Abstract
We have used a specific inhibitor of the malarial aspartic proteinase plasmepsin I and a nonspecific cysteine proteinase inhibitor to investigate the importance of hemoglobin degradation in the mechanism of action of chloroquine, amodiaquine, quinine, mefloquine (MQ), halofantrine, and primaquine. Both proteinase inhibitors antagonized the antiparasitic activity of all drugs tested with the exception of primaquine. An inhibitor of plasmepsin I, Ro40-4388, reduced the incorporation of radiolabelled chloroquine and quinine into malarial pigment by 95%, while causing a 70% reduction in the incorporation of radiolabelled MQ. Cysteine proteinase inhibitor E64 reduced the incorporation of chloroquine and quinine into malarial pigment by 60 and 40%, respectively. This study provides definitive support for the central role of hemoglobin degradation in the mechanism of action of the 4-aminoquinolines and the quinoline and phenanthrene methanol antimalarials.
The 4-aminoquinolines chloroquine (CQ) and amodiaquine (AQ), the quinoline methanols quinine (QN) and mefloquine (MQ), and the phenanthrene methanol halofantrine (HF) all exert selective toxicity towards the erythrocytic stages of malaria parasites and were developed based on a knowledge of quinine structure and activity (29, 31, 41). Although there are structural similarities, QN, MQ, and HF are generally considered to constitute a group distinct from CQ and AQ. This classification is based on a number of reported differences. The 4-aminoquinolines are diprotonated and less lipid soluble at physiological pH, whereas the others, most notably QN and MQ, are much weaker bases (26, 28, 50). Recent reports suggest an inverse relationship between parasite sensitivity to CQ and sensitivity to MQ, HF, and QN (22, 40, 44, 49). CQ and AQ induce pigment clumping in Plasmodium berghei (24, 46). The quinoline methanols do not induce pigment clumping but can inhibit 4-aminoquinoline-induced clumping (20, 27, 30). Based on these observations and spectrophotometric studies it has been suggested that the interactions between the two drug classes and hematin are fundamentally different (48, 49).
Morphological effects following treatment with MQ, QN, and HF are similar to that observed following treatment with CQ, i.e., an initial swelling of the acid food vacuole (20, 27, 30). It is generally accepted that CQ (and AQ) exerts its antimalarial effects by interacting with the hemoglobin degradation process within the parasite, probably through an interaction with hematin (12, 33, 34), although the absolute mechanism of action is still debated (1, 39, 45). The inhibition of hematin polymerization has been used as a surrogate marker of 4-aminoquinoline type antimalarial activities (12, 19, 32, 38), and MQ, QN, and HF, like CQ, and AQ, can inhibit this process in vitro (12, 19, 39). However, there are suggestions that an interaction with hematin polymerization per se may not be enough to explain the activity of drugs such as MQ (14). Although MQ and QN do interact with free hematin, the interaction is relatively weak (6, 7, 12), with correspondingly weaker inhibition of hematin polymerization (12). MQ is a monoprotic weak base which should accumulate less well than CQ; nevertheless, it shows similar 50% inhibitory concentration (IC50) values in vitro. This evidence has been the basis for questioning whether these drugs interact at different points within the hemoglobin degradation process or if MQ has an additional or an independent mechanism of action distinct from that of AQ and CQ (10, 14).
Hemoglobin degradation within the parasite is an ordered process (16, 18) involving at least three proteinases. Aspartic proteinase plasmepsin I is responsible for the initial cleavage of the hemoglobin tetramer at the hinge position, the Phe33-Leu34 bond in the α-globin chain (17). A second aspartic proteinase, plasmepsin II, has also been identified and may have a role in the cleavage of denatured hemoglobin (16). Falcipain, a cysteine proteinase, is also implicated in the cleavage of peptides from the denatured hemoglobin (15, 16). The amino acids resulting from this process are presumably used by the parasite (37, 43). It is generally agreed that the remaining hematin residue, which is potentially toxic, is removed via a polymerization process (11, 38), degradation, or export. Much evidence has accumulated to support the hypothesis that quinoline type blood schizontocides exert their antimalarial activity through interacting with hematin (3, 8, 12, 42). We have recently extended these observations and have provided strong evidence, in the case of CQ, that both the mechanism of action and resistance in the parasite are based on drug access to hematin (5). Further, it has been reported that a specific inhibitor of malarial plasmepsin I, Ro40-4388, antagonizes the actions of CQ (25).
We have used Ro40-4388 and a nonselective inhibitor of cysteine proteinase, E64, as probes to determine if the antimalarial activities of QN, HF, MQ, AQ, and CQ all depend on the efficient degradation of hemoglobin. Primaquine (PQ), an aminoquinoline antimalarial which does not inhibit hematin polymerization (12, 19) and which probably exerts its antimalarial action via a heme-independent mechanism (12), was used as a control.
MATERIALS AND METHODS
Drugs used in the study.
CQ, AQ, QN, PQ, and trans-epoxysuccinyl-l-leucylamido-(4-guanidino)-butane (E64) were purchased from Sigma, Dorset, United Kingdom. MQ and Ro40-4388 were obtained from Hoffmann-La Roche, Basel, Switzerland, and HF was obtained from SmithKline Beecham.
Parasite isolates and cultivation.
A CQ-resistant isolate of Plasmodium falciparum K1 and CQ-sensitive isolate HB3, obtained from D. C. Warhurst, London School of Hygiene and Tropical Medicine, London, United Kingdom, were used throughout this study. Parasite cultures were maintained by an adaptation of the method of Jensen and Trager (21). Cultures were synchronized by the method of Lambros and Vandenburg (23) before use.
In vitro sensitivity assays.
Drug susceptibilities were assessed by the measurement of [3H]hypoxanthine incorporation into parasite nucleic acid as previously described by Desjardins et al. (9). Drug IC50s were calculated from the log of the dose/response relationship, as fitted with Grafit software (Erithacus Software, Kent, United Kingdom). Results are given as the means of at least three separate experiments.
Drug combination assays.
To analyze the combined effect of the antimalarials and proteinase inhibitors (plasmepsin I inhibitor Ro40-4388 and cysteine proteinase inhibitor E64) the IC50 for each drug alone was obtained as described above. From these values, a stock solution of each drug was prepared such that the IC50 of each drug would fall around the fourth serial dilution. Combinations of the stock solutions were prepared in constant ratios of 0:10, 1:9, 3:7, 5:5, 7:3, 9:1, and 10:0. Each combination was serially diluted across a microtiter plate and processed as for the standard sensitivity assay. The fractional inhibitory concentration (FIC; FIC = IC50 of the drug in the combination/IC50 of the drug when tested alone) of each drug was calculated and plotted as an isobologram (4).
Hemozoin purification.
Ring stage parasites were incubated for 24 h in the presence of radiolabelled drug and in the presence or absence of a fixed concentration of proteinase inhibitor Ro40-4388 (300 nM) or E64 (10 μM). The HB3 isolate was used with [3H]CQ (specific activity = 50.4 Ci/mmol) at a concentration of 1 nM and with [3H]QN (specific activity = 14.5 Ci/mmol) at a concentration of 7 nM. The K1 isolate was incubated with [3H]MQ (specific activity = 9.78 Ci/mmol) at a concentration of 5 nM. Hemozoin from the parasite was purified with a sucrose cushion as previously described (42). The cultures were pelleted and washed in RPMI 1640 twice, and the parasites were then lysed with 5 mM sodium phosphate, pH 7.5. The parasite lysate was pelleted and resuspended in 50 mM Tris-HCl, pH 8.0. After sonication the sample was layered on top of a 1.7 M sucrose cushion in 50 mM Tris-HCl (1 ml), pH 8.0, followed by ultracentrifugation at 200,000 × g for 15 min. The pellet was then washed twice with 50 mM Tris-HCl, pH 8.0, and processed for scintillation counting.
RESULTS
In vitro sensitivity of the parasites to antimalarial drugs and proteinase inhibitors.
The IC50 data for all antimalarial drugs tested and for proteinase inhibitors Ro40-4388 and E64, indicative of their activities against CQ-resistant isolate K1 and CQ-sensitive isolate HB3, are shown in Table 1. The ability of specific plasmepsin I inhibitor Ro40-4388 to inhibit parasite growth is shown to be more potent than that of cysteine proteinase inhibitor E64. The CQ-resistant and CQ-sensitive parasite isolates showed no differential susceptibilities to these proteinase inhibitors. PQ displayed antimalarial activity weaker than those of the other quinoline-containing drugs used in this study.
TABLE 1.
In vitro sensitivities of the K1 and HB3 isolates of P. falciparum to the selected antimalarial drugs and proteinase inhibitorsa
| Drug | Mean IC50 (nM) ± SD for isolate:
|
|
|---|---|---|
| K1 | HB3 | |
| Ro40-4388 | 256 ± 26 | 318 ± 22 |
| E64 | 8,982 ± 744 | 9,653 ± 1,677 |
| AQ | 18.3 ± 1.5 | 4.1 ± 1 |
| CQ | 110 ± 10 | 17.7 ± 2.5 |
| HF | 2.6 ± 0.9 | 3.8 ± 0.3 |
| MQ | 14.8 ± 3.2 | 28.7 ± 3.5 |
| QN | 176 ± 25 | 100 ± 20 |
| PQ | 2,027 ± 635 | 8,086 ± 278 |
Data were derived from at least three assays performed at a hematocrit of 1% and a parasitemia of 1%.
The interaction between quinolines and proteinase inhibitors.
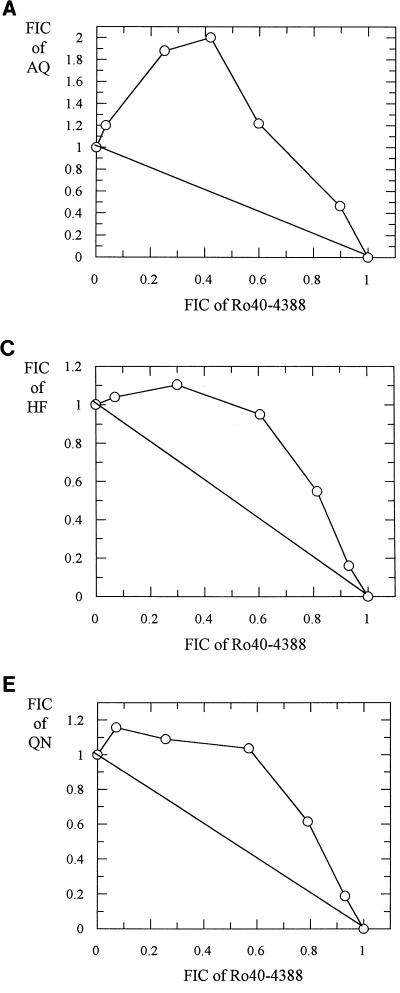
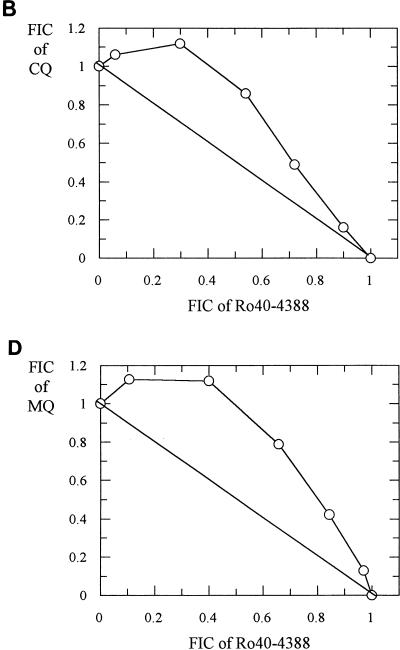
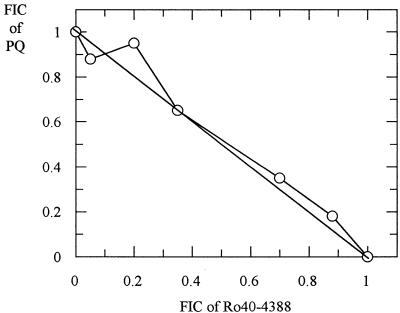
Representative isobolograms for antimalarial drug-proteinase inhibitor combinations are shown in Fig. 1, 2, and 3. The interactions between Ro40-4388 and CQ, AQ, MQ, and HF against the K1 isolate were antagonistic (Fig. 1). Similar antagonism was observed between E64 and these five drugs (Fig. 2). In contrast, the interaction between PQ and Ro40-4388 was additive (Fig. 3). Similar data were obtained with the HB3 isolate (data not shown).
FIG. 1.
Isobolograms showing the relationship between the FICs of Ro40-4388 and AQ (A), CQ (B), HF (C), MQ (D), and QN (E) in the K1 isolate.
FIG. 2.
Isobolograms showing the relationship between the FICs of E64 and AQ (A), CQ (B), HF (C), MQ (D), and QN (E) in the K1 isolate.
FIG. 3.
Isobologram showing the relationship between the FICs of Ro40-4388 and PQ in the K1 isolate.
The effect of proteinase inhibitors on the incorporation of quinolines into hemozoin.
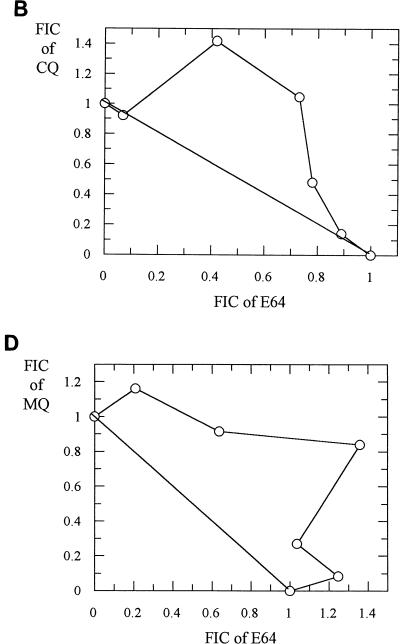
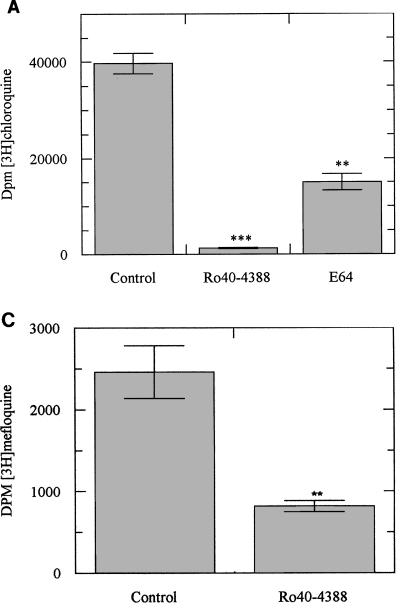
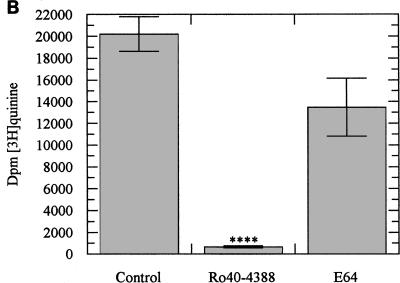
Incubation of ring stage parasites with either radiolabelled CQ, QN, or MQ over 24 h resulted in radiolabelled drug incorporation within the malarial pigment. Ro40-4388 at its IC50 reduced CQ and QN incorporation by more than 95% (Fig. 4A and B) and produced a 70% reduction in MQ incorporation (Fig. 4C). E64 was less efficient in reducing the incorporation of radiolabelled drugs (Fig. 4A and B). The reductions produced by E64 at its IC50 were approximately 60% for CQ and 40% for QN. This effect of E64 is consistent with the observations of Asawamahasakda et al. (1).
FIG. 4.
(A) Incorporation of [3H]CQ into hemozoin in the presence or absence of an inhibitor of plasmepsin I, Ro40-4388 (300 nM), or of cysteine proteinase, E64 (10 μM). Data represent means ± standard deviations of five separate experiments; each experiment was performed in triplicate with 2 × 109 parasitized erythrocytes. ∗∗, P < 0.05; ∗∗∗, P < 0.005. (B) Incorporation of [3H]QN into hemozoin in the presence or absence of Ro40-4388 (300 nM) or E64 (10 μM). Data represent means ± standard deviations of five separate experiments; each experiment was performed in triplicate with 2 × 109 parasitized erythrocytes. ∗∗∗∗, P < 0.001. (C) Incorporation of [3H]MQ into hemozoin in the presence or absence of Ro40-4388 (300 nM). Data represent means ± standard deviations of four separate experiments; each experiment was performed in triplicate with 3 × 109 parasitized erythrocytes. ∗∗, P < 0.05.
DISCUSSION
The malaria parasite needs to degrade hemoglobin for successful growth and development. We believe that this is highlighted by the ability of the two proteinase inhibitors used in this study to inhibit parasite growth, as measured by the incorporation of hypoxanthine. The IC50s of proteinase inhibitors Ro40-4388 and E64 in P. falciparum presented here are comparable to those reported earlier (2, 25). Cysteine proteinase inhibitor E64 has previously been shown to inhibit parasite growth at the trophozoite stage, causing the accumulation of undegraded hemoglobin within the food vacuole (2, 35, 36). E64 was shown to reduce the formation of hemozoin via an inhibition of hemoglobin degradation (1, 35), and this effect was irreversible (35). In contrast, Ro40-4388 has been shown to inhibit the growth of P. falciparum parasites in vitro at nanomolar concentrations (25). Interestingly, the inhibitory effects of Ro40-4388 on hemozoin formation and parasite growth were reversible (unpublished observations). The removal of inhibitor after 24 h of incubation by minimal washing in complete medium was followed by pigment production and parasite growth. This apparent parasitistatic effect may have important implications for the use of these inhibitors as antimalarials.
Moon et al. (25) have shown that Ro40-4388 and CQ interact antagonistically against P. falciparum. We have found similar antagonism between Ro40-4388 and AQ, QN, MQ, and HF. These data suggest that all of the drugs tested have a common mechanism of action based on some component of the hemoglobin degradation process. The fact that [3H]QN and [3H]MQ are incorporated into hemozoin in a manner similar to that of [3H]CQ (42) lends support to this argument but does not provide conclusive proof. Since we have previously shown that Ro40-4388 produces a marked decrease in the number of binding sites, specifically for heme-binding drugs (5), we believe that the antagonism between Ro40-4388 and the quinolines and phenanthrenes is due to a reduction in the amount of heme available for drug binding. Therefore, the observation that the incorporation of a radiolabelled drug (CQ, QN, or MQ) into the growing hemozoin polymers is almost completely arrested in the presence of Ro40-4388 would suggest that it is the interaction of the drug with the heme monomer or polymer which is central to activity, rather than any secondary effect on heme polymerization. This is in keeping with many of the hypotheses put forward to explain the antimalarial activities of these drugs over the years (5, 7, 13) and argues against the need to invoke different mechanisms of action for the 4-aminoquinolines and the quinoline or phenanthrene methanols, as has been suggested (10, 14). The facts that PQ does not inhibit hematin polymerization (12) and that it most likely exerts its antimalarial action via a heme-independent mechanism (12) give support to the use of PQ as a control in this study. As predicted, there was no antagonism between PQ and Ro40-4388. The observations of antagonism between the cysteine proteinase inhibitor E64 highlight, we believe, the importance of the cysteine proteinase falcipain in hemoglobin degradation and help to further confirm the view that all of these drugs (CQ, AQ, QN, MQ, and HF) exert their antimalarial effects via a common heme-dependent mechanism.
We have confirmed the incorporation of radiolabelled CQ into the growing hemozoin reported initially by Sullivan et al. (42) and have extended these observations to QN and MQ. The fact that this incorporation can be reduced with the proteinase inhibitors further supports a role for hemoglobin degradation in their antimalarial activity. It could be argued that, as incorporation was reduced by approximately 50% by E64 at its IC50, some of this effect could be the indirect result of parasite death. However, exposure to this concentration of E64 for an equivalent period has been shown to have little effect on hypoxanthine uptake (1) and, by implication, on parasite viability.
The data presented here confirm the central and common role of hemoglobin degradation in the mechanisms of action of the 4-aminoquinolines, the quinoline methanols, and the phenanthrene methanols. This supports the view that all of these compounds are acting through the same process without the need to invoke additional targets. The data confirm that proteinase inhibition may be a rational target for antimalarial chemotherapy. If future strategies include the use of these inhibitors in combination with other antimalarial drugs, the antagonism seen here would argue against combinations with quinoline type compounds.
ACKNOWLEDGMENTS
This work was supported by a research program grant from The Wellcome Trust. M. Mungthin was supported by the Thai government and Pramongkutklao College of Medicine.
REFERENCES
- 1.Asawamahasakda W, Ittarat S, Chang C C, McElroy P, Meshnick S R. Effects of antimalarials and proteinase inhibitors on plasmodial haemozoin production. Mol Biochem Parasitol. 1994;67:183–191. doi: 10.1016/0166-6851(94)00128-6. [DOI] [PubMed] [Google Scholar]
- 2.Bailly E, Jambou R, Savel J, Jaureguiberry G. Plasmodium falciparum: differential sensitivity in vitro to E-64 (cysteine protease inhibitor) and Pepstatin A (aspartyl protease inhibitor) J Protozool. 1992;39:593–599. doi: 10.1111/j.1550-7408.1992.tb04856.x. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian D, Mohan Roa C, Panjipan B. The malaria parasite monitored by photoacoustic spectroscopy. Science. 1984;223:828–830. doi: 10.1126/science.6695185. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum M C. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 5.Bray P G, Mungthin M, Ridley R G, Ward S A. Access to haematin: the basis of chloroquine resistance. Mol Pharmacol. 1998;54:170–179. doi: 10.1124/mol.54.1.170. [DOI] [PubMed] [Google Scholar]
- 6.Chevli R, Fitsch C D. The antimalarial drug mefloquine binds to membrane phospholipids. Antimicrob Agents Chemother. 1982;21:581–586. doi: 10.1128/aac.21.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou A C, Chevli R, Fitch C D. Ferriprotoporphyrin IX fulfils the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980;19:1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Phifer K, Yielding K. Complex formation between chloroquine and ferrihaemic acid in vitro, and its effect on the antimalarial action of chloroquine. Nature. 1964;202:805–806. doi: 10.1038/202805a0. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desneves J, Thorn G, Berman A, Galatis D, La Greca N, Sinding J, Foley M, Deady L W, Cowman A F, Tilley L. Photoaffinity labelling of mefloquine-binding proteins in human serum, uninfected erythrocytes and Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1996;82:181–194. doi: 10.1016/0166-6851(96)02732-6. [DOI] [PubMed] [Google Scholar]
- 11.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley R G. Malarial haemozoin/β-haematin supports haem polymerization in the absence of protein. Nature. 1995;374:269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 12.Dorn A, Vippagunta S R, Matile H, Jaquet C, Vennerstrom J L, Ridley R G. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem Pharmacol. 1998;55:727–736. doi: 10.1016/s0006-2952(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 13.Fitch C D. Mode of action of antimalarial drugs. In: Evered D, Whelan J, editors. Malaria and the red cell—1983. Ciba Foundation symposium. London, United Kingdom: Pitman; 1983. pp. 222–232. [PubMed] [Google Scholar]
- 14.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance. Int J Parasitol. 1997;27:231–240. doi: 10.1016/s0020-7519(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 15.Francis S E, Gluzman I Y, Oksman A, Banerjee D, Goldberg D E. Characterization of native falcipain, an enzyme involved in Plasmodium falciparum hemoglobin degradation. Mol Biochem Parasitol. 1996;83:189–200. doi: 10.1016/s0166-6851(96)02772-7. [DOI] [PubMed] [Google Scholar]
- 16.Gluzman I Y, Francis S E, Oksman A, Smith C E, Duffin K L, Goldberg D E. Order and specificity of the Plasmodium falciparum haemoglobin degradation pathway. J Clin Invest. 1994;93:1602–1608. doi: 10.1172/JCI117140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg D E, Slater A F G, Beavis R, Chait B, Cerami A, Henderson G B. Haemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J Exp Med. 1991;173:961–969. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg D E, Slater A F G. The pathway of hemoglobin degradation in malaria parasites. Parasitol Today. 1992;8:280–283. doi: 10.1016/0169-4758(92)90146-s. [DOI] [PubMed] [Google Scholar]
- 19.Hawley S R, Bray P G, Mungthin M, Atkinson J D, O’Neill P M, Ward S A. Relationship between antimalarial drug activity, accumulation, and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1998;42:682–686. doi: 10.1128/aac.42.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob G H, Aikawa M, Milhous W K, Rabbege J R. An ultrastructural study of the effects of mefloquine on malaria parasites. Am J Trop Med Hyg. 1987;36:9–14. doi: 10.4269/ajtmh.1987.36.9. [DOI] [PubMed] [Google Scholar]
- 21.Jensen J B, Trager W. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle-jar method. J Parasitol. 1977;63:883–886. [PubMed] [Google Scholar]
- 22.Knowles G, Davidson W L, Jolley D, Alpers M P. The relationship between the in vitro response of Plasmodium falciparum to chloroquine, quinine and mefloquine. Trans R Soc Trop Med Hyg. 1984;78:146–150. doi: 10.1016/0035-9203(84)90260-8. [DOI] [PubMed] [Google Scholar]
- 23.Lambros C, Vandenburg J P. Synchronisation of Plasmodium falciparum erythrocyte stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 24.Macomber P B, Sprinz H, Tousimis A J. Morphological effects of chloroquine on Plasmodium berghei in mice. Nature. 1967;214:937–939. doi: 10.1038/214937a0. [DOI] [PubMed] [Google Scholar]
- 25.Moon R P, Tyas L, Certa U, Rupp K, Bur D, Jaquet C, Matile H, Loetscher H, Grueninger-Leitch F, Kay J, Dunn B M, Berry C, Ridley R G. Expression and characterization of plasmepsin I from Plasmodium falciparum. Eur J Biochem. 1997;244:552–560. doi: 10.1111/j.1432-1033.1997.00552.x. [DOI] [PubMed] [Google Scholar]
- 26.Mu J Y, Israili Z H, Dayton P G. Studies of the disposition and metabolism of mefloquine HCl (WR 142490), a quinolinemethanol antimalarial, in the rat. Limited studies with an analog, WR 30090. Drug Metab Dispos. 1975;3:198–210. [PubMed] [Google Scholar]
- 27.Olliaro P L, Castelli F, Caligaris S, Druihe P, Carosi G. Ultrastructure of Plasmodium falciparum in vitro. II. Morphological patterns of different quinolines’ effects. Microbiologica. 1989;12:15–28. [PubMed] [Google Scholar]
- 28.Perrin D D. Dissociate constants of organic bases in aqueous solution. London, United Kingdom: Butterworth & Co.; 1965. p. 531. [Google Scholar]
- 29.Peters W. Chemotherapy and drug resistance in malaria. London, United Kingdom: Academic Press; 1970. pp. 1–22. [Google Scholar]
- 30.Peters W, Howells R E, Portus J, Robinson B L, Thomas S, Warhurst D C. The chemotherapy of rodent malaria. XXVII. Studies on mefloquine (WR 142490) Ann Trop Med Parasitol. 1977;71:407–418. doi: 10.1080/00034983.1977.11687206. [DOI] [PubMed] [Google Scholar]
- 31.Peters W. Chemotherapy and drug resistance in malaria. 2nd ed. London, United Kingdom: Academic Press; 1987. pp. 1–18. [Google Scholar]
- 32.Raynes K, Foley M, Tilly L, Deady L W. Novel bisquinoline antimalarials: synthesis, antimalarial activity and inhibition of haem polymerisation. Biochem Pharmacol. 1996;52:551–559. doi: 10.1016/0006-2952(96)00306-1. [DOI] [PubMed] [Google Scholar]
- 33.Ridley, R. G. 1997. Haemoglobin degradation and haem polymerization as antimalarial drug targets. J. Pharm. Pharmacol. 49(Suppl. 2):43–48.
- 34.Ridley R G, Dorn A, Vippagunta S R, Vennerstrom J L. Haematin (haem) polymerization and its inhibition by quinoline antimalarials. Ann Trop Med Parasitol. 1997;91:559–566. doi: 10.1080/00034989760932. [DOI] [PubMed] [Google Scholar]
- 35.Rosenthal P J, McKerrow J H, Aikawa M, Nagasawa H, Leech J H. A malarial cysteine protease is necessary for haemoglobin degradation by Plasmodium falciparum. J Clin Investig. 1988;82:1560–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal P J. Plasmodium falciparum: effects of proteinase inhibitors on globin hydrolysis by culture malaria parasites. Exp Parasitol. 1995;80:272–281. doi: 10.1006/expr.1995.1033. [DOI] [PubMed] [Google Scholar]
- 37.Sherman I W, Tanigoshi L. Incorporation of 14C-amino acids by malaria (Plasmodium lophurae) Int J Biochem. 1970;1:635–637. [PubMed] [Google Scholar]
- 38.Slater A F G, Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992;355:167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- 39.Slater A F G. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol Ther. 1993;57:203–255. doi: 10.1016/0163-7258(93)90056-j. [DOI] [PubMed] [Google Scholar]
- 40.Sowunmi A, Oduola A M J, Salako L A, Ogundahunsi O A, Laoye O J, Walker O. The relationship between the response of Plasmodium falciparum malaria to mefloquine in African children and its sensitivity in vitro. Trans R Soc Trop Med Hyg. 1992;86:368–371. doi: 10.1016/0035-9203(92)90221-w. [DOI] [PubMed] [Google Scholar]
- 41.Steck E A. The chemotherapy of protozoan diseases. III, section 4. Washington, D.C: Walter Reed Army Institute of Research; 1972. [Google Scholar]
- 42.Sullivan D J, Gluzman I Y, Russell D G, Goldberg D E. On the molecular mechanism of chloroquine’s antimalarial action. Proc Natl Acad Sci USA. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theakston R D G, Fletcher K A, Maegraith B G. The use of electron microscope autoradiography for examining the uptake and degradation of haemoglobin by Plasmodium falciparum. Ann Trop Med Parasitol. 1970;64:63–71. doi: 10.1080/00034983.1970.11686664. [DOI] [PubMed] [Google Scholar]
- 44.Van der Kaay, H. J., W. H. Wernsdorfer, and F. M. Froeling. 1985. In vitro response of Plasmodium falciparum to mefloquine: studies conducted in West and East-Africa. Ann. Soc. Belge Med. Trop. 65(Suppl. 2):147–153. [PubMed]
- 45.Ward S A, Bray P G, Hawley S R. Quinoline resistance mechanisms in Plasmodium falciparum: the debate goes on. Parasitology. 1997;114:S125–S136. [PubMed] [Google Scholar]
- 46.Warhurst D C, Hockley D J. Mode of action of chloroquine on Plasmodium berghei and Plasmodium cynomolgi. Nature. 1967;214:935–936. doi: 10.1038/214935a0. [DOI] [PubMed] [Google Scholar]
- 47.Warhurst D C. The quinine-haemin interaction and its relationship to antimalarial activity. Biochem Pharmacol. 1981;30:3323–3327. doi: 10.1016/0006-2952(81)90606-7. [DOI] [PubMed] [Google Scholar]
- 48.Warhurst D C. Antimalarial interaction with ferriprotoporphyrin IX monomer and its relationship to activity of blood schizonticides. Ann Trop Med Parasitol. 1987;81:65–67. doi: 10.1080/00034983.1987.11812093. [DOI] [PubMed] [Google Scholar]
- 49.Wernsdorfer W H, Landgraf B, Wiedermann G, Kollaritsch H. Inverse correlation of sensitivity in-vitro of Plasmodium falciparum to chloroquine and mefloquine in Ghana. Trans R Soc Trop Med Hyg. 1994;88:443–444. doi: 10.1016/0035-9203(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 50.Yuthavong Y, Panijpan B, Ruenwongsa P, Sirawaraporn W. Biochemical aspects of drug action and resistance in malaria parasites. Southeast Asian J Trop Med Public Health. 1985;16:459–472. [PubMed] [Google Scholar]