Abstract
Selection of resistance to amoxicillin (with or without clavulanate), cefaclor, cefuroxime, and azithromycin among six penicillin G- and azithromycin-susceptible pneumococcal strains and among four strains with intermediate penicillin sensitivities (azithromycin MICs, 0.125 to 4 μg/ml) was studied by performing 50 sequential subcultures in medium with sub-MICs of these antimicrobial agents. For only one of the six penicillin-susceptible strains did subculturing in medium with amoxicillin (with or without clavulanate) lead to an increased MIC, with the MIC rising from 0.008 to 0.125 μg/ml. Five of the six penicillin-susceptible strains showed increased azithromycin MICs (0.5 to >256.0 μg/ml) after 17 to 45 subcultures. Subculturing in medium with cefaclor did not affect the cefaclor MICs of three strains but and led to increased cefaclor MICs (from 0.5 to 2.0 to 4.0 μg/ml) for three of the six strains, with MICs of other β-lactams rising 1 to 3 twofold dilutions. Subculturing in cefuroxime led to increased cefuroxime MICs (from 0.03 to 0.06 μg/ml to 0.125 to 0.5 μg/ml) for all six strains without significantly altering the MICs of other β-lactams, except for one strain, which developed an increased cefaclor MIC. Subculturing in azithromycin did not affect β-lactam MICs. Subculturing of the four strains with decreased penicillin susceptibility in amoxicillin (with or without clavulanate) or cefuroxime did not select for β-lactam resistance. Subculturing of one strain in cefaclor led to an increase in MIC from 0.5 to 2.0 μg/ml after 19 passages. In contrast to strains that were initially azithromycin susceptible, which required >10 subcultures for resistance selection, three of four strains with azithromycin MICs of 0.125 to 4.0 μg/ml showed increased MICs after 7 to 13 passages, with the MICs increasing to 16 to 32 μg/ml. All azithromycin-resistant strains were clarithromycin resistant. With the exception of strains that contained mefE at the onset, no strains that developed resistance to azithromycin contained ermB or mefE, genes that have been found in macrolide-resistant pneumococci obtained from clinic patients.
Streptococcus pneumoniae strains with reduced susceptibility to penicillin G (MICs, ≥0.125 μg/ml) have become a significant worldwide problem in recent years (1). The problem is exacerbated by spread from country to country and from continent to continent (15). A recent survey in the United States has revealed an increase in penicillin resistance from <5% before 1989 (0.02% of strains with penicillin MICs of ≥2.0 μg/ml) to 6.6% in 1991-1992 (1.3% of strains with penicillin MICs of ≥2.0 μg/ml) (4). In another survey, performed during the winter months of 1994 and 1995, 23.6% of pneumococci were not susceptible to penicillin (8). There is an urgent need for effective outpatient therapy for community-acquired respiratory tract infections, sinusitis, acute exacerbations of chronic bronchitis, and otitis media caused by pneumococci with increased penicillin MICs (11, 13).
Epidemiological studies have documented a significant association between penicillin resistance and overall β-lactam consumption, including the use of oral cephalosporins (2). In addition, the higher the penicillin MIC, the more likely it is that the strain will be resistant to macrolides (8, 9). Pneumococcal macrolide resistance (even in penicillin-susceptible strains) is especially a problem in countries such as France (12) and Spain (2), where these drugs are widely used. Another factor relating to pneumococcal drug resistance which must be considered is the risk of development of resistance during therapy. Carsenti-Etesse and coworkers (5–7) reported that aminopenicillins selected for resistance to this class of antibiotic as well as cephalosporins, whereas cephalosporins tended to select for resistance to their own class, with the exception of cefixime, which led to selection of cross-resistant organisms.
To shed more light on the latter phenomenon, we subcultured 10 strains of S. pneumoniae in media with subinhibitory concentrations of antibiotic to study the selection of resistance to amoxicillin, amoxicillin (with or without clavulanate), cefuroxime, cefaclor, and azithromycin. Drugs were selected to reflect a spectrum of β-lactams with various degrees of activity against pneumococci with increased penicillin MICs, and one representative of the macrolide-azalide group was also studied.
MATERIALS AND METHODS
Bacteria and antimicrobial agents.
Ten strains of S. pneumoniae isolated within the past 3 years were selected for study. Organisms were identified by optochin susceptibility and classified by serotyping. Of these, six were susceptible to penicillin (≤0.06 μg/ml) and azithromycin (0.03 μg/ml) and four showed intermediate penicillin resistance (0.125 to 0.25 μg/ml). Of the four strains with intermediate penicillin resistance, two were susceptible (0.125 to 0.25 μg/ml) and two were resistant (MICs, 2-4 μg/ml) to azithromycin. Azithromycin was chosen empirically as an example of the macrolide-azalide group. Strains were frozen at −70°C in double-strength skim milk (Difco Laboratories, Detroit, Mich.) before being tested. Antimicrobial agents were obtained from their respective manufacturers.
MIC determinations.
MICs were determined by a standardized microdilution method, using Mueller-Hinton broth (Difco Laboratories) supplemented with 5% lysed horse blood (16). When used in combination, clavulanate was added to amoxicillin in a 1:2 ratio. For strains resistant to one or more antimicrobial agents, MIC testing of all agents was performed by the E-test method (AB Biodisk, Solna, Sweden). Macrolide E tests were performed in air and in CO2. Breakpoints for all compounds except cefaclor were those approved by the National Committee for Clinical Laboratory Standards (NCCLS) (16). Strains with azithromycin MICs of ≥1.0 μg/ml at the beginning of the study or after serial passaging were screened for macrolide resistance by side-by-side disk diffusion tests with erythromycin and clindamycin, as described previously (10), and were also tested for susceptibility to clarithromycin by E test in air and in CO2.
Serial passages.
Glass tubes, each containing 1 ml of cation-adjusted Mueller-Hinton broth (Difco) with 5% lysed horse blood and doubling antibiotic dilutions, were initially inoculated with approximately 5 × 105 CFU/ml at antibiotic concentrations three doubling dilutions above and three doubling dilutions below the MIC. The initial inoculum was prepared by suspending growth from an overnight trypticase soy blood agar plate (Difco) in Mueller-Hinton broth. Inocula were diluted to achieve a final concentration of 5 × 105 CFU/ml in each tube. The tubes were incubated at 35°C for 24 h. For each subsequent daily passage, an inoculum was taken from the tube nearest the MIC which had the same opacity as the antibiotic-free controls. When the MIC increased fourfold, strains were subcultured in antibiotic-free medium for 10 serial passages. A maximum of 50 serial passages in antibiotic were performed. Strains were tested with optochin before and after resistance development.
Serotyping.
Serotyping was done by the standard capsule Quellung method. Serogrouping of strains with increased penicillin MICs was performed in our laboratory. Serogrouping of penicillin-susceptible strains and serotyping of all strains were performed in the National Streptococcal Reference Laboratory, Edmonton, Alberta, Canada.
Pulsed-field gel electrophoresis.
To determine whether resistant isolates obtained at the end of serial passaging were identical to those tested at the beginning of the study, the original strain and the resistant strain obtained after the last passage were tested by pulsed-field gel electrophoresis. Bacterial cultures were grown for 6 h in 5 ml of Todd-Hewitt broth supplemented with 5% yeast extract. Preparation of agarose-embedded genomic DNA was done by using a GenePath group I reagent kit (Bio-Rad, Inc., Hercules, Calif.) with the following modification: cells embedded in agarose plugs were lysed by incubation for 1 h at 37°C in 10% sodium deoxycholate–1% disodium phosphate. Agarose-embedded DNA was digested with 20 U of SmaI (New England Biolabs, Beverly, Mass.) for 6 h at 25°C and separated as described by Moissenet et al. (14).
PCR of penicillin-binding protein genes.
To determine whether strains that developed resistance to β-lactams had alterations in penicillin-binding proteins, the pbp2b and pbp2x genes were amplified from purified DNA, using primers specific for these genes (14). Template DNA was prepared from lysed cells by using Prep-A-Gene kit (Bio-Rad) as recommended by the manufacturer. The reaction mixture (100 μl) contained 1× PCR buffer (which contains 1.5 mM MgCl2), 0.2 mM deoxynucleoside triphosphates, 20 pmol of each primer, and 2.5 U of Taq DNA polymerase (Fisher Biotech). Cycling conditions were as follows: one cycle of denaturation (95°C, 5 min), annealing (53°C, 2 min), and extension (72°C, 2 min) followed by 29 cycles of denaturation (95°C, 1 min), annealing (53°C, 2 min), and extension (72°C, 2 min). PCR products were purified from excess primers and nucleotides by using Prep-A-Gene kit as recommended by the manufacturer (Bio-Rad), digested with HinfI (New England Biolabs) for 1 h at 37°C, and separated by electrophoresis for 4 h at 220 V in 0.5× Tris-borate-EDTA on a 3% Metaphor agarose gel (FMC, Rockland, Maine). DNA was visualized by staining with SYBR Green I nucleic acid stain (FMC).
Determination of ermB and mefE by PCR.
To determine the mechanism of macrolide resistance, strains with selected resistance to azithromycin and their parent strains were screened for the presence of ermB and mefE as described by Sutcliffe and coworkers (17, 18), with the following modifications. The ermB downstream primer was 5′-AGTAAYGGTACTTAAATTGTTTAC-3′, and the mefE PCR mixture contained 2 mM MgCl2 instead of 4 mM MgCl2. Two erythromycin-resistant strains (one ermB positive and the other mefE positive) were also tested, as controls.
RESULTS
Results of serial passage studies are summarized in Table 1. As can be seen, only one of the six penicillin-susceptible strains exhibited increased amoxicillin MICs in the combination of amoxicillin-clavulanate upon subculturing, with these MICs increasing from 0.008 to 0.016 μg/ml to 0.125 μg/ml. Subculturing in medium with amoxicillin alone did not select for resistance. For three of the six strains, subculturing in medium with cefaclor did not affect cefaclor MICs, but the other three exhibited increasing cefaclor MICs (0.5 μg/ml to 2 to 4 μg/ml), and the MICs of other β-lactams increased 1 to 3 dilutions, leading to a penicillin MIC of 0.5 μg/ml and a cefuroxime MIC of 2 μg/ml for one strain. Subculturing in cefuroxime led to cefuroxime MICs increasing from 0.03 to 0.06 μg/ml to 0.125 to 0.5 μg/ml for all six strains, without significantly altering the MICs of other β-lactams, except for one strain, whose cefaclor MIC rose to 2 μg/ml. Among the four strains with intermediate penicillin sensitivity, subculturing in amoxicillin, amoxicillin-clavulanate, or cefuroxime did not result in further selection of resistance to the drugs. By contrast, subculturing in cefaclor led to selection of resistance to this antimicrobial agent in one strain after 19 passages, with MICs rising from 0.5 to 2.0 μg/ml.
TABLE 1.
Results of resistance selection studiesa
| Strain no. | Initial MIC (μg/ml)
|
Selected resistance protocol
|
Retest MICb (μg/ml)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pen | Amox | Amox-Cla | Cef | Cefu | Azith | Drug | MIC | No. of passages | Pen | Amox | Amox-Cla | Cef | Cefu | Azith | |
| 1 | 0.016 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Azith | >0.25 | 31 | 0.03 | 0.016 | 0.016 | 1 | 0.03 | 8 |
| 0.016 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Cefu | 0.5 | 24 | 0.06 | 0.03 | 0.03 | 1 | 0.25 | 0.25 | |
| 0.016 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Cef | 4 | 24 | 0.5 | 0.25 | 0.125 | 4 | 2 | 0.125 | |
| 0.016 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Amox | NRc | ||||||||
| 0.016 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Amox-Clav | NR | ||||||||
| 2 | 0.016 | 0.008 | 0.008 | 0.5 | 0.03 | 0.03 | Azith | NR | |||||||
| 0.016 | 0.008 | 0.008 | 0.5 | 0.03 | 0.03 | Cefu | 0.25 | 39 | 0.03 | 0.016 | 0.016 | 0.5 | 0.25 | 0.125 | |
| 0.016 | 0.008 | 0.008 | 0.5 | 0.03 | 0.03 | Cef | 4 | 28 | 0.06 | 0.06 | 0.03 | 2 | 0.125 | 0.25 | |
| 0.016 | 0.008 | 0.008 | 0.5 | 0.03 | 0.03 | Amox | NR | ||||||||
| 0.016 | 0.008 | 0.008 | 0.5 | 0.03 | 0.03 | Amox-Clav | NR | ||||||||
| 3 | 0.03 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Azith | >0.5 | 32 | 0.03 | 0.016 | 0.016 | 0.5 | 0.03 | >256 |
| 0.03 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Cefu | 0.5 | 28 | 0.06 | 0.03 | 0.03 | 1 | 0.5 | 0.125 | |
| 0.03 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Cef | >2 | 35 | 0.06 | 0.06 | 0.06 | 2 | 0.125 | 0.125 | |
| 0.03 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Amox | NR | NR | |||||||
| 0.03 | 0.016 | 0.016 | 0.5 | 0.06 | 0.03 | Amox-Cla | NR | NR | |||||||
| 4 | 0.03 | 0.008 | 0.016 | 0.5 | 0.03 | 0.03 | Azith | 0.25 | 45 | 0.016 | 0.016 | 0.016 | 0.5 | 0.03 | 0.5 |
| 0.03 | 0.008 | 0.016 | 0.5 | 0.03 | 0.03 | Cefu | 0.25 | 44 | 0.016 | 0.016 | 0.03 | 0.5 | 0.125 | 0.25 | |
| 0.03 | 0.008 | 0.016 | 0.5 | 0.03 | 0.03 | Cef | NR | ||||||||
| 0.03 | 0.008 | 0.016 | 0.5 | 0.03 | 0.03 | Amox | NR | ||||||||
| 0.03 | 0.008 | 0.016 | 0.5 | 0.03 | 0.03 | Amox-Cla | NR | ||||||||
| 5 | 0.03 | 0.016 | 0.008 | 0.25 | 0.06 | 0.03 | Azith | 1 | 37 | 0.016 | 0.016 | 0.016 | 0.125 | 0.016 | 2 |
| 0.03 | 0.016 | 0.008 | 0.25 | 0.06 | 0.03 | Cefu | 0.5 | 38 | 0.016 | 0.016 | 0.016 | 2 | 0.5 | 0.125 | |
| 0.03 | 0.016 | 0.008 | 0.25 | 0.06 | 0.03 | Cef | NR | ||||||||
| 0.03 | 0.016 | 0.008 | 0.25 | 0.06 | 0.03 | Amox | NR | ||||||||
| 0.03 | 0.016 | 0.008 | 0.25 | 0.06 | 0.03 | Amox-Cla | 0.25 | 41 | 0.016 | 0.125 | 0.125 | 1 | 0.06 | 0.25 | |
| 6 | 0.03 | 0.016 | 0.016 | 0.5 | 0.03 | 0.03 | Azith | >0.25 | 17 | 0.03 | 0.03 | 0.016 | 0.25 | 0.016 | 32 |
| 0.03 | 0.016 | 0.016 | 0.5 | 0.03 | 0.03 | Cefu | 0.25 | 28 | 0.03 | 0.03 | 0.03 | 1 | 0.125 | 0.125 | |
| 0.03 | 0.016 | 0.016 | 0.5 | 0.03 | 0.03 | Cef | NR | ||||||||
| 0.03 | 0.016 | 0.016 | 0.5 | 0.03 | 0.03 | Amox | NR | ||||||||
| 0.03 | 0.016 | 0.016 | 0.5 | 0.03 | 0.03 | Amox-Cla | NR | ||||||||
| 7 | 0.25 | 0.06 | 0.06 | 2 | 0.5 | 0.25 | Azith | NR | |||||||
| 0.25 | 0.06 | 0.06 | 2 | 0.5 | 0.25 | Cefu | NR | ||||||||
| 0.25 | 0.06 | 0.06 | 2 | 0.5 | 0.25 | Cef | NR | ||||||||
| 0.25 | 0.06 | 0.06 | 2 | 0.5 | 0.25 | Amox | NR | ||||||||
| 0.25 | 0.06 | 0.06 | 2 | 0.5 | 0.25 | Amox-Cla | NR | ||||||||
| 8 | 0.125 | 0.06 | 0.06 | 1 | 0.25 | 0.125 | Azith | 1 | 13 | 0.125 | 0.03 | 0.03 | 1 | 0.5 | 32 |
| 0.125 | 0.06 | 0.06 | 1 | 0.25 | 0.125 | Cefu | NR | ||||||||
| 0.125 | 0.06 | 0.06 | 1 | 0.25 | 0.125 | Cef | NR | ||||||||
| 0.125 | 0.06 | 0.06 | 1 | 0.25 | 0.125 | Amox | NR | ||||||||
| 0.125 | 0.06 | 0.06 | 1 | 0.25 | 0.125 | Amox-Cla | NR | ||||||||
| 9 | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 4 | Azith | 16 | 7 | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | 16 |
| 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 4 | Cefu | NR | ||||||||
| 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 4 | Cef | 8 | 19 | 0.5 | 0.5 | 0.25 | 2 | 0.5 | 8 | |
| 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 4 | Amox | NR | ||||||||
| 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 4 | Amox-Cla | NR | ||||||||
| 10 | 0.25 | 0.125 | 0.125 | 2 | 0.25 | 2 | Azith | 16 | 7 | 0.125 | 0.125 | 0.06 | 1 | 0.25 | 16 |
| 0.25 | 0.125 | 0.125 | 2 | 0.25 | 2 | Cefu | NR | ||||||||
| 0.25 | 0.125 | 0.125 | 2 | 0.25 | 2 | Cef | 16 | 42 | 0.25 | 0.125 | 0.06 | 2 | 0.5 | 8 | |
| 0.25 | 0.125 | 0.125 | 2 | 0.25 | 2 | Amox | NR | ||||||||
| 0.25 | 0.125 | 0.125 | 2 | 0.25 | 2 | Amox-Cla | NR | ||||||||
Abbreviations: Pen, penicillin G; Amox, amoxicillin; Cla, clavulanate; Cef, cefaclor; Cefu, cefuroxime; Azith, azithromycin.
Determined by E test after 10 antibiotic-free subcultures.
NR, no increase in MIC detected.
Although β-lactam resistance was usually stable, in a few cases MICs reverted to baseline after 10 serial subcultures in the absence of antibiotic. This was particularly the case for strains 9 and 10, whose cefaclor MICs dropped from 8 to 16 μg/ml to 2 μg/ml (Table 1). In all cases, MICs that reverted were for organisms that grew at the high concentration; after subculturing, MICs were lower than the selecting concentration.
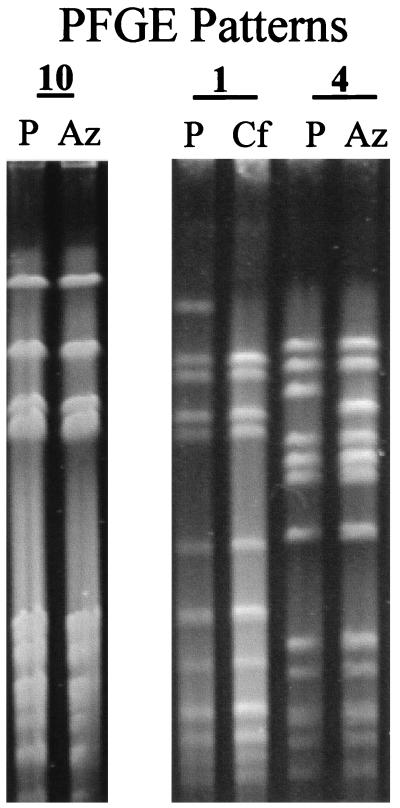
All strains exhibiting selection of resistance to β-lactams showed pulsed-field gel electrophoresis patterns identical (eight strains) or similar (one strain) to those of their parents. The latter, cefaclor-resistant strain differed from the parent by one band (Fig. 1). PCR results for pbp2b and pbp2x in the following parent and selected strains were identical: parent and cefaclor-selected strain 1, parent and cefaclor- and cefuroxime-selected strain 2, parent and cefuroxime-selected strain 3, parent and amoxicillin-clavulanate-selected strain 5, and parent and cefuroxime-selected strain 6. No sequencing of PCR products was performed, so their sequences were not compared to determine if changes had occurred.
FIG. 1.
Pulse-field gel electrophoresis patterns for strains 1, 4, and 10. P, parent strain; Az, after azithromycin selection; Cf, after cefaclor selection.
Five of the six penicillin-susceptible strains showed azithromycin MIC increases from 0.5 to >256.0 μg/ml after 17 to 45 subcultures. Subculturing in azithromycin did not affect β-lactam MICs. Three of the four strains with azithromycin MICs of 0.125 to 4.0 μg/ml showed increased MICs after relatively few passages (7 to 13), with MICs rising to 16 to 32 μg/ml. Azithromycin MICs determined by E test, in the presence of CO2 and in air, were all within 2 dilutions of each other except for those of strain 8 (32 μg/ml in air and >256 μg/ml in CO2) (Table 2). All azithromycin-susceptible strains (no. 1 to 8) (Table 1) were susceptible to erythromycin and clindamycin in the two-disk diffusion test. Parent strains 9 and 10 were erythromycin resistant but clindamycin susceptible. Among strains with selected azithromycin resistance, the resistance patterns were as follows: (i) strains 1 and 5, erythromycin susceptible and clindamycin susceptible; (ii) strains 3, 6, 9, and 10, erythromycin resistant and clindamycin susceptible; (iii) strain 8, erythromycin and clindamycin resistant; and (iv) strain 4, erythromycin and clindamycin susceptible. (Although the MIC for strain 8 rose from 0.03 to 0.5 μg/ml, the latter MIC is still in the susceptible category.) Microdilution MICs for strains 1, 5, and 6 performed in air were within 1 dilution of those obtained with the E test in both air and CO2. Strains with increased azithromycin MICs also had increased clarithromycin MICs. Results of azithromycin and clarithromycin E tests (in air and CO2) and erythromycin-clindamycin double disk tests (9) are presented in Table 2.
TABLE 2.
Macrolide-clindamycin susceptibility of strains for which an increase in azithromycin MIC was selected
| Strain no. | MIC (μg/ml) ofa:
|
Resistance or susceptibility tob:
|
||||
|---|---|---|---|---|---|---|
| Azithromycin
|
Clarithromycin
|
Erythromycin | Clindamycin | |||
| Air | CO2 | Air | CO2 | |||
| 1 | 8 | 16 | 1 | 1 | S | S |
| 3 | >256 | >256 | 2 | 4 | R | S |
| 4 | 0.5 | 1 | 0.03 | 0.125 | S | S |
| 5 | 2 | 4 | 0.5 | 0.5 | S | S |
| 6 | 32 | 64 | >256 | >256 | R | S |
| 8 | 32 | >256 | 32 | >256 | R | R |
| 9 | 16 | 64 | 2 | 4 | R | S |
| 10 | 16 | 64 | 2 | 4 | R | S |
Determined by E test.
Determined by double-disk diffusion (9). S, susceptible; R, resistant.
All azithromycin-resistant derivatives showed pulsed-field patterns identical to those of their parent strains except for strain 4, which differed from its parent by two bands (Fig. 1). Results of ermB and mefE PCR amplification showed that the parent and selected strains 9 and 10 all carry the mefE gene. All other strains remained negative for both genes.
All parental strains were initially typeable. In all cases except the following, serotypes of parent and selected strains were identical and comprised types 1, 6A, 6B, 14, and 19F. Azithromycin-resistant derivatives of strains 4, 5, 9, and 10 were untypeable, as was the cefuroxime-derived strain 2, probably because of selection of capsule-negative strains by repeated subculture.
DISCUSSION
In previous studies (5–7), Carsenti-Etesse and coworkers, utilizing 20 to 35 subcultures, found greater than fourfold increases in MICs of β-lactams for all strains after exposure to subinhibitory concentrations of amoxicillin, amoxicillin-clavulanate, imipenem, or cefixime. Intermediate resistance to these antimicrobial agents developed in almost all strains. With cefadroxil, cefatrizine, cefotiam, cefpodoxime, and cefuroxime, fourfold increases in MIC were noted, and at least 33% of the strains developed intermediate resistance to cephalosporins. More cross-resistance-stable variants were obtained with amoxicillin, amoxicillin-clavulanate, imipenem, and cefixime. With the other three cephalosporins tested, MICs of cephalosporins increased without concomitant changes in aminopenicillin MICs.
In the present study, MICs of amoxicillin or amoxicillin-clavulanate remained in the susceptible range (≤0.5 μg/ml) (16), even for the one strain whose amoxicillin-clavulanate MIC increased from 0.008 to 0.125 μg/ml. The higher MICs of cefuroxime and cefaclor for the mutants were usually 2 to 4 dilutions higher than those for the parent strains. In a few cases, the higher MICs observed after subculturing with β-lactams dropped a few dilutions after 10 sequential subcultures in the absence of antibiotic. The mutation in one of the cefaclor-resistant strains leading to intermediate penicillin resistance and cefuroxime resistance (strain 1 [Table 1]) does not appear, as determined by techniques used in our study, to be due to pbp2b or pbp2x changes. However, sequencing of PCR products may shed light on the resistance mechanisms in this organism; this work is currently in progress.
By comparison, 7 of 10 strains developed high-level resistance (2 to >256.0 μg/ml) to azithromycin. The mechanism of resistance and clinical significance of the azithromycin-resistant strains which did not carry the ermB or mefE gene is unknown. Thus far, all macrolide-resistant pneumococci from clinical specimens have been found to contain the erm or mef gene (17, 18). Only strains with initial high azithromycin MICs yielded increased MICs after relatively few subcultures; the clinical significance of resistance selection after >10 subcultures, as was the case with strains initially susceptible to azithromycin, is unknown. Additionally, we have no explanation for the two strains (no. 1 and 5) which were azithromycin resistant but erythromycin susceptible. In these two strains, azithromycin MICs within one dilution of each other were obtained after E testing in air and CO2 and after performance of the NCCLS microdilution method in air. It is possible that an unknown mechanism for macrolide resistance (possibly ribosomal mutation) exists in pneumococci. Possibly a new gene is present in a latent state and awaits activation by as-yet-unknown factors. The influence of sequential subcultures in media with sub-MIC concentrations on other macrolides, such as erythromycin, clarithromycin, and roxithromycin, has not yet been defined. All derived strains which were azithromycin resistant also showed increased clarithromycin MICs. Before the clinical significance of our azithromycin findings can be assessed, these aspects require study; they are currently being examined by our group. Additionally, the lack of a capsule in azithromycin-resistant selected strains 4, 5, 9, and 10 argues against capsule-related virulence. Virulence studies of all strains with selected resistance need to be done in mice; these studies are planned.
Results in the present study correlate with clinical studies (2) which show that exposure to antimicrobial agents may contribute to emergence of resistance, with overall β-lactam consumption being the most important factor and the ratio of penicillin to oral cephalosporin used being the second-most-important factor. However, in the present study, amoxicillin and amoxicillin-clavulanate selected for less resistance than cefaclor and cefuroxime. Different affinities to penicillin-binding proteins may explain differences between β-lactams. Baquero and Negri (3) have suggested that it takes a long time to select for high-level amoxicillin resistance at low dosages, while at high dosages the repeated challenge may lead to exclusion of the more resistant population. By contrast, a cefixime-like antibiotic may be more selective for high-level resistance.
ACKNOWLEDGMENTS
This study was supported by a grant from SmithKline Beecham Laboratories, Collegeville, Pa.
We thank M. Lovgren (National Streptococcal Reference Laboratory, Edmonton, Alberta, Canada) for help with serotyping of some strains.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, F. 1996. Trends in antibiotic resistance of respiratory pathogens: an analysis and commentary on a collaborative surveillance study. J. Antimicrob. Chemother. 38(Suppl. A):117–132. [DOI] [PubMed]
- 3.Baquero, F., and M. C. Negri. 1997. Strategies to minimise the development of antibiotic resistance. J. Chemother. 9(Suppl. 3):29–37. [PubMed]
- 4.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 5.Carsenti-Etesse H, Durant J, De Salvador F, Bensoussan M, Bensoussan F, Pradier C, Thabaut A, Dellamonica P. In-vitro development of resistance to β-lactam antibiotics in Streptococcus pneumoniae. J Antimicrob Chemother. 1995;36:417–423. doi: 10.1093/jac/36.2.417. [DOI] [PubMed] [Google Scholar]
- 6.Carsenti-Etesse H, Durant J, Roger P M, Bensoussan M, Mancini G, Thabaut A, Dellamonica P. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. In vitro development of resistance of Streptococcus pneumoniae (SP) by spiral method or serial passages in sub-inhibitory (subMICs) concentrations of betalactams, abstr. E11; p. 87. [Google Scholar]
- 7.Carsenti-Etesse H, Roger P M, Mancini G, Thabaut A, Dellamonica P. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. In vitro development of resistance of Streptococcus pneumoniae (SP) by spiral method in sub inhibitory (subMICs) concentrations of betalactams and macrolides, abstr. J13; p. 220. [Google Scholar]
- 8.Doern G V, Brueggemann A, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ednie L M, Visalli M A, Jacobs M R, Appelbaum P C. Comparative activity of clarithromycin, erythromycin, and azithromycin against penicillin-susceptible and penicillin-resistant pneumococci. Antimicrob Agents Chemother. 1996;40:1950–1952. doi: 10.1128/aac.40.8.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasola E L, Bajaksouzian S, Appelbaum P C, Jacobs M R. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob Agents Chemother. 1997;41:129–134. doi: 10.1128/aac.41.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedland I R, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 12.Geslin P, Fremaux A, Sissia G, Spicq C, Aberrane A. Epidémiologie de la résistance aux antibiotiques de Streptococcus pneumoniae en France. Réseau national de surveillance (1984–1993) Med Mal Infect. 1994;24:948–961. [Google Scholar]
- 13.Jacobs M R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992;15:119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 14.Moissenet D, Valcin M, Marchand V, Garabedian E, Gelsin P, Garbarg-Chenon A, Vu-Thien H. Molecular epidemiology of Streptococcus pneumoniae with decreased susceptibility to penicillin in a Paris children’s hospital. J Clin Microbiol. 1997;35:298–301. doi: 10.1128/jcm.35.1.298-301.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz R, Coffey T J, Daniels M, Dowson C G, Laible G, Casal J, Hakenbeck R, Jacobs M, Musser J M, Spratt B G, Tomasz A. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991;164:302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Publication no. M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2251–2255. doi: 10.1128/aac.41.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]