Abstract
Aims
No effective therapy is available in clinics to protect the heart from ischaemia/reperfusion (I/R) injury. Endothelial cells are activated after I/R, which may drive the inflammatory response by releasing ATP through pannexin1 (Panx1) channels. Here, we investigated the role of Panx1 in cardiac I/R.
Methods and results
Panx1 was found in cardiac endothelial cells, neutrophils, and cardiomyocytes. After in vivo I/R, serum Troponin-I, and infarct size were less pronounced in Panx1−/− mice, but leukocyte infiltration in the infarct area was similar between Panx1−/− and wild-type mice. Serum Troponin-I and infarct size were not different between mice with neutrophil-specific deletion of Panx1 and Panx1fl/fl mice, suggesting that cardioprotection by Panx1 deletion rather involved cardiomyocytes than the inflammatory response. Physiological cardiac function in wild-type and Panx1−/− hearts was similar. The time to onset of contracture and time to maximal contracture were delayed in Panx1−/− hearts, suggesting reduced sensitivity of these hearts to ischaemic injury. Moreover, Panx1−/− hearts showed better recovery of left ventricle developed pressure, cardiac contractility, and relaxation after I/R. Ischaemic preconditioning failed to confer further protection in Panx1−/− hearts. Panx1 was found in subsarcolemmal mitochondria (SSM). SSM in WT or Panx1−/− hearts showed no differences in morphology. The function of the mitochondrial permeability transition pore and production of reactive oxygen species in SSM was not affected, but mitochondrial respiration was reduced in Panx1−/− SSM. Finally, Panx1−/− cardiomyocytes had a decreased mitochondrial membrane potential and an increased mitochondrial ATP content.
Conclusion
Panx1−/− mice display decreased sensitivity to cardiac I/R injury, resulting in smaller infarcts and improved recovery of left ventricular function. This cardioprotective effect of Panx1 deletion seems to involve cardiac mitochondria rather than a reduced inflammatory response. Thus, Panx1 may represent a new target for controlling cardiac reperfusion damage.
Keywords: Heart, Mitochondria, Pannexin1, Ischemia/reperfusion, Cardioprotection
Graphical Abstract
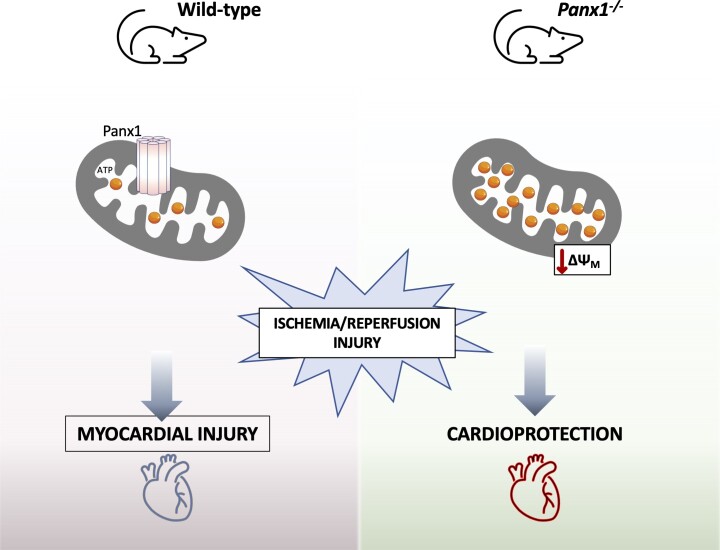
Graphical Abstract.
Time of primary review: 32 days
Translational perspective.
Following acute myocardial infarction, reperfusion is crucial to salvage myocardium but leads by itself to detrimental effects within the heart, like acceleration of cell death and diminished contractile function. No effective therapy is currently available in clinics to protect the heart from ischaemia/reperfusion injury. We studied the role of Pannexin1 channels in ischaemia/reperfusion injury. Pannexin1 deletion confers cardioprotection by decreasing the sensitivity of the heart to ischaemic injury and improving the recovery of cardiac function after reperfusion. The mechanism involves a novel function of Pannexin1 in cardiac mitochondria. Altogether, Pannexin1 may represent a new target for controlling cardiac reperfusion damage.
1. Introduction
Ischaemic heart disease is the leading cause of death worldwide.1 The oxygen and glucose deprivation caused by ischaemia triggers a plethora of cellular responses, contributing to myocardial damage. Reperfusion is crucial to salvage the myocardium but leads by itself to detrimental effects within the heart, involving acceleration of cell death, diminished contractile function, and ventricular arrhythmias.2,3 The massive cardiomyocyte death occurring at the early stages of reperfusion may in some cases lead to heart failure.2 The lack of effective treatment for ischaemia–reperfusion (I/R) injury stimulates research towards the mechanisms underlying this condition to define new cardioprotective strategies.2–5
Increasing evidence shows that an inappropriate inflammatory response in the microcirculation may be the basis of I/R injury. Shortly after the onset of reperfusion, neutrophil activation, and accumulation occurs in the damaged myocardium. Neutrophils are important for the development of reperfusion injury by releasing reactive oxygen species (ROS), proteases, and pro-inflammatory mediators that further amplify the infiltration of leukocytes in the jeopardized myocardium. In addition, ischaemia-induced activation of endothelial cells (ECs) may cause entrapment of leukocytes and platelets in the capillaries, resulting in microvascular plugging and the no-reflow phenomenon.6 Extracellular adenosine triphosphate (ATP) is a key signalling molecule throughout the inflammatory cascade. This danger signal activates the inflammasome and enhances leukocyte infiltration. Moreover, extracellular ATP can be broken down by ectonucleotidases (CD39, CD73) into adenosine diphosphate (ADP), adenosine monophosphate (AMP), and finally adenosine, a critical molecule in the fine-tuning and resolution of inflammation.7 Interestingly, potential cardioprotective effects of adenosine have been already investigated in the clinical setting. Although overall clinical outcomes in patients with ST elevation myocardial infaction (STEMI) undergoing reperfusion therapy with adenosine were not significantly improved at 6 months, infarct size was reduced with the highest dose of adenosine infusion.8 Moreover, post-hoc analysis revealed enhanced early and late survival of STEMI patients presenting within 3 h after the onset of symptoms.9 These observations underscore the need to better understand the microvascular molecular mechanisms during I/R in order to tailor specific cardioprotective therapies to selected groups of patients.
ATP can be released from cells in a controlled manner through pannexin (Panx) channels.10 Pannexins form a three-membered family of channel-building glycoproteins. Panx1 is expressed in most mammalian cells and tissues, including smooth muscle, endothelium, and various types of inflammatory cells. Panx1 is synthesized, N-glycosylated, and oligomerized into heptameric channels in the endoplasmic reticulum before further editing and delivery to the Golgi apparatus. Then, Panx1 channels traffic to the plasma membrane where they appear as functional channels which are known to release nucleotides, such as ATP, but likely serve additional roles in the release or uptake of small molecules.7 Panx1 channels have been suggested as new players in the regulation of inflammation and repair after an ischaemic insult.7,11 A potential role for Panx1 in cardiac I/R might be linked to its involvement in (ATP-mediated) inflammasome activation, in the chemotaxis of neutrophils and macrophages, and in activation of T cells.7 Interestingly, an in vivo role for Panx1 in ischaemic stroke has already been demonstrated in mice subjected to permanent middle cerebral artery occlusion. Deletion of Panx1 was associated with a smaller infarct volume, reduced peri-infarct inflammation, and decreased astrocyte reactivity compared to wild-type (WT) mice.12 A second study examined the role of smooth muscle and endothelial Panx1 on cerebral I/R injury outcomes using conditional cell type-specific knockout mice. Deletion of endothelial Panx1, but not smooth muscle Panx1, reduced cerebral infarct volume after I/R. Infiltration of leukocytes into brain tissue and development of cerebral myogenic tone were both reduced when mice lacked endothelial Panx1.13 Panx1 may also play a role in NETosis and in macrophage apoptosis and clearance from infarcted tissues.14,15 Indeed, apoptotic cells release ‘find me’ signals to recruit phagocytes at the early stages of programmed cell death. Panx1 was identified as the conduit for ATP release from apoptotic cells using siRNA or non-specific pharmacological inhibitors.14 In the present study, we sought to investigate whether Panx1 channels contribute to cardiac I/R injury.
2. Methods
An expanded Materials & Methods section is available as Supplementary material online, Data supplement.
2.1. Animals
All animal experimentation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication Eighth Edition, 2011) and was approved by Swiss veterinary authorities or the animal welfare office of the Justus-Liebig-University Giessen (842_M and 770_M). At the end of each experiment, the mice were euthanized under general anaesthesia by cervical dislocation and bloodletting.
2.1.1. Generation of mice with neutrophil-specific Panx1 deletion
Mice with specific Panx1 deletion in neutrophils [C57BL/6-Ly6G-tm2621 (Cre-dtTomato) Arte × B6.Cg-Gt (ROSA) 26Sortm9 (CAG-tdTomato) Hze/J × Panx1 tm1a (KOMP)Wtsi) were obtained by crossing CatchupIVM−red mice with Panx1fl/fl mice. CatchupIVM−red mice were kindly provided by Dr. Thomas Gunzer (Universitätklinikum Essen, Germany). They have been generated by knock-in of a construct expressing Cre recombinase and tdTomato separated by a self-splicing T2A peptide into the Ly6G locus and tdTomato in the ROSA26 locus triggered by a Cre-activatable CAG promoter.16Panx1fl/fl mice were generated by KOMP at UCDavis17 and kindly provided by Dr. Eliana Scemes (Albert Einstein College of Medicine, New York, USA). Mice were bred to homozygosity (called Panx1Ndel) in the animal facility of the Faculty of Medicine of the University of Geneva and kept under standard housing conditions with a fixed 12 h light/12 h dark cycle.
2.2. In vivo and ex vivo cardiac I/R
In vivo and ex vivo I/R was performed on male C57BL/6J (WT) and Panx1−/−18 or Panx1Ndel and Panx1fl/fl mice using previously published methods.19 For in vivo I/R, anaesthesia was induced with 4% isoflurane and mice were intubated through tracheotomy to allow for mechanical ventilation. Anaesthesia was maintained during the surgical procedure with 2% isoflurane in 100% O2 administrated through a ventilator. Following 24 h of reperfusion and for ex vivo studies, mice were anaesthetized with ketarom (ketamine mixed with xylazine; 120 and 16 mg/kg, respectively) and injected intraperitonally. Quantification was performed with ImageJ (NIH). Area at risk (AAR) was expressed as a percentage of the left ventricle surface. The infarcted area was expressed as a percentage of the AAR. Cardiac Troponin-I (cTnI) is a well-known marker of cardiomyocyte death commonly used in clinics.20 For each mouse, the serum concentration of cTnI was evaluated with the High Sensitivity Mouse Cardiac Troponin-I ELISA kit (Life Diagnostics, Inc.) according to the manufacturer’s instructions.
2.3. Cardiomyocyte and mitochondria isolation from mouse hearts
Cardiomyocytes were isolated from WT and Panx1−/− hearts as described previously.21 Subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria were isolated from WT and Panx1−/− hearts using previously established protocols.22,23 For each mitochondrial preparation, the ventricular tissues of 2 mice were pooled. Purified mitochondria were stored at −80°C.
2.4. RNA extraction, reverse transcription, and real-time quantitative PCR
mRNA was extracted from WT and Panx1−/− hearts, cardiomyocytes or SSM, and from isogenic B16-BL6 and B16-F10 cells. RNA extractions were performed using the NucleoSpin RNA Mini kit (Macherey-Nagel). Reverse-transcription was carried out with the Quantitect Reverse Transcription kit (Qiagen). Real-time quantitative PCR (qPCR) was performed with the ABI Prism StepOnePlus Sequence Detection System (Applied Biosystem). Primers for mouse Panx1 and 18S were purchased from Applied Biosystems. Measurements were performed in duplicate.
2.5. ATP release assay from cardiac mitochondria
After extraction, SSM was distributed in equal volumes and incubated (15 min at 37°C) in Tyrode buffer (124 mM NaCl, 2.44 mM KCl, 10.82 mM NaHCO3, 0.38 mM NaH2PO4, 0.91 mM MgCl2, 1.82 mM CaCl2; pH 7.35; 295 mOsm), osmotic shock solution (30.24 mM NaCl, 10 mM KCl, 10.82 mM NaHCO3, 0.38 mM NaH2PO4, 0.91 mM MgCl2, 1.82 mM CaCl2; pH 7.35; 136 mOsm) or mitochondria were lysed with 50% dimethyl sulfoxide (DMSO). The amount of ATP in the supernatant was measured using an ATP Bioluminescence Assay kit (Sigma–Aldrich) according to the manufacturer’s instructions, and normalized to the protein content of each sample.
2.6. RNA scope
The localization of Panx1 mRNA in cardiac tissue was examined with the RNAscope Fluorescent Multiplex Assay kit (Advanced Cell Diagnosis). Seven μm-thick cryosections from murine hearts were processed according to the manufacturer’s instructions. Panx1 and mitochondrial COX-I mRNA were labelled with RNAscope Probes: Mm-Panx1 and RNAscope Probe Mm-mt-Cox1 (Advanced Cell Diagnosis), respectively. After the labelling of the RNA, sections were mounted in Vectashield Antifade Mounting Medium (VECTOR Laboratories) and imaged using a Zeiss Axio Imager.Z2 Basis LSM 800 microscope with Plan Apochromat 63x/1.40 Oil (420782.9900) objective.
2.7. Cell culture
Mouse cardiac ECs (MCECs; CLU510, Cedarlane/Bioconcept), B16-F10 cells (ATCC CRL-6475), and B16-BL6 mouse melanoma cell line,24 kindly provided by Dr. Lubor Borsig (Institute of Physiology, University of Zürich, Switzerland), were cultured in high-glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (GM). For in vitro experiments, MCECs were placed in a hypoxic chamber (Innova CO-14, New Brunswick Scientific) under 1% O2 for 2 h in GM or a glucose-free medium (DMEM, Gibco). Control MCECs were incubated in parallel in GM under 21% O2.
2.8. Immunofluorescent staining
Immunostaining was performed on 7 μm-thick cryosections or cells using antibodies against Ly6G (1/50, BD Pharmingen), F4/80 (1/250, Proteintech) or Panx1 (1/250, Geneva Antibody Facility25 or anti-mPanx1414–425, 1/500, Aves Labs.26) Briefly, after 15 min fixation with 4% paraformaldehyde, heart sections were permeabilized for 15 min with 0.3% Triton X-100, incubated in 0.5 M NH4Cl and blocked in a PBS solution containing 2% BSA. Incubation of the primary antibody was performed overnight at 4°C. Incubation with the corresponding secondary antibody was performed at room temperature for 2 h. All sections were counterstained with 0.003% Evans blue, and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (10 min, 1/20 000). The number of Ly6G- or F4/80-positive cells was counted and expressed as a percentage of all nuclei present in the given region.
2.9. Electron microscopy
Mouse hearts (three of each genotype) were fixed by retrograde perfusion through the ascending aorta with 2% glutaraldehyde in 50 mM sodium-phosphate buffer (pH 7.2). A tissue block was dissected from the base of the anterior left ventricular (LV) wall, rinsed in 150 mM sodium-phosphate buffer (pH 7.2), and post-fixed with 1% OsO4 in 120 mM sodium-phosphate buffer (pH 7.2) for 2 h. The tissue block was subsequently processed for electron microscopy (EM) using standard procedures. Image fields showing longitudinally cut sarcomere structures as well as sarcolemma were captured and subsequently analysed with ImageJ software. SSM were defined as mitochondria without myofibrils between themselves and the sarcolemma. Conversely, IFM were defined as mitochondria with myofibrils on both sides. In short, the sarcolemma as well as both SSM and IFM were outlined using the free hand tools. From these objects sarcolemmal length, mitochondrial area, and circularity [defined as: 4*π*Area/(Perimeter)2] were obtained for 93–243 SSM and 132–270 IFM per heart. The operator was blinded to genotype during image capture and analysis.
2.10. Western blotting
Proteins were extracted from B16-BL6 and B16-F10 cells, as reported elsewhere.19 Western blotting was performed with rabbit anti-Panx1 (1/1000, 1/500, Cell Signalling), mouse anti-β-actin (1/1000, Sigma), and appropriate secondary antibodies (1/5000, Jackson Laboratories). A luminescent signal was detected using the Immobilon Enhanced Chemiluminecence (ECL) Ultra Western HRP Substrate (Millipore).
SSM and IFM were lysed in 1 × cell lysis buffer (Cell Signalling) supplemented with 1 × PhosStop and complete inhibitors (Roche). Protein concentration was determined using the Lowry assay. Sixty µg mitochondrial proteins were electrophoretically separated on 10% Bis-Tris gels and proteins were transferred to nitrocellulose membranes. After blocking, membranes were incubated with antibodies against Panx1 (as above), manganese superoxide dismutase (MnSOD) as a mitochondrial marker protein (1/1000, Merck), Na+/K + -ATPase (1/1000, Sigma) and Cx43 (1/1000, Sigma). After washing and incubation with the respective secondary antibodies, immunoreactive signals were detected by chemiluminescence (SuperSignal West Pico or SuperSignal West Femto Chemiluminescent Substrate, ThermoFisher). Only mitochondrial preparations, which were negative for marker proteins of the sarcolemma, the nucleus and the cytosol, as determined by Western blot analysis, were used in this study (not shown, see Ref. 23).
2.11. Seahorse Agilent XF cell mito stress
B16-BL6 or B16-F10 cells were plated on Seahorse XF 96-well culture microplate (Agilent) at the concentration of 150 000 cells/well and cultured for 24 h in GM (37°C, 5% CO2). Voltage-dependent anion channel 1 (VDAC1) inhibitors TRO19622 (Tocris) or NSC15463 (MedChemExpress) were used at a concentration of 10 μM, oligomycin at 20 μM, carbonyl cyanide 4−(trifluoromethoxy)phenylhydrazone (FCCP) at 1 μM and rotenone/antimycin at 5 μM. Mitochondrial function was measured by oxygen consumption rate (OCR) using Seahorse XF Mito Stress Test Report Generator (Agilent) using standard procedures. Nuclei were stained with Hoechst allowing for the cell counting and normalization of the data.
2.12. Mitochondrial permeability transition pore opening, oxygen consumption, and ROS formation
Mitochondrial respiration of SSM, ROS formation, and mitochondrial permeability transition pore (mPTP) opening were measured as previously published.23
2.13. Mitochondrial membrane potential measurements
Freshly extracted WT and Panx1−/− cardiomyocytes were loaded for 20 min with 60 nM tetramethylrhodamine methyl ester (TMRE, Abcam) in a buffer containing 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM CaCl2, 10 mM glucose; pH 7.45. Cells were subsequently washed with the same buffer without TMRE for 10 min. Absorbed dye was excited at 545 nm and emission was measured using a long pass 590 filter. Differences in mitochondrial membrane potential (ΔΨm) were assessed by the intensity of fluorescence emission and compared between WT and Panx1−/− cardiomyocytes.
2.14. Statistical analysis
Statistical analyses were performed with GraphPad Prism 9, except for EM data which was analysed in RStudio. Comparisons were performed using a two-tailed unpaired Student’s t-test or two-way analysis of variance (ANOVA), where appropriate. For EM data, a mixed model design was used. Results were reported as mean ± SEM. Significant statistical results are indicated as *P ≤ 0.05.
3. Results
3.1. Panx1 expression in the healthy and injured mouse heart
In healthy ventricular tissue, Panx1 is typically expressed in ECs of the cardiac microvasculature and in the smooth muscle cells (SMCs) of small arterioles (Figure 1A). The signal was absent in negative control immunostainings on WT hearts with the omission of the first antibody (Figure 1B) or in Panx1−/− hearts (not shown). In addition, Panx1 expression was found in ECs and SMCs of coronary arteries (Figure 1C) and in the endocardium (not shown). Twenty-four hours after LAD ligation and reperfusion, Panx1 expression was still observed in the vascular cells and was also seen in leukocytes, including neutrophils, within the microvasculature (Figure 1D) and infiltrating into the ischaemic region (Figure 1E).
Figure 1.
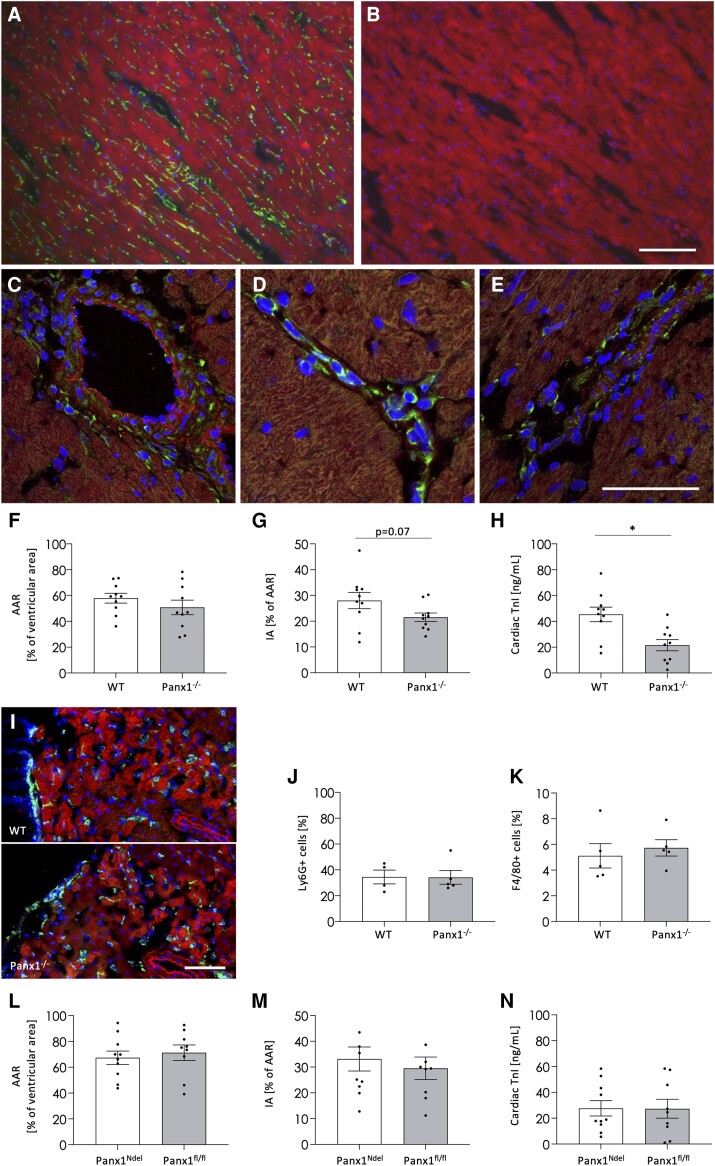
Panx1 deletion protects against I/R injury. Immunofluorescent staining on cardiac cryosections against Panx1 (green) confirmed endothelial expression of Panx1 in the cardiac microvasculature and SMCs of arterioles (A). The signal was absent upon omission of primary antibody (B). After I/R, the Panx1 signal remained present in the endothelium of coronary vessels and the cardiac microvasculature (C, D), as well as in the neutrophils in capillaries and infiltrating the myocardium (D–E). DAPI (blue), Evans blue (red). (A–E) are representative photographs of three independent experiments. Ligation of LAD resulted in a comparable AAR in WT and Panx1−/− mice (F), but Panx1-deficient animals displayed smaller IA (G) and decreased serum cardiac Troponin-I (cTnI; H). N = 10, unpaired t-test. Representative photographs (I) and quantification (J) of immunofluorescent staining against the neutrophil marker Ly6G (green). No differences were observed in neutrophil (J) and macrophage (K) recruitment between WT and Panx1−/− mice (N = 4–5, unpaired t-test). In vivo I/R in mice with neutrophil-specific Panx1 deletion (Panx1Ndel; N = 10) and controls (Panx1fl/fl; N = 9, unpaired t-test) resulted in similar AAR (L), IA (M), and cardiac cTnI (N) between both groups. Scale bars = 50 μm.
To investigate the effects of ischaemia on Panx1 expression in more detail, we performed in vitro studies on MCECs kept under control conditions (normoxia 21% O2; N), exposed for 2 h to hypoxia (1% O2; H) or to oxygen/glucose deprivation (OGD). The correct exposure to hypoxic conditions was confirmed by the induction of hypoxia-inducible factor 1 subunit alpha (HIF-1α) expression (see Supplementary material online, Figure S1A-1B). Panx1 expression levels in MCECs were not affected by hypoxia nor by OGD (see Supplementary material online, Figure S1C), thus corroborating the in vivo observations after LAD ligation and reperfusion.
3.2. Ubiquitous deletion of Panx1 protects against in vivo ischaemia and reperfusion
To investigate the role of Panx1 in leukocyte recruitment and cell death associated with cardiac I/R, we ligated the LAD coronary artery of WT and Panx1−/− mice for 30 min followed by 24 h of reperfusion. The AAR and infarct size were determined based on Evans blue and 2,3,5-Triphenyltetrazolium chloride (TTC) staining. The AAR did not differ between WT (58%) and Panx1−/− (51%) mice (Figure 1F), illustrating the reproducibility of the LAD ligation. Whereas the infarcted area was around 28% of the AAR in WT mice, myocardial cell death was reduced by ∼23% in Panx1−/− mice (Figure 1G). Notably, serum levels of cTnI after I/R were significantly lower in Panx1−/− mice (Figure 1H), further illustrating that Panx1 deletion in mice contributes to the protection against cardiac cell death after I/R.
Next, we investigated the effect of ubiquitous Panx1 deletion on leukocyte recruitment to the site of injury after I/R. The recruitment of neutrophils starts rapidly after the onset of reperfusion with a peak at 1 day of reperfusion.27 Ly6G immunostaining revealed important neutrophil infiltration into the infarcted area 24 h after reperfusion (Figure 1I). However, no difference in neutrophil recruitment was observed between WT and Panx1−/− mice (Figure 1J). Likewise, the number of macrophages in the infarcted area was not different between WT and Panx1−/− mice 24 h after reperfusion (Figure 1K). Next, we performed cardiac I/R on mice with a specific deletion of Panx1 in neutrophils (Panx1Ndel) and their Panx1fl/fl controls. As shown in Figure 1L–N, the AAR, infarct size, and cTnI levels in Panx1Ndel mice were comparable with their controls. The absence of effects on leukocyte recruitment in Panx1−/− mice along with similar damage to the hearts of Panx1Ndel and control mice excludes a major role for neutrophil Panx1 in cardioprotection after I/R.
3.3. Ubiquitous deletion of Panx1 protects against ex vivo ischaemia and reperfusion
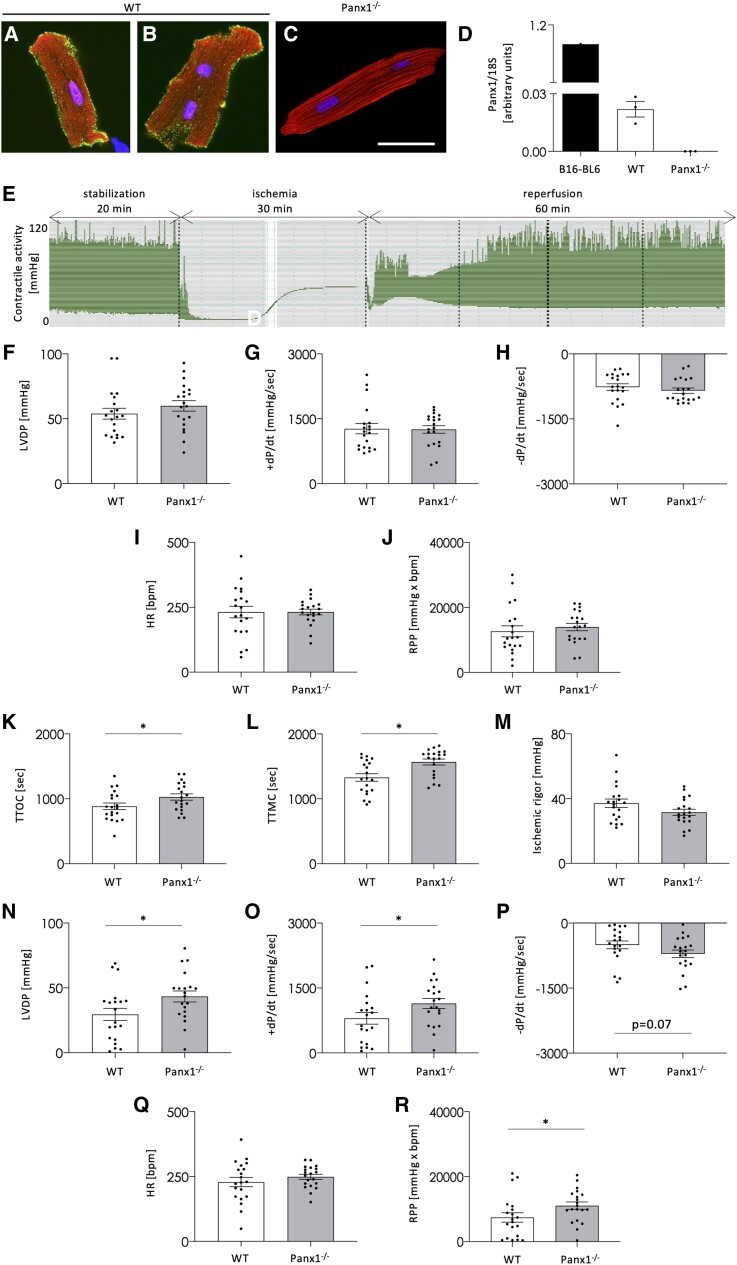
The reduced infarct size observed in Panx1−/− mice after cardiac I/R might be due to the direct effects of Panx1 on cardiomyocyte death. However, the expression of Panx1 in cardiomyocytes of small rodents is under debate.28 Therefore, we have isolated ventricular cardiomyocytes from adult WT mice and immunostained them for Panx1. The protein could be readily detected in the plasma membranes of all cardiomyocytes along with a sparse intracellular signal, which was often more intense in subsarcolemmal areas (Figure 2A–B). Importantly, this signal was absent in negative control immunostainings on WT cardiomyocytes with the omission of the first antibody (not shown) and in Panx1−/− cardiomyocytes (Figure 2C). qPCR on mRNA extracted from isolated ventricular cardiomyocytes confirmed Panx1 expression in WT cardiomyocytes and the absence of Panx1 mRNA in Panx1−/− mice (Figure 2D). Thus, ventricular cardiomyocytes express Panx1, which is localized in plasma membranes but also intracellularly, mostly in subsarcolemmal areas.
Figure 2.
Panx1 deletion improves the recovery of LV function after ex vivo I/R. Immunolabelling of cardiomyocytes isolated from WT adult mice demonstrated Panx1 (green) staining in plasma membranes and subsarcolemmal regions (A, B). The signal was absent in Panx1−/− cardiomyocytes (C). DAPI (blue), Evans blue (red). Scale bar = 50 μm. (A–C) are representative photographs of three independent experiments. qPCR confirmed Panx1 mRNA expression in WT cardiomyocytes while absent in Panx1−/− cardiomyocytes. N = 3. B16-BL6 cells were used as a positive control (D). Representative myocardial contractile activity trace during Langendorff perfusion: WT and Panx1−/− hearts were stabilized for 20 min, and then subjected to global no-flow ischaemia (30 min) and reperfusion (60 min) (E). The physiological myocardial function measured at the end of the stabilization, i.e. left ventricular developed pressure (LVDP; F), contractility (+dP/dt; G), relaxation (–dP/dt; H), HR (I), and RPP (J) were not different between both genotypes. N = 20, unpaired t-test. The sensitivity to ischaemia measured as time to onset of contracture (TTOC; K) and to maximal contracture (TTMC; L) was increased in Panx1−/− mice while the ischaemic rigour (M) remained unchanged. N = 20, unpaired t-test. After I/R, Panx1−/− hearts displayed improved recovery of LVDP (N), +dP/dt (O), –dP/dt (P), and RPP (R), whereas HR (Q) remained similar between both genotypes. N = 20, unpaired t-test.
Cardiomyocytes are responsible for contractile force generation, which enables the heart to work as a pump. To study the effects of ubiquitous Panx1 deletion on LV function, we performed ex vivo functional assays using Langendorff perfusion. The ventricular performance was monitored by introducing a balloon connected to a pressure transducer into the LV of WT and Panx1−/− hearts. Figure 2E illustrates a complete trace of the contractile activity of a WT heart subjected to an I/R protocol. After 20 min stabilization, no differences were observed in the LV developed pressure (LVDP; Figure 2F), contractility (+dP/dt; Figure 2G), relaxation (–dP/dt; Figure 2H), heart rate (HR; Figure 2I) and rate pressure product (RPP; Figure 2J) between the two groups of mice. These results indicate that LV function in Panx1−/− mice was not affected under physiological conditions.
Myocardial sensitivity to 30 min no-flow global ischaemia was evaluated by measuring three parameters characterizing the ischaemic contracture: the time to onset of contracture (TTOC), the time to maximal contracture (TTMC), and the ischaemic rigour. Whereas no differences were observed in ischaemic rigour (Figure 2M), both TTOC (Figure 2K) and TTMC (Figure 2L) were delayed in Panx1−/− hearts, pointing to a reduced sensitivity of the Panx1−/− myocardium to prolonged ischaemia.
The recovery of LV function was measured after 60 min of reperfusion. Panx1 deletion significantly improved the recovery of LVDP (Figure 2N), contractility (Figure 2O), and RPP (Figure 2R). Moreover, a favourable trend was observed for the recovery of relaxation in Panx1−/− hearts (Figure 2P). The HR recovered to similar values in WT and Panx1−/− hearts after I/R (Figure 2Q). Altogether, our data demonstrate that ventricular cardiomyocytes express Panx1 and that deletion of this protein has no effect on physiological cardiac mechanical function but renders the heart less sensitive to myocardial ischaemia and improves functional recovery of the LV after I/R.
3.4. Ischaemic preconditioning abolishes the cardioprotective effect of Panx1 deletion
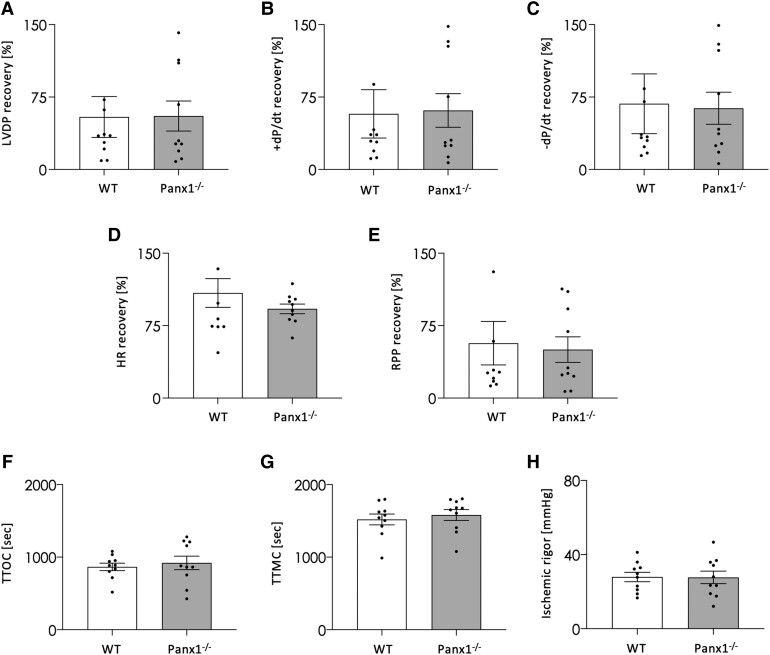
Reperfusion injury can be attenuated by short bursts of non-lethal ischaemia and reperfusion before a lengthy ischaemic period and reperfusion event.29 This ischaemic preconditioning (IPC) initiates the production of autacoids (for example, acetylcholine and adenosine) by cardiomyocytes, which subsequently bind to receptors on the plasma membrane of cardiomyocytes to stimulate various signalling pathways that convey a cardioprotective signal to the mitochondria.2 Indeed, three cycles of 5 min ischaemia/5 min reperfusion before applying 30 min no-flow global ischaemia and 60 min reperfusion, delayed TTMC, reduced ischaemic rigour, and decreased infarct size in WT hearts subjected to IPC (see Supplementary material online, Figure S2). To decipher the mechanism involving Panx1 in cardiac I/R, we have next investigated the effect of Panx1 deletion on IPC-induced cardioprotection. Thus, we subjected hearts from WT and Panx1−/− mice ex vivo to three cycles of IPC before the prolonged I/R. After IPC, the recovery of LVDP (Figure 3A), contractility (Figure 3B), relaxation (Figure 3C), HR (Figure 3D), and RPP (Figure 3E) was comparable between Panx1−/− and WT hearts. Importantly, the difference in sensitivity to ischaemic injury observed between WT and Panx1−/− hearts were no longer present after IPC. Indeed, TTOC (Figure 3F), TTMC (Figure 3G), and ischaemic rigour (Figure 3H) were similar between both genotypes, demonstrating that Panx1 deletion abolishes the protective effects of IPC on cardiac I/R. These results suggest that Panx1 contributes to salvage signalling pathways entailing the mitochondria.
Figure 3.
Panx1 deletion abolishes the cardioprotective effects of IPC. Mouse hearts were subjected to three cycles of brief ischaemia (5 min) followed by reperfusion (5 min) before the period of prolonged ischaemia (30 min) and reperfusion (60 min). The recovery of LVDP (A), +dP/dt (B), –dP/dt (C), HR (D), and RPP (E) was not changed between WT (N = 9) and Panx1−/− (N = 10, unpaired t-test) mice following IPC and I/R. Moreover, no differences were observed in TTOC (F), TTMC (G), and ischaemic rigour (H) under these conditions. N = 10, unpaired t-test.
3.5. Panx1 in cardiac mitochondria affects their ATP content
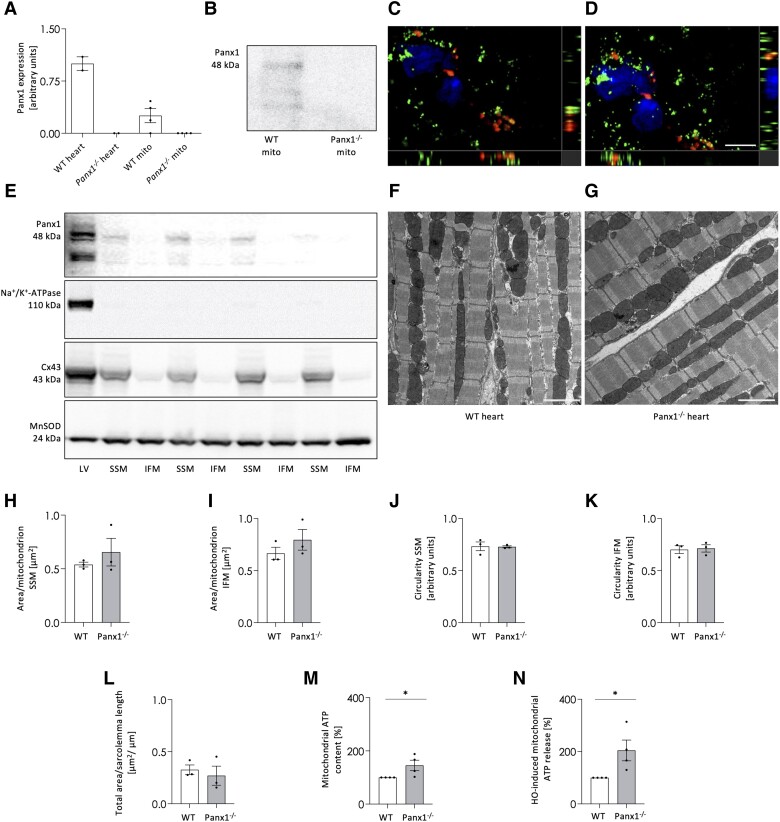
Mitochondria are vital for cellular metabolism as they are the primary source of ATP generated via oxidative phosphorylation. Panx1 mRNA (Figure 4A) and protein (Figure 4B) were detected in mitochondria isolated from cardiac ventricles, whereas they were absent in mitochondria from Panx1−/− ventricles. In addition, co-localization of Panx1 mRNA with mRNA of the mitochondrial marker COX-1 was revealed by RNAscope in situ hybridization (Figure 4C–D). Panx1 mRNA was also abundantly detected in the cytoplasm of cardiomyocytes, suggesting that the mRNA was derived from nuclear transcripts and later localized to this subcellular compartment.30 As intracellular Panx1 mostly localized to subsarcolemmal areas in isolated cardiomyocytes (Figure 2A–B), SSM and IFM were isolated from LV tissue of WT hearts and subjected to Western blot (Figure 4E). MnSOD served as a mitochondrial marker protein and the absence of Na+/K + -ATPase immunoreactivity demonstrated that the SSM and IFM preparations were not contaminated with sarcolemmal proteins. Cx43 is a protein, which is known to be predominantly localized in SSM.31 The prevailing presence of Cx43 in this mitochondrial subpopulation thus indicates the successful separation of SSM and IFM (Figure 4E). Immunoreactivity for Panx1 was detected in SSM, but not in IFM, showing that Panx1 protein is unevenly distributed between mitochondrial subpopulations (Figure 4E).
Figure 4.
Panx1 is present in cardiac SSM. qPCR shows Panx1 mRNA in mitochondria derived from WT ventricular tissue while absent in mitochondria of Panx1−/− ventricles. N = 4. mRNA extracted from WT and Panx1−/− hearts were used as positive and negative control, respectively (A). Panx1 protein is present in mitochondria isolated from WT ventricular tissue while absent in cardiomyocyte mitochondria of Panx1−/− ventricles (B). RNAscope in situ hybridization on murine heart cryosections demonstrated colocalization of Panx1 mRNA (red) with mitochondrial COX-I mRNA (green) (C–D). DAPI (blue). Scale bar = 5 μm. Co-localization is revealed by the yellow signal. (C and D) are representative photographs of three independent experiments. Western blots showing Panx1 protein in SSM fractions and not in IFM fractions isolated from ventricles of WT hearts (E). The absence of Na+/K + -ATPase confirmed the purity of mitochondrial extraction, the presence of Cx43 confirmed the correct subfractioning and MnSOD served as loading control. LV represents a total protein sample of LV tissue of the WT heart. Representative EM images of WT (F) and Panx1−/− (G) hearts. Scale bars = 2 µm. Morphological parameters, i.e. average area per SSM (H) or IFM (I) and circularity of SSM (J) or IFM (K), were not different between genotypes (WT: 44 images analysed from three hearts; Panx1−/−: 43 images analysed from three hearts). The total number of mitochondria analysed was 542 SSM and 668 IFM for WT hearts, and 356 SSM and 635 IFM for Panx1−/− hearts. The total SSM area relative to the length of the sarcolemma (L) was also not different between WT and Panx1−/− hearts. Statistical analysis of EM data was performed using a linear mixed model with either repeated measures and compound symmetry (mitochondrial area per sarcolemmal length) or a nested design (area per mitochondrion and shape circularity). The ATP content in Panx1−/− mitochondria was higher than in WT mitochondria. N = 4 (M). A hypo-osmotic shock induced more ATP release from Panx1−/− mitochondria than from WT mitochondria. N = 4, unpaired t-test (N).
To investigate if the presence of Panx1 in SSM was associated with changes in the morphology of these organelles, hearts from WT and Panx1−/− mice were perfusion-fixed and stained for EM. Figures 4F and G show representative images for WT and Panx1−/− hearts, respectively, and contain both SSM and IFM. A comparison of morphological parameters such as area per mitochondrion (Figures 4H–I) and shape circularity (Figure 4J-4K) revealed no differences between WT and Panx1−/− hearts in either SSM or IFM. The preserved area and circularity of the individual mitochondria indicate no change in fission and fusion processes and thus suggest normal mitochondrial dynamics in Panx1−/− hearts. Likewise, the total area of SSM per sarcolemmal length was not different between WT and Panx1−/− cardiomyocytes (Figure 4L).
Finally, we compared mitochondrial ATP content and release between WT and Panx1−/− cardiomyocytes. Interestingly, under basal conditions, mitochondrial ATP content in cardiomyocytes of Panx1−/− mice was higher than in the mitochondria of WT mice (Figure 4M). Furthermore, exposing cardiac mitochondria to an osmotic shock, a condition that triggers the opening of (plasmalemmal) Panx1 channels in a receptor-independent manner,32 induced more ATP release from Panx1−/− mitochondria than from WT mitochondria (Figure 4N), which rules out a major direct involvement of Panx1 channels in ATP release from these organelles. Overall, our study identified for the first time Panx1 in cardiac SSM. The presence of Panx1 did not affect mitochondrial area and circularity illustrating that fission and fusion processes were not affected, however, Panx1−/− mitochondria had a higher ATP content.
3.6. Panx1 regulates mitochondrial respiration
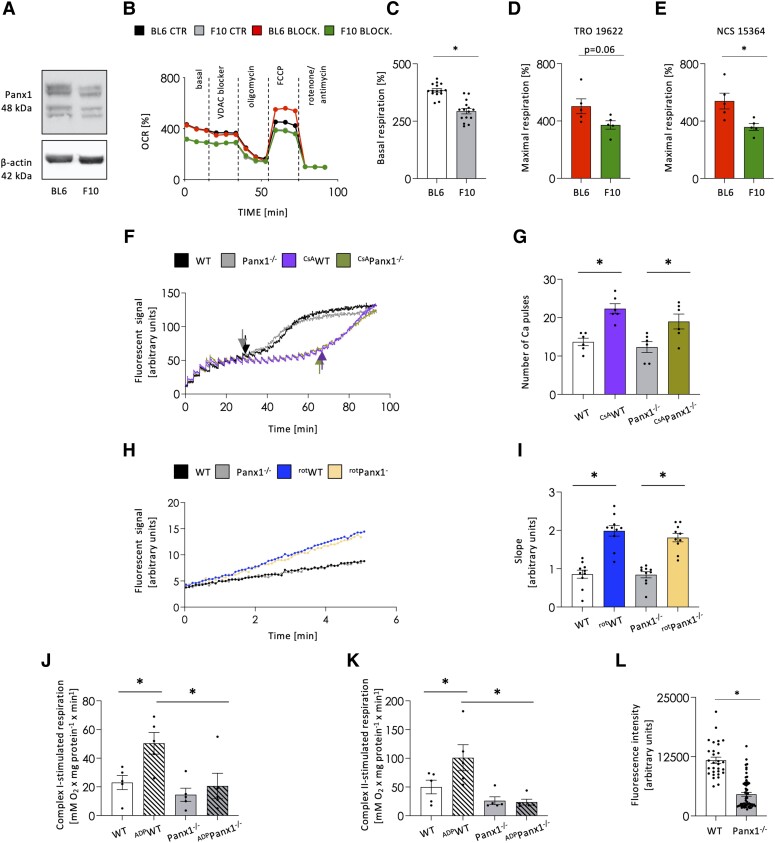
VDAC1, lying in the mitochondrial outer membrane, mediates the transport of ions, ATP, and other metabolites, thus controlling the cross-talk between mitochondria and the cytoplasm.33 To investigate whether Panx1 is involved in mitochondrial function, we first used the isogenic B16-F10 mouse melanoma line, which is known to express low levels of Panx1.34 We confirmed that B16-F10 cells express about half of the Panx1 protein compared with B16-BL6 cells (Figure 5A). The OCR was then monitored in both cell lines under control conditions and revealed that basal respiration was lower in B16-F10 cells (Figure 5B–C). When cells were subsequently exposed to the VDAC1 blocker NSC15364 (10 μM), their basal respiration was not affected and the differences between the two isogenic cell lines were preserved (Figure 5B). As expected, similar proton leak levels were observed in both cell lines after exposure to oligomycin to shut down ATP-linked respiration, and these levels were not affected by the VDAC1 blocker (Figure 5B). Similar results were obtained with 10 μM TRO19622, another VDAC1 blocker (data not shown). After uncoupling of mitochondrial oxidative phosphorylation with 1 μM FCCP, the maximal respiration levels of B16-BL6 cells were enhanced in the presence of either VDAC1 blocker (Figure 5B–E). Interestingly, the maximal respiration levels of B16-F10 cells remained unaffected under those conditions (Figure 5B–E), illustrating that the isogenic cells with higher Panx1 levels could support a higher substrate demand upon VDAC1 inhibition. Of note, the increase in spare capacity of B16-BL6 cells was not observed under control conditions with intact VDAC1 function (Figure 5B). These results suggest that Panx1 modulates OCR under basal conditions and after maximal stimulation.
Figure 5.
Panx1 modulates mitochondrial respiration. Western blots revealed lower Panx1 expression in B16-F10 (F10) cells as compared to isogenic B16-BL6 (BL6) cells (A). Representative traces of OCR measured by Seahorse in untreated B16-BL6 (black) and B16-F10 (grey) cells or after VDAC1 inhibition with 10 μM NSC15364 (B16-BL6: red; B16-F10: green) (B). Basal respiration was reduced in B16-F10 compared to B16-BL6 cells. N = 15, unpaired t-test. (C). Likewise, the maximal respiration rate was reduced in B16-F10 compared to B16-BL6 cells after VDAC1 inhibition with 10 μM of TRO19622 (N = 5, unpaired t-test) (D) or NSC15364 (N = 5, unpaired t-test) (E). Representative traces of calcium-induced mPTP opening in cardiac SSM from WT (black) and Panx1−/− (grey) hearts (F). The mPTP inhibitor—cyclosporin A (CsA) served as internal quality control (WT—violet; Panx1−/−—light green) (F). The quantification of a number of calcium waves before complete mPTP opening (arrows in F) showed no differences between WT and Panx1−/− mitochondria at both basal conditions and after CsA-treatment. N = 6, 2-way ANOVA (G). Representative traces of ROS production from WT and Panx1−/− SSM (H). An increase in slope was observed upon rotenone (rot-) treatment (blue and yellow) compared to the basal level in WT (black) and Panx1-deficient (grey) cardiac mitochondria (H). ROS production, measured as the slope, was comparable between the two genotypes at both conditions. N = 9, two-way ANOVA (I). Basal respiration was similar between WT and Panx1−/− SSM measured at complex 1 (J) and complex 2 (K). The ADP-stimulated respiration showed an increased OCR in WT samples in complex 1 and complex 2, while no differences upon ADP stimulation were observed in Panx1−/− mitochondria (J and K, respectively). N = 5, two-way ANOVA. Panx1−/− cardiomyocytes (N = 61 cardiomyocytes from three heart cell isolations) had a lower mitochondrial membrane potential (ΔΨm) than WT cardiomyocytes (N = 30 cardiomyocytes from three heart cell isolations, unpaired t-test) (L).
To study whether Panx1 regulates cardiac mitochondrial function, SSM were isolated from WT and Panx1−/− ventricles and subjected to various functional tests. Calcium-induced mPTP opening was measured by calcium green 5 N. The calcium concentration that induced mPTP opening was similar in mitochondria from WT and Panx1−/− mice under basal conditions (Figures 5F–G). In the presence of cyclosporine A (CsA), mPTP opening was delayed compared to basal conditions, as expected, but again no differences were observed between WT and Panx1−/− SSM (Figures 5F and G). ROS formation was analysed in SSM isolated from WT and Panx1−/− hearts using Amplex UltraRed. The slope of the fluorescence over time was calculated under basal conditions and in the presence of rotenone, which was used as a positive control as the administration of rotenone enhances ROS formation.35 The slope of the Amplex UltraRed fluorescence was similar between mitochondria from WT and Panx1−/− mice, both under basal conditions and in the presence of rotenone (Figures 5H–I). Finally, mitochondrial oxygen consumption was measured in SSM isolated from WT and Panx1−/− ventricles. Basal respiration was similar between WT and Panx1−/− mitochondria using glutamate and malate as substrates for complex 1 (Figure 5J) and succinate (in the presence of rotenone to inhibit complex 1) as a substrate for complex 2 (Figure 5K). When ADP was added to stimulate mitochondrial respiration, WT mitochondria showed increased oxygen consumption when respiring on substrates for complex 1 and complex 2, whereas such an effect was not detected in Panx1−/− mitochondria (Figures 5J–K). Uncoupled respiration was measured after the addition of FCCP and was similar between WT and Panx1−/− mitochondria (in nmol O2 x min−1 × mg protein−1; complex 1: WT = 263; Panx1−/−=221 (n = 4); complex 2: WT = 314; Panx1−/−=295 (n = 4)). These results demonstrate that Panx1 in cardiac SSM modulates mitochondrial respiration by regulating the function of complex 1 and complex 2.
Finally, we evaluated mitochondrial function in intact WT and Panx1−/− cardiomyocytes by measuring the mitochondrial membrane potential (ΔΨm). In the physiological buffer, the ΔΨm of Panx1−/− mitochondria was reduced as compared to WT mitochondria (Figure 5L), indicating a partial disruption of the proton gradient. Altogether, these data show that deletion of Panx1 in cardiac SSM disturbs ATP fluxes leading to mitochondrial ATP accumulation.
4. Discussion
Our study revealed that Panx1 deletion in mice confers protection against cardiac I/R injury. Surprisingly, we did not observe differences in the acute inflammatory response but revealed a novel role for Panx1 in mitochondria. Although Panx1 deletion did not affect physiologic mechanical cardiac function, Panx1-deficient hearts showed a decreased sensitivity to cardiac I/R injury and a better recovery of cardiac function after the insult, which was explained by an increased basal ATP content in mitochondria of Panx1−/− cardiomyocytes.
The role of Panx1 in ischaemic injury has been explored in several organs, including the heart.13,18,36,37 Using mice with an endothelial cell (EC)-specific deletion of Panx1, Good et al. showed improved cardiac function (ejection fraction) at 14 days post-I/R, although the infarct size was not different at this time point.36 These protective effects in mice with EC-specific deletion of Panx1 correlated with reduced inflammatory cell recruitment to the site of injury.36 They reported decreased monocyte infiltration at 2 days post-I/R, whereas neutrophil infiltration was not altered at this time point. The latter is in accordance with our findings. While neutrophils are known to be recruited to injured area within the first 24 h post-myocardial infaction (MI), massive monocyte infiltration typically occurs at a later phase of the cardiac injury response.27,38 In accordance, we observed only low numbers of F4/80-positive cells at 24 h post-I/R, which were not different between genotypes. Activated TH and Treg cells are also typically found during the inflammatory phase at 2–4 days after the myocardial injury.38 Interestingly, pharmacological blocking of Panx1 with probenecid also improved the ejection fraction at 14 days post-I/R.36 Noteworthy, the ejection fraction measured 24 h after I/R remained unchanged using probenecid as well as upon deletion of Panx1 in ECs. This suggests that the cardioprotective effect of endothelial Panx1 deletion or treatment with probenecid likely took place in the repair phase following I/R. The mouse model with ubiquitous Panx1 deletion used in this study unveils an additional cardioprotective mechanism relevant to potential therapeutic approaches based on Panx1 blockers applied systemically at the onset of reperfusion.
Circulating blood cells can also contribute to cardiac damage after I/R by causing microvascular obstruction inducing no-reflow territories upon reperfusion.39 Ischaemia promotes the adhesion of leukocytes and platelets to the endothelium as well as the formation of platelet-leukocyte aggregates, which can obstruct the coronary microvascular blood flow. Furthermore, erythrocyte aggregates may form at territories with reduced coronary microvascular blood flow, subsequently obstructing the capillary circulation.40 Microvascular obstruction and no-reflow territories are typically restricted to the infarct area (IA) and serial measurements of heart-specific fatty acid binding protein, TnT, or TnI are increasingly recognized as early markers for no-reflow in large animal models.41 Platelets and erythrocytes express relatively high levels of Panx1 and these channels have been proven critical regulators of platelet aggregation.10,26 The single point measurement of plasma TnT at 24 h post-reperfusion used in our study showed a good correlation with infarct size (cell death) determined by TTC at the same time point, but it does unfortunately not allow for conclusions on the role of Panx1 channels in the no-reflow phenomenon.
Although the expression of Panx1 in the myocardium has been long under debate, recent studies have convincingly demonstrated the presence of this channel in cardiomyocytes of various species, including rats,42 mice (this study), dogs, and humans.43 Panx1 channels are typically localized in the sarcolemma, but Panx1 has also been found in other cell compartments such as the endoplasmic reticulum and Golgi apparatus.37,44 Trafficking of Panx1 is regulated by its glycosylation status45 and mediated by its interaction with cytoskeletal proteins, such as actin microfilaments.44 Indeed, the previous proteomic analysis identified cytoskeletal proteins as well as mitochondrial proteins like VDAC1, ANT2, and heat shock protein 70 (Hsc70) as Panx1 partners.34,44,46 Here, we demonstrated for the first time that Panx1 is present in the SSM of murine cardiomyocytes (Figure 4A and E). Altered mitochondrial morphology has been reported in the setting of cardiac I/R (for a review, see Ref. 47) where prevention of mitochondrial fission protects the myocardium and promotes recovery.48,49 Since we found no significant change in mitochondrial size or shape in Panx1−/− mice (Figure 4H–K), the protective effect of Panx1 knockout likely did not involve altered mitochondrial fusion/fission dynamics. However, because we only investigated morphology under normoxic conditions, we cannot exclude that Panx1 could contribute to the initiation or magnitude of mitochondrial fission during ischaemia or reperfusion. Given that ischaemia induces mitochondrial fragmentation, it would be difficult to discern between cause and consequence. The total area of SSM per sarcolemma length was comparable between WT and Panx1-deficient cardiomyocytes (Figure 4L), while the mitochondria of Panx1−/− hearts contained more ATP (Figure 4M). One may hypothesize that this would bring a larger store of ATP close to the sarcolemma and delay the loss of homeostasis by providing energy for active transport.
The accumulation of ATP in Panx1−/− cardiac mitochondria while the mitochondrial membrane potential is decreased can occur during a phenomenon called mitochondrial uncoupling. One of the physiological roles of mitochondrial uncoupling in the heart is to regulate energy metabolism.50,51 During times of high energy demand, such as exercise or stress, mitochondrial uncoupling can increase the rate of electron transport and dissipate the proton gradient, leading to an increase in heat production and a decrease in ATP production. This allows the heart to maintain its energy supply and prevent the build-up of toxic metabolites. Mitochondrial uncoupling would also explain the absence of respiratory response after stimulation with ADP in Panx1−/− cardiac mitochondria (Figure 5J–K).
There is increasing evidence suggesting that mitochondrial uncoupling can protect the heart against I/R injury.51,52 Mild pharmacological uncoupling of mitochondrial oxidative phosphorylation results in a modest depolarization of the mitochondrial membrane potential, which can confer IPC-like cardioprotection in providing an adaptive response to preserve mitochondrial function.50,53,54 The lack of IPC-induced cardioprotection in Panx1−/− hearts may imply that the cardioprotective effect observed in the Panx1-deficient cardiomyocytes involves a similar signalling pathway than the one used by IPC, and further protection cannot be induced in these already maximally adapted cardiomyocytes. Alternatively, the cardioprotective effect observed in Panx1−/− hearts may involve a different signalling pathway than the one used by IPC, but IPC depends on Panx1 and therefore IPC is abolished in Panx1-deficient cardiomyocytes. Although our present data cannot distinguish between these options, the accumulation of ATP in the mitochondrial matrix may result in decreased sensitivity to ischaemic insults as observed in Panx1−/− hearts. Of note, the ATP content and physiology of WT and Panx1−/− SSM have been investigated under basal conditions only in this study. Whether the differences in functioning of WT and Panx1−/− SSM are maintained during cardiac ischaemia or reperfusion will be the subject of future investigations.
In summary, the main finding of this study is that Panx1 deletion does not affect physiological cardiac function but confers cardioprotection against I/R by enhancing mitochondrial ATP content thereby delaying hypercontracture and cell death. We show here for the first time that Panx1 is present in the SSM of cardiomyocytes, contributing to ΔΨm and mitochondrial respiration. As mitochondria have been recognized as major arbiters of cell death and survival in cardiomyocytes, Panx1 might represent a novel target for cardioprotection.
Supplementary Material
Acknowledgements
We thank Christophe Montessuit, Maria Essers, Mehdi Badaoui, Sabrina Böhme, Elvira Ungefug, Bernard Foglia, Graziano Pelli, Viviane Rochemont, and Marc Bachetta for helpful discussions and excellent technical assistance. Also thanks to Zhila Nikrozi and Klaus Qvortrup from the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen as well as to Yves Alexandre Cambet from the READS Core Facility, Faculty of Medicine, and University of Geneva.
Contributor Information
Olga M Rusiecka, Department of Pathology and Immunology, Faculty of Medicine, University of Geneva, Rue Michel-Servet 1, CH-1211 Geneva, Switzerland.
Filippo Molica, Department of Pathology and Immunology, Faculty of Medicine, University of Geneva, Rue Michel-Servet 1, CH-1211 Geneva, Switzerland.
Morten S Nielsen, Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Axel Tollance, Department of Cell Physiology and Metabolism, Faculty of Medicine, University of Geneva, Geneva, Switzerland.
Sandrine Morel, Department of Pathology and Immunology, Faculty of Medicine, University of Geneva, Rue Michel-Servet 1, CH-1211 Geneva, Switzerland.
Maud Frieden, Department of Cell Physiology and Metabolism, Faculty of Medicine, University of Geneva, Geneva, Switzerland.
Marc Chanson, Department of Cell Physiology and Metabolism, Faculty of Medicine, University of Geneva, Geneva, Switzerland.
Kerstin Boengler, Institute of Physiology, Justus-Liebig University, Giessen, Germany.
Brenda R Kwak, Department of Pathology and Immunology, Faculty of Medicine, University of Geneva, Rue Michel-Servet 1, CH-1211 Geneva, Switzerland.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Swiss National Science Foundation (grant numbers 310030_182573, 310030E-176050 and IZCOZ0_189878 to B.R.K., 310030_184756 to M.F., 310030_172909 to M.C.), the Swiss Life Foundation (to F.M.) and the Fondation Carlos et Elsie De Reuter (to F.M.). We also acknowledge the Gabbiani fund for partial salary support (to O.M.R.). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 and IG16225 supported by COST (European Cooperation in Science and Technology).
Data availability
The data underlying this article are available in the article and in its Supplementary material online.
References
- 1. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, Gale CP, Maggioni AP, Petersen SE, Huculeci R, Kazakiewicz D, de Benito Rubio V, Ignatiuk B, Raisi-Estabragh Z, Pawlak A, Karagiannidis E, Treskes R, Gaita D, Beltrame JF, McConnachie A, Bardinet I, Graham I, Flather M, Elliott P, Mossialos EA, Weidinger F, Achenbach S, Atlas Writing Group, European Society of Cardiology . European Society of cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022;43:716–799. [DOI] [PubMed] [Google Scholar]
- 2. Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 2016;13:193–209. [DOI] [PubMed] [Google Scholar]
- 3. Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 2020;17:773–789. [DOI] [PubMed] [Google Scholar]
- 4. Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth 2012;16:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015;65:1454–1471. [DOI] [PubMed] [Google Scholar]
- 6. Davidson SM, Ferdinandy P, Andreadou I, Botker HE, Heusch G, Ibanez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D, Action CC. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 2019;73:89–99. [DOI] [PubMed] [Google Scholar]
- 7. Rusiecka OM, Tournier M, Molica F, Kwak BR. Pannexin1 channels-a potential therapeutic target in inflammation. Front Cell Dev Biol 2022;10:1020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW, Investigators A-I. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol 2005;45:1775–1780. [DOI] [PubMed] [Google Scholar]
- 9. Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J 2006;27:2400–2405. [DOI] [PubMed] [Google Scholar]
- 10. Molica F, Figueroa XF, Kwak BR, Isakson BE, Gibbins JM. Connexins and pannexins in vascular function and disease. Int J Mol Sci 2018;19:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koval M, Cwiek A, Carr T, Good ME, Lohman AW, Isakson BE. Pannexin 1 as a driver of inflammation and ischemia-reperfusion injury. Purinergic Signal 2021;17:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freitas-Andrade M, Bechberger JF, MacVicar BA, Viau V, Naus CC. Pannexin1 knockout and blockade reduces ischemic stroke injury in female, but not in male mice. Oncotarget 2017;8:36973–36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Good ME, Eucker SA, Li J, Bacon HM, Lang SM, Butcher JT, Johnson TJ, Gaykema RP, Patel MK, Zuo Z, Isakson BE. Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight 2018;3:e96272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010;467:863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sofoluwe A, Bacchetta M, Badaoui M, Kwak BR, Chanson M. ATP Amplifies NADPH-dependent and -independent neutrophil extracellular trap formation. Sci Rep 2019;9:16556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasenberg A, Hasenberg M, Mann L, Neumann F, Borkenstein L, Stecher M, Kraus A, Engel DR, Klingberg A, Seddigh P, Abdullah Z, Klebow S, Engelmann S, Reinhold A, Brandau S, Seeling M, Waisman A, Schraven B, Gothert JR, Nimmerjahn F, Gunzer M. Catchup: a mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat Methods 2015;12:445–452. [DOI] [PubMed] [Google Scholar]
- 17. Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PLoS One 2011;6:e25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A 2011;108:20772–20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morel S, Braunersreuther V, Chanson M, Bouis D, Rochemont V, Foglia B, Pelli G, Sutter E, Pinsky DJ, Mach F, Kwak BR. Endothelial Cx40 limits myocardial ischaemia/reperfusion injury in mice. Cardiovasc Res 2014;102:329–337. [DOI] [PubMed] [Google Scholar]
- 20. Frobert A, Valentin J, Magnin JL, Riedo E, Cook S, Giraud MN. Prognostic value of troponin I for infarct size to improve preclinical myocardial infarction small animal models. Front Physiol 2015;6:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chevalier M, Vermij SH, Wyler K, Gillet L, Keller I, Abriel H. Transcriptomic analyses of murine ventricular cardiomyocytes. Sci Data 2018;5:180170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koncsos G, Varga ZV, Baranyai T, Ferdinandy P, Schulz R, Giricz Z, Boengler K. Nagarse treatment of cardiac subsarcolemmal and interfibrillar mitochondria leads to artefacts in mitochondrial protein quantification. J Pharmacol Toxicol Methods 2018;91:50–58. [DOI] [PubMed] [Google Scholar]
- 23. Hirschhauser C, Lissoni A, Gorge PM, Lampe PD, Heger J, Schluter KD, Leybaert L, Schulz R, Boengler K. Connexin 43 phosphorylation by casein kinase 1 is essential for the cardioprotection by ischemic preconditioning. Basic Res Cardiol 2021;116:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hostettler N, Naggi A, Torri G, Ishai-Michaeli R, Casu B, Vlodavsky I, Borsig L. P-selectin- and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. FASEB J 2007;21:3562–3572. [DOI] [PubMed] [Google Scholar]
- 25. Rusiecka OM, Roth CL, Kwak BR, Molica F. RB459 And RB462 antibodies recognize mouse pannexin1 protein by immunofluorescent staining. Antibody Rep 2019;2:39. [Google Scholar]
- 26. Molica F, Morel S, Meens MJ, Denis JF, Bradfield PF, Penuela S, Zufferey A, Monyer H, Imhof BA, Chanson M, Laird DW, Fontana P, Kwak BR. Functional role of a polymorphism in the pannexin1 gene in collagen-induced platelet aggregation. Thromb Haemost 2015;114:325–336. [DOI] [PubMed] [Google Scholar]
- 27. Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 2013;62:24–35. [DOI] [PubMed] [Google Scholar]
- 28. Meens MJ, Kwak BR, Duffy HS. Role of connexins and pannexins in cardiovascular physiology. Cell Mol Life Sci 2015;72:2779–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 30. Das S, Vera M, Gandin V, Singer RH, Tutucci E. Intracellular mRNA transport and localized translation. Nat Rev Mol Cell Biol 2021;22:483–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol 2009;104:141–147. [DOI] [PubMed] [Google Scholar]
- 32. Sandilos JK, Bayliss DA. Physiological mechanisms for the modulation of pannexin 1 channel activity. J Physiol 2012;590:6257–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Camara AKS, Zhou Y, Wen PC, Tajkhorshid E, Kwok WM. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol 2017;8:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Penuela S, Gyenis L, Ablack A, Churko JM, Berger AC, Litchfield DW, Lewis JD, Laird DW. Loss of pannexin 1 attenuates melanoma progression by reversion to a melanocytic phenotype. J Biol Chem 2012;287:29184–29193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 2003;278:8516–8525. [DOI] [PubMed] [Google Scholar]
- 36. Good ME, Young AP, Wolpe AG, Ma M, Hall PJ, Duffy CK, Aronovitz MJ, Martin GL, Blanton RM, Leitinger N, Johnstone SR, Wolf MJ, Isakson BE. Endothelial pannexin 1 regulates cardiac response to myocardial infarction. Circ Res 2021;128:1211–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crespo Yanguas S, Willebrords J, Johnstone SR, Maes M, Decrock E, De Bock M, Leybaert L, Cogliati B, Vinken M. Pannexin1 as mediator of inflammation and cell death. Biochim Biophys Acta Mol Cell Res 2017;1864:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 2015;15:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hausenloy DJ, Chilian W, Crea F, Davidson SM, Ferdinandy P, Garcia-Dorado D, van Royen N, Schulz R, Heusch G. The coronary circulation in acute myocardial ischaemia/reperfusion injury: a target for cardioprotection. Cardiovasc Res 2019;115:1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Driesen RB, Zalewski J, Vanden Driessche N, Vermeulen K, Bogaert J, Sipido KR, Van de Werf F, Claus P. Histological correlate of a cardiac magnetic resonance imaged microvascular obstruction in a porcine model of ischemia-reperfusion. Cardiovasc Pathol 2012;21:129–131. [DOI] [PubMed] [Google Scholar]
- 41. Uitterdijk A, Sneep S, van Duin RW, Krabbendam-Peters I, Gorsse-Bakker C, Duncker DJ, van der Giessen WJ, van Beusekom HM. Serial measurement of hFABP and high-sensitivity troponin I post-PCI in STEMI: how fast and accurate can myocardial infarct size and no-reflow be predicted? Am J Physiol Heart Circ Physiol 2013;305:H1104–H1110. [DOI] [PubMed] [Google Scholar]
- 42. Kienitz MC, Bender K, Dermietzel R, Pott L, Zoidl G. Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J Biol Chem 2011;286:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol 2012;303:H1208–H1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhalla-Gehi R, Penuela S, Churko JM, Shao Q, Laird DW. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J Biol Chem 2010;285:9147–9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boassa D, Qiu F, Dahl G, Sosinsky G. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes 2008;15:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frederiksen SDW-S LE, Sanchez-Arias JC, Swayne LA. Exploring the Pannexin 1 interactome: In silico cross-analyses with postsynaptic proteins and neuropsychiatric disorder susceptibility genes. bioRxiv 2019:1–29.
- 47. Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med 2017;21:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010;121:2012–2022. [DOI] [PubMed] [Google Scholar]
- 49. Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 2014;28:316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenergy 2018;1859:940–950. [DOI] [PubMed] [Google Scholar]
- 51. Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc Res 2006;72:210–219. [DOI] [PubMed] [Google Scholar]
- 52. Kuznetsov AV, Javadov S, Margreiter R, Grimm M, Hagenbuchner J, Ausserlechner MJ. The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants (Basel) 2019;8:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hausenloy DJ, Schulz R, Girao H, Kwak BR, De Stefani D, Rizzuto R, Bernardi P, Di Lisa F. Mitochondrial ion channels as targets for cardioprotection. J Cell Mol Med 2020;24:7102–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minners J, van den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res 2000;47:68–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary material online.