Abstract
LY303366 is a novel semisynthetic derivative of echinocandin B and a potent inhibitor of fungal (1,3)-β-d-glucan synthase. The antifungal efficacy and safety of LY303366 were investigated in treatment and prophylaxis of primary pulmonary aspergillosis due to Aspergillus fumigatus in persistently neutropenic rabbits. Treatment study groups were either not treated (controls) or treated with amphotericin B (AmB) at 1 mg/kg of body weight per day or with LY303366 at 1, 5, 10, and 20 mg/kg/day. In rabbits treated with LY303366, there was a significant improvement in survival and a reduction in organism-mediated pulmonary injury measured by the number of infarcts, total lung weight, and ultrafast computerized tomography scan pulmonary lesion score. Rabbits receiving prophylactic LY303366 also demonstrated significant improvement in survival and reduction in organism-mediated pulmonary injury. AmB and LY303366 had comparable therapeutic efficacies by all parameters with the exception of reduction in tissue burden of A. fumigatus, where AmB was superior to LY303366. LY303366 demonstrated a dose-dependent effect on hyphal injury with progressive truncation, swelling, and vacuolization. LY303366 administered in single doses of 1, 5, 10, and 20 mg/kg demonstrated dose-proportional increases in the maximum concentration of drug in plasma and the area under the concentration-time curve from 0 to 72 h with no changes in plasma drug clearance. The 1-mg/kg dosage maintained plasma drug levels above the MIC for 18 h, and dosages of ≥5 mg/kg maintained plasma drug levels above the MIC for the entire 24-h dosing interval. There was no significant elevation of the concentrations of hepatic transaminases or creatinine in serum in LY303366-treated rabbits. In summary, LY303366 improved survival and decreased pulmonary injury with no apparent toxicity in the treatment and prevention of invasive pulmonary aspergillosis in persistently neutropenic rabbits.
The echinocandins are a new class of semisynthetic lipopeptide antifungal compounds, with potent and relatively broad-spectrum antifungal activity. They act by inhibiting the synthesis of (1,3)-β-d-glucan, an integral component of the fungal cell wall, resulting in cell wall damage and ultimately cell death (13, 15). The novel mode of action and potent antifungal activity in vitro have led to the design of several new compounds for potential clinical development.
Cilofungin was the first echinocandin B derivative developed for clinical trials. This compound had excellent in vitro activity against Candida spp. and was highly effective in animal models of disseminated candidiasis (12–15, 28). The compound also showed activity in a murine model of disseminated aspergillosis (6, 29). However, clinical development of cilofungin was discontinued when toxicity due to the vehicle was observed.
In recent years, a new generation of echinocandins has emerged. LY303366 (LY), a terphenyl-substituted echinocandin B, is the lead compound of this class for clinical investigation (4, 5, 10). Current in vitro studies demonstrate potent and non-cross-resistant antifungal activity against Candida albicans, Candida tropicalis, Candida glabrata, and other Candida species (7, 22, 30). The drug has also been shown to be active against Aspergillus spp. in vitro (20). Little is known, however, about the in vivo efficacy of LY against Aspergillus infections. Zeckner et al. (29) demonstrated improved survival and decreased tissue burden of Aspergillus fumigatus.
Invasive pulmonary aspergillosis is an important cause of morbidity and mortality in patients with persistent neutropenia (18, 24). The in vitro activity and preliminary in vivo antifungal effects in nonneutropenic mice suggest that LY may be an effective agent against this disease (16, 23, 29). Therefore, we investigated the antifungal efficacy and safety of LY in treatment and prophylaxis of primary pulmonary aspergillosis in persistently neutropenic rabbits.
MATERIALS AND METHODS
Animals.
Female New Zealand White rabbits (Hazleton Inc., Deutschland, Pa.), each weighing 2.0 to 3.5 kg at the time of inoculation, were used in all experiments. Rabbits were individually housed and maintained according to the National Institutes of Health (NIH) guidelines for animal care and American Association for Accreditation of Laboratory Animal Care criteria (3). A total of 106 rabbits were used for all experiments. Vascular access was established in each rabbit by the surgical placement of a silastic tunneled central venous catheter (25).
Organism and inoculation.
Pulmonary aspergillosis was established, as previously described (9). Briefly, A. fumigatus (NIH isolate 4215) obtained from a fatal case of pulmonary aspergillosis was used in all the experiments. The MICs by published methods (8), for the organism used in these experiments were 0.125 μg/ml for LY and 2.0 μg/ml for amphotericin B (AmB) deoxycholate. While the term minimal effective concentration (MEC) has been utilized for echinocandins, the MIC, as previously reported by Pfaller et al. (20), is used here. The inoculum of A. fumigatus was prepared from a frozen isolate that was subcultured onto potato dextrose agar slants; these slants were incubated for 24 h at 37°C and then kept at room temperature for 5 days. Conidia were harvested under a laminar airflow hood with a solution of 0.025% Tween 20 (Fisher Scientific, Fair Lawn, N.J.) in normal saline, transferred to a 50-ml conical tube, washed, and counted with a hemacytometer. The concentration was adjusted in order to give each rabbit a predetermined inoculum of 108 conidia of A. fumigatus in a volume of 250 to 350 μl. The concentrations of the inocula were confirmed by serial dilutions, and the aliquots were cultured in Sabouraud glucose agar (SGA) plates.
Inoculation was performed on day 2 of the experiments on rabbits under general anesthesia. Each rabbit was given 0.8 to 1.0 ml of a 2:1 mixture (vol/vol) of ketamine (100 mg/ml) (Fort Dodge Labs, Fort Dodge, Iowa) and xylazine (20 mg/ml) (Mobay Corp., Shawnee, Kans.) intravenously. Once satisfactory anesthesia was obtained, a Flagg O straight-blade laryngoscope (Welch-Allyn, Skaneateles Falls, N.Y.) was inserted in the oral cavity until the vocal cords were clearly visualized. The A. fumigatus inoculum was then administered intratracheally with a tuberculin syringe attached to a 5 1/4-inch Teflon catheter (Becton Dickinson, Sandy, Utah).
Immunosuppression, induction, and maintenance of neutropenia.
Cytarabine (Ara-C) (Cytosar-U; Upjohn, Kalamazoo, Mich.) was initiated 1 day before the endotracheal inoculation of the animals. Profound and persistent granulocytopenia (<100/μl) was achieved by an initial course of 525 mg of Ara-C per m2 for 5 consecutive days. A maintenance dose of 484 mg of Ara-C per m2 was administered for 4 additional days on days 8, 9, 13, and 14 of the experiment. Concomitant thrombocytopenia ranged from 30,000 to 50,000/μl. Methylprednisolone (Abbott, North Chicago, Ill.) at 5 mg/kg of body weight was administered on days 1 and 2 of the experiment to inhibit macrophage activity against conidia and to facilitate establishment of infection. Ceftazidime (Glaxo, Inc., Research Triangle Park, N.C.) (75 mg/kg given intravenously twice daily), gentamicin (Elkins-Sinn, Inc., Cherry Hill, N.J.) (5 mg/kg given intravenously every other day), and vancomycin (Abbott Laboratories) (15 mg/kg given intravenously daily) were administered from day 4 of chemotherapy until study completion for prevention of opportunistic bacterial infections during neutropenia. In order to prevent antibiotic-associated diarrhea due to Clostridium spiriforme, all rabbits continuously received 50 mg of vancomycin per liter of drinking water. Leukocyte counts were monitored twice weekly with a Coulter counter (Coulter Corporation, Miami, Fla.). Absolute neutrophil counts were determined from the product of percent neutrophils and total leukocyte count.
Antifungal compounds and treatment groups.
Rabbits were initially treated with LY (1, 5, 10, or 20 mg/kg/day) or AmB (1 mg/kg/day) for established invasive pulmonary aspergillosis. A third group of rabbits received LY as prophylaxis against invasive pulmonary aspergillosis.
Treatment regimens.
Rabbits were assigned to receive either LY or AmB or no treatment. LY was provided by Eli Lilly and Company (Indianapolis, Ind.) as a 10-mg/ml solution for parenteral administration. LY was administered intravenously at dosages of 1 mg/kg/day (LY1), 5 mg/kg/day (LY5), 10 mg/kg/day (LY10), and 20 mg/kg/day (LY20). LY at dosages of 5, 10, and 20 mg/kg/day was administered directly in a concentration of 10 mg/ml. The dosage of 1 mg/kg was prepared by diluting the initial solution with sterile normal saline (Quality Biological, Inc., Gaithersburg, Md.) to a concentration of 2 mg/ml. LY was given as a slow intravenous bolus. Antifungal therapy was initiated on the next day following endotracheal inoculation. AmB (Squibb, Princeton, N.J.) was started at the same time as LY and administered in a dose of 1 mg/kg/day given intravenously slowly (0.1 ml every 10 s). LY and AmB therapy were continued throughout the course of the experiments for a maximum of 12 days in surviving rabbits.
Prophylactic regimen.
In order to study LY for prevention of aspergillosis in persistently neutropenic hosts, we also performed experiments in a rabbit model which was designed to investigate prophylaxis. The prophylaxis experiments used the same methods as described above with the following exceptions. LY was administered for 4 days before endotracheal inoculation. On the day of inoculation, LY was administered in the morning and the endotracheal inoculum was administered approximately 4 h later. LY was then continued for a maximum of 12 more days after inoculation. In order to simulate the low initial tissue burden of A. fumigatus in the setting of antifungal prophylaxis, the administered inoculum was 5 × 107, or 50%, respectively, of the inoculum size administered for definitive therapy. Based upon assessment of response in the therapeutic model, a single dosage regimen (10 mg/kg/day) was selected for prophylaxis. All other methods, including outcome variables, were identical for both treatment and prophylaxis experiments.
Outcome variables.
A panel of outcome variables was used; these variables included antifungal efficacy, survival, pulmonary infarct score, lung weight, microbiologic lung tissue clearance (in CFU per gram), computerized tomography (CT) scan score, and pathology. Pulmonary infarct score, lung weight, and CT scan score are measures of organism-mediated pulmonary injury.
Survival.
The survival time in days postinoculation was recorded for each rabbit. Surviving rabbits were euthanized by pentobarbital anesthesia on the 13th day postinoculation.
Pulmonary lesion scores.
The entire heart-lung block was carefully resected at autopsy. The heart was then dissected away from the lungs, leaving the tracheobronchial tree and lungs intact. The lungs were weighed and inspected by at least two observers who were blinded to the treatment group and recorded hemorrhagic infarct lesions (if any) in each individual lobe. Positive lobes were added together, and the mean value of all positive lobes was calculated for each treatment group. Hemorrhagic infarcts were dark red consolidated lesions that corresponded histologically to coagulative necrosis and intraalveolar hemorrhage.
BAL.
Bronchoalveolar lavage (BAL) was performed on each lung preparation by the instillation and subsequent withdrawal of 10 ml of sterile normal saline two times into the clamped trachea with a sterile 12-ml syringe. The lavage material was then centrifuged for 10 min at 1,500 × g. The supernatant was discarded, leaving the pellet, which was then resuspended in 1.5 ml of sterile normal saline. A 0.1-ml sample of this fluid and 0.1 ml of a dilution (10−1) of this fluid were cultured on 5% Sabouraud glucose agar.
Histopathology.
Pulmonary lesions were excised and fixed in 10% neutral buffered formalin. Paraffin-embedded tissue sections were stained with periodic acid-Schiff and Gomori methenamine silver stains. Tissues were microscopically examined for pulmonary injury and structural changes in Aspergillus hyphae.
Fungal cultures.
Lung tissue from each rabbit was sampled and cultured by excision of a representative region of the lung. Each fragment was weighed individually, placed in a sterile bag (Tekmar Corp., Cincinnati, Ohio), and homogenized with sterile saline for 15 s per tissue sample (Stomacher 80; Tekmar) (26). Lung homogenate dilutions (10−1 and 10−2) were prepared in sterile saline. Aliquots (100 μl) from homogenates and homogenate dilutions were plated onto SGA and incubated at 37°C for the first 24 h and then at room temperature for another 24 h. The CFU of A. fumigatus were counted and recorded for each lobe, and the CFU per gram were calculated. A finding of one colony of A. fumigatus was considered positive.
CT.
CT of the lungs was performed during all experiments in order to monitor the effects of antifungal treatment on infection-mediated tissue injury during the rabbit’s life. Briefly, rabbits were sedated with ketamine and xylazine and then placed prone, head first, on the scanning couch. CT was performed with the ultrafast electron beam CT scanner (model C-100XL; Imatron, Oyster Point, Calif.), as previously described (27). Ultrafast CT scans (UFCT) were performed by using the high-resolution, table-incremented, volume acquisition mode. Three-millimeter-thick slices were made every 4 s. A small scan circle and a 9-cm-diameter reconstruction circle with a matrix of 512 by 512 were used, which resulted in a pixel size of less than 1 mm. Scan parameters were 130 kV and 630 mA, and scan duration was 100 ms. In virtually all cases, 30 slices were sufficient to scan the entire thorax of the rabbit. Images were photographed using lung windows with a level of −600 HU and a width of 1,800 HU. Each lung was divided into three lobes (upper, middle, and lower), and each lobe was assessed to determine a pulmonary lesion score. The accessory lobe was called the left middle lobe. A mean CT pulmonary lesion score was established by evaluating the infiltrate in each lobe. The pulmonary lesion score in each lobe was initially zero. Each lobe was evaluated and scored independently. A score of +0.5, +1, 0, −1, or −0.5 was assigned to the previous score, if the lobe demonstrated worsening (+1 or +0.5), stabilization (0), or improvement (−0.5 or −1). CT was performed on days 1, 2, 3, 4, 5, 6, 7, 8, and 10 of treatment. The mean CT pulmonary lesion score for that day represents the mean of all lobes of all rabbits in each group.
Toxicity studies.
A sample of blood was collected from each rabbit every other day, starting from the first day after inoculation, and continuing throughout treatment. Plasma samples were stored in Sarsted tubes (Sarsted Inc., Newton, N.C.) at −70°C until all samples were processed simultaneously. Chemical determinations of potassium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine, and total bilirubin (Analytics Inc., Gaithersburg, Md.) concentrations were performed on the next to the last sample drawn from each rabbit.
Pharmacokinetic experiments.
Serial plasma samples were drawn from four groups of three healthy New Zealand White rabbits each from 0.16 to 72 h after administration of a single dose of 1, 5, 10, and 20 mg/kg of LY as an intravenous bolus. Samples were stored at −70°C until assay. The concentrations of LY in plasma were determined after solid-phase extraction by a reverse-phase high-performance liquid chromatographic method.
External standards and quality-control samples were prepared by spiking pooled healthy rabbit serum samples (Gibco Laboratories, Grand Islands, N.Y.) with appropriate amounts of LY. Prior to extraction, 0.5 μg of LY306168, the internal standard, was added to 300 μl of the sample, external standard, or quality-control sample to serve as an internal control for accuracy and precision of the procedure. LY and LY306168 were separated from plasma by utilizing solvents based on acetonitrile–50 mM ammonium acetate (pH 4.0) C8 bonded phase extraction cartridges (Varian, Inc., Harbor City, Calif.), and a vacuum manifold (Supelco, Inc., Bellefonte, Pa.). The eluant was dried in an evaporator (Zymark Corp., Hopkinston, Mass.) under a steady stream of nitrogen at 40°C and reconstituted in 50:50 (vol/vol) methanol–50 mM ammonium acetate pH 4.0 for injection. The average recovery of the extraction procedure in rabbit plasma was >90% compared with unextracted reagent standard. The mobile phase consisted of acetonitrile–50 mM ammonium acetate (pH 4.0) (50:50 [vol/vol]) delivered at 0.5 ml/min. The injection volume was 75 μl. LY and LY306168 eluted at 6.3 and 4.1 min, respectively, using a C8 analytical column (5 μm) (Zorbaz RX-C8; Riceland Technologics, Chadds Ford, Pa.), maintained at 50°C in conjunction with a precolumn filter containing a 2-μm-diameter particle size filter insert. UV detection was utilized at 300 nm. Quantitation was performed using the peak height ratios of LY/LY306168 versus the LY concentrations of the external standard. Standard curves (20 to 5,120 ng/ml) were linear with r2 values of ≥0.999. The lower limit of quantitation was 20 ng/ml. Accuracies were within 0.4 to 3.2%, and intra- and interday variability (precision) ranged from 1.2 to 4.7%.
Standard model-independent techniques were used to calculate the area under the plasma drug concentration-time curve from 0 to 72 h (AUC0–72), apparent volume of distribution (V), total clearance (CL), elimination half-life (t1/2β), and peak plasma drug concentrations at 0 min (Cmax) (11). Trough levels at 24 h post dosing (Cmin24) were obtained directly from the concentration-versus-time profiles.
Statistical analysis.
Comparisons between groups were performed by analysis of variance (ANOVA) with Bonferroni’s correction for multiple comparisons or by the Mann-Whitney U test, as appropriate. Kaplan-Meier survival plots were analyzed by the Mantel-Haenzsel chi-square test. All P values were two sided, and a P value of < 0.05 was considered to be statistically significant. Values are expressed as means ± standard errors of the means (SEMs).
RESULTS
Antifungal therapy.
There was a significant improvement in survival in rabbits treated with LY1 and LY10 compared to that of untreated controls (P = 0.04 and P = 0.03, respectively); however, this was not true for rabbits treated with LY5 and LY20 (Table 1). Although survival was improved in the overall population of LY-treated rabbits, only nine animals survived the entire study. There was a notable decline in survival in LY20-treated rabbits, suggesting an upper threshold of toxicity.
TABLE 1.
Survival of persistently neutropenic rabbits with primary pulmonary aspergillosis treated with AmB or LY compared to untreated controls
| Treatment group | Survival (no. of days)
|
P valueb | |||
|---|---|---|---|---|---|
| Mean ± SEM | Median | Range | 95% CIa | ||
| Control (n = 16) | 7.12 ± 0.72 | 6.5 | 2–13 | 5.59–8.66 | |
| LY1 (n = 8) | 9.62 ± 1.05 | 10.0 | 4–13 | 7.14–12.11 | 0.04 |
| LY5 (n = 8) | 8.50 ± 0.33 | 8.5 | 7–10 | 7.73–9.27 | 0.09 |
| LY10 (n = 16) | 9.75 ± 0.75 | 10.5 | 5–13 | 8.15–11.35 | 0.03 |
| LY20 (n = 16) | 7.06 ± 0.45 | 6.0 | 5–10 | 6.10–8.02 | 0.90 |
| AmB (n = 8) | 8.38 ± 0.78 | 8.0 | 6–13 | 6.54–10.21 | 0.26 |
95% CI, 95% confidence interval.
P values comparing survival to the value for the control group by Mann-Whitney U test.
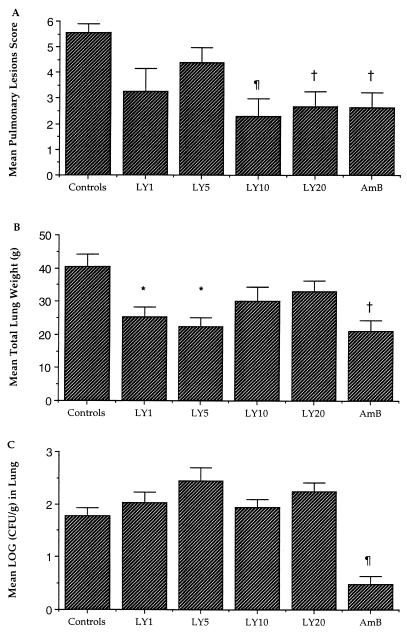
There was a reduction in organism-mediated tissue injury, as measured by the pulmonary infarct score and total lung weight, in rabbits treated with LY and AmB. Animals treated with LY10, LY20, and AmB had significant reductions in the mean pulmonary lesion score compared to those of untreated controls (P < 0.001, P < 0.01, and P < 0.01, respectively) (Fig. 1). The mean lung weights in rabbits treated with LY1, LY5, and AmB were significantly reduced in comparison to those of untreated controls (P < 0.05, P < 0.05, and P < 0.01, respectively); however, no differences in lung weight were noted between untreated animals and animals treated with LY10 and LY20.
FIG. 1.
Response of primary pulmonary aspergillosis in persistently neutropenic rabbits to antifungal therapy measured by mean pulmonary hemorrhage score (A), mean lung weight (B), and mean pulmonary tissue concentration of organism (C) in untreated controls (n = 16) and in rabbits treated with LY1 (n = 8), LY5 (n = 8), LY10 (n = 16), LY20 (n = 16), and AmB (1 mg/kg/day) (n = 8). Values are given as means ± SEMs. Values that are significantly different from the values for untreated controls by ANOVA test with Bonferroni’s correction for multiple comparisons are indicated by the following symbols: ∗, P ≤ 0.05; †, P ≤ 0.01; ¶, P ≤ 0.001.
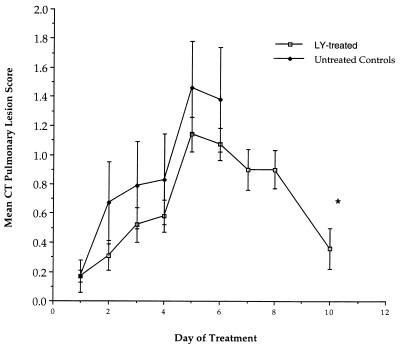
Consistent with the reduction in organism-mediated pulmonary injury, UFCT scan demonstrated resolution of pulmonary infiltrates in rabbits treated with LY (Fig. 2). During the first 5 days of treatment, there was an increase in pulmonary infiltrates. Following day 5 of treatment, there was a significant reduction of infiltrates, with a decline in the mean pulmonary lesion score from 1.14 ± 0.11 to 0.36 ± 0.13 on day 10 (P = 0.005). The mean CT pulmonary lesion score is also depicted for untreated controls; however, due to excess mortality in this group, scanning beyond 6 days was not feasible.
FIG. 2.
Response curve compiled from rabbits monitored with UFCT scan (27), including untreated controls (n = 16) and rabbits treated with LY (n = 39) (all dosage groups). Mortality of untreated controls prevents scanning beyond day 6. There was significant resolution of pulmonary infiltrates between days 5 and 10 in rabbits treated with LY. ∗, P = 0.005 (Mann-Whitney U test). Each point plots the mean and SEM for pulmonary lesion scores on that day. The asterisk indicates statistical significance (P = 0.005).
There was a significant quantitative reduction in A. fumigatus growth in lung tissue from rabbits treated with AmB in comparison to the untreated controls (P = 0.001) (Fig. 1). In contrast, no difference in A. fumigatus growth was observed between LY-treated animals and untreated controls. These results from lung tissue also were reflected in the quantitative cultures of BAL fluid. Rabbits in treatment groups LY1, LY5, LY10, and LY20 demonstrated no significant differences in quantitative cultures of BAL fluid (0.55 ± 0.37, 1.04 ± 0.31, 0.98 ± 0.23, and 0.65 ± 0.25 CFU/ml, respectively) in comparison to those of untreated controls (1.43 ± 0.31 CFU/ml). However, BAL fluid samples from AmB-treated rabbits showed no detectable organisms (P < 0.01 versus controls).
Antifungal prophylaxis.
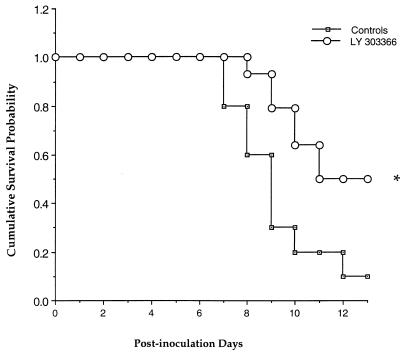
In order to investigate the potential utility of LY in prevention of pulmonary aspergillosis, a model of antifungal prophylaxis with a maximally tolerated dose of LY was subsequently studied. Rabbits pretreated with LY10 (n = 14) showed a significant improvement in survival in comparison to untreated control rabbits (Fig. 3 and Table 2). There also was a significant reduction in organism-mediated tissue injury in LY-treated rabbits, as measured by the mean pulmonary infarct score and mean lung weight, in comparison to untreated controls (Table 2). The pulmonary infarct lesions were significantly reduced in rabbits treated with LY, while lungs from untreated control rabbits consistently had more multilobar infarcts (P = 0.01). The mean lung weights of LY-treated rabbits were also significantly reduced in comparison to those of untreated controls (P = 0.02). However, there was a significant increase in A. fumigatus growth detected in lung tissue in the rabbits treated with LY in comparison to the untreated controls (P = 0.01) (Table 2).
FIG. 3.
Comparative survival of persistently neutropenic rabbits receiving prophylactic LY versus untreated controls. The asterisk indicates statistical significance compared to the value for controls (P = 0.01 by Mantel-Haenzsel chi-square test).
TABLE 2.
Effect of LY in an antifungal prophylaxis model of experimental pulmonary aspergillosis
| Treatment group | No. of pulmonary infarct lesions (mean ± SEM) | Total lung wt (g) (mean ± SEM) | Log CFU/g in lung (mean ± SEM) | Survival (days) (mean ± SEM) |
|---|---|---|---|---|
| Control (n = 10) | 5.00 ± 0.57 | 37.11 ± 3.77 | 1.03 ± 0.22 | 9.3 ± 0.68 |
| LY10 pretreated (n = 14) | 2.50 ± 0.52a | 23.60 ± 3.31b | 1.83 ± 0.16c | 11.4 ± 0.5d |
Significantly different from the value obtained for the control group (P = 0.01 by the Mann-Whitney U test).
Significantly different from the value obtained for the control group (P = 0.02 by the Mann-Whitney U test).
Significantly different from the value obtained for the control group (P = 0.01 by the Mann-Whitney U test).
Significantly different from the value obtained for the control group (P = 0.03 by the Mann-Whitney U test).
Effect on hyphal structure.
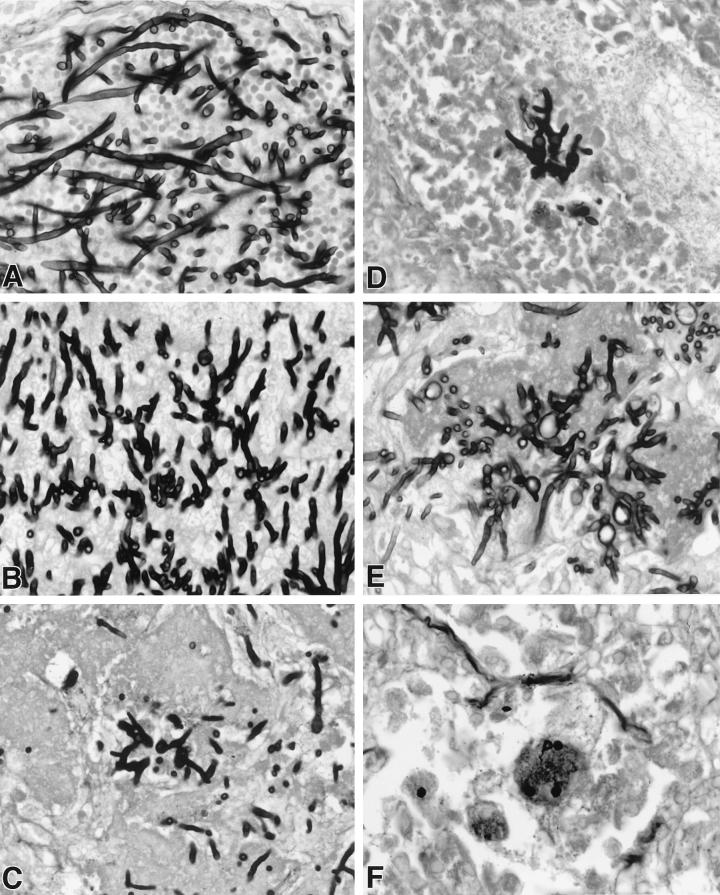
In order to further characterize the persistence of viable colony counts in rabbits treated with LY, the histopathological features were studied in the lungs of all treatment groups. There was dose-dependent damage of hyphal structures in lung tissue of LY303366-treated rabbits. Figure 4 demonstrates a progressive reduction in length and increasing swelling of hyphal elements. Each panel depicts a representative section of hyphal morphology in that dosage group. Organisms from untreated control rabbits (Fig. 4A) demonstrate the typical appearance of elongated branching septate hyphae. Organisms depicted in Fig. 4B (corresponding to LY1) reveal shortening of the hyphal elements. In addition to hyphal shortening, Fig. 4C, D, and E (corresponding to LY5, LY10, and LY20, respectively) also demonstrate progressive hyphal swelling. As depicted in Fig. 4E, hyphae from rabbits treated with 20 mg/kg/day (the maximum dosage administered) had the greatest level of apparent cell wall damage, as evidenced by vacuolization. By comparison, tissues from AmB-treated rabbits seldom revealed hyphal elements (Fig. 4F).
FIG. 4.
Dose-dependent effect on hyphal structure in lung tissue of LY-treated rabbits. Panels A to E demonstrate a progressive reduction in length and increasing swelling and vacuolization of hyphal elements in a representative section of hyphal morphology in the lungs from rabbits in each dosage group. The dosage groups are untreated controls (A), LY1 (B), LY5 (C), LY10 (D), LY20 (E), and AmB (1 mg/kg/day) (Gomori methenamine silver stain; original magnification, ×400).
Safety.
AmB-treated rabbits had a significant increase in the mean serum creatinine concentration compared to that of untreated controls (2.66 ± 0.39 versus 1.04 ± 0.03 mg/dl, respectively) (P < 0.001). By comparison, LY-treated rabbits had no change in the serum creatinine concentration in comparison to untreated controls. There were no differences in serum potassium, AST, ALT, and bilirubin concentrations for any of the treatment groups.
Pharmacokinetics of LY in plasma.
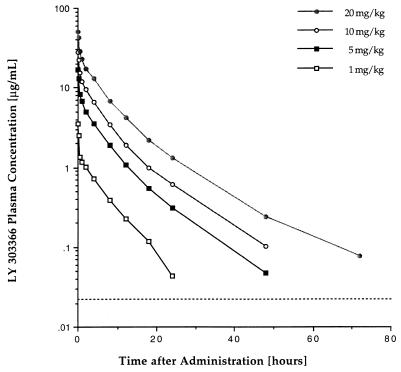
Plasma LY concentration-versus-time profiles after administration of single doses of 1, 5, 10, and 20 mg/kg to healthy rabbits are depicted in Fig. 5, and calculated pharmacokinetic parameters are listed in Table 3. Over the investigated dosage range, the drug demonstrated dose-proportional increases in Cmax and AUC0–72 and no changes in plasma drug clearance, which is consistent with dose-proportional, linear distribution in plasma. There was a significant increase in the apparent V with increasing dosage. By utilizing the MIC for the test organism and by extrapolating the concentration-versus-time profile of healthy rabbits to those used in the infection model, the time spent above the MIC during the experimental dosing interval of 24 h would account for 18 h at the 1-mg/kg dosage level and for 24 h for the remaining dosage levels of LY.
FIG. 5.
Plasma LY concentration-versus-time profiles after the administration of single doses of LY (1, 5, 10, and 20 mg/kg) to healthy rabbits. The broken line indicates the lower limit of quantitation (LLQ) of the analytical assay. At the 1-mg/kg dosage level, values were below LLQ at 48 and 72 h; at the 5- and 10-mg/kg dosage levels, values were below LLQ at 72 h.
TABLE 3.
Noncompartmental pharmacokinetics of LY in plasma after administration of single doses to healthy rabbitsa
| Drug dose (mg/kg) | Cmaxb (ng/ml) | Cmin24b (ng/ml) | AUC0–72c (ng/ml · h) | Vb (liter) | CL (liter/h) | t1/2βc (h) |
|---|---|---|---|---|---|---|
| 1 | 3,563 ± 644 | 44 ± 22 | 10,064 ± 786 | 2.3 ± 0.18 | 0.281 ± 0.02 | 5.8 ± 0.65 |
| 5 | 16,677 ± 2,007 | 312 ± 40 | 53,146 ± 4,430 | 3.1 ± 0.10 | 0.270 ± 0.02 | 8.2 ± 0.86 |
| 10 | 27,552 ± 2,719 | 610 ± 67 | 98,059 ± 3,286 | 3.7 ± 0.22 | 0.286 ± 0.00 | 8.9 ± 0.47 |
| 20 | 51,247 ± 2,100 | 1,294 ± 166 | 197,164 ± 8,091 | 5.0 ± 0.35 | 0.291 ± 0.01 | 11.9 ± 0.35 |
All values are given as means ± SEMs for three rabbits.
P < 0.001 by Kruskal-Wallis nonparametric ANOVA for comparison of all four values.
P < 0.001 by Kruskal-Wallis nonparametric ANOVA for comparison of all four values and P = 0.394 by ANOVA for departure from linearity.
P < 0.005 by Kruskal-Wallis nonparametric ANOVA for comparison of all four values.
DISCUSSION
This study demonstrated that LY administered therapeutically to persistently neutropenic rabbits with primary pulmonary aspergillosis improved survival and reduced organism-mediated pulmonary injury, as measured by pulmonary infarct score, lung weight score, and UFCT scan. These effects on survival and reduced pulmonary infarction were comparable to those of AmB. However, there was no improvement in the clearance of A. fumigatus from the lungs, as measured by the concentrations of drug in tissue samples from LY-treated rabbits. By comparison, organism clearance was significantly reduced in AmB-treated animals. Despite this apparent fungistatic effect, there was a dosage-dependent alteration in the cell wall morphology of Aspergillus hyphae in lung tissue. LY was not associated with any elevation in creatinine, potassium, bilirubin, AST, and ALT concentrations in serum. By comparison, the serum creatinine concentration was significantly increased in rabbits receiving AmB.
Pulmonary infarction and hemorrhagic necrosis due to angioinvasive hyphae are key elements in the pathogenesis of invasive aspergillosis in profoundly neutropenic hosts (2). This organism-mediated pulmonary injury may be measured experimentally by several variables: number of pulmonary infarct lesions, total lung weight, and UFCT scan score. This study reveals that LY interdicts the progression of organism-mediated pulmonary injury in comparison to untreated controls. This effect appears to be comparable to that of AmB; however, the antifungal mechanisms are notably different.
As an echinocandin, LY is a noncompetitive inhibitor of (1-3)-β-d-glucan synthase, which is a key enzyme in fungal cell wall biosynthesis. There was a striking dose-dependent antifungal effect in alteration of cell wall morphology. However, the individual damaged cellular units still appear to be viable, as measured by the lack of reduction in CFU per gram and the absence of a dose-response relationship in reducing pulmonary injury.
As defined by reducing viable CFU over time, AmB in vitro is considered mechanistically to be a fungicidal compound against various fungi. The elimination of histologically evident hyphae and reduction in tissue burden of A. fumigatus in AmB-treated rabbits in the experiments of the current study are also consistent with a fungicidal effect. By comparison, a fungistatic compound in vitro would inhibit the growth of an organism without reducing the number of viable CFU. While there is a clear dose-response relationship of increasing cell wall injury, these damaged cellular units remain viable at all dosage levels. These damaged cells do not appear to invade blood vessels. The persistence of damaged hyphae in tissue without a reduction in the quantitative culture results for LY-treated rabbits suggests that this echinocandin is not uniformly fungicidal or fungistatic against A. fumigatus.
When analyzing the properties of an antifungal compound, one must consider that the operational definitions of a fungicidal and fungistatic agent are critically dependent upon the in vitro or in vivo conditions in which it is studied. An in vivo assessment of fungicidal versus fungistatic activity is dependent upon several key variables, including the immune status of the host, drug delivery to the tissue, dosage, and exposure time.
Nevertheless, the antifungal effect of LY against A. fumigatus in vivo in neutropenic hosts appears to be sufficient to improve survival and to reduce or prevent organism-mediated pulmonary injury.
The antifungal effect of LY against A. fumigatus contrasts with its effect against C. albicans (7). Viable CFU of A. fumigatus persist in these neutropenic animals, while CFU of C. albicans are eradicated in our model of disseminated candidiasis (19). These differences may be due to different affinities of the echinocandin to (1,3)-β-d-glucan synthases from different genera. Alternatively, differences in cell wall structure, biosynthesis, and turnover rates may also be factors contributing to differences between C. albicans and A. fumigatus in response to LY.
The pharmacokinetic results in this study demonstrated that the levels of LY in plasma are maintained above the MIC for the A. fumigatus isolate used in this study in a dose-dependent manner for most of the dosing interval. The MIC obtained for the strain used in these experiments is similar to those for the strains reported by Pfaller et al. (21), where the MIC at which 90% of the strains are inhibited were 0.03 to 0.12 μg/ml. If one utilizes the MEC of 0.02 μg/ml, as reported by Zhanel and colleagues (30), then the time spent above the MEC would be throughout the dosing interval.
The trend toward increased lung weight at 10 and 20 mg/kg/day was not associated with increased pulmonary infarct scores. Instead, there was marked pulmonary edema in lungs at 20 mg/kg/day and to a lesser extent at 10 mg/kg/day. As pulmonary edema is not typically a component of invasive aspergillosis in profoundly neutropenic hosts, the effect may be drug-related pulmonary edema. Consistent with this possibility are the findings that survival was consistently improved in rabbits treated with 1, 5, and 10 mg/kg/day. However, survival decreased precipitously to that of untreated controls in rabbits treated with 20 mg/kg/day.
The efficacy and safety of lipid formulations of AmB have been investigated previously in this rabbit model (1, 9, 17). For example, persistently neutropenic rabbits treated with unilamellar liposomal AmB (AmBisome) at 1, 5, and 10 mg/kg/day demonstrated survival of 80, 100, and 80%, respectively. There was a significant dose-response relationship in the reduction of pulmonary injury, as well as a significant reduction in the levels of A. fumigatus in tissue. Rabbits treated with LY did not achieve this level of survival or reduction of tissue burden. Nevertheless, in comparison to untreated controls, LY improved survival and reduced organism-mediated pulmonary injury with minimal toxicity, particularly when used in a prophylactic model.
The experiments performed to investigate the efficacy of LY in prophylaxis against aspergillosis suggest that this echinocandin may have a useful preventive role. Given the absence of apparent toxicity at dosages of ≤10 mg/kg/day and its broad spectrum of activity against Candida spp. and Aspergillus spp., LY303366 warrants further consideration for prevention of invasive fungal infections in neutropenic patients.
ACKNOWLEDGMENT
We thank Erwin Feuerstein, Department of Radiology, Warren Grant Magnuson Clinical Center, Bethesda, Md., for expert assistance in analysis of UFCT scans.
REFERENCES
- 1.Allende M C, Lee J W, Francis P, Garrett K, Dollenberg H, Berenguer J, Lyman C A, Pizzo P A, Walsh T J. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob Agents Chemother. 1994;38:518–522. doi: 10.1128/aac.38.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berenguer J, Allende M C, Lee J W, Garrett K, Lyman C, Ali N M, Bacher J, Pizzo P A, Walsh T J. Pathogenesis of invasive pulmonary aspergillosis during persistent granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am J Resp Crit Care Med. 1995;152:1079–1086. doi: 10.1164/ajrccm.152.3.7663787. [DOI] [PubMed] [Google Scholar]
- 3.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 4.Debono M, Turner W W, LaGrandeur L M, Molloy R M, Burkhardt F J, Rodriguez M, Zweifel M J, Nissen J S, Clingerman K, Gordee R S, Zeckner D J, Parr T R, Tang J. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. LY303366—a new semi-synthetic lipopeptide antifungal agent related to echinocandin B. Synthetic and structure activity studies, abstr. 359; p. 185. [Google Scholar]
- 5.Debono M, Turner W W, LaGrandeur L, Burkhardt F J, Nissen J S, Nichols K K, Rodriguez M J, Zweifel M J, Zeckner D J, Gordee R S, Tang J, Parr T R., Jr Semisynthetic chemical modification of the antifungal lipopeptide echinocandin B (ECB): structure-activity studies of the lipophilic and geometric parameters of polyarylated acyl analogs of ECB. J Med Chem. 1995;38:3271–3281. doi: 10.1021/jm00017a012. [DOI] [PubMed] [Google Scholar]
- 6.Denning D W, Stevens D A. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother. 1991;35:1329–1333. doi: 10.1128/aac.35.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst M E, Klepser M E, Wolfe E J, Pfaller A. Antifungal dynamics of LY303366, an investigational echinocandin B analog, against Candida ssp. Diagn Microbiol Infect Dis. 1996;26:125–131. doi: 10.1016/s0732-8893(96)00202-7. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis P, Lee J W, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo P A, Walsh T J. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J Infect Dis. 1994;169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 10.Fromtling R A. LY303366. Drugs Future. 1994;19:338–342. [Google Scholar]
- 11.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker; 1982. pp. 455–459. [Google Scholar]
- 12.Gordee R S, Zeckner D J, Ellis L F, Thakkar A L, Howard L C. In vitro and in vivo anti-Candida activity and toxicology of LY121019. J Antibiot (Tokyo) 1984;37:1054–1065. doi: 10.7164/antibiotics.37.1054. [DOI] [PubMed] [Google Scholar]
- 13.Gordee R S, Zeckner D J, Howard L C, Alborn W E, Jr, Debono M. Anti-Candida activity and toxicology of LY121019, a novel semisynthetic polypeptide antifungal antibiotic. Ann N Y Acad Sci. 1988;544:294–309. doi: 10.1111/j.1749-6632.1988.tb40415.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang A, Edwards F, Bernard E M, Armstrong D, Schmitt H J. In vitro activity of the new semi-synthetic polypeptide cilofungin ( LY121019) against Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 1990;9:697–699. doi: 10.1007/BF01964276. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz M B, Douglas C M. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz M B, Heath I B, Marrinan J, Dreikorn S, Onishi J, Douglas C. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob Agents Chemother. 1994;38:1480–1489. doi: 10.1128/aac.38.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J W, Amantea M A, Francis P A, Navarro E E, Bacher J, Pizzo P A, Walsh T J. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob Agents Chemother. 1994;38:713–718. doi: 10.1128/aac.38.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannuti C, Gingrich R, Pfaller M A, Kao C, Wenzel R P. Nosocomial pneumonia in patients having bone marrow transplant. Attributable mortality and risk factors. Cancer. 1992;69:2653–2662. doi: 10.1002/1097-0142(19920601)69:11<2653::aid-cncr2820691106>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Petraitiene R, Petraitis V, Groll A, Candelario M, Sein T, Bell A, Lyman C A, Schaufele R L, Walsh T J. Program and abstracts of the 38th Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy, in press. Washington, D.C: American Society for Microbiology; 1998. Efficacy of LY303366, a novel echinocandin, against disseminated candidiasis in persistently neutropenic rabbits, abstr. J-73; p. 472. [Google Scholar]
- 20.Pfaller M A, Marco F, Messer S A, Jones R N. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis. 1998;30:251–255. doi: 10.1016/s0732-8893(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 21.Pfaller M A, Messer S A, Coffman S. In vitro susceptibilities of clinical yeast isolates to a new echinocandin derivative, LY303366, and other antifungal agents. Antimicrob Agents Chemother. 1997;41:763–766. doi: 10.1128/aac.41.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzun O, Kocagoz S, Cetinkaya Y, Unal S. In vitro activity of a new echinocandin, LY303366, compared with those of amphotericin B and fluconazole against clinical yeast isolates. Antimicrob Agents Chemother. 1997;41:1156–1157. doi: 10.1128/aac.41.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij P E, Oakley K L, Morrissey J, Morrissey G, Denning D W. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Efficacy of LY303,366 against amphotericin B “susceptible” and “resistant” A. fumigatus infection in an immunocompromised murine model, abstr. F-73; p. 158. [Google Scholar]
- 24.Walsh T J, Hiemenz J W, Anaissie E. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin N Am. 1996;10:365–400. doi: 10.1016/s0891-5520(05)70303-2. [DOI] [PubMed] [Google Scholar]
- 25.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Med. 1988;38:467–470. [PubMed] [Google Scholar]
- 26.Walsh T J, McEntee C, Dixon D M. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J Clin Microbiol. 1987;25:931–932. doi: 10.1128/jcm.25.5.931-932.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh T J, Garrett K, Feuerstein E, Girton M, Allende M, Bacher J, Francesconi A, Schaufele R, Pizzo P A. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob Agents Chemother. 1995;39:1065–1069. doi: 10.1128/aac.39.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh T J, Lee J W, Kelly P, Bacher J, Lecciones J, Thomas V, Lyman C, Coleman D, Gordee R, Pizzo P A. Antifungal effects of the nonlinear pharmacokinetics of cilofungin, a 1,3-β-glucan synthetase inhibitor, during continuous and intermittent intravenous infusions in treatment of experimental disseminated candidiasis. Antimicrob Agents Chemother. 1991;35:1321–1328. doi: 10.1128/aac.35.7.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeckner D, Butler T, Boylan C, Boyll B, Lin Y, Raab P, Schmidtke J, Current W. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. LY303366 activity against systemic aspergillosis and histoplasmosis in murine models, abstr. 364; p. 186. [Google Scholar]
- 30.Zhanel G G, Karlowsky J A, Harding G A, Balko T V, Zelenitsky S A, Friesen M, Kabani A, Turik M, Hoban D J. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, and Aspergillus species. Antimicrob Agents Chemother. 1997;41:863–865. doi: 10.1128/aac.41.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]