Abstract
The biosynthetic peptide dolastatin 10 is currently in phase I and II cancer clinical trials. We evaluated the antifungal spectrum of dolastatin 10 and four structural modifications. In broth macrodilution assays, the peptides were fungicidal for American Type Culture Collection strains and clinical isolates (including fluconazole-resistant strains) of Cryptococcus neoformans but no other yeasts or filamentous fungi examined. Specificity for C. neoformans was also demonstrated in the solid-phase disk diffusion assay, and fungicidal activity was confirmed in time-kill experiments. For a methyl ester modification, the MICs at which 50 and 90% of 19 clinical isolates were inhibited (MIC50 and MIC90, respectively) were 0.195 and 0.39 μg/ml, respectively. The MFC50 (50% minimum fungicidal concentration) for this peptide was 0.39 μg/ml, and the MFC90 was 0.78 μg/ml. MICs and MFCs were identical or lower in the presence of human serum but increased with lowered pH. These peptides should be pursued as potential chemotherapeutics for C. neoformans, a leading cause of infection and mortality in immunocompromised patients.
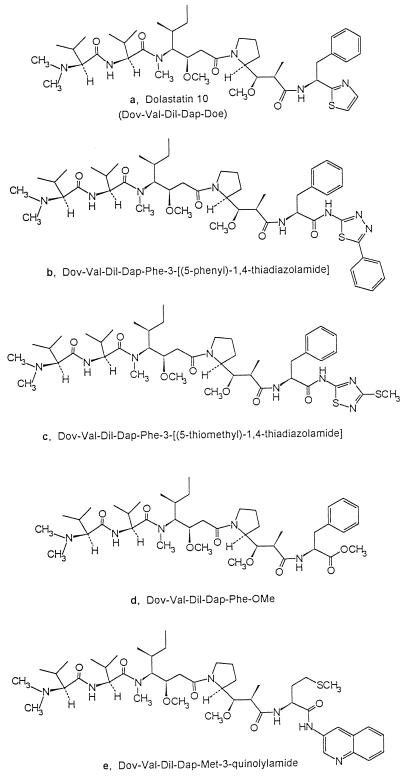
Dolastatin 10, a linear peptide containing three unique amino acid units (Fig. 1), was originally isolated from the Indian Ocean sea hare Dolabella auricularia (15). The synthesis of dolastatin 10 (16, 18) facilitated detailed investigation of its powerful antineoplastic activities (for a review, see reference 14), culminating in the initiation of phase I cancer clinical trials in 1995. In ongoing clinical trials with patients with advanced solid tumors, there is minimal toxicity at doses of up to 200 μg/m2 (2, 9, 20).
FIG. 1.
Structures of dolastatin 10 (a) and analogs (b to e).
In mammalian cells, the intracellular target of dolastatin 10 is tubulin. The peptide inhibits microtubule assembly and tubulin-dependent GTP binding (4) and is a noncompetitive inhibitor of vincristine binding to tubulin (3). Dolastatin 10 causes metaphase arrest in a wide variety of animal and human cancer cell lines and exhibits impressive activity in murine tumor models (for a review, see reference 14). In addition, dolastatin 10 induces apoptosis in certain human lymphoma cell lines (5, 8). The apoptotic mechanism is apparently unrelated to its antimitotic effects (5, 8). The tubulin-binding properties of dolastatin 10 suggested it might also find application as an antifungal agent. We report here that dolastatin 10 and four structural modifications (Fig. 1) are potent and specific fungicidals. Of 13 species of fungi examined, only Cryptococcus neoformans was susceptible to these peptides.
MATERIALS AND METHODS
Antifungal agents.
Dolastatin 10 and modification d (Fig. 1) were synthesized as described elsewhere (16–18). Synthesis of modification e is described in U.S. patent 5,663,149 (published 9/2/97) (18a), and patents are pending on the synthesis of modifications b and c. Compounds were reconstituted in sterile dimethyl sulfoxide (DMSO) immediately prior to all assays.
Fungal strains.
Clinical isolates of C. neoformans were obtained from patient cerebrospinal fluid, blood, bone marrow, sputum, bronchial lavage fluid, and wound infections at the University of Virginia Medical Center. Strains clinically resistant to fluconazole (7) were provided by the Center for Medical Mycology, Case Western Reserve University. Most yeast strains were maintained by single colony transfer on Sabouraud dextrose agar (SDA), pH 5.6, at 35°C. Cryptococcus albidus, C. laurentii, and C. uniguttulatus (66033) were maintained on SDA, pH 6.6, at 25°C, and C. uniguttulatus (34143) and C. ater were maintained on yeast morphology agar at 25°C. Filamentous fungi were maintained on potato dextrose agar (Aspergillus fumigatus and Rhizopus oligosporus) or Emmon’s agar (Pseudallescheria boydii) slants at 35°C.
Disk diffusion susceptibility testing.
Antimicrobial activity was assayed by National Committee for Clinical Laboratory Standards disk susceptibility tests (11). Inocula were adjusted to a density of 0.10 at 625 nm in Sabouraud dextrose broth, and spread on SDA plates. Absorption of excess moisture was allowed to occur for 10 min before application of dried disks containing twofold dilutions of the drugs. Test plates were incubated at 35°C (25°C for C. albidus and C. laurentii), and zones of inhibition were recorded after 48 h. The MIC was defined as the lowest drug concentration resulting in a clear zone of growth inhibition. Disk susceptibility testing of Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Neisseria gonorrhoeae was also performed.
Broth macrodilution susceptibility testing of yeasts.
Dolastatin 10 and modifications were screened against yeasts by the National Committee for Clinical Laboratory Standards broth macrodilution assay (12). Yeasts were suspended and diluted as recommended to yield final inocula ranging from 0.5 × 103 to 2.5 × 103 CFU/ml. Tests were performed in sterile plastic tubes (12 by 75 mm) containing twofold dilutions of the peptides in 0.165 M morpholinepropanesulfonic acid (MOPS)-buffered RPMI 1640 medium (pH 7.0). One tube was left drug free (but contained an equivalent volume of DMSO) for a turbidity control. Tubes were incubated without agitation at 35°C (25°C for C. albidus, C. laurentii, C. ater, and C. uniguttulatus). MICs were determined after 72 h for Cryptococcus and after 48 h for other yeast genera. The MIC was defined as the lowest concentration of a compound that inhibited all visible growth of the test organism (optically clear).
Broth macrodilution susceptibility testing of filamentous fungi.
Broth macrodilution susceptibility testing of A. fumigatus, R. oligosporus, and P. boydii was performed in accordance with a proposed standardized procedure (6) with slight modification. To induce conidium and sporangiospore formation, fungi were grown on potato dextrose agar (A. fumigatus and R. oligosporus) or Emmon’s agar (P. boydii) slants at 35°C for 6 days. Fungal slants were covered with 1 ml of sterile 0.85% NaCl, and suspensions were made by gently probing the colonies with the tip of a sterile Pasteur pipette. The resulting mixture of hyphal fragments and conidia or sporangiospores was withdrawn and transferred to a sterile clear microcentrifuge tube, and heavy particles were allowed to settle for 10 min. The upper homogeneous suspension was transferred to a sterile microcentrifuge tube, vortexed for 15 s, adjusted spectrophotometrically, and diluted in sterile 0.165 M MOPS-buffered RPMI 1640 medium, pH 7.0, to yield final inocula ranging from 0.5 × 103 to 2.5 × 103 CFU/ml. Susceptibility to the peptides was then determined by broth macrodilution assays as described above for the yeast cultures. MICs for the filamentous fungi were read when significant or heavy growth was seen in controls.
MFCs.
Minimum fungicidal concentrations (MFCs) were determined by subculturing 0.1 ml from each tube with no visible growth in the MIC broth macrodilution series onto drug-free SDA plates. The plates were incubated at 35°C for 48 h, and the MFC was defined as the lowest drug concentration that completely inhibited growth on SDA plates.
Effect of host factors.
Broth macrodilution assays were performed with RPMI medium prepared at pHs 5, 6, and 7 and in RPMI medium with and without 50% normal human serum (Lampire Biological Labs). C. neoformans 90112 was used in each case.
Time-kill studies.
Overnight cultures of C. neoformans 90112 in pH 7.0 MOPS-buffered RPMI 1640 medium were inoculated into the same medium containing multiples of the broth macrodilution MIC of the peptides or an equivalent volume of DMSO. Cultures were shaken at 35°C, and aliquots were aseptically removed at various times for dilution plating. Standard errors of the means were calculated from at least two experiments.
RESULTS AND DISCUSSION
Our initial screen for antimicrobial activity, the disk diffusion assay, suggested that dolastatin 10 and four analogs had narrow-spectrum antifungal activity (Table 1). In addition, at 100 μg/disk, there was no inhibition of the bacterial strains tested (see Materials and Methods). The specificity for C. neoformans was also apparent in broth macrodilution assays (Table 2). The parent compound was not growth inhibitory to the related species C. albidus, C. laurentii, C. uniguttulatus, and C. ater (C. albidus 34140, C. laurentii 34142, C. uniguttulatus 34143, and C. ater 14247 are American Type Culture Collection [ATCC] clinical specimens) (Table 2). The MFCs for C. neoformans were typically identical to or twofold greater than the MICs. Exceptions occurred with C. neoformans 14116, for which the MFCs of compounds b and c were 16-fold greater than the MICs. Dolastatin 10 was also fungicidal for strains of C. neoformans that are clinically resistant to fluconazole (7) (Table 2).
TABLE 1.
Antifungal activities of dolastatin 10 and modifications b to e in the disk diffusion assay
| Organism | ATCC no. | MIC (μg/disk) of compound:
|
||||
|---|---|---|---|---|---|---|
| a | b | c | d | e | ||
| Cryptococcus neoformans | 90112 | 25–50 | 3.12–6.25 | 1.56–3.12 | 3.12–6.25 | 25–50 |
| Cryptococcus albidus | 66030 | >100 | >100 | >100 | ||
| Cryptococcus laurentii | 66036 | >100 | >100 | >100 | ||
| Candida albicans | 90028 | >100 | >100 | >100 | >100 | >100 |
| Candida glabrata | 90030 | >100 | >100 | >100 | ||
TABLE 2.
Antifungal activities of dolastatin 10 and modifications b to e in the broth macrodilution assay
| Organism | ATCC no. or strain designation | MIC (MFC)c of compound:
|
||||
|---|---|---|---|---|---|---|
| a | b | c | d | e | ||
| Cryptococcus neoformans | 66031 | 0.78 (1.56) | 0.78 (0.78) | 0.78 (0.78) | 0.195 (0.39) | 0.78 (1.56) |
| Cryptococcus neoformans | 14116 | 3.12 (6.25) | 1.56 (25) | 0.78 (12.5) | 1.56 (3.12) | 12.5 (>50) |
| Cryptococcus neoformans | 32045 | 0.78 (1.56) | 0.78 (0.78) | 0.78 (1.56) | 0.195 (0.39) | 0.78 (0.78) |
| Cryptococcus neoformans | 90112 | 0.78 (1.56) | 1.56 (3.12) | 0.78 (0.78) | 0.0975 (0.195) | 1.56 (6.25) |
| Cryptococcus neoformansa | 94-2406b | 0.0487 (0.0975) | ||||
| Cryptococcus neoformansa | 95-2792b | 0.78 (3.12) | ||||
| Cryptococcus neoformansa | 96-2011b | 0.78 (1.56) | ||||
| Cryptococcus neoformansa | 94-2483b | 0.195 (0.39) | ||||
| Cryptococcus albidus | 66030 | >50 | ||||
| Cryptococcus albidus | 34140 | >50 | ||||
| Cryptococcus albidus | 10666 | >50 | ||||
| Cryptococcus laurentii | 66036 | >50 | ||||
| Cryptococcus laurentii | 18803 | >50 | ||||
| Cryptococcus laurentii | 34142 | >50 | ||||
| Cryptococcus uniguttulatus | 34143 | >50 | ||||
| Cryptococcus uniguttulatus | 66033 | >50 | ||||
| Cryptococcus ater | 14247 | >50 | ||||
| Candida albicans | 90028 | >50 | >25 | >25 | >50 | >50 |
| Candida glabrata | 90030 | >50 | >50 | >50 | >50 | >50 |
| Candida parapsilosis | 22019 | >50 | ||||
| Candida lusitaniae | 42720 | >50 | ||||
| Rhodotorula mucilaginosa | 9449 | >50 | ||||
| Aspergillus fumigatus | 96918 | >50 | ||||
| Rhizopus oligosporus | 22959 | >50 | ||||
| Pseudallescheria boydii | M1.461b | >50 | >50 | |||
Fluconazole-resistant clinical isolate (7).
Strain designation.
All values are in micrograms per milliliter.
As the methyl ester (compound d) was the most potent antifungal peptide in broth macrodilution assays, it was tested against 19 clinical isolates (not including fluconazole-resistant strains) of C. neoformans. No resistant clinical isolates were found. The MICs at which 50 and 90% of the isolates were inhibited (MIC50 and MIC90, respectively) by modification d were 0.195 and 0.39 μg/ml, respectively. The MFC of modification d for 50% of the isolates was 0.39 μg/ml, and the MFC for 90% of the isolates was 0.78 μg/ml. The MICs of analog d for clinical isolates ranged from 0.0975 to 0.78 μg/ml, and the MFCs ranged from 0.0975 to 6.24 μg/ml. For more than 68% of the clinical isolates, the MFC/MIC ratios were less than or equal to 2, and for 26% of the isolates, the ratio equaled 4.
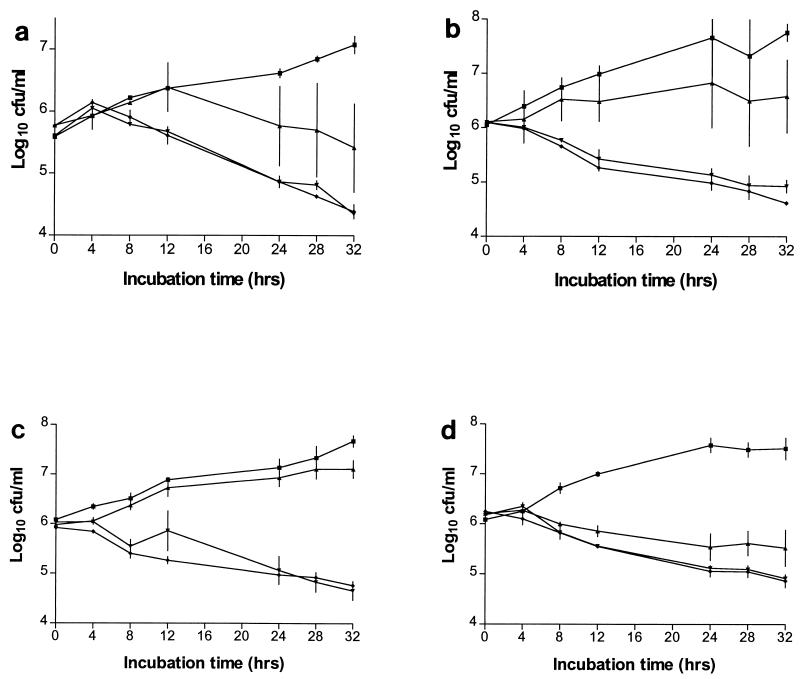
The fungicidal action of four of the peptides was confirmed in time-kill experiments (Fig. 2) (a paucity of modification c prohibited time-kill studies). For all of the peptides tested, killing was concentration dependent between the MIC and four times the MIC but not between four and eight times the MIC.
FIG. 2.
C. neoformans kill curves for dolastatin 10 (a) and modifications b, d, and e (b, c, and d, respectively). Killing was determined with DMSO alone (■) and at the MIC (▴) and four (▾) and eight (⧫) times the MIC of each compound. The results are means ± the standard errors of the means of at least two experiments.
Dolastatin 10 and three of the modifications were available in sufficient quantity for investigation of the effects of two host factors, pH and serum, on broth macrodilution MICs and MFCs. The MICs and MFCs increased in acidified RPMI medium (Table 3). The anticryptococcal activity of modification d was the least affected by a lowered pH. Attempts were made to determine MICs at pH 8, but the strain did not grow in alkaline RPMI medium.
TABLE 3.
Effect of pH or human serum on MICs and MFCs of dolastatin 10 and modifications b, d, and e for C. neoformans
| Treatment | MIC, MFCa of compound:
|
|||
|---|---|---|---|---|
| a | b | d | e | |
| pH 5 | >50, >50 | 50, >50 | 1.56, 3.12 | >50, >50 |
| pH 6 | 6.25, 25 | 1.56, 3.12 | 0.39, 0.78 | 6.25, 25 |
| pH 7 | 0.78, 1.56 | 0.39, 0.78 | 0.0975, 0.195 | 0.78, 1.56 |
| No serum | 0.78, 1.56 | 1.56, 1.56 | 0.195, 0.39 | 1.56, 3.12 |
| 50% human serum | 0.78, 1.56 | 1.56, 3.12 | 0.0975, 0.0975 | 0.78, 0.78 |
All values are in micrograms per milliliter.
To ascertain whether the increased concentration of dolastatin 10 required to kill C. neoformans in acidified medium was due to loss of peptide activity or more rapid growth of the test organism, two controls were performed. Dolastatin 10 was incubated at pHs 5, 6, and 7 under the same conditions as the broth macrodilution assay, and the concentration required to inhibit 50% of the growth of six human cancer cell lines (pancreas BXPC-3, neuroblastoma SK-N-SH, thyroid SW1736, non-small-cell lung NCI-H460, pharynx FADU, and prostate DU-145) were compared. Acid-treated dolastatin 10 had no apparent loss of antiproliferative activity (data not shown). In the second control, optical densities over time were compared in shake flasks containing C. neoformans in pH 5, 6, or 7 RPMI medium. Growth rates at pHs 5 and 6 were identical, and the growth rate at pH 7 was only slightly slower (data not shown). Thus, the increased MICs and MFCs of the peptides in acidified medium cannot be explained at this time. Other possible explanations include (i) more rapid uptake of the peptides at neutral pH owing to increased permeability or activation of transport mechanisms and (ii) the ionization state of dolastatin 10 affects interaction with its fungal target (the human cancer cell line control suggests that the ionization state of dolastatin 10 does not affect interaction with its mammalian tubulin target). Certainly, the increased MICs at lowered pH do not eliminate the potential of these compounds for clinical antifungal development. Widely prescribed antimicrobials with increased MICs in acidic media include streptomycin, erythromycin, gentamicin, and metronidazole (for a review, see reference 1).
In vitro, dolastatin 10 is stable in human, dog, and mouse plasma for at least 24 h at 37°C (13). After intravenous injection into mice, the estimated elimination half-life is 5.6 h (13). In the presence and absence of human serum, the MICs and MFCs of the peptides for C. neoformans were very similar (Table 3). For compounds d and e, the MICs and MFCs were actually lower in the presence of human serum. The activities of the peptides in serum from two different suppliers (Sigma and Lampire) were similar (data not shown).
The incidence of invasive fungal infections in cancer patients ranges from approximately 5 to 30% (10), and C. neoformans is a leading cause of such infections (19). We have described a related series of novel antitumor antibiotics that appear to be specific for C. neoformans. However, susceptibility testing with a large variety of fungal species is required to confirm this observation. We can provide no explanation for the specificity of these peptides. We are investigating the possibility of enhanced drug uptake in C. neoformans and/or extensive homology between C. neoformans tubulin and mammalian tubulin at the dolastatin 10 binding site. If specificity for C. neoformans is confirmed, these peptides should be investigated as a means to rapidly identify C. neoformans in clinical settings. Our immediate goal is to determine if these anticryptococcal peptides are efficacious in vivo.
ACKNOWLEDGMENTS
This work was supported by Outstanding Investigator Grant CA44344-01-09 awarded by the Division of Cancer Treatment, NCI, DHHS; the Arizona Disease Control Research Commission; and the Robert B. Dalton Endowment Fund.
We thank M. Ghannoum (Center for Medical Mycology, Case Western Reserve University, Cleveland, Ohio) for providing fluconazole-resistant strains, F. Hogan for preparing Fig. 1, C. Chapuis for performing the human cancer cell line studies, and L. Crews for technical assistance.
REFERENCES
- 1.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 52–111. [Google Scholar]
- 2.Bagniewski P G, Reid J M, Pitot H C, Sloan J A, Ames M M. Proceedings of the American Association for Cancer Research. Philadelphia, Pa: American Association for Cancer Research; 1997. Pharmacokinetics of dolastatin 10 in adult patients with solid tumors, abstr. 1492. [Google Scholar]
- 3.Bai R, Pettit G R, Hamel E. Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem. 1990;265(28):17141–17149. [PubMed] [Google Scholar]
- 4.Bai R, Pettit G R, Hamel E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal: inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem Pharmacol. 1990;39(12):1941–1949. doi: 10.1016/0006-2952(90)90613-p. [DOI] [PubMed] [Google Scholar]
- 5.Beckwith M, Urba W J, Longo D L. Growth inhibition of human lymphoma cell lines by the marine products, dolastatins 10 and 15. J Natl Cancer Inst. 1993;85(6):483–488. doi: 10.1093/jnci/85.6.483. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds T C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessup C J, Wallace T L, Ghannoum M A. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada. 1997. Evaluation of antifungal activity of nyotran against various pathogenic fungi, poster F-88. [Google Scholar]
- 8.Maki A, Diwakaran H, Redman B, Al-Asfar S, Pettit G R, Mohammad R M, Al-Katib A. The bcl-2 and p53 oncoproteins can be modulated by bryostatin 1 and dolastatins in human diffuse large cell lymphoma. Anti-Cancer Drugs. 1995;6:392–397. doi: 10.1097/00001813-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 9.McElroy E A, Pitot H C, Erlichman C, et al. Proceedings of the American Society of Clinical Oncology. Chicago, Ill: American Society of Clinical Oncology; 1997. Phase I trial of dolastatin 10 in patients with advanced solid tumors, abstr. 782. [Google Scholar]
- 10.Meunier F. Current clinical issues on mycoses in neutropenic patients. Int J Antimicrob Agents. 1996;6:135–140. doi: 10.1016/0924-8579(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Performance standards for Antimicrobial disk susceptibility tests-sixth edition: approved standard M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Newman R A, Fuentes A, Covey J M, Benvenuto J A. Preclinical pharmacology of the natural marine product dolastatin 10 (NSC 376128) Drug Metab Dispos. 1994;22(3):428–432. [PubMed] [Google Scholar]
- 14.Pettit G R. The dolastatins. In: Herz W, Kirby G W, Moore R E, Steglich W, Tamm C, editors. Progress in the chemistry of organic natural products—70th ed. New York, N.Y: Springer-Verlag; 1997. pp. 1–79. [Google Scholar]
- 15.Pettit G R, Kamano Y, Herald C L, Tuinman A A, Boettner F E, Kizu H, Schmidt J M, Baczynskyj L, Tomer K B, Bontems R. The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J Am Chem Soc. 1987;109:6883–6885. [Google Scholar]
- 16.Pettit G R, Singh S B, Hogan F, Lloyd-Williams P, Herald D L, Burkett D D, Clewlow P J. The absolute configuration and synthesis of natural (−)-dolastatin 10. J Am Chem Soc. 1989;111:5463–5465. [Google Scholar]
- 17.Pettit G R, Srirangam J K, Barkóczy J, Williams M D, Boyd M R, Hamel E, Pettit R K, Hogan F, Bai R, Chapuis J, McAllister S C, Schmidt J M. Antineoplastic agents 365. Dolastatin 10 SAR probes. Anti-Cancer Drug Design. 1998;13:243–277. [PubMed] [Google Scholar]
- 18.Pettit G R, Srirangam J K, Singh S B, Williams M D, Herald D L, Barkóczy J, Kantoci D, Hogan F. Dolastatins 24. Synthesis of (−)-dolastatin 10. X-ray molecular structure of N,N-dimethylvalyl-valyl-1. dolaisoleuine tert-butyl ester. J Chem Soc Perkin Trans 1. 1996;5:859–863. [Google Scholar]
- 18a.Pettit, G. R., J. K. Srirangam, and D. Kantoci. September 1997. U. S. patent 5,663,149.
- 19.Samonis G, Bafaloukos D. Fungal infections in cancer patients: an escalating problem. In Vivo. 1992;6:183–194. [PubMed] [Google Scholar]
- 20.Tran H T, Newman R A, Beck D E, et al. Proceedings of the American Association for Cancer Research. Philadelphia, Pa: American Association for Cancer Research; 1997. A phase I, pharmacokinetic/pharmacodynamic study of dolastatin 10 in adult patients with advanced solid tumors, abstr. 2056. [PubMed] [Google Scholar]