Abstract
Purpose:
In many cancers, nivolumab in combination with ipilimumab improves response rates compared to either agent alone, but the combination has not been evaluated in childhood cancer. We conducted a Phase I/II trial of nivolumab plus ipilimumab in children and young adults with recurrent/refractory solid tumors.
Methods:
ADVL1412, Part C assessed safety of nivolumab plus ipilimumab at two dose levels (DL): DL1 1mg/kg of each drug and DL2 3mg/kg nivolumab plus 1mg/kg Ipilimumab. Part D evaluated response at the recommended phase 2 dose (RP2D) in Ewing sarcoma, rhabdomyosarcoma, and osteosarcoma. Part E tested DL3 (1mg/kg nivolumab plus 3mg/kg Ipilimumab) in Ewing sarcoma and rhabdomyosarcoma. Tumor response was measured using RECIST v1.1. Pharmacokinetics and PD-L1 expression on archival tissues were assessed.
Results:
Fifty-five eligible patients enrolled. Based upon safety, tolerability and similar drug exposure to the same doses administered in adults, DL2 was defined as the pediatric RP2D. Among 41 patients treated at the RP2D, 2 patients experienced dose-limiting toxicities during Cycle 1 and 4 patients experienced toxicities beyond that period. Two patients had clinically significant sustained partial responses (1 rhabdomyosarcoma, 1 Ewing sarcoma) and 4 had stable disease. Among 8 patients treated at DL3, 3 DLTs occurred, all immune related adverse events; no objective responses were observed.
Conclusion:
The RP2D of nivolumab (3mg/kg) plus ipilimumab (1mg/kg) is well-tolerated in children and young adults with solid tumors and shows some clinical activity. Increased dose of ipilimumab (3mg/kg) plus nivolumab (1mg/kg) was associated with increased toxicity without clinical benefit.
Introduction
Relapsed and refractory childhood solid tumors are rarely curable with conventional cytotoxic regimens1,2. Some immunotherapies have shown promise in this setting, such as dinutuximab administered in combination with cytotoxic therapy in relapsed/refractory neuroblastoma3,4. Single agent PD-1 or PD-L1 blockade results in significant clinical activity in several common solid tumors of adulthood and single agent CTLA4 blockade mediates durable survival benefit in adults with melanoma5. In contrast, consistent single agent activity of PD-1 or PD-L1 blockade in cancers arising in children and young adults has been limited to Hodgkin and non-Hodgkin lymphoma and cancers arising in the context of biallelic mismatch repair6–8,9. Single agent CTLA-4 blockade showed no significant antitumor activity in a Phase I trial of children with melanoma or other solid tumors10,11.
Combination immune checkpoint inhibition (ICI), using nivolumab plus ipilimumab to mediate dual blockade of PD-1 and CTLA-4 respectively, demonstrates enhanced activity compared to single agent ICI in several adult cancers. In unresectable or metastatic melanoma, overall survival at 5 years is 52% following nivolumab plus ipilimumab compared to 44% for nivolumab alone and 26% for ipilimumab alone12,13. Evidence for benefit of nivolumab plus ipilimumab compared to single agent ICI has also been demonstrated in patients with advanced renal cell cancer, microsatellite high/deficient mismatch repair (MSI-hi/dMMR) metastatic colorectal cancer12,14,15, hepatocellular carcinoma16, and non-small cell, EGFR/ALK wild type lung cancer17–19. Combination ICI may also provide benefit over PD-1/PD-L1 blockade alone in ovarian cancer, small cell lung cancer, castration-resistant prostate cancer, esophageal cancer, sarcoma, and glioblastoma20–29.
Combination ICI regimens are associated with an increased rate and severity of immune related adverse events compared to PD-1/PD-L1 blockade alone, and these appear to be related to the dose of ipilimumab administered. The Checkmate 067 and 069 trials, which tested nivolumab plus ipilimumab in patients with advanced melanoma, led to FDA approval of nivolumab 1mg/kg plus ipilimumab 3mg/kg30. However, toxicity was significant, and thus Checkmate 511 tested nivolumab 3mg/kg plus ipilimumab 1mg/kg, which demonstrated an improved toxicity profile and no reduction in clinical benefit 31. Thus, the optimal dose of combination ICI has not been clearly established for all clinical settings. Given the paucity of clinical responses in pediatric and young adult patients receiving single agent ICI, we explored the safety and tolerability of combination ICI at three dose levels, assessed pharmacokinetics of the combination in children, and assessed activity at the RP2D in patients with sporadic relapsed/refractory childhood sarcomas (osteosarcoma, rhabdomyosarcoma, and Ewing sarcoma).
Methods
Patient Eligibility
Study participants were required to have adequate organ function, recovered from the acute toxic effects of all prior anti-cancer therapies, and be ≥ 42 days from autologous bone marrow transplant, stem cell infusion, or cellular therapy. Patients with known CNS metastases or CNS tumors were not eligible, nor were patients requiring daily systemic corticosteroids or those who had received systemic corticosteroids within 7 days prior to enrollment. If systemic corticosteroids were used to modify immune adverse events related to prior therapy, at least 14 days must have elapsed since last dose of corticosteroid.
Study Design
ADVL1412 comprised parts A-E; parts A/B, a Phase I/II study of single agent nivolumab, were previously reported9. Here we report results of Parts C-E, which undertook Phase I/II testing of nivolumab plus ipilimumab.
Part C was a dose finding arm with the primary goal to determine the recommended phase II dose (RP2D) of nivolumab plus ipilimumab using the schedule described below. Children (age 1–18 years) with recurrent or refractory solid tumors with measurable or evaluable disease were eligible. The trial was approved by the National Cancer Institute’s central institutional review board. Written informed consent and assent were obtained in accordance with federal and institutional guidelines. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Two dose levels (DLs) were tested: DL1 delivered nivolumab 1mg/kg administered intravenously (IV) over 60 minutes followed by ipilimumab 1mg/kg administered IV over 90 minutes on Day 1 every 21 days for four cycles followed by 28-day cycles of single agent nivolumab at 3mg/kg IV over 60 minutes every 14 days. If <33% of 6 patients at DL1 experienced DLT, dose escalation to DL2 occurred, which tested nivolumab 3mg/kg and ipilimumab 1 mg/kg administered according to the same schedule described above. If <33% of 6 patients at DL2 experienced a DLT, DL2 was considered safe for testing in the disease specific cohorts in Part D, and an additional 6 patients were planned to enroll simultaneously in Part C at DL2 to complete a pharmacokinetic cohort. Part D tested DL2 to identify signals of activity in disease specific expansion cohorts and to generate further information regarding toxicity of the agents in this population. Eligibility required age 1–30 years, and measurable rhabdomyosarcoma (RMS) (Part D1), Ewing sarcoma (ES) (Part D2), or osteosarcoma (OS) (Part D3). Each disease-specific cohort in Part D was studied using a Simon optimal two-stage design. For each cohort, 10 response-evaluable patients were enrolled in stage 1. If there were no responders, then the study concludes that the agent does not elicit a sufficient response. Otherwise, an additional 10 patients were enrolled in stage 2. If there were less than 3 responders among 20 evaluable patients overall, then the study concludes that the combination therapy does not elicit a sufficient response.
The therapy combination was not considered of sufficient interest for further evaluation in a disease category if the true response rate was 5% and of sufficient activity if the true response rate was 25%. If the combination therapy has a true response rate of 5%, the rule described above would identify the therapy of sufficient activity for further study with probability 0.07 (type I error), and the trial will have an expected sample size of 14 with 60% probability of early termination. If nivolumab in combination with ipilimumab had a true response rate of 25%, the rule described above would identify the therapy of sufficient activity for further study with probability 0.88 (power).
Based on objective responses in Part D, the protocol was amended to test nivolumab 1mg/kg and ipilimumab 3mg/kg (Part E) administered according to the schedule described for Parts C and D in patients aged 1–30 years with measurable rhabdomyosarcoma (RMS) (Part E1) and Ewing sarcoma (ES) (Part E2). Part E used a similar 10+10 Simon two-stage design as described above. However, both disease cohorts were combined for assessment of response.
Toxicity Evaluation
Patients were evaluable for toxicity if they received at least one dose of each study drug and completed toxicity monitoring or experienced a Cycle 1 dose limiting toxicity (DLT). NCI Common Terminology Criteria for Adverse Events (CTCAE) v5.0 was used for description and grading of all toxicities. Adverse events (AEs) were deemed unrelated, possibly, probably, or definitely related to nivolumab and/or ipilimumab by the treating physician with central confirmation. Review of symptoms, physical examination, and laboratory assessments were conducted weekly during cycle 1, then prior to each cycle and as clinically indicated. Disease specific cohorts enrolled in part E were combined for assessment of toxicity and efficacy, with the rule that if one Cycle 1 DLT was observed in the first 10 patients, the dose would be deemed too toxic for further testing.
Definition of Dose Limiting Toxicity (DLT)
Hematological dose limiting toxicity (DLT) was defined as Grade 4 thrombocytopenia or neutropenia lasting greater than five days. Non-hematological DLT included grade 2 fever that did not resolve to grade ≤ 1 within 7 days, uveitis, eye pain, or blurred vision that did not respond to topical therapy and did not improve to grade 1 prior to next scheduled dose, or any grade 2 toxicity requiring systemic immunosuppressive therapy, including autoimmunity of the lung, heart, kidney, bowel, CNS, pituitary or eye, with the specific exclusion of grade 2 reversible pleural effusion. Other grade 2 toxicities designated as DLTs included adrenal insufficiency, endocrine toxicity requiring hormone replacement, with the exception of grade 2 hypothyroidism, thyroiditis and thyroid dysfunction adequately managed with thyroid hormone replacement. Grade 2 colitis or grade 2 diarrhea of any duration was considered a DLT. Any grade 3 or grade 4 non-hematological toxicity attributable to protocol therapy was considered a DLT with the specific exclusion of: grade 3 rash, oral lesions, or hepatic transaminase elevation (ALT/AST/GGT) that returned to levels meeting protocol eligibility criteria or baseline within 7 days and did not require systemic immunosuppression, grade 3 or 4 serum electrolyte or mineral abnormalities responsive to supplementation, grade 3 or 4 amylase or lipase abnormalities that were not associated with diabetes mellitus, liver or gallbladder inflammation, or clinical manifestations of pancreatitis that resolved to grade ≤ 2 within 7 days, grade 3 fatigue that resolved to grade ≤ 2 within 7 days, grade 3 creatinine increased that resolved to Grade ≤ 1 or baseline within 7 days.
Response Assessment
Radiographic disease assessments were obtained after Cycles 2 and 4, then after every third Cycle. Patients with measurable disease at baseline were evaluable for objective response if they received at least one dose of study drug and had a disease re-evaluation performed or clinical progression of disease was documented by the treating physician. Response was evaluated using revised Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Due to the possibility of pseudoprogression, patients who experienced tumor growth greater than 20% but less than 40% were allowed to remain on study for up to 12 weeks with more frequent disease monitoring, if the patient showed no rapid disease progression or deterioration in performance status, had not experienced a DLT and/or was otherwise demonstrating clinical benefit. Central review was required for all objective responses.
Tumor Analyses
PD-L1 expression was assessed by immunohistochemistry (IHC) on tumor tissue obtained at the time of initial diagnosis or subsequent biopsy. Tumor PD-L1 expression was evaluated centrally using the Dako PD-L1 IHC 28–8 pharmDx assay (Agilent, Santa Clara, CA) in formalin-fixed, paraffin-embedded (FFPE) tumor samples32. Briefly, following incubation with the primary monoclonal antibody to PD-L1 (clone 28–8) or the Negative Control Reagent, specimens were incubated with an anti-rabbit linker antibody followed by a horseradish peroxidase visualization reagent. Control slides containing two formalin-fixed, paraffin-embedded human cell lines known to express PD-1 were included. Results were interpreted using a light microscope. The percentage of tumor cells demonstrating plasma membrane PD-L1 staining at 0, 1+, 2+ or 3+ intensity was quantified in a minimum of 100 evaluable tumor cells. The presence of tumor associated immune cells was assessed by visual inspection by pathologist review. PD-L1 membrane staining on associated lymphocytes or macrophages was assessed qualitatively. Tissue for 2 patients treated in Part D was inevaluable for PD-L1 staining and for one patient from Part C, 2 separate specimens obtained from the same timepoint at diagnosis were tested.
Pharmacokinetics
Blood samples were collected for pharmacokinetic studies prior to the nivolumab infusion and at the end of the ipilimumab infusion (EOI) on Day 1 of Cycles 1–4. Blood samples (2 ml of whole blood) were collected into 2 mL red top serum separator tubes (SST). Samples were allowed to clot for 30–45 minutes and then centrifuged at room temperature for 15 minutes at 1100–1300 x g until clot and serum are well separated. Supernatant serum was transferred into separate tubes and stored at −20°C or −70°C until shipment to the laboratory. Serum samples were analyzed to determine nivolumab and/ or ipilimumab concentration using a validated immunoassay by Pharmaceutical Product Development, LLC, Richmond, VA.
Data Availability
Data generated in this study are available upon request from the corresponding author.
Results
Patients
Enrollment was initiated in February 2015 and data cut off for analysis was September 30, 2020. Patient characteristics are shown in Table 1. Fifty-five eligible patients were enrolled on study and 53 were evaluable for dose limiting toxicity. One patient on part C was not evaluable for DLT due to not having all required evaluations performed and one patient on part D was not evaluable for DLT as this patient did not complete one dose of study drugs; all patients were evaluable for response. As of the data cut-off date, no patient remained on protocol therapy. Eighteen patients enrolled on Part C of whom 6 received DL1. A total of 41 patients received DL2, (n=12 on Part C and n=29 on Part D) and 8 patients received DL3 on Part E. Patients received a median of 2 cycles with a total of 147 patient-cycles delivered on study. Overall, among 55 enrolled patients, there were 51 patient-courses in Part C, 82 patient-courses in Part D and 14 patient-courses in Part E.
Table 1.
Demographics and diagnoses of patients treated on study.
| Demographics | Part C | Part D | Part E | Total |
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | Number (%) | |
| N=18 | N=29 | N=8 | N=55 | |
| Age (years) | ||||
| Median Range |
14 4–17 |
17 5–27 |
19 11–28 |
15 4–28 |
| Sex | ||||
| Male Female |
10 (56) 8 (44) |
21 (72) 8 (28) |
3 (38) 5 (62) |
34 (62) 21 (38) |
| Race | ||||
| White Asian American Indian or Alaska Native Black or African American Unknown |
11 (61) 2 (11) 1 (6) 2 (11) 2 (11) |
23 (79) 0 (0) 0 (0) 2 (7) 4 (14) |
8 (100) 0 (0) 0 (0) 0 (0) 0 (0) |
42 (76) 2 (4) 1 (2) 4 (7) 6 (11) |
| Ethnicity | ||||
| Non-Hispanic Hispanic Unknown |
15 (83) 3 (17) 0 (0) |
22 (76) 5 (17) 2 (7) |
5 (62) 1 (13) 2 (25) |
42 (76) 9 (16) 4 (7) |
| Prior Therapy | ||||
| Chemotherapy Regimens Median Range |
2.5 1–8 |
3 1–7 |
2.5 1–5 |
3 1–8 |
| Radiation Therapy Cycles Median Range |
2 1–4 |
1 1–3 |
1 1–2 |
1 1–4 |
| Diagnosis | ||||
| Adrenal cortical adenoma, NOS | 1 (5.6) | 1 (1.8) | ||
| Alveolar rhabdomyosarcoma | 5 (17.2) | 2 (25) | 7 (12.7) | |
| Carcinoma, NOS | 1 (5.6) | 1 (1.8) | ||
| Desmoplastic small round cell tumor | 2 (11.1) | 2 (3.6) | ||
| Embryonal rhabdomyosarcoma, NOS | 2 (6.9) | 2 (25) | 4 (7.3) | |
| Ewing sarcoma | 2 (11.1) | 9 (31) | 3 (37.5) | 14 (25.5) |
| Hepatoblastoma | 1 (5.6) | 1 (1.8) | ||
| Myofibroblastic tumor, NOS | 1 (5.6) | 1 (1.8) | ||
| Myxoid liposarcoma | 1 (5.6) | 1 (1.8) | ||
| Nephroblastoma, NOS | 2 (11.1) | 2 (3.6) | ||
| Neuroblastoma, NOS | 1 (5.6) | 1 (1.8) | ||
| Osteosarcoma, NOS | 3 (16.7) | 10 (34.5) | 13 (23.6) | |
| Renal cell carcinoma, NOS | 1 (5.6) | 1 (1.8) | ||
| Rhabdomyosarcoma, NOS | 2 (6.9) | 1 (12.5) | 3 (5.5) | |
| Spindle cell rhabdomyosarcoma | 1 (3.4) | 1 (1.8) | ||
| Synovial sarcoma, NOS | 1 (5.6) | 1 (1.8) | ||
| Yolk sac tumor | 1 (5.6) | 1 (1.8) |
Safety and Tolerability
Figure 1 shows all Grade 2 or higher toxicities possibly, probably, or definitely attributable to therapy over time (this data also shown in Supplementary Table 1). Of 53 patients who were evaluable for toxicity, 52 (98%) experienced at least one treatment related adverse event (Supplementary Table 2). Like single-agent nivolumab or ipilimumab, immune-related adverse events (irAE) related to the gastrointestinal system were common, including elevations in ALT, AST, and lipase, nausea, and anorexia. Hematologic toxicities were common with anemia, thrombocytopenia, decreased white blood cell count, including lymphocytes and neutrophils frequently reported. Pleural (n=7) and pericardial (n=2) effusions were observed.
Figure 1.
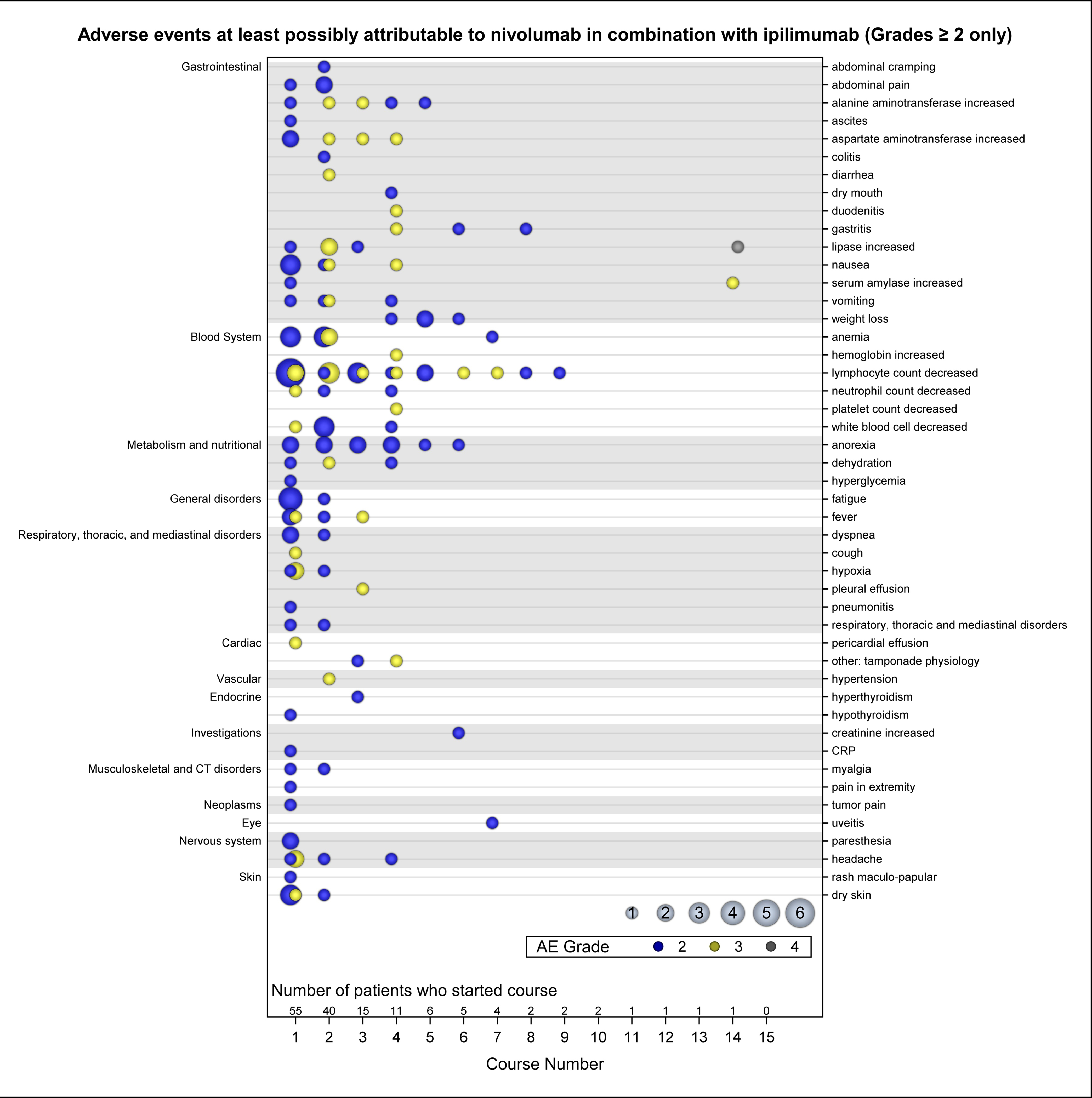
Adverse Events attributable to study agents over time grouped by CTCAE v5.0 organ system. Color indicates AE grade and circle size indicates number of patients.
Seventeen patients in Part C were evaluable for toxicity and only one Cycle 1 DLT was observed in a patient treated at DL2 who experienced a grade 3 creatinine increase. Two additional DLTs occurred beyond Cycle 1 in Part C: a grade 4 increased lipase and a grade 3 alanine aminotransferase elevation (Table 2). Twenty-eight patients enrolled on Part D were evaluable for DLT (97%) and one patient experienced a Cycle 1 DLT, a grade 4 pleural effusion. DLTs beyond Day 28 in Part D were observed in 4 patients with all experiencing more than one DLT (Table 2), all of which were gastrointestinal immune related adverse events. Among 8 patients evaluable for DLT in Part E, one patient experienced a Cycle 1 DLT comprising grade 4 rash and grade 4 fever, which triggered the stopping rule for DL3 and closed Part E to further enrollment. Two additional subjects on Part E experienced cycle 2 DLTs that were immune-related AEs (irAE; Grade 4 diarrhea, elevated AST/ALT, hyperthyroidism) (Table 2). Together, the data suggest that early toxicity (cycle 1) was mild, but additional higher grade irAEs were observed in subsequent cycles, suggesting increased toxicities with longer duration of exposure to these agents (Figure 1; Supplemental Table 1.).
Table 2.
Dose Limiting Toxicities (DLT) of nivolumab plus ipilimumab reported per dosing cohort and subject across all cycles. Each row indicates an individual subject.
| Cycle | Adverse Event | Grade | |
|---|---|---|---|
| Part C N = 17 |
1 | Creatinine increased (DL2) | 2 |
| 2 | Lipase increased (DL2) | 3 | |
| 5 | ALT increased (DL1) | 2 | |
| Part D N = 28 |
4 | AST increased, gastritis, duodenitis, hypertension, nausea, hemoglobin increased | 3 |
| 3 | AST & ALT increased | 3 | |
| 14 | Lipase increased | 4 | |
| amylase increased | 3 | ||
| Follow-Up Period (100-day) | ALT, AST, lipase, and GGT increased | 4 | |
| weight loss, pancreatitis, nausea, anorexia | 3 | ||
| 1 | Pleural effusion | 3 | |
| Part E N = 8 |
2 | ALT, AST increased, hyperthyroidism | 3 |
| 2 | Diarrhea | 3 | |
| 1 | Fever, maculopapular rash | 3 |
Pharmacokinetics
Peak and trough concentration data were available for 48 patients treated with four cycles of nivolumab and ipilimumab in Parts C, D, and E (Supplementary Figure 1 and Supplementary Table 3). A dose proportional increase in peak and trough concentration was observed for both nivolumab and ipilimumab when dose was increased from 1 mg/kg to 3 mg/kg. For nivolumab (3mg/kg), the mean trough concentration was maintained above 10 μg/ml through 4 cycles of treatment in combination with ipilimumab. A mean trough concentration of 17.0±7.9 μg/ml nivolumab (range, 4.8–48.6 μg/ml) was reached after the first 3 mg/kg dose and increased to 32.8±12.6 μg/ml (range, 13.9–54.1 μg/ml) after the third dose. A mean trough concentration of 3.9±1.4 mg/ml (n=32) ipilumumab was reached after the first 1 mg/kg dose of ipilimumab (n = 32). The trough concentration increased to 6.0±2.3 ug/ml after the second dose (n = 12) and 7.7±3.7 mg/ml after the third dose (n = 9).
Response
Response data for all patients are summarized in Figure 2. None of the expansion cohorts studied here met response criteria to proceed to expansion. Two confirmed partial responses as best overall response (BOR) occurred in Part D. A 25-year-old male with alveolar rhabdomyosarcoma, confirmed to have a partial response after the second cycle (Figure 3A), remained on study therapy for twelve cycles, eventually discontinuing due to Grade 3 amylase elevation and grade 4 lipase elevation. An 18-year-old female with Ewing sarcoma (Figure 3B), also with a confirmed partial response after cycle 2, discontinued protocol therapy after cycle 4 due to gastrointestinal irAEs with grade 3 gastritis, duodenitis, nausea and elevated AST. As of the 36-month follow-up (beyond the data cutoff), both patients were alive without disease progression. In addition, four patients had a BOR of stable disease; two patients with rhabdomyosarcoma, one patient with myofibroblastic tumor and one patient with nasopharyngeal carcinoma experienced centrally-reviewed stable disease. These patients continued protocol therapy for a median of 5.7 months (4.5–7.8 months) prior to discontinuing therapy due to progressive disease. There was no activity observed in the osteosarcoma cohort in Part D. In Part E, there were no objective responses in patients with rhabdomyosarcoma (n=3) or Ewing sarcoma (n=4) treated with the higher dose of ipilimumab.
Figure 2.
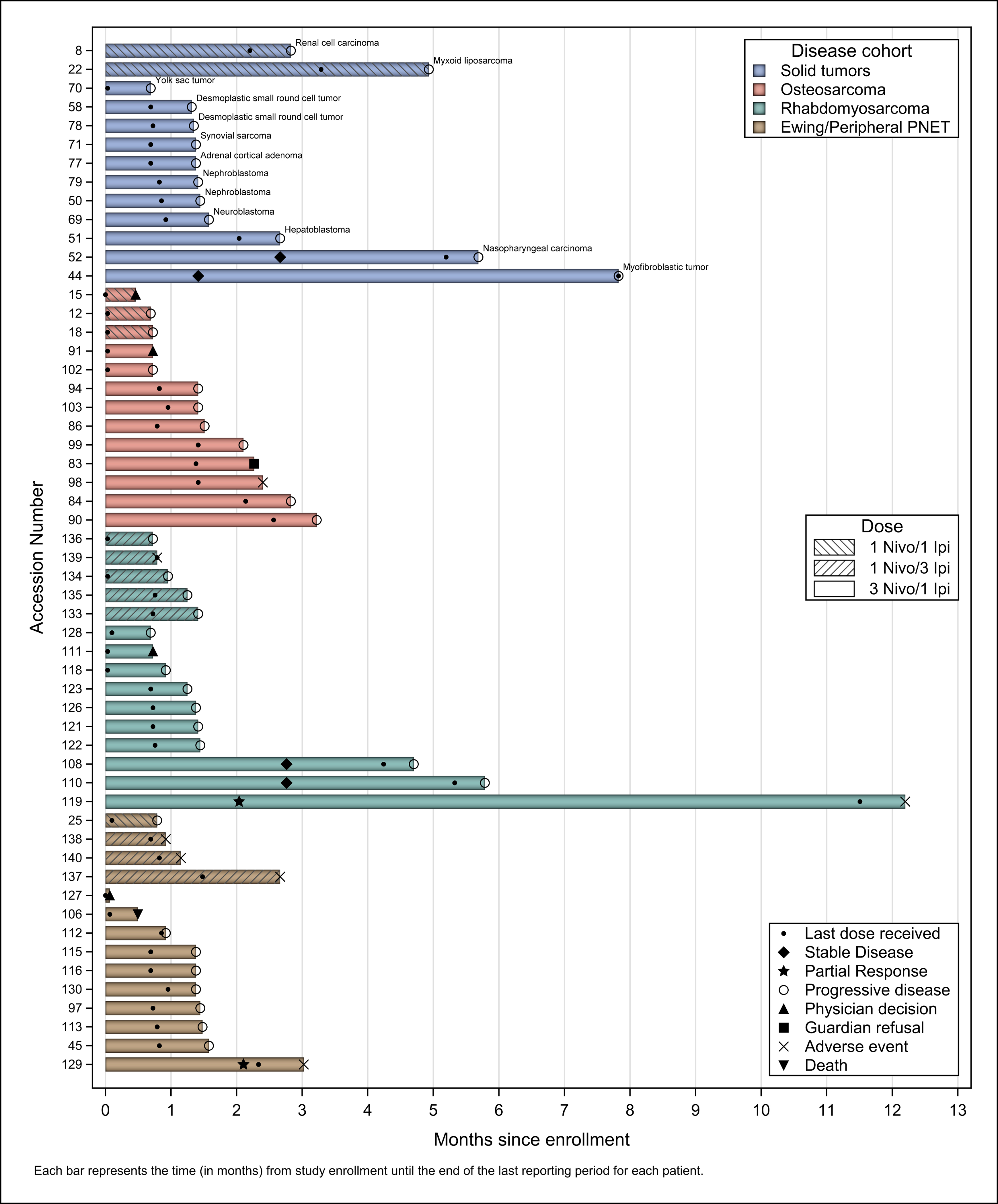
Swimmers plot of response based on dosing cohort and diagnosis across all evaluable patients. Each bar represents the time in months from study enrollment until the end of the last reporting period.
Figure 3.
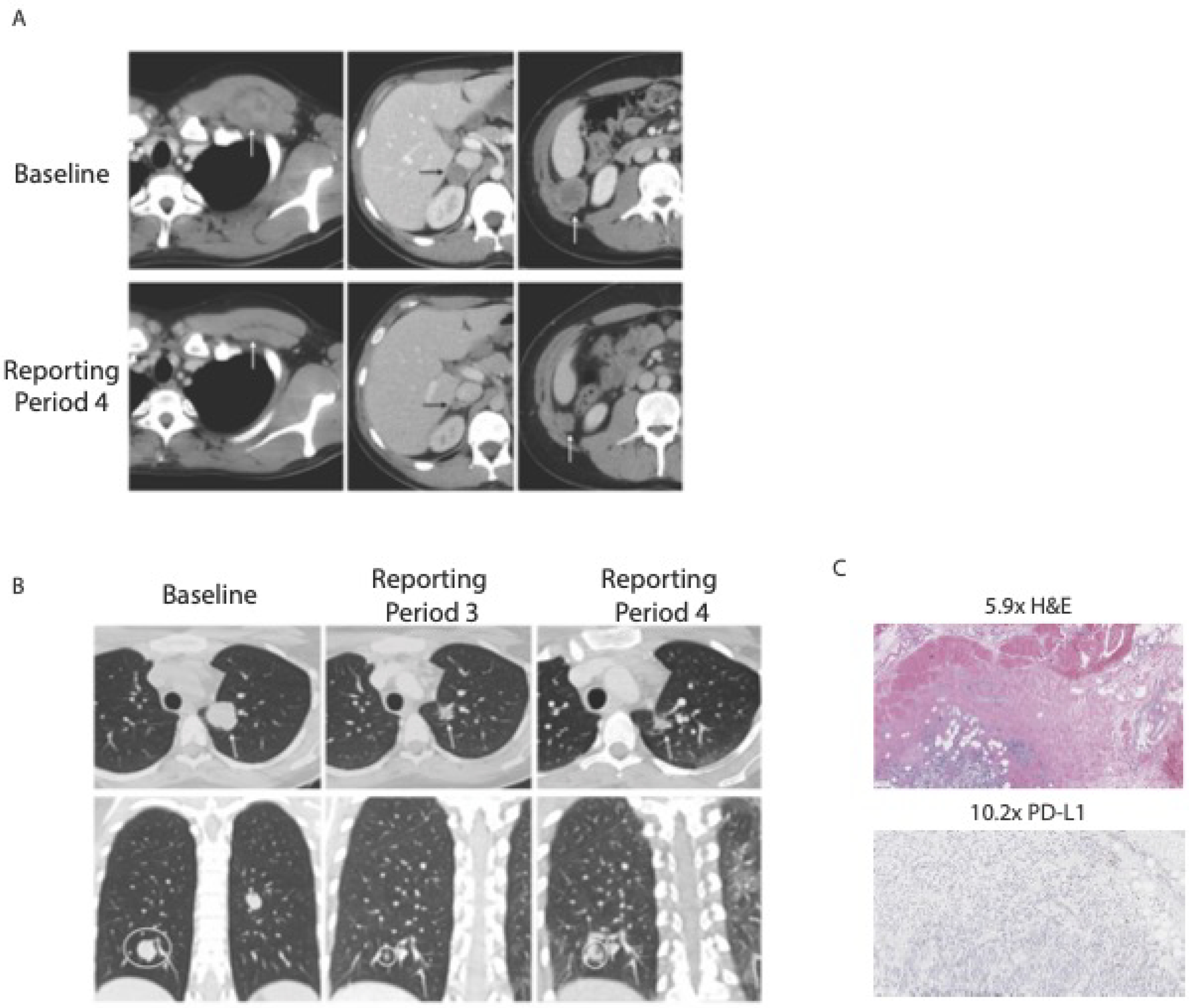
Partial responses in two patients in cohort D (nivolumab 3mg/kg plus ipilimumab 1mg/kg. A) Partial response in rhabdomyosarcoma after 4th cycle B) Partial response in Ewing sarcoma after 3rd cycle. C) Immunohistochemistry demonstrates infrequent PD-L1+ cells in Ewing sarcoma tumor tissue from diagnosis.
Correlative studies: PD-L1 expression
Biomarkers prognostic of response to checkpoint inhibitors have been proposed to include tumor and tumor-associated immune cell expression of PD-L1, the ligand for PD-133,34. Of the 53 patients, 49 patients had archival tumor tissue available for immunohistochemical evaluation of PD-L1 and evaluation of tumor-associated immune cells. The majority of the samples evaluated were obtained at diagnosis. Only 7(14%) specimens demonstrated any staining for PD-L1 which ranged from 3%−100% of positive tumor cells (Table 3). These patients and their BOR are summarized in Table 3. The Ewing sarcoma patient from Part D who had a PR demonstrated 4% of tumor cells PD-L1+ (3% 1+, 1% 2+) (Figure 3C). The remaining tumor specimens evaluated for PD-L1 expression were negative. One notable negative specimen was from the patient with rhabdomyosarcoma who had a partial response.
Table 3.
PD-L1 immunohistochemistry staining intensity and best overall response. Seven of 49 tumors demonstrated PD-L1 staining on tumor cells by immunohistochemistry. SD = stable disease, NR = no response, PR = partial response.
| Histology | PD-L1+ tumor cells (%) | Staining intensity (% cells) | Response | |||
|---|---|---|---|---|---|---|
| +3 | +2 | +1 | 0 | |||
| Nasopharyngeal carcinoma | 100 | 95 | 5 | 0 | 0 | SD |
| Myofibroblastic tumor | 4 | 2 | 2 | 0 | 96 | SD |
| Hepatoblastoma | 3 | 1 | 1 | 1 | 97 | NR |
| Renal cell carcinoma | 97 | 2 | 20 | 75 | 3 | NR |
| Ewing sarcoma | 25 | 0 | 0 | 25 | 75 | NR |
| Ewing sarcoma | 4 | 0 | 1 | 3 | 96 | PR |
| Alveolar RMS | 3 | 0 | 2 | 1 | 97 | NR |
Thirty-six of the evaluable samples demonstrated tumor associated immune cells. These immune cells were evaluated for PD-L1 expression and categorized as lymphocytes, macrophages, or neither. Eighteen specimens demonstrated PD-L1+ immune cells that were neither lymphocytes nor macrophages, 5 demonstrated only PD-L1+ macrophages, 7 demonstrated only PD-L1+ lymphocytes, and 12 demonstrated both. Of the twelve that demonstrated both PD-L1+ lymphocytes and macrophages, all except one were predominantly macrophages. Overall, there did not appear to be any clear relationship between expression of PD-L1 and clinical response or toxicity in these patients.
Discussion
In this pediatric phase I/II trial, we identified 3mg/kg nivolumab and 1mg/kg ipilimumab administered every 21 days for four cycles followed by 3mg/kg nivolumab every 14 days for subsequent cycles as the RP2D of nivolumab plus ipilimumab in children and young adults with relapsed or refractory solid tumors. Mean trough concentrations of nivolumab were maintained above the 10 μg/ml target concentration for the 3 mg/kg dose in the single agent study9. Mean trough concentrations of ipilimumab (1mg/kg) were maintained above the 6 μg/ml concentration that was found to maximally inhibit CTLA-4 binding to its CD80 and CD86 ligands with the 1 mg/kg dose35. Similar to data in adults with cancer demonstrating increased toxicity with nivolumab plus ipilimumab compared to treatment with nivolumab alone, we observed that 15% of patients experienced a DLT with nivolumab plus ipilimumab regimens studied compared to 6.7% with single agent nivolumab9. As with single agent nivolumab, most toxicities were grade 2 or less and did not preclude continued therapy with the combination; grade 3 or 4 adverse events attributable to therapy occurred in 38% of patients, and 38% at the RP2D.
The most common AEs were similar to those observed with single agent nivolumab with fatigue being the most commonly reported toxicity. Hematologic toxicities were not prominent in studies of nivolumab and ipilimumab in adults, but we observed significant rates of hematologic AEs on this study, similar to that previously reported with single agent nivolumab9. It is not clear if the hematologic toxicity observed here was directly attributable to the combination ICI regimen or reflective of the heavily pretreated nature of this patient population. Gastrointestinal toxicities were prominent non-hematologic AEs with transaminitis, nausea, and anorexia commonly reported (Table 2). In contrast to the CheckMate-511 trial where more than a quarter of patients experienced diarrhea with the Nivolumab 3mg/Ipilumumab 1 mg/kg dose regimen, we observed diarrhea in only 4 patients and none more than Grade 2. This difference may be attributable to the relative fewer doses of the combination received in our cohort where patients received a median of 2 cycles of therapy compared to 4 cycles in CheckMate-51131. We also observed pleural effusions in 7 patients, 6 of whom had thoracic involvement of their tumor suggestive of local inflammation in response to the ICI, similar to the high rate of pleural effusions we previously reported with single agent nivolumab9.
We observed a trend toward cumulative irAEs including colitis, gastritis, duodenitis, pancreatitis, and thyroid disorders as patients received subsequent cycles of therapy. This is consistent with irAEs reported in adults treated with this combination27,36–38. Further, individual patients developed multiple irAEs when treatment was continued for additional cycles.
Based upon evidence in the adult literature for improved response rates with increasing doses of ipilimumab in this combination, we sought to determine whether increasing the dose of ipilimumab (Part E), would improve clinical efficacy in patients with rhabdomyosarcoma or Ewing sarcoma. We did not observe clinical responses to this combination in the 8 patients enrolled in this cohort, although evaluation was limited by toxicity as 37.5% of patients (3 of 8) experienced a DLT. One DLT occurred during cycle 1, which triggered our toxicity stopping rule and additional DLTs occurred in Cycle 2. The DLTs were classic immune related AEs (Table 2) that precluded continued evaluation of this combination with the increased dose of ipilimumab. The increased incidence of irAEs observed with the higher dose of ipilimumab was consistent with the reported pediatric and young adult experience with single agent ipilimumab where toxicity was directly correlated with increasing ipilimumab dose10.
Two patients experienced a partial response after treatment with combination nivolumab and ipilimumab which was maintained for 36 months even after discontinuation of study treatment and four additional patients experienced stable disease for a median of 5.6 months. Both patients experiencing PRs discontinued protocol therapy due to the development of irAEs which supports the concept that development of irAEs is correlated with response with ipilimumab. Despite the relatively large size of this Phase I/II study of relapsed childhood tumors, the evaluation of combination ipilimumab and nivolumab was limited to patients with common childhood sarcomas and thus, how this drug combination performs in other histologies of childhood cancer is not addressed. We observed stable disease in one patient with nasopharyngeal carcinoma and one patient with myofibroblastic tumor, suggesting potential utility of nivolumab and ipilimumab in these patients. Further, we had limited enrollment of very young children.
The overall objective response rate on this study was low. Childhood tumors are considered immunologically ‘cold tumors’ with few infiltrating immune cells and low neoantigen burden precluding robust response to immune checkpoint therapies 39,40. Prior studies have demonstrated a paucity of PD-L1 expression on childhood sarcomas (osteosarcoma, Ewing sarcoma and rhabdomyosarcoma) consistent with what we observed in this study39,41. We were unable to evaluate the role of tumor neoantigen burden in this study, however other studies in pediatric patients are systematically addressing this question (NCT04500548).
Pre-treatment biopsies and additional correlative studies would aid discovery of biomarkers of response in these young patients. PD-L1 was demonstrated on a minority of tumor cells in one patient who had a response while another patient with Ewing sarcoma with demonstrable PD-L1 staining of tumor cells had no clinical response. The responding patient had lymphocytes infiltrating the tumor whereas the non-responding patient had both lymphocytes and macrophages. This raises the question regarding the role of tumor-infiltrating macrophages in creating an immune suppressive tumor microenvironment42,43. Correlative PD-L1 IHC was largely performed on specimens from diagnosis, therefore, the expression of PD-L1 at time of immune checkpoint therapy is not known.
Based upon safety, tolerability, and equivalent pharmacokinetics in adults, we defined nivolumab 3 mg/kg plus ipilimumab 1mg/kg as the RP2D in children. Among 41 patients treated at the RP2D, two patients experienced partial responses and four experienced stable disease. Immune related AEs were common but tolerable overall. Similar to increased toxicity observed in adults when higher doses of ipilimumab are administered in the combination regimen, we observed increased irAEs with a regimen employing nivolumab 1 mg/kg plus ipilimumab 3 mg/kg and no improved efficacy.
Despite limited response to immune checkpoint combination in the common sarcoma histologies studied here, durable partial responses were observed in two patients. While not meeting criteria for further enrollment in this study, these responses are clinically meaningful since both patients remain alive at last follow up. Given that the combination is well-tolerated, further study of this combination is warranted in Ewing and rhabdomyosarcoma patients alongside correlative studies including pre- and post-treatment biopsy and tumor mutational burden assessment to identify biologic determinants of response and guide how to integrate these agents into therapy for patients likely to demonstrate response.
Supplementary Material
Statement of Translational Relevance.
This report details, to our knowledge, the first systematic assessment of the safety and pharmacokinetics of dual immune checkpoint inhibition with ipilimumab and nivolumab in pediatric, adolescent, and young adult patients with relapsed or refractory sarcoma. We establish a recommended phase 2 dose and found the combination to be generally well tolerated. In expanded Phase 2 cohorts, we observed two sustained partial responses. We further demonstrate that increased ipilimumab dosing (3mg/kg) in combination with nivolumab (1mg/kg) carries higher toxicity without clinical benefit in this population.
Acknowledgements:
The research reported is supported by Bristol-Myers Squibb (BMS Study ID CA209-070), the Children’s Oncology Group, the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number UM1 CA228823 and the Cookies for Kids’ Cancer Foundation. CLM is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. The work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research (CLM) and by NCI 5P30CA124435 (CLM). KLD was supported in part by the Stanford Maternal and Child Health Research Institute as the Anne T. and Robert M. Bass Endowed Faculty Scholar in Pediatric Cancer and Blood Diseases. JR was supported in part by Grant Number P30 CA015083 from the National Cancer Institute (NCI). EI was supported by National Institute of General Medical Sciences (NIGMS) grant T32GM 08685. We thank the patients and their families for making this study possible. Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: K.L.D has received research funding from Jazz Pharmaceuticals and has acted in advisory role at Novartis, E.F. none, E.I. none J.M.R none, X.L. none, C.M. has a relationship with CHEST journal, S.V. none, S.L.B. is a member of the Children’s Oncology Group Developmental Therapeutics Steering Committee and some clinical trials may be partially industry funded with some funding received at her institution, B.J.W none, C.L.M has stock or other ownership interests in Syncopation Life Sciences, Lyell Immunopharma and Link Cell Therapies, Apricity Health, Ensoma, holds a leadership position in Syncopation Life Sciences and Link Cell Therapyies, has consulting/advisory roles at Apricity Health, Nektar, Lyell Immunopharma, NeoImmune Tech, Syncopation Life Sciences, Bristol-Myers Squibb, Immatics, Glaxo-Smith-Kline, Ensoma, Mammoth. C.L.M receives research funding from Lyell Immunopharma and holds patents related to chimeric antigen receptor therapeutics and receives royalties from NIH for the CD22-CAR licensed to Juno Therapeutics.
References
- 1.Smith MA, Seibel NL, Altekruse SF, et al. : Outcomes for Children and Adolescents With Cancer: Challenges for the Twenty-First Century. Journal of Clinical Oncology 28:2625–2634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith MA, Altekruse SF, Adamson PC, et al. : Declining childhood and adolescent cancer mortality. Cancer 120:2497–2506, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 378:439–448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu AL, Gilman AL, Ozkaynak MF, et al. : Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. New England Journal of Medicine 363:1324–1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS: Overcoming immunological tolerance to melanoma: Targeting CTLA-4. Asia Pac J Clin Oncol 6 Suppl 1:S16–23, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Geoerger B, Kang HJ, Yalon-Oren M, et al. : Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol 21:121–133, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Geoerger B, Zwaan CM, Marshall LV, et al. : Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1–2 study. Lancet Oncol 21:134–144, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Bouffet E, Larouche V, Campbell BB, et al. : Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. Journal of Clinical Oncology 34:2206–2211, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Davis KL, Fox E, Merchant MS, et al. : Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol 21:541–550, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant MS, Wright M, Baird K, et al. : Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clinical Cancer Research 22:1364–1370, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geoerger B, Bergeron C, Gore L, et al. : Phase II study of ipilimumab in adolescents with unresectable stage III or IV malignant melanoma. Eur J Cancer 86:358–363, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 381:1535–1546, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Neyns B, Linette G, et al. : Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11:155–64, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Postow MA, Chesney J, Pavlick AC, et al. : Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–17, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Rini BI, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 20:1370–1385, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau T, Kang YK, Kim TY, et al. : Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol 6:e204564, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. : Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 381:2020–2031, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares L, Ciuleanu TE, Cobo M, et al. : First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 22:198–211, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Ready N, Hellmann MD, Awad MM, et al. : First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol 37:992–1000, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morse MA, Overman MJ, Hartman L, et al. : Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer. Oncologist 24:1453–1461, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overman MJ, Lonardi S, Wong KYM, et al. : Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 36:773–779, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Zamarin D, Burger RA, Sill MW, et al. : Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol 38:1814–1823, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Pachynski RK, Narayan V, et al. : Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 38:489–499 e3, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Omuro A, Vlahovic G, Lim M, et al. : Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol 20:674–686, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Angelo SP, Mahoney MR, Van Tine BA, et al. : Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol 19:416–426, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meindl-Beinker NM, Betge J, Gutting T, et al. : A multicenter open-label phase II trial to evaluate nivolumab and ipilimumab for 2nd line therapy in elderly patients with advanced esophageal squamous cell cancer (RAMONA). BMC Cancer 19:231, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baas P, Scherpereel A, Nowak AK, et al. : First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397:375–386, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Disselhorst MJ, Quispel-Janssen J, Lalezari F, et al. : Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med 7:260–270, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Scherpereel A, Mazieres J, Greillier L, et al. : Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 20:239–253, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. : Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19:1480–1492, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Lebbe C, Meyer N, Mortier L, et al. : Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. J Clin Oncol 37:867–875, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips T, Simmons P, Inzunza HD, et al. : Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol 23:541–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–54, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Taube JM, Anders RA, et al. : Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature Reviews Cancer 16:275–287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng J, Srivastava S, Sanghavi K, et al. : Clinical Pharmacology Considerations for the Development of Immune Checkpoint Inhibitors. J Clin Pharmacol 57 Suppl 10:S26–S42, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Antonia SJ, Lopez-Martin JA, Bendell J, et al. : Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 17:883–895, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Hellmann MD, Rizvi NA, Goldman JW, et al. : Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 18:31–41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ready NE, Ott PA, Hellmann MD, et al. : Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol 15:426–435, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Majzner RG, Simon JS, Grosso JF, et al. : Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer 27:439–9, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Chalmers ZR, Connelly CF, Fabrizio D, et al. : Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9:34, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoki N, Ishibashi Y, Kai K, et al. : Programmed cell death in barley aleurone cells is not directly stimulated by reactive oxygen species produced in response to gibberellin. J Plant Physiol 171:615–8, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Koo J, Hayashi M, Verneris MR, et al. : Targeting Tumor-Associated Macrophages in the Pediatric Sarcoma Tumor Microenvironment. Front Oncol 10:581107, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura K, Smyth MJ: Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol 17:1–12, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in this study are available upon request from the corresponding author.


