Abstract
Nitazoxanide, a thiazolide compound, and its desacetyl derivative, tizoxanide, have antimicrobial properties against anaerobic bacteria, as well as against helminths and protozoa. Because the treatment of Helicobacter pylori infection may be jeopardized by metronidazole resistance, nitazoxanide and tizoxanide were tested in vitro against these bacteria. The MICs of these two compounds were determined by agar dilution and were compared to those of metronidazole. Exposure to subinhibitory concentrations of nitazoxanide was also carried out by the method of Szybalski (W. Szybalski and V. Bryson, J. Bacteriol. 64:489–499, 1952). The MICs of nitazoxanide and tizoxanide for 103 strains ranged from 0.25 to 8 μg/ml, with the MIC at which 50% of strains are inhibited (MIC50) being 1 μg/ml and the MIC90 being 4 μg/ml, and no resistant strain was detected, whereas strains resistant to metronidazole were detected. When 10 strains were successively subcultured on medium containing nitazoxanide, no significant change in the MICs of this compound was observed. A pilot study of nitazoxanide for the treatment of H. pylori infection was carried out with 86 patients in association with 20 mg of omeprazole. An eradication rate of 83% (95% confidence interval, 64% to 94%) was obtained in a per-protocol analysis in the group receiving 1 g of nitazoxanide orally twice daily, and a few side effects were observed. The failures could not be explained by the selection of resistant strains since the MICs of nitazoxanide were similar for six pairs of isolates (proven to be the same strain by random amplified polymorphic DNA analysis in four cases) cultured before and after the treatment failure. Nitazoxanide exhibits good antimicrobial activity against H. pylori without the problem of acquired resistance which is encountered with metronidazole and has been demonstrated to have a satisfactory effect in a dose-ranging pilot study. It is therefore a good candidate to be included in treatment regimens aimed at the eradication of H. pylori.
Fifteen years after the discovery of Helicobacter pylori, the eradication of this organism has become the aim of treatments for the cure of most gastroduodenal diseases, especially peptic ulcer and low-grade mucosa-associated lymphoid tissue lymphoma (1). The most effective regimens associate an antisecretory drug, in particular, a proton pump inhibitor given at a double dose with two of the following antibiotics for 7 days: metronidazole, clarithromycin, and amoxicillin (9). The metronidazole-clarithromycin combination has the advantage that 250 mg of clarithromycin twice daily (b.i.d.) instead of 500 mg b.i.d. can be used when clarithromycin is associated with amoxicillin, limiting the side effects and cost (20). However, resistance to metronidazole, which is more frequent than resistance to clarithromycin, jeopardizes the success of this regimen (11).
Different methods with limited correlation are used to determine H. pylori susceptibility to metronidazole; nevertheless, an association between resistance and treatment failure is found in most studies. Recently, in a large multicenter study performed in Europe, a cure rate of 95% was found when the strains were susceptible to metronidazole and a cure rate of 76% was found when the strains were resistant to metronidazole (MIC, >8 μg/ml, as determined by the agar dilution method) (13). When the combination of metronidazole-amoxicillin is used, the difference is even more striking. In a study in which the MICs were also determined by agar dilution, the cure rates were 90 and 45%, respectively (2). These observations have led to a search for a compound with the same properties as metronidazole but without the problem of resistance.
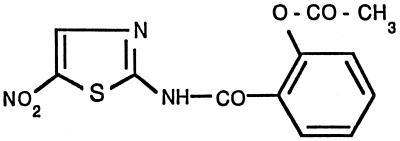
Nitazoxanide is a thiazolide (nitrothiazolamide) compound. Its structure is 2-(acetolyloxy)-N-(5-nitro-2-thiazolyl) benzamide (15) (Fig. 1). The active compound in vivo is its desacetyl derivative, tizoxanide. Antimicrobial properties of nitazoxanide have been shown against helminths, protozoa (cryptosporidia, microsporidia, trichomonas, entamoeba, giardia) (3), and anaerobic bacteria (4).
FIG. 1.
Structure of nitazoxanide.
The aim of this study was to test the susceptibilities of H. pylori strains to nitazoxanide and tizoxanide in comparison to those to metronidazole and to determine the MICs before and after multiple passages on a gradient of nitazoxanide. The results of a pilot study with nitazoxanide administered to humans are also reported.
MATERIALS AND METHODS
Strains.
H. pylori strains were isolated in our laboratory from biopsy specimens obtained from patients with duodenal ulcer or nonulcer dyspepsia consulting different gastroenterologists in France and not having received previous eradication treatment (103 randomly selected strains). In addition, 10 French strains for which MICs of nitazoxanide were high and six pairs of isolates obtained in Egypt before and after a failure of treatment with omeprazole-nitazoxanide were also included.
Briefly, the biopsy specimens were ground and plated on Wilkins Chalgren agar (Oxoid, Basingstoke, Hampshire, United Kingdom) enriched with 10% human blood and containing vancomycin, cefsulodin, trimethoprim, and actidione, as well as on a nonselective medium, Columbia blood agar and pylori agar (bioMérieux, Marcy l’Etoile, France), a commercially available selective medium. The plates were incubated in a microaerobic atmosphere in a jar (GasPak jar without catalyst) for up to 10 days. The colonies were identified by positive catalase, urease, and oxidase tests as well as morphology. The strains were maintained frozen at −70°C before testing.
RAPD testing.
The six pairs of isolates obtained before and after treatment failure were compared by random amplified polymorphic DNA (RAPD) testing. Briefly, genomic DNA was obtained by boiling suspensions of H. pylori with a turbidity equivalent to that of a McFarland no. 5 standard. Amplification was carried out in a 25-μl volume containing 5 μl of H. pylori suspension, 67 mM Tris-HCl (pH 8.8), 16 mM (NH4)2SO4, 0.01% Tween 20, 1.5 mM MgCl2, a 0.4-mM concentration of a deoxynucleoside triphosphate mixture, a 5-μM concentration of primer, 1 U of Taq DNA polymerase (Eurobio, Les Ulis, France), and sterilized water. Each reaction mixture was overlaid with 50 μl of mineral oil. Primer OPH8 (5′-GAA ACA CCC C-3′; Bioprobe Systems, Montreuil, France) was used. A Perkin-Elmer (Norwalk, Conn.) 480 thermal cycler was used for amplification. The cycling program was composed of 1 cycle at 94°C for 10 min; 40 cycles of 94°C for 1 min, 36°C for 1 min, and 72°C for 1 min; and a final incubation at 72°C for 10 min. Then, 20-μl aliquots of the PCR products were separated by electrophoresis in 1.2% agarose gels containing 1 μg of ethidium bromide per ml, and the gel was photographed under UV light. The DNA of φX174 HaeIII and a 1-kb DNA ladder (Promega, Madison, Wis.) were used as size markers in all gels.
Determination of MICs.
MICs were determined by an agar dilution method. Briefly, a suspension with a turbidity equivalent to that of a McFarland no. 3 standard (approximately 109 CFU/ml) was prepared from a 48-h culture on agar. The medium used was Wilkins Chalgren agar enriched with 10% sheep blood and Polyvitex. An appropriate dilution of the compounds (nitazoxanide, tizoxanide, or metronidazole) was added at concentrations ranging from 0.03 to 128 μg/ml. The media were prepared extemporaneously, but no anaerobic preincubation was performed. Nitazoxanide and tizoxanide were obtained from the Romark Institute for Medical Research, Tampa, Fla. The compounds are not commercially available in the United States. In order to test the impact of an acidic medium, the pH was adjusted to 6.5 with a surface microelectrode in another set of experiments. Incubation was performed under the same conditions used for culture. The MIC was defined as the lowest concentration of antimicrobial agent inhibiting the total growth of bacteria. Resistance was considered when the metronidazole MIC was greater than 8 μg/ml.
Exposure to subinhibitory concentrations of nitazoxanide.
A continuous progressive concentration of nitazoxanide or metronidazole was obtained in agar plates by the method of Szybalski and Bryson (19). The gradients tested ranged from 0 to 1 mg/liter and from 0 to 15 mg/liter.
Ten strains from France for which the MICs were the highest were tested. Subcultures were repeated 10 times for each strain, and incubation lasted 7 days each time. Between the different passages, the strains were grown on a medium without antibiotic. MICs were determined before passaging and after 5 and 10 passages of the strains while they were in contact with nitazoxanide or metronidazole.
Pilot study of nitazoxanide in the treatment of H. pylori infections.
An open, phase II dose-ranging study was carried out in Egypt with patients seeking treatment for dyspepsia. Three dosages of 500 mg b.i.d. for 14 days, 500 mg three times daily for 7 days, and 1 g b.i.d. for 7 days were tested, always with 20 mg of omeprazole/day.
At entry, H. pylori infection was diagnosed by histology, culture, and serology by an enzyme-linked immunosorbent assay with blood for the detection of immunoglobulin G antibodies (Quickvue; Quidel, San Diego, Calif.). Patients were included if at least two tests were positive. The same tests except serology were repeated at least 4 weeks after the end of the treatment. In addition, a [13C]urea breath test (Meretek Diagnostics, Inc., Houston, Tex.) was performed with a significant number of patients after treatment.
The patient was considered to be cured of H. pylori infection if all tests were negative. Adverse events were also recorded. The calculation of the eradication rate was performed per protocol analysis only for patients who completed the study protocol and for whom H. pylori tests results were available.
RESULTS
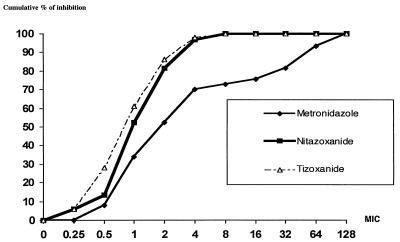
The cumulative MICs of nitazoxanide, tizoxanide, and metronidazole for 103 strains of H. pylori isolated in France are presented in Fig. 2. Nitazoxanide and tizoxanide had similar potencies which were not affected by resistance to metronidazole. The MICs at which 50% of strains are inhibited (MIC50s), MIC90s, and the range of MICs are presented in Table 1. The MICs were also determined for 30 strains at pH 6.5, and the same results were obtained.
FIG. 2.
MICs (micrograms per milliliter) of nitazoxanide, tizoxanide, and metronidazole for 103 strains of H. pylori.
TABLE 1.
MIC50s, MIC90s and ranges of MICs of the different compounds for 103 H. pylori strains
| Compound | MIC (μg/ml)
|
||
|---|---|---|---|
| 50% | 90% | Range | |
| Nitazoxanide | 1 | 4 | 0.25–8 |
| Tizoxanide | 1 | 4 | 0.25–8 |
| Metronidazole | 2 | >32 | 0.25–>32 |
When 10 strains were subcultured 10 times on gradients of nitazoxanide or metronidazole as described by Szybalski and Bryson (19) (Szybalski gradients), it was not possible to detect a significant increase in the MICs of nitazoxanide (Table 2). In contrast, a significant increase in the MICs of metronidazole was observed after contact with either metronidazole or nitazoxanide. The exception was when the strain was already resistant to metronidazole (strains 41, 158, 135, 271, and 306).
TABLE 2.
Susceptibility of H. pylori to metronidazole and nitazoxanide after repeated subculture on Szybalski gradients containing metronidazole or nitazoxanide
| Strain no. | Drug in gradienta | No. of passages | MIC (μg/ml)
|
|
|---|---|---|---|---|
| Metronidazole | Nitazoxanide | |||
| 346 | Metro | 0 | 1 | 1 |
| 5 | 128 | 2 | ||
| 10 | 128 | 2 | ||
| Nitazo | 0 | 1 | 1 | |
| 5 | 128 | 2 | ||
| 10 | 128 | 2 | ||
| T72 | Metro | 0 | 2 | 2 |
| 5 | 128 | 4 | ||
| 10 | 128 | 4 | ||
| Nitazo | 0 | 2 | 2 | |
| 5 | 128 | 4 | ||
| 10 | 128 | 4 | ||
| 117 | Metro | 0 | 8 | 1 |
| 5 | 32 | 2 | ||
| 10 | 32 | 2 | ||
| Nitazo | 0 | 8 | 1 | |
| 5 | 32 | 2 | ||
| 10 | 16 | 2 | ||
| T76 | Metro | 0 | 1 | 4 |
| 5 | 128 | 8 | ||
| 10 | 128 | 4 | ||
| Nitazo | 0 | 1 | 4 | |
| 5 | 2 | 8 | ||
| 10 | 2 | 4 | ||
| T45 | Metro | 0 | 2 | 2 |
| 5 | 128 | 4 | ||
| 10 | 128 | 8 | ||
| Nitazo | 0 | 2 | 2 | |
| 5 | 128 | 2 | ||
| 10 | 128 | 4 | ||
| 41 | Metro | 0 | 64 | 2 |
| 5 | 64 | 2 | ||
| 10 | 64 | 2 | ||
| Nitazo | 0 | 64 | 2 | |
| 5 | 64 | 2 | ||
| 10 | 128 | 4 | ||
| 158 | Metro | 0 | 64 | 2 |
| 5 | 64 | 2 | ||
| 10 | 32 | 2 | ||
| Nitazo | 0 | 64 | 2 | |
| 5 | 64 | 2 | ||
| 10 | 64 | 2 | ||
| 135 | Metro | 0 | 64 | 2 |
| 5 | 64 | 2 | ||
| 10 | 128 | 4 | ||
| Nitazo | 0 | 64 | 2 | |
| 5 | 64 | 8 | ||
| 10 | 128 | 4 | ||
| 271 | Metro | 0 | 32 | 2 |
| 5 | 128 | 4 | ||
| 10 | 64 | 2 | ||
| Nitazo | 0 | 32 | 2 | |
| 5 | 128 | 2 | ||
| 10 | 128 | 4 | ||
| 306 | Metro | 0 | 64 | 4 |
| 5 | 32 | 8 | ||
| 10 | 64 | 4 | ||
| Nitazo | 0 | 64 | 4 | |
| 5 | 32 | 4 | ||
| 10 | 64 | 4 | ||
Metro, metronidazole; Nitazo, nitazoxanide.
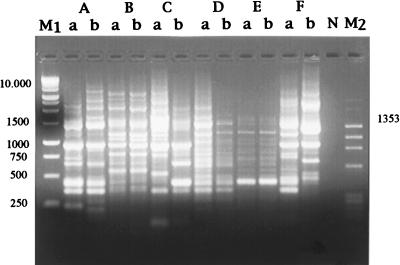
Furthermore, strains isolated from patients with posttreatment failure during the phase II clinical trial in Egypt were tested. The MICs of nitazoxanide, tizoxanide, and metronidazole for these strains were within 1 dilution of the values observed for the pretreatment strains tested (Table 3). Indeed when pre- and posttreatment isolates were compared by RAPD analysis, only four of the six pairs (pairs A, B, D, and E) turned out to be the same strain (Fig. 3).
TABLE 3.
Susceptibilities of pairs of H. pylori strains isolated before and after treatment failure with a nitazoxanide-containing regimen to nitazoxanide and tizoxanide
| Paira | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Nitazoxanide
|
Tizoxanide
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
| A | 4 | 8 | 4 | 8 |
| B | 8 | 8 | 4 | 8 |
| C | 2 | 4 | 4 | 4 |
| D | 4 | 4 | 8 | 4 |
| E | 4 | 4 | 8 | 8 |
| F | 2 | 1 | 2 | 2 |
Pairs A, B, D, and E were identical. Pairs C and F were nonidentical.
FIG. 3.
RAPD patterns (primer OPH8) of six pairs (pairs A to F) of pretreatment (a) and posttreatment (b) H. pylori isolates. Lanes: M1, 1-kb ladder DNA; N, negative control; M2, HaeIII-digested φX174. Numbers on the left and right are base pairs.
Eighty-six patients were analyzed per protocol, including 24 with peptic ulcers and 62 with nonulcer dyspepsia. The results of this pilot study are presented in Table 4. Because of the small number of patients, they were not analyzed according to initial clinical diagnosis. The highest eradication rate was obtained in group 3 (83%). The difference was statistically significant when the eradication rate for group 3 was compared to that for group 2 (58%; P = 0.037) and approached statistical significance when the eradication rate for group 3 was compared to that for group 1 (65%; P = 0.095). Minor side effects were reported by a total of 15 patients. No reason for the higher number of side effects observed in groups 1 and 2 was found.
TABLE 4.
Eradication of H. pylori in a dose-ranging trial of nitazoxanide in association with omeprazolea
| Group (dosage) | No. of evaluable patients |
H. pylori eradication
|
No. (%) of patients with side effectsb | |
|---|---|---|---|---|
| No. (%) of patients | 95% confidence interval | |||
| 1 (500 mg b.i.d. for 14 days) | 26 | 17 (65) | 44–82 | 6 (23) |
| 2 (500 mg t.i.d.c for 7 days) | 31 | 18 (58) | 39–75 | 8 (26) |
| 3 (1 g b.i.d. for 7 days) | 29 | 24 (83) | 64–94 | 1 (3.4) |
Omeprazole was given orally at 20 mg.
Nausea, n = 5; transient diarrhea, n = 2; epigastric pain, n = 4; elevated serum glutamic pyruvic transaminase levels, n = 2.
t.i.d., three times daily.
DISCUSSION
Metronidazole is an attractive compound for use in the treatment of H. pylori infection because it is secreted in the stomach (21) and reaches high concentrations. Furthermore, its activity is not affected by a decrease in pH (10). However, when given as the only antimicrobial agent, acquired resistance is so frequent that the use of metronidazole in association with another drug is mandatory (8). In addition, the high frequency of primary resistance observed makes its use questionable. In developing countries such as Africa, where most of the population is infected with H. pylori, the prevalence of resistance to metronidazole is high and virtually all strains are resistant (5). In developed countries, the prevalence varies between 10 and 50% (6). While there is some controversy with regard to the method to be used to test for metronidazole resistance and the possible recovery of strains with false resistance, a global decrease in the efficacies of treatment regimens containing metronidazole is noted when strains are resistant to this compound compared to the efficacies of these regimens when strains are susceptible (12). Nonetheless, the eradication rate of triple therapies that include metronidazole is still in the range of 50 to 70% due to the activity of the second antibiotic that is present.
A compound similar to metronidazole but without the problem of resistance is needed for the treatment of H. pylori infection. Nitazoxanide seems to fulfill this requirement. We have found that its MICs are in the range of those of metronidazole for the strains which are considered susceptible to metronidazole. Even after long-term exposure of H. pylori strains to nitazoxanide, the MICs are not modified. Ten H. pylori strains were exposed to nitazoxanide and metronidazole in vitro for a total of 2 months, which represents a large number of successive generations of the bacteria, and no resistance was selected.
We have also tested H. pylori strains isolated from patients before treatment and after failure of treatment with nitazoxanide. Four pairs proved to be the same strain, while for the two other pairs we can hypothesize that the patient was infected with two different strains. Again, the selection of resistant mutants was not the cause of failure, and the MICs for the strains were similar before and after the course of treatment.
Nitazoxanide has been shown to have a dose-dependent effect on DNA synthesis. This property has also been found with parasites, but the exact mechanism of action of nitazoxanide is not known, nor is the mechanism of action of metronidazole. Studies show that the nitro group of the antibiotic must be reduced in the target organism (17), and nitazoxanide can be reduced at a redox potential at least 3.3 times lower than that observed for metronidazole (19a).
Nitazoxanide has proved to be nontoxic (14), and its pharmacokinetics have been determined in healthy volunteers (18). The only measurable species in plasma was desacetyl nitazoxanide (tizoxanide), which reached a maximum concentration of 1.9 mg/liter (range, 1.1 to 2.5 mg/liter) 2 to 6 h after ingestion of a single dose of 500 mg of nitazoxanide. For this reason we also tested tizoxanide and found that it has the same activity as nitazoxanide, in contrast to results obtained for anaerobic bacteria except those of the Bacteroides fragilis group (4). No data on the concentration of tizoxanide in gastric juice are currently available.
The high concentration of metronidazole obtained in gastric juice can be explained by the pH partition hypothesis; i.e., weak bases are trapped in acidic compartments such as the stomach (16). When H+ secretion is highly suppressed (when 40 g of omeprazole b.i.d. is used), the concentration of metronidazole in the stomach approaches the range of the concentration in blood (7). Therefore, metronidazole should be used without acid suppression. However, the almost constant development of resistance to metronidazole in vivo renders its use impossible. To avoid the development of resistance, a second antibiotic must be added, with studies recommending the use of either amoxicillin or clarithromycin, both of which have pH-dependent activities. The further addition of an antisecretory drug therefore becomes mandatory. Tizoxanide is also a weak base, and it is most likely that the same phenomenon that occurs with metronidazole occurs with tizoxanide. However, because we are not faced with the problem of the development of resistance, it is possible to use only a low dose of antisecretory drug essentially with the aim of relieving pain, and no other antibiotic is needed.
In the dose-ranging pilot study, nitazoxanide at 1 g/day given with 20 mg of omeprazole led to a promising rate of eradication of 83%, per protocol. Among the antimicrobial agents tested, only amoxicillin and clarithromycin could achieve such a high eradication rate when used with a proton pump inhibitor, but for a treatment duration of 2 weeks. Furthermore, with the dual therapy with amoxicillin, such good results have not been reproduced in all centers (1), the average rate of success being in the range of 50%. Similarly, dual therapy with clarithromycin used high doses (1.5 g/day), which led to taste disturbances. Among the treatment failures, a large proportion of resistant strains was detected (22). In contrast, this problem does not seem to occur with nitazoxanide.
In conclusion, nitazoxanide was a well-tolerated compound, and its use was able to achieve a high rate of eradication of H. pylori when it was administered with omeprazole for 7 days. Its microbiological characteristics are close to those of metronidazole, but resistance could not be observed, despite in vivo exposure during the course of treatments and long-term in vitro exposure.
ACKNOWLEDGMENTS
We thank IPSEN Laboratories (Paris, France) and Romark Laboratories (Tampa, Fla.) for support for this study and S. M. Kabil from the Cairo GIT & Liver Center in Cairo, Egypt, for his contribution to the study of the effects of nitazoxanide in the treatment of H. pylori in Egypt.
REFERENCES
- 1.Anonymous. The report of the Digestive Health Initiative International Update Conference on Helicobacter pylori. Gastroenterology. 1997;113:54–58. doi: 10.1016/s0016-5085(97)80003-0. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard, S., C. Birac, H. Lamouliatte, S. Forestier, and F. Mégraud. 1996. Correlation between the MICs of metronidazole on H. pylori strains and the outcome of a lansoprazole-amoxicillin-metronidazole therapy. Gut 39(Suppl. 2):A6.
- 3.Doumbo O, Rossignol J F, Pichard E, Traore H, Dembele M M, Diakite M, Traore F, Diallo A D. Nitazoxanide in the treatment of cryptosporidiosis in 24 AIDS patients with chronic diarrhea in Mali. Am J Trop Med Hyg. 1997;56:637–639. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 4.Dubreuil L, Houcke X, Mouton Y, Rossignol J F. In vitro evaluation of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob Agents Chemother. 1996;40:2266–2270. doi: 10.1128/aac.40.10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glupczynski Y, Boudeaux L, De Perez C, Devos D, Devreker T, Balegamire B, Goossens H, Van den Borre C, Butzler J-P. Prevalence of Helicobacter pylori in rural Kivu, eastern Zaire: a prospective endoscopic study. Eur J Gastroenterol Hepatol. 1991;3:449–455. [Google Scholar]
- 6.Glupczynski Y the European Study Group on Antibiotic Susceptibility of H. pylori. Results of a multicentre European survey in 1991 of metronidazole resistance in H. pylori. Eur J Clin Microbiol Infect Dis. 1992;11:777–781. [PubMed] [Google Scholar]
- 7.Goddard A F, Jessa M J, Barrett D A, Shaw P N, Idström J P, Cederberg C, Spiller R C. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358–367. doi: 10.1053/gast.1996.v111.pm8690200. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin C S, Marshall B J, Blincow E D, Wilson D H, Blackbourn S, Philipps M. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: clinical and in vitro studies. J Clin Pathol. 1988;41:207–210. doi: 10.1136/jcp.41.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lind T, Veldhuyzen Van Zanten S, Unge P, Lind T, Spiller R, Bayerdörffer E, O’Morain C, Dev Bardhan K, Bradette M, Chiba N, Wrangstadh M. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the Mach 1 study. Helicobacter. 1996;1:138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 10.McNulty C A M, Dent J C, Ford G A, Wilkinson S P. Inhibitory antimicrobial concentration against Campylobacter pylori in gastric mucosa. J Antimicrob Chemother. 1988;22:729–738. doi: 10.1093/jac/22.5.729. [DOI] [PubMed] [Google Scholar]
- 11.Mégraud, F. 1997. Resistance of Helicobacter pylori to antibiotics. Aliment. Pharmacol. Ther. 11(Suppl. 1):43–53. [DOI] [PubMed]
- 12.Megraud F, Doermann H P. Clinical relevance of resistant strains of Helicobacter pylori. A review of current data. Gut. 1998;43:S61–S65. doi: 10.1136/gut.43.2008.s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mégraud F, Lehn N, Lind T, Bayerdörffer E, O’Morain C, Spiller R C, Unger P, Veldhuyzen Van Zanten S, Wrangstadh M, Burman A. The MACH 2 study—Helicobacter pylori resistance to antimicrobial agents and its influence on clinical outcome. Gastroenterology. 1997;112:A216. [Google Scholar]
- 14.Murphy J, Friedman J C. Preclinical toxicology of nitazoxanide—a new antiparasitic compound. J Appl Toxicol. 1985;5:49–52. doi: 10.1002/jat.2550050202. [DOI] [PubMed] [Google Scholar]
- 15.Rossignol J F, Cavier R. New derivative of 2-benzamido-5-nitrothiazoles. Chem Abstr. 1975;83:28216n. [Google Scholar]
- 16.Shore P A, Brodie B B, Hogben C A M. The gastric secretion of drugs: a pH partition hypothesis. J Pharmacol Exp Ther. 1957;119:361–369. [PubMed] [Google Scholar]
- 17.Smith M A, Edwards D I. The influence of microaerophilia and anaerobiosis on metronidazole uptake in Helicobacter pylori. J Antimicrob Chemother. 1995;36:453–461. doi: 10.1093/jac/36.3.453. [DOI] [PubMed] [Google Scholar]
- 18.Stockis A, Lins R, Deroubaix X, Jeanbaptiste B, Calderon P, Rossignol J F. Nitazoxanide pharmacokinetics in healthy volunteers. Int J Clin Pharmacol Ther. 1996;34:349–351. [PubMed] [Google Scholar]
- 19.Szybalski W, Bryson V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952;64:489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Thunus, L. Personal communication.
- 20.Van der Hulst R W M, Keller J J, Rauws E A J, Tytgat G N J. Treatment of Helicobacter pylori infection: a review of the world literature. Helicobacter. 1996;1:6–19. doi: 10.1111/j.1523-5378.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 21.Veldhuyzen Van Zanten S J O, Goldie J, Hollingsworth J, Silletti C, Richardson H, Hunt R H. Secretion of intravenously administered antibiotics in gastric juice: implications for management of Helicobacter pylori. J Clin Pathol. 1992;45:225–227. doi: 10.1136/jcp.45.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurzer H, Rodrigo L, Archambault A, Rokkas T, Skandalis N, Fedorak R, Bazzoli F, Hentschel H, Mora P, Stamler D, Mégraud F. Short-course therapy with amoxicillin-clarithromycin triple therapy for 10 days (ACT-10) eradicates H. pylori and heals duodenal ulcer. Aliment Pharmacol Ther. 1997;11:943–952. doi: 10.1046/j.1365-2036.1997.00223.x. [DOI] [PubMed] [Google Scholar]