Abstract
We determined the MICs of ampicillin, ciprofloxacin, erythromycin, imipenem, and rifampin for two clinical isolates of Legionella pneumophila serogroup 1 by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay and by quantitative culture. To test the influence of subinhibitory concentrations (sub-MICs) of antimicrobial agents on Legionella uptake into Acanthamoeba castellanii and U937 macrophage-like cells, both strains were pretreated with 0.25 MICs of the antibiotics for 24 h. In comparison to that for the untreated control, subinhibitory concentrations of antibiotics significantly reduced Legionella uptake into the host cells. Measurement of the binding of monoclonal antibodies against several Legionella antigens by enzyme-linked immunoassays indicated that sub-MIC antibiotic treatment reduced the expression of the macrophage infectivity potentiator protein (Mip), the Hsp 60 protein, the outer membrane protein (OmpM), an as-yet-uncharacterized protein of 55 kDa, and a few lipopolysaccharide (LPS) epitopes. In contrast, the expression of some LPS epitopes recognized by monoclonal antibodies 8/5 and 30/4 as well as a 45-kDa protein, a 58-kDa protein, and the major outer membrane protein (OmpS) remained unaffected.
Legionella pneumophila, the causative agent of Legionnaires’ disease, is a gram-negative bacterium commonly isolated from lakes, streams, hot-water supplies, and cooling towers (42). In aquatic environments, L. pneumophila is able to parasitize and replicate within free-living amoebae. Thus, these protozoa are important natural reservoirs for the bacteria (11). After transmission of the bacteria to susceptible humans, Legionella pneumonia may occur. In the human lung, L. pneumophila is an intracellular pathogen that replicates within alveolar macrophages (22).
The antimicrobial therapy for Legionnaires’ disease is dictated by the facultative intracellular nature of the causative bacterium (8). Many antibiotics, e.g., beta-lactams and aminoglycosides, are active against legionellae grown on artificial media but have no influence on intracellular legionellae (8). Cell culture models, the course of experimental infections in guinea pigs, and clinical observations confirm that the macrolides, fluoroquinolones, and rifampin are effective in vivo against intracellular L. pneumophila (26, 34).
It is well known that subinhibitory concentrations (sub-MICs) of antibiotics are able to modulate numerous bacterial characteristics, e.g., the morphology of cells, the bacterial surface components such as outer membrane proteins and lipopolysaccharides (LPS), and the production of virulence factors (3, 7, 15, 24, 31, 36, 37, 40).
The aim of this investigation was to determine the influence of sub-MICs of certain antibiotics on the uptake of legionellae into Acanthamoeba castellanii as a model for natural hosts and U937 cells as a model for infected human cells. Our investigations showed that sub-MICs of antimicrobial agents can inhibit the uptake of Legionella into U937 cells and into A. castellanii. A possible explanation for this might be that sub-MIC antibiotic treatment reduces the expression of several virulence-associated Legionella proteins.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Two virulent strains of L. pneumophila serogroup 1 were used in the present study. Strain Philadelphia-1 (ATCC 33152) is the organism that caused the first outbreak of Legionnaires’ disease that occurred in 1976 in Philadelphia, Pa. (26). Strain Corby was isolated from a patient suffering from pneumonia in England and was kindly supplied by M. Tully, Salisbury, United Kingdom (39). Virulent legionellae were isolated from guinea pig spleen suspensions and stored at −80°C. Before testing, the bacteria were passaged fewer than three times on buffered charcoal yeast extract (BCYE) agar (Oxoid, Wesel, Germany) for 48 h at 36°C in humidified air with 2.5% CO2. ACES buffered yeast extract (AYE) broth containing 10 g of N-(2-acetamido)-2-aminoethanesulfonic acid (ACES) buffer, 10 g of bovine serum albumin, 5 g of yeast extract, 0.4 g of l-cysteine-HCl, and 0.25 g of ferric pyrophosphate (all from Sigma, Deisenhofen, Germany) per liter of medium was used for in vitro cultivation of legionellae. All the ingredients were added to sterile distilled water, and the pH was adjusted with potassium hydroxide to 6.9. The AYE broth was filter sterilized and stored at 4°C.
Quantitation of legionellae by MTT assay and quantitative culture.
Quantitation was done by the MTT assay, which is based on the ability of viable bacteria to cleave the yellow tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) by mitochondrial electron transport activity as described by Gebran (12). Briefly, 30 μl of MTT (5 mg per ml phosphate-buffered saline) was added per well to 100 μl of L. pneumophila cells that had been growing for 24 h in AYE broth. After further incubation for 30, 60, or 90 min, the dark formazan derivative was generated. After 30 s of vortexing, the formazan production was quantified by measuring the optical density at 620 nm (OD620) with an enzyme-linked immunosorbent assay (ELISA) reader. In comparative investigations only live legionellae, but not heat-killed or Formalin-inactivated bacteria, were able to cleave the MTT salt and therefore could be quantified by MTT assay.
Quantitative cultures were performed by making logarithmic dilutions of bacteria in double-distilled water, plating 0.1 ml of the resulting dilution onto BCYE agar, and counting CFU after 4 to 5 days of incubation.
Antimicrobial susceptibility testing for the determination of MICs by both quantitative culture and MTT assay.
The following antimicrobial agents were used for susceptibility testing of legionellae: ampicillin (Ratiopharm, Ulm, Germany), ciprofloxacin (Bayer, Leverkusen, Germany), erythromycin (Abbott, Wiesbaden, Germany), imipenem (Merck Sharp & Dohme, Munich, Germany), and rifampin (Pfizer, Karlsruhe, Germany). Shortly before the series of tests, fresh preparations of all the antimicrobial agents were prepared by dissolving them in distilled water. Broth microdilution susceptibility testing was performed in buffered AYE broth, with a final volume of 100 μl. Bacteria (5 × 106 per well) were added to microplates containing diluted antibiotics. The microplates were then incubated at 36°C for 48 h. By MTT assay MICs were defined as the lowest concentration that gave no increase of the OD620 in comparison with the values for the initial inoculum. From these data we calculated the 0.25 MICs of antibiotics for subsequent experiments.
Influence of 0.25 MIC on bacteriological properties of Legionella strains.
Legionella cells were exposed to 0.25 MICs of ampicillin, ciprofloxacin, erythromycin, imipenem, and rifampin in AYE broth, and their morphology was examined by light microscopy after staining with fuchsin or the anti-OmpM monoclonal antibody (MAb) (Fresenius, Oberursel, Germany). To exclude a nonspecific inhibition of the bacteria growing at 0.25 MIC, the growth rates in AYE broth containing 0.25 MIC were determined by quantitative culture and MTT assay and found to be the same as those for the untreated control. The determination of a possible effect after treatment with 0.25 MICs of antibiotics was performed as described previously (5). Briefly, after incubation of the cultures, the antibiotics were removed by three washing steps, and a 10−2 dilution of the bacterial suspension was prepared in fresh antibiotic-free AYE broth. Subsequently, the growth rate of the bacteria in AYE broth was recorded over the next 16 h by quantitative culture (5).
Uptake of L. pneumophila treated with 0.25 MIC into A. castellanii.
Axenic A. castellanii cells (ATCC 30011) were cultured in peptone-yeast extract-glucose broth (PYG 712 medium) at room temperature (35). To estimate the uptake rate of legionellae, acanthamoebae were diluted with PYG 712 medium to 105 cells per ml, distributed into 48-well tissue culture plates (Costar, Cambridge, Mass.), and grown to confluence overnight. Bacteria were incubated in AYE broth medium with 0.25 MIC of ampicillin, ciprofloxacin, erythromycin, imipenem, and rifampin or without antibiotics for 24 h, and the suspensions were centrifuged at 5,500 × g for 5 min. The supernatant was discarded, and the bacterial pellet was diluted with antibiotic-free AYE broth medium. By the MTT assay, cultures of L. pneumophila were diluted in AYE broth to a density of 107 bacteria per ml. Controls were plated in logarithmic dilutions onto BCYE agar. Acanthamoebae were infected with 100 μl of strain of L. pneumophila (Philadelphia-1 or Corby) treated with an antibiotic or left untreated to provide a bacterium-to-cell ratio of 10:1. After the bacteria were added to the culture, they were brought into contact with the acanthamoebae by centrifugation of the 48-well tissue culture plate at low speed (600 × g, 5 min). Following incubation for 2 h at 37°C, extracellular legionellae were killed by adding 80 μg of gentamicin for 1 h (29, 35). After the gentamicin-containing medium was replaced by antibiotic-free medium, the initial uptake into A. castellanii was estimated with a quantitative culture. For this purpose, amoebae were destroyed by thermal breakup (15 min at −80°C, followed by 20 min at 37°C and 15 min at −80°C). The uptake of untreated legionellae was estimated in the same way and set at 100%.
Uptake of L. pneumophila treated with 0.25 MIC into U937 cells.
Undifferentiated U937 monocytic cells were maintained at 37°C in nonadherent, replicative cultures in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffered RPMI 1640 medium (Sigma) which was supplemented with 10% heat-inactivated fetal bovine serum and 4 mM l-glutamine. The nonadherent U937 cells were activated by the addition of 10 nM phorbol 12-myristate-13-acetate (Sigma). Immediately afterward, the suspension was placed into 48-well tissue culture plates (105 cells per well) and incubated for 24 h in order to allow differentiation into adherent confluent cell monolayers.
Infection of U937 cells and determination of the uptake were done essentially as described for acanthamoebae, except that supplemented RPMI medium was used and the cells were lysed by adding sterile distilled water.
Expression of Legionella proteins and LPS epitopes determined by ELISA.
MAbs to react with L. pneumophila were prepared in our laboratory as previously described (18, 19). The specificities of these MAbs are shown in Table 2. Three MAbs (MAb 20/3, MAb 48/6, and MAb 32/4) that recognize proteins of 58, 55, and 45 kDa, respectively, were chosen to elucidate a possible function of these proteins during the initial adherence and/or uptake. Several MAbs did not react with both Legionella strains when live, whole Legionella cells were used in the ELISA. Therefore, MAb 3, MAb 10/5, MAb 30/4, MAb 20/3, and MAb 39/1 were tested only with strain Corby, and MAb 8/4 was tested only with strain Philadelphia-1. A fluorescein isothiocyanate (FITC)-labeled, commercially available MAb against the 26-kDa outer membrane protein OmpM (20) was obtained from Fresenius. To measure the expression of the 29-kDa major outer membrane protein (MOMP) (OmpS), a recombinant antibody was used. Briefly, a single-chain antibody was selected out of a phage library, and the VH and VL genes were inserted into a Fab cloning and expression vector. Functional Fab fragments were obtained by renaturation of insoluble aggregates containing the recombinant antibody. For the ELISA, microtiter plates were coated overnight with 50 μl of live legionellae treated with antibiotic or left untreated at a concentration of 108 per ml. These concentrations were estimated by MTT assay and verified by quantitative culture. After incubation with 1:10 to 1:50 diluted cell culture supernatants of MAbs, bound antibodies were detected with anti-GAM (immunoglobulin G, A, and M) mouse horseradish peroxidase (HPOD) conjugate (Sigma). For the MAb against the OmpM protein and the OmpS protein, we used an HPOD-labeled anti-FITC-conjugate (Boehringer, Mannheim, Germany) and an HPOD-labeled anti-Fab antibody (Sigma), respectively.
TABLE 2.
Legionella antigen expression with antibiotic treatment relative to that of untreated controls
| MAb | Antigen or specificity (%) recognized | Strain | Mean ± SD of OD under 0.25 MIC of the indicated antibiotic
|
||||
|---|---|---|---|---|---|---|---|
| Ampicillin | Ciprofloxacin | Erythromycin | Imipenem | Rifampin | |||
| 8/5 | LPS of all strains of serogroup (sg) 1 (19) | Philadelphia-1 | 97.0 ± 4.9 | 97.8 ± 6.0 | 98.7 ± 5.9 | 95.9 ± 5.8 | 101.6 ± 4.6 |
| 8/5 | Corby | 95.4 ± 4.4 | 87.4 ± 4.2 | 94.3 ± 4.6 | 91.9 ± 4.4 | 102.4 ± 5.0 | |
| 3/1 | LPS of sg 1 strains of the Pontiac subgroup (19) | Philadelphia-1 | 58.1 ± 3.7 | 44.6 ± 2.9 | 54.2 ± 3.1 | 45.4 ± 2.8 | 53.7 ± 2.7 |
| 3/1 | Corby | 51.1 ± 3.5 | 34.4 ± 2.9 | 48.1 ± 3.4 | 32.0 ± 2.6 | 50.5 ± 2.3 | |
| 3 | LPS of several strains of sg 1 (23) | Corby | 62.9 ± 3.6 | 55.4 ± 3.2 | 78.3 ± 4.6 | 52.3 ± 2.1 | 67.4 ± 3.2 |
| 10/5 | LPS of several strains of sg 1 | Corby | 55.7 ± 3.5 | 45.7 ± 3.7 | 55.4 ± 3.3 | 38.5 ± 2.1 | 67.5 ± 3.8 |
| 30/4 | LPS of several strains of sg 1 | Corby | 99.1 ± 7.5 | 96.8 ± 7.0 | 102.4 ± 7.2 | 100.6 ± 6.9 | 96.9 ± 6.8 |
| 8/4 | LPS of several strains of sg 1 (19) | Philadelphia-1 | 71.3 ± 5.3 | 47.5 ± 2.3 | 50.8 ± 3.4 | 69.7 ± 3.3 | 63.7 ± 3.0 |
| 18/1 | Mip protein of several Legionella species (18) | Philadelphia-1 | 56.5 ± 3.2 | 43.1 ± 2.5 | 46.5 ± 2.3 | 47.2 ± 2.6 | 61.5 ± 3.0 |
| 18/1 | Corby | 59.9 ± 6.1 | 47.0 ± 4.8 | 48.0 ± 5.0 | 50.6 ± 4.9 | 66.0 ± 6.2 | |
| 20/2 | Mip protein specific for L. pneumophila (18) | Philadelphia-1 | 63.4 ± 2.5 | 49.2 ± 2.6 | 59.6 ± 2.2 | 58.4 ± 2.5 | 60.0 ± 2.4 |
| 20/2 | Corby | 49.5 ± 3.3 | 44.7 ± 3.1 | 47.6 ± 1.9 | 46.3 ± 3.1 | 56.4 ± 1.9 | |
| 22/1 | Mip protein of all known Legionella species (18) | Philadelphia-1 | 60.7 ± 2.4 | 49.3 ± 1.4 | 54.1 ± 2.1 | 48.4 ± 2.3 | 69.8 ± 3.8 |
| 22/1 | Corby | 51.7 ± 2.4 | 41.9 ± 2.7 | 43.9 ± 2.3 | 55.0 ± 3.1 | 50.9 ± 1.7 | |
| 20/3 | 58-kDa protein of unknown function | Corby | 99.2 ± 7.1 | 99.4 ± 6.7 | 97.1 ± 7.4 | 85.9 ± 6.3 | 101.8 ± 7.9 |
| 48/6 | 55-kDa protein of unknown function | Philadelphia-1 | 76.0 ± 2.5 | 75.0 ± 2.0 | 46.0 ± 1.7 | 61.4 ± 1.5 | 73.0 ± 1.5 |
| 48/6 | Corby | 78.1 ± 2.7 | 74.7 ± 2.8 | 41.7 ± 2.1 | 70.6 ± 3.1 | 99.7 ± 4.0 | |
| 32/4 | 45-kDa protein of unknown function | Philadelphia-1 | 99.2 ± 6.4 | 97.8 ± 6.4 | 100.8 ± 6.2 | 91.5 ± 5.7 | 100.9 ± 7.8 |
| 32/4 | Corby | 97.5 ± 6.2 | 90.9 ± 4.8 | 97.8 ± 6.2 | 79.6 ± 4.5 | 96.6 ± 6.0 | |
| 39/1 | Hsp 60 | Corby | 69.1 ± 4.6 | 57.3 ± 4.6 | 65.9 ± 5.1 | 51.7 ± 3.4 | 70.6 ± 5.3 |
| FITC-labeled | OmpM specific for L. pneumophila (20) | Philadelphia-1 | 72.8 ± 3.9 | 46.8 ± 2.8 | 54.2 ± 4.4 | 55.2 ± 4.3 | 56.3 ± 4.5 |
| FITC-labeled | Corby | 50.8 ± 3.1 | 48.8 ± 3.4 | 42.9 ± 3.0 | 41.1 ± 2.2 | 48.5 ± 2.8 | |
| Phage Ab | OmpS specific for L. pneumophila | Philadelphia-1 | 98.6 ± 6.6 | 100.7 ± 6.2 | 94.0 ± 5.5 | 101.9 ± 6.8 | 98.3 ± 6.3 |
| Phage Ab | Corby | 96.9 ± 5.4 | 100.3 ± 6.2 | 91.7 ± 5.0 | 104.0 ± 5.6 | 99.8 ± 3.9 | |
MAbs 3, 10/5, 30/4, 20/3, and 39/1 were tested only with the Corby strain, and MAb 8/4 was tested only with the Philadelphia-1 strain.
All experiments were performed in quadruplicate and repeated at least three times. The OD values of the antibiotic-free cultures were set at 100%, and the changes in the expression of Legionella antigens were calculated as a percentage of the expression of the untreated controls.
Statistical analysis.
The Student t test was carried out to compare the results for the antibiotic-treated cultures with those for the controls. P values that were <0.05 were considered to be statistically significant. The Poisson distribution was used to estimate the reliability of the quantitative culture determination of Legionella onto BCYE agar after the lysis of host cells.
RESULTS
MIC determination by both quantitative culture and MTT assay.
When serially diluted legionellae were incubated with MTT for 30, 60, and 90 min, a strong linear relationship between the number of bacteria and the amount of the formazan production was found for different incubation periods (R2 ≥ 0.99). Although the level of color generated also increased with a longer MTT incubation period, the sensitivity remained low (5 × 106 L. pneumophila cells).
A comparison between the MTT assay and CFU counting illustrated that the techniques had similar abilities to detect L. pneumophila growth restriction by antibiotic agents (Table 1).
TABLE 1.
MICs of antibiotics for two strains of L. pneumophila by microdilution assay and from previous publications
| Strain | Method | MIC for indicated antibiotic (mg/liter)a
|
Reference or source | ||||
|---|---|---|---|---|---|---|---|
| Ampicillin | Ciprofloxacin | Erythromycin | Imipenem | Rifampin | |||
| Corby | Dilution in AYE broth | 2.4 | 0.014 | 0.25 | 0.45 | 0.0011 | This study |
| Philadelphia-1 | Dilution in AYE broth | 2 | 0.012 | 0.25 | 0.35 | 0.0011 | This study |
| Corby | MTT assay in AYE broth | 2.4 | 0.014 | 0.25 | 0.45 | 0.0011 | This study |
| Philadelphia-1 | MTT assay in AYE broth | 2 | 0.012 | 0.25 | 0.35 | 0.0011 | This study |
| Philadelphia-1 | Microdilution in AYE broth | ND | 0.019 | 0.195 | ND | ≤0.002 | 16 |
| Philadelphia-1 | Agar dilution on BSYEb agar | ND | ≤0.0313 | 0.25 | ND | ≤0.0313 | 34 |
| Philadelphia-1 | Agar dilution on BCYE agar | 0.5 | ND | 0.25 | ND | 0.015 | 38 |
| Philadelphia-1 | Microdilution in AYE broth | ND | 0.0125 | 0.2 | ND | ND | 41 |
ND, not determined.
BSYE, buffered starch yeast extract agar.
Influence of 0.25 MIC on bacteriological properties of Legionella strains.
When Legionella cells exposed to 0.25 MIC were stained with fucsin or the anti-OmpM MAb, the number of filaments did not increase in comparison to that for untreated controls. In AYE broth containing 0.25 MIC, the growth rate was found to be the same as in antibiotic-free AYE broth, as determined by quantitative culture and MTT assay. After the bacteria were grown in AYE broth containing 0.25 MIC of antibiotics for 16 h, the growth rate in antibiotic-free AYE broth was unchanged, suggesting that there was no post-antibiotic effect.
Uptake of L. pneumophila treated with 0.25 MIC into A. castellanii.
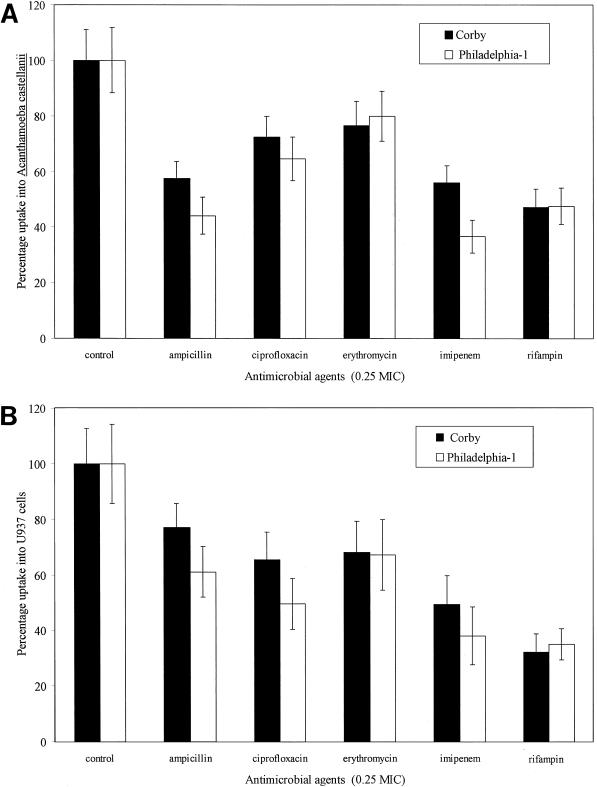
The mean uptake of the untreated control into acanthamoebae amounted to 404 ± 55 (mean ± standard deviation) CFU per culture for the Philadelphia-1 strain and 520 ± 68 CFU per culture for the Corby strain. After pretreatment with subinhibitory concentrations of antibiotics, both Legionella strains showed a significantly inhibited uptake into amoebae (P < 0.01) as illustrated in Fig. 1A. Rifampin leads to a highly significant reduction in the uptake of legionellae, as do the two beta-lactam antibiotics. To a lesser extent, the uptake into acanthamoebae was also influenced by ciprofloxacin and erythromycin. Both strains show slightly different results, but the effect of subinhibitory antibiotic concentrations appears in general to be quite homogeneous.
FIG. 1.
Effects of subinhibitory (0.25 MIC) concentrations of various antibiotics (incubation period, 24 h) on the initial uptake of L. pneumophila strains Corby and Philadelphia-1 into A. castellanii (A) and U937 cells (B). The uptake was executed in antibiotic-free PYG 712 medium for amoebae and in RPMI for U937 cells. The mean initial uptake of the untreated control into host cells was considered to be 100% and amounted to 404 ± 55 CFU per culture for the Philadelphia-1 strain and 520 ± 68 CFU per culture for the Corby strain in the amoebic model. The mean uptake into U937 cells was found to be 1,141 ± 255 CFU per culture for strain Philadelphia-1 and 1,871 ± 268 for strain Corby. Each bar represents the percentage of the mean uptake ± standard deviations (error bars) of the mean of 10-fold cultures.
Uptake of L. pneumophila treated with 0.25 MIC into U937 macrophage-like cells.
An infection model equivalent to uptake into acanthamoebae was used. Compared with the acanthamoeba model, the mean uptake of the untreated control into U937 cells was considerably higher, amounting to 1,141 ± 255 CFU per culture for the Philadelphia-1 strain and 1,871 ± 268 CFU per culture for the Corby strain. Similar to results for the acanthamoeba model, uptake of legionellae into U937 cells was significantly inhibited (P < 0.01) (Fig. 1B) after pretreatment of legionellae with antibiotics. The reduction in uptake from antibiotic-treated Legionella cells showed the same tendency as that for acanthamoebae. The strongest inhibition was again shown for rifampin. Ampicillin-treated legionellae also showed a reduced uptake into U937 cells, although to a lesser extent than for into acanthamoebae.
Effect of 0.25 MIC on the expression of Legionella antigens.
A reduction of more than 15% was considered significant, because the standard deviation of the OD values of the untreated control never exceeded 15%. By this criterion, a reduction of the expression after treatment with 0.25 MIC was found for the macrophage infectivity potentiator (Mip) protein, the OmpM protein, the 55-kDa protein, and a few LPS epitopes (Table 2). For the serogroup 1 strains, levels of reduction of antigen expression were similar. Therefore, a general mechanism for downregulation exists. The expression of the OmpS, the 45-kDa protein, and the 58-kDa protein and that of the LPS epitopes recognized by MAbs 8/5 and 30/4 remained unchanged (Table 2). None of the proteins or LPS epitopes investigated in this study showed any increase in expression after being subjected to subinhibitory concentrations.
DISCUSSION
L. pneumophila is the causative agent of Legionnaires’ disease and Pontiac fever. Risk groups among humans are immunocompromised patients, smokers, and the elderly. The antibacterial therapy used to combat legionellosis is dictated by the intracellular multiplication of the causative bacterium. The treatment of choice for immunocompetent people with community-acquired Legionella pneumonia is still erythromycin or one of the newer macrolide antimicrobial agents alone or in combination with rifampin. For immunosuppressed patients, fluoroquinolones are recommended (8).
No standard method is yet available for determining the MICs of antimicrobial agents for Legionella. In vitro results can be affected by charcoal inactivation in the medium, by the inoculum, the medium, and/or the growth period (8, 16, 33). To minimize such methodological effects and to obtain reproducible results, we used charcoal-free AYE.
Our introductory investigations of the MTT assay for L. pneumophila growth rates yielded results close to those obtained by quantitative culture. But the minimal detectable concentration of legionellae per culture amounted to 5 × 106 by the MTT assay. This result was consistent with the report of Gebran et al. (12). Therefore, the MTT assay was used only with higher bacterial concentrations (>5 × 106/culture). In general, the MTT assay is suitable for evaluating the in vitro susceptibility of L. pneumophila to different antimicrobial agents. The MTT assay allows the processing of large numbers of samples because it can be performed in flat-bottom, 96-well microplates, with measurement done with an ELISA reader.
The MTT assay and quantitative culture showed similar levels of inhibition of Legionella after antibiotic treatment. The MICs determined for strain Philadelphia-1 were in the same range as those reported by other authors (Table 1). No information on MICs for the Corby strain was available, but our results were in the same range as those reported for the Philadelphia-1 strain. The 0.25 MICs of antibiotics for the subsequent uptake experiments were calculated from these results.
We did not observe post-antibiotic effect with 0.25 MIC or find a change in Legionella growth in AYE broth containing 0.25 MIC. Furthermore, the morphology of the bacteria following exposure to 0.25 MIC was the same as that of untreated controls. Thus, methodological errors based on inexact quantitation of legionellae used in the expression assay could be excluded. In summary, neither the reduced uptake of legionellae into acanthamoebae or U937 cells nor the reduced expression of some antigens resulted from growth inhibition of the bacteria due to antibiotic treatment.
In therapeutic use, antibiotics are often present in subinhibitory concentrations at the site of infection. Several factors that influence the adherence and/or uptake of various bacteria can be affected by these levels of antibiotics. Thus, the expression of the K1 antigen, P fimbriae, from the uropathogen Escherichia coli was reduced when the bacteria were treated with subinhibitory concentrations of antibiotics (15, 40). Desnottes et al. (6) reported a reduced adherence of Staphylococcus aureus and Enterococcus faecalis to host cells after pretreatment with the 0.25 MIC of perfloxacin.
We therefore investigated the effect of subinhibitory concentrations of antimicrobial agents on the adhesion to and uptake of legionellae into A. castellanii and human macrophage-like U937 cells. The protozoan model is suited for determining the virulence of L. pneumophila (29, 35). The U937 cell line, which was derived from human histiocytic lymphoma cells, has been shown by other investigators to support the growth of virulent L. pneumophila and to have properties similar to those of human alveolar macrophages (32, 35, 44).
Our study has demonstrated, for two clinical isolates of L. pneumophila serogroup 1, that adhesion to and the uptake into both acanthamoebae and U937 cells can be modulated by treatment with 0.25 MIC of antibiotics. Our study also shows that the rates of inhibition of uptake into U937 cells and acanthamoebae are very similar although the reduction in uptake of the Philadelphia-1 strain was more prominent than that of the Corby strain.
The beta-lactam antibiotics ampicillin and imipenem reduced the uptake of legionellae by 44 to 72% and 37 to 56% of the level without antibiotics, respectively. Both antimicrobial agents have the effect of inhibiting d-alanin transpeptidase, with a subsequent disruption of murein synthesis. Thus, outer membrane proteins and/or LPS structures that may play a role in the adhesion and/or uptake of legionellae could be affected at these sub-MIC levels.
The macrolide erythromycin reversibly affects RNA-dependent protein synthesis by blocking the transpeptidation and/or translocation reactions (14). It is possible that sub-MICs of erythromycin influence the synthesis and/or assembly of bacterial components such as outer membrane proteins or LPS through this mechanism. The inhibition in uptake caused by erythromycin is weaker than the effects of the other antibiotics tested. It might be speculated that erythromycin has a stronger effect on housekeeping proteins in general, not only on proteins whose origins are at the surface of cell membranes, but this possibility has to be proven. Rifampin exerts its bactericidal effect by inhibiting DNA-dependent RNA polymerase at the beta subunit, preventing mRNA elongation. Why rifampin has the smallest uptake of legionellae is not known. The quinolone ciprofloxacin also reduced the uptake of legionellae in comparison to that for the untreated control. Quinolones rapidly initiate cell death by binding to bacterial DNA gyrase and by promoting the cleavage of DNA within the enzyme-DNA complex. At subinhibitory concentrations, DNA synthesis may be affected, leading to a reduction in the production of proteins (7). The adhesion proteins of L. pneumophila could be affected by this mechanism.
To elucidate the mechanisms that cause the reduced adhesion and/or uptake of legionellae, we determined the expression of several antigens by ELISAs with MAbs (Table 2). We found that the expression of several proteins was reduced. Thus, the membrane-associated Mip protein (24 kDa) was downregulated in the presence of 0.25 MICs. The Mip protein was identified as a virulence factor because Mip-negative mutants had a significantly reduced uptake into eukaryotic cells and amoebae (4, 44). The uptake in both sorts of hosts could be restored after complementation with the intact mip gene (4, 44). With the use of three MAbs that recognize different epitopes on the Mip protein, very similar reductions of the OD were obtained. This served as an internal control for reproducibility and the absence of methodological errors. The reduced expression of the Mip protein might be one mechanism that leads to the reduced uptake of legionellae in protozoan and cellular hosts.
Hsp 60, a member of the GroEL family, is upregulated after adhesion and uptake into macrophages (10). Therefore, it could act as an adhesion or uptake molecule. Thus, reduced expression can explain reduced uptake in host cells after treatment with 0.25 MICs.
Another protein that was downregulated after treatment of legionellae with sub-MICs was the outer membrane protein OmpM. High et al. (20) demonstrated that a recombinant E. coli clone carrying the OmpM gene had an increased uptake into embryonated chicken embryos. Therefore, the OmpM protein may also play a role in the uptake of Legionella into host cells.
The function of the 55-kDa protein that was downregulated after sub-MIC treatment is not known. Since it is accessible to MAbs on intact bacteria, it is likely to be a surface protein and may function as an adhesin. We are presently performing experiments to purify the protein with MAb 48/6 and to identify and analyze the corresponding gene by reverse genetics.
In contrast to these results, the expression of a 45-kDa protein recognized by MAb 32/4, a 58-kDa protein recognized by MAb 20/3, and the 29-kDa MOMP (OmpS) remained unaffected by the treatment with 0.25 MICs. The 58-kDa protein is different from the Hsp 60 protein, since MAb 20/3 does not react with a recombinant E. coli clone expressing the latter (17). The functions of the 45-kDa protein and the 58-kDa protein remain to be determined. The MOMP (OmpS) has been cloned and sequenced previously (21). It forms ion-permeable channels in contact with lipid membranes on the L. pneumophila cell surface and functions as a porin. Previous investigations showed that this protein is able to bind the complement components C3b and C3bi and to mediate via complement receptors the uptake of legionellae into macrophages (1, 22). Thus, the MOMP is attributed a role as an adhesion factor. Obviously this protein has important housekeeping functions, and therefore expression was not affected after treatment with subinhibitory concentrations of antibiotics.
The LPS of Legionella has an unusual structure compared with those of other gram-negative bacteria (45). Therefore, the Legionella LPS exhibits only weak endotoxin characteristics. Recently we demonstrated that the epitope on the LPS, recognized by our MAb 3/1, is the O-acetyl group of 5-acetamid-ino-7-acetamido-8-O-acetyl-3,5,7,9-tetradesoxy-d-glycero-l-galactononulosonic acid (legionaminic acid). Strains carrying this epitope are isolated more often than other strains from patients, especially in cases of community-acquired pneumonia affecting immunocompetent people (19). Two LPS epitopes recognized by MAbs 8/5 and 30/4 remained unaffected after treatment with 0.25 MICs. These results again served as a control and excluded nonspecific suppression of growth of the legionellae. We did not observe any LPS epitopes which became more accessible when legionellae were grown in the presence of certain antibiotics, as have been described for E. coli (30). Furthermore, we do not know the exact localization of the epitopes in the LPS that were affected by treatment with sub-MICs. One might speculate that they are located in the outer layer as the epitope recognized by MAb 3/1. The role of Legionella LPS in uptake is not known. Serogroup 1 LPS does not bind C1q (27), and therefore a possible role in complement receptor-mediated uptake is unlikely. Edelstein et al. (9) reported few changes in the LPS epitope pattern during culturing of legionellae at various temperatures. Whether these changes play a role in adhesion and/or uptake remains to be determined. In contrast, the role of several L. pneumophila serogroups and Legionella species as causative agents in Legionella pneumonia (42) makes it unlikely that a limited number of LPS epitopes are involved in the uptake of Legionella into the host cells.
Interestingly, the reduction of surface factors that play a significant role in the adhesion or uptake of legionellae was affected by several classes of antibiotics. Although erythromycin, ciprofloxacin, and rifampin do not influence the composition of the cell wall directly, it could be hypothesized that they impair the synthesis of certain enzymes involved in cell wall synthesis when legionellae are grown in the presence of sub-MICs of these antibiotics. Goldoni et al. (13) also found that subinhibitory concentrations of several antibiotics, i.e., beta-lactams, chloramphenicol, gentamicin, rifampin, erythromycin, and ciprofloxacin, did not affect L. pneumophila growth but did inhibit the hemolytic activities of the strains. Two hemolytic proteins, the legiolysin (Lly, 39 kDa) and the major secretory protein (Msp, 38 kDa), a Zn2+ metalloprotease, have been described previously. Neither protein acts as a virulence factor (2, 28, 43). Since we had no available MAbs against these proteins, we could not test the influence of sub-MICs on the expression of these proteins. Several classes of antibiotics also affected the expression of virulence factors of S. aureus (7), Salmonella typhimurium (25), and Pseudomonas aeruginosa (36). On the other hand, Nichterlein et al. (31) reported reduced expression of listeriolysin in Listeria monocytogenes following treatment with subinhibitory concentrations of antibiotics that inhibit cell wall synthesis.
Further work will be required to elucidate the mechanism by which antimicrobial agents induce alterations of uptake into A. castellanii and U937 macrophage-like cells.
ACKNOWLEDGMENTS
We are grateful to M. Tully, Salisbury, United Kingdom, for providing the serogroup 1 wild-type strain Corby. J. Hacker, Würzburg, Germany, is acknowledged for the recombinant E. coli expressing the Hsp 60 protein. We also thank Sigrid Gäbler, Jutta Möller, and Ines Wolf for technical assistance in producing the MAbs.
This study was supported by the Deutsche Forschungsgemeinschaft (Lu 485/1-2).
REFERENCES
- 1.Bellinger-Kawahara C, Horwitz M A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990;172:1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blander S J, Szeto L, Shuman H A, Horwitz M A. An immunoprotective molecule, the major secretory protein of Legionella pneumophila, is not a virulence factor in a guinea pig model of Legionnaires’ disease. J Clin Investig. 1990;86:817–824. doi: 10.1172/JCI114779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breines D M, Burnham J C. Modulation of Escherichia coli type 1 fimbrial expression and adherence to uroepithelial cells following exposure of logarithmic phase cells to quinolones at subinhibitory concentrations. J Antimicrob Chemother. 1994;34:205–221. doi: 10.1093/jac/34.2.205. [DOI] [PubMed] [Google Scholar]
- 4.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig W A, Gudmundsson S. The postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: Williams and Wilkins; 1986. pp. 403–431. [Google Scholar]
- 6.Desnottes, J. F., N. Diallo, C. Loubeyre, and N. Moreau. 1990. Effect of pefloxacin on microorganisms:host cell interaction. J. Antimicrob. Chemother. 26(Suppl. B):17–26. [DOI] [PubMed]
- 7.Doss S A, Tillotson G S, Amyes S G. Effect of sub-inhibitory concentrations of antibiotics on the virulence of Staphylococcus aureus. J Appl Bacteriol. 1993;75:123–128. doi: 10.1111/j.1365-2672.1993.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein, P. H. 1995. Antimicrobial chemotherapy for Legionnaires’ disease: a review. Clin. Infect. Dis. 21(Suppl. 3):265–276. [DOI] [PubMed]
- 9.Edelstein P H, Beer K B, Deboynton E D. Influence of growth temperature on virulence of Legionella pneumophila. Infect Immun. 1987;55:2701–2705. doi: 10.1128/iai.55.11.2701-2705.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez R C, Logan S M, Lee S H S, Hoffman P S. Elevated levels of Legionella pneumophila stress protein Hsp 60 early in infection of human monocytes and L929 cells correlate with virulence. Infect Immun. 1996;64:1968–1976. doi: 10.1128/iai.64.6.1968-1976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields B S. Legionella and protozoa: interaction as a pathogen and its natural host. In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella—current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 129–136. [Google Scholar]
- 12.Gebran S J, Newton C A, Yamamoto Y, Klein T W, Friedman H. A rapid colorimetric assay for evaluating Legionella pneumophila growth in macrophages in vitro. J Clin Microbiol. 1994;32:127–130. doi: 10.1128/jcm.32.1.127-130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldoni P, Castellani-Pastoris M, Cattani L, Sinibaldi L, Orsi N. Effect of sub-inhibitory concentrations of antibiotics on the hemolytic activity of Legionella. J Chemother. 1993;5:293–296. doi: 10.1080/1120009x.1993.11739247. [DOI] [PubMed] [Google Scholar]
- 14.Hacker J, Hof H. Biology and experimental chemotherapy of Legionella infections. In: Bryskier A, Butzler J P, Neu H C, Tulkens P M, editors. Macrolides. Chemistry, pharmacology and clinical uses. Paris, France: Blackwell Inc.; 1993. pp. 229–234. [Google Scholar]
- 15.Hacker J, Ott M, Hof H. Effects of low, subinhibitory concentrations of antibiotics on expression of a virulence gene cluster of pathogenic Escherichia coli by using a wild type fusion. Int J Antimicrob Agents. 1993;2:263–270. doi: 10.1016/0924-8579(93)90060-i. [DOI] [PubMed] [Google Scholar]
- 16.Havlichek D, Saravolatz L, Pohlod D. Effect of quinolones and other antimicrobial agents on cell-associated Legionella pneumophila. Antimicrob Agents Chemother. 1987;31:1529–1534. doi: 10.1128/aac.31.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helbig, J. H. Unpublished data.
- 18.Helbig J H, Ludwig B, Lück P C, Groh A, Witzleb W, Hacker J. Monoclonal antibodies to Legionella Mip proteins recognize genus- and species-specific epitopes. Clin Diagn Lab Immunol. 1995;2:160–165. doi: 10.1128/cdli.2.2.160-165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helbig J H, Lück P C, Knirel Y A, Witzleb W, Zähringer U. Molecular characterization of a virulence-associated epitope on the lipopolysaccharide of Legionella pneumophila serogroup 1. Epidemiol Infect. 1995;115:71–78. doi: 10.1017/s0950268800058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.High A S, Torosian S D, Rodgers F G. Cloning, nucleotide sequence and expression in Escherichia coli of a gene (OmpM) encoding a 25 kDa major outer membrane protein (MOMP) of Legionella pneumophila. J Gen Microbiol. 1993;139:1715–1719. doi: 10.1099/00221287-139-8-1715. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman P S, Ripley M, Weeratna R. Cloning and nucleotide sequence of a gene (OmpS) encoding the major outer membrane protein of Legionella pneumophila. J Bacteriol. 1992;174:914–920. doi: 10.1128/jb.174.3.914-920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz M A. Toward an understanding of host and bacterial molecules mediating Legionella pneumophila pathogenesis. In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella—current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 55–62. [Google Scholar]
- 23.Joly J R, McKinney R M, Tobin J O, Bibb W F, Watkins I D, Ramsay D. Development of a standardized subgrouping scheme for Legionella pneumophila serogroup 1 using monoclonal antibodies. J Clin Microbiol. 1986;23:768–771. doi: 10.1128/jcm.23.4.768-771.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loubeyre C, Desnottes J F, Moreau N. Influence of sub-inhibitory concentrations of antibacterials on the surface properties and adhesion of Escherichia coli. J Antimicrob Chemother. 1993;31:37–45. doi: 10.1093/jac/31.1.37. [DOI] [PubMed] [Google Scholar]
- 25.Majtanova L, Hostacka A, Majtan V. Effects of subinhibitory concentrations of antibiotics on biological properties of Salmonella typhimurium. Folia Microbiol. 1994;39:141–146. doi: 10.1007/BF02906810. [DOI] [PubMed] [Google Scholar]
- 26.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R the Laboratory Investigation Team. Legionnaires’ disease, isolation of bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 27.Mintz C S, Arnold P I, Johnson W, Schultz D R. Antibody-independent binding of complement component C1q by Legionella pneumophila. Infect Immun. 1995;63:4939–4943. doi: 10.1128/iai.63.12.4939-4943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moffat J F, Edelstein P H, Regula D P, Cirillo J D, Tompkins L S. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol Microbiol. 1994;12:693–705. doi: 10.1111/j.1365-2958.1994.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 29.Moffat J F, Tompkins L S. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson D, Delahooke T E, Poxton I R. Influence of subinhibitory levels of antibiotics on expression of Escherichia coli lipopolysaccharide and binding of anti-lipopolysaccharide monoclonal antibodies. J Med Microbiol. 1993;39:100–106. doi: 10.1099/00222615-39-2-100. [DOI] [PubMed] [Google Scholar]
- 31.Nichterlein T, Domann E, Kretschmar M, Bauer M, Hlawatsch A, Hof H, Chakraborty T. Subinhibitory concentrations of β-lactams and other cell-wall antibiotics inhibit listeriolysin production by Listeria monocytogenes. Int J Antimicrob Agents. 1996;7:75–81. doi: 10.1016/0924-8579(96)00014-3. [DOI] [PubMed] [Google Scholar]
- 32.Pearlman E, Jiwa A H, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 33.Reda C, Quaresima T, Pastoris M C. In-vitro activity of six intracellular antibiotics against Legionella pneumophila strains of human and environmental origin. J Antimicrob Chemother. 1994;33:757–764. doi: 10.1093/jac/33.4.757. [DOI] [PubMed] [Google Scholar]
- 34.Saito A, Sawatari K, Fukuda Y, Nagasawa M, Koga H, Tomonaga A, Nakazato H, Fujita K, Shigeno Y, Suzuyama Y, Yamaguchi K, Izumikawa K, Hara K. Susceptibility of Legionella pneumophila to ofloxacin in vitro and in experimental Legionella pneumophila in guinea pigs. Antimicrob Agents Chemother. 1985;28:15–20. doi: 10.1128/aac.28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinert M, Ott M, Lück P C, Tannich E, Hacker J. Studies on the uptake and intracellular replication of Legionella pneumophila in protozoa and in macrophage-like cells. FEMS Microbiol Ecol. 1994;15:299–308. [Google Scholar]
- 36.Tateda K, Ishii Y, Hirakata Y, Matsumoto T, Ohno A, Yamaguchi K. Profiles of outer membrane proteins and lipopolysaccharide of Pseudomonas aeruginosa grown in the presence of sub-MICs of macrolide antibiotics and their relation to enhanced serum sensitivity. J Antimicrob Chemother. 1994;34:931–942. doi: 10.1093/jac/34.6.931. [DOI] [PubMed] [Google Scholar]
- 37.Trancassini M, Brenciaglia M I, Ghezzi M C, Cipriani P, Filadoro F. Modification of Pseudomonas aeruginosa virulence factors by sub-inhibitory concentrations of antibiotics. J Chemother. 1992;4:78–81. doi: 10.1080/1120009x.1992.11739144. [DOI] [PubMed] [Google Scholar]
- 38.Traub W H, Spor M. In vitro antibiotic susceptibility of Legionellaceae: search for alternative antimicrobial drugs. Chemotherapy. 1984;30:182–187. doi: 10.1159/000238266. [DOI] [PubMed] [Google Scholar]
- 39.Tully M, Williams A, Fitzgeorge R B. Transposon mutagenesis in Legionella pneumophila. II. Mutants exhibiting impaired intracellular growth within macrophages and reduced virulence in vivo. Res Microbiol. 1991;143:481–488. doi: 10.1016/0923-2508(92)90094-5. [DOI] [PubMed] [Google Scholar]
- 40.Vranes J, Zagar Z, Kurbel S. Influence of subinhibitory concentrations of ceftazidime, ciprofloxacin and azithromycin on the morphology and adherence of P-fimbriated Escherichia coli. J Chemother. 1996;8:254–260. doi: 10.1179/joc.1996.8.4.254. [DOI] [PubMed] [Google Scholar]
- 41.Walz A, Nichterlein T, Hof H. Excellent activity of newer quinolones on Legionella pneumophila in J774 macrophages. Zentbl Bakteriol. 1997;285:431–439. doi: 10.1016/s0934-8840(97)80009-6. [DOI] [PubMed] [Google Scholar]
- 42.Winn W C. Legionella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R Y, editors. Manual of clinical microbiology. Washington, D.C: American Society for Microbiology; 1995. pp. 533–544. [Google Scholar]
- 43.Wintermeyer E, Flügel M, Ott M, Steinert M, Rdest U, Mann K H, Hacker J. Sequence determination and mutational analysis of the lly locus of Legionella pneumophila. Infect Immun. 1994;62:1109–1117. doi: 10.1128/iai.62.3.1109-1117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wintermeyer E, Ludwig B, Steinert M, Schmidt B, Fischer G, Hacker J. Influence of site specifically altered Mip proteins on intracellular survival of Legionella pneumophila in eukaryotic cells. Infect Immun. 1995;63:4576–4583. doi: 10.1128/iai.63.12.4576-4583.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zähringer U, Knirel Y A, Lindner B, Helbig J H, Sonesson A, Marre R, Rietschel E T. The lipopolysaccharide of Legionella pneumophila serogroup 1 (strain Philadelphia 1): chemical structure and biological significance. In: Levin J, Alving C R, Munford R S, Redl H, editors. Bacterial endotoxins: lipopolysaccharides from genes to therapy. New York, N.Y: Wiley-Liss Inc.; 1995. pp. 133–139. [PubMed] [Google Scholar]