Abstract
Background:
This survey was designed to study the molecular epidemiology and risk factors of Entamoeba gingivalis and Trichomonas tenax in children with underlying malignancies and those on chemotherapy in Lorestan province, West of Iran.
Methods:
The present cross-sectional descriptive study was performed on children who suffering from different types of malignancies or receiving treatment by chemotherapy referring to oncology section of hospitals of Lorestan Province, Iran during May 2021 to April 2022. The frequency of oral cavity protozoa was investigated using microscopic and conventional polymerase chain reaction (PCR).
Results:
E. gingivalis and T. tenax parasites were found in 23 (25.5%) by microscopic method and 28 (31.1%) using PCR in children with malignancy. Among positive samples, 20 (71.4%) were infected with E. gingivalis; whereas 8 (28.6%) of the participants were positive for T. tenax. In the multivariate model, living in rural regions (OR= 3.437; 95% CI= 1.22–9.63; p=0.019) and using mouthwash (OR= 0.082; 95% CI= 0.018–0.37; p<0.001) were significantly related with the frequency of oral cavity parasites.
Conclusion:
Our results showed the high frequency of oral cavity parasites in children who suffering malignancies or receiving treatment by chemotherapy in Lorestan province, Iran. The awareness of the main risk factors for oral cavity parasites particularly using mouthwash is necessary in improving public and oral health strategies in children with cancer. Consequently, oncologist and dental practitioners must be aware to identify and manage oral health concerns in in children who suffering from different types of malignancies to prevent the oral diseases and infections.
Keywords: Entamoeba gingivalis, Trichomonas tenax, Mouthwash, Malignancy
Introduction
Adults are more likely to develop solid organ cancers, while leukemia and brain tumors are the most common malignancies in children (1). Leukemias and embryonic tumors such as neuroblastoma, Wilms tumor and retinoblastoma and liver tumors are more common in infancy and early childhood, and Hodgkin’s disease and genital malformations and bone tumors are more common in adolescence (2). Today, it has been proven that malignancies, cancers and their drug therapies result in some immune system disorders and predisposes people to many infectious diseases (3). Bacterial, viral, or parasitic infection are observed about 20% of neoplasm patients around the world (4). Chemotherapy and therapeutic radiation for cancer treatment are also associated with some side effects such as immunological disorders (e.g. neutropenia, impaired humoral and cellular immunity) and disrupt the oral mucosa which leading to severe bacterial, viral, and parasitic infections (5, 6).
The human oral cavity contains numerous microorganisms so that a set of different bacterial and viral agents, like the oral microbiome, live inside the gum plaques (7). Entamoeba gingivalis and Trichomonas tenax are considered as anaerobic protozoan parasites observed in human oral cavity (8). Since, these parasites have no cyst forms; thus, they can transmit between individuals, generally through saliva, kissing or contaminated dishes, food, and drinking water, toothpicks, gum or other utensils (8, 9). Both E. gingivalis and T. tenax are oral protozoa that live near teeth, on dental plaque, gums and even tonsils, which can be an indicator of oral health status (10). T. tenax could enter the respiratory tract through respiration and cause pulmonary trichomoniasis (11). In addition, investigations revealed that E. gingivalis might be observed in the progress of periodontal disease, osteomyelitis as well as infections caused by the intrauterine contraceptive device (12).
Considering the prevalence of these parasites, although several studies have been conducted in children with in people with periodontitis and gingivitis, pregnant women, patients undergoing chemotherapy (13–15); nevertheless, the frequency of these oral protozoa and their related risk factors in pediatric oncology children has not been studied. Hence, we aimed to study the molecular epidemiology and risk factors of T. tenax and E. gingivalis in children with underlying malignancies and those on chemotherapy in Lorestan Province, West of Iran.
Materials and Methods
Ethical statement
This work was reviewed and approved by Ethical Committee of Lorestan University of Medical Sciences, Iran (IR.LUMS.REC.1400.317). However, a written informed consent form was obtained from the parents of all participant.
Participants
The present cross-sectional descriptive study was performed on children who suffering from different types of malignancies or receiving treatment by chemotherapy referring to Oncology Section of hospitals of Lorestan Province, Iran during May 2021 to April 2022. Participants who had taken systemic antibiotics in the last three month and immunocompromised patients were discarded.
Questionnaire
Before sampling, a provided questionnaire with a number of demographical information and related risk factors, e.g. age, gender, residence, parent education, toothbrush, mouthwash, was completed for each patient.
Sample collection
Two specimens were obtained from each patient by means of sterile swabs from saliva and dental plaques for microscopic examinations. In addition, the third sample was put into a tube with sterile physiological saline following growth of the trophozoite in the culture for molecular tests.
Microscopic examination
The saliva and dental plaque specimens were smeared on a glass slide, and after staining by Giemsa and trichrome staining technique, they were examined by means of a light microscope (16).
Polymerase Chain Reaction (PCR) assay
Qiagen kits was used to extract DNA from all specimens based on the protocol of producer. The extracted DNA was applied to amplify of the SrRNA gene for E. gingivalis suing the primers of forward (5′-GCGCATTTCGAACAGGAATGTAGA-3′) and reverse (5′- CAAAGCCTTTTCAATAGTATCTTCATTCA- 3′ (17, 18) as well as 18S ribosomal RNA gene for T. tenax using the primers of forward (5′-ATGACCAGTTCCATCGATGCCATTC-3′) and reverse (5′-CTCCAAAGATTCTGCCACTAACAAG -3′) according the previous study (13). The thermal condition was 6 min at 93 °C for early denaturation, 35 cycles of 30 sec at 93 °C, 30 sec at 57 °C, 60 sec at 72 °C, and a last extension phase of 10 min at 72 °C. Then the obtained amplicons using agarose gel (1%) were electrophoresed. The size of the PCR bands for the E. gingivalis and T. tenax was 454 and 496 bp, respectively. Positive and negative controls were DNA of standard strains and distilled water, respectively (17, 18).
Statistical analysis
The analysis of the collected data was performed using SPSS software version 25.0 (IBM Corp., Armonk, NY, USA). Chi-square-test, Fisher exact, univariate and multivariate regression analysis tests were applied to assess the relationship among the variables and the frequency of oral cavity protozoan parasites (E. gingivalis and T. tenax).
Results
Participants
Ninety pediatric oncology children with the mean age of the 9.23 ± 5.2 years were recruited in this study. The most of participants were male participants (48, 53.3%); while the most of participants lived in urban regions (54, 60.0%) and the rest lived in rural region. By age, most of participants 57 (63.3%) were lower than 10 years old. By parent education, 75 (83.3%) of parents had higher education than diploma, while 15 (16.6%) participants had lower education than diploma. By brushing teeth, 52 (57.8%) children brushed their teeth daily. Furthermore, mouthwash was used by just 32 (35.5%) of participants, respectively (Table 1).
Table 1:
Frequency of oral cavity protozoa (E. gingivalis and T. tenax) in pediatric oncology children from Lorestan province, western Iran according to the demographic features and related risk factors by univariate regression analysis
| Group | Totally | Oral cavity parasites | P value Chi-Square | Crude OR | 95%CI | P value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. (%) | Positive No. (%) | Negative No. (%) | ||||||
| Age (yr) | <10 | 57 (63.3) | 20 (35.1) | 37 (64.9) | 0.349 | 1 | 1 | - |
| ≥10 | 33 (36.6) | 8 (24.8) | 25 (75.2) | - | 0.592 | 0.22–1.55 | 0.287 | |
| Parent education ≥diploma | <diploma | 15 (16.6) | 4 (26.7) | 11 (73.3) | - | 1 | 1 | - |
| 75 (83.3) | 24 (32.0) | 51 (68.0) | 0.769 | 0.773 | 0.22–2.67 | 0.684 | ||
| Residence | Rural | 36 (40.0) | 6 (16.7) | 30 (83.3) | - | 1 | 1 | - |
| Urban | 54 (60.0) | 22 (40.7) | 32 (59.3) | 0.02 | 3.437 | 1.22–9.63 | 0.019* | |
| Gender | Male | 48 (53.3) | 14 (29.2) | 34 (70.8) | - | - | - | - |
| Female | 42 (46.7) | 14 (33.3) | 28 (66.7) | 0.820 | 1.214 | 0.49–2.98 | 0.670 | |
| Brushing | Yes | 52 (57.7) | 13 (25.0) | 39 (75.0) | - | - | - | - |
| No | 38 (42.3) | 15 (39.5) | 23 (60.5) | 0.170 | 0.511 | 0.20–1.26 | 0.146 | |
| Mouthwash | Yes | 32 (35.6) | 2 (6.3) | 30 (93.8) | - | - | - | - |
| No | 58 (64.4) | 26 (44.8) | 32 (55.2) | <0.001 | 0.082 | 0.018–0.37 | <0.001* | |
Prevalence of oral cavity protozoan parasites
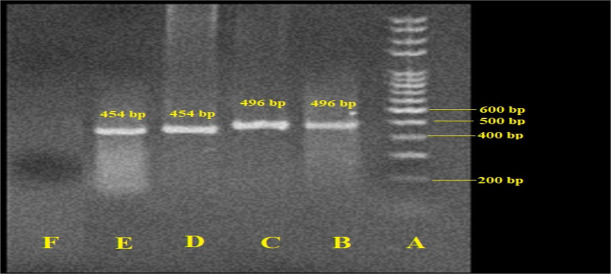
E. gingivalis and T. tenax parasites were found in 23 (25.5%) by microscopic method and 28 (31.1%) using PCR in children with malignancy. Among positive samples, 20 (71.4%) were infected with E. gingivalis; whereas 8 (28.6%) of the participants were positive for T. tenax. Figure 1 shows the gel electrophoresis of the typical E. gingivalis and T. tenax isolates obtained from children with malignancy.
Fig. 1:
Agarose gel electrophoresis of SrRNA and 18s rRNA genes for Entamoeba gingivalis and Trichomonas tenax, respectively. A: Ladder (size marker), 100 bp; B: positive control (T. tenax, 496 bp); C: positive sample of T. tenax; D: positive control (E. gingivalis, 454 bp); E: positive sample of E. gingivalis; E: negative control (distilled water)
Risk factors
The findings of the chi-square analysis showed, there was no significant relationship between age (P=0.349), gender (P=0.670), parent education (P=0.769), brushing teeth (P=0.170) and prevalence of oral protozoa in pediatric oncology children. However, a significant correlation was observed between living in rural regions (P=0.02), use of mouth-wash (P=0.784) and prevalence of oral protozoa in pediatric oncology children. Table 2 shows the prevalence of oral cavity protozoa in pediatric oncology children in Lorestan Province, western Iran according to the demographic features and related risk factors. In the multivariate model, living in rural regions (P=0.022) and brushing teeth (P=<0.001) were significantly related with the frequency of oral cavity parasites.
Table 2:
Comparison of oral cavity protozoa (E. gingivalis and T. tenax) in pediatric oncology children from Lorestan province, western Iran based on the associated risk factors by multivariate regression analysis
| Group | Crude OR | 95%CI | P value |
|---|---|---|---|
| Age(yr) | 1.503 | 0.467–4.837 | 0.494 |
| Parent education | 2.183 | 0.490–9.721 | 0.306 |
| Residence | 0.349 | 0.076–0.816 | 0.022* |
| Gender | 1.261 | 0.414–3.842 | 0.684 |
| Brushing | 1.699 | 0.587–4.918 | 0.328 |
| Mouthwash | 13.01 | 2.67–63.34 | <0.001* |
* P<0.05, difference was statistically significant
Discussion
Pediatric cancer as a stressful event can jeopardize various aspects of the physical, mental and social health of the child and family (19). Prevalence of childhood cancers accounts for 0.5–6.6% of all cancers, and in Iran, about 2.5% of children have cancer (20). This debilitating and common disease is the second leading cause of death of children aged 1–14 years in developed countries and is currently developing; whereas the overall incidence of cancer among Iranian children for girls and boys is reported to be 112-48 and 144-51, respectively, per one million people (21). The most common cancers in children are blood cancers, followed by cancers of the lymph nodes and central nervous system (20, 21). In recent years, due to advances in the treatment of various cancers, the survival rate in children with cancer has increased, and this has led to an increase in the need for parental care at home and at the time of your illness (22).
Since cancer and its therapeutic approaches such as chemotherapy and radiotherapy can lead to some side effects such as immunological disorders (e.g. neutropenia, impaired humoral and cellular immunity) and disrupt the oral mucosa which leading to severe bacterial, viral, and parasitic infections; therefore, oral care in cancer patients should be given special attention (5, 6).
Selecting the appropriate diagnostic method is definitely a very important step in epidemiological studies. Detection of T. tenax and E. gingivalis microorganisms has previously been based on conventional methods such as direct microscopic observation and culture, which, although very rapid, do not produce very reliable results (23). In contrast, today, with the development of molecular methods, PCR is used to identify which has a very high sensitivity and specificity and can determine the presence or absence of microorganisms in a short time and in a small sample (24).
With respect to the prevalence of oral cavity parasites in Iran, in a study conducted on 315 adolescents in Kerman province by culturing and PCR, the findings exhibited that by culture media and PCR the oral cavity parasites was 9.2% and 11.4%, respectively. Whereas, 11.7% and 2.2% of the adolescents were positive E. gingivalis and T. tenax (17). The prevalence of T. tenax in 52 Down syndrome patients with periodontal disease referred to Dental Clinics in Tabriz, Iran was 18.8% by PCR assay (24). In addition, among 240 patients referred to the Dentistry clinics in Tehran, 41.7% and 9.2% of the patients were positive for E. gingivalis and T. tenax, respectively (25). The frequency of E. gingivalis and T. tenax was 0.5% and 0% by microscopic examinations in patients with periodontitis in Khozestan Province, Iran, respectively (26). Considering the prevalence of these parasites in the present studied region, the prevalence rate of E. gingivalis and T. tenax in 76 patients with periodontitis was 17.1% and 14.5%, respectively (16). In a study on 140 patients referred to Khorramabad Dental clinics with at least one decayed tooth, 15.4% and 10.7% were positive by microscopic examinations for E. gingivalis and T. tenax, respectively (27). However, it should be mentioned that the difference between the findings of the current study and previous surveys may related to various factors, e.g. target group, sample size, used diagnostic methods, and lifestyles of studies regions (25, 28).
Our results displayed no significant relationship between age, gender, parent education, brushing teeth and prevalence of oral protozoa in pediatric oncology children. However, in the multivariate model, living in rural regions and brushing teeth were significantly related with the frequency of oral cavity parasites. Similarly, previous studies reported a significant association between mouthwash and the prevalence of E. gingivalis and T. tenax (16, 27). We also found that there was a significant correlation between living in rural regions and the frequency of E. gingivalis and T. tenax. In consistent with these results, it has been exhibited that people living in rural regions poor due to lack of attention to oral health, more frequency of tooth loss and gum diseases was observed (29, 30).
As the main limitation of the present study, the sample size was low, while the use of a larger sample size could lead to better conclusion.
Conclusion
Our results showed the high frequency of oral cavity parasites (E. gingivalis and T. tenax) in children who suffering from different types of malignancies or receiving treatment by chemotherapy in Lorestan province, Western Iran. Our results also showed the awareness of the central risk factors for oral cavity parasites particularly using mouthwash is necessary in improving public and oral health strategies in children with cancer. Consequently, oncologist and dental practitioners must be aware to identify and manage oral health concerns in in children who suffering from different types of malignancies to prevent the oral diseases and infections.
Acknowledgements
The authors thank all the patients and medical staff who contributed to this study.
Footnotes
Funding
None.
Competing interest
The authors declare that they have no competing interest.
References
- 1.Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277–85. [DOI] [PubMed] [Google Scholar]
- 3.Rui L, Schmitz R, Ceribelli M, Staudt LM. Malignant pirates of the immune system. Nature Immunol. 2011;12(10):933–40. [DOI] [PubMed] [Google Scholar]
- 4.Oh JK, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. 2014;80(5):384–92. [DOI] [PubMed] [Google Scholar]
- 5.Olkinuora H, Rahiala J, Anttila VJ, Koskenvuo M, Vettenranta K. Immune deficiency and infections in children having cancer [In Ferench]. Duodecim. 2013;129(12):1233–41. [PubMed] [Google Scholar]
- 6.Mortaz E, Tabarsi P, Mansouri D, et al. Adcock IM. Cancers related to immunodeficiencies: update and perspectives. Front Immunol. 2016;7:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deo PN, Deshmukh R. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23(1):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marty M, Lemaitre M, Kemoun P, Morrier JJ, Monsarrat P. Trichomonas Tenax and Periodontal Diseases: A Concise Review. Parasitology. 2017; 144: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 9.Bonner M, Amard V, Bar-Pinatel C, et al. Detection of the Amoeba Entamoeba gingivalis in Periodontal Pockets. Parasite. 2014;21:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpağ OF, Kaya ÖM. Presence of Trichomonas tenax and Entamoeba gingivalis in peri-implantitis lesions. Quintessence Int. 2020;51(3):212–218. [DOI] [PubMed] [Google Scholar]
- 11.Eslahi AV, Olfatifar M, Abdoli A, et al. The Neglected Role of Trichomonas tenax in Oral Diseases: A Systematic Review and Meta-analysis. Acta Parasitol. 2021;66(3):715–732. [DOI] [PubMed] [Google Scholar]
- 12.Bao X, Wiehe R, Dommisch H, Schaefer AS. Entamoeba gingivalis Causes Oral Inflammation and Tissue Destruction. J Dent Res. 2020;99(5):561–567. [DOI] [PubMed] [Google Scholar]
- 13.Azadbakht K, Baharvand P, Artemes P, Niazi M, Mahmoudvand H. Prevalence and risk factors of oral cavity parasites in pregnant women in Western Iran. Parasite Epidemiol Control. 2022;19:e00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaseen A, Mahafzah A, Dababseh D, et al. Oral Colonization by Entamoeba gingivalis and Trichomonas tenax: A PCR-based study in health, gingivitis, and periodontitis. Front Cell Infect Microbiol. 2021;11:782805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Muathen DM, Yasir LA, Sachit HG. Incidence of Entamoeba gingivalis and Trichomonas tenaxin the oral cavity of periodontal and patients under chemotherapy, confirmed with in vivo study. Indian J Forensic Med Toxicol. 2020;14(3): e00275. [Google Scholar]
- 16.Azadbakht K, Baharvand P, Al-Abodi HR, et al. Molecular epidemiology and associated risk factors of oral cavity parasites in hemodialysis patients in western Iran. J Parasit Dis. 2023;47(1):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharifi M, Jahanimoghadam F, Babaei Z, et al. Prevalence and Associated-Factors for Entamoeba gingivalis in adolescents in Southeastern Iran by culture and PCR, 2017. Iran J Public Health. 2020;49(2):351–59. [PMC free article] [PubMed] [Google Scholar]
- 18.Trim RD, Skinner MA, Farone MB, DuBois JD, Newsome AL. Use of PCR to detect Entamoeba gingivalis in diseased gingival pockets and demonstrate its absence in healthy gingival sites. Parasitol Res. 2011;109:857–864. [DOI] [PubMed] [Google Scholar]
- 19.Kazak AE, Brier M, Alderfer MA, et al. Screening for psychosocial risk in pediatric cancer. Pediatr Blood Cancer. 2012;59(5):822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassanipour S, Fathalipour M, Delam H, et al. The Incidence of Childhood Cancer in Iran: A systematic review and meta-analysis. Iran J Pediat Hematol Oncol. 2019;9(3):193–206. [Google Scholar]
- 21.Hadavand-Siri F, Hassanipour S, Salehiniya H. Epidemiological study of brain cancer in Iran: A systematic review. Adv Human Biol. 2022;12(2):108. [Google Scholar]
- 22.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. [DOI] [PubMed] [Google Scholar]
- 23.Kikuta N, Yamamoto A, Fukura K, Goto N. Specific and sensitive detection of Trichomonas tenax by the polymerase chain reaction. Lett Appl Microbiol. 1997; 24 (3): 193–197. [DOI] [PubMed] [Google Scholar]
- 24.Kashefi Mehr A, Zarandi A, Anush K. Prevalence of oral Trichomonas tenax in periodontal lesions of down syndrome in Tabriz, Iran. J Clin Diagn Res. 2015;9(7):ZC88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharavi MJ, Hekmat S, Ebrahimi A, Jahani MR. Buccal cavity protozoa in patients referred to the faculty of dentistry in Tehran, Iran. Iran J Parasitol. 2006;1(1):43–46. [Google Scholar]
- 26.Maraghi S, Azizi A, Rahdar M, Vazirianzadeh B. A study on the frequency of buccal cavity protozoa in patients with periodontitis and gingivitis in Ahvaz, southwest of Iran in 2009. Jundishapur J Health Sci. 2012;4(4):85–89. [Google Scholar]
- 27.Mahmoudvand H, Sepahvand A, Niazi M, Momeninejad N, Sepahvand SM, Behzadian M. Prevalence and risk factors of oral cavity protozoa (Entamoeba gingivalis and Trichomonas tenax) among patients with dental cavity caries. J Res Med Dent Sci. 2018;6(5):42–46. [Google Scholar]
- 28.Özçelık SE, Gedık T, Gedık R, Malatyali E. Investigation of the relationship between oral and dental health and presence of Entamoeba gingivalis and Trichomonas tenax. Turkiye Parazitolojii Dergisi. 2010;34(4):155–159. [DOI] [PubMed] [Google Scholar]
- 29.Zander A, Sivaneswaran S, Skinner J, Byun R, Jalaludin B. Risk factors for dental caries in small rural and regional Australian communities. Rural Remote Health. 2013;13(3):2492. [PubMed] [Google Scholar]
- 30.Chen MY. Misperception of Oral Health among Adults in Rural Areas: A Fundamental but Neglected Issue in Primary Healthcare. Int J Environ Res Public Health. 2018;15(10):2187. [DOI] [PMC free article] [PubMed] [Google Scholar]