Abstract
The efficacies of dicationic carbazole compounds, nitazoxanide (NTZ), and paromomycin were evaluated against the AUCp1 isolate of Cryptosporidium parvum by using a neonatal mouse model. Compounds were solubilized or suspended in deionized water and administered orally by gavage to neonatal mice at a constant dose rate on days 0 to 5 (treatment started on day 0). Dose rates varied for individual carbazole compounds but ranged from 0.65 to 20 mg/kg of body weight. NTZ was tested at 100 and 150 mg/kg, and paromomycin was tested at 50 mg/kg. Efficacies were determined by comparing numbers of oocysts present in treated versus control mice at necropsy examination on day 6. Demonstrable efficacy was observed for several carbazole compounds, based on significant reductions in the numbers of oocysts recovered from treated mice versus control mice. Compounds 1, 7, and 10 (19.0 mg/kg) reduced oocyst passage in treated mice to less than 5% of that in control mice. Treatment with compounds 6, 8, and 9 (17.0 mg/kg) resulted in reductions of oocyst output to less than 10% of that in controls. Although they were not comparable in efficacy to compounds 1, 6, 7, 8, 9, and 10, treatment with other carbazole compounds resulted in statistically significant reductions in oocyst output in treated versus control mice. Compound 1 retained efficacy resulted in reduction of oocyst output to approximately 6% of that in controls when the dose was reduced to 5 mg/kg. Further reductions in the dose rate resulted in considerable reductions in anticryposporidial activity. Likewise, the efficacies of compounds 9 and 10 were reduced substantially when the doses were lowered to one-half the screening dose. Paromomycin yielded excellent activity (reduction of oocyst output to <2% of that in controls) at a dose of 50 mg/kg. NTZ yielded moderate efficacy as powder and injectable formulations administered at 100 mg/kg orally (reduction of oocyst output to 42 and 26% of that in controls, respectively). Oral administration of the injectable formulation of NTZ at a dose of 150 mg/kg resulted in improved efficacy (oocyst output, <5% of that in controls).
Cryptosporidiosis is a ubiquitous diarrheal disease of animals and humans (6, 10). The causative agent, Cryptosporidium parvum, infects a wide variety of mammalian hosts (10). Its broad host range, together with its refractoriness to most common antiparasitics and disinfectants, has led to its characterization as one of the more important emerging disease agents of the latter two decades. Cryptosporidiosis in immunocompetent humans results in acute, but self-limiting, gastroenteritis. However, in persons with compromised or nonfunctional immune systems, the disease can be protracted and even life-threatening and can involve multiple organ systems. Cryptosporidiosis is also emerging as a major water-borne disease in humans (21). Despite considerable efforts to identify and develop effective cryptosporidicidal agents, none has as yet received regulatory approval for treatment or prevention of human or animal cryptosporidiosis (3, 19).
In studies reported elsewhere (2, 4) we have identified effective cryptosporidicidal compounds among a class of chemical agents known as aromatic dicationic molecules. Although prototype dications such as pentamidine and diminazene often demonstrated undesirable toxic effects, molecules currently under development in our laboratories appear to possess enhanced efficacies against a broader range of parasites and reduced toxicity or a complete lack of toxicity. The broad spectrum of activity of some of these dicationic compounds is noteworthy and includes intracellular and extracellular protozoa, Pneumocystis carinii, Cryptococcus neoformans, Mycobacterium spp., and certain viruses (2, 3–5, 7, 9, 15, 20, 23–26). The purpose of this report is to summarize cryptosporidicidal activities of selected dicationic carbazole compounds determined by use of our neonatal mouse model (2, 4, 16). In addition, we sought to compare the efficacies of the carbazoles, particularly compound 1, to that of nitazoxanide (NTZ) (8, 18) and paromomycin (11–13, 17).
MATERIALS AND METHODS
Chemistry of the carbazole compounds.
The synthesis of carbazoles 1 to 5, 7, 10 to 14, and 16 to 18 and of the dinitrile precursors of carbazoles 6 and 9 has been described previously (20). The synthesis of carbazoles 8 and 15 will be described in a future manuscript. Compounds 6 and 9 were prepared by Pinner syntheses (23) from their respective dinitrile precursors. For dose calculations, compounds were presumed to have a purity of 100% (wt/wt).
General experimental methods.
Uncorrected melting points were measured on a Thomas Hoover capillary melting-point apparatus. 1H nuclear magnetic resonance (NMR) spectra were recorded on Varian XL 400 MHz spectrometers in dimethyl sulfoxide (DMSO)-d6. Anhydrous ethanol was distilled over Mg immediately prior to use. Reactions were monitored by infrared spectrometry (Nujol mull; Perkin-Elmer 1320 spectrophotometer) or by reverse-phase high-pressure liquid chromatography (HPLC). HPLC chromatograms were recorded as previously described (20) with the following modifications. A Dupont Zorbax Rx C8 column (3.0 mm by 15 cm; diameter, 3.5 μm) was used. Mobile phases consisted of mixtures of acetonitrile (3.75 to 67.5% [vol/vol]) in water containing tetramethylammonium chloride, sodium heptanesulfonate, and phosphate buffer (pH 2.5) (10 mM each). The concentration of acetonitrile was maintained at 3.75% for 0.5 min, increased to 45% following a linear gradient over 12.5 min, increased immediately to 67.5% following a linear gradient over 3 min, then maintained at 67.5% for 4.5 min. Fast atom bombardment mass spectra (FAB-MS) were recorded on a VG 70-SEQ Hybrid spectrometer (cesium ion gun; 30 kV). Microanalyses were performed by Atlantic Microlab, Norcross, Ga., and were within ±0.4% of calculated values.
3,6-Bis(N-hydroxyamidino)carbazole hemimaleate hydrochloride (compound 6).
A suspension of 3,6-dicyanocarbazole (2.18 g, 10.0 mmol) and anhydrous ethanol (5.0 ml, 85 mmol) in 1,4-dioxane (150 ml) was saturated with hydrogen chloride at 0°C. The reaction mixture was stirred at room temperature for 34 days before the crude diimidate was filtered off and suspended in anhydrous ethanol (10 ml). An ethanolic solution of hydroxylamine, prepared by treating a solution of the hydrochloride salt (7.00 g, 101 mmol) in hot ethanol (100 ml) with sodium ethoxide (21% [wt/wt] solution in denatured alcohol, 37 ml, 99 mmol) and filtering off the resultant sodium chloride, was added, and the mixture was stirred at reflux for 2 h. The reaction mixture was concentrated, and the crude product was filtered off. Purification was effected sequentially. The crude product was initially treated with excess maleic acid in aqueous ethanol, followed by precipitation of the crude maleate salt with ether. Then an aliquot of the maleate salt was treated with aqueous HCl, followed by precipitation of the hydrochloride salt with acetone. Finally, the maleic acid treatment described above was repeated to give a white solid as the hemimaleate hydrochloride salt (0.34 g; 7.8%): melting point >300°C (dec.); 1H NMR δ 12.23 (s, 1 H), 10.8 (br s, 1 H), 8.60 (s, 2 H), 8.4 (v br s, 2 H), 7.81 (d, J = 8.8 Hz, 2 H), 7.70 (d, J = 8.8 Hz, 2 H), 6.14 (s, 2 H); FAB-MS m/z 284 (MH+ of free base); HPLC tR 9.23 min (93.5 area %). Calculated for C14H13N5O2¥HCl¥C4H4O4: C, 49.61; H, 4.16; N, 16.07; Cl, 8.13. Found: C, 49.36; H, 4.19; N, 15.91; Cl, 8.02.
Synthesis of 2,7-bis(N-isopropylamidino)carbazole dihydrochloride (compound 9).
2,7-Dicyanocarbazole (1.74 g, 8.03 mmol) and anhydrous ethanol (5.0 ml, 86 mmol) were added to 1,4-dioxane (100 ml) saturated with hydrogen chloride. After 4 days the crude diimidate (2.93 g) was filtered off and suspended in dry ethanol (20 ml). Freshly distilled isopropylamine (10 ml, 120 mmol) was added, and the mixture was stirred overnight at room temperature. The excess amine was distilled off, the reaction mixture was diluted with ether, and the crude product was filtered off. A filtered (Celite) solution of the crude product in hot water was treated with NaOH solution to precipitate out the free base. The base was converted back to the dihydrochloride salt by treatment with aqueous HCl. Recrystallization from water-isopropyl alcohol gave a pale yellow powder (1.58 g; 48.3%): melting point >330°C; 1H NMR (400 MHz, DMSO-d6) δ 12.54 (s, 1 H), 9.71 (d, J = 7.9 Hz, 2 H), 9.57 (s, 2 H), 9.21 (s, 2 H), 8.48 (d, J = 8.0 Hz, 2 H), 7.98 (s, 2 H), 7.57 (dd, J = 8.0 and 1.6 Hz, 2 H), 4.15 (m, 2 H), 1.32 (d, J = 6.3 Hz, 12 H); FAB-MS m/z 336 (MH+ of free base); HPLC tR 11.36 min (96.7 area %). Calculated C20H25N5⩵2HCl⩵0.4H2O: C, 57.80; H, 6.74; N, 16.85; Cl, 17.06. Found: C, 57.75; H, 6.67; N, 16.69; Cl, 17.08.
2-Acetyloxy-N-[(5-nitro-2-thiazolyl)] benzamide (NTZ).
The powder formulation of NTZ was obtained from Romark Laboratories, Tampa, Fla. The percentage of active NTZ in the powder formulation was 70.8%. The injectable formulation of NTZ was obtained from Blue Ridge Pharmaceuticals, Greensboro, N.C. The percentage of active NTZ in the injectable formulation was 20.0%.
O-2-Amino-2-deoxy-α-d-gluco-pyranosyl-(1→4)-O-[O-2,6-diamino-2,6-dideoxy- β-l-idopyranosyl-(1→3)-β-d-ribofuranosyl-(1→5)]-2-deoxy-d-streptamine (paromomycin).
Paromomycin was obtained from Sigma Chemical Co., St. Louis, Mo. The percentage of active paromomycin in the formulation was ≥99.9%. The structures of all test compounds are shown in Tables 1 and 2.
TABLE 1.
Efficacies of dicationic carbazoles against C. parvum in the neonatal mouse model
| Compound no. | Compound name | Chemical structure | Dose (mg/kg of body wt)a | Oocyst level (% of control)b (mean ± SE) |
|---|---|---|---|---|
| 1 | 2,7-Diamidinocarbazole dihydrochloride | 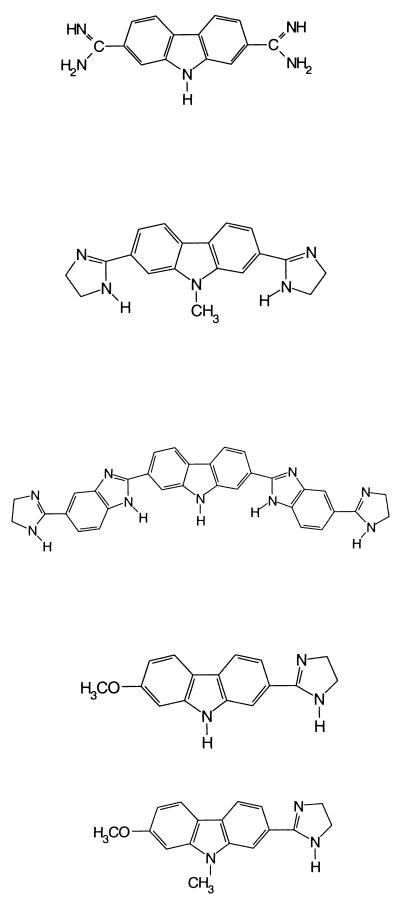 |
19.0c | 0.8 ± 0.3d |
| 2 | 2,7-Bis(2-imidazolinyl)-9-methylcarbazole dihydrochloride | 20.0 | 50.3 ± 3.2d | |
| 3 | 2,7-Bis[5-(2-imidazolinyl)-2-benzimidizoyl] carbazole tetrahydrochloride | 20.0 | 63.5 ± 7.0 | |
| 4 | 2-(2-Imidazolinyl)-7-methoxycarbazole hydrochloride | 17.0 | 102.9 ± 9.9 | |
| 5 | 2-(2-Imidazolinyl)-7-methoxy-9-methycarbazole hydrochloride | 17.0 | 129.0 ± 12.8 | |
| 6 | 3,6-Bis(N-hydroxyamidine)carbazole hemimaleate hydrochloride | 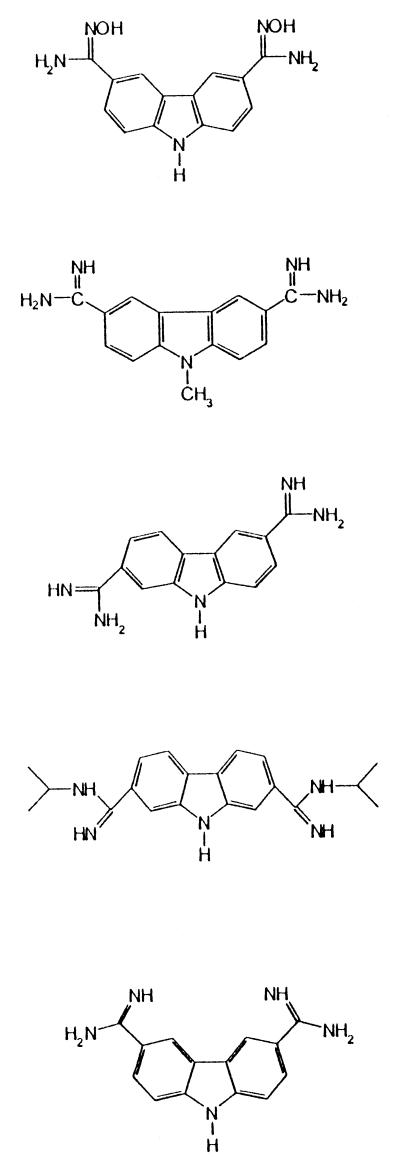 |
17.0 | 5.6 ± 0.9d |
| 7 | 3,6-Diamidino-9-methylcarbazole dihydrochloride | 17.0 | 3.0 ± 0.4d | |
| 8 | 2,6-Diamidinocarbazole dihydrochloride | 17.0 | 6.9 ± 1.1d | |
| 9 | 2,7-Bis(N-isopropylamidino)carbazole dihydrochloride | 8.5 | 30.5 ± 5.0d | |
| 17.0 | 9.1 ± 1.6d | |||
| 10 | 3,6-Diamidinocarbazole dihydrochloride | 9.5 | 28.7 ± 4.6d | |
| 19.0 | 2.2 ± 0.5d | |||
| 11 | 3,6-Bis(N-isopropylamidino)carbazole dihydrochloride | 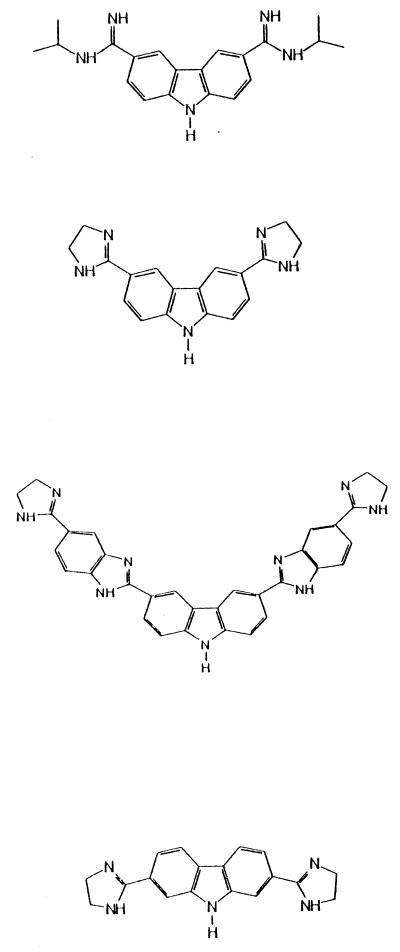 |
20.0 | 94.6 ± 11.3 |
| 12 | 3,6-Bis(2-imidazolinyl)carbazole dihydrochloride | 20.0 | 34.8 ± 7.4d | |
| 13 | 3,6-Bis[5-(2-imidazolinyl)-2 benzimidazoyl] carbazole tetrahydrochloride | 16.4 | 125.5 ± 13.3 | |
| 14 | 2,7-Bis (2-imidazolinyl)carbazole dihydrochloride | 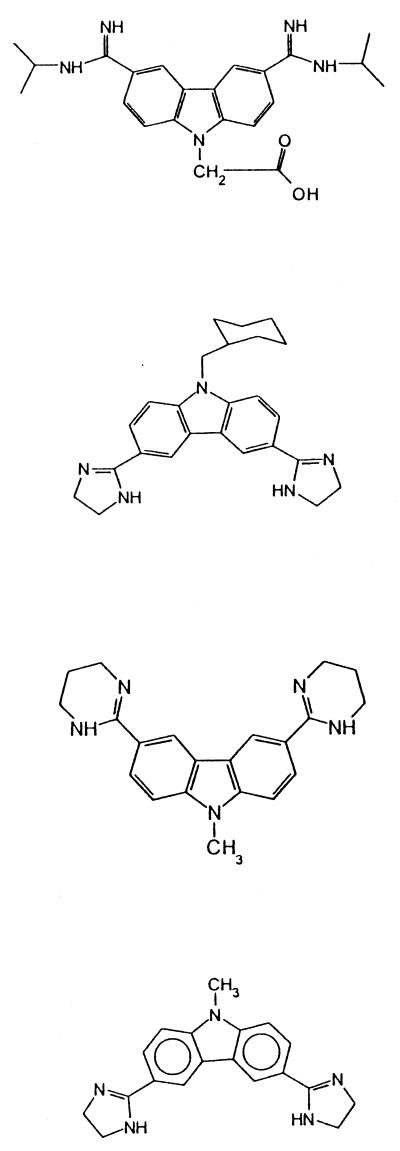 |
14.3 | 54.4 ± 10.7d |
| 15 | 3,6-Bis(N-isopropyl-amidino)-9-carboxy-methylcarbazole dihydrochloride | 17.0 | 55.3 ± 6.2 | |
| 16 | 3,6-Bis(2-imidazolinyl)-9-cyclohexylmethyl carbazole dihydrochloride | 17.0 | 108.5 ± 9.2 | |
| 17 | 3,6-Bis[2-(1,4,5,6-tetrahydropyrimidinyl)]-9-methylcarbazole dihydrochloride | 17.0 | 90.8 ± 10.5 | |
| 18 | 3,6-Bis(2-imidazolinyl)-9-methylcarbazole dihydrochloride | 17.0 | 36.9 ± 4.3d |
Mice were treated at a constant dose rate daily for 6 days.
Mean numbers of oocysts in treated mice are expressed as percentages of the mean number of oocysts recovered from control mice (taken as 100%).
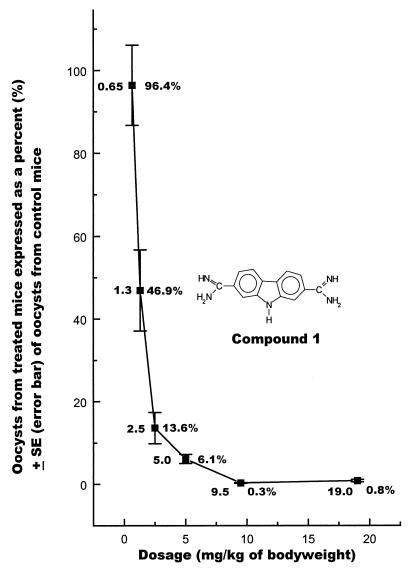
See Fig. 1 for dosage titration of compound 1.
Treated mice and control mice were significantly different (P ≤ 0.05).
TABLE 2.
Efficacies of NTZ and paromomycin against C. parvum in the neonatal mouse model
| Compound | Compound name | Chemical structure | Dose (mg/kg of body wt)a | Oocyst level (% of control)b (mean ± SE) |
|---|---|---|---|---|
| NTZ | 2-Acetyloxy-N-[(5-nitro-2-thiazolyl)] benzamide | 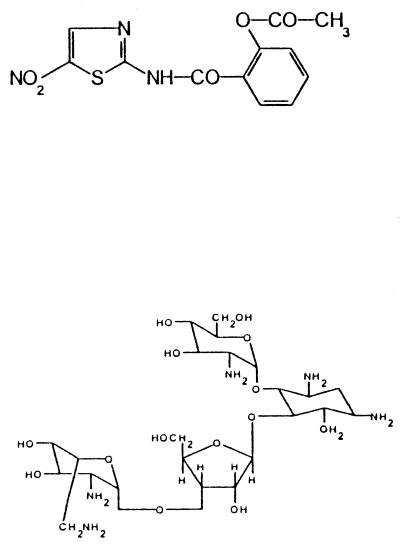 |
100.0c | 42.3 ± 4.6d |
| 100.0e | 26.0 ± 4.5d | |||
| 150.0ef | 4.3 ± 1.0d | |||
| Paromomycin | O-2-Amino-2-deoxy-α-d-gluco-pyranosyl-(1→4)-O-[O-2,6-diamino-2,6-dideoxy-β-l-idopyranosyl-(1→3)-β-d-ribofuranosyl-(1→5)]-2-deoxy-d-streptamine | 50.0 | 1.2 ± 0.6d |
Mice were treated at a constant dose rate daily for 6 days.
Mean numbers of oocysts in treated mice are expressed as percentages of the mean number of oocysts recovered from control mice (taken as 100%).
Oral formulation.
Treated mice and control mice were significantly different (P ≤ 0.05).
Injectable formulation administered orally.
This dosage appeared moderately toxic; 14 of 25 mice survived the treatment period.
Neonatal mouse model.
ICR Swiss mice were purchased from a commercial supplier and delivered to Auburn University as females with 2-day-old litters. Neonatal mice were individually weighed and cross-fostered to females to yield groups of 8 to 10 litters (8 to 12 suckling mice) with approximately equal mean body weights. Females (with litters) were individually housed in plastic box cages containing ground corncobs as bedding and were provided a commercial laboratory block ration and water ad libitum. All test compounds were administered to neonatal mice in each of two litters (8 to 12 mice/litter × 2 litters = 16 to 24 mice per compound). Two litters of mice received the diluent in which drugs were solubilized or dispersed and thus served as nontreated controls (see below). Doses of carbazole compounds were determined initially by solubility characteristics of the compounds. Subsequently, however, we used data from related compounds to establish a screening dose of 17.0 mg/kg of body weight. Multiple dosages were evaluated for compounds 1, 9, and 10 (see Table 1 and Fig. 1). Paromomycin was evaluated at 50 mg/kg. The initial dosage of 100 mg/kg for NTZ was determined by communication with the AIDS Branch of the National Institutes of Health and the supplier of the drug. Subsequent dosages were based on preliminary efficacies obtained in the model.
FIG. 1.
Dosage titration of compound 1 against C. parvum in HsD/ICR Swiss mice.
On the morning of day 0, prior to the start of treatments, mice in each litter were inoculated orally by gavage with 1 × 105 or 2 × 105 oocysts of the AUCp1 isolate of C. parvum (2). Four hours after infection with C. parvum oocysts, each mouse received the first treatment with test compound. Treatments were repeated once daily for 5 additional days (six total treatments). Drugs were solubilized or suspended in deionized water or in a solution of 1% DMSO in deionized water. The concentration of compound in the diluent was calculated to yield the screening dosage when 10 μl of solution or suspension was administered to a 3-g mouse. We could then increase or decrease the dosage by increasing or decreasing the volume of drug administered. The purpose of these procedures was to maintain a constant dose rate throughout the study. Control mice received diluent that did not contain drug at a volumetric rate identical to that for treated animals.
All mice were euthanized by cervical dislocation on day 6. Necropsies, recovery of oocysts, and efficacy evaluations were conducted as previously described (2).
Statistical analyses.
Mean numbers of oocysts recovered from treated and control mice were compared by the nonparametric Wilcoxon Mann-Whitney test. In each instance, a probability level of P < 0.05 was considered significant.
RESULTS AND DISCUSSION
Compound numbers, names, screening doses, and efficacies are presented in Tables 1 and 2. The mean raw hemacytometer oocyst count in diluent-treated mice was 133.1. Counts ranged from 25.7 to 241.5 (standard error [SE] = 12.7). Demonstrable efficacy was observed for several carbazole compounds, based on significant reductions in the numbers of oocysts recovered from treated mice versus control mice. Compounds 1, 7, and 10 (19.0 mg/kg) reduced oocyst passage in treated mice to less than 5% of that in control mice. Treatment with compounds 6, 8, and 9 (17.0 mg/kg) resulted in reductions of oocyst output to less than 10% of that in controls. Although they were not comparable in efficacy to the compounds mentioned above, treatment with carbazoles 2, 12, 14, and 18 resulted in statistically significant reductions in oocyst output in treated versus control mice (Table 1). Treatment with other compounds (i.e., compounds 3, 11, 15, and 17) resulted in numerical but not statistically significant reductions in oocyst counts. Compound 1 retained an efficacy resulting in reduction of oocyst counts to 6% of those in controls when the dose was reduced to 5 mg/kg. Further reductions in the dose resulted in considerable reductions in anticryposporidial activity (Fig. 1). Likewise, the efficacies of compounds 9 and 10 were reduced considerably when the dose was lowered to one-half the screening dose rate. Treatment with some carbazole compounds (e.g., compounds 4, 5, 13, and 16) resulted in no demonstrable numerical reductions in recovered oocysts. Compound 1 is currently under evaluation in other animal models. Paromomycin yielded excellent activity (<2% of the oocyst output in controls) at the screening dose of 50 mg/kg. Treatment with NTZ as a powder or as an injectable formulation administered orally yielded moderate efficacy (42 or 26% of the oocyst output in controls, respectively [Table 2]). Oral administration of the injectable formulation at a dose of 150 mg/kg resulted in a much higher efficacy (<5% of the oocyst output in controls [Table 2]).
We have previously reported the efficacies of pentamidine analogs and diaryl furans against C. parvum (2, 4). However, this is our first report on the efficacies of dicationic carbazole compounds against C. parvum. Not only did we demonstrate substantial efficacies for certain carbazoles, but we also achieved efficacies with certain carbazoles comparable to or exceeding those of paromomycin and NTZ, in both cases at considerably lower doses. The survival and consistent weight gains of treated mice support the safety of the carbazole compounds at the dosages tested.
Compound 1 appears to be the most potent of the effective anticryptosporidial carbazole compounds. Doses of 5.0 mg/kg or more resulted in reductions in oocyst recovery to 6.1% of that in controls or less. Interestingly, increased efficacy was not observed when the dose was increased from 9.5 to 19.0 mg/kg. In other evaluations (4), we have also identified a diaryl furan with excellent activity against C. parvum. In that report, doses of 17.0 and 8.5 mg/kg had efficacies resulting in oocyst reduction to 5.1 and 6.4% of levels in nontreated controls, respectively. It appears that the potency of compound 1 in the present study exceeds that of the diaryl furan.
Our results for paromomycin corroborate efficacies observed against C. parvum in other animal models and in cell cultures (11–13, 17). The effective dose reported here (50 mg/kg) was less than the effective doses reported in other murine models. This could be because most other murine models use chemical immunosuppression to induce and maintain C. parvum infections. Higher doses may be required in such models (e.g., rats) or against established infections. The regimen reported here is a prophylactic regimen because compounds are administered approximately 4 h after infection and before establishment of substantial numbers of developmental stages in the intestinal tract.
This is apparently the first published report of the evaluation of NTZ against C. parvum in a mouse model. Our data are based on the use of two formulations. The powder formulation was moderately effective against C. parvum when tested at 100 mg/kg. These results were probably due to the poor solubility of the drug in an aqueous diluent. Poor solubility could result in clumping in the intestinal lumen and reduced availability of the test compound. This explanation is supported by our results following oral administration of an injectable formulation of NTZ, because improved efficacy (26 versus 42% of the oocyst output in the controls [Table 2]) was obtained with this formulation by using the same dose rate. This is further supported by substantial improvement in efficacy (4.3% of the oocyst output in the controls) when the dose was increased to 150 mg/kg. The observed lack of toxicity of the dicationic carbazoles and paromomycin in this study is likely the result of poor absorption following oral administration. This was initially perceived as a limitation in the use of the carbazoles as antimicrobial agents. However, in the case of intestinal cryptosporidiosis, absorption following oral administration is probably not necessary for activity against cryptosporidial stages in the intestinal tract. Our results support this presumption.
The anticryptosporidial mechanism of action of the dicationic carbazoles is not known with certainty. However, we do know that many of the dicationic-substituted aromatic molecules bind to the minor groove of DNA. We presume that DNA binding of these molecules plays a substantial role in many cases in their antimicrobial activity by inhibiting either DNA-associated enzymes such as topoisomerases (1) or endo- and exonucleases (14) or other processes, such as DNA replication, recombination, and repair.
Although numerous drugs have demonstrated activity against Cryptosporidium, none has received regulatory approval for treatment of either human or animal cryptosporidiosis. In the present report, we demonstrate the efficacies of a new class of dicationic molecules and confirm the efficacies of NTZ and paromomycin against experimental C. parvum infections in our neonatal mouse model. Results reported here indicate that the safety and efficacy of compound 1 either is comparable to or exceeds the safety and efficacies of other anticryptosporidial agents that have been evaluated in other models. Our current efforts focus on further evaluation of compound 1. Although efficacies obtained with certain compounds in our model appear to correlate with results obtained in humans (e.g., with paromomycin), confirmation of the efficacies of compound 1 and other dicationic carbazoles against cryptosporidiosis in humans awaits the results of efficacy studies in humans.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health/NIAID research grant AI33363 and USDA Formula Funds Project 846.
REFERENCES
- 1.Bell C, Dykstra C, Naiman N, Cory M, Fairley T, Tidwell R. Structure-activity studies of dicationically substituted bis-benzimidazoles against Giardia lamblia: correlation of antigiardial activity with DNA binding affinity and giardial topoisomerase II inhibition. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blagburn B, Sundermann C, Lindsay D, Hall J, Tidwell R. Inhibition of Cryptosporidium parvum in neonatal Hsd:(ICR)BR swiss mice by polyether ionophores and aromatic amidines. Antimicrob Agents Chemother. 1991;35:1520–1523. doi: 10.1128/aac.35.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagburn B, Soave R. Prophylaxis and chemotherapy. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 111–128. [Google Scholar]
- 4.Blagburn, B., T. Land, K. Drain, P. Moore, D. Lindsay, D. Boykin, and R. Tidwell. Dicationic furans inhibit development of Cryptosporidium parvum in Hsd/ICR suckling Swiss mice. J. Parasitol., in press. [PubMed]
- 5.Boykin D, Kumar A, Spychala J, Zhou M, Lombardy R, Wilson W, Dykstra C, Jones S, Hall J, Tidwell R, Laughton C, Neidle C. Dicationic diarylfurans as anti-Pneumocystis carinii agents. J Med Chem. 1995;38:912–916. doi: 10.1021/jm00006a009. [DOI] [PubMed] [Google Scholar]
- 6.Casemore D, Wright S, Coop R. Cryptosporidiosis—human and animal epidemiology. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fl. 1997. pp. 65–92. p. 65–92. [Google Scholar]
- 7.Donkor I, Tidwell R, Jones S. Pentamidine congeners. 2. Aromatic diamidines and diimidazolines as potential anti-Pneumocystis carinii agents. J Med Chem. 1994;37:4554–4557. doi: 10.1021/jm00052a014. [DOI] [PubMed] [Google Scholar]
- 8.Doumbo O, Rossignol J, Pichard E, Traore H, Dembele T, Diakite M, Traore F, Diallo D. Nitazoxanide in the treatment of cryptosporidial diarrhea and other intestinal parasitic infections associated with acquired immunodeficiency syndrome in tropical Africa. Am J Trop Med Hyg. 1997;56:637–639. doi: 10.4269/ajtmh.1997.56.637. [DOI] [PubMed] [Google Scholar]
- 9.Dubovi E, Geratz J, Tidwell R. Inhibition of respiratory syncytial virus-host cell interaction by mono- and diamidines. Antimicrob Agents Chemother. 1981;19:649–656. doi: 10.1128/aac.19.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayer R, Speer C, Dubey J. General biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 1–41. [Google Scholar]
- 11.Fayer R, Ellis W. Glycoside antibiotics alone and combined with tetracyclines for prophylaxis of experimental cryptosporidiosis in neonatal BALB/c mice. J Parasitol. 1993;79:553–558. [PubMed] [Google Scholar]
- 12.Fayer R, Ellis W. Paromomycin is effective as prophylaxis for cryptosporidiosis in dairy calves. J Parasitol. 1993;78:771–774. [PubMed] [Google Scholar]
- 13.Healey M, Yang C, Rasmussen S, Jackson M, Du C. Therapeutic efficacy of paromomycin in immunosuppressed adult mice infected with Cryptosporidium parvum. J Parasitol. 1995;81:114–116. [PubMed] [Google Scholar]
- 14.Hildebrandt E, Boykin D, Kumar A, Tidwell R, Dykstra C. Identification and characterization of an endo/exonuclease in P. carinii that is inhibited by dicationic diarylfurans with efficacy against P. carinii. J Eukaryot Microbiol. 1998;45:112–121. doi: 10.1111/j.1550-7408.1998.tb05078.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones S, Hall J, Allen M, Morrison S, Ohemeng K, Reddy V, Geratz J, Tidwell R. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990;34:1026–1030. doi: 10.1128/aac.34.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsay, D., B. Blagburn, and S. Upton. Animal models of Cryptosporidium gastrointestinal infection. In O. Zak and M. Sande (ed.), Handbook of animal models of infection, in press. Academic Press, New York, N.Y.
- 17.Marshall R, Flannigan T. Paromomycin inhibits Cryptosporidium infection of a human enterocyte cell line. J Infect Dis. 1992;165:772–774. doi: 10.1093/infdis/165.4.772. [DOI] [PubMed] [Google Scholar]
- 18.Murphy J, Friedmann J. Pre-clinical toxicology of nitazoxanide—a new antiparasitic compound. J Appl Toxicol. 1985;5:49–52. doi: 10.1002/jat.2550050202. [DOI] [PubMed] [Google Scholar]
- 19.O’Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 20.Patrick D, Boykin D, Wilson W, Bender B, Hall J, Dykstra C, Ohemeng K, Tidwell R. Anti-Pneumocystis carinii pneumonia activity of dicationic carbazoles. Eur J Med Chem. 1997;32:781–793. [Google Scholar]
- 21.Rose J, Lisle J, Lechevallier M. Waterborne cryptosporidiosis: incidence, outbreaks, and treatment strategies. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 93–109. [Google Scholar]
- 22.Tanious F, Ding D, Patrick D, Tidwell R, Wilson W. A new type of DNA minor-groove complex: carbazole dication-DNA interactions, activity. Biochemistry. 1997;36:15315–15325. doi: 10.1021/bi971599r. [DOI] [PubMed] [Google Scholar]
- 23.Tidwell R, Kilgore S, Geratz J, Ohemeng K, Cory M, Hall J. Analogues of 1,5-bis (4-amidophenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J Med Chem. 1990;33:1252–1257. doi: 10.1021/jm00166a026. [DOI] [PubMed] [Google Scholar]
- 24.Tidwell R, Jones S, Geratz J, Ohemeng K, Bell C, Berger B, Hall J. Development of pentamidine analogues as new agents for the treatment of Pneumocystis carinii pneumonia. Ann N Y Acad Sci. 1990;616:421–441. doi: 10.1111/j.1749-6632.1990.tb17862.x. [DOI] [PubMed] [Google Scholar]
- 25.Vonderfect S, Miskuff R, BiWee S, Sato S, Tidwell R, Geratz J, Yolken R. Protease inhibitors suppress the in vitro and in vivo replication of rotavirus. J Clin Investig. 1988;82:2011–2016. doi: 10.1172/JCI113821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson W, Ratmeyer L, Zhao M, Strekowski L, Boykin D. The search for structure-specific nucleic acid-interactive drugs. Effects of compound structure on RNA versus DNA interaction strength. Biochemistry. 1993;32:4098–4104. doi: 10.1021/bi00066a035. [DOI] [PubMed] [Google Scholar]