Abstract
The reduction of predation is a potentially important factor for the evolution of the traits of an island animal species. By relaxed selection, insular animals tend to lose their antipredator behaviors. A monophyletic group of pitvipers (genus Bothrops) in southeastern Brazil, which have high genetic affinity and dwell on the mainland and adjacent islands, provide an appropriate setting to study the evolution of antipredator behavior and how different predatory stimuli can influence this behavior. The mainland Bothrops jararaca has several terrestrial and aerial predators, whereas B. insularis and B. alcatraz, restricted to two small islands, Queimada Grande and Alcatrazes, respectively, have a smaller range of aerial predators. Terrestrial predators are absent on Queimada Grande, but one potential snake predator occurs on Alcatrazes. We observed that the defensive repertoire of island snakes has not been lost, but they display different frequencies of some antipredator behaviors. The type of predatory stimuli (terrestrial and aerial) influenced the defensive response. Bothrops insularis most often used the escape strategies, especially against terrestrial predatory stimuli. Bothrops alcatraz displayed the highest rate of strike for both terrestrial and aerial stimuli. Our results indicate that even though relaxed selection may occur in island environments as compared to mainland environments, these pitvipers still retain their antipredator behaviors but with different response degrees to the two predator types.
Introduction
One of the most relevant interactions between animals in a community is the prey-predator relationship. Predation drives the development of several adaptations in animals. Among these adaptations, the behavioral ones deserve attention, especially the actions that avoid or lessen predation, i.e., the anti-predator behaviors [1]. Like many other animals, snakes have a wide variety of predators, both invertebrates and vertebrates, with different sizes, specialization degrees, capture tactics, metabolic rates, and life habits [2–4]. No single antipredator behavior fits all predation possibilities [5], even if snakes display an extensive defensive repertoire, one of the most elaborate described for reptiles [2].
Antipredator behavior is influenced by intrinsic factors such as age, sex, reproductive condition, and experience or learning as well as extrinsic factors as habitat, temperature, and social context [6,7]. Among these factors, the type of predator and the predation pressure it exerts has great influence [7]. Snakes respond in different ways depending on the type of predatory stimulus, which includes the predator’s color, size, and temperature [8–12]. When isolated from predators, expensive and no longer functional antipredator behaviors tend to be eliminated by relaxed selection [13–15]. Islands generally harbor few predators, as these environments usually have depleted fauna and do not house predators to the same extent as the mainland environments [16]. Therefore, it would be expected that some specific antipredator behaviors would be eliminated by selection if there were no benefits.
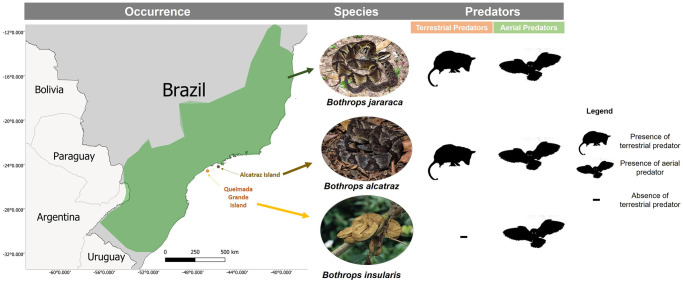
The Alcatrazes lancehead Bothrops alcatraz and the Golden lancehead Bothrops insularis are pitvipers endemic to Alcatrazes Island and Queimada Grande Island, respectively located about 30 to 35 km off the coast of the state of São Paulo, southeast Brazil [17–19]. Both species have similar origins [17–19]. During the Pleistocene, the sea level oscillated many times and the isolation of these islands from the mainland occurred about 11,000 years ago, splitting pitviper populations [17–19]. Genetic studies show that both B. alcatraz and B. insularis bear great similarity to the mainland pitviper Bothrops jararaca [17–20]. The three species experience different predatory pressures: Bothrops jararaca occurs in a wide range of forested habitats on the continent and consequently faces a wider range of predators, both aerial and terrestrial [21,22], whereas B. alcatraz and B. insularis are restricted to small area islands [17–19] with different predation profiles (Fig 1). The Queimada Grande Island has an area of 43 ha with no terrestrial predators, but houses 56 forest bird species, some of which are potential snake predators [18,22]. The Alcatrazes Island has an area of 135 ha and houses a potential terrestrial predator (the large lizard Salvator merianae) and 76 bird species, some of which are potential snake predators [17,18,22,23].
Fig 1. Predator types in the habitats of the three related pitviper species.
These three pitviper species with their respective evolutionary and ecological histories provide an appropriate setting to study the evolution of antipredator behavior, and how different predatory stimuli can influence this behavior. Our aim is to identify the influence that predatory stimulus types have on antipredator behavior in island pitvipers compared to the mainland. Specifically, to evaluate the influence of terrestrial and aerial predatory stimuli on the frequency of each behavior in the antipredator repertoire of each of the three pitviper species. Due to the different set of predators on the two islands and on the mainland, our working hypothesis is that some behavioral reactions of island snakes will be absent or distinct from those displayed by the mainland snake. Additionally, we predict that, due to the different types of predators on each island, the two island pitvipers differ in their defensive behaviors.
Materials and methods
Ethics
All procedures were approved by the Ethics Committee on Animal Use of the Butantan Institute (CEUAIB) and are in accordance with the guidelines of the National Council for the Control of Animal Experimentation (CONCEA) for the care and use of animals in research (CEUA 2705070821).
Study animal
We used 47 snakes, of which 20 B. jararaca individuals (10 males and 10 females), 19 B. insularis individuals (10 males and 9 females) and 8 B. alcatraz individuals (3 males and 5 females). All snakes were born in captivity, from different mothers, and were housed in the Laboratory of Ecology and Evolution at the Instituto Butantan. This procedure removes the effect of previous experience that each individual could have experienced in natural habitats [24]. Although the behaviors of captive animals can be influenced by habituation to laboratory conditions, Araújo & Martins [25] demonstrated no differences between some anti-predator behaviors of captive and wild Bothrops species. All snakes used were adult (i.e., sexually mature) using snout-vent length (SVL) data [26,27]. The snakes were kept at a temperature around 25°C, under a light and dark photoperiod of 12: 12 h.
Behavioral tests
To perform the behavioral tests, we conducted confrontations with the snakes using aerial and terrestrial predator models as stimuli (Supplementary material). The tests were conducted at night (between 6 PM and 10 PM), under artificial lighting. For the confrontations, a 2.025 m2 [1.5m (length) X 1.35m (width) X 1m (height)] arena was set up, with an aluminum plate as a base, and Styrofoam walls. All experiments were carried out between 5 pm and 8 pm at a temperature around 23°C. For each behavioral test, the snakes were left in the arena for up to 15 minutes in order to cease exploratory behaviors and habituate to the test arena.
To simulate a terrestrial predator, we used a cloth and cotton model of the white-eared opossum (Didelphis albiventris) as this mammal preys on B. jararaca [28]. A bottle with water at 37°C was placed inside the model to simulate the typical internal temperature of mammals. Using a 1.5 m wooden stick attached to the predator model, 30 advances of the model toward the snake’s head were performed with a 2 s interval, touching the snake’s body. To simulate an aerial predator, we used a taxidermied burrowing owl (Athene cunicularia) with spread wings. This owl species preys on pitvipers, besides occurring on the two islands [22]. At each behavioral test, the model was warmed with the help of heaters so its temperature was around 37°C to 39°C. With the help of a 2 m long fishing rod, the owl model was attached and confronted the snake 30 times with a 3 s interval, touching the snake’s body. These confrontations were performed from the top downwards simulating an aerial attack. We touched the snake from a distance of about 3 m from the observer at each test and an interval of seven days between the tests to avoid learning and habituation influences [24]. Both predator models had their irradiation temperature measured using an infrared thermometer (ST-620, Incoterm) before and after each behavioral test, ensuring that the models had similar temperatures to mammals and birds. All tests were recorded with a film camera (HDR-PJ200, Sony) for later analysis.
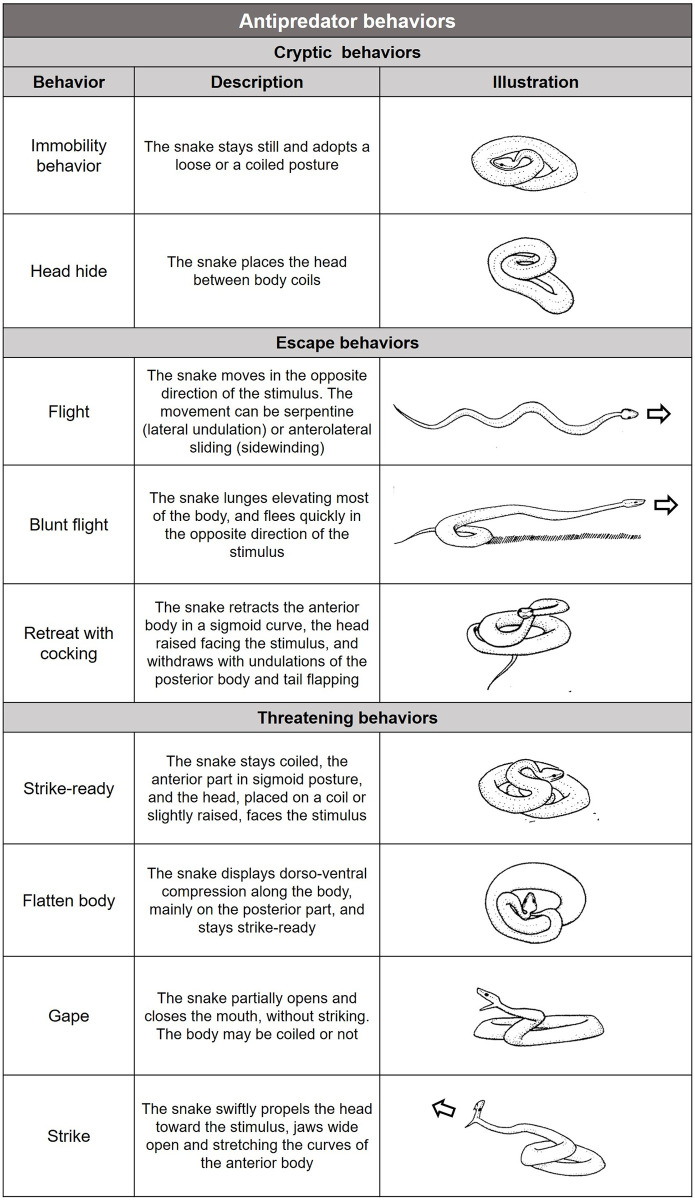
The behaviors displayed by the snakes during the tests were classified by two researchers independently and analyzed for congruence according to Greene [2], Sazima [29], and Araújo & Martins [25] (Fig 2). In addition, following Mori and Burghardt’s [8] apparent function behavioral classification, we grouped the behaviors into three classes to simplify understanding the results. Cryptic behaviors (immobility and head hiding) are an attempt to remain unnoticeable to the predator. Escape behaviors (flee; blunt escape, and cocking) increase the distance between the snake and the predator. Threatening behaviors (gaping; body flatten; strike-ready and strike) are responses that include any behavioral element to prevent or lessen predation by displaying a potential danger to the predator.
Fig 2. Antipredator behaviors of the pitvipers and their explanation.
Descriptions and illustrations partly based on Greene [2], Sazima [29] and Araújo & Martins [25].
Statistical analysis
The fixed categorical predictor variables were snake species (three levels: B. alcatraz, B. insularis, and B. jararaca) and predatory stimuli (two levels: terrestrial and aerial predator models). The response variables were the frequency of each antipredator behavior during the 30 confrontations with each predator model. Due to the nature of these variables and based on the experimental design, we used a generalized linear mixed model (GLMM) with Poisson distribution and log link function for each behavior. Only for body flattening a GlmmTMB model with a negative binomial distribution (nbinom1) was used. The model consisted of the additive and interactive property between the variables (species and predator). As we conducted the tests with the same individual snakes, and the sex was not of interest for our investigation, we included these two variables (ID and sex) as random variables in the models, thereby eliminating pseudoreplication in the experimental design. In addition, we performed Tukey’s test for post-hoc analysis to find significant relationships between the variables, using the "emmeans" package. All models were subjected to data dispersion analysis, homoscedasticity, and delineate tests using model diagnostic values and plots, with the help of the package "DHARMa: residual diagnostics for hierarchical (multilevel/mixed) regression models" in R (version 4.04).
Results
Cryptic behaviors
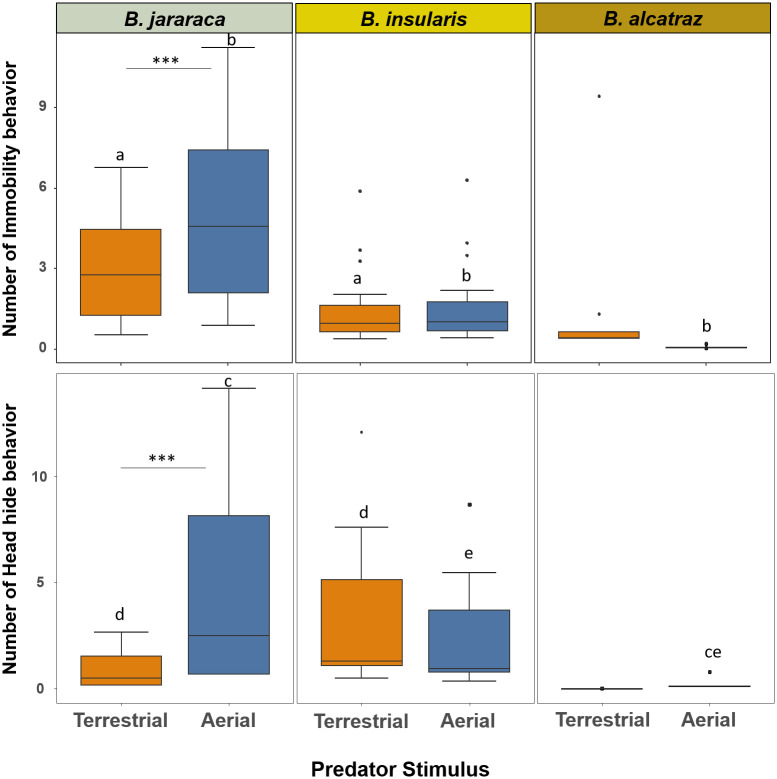
The three pitivipers differed in the frequency of cryptic behaviors such as immobility and head hiding, Bothrops alcatraz showing the least tendency to display cryptic behaviors (Fig 3). Predatory stimulus influenced immobility behavior for B. jararaca only, this species showing a greater tendency to immobilize to aerial predator than to terrestrial predator stimulus by 40% (z = 3.234, p = 0.00122) (S1 Table). In addition, B. jararaca showed the highest rate of immobility among the studied species (Fig 3, S2 Table). The only pitviper species that showed a clear difference in the frequency of head-hiding behavior when exposed to different stimuli was B. jararaca (B. jararaca: z = 6. 349, p < 0.001; B. insularis: z = 1.667, p = 0.5535; B. alcatraz: z = -0.147, p = 1.000) (S3 and S4 Tables). In addition, B. jararaca hid its head more often from aerial predator stimulus than B. alcatraz (z = 3.053, p = 0.0275). However, B. insularis exposed to terrestrial predatory stimulus had a higher rate of head-hiding behavior than B. jararaca (z = 2.792, p = 0.00524) (S4 Table).
Fig 3. Influence of predatory stimulus types on cryptic category behaviors of Bothrops jararaca, B. insularis and B. alcatraz.
Boxplot graph representing the dispersion and asymmetry of groups of data. Box: Represents the second and third quartiles and a central line (median). Whiskers: The lines extending from the box indicate the dispersion of the data, excluding discrepant values. Outliers: The points beyond the whisker’s boxes represent outliers or extreme values in the data distribution. *** indicates that the results show a clear difference in the frequency of behaviors in relation to the type of predatory stimulus among each species (p < 0.001). Corresponding lowercase letters indicate a significant difference between groups (p < 0.05).
Escape behaviors
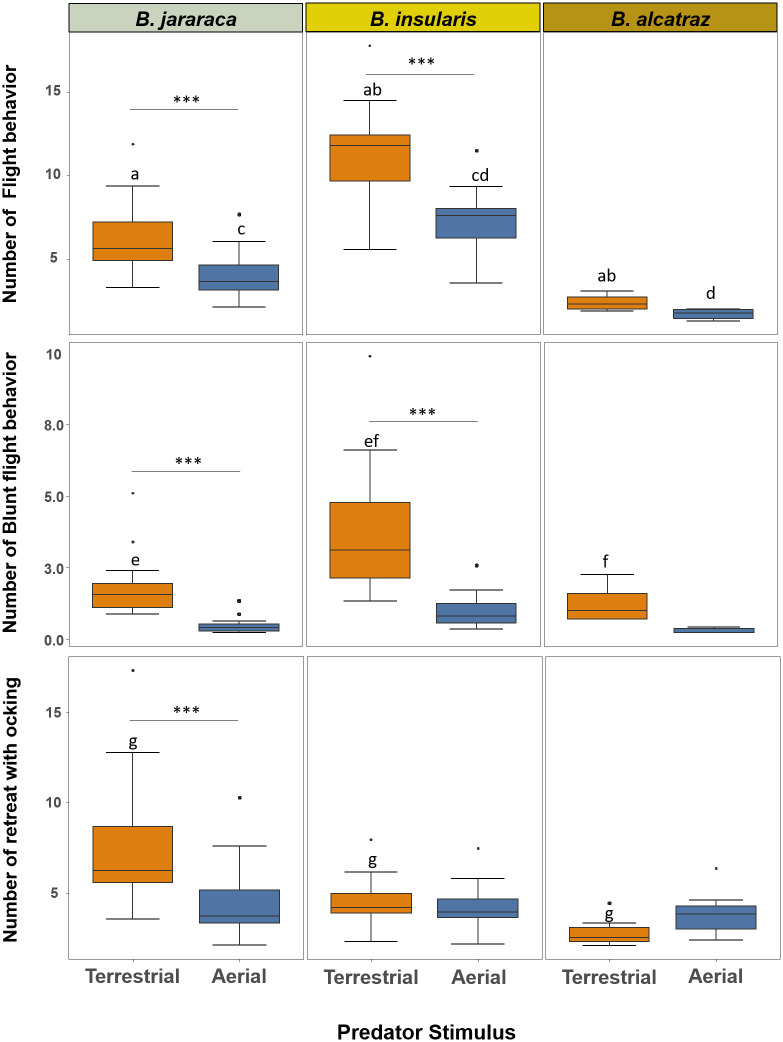
Predatory stimulus type strongly influenced the escape behaviors. Both B. jararaca and B. insularis fled more frequently when confronted with a terrestrial predator stimulus (opossum model) than with an aerial predator (owl model) (B. jararaca: z = -3.458, p = 0.0005, B. insularis: z = 4.288, p = 0.0003) (Fig 4, S5 and S6 Tables). Bothrops insularis, independently of the predatory stimulus, showed a higher flight rate than the other species. For the stimulus of an aerial predator, flight probabilities were found to be approximately 47% and 68% higher than those of B. jararaca and B. alcatraz, respectively (B. insularis—B. jararaca: z = -3.474, p = 0.0068; B. insularis—B. alcatraz: z = 3.972, p = 0.0010) (S6 Table). In the case of terrestrial predator stimulus, chances of flight were observed to be 46% and 85% greater compared to B. jararaca and B. alcatraz, respectively (B. insularis—B. jararaca: z = -3.786, p = 0.0021; B. insularis- B. alcatraz: z = 6.062, p < 0.0001) (S6 Table).
Fig 4. Influence of predatory stimulus types (terrestrial and aerial) on escape category behaviors of Bothrops jararaca, B. insularis and B. alcatraz.
Boxplot graph representing the dispersion and asymmetry of groups of data. Box: Represents the second and third quartiles and a central line (median). Whiskers: The lines extending from the box indicate the dispersion of the data, excluding discrepant values. Outliers: The points beyond the whisker’s boxes represent outliers or extreme values in the data distribution. *** indicates that the results show a clear difference in the frequency of behaviors in relation to the type of predatory stimulus among each species (p < 0.001). Corresponding lowercase letters indicate a significant difference between groups (p < 0.05).
Similarly to the previous behavioral category, only B. alcatraz did not have its blunt flight frequency influenced by the predator type (B. jararaca: z = -3.295, p = 0.0010; B. insularis: z = -5.499, p < 0.0001; B. alcatraz: z = 1.635, p = 0.5753 (Fig 4) (S8 Table). Bothrops jararaca, regardless of the predatory stimuli, had no clear difference in frequency when compared to B. alcatraz (terrestrial: z = 0.872, p = 0.9532; aerial: z = 0.675, p = 0.9847). However, B. insularis, once again, displayed higher frequencies of blunt flight compared to the other species, with approximately 54% and 70% higher likelihood than B. jararaca and B. alcatraz, respectively (B. insularis- B. jararaca: z = -2.470, p = 0.0135; B. insularis- B. alcatraz: z = -2.579, p = 0.0099) (S8 Table).
Cocking behavior showed different patterns depending on the pitviper species and predatory stimuli (Fig 4), and the dependence between variables was significant. For instance, B. insularis and B. alcatraz showed no difference towards the type of predatory stimulus (B. insularis- z = 0.391, p = 0.9988; B. alcatraz- z = -1.169, p = 0.8516), but different predatory stimuli influenced the frequency of cocking by B. jararaca (z = -3,936; p < 0.0001) (S9 Table). In addition, when exposed to the terrestrial predator stimulus B. jararaca showed the highest frequency of retreat with cocking among the three pitvipers (B. jararaca-B. insularis: z = -2.408, p = 0.01604; B. jararaca-B. alcatraz: z = -3.515, p = 0.00044) (S9 and S10 Tables).
Threatening behaviors
All behaviors involving a threat signal, such as body flatten, gape, strike-ready, and strike were influenced by the type of predatory stimulus among the three pitviper species.
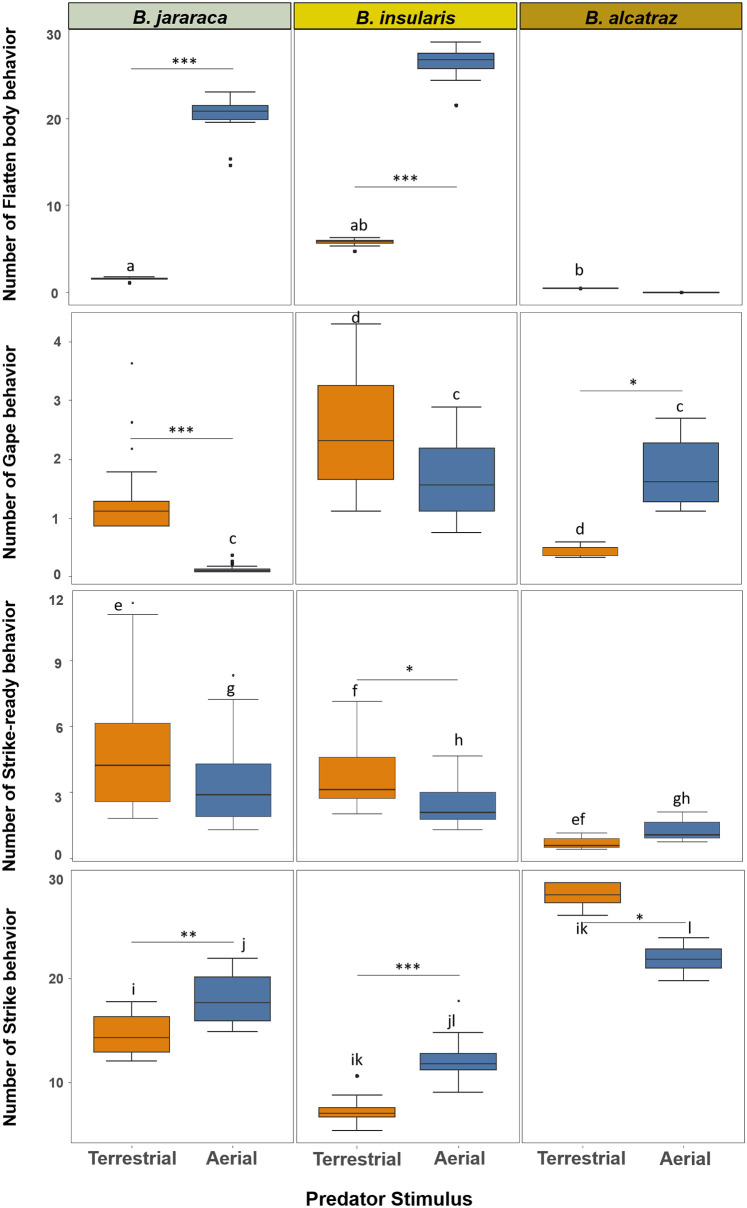
Flatten body was strongly influenced by the type of predator for two of the studied pitvipers. Bothrops alcatraz had a different behavioral pattern than the other species and did not show body flattening. On the other hand, both B. jararaca and B. insularis displayed more body flattening when faced with the aerial predatory stimulus than the terrestrial one (z = -6.638, p < 0.0001). Moreover, B. insularis flattened its body the most among the three species, mainly to terrestrial predator stimulus (B. jararaca-B. insularis: z = 3.169, p = 0.00153; B. insularis- B. alcatraz: z = -2.527, p = 0.0115) (Fig 5; S13 and S14 Tables).
Fig 5. Influence of predatory stimulus types on escape threatening behaviors of Bothrops jararaca, B. insularis and B. alcatraz.
Boxplot graph representing the dispersion and asymmetry of groups of data. Box: Represents the second and third quartiles and a central line (median). Whiskers: The lines extending from the box indicate the dispersion of the data, excluding discrepant values. Outliers: The points beyond the whisker’s boxes represent outliers or extreme values in the data distribution. Asterisk: Indicates that the results show a clear difference in the frequency of behaviors in relation to the type of predatory stimulus among each species (*** p < 0.001; ** p < 0.01; * p < 0.05) Corresponding lowercase letters indicate a significant difference between groups (p < 0.05).
The predator stimulus type strongly influenced gape behavior in all three species, more pronounced in B. insularis. Bothrops jararaca and B. insularis gaped more frequently towards the terrestrial predator stimulus (z = -3.803; p = 0.000143), although the difference was not significant for the latter pitviper (z = -1.905; p = 0.056742). On the other hand, in B. alcatraz gape was elicited more often by the aerial predatory stimulus (z = 2.265; p = 0.02352) (Fig 5). Furthermore, B. jararaca showed less frequency of gape behavior with aerial predator stimulus than the other species (B. jararaca- B. insularis: z = -3.920, p = 0.0012; B. jararaca- B. alcatraz: z = -3.814; p = 0.0019) (S15 and S16 Tables). Bothrops insularis exposed to the terrestrial predator stimulus showed the highest frequency of this behavior among the three species (z = 2.896, p = 0.0439).
Strike-ready behavior had a similar pattern for B. jararaca and B. insularis, with a more pronounced reaction to the terrestrial predator stimulus than the aerial one. Although only B. insularis showed a significant difference (z = -2.257, p = 0.02398), the p-value for the difference in the reaction of B. jararaca was marginally significant (z = -1.853, p = 0.0639). No difference in this behavior was detected in B. alcatraz (z = -0.988, p = 0.3231). However, this pitviper displayed the least strike-ready reaction towards the terrestrial predator stimulus among the three species (B. alcatraz: B. jararaca: z = 3.710, p = 0.000207; B. alcatraz: B. insularis: z = 3.316, p = 0.000915) (S11 and S12 Tables).
Strike was another behavior strongly influenced by the predatory stimulus type. Bothrops jararaca and B. insularis showed the same pattern, with more strikes towards terrestrial than aerial predators stimuli (B. jararaca: z = 2.684, p = 0.007273; B. insularis: z = 4.860, p < 0.0001) (S17 Table). In contrast, B. alcatraz showed the opposite behavior. When faced with the predator stimuli, it displayed more strikes towards the aerial than the terrestrial stimulus (z = -2.217, p = 0.026609). Among the three pitviper species, B. alcatraz displayed the highest strike behavior rates (Fig 5). The odds of B. alcatraz to strike a terrestrial predator were about 75% and 49% higher than B. insularis and B. jararaca, respectively. While for an aerial predator, B. alcatraz had about 44% more odds than B. insularis to display strike behavior (z = -4.715, p < 0.0001). When compared to B. jararaca the difference of 27% chance is not significant (z = -1.604; p = 0.108618).
Discussion
Our study simulating aerial and terrestrial predators demonstrated that these two contrasting predatory stimuli influenced the frequency of each category of antipredator behavior displayed by the three pitviper species. Furthermore, we showed that the mainland B. jararaca and its closest island species reacted diversely to the same predatory stimulus. Our results do not support the hypothesis that the antipredator behavior repertoire of island species was lost due to isolation. However, a quantitative difference in the defensive behaviors displayed by each snake species was evident. Bothrops insularis was the species that displayed the highest frequency of escape behaviors, mainly towards terrestrial predators. Bothrops jararaca displayed more cryptic behaviors among the three pitviper species, the more so towards aerial stimuli. On the other hand, B. alcatraz was the species most prone to bite towards both stimuli types.
A previous study on defensive behavior of five Bothrops species found quantitative differences in behavioral categories that seem to be adaptive reactions to variable predation pressure of aerial predators, as each of the five studied pitvipers dwells in a distinct habitat [29]. However, it must be emphasized that snakes can change their antipredator behavior during a snake’s life depending on previously experienced predation pressure [30]. Adult snakes exposed to a higher risk of predation showed higher bite rates, whereas there was no such association among newborns [30]. All snakes used in our study were born in captivity, had no previous contact with natural predators, and would not change behaviors by ontogeny or plasticity.
Among the escape behaviors we recorded for the three pitvipers, cocking was the most variable. Escape behaviors are strategies that increase the distance between a snake and a potential predator [8], and generally, the snake directs its head to the opposite side of the predatory stimulus. However, cocking is the only behavior that, while increasing the distance between the snake and the stimulus object, the snake keeps its head facing the potential predator. Bothrops jararaca was the only species that displayed changes in frequency of cocking depending on the predatory stimulus. Possibly, this influence is due to the greatest diversity of predators sympatric with B. jararaca, and cocking behavior may be important to avoid predation by a predator type that the other pitvipers do not come across [31]. For the other escape behaviors (flight and blunt flight), all species displayed a similar pattern, which was more frequent when confronted with terrestrial predators. Possibly this is due to this predator type being on the same physical plane as the snake, and can attack more efficiently than an aerial predator. Therefore, snakes would tend to evade as quickly as possible, as behaviors that include moving away from the predatory stimulus are the main ones used by snakes [7,32].
The frequent flight and blunt escape are the defensive traits of B. insularis that differentiates it most from the other two pitvipers. There is an evident predator low richness on Queimada Grande Island and experiments with plasticine snake replicas showed a significantly lower number of attacks on this island than in continental sites [18]. Our hypothesis is that this low predatory pressure on the island reduces the encounter rate with any potential aversive or non-aversive stimulus (terrestrial or aerial), which lowers the sensitivity threshold to respond to something disturbing. However, B. jararaca, which dwells in areas with higher species richness, would tend to be more habituated (with a higher sensitivity threshold for response) to possible stimuli, making it more adaptive to remain motionless and evaluate the predatory stimulus rather than fleeing and thus standing out from the substrate. Consequently, the frequency of other defensive behaviors in the defensive repertoire of B. insularis is reduced compared to those of mainland B. jararaca, making fleeing the main defense strategy of this pit viper on the island. Moreover, the low predator pressure could also explain the uniform yellowish coloration of this island species, apparently out of tune with the environment. The higher frequency of escape behavior displayed by B. insularis may also be explained by is its distinctive coloration. Striped or differently colored snakes have a tendency to escape more frequently than those with spotted coloration, which tend to be more cryptic and stationary as their coloration pattern favors camouflage [33]. Based on this premise, B. alcatraz and B. jararaca with their chevroned pattern should display cryptic behaviors more often than B. insularis, but we found that B. alcatraz did not display any cryptic behavior.
We found that all three pitviper species tend to use cryptic behaviors more towards aerial than towards terrestrial predator stimulus, although this was less evident for B. alcatraz. Absence of movements lessens the detection of a snake by aerial predators, as birds perceive their prey visually mostly by movements of the prey [34]. Furthermore, B. jararaca displayed head hiding more often towards aerial predators than terrestrial predators. Birds of prey, such as the snake-specialist Laughing falcon (Herpetotheres cachinnans), kill their snake prey pecking at its head or tearing the head off [35]. Snakes tend to hide their most vital part (head) during aerial attacks with higher head attack rates [31].
Threat behavior would warn the predator that its potential prey is dangerous [2,8]. Within threat behavior class, strike is the one most influenced by abiotic and biotic factors [8,11,36]. Both B. jararaca and B. insularis displayed higher strike frequency towards aerial stimuli than to terrestrial ones. This tendency of B. insularis is likely influenced by the lack of terrestrial predators on the island, whereas for B. jararaca it could be due to greater pressure from predatory birds than from predatory mammals (29). On the other hand, B. alcatraz displayed more threatening behaviors towards terrestrial predators, possibly due to this pitviper being under greater predation pressure by terrestrial predators than the aerial ones. The large tegu lizard (Salvator merianae) is abundant on the island [18,37], and is able to track and prey on rats and smaller lizards [38,39], which means that it would be a potential snake predator. Among the three species, B. alcatraz displayed the highest strike frequency. This species is very small and shares similarities with juvenile B. jararaca, as it has pedomorphic traits [17,40]. Snake size is one of the main variables that interfere with strike behavior [7,10]. Being more susceptible to predation pressure, small snakes tend to have higher strike rates [29,41–45], which would explain the marked defensive behavior of B. alcatraz. Another important factor is that we have no knowledge of the aerial predation frequency, although there are more potential avian predators on Alcatrazes island than on Queimada Grande island, even though far less than on the mainland [18]. A behavior closely linked to strike is strike-ready. Unexpectedly, B. alcatraz displayed almost no strike-ready behavior towards the predator stimuli. Possibly, higher predation risks on this small pitviper would lead to few strike-ready warnings and more strikes, a hypothesis that merit verification.
Another threatening behavior is gape. Bothrops insularis used this behavior most often among the three pitvipers. Gape behavior is used by arboreal and diurnal snake species [3] and the Golden Lancehead is the most arboreal species among the three studied pitvipers [17,46]. In addition, B. insularis is more diurnal than the two other species, which are predominantly nocturnal [47,48]. Both B. insularis and B. jararaca displayed more gape behavior towards terrestrial predator stimulus than towards the aerial one. Gape is a display that increases the size of the snake on an x-axis, and is possibly more effective for predatory confrontations that occur on the same spatial plane. Both B. jararaca and B. insularis showed very similar patterns of threat behaviors. However, B. alcatraz showed a pattern opposite to the other two species. Tests comparing B. jararaca juveniles and adults could indicate whether this difference is also due to pedomorphic traits. Field observations showed that B. jararaca juveniles and males are more prone to strike than adult female individuals (47). Furthermore, we emphasize that Bothrops jararaca and B. insularis come from the same Bothrops population lineage, whereas B. alcatraz comes from another B. jararaca population lineage [19].
Flatten body behavior expands the snake’s body laterally [2], and when viewed from above the snake appears larger. Bird attacks occur overhead and often during daylight [34]. Both B. jararaca and B. insularis flattened more when confronted with aerial predatory stimuli than with the terrestrial one. Unexpectedly, this behavior was not recorded for B. alcatraz for any predator type stimuli. Our small sample does not allow us to say that B. alcatraz has lost this behavior, but at least it seems to be less frequent in this species. Bothrops insularis hunts during the day and B. jararaca is also observed in daylight with some frequency [17,18,46]. On the other hand, B. alcatraz seems to be more secretive during the day, being usually found within a decomposing trunk or under a fallen leaf [18].
Antipredator behavior can be lost after the loss of a key predator, i.e., loss of a trait after relaxed selection [49], or it can persist for many generations [50]. If unnecessary antipredator behavior has substantial costs when displayed, then loss of predators should lead to rapid trait loss [51]. Thus, island pitviper species that usually have lower predation rates than mainland pitvipers would tend to lose or decrease the frequency of some antipredator behaviors. Essentially, we found that the defensive behavior repertoire persists in island pitvipers but their frequency changes. As emphasized above, the only exception was the absence of flattening in B. alcatraz (apparently lost). The persistence of antipredator behaviors in the three studied pitvipers is consistent with the multipredator hypothesis. This hypothesis attempts to explain the persistence of antipredator behavior under relaxed selection [52]. According to Blumstein [52], the hypothesis emphasizes that antipredator behavior has pleiotropic effects on other traits, which will remain functional regardless of the presence or absence of predators. This implies that a gene associated with defensive behavior can have multiple effects. For example, genes related to strike behavior would be preserved even in the absence of predators, as strike plays a fundamental role in other behaviors such as prey capture. Once this set of genetic behavioral characteristics is established, genes linked to defensive behavior are not lost, even in the absence of a specific predator. Thus, the diversity of predators or the existence of different functional demands would be factors favoring the maintenance of these genetic behavioral traits over time.
The multipredator hypothesis has been studied in some kangaroo and wallaby species (Diprotodontia), in which the loss of all predators apparently led to a rapid loss of antipredator behavior, while the loss of only one or two predators had a limited effect on the expression of antipredator behavior for the missing species [16,50,51]. The evolutionary persistence of the rattlesnakes (Crotalus spp.) recognition by California ground squirrels Spermophilus beecheyi (Rodentia) [53,54] indicates that rattlesnake recognition ability can be maintained for over 70,000 years after rattlesnake isolation. Bothrops insularis and B. alcatraz were isolated from the mainland 11,000 years ago [17–19,55]. Therefore, the integrity of the antipredator behaviors of the isolated island species, based on the multi-predator hypothesis, should be due to some type of predator still exerting pressure on these populations or, if not, they are still relatively young population lineages and would not yet have lost the defensive repertoire.
Another explanation that may clarify the permanence of antipredator behavior is the "phantom predator past hypothesis" [50,56]. This hypothesis states that a species subjected to past selection for antipredator behavior by a predator that exerted strong predatory pressure will maintain antipredator behavior if it is not too costly [56,57]. The ancestral opossums (Didelphimorphia) have been in South America for at least 10 million years and the ancestral lineage of the genus Didelphis for 3 million years [58]. These mammals are important predators of snakes, including pitvipers, as they are resistant to toxins in the venoms [59,60]. Several birds, including raptors, also have been actively preying on snakes on the mainland for millions of years [61,62]. Therefore, even though some of these predators do not occur on the islands, they possibly exerted a strong predation pressure and selection of defensive behaviors on the ancestral lineages of the South American pitvipers since the early Pleistocene, which persists in the current lineages. Other behaviors, such as prey handling by B. jararaca and B. insularis, remain the same while preying on rats, despite the insular species being isolated for more than 11,000 years without access to this prey type [63]. Therefore, we can suppose that, like the feeding behavior conserved in these two species with phylogenetic proximity, the defensive behavior may have been preserved as well.
Our study is the first to investigate the antipredator behavior of island pitviper species in South America and is instructive for the fields of behavioral ecology and evolution of animal behavior. The two island species are endemic and critically endangered. Among the three species, B. alcatraz is the least studied, and this is the first study to investigate some ecological and behavioral aspects of this small species. Further comparative behavioral studies of these three species would be useful, as well as to study other island Bothrops species recently described [64,65]. Experimental studies with snakes in nature would be useful for a better understanding of antipredator behavior, providing information for the conservation of these island species. We encourage integrative studies on natural history (e.g., antipredator behaviors), evolution, and ecology that may help us understand better the behavioral strategies used by a snake species.
Supporting information
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Frequencies of snake behaviors for each species and predatory model.
(XLSX)
Acknowledgments
We thank the editors and referees for the comments and suggestions that greatly improved the manuscript. We thank Selma M. Almeida-Santos, Kelly Kishi, Cintia Fugiwara, Denis Garcia and Bruno Martins for help in the laboratory. JMAN thanks to family (Reinaldo Nunes, Alessandra Nunes and Beatriz Nunes) for their great support, and the Graduate Program in Biodiversity at IBILCE/UNESP. AF thanks to Vivian Martins de Souza for the patience and companionship. IS thanks to Marlies Sazima for her loving support in the field and at home.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
JMAN would like to acknowledge the funding that was provided for this project in part by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)[https://www.gov.br/capes/pt-br]; CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) (00992/79-ZO) and the FAPESP (grant number 20/12658-4). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation and publish, or preparation of the manuscript.
References
- 1.Stubbs M, Edmunds M. Defence in Animals. J Anim Ecol. 1976; 45:607. [Google Scholar]
- 2.Greene HW. Antipredator mechanisms in reptiles. In: Defense and life history (Gans C, Huey RB, eds). New York: Alan R. Liss; 1988. pp. 1–134. [Google Scholar]
- 3.Martins M, Marques OAV, Sazima I. How to be arboreal and diurnal and still stay alive: microhabitat use, time of activity, and defense in neotropical forest snakes. South Am J Herpetol. 2008; 3:58 67. doi: 10.2994/1808-9798(2008)3[58:HTBAAD]2.0.CO;2 [DOI] [Google Scholar]
- 4.Pough FH. et al.Herpetology. 3. Ed. Benjamim Cummings, 2003. [Google Scholar]
- 5.Greene HW, Fogden M. Snakes: the evolution of mystery in nature. Berkeley: Univ. Of California Press; 2000. [Google Scholar]
- 6.Endler JA. Natural selection in the wild. Princeton, N.J.: Princeton University Press; 1986. [Google Scholar]
- 7.Stankowich T, Blumstein DT. Fear in animals: a meta-analysis and review of risk assessment. Proc Biol Sci. 2005; 272:2627–34. doi: 10.1098/rspb.2005.3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori A & Burghardt GM. Thermal effects on the antipredator behavior of snakes: a review and proposed terminology. Herpetol J. 2004; 14:79–87. [Google Scholar]
- 9.Langkilde T, Shine R, Mason RT. Predatory attacks to the head vs. body modify behavioral responses of Garter Snakes. Ethol. 2004; 110:937–47. doi: 10.1111/j.1439-0310.2004.01034.x [DOI] [Google Scholar]
- 10.Shine R, Olsson MM, Lemaster MP, Moore IT, Mason RT. Effects of sex, body size, temperature, and location on the antipredator tactics of free-ranging garter snakes (Thamnophis sirtalis, Colubridae). Behav Eco 2000; 11:239–45. doi: 10.1093/beheco/11.3.239 [DOI] [Google Scholar]
- 11.Whitaker PB, Shine R. Responses of free-ranging brown snakes (Pseudonaja textilis:Elapidae) to encounters with humans. Wildlife Biol. 1999; 26:689. doi: 10.1071/WR98042 [DOI] [Google Scholar]
- 12.Scudder KM, Chiszar D. Effects of six visual stimulus conditions on defensive and exploratory behavior in two species of rattlesnakes. Psychol Rec. 1977. Jul;27(3):519–26. [Google Scholar]
- 13.Kavaliers M. Responsiveness of Deer Mice to a predator, the Short-Tailed Weasel: Population differences and neuromodulatory mechanisms. Physiol Zool. 1990; 63:388–407. [Google Scholar]
- 14.Magurran AE. The inheritance and development of minnow anti-predator behaviour. Anim Behav. 1990; 39:834–42. doi: 10.1016/S0003-3472(05)80947-9 [DOI] [Google Scholar]
- 15.Magurran A. E. The causes and consequences of geographic variation in antipredator behavior: perspectives from fish populations. In: Geographic Variation in Behavior: Perspectives on Evolutionary Mechanisms (Foster S. A. & Endler J. A. eds). Oxford Univ. Press, New York. 1999. pp. 139–163. [Google Scholar]
- 16.Blumstein DT. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J Biogeogr. 2002; 29:685–92. doi: 10.1046/j.1365-2699.2002.00717.x [DOI] [Google Scholar]
- 17.Marques OAV. Martins M. & Sazima I. A jararaca da Ilha da Queimada Grande. Cienc. Hoje.2002; 31: 56–59. [Google Scholar]
- 18.Marques OAV. A ilha das cobras: Biologia, evolução e conservação da jararaca-ilhoa na Queimada Grande. Cotia:Ponto A Editora, 2021. [Google Scholar]
- 19.Barbo F, Grazziotin F, Pereira-Filho G, Freitas MA, Abrantes SH, Kokubum MC. Isolated by dry lands: integrative analyses unveil the existence of a new species and a previously unknown evolutionary lineage of Brazilian Lanceheads (Serpentes: Viperidae: Bothrops) from a Caatinga moist-forest enclave. Can J Zool.2022; 100:147–159. doi: 10.1139/cjz-2021-0131 [DOI] [Google Scholar]
- 20.Zaher H, Murphy RW, Arredondo JC, Graboski R, Machado-Filho PR, Mahlow K, et al. Correction: Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes). PLoS One. 2019; 14: e0217959. doi: 10.1371/journal.pone.0217959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordeiro GAR., Nicolas RAB. Feeding habits of the opossum (Didelphis marsupialis) in northern Venezuela. Field Zool New Ser. 1986, 39: 125–131. [Google Scholar]
- 22.Lillywhite HB. Islands and snakes: isolation and adaptive evolution. New York, Ny, United States Of America: Oxford University Press; 2019. [Google Scholar]
- 23.Muscat E, Saviolli JY, Costa A, Chagas CA, Eugênio M, Rotenberg EL, et al. Birds of the Alcatrazes archipelago and surrounding waters, São Paulo, southeastern Brazil. Check List. 2014; 10:729–39. doi: 10.15560/10.4.729 [DOI] [Google Scholar]
- 24.Glaudas X, Winne CT, Fedewa LA. Ontogeny of anti-predator behavioral habituation in Cottonmouths (Agkistrodon piscivorus). Ethol. 2006; 112:608–15. doi: 10.1111/j.1439-0310.2005.01183.x [DOI] [Google Scholar]
- 25.Araújo MS, Martins M. Defensive behavior in pitvipers of the genus Bothrops (Serpentes, Viperidae). Herpetol J.2006; 16: 297–303. [Google Scholar]
- 26.Matias NR, Alves MLM, de Araujo ML, Jung DMH. Variação morfométrica em Bothropoides jararaca (Serpentes, Viperidae) no Rio Grande do Sul. Iheringia Ser Zool. 2011; 101:275–82. doi: 10.1590/S0073-47212011000300001 [DOI] [Google Scholar]
- 27.Shine R. Reproduction in Australian elapid snakes II. Female reproductive cycles. Aust J Zool 1977; 25: 655–666. doi: 10.1071/ZO9770655 [DOI] [Google Scholar]
- 28.Oliveira ME, Santori RT. Predatory behavior of the opossum Didelphis albiventris on the pitviper Bothrops jararaca. Stud Neotrop Fauna Environ. 1999; 34:72–5. doi: 10.1076/snfe.34.2.72.2105 [DOI] [Google Scholar]
- 29.Sazima I. Natural history of the jararaca pitviper, Bothrops jararaca, in southeastern Brazil. In Campbell J. A. & Brodie E. D. Jr. (Eds.), Biology of the pitvipers. Selva Press, Tyler. 1992. pp. 199–216. [Google Scholar]
- 30.Aubret F, Michniewicz RJ, Shine R. Correlated geographic variation in predation risk and antipredator behavior within a wide-ranging snake species (Notechis scutatus, Elapidae). Austral Ecol. 2010; 36:446–52. doi: 10.1111/j.1442-9993.2010.02171.x [DOI] [Google Scholar]
- 31.Schalk CM, Cove MV. Squamates as prey: Predator diversity patterns and predator-prey size relationships. Food Webs. 2018; 17:e00103. doi: 10.1016/j.fooweb.2018.e00103 [DOI] [Google Scholar]
- 32.Llewelyn J, Webb JK, Shine R. Flexible Defense: Context-dependent antipredator responses of two species of Australian elapid snakes. Herpetologica. 2010; 66:1–11. doi: 10.1655/07-082.1 [DOI] [Google Scholar]
- 33.Jackson JF, Ingram W, Campbell HW. The dorsal pigmentation pattern of snakes as an antipredator strategy: A multivariate approach. Am Nat. 1976; 110:1029–53. [Google Scholar]
- 34.Wilgers DJ, Horne EA. Spatial variation in predation attempts on artificial snakes in a fire-disturbed tallgrass prairie. Southwest Nat. 2007; 52:263–70. doi: 10.1894/0038-4909(2007)52[263:SVIPAO]2.0.CO;2 [DOI] [Google Scholar]
- 35.Medrano-Vizcaíno P. Predating behavior of the Laughing falcon (Herpetotheres cachinnans) on the venomous Amazonian pit viper Bothrops atrox (the use of roads as a prey source). BioRisk. 2019; 14:25–30. doi: 10.3897/biorisk.14.35953 [DOI] [Google Scholar]
- 36.Shine R, Sun L-X, Fitzgerald M, Kearney M. Antipredator responses of free-ranging pitvipers (Gloydius shedaoensis, Viperidae). Copeia. 2002; 3:843–50. doi: 10.1643/0045-8511(2002)002[0843:AROFRP]2.0.CO;2 [DOI] [Google Scholar]
- 37.Muscat E, Olmos F, Rotenberg E. Salvator merianae (black and white tegu) scavenging around the nests of Fregata magnificens (frigatebird). Herpetol Bull. 2016; 135:36–37. [Google Scholar]
- 38.Sazima I. & Haddad CFB. Répteis da Serra do Japi: notas sobre história natural., In: Morellato L. P. C. (Ed.), História natural da Serra do Japi: ecologia e preservação de uma área florestal no sudeste do Brasil. Ed. Unicamp e FAPESP, Campinas.1992. pp. 212–236. [Google Scholar]
- 39.Sazima I. & D’Angelo GB. Range of animal food types recorded for the tegu lizard (Salvator merianae) at an urban park in South-eastern Brazil. Herpetol Notes. 2013; 6: 427–430. [Google Scholar]
- 40.Marques OAV, Martins M, Sazima I. A new insular species of pitviper from Brazil, with comments on evolutionary biology and conservation of the Bothrops jararaca group (Serpentes, Viperidae). Herpetologica. 2002; 3: 303–312. doi: 10.1655/0018-0831(2002)058[0303:ANISOP]2.0.CO;2 [DOI] [Google Scholar]
- 41.Blomberg SP, Shine R. Size-based predation by kookaburras (Dacelo novaeguineae) on lizards (Eulamprus tympanu: Scincidae): what determines prey vulnerability? Behav Ecol Sociobiol. 2000; 6:484–9. doi: 10.1007/s002650000260 [DOI] [Google Scholar]
- 42.Janzen FJ, Tucker JK, Paukstis GL. Experimental analysis of an early life-history stage: selection on size of hatchling turtles. Ecology. 2000; 8:2290–304. doi: 10.1890/0012-9658(2000)081[2290:EAOAEL]2.0.CO;2 [DOI] [Google Scholar]
- 43.Shine R, LeMaster MP, Moore IT, Olsson MM, Mason RT. Bumpus in the snake den: effects of sex, size, and body condition on mortality of red-sided garter snakes. Evolution. 2001; 3:598–604. doi: 10.1554/0014-3820(2001)055[0598:bitsde]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 44.Tucker JK, Filoramo NI, Janzen FJ. Size-biased mortality due to predation in a nesting freshwater turtle, Trachemys scripta. Am Midl Nat. 1999; 1:198–203. [Google Scholar]
- 45.Vitt LJ. Ecological consequences of body size in neonatal and small-bodied lizards in the Neotropics. Herpetol. Monogr. 2000; 14:388. [Google Scholar]
- 46.Amaral A. Contribuição para o conhecimento dos ophideos do Brasil–Parte II: Biologia da nova espécie, Lachesis insularis. Mem Inst Butantan.1921; 1: 40–44. [Google Scholar]
- 47.Sazima I. Um estudo de biologia comportamental da jararaca, Bothrops jararaca, com uso de marcas naturais. Mem. Inst. But.1988; 50:83–99. [Google Scholar]
- 48.Siqueira LHC, Banci KRS, Marques OAV. Seasonal activity of Bothrops jararaca (Serpentes, Viperidae): Optimizing foraging while avoiding predators. S Am J Herpetol. 2021; 20:67–74. doi: 10.2994/SAJH-D-19-00117.1 [DOI] [Google Scholar]
- 49.Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, et al. Relaxed selection in the wild. Trends Ecol Evol. 2009; 24:487–96. doi: 10.1016/j.tree.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 50.Krausman PR, Byers JA. American Pronghorn: Social adaptations and the Ghosts of Predators Past. J Wildl Manage. 1998; 62:1580. [Google Scholar]
- 51.Blumstein DT, Daniel JC, Springett BP. A test of the Multi-Predator hypothesis: Rapid loss of antipredator behavior after 130 years of isolation. Ethol. 2004; 110:919–34. doi: 10.1111/j.1439-0310.2004.01033.x [DOI] [Google Scholar]
- 52.Blumstein DT. The Multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethol. 2006; 112:209–17. doi: 10.1111/j.1439-0310.2006.01209.x [DOI] [Google Scholar]
- 53.Coss RG.Effects of relaxed natural selection on the evolution of behavior. Foster S.A., Endler J.A. (Eds.), Geographic Variation in Behavior, Oxford University Press,1999.pp. 181–208. [Google Scholar]
- 54.Coss RG. & Goldthwaite RO. The persistence of old designs for perception. Persp. Ethol. 1995; 11: 83–148. [Google Scholar]
- 55.Wüster W, Duarte MR, Salomão M da G. Morphological correlates of incipient arboreality and ornithology in island pitvipers, and the phylogenetic position of Bothrops insularis. Zool J. 2005; 266:1–10. [Google Scholar]
- 56.Peckarsky BL, Penton MA. Why do Ephemerella nymphs Scorpion Posture: A “Ghost of Predation Past”?. Oikos. 1988; 53:185. doi: 10.2307/3566061 [DOI] [Google Scholar]
- 57.Neill WE. Induced vertical migration in copepods as a defense against invertebrate predation. Nature. 1990; 345:524–6. doi: 10.1038/345524a0 [DOI] [Google Scholar]
- 58.Dias CAR, Perini FA. Biogeography and early emergence of the genus Didelphis (Didelphimorphia, Mammalia). Zool Scr. 2018; 47:645–54. doi: 10.1111/zsc.12306 [DOI] [Google Scholar]
- 59.Perales J, Munos R, Mousatech H. Isolation and partial characterization of a protein fraction from the opossum (Didelphis marsupialis) serum, with protecting property against the Bothrops jararaca venom. An Acad Brasil Cienc. 1986; 58:155–162. [PubMed] [Google Scholar]
- 60.Melo PA, Suarez-Kurtz G. Release of sarcoplasmic enzymes from skeletal muscle by Bothrops jararacussu venom: antagonism by heparin and by the serum of South American marsupials. Toxicon. 1988; 26:87–95. doi: 10.1016/0041-0101(88)90140-7 [DOI] [PubMed] [Google Scholar]
- 61.Fuchs J, Chen S, Johnson JA., Mindell DP. Pliocene diversification within the South American forest falcons (Falconidae: Micrastur). Mol Phylogenet Evol, 2011; 60:398–407. doi: 10.1007/978-3-319-73745-4_1 [DOI] [PubMed] [Google Scholar]
- 62.Mindell DP, Jérôme F, Johnson JA. Phylogeny, taxonomy, and geographic diversity of diurnal raptors: Falconiformes, Accipitriformes, and Cathartiformes. Birds of prey. Springer, Cham, 2018.pp.3–32. [Google Scholar]
- 63.Sazima I, Marques OAV. Old habits die hard: Mouse handling by a pitviper species on a rodent-free island. Amphibia-Reptilia. 2009; 30:435–8. doi: 10.1163/156853809788795092 [DOI] [Google Scholar]
- 64.Barbo FE, Grazziotin FG, Sazima I, Martins M, Sawaya RJ. A new and threatened insular species of Lancehead from Southeastern Brazil. Herpetologica. 2012; 68:418–29. doi: 10.2307/23255793 [DOI] [Google Scholar]
- 65.Barbo FE, Gasparini JL, Almeida AP, Zaher H, Grazziotin FG, Gusmão RB, et al. Another new and threatened species of lancehead genus Bothrops (Serpentes, Viperidae) from Ilha dos Franceses, Southeastern Brazil. Zootaxa. 2016; 4097:511–29. 10.11646/zootaxa.4097.4.4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Intercept- Species (B. jararaca) and Predator (terrestrial). Bold p-values indicate p < 0.05.
(DOCX)
Bold p-values indicate p < 0.05.
(DOCX)
Frequencies of snake behaviors for each species and predatory model.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.