Abstract
Time-kill studies, even those performed with in vitro dynamic models, often do not provide definitive comparisons of different antimicrobial agents. Also, they do not allow determinations of equiefficient doses or predictions of area under the concentration-time curve (AUC)/MIC breakpoints that might be related to antimicrobial effects (AMEs). In the present study, a wide range of single doses of trovafloxacin (TR) and twice-daily doses of ciprofloxacin (CI) were mimicked in an in vitro dynamic model. The AMEs of TR and CI against gram-negative bacteria with similar susceptibilities to both drugs were related to AUC/MICs that varied over similar eight-fold ranges [from 54 to 432 and from 59 to 473 (μg · h/ml)/(μg/ml), respectively]. The observation periods were designed to include complete bacterial regrowth, and the AME was expressed by its intensity (the area between the control growth in the absence of antibiotics and the antibiotic-induced time-kill and regrowth curves up to the point where viable counts of regrowing bacteria equal those achieved in the absence of drug [IE]). In each experiment monoexponential pharmacokinetic profiles of TR and CI were simulated with half-lives of 9.2 and 4.0 h, respectively. Linear relationships between IE and log AUC/MIC were established for TR and CI against three bacteria: Escherichia coli (MIC of TR [MICTR] = 0.25 μg/ml; MIC of CI [MICCI] = 0.12 μg/ml), Pseudomonas aeruginosa (MICTR = 0.3 μg/ml; MICCI = 0.15 μg/ml), and Klebsiella pneumoniae (MICTR = 0.25 μg/ml; MICCI = 0.12 μg/ml). The slopes and intercepts of these relationships differed for TR and CI, and the IE-log AUC/MIC plots were not superimposed, although they were similar for all bacteria with a given antibiotic. By using the relationships between IE and log AUC/MIC, TR was more efficient than CI. The predicted value of the AUC/MIC breakpoint for TR [mean for all three bacteria, 63 (μg · h/ml)/(μg/ml)] was approximately twofold lower than that for CI. Based on the IE-log AUC/MIC relationships, the respective dose (D)-response relationships were reconstructed. Like the IE-log AUC/MIC relationships, the IE-log D plots showed TR to be more efficient than CI. Single doses of TR that are as efficient as two 500-mg doses of CI (500 mg given every 12 h) were similar for the three strains (199, 226, and 203 mg). This study suggests that in vitro evaluation of the relationships between IE and AUC/MIC or D might be a reliable basis for comparing different fluoroquinolones and that the results of such comparative studies may be highly dependent on their experimental design and datum quantitation.
Over the past few decades development of new antimicrobial agents with improved pharmacokinetics has been a major focus of the pharmaceutical industry. The actual antimicrobial potential as well as the possible advantages of a newly developed drug over its precursors cannot be demonstrated by traditional methods, especially if the comparators have similar intrinsic activities. In fact, the usual in vitro estimates of antimicrobial activity (MIC, minimum bactericidal concentration, results of time-kill studies) determined at constant drug concentrations (static conditions) do not consider pharmacokinetic parameters. Also, data obtained with animal models of infection have limited relevance to clinical practice because of different dosing considerations associated with the scaling up to the doses used for humans.
The true therapeutic potential of pharmacokinetically different antimicrobial agents can be revealed by using in vitro dynamic models that simultaneously consider both intrinsic activity and the pharmacokinetics of antibiotics. These models have been applied in comparative studies with many antimicrobial agents (3, 10, 21). However, all too often, the full potential of this approach is not realized because of inappropriate experimental design and/or suboptimal quantitation of bacterial killing and regrowth curves or the antimicrobial effect itself. More specifically, most of these studies have simulated human pharmacokinetics over a narrow dosage range. Although this approach is reasonable for predicting the antimicrobial effect of a drug on a given organism, it might not be optimal for an accurate comparison of different drugs, especially if the antimicrobial effects are close to either minimum or maximum values (8). Moreover, the one-dose nature of such studies does not provide evaluation of the equiefficient dose of a new drug (the dose of the new drug that produces the same effect observed with a reference drug at its usual dose). Finally, most studies do not include a sufficient duration of observation to cover the entire regrowth phase in time-kill curve studies and therefore do not provide an accurate quantitation of the total antimicrobial effect.
The purpose of this investigation was (i) to compare the kinetics of bacterial killing and regrowth for gram-negative pathogens with similar susceptibilities to trovafloxacin and ciprofloxacin by in vitro simulation of their human pharmacokinetics over a wide range of area under the concentration-time curve (AUC)/MIC ratios; (ii) to establish the relationships between the AUC/MIC ratio and the antimicrobial effect as expressed by its intensity (the area between the control growth and time-kill and regrowth curves estimated up to the point where viable counts on the regrowth curve are close to maximum values observed without drug [IE] [9]) for each drug; (iii) to propose the AUC/MIC breakpoint for predicting an acceptable antimicrobial effect; and (iv) to predict the single dose of trovafloxacin which would be as efficient as two 500-mg doses of ciprofloxacin.
MATERIALS AND METHODS
Antimicrobial agents and bacterial strains.
Trovafloxacin mesylate and ciprofloxacin lactate powders (kindly provided by Roerig, a division of Pfizer, and by Bayer AG, respectively) were used in the study. Stock solutions of the quinolones were prepared in sterile distilled water.
The clinical isolates Escherichia coli 224, Pseudomonas aeruginosa 48, and Klebsiella pneumoniae 121 were used in the study. Susceptibility testing was performed in duplicate in Ca2+- and Mg2+-supplemented Mueller-Hinton broth at an inoculum size of 106 CFU/ml after 24 h of quinolone exposure. The MICs of trovafloxacin for these organisms, 0.25, 0.3, and 0.25 μg/ml, respectively, were comparable to those of ciprofloxacin (0.12, 0.15, and 0.12 μg/ml, respectively).
Simulated pharmacokinetic profiles.
Preliminary examination of the absorption and distribution phases of the pharmacokinetic profiles of trovafloxacin and ciprofloxacin in humans (31, 32) showed that the respective areas contribute less than 10% to the total AUCs (1 and 7%, respectively). Since the contribution of these two phases is relatively small, the comparison of the antimicrobial effects of trovafloxacin and ciprofloxacin was performed by using simulations of simple monoexponential profiles. The simulated half-lives (9.25 h for trovafloxacin and 4.0 h for ciprofloxacin) were consistent with the values reported in humans: 7.2 to 9.9 h (27, 32) and 3.2 to 5.0 h (2, 17, 31), respectively.
In all experiments single doses of trovafloxacin and two doses of ciprofloxacin administered every 12 h were mimicked. The simulated values of the AUC and the respective amounts (A) of the drugs administered in the model were chosen with respect to the MICs for the three organisms to provide similar eightfold ranges of the AUC/MIC ratios. These ratios averaged from 54 to 432 (μg · h/ml)/(μg/ml) for trovafloxacin and from 59 to 473 (μg · h/ml)/(μg/ml) for ciprofloxacin (Table 1). For ciprofloxacin, the AUC values presented in Table 1 reflect the sum of two AUCs provided by two doses of the quinolone administered at a 12-h interval taking into account the residual concentrations at the end of the first interval.
TABLE 1.
Simulated AUCs and the respective values of total amounts of trovafloxacin and ciprofloxacin administered in the model
| Antibiotic |
E. coli
|
P. aeruginosa
|
K. pneumoniae
|
|||
|---|---|---|---|---|---|---|
| AUC (μg·h/ml) | Amt (μg)a | AUC (μg·h/ml) | Amt (μg) | AUC (μg·h/ml) | Amt (μg) | |
| Trovafloxacin | 13.1 | 40 | 16.4 | 50 | 13.7 | 41 |
| 24.5 | 74 | 33.1 | 100 | 27.1 | 81 | |
| 52.7 | 158 | 66.1 | 198 | 54.8 | 164 | |
| 105.3 | 316 | 131.2 | 394 | 109.7 | 329 | |
| Ciprofloxacin* | 7.0 | 24 + 24 | 8.7 | 31 + 31 | 7.3 | 26 + 26 |
| 14.0 | 49 + 49 | 17.5 | 61 + 61 | 14.6 | 51 + 51 | |
| 28.0 | 97 + 97 | 35.0 | 122 + 122 | 29.1 | 102 + 102 | |
| 56.0 | 196 + 196 | 70.0 | 245 + 245 | 58.3 | 204 + 204 | |
The amounts were divided in two portions, according to the twice-daily regimen mimicked.
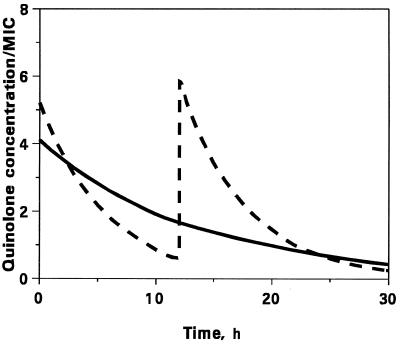
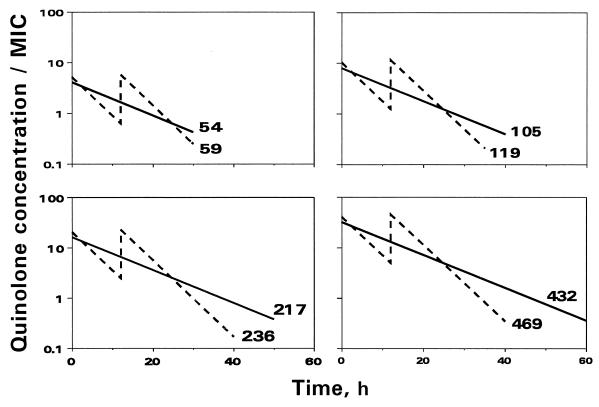
To provide similar AUC/MIC ratios of trovafloxacin and ciprofloxacin, the latter of which has a shorter half-life, the peak concentration/MIC ratios of ciprofloxacin were higher than those of trovafloxacin. This is illustrated by the time (t) courses of trovafloxacin and ciprofloxacin concentrations (C) related to the MIC at one of the AUC/MIC ratios (Fig. 1; natural C/MIC scale) and a series of the pharmacokinetic profiles simulating different AUC/MIC ratios (Fig. 2; logarithmic C/MIC scale).
FIG. 1.
In vitro simulated pharmacokinetic profiles of trovafloxacin (——) and ciprofloxacin (–––) at AUC/MIC ratios of 59 and 54 (μg · h/ml)/(μg/ml), respectively.
FIG. 2.
In vitro simulated pharmacokinetic profiles of trovafloxacin (——) and ciprofloxacin (–––). The averaged value of the simulated AUC/MIC ratios [in (μg · h/ml)/(μg/ml)] is indicated by the number at each plot.
In vitro dynamic model and operating procedure.
The in vitro dynamic model described previously (13) was used in the study. Briefly, the model consisted of two connected flasks, one of them containing fresh Ca2+- and Mg2+-supplemented Mueller-Hinton broth and the other, the central unit, containing the same broth and either a bacterial culture alone (control growth experiments) or a bacterial culture plus antibiotic (killing and regrowth experiments). The central unit was incubated at 37°C in a shaking water bath. Peristaltic pumps (Minipuls 2; Gilson) circulated fresh nutrient medium to the bacteria or the bacteria and antibiotic mixture and from the central 40-ml unit at a flow rate of 3 or 7 ml/h when simulating trovafloxacin or ciprofloxacin pharmacokinetics, respectively. Hence, the clearances provided by the designed flow rates plus the volume of the central unit ensured monoexponential elimination of the quinolones and bacteria from the system with elimination rate constants of 0.075 h−1 (half-life = 9.25 h) and 0.170 h−1 (half-life = 4.0 h), respectively. Accurate simulations of the desired pharmacokinetic profiles were provided by maintaining a constant volume of the central unit and constant flow rates. Validation of the model by determination of ciprofloxacin and trovafloxacin concentrations by high-pressure liquid chromatography showed no systematic deviation of the observed values from the expected values as reported previously (12).
The system was filled with sterile Mueller-Hinton broth and was placed in a temperature-regulated incubator at 37°C. The central unit was inoculated with 18-h cultures of E. coli, P. aeruginosa, or K. pneumoniae, and after a further 2-h incubation, trovafloxacin or ciprofloxacin was injected into the central unit. The resulting exponentially growing cultures approached approximately 106 CFU/ml. Exact values (standard deviations) of the starting inocula of E. coli, P. aeruginosa, and K. pneumoniae were 5.93 (0.10), 5.99 (0.08), and 5.97 (0.05) log CFU/ml, respectively.
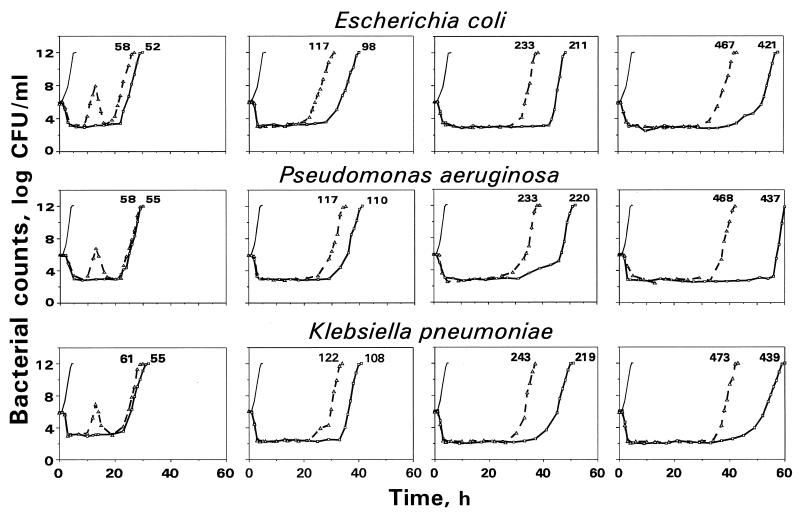
The duration of the experiments was defined in each case as the time until bacteria exposed to antibiotics (NA) reached the maximum numbers observed without antibiotic (control growth [Nc]), i.e., the time when NA becomes equal to NC. In all cases the experiments were stopped when NA reached ≥1011 CFU/ml. Since the experiments that simulated low AUC/MIC ratios met this requirement earlier than those that simulated high AUC/MIC ratios, the duration of the former experiments was shorter than that of the latter: the lower the AUC/MIC ratio, the shorter the observation period (Fig. 2).
Quantitation of bacterial growth and killing.
In each experiment 0.1-ml samples were withdrawn from bacterium-containing media removed from the central unit throughout the observation period, at first every 30 min, later hourly, then every 3 h, and, during the last 6 to 7 h, again hourly. These samples were subjected to serial 10-fold dilutions with chilled, sterile 0.9% NaCl and were plated in duplicate on Mueller-Hinton agar. Antibiotic carryover at low counts was avoided by washing the bacteria with 0.9% NaCl and resuspending them in saline prior to plating. After overnight incubation at 37°C the resulting bacterial colonies were counted, and the numbers of CFU per milliliter were calculated. The limit of detection was 2 × 102 CFU/ml. High within- and interday reproducibilities of the results have been reported previously (12).
To reveal possible changes in susceptibility, the quinolone concentrations (Cregrowth) corresponding to the time when the numbers of surviving organisms in the regrowth curves reached the level of the initial inoculum were determined in each run (10). No AUC/MIC-induced systematic differences in the Cregrowth values were documented with any of the regimens; moreover, the appearance of bacterial regrowth was associated with quinolone concentration-to-MIC ratios of unity.
Quantitative evaluation of the antimicrobial effect.
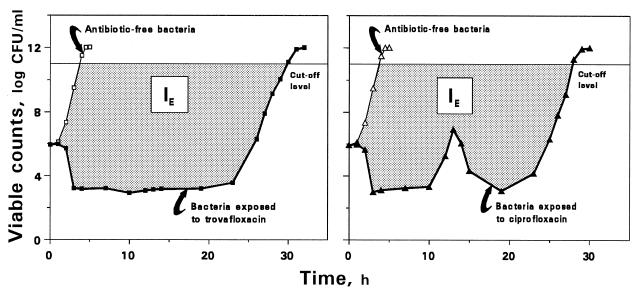
The antimicrobial effect (E) was determined as the difference log NC − log NA. For each pair of bacterial growth-regrowth and control growth curves, IE was estimated as the area between these curves which is equivalent to the area under the E-t curve from the zero point (the moment of drug input into the model) up to the time when viable counts on the regrowth curve are close to the maximum values observed without drug (9). The upper limit of bacterial numbers in the regrowth and control growth curves and the lower limit in the time-kill curve used to determine the IE were 1 × 1011 (13) and 2 × 102 CFU/ml, respectively (Fig. 3).
FIG. 3.
Schematic presentation of the IE determination applied to the kinetics of killing and regrowth of K. pneumoniae exposed to a single dose of trovafloxacin [AUC/MIC = 55 (μg · h/ml)/(μg/ml)] and two doses of ciprofloxacin [AUC/MIC = 61 (μg · h/ml)/(μg/ml)]. IE describes the dashed area between the control growth (empty symbols) and killing and regrowth (filled symbols) curves limited from above by a level of 1011 CFU/ml.
Quantitative relationships between the effect and the AUC/MIC or dose.
The IE versus log AUC/MIC data sets obtained with each quinolone against E. coli, K. pneumoniae, and P. aeruginosa were fitted by the equation IE = a + b log AUC/MIC (equation 1).
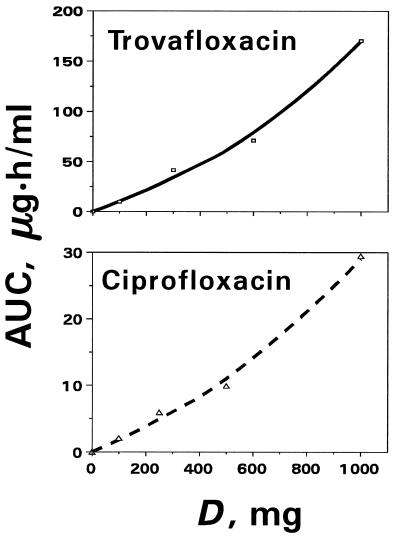
To express the antimicrobial effects as a function of quinolone dose (D), the AUC in the linear relationship between IE and log AUC that corresponds to equation 1 written for a given quinolone-pathogen pair was substituted by D according to the polynomial equation AUC = c + dD + eD2 (equation 2).
The values of c, d, and e for trovafloxacin (−0.01, 7.5 × 10−2, and 9.6 × 10−5, respectively) and for ciprofloxacin (0.10, 1.4 × 10−2, and 7.5 × 10−6, respectively) were calculated by considering the curvilinear pattern of the AUC-D plots (Fig. 4) reconstructed from reported data (2, 27).
FIG. 4.
Dose-dependent changes in the quinolone AUCs fitted by polynomial equations of the second order. The observed AUCs are shown by open symbols, and the respective theoretical values are indicated by the lines.
When predicting the AUC/MIC breakpoint for trovafloxacin, the reported breakpoint value for ciprofloxacin, 125 (μg · h/ml)/(μg/ml), that correlated with bacterial eradication in patients with respiratory tract infections (14) was used. This reference breakpoint reflects the critical value of the area under the inhibitory curve (AUIC) that is very similar to the AUC/MIC, since AUIC is defined by the AUC/MIC measured for the time period where C is greater than the MIC (24).
RESULTS
The time courses of viable counts that reflect killing and regrowth of E. coli, P. aeruginosa, and K. pneumoniae exposed to monoexponentially decreasing concentrations of trovafloxacin and ciprofloxacin as well as the respective control growth curves are shown in Fig. 5. As seen in Fig. 5, the time-kill curves observed with both quinolones against these three bacterial species yielded similar patterns. At the AUC/MIC ratios studied, the regrowth followed a remarkable reduction in bacterial numbers. Unlike the parameter of minimal bacterial numbers after antibiotic exposure, the shift of the regrowth phase to the right along the time axis was distinctly dependent on the simulated AUC/MIC: the higher the AUC/MIC, the later the regrowth. For all three bacterial species exposed to trovafloxacin and at every AUC/MIC ratio simulated, regrowth of bacteria with trovafloxacin was observed later than regrowth of bacteria with ciprofloxacin.
FIG. 5.
The kinetics of killing and regrowth of gram-negative bacteria exposed to trovafloxacin (——) and ciprofloxacin (–––). The simulated AUC/MIC ratio [in (μg · h/ml)/(μg/ml)] is indicated by the number at each curve. The control growth curves are indicated by thin lines.
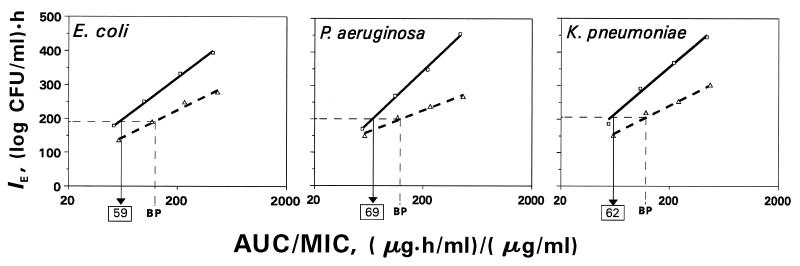
The inherent difference in trovafloxacin and ciprofloxacin efficacies becomes even more evident when the relationships between IE and log AUC/MIC are examined (Fig. 6). As seen in Fig. 6, the antimicrobial effect of trovafloxacin was more pronounced than that of ciprofloxacin against these three microorganisms over the entire AUC/MIC range studied. Regardless of the microorganism, a specific relationship was established for each of the quinolones. The slopes of the IE-log AUC/MIC plots differed in shape and were 1.5- to 2.5-fold higher with trovafloxacin than with ciprofloxacin, i.e., 240 versus 160 (log CFU/ml) · h for E. coli, 309 versus 127 (log CFU/ml) · h for P. aeruginosa, and 282 versus 166 (log CFU/ml) · h for K. pneumoniae. These differences resulted in more striking contrasts between the antimicrobial effects produced by trovafloxacin and ciprofloxacin at high AUC/MIC ratios. For example, at an AUC/MIC ratio of 125 (μg · h/ml)/(μg/ml), which is considered to be a significant breakpoint for predicting acceptable clinical outcome (14), the IEs of trovafloxacin for E. coli, P. aeruginosa, and K. pneumoniae were 1.41-, 1.41-, and 1.42-fold higher, respectively, than those of ciprofloxacin. The respective differences in the antimicrobial effect at an AUC/MIC ratio of 250 (μg · h/ml)/(μg/ml) were more pronounced, with IE values being 1.43-, 1.57-, and 1.47-fold higher, respectively, for trovafloxacin. Thus, despite similar or even lower intrinsic activities, at a given AUC trovafloxacin is more efficient than ciprofloxacin.
FIG. 6.
AUC/MIC-dependent antimicrobial effects of trovafloxacin and ciprofloxacin on gram-negative bacteria as expressed by the IE parameter. The equivalent value of the breakpoint (BP) for trovafloxacin is indicated by the boxed number.
Based on the IE-AUC/MIC relationships, the AUC/MIC breakpoints for trovafloxacin which correspond to the AUC/MIC breakpoints for ciprofloxacin were predicted. Taking the IE produced by the AUC/MIC of 125 (μg · h/ml)/(μg/ml) of ciprofloxacin as acceptable, the AUC/MIC of trovafloxacin which produces the same IE was estimated. The predicted breakpoint values of the trovafloxacin AUC/MIC for E. coli, P. aeruginosa, and K. pneumoniae were close: 58.9, 68.5, and 61.5 (μg · h/ml)/(μg/ml), respectively. Thus, an average AUC/MIC ratio of 63 (μg · h/ml)/(μg/ml) for trovafloxacin administered as a single dose might be equivalent to that corresponding to two doses of ciprofloxacin.
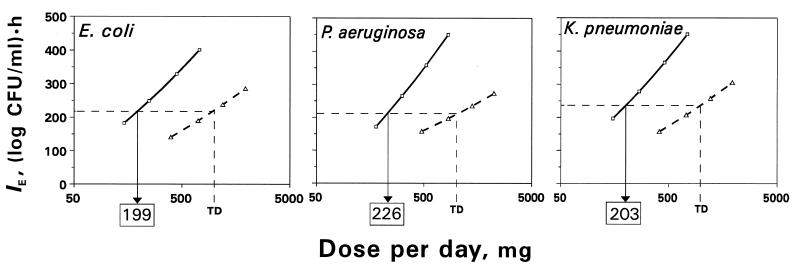
Based on the relationship (equation 1) between IE and log AUC/MIC and the relationship (equation 2) between AUC and D, the respective relationships between IE and log D were reconstructed for each organism. Due to the higher AUCs produced by a given dose of trovafloxacin, the IE-log D curves differ from those for ciprofloxacin more than the respective linear IE-log AUC/MIC plots. As seen in Fig. 7, both the slopes and the positions of the IE-log D plots are quite different for the two quinolones. So, the antimicrobial effect of trovafloxacin is more dose dependent than that of ciprofloxacin. As seen in Fig. 7, the effects produced by the originally proposed dose of trovafloxacin (300 mg) against E. coli, P. aeruginosa, and K. pneumoniae are 20 to 30% greater than those produced by two 500-mg doses of ciprofloxacin. Based on the IE-log D curves, the single dose of trovafloxacin which is as efficient as two 500-mg doses of ciprofloxacin (500 mg given twice daily) was established. Like the predicted AUC/MIC breakpoints, the estimates of the equiefficient dose of trovafloxacin for E. coli, P. aeruginosa, and K. pneumoniae were close to each other: 199, 226, and 203 mg, respectively. Thus, acceptable antimicrobial effects might be provided by a single trovafloxacin dose of approximately 209 mg.
FIG. 7.
Dose-dependent antimicrobial effects of trovafloxacin and ciprofloxacin. TD is the therapeutic dose of ciprofloxacin (500 mg given twice daily). The equiefficient doses of trovafloxacin are indicated by the boxed numbers.
DISCUSSION
Although comparison of pharmacokinetically different antimicrobial agents is frequently cited as a reason to introduce in vitro dynamic models, these sophisticated time-kill studies do not often allow such quantitative comparisons because of inappropriate experimental design and/or suboptimal quantitation of the data. Such shortcomings are also inherent in specific time-kill studies with fluoroquinolones in dynamic models. In most of these studies the antimicrobial effects were compared only when one dose level (4, 30, 33) or a narrow dose range (5, 18, 19) of the quinolones was mimicked. Moreover, the designed doses or the respective simulated AUCs were usually chosen at the “clinically relevant” level without respect to the MICs, even though the AUC/MIC ratio but not the AUC itself or the dose has been suggested as a predictor of a quinolone’s antimicrobial effect (23–25). The design-associated differences in the AUC/MIC ratios for the drugs often resulted in efficacies whose differences were either excessive or negligible (4, 5, 18, 19, 30, 33) for meaningful quantitative comparisons. These factors really prevent accurate interpretation of the observed effects and preclude comparison of quinolones in terms of the AUC/MIC-response curve. On the other hand, such comparisons were not performed even in the most comprehensive study of two fluoroquinolones (ciprofloxacin and ofloxacin) which simulated a 10-fold range of AUC/MIC ratios (20). Because of the investigators’ a priori assumption about the quinolone-independent nature of relationships between the antimicrobial effect and AUC/MIC, only combined data for ciprofloxacin and ofloxacin were analyzed in their report.
Also, in many of the cited studies, an adequate comparison of fluoroquinolones was not possible due to an insufficient duration of the observation period (usually 24 h), which might or might not include the entire regrowth phase. The importance of the full evaluation of this regrowth phase has recently been emphasized in the accurate determination of the antimicrobial effect of ciprofloxacin (13), as assessed by its intensity. In the present comparative study the antimicrobial effects of trovafloxacin and ciprofloxacin were related to a wide range of AUC/MIC ratios for each drug. This approach made possible an accurate comparison of the fluoroquinolones in terms of the relationships between the antimicrobial effect and logarithms of AUC/MIC or dose.
With each bacterial strain studied, a specific linear relationship between the antimicrobial effect (as expressed by its intensity, IE) and log AUC/MIC was inherently associated with a given quinolone (Fig. 6). The IE-log AUC/MIC plots for trovafloxacin and ciprofloxacin differed substantially and were not superimposed. Comparison of these plots showed distinct advantages for trovafloxacin over ciprofloxacin: at each of the AUC/MIC ratios simulated, trovafloxacin produced a greater antimicrobial effect against the three gram-negative bacteria than ciprofloxacin, despite similar intrinsic activities (MICs). These data suggest that a given AUC of trovafloxacin might be “more productive” than the same AUC of ciprofloxacin with respect to the antimicrobial effects which are pharmacokinetic profile dependent in their nature.
Since the slopes of the IE-log AUC/MIC plots were different for the two quinolones, the difference between their effects depends on the AUC/MIC: the higher the AUC/MIC, the greater the difference. To make definitive quantitative comparisons of the quinolones, a certain reference value of AUC/MIC is needed. In this study an AUC/MIC value of 125 (μg · h/ml)/(μg/ml), which has been reported to be a significant breakpoint in an in vivo study with ciprofloxacin (14), was used as the reference value. The respective equivalent values of AUC/MIC of trovafloxacin, i.e., AUC/MICs that provide the same IEs as ciprofloxacin, were twofold lower: 58.9, 68.5, and 61.5 (μg · h/ml)/ (μg/ml) for E. coli, P. aeruginosa, and K. pneumoniae, respectively.
Of course, the average AUC/MIC ratio of 63 (μg · h/ml)/(μg/ml) of trovafloxacin as predicted in this study might or might not correspond to the AUC/MIC breakpoint that remains to be established in an in vivo setting. Indeed, the AUC/MIC breakpoint reported by Forrest et al. (14) was based on clinical data with multiple ciprofloxacin dosing regimens, whereas only single doses of trovafloxacin and two doses of ciprofloxacin were mimicked in our in vitro study. Therefore, further correlations between the AUC/MIC breakpoints based on in vitro and on in vivo data are necessary. However, the described indirect approach to predicting the AUC/MIC breakpoint for a new quinolone might be more useful than providing a direct in vitro evaluation of the AUC/MIC breakpoint without any reference to in vivo data (20). Unlike the cited study (20), our approach provides the ability to operate with both in vitro IE-log AUC/MIC relationships for the two drugs and an in vivo-established AUC/MIC breakpoint for the reference drug. Such an indirect prediction of the AUC/MIC breakpoint does not require knowledge of the correspondence of antimicrobial effects observed in vitro and in vivo or of the critical value of the effect in vitro that corresponds to an acceptable outcome in vivo.
The AUC/MIC analysis of the antimicrobial effect presented in this report reveals differences between drugs that are related to inherent pharmacokinetic properties (half-life, among others). To compare the quinolones in terms of the dose-response relationships, the IE-log AUC/MIC relationships were converted into the respective relationships between IE and log D, taking into account the fact that the organisms studied are representative in terms of the MICs (1, 6, 15). For all three gram-negative bacteria, the antimicrobial effect of trovafloxacin was more dose dependent than that of ciprofloxacin (Fig. 7). Based on the IE-log D curves, the single doses of trovafloxacin which are as efficient as two 500-mg doses of ciprofloxacin (the reference point) were established. As with the predicted equivalent AUC/MICs, estimates of the equiefficient dose of trovafloxacin were bacterial species independent (199 to 226 mg).
Like the predicted AUC/MIC breakpoint, the single dose of trovafloxacin which is as efficient as two doses of ciprofloxacin may or may not be numerically identical to the same equiefficient daily dose. To verify the correspondence between the equiefficient single dose established in this study and the equiefficient daily dose of trovafloxacin, clinical correlations are necessary. In this light, the close correspondence between the equiefficient single dose of trovafloxacin (209 mg) and its clinically proven daily dose (200 mg [16, 22, 26]) in reality might only be encouraging.
It should be noted that in this model neither the IE-log AUC/MIC nor IE-log D relationships could have been established were the observations discontinued at 24 h, as is usual for most reported time-kill studies. Indeed, for three of the four AUC/MIC ratios studied, discrimination between the two quinolones would not have been possible because regrowth did not occur until 24 h (Fig. 5). Therefore, obvious differences between the effects of trovafloxacin and ciprofloxacin could not have been reflected by the numbers of surviving bacteria at 24 h, areas under (area under the bacterial count curve [28]) or above (area above the curve [29]) the log NA-t curve or between this curve and the respective control growth curve (area between the bacterial count curves [11]) measured from 0 to 24 h, or Δlog Nmin, or the times to 100- and 1,000-fold reductions in the initial inoculum (T99% and T99.9%, respectively). As might be expected, no reasonable correlations between dose and T99.9% were reported in a comparative time-kill study with three quinolones (18). Unlike these endpoints, the use of IE allowed the differentiation of the effects of the two quinolones; moreover, the determination of IE by its very definition includes the entire killing and regrowth curve from the onset to the end of the drug’s effect.
In conclusion, the data presented here support the application of the relationships between the antimicrobial effect and the AUC/MIC for comparisons of antimicrobial agents (7). At first glance, the use of a wide range of AUCs (or doses) as reported here might appear to be a contradiction given the small range of the usual clinical doses. However, since a clinical dose given to different patients would be presented to bacteria of different susceptibilities, the AUC/MIC ratios actually do vary widely in vivo. As shown in this study, this situation may be simulated in vitro by varying the AUC with a given organism since the relationships between the antimicrobial effect and AUC/MIC are bacterial species independent (12). Similar time-kill studies in in vitro dynamic models might be applicable to other antibiotic classes.
ACKNOWLEDGMENTS
This study was supported by Roerig, a division of Pfizer.
We are grateful to H. Mattie for useful comments.
REFERENCES
- 1.Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 2.Bergan T, Thorsteinsson S B. Pharmacokinetics and bioavailability of ciprofloxacin. In: Neu H C, Weuta H, editors. Proceedings of the 1st International Ciprofloxacin Workshop, Current clinical practice series 34. Amsterdam, The Netherlands: Elsevier Science Publishers B.V. (Excerpta Medica); 1986. pp. 111–121. [Google Scholar]
- 3.Blaser J, Zinner S H. In vitro models for the study of antibiotic activities. Prog Drug Res. 1987;31:349–381. doi: 10.1007/978-3-0348-9289-6_11. [DOI] [PubMed] [Google Scholar]
- 4.Dalhoff, A. 1995. Activities of ciprofloxacin and sparfloxacin against Streptococcus pneumoniae. Drugs 49(Suppl. 2):194–196. [DOI] [PubMed]
- 5.Dalhoff, A. 1995. Pharmacodynamics of quinolones. Drugs 49(Suppl. 2):197–199. [DOI] [PubMed]
- 6.Felmingham, D., M. J. Robbins, K. Ingley, I. Mathias, H. Bhogal, A. Leakey, G. L. Ridgway, and R. N. Grüneberg. 1997. In-vitro activity of trovafloxacin, a new fluoroquinolone, against recent clinical isolates. J. Antimicrob. Chemother. 39(Suppl. B):43–49. [DOI] [PubMed]
- 7.Firsov A A. In vitro simulated pharmacokinetic profiles: forecasting antibiotic optimal dosage. Eur J Drug Metab Pharmacokinet Special Issue. 1991;3:406–409. [PubMed] [Google Scholar]
- 8.Firsov, A. A. 1993. Critical reappraisal of modern approaches to search determinants of efficacy of antimicrobials. Eur. Bull. Drug Res. 2(Suppl. 1):33–38.
- 9.Firsov A A, Chernykh V M, Navashin S M. Quantitative analysis of antimicrobial effect kinetics in an in vitro dynamic model. Antimicrob Agents Chemother. 1991;34:1312–1317. doi: 10.1128/aac.34.7.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firsov A A, Nazarov A D, Chernykh V M. Advances in science and engineering. Vol. 17. Moscow, Russia: VINITI Publishers; 1989. Pharmacokinetic approaches to rational antibiotic therapy; pp. 1–228. . (In Russian.) [Google Scholar]
- 11.Firsov A A, Savarino D, Ruble M, Gilbert D, Manzano B, Medeiros A A, Zinner S H. Predictors of effect of ampicillin-sulbactam against TEM-1 β-lactamase-producing Escherichia coli in an in vitro dynamic model: enzyme activity versus MIC. Antimicrob Agents Chemother. 1996;40:734–738. doi: 10.1128/aac.40.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firsov A A, Shevchenko A A, Vostrov S N, Zinner S H. Inter- and intraquinolone predictors of antimicrobial effect in an in vitro dynamic model: new insight into a widely used concept. Antimicrob Agents Chemother. 1998;42:659–665. doi: 10.1128/aac.42.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firsov A A, Vostrov S N, Shevchenko A A, Cornaglia G. Parameters of bacterial killing and regrowth kinetics and antimicrobial effect examined in terms of area under the concentration-time curve relationships: action of ciprofloxacin against Escherichia coli in an in vitro dynamic model. Antimicrob Agents Chemother. 1997;41:1281–1287. doi: 10.1128/aac.41.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard A E, Girard D, Gootz T D, Faiella J A, Cimochowski C R. In vivo efficacy of trovafloxacin (CP-99,219), a new quinolone with extended activities against gram-positive pathogens, Streptococcus pneumoniae, and Bacteroides fragilis. Antimicrob Agents Chemother. 1995;39:2210–2216. doi: 10.1128/aac.39.10.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham D R, Klein T, Torres A, Niedermann M the Trovan Nosocomial Pneumonia Study Group. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. A double blind, randomized, multicenter study of nosocomial pneumonia (NOS) comparing trovafloxacin with ciprofloxacin ± clindamycin/metronidazole, abstr. LM-74; p. 378. [Google Scholar]
- 17.Hoffken G, Lode H, Prinzing C, Borner K, Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985;27:375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S L, Rybak M J, McGrath B J, Kaatz G W, Seo S M. Pharmacodynamics of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with rifampin, against methicillin-susceptible and -resistant Staphylococcus aureus in an in vitro infection model. Antimicrob Agents Chemother. 1994;38:2702–2709. doi: 10.1128/aac.38.12.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madaras-Kelly K J, Larsson A J, Rotschafer J C. A pharmacodynamic evaluation of ciprofloxacin and ofloxacin against two strains of Pseudomonas aeruginosa. J Antimicrob Chemother. 1996;37:703–710. doi: 10.1093/jac/37.4.703. [DOI] [PubMed] [Google Scholar]
- 20.Madaras-Kelly K J, Ostergaard B E, Baeker Hovde L, Rotschafer J C. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996;40:627–632. doi: 10.1128/aac.40.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakawa T. In vitro/in vivo kinetic models for evaluating efficacy of antimicrobial agents. In: Kuemmerle H-P, Murakawa T, Nightingale C H, editors. Pharmacokinetics of antimicrobial agents: principles, methods, applications, part IV. Pharmacokinetics and therapeutic efficacy, section IV-1. Landsberg/Lech, Germany: Ecomed Verlags GmbH & Co. KG; 1993. pp. 165–177. [Google Scholar]
- 22.Niederman M, Traub S, Ellison W T, Hopkins D W the Trovan Pneumonia Study Group. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. A double blind, randomized, multicenter, global study in hospitalized community acquired pneumonia (CAP) comparing trovafloxacin with ceftriaxone + erythromycin, abstr. LM-72; p. 377. [Google Scholar]
- 23.Nightingale C H. Pharmacokinetic considerations in quinolone therapy. Pharmacotherapy. 1993;13(2 Pt. 2):34S–38S. [PubMed] [Google Scholar]
- 24.Schentag J J, Nix D E, Adelman M H. Mathematical examination of dual individualization principles. Relationships between AUC above MIC and area under the inhibitory curve (AUC/MIC) for cefmenoxime, ciprofloxacin, and tobramycin. DICP-Ann Pharmacother. 1991;25:1050–1057. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- 25.Schentag J J, Nix D E, Forrest A. Pharmacodynamics of the fluoroquinolones. In: Hooper D C, Wolfson J S, editors. Quinolone antimicrobial agents. 2nd ed. Washington, D.C: American Society for Microbiology; 1993. pp. 259–271. [Google Scholar]
- 26.Sullivan J, Gezon J, Williams Hopkins D the Trovan Community Pneumonia Study Group. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Double blind, randomized, multicenter study in ambulatory community acquired pneumonia (CAP) comparing trovafloxacin with clarithromycin, abstr. LM-73; p. 378. [Google Scholar]
- 27.Teng R, Harris S C, Nix D E, Schentag J J, Foulds G, Liston T E. Pharmacokinetics and safety of trovafloxacin (CP-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemother. 1995;36:385–394. doi: 10.1093/jac/36.2.385. [DOI] [PubMed] [Google Scholar]
- 28.White, C. A., and R. G. Toothaker. 1985. Influence of ampicillin elimination half-life on in-vitro bactericidal effect. J. Antimicrob. Chemother. 15(Suppl. A):257–260. [DOI] [PubMed]
- 29.Wiedemann B, Jansen A. Antibacterial activity of cefpodoxime proxetil in a pharmacokinetic in-vitro model. J Antimicrob Chemother. 1990;26:71–79. doi: 10.1093/jac/26.1.71. [DOI] [PubMed] [Google Scholar]
- 30.Wiedemann, B., C. Rustige-Wiedemann, and B. Kratz. 1995. Comparison of the pharmacodynamic properties of quinolones. Drugs 49(Suppl. 2):269–271. [DOI] [PubMed]
- 31.Wise, R., D. Lister, C. A. M. McNulty, D. Griggs, and J. M. Andrews. 1986. The comparative pharmacokinetics of five quinolones. J. Antimicrob. Chemother. 18(Suppl. D):71–81. [DOI] [PubMed]
- 32.Wise R, Mortiboy D, Child G, Andrews G M. Pharmacokinetics and penetration into inflammatory fluid of trovafloxacin (CP-99,219) Antimicrob Agents Chemother. 1996;40:47–49. doi: 10.1128/aac.40.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabinski R A, Vance-Bryan K, Krinke A J, Walker K J, Moody J A, Rotschafer J C. Evaluation of activity of temafloxacin against Bacteroides fragilis by an in vitro pharmacodynamic system. Antimicrob Agents Chemother. 1993;37:2454–2458. doi: 10.1128/aac.37.11.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]