
- Remillard D, Kaye AD, McAnally H (2019) Oxycodone’s unparalleled addictive potential: is it time for a moratorium? Curr Pain Headache Rep 23:15. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Riley J, Eisenberg E, Müller-Schwefe G, Drewes AM, Arendt-Nielsen L (2008) Oxycodone: a review of its use in the management of pain. Curr Med Res Opin 24:175–192. [DOI] [PubMed] [Google Scholar]
- Riley JL 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB (1998) Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 74:181–187. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN (2002) Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology 43:1258–1268. [DOI] [PubMed] [Google Scholar]
- Robson LE, Paterson SJ, Kosterlitz HW (1983) Opiate receptors, in Handbook of Psychopharmacology, Vol. 17 (Iversen LL, Iversen SD, Snyder SH, eds) pp 13–79, Plenum Press, New York. [Google Scholar]
- Romberg RR, Olofsen E, Bijl H, Taschner PEM, Teppema LJ, Sarton EY, van Kleef JW, Dahan A (2005) Polymorphism of μ-opioid receptor gene (OPRM1:c.118A>G) does not protect against opioid-induced respiratory depression despite reduced analgesic response. Anesthesiology 102:522–530. [DOI] [PubMed] [Google Scholar]
- Ross FB, Smith MT (1997) The intrinsic antinociceptive effects of oxycodone appear to be κ-opioid receptor mediated. Pain 73:151–157. [DOI] [PubMed] [Google Scholar]
- Roux P, Sullivan MA, Cohen J, Fugon L, Jones JD, Vosburg SK, Cooper ZD, Manubay JM, Mogali S, Comer SD (2013) Buprenorphine/naloxone as a promising therapeutic option for opioid abusing patients with chronic pain: reduction of pain, opioid withdrawal symptoms, and abuse liability of oral oxycodone. Pain 154:1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X, Mancuso KF, Kaye AD (2017) Revisiting oxycodone analgesia: a review and hypothesis. Anesthesiol Clin 35:e163–e174. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Zhou Y, Contoreggi NH, Bshesh FK, Gray JD, Kogan JF, Ben KT, McEwen BS, Jeanne Kreek M, Milner TA (2018) Sex differences in the rat hippocampal opioid system after oxycodone conditioned place preference. Neuroscience 393:236–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM (2010) Effects of the NOP receptor agonist Ro65-6570 on the acquisition of opiate- and psychostimulant-induced conditioned place preference in rats. Eur J Pharmacol 645:119–126. [DOI] [PubMed] [Google Scholar]
- Sadée W, Dai Z (2005) Pharmacogenetics/genomics and personalized medicine. Hum Mol Genet 14(Spec No. 2):R207–R214. [DOI] [PubMed] [Google Scholar]
- Sadée W, Wang D, Hartmann K, Toland AE (2023) Pharmacogenomics: driving personalized medicine. Pharmacol Rev 75:789–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury AJ, Blackwood CA, Cadet JL (2021) Prolonged withdrawal from escalated oxycodone is associated with increased expression of glutamate receptors in the rat hippocampus. Front Neurosci 14:617973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, Rossier MF, Hochstrasser D, Dayer P, Desmeules JA (2010a) Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br J Pharmacol 160:919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, Rossier MF, Hochstrasser D, Dayer P, Desmeules JA (2010b) The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol 160:907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson KR, Xu W, Kortagere S, España RA (2022) Intermittent access to oxycodone decreases dopamine uptake in the nucleus accumbens core during abstinence. Addict Biol 27:e13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Carpenter MD, Yohn NL, Blendy JA (2016) Long-lasting effects of adolescent oxycodone exposure on reward-related behavior and gene expression in mice. Psychopharmacology (Berl) 233:3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandweiss AJ, McIntosh MI, Moutal A, Davidson-Knapp R, Hu J, Giri AK, Yamamoto T, Hruby VJ, Khanna R, Largent-Milnes TM, et al. (2018) Genetic and pharmacological antagonism of NK1 receptor prevents opiate abuse potential. Mol Psychiatry 23:1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza F (2003) The DEA perspective on prescription drug diversion and abuse. Proceedings of the 64th annual scientific meeting. The College on Problems of Drug Dependence NIDA Research Monograph vol 183, pp. 83–86. [Google Scholar]
- Schank JR (2014) The neurokinin-1 receptor in addictive processes. J Pharmacol Exp Ther 351:2–8. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM (2017) Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171:1165–1175.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS (2017) Oxycodone for cancer-related pain. Cochrane Database Syst Rev 8:CD003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwienteck KL, Faunce KE, Rice KC, Obeng S, Zhang Y, Blough BE, Grim TW, Negus SS, Banks ML (2019) Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats. Neuropharmacology 150:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seevers MH (1936) Opiate addiction in the monkey I. Methods of study. J Pharmacol Exp Ther 56:147–156. [Google Scholar]
- Seltzer N (2020) The economic underpinnings of the drug epidemic. SSM Popul Health 12:100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal HA, Carlson RG, Kenne DR, Swora MG (2003) Probable relationship between opioid abuse and heroin use. Am Fam Physician 67:942–945, 945. [PubMed] [Google Scholar]
- Shaham Y, Alvares K, Nespor SM, Grunberg NE (1992) Effect of stress on oral morphine and fentanyl self-administration in rats. Pharmacol Biochem Behav 41:615–619. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Klein LC, Alvares K, Grunberg NE (1993) Effect of stress on oral fentanyl consumption in rats in an operant self-administration paradigm. Pharmacol Biochem Behav 46:315–322. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J (1994) Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology (Berl) 114:523–527. [DOI] [PubMed] [Google Scholar]
- Silvasti M, Rosenberg P, Seppälä T, Svartling N, Pitkänen M (1998) Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand 42:576–580. [DOI] [PubMed] [Google Scholar]
- Skarke C, Darimont J, Schmidt H, Geisslinger G, Lötsch J (2003) Analgesic effects of morphine and morphine-6-glucuronide in a transcutaneous electrical pain model in healthy volunteers. Clin Pharmacol Ther 73:107–121. [DOI] [PubMed] [Google Scholar]
- Skolnick P (2015) Biologic approaches to treat substance-use disorders. Trends Pharmacol Sci 36:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivicki RA, Earnest T, Chang YH, Pareta R, Casey E, Li JN, Tooley J, Abiraman K, Vachez YM, Wolf DK, et al. (2023) Oral oxycodone self-administration leads to features of opioid misuse in male and female mice. Addict Biol 28:e13253. [DOI] [PubMed] [Google Scholar]
- Smith MT, Edwards SR, Nielsen CK (2007) Oxycodone’s mechanism of action and potency differences after spinal and systemic routes of administration. Anesthesiology 106:1063–1064, author reply 1064–1065. [DOI] [PubMed] [Google Scholar]
- Sneader W (2005) Drug Discovery: A History, Wiley, Hoboken, NJ. [Google Scholar]
- Spector S (1971) Quantitative determination of morphine in serum by radioimmunoassay. J Pharmacol Exp Ther 178:253–258. [PubMed] [Google Scholar]
- Spector S, Berkowitz B, Flynn EJ, Peskar B (1973) Antibodies to morphine, barbiturates, and serotonin. Pharmacol Rev 25:281–291. [PubMed] [Google Scholar]
- Spector S, Parker CW (1970) Morphine: radioimmunoassay. Science 168:1347–1348. [DOI] [PubMed] [Google Scholar]
- Sproule B, Brands B, Catz-Biro L (2009) Changing patterns in opioid addiction. Characterizing users of oxycodone and other opioids. Can Family Physician 55:68–69.e1-5. [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Woodside B, Shaham Y (1996) Ovarian hormones do not affect the initiation and maintenances of intravenous self-administration of heroin in the female rat. Psychobiology (Austin Tex) 24:154–159. [Google Scholar]
- Stoops WW, Hatton KW, Lofwall MR, Nuzzo PA, Walsh SL (2010) Intravenous oxycodone, hydrocodone, and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology (Berl) 212:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Goto K, Shiizaki K, Omiya Y, Ishige A, Komatsu Y, Kamei J (2001) Antinociceptive effect of U-50488H, a κ-opioid agonist, in streptozotocin-induced diabetic mice. J Pharm Pharmacol 53:521–526. [DOI] [PubMed] [Google Scholar]
- Swain HH, Fly CL, Seevers MH (1977) Evaluation of new compounds for morphine-like physical dependence in the rhesus monkey. Proceedings of the Thirty Ninth Annual Meeting of the Committee on Problems of Drug Dependence, pp. 614–636. [Google Scholar]
- Swedberg MDB (2016) Drug discrimination: a versatile tool for characterization of CNS safety pharmacology and potential for drug abuse. J Pharmacol Toxicol Methods 81:295–305. [DOI] [PubMed] [Google Scholar]
- Takasu K, Ogawa K, Nakamura A, Kanbara T, Ono H, Tomii T, Morioka Y, Hasegawa M, Shibasaki M, Mori T, et al. (2015) Enhanced GABAergic synaptic transmission at VLPAG neurons and potent modulation by oxycodone in a bone cancer pain model. Br J Pharmacol 172:2148–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkkila P, Tuominen M, Lindgren L (1997) Comparison of respiratory effects of tramadol and oxycodone. J Clin Anesth 9:582–585. [DOI] [PubMed] [Google Scholar]
- Tatum AL, Seevers MH, Collins KH (1929) Morphine addiction and its physiological interpretation based on experimental evidences. J Pharmacol Exp Ther 36:447–475. [Google Scholar]
- Thompson CM, Wojno H, Greiner E, May EL, Rice KC, Selley DE (2004) Activation of G-proteins by morphine and codeine congeners: insights to the relevance of O- and N-demethylated metabolites at μ- and δ-opioid receptors. J Pharmacol Exp Ther 308:547–554. [DOI] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Li J-X (2017) Tolerance and cross-tolerance to the antinociceptive effects of oxycodone and the imidazoline I2 receptor agonist phenyzoline in adult male rats. Psychopharmacology (Berl) 234:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Banks ML (2020) Preclinical evaluation of vaccines to treat opioid use disorders: how close are we to a clinically viable therapeutic? CNS Drugs 34:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Naylor JE, Negus SS, Edwards SR, Qureshi HN, McLendon HW, McCurdy CR, Kapanda CN, do Carmo JM, Fernanda SD, et al. (2017) Effects of nalfurafine on oxycodone reinforcement, thermal antinociception and respiration: modeling an abuse-deterrent opioid analgesic in rats. Psychopharmacology (Berl) 234:2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Terzi D, Caldarone BJ, Zachariou V (2009) RGS9-2: probing an intracellular modulator of behavior as a drug target. Trends Pharmacol Sci 30:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong TT, Kosten TR (2022) Current status of vaccines for substance use disorders: a brief review of human studies. J Neurol Sci 434:120098. [DOI] [PubMed] [Google Scholar]
- Umukoro NN, Aruldhas BW, Rossos R, Pawale D, Renschler JS, Sadhasivam S (2021) Pharmacogenomics of oxycodone: a narrative literature review. Pharmacogenomics 22:275–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh AM (1996) Gender variations in clinical pain experience. Pain 65:123–167. [DOI] [PubMed] [Google Scholar]
- Vacca V, Marinelli S, Pieroni L, Urbani A, Luvisetto S, Pavone F (2014) Higher pain perception and lack of recovery from neuropathic pain in females: a behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Pain 155:388–402. [DOI] [PubMed] [Google Scholar]
- Vaille C, Stern G (1954) Drug addiction: medical and social aspects in France. Bull Narcotics 2: 1–4. [Google Scholar]
- van der Schrier R, Jonkman K, van Velzen M, Olofsen E, Drewes AM, Dahan A, Niesters M (2017) An experimental study comparing the respiratory effects of tapentadol and oxycodone in healthy volunteers. Br J Anaesth 119:1169–1177. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Neumark YD, Anthony JC (1999) Male-female differences in the earliest stages of drug involvement. Addiction 94:1413–1419. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Anthony JC (2001) Male-female differences in transitions from first drug opportunity to first use: searching for subgroup variation by age, race, region, and urban status. J Womens Health Gend Based Med 10:797–804. [DOI] [PubMed] [Google Scholar]
- Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ (2014) Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur J Neurosci 40:3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga BR, Streicher JM, Majumdar S (2023) Strategies towards safer opioid analgesics—a review of old and upcoming targets. Br J Pharmacol 180:975–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliu O (2022) Current trends and perspectives in the immune therapy for substance use disorders. Front Psychiatry 13:882491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res 224:25–52. [DOI] [PubMed] [Google Scholar]
- Wade CL, Fairbanks CA (2014) The self-administration of analgesic drugs in experimentally induced chronic pain. Curr Top Behav Neurosci 20:217–232. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF (2015) Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentiny DM, Moisa LT, Beardsley PM (2019) Oxycodone-like discriminative stimulus effects of fentanyl-related emerging drugs of abuse in mice. Neuropharmacology 150:210–216. [DOI] [PubMed] [Google Scholar]
- Walentiny DM, Wiebelhaus JM, Beardsley PM (2018) Nociceptin/orphanin FQ receptors modulate the discriminative stimulus effects of oxycodone in C57BL/6 mice. Drug Alcohol Depend 187:335–342. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Heilig M, Nuzzo PA, Henderson P, Lofwall MR (2013) Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addict Biol 18:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR Jr (2008) The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend 98:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhuang Y, DiBerto JF, Zhou XE, Schmitz GP, Yuan Q, Jain MK, Liu W, Melcher K, Jiang Y, et al. (2023) Structures of the entire human opioid receptor family. Cell 186:413–427.e17. [DOI] [PubMed] [Google Scholar]
- Watson CPN, Babul N (1998) Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology 50:1837–1841. [DOI] [PubMed] [Google Scholar]
- Watson CPN, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J (2003) Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain 105:71–78. [DOI] [PubMed] [Google Scholar]
- Weafer J, Mitchell SH, de Wit H (2014) Recent translational findings on impulsivity in relation to drug abuse. Curr Addict Rep 1:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster LR, Hansen E, Cater J, Smith T (2020) A phase I placebo-controlled trial comparing the effects of buprenorphine buccal film and oral oxycodone hydrochloride administration on respiratory drive. Adv Ther 37:4685–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster LR, Hansen E, Stoddard GJ, Rynders A, Ostler D, Lennon H (2022) Ventilatory response to hypercapnia as experimental model to study effects of oxycodone on respiration depressions. Curr Rev Clin Exp Pharmacol 17:72–80. [DOI] [PubMed] [Google Scholar]
- Webster L, St Marie B, McCarberg B, Passik SD, Panchal SJ, Voth E (2011) Current status and evolving role of abuse-deterrent opioids in managing patients with chronic pain. J Opioid Manag 7:235–245. [DOI] [PubMed] [Google Scholar]
- White JM, Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction 94:961–972. [PubMed] [Google Scholar]
- Wightman R, Perrone J, Portelli I, Nelson L (2012) Likeability and abuse liability of commonly prescribed opioids. J Med Toxicol 8:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withey SL, Doyle RJ, Porter EN, Bergman J, Kangas BD (2020) Discrimination learning in oxycodone-treated nonhuman primates. Drug Alcohol Depend 207:107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withey SL, Paronis CA, Bergman J (2018) Concurrent assessment of the antinociceptive and behaviorally disruptive effects of opioids in squirrel monkeys. J Pain 19:728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff HG, Hardy JD, Goodell H (1940) Measurement of the effect of morphine, codeine, and other opiates on the pain threshold and analysis of their relation to the pain experience. J Clin Invest 19:659–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AK, Somogyi AA, Rubio J, Philip J (2022) The role of pharmacogenomics in opioid prescribing. Curr Treat Options in Oncol 23:1353–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodlief K, Allen MI, Cornelissen JC, Banks ML, Newman AH, Nader MA (2023) Effects of selective dopamine D3 receptor partial agonist/antagonists on oxycodone self-administration and antinociception in monkeys. Neuropsychopharmacol DOI: 10.1038/s41386-023-01590-8 [published ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JH, Ko MC, Winger G, et al. (2003) Evaluation of new compounds for opioid activity. Proceedings of the 64th annual scientific meeting. The College on Problems of Drug Dependence NIDA Research Monograph, vol. 183, pp. 83–86. [Google Scholar]
- Xie K, Masuho I, Brand C, Dessauer CW, Martemyanov KA (2012) The complex of G protein regulator RGS9-2 and Gβ(5) controls sensitization and signaling kinetics of type 5 adenylyl cyclase in the striatum. Sci Signal 5:ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Chockalingam A, Stewart S, Shea K, Matta MK, Narayanasamy S, Pilli NR, Volpe DA, Weaver J, Zhu H, et al. (2020) Developing an animal model to detect drug-drug interactions impacting drug-induced respiratory depression. Toxicol Rep 7:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Krishna A, Stewart S, Shea K, Racz R, Weaver JL, Volpe DA, Pilli NR, Narayanasamy S, Florian J, et al. (2021) Effects of sedative psychotropic drugs combined with oxycodone on respiratory depression in the rat. Clin Transl Sci 14:2208–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P-P, Yeh TK, Loh HH, Law PY, Wang Y, Tao PL (2019) Delta-opioid receptor antagonist naltrindole reduces oxycodone addiction and constipation in mice. Eur J Pharmacol 852:265–273. [DOI] [PubMed] [Google Scholar]
- Yang P-P, Yeh GC, Yeh T-K, Xi J, Loh HH, Law P-Y, Tao P-L (2016) Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res 111:867–876. [DOI] [PubMed] [Google Scholar]
- Yanagidate F, Dohi S (2004) Epidural oxycodone or morphine following gynaecological surgery. Br J Anaesth 93:362–367. [DOI] [PubMed] [Google Scholar]
- Yekkirala AS, Roberson DP, Bean BP, Woolf CJ (2017) Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 16:545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoburn BC, Shah S, Chan K, Duttaroy A, Davis T (1995) Supersensitivity to opioid analgesics following chronic opioid antagonist treatment: relationship to receptor selectivity. Pharmacol Biochem Behav 51:535–539. [DOI] [PubMed] [Google Scholar]
- You Z-B, Bi G-H, Galaj E, Kumar V, Cao J, Gadiano A, Rais R, Slusher BS, Gardner EL, Xi ZX, et al. (2019) Dopamine D3R antagonist VK4-116 attenuates oxycodone self-administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology 44:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z-B, Gao J-T, Bi G-H, He Y, Boateng C, Cao J, Gardner EL, Newman AH, Xi Z-X (2017) The novel dopamine D3 receptor antagonists/partial agonists CAB2-015 and BAK4-54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats. Neuropharmacology 126:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Zhang Y, Liang Y, Zhao C, Randesi M, Kreek MJ (2018) Oxycodone self-administration induces alterations in expression of integrin, semaphoring and ephrin genes in the mouse striatum. Front Psychiatry 9:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D (2001) Federal reports say oxycodone abuse is on the rise. Am J Health Syst Pharm 58:1175–1179. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, et al. (2003) Essential role for RGS9 in opiate action. Proc Natl Acad Sci USA 100:13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C (2003) College on Problems of Drug Dependence Taskforce on Prescription Opioid Non-Medical Use And Abuse: position statement. Drug Alcohol Depend 69:215–232. [DOI] [PubMed] [Google Scholar]
- Zacny JP, de Wit H (2009) The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol Biochem Behav 94:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Drum M (2010) Psychopharmacological effects of oxycodone in healthy volunteers: roles of alcohol-drinking status and sex. Drug Alcohol Depend 107:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S (2003) Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology (Berl) 170:242–254. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S (2009) Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. Drug Alcohol Depend 101:107–114. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S, Kirulus K, McCracken SG (2011) Psychopharmacological effects of oxycodone in volunteers with and without generalized anxiety disorder. Exp Clin Psychopharmacol 19:85–94. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor SA (2008) Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology (Berl) 196:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, McKay MA, Toledano AY, Marks S, Young CJ, Klock PA, Apfelbaum JL (1996) The effects of a cold-water immersion stressor on the reinforcing and subjective effects of fentanyl in healthy volunteers. Drug Alcohol Depend 42:133–142. [DOI] [PubMed] [Google Scholar]
- Zamarripa CA, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB (2018) The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend 192:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa CA, Naylor JE, Huskinson SL, Townsend EA, Prisinzano TE, Freeman KB (2020) Kappa opioid agonists reduce oxycodone self-administration in male rhesus monkeys. Psychopharmacology (Berl) 237:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa CA, Pareek T, Schrock HM, Prisinzano TE, Blough BE, Sufka KJ, Freeman KB (2021) The kappa-opioid receptor agonist, triazole 1.1, reduces oxycodone self-administration and enhances oxycodone-induced thermal antinociception in male rats. Psychopharmacology (Berl) 238:3463–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni G, DeSalle MJ, Deutsch HM, Barr GA, Eisch AJ (2020) Female and male rats readily consume and prefer oxycodone to water in a chronic, continuous access, two-bottle oral voluntary paradigm. Neuropharmacology 167:107978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brownstein AJ, Buonora M, Niikura K, Ho A, Correa da Rosa J, Kreek MJ, Ott J (2015) Self administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience 285:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kahng MW, Elkind JA, Weir VR, Hernandez NS, Stein LM, Schmidt HD (2020) Activation of GLP-1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology 45:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Y, Levran O, Randesi M, Yuferov V, Zhao C, Kreek MJ (2017) Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology (Berl) 234:2259–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A, Ott J, Kreek MJ (2014) Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology (Berl) 231:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ (2009) Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology 34:912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rahematpura S, Ragnini KH, Moreno A, Stecyk KS, Kahng MW, Milliken BT, Hayes MR, Doyle RP, Schmidt HD (2021) A novel dual agonist of glucagon-like peptide-1 receptors and neuropeptide Y2 receptors attenuates fentanyl taking and seeking in male rats. Neuropharmacology 192:108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wang Q, Zheng C, John Rush A, Volkow ND, Xu R (2021) Drug repurposing for opioid use disorders: integration of computational prediction, clinical corroboration, and mechanism of action analyses. Mol Psychiatry 26:5286–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW, Max MB (2003) An old acquaintance: opioids in neuropathic pain. Neurology 60:894–895. [DOI] [PubMed] [Google Scholar]
- Zwisler ST, Enggaard TP, Mikkelsen S, Verstuyft C, Becquemont L, Sindrup SH, Brosen K (2012) Lack of association of OPRM1 and ABCB1 single-nucleotide polymorphisms to oxycodone response in postoperative pain. J Clin Pharmacol 52:234–242. [DOI] [PubMed] [Google Scholar]
- Zwisler ST, Enggaard TP, Noehr-Jensen L, Mikkelsen S, Verstuyft C, Becquemont L, Sindrup SH, Brosen K (2010) The antinociceptive effect and adverse drug reactions of oxycodone in human experimental pain in relation to genetic variations in the OPRM1 and ABCB1 genes. Fundam Clin Pharmacol 24:517–524. [DOI] [PubMed] [Google Scholar]