Abstract
We propose a simple, intuitive model to progressively explain the principles underlying venous return. At stop-flow, mean circulatory filling pressure (MCFP) can be defined by the filling of the cardiovascular system from internal and external environments, blood vessel compliance, and oncotic pressure. The dynamic distribution of blood within a regular cardiac system and the establishment of central venous pressure (CVP) and mean arterial pressure (MAP) can then be explained by cardiac output and factors controlling distribution. Lastly, control of the cardiovascular system can be explained by changes in resistances and compliances of the blood vessels and their effect on CVP and cardiac output while briefly considering how these are controlled.
Keywords: Cardiovascular, Physiology, Education, Instructional design
Introduction
Systemic venous return (SVR) and cardiac output (CO) have long been recognized as essential concepts for the understanding of the cardiovascular system in a clinical setting [1, 2]. The physiology behind such concepts has been modified over the years, with significant contributions from Arthur S. Guyton in 1955 [3]. In a series of experiments on the hearts of dogs, Guyton was able to manipulate the compensatory activity that quickly changes cardiac response with massive transfusions and quick recordings to collect quantitative data regarding cardiac output. This ultimately led to the isolation of several factors contributing to maintenance and fluctuations of cardiac output, the two most notable being the sympathetic system and filling pressure, measured as right atrial pressure.
Guyton’s venous return curves were a seminal finding derived from a computer model of animal experiments. They are significant for revealing the importance of venous return in preloading the heart as it concluded that the combination of venous-cardiac-function curves predicts cardiac output.
The concept of venous return is a vital component of medical education that applies to various clinical situations, including shock [1, 2]. However, it is often difficult for students encountering cardiovascular physiology for the first time to understand and appreciate the significance of venous return curves [4]. Yet concepts such as mean circulatory filling pressure are still crucial to understanding cardiovascular physiology.
We propose a more conceptual model that focuses on two parameters: (A) factors that control the filling of the cardiovascular system and (B) factors that control the distribution of blood within the circulatory system. The advantages of this model are the comprehensiveness and the integration of concrete concepts essential to a clinician’s education. The design of this model also allows for integrating other cardiovascular system concepts or clinical examples identified by other educators. With our model, we anticipate students will find the idea of venous return easier to grasp and more relatable to clinical situations.
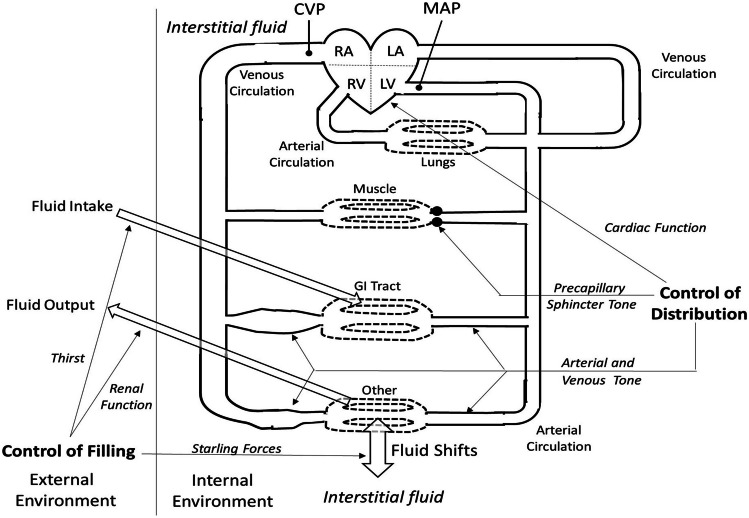
Guyton [3] defined the factors controlling cardiac output in terms of cardiac and venous function curves. Medical physiology texts present these components following discussions of the individual components of the cardiovascular system. The venous function curve is particularly challenging for students to grasp, and it has been suggested that it not be taught [5]. Yet, the mechanisms by which the circulation pre-loads the heart are fundamental to understanding integrated cardiovascular function. Our approach uses a model instead of function curves. To assist in conceptualization, we have divided the factors into those controlling filling of the circulation and those controlling its distribution (Fig. 1).
Fig. 1.
Model for control of venous return. Open arrows represent exchange of fluid between plasma and the external environment (left, the line meant to be a division between the two) and also between plasma and interstitial fluid (bottom). Systemic venous circulation, including venous blood volume reservoirs (represented by dilated areas), enters the right atrium via the right atrioventricular valve. After first transiting the pulmonary circulation, the systemic arterial circuit is pumped from the left ventricle via the aortic valve. Arterial and venous circulations are joined by capillaries. Control of distribution is envisioned as involving the heart (top line on right), pre-capillary sphincters (second line on right), arterioles, and venules (bottom line on right). Control mechanisms are not specified but include neural, hormonal, and metabolic. The pulmonary circuit is only included for completeness
Method
Design of the Model
The extracellular fluid (ECF = plasma plus interstitial fluid (ISF)) is filled or emptied via exchanges between the external and internal environments. These exchanges include regulated (kidneys and gastrointestinal, open arrows on the left side of Fig. 1). There are additional unregulated respiratory, transcutaneous, and perspiratory losses (not shown). ECF is distributed between plasma and ISF by capillaries and lymph, mostly in response to capillary exchange of fluid between plasma and ISF (open arrow on the bottom of Fig. 1).
The Concept of Mean Filling Pressures
Mean circulatory filling pressure (MCFP) in the absence of cardiac output results from elastic recoil of the blood vessels [6]. Here, we propose that oncotic force across capillary walls helps to provide some of the power to create MCFP by distending the elastic blood vessels (capacitance) with additional filling that may result from renal regulation and regulation and regulation of fluid and electrolyte intake. This situation is represented in the upper line in Table 1, where equal but non-zero pressures exist throughout the circulatory system.
Table 1.
Example of effect of cardiac output on central venous pressure (CVP) and mean arterial pressure (MAP)
| Cardiac output (L/min) | Central venous pressure (CVP) | Mean arterial pressure (MAP) |
|---|---|---|
| 0 | 9 mm Hg | 9 mm Hg |
| 5.5 | 1 mm Hg | 95 mm Hg |
In a theoretical situation, if cardiac output goes from 0 to 5.5 L/min (Table 1), the heart pumps blood forward into the elastic arteries, increasing mean arterial pressure (MAP) and decreasing central venous pressure (CVP). A hypothetical average of blood pressure everywhere at this time would be mean systemic filling pressure (MSFP). At this point, we emphasize that a key effect of a positive MCFP/MCSP is to prevent the CVP from dropping so low that the heart will not fill. Furthermore, CVP is an important regulatory factor involved in preloading the heart.
Blood Distribution
Blood distribution is controlled by cardiac output as well as arterial, pre-capillary sphincter, and venous vasomotion. There are neural, hormonal, or local controls of each of these elements [7, 8]. Local factors released in response to oxygen need due to changes in metabolism are especially important in controlling pre-capillary sphincter tone. This latter “metabolic autoregulation” can partially override neural-hormonal controls to match tissue perfusion to tissue needs. Further, widespread autoregulatory sphincter opening — as in exercising muscle — will tend to lower mean arterial pressure (MAP), eliciting increased sympathetic and reduced parasympathetic drive via the baroreceptor reflex. The interplay between these two is seen by the authors as largely responsible for chronic regulation of cardiac output.
Vasomotion, including arterial, metarteriole, and venous constriction as well as per-capillary sphincter tone, can also be controlled by neural, endocrine, and regional factors [7, 9].
The Concept of Filling Pressure
Filling of the extracellular fluid volume (ECF = plasma plus interstitial fluid (ISF)) results from the balance of absorption and excretion of salt and water. Intake is controlled by thirst and salt appetite effects on behavior while excretion is primarily controlled by the net balance of renal filtration plus excretion less reabsorption. Both intake and excretion are regulated by hormones such as angiotensin II and antidiuretic hormone [10, 11] and reflexes elicited by venous, arterial, and intracardiac pressures [12]. Additional losses, mostly unregulated, occur via transcutaneous, respiratory, perspiratory, and/or GI routes.
Transcapillary Balance
Acute distribution of extracellular fluid (ECF) between plasma and interstitial fluid (ISF) is controlled by the balance of forces across the capillary wall. Continuous capillaries make are particularly important because they have pores that largely bar filtration of mid-sized or larger proteins. This effect is amplified by cations retained in the capillary along with the dominantly negatively charged plasma proteins according to the Gibbs-Donnan equilibrium [13], the oncotic pressure. Fluid flows from plasma to ISF when mean capillary pressure is higher than oncotic pressure, and it flows from ISF to plasma when the oncotic pressure is higher. This effect is important for auto-transfusion of fluids in the event of hypovolemia or other acute causes of reduced capillary pressures. Regulation of oncotic pressure is known to occur, but the mechanisms are only partially understood [14].
Vasomotion, Venous Return, and Preload
Arteries are described as resistance because over 70% of the circulatory resistance occurs before the capillary beds. The effects of arterial vasomotion dominate arterial hemodynamics. Veins are described as capacitance vessels because systemic and pulmonary veins contain about 70% of the circulatory blood volume. The venous system contains most of the body’s blood capacity. Consequently, venous return primarily accounts for the Frank-Starling mechanism controlling cardiac output. The output of both ventricles is obligatorily equal in the steady state. This in turn is controlled by the sympathetic nervous system as well as local factors [9, 15]. Constriction of the venous reservoirs (dilated areas in Fig. 1), especially in the gastrointestinal tract, is a key factor in maintaining preload, and therefore cardiac output, by mobilizing stored blood to compensate for the diversion of blood in conditions such as exercise and hemorrhage [16].
One limitation in model is that venous resistance (≈7%, of normal total peripheral resistance) is regarded as negligible. This is true in normal circumstances but in some pathophysiologic conditions, such as heart failure (especially decompensated), venous resistance can be a very significant factor in maintaining cardiac output. That aspect needs to be communicated to students.
Presentation of the Model
In the Fall of 2021–2022 academic year, this integrated model was presented to first-year allopathic medical students enrolled in their 8-week systemic foundations of medicine “B” course, which follows an 8-week (foundations A) cellular-general anatomy-pharmacology course. The B course contains:
-
(A)
Introductory anatomical sciences (anatomy, histology, and embryology), topics covered in detail in systems courses in three following semesters;
-
(B)
Most of the physiology in the curriculum, with systems covering mostly pathophysiology, and;
-
(C)
Introductory hematology autonomic pharmacology, and introductory radiology.
Presentation of the model was spread out over the entire cardiovascular system, as indicated by the asterisks (*) in Table 2.
Table 2.
Cardiovascular section of foundations B course
| Component | Content | Vignette/discussion(s) |
|---|---|---|
| Cardiac | ||
|
Large Group 1 Large Group 2 Large Group 3 PBL Case 1 Clinical Case |
Introduction & Cardiac Mechanics Cardiac Cycle & Pressure–Volume Curves Cardiac Conduction & Electrocardiogram Hypertension resulting in Heart Failure Long Q-T Syndrome |
Myocardial infarction* Pathophysiologic Correlations Pathophysiologic Correlations |
| Circulatory | ||
|
Large Group 3 Large Group 4 PBL Case 2 |
Arterial Circulation & Hemodynamics Venous, Capillary & Lymphatic Circulations Hypertensive Heart Failure |
Isolated Systolic Hypertension* Hypovolemia* |
| Regulatory | ||
|
Large Group 5 Large Group 6 |
Hormonal and Renal Regulation Neural and Autoregulatory Regulation |
Long-Term Cardiac Control* Exercise* |
Large Group 1 (LG 1) started out with presentation of the model to explain the overall circulation and describe the function of its components. The vignette looked at treating myocardial infarction from the standpoint energetic needs and costs. This included pre-hospital abdominal/thoracic compressions (increasing preload*), stenting, and aortic balloon placement (reducing afterload*) as well as positive and negative aspects of inotropes, referring to the model (*) as indicated.
LG 3 vignette used the differential effects of isolated systolic hypertension on systolic versus diastolic blood pressures, with reference to the model, to explain pulse pressure as dependent on both stroke volume and arterial compliance and to explain the dependence of diastolic pressure on the circulatory resistance and time between heart beats.
LG 4 was used to emphasize the importance of maintaining preload in hypovolemia in reference to the model to explain auto-transfusion and the importance of venous constriction elicited by sympathetic stimulation in this situation.
LG 5 was used with the model to describe intermediate-long term control of cardiac output, including the roles of the kidney and the upcoming topic of autoregulation.
LG 6 vignette on exercise was used in conjunction with the model to help students understand the importance of autoregulation and the interplay between autoregulation and nervous regulation via the baroreceptor reflex.
Results
College policies prohibit designing exam items or conducting surveys for educational research purposes. However, we identified two quiz items directly related to this content that were asked in both academic years 2020–2021 and 2021–2022. Question 1 asked which of four maneuvers would most directly increase mean circulatory filling pressure (MCFP). Question 2 asked which of four hemodynamic properties would be most affected by a theoretical veno-selective alpha-adrenergic agonist.
In the 2020–21 quiz, 62.9% of students correctly identified increased venous pressure as most related to increased MCFP. For question 2, 35.6% correctly identified cardiac output as the most likely affected by a veno-selective alpha-adrenergic agonist. After the model had been incorporated, in 2021–22, 73.7% of students chose the correct answer for question 1 and 66.4% chose the correct answer for question 2. Each question had 4 answer choices so 25% might be expected for random guess. Correcting for that, there was approximately 27% improvement in performance on question 1 and 383% improvement in question 2.
However, the authors believe there are cues in choice questions that increase the likelihood of a correct guess and reduce the likelihood of incorrect guess, even for students who do not fully understand the concepts. The lead instructor also uses class discussion vignettes to assess student understanding. The improvement was most evident in the vignette on exercise. In 2020–2021 (and prior years), students viewed the hemodynamic changes during exercise as solely the result of increased sympathetic drive. When the instructor asked why sympathetic drive would simultaneously increase systolic pressure and decrease diastolic pressure, they became confused. Some students referred to sympathetic effects on the heart, the arterial circulation, or both, or even postulated beta-adrenergic vasodilatory effects. After working with the model in 2021–2022, the initial response of some students was the same, but others quickly pointed out the likelihood or exercise-induced metabolic vasodilation.
Later, in the endocrine section of the 2021–2022 course, the instructor facilitated a 2-day problem-based-learning (PBL) case on postpartum hyperthyroidism developing into hypothyroidism in the second session of the PBL case. The 8 students were able to collaboratively identify sympathetic and metabolic effects of thyroid hormone, as well as direct effects of thyroid hormone on the heart.
Discussion
Presentation of integrated cardiovascular function in standard cardiac and venous function curves is usually reserved until the underlying principles have been thoroughly studied. One advantage of this model is that it can be presented early and revisited/expanded as additional underlying physiologic and pathophysiologic principles are studied. It also encourages understanding the cardiovascular as an integrated whole intuitively, free from the struggles some students may have applying formulae or graphical analysis.
The buildable nature of this model, we believe, will help a broader range of students achieve and retain an integrated understanding and thereby better understand how each factor works in the integrated whole. Depending on the student level and program goals, the more quantitative cardiac and venous function curves might then be presented at the conclusion, as they typically are, but hopefully with a better student understanding of their meaning.
We readily acknowledge the limitations of our model. Unlike the cardiac and venous function curves, such a qualitative model cannot quantitatively predict cardiac output. Future studies will provide more complete quantitative data on the impact of this teaching method on students’ understanding on a broader range of topics. Limit of the model in heart failure especially must be addressed during the presentation. Nevertheless, ours and other findings support the increased incorporation of visual and sequential teaching models based on student learning style preferences [8].
Conclusion
We recognize the monumental contributions of Guyton et al. to the field of cardiovascular physiology and attest that the concepts embodied by this model would not be possible without the initial experiments and explanations. However, we challenge the notion that these figures were intended to be used as a teaching tool. Considering this, we believe the integrative and visual nature of the model described in this paper will improve student learning of vital concepts that influence their success academically and as clinical professionals. Future studies intend to prove the success of this model in these spheres.
Acknowledgements
The authors wish to thank Dr. Harold Bell for his helpful comments on this article.
Data Availability
Data on answers are managed by the College of Medicine Office of Medical Education on Top Hat quizzes, for the medical classes entering in 2021 and 2022.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rasha Jawad, Email: jawad1rm@cmich.edu, Email: rasha.jawad@ascension.org.
Richard D. McCabe, Email: mccab1rd@cmich.edu
References
- 1.Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock—part I: physiology. Crit Care Med. 2013;41(1):255–262. doi: 10.1097/CCM.0b013e3182772ab6. [DOI] [PubMed] [Google Scholar]
- 2.Bressack MA, Raffin TA. Importance of venous return, venous resistance, and mean circulatory pressure in the physiology and management of shock. Chest. 1987;92(5):906–912. doi: 10.1378/chest.92.5.906. [DOI] [PubMed] [Google Scholar]
- 3.Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35(1):123–129. doi: 10.1152/physrev.1955.35.1.123. [DOI] [PubMed] [Google Scholar]
- 4.Beard DA, Feigl EO. Understanding Guyton’s venous return curves. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301(3):H629–H633. doi: 10.1152/ajpheart.00228.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard DA, Feigi EO. CrossTalk opposing view: Guyton’s venous return curves should not be taught. J Physiol. 2013;591(23):5795–5797. doi: 10.1113/jphysiol.2013.260034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belz GG. Elastic properties and Windkessel function of the human aorta. Cardiovasc Drugs Ther. 1995;9(1):73–83. doi: 10.1007/BF00877747. [DOI] [PubMed] [Google Scholar]
- 7.Thomas GD. Neural Control of the Circulation. Adv Physiol Educ. 2011;35:29–32. doi: 10.1152/advan.00114.2010. [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Torrano D, Ali S, Chan CK. First year medical students’ learning style preferences and their correlation with performance in different subjects within the medical course. BMC Med Educ. 2017;17(1):1–7. doi: 10.1186/s12909-017-0965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanhoutte PM, Janssens WJ. Local control of venous function. Microvasc Res. 1978;16(2):196–214. doi: 10.1016/0026-2862(78)90055-9. [DOI] [PubMed] [Google Scholar]
- 10.Fitzsimmons JT, Angiotensin, Thirst and Sodium Appetite. Physiol Rev. 1998;78(3):583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 11.Thornton SN. Thirst and hydration: Physiology and consequences of dysfunction. Physio Behav. 2010;100(1):15–21. doi: 10.1016/j.physbeh.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Johnson AK, Thunhorst RL. The Neuroendocrinology, Neurochemistry and Molecular Biology of Thirst and Salt Appetite. In: Lajtha A, Blaustein JD (eds) Handbook of Neurochemistry and Molecular Neurobiology. 2007. Springer, Boston, MA.
- 13.Waniewski J, Pietribiasi M, Pstras L. Calculation of the Gibbs-Donnan factors for multi-ion solutions with non-permeating charge on both sides of a permselective membrane. Sci Rep. 2021;11:22150. doi: 10.1038/s41598-021-00899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levitt D, Levitt MD. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Ing J Gen Med. 2016;9(1):229–255. doi: 10.2147/IJGM.S102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev. 1983;98(4):1281–1342. doi: 10.1152/physrev.1983.63.4.1281. [DOI] [PubMed] [Google Scholar]
- 16.Henderson WR, Griesdale DE, Walley KR, Sheel AWW. Clinical review: Guyton-the role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit Care. 2010;14(6):1–6. doi: 10.1186/cc9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data on answers are managed by the College of Medicine Office of Medical Education on Top Hat quizzes, for the medical classes entering in 2021 and 2022.