Abstract
Sepsis causes significant morbidity and mortality worldwide. Resuscitation is a cornerstone of management. This review covers five areas of evolving practice in the management of early sepsis-induced hypoperfusion: fluid resuscitation volume, timing of vasopressor initiation, resuscitation targets, route of vasopressor administration, and use of invasive blood pressure monitoring. For each topic, we review the seminal evidence, discuss the evolution of practice over time, and highlight questions for additional research. Intravenous fluids are a core component of early sepsis resuscitation. However, with growing concerns about the harms of fluid, practice is evolving toward smaller-volume resuscitation, which is often paired with earlier vasopressor initiation. Large trials of fluid-restrictive, vasopressor-early strategies are providing more information about the safety and potential benefit of these approaches. Lowering blood pressure targets is a means to prevent fluid overload and reduce exposure to vasopressors; mean arterial pressure targets of 60–65 mm Hg appear to be safe, at least in older patients. With the trend toward earlier vasopressor initiation, the need for central administration of vasopressors has been questioned, and peripheral vasopressor use is increasing, although it is not universally accepted. Similarly, although guidelines suggest the use of invasive blood pressure monitoring with arterial catheters in patients receiving vasopressors, blood pressure cuffs are less invasive and often sufficient. Overall, the management of early sepsis-induced hypoperfusion is evolving toward fluid-sparing and less-invasive strategies. However, many questions remain, and additional data are needed to further optimize our approach to resuscitation.
Keywords: sepsis, septic shock, hypotension, fluid therapy, vasoconstrictor agents
Sepsis causes significant morbidity and mortality worldwide, contributing to an estimated 49 million hospitalizations and 11 million deaths in 2017 (1). Resuscitation is a key component of sepsis management, but the optimal approach to resuscitation remains unclear. This review focuses on five key aspects of resuscitation in which practice is evolving: fluid resuscitation volume, vasopressor timing, resuscitation targets, route of vasopressor administration, and use of invasive blood pressure monitoring. For each topic, we review the evidence and current guidelines, discuss practice evolution over time, and highlight questions for future research. In the online supplement, we address additional aspects of resuscitation.
Definitions and Scope
This review focuses on the management of patients with early sepsis-induced hypotension and hyperlactatemia, drawing primarily from clinical trials. Preclinical and clinical physiological studies have also informed current practice but are beyond the scope of this review.
Given the variety and overlap of terms used in practice, we present definitions in Figure 1. We use hypoperfusion to refer to hypotension and/or hyperlactatemia, acknowledging the limitations of this definition. Hypotension and hyperlactatemia are each associated with mortality in sepsis, making them important bedside clinical markers (2). However, their relationship to tissue perfusion is not fully understood (3), as sepsis-induced inflammation can cause microcirculatory dysfunction and disrupt tissue perfusion and oxygen delivery independently of hemodynamics (4, 5). However, given the clinical focus of this review, we define hypoperfusion as hypotension and/or hyperlactatemia, as these widely available clinical markers are used in practice and trials.
Figure 1.
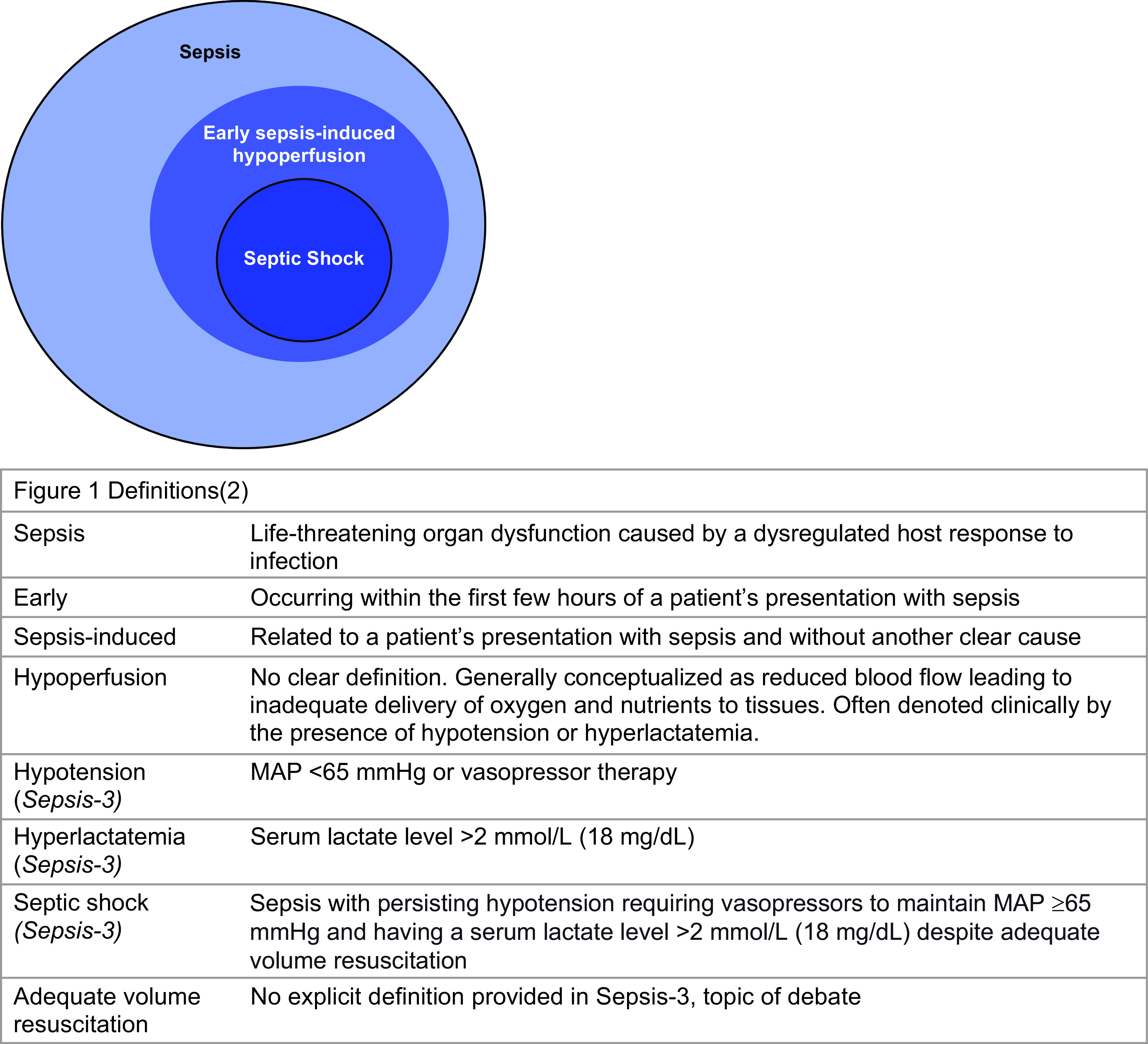
Conceptual diagram of review concepts and their definitions. MAP = mean arterial pressure.
Fluid Resuscitation: How Much Is Enough?
-
•
Conventional teaching: Intravenous fluids are a cornerstone of managing early sepsis-induced hypoperfusion.
-
•
Current guidelines: Several guidelines recommend an initial resuscitation volume of 30 ml/kg (6). However, there are scant recommendations to guide ongoing fluid resuscitation.
-
•
Evolving practice: Practice is evolving toward fluid-sparing approaches to ongoing resuscitation, and there is increasing equipoise about the necessity of the 30 ml/kg initial resuscitation volume.
Fluid resuscitation has been a core component of managing early sepsis-induced hypoperfusion for several decades. After the 2001 Rivers and colleagues trial (7), early goal-directed therapy (EGDT) for septic shock was recommended by the Surviving Sepsis Campaign (SSC) guidelines. The EGDT protocol includes invasive monitoring with central venous and arterial catheters, fluid resuscitation to maintain central venous pressure at 8–12 mm Hg, vasopressors to maintain mean arterial pressure (MAP) ⩾ 65 mm Hg, and blood transfusions and inotropes to maintain central venous oxygen saturation (ScvO2) ⩾ 70%. In the Rivers and colleagues trial, patients randomized to EGDT versus standard therapy received more fluid (4,981 vs. 3,499 ml within 6 h; P < 0.001), blood transfusions (64.1%. vs. 18.5%; P < 0.001), and inotropes (13.7% vs. 0.8%; P < 0.001).
Subsequently, three multicenter trials (Australasian Resuscitation in Sepsis Evaluation [ARISE], Protocolized Care for Early Septic Shock [ProCESS], and Protocolized Management in Sepsis [ProMISe]) tested EGDT versus usual care, which had evolved over the preceding decade in response to the Rivers and colleagues trial (8–10). In these trials, patients randomized to EGDT versus usual care received 200–1,000 ml more fluid within 6 hours after enrollment. Yet, mortality outcomes were neutral in these individual trials and in both standard and individual patient-level meta-analyses (11, 12) (Table 1). Notably, patients in these trials had higher baseline ScvO2 than patients in the Rivers and colleagues trial (70% vs. 49%; Table 1), suggesting they were less sick or enrolled after more resuscitation. However, there was no indication of benefit of EGDT across any of the 59 subpopulations examined in an individual patient-level meta-analysis of ARISE, ProCESS, and ProMISe, including subgroups defined by illness severity and time to randomization (11). Rather, these findings suggest that across all patient populations, usual care and EGDT had equivalent outcomes. Both are reasonable approaches to resuscitation, although EGDT is more invasive and labor intensive.
Table 1.
Trials of Early Goal-directed Therapy
| Study | Population | Intervention | A. Fluids from Presentation to Study Enrollment* | B. Fluids from Study Enrollment to Study Hour 6*† | C. Total Fluids from Presentation to Study Hour 6 (A + B)* | ScvO2 at Study Enrollment | Outcomes‡ (Intervention vs. Control) |
|---|---|---|---|---|---|---|---|
| Rivers et al. (7) | 263 patients with septic shock in 1 U.S. emergency department | EGDT vs. usual care | N/A, prerandomization fluids were included in total reported fluids from Hours 0–6 (column B) | EGDT: 4,981 ml; standard therapy: 3,499 ml; (mean), P < 0.001 | EGDT: 4,981 ml; standard therapy: 3,499 ml; (mean), P < 0.001 | EGDT: 48.6% ± 11.2%; standard therapy: 49.2% ± 13.3%; (mean), P = 0.49 | In-hospital mortality: 30.5% vs. 46.5%; P = 0.009 |
| ARISE (8) | 1,600 patients with septic shock in 51 hospitals in Australia and New Zealand | EGDT vs. usual care | EGDT: 2,515 ml; usual care: 2,591 ml; (mean), P value not reported | EGDT: 1,964 ml; usual care: 1,713 ml; (mean), P < 0.001 | EGDT: 4,479 ml; usual care: 4,304 ml; (mean), P value not calculated | EGDT: 72.7% ± 10.5% (mean); ScvO2 was not monitored in the usual care group | 90-d mortality: 18.6% vs. 18.8%; P = 0.90 |
| ProCESS (9) | 1,341 patients with septic shock in 31 U.S. hospitals | EGDT vs. protocol-based “standard” therapy vs. usual care | EDGT: 2,254 ml; protocol: 2,226 ml; usual care: 2,083 ml; (mean), P = 0.15 | EGDT: 2,805 ml; protocol: 3,285 ml; usual care: 2,279 ml; (mean), P < 0.001 | EDGT: 5,059 ml; protocol: 5,511 ml; usual care: 4,362 ml; (mean), P value not reported | Overall: 71% ± 13% (mean); ScvO2 was not routinely monitored in usual care group | 60-d in-hospital mortality: 21.8% vs. 18.2% vs. 18.9%; P = 0.83 |
| ProMISe (10) | 1,260 patients with septic shock in 56 hospitals in England | EGDT vs. usual care | EDGT: 1,950 ml; usual care: 2,000 ml; (median), P value not reported | EGDT: 2,000 ml; usual care: 1,784 ml; (median), P value not reported | EDGT: 3,950 ml; usual care: 3,784 ml; (median), P value not reported | Overall: 70% ± 12% (mean); ScvO2 was not routinely monitored in usual care group | 90-d mortality: 29.5% vs. 29.2%; P = 0.90; EGDT was associated with higher organ-failure scores, more days of cardiovascular support, and longer ICU stays |
Definition of abbreviations: ARISE = Australasian Resuscitation in Sepsis Evaluation; EGDT = early goal-directed therapy; N/A = not applicable; ProCESS = Protocolized Care for Early Septic Shock; ProMISe = Protocolized Management in Sepsis; ScvO2 = central venous oxygen saturation.
Rivers and colleagues (7) reported the total fluid patients received from presentation to Hour 6, whereas the ARISE, ProCESS, and ProMISe trials reported the fluid patients received before study enrollment and from study enrollment to Study Hour 6 separately, as denoted in columns A and B above. Column C is a summation of total fluid received before enrollment and during the first 6 hours of study enrollment in the ARISE, ProCESS, and ProMISe trials, to facilitate a comparison to the amount of fluid patients received in Rivers and colleagues.
Values do not include fluid received before randomization.
Listed as intervention versus usual care; P value.
After the ARISE, ProCESS, and ProMISe trials, the 2016 SSC Guidelines replaced the recommendation for EGDT with a pragmatic recommendation that patients with sepsis-induced hypoperfusion receive ⩾30 ml/kg crystalloids within 3 hours of presentation, with ongoing resuscitation guided by serial assessments of hemodynamic status. However, most trials have enrolled patients after some initial fluid administration, precluding rigorous evaluation of initial fluid volume. A total of 30 ml/kg was chosen because most patients enrolled in ARISE, ProCESS, and ProMISe received around 30 ml/kg before randomization (Table 1) (11). In addition, 30 ml/kg has been associated with benefit in observational studies. For example, in a multicenter study of patients with sepsis with intermediate lactates (2–4 mmol/L), implementation of a treatment bundle including a 30 ml/kg bolus was associated with increased fluid delivery and decreased mortality over time (13). Importantly, however, no randomized trials have evaluated 30 ml/kg versus other initial fluid volumes, and the SSC downgraded its 30 ml/kg recommendation to a suggestion in 2021 (6).
The SSC’s evolution from recommending EGDT, to recommending 30 ml/kg, to suggesting 30 ml/kg is emblematic of broader shifts in thinking and practice. Intravenous fluids help correct intravascular depletion and restore preload. However, sepsis-induced hypotension and hyperlactatemia do not necessarily imply true hypovolemia. Patients with community-onset sepsis often have decreased oral intake, fever, and insensible losses that may contribute to volume depletion (14), but sepsis also induces an inflammatory response that decreases systemic vascular resistance, increases vascular permeability, and lowers blood pressure in a manner that may not be improved by fluid resuscitation (15, 16).
Over the past 15 years, there has been increasing concern about potential harms from overresuscitation. In observational studies, fluid overload and positive fluid balance have been associated with higher mortality, although the risk of confounding limits strong conclusions (17–20). More compellingly, three randomized controlled trials (RCTs) in lower-resource settings (where negative impacts of fluid overload may be less remediable) showed harm with larger-volume resuscitation, as detailed in Table 2 (21–23).
Table 2.
Trials of Sepsis Resuscitation in Lower-Resource Settings
| Trial | Details | Interventions | Outcomes* |
|---|---|---|---|
| FEAST trial (21) | 3,141 children with fever and organ dysfunction at 6 hospitals in Kenya, Tanzania, and Uganda | Albumin bolus vs. saline bolus vs. usual care | Stopped early owing to increased mortality in the fluid bolus arms; 48-h mortality: 10.6% (albumin arm) vs. 10.6% (saline arm) vs. 7.3% (usual care, no bolus) |
| Simplified Severe Sepsis Protocol-1 (22) | 112 adults with sepsis and hypotension at a single center in Zambia | 6-h sepsis bundle (4,000 ml IV fluids guided by jugular venous pressure, dopamine, and blood transfusion) vs. usual care | Stopped early owing to high mortality in patients with hypoxemic respiratory distress at baseline (8/8 intervention vs. 7/10 control); in-hospital mortality: 64.2% vs. 60.7% (RR, 1.05; 95% CI, 0.79–1.41) |
| Simplified Severe Sepsis Protocol-2 (23) | 209 adults with sepsis and hypotension at a single center in Zambia | 6-h sepsis bundle (IV fluid boluses guided by jugular venous pressure, vasopressors, and blood transfusions) vs. usual care | In-hospital mortality: 48.1% vs. 33.0%, P = 0.03; fluid received within 6 h (median): 3,500 ml vs. 2,000 ml, P < 0.001; fluid received within 24 h (median): 4,000 ml vs. 3,000 ml, P < 0.001; vasopressors received: 14.2% vs. 1.9%, P < 0.001 |
Definition of abbreviations: CI = confidence interval; FEAST = Fluid Expansion as Supportive Therapy trial; RR = relative risk.
Listed as intervention versus usual care, P value.
Several small trials have evaluated fluid-restrictive approaches to ongoing resuscitation, using three general approaches: 1) fluid boluses for limited clinical criteria; 2) fluid boluses guided by serial assessments of fluid responsiveness; and 3) capped total fluid volume (Table 3). Meta-analysis of these trials did not favor fluid-liberal versus fluid-restrictive approaches (6, 24), but the lack of difference should be interpreted with caution because of small sample sizes, differing approaches to fluid limitation, and lack of separation in fluid volume in some trials (25–28).
Table 3.
Trials of Fluid-Restrictive Approaches to Ongoing Resuscitation, Early Vasopressors, and Lower Resuscitation Targets
| Study | Population | Time to Enrollment | Intervention | Differences between Study Arms*† | Outcomes*† |
|---|---|---|---|---|---|
| Fluid resuscitation, strategy 1: Fluid boluses based on select clinical criteria | |||||
| Meyhoff et al. (29)/CLASSIC | 1,554 patients with septic shock in 31 European ICUs | Enrollment after at least 1,000 ml IV fluid, within 12 h of septic shock diagnosis
|
Intervention: 250–500 ml boluses for 4 clinical criteria: 1) lactate ⩾ 4 mmol/L; 2) MAP < 50 mm Hg despite vasopressors; 3) skin mottling; 4) oliguria within 2 h of randomization. Fluids were also allowed to correct fluid losses, dehydration, or electrolyte deficiencies and to ensure a total intake of 1,000 ml/d Control: Usual care |
Fluids within 5 d (median): 1,450 ml vs. 3,077 ml, P value not reported |
90-d mortality: 42.3% vs. 42.1%, P = 0.96 Serious adverse events (including ischemia and kidney injury): 29.4% vs. 30.8%, P = 0.46 |
| Jessen et al. (91)/REFACED feasibility trial | 123 patients with sepsis without shock in 2 Denmark EDs | Enrollment after no more than 500 ml of IV fluid
|
Intervention: 250 ml bolus for lactate ⩾ 4 mmol/l, hypotension, mottling, severe oliguria within 4 h of randomization Control: Usual care |
Fluids within 24 h (mean): 562 ml vs. 1,370 ml, P = 0.001 | No difference in use of mechanical ventilation, vasopressors, or new kidney injury |
| Hjortrup et al. (92)/CLASSIC feasibility trial | 151 patients with septic shock in 9 Scandinavian ICUs | Enrollment after 30 ml/kg bolus, within 12 h of septic shock diagnosis
|
Intervention: 250–500 ml boluses for 4 clinical criteria: 1) lactate ⩾ 4 mmol/L; 2) MAP < 50 mm Hg despite vasopressors; 3) skin mottling; 4) oliguria within 2 h of randomization Control: Usual care |
Fluids within 5 d (median): 500 ml vs. 2,000 ml, P < 0.001 | 90-d mortality: 33% vs. 41%, P = 0.32 AKI: 37% vs. 54%, P = 0.03 |
| Semler et al. (25)/BALANCE pilot trial‡ | 30 patients with SIRS and shock or respiratory insufficiency in 1 U.S. medical ICU | Enrollment within 12 h of ICU admission
|
Intervention: IV fluid only for oliguria or increasing vasopressor requirement Control: Usual care |
Difference in daily fluid balance (mean): −398 ml, P = 0.33 | In-hospital mortality: 30.0% vs. 26.7%, P > 0.99 Neutral results for secondary outcomes, including mortality, support-free days, AKI |
| Fluid resuscitation, strategy 2: Fluid boluses based on evaluation of fluid responsiveness | |||||
| Douglas et al. (33) | 124 patients with septic shock at 13 hospitals in the United States and United Kingdom | Enrollment within 24 h of hospital arrival
|
Intervention: PLR assessment before any clinician-desired fluid bolus; fluids given only if PLR positive Control: Usual care (2:1 randomization) |
Fluid balance at 72 h or ICU discharge (mean): 650 ml vs. 2,020 ml, P = 0.021 | 30-d mortality: 15.7% vs. 22.0%, not significant RRT: 5.1% vs. 17.5%, P = 0.04 Mechanical ventilation: 17.7% vs. 34.1%, P = 0.04 |
| Lanspa et al. (26)/feasibility trial‡ | 30 patients with septic shock in 1 U.S. medical ICU | Enrollment within 6 h of septic shock diagnosis
|
Intervention: Echocardiogram-guided resuscitation every 1 h for 6 h Control: Modified EGDT for 6 h |
Fluids received during study (median): 0 ml vs. 1,000 ml, P = 0.61 |
Change in SOFA score at 48 h: −4 vs. −6 points, P = 0.10 28-d mortality: 33% vs. 20%, P = 0.68 |
| Cronhjort et al. (28)‡ | 34 patients with septic shock in 1 Swedish surgical ICU | Enrollment within 12 h of septic shock diagnosis
|
Intervention: PLR assessment before any clinician-desired fluid bolus; fluids given only if PLR positive Control: Usual care |
Fluids during study (median): 2,103 ml vs. 2,408 ml, P = 0.38 |
Weight difference from enrollment to Day 3 (mean): 0.6 kg vs. 1.3 kg, P = 0.59 30-d mortality: 12.5% vs. 11.1%, P = 1.00 |
| Chen and Kollef (27)/pilot trial‡ | 82 patients with septic shock in 1 U.S. medical ICU | Enrollment within 12 h of initial fluid bolus
|
Intervention: Targeted fluid minimization, defined as daily PLR assessments with fluids only if positive Control: Usual care |
Fluid balance by Day 3 (median): 1,952 ml vs. 3,124 ml, P = 0.20 Fluid balance by Day 5 (median): 2,641 ml vs. 3,616 ml, P = 0.40 |
In-hospital mortality: 56.1% vs. 48.8%, P = 0.51 Neutral results for other secondary outcomes, including ventilator days, need for RRT |
| Richard et al. (93) | 60 patients with septic shock in 1 French medical ICU | Enrollment within 12 h of initial hypotension
|
Intervention: Fluids guided by preload dependence indices (pulse pressure variation or PLR) every 1 h for 6 h, then every 4 h until vasopressor weaning Control: CVP-guided fluids every 1 h for 6 h, then every 4 h until vasopressor weaning |
Daily fluids (median): 383 ml/d vs. 917 ml/d, P = 0.04 |
Time to shock resolution: 2.3 d vs. 2.0 d, P = 0.29 28-d mortality: 23% vs. 47%, P = 0.10 |
| Fluid resuscitation, strategy 3: Restricting total fluid volume | |||||
| Corl et al. (94)/RIFTS pilot trial | 109 patients with septic shock in 2 U.S. medical ICUs | Enrollment after 1,000 ml initial bolus
|
Intervention: Limit of ⩽60 ml/kg fluid within 72 h Control: Usual care |
Fluids within 72 h of enrollment (mean): 47 ml/kg vs. 61 ml/kg, P = 0.01 | 30-d mortality: 21.8% vs. 22.2%, P > 0.99 |
| Early vasopressor initiation | |||||
| NHLBI PETAL (Prevention and Early Treatment of Acute Lung Injury) Trial Network (30)/CLOVERS | 1,563 patients with sepsis-induced hypotension across 60 U.S. hospitals | Enrollment after 1,000–3,000 ml initial bolus
|
Intervention: Fluid restriction, with vasopressors for ongoing hypotension and rescue fluid boluses only for select clinical criteria Control: Fluid liberal, with fluid boluses for ongoing hypotension and rescue vasopressors only for select clinical criteria |
Fluids in first 24 h: 1,267 ml vs. 3,400 ml; difference, −2,134; 95% CI, −2,318 to −1,949 ml Vasopressor administration in first 24 h: 59.0% vs. 37.2%; difference, 21.7%; 95% CI, 16.9% to 26.6% |
90-d mortality: 14.0% vs. 14.9%; difference, −0.9; 95% CI, −4.4 to 2.6 |
| Permpikul et al. (49)/CENSER | 320 patients with sepsis-induced hypotension in 1 Thailand hospital | Enrollment within 1 h of hypotension
|
Intervention: Fixed-dose norepinephrine (0.05 μg/kg/min for 24 h) Control: Placebo infusion |
Total fluids within 6 h (median): 2,450 ml vs. 2,600 ml, P = 0.33 |
Resuscitation targets achieved within 6 h: 76.1% vs. 48.4%, P < 0.001 28-d mortality: 15.5% vs. 21.9%, P = 0.15 |
| MacDonald et al. (48)/REFRESH pilot trial | 99 patients with sepsis-induced hypotension in 8 Australian EDs | Enrollment after 1,000 ml initial bolus
|
Intervention: Vasopressors for MAP < 65 mm Hg; 250 ml boluses at physician discretion Control: Usual care, defined as 1,000 ml initial fluid bolus and further 500 ml boluses at physician discretion, vasopressors for sustained MAP < 65 mm Hg despite fluids |
Total fluids within 6 h (median): 2,387 ml (30 ml/kg) vs. 3,000 ml (43 ml/kg), P < 0.001 | 90-d mortality: 8% vs. 6%, P value not reported Neutral results in other secondary outcomes, including ICU admission, LOS, and vasopressor-free, ventilator-free, and RRT-free days |
| Resuscitation targets† | |||||
| SEPSISPAM (54) | 776 patients with septic shock across 29 hospitals in France | Enrollment after at least 30 ml/kg fluids and within 6 h of vasopressor initiation | Low-target arm: 65–70 mm Hg High-target arm: 80–85 mm Hg |
Norepinephrine dose (Day 1, median): 0.45 μg/kg/min vs. 0.58 μg/kg/min, P < 0.001 Norepinephrine duration (mean): 3.7 d vs. 4.7 d, P < 0.001 |
28-d mortality: 34.0% vs. 36.6%, P = 0.57 Atrial fibrillation: 2.8% vs. 6.7%, P = 0.02 Among patients with chronic hypertension, rate of renal replacement therapy: 42.2% vs. 31.7%, P = 0.046 |
| OVATION Pilot Trial (56) | 118 patients with vasodilatory shock across 11 ICUs in Canada and the United States | Enrollment after “adequate fluid resuscitation” per treating physician and within 24 h of vasopressor initiation | Low-target arm: 60–65 mm Hg High-target arm: 75–80 mm Hg |
Vasopressor dose (norepinephrine equivalents, median): 10 mg vs. 14 mg, P = 0.017 Vasopressor duration (median): 3 d vs. 5 d, P = 0.0075 |
Separation in MAP between groups: 9 mm Hg; 95% CI, 7 mm Hg to 11 mm Hg Composite mortality or persistent organ dysfunction at 28 d: 44% vs. 46%, P = 0.21 Cardiac arrhythmia: 20% vs. 26%, P = 0.07 |
| The 65 Trial (58) | 2,600 patients ⩾65 yr old with vasodilatory shock across 65 ICUs in the United Kingdom | Enrollment after “adequate fluid resuscitation” per treating physician and within 6 h of vasopressor initiation | Low-target arm: 60–65 mm Hg High-target arm: Usual care |
Vasopressor dose (norepinephrine equivalents, median): 17.7 mg vs. 26.4 mg; difference, −8.7 mg; 95% CI, −12.8 mg to −4.6 mg Vasopressor duration (median): 33 h vs. 38 h; difference, −5 h; 95% CI, −7.8 h to −2.2 h |
Unadjusted 90-d mortality: 41.0% vs. 43.8%, P = 0.15; OR, 0.89; 95% CI, 0.76 to 1.04 Adjusted 90-d mortality: aOR, 0.82; 95% CI, 0.68 to 0.98 90-d mortality in patients with chronic hypertension: 38.2% vs. 44.3%, P = 0.047; aOR, 0.67; 95% CI, 0.51 to 0.88 |
| ANDROMEDA-SHOCK (62) | 424 patients with septic shock and lactate ⩾ 2.0 mmol/L across 28 ICUs in 5 countries | Enrollment after at least 20 ml/kg fluids and within 4 h of vasopressor initiation | MAP ⩾ 65 mm Hg with additional fluids, higher MAP targets, and inotropes in patients who failed to meet the randomized resuscitation target Arm 1: Capillary refill time normalization Arm 2: Decrease in serum lactate of 20% |
Vasopressor doses not reported Vasopressor-free days within 28 d (mean): 16.7 d vs. 15.1 d, P = 0.18 |
28-d mortality: 34.9% vs. 43.4%, P = 0.06 28-d mortality among patients with APACHE II scores < 25: 24.6% vs. 36.3%; HR, 0.61; 95% CI, 0.39 to 0.96 |
Definition of abbreviations: AKI = acute kidney injury; ANDROMEDA-SHOCK = The ANDROMEDA-SHOCK Study; aOR = adjusted odds ratio; APACHE = Acute Physiology and Chronic Health Evaluation; BALANCE = A Randomized Controlled Trial of a Conservative Fluid Balance Strategy for Patients With Sepsis and Cardiopulmonary Dysfunction (BALANCE Study); CENSER = Early Use of Norepinephrine in Septic Shock Resuscitation; CI = confidence interval; CLASSIC = Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care; CLOVERS = Crystalloid Liberal vs Vasopressors Early Resuscitation in Sepsis; ED = emergency department; LOS = length of stay; MAP = mean arterial pressure; OR = odds ratio; PLR = passive leg raise; REFACED = Restrictive Fluid Administration vs. Standard of Care in Emergency Department Sepsis Patients; REFRESH = Restricted Fluid Resuscitation in Suspected Sepsis Associated Hypotension; RIFTS = The Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock; RRT = renal replacement therapy; SEPSISPAM = Assessment of Two Levels of Arterial Pressure on Survival in Patients With Septic Shock; SIRS = Systemic Inflammatory Response Syndrome; SOFA = Sepsis Related Organ Failure Assessment.
Primary outcomes are reported in italics.
Results listed as intervention versus control, P value.
Results presented as lower-MAP target arm versus higher-MAP target arm for SEPSISPAM, OVATION, and the 65 Trial and as capillary refill time arm versus lactate arm for ANDROMEDA-SHOCK.
Denotes studies that did not meet prespecified targets for separation in fluid delivery between study arms.
Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care (CLASSIC), the first multicenter trial of fluid-restrictive resuscitation powered to assess patient outcomes, enrolled 1,554 patients with septic shock across 31 European ICUs after initial fluid resuscitation (29). Patients were randomized to usual care versus fluid restriction, in which 250–500 ml crystalloid boluses were allowed for select clinical markers of hypoperfusion (lactate ⩾ 4 mmol/L, MAP < 50 mm Hg, skin mottling, oliguria within 2 h); to correct fluid losses, dehydration, or electrolyte deficiencies; and to ensure a total intake of 1,000 ml/d. Patients randomized to fluid restriction received less fluid (median difference, −813 ml, Day 1), but mortality and secondary outcomes were similar (Table 3). Interpretation of these results is complicated by several factors. First, although prerandomization fluid volume was notably lower in this trial than in the pilot trial 6 years earlier, indicative of recent trends toward fluid restriction (median, 3,000–3,200 ml vs. 4,200–4,790 ml), it was still high. By comparison, the separation in fluid between arms was small and of uncertain clinical significance. 21.5% had a protocol violation in the restriction arm, and although small (median 97 ml/d), this further reduced the difference between arms. Subgroup analysis of patients on respiratory support revealed numerically lower 90-day mortality in the fluid-restriction arm (46.5% vs. 52.0%; P value for heterogeneity = 0.03), suggesting a potential benefit of fluid restriction in these patients that may have been masked by suboptimal separation in study arms and heterogeneity of treatment effect.
In the recent Crystalloid Liberal vs Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial, 1,563 patients with early sepsis-induced hypotension in 60 U.S. hospitals were randomized to a fluid-restrictive, vasopressor-early versus fluid-liberal approach (30). The trial was stopped early in February 2022 for futility. There was high protocol adherence (97% vs. 96%) and good treatment separation between arms (24-h median differences: fluids −2,134 ml, vasopressors 21.7%). However, outcomes were similar (Table 3). Hypothesized effect sizes were large and led to early stopping for futility, which results in wide confidence intervals and difficulty interpreting adverse events and subgroup analyses.
The neutral results of CLASSIC and CLOVERS despite statistically significant separation between arms present a few possible interpretations: 1) fluid-restrictive, vasopressor-early strategies may not be better than traditional fluid-liberal strategies; 2) the clinical criteria used to guide fluid boluses and vasopressor initiation in these studies do not represent the optimal approach; and 3) the magnitude of the treatment effect included in the sample size calculations was unrealistically large, particularly given limitations in clinically meaningful group separation and patient heterogeneity.
Dynamic measures of fluid responsiveness (e.g., changes in cardiac output or stroke volume in response to passive leg raise or fluid challenges) can help inform ongoing fluid administration and avoid under- or overresuscitation. Meta-analyses have yielded conflicting results on whether these approaches improve clinical outcomes (31, 32). More recently, however, in a multicenter RCT of 124 patients with sepsis-induced hypotension, randomization to fluid boluses guided by stroke volume change after passive leg raise resulted in lower ICU fluid balance, less renal replacement therapy, and less mechanical ventilation than usual care (33) (Table 3).
Overall, recent trials comparing fluid resuscitation approaches in higher-resource settings have all yielded neutral results (8–10, 29), suggesting any of the tested approaches are reasonable in these settings. In bedside practice, clinicians should consider individual conditions that may require more or less resuscitation (e.g., dehydration and respiratory failure, respectively) and assess dynamic measures of fluid responsiveness through fluid challenges to target resuscitation to individual patient needs. A reasonable rule of thumb for initial fluid volume is 30 ml/kg, but this should be tailored based on patient factors and clinical response to fluid administration. Finally, it is important to note that existing resuscitation trials enrolled patients after fluid volumes of ⩾30 ml/kg (Tables 1 and 3). Thus, although the evidence behind 30 ml/kg fluid volume is weak and primarily drawn from observational studies, existing trials do not support limiting initial resuscitation to <30 ml/kg. Two ongoing trials of early sepsis resuscitation are enrolling patients even earlier and will further inform practice: Australasian Resuscitation in Sepsis Evaluation: Fluids or Vasopressors in Emergency Department Sepsis (ARISE FLUIDS) (NCT 04569942) and Early Vasopressors in Sepsis (EVIS) (NCT 05179499) (Table 4).
Table 4.
Outstanding Clinical Questions in the Management of Early Sepsis-induced Hypoperfusion, Related to Topics Covered in This Review
| Topic | Outstanding Clinical Questions | Ongoing Trials | Trial Details | Status |
|---|---|---|---|---|
| Fluid resuscitation | Should patients with sepsis-induced hypoperfusion receive an initial fluid bolus volume of 30 ml/kg vs. other volumes (e.g., 20 ml/kg) vs. initial vasopressors without IV fluid? | None | — | — |
| Fluid resuscitation | For patients with ongoing sepsis-induced hypoperfusion despite an initial fluid bolus, should subsequent fluid boluses be guided by total volume goals, clinical criteria, serial evaluations of fluid responsiveness, or all of the above? | None | — | — |
| Vasopressor timing | For patients with sepsis- induced hypotension, should blood pressure be treated with additional fluid resuscitation vs. initiation of vasopressors? | CLOVERS (NCT 03434028) | 1,563 patients with sepsis-induced hypotension in U.S. EDs and ICUs randomized to early vasopressors and restrictive fluids vs. liberal fluids | Completed, see Table 3 |
| ARISE FLUID (NCT 04569942) | 1,000 patients with sepsis-induced hypotension in New Zealand and Australia EDs randomized to early vasopressors and restrictive fluids vs. liberal fluids | Recruiting | ||
| Vasopressor timing | For patients with sepsis- induced hypotension, should vasopressors be started before an initial fluid bolus, concurrently with an initial fluid bolus, or only if blood pressure fails to respond to an initial fluid bolus? | EVIS (NCT 05179499) | 3,286 patients with sepsis-induced hypotension in the United Kingdom randomized to early, peripheral vasopressors vs. standard care | Recruiting |
| Resuscitation targets | For patients with sepsis- induced hypotension, should the target MAP be ⩾65 mm Hg, 60–65 mm Hg, or another target? | None | — | — |
| Resuscitation targets | For patients with sepsis- induced hypoperfusion, should resuscitation be guided by targets other than MAP, such as diastolic blood pressure or tissue perfusion markers? | ANDROMEDA-2 (NCT 05057611) | 1,500 patients with septic shock across multiple hospitals on 4 continents randomized to resuscitation guided by capillary refill time combined with clinical hemodynamic phenotyping (using pulse pressure variation to guide additional fluid and diastolic blood pressure to guide vasopressors) vs. usual care | Recruiting |
| TARTARE-2S (NCT 02579525) | 200 patients with septic shock in 4 European ICUs randomized to tissue perfusion targeted resuscitation (capillary refill time, skin mottling, lactate, peripheral temperature, urine output, MAP, and ScvO2) vs. standard MAP targets | Recruiting | ||
| Route of vasopressor administration | For patients with sepsis- induced hypotension on vasopressor therapy, under what circumstances should central venous access be obtained? | None | — | — |
| Blood pressure monitoring | For patients with sepsis- induced hypotension on vasopressors, should blood pressure be monitored invasively with an arterial catheter vs. noninvasively with a blood pressure cuff vs. noninvasively with other novel blood pressure monitoring strategies? | None | — | — |
Definition of abbreviations: ANDROMEDA-2 = Hemodynamic Phenotype and Capillary Refill Time-targeted Resuscitation Strategy; ARISE FLUID = Australasian Resuscitation in Sepsis Evaluation: Fluids or Vasopressors in Emergency Department Sepsis; CLOVERS = Crystalloid Liberal vs Vasopressors Early Resuscitation in Sepsis; ED = emergency department; EVIS = Early Vasopressors in Sepsis; MAP = mean arterial pressure; ScvO2 = central venous oxygen saturation; TARTARE-2S = Targeted Tissue Perfusion Versus Macrocirculatory-guided Standard Care in Patients With Septic Shock.
Resuscitation Timing: When Should We Add Vasopressors?
-
•
Conventional teaching: Vasopressors are reserved for patients who remain hypotensive despite fluid resuscitation.
-
•
Current guidelines: Guidelines recommend initiating vasopressors before completing initial fluid resuscitation in patients with severe hypotension (34, 35).
-
•
Evolving practice: Earlier initiation of vasopressors, concurrent with initial fluids and often paired with fluid restriction.
The most common vasopressors (e.g., norepinephrine) are potent catecholamines with side effects including tachyarrhythmias, myocardial cell damage, immunomodulation, and potential rare organ or limb ischemia (36, 37). There is a theoretical concern that initiating vasopressors before intravenous fluids could mask ongoing volume deficits if present (38). Therefore, traditional practice has been to initiate vasopressors only if patients remain hypotensive after initial fluid resuscitation. In a 2017 survey of 839 physicians in Europe, only 12% used vasopressors “early, before complete resuscitation” in sepsis-induced hypotension (34).
However, vasopressors have potential benefits. They raise blood pressure by increasing preload (like fluids), cardiac contractility, and systemic vascular resistance, although their effect on microcirculation and tissue perfusion is less clear (39, 40). In animal models of shock, norepinephrine helps restore blood pressure, mesenteric blood flow, and tissue oxygenation and limits fluid volume (41, 42). Prompt restoration of blood pressure may be important because duration of low MAP in early sepsis is associated with increased mortality (43). These preclinical and observational data have limitations but have spurred interest in earlier vasopressor initiation to expedite shock resolution and minimize fluid resuscitation volumes.
Cohort studies and secondary analyses of trials have yielded conflicting results about the effects of early vasopressor initiation (44–47), and interpretation is limited by the high risk for confounding.
Before CLOVERS, only three small RCTs had evaluated early vasopressor initiation in sepsis-induced hypotension (48–50). The largest, the Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER) trial, was a single-center trial in Thailand that randomized 320 patients with sepsis-induced hypotension to early, fixed-dose norepinephrine (0.05 μg/kg/min for 24 h) versus placebo infusion (Table 3) (49). Time to open-label norepinephrine and fluid administration within 6 hours were similar between study arms. However, patients randomized to early norepinephrine were more likely to achieve resuscitation targets (MAP > 65 mm Hg, urine output > 0.5 ml/kg, and decrease in lactate > 10%) within 6 hours, suggesting early, low-dose norepinephrine is safe and may hasten resolution of shock. The impact of early vasopressors on patient-centered outcomes is unclear, with recent trials of fluid-restrictive, vasopressor-early regimens in sepsis (CLASSIC and CLOVERS) yielding neutral results, as discussed above.
When considering timing of vasopressor initiation, it is important to acknowledge the potential downstream impacts of vasopressor-early strategies. In CENSER, 47% of patients were managed on the general ward, but many institutions require ICU admission or central venous access for patients receiving vasopressors. In CLOVERS, patients randomized to the vasopressor-early arm were more likely to be admitted to an ICU than patients in the fluid-liberal arm (67.3% vs. 59.2%; difference, 8.1%; 95% confidence interval [CI], 3.3–12.8) (30). Therefore, earlier vasopressor initiation could impact ICU use and must be weighed against potential benefits of faster shock control and minimizing fluid volume.
Although the benefit of early vasopressors is unclear, CLOVERS suggests a fluid-restrictive, vasopressor-early strategy is a safe and reasonable alternative to liberal fluids. Additional guidance on timing of vasopressor initiation may be provided by two ongoing multicenter trials: ARISE FLUIDS (NCT 04569942) and EVIS (NCT 05179499) (Table 4).
Moving the Target: Reframing our Resuscitation Goals
-
•
Conventional teaching: Maintain MAP ⩾ 65 mm Hg.
-
•
Current guidelines: An initial MAP target ⩾ 65 mm Hg is broadly recommended (6, 34).
-
•
Evolving practice: Use of lower MAP goals and adjunctive resuscitation targets.
Lowering blood pressure targets is one way to prevent fluid overload while also avoiding vasopressors and associated line placement to facilitate vasopressor delivery.
MAP is the most widely accepted and studied target for resuscitation and vasopressor titration. However, tissue hypoperfusion may also occur in the absence of systemic hypotension and has independent implications for mortality (2, 4). Therefore, more direct markers of tissue perfusion (e.g., lactate, capillary refill time) are sometimes used as adjunctive resuscitation targets.
Most studies of MAP targets in sepsis have compared ⩾65 mm Hg to ⩾75–85 mm Hg, with the hypothesis that higher MAPs improve tissue perfusion and organ function. Although higher MAPs may increase cardiac output and potentially microcirculation, they do not consistently improve renal function or lactate concentration (51, 52). This finding may be explained by alternative, poorly understood causes of sepsis-induced organ dysfunction. For example, animal models of sepsis suggest that acute kidney injury occurs independently of renal blood flow, oxygen delivery, or histologic injury (53).
The Assessment of Two Levels of Arterial Pressure on Survival in Patients With Septic Shock (SEPSISPAM) trial was the first to evaluate the impact of MAP targets on mortality, randomizing 776 patients with septic shock to a MAP target 65–70 mm Hg versus 80–85 mm Hg (54). There was significant separation in observed MAPs between arms (P = 0.02). Among patients with chronic hypertension, randomization to the lower MAP target was associated with increased incidence of renal replacement therapy. However, overall patients randomized to the lower MAP target received less norepinephrine, had lower incidence of atrial fibrillation, and had similar 28-day mortality (Table 3). Based on these results, SSC guidelines recommend an initial MAP target of ⩾65 mm Hg over higher targets (6).
Some experts have suggested further lowering MAP targets, given the potential risks of fluids and vasopressors. Although difficult to extrapolate to sepsis, permissive hypotension is guideline recommended in trauma patients with hemorrhagic shock, where overresuscitation and high MAPs may propagate bleeding and contribute to complications (55). In sepsis, exploratory analyses of SEPSISPAM and the Optimal Vasopressor Titration (OVATION) pilot trial found decreased mortality in older patients randomized to lower MAPs (56, 57). These findings motivated the 65 Trial, a pragmatic, multicenter RCT that randomized 2,600 ICU patients aged ⩾65 years with vasodilatory shock to permissive hypotension (MAP target, 60–65 mm Hg) versus usual care (58). There was separation between arms in observed MAP (median, 66.7 vs. 72.6 mm Hg), and randomization to permissive hypotension resulted in less vasopressor exposure and lower adjusted 90-day mortality. Unadjusted 90-day mortality findings were neutral (Table 3). In a prespecified subgroup analysis, patients with chronic hypertension randomized to permissive hypotension had lower 90-day mortality, suggesting that lower MAP targets in chronically hypertensive older patients may be beneficial, or are at least unlikely to be harmful—a long-held concern bolstered by the SEPSISPAM trial. Overall, the 65 Trial suggests that targeting a MAP of 60–65 mm Hg decreases vasopressor exposure, is likely safe, and may be beneficial in older patients. Indeed, a recent meta-analysis of SEPSISPAM, OVATION, and the 65 Trial, although negative overall, found lower MAP targets were associated with lower mortality in the sepsis subgroup (Risk Ratio (RR), 0.91; 95% CI, 0.83–0.99) (59).
Adjunctive markers of tissue perfusion, such as lactate and capillary refill time, provide additional data to guide resuscitation. A meta-analysis of four small RCTs found that targeting resuscitation to a 10–20% reduction in lactate, in addition to traditional hemodynamic targets, was associated with decreased mortality (60). Capillary refill time may provide an even more direct bedside measurement of tissue perfusion than MAP or lactate (61). The largest trial to assess capillary refill time was the ANDROMEDA-SHOCK Study, a multicenter trial that randomized 424 patients with septic shock to receive fluids, higher MAPs, and inotropes if they failed to meet resuscitation targets by capillary refill versus serial lactate measurements despite maintaining MAP ⩾ 65 mm Hg (62). Trial adherence was high (protocol deviations: 13.7% capillary refill arm, 10.8% lactate arm), although the difference in resuscitation was small: compared with the lactate arm, patients in the capillary refill arm received 408 ml less fluid within 8 hours (P = 0.01), with no difference in vasopressor-free days or inotrope use. The point estimate for 28-day mortality favored the capillary refill arm and—although 95% CIs were wide and crossed the line of no effect (Table 3)—a Bayesian reanalysis found >90% probability that capillary refill–guided resuscitation improved 28-day mortality versus lactate across all priors (63). We suggest that both lactate and capillary refill can be helpful to inform resuscitation, but clinicians should be cognizant that these markers may be influenced by factors unrelated to perfusion, such as liver function and temperature, respectively (64). Clinicians should not rely on any single marker in isolation to guide resuscitation but rather must consider the overall clinical picture to inform decision making.
Beyond capillary refill time and lactate, markers of microcirculatory changes, such as sublingual orthogonal polarization spectral imaging, aim to measure tissue perfusion more directly at the bedside but are not widely available, and their role in targeting resuscitation has not been established (5). There are ongoing efforts to develop additional bedside measures to individualize resuscitation approaches by identifying which patients with sepsis-induced hypoperfusion need fluid, vasopressors, or both; measures could include, for example, diastolic shock index (ratio between heart rate and diastolic blood pressure) (65) or dynamic arterial elastance (calculated using bedside ultrasound) (66).
Overall, we suggest an initial MAP target of ⩾65 mm Hg in younger patients and 60–65 mm Hg in older patients. Clinician exam, lactate, capillary refill time, and other measures of end-organ function (e.g., mentation, urine output) should be monitored to assess the adequacy of resuscitation, guide additional resuscitation, and inform subsequent resuscitation targets (67). It should be noted that capillary refill time and lactate have only been tested for intensifying therapy in refractory shock. The use of these and other markers to evaluate adequacy of different MAP goals warrants further study. A large multinational, multicenter trial (ANDROMEDA-2; NCT 05057611) and a smaller trial (Targeted Tissue Perfusion Versus Macrocirculatory-guided Standard Care in Patients With Septic Shock [TARTARE-2S]; NCT 02579525), will provide more data about possible benefits of targeting resuscitation to multiple markers of tissue perfusion and fluid responsiveness (Table 4).
Challenging a Paradigm: Must Vasopressors Be Administered Centrally?
-
•
Conventional teaching: Vasopressors must be administered via central venous access.
-
•
Current guidelines: 2021 SSC guidelines suggest initiating vasopressors peripherally rather than delaying initiation until central access is obtained but advise central administration as soon as feasible (6).
-
•
Evolving practice: Primary peripheral administration of vasopressors.
In the 1950 s, several case reports described catastrophic tissue injury from peripheral extravasation of vasopressors (68). Based on these reports, central administration became standard. After the 2001 Rivers trial of EGDT, placement of central venous catheters (CVCs) was further justified to facilitate ScvO2 monitoring.
Over the past 5 years, however, the long-held teaching that vasopressors must be delivered centrally has been questioned. CVCs provide secure access for medication delivery and a means of hemodynamic monitoring that is critical for some patients. Although ScvO2 monitoring can be useful, ARISE, ProCESS, and ProMISe indicate it is not required for all patients, eliminating one indication for routine CVC placement (8–10). Furthermore, requiring CVCs in all patients receiving vasopressors may cause more harm than benefit. CVC placement requires time and expertise, which can delay vasopressor initiation (69). CVC placement also carries a risk for mechanical complications, line infections, and thrombosis (70). Given that some patients need vasopressors for only short durations, requiring CVCs for vasopressor administration may introduce unnecessary risk for these patients (71). Finally, modern medication pumps permit tight control of vasopressor infusion rates, and ultrasound is widely available to confirm appropriate placement of peripheral venous access, lowering the risk of extravasation since the original case reports of harm.
Indeed, peripheral vasopressor administration seems to be increasing in practice. In ARISE, 42% of early vasopressor-treated patients had vasopressors initiated through a peripheral intravenous line (PIV), which was associated with decreased time to vasopressor initiation compared with central administration (median, 2.4 vs. 4.9 h from emergency department arrival; P < 0.001) (69). Furthermore, trial protocols increasingly allow for peripheral vasopressor administration, such as CLOVERS (30), ARISE FLUIDS (NCT 04569942), and EVIS (NCT 05179499).
Despite the increased use of peripheral vasopressors, only one RCT has indirectly addressed central versus peripheral vasopressor administration. In this trial, 266 patients in three French ICUs who needed venous access (70% for the indication of low-dose vasopressors) were randomized to receive peripheral versus central venous access (72). Complications were more common among patients randomized to peripheral access, although PIV complications (e.g., erythema, extravasation) tended to be less serious than complications from central access (e.g., pneumothorax, arterial puncture). Importantly, 61 (47.7%) patients randomized to PIV never received central access, suggesting it may be feasible to avoid central access for at least some patients receiving low-dose vasopressors.
A growing body of literature supports the safety of peripheral vasopressor administration within certain limitations. In a review of 318 peripheral vasopressor adverse events (114 extravasations, 204 tissue injuries), few events were reported with short-term infusion (<24 h) or with PIVs proximal to the antecubital or popliteal fossae (73). In a meta-analysis of 11 studies including 16,055 adult patients receiving peripheral vasopressors, the pooled incidence of adverse events (infiltration, extravasation, or erythema) was 1.8%, and there were no cases of tissue necrosis (74). After excluding a large perioperative study, in which patients received vasopressors in a controlled manner for short durations during surgery, the pooled incidence of adverse events remained low (2.1%). Two other systematic reviews of peripheral vasopressor use in emergency departments and ICUs estimated extravasation and infiltration rates closer to 3%, but likewise found no episodes of tissue necrosis (75, 76). The most important factor associated with extravasation was lack of safety guidelines for PIV monitoring, underscoring the importance of monitoring peripheral vasopressor infusions (76). Notably, peripheral vasopressor complication rates with monitoring are similar to current complication rates of CVC placement, which ranged from 3.1% to 3.7% in a multicenter trial of CVC insertion (70).
Overall, extravasation of peripheral vasopressors is uncommon, and tissue injury is rare with monitored peripheral administration. Therefore, in patients with secure PIVs, we recommend initiating vasopressors peripherally to expedite vasopressor initiation and suggest vasopressors can be continued peripherally at lower doses and with regular monitoring for extravasation. Clinicians should consider the vasopressor dose, clinical trajectory, size/location of the PIV, and other indications for CVC placement when deciding whether to continue vasopressors peripherally versus transition to central access. There are no universally agreed-upon thresholds dictating transition to central administration, so institutional policies and practices vary widely (77). More research is needed to understand the safety of longer-term and higher-dose peripheral vasopressor administration, as well as risks for complications other than extravasation and tissue injury (e.g., thrombosis).
Always Necessary? The Role of Arterial Catheters
-
•
Conventional teaching: Patients receiving vasopressors should have arterial catheters for blood pressure monitoring.
-
•
Current guidelines: Multiple societies suggest invasive blood pressure monitoring with arterial catheters for patients receiving vasopressors (6, 34).
-
•
Evolving practice: Use of noninvasive blood pressure (NIBP) monitoring with a blood pressure cuff, in absence of other indications for arterial catheters.
Despite recommendations for invasive blood pressure monitoring in patients with sepsis requiring vasopressors, arterial catheter use varies widely in practice. In a 2017 survey of physicians in Europe, 84% of respondents “always” used arterial catheters to measure blood pressure in septic shock (34). However, in a study of 168 U.S. ICUs, arterial catheter placement among patients receiving vasopressors was lower (51.7% in the median hospital) and varied widely across hospitals (interquartile range, 30.8%–76.2%) (78).
Arterial catheters are more accurate than blood pressure cuffs and provide continuous measurements, facilitating vasopressor titration (79). They also allow for arterial blood sampling. However, both blood pressure cuffs and arterial catheters are susceptible to artifacts that can limit their interpretation, and NIBP monitoring is accurate in detecting MAPs <65 mm Hg and clinically meaningful MAP changes (80). Therefore, arterial catheters may not improve detection or treatment of hypotension over NIBP monitoring, particularly in less severely ill patients with reliable blood pressure cuff readings.
Although arterial catheters are generally considered safer than CVCs, they may carry risk for catheter-associated infections and colonization of similar magnitude to CVCs (81). Arterial catheter placement also carries mechanical risks, including hematomas, thrombosis, and rare arterial complications, such as ischemia and pseudoaneurysms (82). Although complication rates are similar among all arterial catheter sites (82), complications occurring at central sites, such as femoral and axillary arteries, may have more serious consequences, which must be weighed against the potential increase in measurement accuracy of central versus radial arterial catheters (83).
The only study evaluating the clinical impact of arterial catheter use in vasopressor-treated patients was a propensity-matched cohort study, which did not find benefit (84). Interpretation of these findings is limited by the observational design but underscores the need for RCTs to assess the utility of arterial catheters and target invasive interventions to patients most likely to benefit (85).
The field of NIBP monitoring is growing, and there may be alternatives to blood pressure cuffs in the future. Ongoing trials are testing novel monitoring devices, although these devices are not yet widely available and will need to be tested in critically ill patients (86).
In the meantime, we suggest that arterial catheter placement is not necessary for all patients receiving vasopressors and should be prioritized in patients with labile vasopressor requirements, unreliable blood pressure cuff readings, or other indications for arterial catheter placement (e.g., blood draws).
Future Directions
There is a growing body of literature informing management of early sepsis-induced hypoperfusion. Although most trials discussed in this review yielded neutral results, they have informed practice by showing that less-invasive or less-intensive approaches to resuscitation often yield similar outcomes, at least in the overall study population (Figure 2). The next step in sepsis resuscitation research is to understand how we can individualize care. Advanced statistical approaches have been used post hoc to identify patients most likely to benefit from tested interventions, which can help overcome the heterogeneity of treatment effects inherent to existing ICU trials (87). Going forward, however, trials must prospectively consider the heterogenous nature of sepsis by identifying sepsis phenotypes and treatment-responsive subgroups to inform and test personalization of care within trials (88). In addition, in defining subgroups it may be beneficial to shift from studying septic shock separately to focusing on broader shock phenotypes (e.g., defined by cardiac function, fluid status, or the presence of vasodilation, as in the 65 Trial [58]). Future trials must be sufficiently large to detect small but clinically meaningful differences in patient-important outcomes. Trials should avoid stopping early based on unrealistically large estimated effect sizes, which limits the power of subgroup analyses and the ability to assess heterogeneity. Finally, to inform early resuscitation practices, trials of resuscitation must incorporate novel trial designs and consent structures that facilitate earlier enrollment (89), drawing on experiences with alterations to informed consent processes in cardiac arrest and brain injury trials (90).
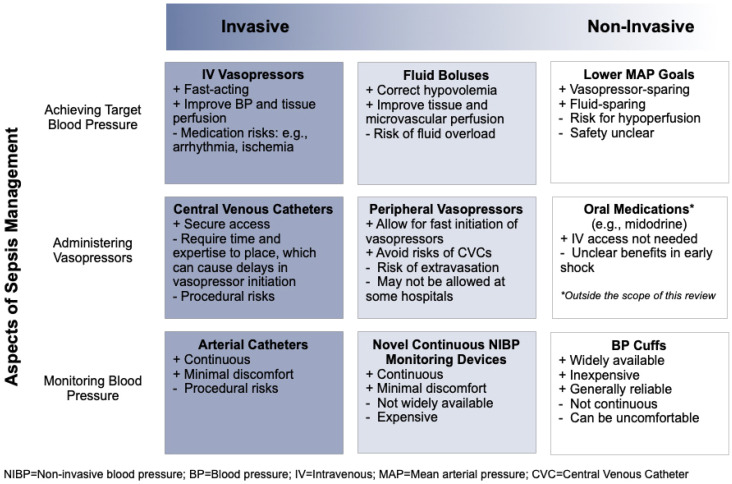
Figure 2.
Overview of invasive versus noninvasive approaches to the management of early sepsis-induced hypoperfusion.
Conclusions
Sepsis is a major driver of morbidity and mortality worldwide, and resuscitation is a critical component of management. In this review, we summarize the evidence behind current resuscitation practices, discuss practice evolution toward less intensive approaches, and highlight gaps and limitations of our current evidence base.
Footnotes
Supported by National Institutes of Health Multidisciplinary Training Program in Lung Disease grant number T32 HL 007749 (E.S.M.) and National Institute for Health Research Clinician Scientist Award NIHR-CS-2016-16-011 (M.S.-H.). This research was completed during the tenure of a Clinical Practitioner Research Fellowship from the Health Research Council of New Zealand (P.J.Y.). The Medical Research Institute of New Zealand is supported by Independent Research Organization funding from the Health Research Council of New Zealand. This material is the result of work supported with resources and use of facilities at the Ann Arbor VA Medical Center. This manuscript does not represent the views of the Department of Veterans Affairs or the U.S. government. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service (NHS), the U.K. National Institute for Health Research, or the Department of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: E.S.M. and H.C.P. contributed to the conception and drafting of this review. R.C.H., M.W.S., M.S.-H., P.J.Y., and F.G.Z. contributed to the writing and substantive revisions. All authors have read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
CME will be available for this article at https://shop.thoracic.org/collections/cme-moc/ethos-format-type-journal.
Originally Published in Press as DOI: 10.1164/rccm.202209-1831CI on February 22, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet . 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA . 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med . 2013;41:791–799. doi: 10.1097/CCM.0b013e3182742e8b. [DOI] [PubMed] [Google Scholar]
- 4. Hinshaw LB. Sepsis/septic shock: participation of the microcirculation: an abbreviated review. Crit Care Med . 1996;24:1072–1078. doi: 10.1097/00003246-199606000-00031. [DOI] [PubMed] [Google Scholar]
- 5. De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med . 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 6. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med . 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 7. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med . 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 8. Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, et al. ARISE Investigators, ANZICS Clinical Trials Group Goal-directed resuscitation for patients with early septic shock. N Engl J Med . 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 9. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. ProCESS Investigators A randomized trial of protocol-based care for early septic shock. N Engl J Med . 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. ProMISe Trial Investigators Trial of early, goal-directed resuscitation for septic shock. N Engl J Med . 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 11. Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, et al. PRISM Investigators Early, goal-directed therapy for septic shock: a patient-level meta-analysis. N Engl J Med . 2017;376:2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 12. Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med . 2015;41:1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 13. Liu VX, Morehouse JW, Marelich GP, Soule J, Russell T, Skeath M, et al. Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med . 2016;193:1264–1270. doi: 10.1164/rccm.201507-1489OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warren JL, Bacon WE, Harris T, McBean AM, Foley DJ, Phillips C. The burden and outcomes associated with dehydration among US elderly, 1991. Am J Public Health . 1994;84:1265–1269. doi: 10.2105/ajph.84.8.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carrara M, Antenucci P, Liu S, Kohler A, Langer R, Jakob SM, et al. Autonomic and circulatory alterations persist despite adequate resuscitation in a 5-day sepsis swine experiment. Sci Rep . 2022;12:19279. doi: 10.1038/s41598-022-23516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, et al. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med . 2010;36:2019–2029. doi: 10.1007/s00134-010-2045-8. [DOI] [PubMed] [Google Scholar]
- 17. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med . 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 18. Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med . 2017;43:625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- 19. Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med . 2014;29:213–217. doi: 10.1177/0885066613478899. [DOI] [PubMed] [Google Scholar]
- 20. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis Occurrence in Acutely Ill Patients Investigators Sepsis in European intensive care units: results of the SOAP study. Crit Care Med . 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 21. Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. FEAST Trial Group Mortality after fluid bolus in African children with severe infection. N Engl J Med . 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 22. Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med . 2014;42:2315–2324. doi: 10.1097/CCM.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA . 2017;318:1233–1240. doi: 10.1001/jama.2017.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyhoff TS, Møller MH, Hjortrup PB, Cronhjort M, Perner A, Wetterslev J. Lower vs higher fluid volumes during initial management of sepsis: a systematic review with meta-analysis and trial sequential analysis. Chest . 2020;157:1478–1496. doi: 10.1016/j.chest.2019.11.050. [DOI] [PubMed] [Google Scholar]
- 25. Semler MW, Janz DR, Casey JD, Self WH, Rice TW. Conservative fluid management after sepsis resuscitation: a pilot randomized trial. J Intensive Care Med . 2020;35:1374–1382. doi: 10.1177/0885066618823183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lanspa MJ, Burk RE, Wilson EL, Hirshberg EL, Grissom CK, Brown SM. Echocardiogram-guided resuscitation versus early goal-directed therapy in the treatment of septic shock: a randomized, controlled, feasibility trial. J Intensive Care . 2018;6:50. doi: 10.1186/s40560-018-0319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen C, Kollef MH. Targeted fluid minimization following initial resuscitation in septic shock: a pilot study. Chest . 2015;148:1462–1469. doi: 10.1378/chest.15-1525. [DOI] [PubMed] [Google Scholar]
- 28. Cronhjort M, Bergman M, Joelsson-Alm E, Divander MB, Jerkegren E, Balintescu A, et al. Fluid responsiveness assessment using passive leg raising test to reduce fluid administration and weight gain in patients with septic shock. J Anesth Perioper Med . 2017;4:169–178. [Google Scholar]
- 29. Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med . 2022;386:2459–2470. doi: 10.1056/NEJMoa2202707. [DOI] [PubMed] [Google Scholar]
- 30. Shapiro NI, Douglas IS, Brower RG, Brown SM, Exline MC, Ginde AA, et al. National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med . 2023;388:499–510. doi: 10.1056/NEJMoa2212663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ehrman RR, Gallien JZ, Smith RK, Akers KG, Malik AN, Harrison NE, et al. Resuscitation guided by volume responsiveness does not reduce mortality in sepsis: a meta-analysis. Crit Care Explor . 2019;1:e0015. doi: 10.1097/CCE.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bednarczyk JM, Fridfinnson JA, Kumar A, Blanchard L, Rabbani R, Bell D, et al. Incorporating dynamic assessment of fluid responsiveness into goal-directed therapy: a systematic review and meta-analysis. Crit Care Med . 2017;45:1538–1545. doi: 10.1097/CCM.0000000000002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Douglas IS, Alapat PM, Corl KA, Exline MC, Forni LG, Holder AL, et al. Fluid response evaluation in sepsis hypotension and shock: a randomized clinical trial. Chest . 2020;158:1431–1445. doi: 10.1016/j.chest.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scheeren TWL, Bakker J, De Backer D, Annane D, Asfar P, Boerma EC, et al. Current use of vasopressors in septic shock. Ann Intensive Care . 2019;9:20. doi: 10.1186/s13613-019-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign bundle: 2018 update. Intensive Care Med . 2018;44:925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 36. Hollenberg SM. Vasoactive drugs in circulatory shock. Am J Respir Crit Care Med . 2011;183:847–855. doi: 10.1164/rccm.201006-0972CI. [DOI] [PubMed] [Google Scholar]
- 37. Stolk RF, van der Poll T, Angus DC, van der Hoeven JG, Pickkers P, Kox M. Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med . 2016;194:550–558. doi: 10.1164/rccm.201604-0862CP. [DOI] [PubMed] [Google Scholar]
- 38. Nouira S, Elatrous S, Dimassi S, Besbes L, Boukef R, Mohamed B, et al. Effects of norepinephrine on static and dynamic preload indicators in experimental hemorrhagic shock. Crit Care Med . 2005;33:2339–2343. doi: 10.1097/01.ccm.0000182801.48137.13. [DOI] [PubMed] [Google Scholar]
- 39. Hamzaoui O, Georger JF, Monnet X, Ksouri H, Maizel J, Richard C, et al. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care . 2010;14:R142. doi: 10.1186/cc9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Loon LM, Stolk RF, van der Hoeven JG, Veltink PH, Pickkers P, Lemson J, et al. Effect of vasopressors on the macro- and microcirculation during systemic inflammation in humans in vivo. Shock . 2020;53:171–174. doi: 10.1097/SHK.0000000000001357. [DOI] [PubMed] [Google Scholar]
- 41. Sennoun N, Montemont C, Gibot S, Lacolley P, Levy B. Comparative effects of early versus delayed use of norepinephrine in resuscitated endotoxic shock. Crit Care Med . 2007;35:1736–1740. doi: 10.1097/01.CCM.0000269028.28521.08. [DOI] [PubMed] [Google Scholar]
- 42. Libert N, Laemmel E, Harrois A, Laitselart P, Bergis B, Isnard P, et al. Renal microcirculation and function in a pig model of hemorrhagic shock resuscitation with norepinephrine. Am J Respir Crit Care Med . 2022;206:34–43. doi: 10.1164/rccm.202109-2120OC. [DOI] [PubMed] [Google Scholar]
- 43. Vincent JL, Nielsen ND, Shapiro NI, Gerbasi ME, Grossman A, Doroff R, et al. Mean arterial pressure and mortality in patients with distributive shock: a retrospective analysis of the MIMIC-III database. Ann Intensive Care . 2018;8:107. doi: 10.1186/s13613-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Udy AA, Finnis M, Jones D, Delaney A, Macdonald S, Bellomo R, et al. ARISE Investigators Incidence, patient characteristics, mode of drug delivery, and outcomes of septic shock patients treated with vasopressors in the Arise trial. Shock . 2019;52:400–407. doi: 10.1097/SHK.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 45. Waechter J, Kumar A, Lapinsky SE, Marshall J, Dodek P, Arabi Y, et al. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group Interaction between fluids and vasoactive agents on mortality in septic shock: a multicenter, observational study. Crit Care Med . 2014;42:2158–2168. doi: 10.1097/CCM.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 46. Ospina-Tascón GA, Hernandez G, Alvarez I, Calderón-Tapia LE, Manzano-Nunez R, Sánchez-Ortiz AI, et al. Effects of very early start of norepinephrine in patients with septic shock: a propensity score-based analysis. Crit Care . 2020;24:52. doi: 10.1186/s13054-020-2756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeo HJ, Lee YS, Kim TH, Jang JH, Lee HB, Oh DK, et al. Vasopressor initiation within 1 hour of fluid loading is associated with increased mortality in septic shock patients: analysis of National Registry data. Crit Care Med . 2022;50:e351–e360. doi: 10.1097/CCM.0000000000005363. [DOI] [PubMed] [Google Scholar]
- 48. Macdonald SPJ, Keijzers G, Taylor DM, Kinnear F, Arendts G, Fatovich DM, et al. REFRESH trial investigators Restricted fluid resuscitation in suspected sepsis associated hypotension (REFRESH): a pilot randomised controlled trial. Intensive Care Med . 2018;44:2070–2078. doi: 10.1007/s00134-018-5433-0. [DOI] [PubMed] [Google Scholar]
- 49. Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early use of norepinephrine in septic shock resuscitation (CENSER): a randomized trial. Am J Respir Crit Care Med . 2019;199:1097–1105. doi: 10.1164/rccm.201806-1034OC. [DOI] [PubMed] [Google Scholar]
- 50. Elbouhy MA, Soliman M, Gaber A, Taema KM, Abdel-Aziz A. Early use of norepinephrine improves survival in septic shock: earlier than early. Arch Med Res . 2019;50:325–332. doi: 10.1016/j.arcmed.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 51. Thooft A, Favory R, Salgado DR, Taccone FS, Donadello K, De Backer D, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care . 2011;15:R222. doi: 10.1186/cc10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bourgoin A, Leone M, Delmas A, Garnier F, Albanèse J, Martin C. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med . 2005;33:780–786. doi: 10.1097/01.ccm.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- 53. Maiden MJ, Otto S, Brealey JK, Finnis ME, Chapman MJ, Kuchel TR, et al. Structure and function of the kidney in septic shock: a prospective controlled experimental study. Am J Respir Crit Care Med . 2016;194:692–700. doi: 10.1164/rccm.201511-2285OC. [DOI] [PubMed] [Google Scholar]
- 54. Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. SEPSISPAM Investigators High versus low blood-pressure target in patients with septic shock. N Engl J Med . 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 55. Carrick MM, Leonard J, Slone DS, Mains CW, Bar-Or D. Hypotensive resuscitation among trauma patients. BioMed Res Int . 2016;2016:8901938. doi: 10.1155/2016/8901938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lamontagne F, Meade MO, Hébert PC, Asfar P, Lauzier F, Seely AJE, et al. Canadian Critical Care Trials Group Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med . 2016;42:542–550. doi: 10.1007/s00134-016-4237-3. [DOI] [PubMed] [Google Scholar]
- 57. Lamontagne F, Day AG, Meade MO, Cook DJ, Guyatt GH, Hylands M, et al. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med . 2018;44:12–21. doi: 10.1007/s00134-017-5016-5. [DOI] [PubMed] [Google Scholar]
- 58. Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, et al. 65 trial investigators Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA . 2020;323:938–949. doi: 10.1001/jama.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richards-Belle A, Hylands M, Muttalib F, Taran S, Rochwerg B, Day A, et al. Lower versus higher exposure to vasopressor therapy in vasodilatory hypotension: a systematic review with meta-analysis. Crit Care Med . 2022;51:254–266. doi: 10.1097/CCM.0000000000005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med . 2015;41:1862–1863. doi: 10.1007/s00134-015-3955-2. [DOI] [PubMed] [Google Scholar]
- 61. Monteerarat Y, Limthongthang R, Laohaprasitiporn P, Vathana T. Reliability of capillary refill time for evaluation of tissue perfusion in simulated vascular occluded limbs. Eur J Trauma Emerg Surg . 2022;48:1231–1237. doi: 10.1007/s00068-020-01594-9. [DOI] [PubMed] [Google Scholar]
- 62. Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN) Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA . 2019;321:654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zampieri FG, Damiani LP, Bakker J, Ospina-Tascón GA, Castro R, Cavalcanti AB, et al. Effects of a resuscitation strategy targeting peripheral perfusion status versus serum lactate levels among patients with septic shock: a Bayesian reanalysis of the ANDROMEDA-SHOCK trial. Am J Respir Crit Care Med . 2020;201:423–429. doi: 10.1164/rccm.201905-0968OC. [DOI] [PubMed] [Google Scholar]
- 64. Cecconi M, Hernandez G, Dunser M, Antonelli M, Baker T, Bakker J, et al. Fluid administration for acute circulatory dysfunction using basic monitoring: narrative review and expert panel recommendations from an ESICM task force. Intensive Care Med . 2019;45:21–32. doi: 10.1007/s00134-018-5415-2. [DOI] [PubMed] [Google Scholar]
- 65. Ospina-Tascón GA, Teboul JL, Hernandez G, Alvarez I, Sánchez-Ortiz AI, Calderón-Tapia LE, et al. Diastolic shock index and clinical outcomes in patients with septic shock. Ann Intensive Care . 2020;10:41. doi: 10.1186/s13613-020-00658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou X, Pan W, Chen B, Xu Z, Pan J. Predictive performance of dynamic arterial elastance for arterial pressure response to fluid expansion in mechanically ventilated hypotensive adults: a systematic review and meta-analysis of observational studies. Ann Intensive Care . 2021;11:119. doi: 10.1186/s13613-021-00909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De Backer D, Cecconi M, Chew MS, Hajjar L, Monnet X, Ospina-Tascón GA, et al. A plea for personalization of the hemodynamic management of septic shock. Crit Care . 2022;26:372. doi: 10.1186/s13054-022-04255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Humphreys J, Johnston JH, Richardson JC. Skin necrosis following intravenous noradrenaline. BMJ . 1955;2:1250–1252. doi: 10.1136/bmj.2.4950.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Delaney A, Finnis M, Bellomo R, Udy A, Jones D, Keijzers G, et al. Initiation of vasopressor infusions via peripheral versus central access in patients with early septic shock: a retrospective cohort study. Emerg Med Australas . 2020;32:210–219. doi: 10.1111/1742-6723.13394. [DOI] [PubMed] [Google Scholar]
- 70. Parienti JJ, Mongardon N, Mégarbane B, Mira JP, Kalfon P, Gros A, et al. 3SITES Study Group Intravascular complications of central venous catheterization by insertion site. N Engl J Med . 2015;373:1220–1229. doi: 10.1056/NEJMoa1500964. [DOI] [PubMed] [Google Scholar]
- 71. Haimovich AD, Jiang R, Taylor RA, Belsky JB. Risk factor identification and predictive models for central line requirements for patients on vasopressors. Anaesth Intensive Care . 2021;49:275–283. doi: 10.1177/0310057X211024258. [DOI] [PubMed] [Google Scholar]
- 72. Ricard JD, Salomon L, Boyer A, Thiery G, Meybeck A, Roy C, et al. Central or peripheral catheters for initial venous access of ICU patients: a randomized controlled trial. Crit Care Med . 2013;41:2108–2115. doi: 10.1097/CCM.0b013e31828a42c5. [DOI] [PubMed] [Google Scholar]
- 73. Loubani OM, Green RS. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. J Crit Care . 2015;30:653.e9–653.e17. doi: 10.1016/j.jcrc.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 74. Owen VS, Rosgen BK, Cherak SJ, Ferland A, Stelfox HT, Fiest KM, et al. Adverse events associated with administration of vasopressor medications through a peripheral intravenous catheter: a systematic review and meta-analysis. Crit Care . 2021;25:146. doi: 10.1186/s13054-021-03553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tian DH, Smyth C, Keijzers G, Macdonald SP, Peake S, Udy A, et al. Safety of peripheral administration of vasopressor medications: a systematic review. Emerg Med Australas . 2020;32:220–227. doi: 10.1111/1742-6723.13406. [DOI] [PubMed] [Google Scholar]
- 76. Tran QK, Mester G, Bzhilyanskaya V, Afridi LZ, Andhavarapu S, Alam Z, et al. Complication of vasopressor infusion through peripheral venous catheter: a systematic review and meta-analysis. Am J Emerg Med . 2020;38:2434–2443. doi: 10.1016/j.ajem.2020.09.047. [DOI] [PubMed] [Google Scholar]
- 77. Munroe E, Claar D, Tamae-Kakazu M, Tatem G, Blamoun J, McSparron JI, et al. Hospital policies on intravenous vasopressor administration and monitoring: a survey of Michigan hospitals. Ann Am Thorac Soc . 2022;19:1769–1772. doi: 10.1513/AnnalsATS.202203-197RL. [DOI] [PubMed] [Google Scholar]
- 78. Gershengorn HB, Garland A, Kramer A, Scales DC, Rubenfeld G, Wunsch H. Variation of arterial and central venous catheter use in United States intensive care units. Anesthesiology . 2014;120:650–664. doi: 10.1097/ALN.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Riley LE, Chen GJ, Latham HE. Comparison of noninvasive blood pressure monitoring with invasive arterial pressure monitoring in medical ICU patients with septic shock. Blood Press Monit . 2017;22:202–207. doi: 10.1097/MBP.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 80. Lakhal K, Macq C, Ehrmann S, Boulain T, Capdevila X. Noninvasive monitoring of blood pressure in the critically ill: reliability according to the cuff site (arm, thigh, or ankle) Crit Care Med . 2012;40:1207–1213. doi: 10.1097/CCM.0b013e31823dae42. [DOI] [PubMed] [Google Scholar]
- 81. Koh DBC, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med . 2008;36:397–402. doi: 10.1097/CCM.0b013e318161f74b. [DOI] [PubMed] [Google Scholar]
- 82. Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care . 2002;6:199–204. doi: 10.1186/cc1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dorman T, Breslow MJ, Lipsett PA, Rosenberg JM, Balser JR, Almog Y, et al. Radial artery pressure monitoring underestimates central arterial pressure during vasopressor therapy in critically ill surgical patients. Crit Care Med . 1998;26:1646–1649. doi: 10.1097/00003246-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 84. Gershengorn HB, Wunsch H, Scales DC, Zarychanski R, Rubenfeld G, Garland A. Association between arterial catheter use and hospital mortality in intensive care units. JAMA Intern Med . 2014;174:1746–1754. doi: 10.1001/jamainternmed.2014.3297. [DOI] [PubMed] [Google Scholar]
- 85. Garland A. Arterial lines in the ICU: a call for rigorous controlled trials. Chest . 2014;146:1155–1158. doi: 10.1378/chest.14-1212. [DOI] [PubMed] [Google Scholar]
- 86. Quan X, Liu J, Roxlo T, Siddharth S, Leong W, Muir A, et al. Advances in non-invasive blood pressure monitoring. Sensors (Basel) . 2021;21:4273. doi: 10.3390/s21134273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pirracchio R, Hubbard A, Sprung CL, Chevret S, Annane D, Rapid Recognition of Corticosteroid Resistant or Sensitive Sepsis (RECORDS) Collaborators Assessment of machine learning to estimate the individual treatment effect of corticosteroids in septic shock. JAMA Netw Open . 2020;3:e2029050. doi: 10.1001/jamanetworkopen.2020.29050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, et al. Redefining critical illness. Nat Med . 2022;28:1141–1148. doi: 10.1038/s41591-022-01843-x. [DOI] [PubMed] [Google Scholar]
- 89. Semler MW, Bernard GR, Aaron SD, Angus DC, Biros MH, Brower RG, et al. Identifying clinical research priorities in adult pulmonary and critical care: NHLBI working group report. Am J Respir Crit Care Med . 2020;202:511–523. doi: 10.1164/rccm.201908-1595WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kjaergaard J, Møller JE, Schmidt H, Grand J, Mølstrøm S, Borregaard B, et al. Blood-pressure targets in comatose survivors of cardiac arrest. N Engl J Med . 2022;387:1456–1466. doi: 10.1056/NEJMoa2208687. [DOI] [PubMed] [Google Scholar]
- 91. Jessen MK, Andersen LW, Thomsen MH, Kristensen P, Hayeri W, Hassel RE, et al. Restrictive fluids versus standard care in adults with sepsis in the emergency department (REFACED): a multicenter, randomized feasibility trial. Acad Emerg Med . 2022;29:1172–1184. doi: 10.1111/acem.14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettilä V, et al. CLASSIC Trial Group, Scandinavian Critical Care Trials Group Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med . 2016;42:1695–1705. doi: 10.1007/s00134-016-4500-7. [DOI] [PubMed] [Google Scholar]
- 93. Richard JC, Bayle F, Bourdin G, Leray V, Debord S, Delannoy B, et al. Preload dependence indices to titrate volume expansion during septic shock: a randomized controlled trial. Crit Care . 2015;19:5. doi: 10.1186/s13054-014-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Corl KA, Prodromou M, Merchant RC, Gareen I, Marks S, Banerjee D, et al. The restrictive IV fluid trial in severe sepsis and septic shock (RIFTS): a randomized pilot study. Crit Care Med . 2019;47:951–959. doi: 10.1097/CCM.0000000000003779. [DOI] [PMC free article] [PubMed] [Google Scholar]