Abstract
Background
The effectiveness of inhaled glucocorticoids in shortening the time to symptom resolution or preventing hospitalization or death among outpatients with mild-to-moderate coronavirus disease 2019 (Covid-19) is unclear.
Methods
We conducted a decentralized, double-blind, randomized, placebo-controlled platform trial in the United States to assess the use of repurposed medications in outpatients with confirmed coronavirus disease 2019 (Covid-19). Nonhospitalized adults 30 years of age or older who had at least two symptoms of acute infection that had been present for no more than 7 days before enrollment were randomly assigned to receive inhaled fluticasone furoate at a dose of 200 μg once daily for 14 days or placebo. The primary outcome was the time to sustained recovery, defined as the third of 3 consecutive days without symptoms. Key secondary outcomes included hospitalization or death by day 28 and a composite outcome of the need for an urgent-care or emergency department visit or hospitalization or death through day 28.
Results
Of the 1407 enrolled participants who underwent randomization, 715 were assigned to receive inhaled fluticasone furoate and 692 to receive placebo, and 656 and 621, respectively, were included in the analysis. There was no evidence that the use of fluticasone furoate resulted in a shorter time to recovery than placebo (hazard ratio, 1.01; 95% credible interval, 0.91 to 1.12; posterior probability of benefit [defined as a hazard ratio >1], 0.56). A total of 24 participants (3.7%) in the fluticasone furoate group had urgent-care or emergency department visits or were hospitalized, as compared with 13 participants (2.1%) in the placebo group (hazard ratio, 1.9; 95% credible interval, 0.8 to 3.5). Three participants in each group were hospitalized, and no deaths occurred. Adverse events were uncommon in both groups.
Conclusions
Treatment with inhaled fluticasone furoate for 14 days did not result in a shorter time to recovery than placebo among outpatients with Covid-19 in the United States. (Funded by the National Center for Advancing Translational Sciences and others; ACTIV-6 ClinicalTrials.gov number, NCT04885530.)
As the coronavirus disease 2019 (Covid-19) pandemic continues, the need for early therapies to prevent progression to severe disease is ongoing. New oral antiviral therapies are being used to an increasing degree in high-income countries, with a resulting benefit in unvaccinated persons.1 However, antivirals are unavailable in most low- and middle-income countries, and vaccination rates are variable. Thus, effective therapies are still needed for persons with symptomatic infection to hasten clinical recovery.
Numerous repurposed drugs have been investigated for potential therapeutic effects.2-5 Dexamethasone has been effective among hospitalized patients with hypoxemia,6 and inhaled glucocorticoids have been tested as a possible early outpatient therapy, with inconsistent results.7-11 Two open-label randomized trials involving the use of inhaled budesonide at a dose of 800 μg twice daily for 14 days showed benefits for faster time to recovery and strong trends in decreased hospitalizations or deaths.10,11 Conversely, three randomized trials of inhaled ciclesonide (at a dose of 640 μg per day), two of which were double-blind trials, showed no change in symptom duration, and the analyses showed variable decreases — or possible increases — in the use of health care resources.7-9 These five trials of inhaled glucocorticoids were conducted among predominantly unvaccinated persons.7-11 Trial sizes ranged from 146 to 2530 participants, with both the largest and smallest trials reporting a significant benefit of budesonide over placebo.10,11 The conflicting results led regulatory authorities and guideline committees not to recommend inhaled glucocorticoid therapy for use as an early treatment option in Covid-19.
We sought to investigate inhaled fluticasone furoate in a double-blind, randomized, placebo-controlled platform trial to assess the use of repurposed drugs in nonhospitalized persons with mild-to-moderate Covid-19. Fluticasone propionate has approximately four times the relative systemic steroid potency of budesonide,12 and fluticasone furoate has greater receptor affinity than the propionate ester, allowing for once-daily dosing.13 With enrollment to date having been conducted in the postvaccine era and largely representing persons infected with the delta and omicron (BA.1.1) variants, we report the efficacy of inhaled fluticasone furoate at a dose of 200 μg daily for 14 days as compared with placebo for the treatment of early mild-to-moderate Covid-19.
Methods
Trial Design and Oversight
The Accelerating Covid-19 Therapeutic Interventions and Vaccines (ACTIV-6) trial is an ongoing double-blind, randomized, placebo-controlled, platform-protocol trial conducted with the use of a decentralized approach. ACTIV-6 enrolls outpatients with mild-to-moderate Covid-19 who have a confirmed positive polymerase-chain-reaction (PCR) test or antigen test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, including home-based testing. The protocol and statistical analysis plan are available with the full text of this article at NEJM.org.
The protocol was approved by the institutional review board at each trial site. Informed consent was obtained from all the participants either as written consent or by means of an electronic consent process. An independent data and safety monitoring committee oversaw participant safety and trial performance. GSK donated the inhaled fluticasone furoate used in the trial.
Participants
The ongoing overall platform trial began recruitment on June 11, 2021. Participants were enrolled from August 6, 2021, through February 9, 2022, at 91 sites in the United States. The participants were assigned to the group that received inhaled fluticasone furoate or into groups that received matched placebo inhaler or contributing placebo (matched placebo for a different active study drug, with data from those groups “contributing” to the pooled analyses) from a concurrent study group on the platform. Participants were recruited by trial sites or independently applied to the trial online or by calling a central study telephone hotline. Trial participation of persons who did not live near a local site was managed centrally.
Trial personnel at sites verified that participants met the eligibility criteria: they were at least 30 years of age, had SARS-CoV-2 infection that had been confirmed within the past 10 days, and had had at least two symptoms of Covid-19 for up to 7 days before enrollment. Symptoms included fatigue, dyspnea, fever, cough, nausea, vomiting, diarrhea, body aches, chills, headache, sore throat, nasal symptoms, and new loss of sense of taste or smell. Key exclusion criteria were hospitalization, known allergy or contraindication to the trial drug (including the use of prohibited concomitant medications), or use of the trial drug within 14 days before enrollment. The full list of inclusion and exclusion criteria are provided in the protocol. Fluticasone-furoate–specific exclusion criteria reported by participants were pregnancy, breast-feeding, milk-protein hypersensitivity, and use of inhaled or systemic glucocorticoids within the 30 days before enrollment. Vaccination was not an exclusion criterion. The use of standard-care therapies for Covid-19 that were available under Food and Drug Administration (FDA) approval or emergency use authorization was allowable.
Randomization and Interventions
Within the platform trial, active trial drugs could be added or removed according to adaptive design or emerging evidence (or both). We randomly assigned participants using a random number generator in a two-step process. First, they underwent randomization with equal probability for assignment to receive any trial drug for which participants were actively being enrolled within the platform (i.e., fluticasone furoate at a dose of 200 μg per day, ivermectin at a dose of 400 μg per kilogram of body weight per day, or fluvoxamine at a dose of 50 mg twice daily) and for which the participants were eligible. Participants could choose to opt out of assignment to receive specific trial drugs if they or the site investigators did not believe there was equipoise regarding the effectiveness of any of the active study interventions for treatment of Covid-19. Allowing choice may have complicated direct comparisons among active trial agents, but the comparison between an active agent and placebo remained valid because participants could not opt out of randomization to placebo.
After randomization to potential assignment to one of the trial drugs, participants were randomly assigned to receive either the active agent or placebo in a ratio of m:1, in which m was the number of trial drugs a participant was eligible to receive. The more trial drugs that a participant was eligible for, the greater was that participant’s chance of receiving an active trial drug. Only data from participants who were eligible to receive fluticasone furoate (and who had agreed to be included in randomization for that group) but who were assigned to receive placebo for a different trial drug for which participants were currently being enrolled on the platform were included in the analysis of the concurrent pooled placebo group.
The trial drug or placebo was shipped directly to participants by means of home delivery from a central pharmacy. Participants received an inhaler with a 14-day supply of either fluticasone furoate provided as a dry powder in a foil blister strip or identical matched placebo in a foil blister strip, both provided by the manufacturer. Using the inhaler, participants self-administered 200 μg (packaged as 1 blister) of fluticasone furoate or matched placebo once daily for 14 days. Other concurrent placebo regimens included ivermectin-matched placebo for 3 days or fluvoxamine-matched placebo for 14 days.
Outcome Measures
The primary effectiveness outcome was time to recovery, defined as the third of 3 consecutive days without symptoms. This was selected a priori from among the two coprimary outcomes that remained available to other platform trial drugs (see the statistical analysis plan in the protocol). The key secondary outcome was hospitalization or death by day 28. Other secondary outcomes were the number of days unwell with ongoing symptoms and scores on the Covid-19 clinical progression ordinal outcome scale (based on the World Health Organization Ordinal Scale for Clinical Improvement) on days 7, 14, and 28.
Trial Procedures
ACTIV-6 is a decentralized trial; therefore, all trial visits are prespecified as occurring by telephone or through the study portal accessed with a computer or other device. Screening and eligibility confirmation were reported by the participant and confirmed by trial-site personnel. Positive SARS-CoV-2 test results were verified by site personnel before participants underwent randomization. At the screening visit, participants reported demographic information, eligibility criteria, medical history, concomitant medications, and symptoms, and they completed quality-of-life questionnaires.
A central investigational pharmacy distributed the trial drug or placebo to residential addresses provided by the participants, and shipping and delivery were tracked. The participants must have received the trial drug to have been included in the analysis. The day of receipt of the trial drug was designated as day 1.
Participants were asked to complete assessments and report safety events daily through the first 14 days of the trial. From day 15 through day 28, participants continued to report whether they had symptoms until they had 3 consecutive days without symptoms. Follow-up visits occurred at day 28 and day 90. At each trial assessment, participants reported symptoms and symptom severity, health care visits, and any new medications.
The daily and follow-up assessments were monitored, and sites were actively notified of events necessitating review, including events that met the criteria for serious adverse events or unanticipated adverse device effects associated with the inhaler. In addition, participants were invited during assessments to request contact from the trial team or to report any unusual circumstances. Uncompleted daily assessments also triggered a review for any possible serious adverse events. A missed assessment on the day after the first dose of study medication was received (day 2) or any day of missed assessments up to day 14 prompted a notification to the site to contact the participant. All participants were instructed to report concerns either by accessing the online event reporting system or by calling their trial site or a 24-hour central telephone hotline.
Events of special interest and serious adverse events were obtained by site investigators from the participants’ medical records if participants sought medical care or were hospitalized. Any medical events that occurred after enrollment but began before the receipt of the trial drug (day 1) were not considered to be adverse events or outcome events.
Statistical Analysis
We designed the ACTIV-6 trial using a Bayesian approach. A goal sample size of approximately 1200 participants per trial group was estimated to be sufficient at the outset of this platform trial to show the primary outcome of symptom burden and clinical events.
The planned primary outcome analysis was a Bayesian proportional-hazards model. At the request of regulatory agencies, decision thresholds and other analysis variables were set to balance overall statistical power with strict control of the type I error rate for the planned schedule of interim analyses in the context of a trial-specific outcome.
The primary inferential (decision-making) quantity was the posterior distribution for the treatment-assignment hazard ratio, with a hazard ratio of more than 1.0 considered to indicate benefit. A posterior probability of benefit that exceeded 0.95 at any of the interim or final analyses would be considered to show efficacy of the intervention. The simulations described in the statistical analysis plan show a preserved type I error of less than 0.05, when the prior for the treatment effect parameter (on the log relative-hazard scale) was a normal distribution centered at 0 and scaled to a standard deviation of 0.1. The trial was designed to have 80% power to detect a hazard ratio of more than 1.2 for the primary outcome of time to recovery.
We analyzed secondary outcomes with Bayesian regression models (either proportional hazards or proportional odds) using weakly informative priors for all parameters. We did not use secondary outcomes for formal decision making, and we selected no decision threshold. The same set of covariates that we used in the primary outcome model was used in the analysis of secondary outcomes, provided that enough outcome events accrued to be analyzed with covariate adjustment.
In this platform trial, the primary analysis is implemented separately for each trial drug, in which the placebo group consists of concurrent randomization of participants who met enrollment criteria for the trial drug, including consent to undergo randomization to treatment with fluticasone furoate. A modified intention-to-treat approach was specified for the primary analyses, which included all participants who received the trial drug. On the basis of results from other remote trials,4,14 we recognized that medication delivery (placebo or trial drug) might not always occur (e.g., because of failure of delivery, participant withdrawal, or intervening hospitalization before receipt of delivery). Any participant who did not receive delivery of the drug or placebo was excluded from the modified intention-to-treat population. All available data were used to assess fluticasone furoate as compared with a concurrent placebo control, regardless of postrandomization adherence to the assigned regimen. The safety population included the participants in the modified intention-to-treat population who reported taking at least one dose of trial drug or matching placebo. Heterogeneity in treatment effect was assessed in preselected subgroups and for the following covariates: age, sex, duration of symptoms, body-mass index (BMI, the weight in kilograms divided by the square of the height in meters), symptom severity, calendar time (corresponding to the predominant SARS-CoV-2 variant), and vaccination status.
Results
Participants
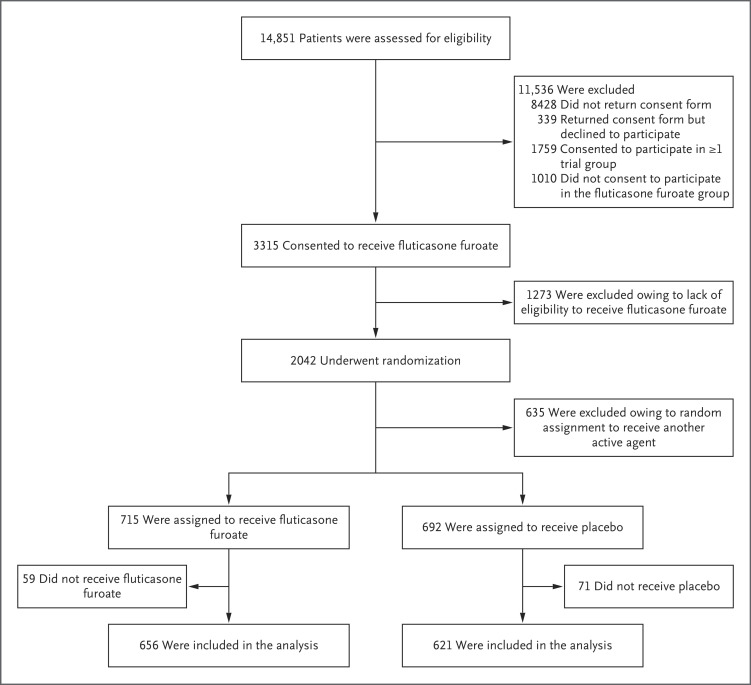
Among the 3750 participants in the platform trial, 715 were randomly assigned to receive inhaled fluticasone furoate at a dose of 200 μg per day and 692 to receive placebo, and 656 and 621, respectively, were included in the analysis. (Figure 1). Of the 621 participants in the placebo group, 350 (56%) received matching placebo and 271 (44%) from concurrent placebo groups were included for analysis.
Figure 1. Randomization and Follow-up of the Patients.
The mean (±SD) age of the participants was 47±12 years, and 39% were at least 50 years of age (Table 1 and Table S1 in the Supplementary Appendix, available at NEJM.org). The population was 63% female; 80.1% of the participants identified as White, 7.1% as Black or African American, and 5.1% as Asian, and 12.6% were of Latino or Hispanic ethnic groups. Although the presence of coexisting conditions was not required for enrollment, they were prevalent, including a BMI of more than 30 (in 39% of all the participants), diabetes (in 10%), hypertension (in 25%), and asthma (in 13%).
Table 1. Characteristics of the Participants at Baseline.*.
| Variable | Inhaled Fluticasone Furoate (N=656) |
Placebo (N=621) |
Total (N=1277) |
|---|---|---|---|
| Median age (IQR) — yr | 45 (37–55) | 46 (38–56) | 45 (37–55) |
| Age <50 yr — no. (%) | 405 (61.7) | 370 (59.6) | 775 (60.7) |
| Female sex — no. (%)† | 431 (65.7) | 376 (60.5) | 807 (63.2) |
| Race — no. (%)‡ | |||
| Black or African American | 47 (7.2) | 44 (7.1) | 91 (7.1) |
| White | 523 (79.7) | 500 (80.5) | 1023 (80.1) |
| Hispanic or Latino ethnic group — no./total no. (%)§ | 78/655 (11.9) | 83/620 (13.4) | 161/1275 (12.6) |
| United States region — no. (%) | |||
| Midwest | 141 (21.5) | 115 (18.5) | 256 (20.0) |
| Northeast | 52 (7.9) | 56 (9.0) | 108 (8.5) |
| South | 368 (56.1) | 371 (59.7) | 739 (57.9) |
| West | 95 (14.5) | 79 (12.7) | 174 (13.6) |
| Call center¶ | 96 (14.6) | 97 (15.6) | 193 (15.1) |
| Body-mass index‖ | |||
| Median (IQR) | 28.1 (24.4–33.6) | 28.1 (24.6–32.9) | 28.1 (24.4–33.4) |
| >30 — no./total no. (%) | 260/656 (39.6) | 239/620 (38.5) | 499/1276 (39.1) |
| Heart disease — no./total no. (%) | 25/640 (3.9) | 33/606 (5.4) | 58/1246 (4.7) |
| Diabetes — no./total no. (%) | 56/640 (8.8) | 65/606 (10.7) | 121/1246 (9.7) |
| Hypertension — no./total no. (%) | 156/640 (24.4) | 169/606 (27.9) | 325/1246 (26.1) |
| COPD — no./total no. (%) | 7/640 (1.1) | 11/606 (1.8) | 18/1246 (1.4) |
| Asthma — no./total no. (%) | 76/640 (11.9) | 86/606 (14.2) | 162/1246 (13.0) |
| Chronic kidney disease — no./total no. (%) | 6/640 (0.9) | 4/606 (0.7) | 10/1246 (0.8) |
| Smoker in past year — no./total no. (%) | 83/640 (13.0) | 72/606 (11.9) | 155/1246 (12.4) |
| Cancer — no. (%) | 20 (3.0) | 23 (3.7) | 43 (3.4) |
| Vaccine status — no. (%) | |||
| Not vaccinated | 220 (33.5) | 211 (34.0) | 431 (33.8) |
| Vaccinated — 1 dose | 8 (1.2) | 11 (1.8) | 19 (1.5) |
| Vaccinated — 2+ doses | 428 (65.2) | 399 (64.3) | 827 (64.8) |
| Median no. of days between symptom onset and receipt of fluticasone furoate or placebo (IQR)** | 6 (4–7) | 5 (4–7) | 5 (4–7) |
COPD denotes chronic obstructive pulmonary disease, and IQR interquartile range.
Participants had the option to report sex as male, female, or unknown.
Participants had the option to choose any (or a combination) of the following race and ethnic-group descriptors: American Indian or Alaska Native; Asian; Black, African American, or African; Middle Eastern or North African; Native Hawaiian or Other Pacific Islander; White; None of the above; and Prefer not to answer.
Participants had the option to report their ethnic group as “Hispanic or Latino” or “Not Hispanic or Latino.”
Participants could enroll in the trial or participate in trial visits by contacting a 24-hour call center.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The distribution of symptoms on trial day 1 at the time of receipt of fluticasone furoate or placebo is shown in Table S2.
Vaccination was common, with 65% of the participants reporting having received at least two doses of a vaccine series. The median time from symptom onset to receipt of the trial drug or placebo was 5 days (interquartile range, 4 to 7) (Fig. S1). Symptom prevalence and severity at baseline are described in Table S2. Although prespecified as allowable, therapeutics available under FDA approval or emergency use authorization were uncommonly used (i.e., remdesivir in 0.1% of participants, monoclonal antibody in 2.4%, and ritonavir-boosted nirmatrelvir in 0.1%) (Table S3).
Primary and Secondary Outcomes
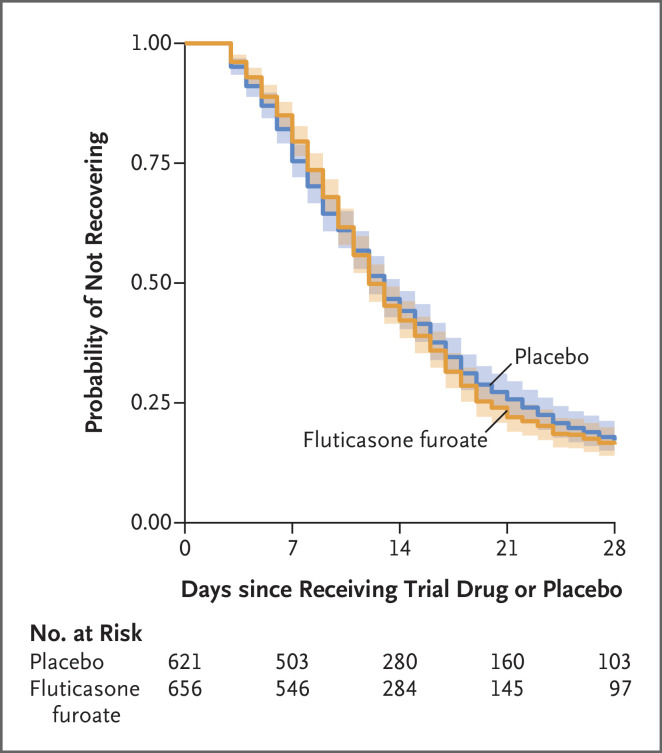
In analyses involving data from the modified intention-to-treat population, we observed no significant difference in the primary outcome of time to recovery between the inhaled fluticasone furoate group and placebo group (hazard ratio, 1.01; 95% credible interval, 0.91 to 1.12; posterior probability of benefit [defined as a hazard ratio >1], 0.56) (Table 2, Figure 2, and Figs. S2 and S3). Results of an exploratory analysis suggested that observations regarding the time to recovery may have differed when active treatment was compared with a matched placebo and when active treatment was compared with an unmatched placebo; participants who received unmatched placebo had faster recovery than their counterparts who received matched placebo (Fig. S4).
Table 2. Primary and Secondary Outcomes through Day 28.*.
| Outcome | Inhaled Fluticasone Furoate (N=621) |
Placebo (N=656) |
Estimated Difference or Ratio (95% Credible Interval or Confidence Interval) |
Posterior Probability of Efficacy |
|---|---|---|---|---|
| Time to recovery† | ||||
| Skeptical prior: primary analysis | 1.01 (0.91 to 1.12)‡ | 0.56 | ||
| Weakly informative prior: sensitivity analysis | 1.01 (0.89 to 1.14)‡ | 0.57 | ||
| No prior: sensitivity analysis | 1.01 (0.89 to 1.14)§ | — | ||
| Mean no. of days unwell¶ | 11.2 (11.0 to 11.4) | 11.3 (11.1 to 11.5) | −0.10 (−0.45 to 0.26)‖ | 0.70 |
| Hospitalization or death through day 28 — no. (%) | 3 (0.5) | 3 (0.5) | 0.94 (0.19 to 4.68)** | — |
| Composite of hospitalization, urgent-care visit, emergency department visit, or death — no. (%) | 24 (3.7) | 13 (2.1) | 1.9 (0.8 to 3.5)‡ | 0.04 |
| Value on Covid-19 clinical progression ordinal outcome scale†† | ||||
| Day 7 | 1.10 (0.62 to 1.63) | 0.41 | ||
| Day 14 | 0.91 (0.42 to 1.50) | 0.67 | ||
| Day 28 | 2.74 (0.50 to 5.94) | 0.06 |
There were no deaths reported in either group. Covid-19 denotes coronavirus disease 2019.
The skeptical and weakly informative priors were zero-centered normal distributions with standard deviations of 0.1 and 2.5, respectively. No prior indicates that the hazard ratio was estimated with maximum partial likelihood methods, which do not incorporate a prior distribution. A hazard ratio of more than 1.0 is favorable for faster recovery.
The value is a hazard ratio and the range is the credible interval.
The value is a hazard ratio and the range is the confidence interval (CI).
The mean number of days unwell with ongoing symptoms was estimated from the day of receipt of the trial drug through trial day 14.
The value is the mean difference, and the range is the credible interval. Figure S5C shows the posterior distribution of the difference in mean days unwell.
A low incidence of events precluded covariate adjustment.
The clinical progression ordinal outcome scale is based on the World Health Organization Ordinal Scale for Clinical Improvement. The value is an odds ratio, and the range is the credible interval. Values greater than 1 indicate greater odds of progression with fluticasone than with placebo.
Figure 2. Times to Sustained Recovery with Inhaled Fluticasone Furoate or Placebo.
Shown is the Kaplan–Meier curve for the primary outcome of time to recovery with pointwise 95% confidence intervals indicated with shading. Time to sustained recovery was defined as the number of days between the day of receipt of the trial drug or placebo and 3 consecutive days without Covid-19 symptoms, as affirmatively reported by the trial participants. Time-to-recovery data were administratively censored at 28 days. The posterior probability was 0.56 for a faster recovery with inhaled fluticasone furoate than with placebo. The posterior distribution of treatment effect is shown in Figure S2 in the Supplementary Appendix, sensitivity analyses for alternative methods of handling missing daily symptom data are shown in Figure S3, the Kaplan–Meier curve for stratification of matched and unmatched placebo groups is shown in Figure S7, and results of an unplanned analysis in which participants who received unmatched placebos appeared to recover faster than participants who received matched placebos are shown in Figure S4.
Secondary outcomes that were measured through day 28 (Fig. S5A through S5C) were similar in the two groups. There were no deaths, and hospitalizations were uncommon, occurring in only three participants (0.5%) in each of the two groups. There were numerically more composite secondary outcome events of urgent-care or emergency department visits, hospitalizations, or death in the fluticasone furoate group (24 participants [3.7%]) than in the placebo group (13 participants [2.1%]) (Table 2). We did not observe any benefit with regard to scores on the Covid-19 clinical progression ordinal outcome scale (Fig. S6) at days 7, 14, and 28, although slightly fewer participants in the fluticasone furoate group responded to surveys on days 14 and 28. For example, by day 7, a total of 567 participants (86.4%) in the fluticasone furoate group and 546 participants (87.9%) in the placebo group were not hospitalized and did not have any limitation of activities (Table S4).
Heterogeneity of Treatment-Effect Analyses
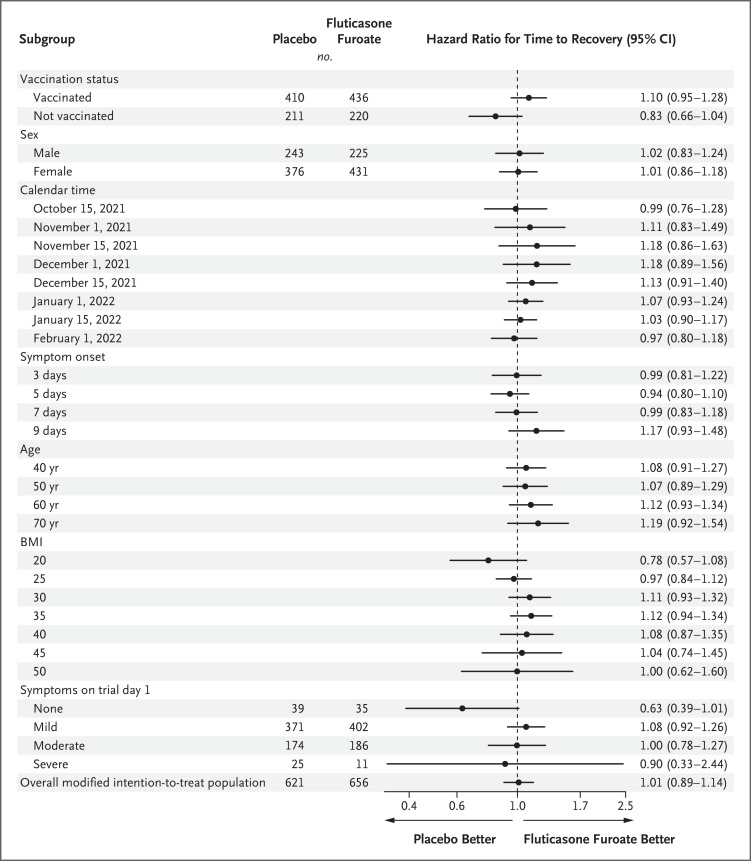
We found no evidence of a treatment effect for time to recovery with fluticasone furoate as compared with placebo with regard to timing of symptom onset, severity of symptoms (classified as none, none more than mild, none more than moderate, and severe), BMI, age, sex, or calendar period (Figure 3 and Fig. S7). Possible differential treatment effects were noted for vaccination status. Among the 845 participants who were vaccinated, those in the fluticasone furoate group tended to have faster recovery than participants in the placebo group (hazard ratio, 1.10, 95% confidence interval [CI], 0.95 to 1.28). However, among unvaccinated participants, those in the fluticasone furoate group tended to have longer times to recovery than participants in the placebo group (hazard ratio, 0.83; 95% CI, 0.66 to 1.03), a finding that suggests possible heterogeneity in the treatment response (Figure 3).
Figure 3. Heterogeneity of the Treatment Effect of Time to Recovery.
A hazard ratio greater than 1.0 indicates a faster time to recovery. Trial day 1 was the day receipt of trial medication or placebo began. Modified intention-to-treat data reflects an analysis of participants who underwent randomization and were enrolled within 7 days after symptom onset and received the trial drug or placebo. Shown are the covariate-adjusted and model-based estimates of the treatment effect for selected subgroups.
Safety
When we excluded from analysis the participants who did not report taking at least one dose of the trial medication or placebo (16 participants in each group), the incidence of adverse events was similar in the two groups (13 of 640 participants [2.0%] in the fluticasone furoate group vs. 16 of 605 participants [2.5%] in the placebo group) (Tables S5 and S6).
Discussion
No beneficial treatment effects with inhaled fluticasone furoate at a daily dose of 200 μg were identified in this randomized, double-blind trial involving 1277 participants. The lack of treatment effect was consistent for the primary outcome of time to recovery with symptom resolution. Although no deaths occurred and hospitalizations were rare and similar in both groups, the number of urgent-care and emergency department visits was higher in the fluticasone furoate group than in the placebo group. Overall, the lack of treatment effect and the possible increase in health care visits observed with inhaled fluticasone furoate as compared with placebo (3.2% vs. 1.6%) suggest that inhaled fluticasone furoate is not a favorable Covid-19 therapy.
Conflicting results have been reported in numerous trials of inhaled glucocorticoids, differences that may have been the result of varying trial populations or sample sizes.7-10 ACTIV-6 has some similarities to the United Kingdom PRINCIPLE trial as well as some differences.11 The timing of treatment was similar: the PRINCIPLE trial initiated treatment with inhaled budesonide at a median of 6 days (interquartile range, 4 to 9) from symptom onset. In theory, the relative daily dose of glucocorticoids that was specified in the PRINCIPLE trial was similar to the 200-μg daily dose of fluticasone furoate. The fluticasone furoate therapy at 200 μg daily is approximately four times more potent than budesonide at a twice-daily dose of 800 μg, and the fluticasone furoate formulation has a longer half-life.12,13 However, fluticasone and budesonide are different glucocorticoids, and there could be a budesonide-specific effect. Two other notable differences are the methods used and the trial populations. ACTIV-6 used a double-blind, placebo-controlled trial design, and PRINCIPLE was a pragmatic, open-label trial in which the control group received usual care and did not receive placebo.11 Whether a placebo effect could account for the faster (by 2.9 days) time to recovery in the PRINCIPLE trial seems unlikely; however, ACTIV-6 investigators observed no improvement in time to recovery. Finally, these trials enrolled different populations. The PRINCIPLE trial enrolled unvaccinated persons who were older than 65 years of age or older than 50 years of age with a coexisting condition (overall mean age, 65 years), whereas ACTIV-6 enrolled any adult at least 30 years of age (mean age, 47 years), of whom 65% were vaccinated. The differences in age and vaccination status might have resulted in differences with regard to the risk of severe disease and opportunities for treatment effects. In ACTIV-6, participants who were older than 70 years had the most favorable benefit with regard to faster symptom resolution (hazard ratio, 1.19; 95% credible interval, 0.93 to 1.54), but in the PRINCIPLE trial, there was no observed differential effect according to age.11 Similarly, participants in ACTIV-6 who reported completion of any Covid-19 vaccine series were more likely to have a benefit (hazard ratio, 1.10; 95% credible interval, 0.95 to 1.28), but the PRINCIPLE trial did not include persons who were vaccinated against Covid-19. Thus, differing vaccination status between the two trials does not appear to account for the differing results.
Our trial has several strengths. As a nationwide trial, ACTIV-6 findings are generalizable to all adults in the United States who are at least 30 years of age with Covid-19. This trial had rapid enrollment during the delta- and omicron-variant surges and included vaccinated patients, thus remaining a relevant population.
Our trial also has several limitations. First, owing to the broadly inclusive trial population, few clinical events occurred, which limited the power to study the treatment effect on clinical outcomes such as hospitalization. Second, although the inclusion criteria allowed for a broad study population, the ACTIV-6 trial did not reach the level of representation desired for underrepresented populations. Third, owing to the remote nature of the trial, the median time from symptom onset to receipt of the trial drug was 6 days, which is longer than the recommended target of 5 days or less for the start of antiviral therapy.1,15 However, evidence for an interaction with respect to the duration of symptoms from the time of the start of therapy was not strong. Finally, the different administration methods of placebo (oral vs. inhaled) resulted in possible heterogeneity in the placebo groups.
Our trial did not identify a clinically relevant effect associated with inhaled fluticasone furoate at a daily dose of 200 μg for 14 days as outpatient treatment for Covid-19 when delivered directly to the participant along with written instructions for use of the inhaler. We also did not observe a faster time to clinical recovery with fluticasone furoate as compared with placebo in the population studied, unlike results shown in previous open-label trials of inhaled steroids, nor did we observe an effect on prevention of clinical progression.10,11
Acknowledgments
We thank Samuel Bozzette, M.D., Ph.D., and Eugene Passamani, M.D., both of NCATS, for their contributions to the trial design and protocol development.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was updated on September 21, 2023, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by a grant (3U24TR001608-06S1) from the National Center for Advancing Translational Sciences (NCATS), a contract (no.75A50122C00037) with the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, a Vanderbilt University Medical Center Clinical and Translational Science award from NCATS (UL1TR002243), and a core grant (U24TR001579) from the Vanderbilt University Medical Center Recruitment Innovation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022;386:1397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022;10(1):e42-e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramante CT, Huling JD, Tignanelli CJ, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med 2022;387:599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med 2020;173:623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis G, Silva EASM, Silva DCM, et al. Effect of early treatment with ivermectin among patients with Covid-19. N Engl J Med 2022;386:1721-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern Med 2022;182:42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvignaud A, Lhomme E, Onaisi R, et al. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE). Clin Microbiol Infect 2022;28:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezer N, Belga S, Daneman N, et al. Inhaled and intranasal ciclesonide for the treatment of Covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ 2021;375:e068060-e068060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramakrishnan S, Nicolau DV Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med 2021;9:763-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L-M, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021;398:843-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boorsma M, Andersson N, Larsson P, Ullman A. Assessment of the relative systemic potency of inhaled fluticasone and budesonide. Eur Respir J 1996;9:1427-1432. [DOI] [PubMed] [Google Scholar]
- 13.Biggadike K. Fluticasone furoate/fluticasone propionate — different drugs with different properties. Clin Respir J 2011;5:183-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020;383:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022;386:509-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.