Abstract
GM 193663, GM 211676, GM 222712, and GM 237354 are new semisynthetic derivatives of the sordarin class. The in vitro antifungal activities of GM 193663, GM 211676, GM 222712, and GM 237354 against 111 clinical yeast isolates of Candida albicans, Candida kefyr, Candida glabrata, Candida parapsilosis, Candida krusei, and Cryptococcus neoformans were compared. The in vitro activities of some of these compounds against Pneumocystis carinii, 20 isolates each of Aspergillus fumigatus and Aspergillus flavus, and 30 isolates of emerging less-common mold pathogens and dermatophytes were also compared. The MICs of GM 193663, GM 211676, GM 222712, and GM 237354 at which 90% of the isolates were inhibited (MIC90s) were 0.03, 0.03, 0.004, and 0.015 μg/ml, respectively, for C. albicans, including strains with decreased susceptibility to fluconazole; 0.5, 0.5, 0.06, and 0.12 μg/ml, respectively, for C. tropicalis; and 0.004, 0.015, 0.008, and 0.03 μg/ml, respectively, for C. kefyr. GM 222712 and GM 237354 were the most active compounds against C. glabrata, C. parapsilosis, and Cryptococcus neoformans. Against C. glabrata and C. parapsilosis, the MIC90s of GM 222712 and GM 237354 were 0.5 and 4 μg/ml and 1 and 16 μg/ml, respectively. The MIC90s of GM 222712 and GM 237354 against Cryptococcus neoformans were 0.5 and 0.25 μg/ml, respectively. GM 193663, GM 211676, GM 222712, and GM 237354 were extremely active against P. carinii. The efficacies of sordarin derivatives against this organism were determined by measuring the inhibition of the uptake and incorporation of radiolabelled methionine into newly synthesized proteins. All compounds tested showed 50% inhibitory concentrations of <0.008 μg/ml. Against A. flavus and A. fumigatus, the MIC90s of GM 222712 and GM 237354 were 1 and 32 μg/ml and 32 and >64 μg/ml, respectively. In addition, GM 237354 was tested against the most important emerging fungal pathogens which affect immunocompromised patients. Cladosporium carrioni, Pseudallescheria boydii, and the yeast-like fungi Blastoschizomyces capitatus and Geotrichum clavatum were the most susceptible of the fungi to GM 237354, with MICs ranging from ≤0.25 to 2 μg/ml. The MICs of GM 237354 against Trichosporon beigelii and the zygomycetes Absidia corymbifera, Cunninghamella bertholletiae, and Rhizopus arrhizus ranged from ≤0.25 to 8 μg/ml. Against dermatophytes, GM 237354 MICs were ≥2 μg/ml. In summary, we concluded that some sordarin derivatives, such as GM 222712 and GM 237354, showed excellent in vitro activities against a wide range of pathogenic fungi, including Candida spp., Cryptococcus neoformans, P. carinii, and some filamentous fungi and emerging invasive fungal pathogens.
During the past two decades, the incidence of infections caused by opportunistic fungal pathogens in immunocompromised patients has increased substantially (1, 11, 30, 31, 36). Candida albicans is the major opportunistic pathogen, although the incidence of fungal infections caused by non-C. albicans species is increasing (37). Pneumocystis carinii remains an important pathogen in AIDS patients and other immunocompromised individuals (17), and invasive pulmonary aspergillosis remains a frequently fatal complication of bone marrow transplantation and of cancer chemotherapy in patients with hematologic neoplasms (23, 26, 29). Although there has been an expansion in the number of antifungal drugs available (8–10), in many cases, treatment of fungal diseases remains unsatisfactory. This situation has led to an ongoing search for fungicidal agents with different modes of action and fewer side effects and which can be administered both orally and parenterally.
One of the major challenges to finding a potent yet safe antifungal agent is the similarity between fungal and mammalian cells. Like mammalian cells, fungi are eukaryotic, so they have many of the same structures and metabolic pathways as mammalian cells, making it more difficult to find targets of differential toxicity. Although protein synthesis is a universal process in living cells, it has always been considered as one of the more attractive targets for the development of antimicrobial agents (8, 12, 36). It is known that fungal protein has exploitable differences relative to its mammalian counterpart, e.g., the two soluble protein factors elongation factor 3 (EF-3) (19, 34) which is absent from mammalian cells, EF-2, which is functionally distinct from its mammalian counterpart (5, 6). On the basis of these differences, a target-based screening program was established, with the objective of isolating selective protein synthesis inhibitors of the fungal machinery (2). As part of this screening program, a novel antifungal compound, GR 135402, was isolated from fermentation broth of Graphium putredinis and characterized (20). This new compound is the first natural product described to date which possesses antifungal activity through inhibition of fungal but not mammalian protein synthesis (20). GR 135402 belongs to the sordarin class (13), and although it has some structural similarity to zofimarin (27), sordarin (33), and sordarin derivatives (3, 28), no mode of action was described for the antifungal activity of these compounds. A synthetic chemical program was initiated to improve the biological properties of GR 135402, and four compounds, designated GM 193663, GM 211676, GM 222712, and GM 237354, were selected for evaluation.
In this study, we analyze the in vitro antifungal activities of these four new sordarin derivatives against several groups of clinical isolates.
(This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997 [15, 16].)
MATERIALS AND METHODS
Antifungal agents.
GM 193663, GM 211676, GM 222712, and GM 237354 were synthesized at Glaxo Wellcome S.A. (Tres Cantos, Madrid, Spain). Compounds, as sodium salts, were initially solubilized in sterile distilled water at a starting concentration of 5 mg/ml and then diluted in medium to the appropriate concentration. Solutions were prepared just before use.
Organisms.
The organisms used for susceptibility testing were unique, unselected clinical isolates collected from various separate medical centers in Spain. A total of 111 clinical yeasts isolates were tested under a single set of standardized conditions. This group consisted of 40 isolates of C. albicans, including 10 strains with decreased susceptibility to fluconazole (a generous gift of J. V. Martinez-Suarez and J. L. Rodriguez Tudela [21]), 10 isolates of Candida tropicalis, 10 Candida kefyr isolates, 11 strains of Candida glabrata, 10 Candida parapsilosis isolates, 10 Candida krusei strains, and 20 isolates of Cryptococcus neoformans. A total of 40 Aspergillus spp. clinical isolates were used for susceptibility studies, 20 isolates each of Aspergillus fumigatus and Aspergillus flavus. In addition, 24 emerging and less-common mold pathogens and 6 dermatophyte strains were tested. C. albicans ATCC 90028, C. tropicalis ATCC 750, C. parapsilosis ATCC 90018, and C. krusei ATCC 6258, obtained from the American Type Culture Collection (Rockville, Md.), were used as reference strains. Organisms were identified by standard microbiological methods and stored in Sabouraud dextrose (SAB) broth (Difco, Detroit, Mich.) with 15% glycerol at −70°C until required. Prior to antifungal susceptibility testing, each isolate was passaged on SAB agar (Difco) to ensure optimal growth characteristics. P. carinii organisms were obtained from the lungs of spontaneously infected immunosuppressed Wistar rats just before each experiment, as is described below.
Media and buffers.
RPMI–2% glucose was used in most studies (32). The basal medium RPMI 1640 (GIBCO BRL, Life Technologies, Renfrewshire, United Kingdom) with l-glutamine (Merck, Darmstadt, Germany), buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Sigma Chemical Co., St. Louis, Mo.), was supplemented with 18 g of glucose (Sigma-Aldrich S.A., Madrid, Spain) per liter. For Cryptococcus neoformans, RPMI–2% glucose was replaced by yeast nitrogen base medium (Difco) with 2% glucose. P. carinii was extracted and purified in Dulbecco modified Eagle’s medium (DMEM; BioWhittaker, Boehringer Ingelheim, Brussels, Belgium) with l-glutamine, supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml). Determinations of in vitro activity against P. carinii were performed in modified Eagle’s medium (MEM) without l-methionine (GIBCO BRL, Life Technologies) supplemented with 10% of fetal calf serum (GIBCO BRL, Life Technologies) and the same antibiotics as used in DMEM.
Antifungal susceptibility studies.
Susceptibility testing was performed by broth microdilution and/or by the agar dilution method.
(i) Broth microdilution method.
For yeasts, MICs were determined by the broth microdilution technique according to National Committee for Clinical Laboratory Standards (NCCLS) reference document M27-A (25) with minor modifications. A Microlab AT Plus robot (Hamilton Bonaduz, Bonaduz, Switzerland) was used to prepare microdilution panels containing twofold dilutions of the drugs in 0.1 ml of medium, with concentrations ranging from 0.001 to 32 μg/ml. Starting inocula were adjusted by the spectrophotometric method to 106 CFU/ml. Then, the adjusted yeast suspensions were diluted 1:10 with medium, and microtiter plates were inoculated with this dilution (by using the Hamilton system to dispense 10 μl into each well) to obtain a final inoculum of approximately 104 yeast cells per ml. The inoculated plates were incubated at 35°C without agitation for 24 h (Candida spp.) or for 48 h (Cryptococcus neoformans) in a humid atmosphere. Following incubation and after agitation with a microtiter plate shaker for 5 min, the plates were read visually with the aid of a reading mirror and spectrophotometrically with an automatic plate reader (IEMS; Labsystems, Helsinki, Finland) set at 620 nm. MICs were defined as the lowest concentrations of antifungal agents which prevented any visible growth or which inhibited growth by 95% compared with drug-free control wells. The MICs determined by visual and spectrophotometric evaluations demonstrated excellent agreement in all the cases.
For filamentous fungi, susceptibility testing was performed in RPMI–2% glucose medium as described by Espinel-Ingroff et al. (7). To induce conidium formation, filamentous fungi and dermatophytes were grown on SAB agar slants at 27°C until they were judged to have formed maximal numbers of conidia. Then, each fungal culture was covered with 1 ml of sterile saline containing 0.1% Tween 80, and spores were washed off by gently probing the colonies with the tip of a pipette. Finally, the suspension was vortexed 10 s to break up clumps of cells and then filtered through four layers of sterile gauze. The conidia were counted by using a hemocytometer, adjusted to a density of 106/ml, and stored at −70°C in small lots until required.
MICs were determined by performing microdilution tests as described above for yeasts but using double dilutions of drugs, with concentrations ranging from 0.25 to 64 μg/ml. Stock conidial suspensions were diluted with medium to obtain the final desired inoculum size of approximately 104 conidia/ml. Inoculum quantitation was performed by plating dilutions of the conidia on SAB agar to determine the viable number of CFU per milliliter. Plates were incubated at 35°C and read as soon as growth became visible in control wells, using a microplate mirror. Except in the case of Aspergillus spp., MICs were defined as the lowest concentrations of antifungal agents that inhibited the development of visible growth. For Aspergillus spp., MICs were defined as the lowest compound concentrations which produced a reduction of growth of approximately 75% compared to that of the growth control. That value is equivalent to the number 1 of the numerical score proposed by Espinel-Ingroff et al. (0, optically clear; 1, slight growth or ∼75% reduction in growth; 2, prominent reduction in growth or ∼50% reduction in growth; 3, slight reduction in growth or ∼25% reduction in growth; and 4, no reduction in growth [7]).
Aspergillus spp. MICs were determined for two additional end points by using the colorimetric indicators Alamar blue and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyldiphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS), a water-soluble salt. In both cases, microtiter plates were prepared as in the conventional method. Alamar blue solution (Accumed International Ltd.) was added to each well (10 μl/well) at the time of inoculation. The plates were then incubated at 35°C and read after 24 h with a microplate reader set at 570 nm. MTS assays were performed as described by the supplier (Promega Corporation). After 24 h of incubation with antifungal agents, 20 μl of a solution containing MTS and phenazine methosulfate (PMS) was added to each well (333 μg of MTS per ml and 25 μM PMS [final concentrations]). Incubations were continued for another 3 h at 35°C, and the plates were read at 490 nm.
(ii) Agar dilution procedure.
Susceptibility testing of yeast-like fungi, filamentous fungi, and dermatophytes was also performed by the agar dilution procedure (24). Six-well plates (Nunclon Multidishes; Nalge Nunc International) were prepared with RPMI–2% glucose agar medium containing the corresponding dilution of drug (0.12 to 64 μg/ml). Final inocula consisted of 103 CFU per spot. The MICs on solid medium were defined as the lowest concentrations that inhibited the development of visible growth, except for Aspergillus spp., in which case it was the lowest concentration of drug which produced a reduction of growth of approximately 75% compared to that of the growth control.
Antifungal activity against P. carinii.
Corticosteroid-treated Wistar rats were used as the source of P. carinii organisms. Extraction was performed before each experiment as previously described (22). Briefly, lungs were removed aseptically and minced in sterile DMEM. Organisms were released by agitation of the lung pieces with a magnetic stirrer. The resultant homogenate was sieved successively through sterile gauze and two stainless steel meshes of 250 and 63 μm, respectively. Finally, a Ficoll-Hypaque gradient (Histopaque-1077; Sigma-Aldrich S.A.) was used to purify P. carinii from host cell debris. Gradients were centrifuged at 1,000 × g for 15 min at 4°C. Organism numbers were assessed by Giemsa staining, and the final suspension was plated on blood agar and SAB agar for detection of bacterial or fungal contamination.
Activities of sordarins against P. carinii were assayed by determining the inhibition of uptake and incorporation of [35S]methionine, using a procedure previously developed in our laboratory (14). Microtiter wells with 200 μl of methionine-free MEM supplemented with 10% fetal calf serum and antibiotics plus the corresponding dilution of drug were inoculated with P. carinii to give a final concentration of 5 × 106 organisms per ml. Compounds were evaluated within the range from 0.008 to 1 μg/ml. P. carinii organisms were pulsed with 5 μCi of [35S]methionine per ml and then incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 h. Following incubation, the organisms were harvested on glass fiber filters by using a cell harvester (Tomtec; Wallac, Turku, Finland). Filter radioactivity was counted in a microplate scintillation counter (1450 Microbeta liquid scintillation counter; Wallac) after addition of Optiphase Hisafe 2 liquid (Wallac). Studies were performed in triplicate, and positive (parasites in free-drug medium) and negative (boiled P. carinii inoculum) control wells were included. Results were expressed as 50% inhibitory concentrations (IC50s), defined as the compound concentration at which incorporation of [35S]methionine was decreased by 50% in comparison with positive-control wells.
Aspergillus ATP assay.
ATP determination has been previously reported as a method to evaluate antifungal activity for microorganisms of slow growth, such as dermatophytes (38). For A. flavus CM 74 and A. fumigatus 48238, intracellular ATP was determined by a method adapted for Aspergillus spp. in our laboratory. Microtiter plates containing spores and doubling dilutions of drugs were prepared as described for susceptibility tests. After an overnight incubation (16 h), ATP pools were released by adding 50 μl of extractant (1.53 M trichloroacetic acid and 51 mM EDTA in water) to each well. The plates were incubated 15 min at room temperature with agitation. Immediately afterward, samples were diluted 1/20 in water, and 40-μl aliquots of the diluted extracts were transferred to another previously prepared plate containing 100 μl of Tris-acetate buffer (70 mM Tris-acetate–2 mM EDTA [pH 7.75], 60 μM dithiothreitol, 0.125% bovine serum albumin) and 20 μl of 100 mM magnesium acetate per well. Following addition of 40 μl of luciferin-luciferase (Boehringer Mannheim S.A.) to each well, the light generated was measured with a microplate luminometer (Victor 1420 Multilabel counter; Wallac) for 6 s. Experiments were carried out in duplicate, and results were expressed as percentages of relative light units in comparison with drug-free control wells. The ATP IC75 of an antifungal drug was defined as the lowest concentration at which the ATP content was decreased by 75%.
RESULTS
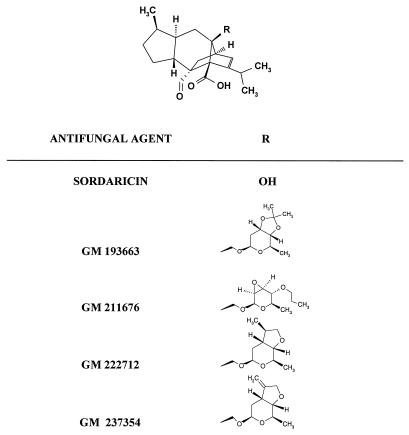
The molecular structure of sordaricin and the differences in the structures of the four new derivative antifungal agents are shown in Fig. 1. GM 193663, GM 222712, and GM 237354 are structurally related compounds which have different types of fused rings at positions C-3′ and C-4′ of the sugar moiety of the sordarin molecule. GM 193663 contains a 3′,4′-fused dioxolane ring. GM 222712 and GM 237354 contain a 3′,4′-fused tetrahydrofurane ring with a methyl and an exomethylene group, respectively. GM 211676 is chemically characterized by the presence of an oxirane ring at positions 2′ and 3′ of the sugar moiety.
FIG. 1.
Chemical structures of sordaricin and four new sordarin derivatives.
Antifungal agent activity against yeasts.
The MICs of GM 193663, GM 211676, GM 222712, and GM 237354 against groups of yeast pathogens of humans are summarized in Table 1. For sordarins, end points corresponding to a total absence of growth were clearly defined; for that reason, MICs read visually and spectrophotometrically showed an excellent agreement in all cases. Against various species of the genus Candida, such as C. albicans (including strains with decreased susceptibility to the azole derivatives), C. tropicalis, and C. kefyr, the MIC90s of the compounds were as follows: 0.06 μg/ml for GM 222712, 0.12 μg/ml for GM 237354, and 0.5 μg/ml for GM 193663 and GM 211676. For C. albicans, the MIC90s were 0.004 for GM 222712, 0.015 for GM 237354, and 0.03 for GM 193663 and GM 211676. Against C. albicans, GM 222712 was fourfold more active than GM 237354 and GM 237354 was twofold more active than either GM 193663 or GM 211676. Against C. tropicalis, GM 222712 was twofold more active than GM 237354 and GM 237354 was fourfold more active than either GM 193663 or GM 211676. GM 193663 was the most active compound against C. kefyr. The MIC90 of GM 193663 against C. kefyr isolates was 0.004 μg/ml, which was twofold lower than that of GM 222712, fourfold lower than that of GM 211676, and eightfold lower than that of GM 237354. The antifungal activity of GM 222712 against isolates of C. glabrata (MIC90, 0.5 μg/ml) was twofold higher than that of GM 237354. However, against this organism, the activity of GM 237354 (MIC90, 1 μg/ml) was 16-fold and up to 32-fold higher than those of GM 211676 and GM 193663, respectively. GM 193663 and GM 211676 were not active against C. parapsilosis.
TABLE 1.
Antifungal activities of four sordarin derivatives against groups of clinical isolates
| Organism (no. of isolates) | Antifungal agent | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| C. albicans (30) | GM 193663 | ≤0.001–0.03 | 0.001 | 0.03 |
| GM 211676 | ≤0.001–0.03 | 0.004 | 0.03 | |
| GM 222712 | ≤0.001–0.03 | 0.002 | 0.004 | |
| GM 237354 | ≤0.001–0.03 | 0.008 | 0.015 | |
| C. albicans, Flu (10) | GM 193663 | ≤0.001–0.03 | 0.001 | 0.03 |
| GM 211676 | ≤0.001–0.03 | 0.015 | 0.03 | |
| GM 222712 | ≤0.001–0.004 | 0.001 | 0.004 | |
| GM 237354 | ≤0.001–0.002 | 0.001 | 0.002 | |
| C. tropicalis (10) | GM 193663 | 0.03–1 | 0.25 | 0.5 |
| GM 211676 | 0.015–0.5 | 0.06 | 0.5 | |
| GM 222712 | 0.008–0.12 | 0.06 | 0.06 | |
| GM 237354 | 0.002–0.12 | 0.06 | 0.12 | |
| C. kefir (10) | GM 193663 | 0.002–0.015 | 0.004 | 0.004 |
| GM 211676 | 0.004–0.015 | 0.008 | 0.015 | |
| GM 222712 | ≤0.001–0.008 | 0.004 | 0.008 | |
| GM 237354 | ≤0.001–0.03 | 0.015 | 0.03 | |
| C. glabrata (11) | GM 193663 | 32–>32 | >32 | >32 |
| GM 211676 | 4–16 | 8 | 16 | |
| GM 222712 | 0.03–0.5 | 0.25 | 0.5 | |
| GM 237354 | 0.25–1 | 1 | 1 | |
| C. parapsilosis (10) | GM 193663 | >32 | >32 | >32 |
| GM 211676 | >32 | >32 | >32 | |
| GM 222712 | 1–4 | 2 | 4 | |
| GM 237354 | 0.25–16 | 8 | 16 | |
| Cryptococcus neo-formans (20) | GM 193663 | 2–8 | 4 | 8 |
| GM 211676 | 2–8 | 4 | 8 | |
| GM 222712 | 0.25–1 | 0.12 | 0.5 | |
| GM 237354 | 0.015–0.25 | 0.12 | 0.25 | |
The MICs of GM 193663, GM 211676, GM 222712, and GM 237354 for NCCLS reference Candida spp. strains were as follows: 0.004, 0.015, 0.004, and 0.004 μg/ml, respectively, against C. albicans ATCC 90028; 0.12, 0.12, 0.03, and 0.015 μg/ml, respectively, against C. tropicalis ATCC 750; and >32, >32, 1, and 8 μg/ml, respectively, against C. parapsilosis ATCC 90018. Against C. krusei ATCC 6258, the activities of the four compounds tested were all >32 μg/ml.
GM 237354 inhibited growth of 90% of the Cryptococcus neoformans isolates at concentrations of 0.25 μg/ml. The activity of GM 237354 against C. neoformans was twofold higher than that of GM 222712 and 32-fold higher than that of either GM 193663 or GM 211676.
Activity against P. carinii.
The activity of sordarin derivatives against P. carinii was determined by measuring the inhibition of the uptake and incorporation of radiolabelled methionine into newly synthesized proteins. As is summarized in Table 2, sordarins proved to be highly potent inhibitors of P. carinii protein synthesis. After 24 h of incubation, all of the compounds tested showed IC50s of <0.008 μg/ml, whereas the pentamidine IC50 was 0.1 μg/ml. A concentration of 0.008 μg of GM 222712 or GM 237354 per ml resulted in practically a total inhibition of protein synthesis (92 and 95%, respectively), while GM 193663 and GM 211676 produced inhibitions of 70 and 80%, respectively, at the same concentration.
TABLE 2.
In vitro activities of sordarins against P. cariniia
| Antifungal agent | IC50 (μg/ml) | % Inhibition at 0.008 μg/ml |
|---|---|---|
| Sordarins | ||
| GM 193663 | <0.008 | 70 |
| GM 211676 | <0.008 | 80 |
| GM 222712 | <0.008 | 92 |
| GM 237354 | <0.008 | 95 |
| Pentamidine (control) | 0.1 | NTb |
Results are the means of triplicate values.
NT, not tested.
Activity against Aspergillus spp.
Susceptibility of Aspergillus spp. to the sordarin derivatives was tested by the broth microdilution and the agar dilution methods (Table 3). Results were read after 48 h of incubation and were equivalent in broth and in agar. Some sordarins produced a marked reduction of Aspergillus growth; however, end points were less sharp than those obtained with yeasts, and slight growth was observed at concentrations above the MIC, which was defined as the lowest concentration of compound that inhibited mycelium growth by 75%. According to this criterion, GM 222712 and GM 237354 were the most potent compounds against Aspergillus spp. As is summarized in Table 4, the MIC50 and MIC90 of GM 222712 against A. flavus isolates were 0.25 and 1 μg/ml, respectively. GM 237354’s MIC50 and MIC90 were 8 and 32 μg/ml, respectively. Against A. fumigatus, the MIC50 and MIC90 of GM 222712 were both 32 μg/ml, while those of GM 237354 were 64 and >64 μg/ml, respectively.
TABLE 3.
Susceptibility of A. flavus and A. fumigatus to sordarin derivatives: MICs and intracellular ATP levels
| Aspergillus sp. | Antifungal agent | MICa (μg/ml)
|
ATP IC75b (μg/ml) | |
|---|---|---|---|---|
| Broth | Agar | |||
| A. flavus CM 74 | GM 193663 | >64 | >64 | >64 |
| GM 211676 | 16 | 32 | 8 | |
| GM 222712 | 0.25 | 0.5 | 0.25 | |
| GM 237354 | 4 | 4 | 2 | |
| A. fumigatus 48238 | GM 193663 | >64 | >64 | >64 |
| GM 211676 | 64 | 64 | 64 | |
| GM 222712 | 32 | 32 | 32 | |
| GM 237354 | 64 | 64 | 64 | |
Minimum concentration which inhibits growth 75% compared to control.
Concentration which reduces measured ATP levels by 75% (average of five assays).
TABLE 4.
MICs of GM 222712 and GM 237354 against A. flavus and A. fumigatus
| Organism (no. of isolates) | Antifungal agent | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| A. flavus (20) | GM 222712 | 0.25–2 | 0.25 | 1 |
| GM 237354 | 4–62 | 8 | 32 | |
| A. fumigatus (20) | GM 222712 | 16–64 | 32 | 32 |
| GM 237354 | 64–>64 | 64 | >64 | |
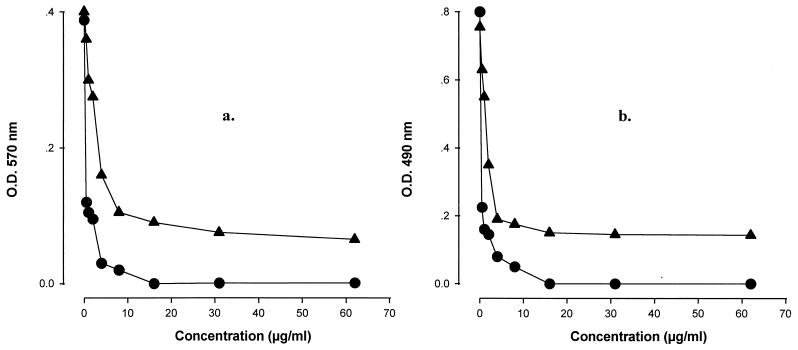
MICs were also determined by using oxidation-reduction indicators. Plates with RPMI–2% glucose medium supplemented with Alamar blue or MTS were easily read after 24 h of incubation, while by the conventional method we were unable to clearly detect growth until 48 h of incubation. As is shown in Fig. 2, after 24 h, the optical densitities of control wells with Alamar blue and MTS were approximately 0.4 and 0.8, respectively. In comparison with the optical densities obtained by the standard method (about 0.1), the values with Alamar blue and MTS were four- and eightfold higher, respectively. The in vitro sordarin activities determined by the colorimetric methods clearly agreed with the MICs obtained by conventional methods (± two doubling dilutions).
FIG. 2.
In vitro activities of GM 222712 (•) and GM 237354 (▴) against A. flavus CM 48, determined by using colorimetric reagents. (a) Alamar Blue assay; (b) MTS assay. O.D., optical density.
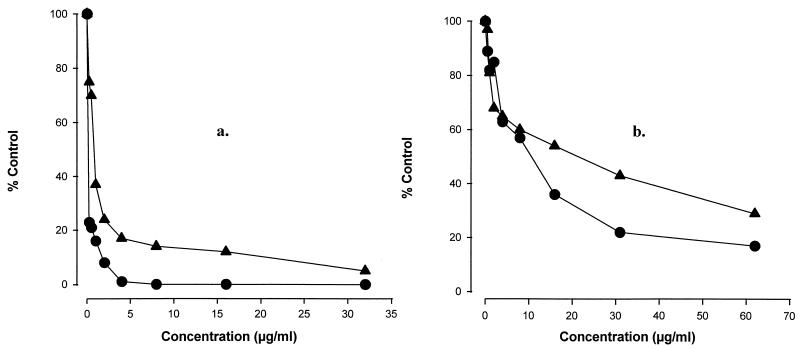
MICs were also consistant with reductions in intracellular ATP levels. After treatment with sordarins (16 h), A. flavus CM 74 and A. fumigatus 48238 mycelia were destroyed and ATP pools were determined as an indicator of biomass. As is presented in Fig. 3, sordarins significantly diminished Aspergillus ATP levels, and the results corresponded with the MICs obtained by visual readings (Table 3).
FIG. 3.
Effect of GM 222712 (•) and GM 237354 (▴) on A. flavus CM 48 (a) and A. fumigatus 48238 (b) ATP levels.
Activities against other, emerging fungal pathogens and dermatophytes.
GM 237354, one of the two most-active compounds, was also evaluated against a broad range of emerging, less-common mold pathogens and dermatophytes in broth and in agar medium. Table 5 shows the GM 237354 susceptibilities of a variety of organisms, which were selected at random from pathogenic isolates of the respective species. In general, GM 237354 was more active in solid medium than in broth.
TABLE 5.
Activity of GM 237354 against emerging, less-common mold pathogens and dermatophytes
| Organism (no. of isolates) | MIC (μg/ml)a
|
|
|---|---|---|
| Broth | Agar | |
| Yeast-like fungi | ||
| Blastoschizomyces capitatus (2) | 1 (2) | 2 (2) |
| Geotrichum clavatum (2) | 1 (2) | ≤0.25 (2) |
| Trichosporon beigelii (5) | 0.5 (2), 2 (1), 4 (1), 8 (1) | ≤0.25 (2), 2 (2), 4 (1) |
| Dematiaceous fungi | ||
| Alternaria alternata (1) | >64 | >64 |
| Curvularia lunata (1) | >64 | >64 |
| Cladosporium carrioni (2) | 1 (2) | ≤0.25 (2) |
| Hyaline fungi | ||
| Fusarium spp. (3)b | >64 | >64 |
| Pseudallescheria boydii (5) | 0.5 (1), 2 (4) | ≤0.25 (3), 1 (1), 2 (1), |
| Zygomycetes | ||
| Absidia corymbifera (1) | 4 | 4 |
| Cunninghamella bertholletiae (1) | 2 | 4 |
| Rhizopus arrhizus (1) | 2 | 4 |
| Dermatophytes | ||
| Epidermophyton floccosum (1) | 32 | 2 |
| Microsporum canis (1) | 8 | 8 |
| Microsporum gypseum (1) | 32 | >64 |
| Trichophyton mentagrophytes (1) | 64 | 16 |
| Trichophyton rubrum (1) | 64 | >64 |
| Trichophyton verrucosum (1) | >64 | >64 |
Values in parentheses indicate the number of isolates for which the MIC was as indicated.
Fusarium spp. were represented by three strains each of F. moniliforme, F. oxyporum, and F. solani.
All T. beigelii strains tested were susceptible to GM 237354 (MICs ranged from 0.5 to 8 μg/ml in broth and from ≤0.25 to 4 μg/ml in agar), as were the two strains of B. capitatus (MICs, ≤2 μg/ml) and the two isolates of G. clavatum (MICs, ≤2 μg/ml). Against strains of dematiaceous fungi such as Alternaria alternata and Curvularia lunata, MICs of GM 237354 were >64 μg/ml. However, GM 237354 was active against the two strains of Cladosporium carrioni, with MICs of ≤1 on broth and ≤0.25 μg/ml on agar. Against hyaline hyphomycetes, GM 237354 MICs ranged from ≤0.25 to 2 μg/ml for Pseudallescheria boydii and were >64 μg/ml against Fusarium spp. MICs of GM 237354 against zygomycetes (including one isolate each of Absidia corymbifera, Cunninghamella bertholletiae, and R. arrhizus) were ≤4 μg/ml. Against a set of dermatophytes, GM 237354 MICs ranged from 16 to >64 μg/ml in broth and from 2 to >64 μg/ml in agar, with Epidermophyton floccosum and Microsporum canis being the most susceptible strains.
DISCUSSION
Sordarins are a class of antifungal agents distinguished from other antifungals, such as polyenes, azole derivatives, or allylamines, by their different mechanism of action. GM 193663, GM 211676, GM 222712, and GM 237354 are highly selective fungal protein synthesis inhibitors (20); they interact with the translation elongation factor EF-2 (6), inhibiting translation elongation in fungal cells (5, 20). EF-2 is a large (more than 800 residues), probably multifunctional protein that apparently binds to the same ribosomal structure as the EF-1–GTP–aminoacyl-tRNA complex. During protein synthesis, amino acid residues are added to the growing peptide chain in an elongation process that involves two GTP-switched elongation factors, denominated EF-1 and EF-2 in eukaryotes.
To define the spectrum of action of these new antifungal agents, the in vitro activities of GM 193663, GM 211676, GM 222712, and GM 237354 against a wide range of pathogenic yeasts and filamentous fungi, including P. carinii, emerging fungal pathogens, and dermatophytes, were evaluated. The nature of the R group (Fig. 1) had a marked effect on the in vitro potency and spectrum of activity of these new sordarin agents. GM 222712 and GM 237354, containing a 3′,4′-fused tetrahydrofurane ring with a methyl or an exomethylene group, displayed activities higher than those of GM 193663 and GM 211676, which contain a 3′,4′-fused dioxolane ring or an oxirane ring at positions 2′ and 3′ of the sugar moiety, respectively. In spite of their structural differences, all of the new sordarin derivatives tested showed remarkable in vitro antifungal activity against the key yeast pathogen C. albicans, including azole-resistant isolates. Against the azole-resistant C. albicans isolates, the MIC90s were 0.03 μg/ml for GM 193663 and GM 211676, 0.004 μg/ml for GM 222712, and 0.002 μg/ml for GM 237354. C. krusei was intrinsically resistant to these new sordarin derivatives. However, GM 222712 and GM 237354 were characterized by their high levels of activity against non-C. albicans species such as C. tropicalis, C. kefyr, and C. glabrata, which are emerging as serious opportunistic fungal pathogens among immunocompromised patients in clinics. The MIC90s of GM 222712 and GM 237354 against these three Candida species were 0.008 to 0.5 and 0.03 to 1 μg/ml, respectively. Against C. parapsilosis, the MIC90s of GM 222712 and GM 237354 were 4 and 16 μg/ml, respectively. C. neoformans isolates were remarkably susceptible to GM 222712 and GM 237354 (MIC90s, 0.5 and 0.25 μg/ml, respectively).
Sordarin derivatives were found to have extremely potent activity against P. carinii (Table 2). After 24 h of incubation, all of the compounds dramatically inhibited the synthesis of proteins, showing IC50s of <0.008 μg/ml, while the IC50 of pentamidine was 0.1 μg/ml. These results reveal a clear advantage of using sordarins instead of the three major classes of systemic antifungal agents currently in clinical use. The polyenes, the azoles, and the allylamines are targeted against ergosterol, the major fungal sterol in the plasma membrane. They are thus ineffective against P. carinii, which has cholesterol instead of ergosterol, possibly acquired from its mammalian host (9). A clear correlation between P. carinii in vitro susceptibility results and therapeutic efficacy in animal models of infection has been demonstrated (4).
The in vitro activities of new sordarin derivatives against filamentous fungal isolates were also tested by the broth microdilution assay that is under the evaluation by the NCCLS, as well as by an agar dilution assay. Because a standard method for testing of filamentous fungi is not available, Aspergillus spp. MICs were also determined for two additional end points by using colorimetric indicators and by determination of intracellular ATP levels. Colorimetric methods have been previously used for Aspergillus spp. (7, 18). In our study using RPMI–2% glucose and Alamar blue or MTS, we observed a change of color at 24 h of incubation and good correlation with the data obtained by conventional methods. The determination of intracellular ATP levels was used by Yoshida et al. in dermatophytes (38). We demonstrated the applicability of a similar system to Aspergillus susceptibility testing. The technique is extremely sensitive, permitting the determination of reproducible and accurate MIC end points after shorter periods of incubation, especially with drugs which produce partial inhibition of growth (trailing). In general, excellent agreement was observed between the results obtained by colorimetric methods, determination of intracellular ATP levels, and conventional methods.
Although aspergillosis is still the most common form of mold infection in immunocompromised patients, a growing number of other organisms have been reported to cause lethal infection in these individuals. For that reason, one of the most active compounds, GM 237354, was tested against a broad range of yeast-like and filamentous fungi responsible for such infections (Table 5). GM 237354 demonstrated significant in vitro activity against yeast-like fungi such as B. capitatus, G. clavatum, and T. beigelii and against dematiaceous fungi such as Cladosporium carrioni. GM 237354 was inactive against Fusarium spp., but Pseudallescheria boydii and R. arrhizus, some strains of which are resistant to current antifungal therapy, were remarkably susceptible to GM 237354. Further studies with larger and more diverse panels of filamentous fungal isolates are required before more definitive conclusions can be made on the spectrum and potency of sordarin antifungal agents against filamentous fungal pathogens.
Recently, Stevens (35) demonstrated that sordarin derivatives had potent fungicidal activity against important dimorphic endemic fungal pathogens such as Histoplasma capsulatum, Paracoccidioides brasiliensis, Blastomyces dermatitidis, and Coccidioides immitis.
Much effort has been directed toward the development of new antifungal agents with unique modes of action and good spectra that are fungicidal and have fewer side effects. The in vitro profiles of sordarin derivatives justify the performance of additional studies to determine the potential of this class of antifungal agents.
ACKNOWLEDGMENTS
We thank Sonia Lozano for expert technical assistance and members of the Organic Chemistry Group for compound synthesis.
REFERENCES
- 1. Anaissie, E. J. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14(Suppl. 1):43–53. [DOI] [PubMed]
- 2.Colthurst D R, Chalk P, Hayes M, Tuite M F. Efficient translation of synthetic and natural mRNAs in an mRNA-dependent cell free system from the dimorphic fungus Candida albicans. J Gen Microbiol. 1991;137:851–857. doi: 10.1099/00221287-137-4-851. [DOI] [PubMed] [Google Scholar]
- 3.Coval S J, Puar M S, Phife D W, Terracciano J S, Patel M. SCH57404, an antifungal agent possessing the rare sordaricin skeleton and tricyclin moiety. J Antibiot. 1995;48:1171–1172. doi: 10.7164/antibiotics.48.1171. [DOI] [PubMed] [Google Scholar]
- 4.Dei-Cas E, Aliouat E M, Mullet C, Mazars E, Gargallo D. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. A new antimicrobial molecule (GM 237354) highly active against Pneumocystis carinii: in vivo studies and ultrastructural data, abstr. F-65; p. 157. [Google Scholar]
- 5.Domínguez J M, Kelly V A, Kinsman O S, Marriott M S, Gómez de las Heras F, Martín J J. Sordarins: a new class of antifungals with selective inhibition of the protein synthesis elongation cycle in yeasts. Antimicrob Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domínguez J M, Martín J J. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob Agents Chemother. 1998;42:2279–2283. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgopapadakou N H, Walsh T J. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgopapadakou N H, Walsh T J. Human mycoses: drugs and targets for emerging pathogens. Science. 1994;264:371–373. doi: 10.1126/science.8153622. [DOI] [PubMed] [Google Scholar]
- 10.Gold W, Stout H A, Pagano J F, Donovick R. –1956. Amphotericins A and B, antifungal antibiotics produced by a streptomycete. I. In vitro studies. Antibiot Ann. 1955;1955/1956:579–586. [PubMed] [Google Scholar]
- 11.Groll A H, Shah P M, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the post-mortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33:23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 12.Hall C C, Bertasso A B, Watkins J D, Georgopapadakou N H. Screening assays for protein synthesis inhibitors. J Antibiot. 1992;45:1697–1699. doi: 10.7164/antibiotics.45.1697. [DOI] [PubMed] [Google Scholar]
- 13.Hauser D, Sigg H P. Isolietung und abbau von Sordarin. Helv Chim Acta. 1971;54:1178–1190. doi: 10.1002/hlca.19710540427. [DOI] [PubMed] [Google Scholar]
- 14.Herreros E, Almela M J, Martinez M, Lozano S, Jackson H, Aliouat E M, Gargallo-Viola D. Microplate assays for in vitro evaluation of anti-Pneumocystis drugs. J Eukaryot Microbiol. 1997;44:43S–44S. doi: 10.1111/j.1550-7408.1997.tb05766.x. [DOI] [PubMed] [Google Scholar]
- 15.Herreros E, Martinez A, Jimenez E, Aviles P, Almela M J, Lozano S, Martinez C M, San Roman R, Aliouat E M, Alvarez A, Gomez de las Heras F, Gargallo D. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Anti-Pneumocystosis activity of GM 237354 in vitro and in vivo, abstr. F-64; p. 156. [Google Scholar]
- 16.Herreros E, Martinez C M, Almela M J, Lozano S, Gomez de las Heras F, Gargallo D. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. In vitro activity of GM 237354 against a broad range of fungi, abstr. F-57; p. 155. [Google Scholar]
- 17.Hughes W T. Pneumocystis carinii pneumonia: new approaches to diagnosis, treatment and prevention. Pediatr Infect Dis J. 1991;10:391–399. [PubMed] [Google Scholar]
- 18.Jahn B, Martin E, Stueben A, Bhakdi S. Susceptibility testing of Candida albicans and Aspergillus species by a simple microtiter menadi-one-augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay. J Clin Microbiol. 1995;33:661–667. doi: 10.1128/jcm.33.3.661-667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamath A, Chakraburtty K. Role of yeast elongation factor 3 in the elongation cycle. J Biol Chem. 1989;264:15423–15428. [PubMed] [Google Scholar]
- 20.Kinsman O S, Chalk P A, Jackson H C, Middleton R F, Shuttleworth A, Rudd B A M, Jones C A, Noble H M, Wildman H G, Dawson M J, Lynn S, Hayes M V. Isolation and characterisation of an antifungal antibiotic (GR 135402) with protein synthesis inhibition. J Antibiot. 1998;51:41–49. doi: 10.7164/antibiotics.51.41. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Suarez J V, Rodriguez-Tudela J L. Patterns of in vitro activity of itraconazole and imidazole antifungal agents against Candida albicans with decreased susceptibility to fluconazole from Spain. Antimicrob Agents Chemother. 1995;39:1512–1516. doi: 10.1128/aac.39.7.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Members of the European Concerted Action on Pneumocystis carinii. In vitro systems in Pneumocystis research. Parasitol Today. 1996;12:245–249. doi: 10.1016/0169-4758(96)80812-x. [DOI] [PubMed] [Google Scholar]
- 23.Meyer R D, Yong L S, Armstrong D, Yu B. Aspergillosis complicating neoplasic disease. Am J Med. 1973;56:6–15. doi: 10.1016/0002-9343(73)90077-6. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. 10, no. 8. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M27-A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 26.O’Donell M R, Schmitt G M, Tegtmeier B R, Faucett C, Fahey J L, Ito J, Nadamanec A, Niland J, Parker P, Smith E P, Snyder D S, Stein A S, Blume K G, Forman S J. Prediction of systemic fungal infection in allogenic marrow transplant recipients: impact of amphotericin B prophylaxis in high-risk patients. J Clin Oncol. 1994;12:827–834. doi: 10.1200/JCO.1994.12.4.827. [DOI] [PubMed] [Google Scholar]
- 27.Ogita, T., A. Hayashi, S. Sato, and W. Furutani. February 1987. Japanese patent 62-40292.
- 28.Okada Y, Nagabato M, Kamitami M. Antifungal agent BE-31405. Japanese patent 157; 1994. p. 582. [Google Scholar]
- 29.Pannuti C S, Gingrich R, Pfaller M A, Kao C, Wenzel R P. Nosocomial pneumonia in patients having bone marrow transplant. Cancer. 1992;69:2653–2662. doi: 10.1002/1097-0142(19920601)69:11<2653::aid-cncr2820691106>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller M A, Wenzel R. The impact of changing epidemiology of fungal infections in the 1990s. Eur J Clin Microbiol Infect Dis. 1992;11:287–291. doi: 10.1007/BF01962067. [DOI] [PubMed] [Google Scholar]
- 31.Richardson, M. D. 1991. Opportunistic and pathogenic fungi. J. Antimicrob. Chemother. 28(Suppl. A):1–11. [DOI] [PubMed]
- 32.Rodriguez-Tudela J L, Martinez-Suarez J V. Improved medium for fluconazole susceptibility testing of Candida albicans. Antimicrob Agents Chemother. 1994;38:45–48. doi: 10.1128/aac.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigg, H. P., and C. Stoll. August 1969. U.K. patent 1,162,027.
- 34.Skogerson L, Engelhardt D. Dissimilarity in chain elongation factor requirements between yeast and rat liver ribosomes. J Biol Chem. 1977;252:1471–1475. [PubMed] [Google Scholar]
- 35.Stevens D A. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Screening of sordaricin derivatives against endemic fungal pathogens, abstr. F-58; p. 155. [Google Scholar]
- 36.Walsh T J. Invasive fungal infections: problems and challenges in developing new antifungal compounds. In: Sutcliffe J, Georgopapadakou N H, editors. Emerging targets in antibacterial and antifungal chemotherapy. New York, N.Y: Chapman and Hall; 1992. pp. 349–373. [Google Scholar]
- 37.Walsh T J, Pizzo P A. Nosocomial fungal infections. Annu Rev Microbiol. 1988;42:517–545. doi: 10.1146/annurev.mi.42.100188.002505. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida T, Uchida K, Yamaguchi H. An ATP bioluminescence assay applicable to rapid fluconazole susceptibility testing of dermatophytes. Microbiol Immunol. 1997;41:377–386. doi: 10.1111/j.1348-0421.1997.tb01868.x. [DOI] [PubMed] [Google Scholar]