Highlights
-
•
The effectiveness of induction chemotherapy (IC) is a significant prognosis factor for locally advanced nasopharyngeal carcinoma (LANPC). Nimotuzumab (NTZ) is an EGFR-targeting antibody which has shown promising efficacy combined with RT in the treatment of LANPC.
-
•
We designed this single-arm, open-label phase II clinical trial investigating the efficacy and safety of NTZ combined with CCRT in IC resistant LANPC. The 3-year and 5-year PFS and OS rates were 79.3 % and 72.1 %, 94.0 % and 87.2 %, respectively. Patients treated with ≥6 weeks of NTZ showed improved 3-year PFS rate (83.0% % vs. 73.9 %, p > 0.05) and 5-year PFS rate (83.0% % vs. 61.6 %, P>0.05) compared with <6 weeks NTZ.
-
•
Combination NTZ-CCRT chemotherapy is efficacious and tolerable in IC resistance LANPC, particularly in patients who received ≥6 weeks NTZ.
Keywords: Nasopharyngeal carcinoma, Anti-epidermal growth factor receptor (EGFR) monoclonal antibody, Induction chemotherapy resistant, Chemoradiotherapy, a prospective phase II study
Abstract
Objectives
To investigate the efficacy and safety of nimotuzumab (NTZ) combined with concurrent chemo-radiotherapy (CCRT) in induction chemotherapy (IC) resistant locally advanced nasopharyngeal carcinoma (LANPC).
Materials and methods
A single-arm, open-label phase II clinical trial was conducted (NCT04508816). Eligible patients were 18–70 years old, pathologically confirmed NPC at stage III-IVA, stable disease or progressive disease after IC by imaging evaluation, and ECOG performance status with 0–1. All patients received intensity-modulated radiotherapy (IMRT) concurrent with chemotherapy and NTZ (200 mg/w). The primary endpoint was progression-free survival (PFS). The secondary endpoints were overall survival (OS), objective response rate (ORR) and safety.
Results
From May 2015 to July 2020, 56 NPC patients were enrolled. With the median follow-up of 34 months (range from 8 to 77 months), the 3-year and 5-year PFS and OS rates were 79.3 % and 72.1 %, 94.0 % and 87.2 %, respectively. ORR of the nasopharynx and cervical lymph nodes involvement were 98.2 % and 98.1 % three months after IMRT. Univariate analysis revealed that pretreatment PET/CT was the factor that influenced PFS (P = 0.038). Patients treated with ≥6 weeks of NTZ showed improved 3‐year PFS rate (83.0% vs. 73.9 %, P > 0.05) and 5-year PFS rate (83.0% vs. 61.6 %, P>0.05) compared with <6 weeks NTZ. The acute toxicities were mainly grade 1/2 hematologic. Severe toxicities were uncommon. The major grade 3/4 AE was neutropenia (26.8 %).
Conclusions
The results demonstrated that NTZ combined with CCRT in IC resistant LANPC was effective with mild toxicity.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant neoplasm of the epithelial tissue, which has unique heterogeneous racial and geographical distributions. Majorite of new cases (>70 %) occur in East and Southeast Asia. The (world) age-standardized incidence is 3.0 cases per 100,000 in China and 0.4 cases per 100,000 people in predominantly white populations. [1] Owing to its challenging anatomical location, radiotherapy (RT) plays an essential role for non-metastatic disease. A large majority of newly diagnosed NPC cases are classified as loco-regionally advanced disease and the combination of RT with chemotherapy is generally considered. [1], [2], [3] Due to the advantages of induction chemotherapy (IC) in improving survival, reducing the gross volume of the tumor, and improving tumor coverage during RT, IC followed by concurrent chemo-radiotherapy (CCRT) has become a new standard of treatment for non-metastatic stage III–IVA NPC according to CSCO/ASCO guidelines. [4,5] Moreover, the effectiveness of IC is a significant prognosis factor for locally advanced nasopharyngeal carcinoma (LANPC). An unsatisfactory tumor response (SD, stable disease or PD, progressive disease) after IC correlated with poor clinical outcome. Peng et al. [6] retrospectively reviewed 399 NPC patients with pre- and post-IC MRI. The 4-year disease-free survival (DFS) and overall survival (OS) for CR vs. PR vs. SD were 90.0% vs. 79.0% vs. 58.2 % (CR vs. PR: P1 = 0.007; CR vs. SD: P2 < 0.001; PR vs. SD: P3 = 0.004) and 95.7% vs. 88.7% vs. 70.2 % (P1 = 0.017, P2 < 0.001, P3 = 0.005), respectively. Multivariate analysis identified that the tumor response to IC was an independent prognostic factor for DFS and OS. Similar results were also confirmed in Liu's study [7]. How to improve the therapeutic effect of IC resistant NPC is a major challenge for clinical oncologists.
Epidermal growth factor receptor (EGFR), also named HER1 or ErbB1, is a member of the ErbB family of receptor tyrosine kinases highly expressed in most human epithelial carcinomas [8,9]. It has been demonstrated that EGFR is highly expressed in NPC patients and is a potential negtive prognostic factor. [10] Nimotuzumab (NTZ) is an EGFR-targeting antibody which has shown promising efficacy combined with RT in the treatment of LANPC. Furthermore, compared with another EGFR-targeting cetuximab, NTZ was with minimal toxicity. [11], [12], [13] Cetuximab/NTZ plus CCRT was associated with a significantly increased overall survival (3-year OS, 96.6% vs. 92.9 %, P = 0.015), disease-free survival (3-year DFS, 93.5% vs 86.9 %, P = 0.028), and distant metastasis-free survival (3-year DMFS, 94.6% vs 89.3 %, P = 0.030) compared with CCRT alone. [13] A propensity score matched study found the addition of NTZ to CCRT after IC could obtain higher survival benefits. Additionally, CCRT plus NTZ was well-tolerated and did not increase treatment related toxicities. [14] The results of RTOG 0522 [15] demonstrated that adding cetuximab to CCRT did not improve outcome for stage III to IV head and neck carcinoma, possibly due to the failure of selection for IC. So, we designed a single-arm, open-label phase II clinical trial investigating the efficacy and safety of NTZ combined with CCRT in IC resistant LANPC.
Materials and methods
Patients and study design
Eligibility criteria included: (1) pathologically confirmed NPC at stage III-IVA according to the American Joint Committee on Cancer Staging System (AJCC 7 ed); (2) IC regimen: TP (docetaxel 75 mg/m2 d1+DDP/edaplatin 25 mg/m2 d1–3) or GP (gemcitabine 1.0 g/m2 d1, d8+DDP 25 mg/m2 d1–3); (3) stable disease (SD) or progressive disease (PD) after IC by imaging evaluation; (4) 18–70 years old; (5) the Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; (6) adequate hematological, hepatic and renal functions. Exclusion criteria included: (1) a history of any other type of malignant disease; (2) pregnancy or lactation; (3) failure of cardiac, lung, liver or renal function; (4) distant metastasis.
This study was a single-arm, open-label phase II study. The primary endpoint was progression-free survival (PFS), and secondary endpoints were overall survival (OS), objective response rate (ORR), and safety. All patients provided written informed consent before enrollment. The study protocol was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center. Initial assessment consisted of medical history and physical examination, blood routine and biochemistry tests, enhanced magnetic resonance imaging (MRI) of the nasopharynx, enhanced MRI/CT of the neck. Other assessment included positron emission tomography-CT (PET-CT), or replaced by chest CT, abdominal ultrasound/CT and bone emission CT.
Treatment
All patients received IC with TP or GP regimen. Generally, the IC regimens were delivered every 21 days: TP (docetaxel 75 mg/m2 d1+DDP/Nedaplatin 25 mg/m2 d1–3) or GP (gemcitabine 1.0 g/m2 d1, d8+DDP 25 mg/m2 d1–3). NTZ was administered concomitantly with IMRT at a dose of 200 mg weekly. If imaging evaluation was SD or PD in the first or second IC, NTZ could be administered during IC. All patients were treated with IMRT. The details of the tumor volume delineation have been described previously [16]. The prescribed dose given to primary tumor was 70.4 Gy in 32 fractions (PTV-NX: GTV-NX +3 mm). A total dose of 67.2–70.4 Gy was given to the planned target volume of the lymph nodes (PTV-LN: GTV-LN +3 mm) in 32 fractions. The PTV covering the high-risk CTV and a 3-mm margin was prescribed 57.6 Gy/32 F. The PTV covering the low-risk CTV and a 3-mm margin was prescribed 54.4 Gy/32 F. Radiotherapy was given once daily, 5 fractions per week.
During the IMRT, CCRT was administrated DDP/Nedaplatin 25 mg/m2 d1–3 every 21 days or S-1 orally bid (BSA < 1.25 m2, 30 mg; BSA: 1.25– 1.5 m2, 40 mg; BSA > 1.5 m2, 50 mg). The hematologic parameters, skin and mucosal reactions were assessed during CCRT weekly. Treatment would be interrupted and the dose of CCRT would be reduced by 20 % in case of grade 4 toxicity.
Assessment and follow-up
Toxicities were assessed after each cycle of chemotherapy and during RT weekly. Drug-related toxicities were graded by the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0. Radiotherapy-related toxicities were assessed according to the Radiation Therapy Oncology Group (RTOG). Short-term efficacy was evaluated as complete remission (CR), partial remission (PR), SD, and PD according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) after 2–3 cycles of IC, IMRT, and 3 months after IMRT. After treatment completion, follow-ups occurred every 3 months in the first 2 years, every 6 months from the third to the fifth year and annually thereafter.
Statistical analysis
SPSS 23.0 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis in this study. The estimated PFS, OS, loco-regional progression-free survival (LRPFS), and distant metastasis-free survival (DMFS) were calculated by the Kaplan–Meier method. PFS was calculated from the date of enrollment to the date of disease progression or death for any cause. OS was calculated from the date of enrollment to death for any cause. LRPFS was calculated from the date of enrollment to loco-regional recurrence or the date of death for any cause. DMFS was calculated from the date of enrollment to distant metastasis or death for any cause. The duration of survival was measured from the time of enrollment until death or the last follow up. A two-sided P<0.05 was considered statistically significant.
The 3-year PFS rates for IC resistant and IC sensitivity LANPC were 61.4 % and 83.2 % [7]. According to reference 14, we assumed that our treatment regimen would increase 3-year PFS to 80 %. For a significance level of 0.05 (two-side) with 80 % statistical power and a 10 % drop-out or loss of follow-up, 45 patients were required.
Results
Patients’ characteristics
From May 2015 to July 2020, 56 NPC patients were enrolled in this study. The characteristics of patients were summarized in Table 1.
Table 1.
Characteristics of patients.
| Characteristic | No. of patients (N = 56) | Percent (%) |
|---|---|---|
| Age (years) | ||
| Median 47 (Range 19–68) | ||
| Gender | ||
| Male | 43 | 76.8 |
| Female | 13 | 23.2 |
| T Stage | ||
| 1 | 4 | 7.1 |
| 2 | 12 | 21.4 |
| 3 | 16 | 28.6 |
| 4 | 24 | 42.9 |
| N Stage | ||
| 0 | 2 | 3.6 |
| 1 | 11 | 19.6 |
| 2 | 21 | 37.5 |
| 3 | 22 | 39.3 |
| Total Stage | ||
| III | 14 | 25.0 |
| IVA | 42 | 75.0 |
| PET-CT | ||
| Yes | 34 | 60.7 |
| No | 22 | 39.3 |
Efficacy
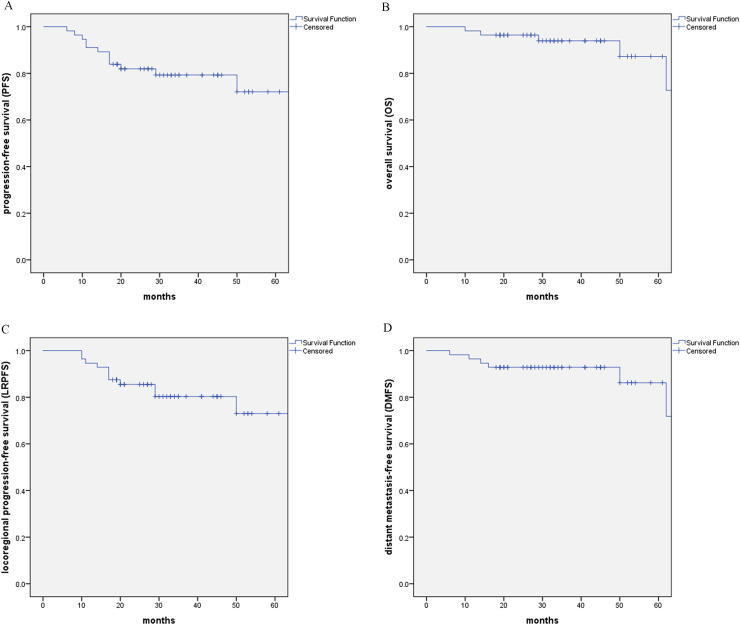
Treatment summary of patients were listed in Table 2. The follow-ups continue until death or the last follow up. With the median follow-up of 34 months (range: 8 to 77 months), 4 patients exhibited nasopharyngeal recurrence, 4 patients developed failure in the neck, 3 patients had distant metastases, and 6 patients died. The 3-year and 5-year PFS and OS rates were 79.3 % (95 % CI: 68.3–90.3) and 72.1 % (95 % CI: 55.2–89.0), 94.0 % (95 % CI: 87.3–100) and 87.2 % (95 % CI: 73.1–100), respectively. The 3-year and 5-year LRPFS and DMFS rates were 80.3 % (95 % CI: 69.1–91.5) and 73.0 % (95 % CI: 56.0–90.1), 92.9 % (95 % CI: 86.2–99.6) and 86.2 % (95 % CI: 72.3–100), respectively. Fig. 1.
Table 2.
Treatment summary of patients.
| Treatment summary | No. of patients (N = 56) | Percent (%) |
|---|---|---|
| Induction chemotherapy | ||
| Docetaxel + Cisplatin | 15 | 26.8 |
| Docetaxel + Nedaplatin | 40 | 71.4 |
| Gemcitabine +Cisplatin | 1 | 1.8 |
| Cycles of IC | ||
| 1 | 2 | 3.6 |
| 2 | 25 | 44.6 |
| 3 | 26 | 46.4 |
| 4 | 3 | 5.4 |
| Cycles of NTZ | ||
| <6 | 23 | 41.1 |
| ≥6 | 33 | 58.9 |
| CCRT | ||
| DDP | 14 | 25.0 |
| NDP | 29 | 51.8 |
| S-1 | 13 | 23.2 |
Fig. 1.
Kaplan–Meier estimate of (A) PFS, (B) OS, (C) LRPFS, and (D) DMFS curves for all the patients.
After IC, efficacy was evaluated by enhanced MRI of the nasopharynx/neck. Response for the nasopharynx in the 56 patients were as follows: CR in 1 patient (1.8 %), PR in 23 patients (41.1 %), SD in 31 patients (55.4 %) and PD in 1 patient (1.8 %). Response for the cervical lymph nodes in the 54 evaluated N1–3 patients were as follows: PR in 19 patients (35.2 %), SD in 33 patients (61.1 %) and PD in 2 patients (3.7 %). After IMRT, response rates evaluated by MRI and nasopharyngoscopy were as follows: ORR of the nasopharynx and cervical lymph nodes involvement were 96.4 % in 56 patients and 96.3 % in 54 patients. ORR of the nasopharynx and cervical lymph nodes involvement were 98.2 % and 98.1 % three months after IMRT.
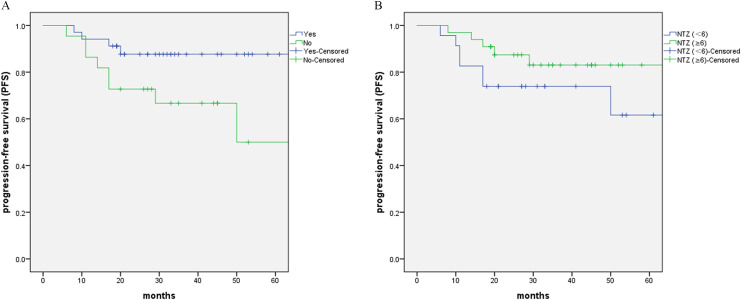
Pretreatment of PET/CT (No. of patients: 34; P = 0.038) was the factor that significantly influenced PFS from univariate analyses. Duration of NTZ ≥6 weeks showed improved 3‐year and 5-year PFS rates compared with NTZ <6 weeks (83.0% vs. 73.9 % and 83.0% vs. 61.6 %, P = 0.191). (Fig. 2. A and B)
Fig. 2.
Kaplan-Meier estimates of PFS (A) between patients with/without pretreatment PET/CT, (B) between patients with NTZ (≥6) and NTZ (<6).
Toxicities
There were no treatment-related deaths. During the IC phase, neutropenia was the most common acute treatment toxicities in our study. 7 (12.5 %), 10 (17.9 %) and 5 (8.9 %) patients had grade 2, 3 and 4 neutropenia, respectively. Among them, one patient had neutropenic fever. 12 (21.4 %) patients had 1–2 hepatic toxicity. The most common nonhematological toxicities were anorexia, nausea, vomiting, and fatigue. Moreover, no renal toxicity or ototoxicity were observed.
During the RT phase, no grade 4 toxicities were observed. The most common hematological toxicities were thrombocytopenia. 10 (17.9 %) and 4 (7.1 %) patients had grade 1 and 2 thrombocytopenia, respectively. Mucositis was the most common nonhematological acute treatment toxicities. Among all the patients, 8 (14.3 %), 36 (64.3 %), and 12 (21.4 %) had grade 1, 2 and 3 mucositis, respectively. None of these patients required tube feeding support. Overall, late injuries were assessed as grades 0 to 1 and xerostomia was the most common late effects. One patient had the second primary rectal cancer.
Discussion
In this study, we reported on the efficacy and safety of a combination of NTZ, an anti-EGFR monoclonal antibody, and CCRT in an unfavorable subgroup of patients who was IC resistant in LANPC. Multivariate analysis identified that chemo-resistance was an independent negative prognostic factor, which may be related in part to the EGFR signaling pathway. [17,18] Our hypothesis was that targeting EGFR pathway could resensitize these chemotherapy-resistant tumor clones. NTZ has shown promising efficacy with minimal toxicity in the treatment of LANPC. We therefore speculated that NTZ might enhance the efficacy of CCRT in IC resistance LANPC. Our study closely met the primary endpoint of 3-year PFS in 79.3 % of 80.0 %.
PFS rates were linked to the total dose of targeted therapy (58.9 % in patients received ≥1200 mg NTZ). Cycles of NTZ ≥6 weeks showed improved 3‐year and 5-year PFS rates compared with NTZ <6 weeks (83.0% vs. 73.9 % and 83.0% vs. 61.6 %) in our study. Similar to Zhao's research [19], patients who received ≥2400 mg nimotuzumab had superior ORR, PFS, and OS in recurrent metastatic NPC. Our results showed that NTZ may be efficacious with CCRT targeting chemoresistance to IC. Moreover, analyses suggested that patients with pretreatment PET/CT (P = 0.038) was the factor that demonstrated significantly inferior survival outcomes. The reasons might be that PET-CT was more sensitive for the detection of distant metastases, and therefore in patients without an initial PET-CT examination the presence of distant metastases could have been underdiagnosed. In addition, pretreatment PET-CT was helpful to determine the extent of primary tumor and positive lymph nodes in NPC. [20,21]
The combination of NTZ-CCRT was well tolerated. The acute toxicities were mainly grade 1/2 hematologic toxicities and severe toxicities were uncommon. During the IC phase, the only major grade 3/4 AE was neutropenia (26.8 %). During the NTZ-CCRT phase, no grade 4 toxicities were observed. The most common hematological toxicities were grade 1–2 thrombocytopenia and mucositis was the most common non-hematological acute treatment toxicities. Consistent with previous studies, NTZ was with minimal toxicity [22]. On the other hand, nedaplatin was used in 71.4 % patients during the IC phase and non-cisplatin-CCRT (nedaplatin or S1) was administrated in 75.0 % patients during the NTZ-CCRT phase. Though cisplatin-based CCRT was considered to be the standard treatment regimen for patients with LANPC, side-effects such as gastrointestinal reactions, nephrotoxicity and ototoxicity had also been well known. Nedaplatin-based CCRT was developed to decrease the toxic effects induced by cisplatin, which represents an alternative doublet treatment strategy to cisplatin-based CCRT for patients with LANPC. [23,24] Also, IMRT combined with S-1 CCRT demonstrated favorable efficacy and mild toxicity in LANPC patients in our previous research. [16] With such a favorable toxicity profile of NTZ-CCRT, could NTZ be used in advance to the start of IC and get better survival? More prospective trials should be considered in the future research.
The limitation of our study was a single-arm design. Given that we exclusively recruited LANPC patients who were evaluated SD or PD after IC, it was a challenge to recruit a larger sample size for a randomized phase II comparison because the rates of SD/PD after IC were only 9.0 %−23.1 %. [6,7] In addition, there was lack of information on the EGFR expression status in our cohort. Though it has been previously reported that EGFR is highly expressed in nearly 85% of NPC patients, more molecular phenotype would support other independent mechanisms likely underpin the treatment efficacy of anti-EGFR antibody. [25] Moreover, there was a relatively short follow-up time in our study, but we had almost achieved our primary endpoints. Long time follow-up was needed to assess late toxicities and survival outcomes.
Conclusions
Combination NTZ-CCRT chemotherapy is efficacious and tolerable in IC resistance LANPC, particularly in patients who received ≥6 weeks NTZ. If this result is confirmed in the phase III trial, it could open avenues for the therapeutic approaches for LANPC.
Funding
This study was supported by the Medical Guidance Project of the Shanghai Science and Technology Commission [number 21Y11900300] and the National Key R&D Program of China [number 2020YFE0205500].
CRediT authorship contribution statement
Xiaoshuang Niu: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Peiyao Liu: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. Xin Zhou: Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing. Dan Ou: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. Xiaoshen Wang: Conceptualization, Data curation, Formal analysis, Funding acquisition, Resources, Writing – review & editing. Chaosu Hu: Conceptualization, Resources, Writing – review & editing.
Declaration of Competing Interest
No conflicts of interest to disclose.
Contributor Information
Xiaoshen Wang, Email: xiaoshen.wang@fdeent.org.
Chaosu Hu, Email: hucsu62@163.com.
References
- 1.Chen Y.P., Chan A., Le Q.T., et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Wong K., Hui E.P., Lo K.W., et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat. Rev. Clin. Oncol. 2021;18(11):679–695. doi: 10.1038/s41571-021-00524-x. [DOI] [PubMed] [Google Scholar]
- 3.Ou X., Zhou X., Shi Q., et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boost. Oncotarget. 2015;6(35):38381–38397. doi: 10.18632/oncotarget.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C., Miao J.J., Hua Y.J., et al. Locoregional control and mild late toxicity after reducing target volumes and radiation doses in patients with locoregionally advanced nasopharyngeal carcinoma treated with induction chemotherapy (IC) followed by concurrent chemoradiotherapy: 10-year results of a phase 2 study. Int. J. Radiat. Oncol. Biol. Phys. 2019;104(4):836–844. doi: 10.1016/j.ijrobp.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y.P., Ismaila N., Chua M., et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J. Clin. Oncol. 2021;39(7):840–859. doi: 10.1200/JCO.20.03237. [DOI] [PubMed] [Google Scholar]
- 6.Peng H., Chen L., Zhang Y., et al. The tumour response to induction chemotherapy has prognostic value for long-term survival outcomes after intensity-modulated radiation therapy in nasopharyngeal carcinoma. Sci. Rep. 2016;6:24835. doi: 10.1038/srep24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S.L., Sun X.S., Yan J.J., et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on induction chemotherapy response. Radiother. Oncol. 2019;137:83–94. doi: 10.1016/j.radonc.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Arteaga C.L., Engelman J.A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J. Clin. Oncol. 2002;20(18 Suppl):1S–13S. [PubMed] [Google Scholar]
- 10.Ciardiello F., Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin. Cancer Res. 2001;7(10):2958–2970. [PubMed] [Google Scholar]
- 11.Chua D.T., Nicholls J.M., Sham J.S., et al. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004;59(1):11–20. doi: 10.1016/j.ijrobp.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Ma B.B., Kam M.K., Leung S.F., et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann. Oncol. 2012;23(5):1287–1292. doi: 10.1093/annonc/mdr401. [DOI] [PubMed] [Google Scholar]
- 13.You R., Hua Y.J., Liu Y.P., et al. Concurrent chemoradiotherapy with or without Anti- EGFR- targeted treatment for stage II- IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and long follow- up. Theranostics. 2017;7(8):2314–2324. doi: 10.7150/thno.19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F., Sun Q., Jiang C., et al. Additional induction chemotherapy to concurrent chemotherapy and intensity-modulated radiotherapy with or without nimotuzumab in first-line treatment for locoregionally advanced nasopharyngeal carcinoma: a propensity score matched analysis. J. Cancer. 2018;9(3):594–603. doi: 10.7150/jca.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ang K.K., Zhang Q., Rosenthal D.I., et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 2014;32(27):2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv T., Wang Y., Ou D., et al. IMRT combined with S-1 concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a prospective phase II study. Invest. New Drugs. 2019;37(2):352–359. doi: 10.1007/s10637-018-00720-0. [DOI] [PubMed] [Google Scholar]
- 17.Wykosky J., Fenton T., Furnari F., et al. Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin. J. Cancer. 2011;30(1):5–12. doi: 10.5732/cjc.010.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talukdar S., Emdad L., Das S.K., et al. EGFR: an essential receptor tyrosine kinase-regulator of cancer stem cells. Adv. Cancer. Res. 2020;147:161–188. doi: 10.1016/bs.acr.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C., Miao J., Shen G., et al. Anti-epidermal growth factor receptor (EGFR) monoclonal antibody combined with cisplatin and 5-fluorouracil in patients with metastatic nasopharyngeal carcinoma after radical radiotherapy: a multicentre, open-label, phase II clinical trial. Ann. Oncol. 2019;30(4):637–643. doi: 10.1093/annonc/mdz020. [DOI] [PubMed] [Google Scholar]
- 20.Chen C., Xu T., Qiu X., et al. Selectively recommend (18)F-FDG PET/CT for patients with de novo nasopharyngeal carcinoma in endemic areas. Radiat. Oncol. 2021;16(1):229. doi: 10.1186/s13014-021-01954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng H., Dong D., Fang M.J., et al. Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin. Cancer Res. 2019;25(14):4271–4279. doi: 10.1158/1078-0432.CCR-18-3065. [DOI] [PubMed] [Google Scholar]
- 22.Zhai R.P., Ying H.M., Kong F.F., et al. Experience with combination of nimotuzumab and intensity-modulated radiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Onco. Target. Ther. 2015;8:3383–3390. doi: 10.2147/OTT.S93238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang L.Q., Chen D.P., Guo L., et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19(4):461–473. doi: 10.1016/S1470-2045(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 24.Tang Q.N., Liu L.T., Qi B., et al. Effect of concurrent chemoradiotherapy with nedaplatin vs cisplatin on the long-term outcomes of survival and toxic effects among patients with stage II to IVB nasopharyngeal carcinoma: a 5-year follow-up secondary analysis of a randomized clinical trial. JAMA Netw. Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.38470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X.S., Liang R.B., Zhu X.D. Anti-EGFR therapies in nasopharyngeal carcinoma. Biomed. Pharmacother. 2020 Nov;131 doi: 10.1016/j.biopha.2020.110649. [DOI] [PubMed] [Google Scholar]