Abstract
A randomized, double-blind, clinic-initiated, sequential dose-escalation pilot study was performed to compare the safety and efficacy of single applications of 1, 3, and 5% cidofovir gel with placebo in the treatment of early, lesional, recurrent genital herpes at five Canadian outpatient sites. Ninety-six patients began treatment within 12 h of lesion appearance and were evaluated twice daily until healing of the lesion occurred. Cidofovir gel at all strengths significantly decreased the median time to negative virus culture in a dose-dependent fashion (3.0 days in the placebo group versus 2.2, 1.3, and 1.1 days in the 1, 3, and 5% cidofovir gel treatment groups, respectively; P = 0.02, 0.0001, and 0.0003, respectively). A trend toward a reduction in the median time to complete healing in association with treatment was present, but the differences were not statistically significant (5.0 days in the placebo group versus 4.3, 4.1, and 4.6 days in the 1, 3, and 5% cidofovir gel treatment groups, respectively). Application site reactions occurred in 3, 5, 19, and 22% of the patients in these four groups, respectively. Treatment-associated lesion recrudescence with delayed healing, which is suggestive of local toxicity, was observed in three patients treated with 5% cidofovir gel and one patient treated with 3% cidofovir gel. In summary, single-dose application of cidofovir gel confers a significant antiviral effect on lesions of recurrent genital herpes. Additional studies are warranted to further identify the optimal efficacious dose of cidofovir in association with the maximum gel strength that can be tolerated.
Genital herpes is a sexually transmitted disease characterized by episodic genital lesions and persistent latent infection (7). More than 30 million persons in North America have genital herpes simplex virus (HSV) infection, the incidence of which appears to be rising (3). Although long-term suppressive antiviral therapy has become the standard of care for those patients with frequent recurrences, chronic therapy may not be practical for the majority of infected individuals with recurrent infection who experience only mild symptoms or infrequent recurrences. Currently, three oral antiviral agents, acyclovir, valacyclovir, and famciclovir, have been shown to confer significant effects on the cessation of virus shedding, lesion healing, and symptomatic relief when used episodically early in the course of recurrent genital infection (6, 8, 13). To date, topical antiviral agents such as 5% acyclovir ointment (5), foscarnet cream (9), edoxudine (10), and alpha interferon gel (11) have not provided results comparable to those provided by systemic agents. A topical treatment which attenuates the severity and duration of recurrent episodes in a fashion comparable to those by which systemic agents attenuate the severity and duration of recurrent episodes would add significantly to currently available therapies.
Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxy propyl) cytosine] is an acyclic nucleotide analog that is phosphorylated in cells to the active metabolite cidofovir diphosphate, and this is done independently of viral thymidine kinase (12). Cidofovir has a broad antiviral spectrum, including activity against HSV type 1 (HSV-1) and HSV-2, varicella-zoster virus, Epstein-Barr virus, cytomegalovirus, human herpesviruses 6 and 8, human papillomavirus, polyomavirus, and poxviruses (12). An intravenous formulation (Vistide) is approved for the treatment of cytomegalovirus retinitis in AIDS patients on the basis of three controlled clinical trials (12). The prolonged intracellular half-lives of the active metabolites (up to 65 h) permit systemic dosing at intervals of every 1 to 2 weeks and suggest that the administration of single topical doses might be effective for the treatment of cutaneous HSV infections.
In animal models of genital HSV infection, successful outcomes have been observed in guinea pigs that have been inoculated with HSV-2 and that have received single-dose therapy (1). Additionally, cidofovir gel had significant antiviral and clinical efficacies compared with those of a placebo in the treatment of acyclovir-resistant HSV infection in patients with AIDS (4). We report here the results of a randomized, double-blind, placebo-controlled, dose-escalation pilot study designed to determine whether antiviral and/or clinical benefits of single-dose cidofovir gel could be achieved in immunocompetent patients treated early in the course of lesional recurrent genital herpes.
MATERIALS AND METHODS
Patient population.
Patients at five Canadian centers were enrolled in the study between May 1995 and June 1996. All subjects were older than 18 years, had a history of genital herpes previously confirmed by culture or serology, and had recurrent anogenital herpes lesions that were less than 12 h in duration and that had not progressed beyond the ulcer stage. Subjects were in good general health and were willing to abstain from sexual contact for the duration of the study. All patients provided written, informed consent, as approved by the ethical review board at each center. Excluded patients were those who had received antiviral therapy in the preceding 2 weeks or immunomodulator therapy within 30 days, had a history of immunodeficiency or other conditions which could interfere with study assessments, or were pregnant or breast-feeding.
Study design.
Eligible patients were stratified by gender and randomized in a 2:1 ratio to receive a single dose of either cidofovir gel or matching placebo. Sequential cohorts received 1, 3, or 5% cidofovir gel strengths. Active and placebo gels were supplied by Gilead Sciences Inc. and were formulated in propylene glycol, hydroxyethylcellulose, methylparaben, propylparaben, and disodium EDTA, with the pH adjusted to 7.0. Samples of all visible genital lesions were obtained for culture prior to treatment. Active or placebo gel was administered by study personnel and was permitted to dry before clothing was replaced. The gel was applied in a thin layer to cover all visible lesions and extend beyond the edge of each by a margin of 5 mm.
New lesions appearing subsequent to day 1 were not treated, nor were any original lesions retreated. Patients returned twice daily at 6- to 16-h intervals for lesion examination and virus culture until complete healing (i.e., reepithelialization) had occurred. At each visit, the following were collected: data on symptom(s) (itching, pain or burning, tingling, and swab-associated tenderness), data on lesional stage (edema or papule, vesicle or pustule, ulcer, soft crust, hard crust), lesion swab for HSV culture, data on the incidence of new lesions within the treatment area, and data on adverse events. The times at which all observations were made were recorded. Lesion staging and culture techniques were standardized across study sites by means of investigator training, color illustrations, and photographs provided by Viridae Clinical Sciences, Inc. Complete blood count, serum chemistries, and urinalyses were performed on day 1 (predosing) and day 5.
Prior to dose escalation, adverse event data for the current group were assessed in a blinded fashion. Advancement to the next dosing cohort required that fewer than 30% of patients within a given stratum experienced dose-limiting toxicity. No patient was reenrolled into subsequent dosing cohorts.
Virology.
African green monkey kidney cells were provided by Connaught Laboratories (North York, Ontario, Canada) or Viromed Laboratories (Minneapolis, Minn.). HSV cultures were performed at each local laboratory with standardized Dacron swabs, transport medium, and culture method. Lesions at the hard crust stage were not unroofed for swabbing.
Statistical methods.
The primary efficacy endpoint was time to complete healing of all lesions. Secondary efficacy endpoints included safety, time to conversion to HSV-negative culture, and duration of lesion-associated symptoms.
Efficacy and safety analyses included all randomized patients and were performed by an intent-to-treat method comparing treated and placebo patients. The study was not powered to detect statistical differences between treatment groups. Patients were censored in time-to-event analyses at the last follow-up without an event. Only patients with a positive culture within 24 h of enrollment were analyzed for time to HSV-negative culture, defined as the time from randomization to the time that the first negative culture without a subsequent positive culture.
Time-to-event comparisons of the treatment groups (e.g., median time to complete healing, median time to cessation of virus shedding, and median duration of symptoms) were performed by the log-rank test (SAS version 6.07; SAS, Cary, N.C.).
RESULTS
Patient characteristics and dispositions.
Ninety-six patients (49 females and 47 males) with a median age of 35 years were randomized into the study as follows: 20 patients to the 1% cidofovir gel, 21 to the 3% cidofovir gel, 23 to the 5% cidofovir gel, and 32 to the combined placebo groups (Table 1). The four treatment groups were comparable with respect to demographics, past herpes history, and baseline characteristics. Ninety-four percent of patients were Caucasian. Over the preceding 6 months, the subjects had a median number of three recurrences of HSV that lasted a median of 6 days and at study entry had one to three lesions with a median duration of 7 h prior to application of the study drug. Eighty-one percent had at least one positive virus culture during the study, of which all but one was HSV-2.
TABLE 1.
Patient demography and disease characteristics
| Characteristic | Placebo group | 1% cidofovir group | 3% cidofovir group | 5% cidofovir group | All patients |
|---|---|---|---|---|---|
| Total no. of patients | 32 | 20 | 21 | 23 | 96 |
| Gender (no. [%]) | |||||
| Female | 14 (44) | 11 (55) | 11 (52) | 13 (57) | 49 (51) |
| Male | 18 (56) | 9 (45) | 10 (48) | 10 (43) | 47 (49) |
| Age (yr)a | 33 (23–51) | 37 (22–58) | 33 (21–52) | 37 (23–66) | 35 (21–66) |
| Duration of herpes (yr)a | 3 (0–19) | 7 (0–34) | 6 (0–20) | 4 (0–20) | 4 (0–34) |
| No. of recurrences in past 6 moa | 3 (0–12) | 3 (0–12) | 4 (0–20) | 3 (0–12) | 3 (0–20) |
| No. of lesions at entry (range) | 1–2 | 1–2 | 1–3 | 1–2 | 1–3 |
| Lesion stage at entry (no. [%] of patients) | |||||
| Papule or edema | 28 (88) | 19 (95) | 19 (90) | 18 (78) | 84 (88) |
| Vesicle or pustule | 24 (75) | 13 (65) | 15 (71) | 14 (61) | 66 (69) |
| Ulcer | 12 (38) | 9 (45) | 4 (19) | 10 (43) | 35 (36) |
| Time (h) from lesion onset to drug application (range) | 8 (2–13) | 5 (2–12) | 8 (2–12) | 6 (1–13) | 7 (1–13) |
Median.
One patient was lost to follow-up after day 1, and three patients were withdrawn from study observation because of treatment-related ulceration (3% cidofovir, one patient; 5% cidofovir, two patients). Overall, compliance with the twice-daily clinic visit schedule was excellent: of 1,096 potential clinic visits, 1,012 (92%) were completed. Seventy-six patients (79%) did not miss a visit.
Lesion healing.
The median time to complete healing was shorter across all cidofovir groups compared with that across all placebo groups, reaching statistical significance only for women receiving 3% cidofovir (P = 0.04; Table 2). Time to complete healing appeared to be dose dependent, with a suggestion of faster healing with 1 and 3% cidofovir compared with that with placebo. Recrudescence of ulceration was observed in two men receiving 5% cidofovir, leading to a lessened improvement in healing time relative to those for subjects in the 1% and 3% cidofovir groups. New lesions in the original treatment area appeared only in patients receiving 3 or 5% cidofovir gel and appeared in a total of six patients (one woman and five men). No patient in the placebo or 1% cidofovir gel groups developed new lesions within the original treatment area.
TABLE 2.
Lesion healing and virologic response
| Group | No. of patients | Median time to complete healing (95% CIa; P value)
|
Median time (days) to virus shedding cessation (95% CI; P value)
|
||||
|---|---|---|---|---|---|---|---|
| All patients | Females | Males | All patients | Females | Males | ||
| Placebo | 32 | 5.0 (4–6) | 5.3 (4–7) | 4.9 (4–6) | 3.0 (2–4) | 2.6 (2–4) | 3.2 (2–4) |
| 1% cidofovir | 20 | 4.3 (3–5; NSb) | 4.3 (4–6; NS) | 5.0 (3–6; NS) | 2.2 (1–3; 0.02) | 1.7 (1–2; NS) | 2.9 (1–3; NS) |
| 3% cidofovir | 21 | 4.1 (4–5; NS) | 3.8 (3–5; 0.04) | 4.4 (4–6; NS) | 1.3 (1–2; 0.0001) | 1.3 (1–2; 0.004) | 1.4 (1–2; 0.01) |
| 5% cidofovir | 23 | 4.6 (4–7; NS) | 4.0 (4–7; NS) | 6 (4–NRc; NS) | 1.1 (1–2; 0.0007) | 1.1 (1–2; 0.02) | 1.1 (1–2; 0.01) |
| Combined cidofovir | 64 | 4.2 (4–5; NS) | 3.9 (4–5; NS) | 4.7 (4–6; NS) | 1.3 (1–2; 0.0001) | 1.3 (1–2; 0.007) | 1.4 (1–2; 0.003) |
CI, confidence interval.
NS, not significant.
NR, not reached.
Virus shedding.
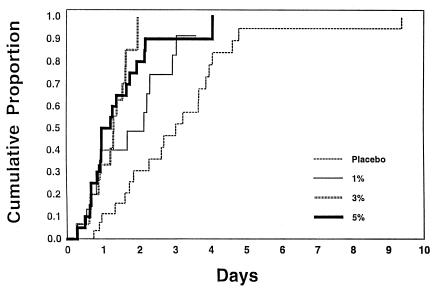
The median time to conversion to negative HSV culture was 3.0 days for patients receiving placebo, whereas they were 2.2, 1.3, and 1.1 days for patients receiving 1, 3, and 5% cidofovir, respectively (P = 0.02, 0.0001, and 0.0003, respectively) (Table 2 and Fig. 1).
FIG. 1.
Cumulative proportions of patients with cessation of virus shedding.
Lesion symptoms.
The median duration of all symptoms was 2.9 days for patients receiving placebo, whereas they were 2.3, 2.4, and 3.5 days for patients receiving 1, 3, and 5% gel, respectively (P was not significant for all comparisons). In general, men had longer median durations of symptoms (2.9 to 3.6 days) than women (2.0 to 4.0 days).
Adverse events.
Fifty-five percent of all patients reported at least one adverse event or intercurrent illness during the study period. The most frequent systemic adverse events included headache (11% of patients treated with cidofovir versus 13% of patients treated with placebo), pharyngitis (11 versus 6%), and nausea (6 versus 6%). There were no laboratory findings suggestive of systemic toxicity in any patient during the study.
Application site reactions consisted of pain, pruritus, skin changes, or ulceration and appeared to be dose dependent (Table 3). Application site reactions occurred in one patient receiving placebo (3%), one patient receiving 1% cidofovir (5%), four patients receiving 3% cidofovir (19%), and five patients receiving 5% cidofovir (22%).
TABLE 3.
Application site reactions
| Reaction | No. (%) of patients
|
||||
|---|---|---|---|---|---|
| Placebo (n = 32a) | 1% cidofovir (n = 20) | 3% cidofovir (n = 21) | 5% cidofovir (n = 23) | Combined cidofovir (n = 64) | |
| Any application site reaction | 1 (3) | 1 (5) | 4 (19) | 5 (22) | 10 (16) |
| Dry skin | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 1 (2) |
| Pain | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 1 (2) |
| Paresthesia | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (2) |
| Pruritus | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (2) |
| Rash | 1 (3) | 0 (0) | 1 (5) | 2 (9) | 3 (5) |
| Skin discoloration | 0 (0) | 0 (0) | 1 (5) | 2 (9) | 3 (5) |
| Skin disorder | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 1 (2) |
| Skin hypertrophy | 1 (3) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Ulcer | 0 (0) | 0 (0) | 1 (5) | 3 (13) | 4 (6) |
Total number of patients in the group.
The ability to distinguish local toxicity (e.g., ulceration) from the natural course of genital herpes complicated the ability to understand drug-related effects. The demonstration of negative virus cultures and the delayed time to healing were suggestive of local toxicity. For example, a single ulcer observed in a man receiving 3% cidofovir was small and short-lived; it was originally thought to represent a new herpes lesion but was culture negative for virus. Genital ulcers in three men receiving 5% cidofovir were larger, lasted for several weeks, and were also culture negative for virus. Each of these men experienced a similar course, consisting of initial healing of the original herpes lesion(s), followed by an apparent relapse (i.e., renewed ulceration, pruritus, and/or erythema) of mild to moderate severity 3 to 7 days later. Two patients were empirically started on oral acyclovir, and two patients were also treated with topical corticosteroids, without benefit.
DISCUSSION
The pilot study described here demonstrates that a single topical dose of cidofovir gel applied in a clinic within 12 h of the onset of a recurrent genital herpes outbreak significantly decreases the time to cessation of virus shedding in a dose-dependent manner compared with that after application of a placebo. Additionally observed was a trend toward faster healing of herpes lesions in cidofovir-treated versus placebo-treated patients, although this did not reach statistical significance. The relationship of application site reactions following the use of different doses of the study drug suggests that the optimal dose of cidofovir gel for this indication may be 1%.
Previous studies of topical treatments for genital herpes have yielded mixed results. Trials of topical acyclovir and foscarnet cream did not show a clinical benefit compared with that from placebo (5, 9). A study of topical alpha interferon demonstrated both antiviral and lesion healing effects, particularly in men (11); a 5-day application of topical edoxudine showed significant antiviral effects without improvement of lesion healing (10). Cidofovir gel had significant antiviral effects in both men and women after a single application in the current study, and within the limits of the small sample size, a trend toward improvement in clinical parameters was suggested as well. An efficacious, well-tolerated topical preparation requiring infrequent administration would represent a convenient site-directed alternative for patients.
A comparison of the antiviral effects of topical cidofovir with those of oral antiviral agents for the treatment of recurrent genital herpes is encouraging. In previous studies, the median times to cessation of virus shedding were reduced from 3.3 days to 1.3 to 1.7 days by twice-daily oral famciclovir treatment for 5 days (8) and from 4.0 to 2.0 days following once-daily oral valacyclovir therapy for 5 days (13). In comparison, single-dose treatment with cidofovir gel reduced the median time to cessation of virus shedding from 3.0 days to 1.1 to 2.2 days. Potential differences in patient populations as well as the noncontemporaneous nature of these studies prevent a true comparison; additionally, a local effect of topical agents may render virus cultures more difficult to perform during clinical trials and, thus, makes a comparison with clinical trials of systemic agents potentially misleading. However, the results of our pilot study suggest that larger future trials with topical cidofovir are warranted to further assess antiviral and clinical efficacies.
The unique single-dose format used in the trial described here is different from the format with repeated doses of cidofovir gel used for the treatment of acyclovir-resistant herpes simplex virus (4) or for the treatment of human papillomavirus infection in immunocompromised hosts (2). The lack of relapse of virus shedding following a single application of gel suggests that cellular uptake and the half-lives of cidofovir metabolites were adequate for prolonged suppression of replication, thus providing further support for the role of intracellular inhibition of viral DNA polymerase as the key determinant of a clinical antiviral effect. It is not clear whether lower strengths of cidofovir gel used more frequently would retain efficacy while limiting local adverse events.
These findings support the further development of cidofovir gel as a therapy for recurrent genital herpes in the immunocompetent host, and they also highlight the need to identify a uniformly well-tolerated dose that will achieve optimal antiviral and clinical effects.
ACKNOWLEDGMENTS
This study was supported by a grant from Gilead Sciences, Inc.
We thank Denise Galipeau for assistance with the preparation of the manuscript, Shiao-ping Lu and Henry Liu for statistical analyses, Bruce Rennie for on-site teaching of virology methods, and the following clinical research personnel: Chantal Bergeron, Pat Coutts, Louise Gosselin, Rashieda Gluck, Lyne Lapointe, Lianne Martin, Sharon Roberts, and Joseph J. Sasadeusz.
REFERENCES
- 1.De Clerq E, Holy A. Efficacy of (S)-2-(3-hydroxy-2-phosphonylmethoxy propyl)cytosine in various models of herpes simplex virus infection in mice. Antimicrob Agents Chemother. 1991;35:701–706. doi: 10.1128/aac.35.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas J, Corey L, Tyring S, Kriesel J, Bowden B, Crosby D, Berger T, Conant M, McGuire B, Jaffe H S. Program and abstracts of the 4th Conference on Retroviruses and Opportunistic Infections. 1997. A phase I/II study of cidofovir topical gel for refractory condyloma acuminatum in patients with HIV infection, abstr. 334. [Google Scholar]
- 3.Fleming D T, McQuillan G M, Johnson R E, Nahmias A J, Aral S O, Lee F K, St. Louis M E. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 4.Lalezari J, Schacker T, Feinberg J, Gathe J, Lee S, Cheung T, Kramer F, Kessler H, Corey L, Drew W L, Boggs J, McGuire B, Jaffe H S, Safrin S. A randomized, double-blind, placebo-controlled trial of cidofovir gel for the treatment of acyclovir-unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. J Infect Dis. 1997;176:892–898. doi: 10.1086/516542. [DOI] [PubMed] [Google Scholar]
- 5.Reichman R, Badger G J, Guinan M E, Nahmias A J, Keeney R E, Davis L G, Ashikaga T, Dolin R. Topically administered acyclovir in the treatment of recurrent herpes simplex genitalis: a controlled trial. J Infect Dis. 1983;147:336–340. doi: 10.1093/infdis/147.2.336. [DOI] [PubMed] [Google Scholar]
- 6.Reichman R C, Badger G J, Mertz G J, Corey L, Richman D D, Connor J D, Redfield D, Savoia M C, Oxman M N, Bryson Y. Treatment of recurrent genital herpes simplex infections with oral acyclovir. JAMA. 1984;251:2103–2107. [PubMed] [Google Scholar]
- 7.Sacks S L. Genital herpes simplex virus infection and treatment. In: Sacks S L, Straus S E, Whitley R A, Griffiths P A, editors. Clinical management of herpes virus infections. Amsterdam, The Netherlands: IOS Press; 1995. pp. 55–74. [Google Scholar]
- 8.Sacks S L, Aoki F Y, Diaz-Mitoma F, Sellors J, Shafran S D for the Canadian Famciclovir Study Group. Patient-initiated, twice-daily oral famciclovir for early recurrent genital herpes. JAMA. 1996;276:44–49. [PubMed] [Google Scholar]
- 9.Sacks S L, Portony J, Lawee D, Schlech III W I, Aoki F Y, Tyrrell D L, Poisson M, Bright C, Kaluski J the Canadian Cooperative Study Group. Clinical course of recurrent genital herpes and treatment with foscarnet cream: results of a Canadian multicenter trial. J Infect Dis. 1987;155:178–186. doi: 10.1093/infdis/155.2.178. [DOI] [PubMed] [Google Scholar]
- 10.Sacks S L, Tyrrell L D, Lawee D, Schlech III W, Gill M J, Aoki F Y, Martel A Y, Singer J the Canadian Cooperative Study Group. Randomized, double-blind, placebo-controlled, clinic-initiated, Canadian multicenter trial of topical edoxudine 3.0% cream in the treatment of recurrent genital herpes. J Infect Dis. 1991;164:665–672. doi: 10.1093/infdis/164.4.665. [DOI] [PubMed] [Google Scholar]
- 11.Sacks S L, Varner T L, Davies K S, Rekart M L, Stiver H G, Delong E R, Sellers P W. Randomized, double-blind, placebo-controlled, patient-initiated study of topical high- and low-dose interferon-alpha with nonoxynol-9 in the treatment of recurrent genital herpes. J Infect Dis. 1990;161:692–698. doi: 10.1093/infdis/161.4.692. [DOI] [PubMed] [Google Scholar]
- 12.Safrin S, Cherrington J, Jaffe H S. Clinical uses of cidofovir. Rev Med Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Spruance S L, Tyring S K, DeGregorio B, Miller C, Beutner K the Valaciclovir HSV Study Group. A large-scale, placebo-controlled, dose-ranging trial of peroral valaciclovir for episodic treatment of recurrent herpes genitalis. Arch Intern Med. 1996;156:1729–1735. [PubMed] [Google Scholar]