Abstract
An analytical methodology was developed to quantitate the intracellular nucleotides including mono-, di-, and triphosphates and the diphosphocholine derivative of (−)-2′,3′-deoxy-3′-thiacytidine (3TC) in human peripheral blood mononuclear cells (PBMCs). The procedure includes the resolution of 3TC nucleotides by solid-phase extraction (SPE) on an anion-exchange cartridge, with subsequent enzyme digestion of the resulting phosphates to the parent drug that is ultimately quantitated by high-performance liquid chromatography with UV detection (HPLC-UV). Validation was performed with PBMCs from healthy donors exposed to [3H]3TC, leading to the formation of intracellular nucleotides that were quantitated by anion-exchange HPLC with radioactive detection (HPLC-RA). These nucleotide levels served as reference values and were used for cross-validation with data obtained by HPLC-UV. An excellent correlation was established between the results obtained by HPLC-RA and those obtained by HPLC-UV, with a slope of the regression lines close to unity and intercepts near nullity as well as a correlation coefficient close to unity for all 3TC phosphates. The assay was characterized by a limit of quantitation below 1 ng (amount on column) with a precision (percentage of coefficient of variation of repeated measurement) ranging from 0.8 to 18.1% and an accuracy (deviation of the amount determined by HPLC-UV from the nominal reference value) varying from −14.8 to 19.4%. This methodology was successfully applied to determine the quantity of 3TC nucleotides in PBMCs of a patient infected with human immunodeficiency virus after oral administration of 3TC and stavudine.
2′,3′-Dideoxynucleoside analogs are a major class of antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV) infections. 3′-Azido-3′-deoxythymidine (zidovudine [ZDV]) was the first approved nucleoside analog, followed by 2′,3′-dideoxyinosine (didanosine [ddI]), 2′,3′-dideoxycytidine (zalcitabine), 2′,3′-didehydro-3′-deoxythymidine (stavudine [d4T]), and (−)-2′,3′-dideoxy-3′-thiacytidine (3TC) (2). While the parent drugs are not active, these compounds are anabolized in the host cells by multiple kinases to their respective 5′-mono- (MP), di- (DP), and triphosphate (TP) derivatives. The triphosphates are competitive inhibitors of HIV reverse transcriptase (RT) and can also be incorporated into the viral genome causing DNA chain termination. Although these nucleoside RT inhibitors (NRTIs) have the same mechanism of action, they differ substantially in anti-HIV activity—cytotoxicity as well as viral resistance pattern (19, 20).
Anti-HIV therapy was initiated with ZDV monotherapy but now consists of triple-drug combinations including NRTIs, nonnucleoside RT inhibitors, and HIV protease inhibitors. Major clinical trials have demonstrated an enhanced antiviral efficacy with reduced toxicity for appropriate combinations (5). So far, most clinical pharmacokinetic and drug-drug interaction studies have been performed with data from plasma or serum. While a relationship between plasma pharmacokinetics and antiviral activity and/or toxicity has recently been suggested for non-nucleoside RT inhibitors (9, 12) and HIV protease inhibitors (11, 16), the only NRTI for which such a correlation has been demonstrated is ddI (7). A relationship between ddI plasma PK and the suppression of HIV p24 antigen has previously been shown in patients (7). Since NRTIs require intracellular activation, it has previously been hypothesized that the intracellular level of the active 5′-TP metabolite of NRTIs might be a better predictor of virologic effects (8, 25, 28, 30). This hypothesis has recently been proven by the demonstration that intracellular concentrations of the active 5′-TP metabolite of 3TC and d4T, rather than levels of unchanged drugs in plasma, correlated with virologic response in HIV-infected patients (22, 23). In addition to the triphosphate derivatives, kinetic studies of the other intracellular anabolites should provide a better understanding of the different steps involved in the activation of NRTIs and of the contribution of these intermediate anabolites to antiviral effects and/or toxicity. Recent in vitro data have suggested that accumulation of intracellular ZDV-MP may lead to cytotoxicity associated with ZDV (1, 26).
Cellular pharmacology of NRTIs has primarily been investigated in vitro in target cells such as peripheral blood mononuclear cells (PBMCs) with radiolabeled drugs. Data from these studies, however, cannot always be reliably extrapolated to situations with HIV-infected patients. Moreover, radiolabeled drugs are not recommended in large-scale clinical trials. Development of novel analytical methodologies capable of measuring intracellular phosphates of nonradioactive NRTIs is therefore a high priority. Analytical methods published so far have focused only on the determination of intracellular nucleotides of ZDV, the first available anti-HIV drug (10, 14, 15, 18, 27). These methods can be classified into two major categories, enzymatic assay and chromatographic separation followed by enzyme digestion and radioimmunoassay, respectively. The first procedure was based on the inhibition by ZDV-TP of HIV RT activity (14). This method, although sensitive, was limited to quantitating ZDV-TP. By the second methodology, the separation processes of ZDV-MP, -DP, and -TP were critical and were initially performed by strong anion-exchange high-performance liquid chromatography (HPLC), which was highly selective but time-consuming and hence difficult to apply practically to a large number of samples (18). The method of separation was recently improved by the use of anion-exchange solid-phase extraction (SPE), a procedure that is preferred for large clinical trials, since multiple samples can be simultaneously processed (15).
3TC is a novel synthetic 2′,3′-dideoxynucleoside analog which has demonstrated a potent in vitro antiretroviral activity against both HIV type 1 (HIV-1) and HIV-2 isolates (4, 17), including ZDV-resistant strains (24), and inhibition of human hepatitis B virus replication (3). 3TC has also been shown to be much less toxic towards human bone marrow cells compared to nucleoside analogs currently used in HIV therapy (21). In addition, a high selectivity was observed in liver cells, which represent a critical target site for anti-hepatitis drugs (6). Recent clinical trials evaluating the anti-HIV and anti-hepatitis B virus effects of 3TC have confirmed these in vitro findings, with an excellent safety profile and without major dose-limiting toxicity at doses ranging from 0.5 to 20 mg/kg of body weight per day (13).
In this study we describe an SPE procedure combined with an HPLC assay with UV detection to measure intracellular 3TC nucleotides, including its MP, DP, TP, and DP-choline derivatives in human PBMCs. Assay performance was evaluated. This method was successfully applied to determine the quantity of 3TC phosphates in PBMCs isolated from an HIV-infected patient receiving an oral administration of the drug in combination with d4T.
MATERIALS AND METHODS
Chemicals.
Nonlabeled 3TC and authentic standards of 3TC-5′-phosphates (3TC-TP and 3TC-MP) were kindly provided by R. Schinazi (Emory University, Atlanta, Ga.). [methyl-3H]3TC (12 Ci/mmol) was purchased from Moravek Biochemicals (Brea, Calif.) and was more than 98% pure as ascertained by the HPLC methods described below. Alkaline phosphatase (3.100 U/mg of protein) and phosphodiesterase I (31.0 U/mg [dry weight]), for enzyme digestion, were purchased from Worthington Biochemical Corporation (Freehold, N.J.). HPLC-grade potassium phosphate monobasic, orthophosphoric acid (85%), and methanol were obtained from Fisher Scientific (Fair Lawn, N.J.). All other chemicals used were of analytical grade.
Cell culture and incubation.
Human PBMCs were isolated from whole blood of healthy donors. Briefly, blood was diluted with an equal volume of phosphate-buffered saline (PBS) and laid onto a Ficoll-Histopaque gradient in 50-ml conical tubes. After centrifugation at 500 × g for 30 min, the layer containing PBMCs was carefully recovered and washed three times with PBS. Isolated PBMCs were then suspended in RPMI 1640 medium supplemented with 20% fetal bovine serum, 1% penicillin-streptomycin, and 1% l-glutamine and stimulated 48 h with phytohemagglutinin at a final concentration of 10 μg/ml. All cultures were maintained at 37°C under an atmosphere of 5% CO2.
After stimulation, cells were resuspended in phytohemagglutinin-free medium at a cytocrit of 2 × 106 cells/ml. Isotopic preparations (specific activity, 200 dpm/pmol) of 3TC were added to the culture at final concentrations of 5 and 10 μM and incubated 24 h. The final incubation volume was 50 ml. Following incubation, cells were pelleted by centrifugation, rinsed three times with cold PBS, and extracted with 2 × 4 ml of 60% methanol at −70°C overnight. Cellular debris was then removed by centrifugation at 2,000 × g for 10 min. The resulting supernatant, containing 3TC nucleotides, was collected and stored as 500-μl aliquots at −70°C until analysis. These aliquots were to serve as validation samples.
Prior to analysis, methanol was evaporated under a gentle nitrogen flow, and the volume of the remaining aqueous phase was carefully adjusted to 200 μl with deionized water. This phase was then divided into one portion of 180 μl and one portion of 20 μl. The 20-μl portion was counted to assess total radioactivity. The 180-μl portion was to be analyzed by anion-exchange HPLC, as described later in this section, to directly quantitate intracellular levels of 3TC derivatives based on the radioactivity of each peak and the specific activity. Usually, five aliquots were processed simultaneously, and the mean levels of 3TC phosphates obtained by this method served as reference values. Typically, based on these reference levels, the volume of the remaining aliquots was adjusted by division or combination so that a range of levels of 3TC phosphates could be covered. These aliquots were subjected to anion-exchange SPE followed by enzyme digestion to hydrolyze 3TC phosphates. Fractions containing 3TC were subsequently quantitated by reverse-phase HPLC with UV detection (HPLC-UV) as previously reported (29).
PBMCs from an HIV-infected patient treated with 3TC.
This methodology was applied to measure levels of 3TC phosphates in an HIV-infected patient enrolled in the ALTIPHAR study (22). This study was designed to evaluate the pharmacologic mechanisms underlying the differences in virologic response of antiretroviral naïve versus experienced (mainly long-term ZDV therapy) patients to a combination of 3TC and d4T 24 weeks after the initiation of therapy. After giving their written informed consent, 19 patients were enrolled in the study and received the combination therapy at standard doses of 3TC (150 mg twice daily) and d4T (40 mg twice daily). This study was approved by the Institutional Review Board of the Hôpital Pitié-Salpetrière, Paris, France. At least 15 ml of blood was drawn into two Vacutainer CPT cell preparation tubes (Becton Dickinson, Franklin Lakes, N.J.) prior to and at 2, 4, 6, and 10 h after oral administration of 3TC. The tubes were centrifuged at 1,500 × g for 20 min at room temperature. The upper layer, representing plasma and PBMCs, was recovered and centrifuged at 500 × g for 10 min to pellet the cells. Plasma was removed, and 200 μl of 60% methanol was added to the PBMCs. The samples were shipped frozen in dry ice to our institution and stored at −70°C until analyzed.
Anion-exchange SPE.
SPE was performed with anion-exchange cartridges (Sep-Pack VAC [100-mg phase]; Waters, Milford, Mass.). Cartridges were preconditioned with 500 μl of deionized water. Cell extracts of validation or patients’ samples were loaded onto the cartridge and eluted under reduced pressure. The cartridge was then washed twice with water (200 and 500 μl). These fractions representing the unchanged nucleoside were combined, and the cartridge was rinsed with 500 μl of water and 200 μl of 20 mM KCl. The nucleotides of 3TC, including its DP-choline, MP, DP, and TP, were successively resolved with a KCl gradient. Briefly, 3TC-DP-choline was eluted with 300 μl of 60 mM KCl, and the cartridge was rinsed with 100 μl of the buffer. The 3TC-MP, -DP, and -TP were eluted with 400 μl of 100 mM KCl, 500 μl of 120 mM KCl, and 500 μl of 400 mM KCl, respectively. The cartridge was rinsed with 100 μl of the corresponding buffer between each step.
Following SPE, the purity of the unchanged drug and each derivative was checked by anion-exchange HPLC analysis as described below.
Enzyme digestion.
The phosphates of 3TC resolved from the SPE step were then subjected to enzyme digestion to free the nucleoside. Fractions containing the phosphates were incubated with alkaline phosphatase (50 U/fraction) at 37°C overnight. Phosphodiesterase (1 U/fraction) was added to the fraction containing 3TC-DP-choline in addition to alkaline phosphatase. Following digestion, acetonitrile (3 volumes) was added to precipitate proteins. Supernatant was recovered after centrifugation and dried under nitrogen. The residue was dissolved in 150 μl of water and analyzed by reverse-phase HPLC.
Anion-exchange HPLC with radioactive detection (HPLC-RA).
Validation samples were analyzed by HPLC with a model 1090M chromatograph (Hewlett-Packard Company, Palo Alto, Calif.) equipped with an automatic injector and a diode array detector. Anion-exchange HPLC was performed with a 4.6 × 250 mm Partisil-10-μm SAX column (Jones Chromatography, Lakewood, Colo.) with a 65-min linear gradient of potassium phosphate buffer (pH 3.5) from 8 mM to 1 M starting at 10 min. For qualitative analysis such as purity assessment, eluent from the column was directed to a model 525TR Flo-one online radioactivity detector (Packard Instrument Company, Inc., Meriden, Conn.), with a 3:1 flow ratio of scintillation liquid to HPLC buffer. For quantitative determination, eluent from the column was fractionated at 1-min intervals with a RediFrac fraction collector (Pharmacia LKB, Uppsala, Sweden). After the addition of 5 ml of scintillation fluid, the vials were counted with a LS 5000 TA scintillation counter (Beckman Instruments, Inc., Fullerton, Calif.). Intracellular levels of 3TC and its derivatives were calculated based on the radioactivity of each peak and the specific activity. These levels served as references to validate those quantitated by HPLC-UV.
HPLC-UV.
Fractions representing the DP-choline, MP, DP, and TP derivatives of 3TC, obtained after SPE and enzyme digestion, were analyzed by reverse-phase HPLC with the Hewlett-Packard 1090 liquid chromatography system as previously described (29). Briefly, portions (150 μl each) of the reconstituted dry residue were injected. 3TC was isocratically chromatographed on a reverse-phase C18 column (Columbus [5-μm particle size, 4.6 by 250 mm]; Phenomenex, Inc., Torrance, Calif.) with a mixture of phosphate buffer (43 mM [pH 7.0])-methanol (90/10 [vol/vol]) and monitored at 280 nm. The lower limit of quantitation was 1 ng (amount on column). Standards ranging from 1 to 100 ng (amount on column) were processed in the same way as the samples, including SPE and enzyme digestion. Standard curve parameters were obtained from an unweighted least-squares linear regression analysis of the standard concentrations as a function of peak area. Unknown concentrations were calculated by interpolation with each observed peak area and standard curve parameters. Intra- and interday variation of the assay determined by using the calibration standards was less than 15% (29).
Data analysis.
The data were analyzed by using two statistical tests. First, for each derivative, results obtained from HPLC-RA and HPLC-UV were correlated by using least-squares linear regression analysis. Coefficient of correlation (r2), intercept, and slope were calculated. The unity of the slope and the nullity of the intercept of the regression lines were ascertained by Student’s t test. In addition, bias, as defined by the mean percentage of deviation of HPLC-UV from HPLC-RA for each 3TC nucleotide, was calculated. Second, the ratio of the paired levels obtained from the two methods was calculated and compared with unity (the expected value) by using Student’s t test.
Intracellular pharmacokinetic analysis.
Intracellular pharmacokinetics of the active metabolite of 3TC, 3TC-TP, were characterized by its half-life, average levels over the sampling period (10 h), and the area under the time curve for intracellular levels from 0 to 10 h (AUC0–10). Intracellular half-life of 3TC-TP was estimated by using the slope (λ) of the terminal linear phase as 0.693/λ, where λ was obtained by using linear least-squares regression analysis, as implemented with the pharmacokinetic software SIPHAR (Simed, Creteil, France), with 1/x2 as the weighting factor. Intracellular AUC was calculated according to the trapezoidal rule.
RESULTS AND DISCUSSION
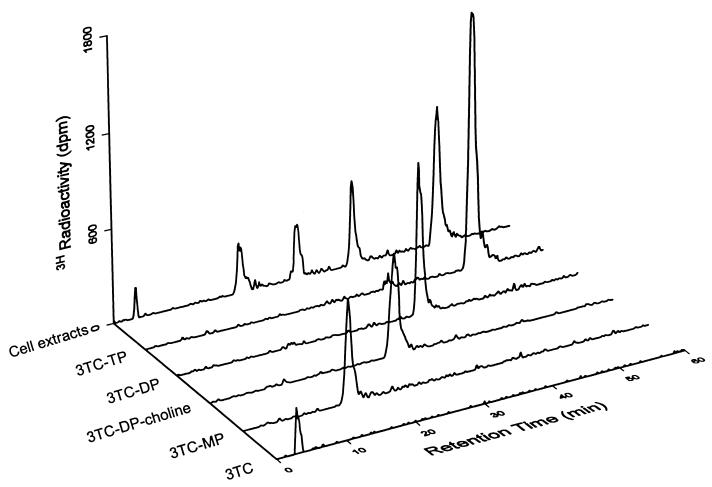
The assay validation strategy included the use of PBMCs from healthy human donors as an in vitro approach to produce intracellular 3TC nucleotides. Concentrations of 5 and 10 μM 3TC were physiologically relevant and expected to lead to levels of intracellular formation of 3TC phosphates comparable to those obtained in 3TC-treated patients. The levels of the intracellular derivatives were then measured by anion-exchange HPLC-RA and served as references to validate the nonradioactive HPLC assay with UV detection. Figure 1 depicts a typical phosphorylation profile of 3TC in human PBMCs, illustrating the formation of its MP, DP, TP, and DP-choline derivatives. The identity of 3TC-MP and -TP was confirmed by peak retention time of authentic standards and enzyme digestion for the original nucleoside. Since authentic standards for 3TC-DP and DP-choline were not available, the identities of these anabolites were assessed by their retention times relative to those of 3TC-MP and -TP and by enzyme digestion with alkaline phosphatase for the diphosphate and alkaline phosphatase plus phosphodiesterase for the DP-choline derivative.
FIG. 1.
Intracellular phosphorylation profile of 3TC in human PBMCs and purity check by anion-exchange HPLC-RA of the 3TC nucleotides resolved by anion-exchange SPE.
Purity of 3TC nucleotides after anion-exchange SPE.
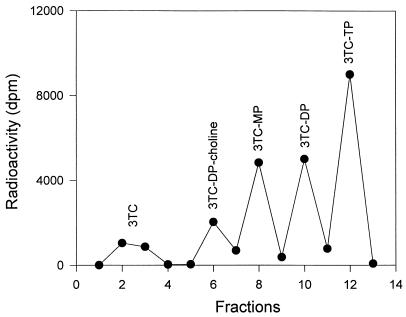
Following SPE, the five fractions representing unchanged 3TC and its MP, DP, TP, and DP-choline derivatives were separately injected onto the anion-exchange HPLC and checked for purity. As shown in Fig. 1, only a single peak was obtained in each case. This peak was identified as the expected phosphate by comparing peak retention time, indicating that the SPE procedure led to the resolution of pure 3TC nucleotides. Figure 2 shows a representative SPE profile of 3TC phosphates. Of note, the order of elution from the anion-exchange HPLC was 3TC, -MP, DP-choline, -DP, and -TP (Fig. 1). The order of elution of MP and DP-choline was reversed by anion-exchange SPE (Fig. 2). This phenomenon may be explained by the differences in analyte retention between the two types of columns.
FIG. 2.
Separation of the 3TC phosphates from human PBMCs by anion-exchange SPE.
Recovery of 3TC nucleotides.
The overall recovery of 3TC nucleotides was assessed by comparing the amount of each phosphate as measured by reverse-phase HPLC-UV to that determined by direct anion-exchange HPLC-RA. Authentic replicates (n = 3 to 5) of the aliquots of cell extracts obtained after exposure of human PBMCs to 5 and 10 μM 3TC were analyzed either by HPLC-RA or by HPLC-UV following SPE and enzyme digestion. Data obtained by direct HPLC-RA analysis were considered to represent 100% recovery (by definition), and the overall recovery of 3TC nucleotides measured by HPLC-UV after SPE and enzyme digestion was calculated as the ratio. Results are presented in Table 1. The recovery of 3TC nucleotides was high, ranging from 85.2 to 119.4%.
TABLE 1.
Recovery of 3TC nucleotides following anion-exchange SPE and enzyme digestion
| Nucleotide | 5 μM concn
|
10 μM concn
|
||
|---|---|---|---|---|
| n | Mean ± SD of recovery (%) | n | Mean ± SD of recovery (%) | |
| 3TC | 5 | 91.3 ± 2.7 | 4 | 86.0 ± 0.7 |
| 3TC-DP-choline | 5 | 99.8 ± 40.3 | 3 | 119.4 ± 1.3 |
| 3TC-MP | 5 | 94.2 ± 13.8 | 4 | 85.2 ± 7.1 |
| 3TC-DP | 5 | 94.3 ± 11.8 | 4 | 105.8 ± 11.1 |
| 3TC-TP | 5 | 99.7 ± 30.2 | 3 | 93.6 ± 19.6 |
Standard curve.
Due to the unavailability of some of the standard 3TC phosphates, a reference curve could not be set up for each of the nucleotides. Therefore, the amounts of all 3TC derivatives were derived from a standard curve established with unchanged drug, assuming consistent recovery among the nucleotides. Cell extracts equivalent to 20 × 106 PBMCs from healthy human donors were spiked with increasing amounts of 3TC and processed the same way as the validation samples. After SPE and enzyme digestion, portions of the standard samples were analyzed by HPLC-UV. A standard curve from 1 to 100 ng (amount on column) of 3TC was routinely used. The limit of quantitation was 1 ng on the column, which was lower than the limit of quantitation of 2 ng achieved with human serum samples (29), presumably due to the fact that less interference with endogenous substances was present in PBMCs. The ranges of 3TC phosphates observed in the validation samples were 1.3 to 20.0, 2.0 to 28.6, 2.6 to 30.0, and 1.0 to 11.0 ng for the TP, DP, MP, and DP-choline derivatives, respectively.
Precision and accuracy.
Assay precision (coefficient of variation) and accuracy (deviation of HPLC-UV results from those by HPLC-RA) were assessed with the validation samples at two concentrations for each derivative. Results are presented in Table 2. Assay performance was characterized by using a coefficient of variation ranging from 0.8 to 18.1% and a deviation from −14.8 to 19.4% across the 3TC nucleotides.
TABLE 2.
Data for assay performance
| Nucleotide | HPLC-RA
|
HPLC-UV
|
|||
|---|---|---|---|---|---|
| Nominal amt (ng) | n | Measured amt (ng) | Deviation (%) | Coefficient of variation (%) | |
| 3TC-TP | 3.7 | 5 | 3.6 | −0.3 | 13.6 |
| 8.5 | 3 | 8.0 | −6.4 | 12.3 | |
| 3TC-DP | 20.1 | 5 | 19.0 | −5.7 | 5.6 |
| 25.2 | 4 | 26.6 | 5.8 | 5.2 | |
| 3TC-MP | 11.4 | 5 | 10.8 | −5.8 | 6.7 |
| 18.0 | 4 | 15.4 | −14.8 | 4.2 | |
| 3TC-DP-choline | 3.4 | 5 | 3.3 | −0.2 | 18.1 |
| 2.4 | 3 | 2.9 | 19.4 | 0.8 | |
Statistical analyses.
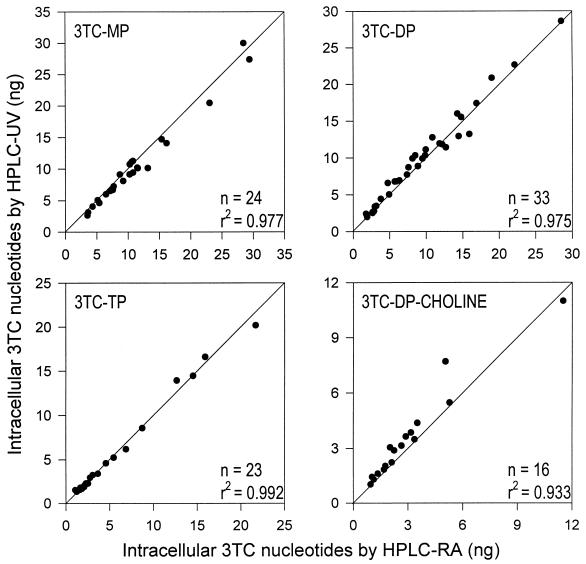
Least-squares linear regression analysis was used to evaluate the correlation between the amount of 3TC phosphates obtained by HPLC-UV after SPE and that determined by direct HPLC-RA (reference method). As depicted in Fig. 3, the two methodologies led to nearly identical results, with squared coefficients of correlation between 0.933 and 0.992. Bias, defined as the mean percentage of deviation of HPLC-UV results from HPLC-RA data used in the correlation analysis, varied from −7.7 to 19.3% across all 3TC nucleotides. The parameters of the regression equations are summarized in Table 3. For all 3TC phosphates, regression lines were characterized by a slope close to unity and an intercept near zero ascertained by t test (Table 3). In order to confirm these results, the ratio of the paired data (HPLC-UV to HPLC-RA) for each phosphate was calculated. The latter, with a mean value ranging from 0.92 to 1.20, should be unity if the two analytical approaches give the same results. Indeed, Student’s t test indicated no statistically significant difference between the mean ratio and unity for any 3TC phosphate (P = 0.24 to 0.94).
FIG. 3.
Least-squares linear regression analysis of the correlation between levels of 3TC nucleotides determined by anion-exchange HPLC-RA (reference method) and those quantitated by HPLC-UV following anion-exchange SPE and enzyme digestion, respectively. The solid lines are lines of identity.
TABLE 3.
Comparison of data obtained by HPLC-RA and HPLC-UV by regression analysis
| Intracellular nucleotide | Results of linear regression analysisa
|
Results of t test
|
Average bias of HPLC-UV vs HPLC-RA (% deviation) | ||||
|---|---|---|---|---|---|---|---|
| Slope | 95% CI | Intercept | 95% CI | P (slope vs 1) | P (intercept vs 0) | ||
| 3TC-TP | 0.98 | 0.94–1.02 | 0.05 | −0.25–0.35 | 0.3 | 0.7 | −1.2 |
| 3TC-DP | 0.98 | 0.93–1.04 | 0.64 | −0.01–1.30 | 0.5 | 0.1 | 8.0 |
| 3TC-MP | 0.97 | 0.91–1.03 | −0.42 | −1.28–0.44 | 0.5 | 0.3 | −7.7 |
| 3TC-DP-choline | 0.98 | 0.83–1.21 | 0.57 | 0.01–1.13 | 0.5 | 0.1 | 19.3 |
CI, confidence interval.
Application to biological samples.
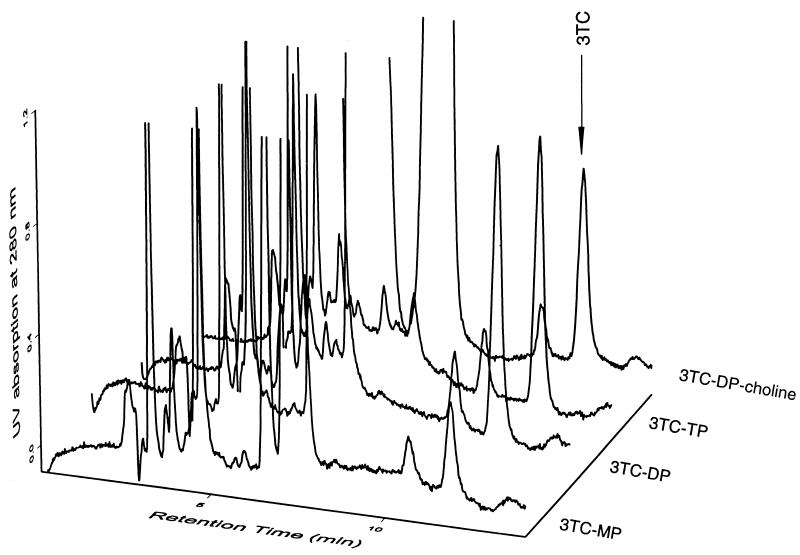
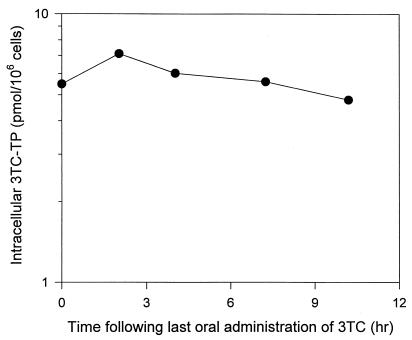
By using this new combined HPLC and SPE approach, levels of 3TC nucleotides were determined in the PBMCs of an HIV-infected patient on a 150-mg oral regimen of the drug twice daily as part of the ALTIPHAR study. Five blood samples were obtained up to 10 h after drug dosing, and PBMCs were isolated and processed as described above. Representative chromatograms for 3TC phosphates from the 2-h PBMC sample of this patient are shown in Fig. 4. Measurements of these anabolite levels were 0.61 (2.65), 0.68 (2.95), 0.20 (0.85), and 0.48 (2.11) ng/106 cells (pmol/106 cells) for 3TC-TP, -DP, -MP, and -DP-choline, respectively. The intracellular time course of the active 3TC-TP in this patient is depicted in Fig. 5. The 3TC-TP exhibited a long intracellular half-life of 15.5 h (95% confidence interval, 14.9 to 16.1 h) in that patient. The average intracellular level of 3TC-TP from the above-described kinetics was 1.33 ± 0.19 ng/106 cells (mean ± standard deviation) or 5.79 ± 0.85 pmol/106 cells. Intracellular AUC0–10, considered as a measure of exposure, was estimated to be 13.7 ng/106 cells × h or 59.8 pmol/106 cells × h.
FIG. 4.
Chromatographic profiles of the 3TC nucleotides present in a 2-h PBMC sample from a patient receiving 150 mg of the indicated drug orally twice a day. The HPLC chromatograms were obtained by UV detection following anion-exchange SPE and enzymatic digestion.
FIG. 5.
Time course of intracellular 3TC-TP in a patient receiving 150 mg of 3TC twice a day orally.
In summary, an analytical methodology combining SPE and HPLC was developed and validated for the quantitation of intracellular phosphates of 3TC. While this methodology is rather complex and only measures nucleotides after anion-exchange SPE and enzyme digestion indirectly, it has successfully been applied to quantitate intracellular 3TC phosphates in HIV-infected patients. This new methodology should allow the determination of intracellular pharmacokinetics of the active triphosphate and other anabolites of NRTIs, which will be critical to the establishment of a reliable pharmacokinetic-pharmacodynamic relationship in anti-HIV therapy and, it is hoped, to the design of optimal combination regimens involving antiviral nucleoside analogs.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants.
We thank Christine Katlama and Marc Valentin, of Hôpital Pitié-Salpetrière, Paris, France, for providing samples from an HIV-infected patient who was being treated with 3TC.
REFERENCES
- 1.Bridges E G, Faraj A, Sommadossi J P. Inhibition of mammalian DNA polymerase-associated 3′ to 5′ exonuclease activity by 5′-monophosphates of 3′-azido-3′-deoxythymidine. Biochem Pharmacol. 1993;45:1571–1576. doi: 10.1016/0006-2952(93)90296-9. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. JAMA. 1996;276:146–154. [PubMed] [Google Scholar]
- 3.Chang C N, Doong S L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C H, Cheng Y C, Liotta D, Schinazi R F. Deoxycytidine deaminase-resistant stereoisomer is the active form of (+/−)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. [PubMed] [Google Scholar]
- 4.Coates J A V, Cammack N, Jenkinson H J, Mutton I M, Pearson B A, Storer R, Cameron J M, Penn C R. The separated enantiomers of 2′-deoxy-3′-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother. 1992;36:202–205. doi: 10.1128/aac.36.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collier A C. Efficacy of combination antiretroviral therapy. Adv Exp Med Biol. 1996;394:355–372. doi: 10.1007/978-1-4757-9209-6_33. [DOI] [PubMed] [Google Scholar]
- 6.Cui L, Yoon S, Schinazi R F, Sommadossi J P. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-1-beta-d-arabinofuranosyl)-5-iodouracil in human liver cells. J Clin Investig. 1995;95:555–563. doi: 10.1172/JCI117698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drusano G L, Yuen G L, Lambert J S, Seidlin M, Dolin R, Valentine F T. Relationship between dideoxyinosine exposure, CD4 counts, and p24 antigen levels in human immunodeficiency virus infection. A phase I trial. Ann Intern Med. 1992;116:562–566. doi: 10.7326/0003-4819-116-7-562. [DOI] [PubMed] [Google Scholar]
- 8.Gao W Y, Shirasaka T, Johns D G, Broder S, Mitsuya H. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J Clin Investig. 1993;91:2326–2333. doi: 10.1172/JCI116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havlir D, Cheeseman S H, McLaughlin M, Murphy R, Erice A, Spector S A, Greenough T C, Sullivan J L, Hall D, Myers M, Lamson M, Richman D D. High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J Infect Dis. 1995;171:537–545. doi: 10.1093/infdis/171.3.537. [DOI] [PubMed] [Google Scholar]
- 10.Kuster H, Vogt M, Joos B, Nadai V, Luthy R. A method for the quantification of intracellular zidovudine nucleotides. J Infect Dis. 1991;164:773–776. doi: 10.1093/infdis/164.4.773. [DOI] [PubMed] [Google Scholar]
- 11.Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper P J, Mo H M, Markowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman G R, Ho D D, Boucher C A, Leonard J M, Norbeck D W, Kempf D J. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 12.Murphy, R., J. P. Sommadossi, M. Lamson, P. Gagnier, D. Hall, M. Myers, and A. Dusek. Antiviral effect and pharmacokinetic interaction between nevirapine and indinavir in HIV-1 infected individuals. Submitted for publication. [DOI] [PubMed]
- 13.Perry C M, Faulds D. Lamivudine—a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV. Drugs. 1997;53:657–680. doi: 10.2165/00003495-199753040-00008. [DOI] [PubMed] [Google Scholar]
- 14.Robbins B L, Rodman J, McDonald C, Srinivas R V, Flynn P M, Fridland A. Enzymatic assay for measurement of zidovudine triphosphate in peripheral blood mononuclear cells. Antimicrob Agents Chemother. 1994;38:115–121. doi: 10.1128/aac.38.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins B L, Waibel B H, Fridland A. Quantitation of intracellular zidovudine phosphates by use of combined cartridge-radioimmunoassay methodology. Antimicrob Agents Chemother. 1996;40:2651–2654. doi: 10.1128/aac.40.11.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schapiro J M, Winters M A, Stewart F, Efron B, Norris J, Kozal M J, Merigan T C. The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann Intern Med. 1996;124:1039–1050. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Schinazi R F, Chu C K, Peck A, McMillan A, Mathis R, Cannon D, Jeong L-S, Beach J W, Choi W-B, Yeola S, Liotta D C. Activities of the four optical isomers of 2′,3′-dideoxy-3′-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrob Agents Chemother. 1992;36:672–676. doi: 10.1128/aac.36.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slusher J T, Kuwahara S K, Hamzeh F M, Lewis L D, Kornhauser D M, Lietman P S. Intracellular zidovudine (ZDV) and ZDV phosphates as measured by a validated combined high-pressure liquid chromatography-radioimmunoassay procedure. Antimicrob Agents Chemother. 1992;36:2473–2477. doi: 10.1128/aac.36.11.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommadossi J P. Nucleoside analogs: similarities and differences. Clin Infect Dis. 1993;16:S7–S15. doi: 10.1093/clinids/16.supplement_1.s7. [DOI] [PubMed] [Google Scholar]
- 20.Sommadossi, J. P. 1997. Pharmacology of anti-HIV therapies. Antiviral Ther. 2(Suppl. 3):55–60.
- 21.Sommadossi J P, Schinazi R F, Chu C K, Xie M Y. Comparison of cytotoxicity of the (−)- and (+)-enantiomer of 2′,3′-dideoxy-3′-thiacytidine in normal human bone marrow progenitor cells. Biochem Pharmacol. 1992;44:1921–1925. doi: 10.1016/0006-2952(92)90093-x. [DOI] [PubMed] [Google Scholar]
- 22.Sommadossi J P, Valentin M A, Zhou X J, Xie M Y, Moore J, Calvez V, Desa M, Katlama C. Program and abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Intracellular phosphorylation of stavudine (d4T) and 3TC correlates with their antiviral activity in naïve and zidovudine (ZDV)-experienced HIV-infected patients, abstr. 262; p. 146. [Google Scholar]
- 23.Sommadossi J P, Zhou X J, Moore J, Havlir D V, Friedland G, Tierney C, Smeaton L, Fox L, Richman D, Pollard R the National Institute of Allergy and Infectious Diseases ACTG 290 investigators. Program and Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Impairment of stavudine (d4T) phosphorylation in patients receiving a combination of zidovudine and d4T (ACTG 290), abstr. 3; p. 79. [Google Scholar]
- 24.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tornevik Y, Jacobsson B, Britton S, Eriksson S. Intracellular metabolism of 3′-azidothymidine in isolated human peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 1991;7:751–759. doi: 10.1089/aid.1991.7.751. [DOI] [PubMed] [Google Scholar]
- 26.Tornevik Y, Ullman B, Balzarini J, Wahren B, Eriksson S. Cytotoxicity of 3′-azido-3′-deoxythymidine correlates with 3′-azidothymidine-5′-monophosphate (AZTMP) levels, whereas anti-human immunodeficiency virus (HIV) activity correlates with 3′-azidothymidine-5′-triphosphate (AZTTP) levels in cultured CEM T-lymphoblastoid cells. Biochem Pharmacol. 1995;49:829–837. doi: 10.1016/0006-2952(94)00453-s. [DOI] [PubMed] [Google Scholar]
- 27.Toyoshima T, Kimura S, Muramatsu S, Takahagi H, Shimada K. A sensitive nonisotopic method for the determination of intracellular azidothymidine 5′-mono-, 5′-di-, and 5′-triphosphate. Anal Biochem. 1991;196:302–307. doi: 10.1016/0003-2697(91)90470-e. [DOI] [PubMed] [Google Scholar]
- 28.Yarchoan R, Mitsuya H, Thomas R V, Pluda J M, Hartman N R, Perno C F, Marczyk K S, Allain J P, Johns D G, Broder S. In vivo activity against HIV and favorable toxicity profile of 2′,3′-dideoxyinosine. Science. 1989;245:412–415. doi: 10.1126/science.2502840. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X J, Sommadossi J P. Rapid quantitation of (−)-2′-deoxy-3′-thiacytidine in human serum by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B. 1997;691:417–424. doi: 10.1016/s0378-4347(96)00467-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Z, Ho H T, Hitchcock M J, Sommadossi J P. Cellular pharmacology of 2′,3′-didehydro-2′,3′-dideoxythymidine (D4T) in human peripheral blood mononuclear cells. Biochem Pharmacol. 1990;39:R15–R19. doi: 10.1016/0006-2952(90)90418-k. [DOI] [PubMed] [Google Scholar]