Abstract
Fluoroquinolones trap gyrase on DNA as bacteriostatic complexes from which lethal DNA breaks are released. Substituents at the C-8 position increase activities of N-1-cyclopropyl fluoroquinolones against several bacterial species. In the present study, a C-8-methoxyl group improved bacteriostatic action against gyrA (gyrase-resistant) strains of Mycobacterium tuberculosis and M. bovis BCG. It also enhanced lethal action against gyrase mutants of M. bovis BCG. When cultures of M. smegmatis, M. bovis BCG, and M. tuberculosis were challenged with a C-8-methoxyl fluoroquinolone, no resistant mutant was recovered under conditions in which more than 1,000 mutants were obtained with a C-8-H control. A C-8-bromo substituent also increased bacteriostatic and lethal activities against a gyrA mutant of M. bovis BCG. When lethal activity was normalized to bacteriostatic activity, the C-8-methoxyl compound was more bactericidal than its C-8-H control, while the C-8-bromo fluoroquinolone was not. The C-8-methoxyl compound was also found to be more effective than the C-8-bromo fluoroquinolone at reducing selection of resistant mutants when each was compared to a C-8-H control over a broad concentration range. These data indicate that a C-8-methoxyl substituent, which facilitates attack of first-step gyrase mutants, may help make fluoroquinolones effective antituberculosis agents.
As disease caused by the human immunodeficiency virus spreads to regions of the world where infection with Mycobacterium tuberculosis is common, the incidence of active cases of tuberculosis is increasing dramatically due to weakened host immunity (3, 22). The magnitude of this problem is emphasized by the fact that more than one billion persons are already infected with M. tuberculosis (22, 27). Effective antituberculosis agents are available, but assuring patient compliance to therapy regimens is expensive (5). Moreover, drug-resistant strains of M. tuberculosis, which cause disease that is often very difficult to treat, occasionally arise and spread (1). These considerations suggest that tuberculosis will become an increasingly serious medical problem.
In an attempt to develop more effective antituberculosis agents, we have been studying the fluoroquinolones. These compounds are generally used only as second-line therapeutics because resistance of M. tuberculosis often renders them ineffective (for reviews, see references 2 and 8). However, studies with other bacteria have suggested ways to seek more effective derivatives. For example, it has been proposed that two distinct events occur when cells are treated with fluoroquinolones (7, 10). The drugs first trap gyrase or topoisomerase IV on the chromosome as fluoroquinolone-enzyme-DNA complexes in which the DNA is broken but constrained by protein. These trapped complexes reversibly block DNA synthesis and bacterial growth. In a second event, some of the complexes release DNA ends, resulting in cell death. While inhibition of growth is a convenient assay for drug potency, it sometimes fails to identify the most lethal fluoroquinolone (29). Lethality is likely to be important for minimizing the mutagenic action of fluoroquinolones (20, 21) and the appearance of resistant strains. Thus, fluoroquinolones should be evaluated on the basis of lethal as well as bacteriostatic action. Another issue concerns the finding that resistance to fluoroquinolones arises stepwise, usually through mutations accumulating in the genes encoding DNA gyrase and DNA topoisomerase IV (for reviews, see references 10 and 19). It has been possible to identify compounds that have an increased ability to kill moderately resistant, first-step mutants, and such compounds reduce the ability of resistant mutants to be selected in wild-type populations (29). Those same compounds may restrict the ability of mycobacteria to acquire resistance mutations, since resistance in mycobacteria also arises stepwise from mutations in gyrase (16, 26, 28).
In the present study we examined fluoroquinolone resistance and intracellular activity in several species of Mycobacterium. With M. bovis BCG, both first- and second-step mutations conferring resistance to ciprofloxacin appeared to be located on alleles of gyrA, one of the genes encoding gyrase. These strains, as well as wild-type M. smegmatis and clinical isolates of M. tuberculosis, were used to study the effects of fluoroquinolone structure. It had been suggested that C-8 alkoxyl groups might increase fluoroquinolone activity against M. avium (14), and both Pseudomonas aeruginosa and Staphylococcus aureus were known to be more susceptible to C-8-modified fluoroquinolones (11, 13, 30). We focused on a methoxyl (OMe) group at position C-8 because fluoroquinolones having this substituent exhibit low mammalian phototoxicity and only moderate cytotoxicity (18, 23, 24). Against M. bovis BCG the C-8-OMe group increased both bacteriostatic and lethal action, especially against first-step gyrA resistant mutants. A C-8-bromo (C-8-Br) moiety was not as active as the C-8-OMe group, particularly when lethal action was normalized to bacteriostatic activity. These data suggested that the C-8-OMe substituent may be especially effective at facilitating the release of lethal DNA breaks from drug-gyrase-DNA complexes. When wild-type cultures of all three species were tested for the ability to acquire resistance mutations, fluoroquinolone concentrations were found at which no mutant was recovered following challenge with a C-8-OMe compound, while many were recovered following challenge with a C-8-H control. Thus, a C-8-OMe substituent makes fluoroquinolones more effective at attacking gyrase mutants and at preventing selection of resistant mutants from wild-type populations of mycobacteria.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. smegmatis mc2155 was obtained from I. Smith, Public Health Research Institute. M. bovis BCG (Bacille Calmette-Guerin substrain Pasteur) was provided by Mounsef Tiza, Public Health Research Institute, and was called KD1295. Strain CX1 (9) was a derivative of KD1295 that contained a gyrA mutation with a GAC to AAC change at codon 94, which would change aspartic acid to asparagine; strain CX2 was a derivative of CX1 that contained an additional gyrA mutation with a GCG to GTG change at codon 90, changing alanine to valine. Clinical isolates of M. tuberculosis were obtained from the Public Health Research Institute Tuberculosis Center. All M. tuberculosis strains were identified by IS6110 DNA typing as members of the W group, a set of clonal isolates recovered from persons affected during a recent outbreak of multidrug-resistant tuberculosis in New York City (4). Middlebrook 7H9 medium, enriched with 10% ADC (albumin-dextrose complex) and 0.05% Tween 80 (12), was used to grow mycobacteria in liquid culture. Middlebrook 7H10 agar plates were used for single-colony isolation, MIC determination, and measurement of CFU. Agar plates containing fluoroquinolones were prepared by adding concentrated solutions to molten agar. All experiments with M. tuberculosis were carried out in a BSL3 containment facility.
Fluoroquinolones.
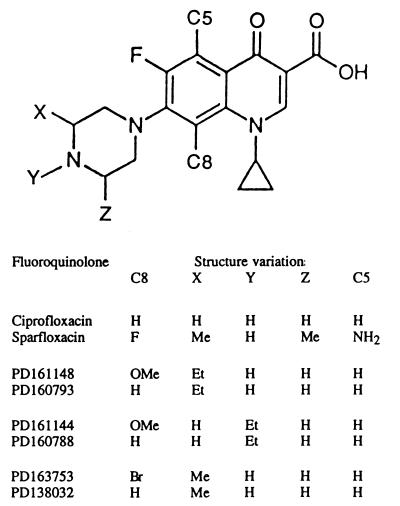
Ciprofloxacin was purchased from Miles Pharmaceutical Company. Additional fluoroquinolones were provided by Parke-Davis Pharmaceutical Company (see Fig. 1 for structures). Fluoroquinolones were first dissolved in 1 N NaOH (1/10 final volume), and then sterile water was added to yield a final concentration of 10 mg/ml. This stock solution was divided into 50-μl aliquots and stored at −80°C. Dilution series were prepared with sterile water. Fluoroquinolones PD163753 and PD161144 correspond to compounds 3b and 5d, respectively, in a previous study (23).
FIG. 1.
Structures of fluoroquinolones examined. The structure common to the compounds is shown, with positions of variable groups indicated by C5, C8, X, Y, or Z. Specific groups that distinguish the compounds are shown at the bottom of the figure. Abbreviations: H, hydrogen; Br, bromo; OMe, methoxyl; Me, methyl; Et, ethyl; NH2, amino.
Determination of mycobacterial sensitivity to fluoroquinolones.
Bacteriostatic concentrations of the fluoroquinolones were estimated in two ways. For growth in liquid culture, cells were diluted in tubes containing various concentrations of fluoroquinolone and then incubated until an untreated control culture reached stationary phase (monitored by culture turbidity, A600). The drug concentration required to inhibit bacterial growth by 50% relative to the untreated control was defined as the 50% inhibitory dose (ID50) (28). The MIC was determined by plating dilutions of cultures on agar containing various concentrations of fluoroquinolone. The concentration that reduced the number of colonies by at least 99% relative to untreated controls was taken as the MIC. Growth was at 37°C in the presence of 5% CO2.
Bactericidal effects against M. bovis BCG were assessed by determination of the number of CFU following fluoroquinolone treatment. Cultures were grown with horizontal rolling to mid-log phase (approximately 3 × 108 cells/ml), and 1-ml aliquots were transferred to sterile polystyrene tubes containing various fluoroquinolones. After 48 h of incubation at 37°C, 100-μl portions were diluted in 900 μl of fresh medium lacking drug. Serial dilutions were made, aliquots were spread on 7H10 agar lacking drug, and colonies were counted after 3 to 4 weeks’ incubation.
Selection of resistant mutants.
Resistant mutants were obtained by incubation of cultures spread on 7H10 agar plates containing fluoroquinolone. A spontaneous gyrA (Cipr) mutant, strain CX1, was obtained (9) by growth of M. bovis BCG on agar containing 1 μg of ciprofloxacin/ml. A culture of strain CX1 was then plated on agar containing 10 μg of ciprofloxacin/ml to select a second-step gyrA mutant, strain CX2. Nucleotide sequences in the quinolone-resistance-encoding region of gyrA were determined by automated DNA sequencing following amplification by PCR (26). For measurement of the ability of a fluoroquinolone to reduce the selection of resistant mutants, mycobacterial cultures were grown to early stationary phase in 7H9 medium. For M. smegmatis, cells (1 ml) were then spread directly on 7H10 agar plates containing fluoroquinolone. For M. bovis BCG and M. tuberculosis, cells (60 to 100 ml) were grown in roller bottles, concentrated by centrifugation (3,000 × g for 10 min), resuspended in 20 ml of medium, and applied to agar plates in 1-ml aliquots at an estimated concentration of 2 × 109 to 30 × 109 CFU/ml. Colonies were counted after incubation at 37°C for 7 to 10 days (M. smegmatis) or 21 to 28 days (M. bovis BCG and M. tuberculosis).
RESULTS
Bacteriostatic effects of fluoroquinolones.
Formation of fluoroquinolone-gyrase-DNA complexes blocks bacterial growth; consequently, the abilities of different fluoroquinolones to trap gyrase on DNA can be compared by determining the drug concentration required to block colony formation or inhibit growth in a liquid culture. In a preliminary search for fluoroquinolones that are more effective than ciprofloxacin against moderately resistant gyrase mutants of M. tuberculosis, we compared the bacteriostatic activities of sparfloxacin and ciprofloxacin using several clonal isolates of the multidrug-resistant W IS6110 restriction fragment length polymorphism type (28). Against a gyrA+ strain (TN1626), sparfloxacin had the same effect as ciprofloxacin at one-half the dose; against two resistant gyrA mutants, TN606 and TN1625, only one-quarter the dose of sparfloxacin was required (Table 1). Thus, sparfloxacin was more effective than ciprofloxacin at overcoming the protective action of a gyrA mutation.
TABLE 1.
ID50s of fluoroquinolones for M. tuberculosis
| Fluoroquinolonea | ID50 (μg/ml) forb:
|
||
|---|---|---|---|
| TN1626 (wild type) | TN1625 (A90V) | TN606 (D94G) | |
| Ciprofloxacin | 0.073 ± 0.005 | 1.2 ± 0.18 | 4.5 ± 0.34 |
| Sparfloxacin (ratioc) | 0.035 ± 0.004 (2.1) | 0.34 ± 0.03 (3.5) | 1.1 ± 0.12 (4.1) |
For structures, see Fig. 1.
Mutations are indicated as the amino acid at either position 90 or 94 of the GyrA protein. The amino acid preceding the position number refers to the wild-type amino acid, and that following the position number refers to the mutant amino acid. Abbreviations: A, alanine; D, aspartic acid; G, glycine; H, histidine; N, asparagine; V, valine.
Of the ID50 for ciprofloxacin to the ID50 for sparfloxacin.
Since sparfloxacin and ciprofloxacin differ in several aspects (structures are shown in Fig. 1), the structural basis for the greater activity by sparfloxacin was not clear. However, we suspected that the additional fluorine at position C-8 in sparfloxacin was important, because substituents at this position are known to increase the activity of fluoroquinolones against other bacteria (11, 13, 23, 29, 30). To focus more closely on C-8 substituents, we next compared two new compounds, a C-8-OMe fluoroquinolone (PD161148) and its C-8-H control (PD160793), for the ability to block growth of W type isolates of M. tuberculosis. These compounds and ciprofloxacin showed equal ability to block growth of a gyrA+ strain (TN1626) (Table 2); however, the C-8-OMe compound inhibited growth at four-to-seven-times-lower concentration than its C-8-H control or ciprofloxacin when gyrA mutants were examined (Table 2). Thus, a C-8-OMe group increased the bacteriostatic activities of fluoroquinolones against moderately resistant, clinical isolates of M. tuberculosis.
TABLE 2.
MICs of fluoroquinolones for M. tuberculosis
| Fluoroquinolonea | MIC (μg/ml) forb:
|
|||
|---|---|---|---|---|
| TN1626 (wild type) | TN1625 (A90V) | TN565 (D94H) | TN3033 (D94N) | |
| PD161148 (C-8-OMe) | 0.3 | 0.7 | 0.7 | 0.7 |
| PD160793 (C-8-H) | 0.3 | 3.0 | 5.0 | 5.0 |
| Ciprofloxacin | 0.3 | 3.0 | 3.0 | 5.0 |
For structures, see Fig. 1.
Mutations are indicated as the amino acid at either position 90 or 94 of the GyrA protein. The amino acid preceding the position number refers to the wild-type amino acid, and that following the position number refers to the mutant amino acid. Abbreviations for mutations as indicated in Table 1.
A more extensive comparison of C-8 substituents was carried out with strains of M. bovis BCG. Against the wild type, a C-8-OMe group doubled fluoroquinolone potency for growth inhibition while a C-8-Br substituent had little effect (Table 3). Ciprofloxacin exhibited the least activity. As expected, a gyrA (Cipr) mutation (asparagine substituted for aspartic acid at codon 94) increased the fluoroquinolone concentration required to inhibit growth (strain CX1, Table 3). The ID50 ratio for first-step mutant to wild type was 7- to 10-fold lower for compounds carrying C-8-OMe and C-8-Br groups than for C-8-H derivatives (Table 3). Thus, C-8-OMe and C-8-Br groups increase the bacteriostatic action of fluoroquinolones against first-step gyrA resistant mutants for slow-growing mycobacteria as they do for Escherichia coli (reference 29 and data not shown). The presence of an additional, second-step gyrA mutation, which results in substitution of valine for alanine at codon 90 (strain CX2), provided roughly the same additional (two- to fourfold) protection against all of the compounds tested (Table 3). Thus, the enhancing effect of C-8 substituents on bacteriostatic activity of fluoroquinolones appears to be focused primarily on first-step mutants.
TABLE 3.
Bacteriostatic action of fluoroquinolones against M. bovis BCG
| Fluoroquinolone | Structureb
|
ID50 (μg/ml) fora:
|
Ratio
|
|||||
|---|---|---|---|---|---|---|---|---|
| C-8 | X | Y | KD1295 (wild type) | CX1 (D94N) | CX2 (A90V-D94N) | CX1 to KD1295 | CX2 to CX1 | |
| Ciprofloxacin | H | H | H | 0.15 ± 0.013 | 6.1 ± 1.4 | 16 ± 0.54 | 41 | 2.7 |
| PD161148 | OMe | Et | H | 0.048 ± 0.005 | 0.61 ± 0.14 | 2.0 ± 0.26 | 13 | 3.3 |
| PD160793 | H | Et | H | 0.08 ± 0.015 | 7.0 ± 0.82 | 14 ± 0.58 | 87.5 | 2.0 |
| PD161144c | OMe | H | Et | 0.058 ± 0.005 | 1.2 ± 0.48 | 5.5 ± 0.34 | 21 | 4.6 |
| PD160788 | H | H | Et | 0.11 ± 0.01 | 10.5 ± 0.5 | 35 ± 2.4 | 195 | 3.3 |
| PD163753d | Br | Me | H | 0.058 ± 0.008 | 0.76 ± 0.19 | 2.1 ± 0.15 | 13 | 2.8 |
| PD138032 | H | Me | H | 0.053 ± 0.001 | 5.6 ± 0.16 | 25 ± 0.5 | 105 | 4.5 |
Bactericidal effects of fluoroquinolones.
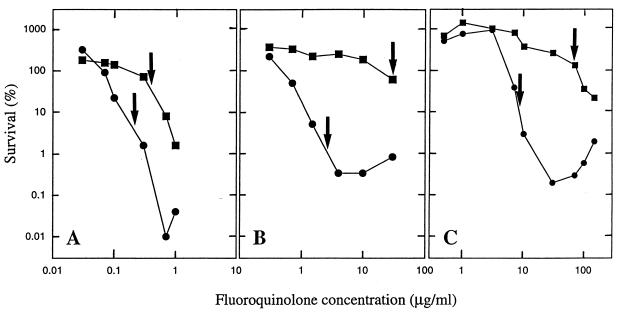
Since bactericidal action is not always predictable from bacteriostatic activity (29), we measured the effects of C-8 substituents on survival of M. bovis BCG during fluoroquinolone treatment. Examination of Fig. 2 shows that a C-8-OMe group enhanced fluoroquinolone lethality, particularly against a first-step gyrA mutant. Similar data were obtained for another C-8-OMe derivative, PD161144, when it was compared to the C-8-H control compound PD160788 (data not shown).
FIG. 2.
Effect of a C-8-OMe group on bactericidal action of fluoroquinolones. (A) Wild-type M. bovis BCG (strain KD1295) was incubated at the indicated concentrations of fluoroquinolone for 48 h. Cultures were then diluted in drug-free medium, and the number of viable colonies was determined by growth on agar. Survival was calculated as the number of colonies recovered for each fluoroquinolone concentration normalized to the number observed in samples taken at the time of drug addition. (B) M. bovis BCG strain CX1 (first-step gyrA mutant) was treated as described for panel A. (C) M. bovis BCG strain CX2 (second-step gyrA mutant) was treated as described for panel A. Arrows indicate concentrations that are 4 times the ID50 for each compound. Symbols: circles, PD161148 (C-8-OMe); squares, PD160793 (C-8-H).
We have argued that fluoroquinolones can be compared for their abilities to stimulate release of DNA breaks from drug-gyrase-DNA complexes by normalizing lethal action to bacteriostatic effects (29). Since survival curves are complex, we have chosen to compare the fractions of survivors for each compound at a concentration that is a fixed multiple of the bacteriostatic parameter, ID50. In the present case, 4 times the ID50 was used. Of wild-type cells, 4% survived treatment with PD161148 (C-8-OMe) at 4 times the ID50 (0.19 μg/ml), while 50% survived incubation with its C-8-H derivative (4 times the ID50, 0.32 μg/ml; arrows in Fig. 2A). With the first-step mutant (Fig. 2B), the difference in survival between the two compounds at 4 times the ID50 was about 60-fold. For the other C-8-OMe/C-8-H pair (PD161144 and PD160788), the C-8-OMe substituent increased lethal activity at 4 times the ID50 by about 5-fold for wild-type cells and about 50-fold for the first-step gyrase mutant (strain CX1; data not shown). These data are consistent with the C-8-OMe group enhancing the release of lethal DNA breaks, particularly in a gyrA mutant.
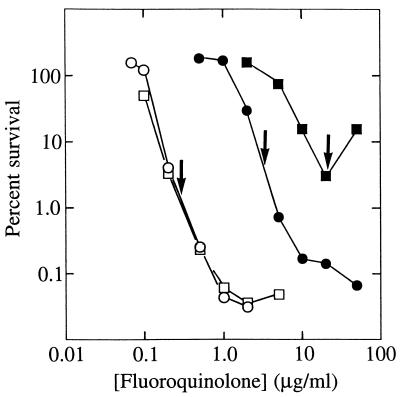
A C-8-Br moiety did not increase fluoroquinolone potency as much as a C-8-OMe group. With wild-type M. bovis BCG, the C-8-Br compound (PD163753) and its C-8-H control (PD138032) exhibited similar dose responses for both bacteriostatic (Table 3) and bactericidal (Fig. 3) activity. Against the first-step mutant CX1, the C-8-Br compound was more bacteriostatic (Table 3) and allowed fewer survivors (Fig. 3) than its cognate C-8-H derivative at a given concentration. However, the lethal actions of the two compounds were equal at 4 times the ID50 (Fig. 3). Thus, the enhancing effect of the C-8-Br substituent is exerted largely at the level of bacteriostatic action, unlike the situation described above for the C-8-OMe group. Nevertheless, the absolute bacteriostatic and bactericidal activities of the C-8-OMe (PD161148) and C-8-Br (PD163753) are similar (Table 2 and Fig. 2 and 3). This similarity probably arises from enhanced activity of the C-8-Br compound due to the presence of a methyl group rather than an ethyl group on its C-7-piperizinyl ring (see Fig. 1). The methyl substituent improved both bacteriostatic and lethal activities, as seen in comparisons of the C-8-H compounds PD160793 and PD138032 (Table 3 and Fig. 2 and 3).
FIG. 3.
Effect of a C-8-Br group on bactericidal action of fluoroquinolones. M. bovis BCG strain KD1295 (wild type; open symbols) or strain CX1 (first-step gyrA mutant; filled symbols) was treated as in Fig. 2. Arrows indicate concentrations that are 4 times the ID50 for each compound. Symbols: circles, PD163753 (C-8-Br); squares, PD138032 (C-8-H).
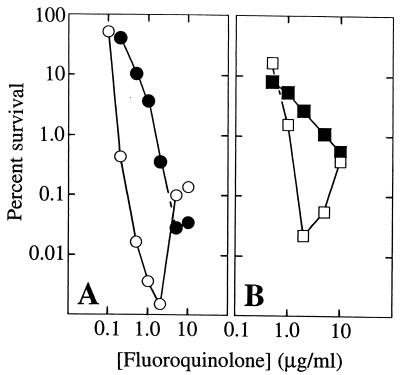
Based on other work it has been argued that two forms of lethal action can be distinguished by using antagonists of RNA synthesis or protein synthesis (7, 17). Inhibitors such as rifampin and chloramphenicol block one pathway of fluoroquinolone lethality but not the other (10). When chloramphenicol was tested for the ability to protect M. bovis BCG from fluoroquinolone action, the lethal action of the C-8-OMe compound PD161148 was shifted to higher concentrations, but the compound still killed M. bovis BCG (Fig. 4A). These data indicate that two pathways of lethal action exist for C-8-OMe fluoroquinolones and suggest that these agents may have considerable activity even against nongrowing cells. Since a similar protective effect was seen with the C-8-H control (Fig. 4B), activity in the presence of chloramphenicol is not unique to fluoroquinolones containing a C-8-OMe group.
FIG. 4.
Effect of chloramphenicol on bactericidal action of C-8-OMe and C-8-H fluoroquinolones. (A) Wild-type M. bovis BCG was treated with the indicated concentrations of PD161148 (C-8-OMe) in the presence (filled circles) or absence (open circles) of 20 μg of chloramphenicol/ml added 60 min prior to the fluoroquinolone. Incubation was continued for 48 h, after which percent survival was determined as described in the legend for Fig. 2. (B) Conditions were as described for panel A except that cells were treated with PD160793 (C-8-H), in the presence (filled squares) or absence (open squares) of chloramphenicol.
Selection of fluoroquinolone resistance.
The data presented above show that C-8 substituents make fluoroquinolones more bacteriostatic and bactericidal against mycobacteria, especially when the cells already contain fluoroquinolone-resistant gyrA mutations. This raised the possibility that C-8 substituents would reduce the selection of resistance when wild-type cells were challenged with moderate fluoroquinolone concentrations, since the gyrA mutation plus a second mutation would be required for significant resistance. As a preliminary test of this idea, we examined the rapidly growing species M. smegmatis by plating cells on agar containing PD161148 (C-8-OMe) or its C-8-H control, PD160793 (for this organism the two compounds inhibit growth equally). As shown in Table 4, the C-8-OMe group greatly reduced the selection of resistant mutants. Restriction of mutant selection was not limited to C-8-OMe derivatives: no mutants were obtained with the C-8-Br derivative under conditions in which hundreds of mutants were obtained with its C-8-H control (data not shown). The C-8-OMe group also reduced the selection of resistant mutants in M. bovis BCG (Table 4). When the test was applied to M. tuberculosis (clinical isolate TN1626), no mutant was obtained with the C-8-OMe compound (PD161148) while more than a thousand resistant mutants were recovered with the same concentration of its C-8-H control (PD160793; Table 4). As expected, the resistance mutations were mapped in gyrA (the nucleotide sequences were determined for the regions of gyrA determining quinolone resistance in two M. tuberculosis isolates following amplification by PCR, and in both cases a substitution of glycine for aspartic acid was found at codon 94).
TABLE 4.
Selection of resistant mutants
| Organism and fluoro-quinolone (3.75 μg/ml) | Number of resistant mutants recovered in experiment
|
Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| M. smegmatis | ||||
| PD160793 (C-8-H) | 285 | 250 | 646 | 1,181 |
| PD161148 (C-8-OMe) | 0 | 0 | 0 | 0 |
| M. bovis BCG | ||||
| PD160793 (C-8-H) | 65 | 271 | 716 | 1,052 |
| PD161148 (C-8-OMe) | 0 | 0 | 0 | 0 |
| M. tuberculosis | ||||
| PD160793 (C-8-H) | 334 | 200 | 840 | 1,334 |
| PD161148 (C-8-OMe) | 0 | 0 | 0 | 0 |
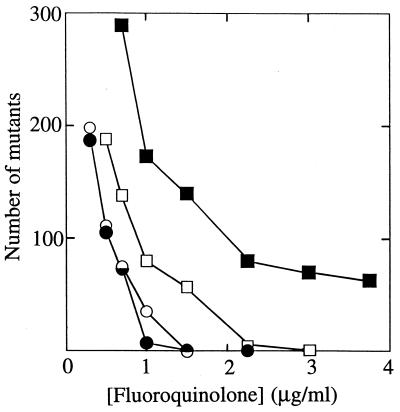
To assess the effect of drug concentration on mutant selection, we determined the numbers of M. bovis BCG mutants that arose on agar plates containing various fluoroquinolone concentrations (Fig. 5). The number of resistant mutants declined as concentration increased, with the more potent compounds generally allowing fewer mutations to arise at a given concentration. Two features of Fig. 5 merit attention. First, the C-8-Br derivative was more restrictive for mutant selection than its C-8-H control (Fig. 5), even though the two were equally potent against wild-type cells. These two compounds differ in their activities against first-step gyrA mutants (Table 3 and Fig. 3), and that is probably why the C-8-Br compound allows fewer resistant colonies to arise. The second noteworthy observation is that one C-8-H compound, PD138032, was more effective than the other, PD160793, at reducing the selection of resistant mutants (Fig. 5). This difference probably arises from substituents on the C-7 ring, since they constitute the only difference between PD138032 and PD160793 (Fig. 1).
FIG. 5.
Effect of fluoroquinolone concentration on selection of resistant mutants of M. bovis BCG. Wild-type cells were spread on agar plates containing the indicated concentrations of PD161148 (C-8-OMe; filled circles), PD160793 (C-8-H; filled squares), PD163753 (C-8-Br; open circles), or PD138032 (C-8-H; open squares). The plates were then incubated for 28 days at 37°C, and the number of fluoroquinolone-resistant colonies was recorded.
DISCUSSION
C-8-OMe and C-8-Br substituents, when compared with C-8-H controls, improved fluoroquinolone activity against mycobacteria, particularly against moderately resistant gyrA mutants. With respect to inhibition of growth, a C-8-OMe group had little enhancing action against a wild-type isolate of M. tuberculosis, but the moiety made fluoroquinolones four- to sevenfold more bacteriostatic against gyrA mutants (Tables 1 and 2). With M. bovis BCG, a C-8-OMe group doubled the activity against wild-type cells and increased it by almost 10-fold against a first-step gyrA mutant (Table 3). A C-8-Br group did not enhance bacteriostatic action with wild-type cells, but it did with first-step mutants (Table 3). The C-8-OMe and C-8-Br substituents probably increase the ability of fluoroquinolones to block growth by facilitating formation of fluoroquinolone-gyrase-DNA complexes (7). There is no evidence that complexes form with topoisomerase IV in mycobacteria, since first-step and second-step mutations map in the gyrase genes for both M. tuberculosis (16) and M. bovis BCG (strains CX1 and CX2 of the present study). In this respect mycobacteria may differ from bacteria such as E. coli, S. aureus, Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae, since parC (topoisomerase IV) mutants are readily obtained in the latter organisms (reviewed in reference 10).
Enhancement of lethal action by a C-8-OMe group was readily observed with wild-type and resistant mutants of M. bovis BCG (Fig. 2). Enhancement was probably due to an increase in the release of double-stranded DNA breaks from drug-gyrase-DNA complexes (7). Increased lability of the complexes was estimated indirectly by determining percent survival at fluoroquinolone concentrations normalized to correct for differences in growth inhibition. For example, at 4 times the ID50, a C-8-OMe group increased by 60-fold the fraction of cells killed for a first-step gyrA mutant (Fig. 2). Apparently many more drug-gyrase-DNA complexes form than release lethal double-stranded DNA ends; a C-8-OMe group stimulates this release. Two forms of release can be distinguished by sensitivity to chloramphenicol (7). The activity enhancement due to a C-8-OMe group appears to affect both, because chloramphenicol provided partial protection against both C-8-OMe and C-8-H fluoroquinolones (Fig. 4).
The enhancing effect of the C-8-Br group, which is seen only with the first-step gyrA mutant (Fig. 3), appears to differ qualitatively from the effect of the C-8-OMe group: the fraction of surviving cells is the same for C-8-Br and C-8-H compounds when the data are normalized for bacteriostatic action (Fig. 3), while the C-8-OMe compound is more lethal than its C-8-H control in this comparison (Fig. 2). Lethality enhancement by the C-8-Br group probably reflects an increase in trapping of gyrase-DNA complexes rather than in facilitated release of DNA breaks. Additional experiments are required to determine whether alkyl groups on the C-7 ring, which differ in the C-8-OMe and C-8-Br compounds, influence the action of C-8 moieties.
Since C-8 substituents facilitate attack of gyrA mutants, we expected fewer resistant mutants to be recovered when cells were treated with C-8-OMe fluoroquinolones than with C-8-H derivatives. Two tests were performed. In one, susceptible populations of M. smegmatis, M. bovis BCG, and M. tuberculosis were plated on agar containing a moderately high concentration of fluoroquinolone (3.75 μg/ml). The presence of a C-8-OMe group reduced the selection of resistant mutants (Table 4). For the C-8-OMe compound this concentration was sixfold greater than the ID50 of a gyrA mutant of M. bovis BCG, but for the C-8-H derivative it was only half the ID50. Thus, there is a concentration window in which only the C-8-OMe compound prevents growth of mutants already present in the susceptible population. The concentration window was clearly seen when fluoroquinolone concentration was varied (Fig. 5).
The data shown in Fig. 5 also draw attention to the C-7 piperizinyl ring: the C-8-H compound having a methyl group on its C-7 ring (PD138032) was more effective than the compound having an ethyl group (PD160793) (Fig. 5). Previous work indicates that alkylation of this ring can influence bacteriostatic action against M. smegmatis (23) and M. avium (15). We are now determining whether substituting a methyl group for the ethyl group of PD161148 (C-8-OMe) will make a C-8-OMe fluoroquinolone even more effective.
The activity enhancement by C-8 substituents, described above for mycobacteria, is also observed with other bacteria. For example, compounds containing a C-8-OMe group are more bactericidal against quinolone-resistant S. aureus than are C-8-H derivatives, while C-8-Br and C-8-F derivatives are of intermediate potency (11, 30). A compound containing a C-8-Cl is more bacteriostatic than its C-8-H derivative against quinolone-resistant gyrA mutants of P. aeruginosa, a feature that is also reflected in potency against gyrase purified from the strains (13). With E. coli, a C-8-OMe group increases fluoroquinolone lethality against gyrA mutants and reduces the ability of the compound to select resistant mutants in wild-type populations (29). Additional responses to quinolone treatment shared by mycobacteria and other bacteria are rapid inhibition of DNA synthesis (9, 25), fragmentation of DNA (9, 25), and stepwise selection of resistance mutations in the same regions of the gyrase genes (10, 16, 26) at about the same frequency (10−8 to 10−9, data not shown). Mycobacteria also display the unexplained phenomenon of moderate concentrations of fluoroquinolone being more lethal than very high ones (Fig. 2). This property is seen with many quinolones and many bacterial species (reviewed in reference 10). Taken together, these observations indicate that our general understanding of fluoroquinolone action (10), obtained largely from studies with E. coli, applies to mycobacteria.
A difference between gyrase from E. coli and that from many mycobacteria is the alanine at position 90 of the mycobacterial GyrA protein (6, 26). In E. coli and many other bacteria, the equivalent position contains a serine or threonine, and mutation of either to a hydrophobic amino acid is associated with resistance. This may be why gyrase in many mycobacterial species is moderately resistant to the fluoroquinolones (6). The ease with which resistant M. tuberculosis strains arise when patients are treated with compounds such as ciprofloxacin may result from these agents blocking growth without effectively killing cells. As a result, the mutagenic SOS response (20, 21) will be induced, and new gyrA mutants will add to those already existing in the population. Many (80%) of the gyrA mutations map at codon 94 (28), making the cells effectively double mutants. If this idea is correct, a key to developing more effective antituberculosis fluoroquinolones is finding compounds that are so lethal that first-step mutants are readily attacked and few wild-type cells survive to spawn such mutants. Examination of C-8-Br and C-8-OMe derivatives for bacteriostatic activity (Table 3), lethal action (Fig. 2 and 3), and reduction of resistant mutant selection (Fig. 5) shows that adding C-8 substituents to fluoroquinolones is a step in the right direction.
ACKNOWLEDGMENTS
We thank S.-W. Lee and S. Moghazeh for technical assistance and M. Gennaro, S. Kayman, B. Kreiswirth, T. Lu, and R. Pine for critical comments on the manuscript.
This work was supported by Public Health Service grant AI 35257.
REFERENCES
- 1.Agerton T, Valway S, Gore B, Pozsik C, Plikaytis B, Woodley C, Onorato I. Transmission of a highly drug-resistant strain (strain W1) of Mycobacterium tuberculosis. JAMA. 1997;278:1073–1077. [PubMed] [Google Scholar]
- 2.Alangaden G J, Lerner S A. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases. Clin Infect Dis. 1997;25:1213–1221. doi: 10.1086/516116. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P F, Bloch A B, Davidson P T, Snider D E. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 4.Bifani P, Plikaytis B B, Kapur V, Stockbauer K, Pan X, Lusfty M, Moghazeh S, Eisner W, Daniel T, Kaplan M, Crawford J T, Musser J M, Kreiswirth B N. Origin and interstate spread of a New York City multidrug resistant Mycobacterium tuberculosis clone family: adverse implications for tuberculosis control in the 21st century. JAMA. 1996;275:452–457. [PubMed] [Google Scholar]
- 5.Bloom B, Murray C. Tuberculosis: commentary on a reemergent killer. Science. 1992;251:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 6.Cambau E, Jarlier V. Resistance to quinolones in mycobacteria. Res Microbiol. 1996;147:52–59. doi: 10.1016/0923-2508(96)80204-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-R, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–637. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]
- 8.Drlica K, Malik M, Wang J-Y, Levitz R, Burger R. The fluoroquinolones as antituberculosis agents. In: Rom W, Garay S, editors. Tuberculosis. Boston, Mass: Little, Brown and Co.; 1995. pp. 817–827. [Google Scholar]
- 9.Drlica K, Xu C, Wang J-Y, Burger R M, Malik M. Fluoroquinolone action in mycobacteria: similarity with effects in Escherichia coli and detection by cell lysate viscosity. Antimicrob Agents Chemother. 1996;40:1594–1599. doi: 10.1128/aac.40.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Matsumoto M, Nishino T. Improved bactericidal activity of Q-35 against quinolone-resistant staphylococci. Antimicrob Agents Chemother. 1995;39:1522–1525. doi: 10.1128/aac.39.7.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs W R. Genetic systems in mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura A, Hoshino K, Kimura Y, Hayakawa I, Sato K. Contribution of the C-8 substituent of DU-6859a, a new potent fluoroquinolone, to its activity against DNA gyrase mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1467–1471. doi: 10.1128/aac.39.7.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klopman G, Fercu D, Li J-Y, Rosenkranz H S, Jacobs M R. Antimycobacterial quinolones: a comparative analysis of structure-activity and structure-cytotoxicity relationships. Res Microbiol. 1996;147:86–96. doi: 10.1016/0923-2508(96)80209-9. [DOI] [PubMed] [Google Scholar]
- 15.Klopman G, Fercu D, Renau T E, Jacobs M R. N-1-tert-butyl-substituted quinolones: in vitro anti-Mycobacterium avium activities and structure-activity relationship studies. Antimicrob Agents Chemother. 1996;40:2637–2643. doi: 10.1128/aac.40.11.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocagoz T, Hackbarth C J, Unsal I, Rosenberg E, Nikaido H, Chambers H F. Gyrase mutations in laboratory-selected fluoroquinolone-resistant mutants of Mycobacterium tuberculosis H37Ra. Antimicrob Agents Chemother. 1996;40:1768–1774. doi: 10.1128/aac.40.8.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewin C, Howard B, Smith J. Protein- and RNA-synthesis independent bactericidal activity of ciprofloxacin that involves the A subunit of DNA gyrase. J Med Microbiol. 1991;34:19–22. doi: 10.1099/00222615-34-1-19. [DOI] [PubMed] [Google Scholar]
- 18.Marutani K, Matsumoto M, Otabe Y, Nagamuta M, Tanaka K, Miyoshi A, Hasegawa T, Nagano H, Matsubara S, Kamide R, Yokota T, Matsumoto F, Ueda Y. Reduced phototoxicity of a fluoroquinolone antibacterial agent with a methoxy group at the 8 position in mice irradiated with long-wavelength UV light. Antimicrob Agents Chemother. 1993;37:2217–2223. doi: 10.1128/aac.37.10.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura S. Mechanisms of quinolone resistance. J Infect Chemother. 1997;3:128–138. [Google Scholar]
- 20.Phillips I, Culebras E, Moreno F, Baquero F. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother. 1987;20:631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- 21.Piddock L, Wise R. Induction of the SOS response in Escherichia coli by 4-quinolone antimicrobial agents. FEMS Microbiol Lett. 1987;41:289–294. [Google Scholar]
- 22.Porter J. Mycobacteriosis and HIV infection: the new public health challenge. J Antimicrob Chemother. 1996;37:113–120. doi: 10.1093/jac/37.suppl_b.113. [DOI] [PubMed] [Google Scholar]
- 23.Renau T, Gage J, Dever J, Roland G, Joannides E T, Shapiro M, Sanchez J, Gracheck S, Domagala J, Jacobs M, Reynolds R. Structure-activity relationships of quinolone agents against mycobacteria: effect of structural modifications at the 8 position. Antimicrob Agents Chemother. 1996;40:2363–2368. doi: 10.1128/aac.40.10.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez J P, Gogliotti R D, Domagala J M, Gracheck S J, Huband M D, Sesnie J A, Cohen M A, Shapiro M A. The synthesis, structure-activity, and structure-side effect relationships of a series of 8-alkoxy- and 5-amino-8-alkoxyquinolone antibacterial agents. J Med Chem. 1995;38:4478–4487. doi: 10.1021/jm00022a013. [DOI] [PubMed] [Google Scholar]
- 25.Snyder M, Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979;131:287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- 26.Takiff H, Salazar L, Guerrero C, Philipp W, Huang W M, Kreiswirth B, Cole S T, Jacobs W R, Jr, Telenti A. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob Agents Chemother. 1994;38:773–780. doi: 10.1128/aac.38.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO report on the tuberculosis epidemic. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 28.Xu C, Kreiswirth B N, Sreevatsan S, Musser J M, Drlica K. Fluoroquinolone resistance associated with specific gyrase mutations in clinical isolates of multidrug resistant Mycobacterium tuberculosis. J Infect Dis. 1996;174:1127–1130. doi: 10.1093/infdis/174.5.1127. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Xu C, Domagala J, Drlica K. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc Natl Acad Sci USA. 1997;94:13991–13996. doi: 10.1073/pnas.94.25.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Xu C, Dong Y, Wang J-Y, Zhou J, Domagala J, Drlica K. Killing of Staphylococcus aureus with C-8-methoxy fluoroquinolones. Antimicrob Agents Chemother. 1998;42:956–958. doi: 10.1128/aac.42.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]